- 1 Faculty of Health Sciences, Hokkaido University, Sapporo, Japan
- 2 Department of Physiology, Hokkaido University School of Medicine, Sapporo, Japan
- 3 Clinical Brain Research Laboratory, Department of Neurology, Sapporo Yamanoue Hospital, Sapporo, Japan
Smooth-pursuit eye movements are voluntary responses to small slow-moving objects in the fronto-parallel plane. They evolved in primates, who possess high-acuity foveae, to ensure clear vision about the moving target. The primate frontal cortex contains two smooth-pursuit related areas; the caudal part of the frontal eye fields (FEF) and the supplementary eye fields (SEF). Both areas receive vestibular inputs. We review functional differences between the two areas in smooth-pursuit. Most FEF pursuit neurons signal pursuit parameters such as eye velocity and gaze-velocity, and are involved in canceling the vestibulo-ocular reflex by linear addition of vestibular and smooth-pursuit responses. In contrast, gaze-velocity signals are rarely represented in the SEF. Most FEF pursuit neurons receive neck velocity inputs, while discharge modulation during pursuit and trunk-on-head rotation adds linearly. Linear addition also occurs between neck velocity responses and vestibular responses during head-on-trunk rotation in a task-dependent manner. During cross-axis pursuit–vestibular interactions, vestibular signals effectively initiate predictive pursuit eye movements. Most FEF pursuit neurons discharge during the interaction training after the onset of pursuit eye velocity, making their involvement unlikely in the initial stages of generating predictive pursuit. Comparison of representative signals in the two areas and the results of chemical inactivation during a memory-based smooth-pursuit task indicate they have different roles; the SEF plans smooth-pursuit including working memory of motion–direction, whereas the caudal FEF generates motor commands for pursuit eye movements. Patients with idiopathic Parkinson’s disease were asked to perform this task, since impaired smooth-pursuit and visual working memory deficit during cognitive tasks have been reported in most patients. Preliminary results suggested specific roles of the basal ganglia in memory-based smooth-pursuit.
Introduction
Contrary to the traditional view that vestibular signals are projected to restricted regions of the cerebral cortex, recent studies have shown extensive vestibular–cortical projections in rats, cats, monkeys, and humans (see Goldberg et al., 2011 for review; also de Waele et al., 2001). Vestibular information is necessary for virtually every aspect of our daily life; indispensable for the control of eyes, head, or whole-body through various vestibular reflexes. Vestibular receptors resolve the head motion and its orientation in the Earth’s gravitational field into fundamental components, each of which is conveyed to the brainstem by specific channels from the three semi-circular canals and two otolith organs. These distinct channels provide different pathways for various vestibular reflexes such as the vestibulo-ocular reflex (VOR) and vestibulo-collic reflex. Most of these functions depend on projection of vestibular signals primarily to the brainstem/spinal cord and to the cerebellum. In addition, many cognitive functions rely on vestibular signals for appropriate behavior in three dimensional space such as self-motion perception, spatial perception and memory, visual spatial constancy, and visual object motion perception. Undoubtedly, these functions require projection of vestibular signals to the cerebral cortex.
Second-order vestibular neurons project to the thalamus, which, in turn, sends projections to various regions of the cerebral cortex including two frontal cortical areas involved in controlling eye movements, the caudal part of the frontal eye fields (caudal FEF) in the fundus of the arcuate sulcus and the supplementary eye fields (SEF) in the dorsomedial frontal cortex (Fukushima et al., 2006 for review). Individual vestibular signals must be integrated to reconstruct the head motion and its orientation in space. In addition, precise reconstruction requires exteroceptive inputs especially vision and proprioceptive inputs from the neck. What are the differing functional roles of the vestibular signals represented in various cortical areas? Understanding this difference in roles of the vestibular signals represented in the two areas requires an understanding of the differences in those areas in controlling eye movements.
The caudal FEF and SEF of non-human primates contain smooth-pursuit related neurons (pursuit neurons). Smooth-pursuit eye movements are voluntary movements used to track small objects (target) moving slowly in the fronto-parallel plane. They evolved in primates, who possess high-acuity foveae, to ensure clear vision of the moving target while the animals visually pursue it. Major pathways for smooth-pursuit are schematically summarized in Figure 1A (for reviews, see Lisberger et al., 1987; Leigh and Zee, 2006). Since the foveal projection field covers only about 2° of the visual fields of each eye, accurate pursuit requires precise eye movement control.
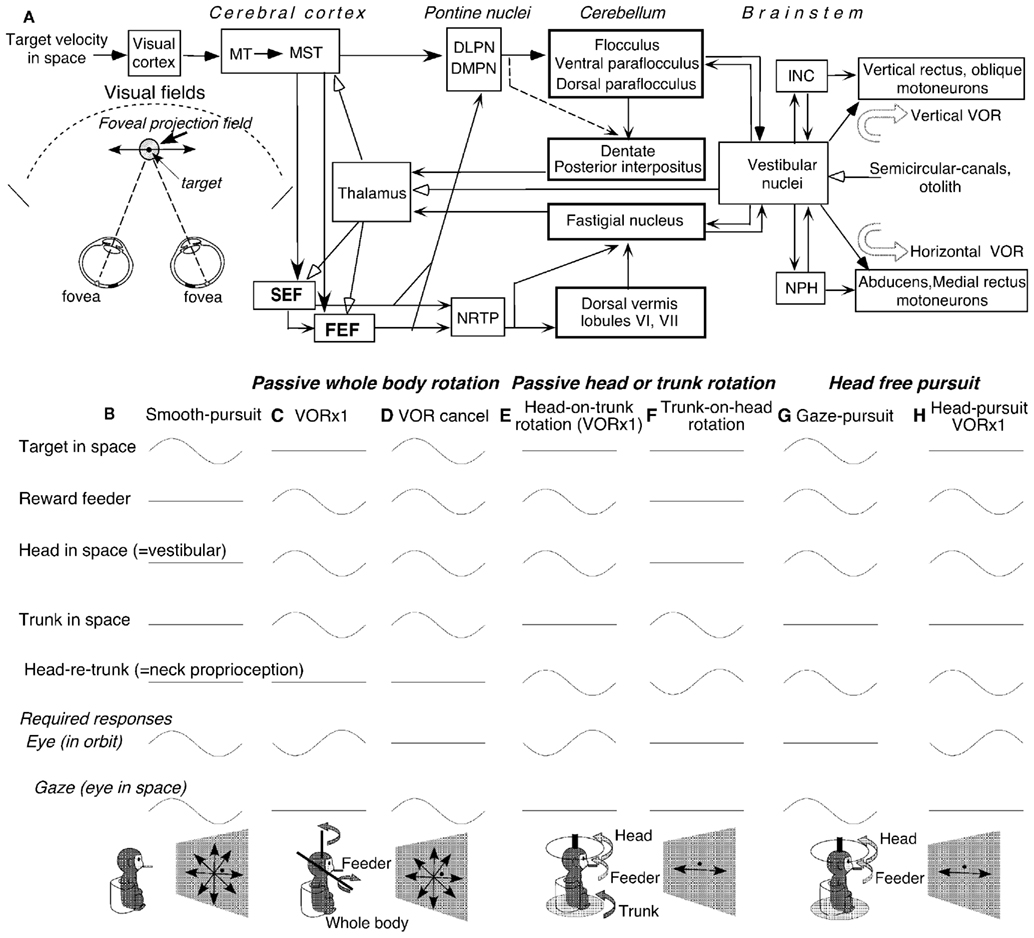
Figure 1. Major pathways related to smooth-pursuit eye movements and behavioral tasks. (A) A schematic top view of visual fields and foveal projection field in three dimensional space, major pathways related to smooth-pursuit eye movements and vestibular inputs. (B–H) Behavioral tasks. Only sinusoidal stimulus condition is shown for simplicity. DLPN, dorsolateral pontine nucleus; DMPN, dorsomedial pontine nucleus; INC, interstitial nucleus of Cajal; MT, middle temporal visual area; MST, medial superior temporal visual area; NRTP, nucleus reticularis tegmenti pontis; NPH, nucleus prepositus hypoglossi. For further explanation, see text. (A) Modified from Fukushima (2003a,b) and Fukushima et al. (2006) with permission. (B–H) Modified from Fukushima et al. (2009, 2010) with permission.
We recently reviewed the roles of vestibular signals in the frontal cortex related to smooth-pursuit (Fukushima et al., 2004b, 2005, 2006). In this article, we first update our recent reviews with additional information obtained from our laboratory on pursuit–vestibular and neck–vestibular interactions during passive and active head rotation (see Vestibular Inputs, Neck Proprioceptive Inputs, Active Head-Pursuit and Discharge of Caudal FEF Pursuit Neurons). Coordinate frames of smooth-pursuit signals will also be reviewed (see Coordinate Frames). We, then, describe the different roles of the caudal FEF and SEF in smooth-pursuit, based on data obtained with a new memory-based smooth-pursuit task (see Memory-Based Smooth-Pursuit). This task was applied to patients with idiopathic Parkinson’s disease (see Preliminary Results of Clinical Application). Preliminary results suggested specific roles of the basal ganglia in memory-based pursuit. Pursuit-in-depth (i.e., vergence-pursuit, Fukushima, 2003a) will not be considered.
A Close Functional Relationship between Smooth-Pursuit and the Vestibular Systems is Essential for Efficient and Accurate Smooth-Pursuit
To understand the roles of vestibular signals in the smooth-pursuit related areas of the cerebral cortex, the conditions required for efficient and accurate smooth-pursuit must first be understood and these will be described in more detail in Section “Memory-Based Smooth-Pursuit.” Here we list what is required for accurate execution of smooth-pursuit in the presence of head motion. The smooth-pursuit system maintains the target within the foveal projection field to maximize tracking accuracy by matching the eye velocity in space (i.e., gaze-velocity) to target velocity (Figure 1A). Efficient and accurate pursuit performance must meet several criteria.
First, any head movement must be compensated during smooth-pursuit. Head rotation is detected by the semi-circular canals and induces the rotational VOR, whereas head-translation is detected by otolith receptors and induces the translational VOR (tVOR). The required magnitude of the translational (e.g., leftward–rightward) VOR depends critically on the target distance and is calculated trigonometrically as the angle between the target distance and the translation distance. If the stationary target is close to the observer (e.g., within arm’s reach), the required magnitude of the tVOR is similar to that of the rotational VOR. However, for distant objects, virtually no compensatory eye movement is required for identical otolith inputs.
Second, if the target moves in space with the observer (i.e., in the same direction with the same magnitude), the VOR must be canceled to keep the target within the foveal projection field (Figure 1A). How this is done is still controversial (see VOR Cancelation: Addition of Vestibular and Smooth-Pursuit Related Signals in the Caudal FEF), but the smooth-pursuit system contributes. Since the required magnitude for VOR cancelation is distance dependent, the pursuit system must adjust the magnitude of the cancelation signal to attenuate or augment the resulting VOR.
Third, vestibular signals alone cannot distinguish dissociated head movements from those associated with trunk or whole-body movement. Inputs from neck proprioceptors help the brain distinguish between the two, emphasizing the need to understand how neck proprioceptive responses and vestibular responses interact (see Neck Proprioceptive Inputs).
Fourth, compared to the short latencies of VOR (∼10 ms), smooth-pursuit have long latencies (∼100 ms) between changes in target motion and the initiation of changes in pursuit eye movements. Prediction must compensate for these delays between processing visual motion and eye velocity commands to maintain the target within the foveal projection field during pursuit (e.g., Barnes, 1993). Prediction is influenced by various factors such as cues and memory of stimulus trajectory (see Higher Functions of the Caudal FEF and SEF). Efficient performance in daily life such as sports requires training.
Fifth, vestibular receptors are unable to respond to constant velocity motion, which in the light must depend on visual inputs. Since visual–vestibular interactions have been extensively reviewed elsewhere (e.g., Leigh and Zee, 2006), they will not be considered in this article.
Thus, processing of vestibular signals requires interactions with visual, neck proprioceptive, and motor command signals appropriate for the desired action. Moreover, within these processes, coordinate transformations are necessary since motion detected by the vestibular end organs and visual information derived from the retina are encoded in different coordinate systems and since final motor output signals are coded in the effector coordinates. Signals in various brain areas are thought to be represented in intermediate coordinates, such as head-centered or body-centered coordinates (see Andersen et al., 1997 for review). Vestibular signals represented in various cortical areas may be used for different aspects of processing vestibular information for each of these separate requirements. To better understand the roles of the caudal FEF and SEF in coordinating smooth-pursuit, it is necessary to examine coordinate frames representing smooth-pursuit signals in these areas (see Coordinate Frames).
The importance of vestibular inputs to the smooth-pursuit system is also demonstrated by the observation that virtually all brain areas known to be related to smooth-pursuit (Figure 1A) also respond to whole-body rotation (see Leigh and Zee, 2006 for review). These areas include the cerebellar floccular region, dorsal vermis, caudal fastigial nucleus, dorsolateral pontine nucleus (DLPN) and nucleus reticularis tegmenti pontis (NRTP), medial superior temporal (MST) cortical area, caudal FEF, and SEF. Discharge induced by translation, which activates otolith receptors, is also observed in neurons in MST (Duffy, 1998) and caudal FEF (Akao et al., 2009).
Smooth-Pursuit Related Frontal Cortical Areas: The Caudal FEF and SEF
Most pursuit neurons in the caudal FEF have a preferred direction and the preferred directions of individual neurons are distributed evenly for all directions (e.g., MacAvoy et al., 1991). For target motion in the preferred direction, most FEF pursuit neurons exhibit discharge modulation that is linearly correlated with peak eye velocity, indicating that FEF pursuit neurons code direction and velocity of pursuit eye movements. About half of FEF pursuit neurons also exhibit visual responses to test-spot motion during fixation of a stationary spot (Fukushima et al., 2000b, 2002a). The preferred direction of visual response is similar to the pursuit-preferred direction for each FEF neuron. These neurons are thought to issue a pursuit command for the following reasons; (1) most of them discharge before pursuit eye movements begin; (2) electrical microstimulation of the caudal FEF induces smooth eye movements, and (3) lesions or chemical inactivation of the caudal FEF impairs smooth-pursuit (Lynch, 1987; Keating, 1991, 1993; MacAvoy et al., 1991; Gottlieb et al., 1993, 1994; Tian and Lynch, 1996; Shi et al., 1998; Tanaka and Fukushima, 1998; Tanaka and Lisberger, 2002; Akao et al., 2005, 2007a; Lynch and Tian, 2006; Fukushima et al., 2011a; Mahaffy and Krauzlis, 2011).
Further support for the notion that the caudal FEF issues pursuit commands was obtained recently in two series of experiments. In the laboratory, all tested monkeys must be trained for extended periods in order to ensure excellent pursuit eye movements. As a result, a pursuit command signal is usually difficult to differentiate from an actual eye velocity signal during pursuit which could come from various sources including eye muscle proprioception (Wang et al., 2007). A critical question of whether caudal FEF pursuit neurons signal actual eye velocity was examined using young monkeys that exhibited intrinsic differences between upward and downward smooth-pursuit capabilities; their upward eye velocity was significantly lower than downward eye velocity (Akao et al., 2007b). Discharge modulation vs. target velocity was similar between downward and upward pursuit neurons, but a clear dissociation was observed between the discharge modulation of upward pursuit neurons and upward eye velocity, indicating that upward FEF pursuit neurons did not signal the actual eye velocity during pursuit (Kurkin et al., 2009). These results suggest that their activity primarily reflected the required eye velocity. The second set of supporting data was obtained using a memory-based smooth-pursuit task (Fukushima et al., 2011a), and will be described in Section “Memory-Based Smooth-Pursuit.” FEF pursuit neurons are multimodal neurons that receive various inputs from sources such as visual, vestibular, and neck proprioceptors (see Vestibular Inputs, Neck Proprioceptive Inputs, Higher Functions of the Caudal FEF and SEF).
Although the SEF also contains pursuit related neurons (Schall, 1991; Heinen, 1995; Heinen and Liu, 1997), their role in pursuit eye movements was largely unrecognized since; (1) electrical microstimulation of the SEF does not induce smooth-pursuit, although it facilitates smooth-pursuit initiation and enhances anticipatory pursuit eye velocity (Missal and Heinen, 2001, 2004). This contrasts with the effect of electrical microstimulation on the saccadic system, which induces saccadic eye movements (e.g., Schlag and Schlag-Rey, 1987). (2) Over half of smooth-pursuit related SEF neurons do not signal eye velocity during pursuit, and most of them do not exhibit visual motion responses to test-spot motion during fixation of another stationary spot (Fukushima et al., 2004a), and (3) SEF lesions have minimal effects on pursuit eye movements (see Tehovnik et al., 2000 for review). Possibly, the SEF does not form part of the postulated smooth-pursuit pathways (e.g., Lisberger, 2010) but is involved in the process of guiding anticipatory pursuit (Leigh and Zee, 2006). Recent studies have revealed different roles of the caudal FEF and SEF in smooth-pursuit (see Memory-Based Smooth-Pursuit).
General Methods to Examine Pursuit–Vestibular and Neck–Vestibular Interactions
Vertical and horizontal components of eye movements were recorded in monkeys (Macaca fuscata) using the scleral search coil method. Extracellular recordings of pursuit related neurons were made in the caudal FEF and SEF while the head-fixed monkeys pursued a moving target (Figure 1B). During passive rotation of the head and/or whole-body (Figures 1C–E), monkeys’ heads were firmly restrained in the primate chair. Monkeys were rewarded for fixating a spot and pursuing it when it moved along various directions (e.g., Fukushima et al., 2000b, 2004a).
Since smooth-pursuit with the head restrained cannot dissociate eye movement in the orbit from eye movement in space (i.e., gaze = eye in orbit + head in space, Figure 1B), two other tasks have routinely been used to distinguish eye movement per se from gaze movement (e.g., Lisberger and Fuchs, 1978). In a VOR ×1 task (Figure 1C), a target stayed stationary in space during passive whole-body rotation that moved both the head (head in space) and trunk (trunk in space). The monkeys fixated the stationary spot, which required a perfect VOR and gaze remained stationary in space. In a VOR cancelation task (Figure 1D), the monkeys tracked a target that moved in space with the same amplitude, direction, and phase as the whole-body rotation. This condition required the monkeys to cancel the VOR so that the eyes remained virtually motionless in the orbit while gaze moved with the target and whole-body.
To examine the effects of neck proprioceptive inputs, passive horizontal rotation was applied to the head alone or trunk alone (Figures 1E,F). During head-on-trunk rotation (Figure 1E), the trunk was kept stationary in space. During trunk-on-head rotation (Figure 1F), the head was kept stationary in space. During these tasks, the target stayed stationary in space straight ahead of the monkeys’ eyes to minimize the contribution of gaze movement-related discharge modulation. During passive trunk-on-head rotation (Figure 1F), the direction of head rotation relative to the trunk (i.e., head re trunk) is opposite to the direction of trunk rotation in space. During passive whole-body rotation and passive head-on-trunk rotation (Figures 1C–E), a feeder for juice reward was moved together with the head.
To examine whether FEF pursuit neurons were specifically involved in generating smooth-pursuit eye movement per se or smooth head movement as well, the reward feeder was moved. Two tasks were used (Figures 1G,H). In a gaze-pursuit task, the target and juice-feeder moved together with the same phase, amplitude and direction, and the monkey tracked the feeder by moving the head and the spot with the eyes which required canceling the VOR (Figure 1G). In a head-pursuit (VOR ×1) task (Figure 1H), the monkey was required to track the reward feeder by moving the head while the spot remained stationary in space. Further stimulus conditions including visual stimuli during a memory-based smooth-pursuit task will be described in the related sections (see Otolith Inputs to Caudal FEF Pursuit Neurons, Linear Addition of Discharge Modulation During Smooth-Pursuit and Trunk-on-Head Rotation, Coordinate Frames, Higher Functions of the Caudal FEF and SEF).
Vestibular Inputs
Semi-Circular Canal Inputs to the Caudal FEF and Pursuit–Vestibular Interactions: Gaze-Velocity and Eye/Head-Velocity Neurons
During passive whole-body rotation, most FEF pursuit neurons (66/100 = 66%, Fukushima et al., 2000b) exhibit a discharge pattern similar to the gaze-velocity response of Purkinje cells in the cerebellar floccular region (Miles and Fuller, 1975; Lisberger and Fuchs, 1978; Miles et al., 1980). We have called such neurons gaze-velocity neurons. These neurons respond during VOR cancelation and have preferred directions similar to the pursuit-preferred direction. Preferred directions for VOR cancelation directions are distributed virtually evenly for all directions, and discharge modulation of individual neurons during VOR cancelation along the preferred direction is linearly correlated with peak gaze-velocity, but modulation is much weak during VOR ×1 that eliminates gaze movement. It also responds, albeit weakly, during VOR in complete darkness with the same preferred direction for vestibular stimulation, suggesting that vestibular inputs contribute to the VOR cancelation responses (Fukushima et al., 2000b).
In contrast, a minority (32%) of FEF pursuit neurons are called eye/head-velocity neurons (Fukushima et al., 2000b). Although these neurons also respond during VOR cancelation, they clearly respond during VOR ×1 with a magnitude comparable to their response during smooth-pursuit, and more robustly than gaze-velocity neurons during VOR ×1. Furthermore, preferred directions of some of this group of neurons during VOR cancelation are opposite to pursuit-preferred directions but similar to the direction during VOR in complete darkness.
VOR Cancelation: Addition of Vestibular and Smooth-Pursuit Related Signals in the Caudal FEF
To clarify the role of FEF pursuit neurons in pursuit–vestibular interactions, we examined the latency and time course of discharge modulation to horizontal target motion and/or whole-body rotation applied in a position-ramp (i.e., velocity step) trajectory in head-stabilized monkeys. Figures 2A,B plots mean discharge of FEF gaze-velocity neurons (Figure 2B) and mean de-saccaded and averaged eye velocity (Figure 2A) during smooth-pursuit (green), VOR cancelation (red), and VOR ×1 (blue). For comparison, responses during whole-body rotation in complete darkness without a target are also shown (Figures 2A,B, black, VORd, Akao et al., 2007a).
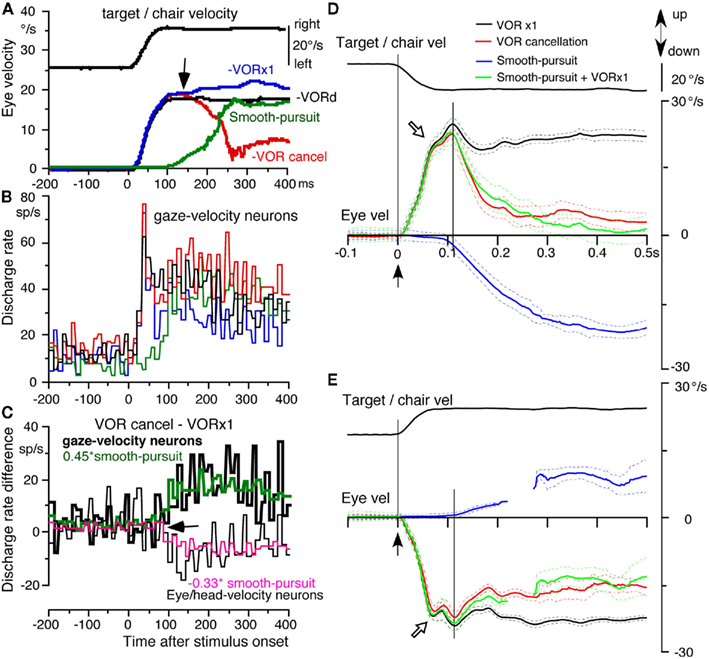
Figure 2. Whole-body rotation, population responses of caudal FEF pursuit neurons and vertical eye velocity of juvenile monkeys. (A–C) Horizontal whole-body rotation and target motion were applied in a velocity step trajectory (top). (A) Plots de-saccaded and averaged eye velocity during smooth-pursuit (green), VOR cancelation (red), VOR ×1 (blue), and during whole-body rotation in complete darkness without a target (VORd, black). Arrow indicates the divergent point for eye velocity between VOR cancelation and ×1. (B) Averaged discharge rates of nine gaze-velocity neurons during VOR ×1 (blue), VOR cancelation (red), and VORd (black) and smooth-pursuit (green). (C) Plots difference in mean discharge rate between VOR cancelation and ×1 for nine gaze-velocity neurons (thick line) and nine eye/head-velocity neurons (thin line). Green trace in (C) shows that the discharge rate difference of gaze-velocity neurons (thick line) was well predicted by 0.45* smooth-pursuit responses, whereas pink trace in (C) shows that the discharge rate difference of eye/head-velocity neurons was predicted by −0.33* smooth-pursuit responses. (D) De-saccaded and averaged vertical eye velocity (mean ±SD) during downward smooth-pursuit (blue), upward mean ± SD eye velocity induced by downward whole-body rotation (VOR ×1, black), and by downward target motion and whole-body rotation (VOR cancelation, red). Green line is predicted eye velocity during VOR cancelation by addition of eye velocity during downward smooth-pursuit and VOR ×1 induced by downward rotation. (E) De-saccaded and averaged eye velocity (mean ± SD) during upward smooth-pursuit (blue), downward mean ± SD eye velocity induced by upward whole-body rotation (VOR ×1, black) and by upward target motion and whole-body rotation (VOR cancelation, red). Green lines are predicted eye velocity during VOR cancelation by addition of eye velocity during upward smooth-pursuit and VOR ×1 induced by upward rotation. In (E), eye velocity trace during smooth-pursuit (blue) and predicted eye velocity (green) is not continuous because of corrective saccades. Vel indicates velocity. (A–C) Modified from Akao et al. (2007a) with permission. (D–E) Modified from Akao et al. (2007b) with permission.
Latencies of eye movement responses to whole-body rotation during VOR cancelation, VOR ×1, and VORd were ∼10 ms and were identical during the three conditions (Figure 2A, eye velocity directions were corrected as positive for all traces). Latencies of FEF pursuit neurons to whole-body rotation were 20 ms and were identical during the three conditions (Figure 2B). Initial trajectories (<∼80 ms after whole-body rotation) of discharge modulation and eye velocity were identical during the three conditions, suggesting that these are vestibular responses (Figures 2A,B). Initial responses by gaze-velocity neurons and eye/head-velocity neurons were also similar. In contrast, mean latencies of eye movement responses and of FEF pursuit neurons to the onset of target motion during smooth-pursuit were 100 and 80 ms, respectively (Figures 2A,B, green).
To examine how smooth-pursuit related discharge contributes to VOR cancelation, Figure 2C plots the difference in discharge modulation between VOR cancelation and VOR ×1 for gaze-velocity neurons (thick black line) and eye/head-velocity neurons (thin black line). The mean discharge rate difference during the initial vestibular responses (i.e., ∼<80 ms following the onset of whole-body rotation) was similar to the mean rate before stimulus onset, and there was no significant difference between the two groups of neurons. However, at ∼>90 ms following the onset of whole-body rotation, the difference in discharge rate between the two groups of neurons diverged (Figure 2C, arrow); the difference significantly increased for gaze-velocity neurons (Figure 2C, thick black line), whereas the difference significantly decreased for eye/head-velocity neurons (Figure 2C, thin black line).
These differences in discharge modulation between the two groups (Figure 2C) were most probably induced by modulation associated with smooth-pursuit, since by subtracting discharge rate during VOR ×1 from discharge rate during VOR cancelation, vestibular components should have been nullified and the main remaining components should be pursuit related modulation. This possibility was confirmed by comparing the discharge rate difference and modulation during smooth-pursuit for the two groups of neurons. As illustrated in Figure 2C, the time course of discharge rate difference of the two groups of neurons between VOR cancelation and ×1 was well predicted by the modulation associated with smooth-pursuit with a gain factor of 0.45 and −0.33 (green and pink). In contrast, divergence of eye velocity between VOR cancelation and ×1 occurred at 115 ms (Figure 2A, arrow), consistent with the appearance of smooth-pursuit (Figure 2A, green). These results indicate that FEF pursuit neurons contribute to the addition of vestibular and smooth-pursuit related modulation.
Neural mechanisms of VOR cancelation still remain controversial (see Leigh and Zee, 2006 for review). Lisberger (1990) suggested that two neural mechanisms are used during VOR cancelation: the addition mechanism and the parametric adjustment mechanism. In the addition mechanism, the smooth-pursuit system and the VOR operate entirely independently and the two signals sum or cancel each other (also Misslisch et al., 1996). The parametric adjustment mechanism is a non-pursuit mechanism, and there is a momentary adjustment of transmission in the VOR pathways to suppress the VOR itself (McKinley and Peterson, 1985; Lisberger, 1990; Roy and Cullen, 1998; Belton and McCrea, 2000; also Takeichi et al., 2000). The results summarized in Figures 2A–C favor the addition mechanism. To further verify this mechanism, we examined vertical eye movements of juvenile monkeys.
Many juvenile Japanese monkeys have upward and downward pursuit asymmetry as described above (Fukushima et al., 2003; Takeichi et al., 2003; Kasahara et al., 2006; Kurkin et al., 2009; cf., Grasse and Lisberger, 1992; Marti et al., 2006) and provide an excellent opportunity to examine the contribution of smooth-pursuit to VOR cancelation. If the properties of VOR cancelation correlate closely with the unique properties of smooth-pursuit eye movements, we can conclude that pursuit is a major contributor (Akao et al., 2007b).
Figures 2D,E summarizes mean (±SD) eye velocity of a representative monkey during downward (Figure 2D) and upward (Figure 2E) whole-body velocity step rotation (black and green, VOR ×1, and VOR cancelation) and/or target motion (blue, smooth-pursuit). Initial responses (∼<0.1 s) were nearly identical to the mean responses except for the direction (Figures 2D,E, open arrow heads). Upward eye velocity during VOR cancelation induced by downward whole-body rotation decreased sharply after 110 ms (Figure 2D, green) with a time course similar to that of downward smooth-pursuit (Figure 2D, blue). This contrasts with the much smaller decrease of downward eye velocity during VOR cancelation induced by upward whole-body rotation (Figure 2E, green), and this time course was similar to that of upward smooth-pursuit (Figure 2E, blue). Comparison of actual eye velocity during VOR cancelation (Figures 2D,E, green) with predicted eye velocity that was the sum of eye velocity during smooth-pursuit and VOR ×1 (Figures 2D,E, red) indicates that the predicted eye velocity in both upward and downward directions was nearly identical to the actual eye velocity. Thus, these results indicate that the eye velocity during VOR cancelation can be explained primarily by addition of eye velocity during smooth-pursuit and VOR ×1. Lisberger (1990) pointed out that parametric modulation of the VOR is a strategy that is invoked by monkeys voluntarily and whether a monkey employs parametric modulation of the VOR depends on training procedures, experimental conditions, and the level of motivation.
Semi-Circular Canal Inputs to SEF Pursuit Neurons
Although most SEF pursuit neurons also respond to passive whole-body rotation, they were not simply classified as gaze-velocity or eye/head-velocity neurons. Compared to the vestibular responses of FEF gaze-velocity neurons that exhibited clear preferred directions similar to their pursuit-preferred directions, vestibular preferred directions were indeterminate in most SEF pursuit neurons, suggesting a functional difference between pursuit and vestibular systems between FEF and SEF neurons. We have, therefore, called these SEF neurons pursuit plus vestibular neurons (Fukushima et al., 2004a). At present, the role of vestibular signals in the SEF is unknown. However, the absence of preferred vestibular directions may suggest a role other than signaling gaze-velocity. Vestibular signals may also participate in coordinate transformation (see Coordinate Frames), and in learning-related activity in the SEF (cf., Chen and Wise, 1995; Nakamura et al., 1998).
Otolith Inputs to Caudal FEF Pursuit Neurons
Most FEF pursuit neurons (44/68 = 65%) respond to passive whole-body translation in the horizontal plane that activates utriculus receptors (Akao et al., 2009). Preferred translation-directions were examined by positioning the primate chair (and hence the monkeys) at different orientations (Figure 3A) and by oscillating the monkeys along the same earth-horizontal direction (arrows) in complete darkness. An example is shown in Figures 3B,C for a representative neuron that responded during rightward pursuit and convergence (Figures 3B1,B2). It responded during whole-body translation in complete darkness with the preferred translation-direction near +45° (Figure 3C). Preferred directions of FEF pursuit neurons (n = 23) for translational responses were distributed nearly evenly in front of the monkeys (Figure 3D).
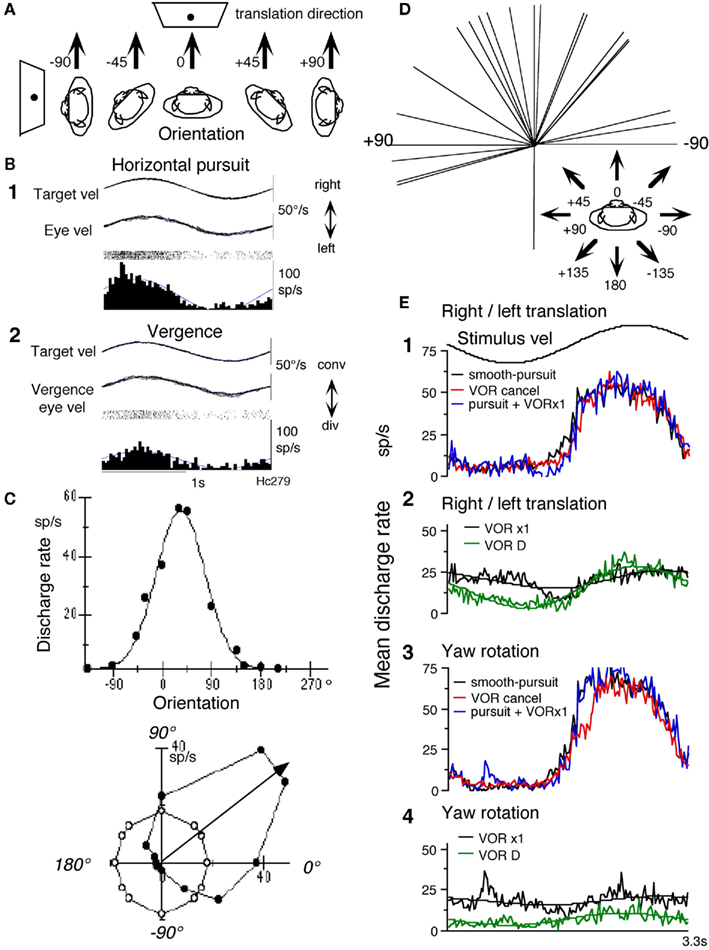
Figure 3. Otolith inputs to caudal FEF pursuit neurons. (A) Methods to test whole-body translation. Schematic illustration of the direction of passive whole-body translation and orientation of monkeys while identical sinusoidal translation was applied along the same direction pointed by arrows in complete darkness without a target. (B,C) Responses of a single neuron. (B) Discharge during horizontal pursuit (B1) and vergence-pursuit (B2). (C) Directional tuning and Gaussian fit. Filled circles plot discharge rate against translation-direction with respect to the monkey. Open circles are resting discharge rate while the whole-body was stationary in space. Preferred direction calculated by Gaussian fit is indicated by arrow. (D) Preferred directions of translational responses. Preferred directions were calculated for 23 FEF pursuit neurons and are plotted in the monkey coordinate as indicated by arrows. (E) Mean discharge rate of FEF pursuit neurons during translational and rotational VOR cancelation, VOR ×1, and smooth-pursuit. (E1,E2) Show mean response of seven neurons. (E3,E4) Show mean response of six of the seven neurons, during smooth-pursuit [(E1,E2), black], right/left translational VOR (tVOR) cancelation [(E1), red] and tVOR ×1 [(E2), black], right/left translation in complete darkness without a target [(E2), VOR D, green], rotational VOR cancelation [(E3), red] and VOR ×1 [(E4), black], and yaw rotation in complete darkness without a target [(E4), VOR D, green]. Predicted modulation during translational VOR cancelation is shown in (E1) in blue by adding discharge during smooth-pursuit and tVOR ×1. Predicted modulation during rotational VOR cancelation is shown in (E3) in blue by adding discharge during smooth-pursuit and rotational VOR ×1. Mean resting discharge rate during fixation of a stationary target without vestibular stimulation was subtracted from the adding discharge in (E1,E3). In (E2,E4), fit sine function is shown for each discharge. Reproduced from Akao et al. (2009) with permission.
To understand how otolith responses interact with pursuit responses, we examined responses during rightward/leftward translation under two conditions (Figure 3A, −90°). In one, the target moved with the monkeys, and in the other, the target remained stationary in space. The former required the monkeys to cancel the tVOR so that the eyes remained stationary in the orbit and gaze moved with the target/whole-body, whereas the latter required compensatory eye movements and no gaze movement during translation (tVOR ×1). The mean discharge rates of FEF pursuit neurons (n = 7) are summarized in Figure 3E during rightward/leftward tVOR cancelation (Figure 3E1, red), tVOR ×1 (Figure 3E2, black), and horizontal pursuit (Figure 3E1, black). Discharge modulation during horizontal pursuit and tVOR cancelation was similar and was clearly larger than the modulation during tVOR ×1 (Figure 3E2). For comparison, the green trace in Figure 3E2 plots mean discharge rate during rightward/leftward translation in complete darkness. Modulation was clear, although it was smaller than the modulation during tVOR cancelation (Figure 3E2: green vs. Figure 3E1: red). These results suggest that some FEF pursuit neurons carry gaze-velocity signals not only during passive whole-body rotation (yaw) which activates semi-circular canals (Figures 3E3,E4) but also during rightward/leftward translation which activates otolith organs (Figures 3E1,E2). Similar analysis has not been done for SEF pursuit neurons.
Otolith inputs to the cerebral cortex were also examined by using functional magnetic resonance imaging (fMRI) and the effect of loud clicks (Miyamoto et al., 2007) that selectively stimulate the sacculus (Murofushi et al., 1995; Vidal et al., 1999). High intensity clicks activated wide areas of the cortex, i.e., the frontal lobe (prefrontal cortex, premotor cortex, and FEF), parietal lobe (the region around the intraparietal sulcus, temporo-parietal junction, and paracentral lobule), and cingulate cortex. These areas are similar to previous imaging findings analyzing the cortical responses to semi-circular canal activation, and suggest that semi-circular canal and otolith (i.e., saccular) signals may be processed in the similar regions (Miyamoto et al., 2007; also Schlindwein et al., 2008).
Neck Proprioceptive Inputs
Neck Velocity Inputs to Caudal FEF Pursuit Neurons
Frontal eye fields pursuit neurons receive direction-specific neck inputs during trunk-on-head rotation (Figure 1F; Fukushima et al., 2010). Figure 4A illustrates discharge of a representative horizontal pursuit neuron at different frequencies of trunk-on-head rotation in the horizontal plane. Response magnitudes clearly increased as peak trunk-on-head-velocity increased from 0.3 to 1.0 Hz. Amplitude of discharge modulation is linearly correlated with trunk-on-head-velocity (Figure 4B). Similar discharge modulation was also observed during trunk-on-head rotation in complete darkness (Figure 4A, no target, Figure 4C), indicating minimal impact of target existence on discharge modulation. In both conditions, eye velocity responses (i.e., cervico-ocular reflex, COR) were rarely induced in our monkeys (gain = eye velocity/trunk-on-head rotation velocity <0.1), indicating that the modulation was not due to eye movement-related responses.
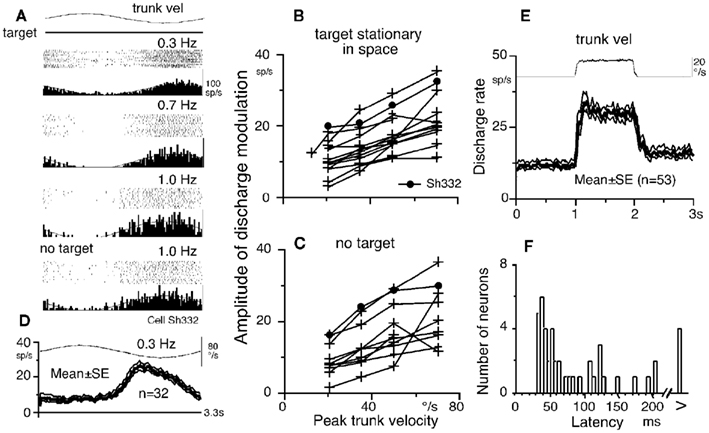
Figure 4. Neck proprioceptive inputs to FEF pursuit neurons. (A) Discharge of a single neuron during passive trunk-on-head rotation at different stimulus frequencies with constant amplitude (±10°) while a target was stationary. No target indicates that trunk-on-head rotation was applied in complete darkness without a target. (B,C) Amplitude of discharge modulation plotted against peak trunk-on-head-velocity. In (B), the monkeys fixated a stationary spot in space. In (C), trunk-on-head rotation was applied in complete darkness without a target. (D) Mean ± SE discharge of population of 32 pursuit neurons during passive trunk-on-head rotation while the monkeys fixated a stationary spot in space. (E) Mean ± SE discharge of population of 53 pursuit neurons to velocity step rotation. (F) Latency histogram of pursuit neurons to velocity step trunk-on-head rotation. Reproduced from Fukushima et al. (2010) with permission.
The averaged discharge of a population of pursuit neurons during trunk-on-head rotation with a stationary target (Figure 4D) indicates that peak discharge modulation was observed between peak trunk-on-head-velocity and position, suggesting it contained both velocity and position components. This was confirmed by testing discharge during velocity step trunk-on-head rotation (Figure 4E). Velocity components predominated in the modulation, suggesting that it primarily depended on neck proprioceptive inputs. The modal latency (=35 ms, Figure 4F) is longer than the latency of the vestibular responses induced by passive whole-body step rotation (=20 ms, Akao et al., 2007a) but shorter than the typical visual responses of FEF pursuit neurons induced by target motion (∼80 ms, Figure 2B). Since similar analysis has not been done for SEF pursuit neurons, whether SEF pursuit neurons receive neck proprioceptive inputs remains undetermined.
In humans neck proprioceptive inputs to the FEF have been detected by fMRI studies using neck muscle vibration that selectively activates intramuscular spindle receptors (Fasold et al., 2008). Like our monkey studies, COR is rarely induced by neck muscle vibration in normal human subjects, though COR is consistently evoked in patients with peripheral vestibular deficit (Bronstein and Hood, 1986; Strupp et al., 1998), suggesting a compensatory role of neck proprioceptive inputs for vestibular loss (Barmack and Pettorossi, 1988).
Linear Addition of Discharge Modulation during Smooth-Pursuit and Trunk-on-Head Rotation
Discharge modulation of FEF pursuit neurons during smooth-pursuit and trunk-on-head rotation adds linearly. This has been shown by testing modulation induced by trunk-on-head rotation and smooth-pursuit separately and by comparing each modulation with the modulation induced by applying both stimuli together. The actual modulation during the latter condition was clearly larger than each modulation and was similar to the predicted modulation calculated as the sum of each modulation (Fukushima et al., 2010). Linear addition of discharge modulation during smooth-pursuit and trunk-on-head rotation was also shown by applying target motion and trunk-on-head rotation at different frequencies. As illustrated in Figure 5, discharge modulation during combined stimulation (Figures 5C,D) was predicted by the simple linear addition of each modulation (Figure 5A: pursuit only, Figure 5B: trunk rotation only).
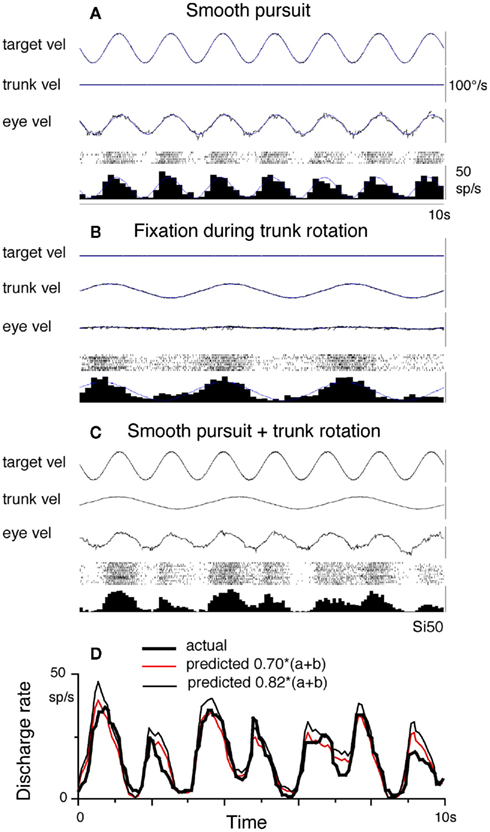
Figure 5. Linear addition of smooth-pursuit and neck input related modulation. Discharge is shown for a representative FEF eye/head-velocity neuron. (A) Smooth-pursuit at 0.7 Hz. (B) Passive trunk-on-head rotation at 0.3 Hz while the monkey fixated a stationary spot in space. (C) The combination of the two stimuli. (D) Compares actual (thick line) and predicted modulation calculated by addition of modulation in (A,B) with a gain factor as indicated (red and thin black). Thin lines on averaged histograms of cell discharge in (A,B) are superimposed fit sine waves. Eye vel indicates de-saccaded and averaged eye velocity. Target vel and trunk vel indicate target velocity in space and trunk velocity in space. Reproduced from Fukushima et al. (2010) with permission.
Addition of Neck Velocity Responses and Vestibular Responses during Head-on-Trunk Rotation
Since passive head-on-trunk rotation activates both vestibular and neck proprioceptive afferents (Figure 1E), we asked whether the discharge modulation of FEF pursuit neurons during head-on-trunk rotation (VOR ×1) was predicted by the sum of modulation due to vestibular and neck velocity responses. For this, we compared actual modulation during passive head-on-trunk rotation (VOR ×1) to predicted modulation due to the two inputs. Figures 6A–F illustrates responses of two representative neurons (Figures 6B,C, gaze-velocity neuron; Figures 6E,F, eye/head-velocity neuron) when whole-body rotation (VOR ×1) and trunk-on-head rotation were given separately (Figures 6B,E) and when the two were applied together (Figures 6C,F, thick lines). During passive head-on-trunk rotation, the actual modulation of eye/head-velocity neurons was similar to, but slightly smaller than, the modulation calculated simply by adding the two responses (Figure 6F, thin line).
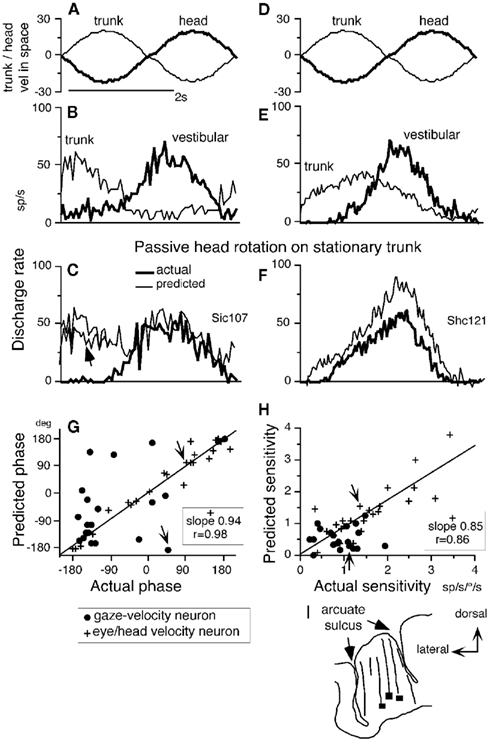
Figure 6. Linear addition of discharge modulation to neck and vestibular inputs. (A,D) Stimulus velocity in space during passive trunk-on-head rotation and whole-body rotation. (B,C) and (E,F) Averaged discharge of two FEF pursuit neurons. In (A,B) and (D,E), passive trunk-on-head rotation (thin lines) and whole-body rotation (thick lines) were applied separately while, in each condition, the monkeys fixated a stationary spot in space. In (C,F), passive head-on-trunk rotation was applied (thick lines, actual) while the monkeys fixated a stationary target. Thin lines in (C,F) are predicted modulation calculated by adding neck and vestibular modulation (B,E). Directions of chair rotation during whole-body rotation (vestibular) and trunk-on-head rotation [(neck (A,D)] are shown oppositely, since during passive head-on-trunk rotation, neck movement direction relative to the trunk is opposite to trunk movement direction induced by chair rotation (see Figures 1E,F). Resting discharge rate was subtracted from the predicted discharge in (C,F). In (G,H), phase (G) and sensitivity (H) of predicted modulation (re stimulus velocity) that was calculated by adding neck and vestibular modulation are plotted against actual modulation (re stimulus velocity) during passive head-on-trunk rotation for gaze-velocity neurons and eye/head-velocity neurons. Responses of the two example neurons (A–F) are indicated by arrows in (G,H). (I) Transverse section of representative recording tracks of monkey Si and locations of three pursuit neurons responding to trunk-on-head rotation (squares). Reproduced from Fukushima et al. (2010) with permission.
In contrast, the actual modulation of most gaze-velocity neurons during head-on-trunk rotation was different from the predicted modulation (Figure 6C, thin line), but was similar to the modulation during whole-body rotation alone (Figures 6C vs. 6B, thick lines). Figures 6G,H compares phase and sensitivity of actual and predicted modulation (re stimulus velocity). Eye/head-velocity neurons exhibited significant correlation between predicted and actual modulation with slopes of both phase and amplitude of modulation close to one (Figures 6G,H, crosses). In about half of gaze-velocity neurons, however, addition of the two responses suggested significantly smaller sensitivity to head-on-trunk velocity than actual sensitivity, and phases between the two were considerably different (Figures 6G,H, dots). These results show that neck proprioceptive signals reaching FEF pursuit neurons can be strongly suppressed by the context in which neck rotation occurs (Figure 6C, arrow).
At present we can only speculate about the possible roles neck proprioceptive inputs to eye/head-velocity and gaze-velocity neurons might play (Fukushima et al., 2010). We think that neck velocity inputs could contribute to representing target-, eye-, and gaze-velocity in trunk coordinates (see Coordinate Frames) in a context-dependent manner. Where both eye/head-velocity and gaze-velocity signals are active during head-on-trunk rotation, they tend to combine in a way that generates a target velocity with respect to the trunk signal. In the case of gaze-velocity neurons that do not suppress neck signals this would lead to a gaze with respect to trunk signal. This would be useful in contexts where the animals were following a target that moved with his body (eye–hand coordination for instance, e.g., Maioli et al., 2007). Animals also have to follow targets in the external world. Gaze-velocity neurons where the neck rotation signal is suppressed during head-on-trunk rotations would serve this purpose. Activity of eye/head-velocity neurons does not change greatly when an animal stabilizes its gaze using the VOR as opposed to active pursuit. In both cases, the neurons’ discharge follows eye velocity. The neck input received by all of these neurons during passive head-on-trunk rotation tends to convert this to an eye velocity with respect to trunk signal. This could be useful for signaling required smooth eye velocity with respect to the trunk velocity during head-on-trunk rotation.
Active Head-Pursuit and Discharge of Caudal FEF Pursuit Neurons
In daily life where the head is free to move, eye and head movements are coordinated during pursuit of a slowly moving visual target (i.e., gaze-pursuit). Eye and head coordination is well understood during saccadic gaze shifts, although the responsible neural mechanisms still remain controversial. There are two competing hypotheses (for review, see Chen, 2006); (1) the brain issues an integrated gaze command that is then decomposed into separate eye- and head-commands (also van der Steen et al., 1986); and (2) the brain issues independent eye- and head-commands at all levels. For gaze-pursuit also, Lanman et al. (1978) suggested that the brain issues gaze-pursuit commands driving both eye- and head movements and further that the eye-pursuit command is combined with vestibular feedback from head movement to maintain gaze by compensating for variations in the amount of head movement (also Miles and Lisberger, 1981), but independent commands have also been suggested (e.g., Belton and McCrea, 1999, 2000) for gaze-velocity Purkinje cells in the cerebellar floccular region (Miles and Fuller, 1975).
Frontal eye fields saccade neurons are thought to signal both rapid eye movement commands and rapid head movement commands for head free gaze shifts (e.g., Tu and Keating, 2000; Knight and Fuchs, 2007; however, Chen, 2006). During passive whole-body rotation, most FEF pursuit neurons carry gaze-velocity signals as described above (e.g., Figures 2B, red, Figures 3E3,E4). We speculated whether FEF pursuit neurons carry gaze-pursuit commands that drive both eye-pursuit (i.e., smooth-pursuit) and head-pursuit during head free gaze-pursuit. To examine this, monkeys whose heads were free to rotate about a vertical axis were trained to pursue a juice-feeder with their head and a target with their eyes (Figures 1G,H, Fukushima et al., 2009). The feeder and target moved synchronously with the same visual angle. FEF neurons responding to this gaze-pursuit were tested for eye-pursuit of target motion while the feeder was stationary and for head-pursuit while the target was stationary.
Most pursuit neurons exhibited modulation during head-pursuit, but their preferred directions during eye-pursuit and head-pursuit differed. Although peak modulation occurred during head movements, usually discharge onset was not aligned with the head movement-onset. The minority of neurons whose discharge onset was so aligned, discharged after the head movement-onset. These results do not support the idea that the head-pursuit related modulation reflects head-pursuit commands. Furthermore, modulation similar to that during head-pursuit was obtained by passive head rotation on a stationary trunk. These results together with the above results regarding vestibular and neck velocity responses suggest that FEF pursuit neurons issue gaze- or eye movement commands during gaze-pursuit but that the head-pursuit related modulation primarily reflects re-afferent signals resulting from head movements (Fukushima et al., 2009). A similar analysis has not been done for SEF pursuit neurons.
We have shown earlier that pursuit signals are represented three dimensionally (3D) in the caudal FEF by combining fronto-parallel pursuit (i.e., smooth-pursuit) and vergence-pursuit (pursuit-in-depth) velocity components (Fukushima et al., 2002b). Representation of pursuit velocity signals relative to trunk velocity during head movement (see Neck Proprioceptive Inputs) would be useful for coordinating pursuit eye movements with hand- and/or arm-movements in primates during erect posture for reaching a moving target in 3D extrapersonal space (cf., Chen et al., 2010). Development of the fovea and the necessity for 3D pursuit in primates may have contributed to the possible representation of these signals in the frontal cortex in trunk coordinates (see Coordinate Frames).
Coordinate Frames
Spatial (e.g., direction) signals about visual object motion and/or pursuit eye movements could be represented in various coordinate systems such as eye-centered, head-centered, or earth-centered coordinates. While upright, these coordinates are mostly congruent, making it difficult to distinguish which coordinates represent which signals. During static whole-body roll-tilt, however, the earth-centered coordinate can be distinguished from the other two, since ocular counter-rolling during static roll-tilt is minimal (gain = 0.1–0.2) in primates (Krejcova et al., 1971; Suzuki et al., 1997; see Leigh and Zee, 2006 for review).
The subjective direction of gravity under static whole-body roll-tilt is called the subjective visual vertical (SVV), and otolith inputs must substantially contribute to the SVV (Kaptein and van Gisbergen, 2004). Human subjects can align the perceived motion–direction of a random-dot pattern to the direction of gravity in a laterally tilted position (de Vrijer et al., 2008). For this, retinal motion signals must be combined with head position signals in the earth-centered coordinates. By measuring the SVV, Daddaoua et al. (2008) and Lewis et al. (2008) have shown that rhesus macaques can also perceive the earth-centered axis as accurately as humans. These results suggest that in the brain areas involved in perceiving motion–direction, coordinates representing visual motion signals should be earth-centered, not eye- or head-centered.
We asked whether the discharge coding smooth-pursuit eye movements and visual motion responses in the caudal FEF is in the head-centered coordinate or earth-centered coordinate (Kurkin et al., 2007). This question is fundamental to understanding the role of the caudal FEF in coordinating smooth-pursuit. Similarly, we explored the same question about visual motion and/or smooth-pursuit related signals carried by neurons in the dorsomedial MST (MSTd, Desimone and Ungerleider, 1986), since MST also contains pursuit neurons and send direct projections to the FEF (e.g., Leigh and Zee, 2006) and since MSTd has been suggested to be involved in perception and memory of visual motion (e.g., Celebrini and Newsome, 1994; Britten and Van Wezel, 2002; Gu et al., 2007; Liu and Angelaki, 2009; cf., Heuer and Britten, 2004).
To examine coordinate frames of smooth-pursuit and/or visual motion-related discharge, the computer monitor, the monkey and coil frame for detection of eye movements were tilted in the roll plane to 40° from the earth-vertical (either rightwards or leftwards). The results were basically similar for all neurons tested in the caudal FEF (n = 29, Kurkin et al., 2007) and MSTd (n = 51, Fujiwara et al., 2011).
Discharge of a representative neuron in the caudal FEF is shown in Figure 7 as the monkey pursued in the neuron’s preferred direction (i.e., downward) while upright (Figure 7C or during static whole-body roll-tilt (Figures 7A,E). This neuron discharged similarly during the three conditions (Figures 7A,C,E). The preferred direction was 266° during upright (Figure 7D). If preferred directions of FEF pursuit neurons are coded in the earth-vertical coordinates, the expected preferred direction of this neuron during 40° right ear down would be 306° (=266 + 40, Figure 7B) and 226° (=266 − 40, Figure 7F) during 40° left ear down. Actual preferred directions (relative to monkey’s head/trunk axis) during the right/left ear down conditions (271°/263°, respectively, solid lines in Figures 7B,F) were clearly different from the directions expected from the earth-vertical coordinates (dashed lines in Figures 7B,F).
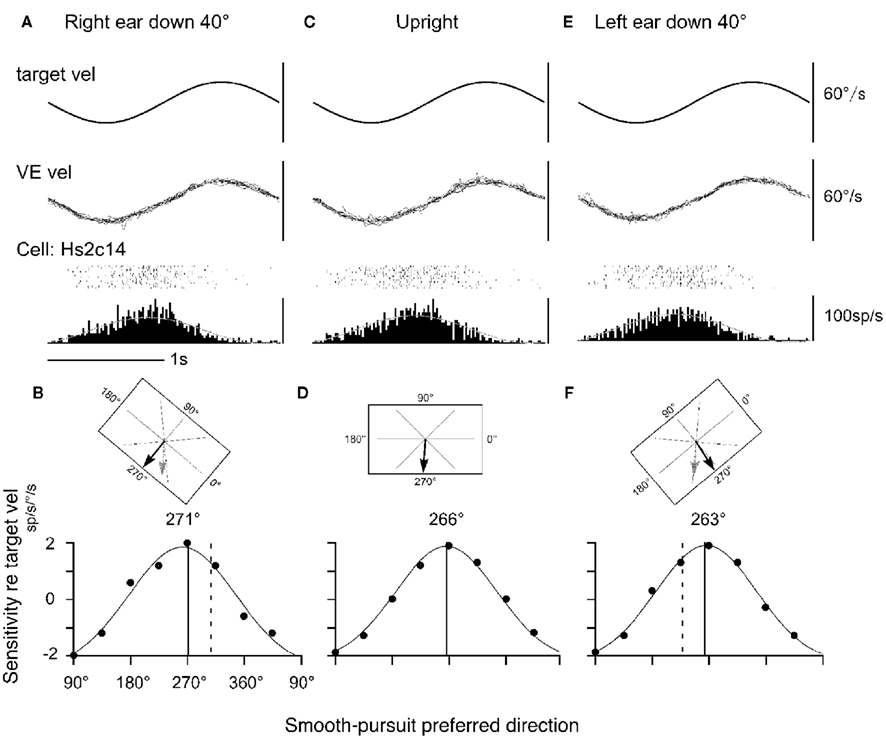
Figure 7. Static whole-body roll-tilt and preferred direction of a representative FEF pursuit neuron. (A,B) Forty degrees whole-body roll-tilt toward right (right ear down). (C,D) Upright. (E,F) Forty degrees whole-body roll-tilt toward left (left ear down). (B,D,F; top) illustrate computer monitor and target motion–directions from the monkey’s view for the three task conditions. Actual preferred directions during pursuit are indicated by black arrows. Expected preferred directions in the earth-vertical coordinates are indicated by gray arrows in (B,F). (B,D,F; bottom) illustrate directional tning and Gaussian fits for actual responses for each condition. Solid and dashed lines are actual and expected preferred directions, respectively. Dots indicate actual sensitivity values. VE vel indicates vertical eye velocity. Reproduced from Kurkin et al. (2007) with permission.
In virtually all neurons tested, shifts of preferred directions during the right/left ear down conditions were small with the mean absolute difference of 5–6°, indicating that none of the FEF pursuit neurons or MSTd neurons tested signal pursuit eye velocity and/or visual motion–directions in the earth-centered coordinates. These results plus our results showing neck velocity inputs and linear addition of neck velocity and pursuit signals in the caudal FEF (Figures 5 and 6) suggest that the preferred direction of FEF pursuit neurons are coded in the trunk coordinates (see Neck Proprioceptive Inputs).
Similar analysis has not been done for SEF pursuit neurons; coordinate frames in which SEF pursuit signals are represented are unknown. As for saccadic eye movements and gaze shifts, the SEF has been implicated as the area that integrates complex visuo-spatial information and controls eye–head gaze shifts (e.g., Martinez-Trujillo et al., 2004). Saccade-related SEF neurons encode visual targets in both eye-centered and object-centered coordinates in the task context (Schlag-Rey et al., 1997; Olson and Gettner, 1999; Olson et al., 2000; Park et al., 2006).
The importance of vestibular signals for representing target position in space has been suggested by clinical studies using the vestibular contingent memory-guided saccade tasks (Bloomberg et al., 1988). Briefly, human subjects fixated a stationary target and then changed fixation to a very low intensity, small light-emitting diode (LED) that was fixed to the head. The subjects were instructed to continue fixating the LED during sudden whole-body rotation in the horizontal plane either to the left or right in complete darkness. After completing rotation, the LED was extinguished, and this was the signal that the subject had to make a voluntary saccadic eye movement to the remembered location of the original earth-fixed target. Normal subjects could accurately locate the target position in space in this task (Bloomberg et al., 1988, 1991), whereas labyrinthine-defective subjects could not (Nakamura and Bronstein, 1995), indicating the necessity for vestibular information for accurate performance of this task. Israël et al. (1992, 1993) and Pierrot-Deseilligny et al. (1993, 1995) reported that vestibular contingent memory-guided saccades were impaired in patients with SEF lesions, although they did not exhibit abnormalities during memory-guided saccades without vestibular stimulation. These observations suggest that the SEF and vestibular signals are necessary for representing target position in space in the earth-centered coordinate during memory-guided saccades.
Higher Functions of the Caudal FEF and SEF
Cross-Axis Pursuit–Vestibular Interactions
Vestibular signals effectively initiate predictive pursuit (Fukushima et al., 2001a,b; Tsubuku et al., 2006) as shown in the following experiments. Monkeys were trained to pursue a spot moving in a trapezoidal position trajectory (20°/s, ±10°) either vertically or horizontally during whole-body rotation with the same trajectory but in the orthogonal plane. When the target simultaneously moved with whole-body rotation, latencies of initial pursuit eye movements to vertical spot motion during horizontal rotation were adaptively shortened from about 100 ms (i.e., normal pursuit latency) to less than 50 ms (a latency too short for visual feedback) and initial eye velocities increased within 30 min of training. This initial eye movement response was induced even without a target and the latencies depended on the training conditions, consistent with the interpretation that it was induced predictively.
The predictive nature of the initial eye movements is made clear by changing the delay between target motion onset and whole-body rotation from 100 to 700 ms (Tsubuku et al., 2006). Pursuit eye movements after training were initiated before the onset of target motion. The latencies were proportional to (but shorter by 22–36% than) the actual delays used for training. Even without the presence of the target, the latencies and velocity of pursuit were similar. These results indicate that vestibular signals specifically contributed to the timing of predictive pursuit eye movement initiation in cross-axis vestibular–pursuit interactions.
Frontal eye fields pursuit neuron activity was recorded to determine whether they were involved in predictive pursuit induced by pursuit–vestibular interaction training (Fujiwara et al., 2009). The latencies of discharge modulation of 61% of the FEF pursuit neurons tested (14/23) shortened after the training in association with a shortening of pursuit latency. However, their discharge modulation occurred with a mean lag of 12 ms after the onset of pursuit eye velocity after training, although most of them discharged before the onset of eye velocity before training. Only a minority of neurons (4/23 = 17%) discharged before the eye movement onset. Thus, most FEF pursuit neurons are unlikely to be involved in the initial stage of generating predictive eye movements during cross-axis pursuit–vestibular interactions; they may participate in maintaining predictive pursuit. SEF pursuit neurons have not been examined during cross-axis pursuit–vestibular interactions.
Memory-Based Smooth-Pursuit
Our understanding of the different roles of the SEF and caudal FEF during pursuit–vestibular and neck–vestibular interactions is limited by our lack of detailed information for SEF activity as described above. However, in addition to its well-known saccade-related activity (Schlag and Schlag-Rey, 1987; Schall, 1991; Russo and Bruce, 1996), the SEF has learning-related activity (Chen and Wise, 1995; Nakamura et al., 1998) and is crucial in complex behaviors such as planning of saccades (Olson et al., 2000), decision-making processes (Coe et al., 2002), sequential performance of saccades (Russo and Bruce, 1996; Isoda and Tanji, 2002, 2003; Lu et al., 2002), antisaccades (Schlag-Rey et al., 1997), and eye–hand reach coordination (Mushiake et al., 1996). Reward-predicting activity has also been reported (Amador et al., 2000). Recent studies using a memory-based smooth-pursuit task have revealed clear differences in the roles of the SEF and caudal FEF in controlling smooth-pursuit (Fukushima et al., 2008, 2011a; Shichinohe et al., 2009).
Memory-based smooth-pursuit task separates visual motion–memory from movement–preparation
During smooth-pursuit, a moving target must be maintained within the foveal projection field (Figure 1A) to ensure clear vision about the target by predictive compensation for the inherent delays (∼100 ms lag) in responses to target motion. Prediction is influenced by various factors such as cues and working memory of stimulus trajectory (e.g., Badler and Heinen, 2006; Barnes and Collins, 2011; see Barnes, 2008 for review). Prediction could occur not only in motor commands to prepare for and maintain ongoing movements but also in the sensory and/or perception pathways (e.g., Barboroica and Ferrera, 2003). Such a mechanism may use memory (e.g., Assad and Maunsell, 1995); however, neural mechanisms of predictive pursuit are poorly understood. Also, it remains unknown where the visual motion–memory for predictive pursuit is stored.
Prediction-related neuronal discharge during smooth-pursuit was reported in the SEF (Heinen, 1995; Heinen and Liu, 1997; Kim et al., 2005; de Hemptinne et al., 2008) and caudal FEF (e.g., MacAvoy et al., 1991; Fukushima et al., 2002a). Prediction-related activation of these areas during smooth-pursuit was also reported by fMRI in humans (e.g., Schmid et al., 2001; Burke and Barnes, 2008). However, in these studies, activation related to preparation for pursuit eye movements could not be separated from activation related to processing of target motion signals or their working memory. Moreover, in daily life, a specific target must be selected from multiple moving objects, requiring decisions and selection of whether and what to pursue. Although the caudal FEF is involved in initiating and executing smooth-pursuit in monkeys, the roles of the two areas in predictive pursuit are largely unknown.
To examine neuronal substrates for predictive aspects of pursuit, we employed a memory-based smooth-pursuit task that uses two cues and two delay periods (Figure 8A): cue 1 indicates the visual motion–direction and cue 2 instructs whether to prepare to pursue (i.e., go) or not to pursue (i.e., no-go). Based on the memory of visual motion–direction presented at cue 1 (Figure 8A2) and the go/no-go instruction presented at cue 2 (Figure 8A4), monkeys must select the correct spot and pursue it or not by maintaining fixation of a stationary spot during the action period (Figure 8A6, for further task explanation, see legend of Figure 8).
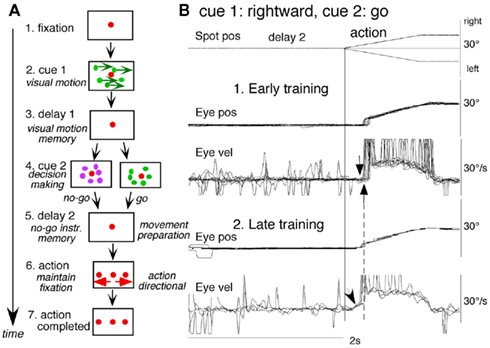
Figure 8. Memory-based smooth-pursuit task and representative eye movements. (A) Schematic illustration of the task. A red stationary spot appeared at the screen center and the monkeys were required to fixate it [(A1) fixation]. Cue 1 consisted of a random-dot pattern of 10° diameter. All 150 dots moved along one of 8 directions at 10°/s for 0.5 s [(A2) cue 1, 100% correlation of Newsome and Pare, 1988]. Visual motion–direction was randomly presented. The monkeys were required to remember both the color of the dots and their movement direction while fixating the stationary spot. After a delay [(A3) delay 1], a stationary random-dot pattern was presented as the second cue for 0.5 s [(A4) cue 2]. If the color of the stationary cue 2 dots was the same as the cue 1 color, it instructed the monkeys to prepare to pursue a spot that would move in the direction instructed by cue 1 (i.e., go). If the color of cue 2 differed from cue 1, it instructed the monkeys not to pursue (i.e., no-go) but to maintain fixation of a stationary spot which required remembering the no-go instruction during the second delay [(A5) delay 2]. Go/no-go cue was randomly presented. After the delay, the monkeys were required to execute the correct action by selecting one of three spots and either pursuing the correct spot in the correct direction or maintaining fixation [(A6) action]. For this, the stationary spot remained centered, but spawned two identical spots; one that moved in the direction instructed by cue 1 and the other moved in the opposite direction at 10°/s. For correct performance, the monkeys were rewarded. For analysis, all trials were sorted by cue 1, cue 2 direction/instructions. (B) Eye movement records during early and late training when cue 1 was rightward and cue 2 was go. Pos and vel indicate position and velocity. For further explanation, see text. Modified from Fukushima et al. (2008) and Shichinohe et al. (2009) with permission.
Figure 8B shows representative eye movement records of a monkey during early and late training when cue 1 was rightward and cue 2 was go. Early in their training (typically after 6–8 months of training), monkeys learned the task basics with error rates of less than 10% for go and no-go trials. As illustrated in Figure 8B1, the monkey initiated the final action by saccades (but not by smooth-pursuit) with latencies typically 260–300 ms (Figure 8B1, upward arrow), and these saccades were followed by smooth-pursuit. The lack of an initial smooth-pursuit component before saccades (Figure 8B1, downward arrow) is consistent with the finding that vector averaging is used to combine visual inputs arising from two moving spots (Lisberger and Ferrera, 1997); in our task, visual motion inputs arising from the two oppositely moving spots with the same speed during the action period (Figure 8A6, e.g., leftward vs. rightward) would be nullified. Saccades to the cued direction during early training (Figure 8B1) must have enhanced visual motion processing of the pursuit target in that direction so that smooth-pursuit were effectively induced after saccades (i.e., postsaccadic enhancement of pursuit initiation, Lisberger, 1998; Ogawa and Fujita, 1998).
Later (typically after a year of training), saccade latency to spot motion shortened usually to about 220 ms, and preceding the saccades, initial smooth-pursuit appeared with latencies typically of 130–150 ms (Figure 8B2, arrow), indicating that appearance of the initial smooth-pursuit component requires further training for efficient tracking performance even after the monkeys learned the task basics. This training may have enhanced spot motion responses in the cued direction during the action period possibly due to priming effects by cue 1 direction memory and cue 2 go instruction (e.g., Bichot and Schall, 2002; Garbutt and Lisberger, 2006; see below).
Representation of directional visual motion–memory and movement–preparation signals
Using the memory-based smooth-pursuit task, signals for directional visual motion–memory and movement–preparation have been identified in the SEF and caudal FEF. Three groups of neurons were found; two of them carried these signals separately (visual memory neurons, movement–preparation neurons) and the third carried both signals (visual memory + movement–preparation neurons). Although the two regions carried qualitatively similar signals, there were significant quantitative differences in the signals represented in the two (see below). SEF visual memory neurons were unrelated to pursuit, whereas some FEF visual memory neurons were pursuit neurons.
Visual memory neurons. visual memory neurons exhibited direction-specific discharge during delay 1. An example SEF neuron (Figure 9) responded when rightward (not leftward) visual motion was presented at cue 1 during go and no-go trials (Figures 9B1,B2 vs. 9C1,C2). The delay 1 discharge was not significantly influenced by the monkey’s preparation of pursuit (Figures 9B1 vs. 9C1). This is also seen when the monkey erred (Figure 9B1, red trace in eye pos) by performing leftward (instead of rightward) pursuit. Despite this error, discharge similar to that during correct trials was clearly observed during delay 1 (Figure 9B1, red raster). Moreover, it did not exhibit directional responses during delay 2 during go (Figure 9B3, blue vs. black) or no-go trials (Figure 9C3, blue vs. black). These results suggest that the delay 1 activity of visual memory neurons reflected memory of the visual motion–direction presented by cue 1.
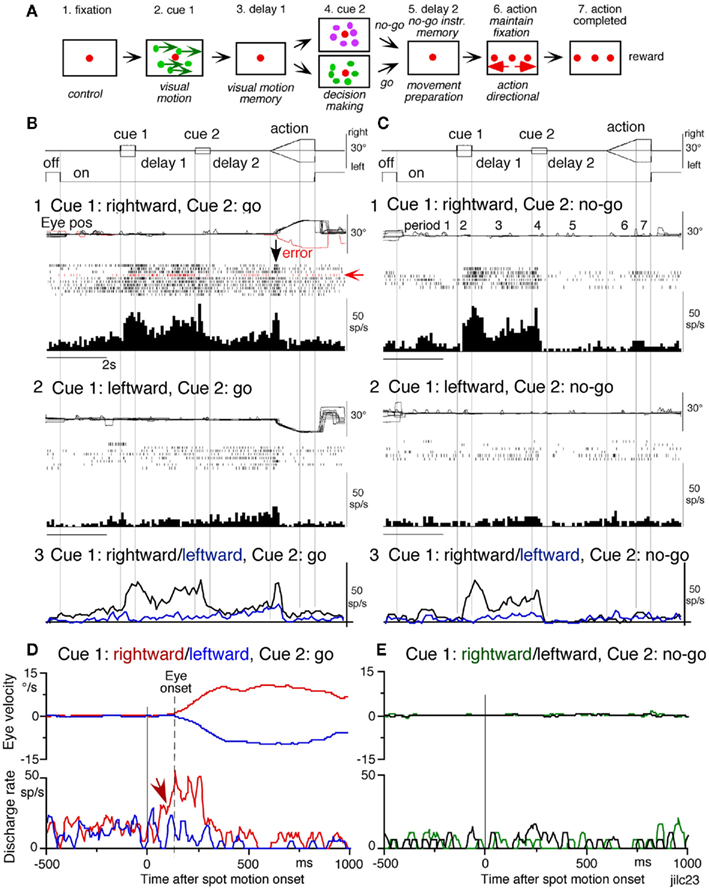
Figure 9. Discharge of a representative SEF visual memory neuron. (A) Task conditions. (B1,B2) Go trials when rightward (B1) and leftward (B2) visual motion was applied as cue 1. (C1,C2) No-go trials when rightward (C1) and leftward (C2) visual motion was applied as cue 1. Red trace in eye position (pos) record and arrow in spike raster in (B1) highlight an error trial. (B3,C3) Compare mean discharge rate during rightward (black)/leftward (blue) cue 1 visual motion for go and no-go trials, respectively. To assess which period(s) of the task (A2–A7) were associated with modulated neuronal activity, mean discharge rates of individual neurons were measured during the different task periods for the correct response [e.g. (C1) periods 2–7], and were compared with the mean rate (±SD) during the initial fixation [(C1), period 1] for each neuron. Significant differences were defined as those having a p-value <0.05 using Student’s t test with the Bonferroni correction for multiple comparisons. Neurons that exhibited significant modulation during this task were defined as task-related neurons. (D,E) De-saccaded and averaged eye velocity and discharge of this neuron 500 ms before and 1000 ms after spot motion onset (vertical straight line) during the action period. Smooth-pursuit onset is indicated by a dashed line. Only correct trials were averaged for go(D) and no-go conditions (E) as indicated by colors. See text for further explanation. Modified from Shichinohe et al. (2009) with permission.
Possible neural correlates for the putative priming effects by cues during the action period (Figure 8B2, arrow, see above) are suggested in Figures 9B,C for this SEF visual memory neuron that had rightward preferred direction to cue 1 visual motion (Figures 9B1,C1). Since this neuron was unrelated to pursuit (Figures 9B1,B2, action), the initial burst during the action period of go trials (Figure 9B1, downward arrow) must have reflected visual response to rightward spot motion. Notice selective burst discharge to identical visual motion stimuli during the action period, i.e., the clear burst during the action period appeared only in Figures 9B1 vs. 9B2, C1,C2), indicating that the spot motion responses clearly depended on the visual motion–direction memory and go/no-go instructions. This interpretation is confirmed in Figure 9D; discharge to spot motion clearly occurred before the onset of the initial smooth eye velocity (Figure 9D, red arrow before eye onset vs. other conditions Figures 9D,E). Similar modulation of spot motion responses during the action period by cues was also observed in visual motion responses of some caudal FEF pursuit neurons (Figures 2F–I of Fukushima et al., 2011a).
Visual memory + movement–preparation neurons. visual memory + movement–preparation neurons exhibited direction-specific discharge during both delay 1 and delay 2. An example SEF neuron (Figures 10A1–A4) showed clear discharge during the late period of delay 1 when leftward visual motion was presented at cue 1 during go and no-go trials (Figures 10A1 vs. Figures 10A2, Figures 10A3 vs. 10A4). In addition, when the cue 2 instructed go to prepare to pursue in the congruent direction (Figure 10A1), it exhibited robust discharge during the late period of delay 2. Figure 10B plots a difference in time course of mean discharge of visual memory neurons (red) and visual memory + movement–preparation neurons (blue) in the SEF during go trials in their preferred directions. While the initial response to cue 1 for visual memory neurons (Figure 10B, red) was larger, the two groups of neurons displayed similar discharge during the delay 1 and cue 2. During delay 2, the discharge of the two groups of neurons diverged.
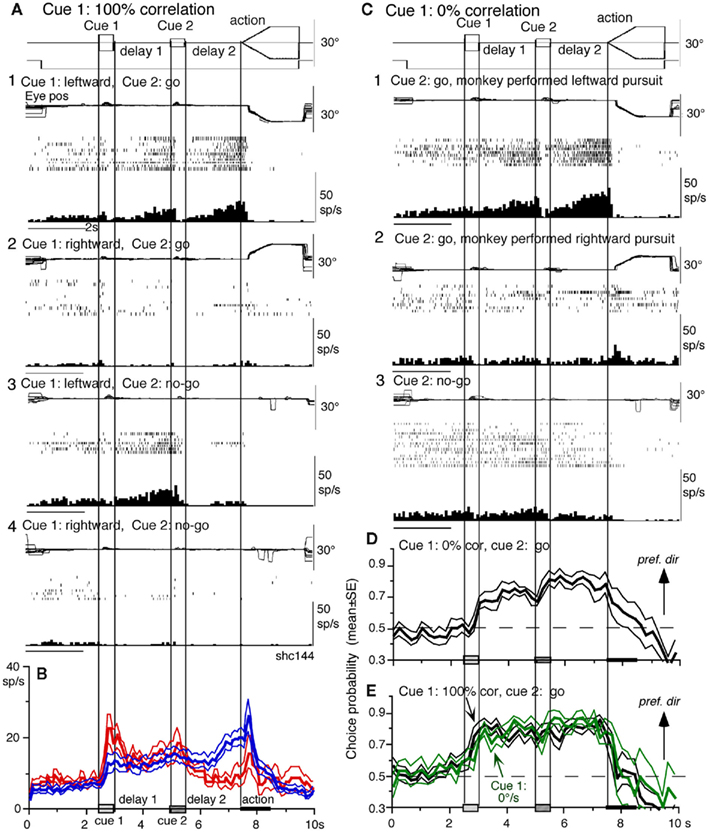
Figure 10. Visual memory + movement–preparation neurons and comparison with visual memory neurons. (A,C) Discharge of a representative SEF visual memory + movement–preparation neuron. Cue 1 motion–direction was presented as 100% correlation (A) and 0% correlation (C). (A1,A2) Go trials when cue 1 motion was leftward (A1) and rightward (A2). (A3,A4) No-go trials when cue 1 motion was leftward (A3) and rightward (A4). (B) Time course of mean (±SE) discharge modulation of visual memory neurons (red, n = 13) and visual memory + movement–preparation neurons (blue, n = 22) during go trials in their preferred directions. In (C1,C2), go trials were sorted into leftward pursuit (C1) and rightward pursuit (C2) during action period. (C3), no-go trials. (D,E) Plot mean (±SE) choice probability time course of 10 SEF visual memory + movement–preparation neurons during go trials based on whether the monkeys pursued in the preferred directions of individual neurons during delay 2 when cue 1 was presented with 0% correlation (D) and 100% correlation [(E), black]. Green traces in (E) are mean (±SE) choice probability time course of the same 10 neurons when a stationary pattern was presented at cue 1 (0°/s). For further explanation, see text. Modified from Shichinohe et al. (2009) with permission.
Visual memory + movement–preparation neurons exhibited congruent directionality during delay 1 and delay 2 of go trials (Figures 10A1,B, blue). Our results suggest that the delay 1 information about the visual motion–direction is used for further processing in preparing for pursuit direction in the SEF (Shichinohe et al., 2009). This was examined in the following experiments. First, to examine how delay 1 and 2 responses were correlated, we let the monkeys choose the pursuit direction and examined how these neurons discharged during these periods. For this, we used the paradigm devised by Newsome and Pare(1988, 0% correlation) that moved each dot randomly in different directions at cue 1. In this condition, cue 1 does not provide the necessary information about the visual motion–direction. If the color of cue 2 was the same as cue 1, it instructed go and the monkey followed one of the two moving spots. If the color of cue 2 was different from that of cue 1, it instructed no-go, and the monkeys’ maintained fixation. Each trial was sorted based on the monkeys’ choice of either the preferred direction of delay 2 activity or the anti-preferred direction of the neuron (tested by 100% correlation).
Figure 10C plots sorted trials during 0% correlation for leftward pursuit (Figure 10C1), rightward pursuit (Figure 10C2), and no-go (Figure 10C3) of the same neuron (Figure 10A). When the monkey made leftward pursuit (i.e., in the preferred direction of this neuron, Figure 10A), discharge during delay 2 was much stronger compared to the trials where the monkey made rightward pursuit (Figures 10C1 vs. 10C2), indicating that the delay 2 activity indeed reflected preparation for pursuit. In addition, the stronger discharge during the delay 1 in the same trials (Figures 10C1 vs. 10C2) suggests that this discharge was also related to the monkey’s choice and preparation for the subsequent pursuit direction independent of the cue 1 stimulus itself, which was non-directional.
Second, to evaluate these results, we calculated choice probability (Britten et al., 1996) and its time course based on whether the monkeys pursued in the preferred direction of the neuron (tested by 100% correlation) or anti-preferred direction. The results for 10 SEF visual memory + movement–preparation neurons are plotted in Figure 10D. Mean choice probability values (which were ∼0.5 before cue 1) increased above 0.7 during delay 1 and delay 2. For comparison, the time course of choice probability of the 10 neurons during 100% is plotted in Figure 10E (black). Also plotted in green (Figure 10E) is choice probability time course of the 10 neurons when a stationary pattern (i.e., 0°/s) was presented at cue 1. The three curves (Figures 10D,E) were basically similar, indicating that delay 1 discharge is not a simple holding of visual motion response; the delay 1 response does not require visual motion stimuli, but reflects motion–direction assessment and memory.
Different signals represented in the SEF and caudal FEF
To compare direction-specific discharge modulation during different task periods of go trials in the caudal FEF and SEF, Figure 11A plots the percent of modulated neurons (out of the total number of task-related neurons in each area) that showed direction-specific modulation in each period (e.g., Figure 9C1, periods 2–7, see legend of Figure 9 for the definition of task-related neurons). Qualitatively similar signals were found in both areas (Figure 11A), consistent with the anatomical studies that show reciprocal connections between the SEF and FEF (Huerta et al., 1987). However, there were quantitatively significant differences between the two areas during delay 1 and action period (Figure 11A, *); the percent of modulated neurons in the caudal FEF was significantly lower than that of the SEF during delay 1 but higher than that of the SEF during the action period. No significant difference between the two areas was detected in other periods including the delay 2 of go trials where movement–preparation is required.
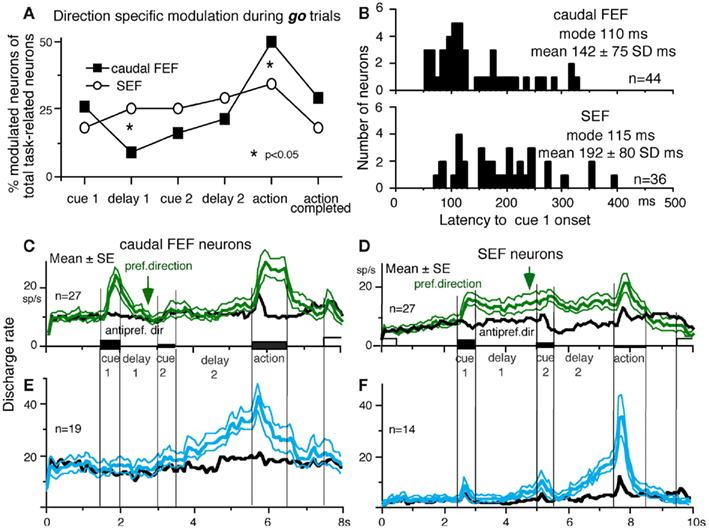
Figure 11. Comparison of SEF and caudal FEF neuron discharge during memory-based pursuit. (A) Comparison of percent of modulated neurons in caudal FEF and SEF (of total task-related neurons) that exhibited direction-specific modulation during go trials. See legend of Figure 10 for the definition of task-related neurons. (B) Comparison of latencies of visual motion responses of caudal FEF and SEF neurons to cue 1. Neurons with shorter visual latencies were significantly more frequent in caudal FEF than SEF (p < 0.01). (C) Mean ± SE discharge of 27 caudal FEF neurons that exhibited directional visual motion response to cue 1 during go trials. (D) Mean ± SE discharge of 27 SEF neurons that exhibited directional visual motion response to cue 1 during go trials. In (C,D), green and black traces are discharge modulation in the preferred direction and anti-preferred direction, respectively. (E,F) Mean ± SE discharge of movement–preparation neurons in the caudal FEF (E) and SEF (F) during go trials. Blue and black traces are discharge modulation in the preferred direction and anti-preferred direction, respectively. (D,F) Reproduced from Shichinohe et al. (2009) with permission. Others reproduced from Fukushima et al. (2011a) with permission.
Frontal eye fields neurons exhibit visual latencies comparable with those in the middle temporal area (MT) and MST and sometimes even as early as some neurons in V1 (Schmolesky et al., 1998). Comparison of visual latencies of neurons that exhibited directional visual motion responses to cue 1 indicates that neurons with shorter visual latencies were significantly more frequent in the caudal FEF than the SEF (Figure 11B, Fukushima et al., 2011a). To examine how the difference between the two areas during delay 1 that signals directional visual motion–memory was reflected in the time course of mean discharge, Figure 11C plots discharge of caudal FEF neurons that exhibited directional responses to cue 1 in their preferred (green) and anti-preferred direction (black) during go trials. Although caudal FEF neurons exhibited a residual visual motion response to cue 1 at the beginning of delay 1, the responses returned to control level near the end of delay 1 before cue 2 onset (Figure 11C, arrow). This contrasts with the discharge of SEF neurons that exhibited directional responses to cue 1 visual motion; cue 1 discharge was maintained during the whole delay 1 period (Figure 11D, arrow).
Movement–preparation neurons. movement–preparation neurons exhibited direction-specific discharge during the delay 2 of go trials (Shichinohe et al., 2009). Figures 11E,F compares discharge modulation of movement–preparation neurons in the caudal FEF (Figure 11E) and SEF (Figure 11F); their time courses were similar. There was no significant difference in the percent of movement–preparation neurons (Figure 11A, delay 2) between the two areas.
No-go neurons. no-go neurons exhibited no-go instruction-specific discharge during delay 2 no-go trials. The proportion of no-go neurons was significantly higher in the SEF than caudal FEF (Fukushima et al., 2011a). As shown in Figure 12A, this SEF no-go neuron exhibited discharge during the action period of go trials, regardless of the pursuit direction (Figure 12A1). When the cue 2 instruction was no-go (Figure 12A2), it exhibited a stronger discharge during cue 2 and delay 2. The difference in discharge modulation during these periods is clear in the mean discharge rates during no-go and go trials (Figure 12B, red vs. black). Furthermore, when the monkey erred during the action period of a no-go trial by pursuing a leftward moving spot (Figure 12A2, red trace), this no-go neuron nearly stopped discharging at cue 2 and during delay 2, suggesting that the discharge during these periods reflected the monkey’s decision to maintain fixation and not to pursue during go trials. This interpretation was supported by the analysis of choice probability during delay 2 with respect to the monkeys’ choice based on whether they maintained fixation (i.e., no-go) or if they pursued a moving spot, regardless of its directions (Figures 12A1 vs. 12A2). The choice probability increased to ∼0.8 after cue 2 and decreased during the action period (Figure 12C).
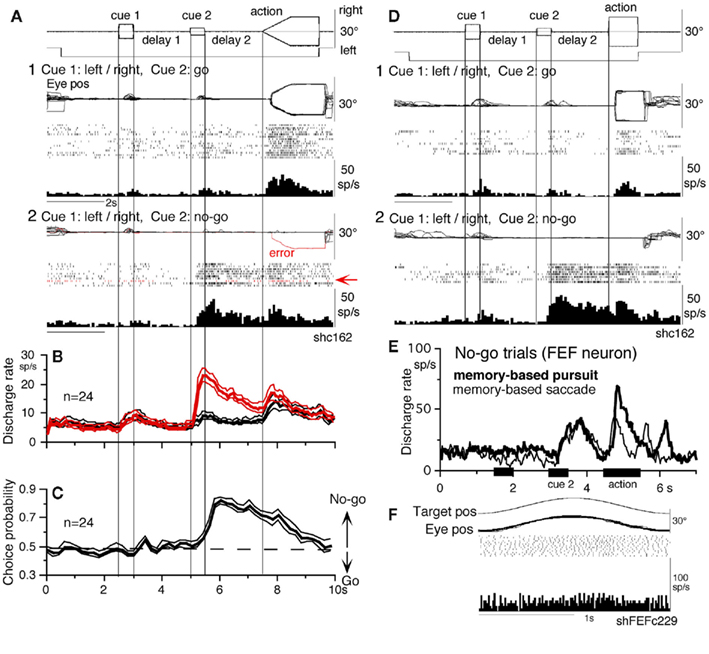
Figure 12. No-go neurons in the SEF and caudal FEF. (A,D) A representative SEF neuron during memory-based pursuit (A) and memory-based saccades (D). (A1) Go trials when rightward and leftward visual motion was applied as cue 1. (A2) No-go trials. Red trace in eye position record (arrow) and arrow in spike raster highlight an error trial. (B) Time course of mean (±SE) discharge of the 24 no-go SEF neurons during no-go (red) and go (black) trials. (C) Choice probability time course for the 24 SEF no-go neurons during no-go and go trials. (D1,D2) Go and no-go trials during memory-based saccades, respectively. (E,F) Discharge of a representative FEF no-go neuron during no-go trials of memory-based pursuit [(E), thick] and memory-based saccades [(E), thin]. (F) Simple pursuit of a single spot that moved sinusoidally. None of no-go neurons responded during simple pursuit of a single spot. (A–D) Reproduced from Shichinohe et al. (2009) with permission. (E–F) Reproduced from Fukushima et al. (2011a) with permission.
No-go related discharge was also observed when the monkeys performed memory-based saccades (Figures 12D1 vs. 12D2). No-go neurons in the caudal FEF behaved similarly (Figure 12E), indicating that no-go signals are common during delay 2 that requires no-go instruction memory (Figure 8A5) for both types of eye movements (Figures 12A,D,E). However, none of no-go neurons tested exhibited discharge modulation during simple pursuit using a single spot (Figure 12F), indicating that no-go neurons differ from pursuit neurons. No-go neurons were reported in a saccadic go/no-go task in the dorsomedial frontal cortex (Mann et al., 1988) and prefrontal cortex and FEF (Hasegawa et al., 2004).
Different effects after chemical inactivation of the SEF and caudal FEF
Significant differences in signals represented in the two areas (see Representation of Directional Visual Motion–Memory and Movement–Preparation Signals and Different Signals Represented in the SEF and Caudal FEF) are consistent with the effects of chemical inactivation. As illustrated in Figure 13B, infusion of GABA agonist muscimol into the SEF resulted in significantly higher direction errors during go trials and go/no-go selection errors during no-go trials (vs. Figure 13A, before infusion). Such errors were not induced by caudal FEF inactivation (Figure 13C). Also, consistent with the existence of movement–preparation neurons in both areas (Figures 11E,F), chemical inactivation of either area impaired an initial smooth-pursuit component before saccades (Figures 13E,F, after, arrows). Furthermore, since both areas contained neurons (visual memory neurons and pursuit neurons) that showed visual motion response enhancement to the cued spot during the action period (e.g., Figures 9B,D, arrows), loss of their activity may also have contributed to the impaired initial pursuit. In addition, consistent with the significant difference in percent of pursuit neurons in the two areas (Figure 11A, action), caudal FEF inactivation significantly decreased pursuit eye velocity during pursuit maintenance, resulting in saccadic tracking (Figures 13D,F), whereas SEF inactivation did not impair pursuit maintenance (Figure 13E, *). In particular, caudal FEF inactivation not only decreased eye velocity gain, but impaired delay compensation of pursuit eye movements during sinusoidal pursuit of a singe spot at frequencies ∼1 Hz (Figures 13G,H), suggesting that the caudal FEF is necessary for response delay compensation during sinusoidal pursuit.
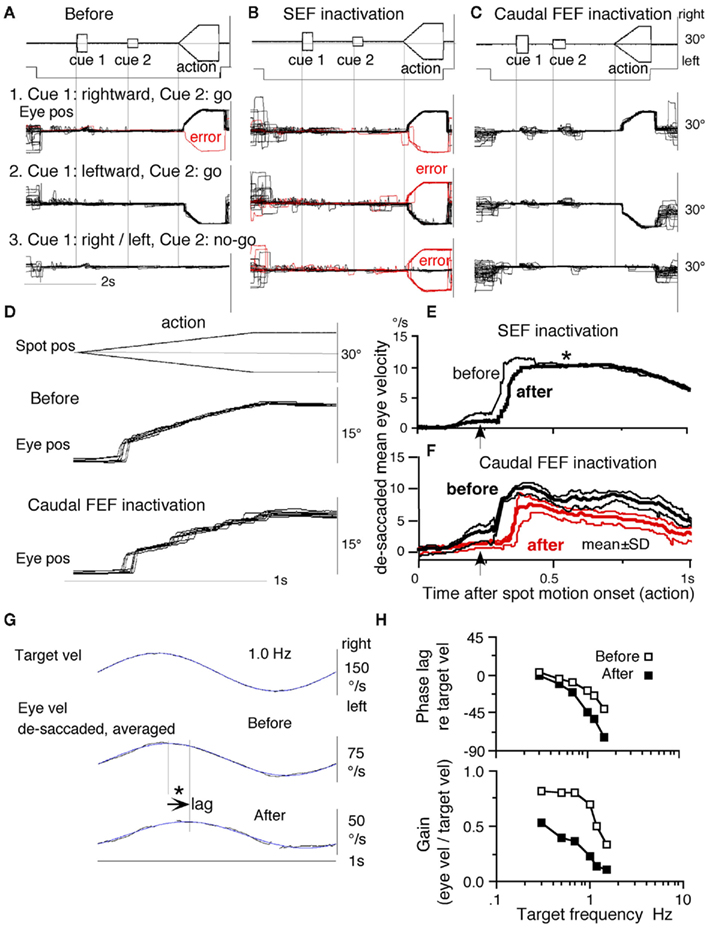
Figure 13. Comparison of effects of muscimol infusion into the SEF and caudal FEF. (A) Before infusion. (B) After SEF inactivation. (C) After caudal FEF inactivation. (D) Tracking eye movements during go trials before and after caudal FEF inactivation. (E) De-saccaded and averaged mean eye velocity before and after SEF inactivation. (F) Mean ± SD eye velocity before and after caudal FEF inactivation. Eye pos = eye position. Red traces in (A,B) show errors. (G) Compares de-saccaded and averaged eye velocity before and after caudal FEF inactivation. Arrow indicates that peak eye velocity lagged after muscimol infusion. (H) Plots phase and gain of eye velocity (relative to target velocity) before (open) and after (filled) caudal FEF inactivation. (A,B,E) Reproduced from Shichinohe et al. (2009) and Fukushima et al. (2011a) with permission. (C,D,F,G,H) Reproduced from Fukushima et al. (2011a,b) with permission.
These results indicate that the SEF is primarily involved in planning smooth-pursuit, whereas the caudal FEF is primarily involved in generating motor commands for pursuit execution. The existence of no-go neurons along with impairment in performing no-go trials after chemical inactivation (Figures 12 and 13B) suggests that the SEF is necessary for decision-process of whether or not to pursue moving spots including working memory of no-go instructions (Shichinohe et al., 2009; Fukushima et al., 2011a).
After inactivation of either area, postsaccadic enhancement of smooth-pursuit (Lisberger, 1998) was still observed (Figures 13B–F), indicating involvement of different neural mechanisms in generating the initial pursuit component and postsaccadic pursuit enhancement. Mahaffy and Krauzlis (2011) reported that inactivation and stimulation of the frontal pursuit area change pursuit metrics without affecting pursuit target selection.
Our knowledge of where the SEF visual memory signals are generated is imprecise. The dorsolateral prefrontal cortex has been linked to temporal storage of sensory signals (i.e., working memory, Goldman-Rakic, 1995). Kim and Shadlen (1999) demonstrated that visual motion responses could be maintained during a delay period in prefrontal cortex neurons. However, in their studies, discharge related to the memory of visual motion could not be separated from discharge related to movement–preparation (also Zaksas and Pasternak, 2006).
Another potential site is MST, since this region, especially MSTd (Desimone and Ungerleider, 1986), sends direct projections to the SEF (Huerta and Kaas, 1990), and MSTd is involved in perception and memory of visual motion (see Coordinate Frames). However, representative signals in MSTd clearly differed from those in the SEF during memory-based smooth-pursuit; MSTd neurons signaled visual motion accurately, but none of the 108 MSTd neurons that showed directional visual motion response to cue 1 exhibited direction- and/or instruction-specific discharge during the delay periods (Kurkin et al., 2011). Although we do not exclude possible alternative types of MSTd neurons coding assessment and memory of visual motion–direction (Ferrera and Lisberger, 1997), it seems more likely that visual motion–direction information sent from MSTd and caudal FEF to the SEF is further processed to create assessment and the memory of visual motion–direction within the SEF (Fukushima et al., 2011a).
Preliminary Results of Clinical Application
Smooth-pursuit eye movements are impaired in most patients with Parkinson’s disease, though the nature of the impairment is poorly understood (see Leigh and Zee, 2006 for review). Working memory impairment during cognitive tasks has been reported (e.g., Possin et al., 2008; Lee et al., 2010). Application of the memory-based smooth-pursuit task to patients with Parkinson’s disease, however, revealed that error rates during go and no-go trials were normal, indicating that the working memory of visual motion–direction and go/no-go selection of the patients tested during this task was normal (Fukushima et al., 2011b).
Clear differences from normal controls were observed during go trials. Normal controls exhibited initial smooth-pursuit component in the cued direction with a mean latency of 155 ms (Figure 14B1, *) followed by corrective saccades (Fukushima et al., 2011b; cf., Garbutt and Lisberger, 2006) which were further followed by enhanced smooth-pursuit responses (cf., Figure 8B2, Lisberger, 1998). In contrast, most patients tracked the correct spot with saccades; initial pursuit was rarely induced before the saccades (Figure 14A1, *), and postsaccadic enhancement of smooth-pursuit was rarely observed. Moreover, consistent with many previous reports, peak pursuit eye velocities after saccades were significantly lower than those of controls during pursuit maintenance (Figures 14A1 vs. 14B1, de-saccaded, averaged).
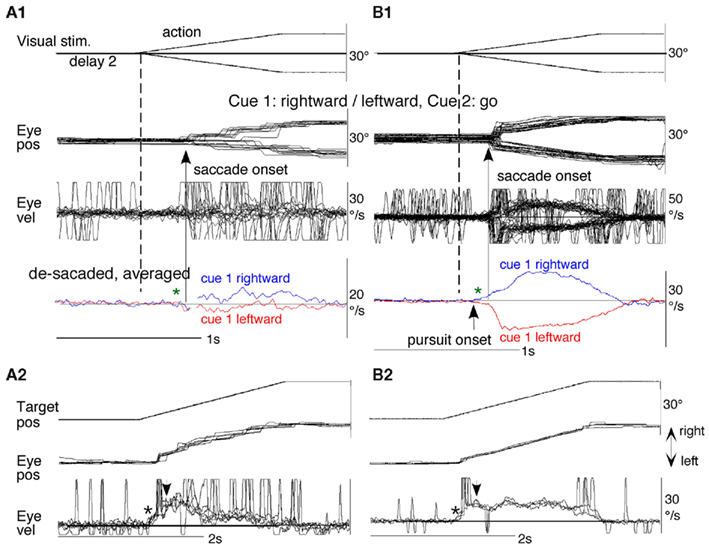
Figure 14. Eye movements of a patient with Parkinson’s disease and an age-matched normal control. (A1,A2) Memory-based pursuit (A1) and ramp pursuit using a single spot (A2) of a Hoehn-Yahr stage III patient (73 years old). (B1,B2) Normal control. In (A1,B1) Go trials with rightward and leftward cue 1 motion were combined. Eye velocity during saccades was clipped. Bottom traces in (A1,B1) are de-saccaded, averaged eye velocity for cue 1 rightward and cue 1 leftward as indicated by colors. Modified from Fukushima et al. (2011b) with permission.
The lack of initial pursuit and deficient postsaccadic enhancement in patients with Parkinson’s disease are unlikely to be due to impairments of smooth-pursuit eye movements per se, since during simple pursuit of a single spot the same patients clearly exhibited an initial pursuit component before saccades, similar to normal controls (Figures 14A2 vs. 14B2, *), and since postsaccadic enhancement of smooth-pursuit was also seen at least for the first saccades after spot motion (Figures 14A2,B2, arrows).
To understand the nature of the lack of initial pursuit during memory-based pursuit in patients, it must first be understood why the initial pursuit appeared in normal controls; visual motion inputs arising from the two oppositely moving spots with the same speed during the action period would be nullified (see Memory-Based Smooth-Pursuit Task Separates Visual Motion–Memory from Movement–Preparation; Lisberger and Ferrera, 1997; Garbutt and Lisberger, 2006). The appearance of the initial pursuit during the action period of memory-based pursuit in controls (Figure 14B1) most probably reflects priming effects by cues and depends on normal activity of the SEF and caudal FEF for the following reasons in monkey studies (see Memory-Based Smooth-Pursuit Task Separates Visual Motion–Memory from Movement–Preparation, Representation of Directional Visual Motion–Memory and Movement–Preparation Signals); (1), cue 1 direction memory and cue 2 go instruction enhance visual motion responses of SEF and caudal FEF neurons in the cued direction (e.g., Figures 9B,D), and (2) chemical inactivation of these frontal cortical areas impairs initial pursuit before saccades (Figures 13E,F). Note that, in monkeys, the acquisition of working memory in this task and the appearance of the initial smooth-pursuit before saccades are separate processes (Figures 8B1,B2); the latter required further training for efficient and nearly “automatic” tracking performance even after the monkeys learned the task basics.
Conversely, the lack of initial pursuit in patients with Parkinson’s disease suggests that they have difficulty in inducing priming effects during memory-based pursuit (Figures 14A1 vs. 14B1, *) which required the patients to prepare and execute smooth-pursuit to a selected spot using the cue information (see Functional Considerations, cf., Ladda et al., 2008). Basal ganglia outputs project to the FEF and SEF through the thalamus, and Cui et al. (2003) described a possible pursuit loop between the caudal FEF and basal ganglia (Figure 15, thick dashed lines, also Lynch and Tian, 2006). Yoshida and Tanaka (2009) suggested that this loop may contribute to maintaining normal pursuit gain.
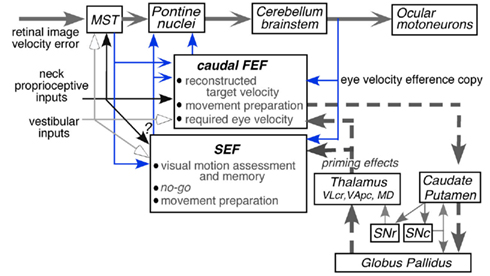
Figure 15. Proposed signal flow diagram for smooth-pursuit eye movements and major signals in the SEF and caudal FEF. Gray thick arrows indicate the direct pathways. Thin arrows indicate indirect pathways through the SEF and caudal FEF. Thick gray dashed lines indicate the pursuit loop between the caudal FEF and basal ganglia through the thalamus proposed by Cui et al. (2003). ? Indicates whether SEF pursuit neurons receive neck proprioceptive inputs remains undetermined. MD, medial dorsal nucleus; SNc, substantia nigra pars compacta; SNr, substantia nigra, pars reticularis; VApc, ventral anterior nucleus, pars parvocellularis; VLcr, rostral portion of the ventral lateral nucleus, pas caudalis. See text for further explanation.
In contrast to normal working memory during memory-based pursuit in patients with Parkinson’s disease who were tested, significantly higher error rates were observed in patients with frontal cortical dysfunction using the identical task (Ito et al., 2011), suggesting that patients with Parkinson’s disease with working memory impairment may have frontal cortical dysfunction that includes the SEF. These preliminary observations taken together suggest that the memory-based smooth-pursuit task is applicable for clinical studies.
Functional Considerations
Figure 15 is a proposed signal flow diagram for smooth-pursuit and major signals in the SEF and caudal FEF. MST sends visual motion signals directly, and indirectly through the caudal FEF and SEF, to the pontine nuclei (Figure 1A). The direct and indirect pathways have different roles (cf., Figure 10A of Shichinohe et al., 2011). The direct pathways (Figure 15, gray thick arrows) may primarily be active during simple pursuit using a single spot in patients with Parkinson’s disease (e.g., Figure 14A2). Reconstruction of target velocity in space with respect to the body is done in the indirect pathways (Shichinohe et al., 2011), primarily in caudal FEF neurons; these neurons are multimodal neurons that receive various signals from sources such as visual, vestibular, and neck proprioceptors (see Vestibular Inputs, Neck Proprioceptive Inputs, Memory-Based Smooth-Pursuit, also Fukushima et al., 2000b, 2002a; Lisberger, 2010). Part of eye velocity efference copy feedback to the caudal FEF and SEF may originate from the outputs of the cerebellar floccular region (section 8.2 of Fukushima et al., 2006; also Figure 10 of Lisberger, 2010). The SEF is needed for more complex smooth-pursuit that requires planning (see Memory-Based Smooth-Pursuit).
The basal ganglia are necessary for efficient and accurate pursuit performance possibly by contributing to maintaining normal pursuit gain (Cui et al., 2003; Lynch and Tian, 2006; Yoshida and Tanaka, 2009) and by inducing the priming effects on visual motion responses of SEF and caudal FEF neurons (Figure 15, thick dashed lines). The latter may be necessary for fast and “automatic” selection of pursuit target during memory-based pursuit (see Memory-Based Smooth-Pursuit; Figure 14B1, *). Perhaps, degeneration of the substantia nigra in Parkinson’s disease may cause dysfunction of the pursuit loop between the basal ganglia and caudal FEF (Figure 15, Cui et al., 2003), resulting in deficient priming effects and pursuit gain drop to the cued direction (Figures 14A1 vs. 14B1, *). Further studies are needed to understand how the basal ganglia contribute to the priming effects. Recently, Warabi et al. (2011) have shown that the prolonged latency of visually induced saccades and wrist movements in Parkinson’s disease is primarily due to the difficulty in terminating the preceding movement/posture. A similar difficulty in terminating ocular fixation may also contribute to the prolonged latency of smooth-pursuit eye movements during our memory-based smooth-pursuit task.
For the primates to behave adequately in three dimensional space, the brain not only receives accurate sensory information but must also place it into the correct spatial coordinates which are most probably provided by the vestibular system (see review for Fuchs and Phillips, 1989; also Fukushima, 1997; see Coordinate Frames). Hemi-spatial neglect is common in patients with lesions of the posterior parietal cortex. They neglect events occurring in the contralateral portion of the extrapersonal space, and neglect encompasses a variety of deficits, which may be motor, sensory, cognitive, or attentional in nature (for reviews, see Bisley and Goldberg, 2010; Corbetta and Shulman, 2011). Spatial neglect also occurs in patients with frontal cortical dysfunction and/or basal ganglia damage (see Fuster, 2008 for review; alsoDamasio et al., 1980; Sakashita, 1991; Corbetta and Shulman, 2011). Vestibular caloric stimulation reduces, in a specific manner, neglect in mental images of representational space (Rode and Perenin, 1994; Pizzamiglio et al., 1995) as well as somatosensory and visuo-spatial hemi-neglect (Vallar et al., 1993, 1995). Similar effects have also been reported to occur by neck muscle vibration that activates neck muscle proprioceptors (Biguer et al., 1988; Karnath et al., 1993; cf., Popov et al., 1999). These observations suggest that, in addition to eye movement-related signals, vestibular and neck proprioceptive information contributes to representation of self-centered space in the frontal cortex (also de Waele et al., 2001).
Concluding Comments
Tanji (1996) stated that the use of the SEF depends more on the behavior or conditional state than the use of the FEF (also Mushiake et al., 1996; Isoda and Tanji, 2002, 2003). We have reviewed examples of many differences in discharge between the caudal FEF and SEF that are consistent with this view, and further extended Tanji’s insight using the memory-based smooth-pursuit task that distinguishes discharges related to movement–preparation from discharges related to processing and/or the memory of target motion signals. While both areas are involved in preparing smooth-pursuit, the SEF is primarily involved in higher planning functions such as working memory of visual motion–direction, deciding for or against it, whereas the caudal FEF is primarily involved in generating motor commands for executing smooth-pursuit eye movements.
There are a number of similarities in eye movement properties and its underlying neural control between monkeys and humans, and these similarities allow eye movement tests as a useful tool for clinical research (Leigh and Zee, 2006). However, there are also many differences between the two primate species especially in the tasks that require cognitive processes such as distractor interference and cuing effects (e.g., Garbutt and Lisberger, 2006; Spering et al., 2006). The functions using these processes in non-human primates are achieved by training monkeys to learn the significance of cues in the task context over many months (e.g., see Memory-Based Smooth-Pursuit Task Separates Visual Motion–Memory from Movement–Preparation). Conversely, distinct neuronal activity in the frontal cortical areas that reflects higher functions is a result of learning to which these areas contribute (e.g., Bichot and Schall, 2002). Human subjects, in contrast, can perform various cognitive tasks including memory-based smooth-pursuit easily after several trials, suggesting a fundamental difference in the way brain processes higher functions. Note that even in humans, functions for accurate pursuit mature later, and higher functions including effective use of cue information for subsequent eye movements also mature later, after preadolescence (e.g., Fukushima et al., 2000a; Takeichi et al., 2003). Furthermore, in most brain diseases, patients’ performance may include adopting new strategies that may also reflect compensation for difficulty in their task performance. Animal and human studies including patients using various cognitive tasks must bear these points in mind for further understanding of the pathophysiology of patients’ eye movements including vestibular/neck interactions and the underlying neural control.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Peter M. Olley of Sapporo Medical University for his valuable comments on the manuscript. Supported by Grant-in-Aid for Scientific Research on Priority Areas (System study on higher-order brain functions) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (17022001).
References
Akao, T., Kurkin, S., Fukushima, J., and Fukushima, K. (2005). Visual and vergence eye movement related responses of pursuit neurons in the caudal frontal eye fields to motion-in-depth stimuli. Exp. Brain Res. 164, 92–108.
Akao, T., Kurkin, S., Fukushima, J., and Fukushima, K. (2009). Otolith inputs to pursuit neurons in the frontal eye fields of alert monkeys. Exp. Brain Res. 193, 455–466.
Akao, T., Saito, H., Fukushima, J., Kurkin, S., and Fukushima, K. (2007a). Latency of vestibular responses of pursuit neurons in the caudal frontal eye fields to whole body rotation. Exp. Brain Res. 177, 400–410.
Akao, T., Kumakura, Y., Kurkin, S., Fukushima, J., and Fukushima, K. (2007b). Directional asymmetry in vertical smooth-pursuit and cancellation of the vertical vestibulo-ocular reflex in juvenile monkeys. Exp. Brain Res. 182, 469–478.
Amador, N., Schlag-Rey, M., and Schlag, J. (2000). Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. J. Neurophysiol. 84, 2166–2170.
Andersen, R. A., Snyder, L. H., Bradley, D. C., and Xing, J. (1997). Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu. Rev. Neurosci. 20, 303–330.
Assad, J. A., and Maunsell, J. H. R. (1995). Neuronal correlates of inferred motion in primate posterior parietal cortex. Nature 37, 518–521.
Badler, J. B., and Heinen, S. J. (2006). Anticipatory movement timing using prediction and external cues. J. Neurosci. 26, 4519–4525.
Barboroica, A., and Ferrera, V. P. (2003). Estimating invisible target speed from neuronal activity in monkey frontal eye field. Nat. Neurosci. 6, 66–74.
Barmack, N. H., and Pettorossi, V. E. (1988). The induction and compensation of asymmetric eye movements following unilateral blockage of a horizontal semicircular canal in the rabbit. J. Neurosci. 8, 2836–2843.
Barnes, G. (1993). Visual-vestibular interaction in the control of head and eye movement: the role of visual feedback and predictive mechanisms. Prog. Neurobiol. 41, 435–472.
Barnes, G. R. (2008). Cognitive processes involved in smooth pursuit eye movements. Brain Cogn. 68, 309–326.
Barnes, G. R., and Collins, C. J. S. (2011). The influence of cues and stimulus history on the non-linear frequency characteristics of the pursuit response to randomized target motion. Exp. Brain Res. 212, 225–240.
Belton, T., and McCrea, R. A. (1999). Contribution of the cerebellar flocculus to gaze control during active head movements. J. Neurophysiol. 81, 3105–3109.
Belton, T., and McCrea, R. A. (2000). Role of the cerebellar flocculus region in cancellation of the VOR during passive whole body rotation. J. Neurophysiol. 84, 1599–1613.
Bichot, N. P., and Schall, J. D. (2002). Priming in macaque frontal cortex during popout visual search: feature-based facilitation and location-based inhibition of return. J. Neurosci. 22, 4675–4685.
Biguer, B., Donaldson, I. M. L., Hein, A., and Jeannerod, M. (1988). Neck muscle vibration modifies the representation of visual motion and direction in man. Brain 111, 1405–1424.
Bisley, J. M., and Goldberg, M. E. (2010). Attention, intention and priority in the parietal lobe. Annu. Rev. Neurosci. 33, 1–21.
Bloomberg, J., Melvill, G. J., and Segal, B. (1991). Adaptive modification of vestibularly perceived rotation. Exp. Brain Res. 84, 47–56.
Bloomberg, J., Melvill, G. J., Segal, B., McFarlane, S., and Soul, J. (1988). Vestibular-contingent voluntary saccades based on cognitive estimates of remembered vestibular information. Adv. Otorhinolaryngol. 41, 71–75.
Britten, K. H., Newsome, W. T., Shadlen, M. N., Celebrini, S., and Movshon, J. A. (1996). A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis. Neurosci. 13, 87–100.
Britten, K. H., and Van Wezel, RJ. (2002). Area MST and heading perception in macaque monkeys. Cereb. Cortex 1, 692–701.
Bronstein, A. M., and Hood, J. D. (1986). The cervico-ocular reflex in normal subjects and patients with absent vestibular function. Brain Res. 373, 399–408.
Burke, M. R., and Barnes, G. R. (2008). Brain and behavior: a task-dependent eye movement study. Cereb. Cortex 18, 126–135.
Celebrini, S., and Newsome, W. T. (1994). Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. J. Neurosci. 14, 4109–4124.
Chen, A. L., Riley, D. E., King, S. A., Joshi, A. C., Serra, A., Liao, K., Cohen, M. L., Otero-Millan, J., Martinez-Conde, S., Strupp, M., and Leigh, R. J. (2010). The disturbance of gaze in progressive supranuclear palsy: implications for pathogenesis. Front. Neurol. 1:147. doi: 10.3389/fneur.2010.00147
Chen, L. L. (2006). Head movements evoked by electrical stimulation in the frontal eye field of the monkey: evidence for independent eye and head control. J. Neurophysiol. 95, 3528–3542.
Chen, L. L., and Wise, S. P. (1995). Neuronal activity in the supplementary eye field during acquisition of conditional oculomotor associations. J. Neurophysiol. 73, 1101–1121.
Coe, B., Tomihara, K., Matsuzawa, M., and Hikosaka, O. (2002). Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J. Neurosci. 22, 5081–5090.
Corbetta, M., and Shulman, G. L. (2011). Apatial neglect and attention network. Annu. Rev. Neurosci. 34, 569–599.
Cui, D.-M., Yan, Y.-J., and Lynch, J. C. (2003). Pursuit subregion of the frontal eye field projects to the caudate nucleus in monkeys. J. Neurophysiol. 89, 2678–2684.
Daddaoua, N., Dicke, P. W., and Thier, P. (2008). The subjective visual vertical in a nonhuman primate. J. Vis. 8, 1–8.
Damasio, A. R., Damasio, H., and Chui, H. C. (1980). Neglect following damage to frontal lobe or basal ganglia. Neuropsychologia 18, 123–132.
de Hemptinne, C., Lefevre, P., and Missal, M. (2008). Neuronal basis of directional expectation and anticipatory pursuit. J. Neurosci. 28, 4298–4310.
de Vrijer, M., Medendorp, W. P., and van Gisbergen, J. A. (2008). Shared computational mechanism for tilt compensation accounts for biased verticality percepts in motion and pattern vision. J. Neurophysiol. 99, 915–930.
de Waele, C., Baudonniere, P. M., Lepecq, J. C., Huy, P. T. B., and Vidal, P. P. (2001). Vestibular projections in the human cortex. Exp. Brain Res. 141, 541–551.
Desimone, R., and Ungerleider, L. G. (1986). Multiple visual areas in the superior temporal sulcus of the macaque. J. Comp. Neurol. 248, 164–189.
Duffy, C. J. (1998). MST neurons respond to optic flow and translational movement. J. Neurophysiol. 80, 1816–1827.
Fasold, O., Heinau, J., Trenner, M. U., Villringer, A., and Wenzel, R. (2008). Proprioceptive head posture-related processing in human polysensory cortical areas. Neuroimage 40, 1232–1242.
Ferrera, V. P., and Lisberger, S. G. (1997). Neuronal responses in visual areas MT and MST during smooth pursuit target selection. J. Neurophysiol. 78, 1433–1446.
Fuchs, A. F., and Phillips, J. O. (1989). “Association cortex,” in Textbook of Physiology, Vol. 1, eds H. D. Patton, A. F. Fuchs, B. Hille, A. M. Scher, and R. Steiner (Philadelphia: W. B. Saunders Company), 663–692.
Fujiwara, K., Akao, T., Kurkin, S., and Fukushima, K. (2009). Discharge of pursuit neurons in the caudal part of the frontal eye fields during cross-axis vestibular-pursuit training in monkeys. Exp. Brain Res. 195, 229–240.
Fujiwara, K., Akao, T., Kurkin, S., and Fukushima, K. (2011). Activity of pursuit-related neurons in medial superior temporal area (MST) during static roll-tilt. Cereb. Cortex 21, 155–165.
Fukushima, J., Akao, T., Kurkin, S., Kaneko, C. R. S., and Fukushima, K. (2006). The vestibular-related frontal cortex and its role in smooth-pursuit eye movements and vestibular-pursuit interactions. J. Vestib. Res. 16, 1–22.
Fukushima, J., Akao, T., Shichinohe, N., Kurkin, S., Kaneko, C. R. S., and Fukushima, K. (2011a). Neuronal activity in the caudal frontal eye fields of monkeys during memory-based smooth-pursuit eye movements: comparison with the supplementary eye fields. Cereb. Cortex 21, 1910–1924.
Fukushima, K., Fukushima, J., Kaneko, C. R. S., Belton, T., Ito, N., Olley, P. M., and Warabi, T. (2011b). Memory-based smooth-pursuit: neuronal mechanisms and preliminary results of clinical application. Ann. N. Y. Acad. Sci. 1233, 117–126.
Fukushima, J., Akao, T., Takeichi, N., Kaneko, C. R. S., and Fukushima, K. (2003). Involvement of the frontal oculomotor areas in developmental compensation for the directional asymmetry in smooth pursuit eye movements in young primates. Ann. N. Y. Acad. Sci. 1004, 451–456.
Fukushima, J., Akao, T., Takeichi, N., Kurkin, S., Kaneko, C. R. S., and Fukushima, K. (2004a). Pursuit-related neurons in the supplementary eye fields: discharge during pursuit and passive whole body rotation. J. Neurophysiol. 91, 2809–2825.
Fukushima, K., Yamanobe, T., Shinmei, Y., Fukushima, J., and Kurkin, S. (2004b). Role of the frontal eye fields in smooth gaze tracking. Prog. Brain Res. 143, 391–401.
Fukushima, J., Hatta, T., and Fukushima, K. (2000a). Development of voluntary control of saccadic eye movements. (I) Age-related changes in normal children. Brain Dev. 22, 173–180.
Fukushima, K., Sato, T., Fukushima, J., Shinmei, Y., and Kaneko, C. R. S. (2000b). Activity of smooth pursuit-related neurons in the monkey periarcuate cortex during pursuit and passive whole body rotation. J. Neurophysiol. 83, 563–587.
Fukushima, K. (1997). Cortico-vestibular interactions: anatomy, electro-physiology and functional considerations. Exp. Brain Res. 117, 1–16.
Fukushima, K. (2003a). Frontal cortical control of smooth-pursuit. Curr. Opin. Neurobiol. 13, 647–654.
Fukushima, K. (2003b). Roles of the cerebellum in pursuit-vestibular interactions. Cerebellum 2, 223–232.
Fukushima, K., Akao, T., Kurkin, S., and Fukushima, J. (2005). Role of vestibular signals in the caudal part of the frontal eye fields in pursuit eye movements in three-dimensional space. Ann. N. Y. Acad. Sci. 1039, 272–282.
Fukushima, K., Akao, T., Saito, H., Kurkin, S., Fukushima, J., and Peterson, B. W. (2010). Representation of neck velocity and neck-vestibular interactions in pursuit neurons in the simian frontal eye fields. Cereb. Cortex 20, 1195–1207.
Fukushima, K., Akao, T., Shichinohe, N., Nitta, T., Kurkin, S., and Fukushima, J. (2008). Predictive signals in the pursuit area of the monkey frontal eye fields. Prog. Brain Res. 171, 433–440.
Fukushima, K., Kasahara, S., Akao, T., Kurkin, S., Fukushima, J., and Peterson, B. W. (2009). Eye-pursuit and re-afferent head movement signals carried by pursuit neurons in the caudal part of the frontal eye fields during head-free pursuit. Cereb. Cortex 19, 263–275.
Fukushima, K., Wells, S. G., Takeichi, N., Yamanobe, T., and Fukushima, J. (2001a). Adaptive changes in smooth pursuit eye movement induced by pursuit-vestibular interaction training in monkeys. Exp. Brain Res. 139, 473–481.
Fukushima, K., Fukushima, J., Yamanobe, T., Shinmei, Y., and Kurkin, S. (2001b). Adaptive eye movements induced by cross-axis pursuit-vestibular interactions in trained monkeys. Acta Otolaryngol. Suppl. 545, 73–79.
Fukushima, K., Yamanobe, T., Shinmei, Y., and Fukushima, J. (2002a). Predictive responses of peri-arcuate pursuit neurons to visual target motion. Exp. Brain Res. 145, 104–120.
Fukushima, K., Yamanobe, T., Shinmei, Y., Fukushima, J., Kurkin, S., and Peterson, B. W. (2002b). Coding of smooth eye movements in three-dimensional space by frontal cortex. Nature 419, 157–162.
Garbutt, S., and Lisberger, S. G. (2006). Directional cuing of target choice in human smooth pursuit eye movements. J. Neurosci. 26, 12479–12486.
Goldberg, J. M., Wilson, V. J., Cullen, K. E., Angelaki, D. E., Broussard, D. M., Büttner-Ennever, J. A., Fukushima, K., and Minor, L. B. (2011). The Vestibular System. A Sixth Sense. New York: Oxford University Press.
Gottlieb, J. P., Bruce, C. J., and MacAvoy, M. G. (1993). Smooth eye movements elicited by microstimulation in the primate frontal eye field. J. Neurophysiol. 69, 786–799.
Gottlieb, J. P., MacAvoy, M. G., and Bruce, C. J. (1994). Neural responses related to smooth pursuit eye movements and their correspondence with electrically elicited slow eye movements in the primate frontal eye field. J. Neurophysiol. 72, 1634–1653.
Grasse, K. L., and Lisberger, S. G. (1992). Analysis of a naturally occurring asymmetry in vertical smooth pursuit eye movements in a monkey. J. Neurophysiol. 67, 164–179.
Gu, Y., DeAngelis, G. C., and Angelaki, D. E. (2007). A functional link between area MSTd and heading perception based on vestibular signals. Nat. Neurosci. 10, 1038–1047.
Hasegawa, R. P., Peterson, B. W., and Goldberg, M. E. (2004). Prefrontal neurons coding suppression of specific saccades. Neuron 42, 415–425.
Heinen, S. J. (1995). Single neuron activity in the dorsomedial frontal cortex during smooth pursuit eye movements. Exp. Brain Res. 104, 357–361.
Heinen, S. J., and Liu, M. (1997). Single-neuron activity in the dorsomedial frontal cortex during smooth-pursuit eye movements to predictable target motion. Vis. Neurosci. 14, 853–865.
Heuer, H. W., and Britten, K. H. (2004). Optic flow signals in extrastriate area MST: comparison of perceptual and neuronal sensitivity. J. Neurophysiol. 91, 1314–1326.
Huerta, M. F., and Kaas, J. H. (1990). Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J. Comp. Neurol. 293, 299–330.
Huerta, M. F., Krubitzer, L. A., and Kaas, J. H. (1987). Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J. Comp. Neurol. 265, 332–361.
Isoda, M., and Tanji, J. (2002). Cellular activity in the supplementary eye field during sequential performance of multiple saccades. J. Neurophysiol. 88, 3541–3545.
Isoda, M., and Tanji, J. (2003). Contrasting neuronal activity in the supplementary and frontal eye fields during temporal organization of multiple saccades. J. Neurophysiol. 90, 3054–3065.
Israël, I., Fetter, M., and Koenig, E. (1993). Vestibular perception of passive whole-body rotation about horizontal and vertical axes in humans: goal-directed vestibulo-ocular reflex and vestibular memory-contingent saccades. Exp. Brain Res. 96, 335–346.
Israël, I., Rivaud, S., Berthoz, A., and Pierrot-Deseilligny, C. (1992). Cortical control of vestibular memory-guided saccades. Ann. N. Y. Acad. Sci. 656, 472–484.
Ito, F., Ikeno, K., Kobayashi, N., Takei, H., Olley, P. M., Chiba, S., Inoue, K., Fukushima, K., and Warabi, T. (2011). Clinical application of a memory-based smooth pursuit eye movement (SPEM) task to patients with idiopathic Parkinson’s disease (PD) and patients with frontal dysfunction. Neurosci. Res. 71(Suppl.), :e145.
Kaptein, R. G., and van Gisbergen, J. A. (2004). Interpretation of a discontinuity in the sense of verticality at large body tilt. J. Neurophysiol. 91, 2205–2214.
Karnath, H. O., Christ, K., and Hartje, W. (1993). Decrease of contralateral neglect by neck muscle vibration and spatial orientation of trunk midline. Brain 116, 383–396.
Kasahara, S., Akao, T., Fukushima, J., Kurkin, S., and Fukushima, K. (2006). Further evidence for selective difficulty of upward eye pursuit in young monkeys: effects of optokinetic stimulation, static roll tilt, and active head movements. Exp. Brain Res. 171, 306–321.
Keating, E. G. (1991). Frontal eye field lesions impair predictive and visually-guided pursuit eye movements. Exp. Brain Res. 86, 311–323.
Keating, E. G. (1993). Lesions of the frontal eye field impair pursuit eye movements, but preserve the predictions driving them. Behav. Brain Res. 53, 91–104.
Kim, J.-M., and Shadlen, M. N. (1999). Neural correlates of decision in the dorsolateral prefrontal cortex of the macaque. Nat. Neurosci. 2, 176–185.
Kim, Y.-G., Badler, J. B., and Heinen, S. J. (2005). Trajectory interpretation by supplementary eye field neurons during ocular baseball. J. Neurophysiol. 94, 1385–1391.
Knight, T. A., and Fuchs, A. F. (2007). Contribution of the frontal eye field to gaze shifts in the head-unrestrained monkey: effects of microstimulation. J. Neurophysiol. 97, 618–634.
Krejcova, H., Highstein, S., and Cohen, B. (1971). Labyrinthine and extra-labyrinthine effects on ocular counter-rolling. Acta Otolaryngol. 72, 165–171.
Kurkin, S., Akao, T., Fukushima, J., and Fukushima, K. (2009). Discharge of pursuit-related neurons in the caudal part of the frontal eye fields in juvenile monkeys with up-down pursuit asymmetry. Exp. Brain Res. 193, 181–188.
Kurkin, S., Akao, T., Shichinohe, N., Fukushima, J., and Fukushima, K. (2011). Neuronal activity in medial superior temporal area (MST) during memory-based smooth pursuit eye movements in monkeys. Exp. Brain Res. 214, 293–301.
Kurkin, S. A., Akao, T., Fukushima, J., and Fukushima, K. (2007). Activity of pursuit neurons in the caudal part of the frontal eye fields during static roll-tilt. Exp. Brain Res. 176, 658–664.
Ladda, J., Valkovič, P., Eggert, T., and Straube, A. (2008). Parkinsonian patients show impaired predictive smooth pursuit. J. Neurol. 255, 1071–1078.
Lanman, J., Bizzi, E., and Allum, J. (1978). The coordination of eye and head movement during smooth pursuit. Brain Res. 153, 39–53.
Lee, E. Y., Cowan, N., Vogel, E. K., Rolan, T., Valle-Inclan, F., and Hackley, S. A. (2010). Visual working memory deficits in patients with Parkinson’s disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain 133, 2677–2689.
Leigh, R., and Zee, D. S. (2006). The Neurology of Eye Movements, 4th Edn. New York: Oxford University Press.
Lewis, R. F., Haburcakova, C., and Merfeld, D. M. (2008). Roll tilt psychophysics in rhesus monkeys during vestibular and visual stimulation. J. Neurophysiol. 100, 140–153.
Lisberger, S., and Fuchs, A. F. (1978). Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J. Neurophysiol. 41, 733–763.
Lisberger, S. G. (1990). Visual tracking in monkeys: evidence for short-latency suppression of the vestibuloocular reflex. J. Neurophysiol. 63, 676–688.
Lisberger, S. G. (1998). Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J. Neurophysiol. 79, 1918–1930.
Lisberger, S. G. (2010). Visual guidance of smooth-pursuit eye movements: sensation, action, and what happens in between. Neuron 66, 477–491.
Lisberger, S. G., and Ferrera, V. P. (1997). Vector averaging for smooth pursuit eye movements initiated by two moving targets in monkeys. J. Neurosci. 17, 7490–7502.
Lisberger, S. G., Morris, E. J., and Tychsen, L. (1987). Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu. Rev. Neurosci. 10, 97–129.
Liu, S., and Angelaki, D. E. (2009). Vestibular signals in macaque extrastriate visual cortex are functionally appropriate for heading perception. J. Neurosci. 29, 8936–8945.
Lu, X., Matsuzawa, M., and Hikosaka, O. (2002). A neural correlate of oculomotor sequences in supplementary eye field. Neuron 34, 317–325.
Lynch, J. C. (1987). Frontal eye field lesions in monkeys disrupt visual pursuit. Exp. Brain Res. 68, 437–441.
Lynch, J. C., and Tian, J.-R. (2006). Cortico-cortical networks and cortico-subcortical loops for the higher control of eye movements. Prog. Brain Res. 151, 461–501.
MacAvoy, M. G., Gottlieb, J. P., and Bruce, C. J. (1991). Smooth pursuit eye movement representation in the primate frontal eye field. Cereb. Cortex 1, 95–102.
Mahaffy, S., and Krauzlis, R. A. (2011). Inactivation and stimulation of the frontal pursuit area change pursuit metrics without affecting pursuit target selection. J. Neurophysiol. 106, 347–360.
Maioli, C., Falciati, L., and Gianesini, T. (2007). Pursuit eye movements involve a covert motor plan for manual tracking. J. Neurosci. 27, 7168–7173.
Mann, S. E., Thau, R., and Schiller, P. H. (1988). Conditional task-related responses in monkey dorsomedial frontal cortex. Exp. Brain Res. 69, 460–468.
Marti, S., Bockisch, C. J., and Straumann, D. (2006). Asymmetric short-term adaptation of the vertical vestibulo-ocular reflex in humans. Exp. Brain Res. 172, 343–350.
Martinez-Trujillo, J. C., Mendendorp, W. P., Wang, H., and Crawford, J. D. (2004). Frames of reference for eye-head gaze commands in primate supplementary eye fields. Neuron 44, 1057–1066.
McKinley, P. A., and Peterson, B. W. (1985). Voluntary modulation of the vestibuloocular reflex in humans and its relation to smooth pursuit. Exp. Brain Res. 60, 454–496.
Miles, F. A., and Fuller, J. H. (1975). Visual tracking and the primate flocculus. Science 189, 1000–1003.
Miles, F. A., Fuller, J. H., Braitman, D. J., and Dow, B. M. (1980). Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J. Neurophysiol. 43, 1437–1476.
Miles, F. A., and Lisberger, S. G. (1981). The “error” signals subserving adaptive gain control in the primate vestibulo-ocular reflex. Ann. N. Y. Acad. Sci. 374, 513–525.
Missal, M., and Heinen, S. J. (2001). Facilitation of smooth pursuit initiation by electrical stimulation in the supplementary eye fields. J. Neurophysiol. 86, 2413–2425.
Missal, M., and Heinen, S. J. (2004). Supplementary eye fields stimulation facilitates anticipatory pursuit. J. Neurophysiol. 92, 1257–1262.
Misslisch, H., Tweed, D., Fetter, M., Dichgans, J., and Vilis, T. (1996). Interaction of smooth pursuit and the vestibuloocular reflex in three dimensions. J. Neurophysiol. 75, 2520–2532.
Miyamoto, T., Fukushima, K., Takada, T., de Waele, C., and Vidal, P.-P. (2007). Saccular stimulation of the human cortex: a functional magnetic resonance imaging study. Neurosci. Lett. 423, 68–72.
Murofushi, T., Curthoys, I. S., Topple, A. N., Colebatch, J. G., and Halmagyi, G. M. (1995). Responses of guinea pig primary vestibular neurons to clicks. Exp. Brain Res. 103, 174–178.
Mushiake, H., Fujii, N., and Tanji, J. (1996). Visually guided saccade versus eye-hand reach: contrasting neuronal activity in the cortical supplementary and frontal eye fields. J. Neurophysiol. 75, 2187–2191.
Nakamura, K., Sakai, K., and Hikosaka, O. (1998). Neuronal activity in medial frontal cortex during learning of sequential procedures. J. Neurophysiol. 80, 2671–2687.
Nakamura, T., and Bronstein, A. (1995). The perception of head and neck angular displacement in normal and labyrinthine-defective subjects: a quantitative study using a “remembered saccade” technique. Brain 118, 1157–1168.
Newsome, W. T., and Pare, E. B. (1988). A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J. Neurosci. 8, 2201–2211.
Ogawa, T., and Fujita, M. (1998). Velocity profile of smooth pursuit eye movements in humans: pursuit velocity increase linked with the initial saccade occurrence. Neurosci. Res. 31, 201–209.
Olson, C. R., and Gettner, S. N. (1999). Macaque SEF neurons encode object-centered directions of eye movements regardless of the visual attributes of instructional cues. J. Neurophysiol. 81, 2340–2346.
Olson, C. R., Gettner, S. N., Ventura, V., Carta, R., and Kass, R. E. (2000). Neuronal activity in macaque supplementary eye field during planning of saccades in response to pattern and spatial cues. J. Neurophysiol. 84, 1369–1384.
Park, J., Schlag-Rey, M., and Schlag, J. (2006). Frames of reference for saccadic command tested by saccade collision in the supplementary eye field. J. Neurophysiol. 95, 159–1706.
Pierrot-Deseilligny, C., Israël, I., Berthoz, A., Rivaud, S., and Gaymard, B. (1993). Role of the different frontal lobe areas in the control of the horizontal component of memory-guided saccades in man. Exp. Brain Res. 95, 166–171.
Pierrot-Deseilligny, C., Rivaud, S., Gaymond, B., Müri, R., and Vermersch, A.-I. (1995). Cortical control of saccades. Ann. Neurol. 37, 557–567.
Pizzamiglio, L., Vallar, G., and Doricchi, F. (1995). Gravity and hemineglect. Neuroreport 7, 370–371.
Popov, K. E., Lekhel, H., Faldon, M., Bronstein, A. M., and Gresty, M. A. (1999). Visual and oculomotor responses induced by neck vibration in normal subjects and labyrinthine-defective patients. Exp. Brain Res. 128, 343–352.
Possin, K. L., Filoteo, J. V., Song, D. D., and Salmon, D. P. (2008). Spatial and object working memory deficits in Parkinson’s disease are due to impairment in different underlying processes. Neuropsychology 22, 585–595.
Rode, G., and Perenin, M. T. (1994). Temporary remission of representational hemineglect through vestibular stimulation. Neuroreport 5, 869–872.
Roy, J. E., and Cullen, K. E. (1998). A neural correlate for vestibulo-ocular reflex suppression during voluntary eye-head gaze shifts. Nat. Neurosci. 1, 404–410.
Russo, G. S., and Bruce, C. J. (1996). Neurons in the supplementary eye field of rhesus monkeys code visual targets and saccadic eye movements in an oculocentric coordinate system. J. Neurophysiol. 76, 825–848.
Sakashita, Y. (1991). Visual attentional disturbance with unilateral lesions in the basal ganglia and deep white matter. Ann. Neurol. 30, 673–677.
Schall, J. D. (1991). Neuronal activity related to visually guided saccadic eye movements in the supplementary motor area of rhesus monkeys. J. Neurophysiol. 66, 530–558.
Schlag, J., and Schlag-Rey, M. (1987). Evidence for a supplementary eye field. J. Neurophysiol. 57, 179–200.
Schlag-Rey, M., Amador, N., Sanchez, H., and Schlag, J. (1997). Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature 390, 398–340.
Schlindwein, P., Mueller, M., Bauermann, T., Brandt, T., Stoeter, P., and Dieterich, M. (2008). Cortical representation of saccular vestibular stimulation: VEMPs in fMRI. Neuroimage 39, 19–31.
Schmid, A., Rees, G., Frith, C., and Barnes, G. (2001). An fMRI study of anticipation and learning of smooth pursuit eye movements in humans. Neuroreport 12, 1409–1414.
Schmolesky, M. T., Wang, Y., Hanes, D. P., Thompson, K. G., Leutgeb, S., Schall, J. D., and Leventhal, A. G. (1998). Signal timing across the macaque visual system. J. Neurophysiol. 79, 3272–3278.
Shi, D., Friedman, H. R., and Bruce, C. J. (1998). Deficits in smooth pursuit eye movements after muscimol inactivation within the primate frontal eye field. J. Neurophysiol. 80, 458–464.
Shichinohe, N., Akao, T., Kurkin, S., Fukushima, J., Kaneko, C. R. S., and Fukushima, K. (2009). Memory and decision-making in the frontal cortex during visual motion-processing for smooth pursuit eye movements. Neuron 62, 717–732.
Shichinohe, N., Barnes, G., Akao, T., Kurkin, S., Fukushima, J., Kase, M., Leigh, R. J., Belton, T., and Fukushima, K. (2011). Oscillatory eye movements resembling pendular nystagmus in normal juvenile macaques. Invest. Ophthalmol. Vis. Sci. 52, 3458–3467.
Spering, M., Gegenfurtner, K. R., and Kerzel, D. (2006). Distractor interference during smooth pursuit eye movements. J. Exp. Psychol. Hum. Percept. Perform. 32, 1136–1154.
Strupp, M., Arbusow, V., Dieterich, M., Sautier, W., and Brandt, T. (1998). Perceptual and oculomotor effects of neck muscle vibration in vestibular neuritis. Ipsilateral somatosensory substitution of vestibular function. Brain 121, 677–685.
Suzuki, Y., Kase, M., Kato, H., and Fukushima, K. (1997). Stability of ocular counterrolling and Listings plane during static roll tilts. Invest. Ophthalmol. Vis. Sci. 38, 2103–2111.
Takeichi, N., Fukushima, J., Kurkin, S., Yamanobe, T., Shinmei, Y., and Fukushima, K. (2003). Directional asymmetry in smooth ocular tracking in the presence of visual background in young and adult primates. Exp. Brain Res. 149, 380–390.
Takeichi, N., Fukushima, K., Sasaki, H., Yabe, I., Tashiro, K., and Inuyama, Y. (2000). Dissociation of smooth pursuit and VOR-cancellation in spinocerebellar ataxia type 6. Neurology 54, 860–866.
Tanaka, M., and Fukushima, K. (1998). Neuronal responses related to smooth pursuit eye movements in the periarcuate cortical area of monkeys. J. Neurophysiol. 80, 28–47.
Tanaka, M., and Lisberger, S. G. (2002). Enhancement of multiple components of pursuit eye movement by microstimulation in the arcuate frontal pursuit area in monkeys. J. Neurophysiol. 87, 802–818.
Tehovnik, E. J., Sommer, M. A., Chou, I.-H., Slocum, W. M., and Schiller, P. H. (2000). Eye fields in the frontal lobes of primates. Brain Res. Rev. 32, 413–448.
Tian, J., and Lynch, J. C. (1996). Functionally defined smooth and saccadic eye movement subregions in the frontal eye field of cebus monkeys. J. Neurophysiol. 76, 2740–2771.
Tsubuku, T., Akao, T., Kurkin, S. A., and Fukushima, K. (2006). Prediction in the timing of pursuit eye movement initiation revealed by cross-axis vestibular-pursuit training in monkeys. Exp. Brain Res. 168, 427–435.
Tu, T. A., and Keating, G. (2000). Electrical stimulation of the frontal eye field in a monkey produces combined eye and head movements. J. Neurophysiol. 84, 1103–1106.
Vallar, G., Bottini, G., Rusconi, M. L., and Sterzi, R. (1993). Exploring somatosensory hemineglect by vestibular stimulation. Brain 116, 71–86.
Vallar, G., Papagno, C., Rusconi, M. L., and Bisiach, E. (1995). Vestibular stimulation, spatial hemineglect and dysphasia, selective effects. Cortex 31, 589–593.
van der Steen, J., Russel, I. S., and James, G. O. (1986). Effects of unilateral frontal eye-field lesions on eye-head coordination in monkey. J. Neurophysiol. 55, 696–714.
Vidal, P. P., de Waele, C., Baudonniere, P. M., Lepecq, J. C., and Ba Huy, P. T. (1999). Vestibular projections in the human cortex. Ann. N. Y. Acad. Sci. 871, 455–457.
Wang, X., Zhang, M., Cohen, I. S., and Goldberg, M. E. (2007). The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nat. Neurosci. 10, 640–646.
Warabi, T., Fukushima, K., Olley, P. M., Chiba, S., and Yanagisawa, N. (2011). Difficulty in terminating the preceding movement/posture explains the impaired initiation of new movements in Parkinson’s disease. Neurosci. Lett. 496, 84–89.
Yoshida, A., and Tanaka, M. (2009). Neuronal activity in the primate globus pallidus during smooth pursuit eye movements. Neuroreport 20, 121–125.
Keywords: gaze-velocity, neck proprioception, frontal eye fields, supplementary eye fields, coordinate frames, visual motion–memory, no-go, movement–preparation
Citation: Fukushima K, Fukushima J and Warabi T (2011) Vestibular-related frontal cortical areas and their roles in smooth-pursuit eye movements: representation of neck velocity, neck-vestibular interactions, and memory-based smooth-pursuit. Front. Neur. 2:78. doi: 10.3389/fneur.2011.00078
Received: 07 October 2011; Paper pending published: 11 November 2011;
Accepted: 20 November 2011; Published online: 14 December 2011.
Edited by:
Pierre-Paul Vidal, Universite Rene Descartes, FranceReviewed by:
Hans van der Steen, Erasmus Medical Center, NetherlandsGiacinto Asprella-Libonati, Madonne delle Grazie Hospital ASM Matera, Italy
Copyright: © 2011 Fukushima, Fukushima and Warabi. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Kikuro Fukushima, Clinical Brain Research Laboratory, Department of Neurology, Toyokura Memorial Hall, Sapporo Yamanoue Hospital, Yamanote 6-9-1-1, Nishiku, Sapporo 063-0006, Japan. e-mail: kikuro@med.hokudai.ac.jp
