- Medical Research Council Institute of Hearing Research, University of Nottingham, Nottingham, UK
Tinnitus is highly complex, diverse, and difficult to treat, in part due to the fact that the underlying causes and mechanisms remain elusive. Tinnitus is generated within the auditory brain; however, consolidating our understanding of tinnitus pathophysiology is difficult due to the diversity of reported effects and the variety of implicated brain nuclei. Here, we focus on the inferior colliculus (IC), a midbrain structure that integrates the vast majority of ascending auditory information and projects via the thalamus to the auditory cortex. The IC is also a point of convergence for corticofugal input and input originating outside the auditory pathway. We review the evidence, from both studies with human subjects and from animal models, for the contribution the IC makes to tinnitus. Changes in the IC, caused by either noise exposure or drug administration, involve fundamental, heterogeneous alterations in the balance of excitation and inhibition. However, differences between hearing loss-induced pathology and tinnitus-related pathology are not well understood. Moreover, variability in tinnitus induction methodology has a significant impact on subsequent neural and behavioral changes, which could explain some of the seemingly contradictory data. Nonetheless, the IC is likely involved in the generation and persistence of tinnitus perception.
Introduction
The most common etiology of chronic tinnitus in the human population arises from exposure to excessive levels of noise (1). Tinnitus is suggested to affect between 8 and 15% of the population and is extremely debilitating in ~1% (2). It is often perceived as a ringing sound (the word tinnitus originates from the Latin tinnire, which translates as “to ring”), but characteristics vary across individuals (3). While the cause of chronic tinnitus was believed to reside within the inner ear (4), it is now widely accepted that changes in the central auditory system are pivotal in generating the phantom percept, as symptoms persist following cochlea ablation (5) or severance of the auditory nerve (AN) (6). However, in the early stages of tinnitus development, central changes still appear to be dependent upon peripheral activity (7, 8).
The inferior colliculus (IC) is a near-obligatory relay in the ascending auditory pathway, a point at which virtually all lemniscal and extra-lemniscal ascending inputs converge (9). As such, pathophysiological changes in the IC can alter all aspects of auditory perception. Thus, the IC has been putatively proposed as an important structure in central mechanisms that underlie subjective tinnitus, and has been widely studied in this context (10).
In this review, we concentrate on research focused on the role of the IC in tinnitus. This translates – in the main – to animal models of tinnitus, which have been extensively used to identify putative neural correlates of tinnitus in the midbrain, such as changes in patterns of neural activity and alterations in neurotransmission. While acoustic over-exposure (AOE), or damage caused by intense sound, is the most prevalent cause of tinnitus in humans, here we also consider animal studies examining the effects of pharmacologically induced tinnitus in an attempt to consolidate the nuanced similarities and differences between models. In addition, we consider data derived from imaging studies with tinnitus patients. Finally, we discuss IC pathophysiology as a contributing factor in the context of different tinnitus models, and the likelihood of tinnitus generation occurring through interactions with other neural circuitries, such as limbic and somatosensory systems.
Changes in the IC Following AOE
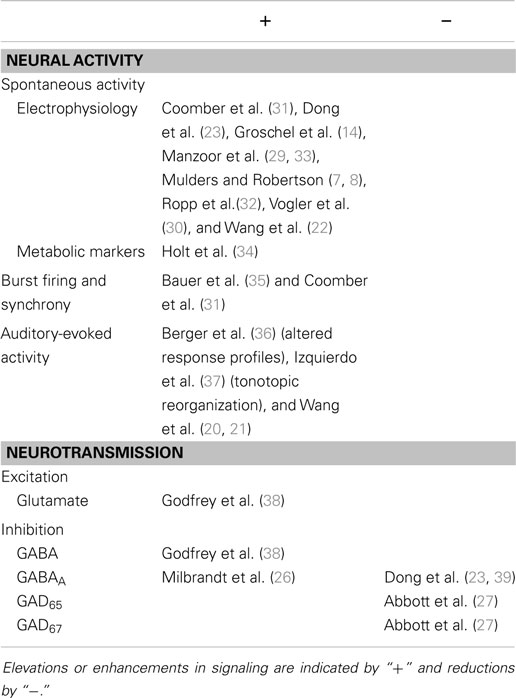
Short-term changes following noise exposure are summarized in Table 1. In the immediate aftermath of intense noise exposure, a number of studies have demonstrated altered patterns of neural activity in the IC. In mouse brain slice recordings spontaneous firing rates (SFRs) of IC neurons decreased (11). Furthermore, auditory-evoked responses in the IC of awake guinea pigs were reduced immediately after noise exposure (12, 13). In contrast, other groups have found no immediate change in IC firing rates recorded in vitro (14), or increased neuronal firing in vivo 12 h post-AOE (15). Increased neural activity in the IC was implied by elevated c-Fos (a gene associated with neuronal depolarization) in rats (16). The most likely explanation for immediate decreases in neural activity in the IC is the well-documented decrease in cochlear and AN output (17–20). On the other hand, elevated activity in the IC might reflect rapid plastic changes to compensate for diminished input to the IC, such as a suppression of lateral inhibition (21). The spectrum of changes caused by AOE probably arises from variations in exposure duration/sound level, differences in measurement time-points following AOE, or possibly species differences.
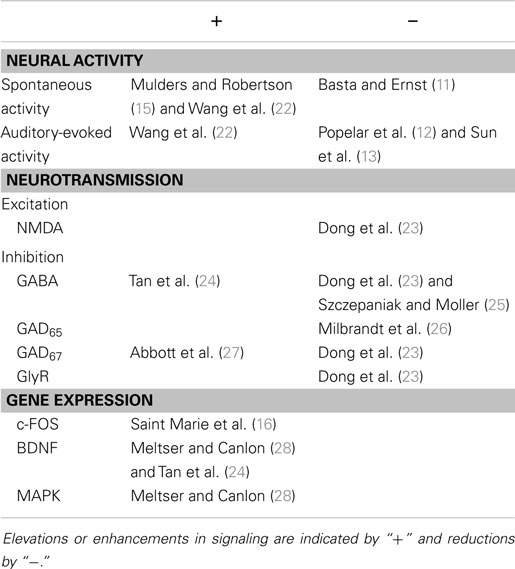
Table 1. Pathophysiology in the IC associated with the short-term effects of acoustic over-exposure.
Subsequently, within 2 weeks post-AOE, there is general and widespread agreement that SFRs in the IC increase (14, 22, 23, 29, 30). This hyperactivity is often restricted to regions that respond preferentially either to the exposure frequency or frequencies above, but recent evidence suggests that this is not always the case (31, 32). Long-term changes following noise exposure are summarized in Table 2.
Changes in Neurotransmission Following Acoustic Trauma
Early work examining γ-aminobutyric acid (GABA)-mediated neural signaling indicated decreased GABAergic inhibition in the central nucleus of the inferior colliculus (CNIC) following tonal noise exposure (25). Thus, a potential mechanism by which chronic, AOE-induced hyper-excitability could be mediated in the midbrain was identified. GABAergic inhibition in the IC is widespread, shapes acoustically evoked non-monotonicity, offset inhibition and binaural inhibition [for a review, see Ref. (40)], and synaptic responses in rat brain slices (41).
There is a reasonable body of evidence suggesting that inhibition in the IC is altered rapidly following AOE, although the time scales vary considerably between studies. Furthermore, in the longer term, inhibition is altered permanently, affecting the balance of inhibition and excitation.
Levels of glutamic acid decarboxylase (GAD), the main enzyme responsible for GABA production, are altered following AOE. Immediately after noise exposure, the GAD67 isoform, which is widespread within neurons and produces GABA for wide-ranging functions (42, 43), was elevated in terms of protein levels and the density of stained cells (27). After 30 days, GAD67 protein levels were reduced relative to unexposed controls, but with no significant changes in stained cell densities. At the same time point, protein levels of GAD65 (localized to synaptic terminals and responsible for transiently synthesizing GABA for fast neurotransmission) were also reduced, but to a lesser extent.
Suneja et al. (44) found that GABA release in CNIC was immediately augmented by both ossicle removal and cochlear ablation, although in their study GABA release remained elevated. Meanwhile GABA uptake was depressed, providing further evidence for pathologically altered inhibition in the IC, in this case caused by invasive peripheral trauma.
In contrast, Milbrandt et al. (26) observed a significant decrease in GAD65 in rats in the short-term when using a noise exposure paradigm specifically designed to target high frequencies, but this recovered to near-normal levels when examined 30 days after AOE. Furthermore, GAD65 recovery at 30 days coincided with a significant increase in [3H]muscimol binding, indicative of increased GABAA receptor binding sites. Dong et al. (23) also found immediate reductions in gene expression related to inhibition after noise exposure, but this was also the case for genes related to excitation, despite the absence of a change in spontaneous firing. In both cases, expression generally returned to near-normal levels over time. Moreover, GABAAα1 expression, a receptor subunit involved in fast inhibitory neurotransmission, decreased in tonotopically organized regions of the IC that responded to frequencies close to the exposure frequency (39).
Rats subjected to a cochleotomy, on the other hand, displayed more consistent reductions in GAD67 expression over time (45). In the same study, decreased expression of the α1 subunit of glycine receptors was also observed under certain conditions, yet there were no changes in other glycine receptor subunits, or in a variety of components that make up GABAA or N-methyl-d-aspartate (NMDA) receptors. Interestingly, Argence et al. (46) were subsequently able to reverse the down-regulation of GAD67 and GlyRα1 by electrically stimulating the deafferented AN. This effect proved to be temporary, disappearing within 5 days after stimulation ceased. However, it should be noted that the breadth of changes induced by a complete cochleotomy are likely to differ significantly from a more selective approach, such as AOE.
In hamsters, moderate yet significant elevations of both glutamate and GABA were observed in the IC after bilateral AOE, yet no change was seen in glycinergic signaling, or in levels of neurotransmitters in other parts of the auditory system, including the cochlear nucleus, medial geniculate body (MGB), and auditory cortex (38). These changes were concurrent with elevations in aspartate (relating to glutamate synthesis) and decreased levels of taurine (which can be linked with GABA or glycine function). Of particular interest was the time-span over which a presumed shift in the balance of inhibition and excitation occurred; in this study, hamsters were examined 5 months after AOE and compared with unexposed controls. In an earlier paper, Tan et al. (24) also demonstrated an increase in GABA-positive neurons, combined with elevated brain-derived neurotrophic factor (or BDNF) 6 days following AOE. Contrastingly, using an imaging technique to quantify GABA and glutamate, Brozoski et al. (47) demonstrated no excitatory or inhibitory changes in the IC, but significant changes in the dorsal cochlear nucleus (DCN), MGB, and primary auditory cortex (AI) of normal-hearing rats with tinnitus (induced by unilateral AOE and confirmed behaviorally).
Chronic Hyperactivity in the IC
Changes in inhibitory neurotransmission, such as those described above, could result in the unmasking of previously dormant inputs within these regions. Such a mechanism might feasibly contribute to maintaining hyperactivity induced by AOE, as well as contributing to tonotopic reorganization in the IC, which in some instances has been found to occur (22, 37). However, the origin of increased spontaneous activity in the IC, in terms of generation and persistence, and whether it depends upon intrinsic processes or external input, has only been explored more recently.
Manzoor et al. (33) demonstrated that IC hyperactivity was significantly reduced by ablation of the DCN 2–3 weeks following AOE, suggesting that an increase in SFRs at the level of the midbrain occurred as a result of increased activity extrinsic to this structure. This suggests that IC hyperactivity, at least in the early stages following AOE, is not a result of intrinsic plasticity. In support of this, Brozoski et al. (48) demonstrated that bilateral DCN lesions prior to AOE prevented the development of behavioral evidence of tinnitus, although this was not the case for unilateral lesions.
However, these results do not rule out the possibility that IC hyperactivity becomes an intrinsic process over a period of time, independent of ascending input. Bilateral DCN ablation 3–5 months after AOE did not abolish behavioral evidence of tinnitus (49), while cochlear ablation only modulated IC hyperactivity within 6 weeks of AOE (7, 8). In light of the current evidence, IC hyperactivity likely depends on ascending input in the early stages post-AOE, but subsequently becomes self-sustaining.
Interestingly, recent findings by Ropp et al. (32) suggest that the ventral cochlear nucleus (VCN) may play a substantial role in IC hyperactivity; recording 1–4 months after AOE, IC neurons receiving input from VCN (identified by electrophysiological response profiles) were hyperactive, while IC neurons receiving input from DCN were not. Thus, at a later stage, IC hyperactivity appears to be independent of input from DCN, but the VCN may be implicated in the persistence of increased SFRs. However, more research examining how VCN modulates IC firing following AOE is necessary to draw definite conclusions.
Linking Neural Pathophysiology to Tinnitus Perception
Some of the studies outlined above highlight a series of consequences of hearing loss that could underlie tinnitus generation. However, the implementation of a complementary behavioral test in animals is an essential step in correlating neural changes with evidence of tinnitus perception. This enables delineation of changes that might relate to tinnitus from those that occur as a by-product of hearing loss. This is a pertinent point, as hearing loss does not invariably lead to the generation of tinnitus (50). Early behavioral tests relied on extensive training to produce a conditioned response (51–53), although more recent studies have exploited unconscious reflexes to determine tinnitus-like behavior, often known as the gap prepulse inhibition of acoustic startle (GPIAS) test (54–58). While the efficacy of these behavioral tests for detecting tinnitus per se has recently been questioned (59, 60), an effective, objective test is vital if we are to be confident in attributing neural changes to tinnitus rather than to hearing loss.
Behavioral evidence of chronic tinnitus emerges at a period beginning five weeks after AOE (61). Around the same time, hyperactivity is evident in the IC (7, 8). However, two recent studies have suggested that this hyperactivity may not be sufficient as a sole generator of tinnitus. In AOE-treated guinea pigs and rats, increased spontaneous neural activity was present even in the absence of behavioral evidence of tinnitus (31, 32), although these findings may be influenced by interpretation of behavioral data. Given that the majority of studies demonstrate that IC hyperactivity is evident following noise trauma, but can also be present in the absence of tinnitus, this suggests that increased SFRs, at least at the level of the IC, may be necessary but not sufficient to explain tinnitus generation.
Other Changes Occurring within the IC
In addition to elevated spontaneous activity, a number of other changes in neuronal firing properties induced by noise exposure have been linked to tinnitus, such as increased burst firing in chinchillas (35) and guinea pigs (31). Burst firing patterns have previously been associated with neural synchrony (62); that is, correlated firing across a population of neurons. Increased neural synchrony has also been directly measured by correlating firing patterns across different IC neurons (35). It has been suggested that neural synchrony underlies auditory perception [for a review, see Ref. (63)] so it is entirely plausible that synchronous activity in the absence of auditory input could manifest as a tinnitus percept. Indeed, some treatment options focus solely on disrupting neural synchrony [see Ref. (64)]. However, the importance of synchrony in the IC to tinnitus generation and maintenance is yet to be elucidated.
The uncertainty surrounding the role of the IC in tinnitus generation can in part be attributed to differences between the three major divisions: the CNIC, the external nucleus of the inferior colliculus (ICx), and the dorsal cortex (ICd). The responses of these divisions are relatively distinct, with different proposed functional roles (65). It is therefore reasonable to assume that responses to reduced input following AOE might also differ. Although IC sub-divisions can be identified physiologically or histologically (66, 67), the delineations and borders are often not clear and thus many studies do not explicitly state which area was examined. Interestingly, using manganese-enhanced magnetic resonance imaging (MRI) in rats to examine a variety of auditory and non-auditory nuclei, 2 days post-AOE, Holt et al. (34) found that the ICd was the only area that exhibited consistent increases in activity following two different tinnitus inducers. ICd is the predominant site of corticocollicular descending input (68–72), although there is some overlap near the border with CNIC where ascending connections are also present (68, 73). Furthermore, there are well-defined intrinsic connections between ICd and CNIC [for a review, see Ref. (74)]. Nonetheless, these data could implicate the descending forebrain in altering spontaneous activity in the IC (75). Indeed, when focal electrical stimulation was applied to the auditory cortex, thus activating corticofugal pathways, this caused temporary shifts in IC frequency representation (76). Thus, tonotopic restructuring in the IC following acoustic trauma (22, 37) could feasibly be mediated, at least in part, by descending input.
Within the CNIC, AOE-induced changes were not restricted to particular response profiles (30). We recently demonstrated that the proportional balance of response profiles can be altered by AOE; the proportion of onset-type responses increased significantly, while the proportion of single-units with sustained firing patterns decreased (36). However, the presence or absence of behavioral tinnitus had no bearing on the balance of onset and sustained profiles; in other words, this effect likely reflected long-term changes induced by AOE.
A potential confound to any study examining changes following AOE pertains to the exposure paradigm itself; that is, different sound levels, durations, and frequencies of noise exposure could result in diverse neural changes. Suggestive of this, hamsters were more likely to develop behavioral evidence of tinnitus when subjected to an increased duration of an otherwise identical noise exposure (52). Moreover, Meltser and Canlon (28) found that an AOE paradigm designed to cause “permanent” damage resulted in transient activation of BDNF and a variety of mitogen-activated protein kinases, whereas temporary damage was only associated with activation of selected p38 kinases. They reasoned that these effects were indicative of plastic changes resulting from reduced sensory input, dependent on the magnitude of insult. AOE paradigms vary substantially between studies, while species differences in susceptibility to AOE-induced damage (77) prevent implementation of a standardized protocol. Accordingly, disparity in AOE protocols provides a possible explanation for seemingly conflicting results.
The short-term and long-term effects of AOE, specifically, are summarized in Tables 1 and 2. To conclude, the short-term effects could be described as: (1) immediate changes in spontaneous and auditory-evoked neural activity, which are variable perhaps due to experimental protocol, (2) predominantly, reductions in mediators of both excitation and inhibition, with a couple of exceptions, and (3) elevated immediate-early gene expression, indicative of altered patterns of neuronal activity. In terms of long-term changes after AOE, studies indicate, overwhelmingly, that (1) spontaneous, synchronous, and auditory-evoked activity are elevated, and (2) changes in components of inhibition in particular are complex, but probably underlie an overall change in the balance of excitation and inhibition.
IC Pathology in Models of Pharmacologically Induced Tinnitus
Sodium salicylate, an analog of acetylsalicylic acid (the active ingredient in aspirin), is ototoxic at high doses and induces transient tinnitus in humans (78). Salicylate is used experimentally to induce tinnitus in animal models (53, 54, 79–81). A significant benefit in using salicylate as a tinnitus-inducing agent, is that – compared with AOE – the behavioral effects are largely homogeneous. Thus, one can reliably predict that nearly all of the animals will exhibit behavioral evidence of tinnitus.
The definitive mechanisms by which salicylate causes tinnitus are unknown, although neural activity is affected at multiple levels of the auditory pathway. This includes peripheral effects, such as altered outer hair cell electromotility (82), as well as central effects, from AN fibers through to auditory cortex (83–85). Early work indicated that – in cats at least – secondary auditory cortex (AII) exhibited increased firing rates, while neuronal firing in AI and anterior auditory field (AAF) decreased (86). Moreover, elevated firing was detected in neurons tuned to higher frequencies, while at low frequency sites the opposite was true. These data implied that salicylate may exert effects via extra-lemniscal pathways, which provide an input for AII.
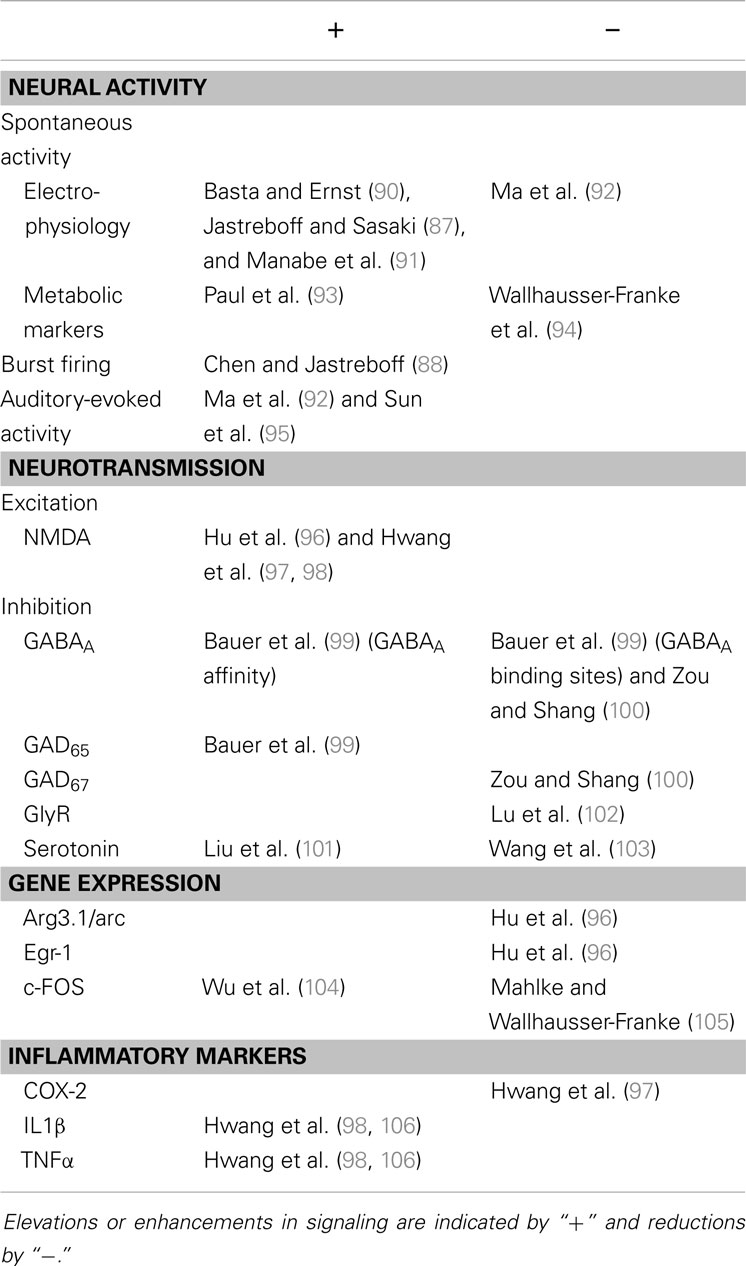
In the IC, the earliest evidence for salicylate-induced neural hyper-excitability came from Jastreboff and Sasaki (87), who measured spontaneous neuronal firing in the ICx of guinea pigs. Subsequently, bursting patterns of activity were discovered in the ICx in salicylate-administered rats (88), an effect most pronounced in neurons tuned to high frequencies. This is consistent with the finding that salicylate treatment often results in behavioral evidence of a high-frequency tinnitus percept (89). Changes in the IC following salicylate treatment are summarized in Table 3.
Increased excitability or enhanced metabolic activity in the IC were also demonstrated by others in vivo in guinea pigs (91), and in rats (93), as well as hyper-excitability in mouse brain slices (90). The latter study confirmed that salicylate acts at central targets, i.e., hyper-excitability does not occur simply as a result of peripheral effects. Indeed, in a later study, Basta et al. (107) showed that deafferented CN, MGB, and auditory cortex preparations were all susceptible to modulation of firing rates by salicylate, albeit with a lower sensitivity than the IC. The propensity for salicylate to directly alter central activity was further shown in vivo by Sun et al. (95). In their study, systemically administered salicylate increased the amplitude of sound-evoked field potentials in auditory cortex in awake rats, while reduced IC and cortical potentials were seen after direct administration of salicylate to the cochlea. Moreover, systemic administration also reduced the sound-evoked output of the cochlea, which – when considered alongside elevated cortical responses – suggests a change in central gain.
Contrastingly, Ma et al. (92) found a decrease in SFRs in the CNIC in mice following acute salicylate treatment. Moreover, this effect was strongest in neurons preferentially tuned to low frequencies. It should be noted that the salicylate dose administered by Ma et al. was somewhat lower than that used previously by others, although this was still sufficient to induce behavioral evidence of tinnitus in other rodents (108). Further evidence of decreased neural activity in the IC comes from a study in which salicylate treatment resulted in a reduction of 2-deoxyglucose activity in the IC, particularly in high-frequency regions (94).
Interestingly, Kumagai (109) demonstrated that salicylate-induced SFRs in AN fibers were only significantly elevated following administration of a high dose of salicylate, but not a lower dose. This provides a possible explanation for the disparity and heterogeneity between studies, with respect to IC hyper-excitability. However, given that relatively low doses result in behavioral evidence of tinnitus, it seems that increased excitation may be overly simplistic as a mechanism for salicylate-induced tinnitus.
A number of studies have examined the effects of salicylate on the balance of excitation and inhibition in the IC, in an attempt to understand the mechanisms underlying direct salicylate-induced hyper-excitability. Using the GPIAS approach to confirm tinnitus, Hu et al. (96) found that salicylate-induced reversible plastic changes in the IC. Specifically, they demonstrated an increase in the NR2B subunit of NMDA receptors, yet decreased expression of the immediate-early genes for Arc (activity-regulated cytoskeleton-associated protein) and Egr-1 (early growth response protein 1) in both the IC and auditory cortex of rats. The latter effect was somewhat surprising, given that both are normally associated with sensory-evoked neuronal activity. Salicylate-mediated changes in NR2B expression, as well as inflammatory mediators including tumor necrosis factor α (TNFα), cyclooxygenase-2 (COX-2), and interleukin-1β (IL1β), were also found in mice with behaviorally confirmed tinnitus (97, 98, 106).
Immediate-early gene expression was previously also examined in structures of the auditory and limbic systems of salicylate-treated gerbils (105). Although frequency-specific patterns of the arg3.1 gene (which translates to Arc) and c-Fos were apparent in auditory cortex, expression of these genes was limited in sub-cortical auditory structures, suggesting a lack of neuronal hyperactivity in the IC. Intriguingly, however, in the central nucleus of the amygdala both c-Fos and Arg3.1 were elevated following salicylate treatment, compared with saline-treated controls. Brainstem measurements of c-Fos from another study indicated an increase in the CNIC, while expression was negligible in the CN, in rats chronically dosed with salicylate (104), data which correlate with previous reports of salicylate-mediated increases in spontaneous activity in the IC.
With respect to components of inhibitory neurotransmission, early work involving chronic dosing of rats with salicylate demonstrated a number of changes to GABA signaling in the IC (99). Levels of GAD65 were elevated, while binding studies indicated increased GABAA receptor affinity in CNIC, as well as in combined samples of ICx and ICd, which was coincident with a reduction in the number of GABAA binding sites in CNIC. These effects correlated with behavioral evidence of salicylate-induced tinnitus in the same animals. This relationship between tinnitus behavior and changes in GABAergic signaling suggested that salicylate affected the balance of excitation and inhibition in the central auditory system, manifesting as a perceived tinnitus.
Contrastingly, Zou and Shang (100) found decreases in GABAAα1 and GAD67 expression in large GABAergic neurons in the IC 1 day after 5 days of chronic salicylate treatment, yet no change in levels of GAD65. This coincided with unchanged levels of the vesicular glutamate transporter VGLUT2, suggesting that glutamatergic input remained constant. It is worth highlighting that this study used a lower dose of salicylate than Bauer et al. (99) and measured levels of both GAD isoforms. Furthermore, Zou and Shang (100) concentrated on large GABAergic neurons in CNIC, thought to be the primary source of GABAergic input to the MGB from the IC. These factors do not necessarily explain the inconsistent results in GAD expression, but variability in methodology could underlie these differences.
Memantine, an NMDA receptor antagonist, did not abolish behavioral or neural evidence of tinnitus (108), which also suggests altered inhibitory drive in the midbrain is a contributing factor to tinnitus generation. In physiological terms, altered inhibitory neurotransmission may underlie enhancement of sound-evoked local field potentials (95) and broadening of excitatory receptive fields (92) in the IC. Others have shown the capacity for salicylate to interact with a number of neurotransmitter and neuromodulation systems. In vitro recordings from cultured rat IC neurons indicated non-competitive antagonism of α1 glycine receptor subunits (102). Furthermore, both in vivo and in vitro studies in rats point toward salicylate modulating serotonergic input (101, 103).
Salicylate-induced tinnitus is transient and hence studies that use salicylate are limited in translational value, in terms of providing signposts to understanding the chronic human condition, which is most often caused by noise exposure. However, salicylate remains a useful tool for making comparisons between a behavioral effect likened to tinnitus perception in humans, and potential underlying neural mechanisms. At this point in time – as discussed – the literature contains a variety of possible theories for salicylate-induced tinnitus, not least the effects this drug has in the IC. What seems clear, however, is that salicylate does induce central effects, and can directly affect both excitatory and inhibitory neurotransmission and plasticity in the IC. The effects of salicylate on the IC are summarized in Table 3. To generalize, studies suggest that salicylate (1) probably increases neural activity in the IC, (2) enhances excitation, (3) causes complex changes in inhibition, (4) reduces expression of immediate-early genes associated with neuronal activity (with one exception), and (5) initiates the production of some inflammatory mediators.
Evidence from Tinnitus Patients That Implicates the IC
Collectively, data acquired using animal models of tinnitus implicate the IC in generating or maintaining the tinnitus percept. A degree of support for this idea is evident from work conducted with human subjects. Differences in the patterns of sound-evoked brain activation in the IC have been demonstrated in tinnitus patients with near-normal hearing (110), and in some instances, this was asymmetrical in patients with lateralized tinnitus, whereas bilateral tinnitus subjects exhibited symmetrical sound-evoked activation (111, 112). In a later study, Melcher et al. (113) suggest that asymmetrical activation coinciding with lateralized tinnitus actually constitutes a sub-group in terms of tinnitus classification. However, no discernible differences were apparent between controls and tinnitus patients when PET imaging was used to investigate previously reported hemispheric metabolic asymmetries in auditory cortex and the IC (114).
A reduction in functional connectivity between the IC and auditory cortices was also demonstrated in subjects with tinnitus, and interpreted as evidence to support failed thalamic gating in tinnitus patients (115), while no differences in either the magnitude or lateralization of functional MRI (fMRI) responses to auditory stimuli were seen in the IC. The idea of dysfunctional thalamic gating can be tentatively linked to the thalamocortical dysrhythmia model of tinnitus, proposed by Llinas et al. (116), which argues that disinhibition of auditory cortex as a consequence of abnormal thalamic input represents a putative mechanism for tinnitus. Indeed, using fMRI, patients with gaze-evoked tinnitus were found to exhibit less gaze-evoked inhibition of the auditory cortex, compared with controls (117). This was coupled with abnormal patterns of activation in the IC and inhibition in the MGB, and correlated with a perceived increase in tinnitus loudness. Elevations in IC neural activity measured directly in animals, and by more indirect means in humans, may be a prerequisite or a contributing component of the thalamocortical dysrhythmia model.
In addition to studies examining metabolic changes – suggestive of neural activity – a number of studies examining structural changes in the brain in tinnitus patients have identified altered morphology in the IC. Landgrebe et al. (118) identified a significant increase in gray matter in both the right IC and left hippocampus of their tinnitus group using structural MRI, compared with controls. This study originally aimed to replicate the findings of an earlier study (119), which showed subcallosal and thalamic volume changes; although regions of interest differed between studies, data from both suggest morphological volume changes in auditory and limbic brain areas relating to tinnitus pathophysiology, although others have failed to demonstrate volume changes in tinnitus patients [e.g., Ref. (120)]. White matter differences in the IC have also been examined in tinnitus patients, specifically comparing fiber tracts between IC and auditory cortex, IC and amygdala, and also between auditory cortex and the amygdala (121). Significant differences in this study were evident between the left IC and amygdala, right auditory cortex and IC, as well as bilateral auditory cortex and amygdala.
Several case reports also support a role for IC pathology in tinnitus. For example, Stimmer et al. (122) reported the sudden onset of a unilateral, right-sided tinnitus in a patient who exhibited prolonged auditory brainstem response (ABR) inter-peak latencies – which suggests the presence of pathology in the auditory pathway – and a small lesion in the left IC, presumed to have resulted from a transient, acute hemorrhage. Moreover, infarction in an area located near to the IC also coincided with a sudden worsening of reported tinnitus (123).
Changes in ABRs have been extensively studied and further implicate the IC and other brainstem structures in tinnitus pathology. Schaette and McAlpine (124) reported a significant reduction in wave I ABR amplitudes (generated by the auditory periphery), relative to the centrally generated wave V, thought to represent activity in the lateral lemniscus and IC. The authors proposed that this provided evidence for elevated central gain in the presence of reduced peripheral input. Subsequently, Gu et al. (125) also reported reduced wave I and augmented wave V amplitudes in tinnitus patients when compared with controls matched in age, sex, and hearing thresholds, and that ratio differences were most pronounced when comparing V:I and V:III (thought to represent activity in outputs from spherical bushy cells of the VCN). Interestingly, when these two groups were compared with a third cohort of younger, non-tinnitus subjects, elevated thresholds at mid-to-high frequencies were apparent in both groups, as was a reduction in wave I amplitudes, such that the observed peripheral dysfunction was not a unique indicator of tinnitus. Despite this, however, brainstem recordings performed in humans support a role for the IC and other brainstem structures in tinnitus pathology.
Somatosensory modulation of tinnitus is a well-established phenomenon (126). In animals, data suggest that the DCN is a key brainstem structure for the integration of somatosensory and auditory neural information that represents a neural correlate for such tinnitus modulation (127). Lanting et al. (128) examined neural correlates for somatosensory modulation of tinnitus by conducting fMRI experiments in patients capable of modulating tinnitus with jaw movements. Interestingly, this study found that jaw movements increased metabolic activity in both the CN and IC of tinnitus patients. Brain regions responsible for integrating somatosensory and auditory information, which includes the DCN and to a lesser extent the IC (129), were further implicated in tinnitus pathophysiology by a report from Gritsenko et al. (130), whereby lateralized tinnitus in an individual also exhibiting medial branch nerve degeneration was abolished by temporarily blocking C2–C3 nociceptive input.
To summarize, the majority of studies in humans have used either fMRI or structural MRI to examine the IC and have demonstrated the following: (1) changes in evoked activity in IC, (2) reductions in functional connectivity between IC and auditory cortex, (3) disparate morphological changes in the IC and other brain regions, and (4) altered brainstem responses implicating the IC. However, drawing firm conclusions regarding the role of the IC in the human condition is not feasible with the evidence available currently.
Targeting IC Pathophysiology to Eliminate Tinnitus
There is currently no universally effective treatment for tinnitus. Consequently, a number of studies have addressed whether a range of interventions affect pathophysiological changes in the IC, caused by AOE. One approach has been to focus on GABA-enhancing drugs, aimed at restoring inhibition to suppress hyperactivity. Szczepaniak and Moller (131) demonstrated that l-baclofen, an antispasmodic GABAB agonist, successfully attenuated hyper-excitability in the IC of AOE-treated rats. No behavioral testing was performed in this study to determine effectiveness on tinnitus. However, Zheng et al. (132) later demonstrated that a high dose of l-baclofen diminished tinnitus-like behavior in rats. While these animal data appear promising, the efficacy of this drug in eliminating tinnitus in humans is highly variable [for a review, see Ref. (133)].
Previous studies have demonstrated that tinnitus can persist following AN sectioning (6), which may even induce tinnitus in subjects that previously did not experience it (134, 135). In guinea pigs, Mulders and Robertson (7) demonstrated that, although IC hyperactivity could be reduced by cochlear ablation up to 6 weeks following AOE, there was no effect from 8 weeks onward (8). This suggests that central activity is dependent on peripheral drive in the early stages following AOE and later becomes centralized.
Recently, the loop diuretic furosemide was shown to reduce AOE-induced IC hyperactivity and behavioral evidence of tinnitus in guinea pigs within 6 weeks of acoustic trauma (136, 137). Putatively, this has been suggested to work via a reduction in the endolymphatic potential (138). Thus, it is likely to be most effective during the early stages of tinnitus development, when IC hyperactivity is dependent on cochlear input (7). Indeed, ~50% of patients experienced a reduction in tinnitus symptoms following intravenous administration of furosemide (139), an effect attributed to tinnitus being of cochlear origin. Paradoxically, however, high doses of furosemide actually appear to cause tinnitus in humans [see Ref. (140), for a review], so the efficacy for reducing tinnitus is as yet unclear.
An alternative and intriguing approach for modulating tinnitus-related pathology in the IC has recently been proposed as a result of work by Offutt et al. (141). These authors demonstrated, in guinea pigs, that electrical stimulation of the ICd resulted in either suppression or facilitation of firing rates in the CNIC, and postulated that a midbrain implant could be used to reduce or even eliminate tinnitus. As yet, these effects have not been demonstrated in AOE-treated animals, or indeed in animals displaying evidence of tinnitus, so the viability of this intervention remains to be determined.
Tinnitus can briefly be reduced or eliminated following the presentation of a masking sound stimulus, a phenomenon referred to as residual inhibition (142). Voytenko and Galazyuk (143) suggested that suppression of activity in awake mouse IC neurons by a preceding sound stimulus represents a possible underlying mechanism for residual inhibition. While this intervention only produces a temporary cessation of tinnitus, it nonetheless provides a useful tool for comparing neural pathology underlying tinnitus to a brain state wherein tinnitus is absent.
To date, intervention-centered research has been hampered by a lack of differentiation between underlying tinnitus pathology and effects that simply relate to AOE. Without this, the efficacy of treatment approaches is difficult to appraise.
The IC as a Component in Putative Models of Tinnitus
The central gain hypothesis proposes a reduction in cochlear output concurrent with a paradoxical sustained enhancement of central activity [for a recent review, see Ref. (144)]. This likely reflects homeostatic mechanisms initiated to sustain the mean level of firing within the auditory brain (145, 146). Increases in the steepness of local field potential amplitude-sound level functions, despite a loss of peripheral sensitivity, implied that this central enhancement was evident at the level of the IC (21, 147).
While the central gain hypothesis is persuasive, and is perhaps most pertinent when considering IC involvement, it is highly likely that tinnitus perception involves complex interactions with other brain areas (148). Given the strong emotional aspects of tinnitus, it has previously been suggested that input from limbic areas (149), specifically to the MGB (150), likely underlie the awareness of tinnitus. Connections between the MGB and limbic areas are prevalent, and presumably are involved in modulating responses to auditory stimuli (150). Moreover, there are also direct connections between the CNIC and the amygdala (151). However, it is as yet unclear whether limbic-auditory interactions at the level of the IC are altered in a way that correlates with tinnitus. Examining limbic-auditory interactions is currently en vogue in tinnitus research, although clearly this is a challenging question to pose in animal models. Nevertheless, limbic-auditory interactions remain an intriguing avenue for inquiry. In particular, pathological interactions could underlie differences in tinnitus susceptibility, since some people with peripheral damage do not develop tinnitus, while others do (152).
A large proportion of patients demonstrate tinnitus modulation by jaw movements or neck muscle contractions (153, 154). This phenomenon is likely the result of interactions between auditory and somatosensory neural circuitry [for a review, see Ref. (155)]. It has previously been shown that stimulation of somatosensory areas modulated firing in cochlear nucleus neurons (156–158), and that this modulation was altered in the presence of behavioral evidence of tinnitus (127). There are also direct and prominent connections to the ICx from somatosensory areas (159, 160), and IC activity was modulated by somatosensory stimulation (161). Thus, it would be of considerable interest to determine whether changes in IC-somatosensory system interactions correlate with behavioral evidence of tinnitus; this may also further elucidate the mechanisms underlying somatosensory modulation of tinnitus.
Conclusion
Studies using animal models imply that the IC plays an important role in tinnitus pathology. This is the case regardless of whether tinnitus is induced by AOE or salicylate. Animal models allow for invasive studies, but carry with them the fundamental difficulty of establishing whether animals actually perceive tinnitus. The introduction of behavioral tests attempting to identify tinnitus allows researchers to correlate neural changes with the presence of a tinnitus percept, although existing tests have their caveats. Human studies are important for directly relating changes in the brain to the human condition, although generally do not allow for invasive recording techniques and current measures are limited in their spatial resolution (e.g., EEG) or temporal resolution (e.g., fMRI or PET). Currently, there are clear disparities in the literature that need to be resolved, namely, clarifying the time-course of changes post-AOE, and separating tinnitus-related effects from those attributable simply to noise exposure. There is also a lack of clarity in the contributions of different IC sub-divisions to tinnitus pathophysiology. Elucidating these characteristics of tinnitus pathology will be undeniably difficult, but will likely prove essential to facilitate development of a treatment to eliminate the tinnitus percept.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Professor Alan Palmer, Dr. Mark Wallace, and Dr. Leonie Norris for their helpful comments.
References
1. Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci (2004) 27:676–82. doi: 10.1016/j.tins.2004.08.010
2. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med (2010) 123:711–8. doi:10.1016/j.amjmed.2010.02.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Dobie RA. Overview: suffering from tinnitus. In: Snow JB, editor. Tinnitus: Theory and Management. Hamilton, ON: BC Decker Inc (2004). p. 1–7.
4. Zeng FG, Tang Q, Dimitrijevic A, Starr A, Larky J, Blevins NH. Tinnitus suppression by low-rate electric stimulation and its electrophysiological mechanisms. Hear Res (2011) 277:61–6. doi:10.1016/j.heares.2011.03.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Zacharek MA, Kaltenbach JA, Mathog TA, Zhang J. Effects of cochlear ablation on noise induced hyperactivity in the hamster dorsal cochlear nucleus: implications for the origin of noise induced tinnitus. Hear Res (2002) 172:137–43. doi:10.1016/S0378-5955(02)00575-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. House JW, Brackmann DE. Tinnitus: surgical treatment. Ciba Found Symp (1981) 85:204–16. doi:10.1002/9780470720677.ch12
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Mulders WH, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience (2009) 164:733–46. doi:10.1016/j.neuroscience.2009.08.036
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Mulders WH, Robertson D. Progressive centralization of midbrain hyperactivity after acoustic trauma. Neuroscience (2011) 192:753–60. doi:10.1016/j.neuroscience.2011.06.046
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Aitkin LM, Phillips SC. Is the inferior colliculus an obligatory relay in the cat auditory system? Neurosci Lett (1984) 44:259–64. doi:10.1016/0304-3940(84)90032-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Robertson D, Mulders W. The inferior colliculus: involvement in hyperactivity and tinnitus. In: Eggermont JJ, Zeng F-G, Popper AN, Fay RR, editors. Tinnitus. New York, NY: Springer (2012). p. 121–35.
11. Basta D, Ernst A. Erratum to “Noise-induced changes of neuronal spontaneous activity in mice inferior colliculus brain slices”. Neurosci Lett (2005) 374:74–9. doi:10.1016/j.neulet.2004.11.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Popelar J, Syka J, Berndt H. Effect of noise on auditory evoked responses in awake guinea pigs. Hear Res (1987) 26:239–47. doi:10.1016/0378-5955(87)90060-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Sun W, Deng A, Jayaram A, Gibson B. Noise exposure enhances auditory cortex responses related to hyperacusis behavior. Brain Res (2012) 1485:108–16. doi:10.1016/j.brainres.2012.02.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Groschel M, Ryll J, Gotze R, Ernst A, Basta D. Acute and long-term effects of noise exposure on the neuronal spontaneous activity in cochlear nucleus and inferior colliculus brain slices. Biomed Res Int (2014) 2014:909260. doi:10.1155/2014/909260
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Mulders WH, Robertson D. Development of hyperactivity after acoustic trauma in the guinea pig inferior colliculus. Hear Res (2013) 298:104–8. doi:10.1016/j.heares.2012.12.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Saint Marie RL, Luo L, Ryan AF. Effects of stimulus frequency and intensity on c-fos mRNA expression in the adult rat auditory brainstem. J Comp Neurol (1999) 404:258–70. doi:10.1002/(SICI)1096-9861(19990208)404:2<258::AID-CNE9>3.0.CO;2-U
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Dallos P, Harris D, Ozdamar O, Ryan A. Behavioral, compound action potential, and single unit thresholds: relationship in normal and abnormal ears. J Acoust Soc Am (1978) 64:151–7. doi:10.1121/1.381980
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl (1978) 358:1–63.
19. Salvi RJ, Ding D, Wang J, Jiang HY. A review of the effects of selective inner hair cell lesions on distortion product otoacoustic emissions, cochlear function and auditory evoked potentials. Noise Health (2000) 2:9–26.
20. Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol (2002) 3:248–68. doi:10.1007/s101620020028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Wang JA, Ding DL, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res (2002) 168:238–49. doi:10.1016/S0378-5955(02)00360-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Wang F, Zuo L, Hong B, Han D, Range EM, Zhao L, et al. Tonotopic reorganization and spontaneous firing in inferior colliculus during both short and long recovery periods after noise overexposure. J Biomed Sci (2013) 20:91. doi:10.1186/1423-0127-20-91
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Dong S, Mulders WH, Rodger J, Woo S, Robertson D. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. Eur J Neurosci (2010) 31:1616–28. doi:10.1111/j.1460-9568.2010.07183.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Tan J, Ruttiger L, Panford-Walsh R, Singer W, Schulze H, Kilian SB, et al. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience (2007) 145:715–26. doi:10.1016/j.neuroscience.2006.11.067
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Szczepaniak WS, Moller AR. Evidence of decreased GABAergic influence on temporal integration in the inferior colliculus following acute noise exposure: a study of evoked potentials in the rat. Neurosci Lett (1995) 196:77–80. doi:10.1016/0304-3940(95)11851-M
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res (2000) 147:251–60. doi:10.1016/S0378-5955(00)00135-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Abbott SD, Hughes LF, Bauer CA, Salvi R, Caspary DM. Detection of glutamate decarboxylase isoforms in rat inferior colliculus following acoustic exposure. Neuroscience (1999) 93:1375–81. doi:10.1016/S0306-4522(99)00300-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Meltser I, Canlon B. The expression of mitogen-activated protein kinases and brain-derived neurotrophic factor in inferior colliculi after acoustic trauma. Neurobiol Dis (2010) 40:325–30. doi:10.1016/j.nbd.2010.06.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Manzoor NF, Gao Y, Licari F, Kaltenbach JA. Comparison and contrast of noise-induced hyperactivity in the dorsal cochlear nucleus and inferior colliculus. Hear Res (2013) 295:114–23. doi:10.1016/j.heares.2012.04.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Vogler DP, Robertson D, Mulders WH. Hyperactivity following unilateral hearing loss in characterized cells in the inferior colliculus. Neuroscience (2014) 265:28–36. doi:10.1016/j.neuroscience.2014.01.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Coomber B, Berger JI, Kowalkowski VL, Shackleton TM, Palmer AR, Wallace MN. Neural changes accompanying tinnitus following unilateral acoustic trauma in the guinea pig. Eur J Neurosci (2014) 40(2):2427–41. doi:10.1111/ejn.12580
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Ropp TJ, Tiedemann KL, Young ED, May BJ. Effects of unilateral acoustic trauma on tinnitus-related spontaneous activity in the inferior colliculus. J Assoc Res Otolaryngol (2014) 15(6):1007–22. doi:10.1007/s10162-014-0488-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Manzoor NF, Licari FG, Klapchar M, Elkin RL, Gao Y, Chen G, et al. Noise-induced hyperactivity in the inferior colliculus: its relationship with hyperactivity in the dorsal cochlear nucleus. J Neurophysiol (2012) 108:976–88. doi:10.1152/jn.00833.2011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Holt AG, Bissig D, Mirza N, Rajah G, Berkowitz B. Evidence of key tinnitus-related brain regions documented by a unique combination of manganese-enhanced MRI and acoustic startle reflex testing. PLoS One (2010) 5:e14260. doi:10.1371/journal.pone.0014260
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res (2008) 86:2564–78. doi:10.1002/jnr.21699
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Berger JI, Coomber B, Wells TT, Wallace MN, Palmer AR. Changes in the response properties of inferior colliculus neurons relating to tinnitus. Front Neurol (2014) 5:203. doi:10.3389/fneur.2014.00203
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Izquierdo MA, Gutierrez-Conde PM, Merchan MA, Malmierca MS. Non-plastic reorganization of frequency coding in the inferior colliculus of the rat following noise-induced hearing loss. Neuroscience (2008) 154:355–69. doi:10.1016/j.neuroscience.2008.01.057
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Godfrey DA, Kaltenbach JA, Chen K, Ilyas O, Liu X, Licari F, et al. Amino acid concentrations in the hamster central auditory system and long-term effects of intense tone exposure. J Neurosci Res (2012) 90:2214–24. doi:10.1002/jnr.23095
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Dong S, Rodger J, Mulders WH, Robertson D. Tonotopic changes in GABA receptor expression in guinea pig inferior colliculus after partial unilateral hearing loss. Brain Res (2010) 1342:24–32. doi:10.1016/j.brainres.2010.04.067
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Faingold CL. Role of GABA abnormalities in the inferior colliculus pathophysiology – audiogenic seizures. Hear Res (2002) 168:223–37. doi:10.1016/S0378-5955(02)00373-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Li Y, Evans MS, Faingold CL. Synaptic response patterns of neurons in the cortex of rat inferior colliculus. Hear Res (1999) 137:15–28. doi:10.1016/S0378-5955(99)00129-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Kanaani J, Kolibachuk J, Martinez H, Baekkeskov S. Two distinct mechanisms target GAD67 to vesicular pathways and presynaptic clusters. J Cell Biol (2010) 190:911–25. doi:10.1083/jcb.200912101
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Pinal CS, Tobin AJ. Uniqueness and redundancy in GABA production. Perspect Dev Neurobiol (1998) 5:109–18.
44. Suneja SK, Potashner SJ, Benson CG. Plastic changes in glycine and GABA release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol (1998) 151:273–88. doi:10.1006/exnr.1998.6812
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Argence M, Saez I, Sassu R, Vassias I, Vidal PP, De Waele C. Modulation of inhibitory and excitatory synaptic transmission in rat inferior colliculus after unilateral cochleectomy: an in situ and immunofluorescence study. Neuroscience (2006) 141:1193–207. doi:10.1016/j.neuroscience.2006.04.058
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
46. Argence M, Vassias I, Kerhuel L, Vidal PP, De Waele C. Stimulation by cochlear implant in unilaterally deaf rats reverses the decrease of inhibitory transmission in the inferior colliculus. Eur J Neurosci (2008) 28:1589–602. doi:10.1111/j.1460-9568.2008.06454.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
47. Brozoski T, Odintsov B, Bauer C. Gamma-aminobutyric acid and glutamic acid levels in the auditory pathway of rats with chronic tinnitus: a direct determination using high resolution point-resolved proton magnetic resonance spectroscopy (H-MRS). Front Syst Neurosci (2012) 6:9. doi:10.3389/fnsys.2012.00009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
48. Brozoski TJ, Wisner KW, Sybert LT, Bauer CA. Bilateral dorsal cochlear nucleus lesions prevent acoustic-trauma induced tinnitus in an animal model. J Assoc Res Otolaryngol (2012) 13:55–66. doi:10.1007/s10162-011-0290-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res (2005) 206:227–36. doi:10.1016/j.heares.2004.12.013
50. Carpenter-Thompson JR, Akrofi K, Schmidt SA, Dolcos F, Husain FT. Alterations of the emotional processing system may underlie preserved rapid reaction time in tinnitus. Brain Res (2014) 1567:28–41. doi:10.1016/j.brainres.2014.04.024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
51. Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J Assoc Res Otolaryngol (2001) 2:54–64. doi:10.1007/s101620010030
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
52. Heffner HE, Harrington IA. Tinnitus in hamsters following exposure to intense sound. Hear Res (2002) 170:83–95. doi:10.1016/S0378-5955(02)00343-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
53. Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT. Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci (1988) 102:811–22. doi:10.1037/0735-7044.102.6.811
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
54. Berger JI, Coomber B, Shackleton TM, Palmer AR, Wallace MN. A novel behavioural approach to detecting tinnitus in the guinea pig. J Neurosci Methods (2013) 213:188–95. doi:10.1016/j.jneumeth.2012.12.023
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
55. Dehmel S, Eisinger D, Shore SE. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front Syst Neurosci (2012) 6:42. doi:10.3389/fnsys.2012.00042
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
56. Longenecker RJ, Galazyuk AV. Methodological optimization of tinnitus assessment using prepulse inhibition of the acoustic startle reflex. Brain Res (2012) 1485:54–62. doi:10.1016/j.brainres.2012.02.067
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Pace E, Zhang J. Noise-induced tinnitus using individualized gap detection analysis and its relationship with hyperacusis, anxiety, and spatial cognition. PLoS One (2013) 8:e75011. doi:10.1371/journal.pone.0075011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
58. Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, et al. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci (2006) 120:188–95. doi:10.1037/0735-7044.120.1.188
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
59. Campolo J, Lobarinas E, Salvi R. Does tinnitus “fill in” the silent gaps? Noise Health (2013) 15:398–405. doi:10.4103/1463-1741.121232
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
60. Eggermont JJ. Hearing loss, hyperacusis, or tinnitus: what is modeled in animal research? Hear Res (2013) 295:140–9. doi:10.1016/j.heares.2012.01.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
61. Turner J, Larsen D, Hughes L, Moechars D, Shore S. Time course of tinnitus development following noise exposure in mice. J Neurosci Res (2012) 90:1480–8. doi:10.1002/Jnr.22827
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
63. Eggermont JJ. Between sound and perception: reviewing the search for a neural code. Hear Res (2001) 157:1–42. doi:10.1016/S0378-5955(01)00259-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
64. Roberts LE. Neural synchrony and neural plasticity in tinnitus. In: Møller A, Langguth B, De Ridder D, Kleinjung T, editors. Textbook of Tinnitus. New York, NY: Springer (2011). p. 103–12.
65. Huffman RF, Henson OW Jr. The descending auditory pathway and acousticomotor systems: connections with the inferior colliculus. Brain Res Brain Res Rev (1990) 15:295–323. doi:10.1016/0165-0173(90)90005-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
66. Aitkin LM, Webster WR, Veale JL, Crosby DC. Inferior colliculus. I. Comparison of response properties of neurons in central, pericentral, and external nuclei of adult cat. J Neurophysiol (1975) 38:1196–207.
67. Faye-Lund H, Osen KK. Anatomy of the inferior colliculus in rat. Anat Embryol (Berl) (1985) 171:1–20. doi:10.1007/BF00319050
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
68. Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. The ferret auditory cortex: descending projections to the inferior colliculus. Cereb Cortex (2007) 17:475–91. doi:10.1093/cercor/bhj164
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
69. Druga R, Syka J. Ascending and descending projections to the inferior colliculus in the rat. Physiol Bohemoslov (1984) 33:31–42.
70. Druga R, Syka J. Neocortical projections to the inferior colliculus in the rat. (An experimental study using anterograde degeneration techniques). Physiol Bohemoslov (1984) 33:251–3.
71. Druga R, Syka J, Rajkowska G. Projections of auditory cortex onto the inferior colliculus in the rat. Physiol Res (1997) 46:215–22.
72. Winer JA, Larue DT, Diehl JJ, Hefti BJ. Auditory cortical projections to the cat inferior colliculus. J Comp Neurol (1998) 400:147–74. doi:10.1002/(SICI)1096-9861(19981019)400:23.0.CO;2-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
73. Saldana E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol (1996) 371:15–40. doi:10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
74. Malmierca MS. The inferior colliculus: a center for convergence of ascending and descending auditory information. Neuroembryol Aging (2006) 3:215–29. doi:10.1159/000096799
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
75. Popelar J, Nwabueze-Ogbo FC, Syka J. Changes in neuronal activity of the inferior colliculus in rat after temporal inactivation of the auditory cortex. Physiol Res (2003) 52:615–28.
76. Ma X, Suga N. Plasticity of bat’s central auditory system evoked by focal electric stimulation of auditory and/or somatosensory cortices. J Neurophysiol (2001) 85:1078–87.
77. Duan M, Laurell G, Qiu J, Borg E. Susceptibility to impulse noise trauma in different species: guinea pig, rat and mouse. Acta Otolaryngol (2008) 128:277–83. doi:10.1080/00016480701509941
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
78. Myers EN, Bernstein JM. Salicylate ototoxicity; a clinical and experimental study. Arch Otolaryngol (1965) 82:483–93. doi:10.1001/archotol.1965.00760010485006
79. Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M. Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg (1999) 121:457–62. doi:10.1016/S0194-5998(99)70237-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
80. Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC). Hear Res (2004) 190:109–14. doi:10.1016/S0378-5955(04)00019-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
81. Ruttiger L, Ciuffani J, Zenner HP, Knipper M. A behavioral paradigm to judge acute sodium salicylate-induced sound experience in rats: a new approach for an animal model on tinnitus. Hear Res (2003) 180:39–50. doi:10.1016/S0378-5955(03)00075-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
82. Shehata WE, Brownell WE, Dieler R. Effects of salicylate on shape, electromotility and membrane characteristics of isolated outer hair cells from guinea pig cochlea. Acta Otolaryngol (1991) 111:707–18. doi:10.3109/00016489109138403
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
83. Evans EF, Borerwe TA. Ototoxic effects of salicylates on the responses of single cochlear nerve fibres and on cochlear potentials. Br J Audiol (1982) 16:101–8. doi:10.3109/03005368209081454
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
84. Wei L, Ding D, Salvi R. Salicylate-induced degeneration of cochlea spiral ganglion neurons-apoptosis signaling. Neuroscience (2010) 168:288–99. doi:10.1016/j.neuroscience.2010.03.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
85. Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, et al. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res (2007) 226:244–53. doi:10.1016/j.heares.2006.06.013
86. Eggermont JJ, Kenmochi M. Salicylate and quinine selectively increase spontaneous firing rates in secondary auditory cortex. Hear Res (1998) 117:149–60. doi:10.1016/S0378-5955(98)00008-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
87. Jastreboff PJ, Sasaki CT. Salicylate-induced changes in spontaneous activity of single units in the inferior colliculus of the guinea pig. J Acoust Soc Am (1986) 80:1384–91. doi:10.1121/1.394391
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
88. Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear Res (1995) 82:158–78. doi:10.1016/0378-5955(94)00174-O
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
89. Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol (2000) 62:583–631. doi:10.1016/S0301-0082(00)00027-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
90. Basta D, Ernst A. Effects of salicylate on spontaneous activity in inferior colliculus brain slices. Neurosci Res (2004) 50:237–43. doi:10.1016/j.neures.2004.07.003
91. Manabe Y, Yoshida S, Saito H, Oka H. Effects of lidocaine on salicylate-induced discharge of neurons in the inferior colliculus of the guinea pig. Hear Res (1997) 103:192–8. doi:10.1016/S0378-5955(96)00181-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
92. Ma WL, Hidaka H, May BJ. Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear Res (2006) 212:9–21. doi:10.1016/j.heares.2005.10.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
93. Paul AK, Lobarinas E, Simmons R, Wack D, Luisi JC, Spernyak J, et al. Metabolic imaging of rat brain during pharmacologically-induced tinnitus. Neuroimage (2009) 44:312–8. doi:10.1016/j.neuroimage.2008.09.024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
94. Wallhausser-Franke E, Braun S, Langner G. Salicylate alters 2-DG uptake in the auditory system: a model for tinnitus? Neuroreport (1996) 7:1585–8. doi:10.1097/00001756-199607080-00010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
95. Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, et al. Salicylate increases the gain of the central auditory system. Neuroscience (2009) 159:325–34. doi:10.1016/j.neuroscience.2008.12.024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
96. Hu SS, Mei L, Chen JY, Huang ZW, Wu H. Expression of immediate-early genes in the inferior colliculus and auditory cortex in salicylate-induced tinnitus in rat. Eur J Histochem (2014) 58:2294. doi:10.4081/ejh.2014.2294
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
97. Hwang JH, Chen JC, Yang SY, Wang MF, Liu TC, Chan YC. Expression of COX-2 and NMDA receptor genes at the cochlea and midbrain in salicylate-induced tinnitus. Laryngoscope (2011) 121:361–4. doi:10.1002/Lary.21283
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
98. Hwang JH, Chen JC, Chan YC. Effects of C-phycocyanin and spirulina on salicylate-induced tinnitus, expression of NMDA receptor and inflammatory genes. PLoS One (2013) 8:e58215. doi:10.1371/journal.pone.0058215
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
99. Bauer CA, Brozoski TJ, Holder TM, Caspary DM. Effects of chronic salicylate on GABAergic activity in rat inferior colliculus. Hear Res (2000) 147:175–82. doi:10.1016/S0378-5955(00)00130-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
100. Zou QZ, Shang XL. Effect of salicylate on the large GABAergic neurons in the inferior colliculus of rats. Acta Neurol Belg (2012) 112:367–74. doi:10.1007/s13760-012-0090-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
101. Liu J, Li X, Wang L, Dong Y, Han H, Liu G. Effects of salicylate on serotoninergic activities in rat inferior colliculus and auditory cortex. Hear Res (2003) 175:45–53. doi:10.1016/S0378-5955(02)00708-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
102. Lu YG, Tang ZQ, Ye ZY, Wang HT, Huang YN, Zhou KQ, et al. Salicylate, an aspirin metabolite, specifically inhibits the current mediated by glycine receptors containing alpha1-subunits. Br J Pharmacol (2009) 157:1514–22. doi:10.1111/j.1476-5381.2009.00321.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
103. Wang HT, Luo B, Huang YN, Zhou KQ, Chen L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear Res (2008) 236:42–51. doi:10.1016/j.heares.2007.11.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
104. Wu JL, Chiu TW, Poon PWF. Differential changes in Fos-immunoreactivity at the auditory brainstem after chronic injections of salicylate in rats. Hear Res (2003) 176:80–93. doi:10.1016/S0378-5955(02)00747-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
105. Mahlke C, Wallhausser-Franke E. Evidence for tinnitus-related plasticity in the auditory and limbic system, demonstrated by arg3.1 and c-fos immunocytochemistry. Hear Res (2004) 195:17–34. doi:10.1016/j.heares.2004.03.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
106. Hwang JH, Chen JC, Yang SY, Wang MF, Chan YC. Expression of tumor necrosis factor-alpha and interleukin-1beta genes in the cochlea and inferior colliculus in salicylate-induced tinnitus. J Neuroinflammation (2011) 8:30. doi:10.1186/1742-2094-8-30
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
107. Basta D, Goetze R, Ernst A. Effects of salicylate application on the spontaneous activity in brain slices of the mouse cochlear nucleus, medial geniculate body and primary auditory cortex. Hear Res (2008) 240:42–51. doi:10.1016/j.heares.2008.02.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
108. Lobarinas E, Yang G, Sun W, Ding D, Mirza N, Dalby-Brown W, et al. Salicylate-and quinine-induced tinnitus and effects of memantine. Acta Otolaryngol Suppl (2006) 556:13–9. doi:10.1080/03655230600895408
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
109. Kumagai M. [Effect of intravenous injection of aspirin on the cochlea]. Hokkaido Igaku Zasshi (1992) 67:216–33.
110. Lanting CP, De Kleine E, Bartels H, Van Dijk P. Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngol (2008) 128:415–21. doi:10.1080/00016480701793743
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
111. Melcher JR, Sigalovsky IS, Guinan JJ Jr, Levine RA. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol (2000) 83:1058–72.
112. Smits M, Kovacs S, De Ridder D, Peeters RR, Van Hecke P, Sunaert S. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology (2007) 49:669–79. doi:10.1007/s00234-007-0231-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
113. Melcher JR, Levine RA, Bergevin C, Norris B. The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear Res (2009) 257:63–74. doi:10.1016/j.heares.2009.08.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
114. Geven LI, De Kleine E, Willemsen AT, Van Dijk P. Asymmetry in primary auditory cortex activity in tinnitus patients and controls. Neuroscience (2014) 256:117–25. doi:10.1016/j.neuroscience.2013.10.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
115. Boyen K, De Kleine E, Van Dijk P, Langers DR. Tinnitus-related dissociation between cortical and subcortical neural activity in humans with mild to moderate sensorineural hearing loss. Hear Res (2014) 312:48–59. doi:10.1016/j.heares.2014.03.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
116. Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A (1999) 96:15222–7. doi:10.1073/pnas.96.26.15222
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
117. van Gendt MJ, Boyen K, De Kleine E, Langers DRM, Van Dijk P. The relation between perception and brain activity in gaze-evoked tinnitus. J Neurosci (2012) 32:17528–39. doi:10.1523/Jneurosci.2791-12.2012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
118. Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, et al. Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage (2009) 46:213–8. doi:10.1016/j.neuroimage.2009.01.069
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
119. Muhlau M, Rauschecker JP, Oestreicher E, Gaser C, Rottinger M, Wohlschlager AM, et al. Structural brain changes in tinnitus. Cereb Cortex (2006) 16:1283–8. doi:10.1093/cercor/bhj070
120. Melcher JR, Knudson IM, Levine RA. Subcallosal brain structure: correlation with hearing threshold at supra-clinical frequencies (> 8 kHz), but not with tinnitus. Hear Res (2013) 295:79–86. doi:10.1016/j.heares.2012.03.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
121. Crippa A, Lanting CP, Van Dijk P, Roerdink JB. A diffusion tensor imaging study on the auditory system and tinnitus. Open Neuroimag J (2010) 4:16–25. doi:10.2174/1874440001004010016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
122. Stimmer H, Borrmann A, Loer C, Arnold W, Rummeny EJ. Monaural tinnitus from a contralateral inferior colliculus hemorrhage. Audiol Neurootol (2009) 14:35–8. doi:10.1159/000152854
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
123. Choi SY, Song JJ, Hwang JM, Kim JS. Tinnitus in fourth nerve palsy: an indicator for an intra-axial lesion. J Neuroophthalmol (2010) 30:325–7. doi:10.1097/WNO.0b013e3181e4e03e
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
124. Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci (2011) 31:13452–7. doi:10.1523/JNEUROSCI.2156-11.2011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
125. Gu JW, Herrmann BS, Levine RA, Melcher JR. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J Assoc Res Otolaryngol (2012) 13:819–33. doi:10.1007/s10162-012-0344-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
126. Sanchez TG, Rocha CB. Diagnosis and management of somatosensory tinnitus: review article. Clinics (Sao Paulo) (2011) 66:1089–94. doi:10.1590/S1807-59322011000600028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
127. Dehmel S, Pradhan S, Koehler S, Bledsoe S, Shore S. Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus – possible basis for tinnitus-related hyperactivity? J Neurosci (2012) 32:1660–71. doi:10.1523/JNEUROSCI.4608-11.2012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
128. Lanting CP, De Kleine E, Eppinga RN, Van Dijk P. Neural correlates of human somatosensory integration in tinnitus. Hear Res (2010) 267:78–88. doi:10.1016/j.heares.2010.04.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
129. Aitkin LM, Dickhaus H, Schult W, Zimmermann M. External nucleus of inferior colliculus: auditory and spinal somatosensory afferents and their interactions. J Neurophysiol (1978) 41:837–47.
130. Gritsenko K, Caldwell W, Shaparin N, Vydyanathan A, Kosharskyy B. Resolution of long standing tinnitus following radiofrequency ablation of C2-C3 medial branches – a case report. Pain Physician (2014) 17:E95–8.
131. Szczepaniak WS, Moller AR. Effects of (-)-baclofen, clonazepam, and diazepam on tone exposure-induced hyperexcitability of the inferior colliculus in the rat: possible therapeutic implications for pharmacological management of tinnitus and hyperacusis. Hear Res (1996) 97:46–53. doi:10.1016/S0378-5955(96)80006-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
132. Zheng Y, Vagal S, Mcnamara E, Darlington CL, Smith PF. A dose-response analysis of the effects of L-baclofen on chronic tinnitus caused by acoustic trauma in rats. Neuropharmacology (2012) 62:940–6. doi:10.1016/j.neuropharm.2011.09.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
133. Smith PF, Zheng Y, Darlington CL. Revisiting baclofen for the treatment of severe chronic tinnitus. Front Neurol (2012) 3:34. doi:10.3389/fneur.2012.00034
134. Baguley DM, Phillips J, Humphriss RL, Jones S, Axon PR, Moffat DA. The prevalence and onset of gaze modulation of tinnitus and increased sensitivity to noise after translabyrinthine vestibular schwannoma excision. Otol Neurotol (2006) 27:220–4. doi:10.1097/01.mao.0000172412.87778.28
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
135. Berliner KI, Shelton C, Hitselberger WE, Luxford WM. Acoustic tumors: effect of surgical removal on tinnitus. Am J Otol (1992) 13:13–7. doi:10.1097/00129492-199201000-00005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
136. Mulders WH, Barry KM, Robertson D. Effects of furosemide on cochlear neural activity, central hyperactivity and behavioural tinnitus after cochlear trauma in guinea pig. PLoS One (2014) 9:e97948. doi:10.1371/journal.pone.0097948
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
137. Mulders WH, Mcmahen C, Robertson D. Effects of chronic furosemide on central neural hyperactivity and cochlear thresholds after cochlear trauma in guinea pig. Front Neurol (2014) 5:146. doi:10.3389/fneur.2014.00146
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
138. Rybak LP, Morizono T. Effect of furosemide upon endolymph potassium concentration. Hear Res (1982) 7:223–31. doi:10.1016/0378-5955(82)90015-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
139. Risey JA, Guth PS, Amedee RG. Furosemide distinguishes central and peripheral tinnitus. Int Tinnitus J (1995) 1:99–103.
140. Salvi R, Loborinas E, Sun W. Pharmacological treatments for tinnitus: new and old. Drugs Future (2009) 34:381–400. doi:10.1358/dof.2009.34.5.1362442
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
141. Offutt SJ, Ryan KJ, Konop AE, Lim HH. Suppression and facilitation of auditory neurons through coordinated acoustic and midbrain stimulation: investigating a deep brain stimulator for tinnitus. J Neural Eng (2014) 11:066001. doi:10.1088/1741-2560/11/6/066001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
142. Roberts LE. Residual inhibition. Prog Brain Res (2007) 166:487–95. doi:10.1016/S0079-6123(07)66047-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
143. Voytenko SV, Galazyuk AV. mGluRs modulate neuronal firing in the auditory midbrain. Neurosci Lett (2011) 492:145–9. doi:10.1016/j.neulet.2011.01.075
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
144. Auerbach BD, Rodrigues PV, Salvi R. Central gain control in tinnitus and hyperacusis. Front Neurol (2014) 5:206. doi:10.3389/fneur.2014.00206
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
145. Norena AJ. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev (2011) 35:1089–109. doi:10.1016/j.neubiorev.2010.11.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
146. Schaette R, Kempter R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. Eur J Neurosci (2006) 23:3124–38. doi:10.1111/j.1460-9568.04774.x
147. Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N. Enhanced evoked-response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hear Res (1990) 50:245–58. doi:10.1016/0378-5955(90)90049-U
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
148. De Ridder D, Vanneste S, Weisz N, Londero A, Schlee W, Elgoyhen AB, et al. An integrative model of auditory phantom perception: tinnitus as a unified percept of interacting separable subnetworks. Neurosci Biobehav Rev (2014) 44:16–32. doi:10.1016/j.neubiorev.2013.03.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
149. Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res (1990) 8:221–54. doi:10.1016/0168-0102(90)90031-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
150. Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron (2010) 66:819–26. doi:10.1016/j.neuron.2010.04.032
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
151. Marsh RA, Fuzessery ZM, Grose CD, Wenstrup JJ. Projection to the inferior colliculus from the basal nucleus of the amygdala. J Neurosci (2002) 22:10449–60.
152. Ahlf S, Tziridis K, Korn S, Strohmeyer I, Schulze H. Predisposition for and prevention of subjective tinnitus development. PLoS One (2012) 7:e44519. doi:10.1371/journal.pone.0044519
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
153. Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol (1999) 20:351–62. doi:10.1016/S0196-0709(99)90074-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
154. Pinchoff RJ, Burkard RF, Salvi RJ, Coad ML, Lockwood AH. Modulation of tinnitus by voluntary jaw movements. Am J Otol (1998) 19:785–9.
155. Shore SE. Plasticity of somatosensory inputs to the cochlear nucleus – implications for tinnitus. Hear Res (2011) 281:38–46. doi:10.1016/j.heares.2011.05.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
156. Koehler SD, Pradhan S, Manis PB, Shore SE. Somatosensory inputs modify auditory spike timing in dorsal cochlear nucleus principal cells. Eur J Neurosci (2011) 33:409–20. doi:10.1111/j.1460-9568.2010.07547.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
157. Shore SE, El Kashlan H, Lu J. Effects of trigeminal ganglion stimulation on unit activity of ventral cochlear nucleus neurons. Neuroscience (2003) 119:1085–101. doi:10.1016/S0306-4522(03)00207-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
158. Shore SE. Multisensory integration in the dorsal cochlear nucleus: unit responses to acoustic and trigeminal ganglion stimulation. Eur J Neurosci (2005) 21:3334–48. doi:10.1111/j.1460-9568.2005.04142.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
159. Kunzle H. Origin and terminal distribution of the trigeminal projections to the inferior and superior colliculi in the lesser hedgehog tenrec. Eur J Neurosci (1998) 10:368–76. doi:10.1046/j.1460-9568.1998.00020.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
160. Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol (2006) 495:100–12. doi:10.1002/cne.20863
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
161. Aitkin LM, Kenyon CE, Philpott P. The representation of the auditory and somatosensory systems in the external nucleus of the cat inferior colliculus. J Comp Neurol (1981) 196:25–40. doi:10.1002/cne.901960104
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: acoustic over-exposure, salicylate, auditory, inferior colliculus, tinnitus, midbrain
Citation: Berger JI and Coomber B (2015) Tinnitus-related changes in the inferior colliculus. Front. Neurol. 6:61. doi: 10.3389/fneur.2015.00061
Received: 17 December 2014; Accepted: 09 March 2015;
Published online: 30 March 2015.
Edited by:
Jinsheng Zhang, Wayne State University, USAReviewed by:
Rony H. Salloum, Cleveland Clinic, USAAugustine Changdae Lee, University of Toledo, USA
Donald Albert Godfrey, University of Toledo, USA
Hao Luo, Wayne State University, USA
Copyright: © 2015 Berger and Coomber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joel I. Berger and Ben Coomber, Medical Research Council Institute of Hearing Research, University of Nottingham, Science Road, Nottingham NG7 2RD, UK e-mail: joel@ihr.mrc.ac.uk; ben@ihr.mrc.ac.uk
 Joel I. Berger
Joel I. Berger Ben Coomber
Ben Coomber