- 1Reference Center for Biological Markers of Dementia (BIODEM), Institute Born-Bunge, University of Antwerp, Antwerp, Belgium
- 2StatUa Center for Statistics, University of Antwerp, Antwerp, Belgium
- 3Biobank, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium
- 4Department of Neurology and Memory Clinic, Hospital Network Antwerp (ZNA) Middelheim and Hoge Beuken, Antwerp, Belgium
- 5Department of Neurology and Alzheimer Research Center, University Medical Center Groningen (UMCG), Groningen, Netherlands
The goal of this study is to investigate the value of tau phosphorylated at threonine 181 (P-tau181P) in the Alzheimer’s disease (AD) cerebrospinal fluid (CSF) biomarker panel for differential dementia diagnosis in autopsy confirmed AD and non-AD patients. The study population consisted of 140 autopsy confirmed AD and 77 autopsy confirmed non-AD dementia patients. CSF concentrations of amyloid-β peptide of 42 amino acids (Aβ1–42), total tau protein (T-tau), and P-tau181P were determined with single analyte ELISA-kits (INNOTEST®, Fujirebio, Ghent, Belgium). Diagnostic accuracy was assessed through receiver operating characteristic (ROC) curve analyses to obtain area under the curve (AUC) values and to define optimal cutoff values to discriminate AD from pooled and individual non-AD groups. ROC curve analyses were only performed on biomarkers and ratios that differed significantly between the groups. Pairwise comparison of AUC values was performed by means of DeLong tests. The Aβ1–42/P-tau181P ratio (AUC = 0.770) performed significantly better than Aβ1–42 (AUC = 0.677, P = 0.004), T-tau (AUC = 0.592, P < 0.001), and Aβ1–42/T-tau (AUC = 0.678, P = 0.001), while P-tau181P (AUC = 0.720) performed significantly better than T-tau (AUC = 0.592, P < 0.001) to discriminate between AD and the pooled non-AD group. When comparing AD and the individual non-AD diagnoses, Aβ1–42/P-tau181P (AUC = 0.894) discriminated AD from frontotemporal dementia significantly better than Aβ1–42 (AUC = 0.776, P = 0.020) and T-tau (AUC = 0.746, P = 0.004), while P-tau181P/T-tau (AUC = 0.958) significantly improved the differentiation between AD and Creutzfeldt-Jakob disease as compared to Aβ1–42 (AUC = 0.688, P = 0.004), T-tau (AUC = 0.874, P = 0.040), and Aβ1–42/P-tau181P (AUC = 0.760, P = 0.003). In conclusion, this study demonstrates P-tau181P is an essential component of the AD CSF biomarker panel, and combined assessment of Aβ1–42, T-tau, and P-tau181P renders, to present date, the highest diagnostic power to discriminate between AD and non-AD dementias.
Introduction
The clinical diagnosis of Alzheimer’s disease (AD) is mainly based on the exclusion of other diseases (1). Relative to autopsy confirmation, the clinical diagnostic criteria of AD (1) reach on average 81% sensitivity and 70% specificity (2). However, these figures mostly originate from specialized clinical centers and from diagnoses based on follow-up periods of several years. In the earliest stages of the disease and when the diagnostic work-up is performed in non-specialized centers, far lower diagnostic accuracy can be expected. Diagnosis of definite AD can therefore only be made through postmortem pathological examination of the brain.
Analyzing cerebrospinal fluid (CSF) levels of amyloid-β peptide of 42 amino acids (Aβ1–42), total tau protein (T-tau) and tau phosphorylated at threonine 181 (P-tau181P) increases diagnostic certainty for AD (3). Based on autopsy confirmation, it was shown that in the majority of patients with a clinically ambiguous diagnosis (when the clinical diagnostic work-up was not able to discriminate between AD and a non-AD dementia), a correct diagnosis would have been established in 82% by using these CSF biomarkers, indicating that CSF biomarkers may have a particular added diagnostic value in patients with ambiguous clinical diagnoses (4).
Compared to controls, decreased Aβ1–42 and increased T-tau and/or P-tau181P concentrations are found in AD. However, when compared to non-AD dementias, the differences are less obvious as the concentrations in patients with non-AD dementias are generally intermediate compared to those found between controls and AD patients, thus pointing to an overlap between AD and non-AD patients, especially in dementia with Lewy bodies (DLB) and to a lesser extent in frontotemporal dementia (FTD), vascular dementia (VaD), and Creutzfeldt-Jakob’s disease (CJD) (5). This overlap may partly be explained by the presence of mixed pathologies as well as the low sensitivity and specificity of the clinical diagnosis as most biomarker studies rely on clinically diagnosed patients.
The goal of this study is to investigate the value of P-tau181P in the AD CSF biomarker panel for differential dementia diagnosis in autopsy confirmed AD and non-AD patients.
Materials and Methods
Study Population
In brief, the study population consisted of 140 and 77 CSF samples from dementia patients with pathologically confirmed diagnoses of AD and non-AD, respectively. All CSF samples were selected from the Biobank, Institute Born-Bunge, Antwerp, Belgium. Samples from 173 dementia patients were collected in the Memory Clinic of the Hospital Network Antwerp (ZNA, Antwerp, Belgium) between January 1992 and May 2008, whereas samples from 44 dementia patients were collected in referring centers between April 1992 and May 2005.
The study was approved by the local ethics committee (CME Middelheim) and all subjects gave written informed consent.
Pathological Criteria
All pathological diagnoses were established according to standard neuropathological criteria by the same neuropathologist (Jean-Jacques Martin). Although the neuropathologist was blinded for the CSF biomarker data, he had access to all neuroimaging data and the clinical files of the patients included. For the diagnosis of AD, VaD (n = 18), and DLB (n = 24), the neuropathological criteria of Montine et al. (6) were applied. FTD (n = 17) was neuropathologically diagnosed according to the Cairns criteria (7) and Mackenzie criteria (8, 9). CJD (n = 13) was diagnosed according to the criteria of Markesbery (10). Mixed dementia (MXD) was diagnosed when the patient fulfilled the neuropathological criteria of AD in combination with minor pathology suggestive of cerebrovascular disease (n = 12), DLB (n = 1), or Parkinson’s disease (n = 1). For statistical analyses, the MXD group (n = 14) was pooled with the AD group. The pooled non-AD group furthermore consisted of few patients with progressive nuclear palsy (n = 3), spinocerebellar ataxia (n = 1), and normal pressure hydrocephalus combined with VaD (n = 1). Neuropathology was performed on the right hemisphere of the brain.
CSF Analyses
All subjects underwent a lumbar puncture (LP) in order to collect CSF. LP was performed between the intervertebral space L3/L4 or L4/L5 (11). CSF was sampled according to a standard protocol (12). All samples were stored in polypropylene vials to avoid adsorption of Aβ to the wall of the vial. The samples were frozen in liquid nitrogen and stored at −80°C until analysis.
CSF concentrations of Aβ1–42, T-tau, and P-tau181P were determined with commercially available single analyte ELISA-kits (respectively, INNOTEST® β-AMYLOID(1–42), INNOTEST® hTAU-Ag, and INNOTEST® PHOSPHO-TAU(181P); Fujirebio, Ghent, Belgium). A complete description of the CSF analysis has been published previously (13).
Statistical Analyses
Statistical analyses were performed using SPSS 20. As most variables were not normally distributed, non-parametric tests were used. To compare gender distribution between the groups, a Chi-square test was performed. Subsequently, Mann–Whitney U tests were performed to compare clinical and biomarker data between the groups. Receiver operating characteristic (ROC) curve analyses were used to obtain area under the curve (AUC) values and to define optimal cutoff values to discriminate AD from the pooled and individual non-AD groups. ROC curve analyses were only performed on biomarkers and ratios that were significantly different (P < 0.05), based on the Mann–Whitney U tests. The cutoff values were determined by calculating the maximal sum of sensitivity and specificity (i.e., maximizing the Youden index). In order to pairwise compare AUC values, DeLong tests were performed using the pROC package (14) in the statistical software package R (R Core Team).
Systematic Review
To be able to compare the results of this study, a systematic review on the diagnostic accuracy of P-tau181P for differential dementia diagnosis was performed. A PubMed search (until May 2015) was performed using the following terms: (Cerebrospinal fluid OR CSF) AND diagnos* AND (Alzheimer* OR AD OR dementia) AND (tau OR beta amyloid OR abeta) AND (sensitivity OR specificity). Only publications in the English language were evaluated. Subsequently, relevant publications were searched for in reference lists. Publications were included when: (a) their aim was to improve the diagnostic accuracy of diagnosis of dementia by means of CSF biomarkers, (b) AD patients and pooled non-AD patients or patients with DLB, FTD, VaD and/or CJD were included, (c) P-tau181P together with Aβ1–42 and/or T-tau was measured in CSF, and (d) diagnostic accuracy values were reported (AUC, sensitivity, and/or specificity). Publications comparing only AD to healthy control subjects were not considered.
Results
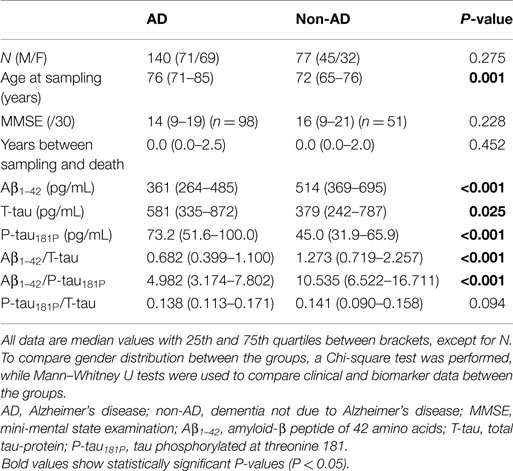
Table 1 shows the demographic, clinical, and biomarker data of the studied population. The AD and non-AD groups were not age-matched. However, based on co-variate analyses, confounding effects of age on differences in biomarker concentrations were excluded. Therefore, no corrections for age were included in the subsequent analyses. Boxplots of the individual biomarkers and ratios are presented in Figure 1.
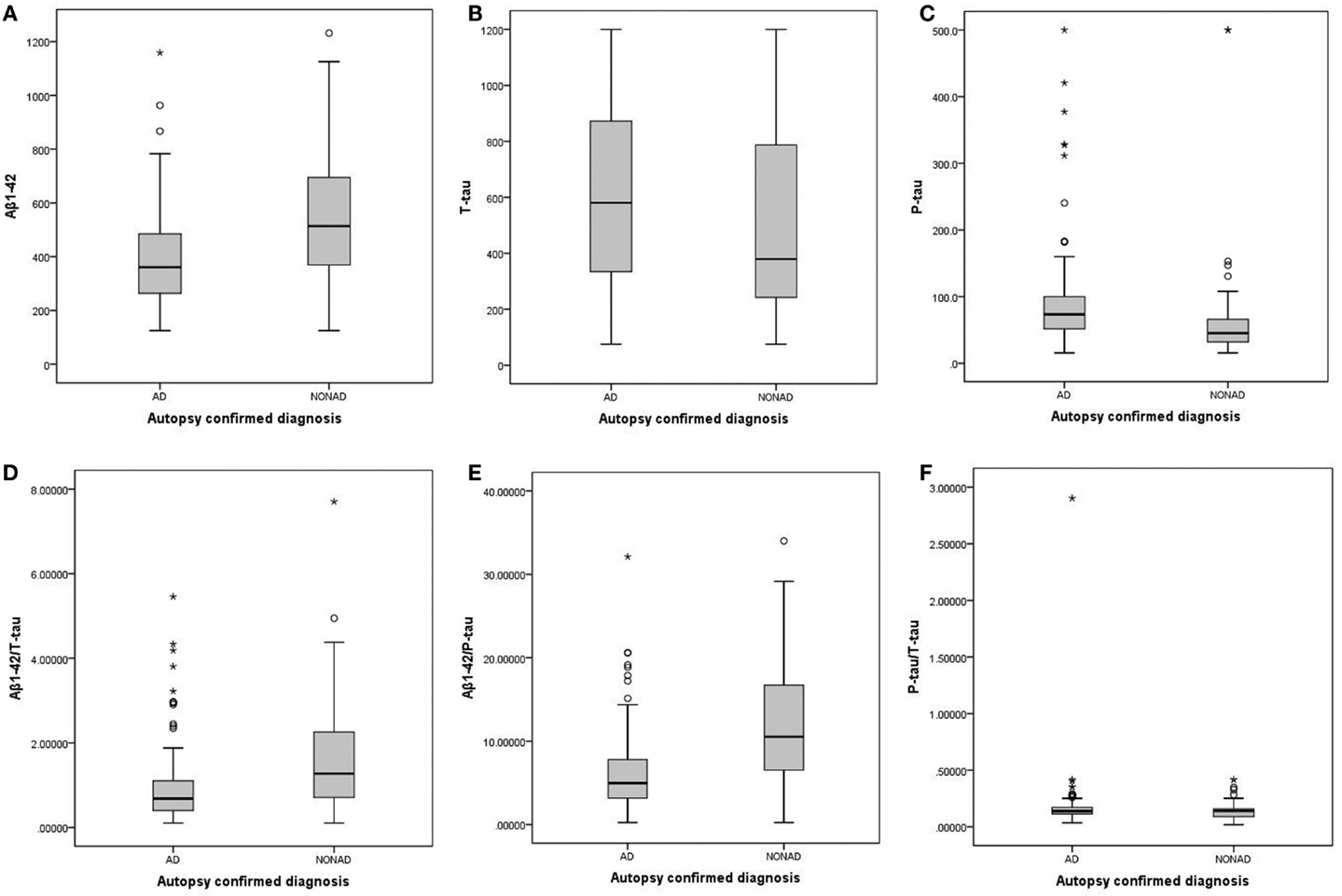
Figure 1. Boxplots of the individual biomarkers and ratios, comparing AD and non-AD. (A) Aβ1–42; (B) T-tau; (C) P-tau181P; (D) Aβ1–42/T-tau; (E) Aβ1–42/P-tau181P; (F) P-tau181P/T-tau. AD, Alzheimer’s disease; non-AD, dementia not due to Alzheimer’s disease; Aβ1–42, amyloid-β peptide of 42 amino acids; T-tau, total tau-protein; P-tau181P, tau phosphorylated at threonine 181.
The diagnostic powers to discriminate between AD and non-AD of the individual biomarkers and ratios that were significantly different are shown in Table 2. Based on the DeLong tests (Table 3), the AUC of the Aβ1–42/P-tau181P ratio was significantly different from those of Aβ1–42, T-tau, and Aβ1–42/T-tau, while the AUC of P-tau181P differed significantly from the AUC of T-tau.
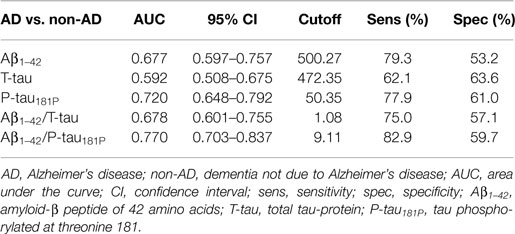
Table 2. Diagnostic power of the significantly different individual biomarkers and ratios to discriminate between AD and non-AD, measured by ROC curve analyses.
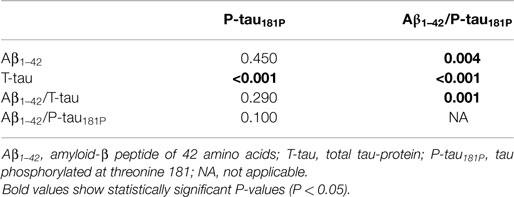
Table 3. P values of pairwise comparisons of AUC values of the ROC curve analyses to discriminate between AD and non-AD, using DeLong tests.
When comparing AD and the different non-AD diagnoses, the Aβ1–42/P-tau181P ratio was significantly different in every differential diagnosis (Table 4). This also held true for P-tau181P, except for AD vs. CJD. On the other hand, P-tau181P/T-tau was found to be significantly different when comparing AD to CJD.
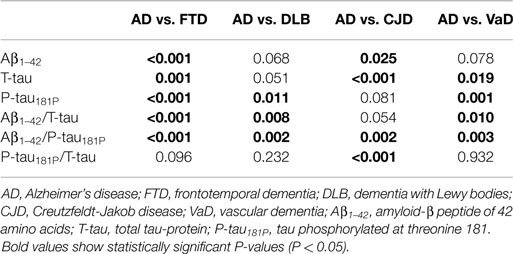
Table 4. P values of pairwise comparisons of the individual biomarkers and ratios, measured by Mann–Whitney U tests.
The diagnostic powers to discriminate between AD and the different non-AD diagnoses of the individual biomarkers and ratios that differed significantly are shown in Table 5. Based on the DeLong tests (Table 6), the Aβ1–42/P-tau181P ratio performed significantly better than Aβ1–42 and T-tau to discriminate AD from FTD, while the AUC of P-tau181P/T-tau was significantly better than those of Aβ1–42, T-tau, and Aβ1–42/P-tau181P to differentiate between AD and CJD.
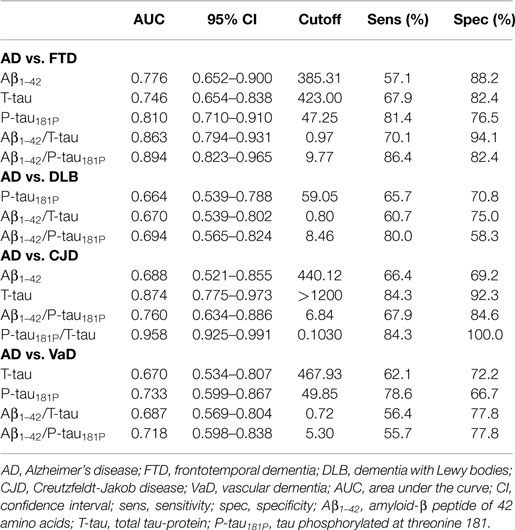
Table 5. Diagnostic power of the significantly different individual biomarkers and ratios to discriminate between AD and individual non-AD diagnoses, measured by ROC curve analyses.
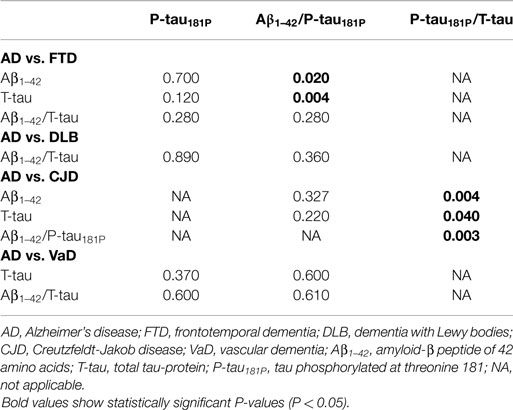
Table 6. P values of pairwise comparisons of AUC values of the ROC curve analyses to discriminate between AD and individual non-AD diagnoses, using DeLong tests.
The results of the systematic review are summarized in Table S1 in Supplementary Material. Only results comparing AD to non-AD, FTD, DLB, CJD, and/or VaD were included in this table.
Discussion
The goal of this study was to investigate the value of P-tau181P in the AD biomarker panel for differential dementia diagnosis. First of all, the ratio of Aβ1–42/P-tau181P was shown to have a significantly higher diagnostic power than Aβ1–42, T-tau, and the Aβ1–42/T-tau ratio, while P-tau181P was found to perform significantly better than T-tau to discriminate between AD and non-AD dementia. This clearly signifies the importance of P-tau181P in the biomarker panel for differential dementia diagnosis. Our results are in line with previously reported findings of (combinations with) P-tau181P having most power to discriminate between AD and non-AD dementias (12, 15–29).
However, in contrast to former studies performed in clinically diagnosed AD and pooled non-AD dementia patients (15, 21, 22, 26–28), the AUC, sensitivity, and specificity of neither P-tau181P nor Aβ1–42/P-tau181P reached the minimal level of 0.80, as established by the Consensus Report of the Working Group on Molecular and Biochemical Markers of AD (30). This is probably not due to the accuracy of the diagnoses used in this study, as autopsy confirmation was used. A possible explanation of the discrepancy in accuracy levels between this study and former studies could be the composition of the non-AD groups. As shown in this study, the accuracy levels of, for example, AD vs. FTD are substantially higher than those of AD vs. DLB. Therefore, if a non-AD group is primarily composed of FTD patients, the AUC levels may be higher than when DLB patients prevail in the non-AD group.
When focusing on the discrimination between AD and FTD, our results showed that the diagnostic power of Aβ1–42/P-tau181P was significantly higher than those of Aβ1–42 and T-tau. These results confirm earlier studies performed in clinically diagnosed AD and FTD patients (16, 17, 24, 25, 29).
With regard to the differentiation between AD and CJD, the diagnostic power of P-tau181P/T-tau was significantly higher than those of Aβ1–42, T-tau, and Aβ1–42/P-tau181P. Our results confirm those of former studies performed in clinically diagnosed AD and CJD patients, and partly performed in autopsy confirmed cases (31–34).
In these latter two comparisons with individual non-AD groups, the AUCs did reach the minimal level of 0.80. This indicates that the pathophysiological variability in the pooled non-AD group lowers the diagnostic accuracy of the CSF biomarkers.
It should be noted that the ratios and other combinations of the AD CSF biomarkers should be used with care. Due to (pre-)analytical issues (35), concentrations differ exceedingly between laboratories. External quality controls and reference material might be able to reduce this variability, which would enable the general use of the same cutoff that was validated in a multicenter setting. At this moment, cutoffs for individual biomarkers as well as ratios and other combinations should be validated in-house before they can be used in clinical practice (36, 37).
In order to further increase diagnostic accuracy, other biomarkers should be included in the biomarker panel in the future. Examples of possible fluid biomarkers for features of Aβ processing in AD are β-site APP cleaving enzyme-1 (BACE1) activity (38–44), soluble amyloid precursor protein (sAPP) α and β (42, 44–51), and Aβ oligomers (52–60). Some fluid biomarkers that are still being investigated seem more specific for non-AD dementias and could also increase diagnostic accuracy when added to the biomarker panel. Examples of possible such non-AD biomarkers are TAR DNA-binding protein 43 (TDP-43) (61–63), TPD-43 phosphorylated at S409 (pTDP-43) (63), and progranulin (64–66) for FTD, α-synuclein (67–71) and neurosin (72) for DLB, metalloproteinases-9 for VaD (73, 74), and total CSF prion protein for CJD (75). For reviews on these biomarkers, see Ref. (76–80). Most of these biomarkers need extensive validation as well as validated ready-to-use analytical methods before they can be used in combination with Aβ1–42, T-tau, and P-tau181P for differential dementia diagnosis in clinical practice.
Another highly promising approach is combining fluid biomarkers and imaging, such as magnetic resonance imaging (MRI) and positron emission tomography (PET) imaging. Several studies have shown that combinations of fluid and imaging biomarkers render higher diagnostic power than these modalities alone (81–85).
In conclusion, this study demonstrates P-tau181P is a fundamental component of the AD biomarker panel and the combined assessment of Aβ1–42, T-tau, and P-tau181P renders, to present date, the highest diagnostic power to discriminate between AD and non-AD dementias. New biomarkers more specifically targeted at non-AD dementia pathology should further increase diagnostic power in the future.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the University of Antwerp Research Fund; the Alzheimer Research Foundation (SAO-FRA); the central Biobank facility of the Institute Born-Bunge/University Antwerp; the Research Foundation Flanders (FWO); the Agency for Innovation by Science and Technology (IWT); the Belgian Science Policy Office Interuniversity Attraction Poles (IAP) program; the Flemish Government initiated Methusalem excellence grant; the University of Antwerp Research Fund, Belgium. This work is part of the BIOMARKAPD project within the EU Joint Programme for Neurodegenerative Disease Research (JPND). The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115372, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fneur.2015.00138
References
1. Mckhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology (1984) 34:939–44. doi: 10.1212/WNL.34.7.939
2. Knopman DS, Dekosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the quality standards subcommittee of the american academy of neurology. Neurology (2001) 56:1143–53. doi:10.1212/WNL.56.9.1143
3. Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol (2010) 6:131–44. doi:10.1038/nrneurol.2010.4
4. Le Bastard N, Martin JJ, Vanmechelen E, Vanderstichele H, De Deyn PP, Engelborghs S. Added diagnostic value of CSF biomarkers in differential dementia diagnosis. Neurobiol Aging (2010) 31:1867–76. doi:10.1016/j.neurobiolaging.2008.10.017
5. Engelborghs S, Le Bastard N. The impact of cerebrospinal fluid biomarkers on the diagnosis of Alzheimer’s disease. Mol Diagn Ther (2012) 16:135–41. doi:10.2165/11634170-000000000-00000
6. Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol (2012) 123:1–11. doi:10.1007/s00401-011-0910-3
7. Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol (2007) 114:5–22. doi:10.1007/s00401-007-0237-2
8. Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol (2010) 119:1–4. doi:10.1007/s00401-009-0612-2
9. Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol (2011) 122:111–3. doi:10.1007/s00401-011-0845-8
11. Niemantsverdriet E, Struyfs H, Duits F, Teunissen C, Engelborghs S. Techniques, contraindications and complications of CSF collection procedures. In: Deisenhammer F, Sellebjerg F, Teunissen CE, Tumani H, editors. Cerebrospinal Fluid in Clinical Neurology. Cham, Switzerland: Springer International Publishing (2015). p. 35–57.
12. Engelborghs S, De Vreese K, Van De Casteele T, Vanderstichele H, Van Everbroeck B, Cras P, et al. Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging (2008) 29:1143–59. doi:10.1016/j.neurobiolaging.2007.02.016
13. Le Bastard N, Aerts L, Sleegers K, Martin JJ, Van Broeckhoven C, De Deyn PP, et al. Longitudinal stability of cerebrospinal fluid biomarker levels: fulfilled requirement for pharmacodynamic markers in Alzheimer’s disease. J Alzheimers Dis (2013) 33:807–22. doi:10.3233/JAD-2012-110029
14. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics (2011) 12:77. doi:10.1186/1471-2105-12-77
15. Maddalena A, Papassotiropoulos A, Muller-Tillmanns B, Jung HH, Hegi T, Nitsch RM, et al. Biochemical diagnosis of Alzheimer disease by measuring the cerebrospinal fluid ratio of phosphorylated tau protein to beta-amyloid peptide42. Arch Neurol (2003) 60:1202–6. doi:10.1001/archneur.60.9.1202
16. Schoonenboom NS, Pijnenburg YA, Mulder C, Rosso SM, Van Elk EJ, Van Kamp GJ, et al. Amyloid beta(1-42) and phosphorylated tau in CSF as markers for early-onset Alzheimer disease. Neurology (2004) 62:1580–4. doi:10.1212/01.WNL.0000123249.58898.E0
17. Blasko I, Lederer W, Oberbauer H, Walch T, Kemmler G, Hinterhuber H, et al. Measurement of thirteen biological markers in CSF of patients with Alzheimer’s disease and other dementias. Dement Geriatr Cogn Disord (2006) 21:9–15. doi:10.1159/000089137
18. De Jong D, Jansen RW, Kremer BP, Verbeek MM. Cerebrospinal fluid amyloid beta42/phosphorylated tau ratio discriminates between Alzheimer’s disease and vascular dementia. J Gerontol A Biol Sci Med Sci (2006) 61:755–8. doi:10.1093/gerona/61.7.755
19. Vanderstichele H, De Vreese K, Blennow K, Andreasen N, Sindic C, Ivanoiu A, et al. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU181P assay for discrimination between Alzheimer’s disease and dementia with Lewy bodies. Clin Chem Lab Med (2006) 44:1472–80. doi:10.1515/CCLM.2006.258
20. Reijn TS, Rikkert MO, Van Geel WJ, De Jong D, Verbeek MM. Diagnostic accuracy of ELISA and xMAP technology for analysis of amyloid beta(42) and tau proteins. Clin Chem (2007) 53:859–65. doi:10.1373/clinchem.2006.081679
21. Welge V, Fiege O, Lewczuk P, Mollenhauer B, Esselmann H, Klafki HW, et al. Combined CSF tau, p-tau181 and amyloid-beta 38/40/42 for diagnosing Alzheimer’s disease. J Neural Transm (2009) 116:203–12. doi:10.1007/s00702-008-0177-6
22. Yakushev I, Bartenstein P, Siessmeier T, Hiemke C, Scheurich A, Lotz J, et al. Cerebrospinal fluid tau protein levels and 18F-fluorodeoxyglucose positron emission tomography in the differential diagnosis of Alzheimer’s disease. Dement Geriatr Cogn Disord (2010) 30:245–53. doi:10.1159/000320206
23. Aerts MB, Esselink RA, Claassen JA, Abdo WF, Bloem BR, Verbeek MM. CSF tau, Abeta42, and MHPG differentiate dementia with Lewy bodies from Alzheimer’s disease. J Alzheimers Dis (2011) 27:377–84. doi:10.3233/JAD-2011-110482
24. De Souza LC, Lamari F, Belliard S, Jardel C, Houillier C, De Paz R, et al. Cerebrospinal fluid biomarkers in the differential diagnosis of Alzheimer’s disease from other cortical dementias. J Neurol Neurosurg Psychiatry (2011) 82:240–6. doi:10.1136/jnnp.2010.207183
25. Gabelle A, Roche S, Geny C, Bennys K, Labauge P, Tholance Y, et al. Decreased sAbetaPPbeta, Abeta38, and Abeta40 cerebrospinal fluid levels in frontotemporal dementia. J Alzheimers Dis (2011) 26:553–63. doi:10.3233/JAD-2011-110515
26. Gabelle A, Dumurgier J, Vercruysse O, Paquet C, Bombois S, Laplanche JL, et al. Impact of the 2008-2012 French Alzheimer Plan on the use of cerebrospinal fluid biomarkers in research memory center: the PLM Study. J Alzheimers Dis (2013) 34:297–305. doi:10.3233/JAD-121549
27. Shea YF, Chu LW, Zhou L, Li WM, Lin OY, Chan MN, et al. Cerebrospinal fluid biomarkers of Alzheimer’s disease in Chinese patients: a pilot study. Am J Alzheimers Dis Other Demen (2013) 28:769–75. doi:10.1177/1533317513504615
28. Duits FH, Teunissen CE, Bouwman FH, Visser PJ, Mattsson N, Zetterberg H, et al. The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement (2014) 10(713–723):e712. doi:10.1016/j.jalz.2013.12.023
29. Ewers M, Mattsson N, Minthon L, Molinuevo JL, Antonell A, Popp J, et al. CSF biomarkers for the differential diagnosis of Alzheimer’s disease. A large-scale international multicenter study. Alzheimers Dement (2015). doi:10.1016/j.jalz.2014.12.006
30. The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group. Consensus report of the working group on: “molecular and biochemical markers of Alzheimer’s disease”. Neurobiol Aging (1998) 19:109–16.
31. Riemenschneider M, Wagenpfeil S, Vanderstichele H, Otto M, Wiltfang J, Kretzschmar H, et al. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol Psychiatry (2003) 8:343–7. doi:10.1038/sj.mp.4001220
32. Satoh K, Shirabe S, Eguchi H, Tsujino A, Eguchi K, Satoh A, et al. 14-3-3 protein, total tau and phosphorylated tau in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease and neurodegenerative disease in Japan. Cell Mol Neurobiol (2006) 26:45–52. doi:10.1007/s10571-006-9370-z
33. Bahl JM, Heegaard NH, Falkenhorst G, Laursen H, Hogenhaven H, Molbak K, et al. The diagnostic efficiency of biomarkers in sporadic Creutzfeldt-Jakob disease compared to Alzheimer’s disease. Neurobiol Aging (2009) 30:1834–41. doi:10.1016/j.neurobiolaging.2008.01.013
34. Skillback T, Rosen C, Asztely F, Mattsson N, Blennow K, Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish mortality registry. JAMA Neurol (2014) 71:476–83. doi:10.1001/jamaneurol.2013.6455
35. Le Bastard N, De Deyn PP, Engelborghs S. Importance and impact of preanalytical variables on Alzheimer disease biomarker concentrations in cerebrospinal fluid. Clin Chem (2015) 61:734–43. doi:10.1373/clinchem.2014.236679
36. Lehmann S, Gabelle A, Paquet C. Can we rely only on ratios of cerebrospinal fluid biomarkers for AD biological diagnosis? Alzheimers Dement (2014). doi:10.1016/j.jalz.2014.09.003
37. Lehmann S, Delaby C, Paquet C, Gabelle A. Analytical challenges related to the use of biomarker ratios for the biological diagnosis of Alzheimer’s disease. Clin Chem Lab Med (2015). doi:10.1515/cclm-2014-1232
38. Holsinger RM, Mclean CA, Collins SJ, Masters CL, Evin G. Increased beta-secretase activity in cerebrospinal fluid of Alzheimer’s disease subjects. Ann Neurol (2004) 55:898–9. doi:10.1002/ana.20144
39. Holsinger RM, Lee JS, Boyd A, Masters CL, Collins SJ. CSF BACE1 activity is increased in CJD and Alzheimer disease versus [corrected] other dementias. Neurology (2006) 67:710–2. doi:10.1212/01.wnl.0000229925.52203.4c
40. Verheijen JH, Huisman LG, Van Lent N, Neumann U, Paganetti P, Hack CE, et al. Detection of a soluble form of BACE-1 in human cerebrospinal fluid by a sensitive activity assay. Clin Chem (2006) 52:1168–74. doi:10.1373/clinchem.2006.066720
41. Zhong Z, Ewers M, Teipel S, Burger K, Wallin A, Blennow K, et al. Levels of beta-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatry (2007) 64:718–26. doi:10.1001/archpsyc.64.6.718
42. Zetterberg H, Andreasson U, Hansson O, Wu G, Sankaranarayanan S, Andersson ME, et al. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol (2008) 65:1102–7. doi:10.1001/archneur.65.8.1102
43. Mulder SD, Van Der Flier WM, Verheijen JH, Mulder C, Scheltens P, Blankenstein MA, et al. BACE1 activity in cerebrospinal fluid and its relation to markers of AD pathology. J Alzheimers Dis (2010) 20:253–60. doi:10.3233/JAD-2010-1367
44. Rosen C, Andreasson U, Mattsson N, Marcusson J, Minthon L, Andreasen N, et al. Cerebrospinal fluid profiles of amyloid beta-related biomarkers in Alzheimer’s disease. Neuromolecular Med (2012) 14:65–73. doi:10.1007/s12017-012-8171-4
45. Olsson A, Hoglund K, Sjogren M, Andreasen N, Minthon L, Lannfelt L, et al. Measurement of alpha- and beta-secretase cleaved amyloid precursor protein in cerebrospinal fluid from Alzheimer patients. Exp Neurol (2003) 183:74–80. doi:10.1016/S0014-4886(03)00027-X
46. Gabelle A, Roche S, Geny C, Bennys K, Labauge P, Tholance Y, et al. Correlations between soluble alpha/beta forms of amyloid precursor protein and Abeta38, 40, and 42 in human cerebrospinal fluid. Brain Res (2010) 1357:175–83. doi:10.1016/j.brainres.2010.08.022
47. Hertze J, Minthon L, Zetterberg H, Vanmechelen E, Blennow K, Hansson O. Evaluation of CSF biomarkers as predictors of Alzheimer’s disease: a clinical follow-up study of 4.7 years. J Alzheimers Dis (2010) 21:1119–28. doi:10.3233/JAD-2010-100207
48. Lewczuk P, Kamrowski-Kruck H, Peters O, Heuser I, Jessen F, Popp J, et al. Soluble amyloid precursor proteins in the cerebrospinal fluid as novel potential biomarkers of Alzheimer’s disease: a multicenter study. Mol Psychiatry (2010) 15:138–45. doi:10.1038/mp.2008.84
49. Johansson P, Mattsson N, Hansson O, Wallin A, Johansson JO, Andreasson U, et al. Cerebrospinal fluid biomarkers for Alzheimer’s disease: diagnostic performance in a homogeneous mono-center population. J Alzheimers Dis (2011) 24:537–46. doi:10.3233/JAD-2011-101878
50. Perneczky R, Tsolakidou A, Arnold A, Diehl-Schmid J, Grimmer T, Forstl H, et al. CSF soluble amyloid precursor proteins in the diagnosis of incipient Alzheimer disease. Neurology (2011) 77:35–8. doi:10.1212/WNL.0b013e318221ad47
51. Lewczuk P, Popp J, Lelental N, Kolsch H, Maier W, Kornhuber J, et al. Cerebrospinal fluid soluble amyloid-beta protein precursor as a potential novel biomarkers of Alzheimer’s disease. J Alzheimers Dis (2012) 28:119–25. doi:10.3233/JAD-2011-110857
52. Pitschke M, Prior R, Haupt M, Riesner D. Detection of single amyloid beta-protein aggregates in the cerebrospinal fluid of Alzheimer’s patients by fluorescence correlation spectroscopy. Nat Med (1998) 4:832–4. doi:10.1038/nm0798-832
53. Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL, et al. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc Natl Acad Sci U S A (2005) 102:2273–6. doi:10.1073/pnas.0409336102
54. Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med (2008) 14:837–42. doi:10.1038/nm1782
55. Fukumoto H, Tokuda T, Kasai T, Ishigami N, Hidaka H, Kondo M, et al. High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J (2010) 24:2716–26. doi:10.1096/fj.09-150359
56. Gao CM, Yam AY, Wang X, Magdangal E, Salisbury C, Peretz D, et al. Abeta40 oligomers identified as a potential biomarker for the diagnosis of Alzheimer’s disease. PLoS One. (2010) 5:e15725. doi:10.1371/journal.pone.0015725
57. Santos AN, Ewers M, Minthon L, Simm A, Silber RE, Blennow K, et al. Amyloid-beta oligomers in cerebrospinal fluid are associated with cognitive decline in patients with Alzheimer’s disease. J Alzheimers Dis (2012) 29:171–6. doi:10.3233/JAD-2012-111361
58. Bruggink KA, Jongbloed W, Biemans EA, Veerhuis R, Claassen JA, Kuiperij HB, et al. Amyloid-beta oligomer detection by ELISA in cerebrospinal fluid and brain tissue. Anal Biochem (2013) 433:112–20. doi:10.1016/j.ab.2012.09.014
59. Handoko M, Grant M, Kuskowski M, Zahs KR, Wallin A, Blennow K, et al. Correlation of specific amyloid-beta oligomers with tau in cerebrospinal fluid from cognitively normal older adults. JAMA Neurol (2013) 70:594–9. doi:10.1001/jamaneurol.2013.48
60. Yang T, Hong S, O’malley T, Sperling RA, Walsh DM, Selkoe DJ. New ELISAs with high specificity for soluble oligomers of amyloid beta-protein detect natural Abeta oligomers in human brain but not CSF. Alzheimers Dement (2013) 9:99–112. doi:10.1016/j.jalz.2012.11.005
61. Steinacker P, Hendrich C, Sperfeld AD, Jesse S, Von Arnim CA, Lehnert S, et al. TDP-43 in cerebrospinal fluid of patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Arch Neurol (2008) 65:1481–7. doi:10.1001/archneur.65.11.1481
62. Feneberg E, Steinacker P, Lehnert S, Schneider A, Walther P, Thal DR, et al. Limited role of free TDP-43 as a diagnostic tool in neurodegenerative diseases. Amyotroph Lateral Scler Frontotemporal Degener (2014) 15:351–6. doi:10.3109/21678421.2014.905606
63. Suarez-Calvet M, Dols-Icardo O, Llado A, Sanchez-Valle R, Hernandez I, Amer G, et al. Plasma phosphorylated TDP-43 levels are elevated in patients with frontotemporal dementia carrying a C9orf72 repeat expansion or a GRN mutation. J Neurol Neurosurg Psychiatry (2014) 85:684–91. doi:10.1136/jnnp-2013-305972
64. Ghidoni R, Benussi L, Glionna M, Franzoni M, Binetti G. Low plasma progranulin levels predict progranulin mutations in frontotemporal lobar degeneration. Neurology (2008) 71:1235–9. doi:10.1212/01.wnl.0000325058.10218.fc
65. Van Damme P, Van Hoecke A, Lambrechts D, Vanacker P, Bogaert E, Van Swieten J, et al. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol (2008) 181:37–41. doi:10.1083/jcb.200712039
66. Philips T, De Muynck L, Thu HN, Weynants B, Vanacker P, Dhondt J, et al. Microglial upregulation of progranulin as a marker of motor neuron degeneration. J Neuropathol Exp Neurol (2010) 69:1191–200. doi:10.1097/NEN.0b013e3181fc9aea
67. Mollenhauer B, El-Agnaf OM, Marcus K, Trenkwalder C, Schlossmacher MG. Quantification of alpha-synuclein in cerebrospinal fluid as a biomarker candidate: review of the literature and considerations for future studies. Biomark Med (2010) 4:683–99. doi:10.2217/bmm.10.90
68. Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol (2011) 69:570–80. doi:10.1002/ana.22311
69. Aerts MB, Esselink RA, Abdo WF, Bloem BR, Verbeek MM. CSF alpha-synuclein does not differentiate between parkinsonian disorders. Neurobiol Aging (2012) 33(430):e431–3. doi:10.1016/j.neurobiolaging.2010.12.001
70. Kapaki E, Paraskevas GP, Emmanouilidou E, Vekrellis K. The diagnostic value of CSF alpha-synuclein in the differential diagnosis of dementia with Lewy bodies vs. normal subjects and patients with Alzheimer’s disease. PLoS One (2013) 8:e81654. doi:10.1371/journal.pone.0081654
71. Slaets S, Vanmechelen E, Le Bastard N, Decraemer H, Vandijck M, Martin JJ, et al. Increased CSF alpha-synuclein levels in Alzheimer’s disease: correlation with tau levels. Alzheimers Dement (2014) 10(5 Suppl):S290–8. doi:10.1016/j.jalz.2013.10.004
72. Wennstrom M, Surova Y, Hall S, Nilsson C, Minthon L, Bostrom F, et al. Low CSF levels of both alpha-synuclein and the alpha-synuclein cleaving enzyme neurosin in patients with synucleinopathy. PLoS One (2013) 8:e53250. doi:10.1371/journal.pone.0053250
73. Adair JC, Charlie J, Dencoff JE, Kaye JA, Quinn JF, Camicioli RM, et al. Measurement of gelatinase B (MMP-9) in the cerebrospinal fluid of patients with vascular dementia and Alzheimer disease. Stroke (2004) 35:e159–62. doi:10.1161/01.STR.0000127420.10990.76
74. Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol (2009) 8:205–16. doi:10.1016/S1474-4422(09)70016-X
75. Dorey A, Tholance Y, Vighetto A, Perret-Liaudet A, Lachman I, Krolak-Salmon P, et al. Association of cerebrospinal fluid prion protein levels and the distinction between Alzheimer disease and Creutzfeldt-Jakob disease. JAMA Neurol (2015) 72:267–75. doi:10.1001/jamaneurol.2014.4068
76. Fagan AM, Perrin RJ. Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomark Med (2012) 6:455–76. doi:10.2217/bmm.12.42
77. Rosen C, Hansson O, Blennow K, Zetterberg H. Fluid biomarkers in Alzheimer’s disease – current concepts. Mol Neurodegener (2013) 8:20. doi:10.1186/1750-1326-8-20
78. Roh JH, Lee JH. Recent updates on subcortical ischemic vascular dementia. J Stroke (2014) 16:18–26. doi:10.5853/jos.2014.16.1.18
79. Schade S, Mollenhauer B. Biomarkers in biological fluids for dementia with Lewy bodies. Alzheimers Res Ther (2014) 6:72. doi:10.1186/s13195-014-0072-3
80. Oeckl P, Steinacker P, Feneberg E, Otto M. Cerebrospinal fluid proteomics and protein biomarkers in frontotemporal lobar degeneration: current status and future perspectives. Biochim Biophys Acta (2015) 1854:757–68. doi:10.1016/j.bbapap.2014.12.010
81. Schoonenboom NS, Van Der Flier WM, Blankenstein MA, Bouwman FH, Van Kamp GJ, Barkhof F, et al. CSF and MRI markers independently contribute to the diagnosis of Alzheimer’s disease. Neurobiol Aging (2008) 29:669–75. doi:10.1016/j.neurobiolaging.2006.11.018
82. Brys M, Glodzik L, Mosconi L, Switalski R, De Santi S, Pirraglia E, et al. Magnetic resonance imaging improves cerebrospinal fluid biomarkers in the early detection of Alzheimer’s disease. J Alzheimers Dis (2009) 16:351–62. doi:10.3233/JAD-2009-0968
83. Vos S, Van R I, Burns L, Knol D, Scheltens P, Soininen H, et al. Test sequence of CSF and MRI biomarkers for prediction of AD in subjects with MCI. Neurobiol Aging (2012) 33:2272–81. doi:10.1016/j.neurobiolaging.2011.12.017
84. Westman E, Muehlboeck JS, Simmons A. Combining MRI and CSF measures for classification of Alzheimer’s disease and prediction of mild cognitive impairment conversion. Neuroimage (2012) 62:229–38. doi:10.1016/j.neuroimage.2012.04.056
Keywords: Alzheimer’s disease, dementia, differential diagnosis, biomarkers, cerebrospinal fluid, neuropathology, tau
Citation: Struyfs H, Niemantsverdriet E, Goossens J, Fransen E, Martin J-J, De Deyn PP and Engelborghs S (2015) Cerebrospinal fluid P-tau181P: biomarker for improved differential dementia diagnosis. Front. Neurol. 6:138. doi: 10.3389/fneur.2015.00138
Received: 26 March 2015; Accepted: 01 June 2015;
Published: 17 June 2015
Edited by:
Sylvain Lehmann, Montpellier University Hospital, FranceReviewed by:
Zhihui Yang, University of Florida, USADavide Chiasserini, University of Perugia, Italy
Copyright: © 2015 Struyfs, Niemantsverdriet, Goossens, Fransen, Martin, De Deyn and Engelborghs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastiaan Engelborghs, Reference Center for Biological Markers of Dementia (BIODEM), University of Antwerp, Universiteitsplein 1, Antwerp 2610, Belgium, sebastiaan.engelborghs@uantwerpen.be
 Hanne Struyfs
Hanne Struyfs Ellis Niemantsverdriet
Ellis Niemantsverdriet Joery Goossens1
Joery Goossens1 Erik Fransen
Erik Fransen Jean-Jacques Martin
Jean-Jacques Martin Sebastiaan Engelborghs
Sebastiaan Engelborghs