- 1Department of Psychology, University of Graz, Graz, Austria
- 2BioTechMed Graz, Graz, Austria
- 3Laboratory of Brain-Computer Interfaces, Institute for Knowledge Discovery, Graz University of Technology, Graz, Austria
- 4Klinik Judendorf-Straßengel, Gratwein-Straßengel, Austria
In the present multiple case study, we examined hemodynamic changes in the brain in response to motor execution (ME) and motor imagery (MI) of swallowing in dysphagia patients compared to healthy matched controls using near-infrared spectroscopy (NIRS). Two stroke patients with cerebral lesions in the right hemisphere, two stroke patients with lesions in the brainstem, and two neurologically healthy control subjects actively swallowed saliva (ME) and mentally imagined to swallow saliva (MI) in a randomized order while changes in concentration of oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb) were assessed. In line with recent findings in healthy young adults, MI and ME of swallowing led to the strongest NIRS signal change in the inferior frontal gyrus in stroke patients as well as in healthy elderly. We found differences in the topographical distribution and time course of the hemodynamic response in dependence on lesion location. Dysphagia patients with lesions in the brainstem showed bilateral hemodynamic signal changes in the inferior frontal gyrus during active swallowing comparable to healthy controls. In contrast, dysphagia patients with cerebral lesions in the right hemisphere showed more unilateral activation patterns during swallowing. Furthermore, patients with cerebral lesions showed a prolonged time course of the hemodynamic response during MI and ME of swallowing compared to healthy controls and patients with brainstem lesions. Brain activation patterns associated with ME and MI of swallowing were largely comparable, especially for changes in deoxy-Hb. Hence, the present results provide new evidence regarding timing and topographical distribution of the hemodynamic response during ME and MI of swallowing in dysphagia patients and may have practical impact on future dysphagia treatment.
Introduction
Dysphagia is a difficulty in swallowing that often occurs in neurological patients (1). Especially, dysphagia is a very common consequence of stroke, estimated to occur in up to 76% of patients with acute stroke (2). Dysphagia can have tremendous consequences, such as chest infection, malnutrition, prolonged hospital stay, slower rate of recovery, poorer rehabilitation potential, mortality, and reduced quality of life (3, 4). In the present study, we aimed at identifying brain correlates of active swallowing and mental imagination of swallowing in single stroke patients with dysphagia compared to healthy matched controls, which might be the basis for upcoming dysphagia rehabilitation (5).
Brain activation patterns associated with mental imagination of swallowing have been scarcely investigated. Based on numerous studies investigating brain correlates of motor imagery (MI) and motor execution (ME) of limb movements, it is well known that both tasks lead to similar brain activation patterns. Moreover, mental practice with MI can improve motor functions. Hence, MI strategies can be potentially used for motor rehabilitation (5–16). In a recent study performed in our lab, we investigated brain activation patterns associated with ME and mental imagery of swallowing in healthy young adults using near-infrared spectroscopy (NIRS). We found the strongest activation during both tasks in the inferior frontal gyrus bilaterally. Hence, this prior NIRS study provided first evidence that the hemodynamic response during ME and MI of swallowing is largely comparable in healthy people (5).
Brain activation patterns associated with active swallowing have been thoroughly investigated (17–37). Generally, swallowing is a complex and fundamental neuromuscular activity consisting of voluntary (oral phase) and involuntary (pharyngeal and esophageal phase) phases (38). Reflexive components of the swallowing process are controlled by swallowing centers in the brainstem (20). Acute focal brainstem infarct may produce dysphagia symptoms, especially affecting reflexive phases of the swallowing process, with little or no further neurocognitive deficit. About 62.5% of stroke patients with lesions in the brainstem, i.e., medulla oblongata or pons, aspirate (2). In contrast, volitional swallowing is represented in multiple regions of the cerebral cortex, such as the sensorimotor cortex, the lateral motor cortex, insula, the cerebellum, the superior temporal gyrus, middle and inferior frontal gyri, anterior cingulate cortex, and frontal operculum including the Broca’s area (21, 25, 38–40). Lesions in these cerebral regions impair voluntary swallowing movements, which may lead to aspiration, too (2, 41). On the basis of clinical and neuroimaging studies, especially unilateral hemispheric stroke can result in dysphagia (2, 42). A number of studies confirmed that about 40% of patients with unilateral hemispheric stroke may have swallowing difficulties (20).
These prior neuroimaging studies that investigated brain activation patterns underlying active swallowing were mainly based on functional magnetic resonance imaging (fMRI). In the present study, we used NIRS, which is a relatively new, non-invasive optical neuroimaging technique. NIRS measures concentration changes of oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb) in the cerebral vessels based on their different absorption spectra for light in the near-infrared range (43). It is based on the same physiological signal as fMRI, since it measures the blood oxygenation level dependent (BOLD) effect in cortical areas (44). There is evidence that the oxy-Hb signal is the most sensitive indicator of changes in cerebral blood flow (CBF). The CBF is an indicator of cortical activation. In contrast, the direction of changes in deoxy-Hb is determined by the degree of changes in venous blood oxygenation and volume (45). Compared to fMRI, NIRS has low costs, is more flexible, and portable. Furthermore, NIRS has a higher temporal resolution than most commonly used fMRI scanners (46, 47) and also wireless, and portable instruments are available (47, 48). A further big advantage of NIRS over fMRI is that NIRS has a lower sensitivity to motion-artifacts (49, 50), allowing measurements of brain activation also in natural and realistic environments. However, with fMRI, the whole brain including sub-cortical areas can be recorded with a high spatial resolution (44). When using NIRS, only changes in hemodynamic responses a few centimeters (0.5–2 cm) below the surface of the head can be assessed (46). Summing up, the main advantages of NIRS are that it is not locally bounded to the installation site and can accommodate a higher degree of movement. Therefore, NIRS turned out to be an adequate neurophysiological method enabling the assessment of brain activation patterns of neurological patients in clinics or at the patients’ home (51).
The aim of the present multiple single-case study was to use NIRS to examine the cortical correlates of swallowing in patients with dysphagia. We focused on the comparability of brain activation patterns associated with saliva swallowing between dysphagia patients and healthy-matched controls. More precisely, we were interested in the time course and the topographical distribution of the hemodynamic signal change (oxy-Hb and deoxy-Hb) during swallowing in dysphagia patients compared to healthy controls. Special emphasis lay on the question whether there are differences in brain activation patterns during swallowing movements between dysphagia patients with lesions in the cerebral cortex and dysphagia patients with lesions in the brainstem. Since there is evidence that lesions in cerebral regions impair voluntary swallowing movements whereas lesions in the brainstem impair more involuntary phases of the swallowing process, we expected differences in cerebral activation patterns during swallowing in dependence on lesion location (2, 41). Furthermore, we wanted to determine the extent to which MI and ME of swallowing lead to comparable brain activation patterns in stroke patients, such as we have already observed in healthy young adults (5).
Materials and Methods
Participants
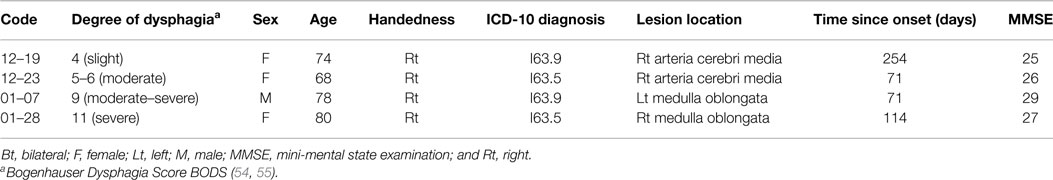
This study used a multiple case design (52, 53). ME and MI of swallowing were explored in four stroke patients with dysphagia matched for gender and age to two healthy control subjects. Patient details are summarized in Table 1. Recruitment of stroke patients and NIRS measurements were performed at the rehabilitation clinic Judendorf-Strassengel, Austria. Patients 12–19 and 12–23 had cerebral lesions in the right hemisphere, patients 01–07 and 01–28 had lesions in the brainstem. Assessment of swallowing abilities and determination of the degree of dysphagia were performed by speech and language therapists of the rehabilitation clinic Judendorf-Strassengel. Dysphagia was scored using the Bogenhauser Dysphagia Score BODS (54, 55). The BODS is a psychometric assessment of clinical dysphagia implying swallowing saliva as well as oral intake of food and liquids. It is a systematic rating of the functional severity of dysphagia. Content validity and inter-rater reliability were investigated. Psychometric data indicate that the BODS is an appropriate and reliable tool for the clinical evaluation of swallowing abilities of patients with neurogenic dysphagia (56). This study was approved by the local ethics committee of the University of Graz and conforms to the Declaration of Helsinki.
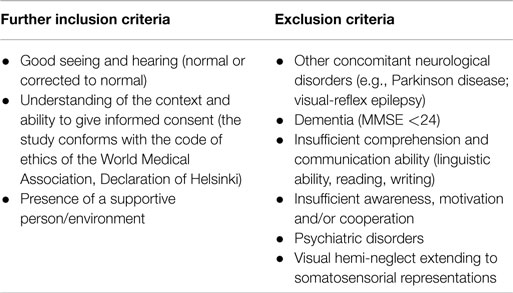
Selection of patients was based on the following criteria: in the first step and with the prior consent of the attending medical doctors or therapists, neurological patients who showed severe impairments in swallowing, with any site of brain lesion and motor deficit and with a time laps from the event of at least 4 weeks, were invited to participate in this investigation. Further inclusion and exclusion criteria are listed in Table 2.
Two neurologically healthy control subjects with no swallowing problems performed the swallowing task as well, one 67 years old man, and one 64 years old woman. The healthy control subjects were largely comparable to the stroke patients regarding age and gender.
MI/ME Task Swallowing
The MI/ME task was performed in the rehabilitation clinic Judendorf-Strassengel. During the task, participants were sitting in front of a computer screen. The portable NIRS equipment was placed behind the participants. NIRS montage took about 5 min. In sum, all participants performed 10 trials of MI of swallowing and 10 trials of ME of swallowing in a random order. For the ME task, participants were instructed to successively swallow their own saliva two to three times, indicated by a sign on the computer screen. We did not use a water swallowing task in this study to prevent aspiration in dysphagia patients. For the MI task, participants were instructed to imagine to swallow saliva but not to execute any swallowing movement. Each trial lasted 15 s. Between task trials, a fixation cross appeared at the screen with a variable duration from 28 to 32 s. During these resting trials, participants were instructed to relax and to avoid swallowing as much as they could. Participants trained both tasks at the beginning of the experimental session to accustom oneself to the timing of the trials before starting the MI/ME tasks. The whole procedure including the NIRS montage took approximately 30 min.
NIRS Recordings and Analysis
To assess the relative concentration changes of oxy-Hb and deoxy-Hb during MI and ME of swallowing, measurements were performed on a continuous wave system, the NIRSport 88 system from NIRx Medical Technologies (Glen Head, NY, USA), consisting of 8 photo-detectors and 8 light emitters resulting in a total of 20 channels (see Figure 1). The sampling rate of the NIRS system was set to 10 Hz. The channel configuration of the NIRS probe set is illustrated in Figure 1. Based on the results of a recent study (5), channels were positioned over the inferior frontal gyrus bilaterally.
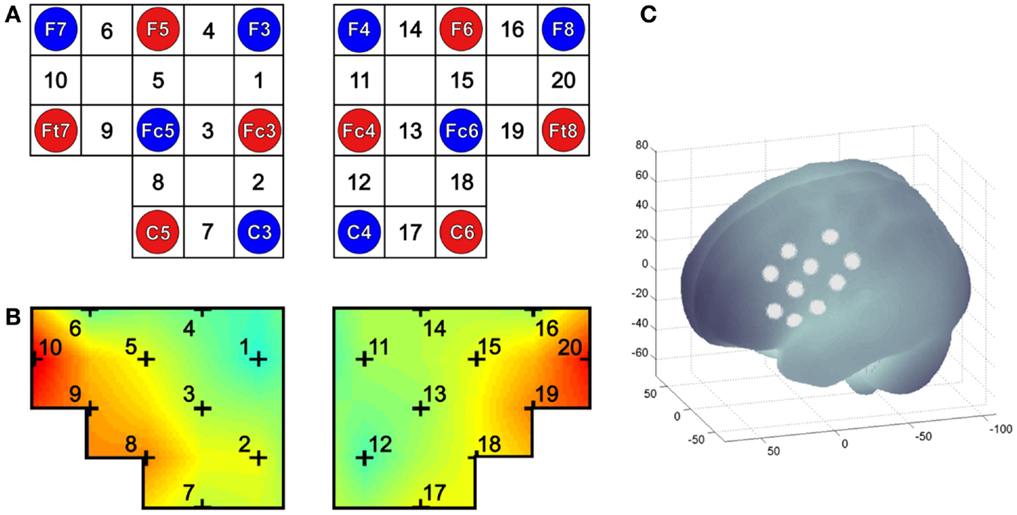
Figure 1. (A) Channel configuration of the optode probe set. The NIRS probe set of 20 channels was positioned over the right and left inferior frontal gyrus. Red and blue circles illustrate positions of NIRS sensors and detectors, respectively, and the white rectangles mark the measured 20 channels. (B) Example of a topographical map, in which the 20 NIRS channel locations are additionally marked. (C) Projections of 10 NIRS channel positions (white points) on the cortical surface over the left hemisphere. NIRS positions are overlaid on an MNI-152 compatible canonical brain that is optimized for NIRS analysis according to a procedure by Singh et al. (57).
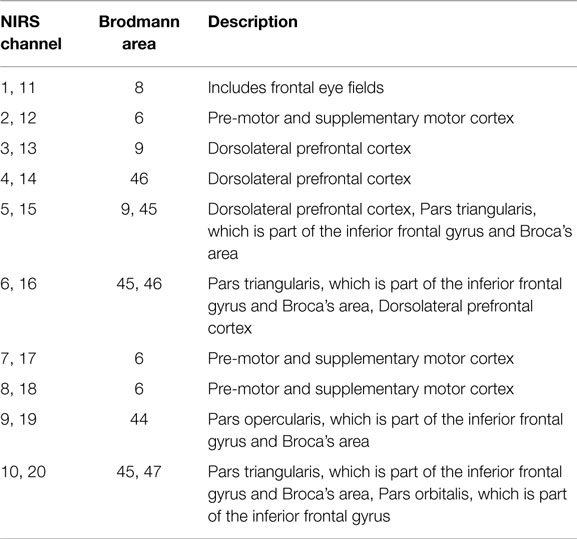
The MNI coordinates of the NIRS channels were assessed using ELPOS (zebris Medical GmbH), a system to determine 3D coordinates of EEG/NIRS electrode/optode positions with high accuracy based on the run time measurement of ultrasonic pulses. Afterwards, a coordinate-based system was used to retrieve brain labels from the 1988 Talairach Atlas, called the Talairach Daemon (58, 59). In Table 3, the anatomic labeling (Brodmann areas, Talairach Daemon) are listed for each NIRS channel.
For offline analyses of the NIRS signal, we investigated changes in oxy-Hb and deoxy-Hb separately. Before statistical analyses, the NIRS raw signal was preprocessed. Specifically, the raw data were corrected for artifacts (criterion for rejection: amplitude of Hb signal >±3 SD; visual inspection) and filtered with a 0.01 Hz high-pass filter to remove baseline drifts and a 0.90 Hz low-pass filter. The time courses of oxy-Hb and deoxy-Hb were averaged task-related separately for the MI and execution condition. Task-related concentration changes of oxy-Hb and deoxy-Hb were referred to a 5 s baseline interval prior to the MI/ME task (−5 to 0 s). Furthermore, oxy-Hb and deoxy-Hb were averaged for the time period around the peak activation during the MI/ME task (10–20 s after task onset).
Results
Topographical Distribution
First, we were interested in the topographical distribution of changes in oxy- and deoxy-Hb during ME and MI of swallowing in each single participant (Figures 2 and 3). To identify the NIRS channels that showed the strongest NIRS signal change (oxy- and deoxy-Hb) during ME/MI of swallowing compared to the baseline interval in each individual participant, we used the false discovery rate (FDR) method to control the proportion of false positives among the channels that are detected as significant (60).
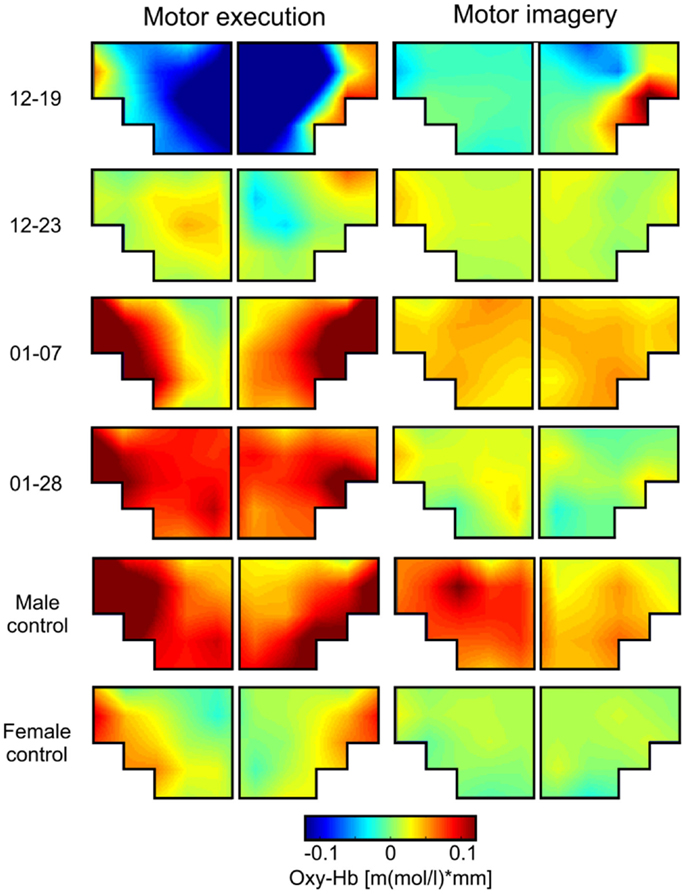
Figure 2. Grand average topographical maps of oxy-Hb during motor execution (ME) and motor imagery (MI) of swallowing, presented separately for each participant [averaged time interval (second) 10–20 after task onset]. The upper two panels show topographical maps of dysphagia patients with cerebral lesions (12–19 and 12–23), the middle two panels show topographical maps of dysphagia patients with lesions in the brainstem (01–07 and 01–28), and the lower two panels show maps of healthy controls.
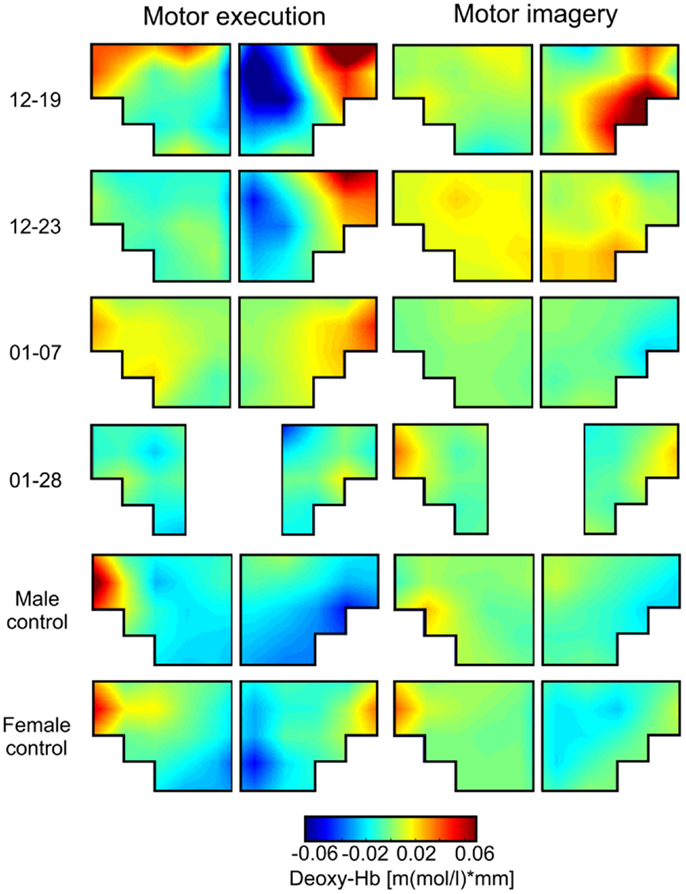
Figure 3. Grand average topographical maps of deoxy-Hb during motor execution (ME) and motor imagery (MI) of swallowing, presented separately for each participant [averaged time interval (second) 10–20 after task onset]. The upper two panels show topographical maps of dysphagia patients with cerebral lesions (12–19 and 12–23), the middle two panels show topographical maps of dysphagia patients with lesions in the brainstem (01–07 and 01–28), and the lower two panels show maps of healthy controls. Note that for patient 01–28 channel 1, 2, 11, and 12 had to be excluded because of bad signal quality.
This analysis revealed that during ME, relative concentration changes in oxy- (Figure 2, left panel) and deoxy-Hb (Figure 3, left panel) were strongest bilaterally over channels 9, 10, 19, and 20 in healthy controls as well as in patients 01–07 and 01–28 with lesions in the brainstem (p < FDR 0.10), except for the healthy male control subject, who showed the strongest deoxy-Hb increase only over left channels (9, 10) during ME. In contrast, patients 12–19 and 12–23 with cerebral lesions showed more unilateral activation patterns during active swallowing. In these two patients, relative concentration changes in oxy- and deoxy-Hb during ME of swallowing were strongest over the right hemisphere including channels 16, 19, and 20 (p < FDR 0.10).
During the MI task (Figures 2 and 3, right panel), patients 12–19 and 12–23 showed more unilateral activation patterns, too. Comparable to the ME task, changes in deoxy-Hb were strongest over the right hemisphere including channels 16, 18, 19 (p < FDR 0.10). For patients 12–19, changes in oxy-Hb during MI of swallowing were strongest over the right hemisphere including channel 18, 19, 20 (p < FDR 0.10), whereas for patients 12–23 changes in oxy-Hb during MI were strongest over the left hemisphere including channels 9 and 10 (p < FDR 0.10). For patients 01–07, no significant differences in oxy-/deoxy-Hb between NIRS channels could be observed during MI of swallowing. Patients 01–28 showed the strongest increase in oxy-Hb during MI over left hemispheric channels (channel 10), whereas changes in deoxy-Hb were more bilaterally distributed (channels 10, 20) (p < FDR 0.10). For the two healthy control subjects, the strongest activation changes during MI of swallowing were found over the left hemisphere including channel 5, 9, and 10 (p < FDR 0.10). As already observed for the ME task, topographical distribution patterns during MI of swallowing were largely comparable between healthy controls and patients with lesions in the brainstem. These results are summarized in Table 4.
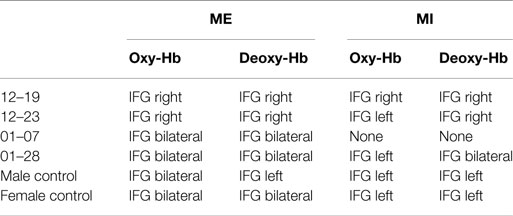
Table 4. Summary of the strongest relative concentration changes in oxy- and deoxy-Hb during ME and MI of swallowing over the inferior frontal gyrus (IFG).
Brain activation patterns during MI and ME of swallowing were largely comparable, especially for changes in deoxy-Hb (see Figure 3). Both tasks led to the strongest NIRS signal change over the inferior frontal gyrus. Generally, the absolute amplitude of the NIRS signal was larger for active swallowing than for imagery of swallowing. Nevertheless, the topographical distribution of the strongest relative concentration change of oxy- and deoxy-Hb was comparable between both tasks.
Time Course of Oxy- and Deoxy-Hb during Motor Execution and Imagery of Swallowing
The topographical analysis revealed that changes in oxy- and deoxy-Hb during both tasks (MI and ME) were most pronounced in the inferior frontal gyrus including the Broca’s area (channels 6, 9, 10, 16, 19, 20). Based on the results of the FDR analysis, these channels were thus averaged to analyze differences in the time course of the NIRS signal during MI and ME of swallowing. Preliminary results revealed no hemispherical differences in the NIRS time course, therefore, channels of the right and left hemisphere were averaged for further analysis.
In Figure 4, the time course of oxy- and deoxy-Hb during MI and ME of swallowing is presented separately for each participant. The time courses of the NIRS signal of patients 01–07 and 01–28 with brainstem lesions were comparable to the time courses of the NIRS signal of the two healthy controls. The healthy controls and patients with brainstem lesions showed an earlier peak activation level in oxy- and deoxy-Hb during ME compared to patients 12–19 and 12–23. Hence, compared to the baseline interval, the NIRS signal increased faster in healthy controls and patients with brainstem lesions than in patients with cerebral lesions during ME of swallowing. The amplitudes of the NIRS signal of patients 01–07 and 01–28 were comparable to the absolute amplitude of the NIRS signal of the male control subject. The absolute amplitude level of oxy-Hb was generally lower during MI of swallowing than during ME in all participants. However, the time course of oxy-Hb during MI was comparable to the time course during ME. For the MI task, the peak of oxy-Hb seems to appear earlier in healthy controls and patients 01–07 and 01–28 compared to patients 12–19 and 12–23, too. No clear peak was detectable in deoxy-Hb during MI. In Table 5, the means and SE of the peak latency values for patients with cerebral lesions, patients with brainstem lesions, and healthy controls are presented separately for oxy- and deoxy-Hb and the ME and MI tasks.
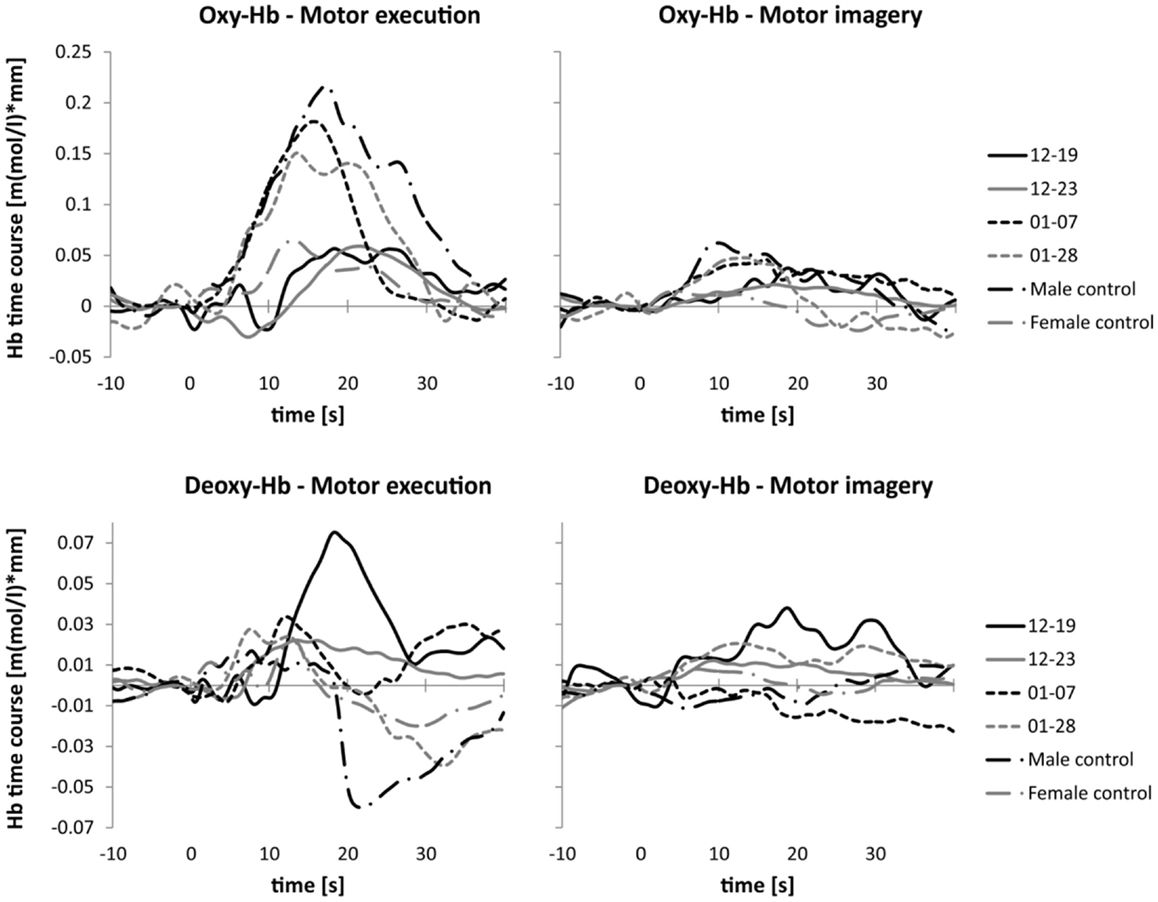
Figure 4. Time course of the NIRS signal. Mean activation changes in oxy- (upper panel) and deoxy-Hb (lower panel) in response to motor execution (left panel) and motor imagery (right panel), presented separately for each participant.
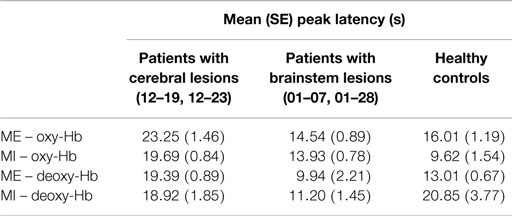
Table 5. Means and SE of peak latency values for patients with cerebral lesions, patients with brainstem lesions, and healthy controls, presented separately for oxy- and deoxy-Hb and the ME and MI tasks.
Discussion
In the present study, we investigated hemodynamic signal changes accompanying ME and imagery of swallowing in stroke patients with dysphagia when compared to healthy controls using NIRS. We found differences in the topographical distribution and the time course of the hemodynamic response depending on lesion location. Dysphagia patients with lesions in the brainstem showed bilateral hemodynamic signal changes in the inferior frontal gyrus during active swallowing comparable to healthy controls. In contrast, dysphagia patients with cerebral lesions in the right hemisphere showed more unilateral activation patterns during swallowing. Furthermore, patients with cerebral lesions showed a prolonged time course of the hemodynamic response during MI and ME of swallowing compared to healthy controls and stroke patients with brainstem lesions. Brain activation patterns associated with ME and MI of swallowing were largely comparable, especially for changes in deoxy-Hb. In the following paragraphs, these results are discussed in more detail.
Topographical Distribution
In line with previous studies that investigated cortical correlates of swallowing (5, 18, 19, 21, 25, 61), we found an increased activation during swallowing in the inferior frontal gyrus including the Broca’s area. Generally, Broca’s area is considered to subserve motor speech production. Sensation of the mouth and pharynx was localized to BA 44, too (62), which indicates that the Broca’s area is also involved in the control of non-speech orofacial sensorimotor behaviors (21, 40). Furthermore, the increased activation around the Broca’s area during swallowing might be explained by the activation in deeper brain structures. For instance, the insula (i.e., BA 13) lies proximal to BA 44/45. Therefore, activation in BA 44 and 45 during the swallowing task might be caused by an increased activation in the insula. Various studies consistently report an involvement of the insula in the swallowing process (18, 19, 21, 40). Anatomical evidence shows that the insula receives afferent inputs from various brain regions involved in the swallowing process [e.g., BA 6, 1, 2, 3, 24, etc., for review, see Ref. (63)]. Furthermore, the inner side of BA 45 and the insular cortex are assumed to represent a primary gustatory cortex (21, 25, 40, 64–67). Damage to the insula and inner side of the operculum (BA 44, 45) often leads to dysphagia in humans underlining the importance of these brain regions for the swallowing process (2, 26, 68). However, activation levels of deeper brain structures, such as the insular cortex (BA 13), cannot be assessed by using NIRS, since NIRS measures change in hemodynamic responses only a few centimeters (0.5–2 cm) from the surface of the head (46).
Regarding lateralization effects during swallowing, we found a more bilateral activation in healthy controls and patients with lesions in the brainstem. Prior studies report on heterogeneous results. Some studies found that cortical activation patterns during swallowing were mainly bilateral (5, 18), whereas other studies found strong degrees of interhemispheric asymmetry (19, 40). Lateralization may also change with age. As people age, the cortical hemispheric control of swallowing seems to start becoming more bilateral (69). Participants of the present study were over 60 years old. Probably, the advanced age of the present cases might be a reason for the more bilateral activation observed during swallowing.
In contrast, stroke patients with lesions in the right ACM showed a more unilateral activation during swallowing. They showed a stronger increase in oxy- and deoxy-Hb during ME of swallowing in the right hemisphere, which was the affected hemisphere. The healthy left hemisphere was less active during swallowing. Generally, natural recovery of swallowing after stroke is associated with increased cerebral activation in the unaffected hemisphere (40). For instance, using transcranial magnetic stimulation (TCMS), Hamdy et al. (17) investigated functional changes in swallowing-related brain areas in stroke patients with lesions in the right hemisphere showing dysphagia symptoms 3 months after stroke. Comparable to the present findings in stroke patients with lesions in the right hemisphere, they found that dysphagia was associated with a smaller cortical activation in the intact hemisphere. The cortical activation in the unaffected hemisphere increased in size with recovery of swallowing (17). Furthermore, there are areas of hypometabolism at sites not directly affected by the stroke. This reduction in metabolism in unaffected areas may explain the representation and subsequent recovery as the metabolic state returns to baseline (2). Khedr et al. (70) confirmed that both severity of stroke and neuroplasticity of the unaffected hemisphere have implications for the development of dysphagia (70). In summary, natural recovery of dysphagia after a brain lesion, e.g., caused by a stroke, seems to be due to an improvement in the functional control of swallowing in the unaffected hemisphere. Hence, swallowing might recover by improving the output from the healthy side of the brain (2, 71, 72). Our findings are in line with these previous studies, since we found a lower cortical activation level during swallowing in the healthy hemisphere compared to the affected hemisphere in patients 12–19 and 12–23.
Time Course of Oxy-Hb during Motor Execution and Imagery of Swallowing
In line with previous NIRS studies examining healthy young participants, the mean peak latency of oxy-Hb during ME of swallowing was around 15 s after task onset in healthy elderly controls as well as in patients 01–07 and 01–28, who had lesions in the brainstem (5, 61). A decrease in deoxy-Hb after ME around 10–15 s, which can be seen in the healthy controls and in patients with brainstem lesions, is also comparable to the deoxy-Hb time course of healthy young participants (5). Furthermore, the time course of the NIRS signal during active swallowing is comparable to the time course of the BOLD signal found in prior fMRI studies investigating neuronal correlates of swallowing (18, 21).
During MI of swallowing, oxy- and deoxy-Hb increased in the inferior frontal gyrus in healthy controls as well as in patients with lesions in the brainstem. An increase in deoxy-Hb during MI of swallowing was also found in healthy young adults (5). However, in healthy elderly participants, we found an increase in oxy-Hb during MI, whereas in a previous study on younger participants, oxy-Hb decreased during MI of swallowing (5). A decrease in oxy-Hb during MI of swallowing compared to a rest period was explained by possible motor inhibition mechanisms (5, 73). Generally, MI contains on the one hand ME programs for muscle contractions, but, on the other hand, these programs might be blocked at some level of the motor system by inhibitory mechanisms, which might in turn lead to a decreased activation level in swallowing-related brain areas (7, 74). In the present pilot study, we did not have the opportunity to assess the activity of muscles involved in swallowing, e.g., suprahyoid muscles, by using, for instance, the electromyogram EMG (75). Possible muscle activity during MI of swallowing was controlled by visual inspection. However, we cannot exclude that participants produced small movements during MI of swallowing, which could not be detected by eye. An incomplete inhibition of the motor command might explain that MI and ME of swallowing lead to an increase in oxy-Hb during both tasks (7). Prior studies in neurological patients also showed diminished inhibitory control, which led to involuntarily movements while imaging motor actions (7).
Patients with cerebral lesions showed a prolonged time course of the NIRS signal compared to healthy controls and patients with brainstem lesions. This prolongation probably reflects problems in initiating the voluntary swallowing process in these patients, or afferent/efferent feedback loops from the cortex to the periphery may work slower and lead to this sustained cerebral response (18, 21).
MI vs. ME
Generally, both ME and MI tasks lead to the strongest NIRS signal change over the inferior frontal gyrus in all participants. Hence, MI and ME of swallowing lead to comparable brain activation patterns in stroke patients. Especially, changes in deoxy-Hb were most comparable between the ME and MI tasks. This result is in line with a previous NIRS study in healthy young adults. In that study, a stronger congruency was found in deoxy-Hb changes between ME and MI of swallowing than in oxy-Hb changes (5).
For oxy-Hb, the relative concentration change between resting period and task period was overall stronger for the ME than for the MI task. However, ME of any movement task generally leads to stronger brain activation patterns compared to mental imagery of the corresponding movements (5, 14, 16).
Impact
The results of the present study may have practical impact on the future treatment of dysphagia. MI can be potentially used for motor rehabilitation since MI activates brain areas comparable to those during ME (5, 6, 16, 76). There is evidence that mental imagery of a specific motor task leads to a neuronal reorganization and consequently to improvements in motor functions (22, 77–81). However, prior MI studies focused on hand and food movements (14–16, 78). MI of swallowing movements might have comparable effects and might harness plasticity of the brain to improve the recovery of swallowing functions after brain injury (6, 82). However, this is a matter of future investigations.
Limitations
Generally, multiple case studies enable the researcher to explore differences within and between cases. The goal is to replicate findings across cases, as we could show for patients with cerebral lesions and brainstem lesions. It is necessary that the cases are chosen carefully so that one can predict similar results across cases, or predict contrasting results based on a theory (52). We tried to select our cases carefully with comparable brain lesion sites, time since onset, cognitive abilities (MMSE), and degree of dysphagia (53). Case studies are valuable only if the case definition is precise and often raise issues of patient selection and comparability with other populations (83). However, they offer a practical and more rapid method of neuroscientific investigations. We believe that this design is appropriate for the initial phase of the scientific evaluation of cortical correlates of MI and ME of swallowing in dysphagia patients. However, the present results are based on a small sample of stroke patients. Hence, our results are more hypothesis generating and future studies with larger samples are needed to demonstrate the potential usefulness of mental imagery of swallowing for the treatment of dysphagia.
Conclusion
In the present study, we revealed changes in hemodynamic responses accompanying MI and ME of swallowing in dysphagia patients using a relatively new imaging technique, the NIRS. Comparable to findings in healthy participants, we found the strongest hemodynamic changes in the inferior frontal gyrus during both ME and MI of swallowing in dysphagia patients. Furthermore, we could provide first evidence that localization of hemodynamic correlates of swallowing differ in dependence on brain lesion location. Hence, the present results provide new evidence concerning timing and topographical distribution of the hemodynamic response during ME and MI of swallowing in dysphagia patients, which might have practical impact on future dysphagia treatment. To the best of our knowledge, this is the first study investigating cortical correlates of MI and ME of swallowing in stroke patients with dysphagia symptoms.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was partially supported by the European STREP Program – Collaborative Project no. FP7-287320 – CONTRAST. Possible inaccuracies of information are under the responsibility of the project team. The text reflects solely the views of its authors. The European Commission is not liable for any use that may be made of the information contained therein. Published with the support of the University of Graz, Austria.
References
1. Smithard DG, Smeeton NC, Wolfe CDA. Long-term outcome after stroke: does dysphagia matter? Age Ageing (2006) 36(1):90–4. doi:10.1093/ageing/afl149
2. Khedr EM, Abo-Elfetoh N. Noninvasive brain stimulation for treatment of post-stroke dysphagia. Neuroenterology (2013) 2:1–9. doi:10.4303/ne/235663
3. Haider C, Zauner H, Gehringer-Manakamatas N, Kadar K, Wood G, Wallner K, et al. Neurogenic dysphagia: nutrition therapy improves rehabilitation. Journal für Ernährungsmedizin (2008) 10(3):6–11.
4. Bours GJ, Speyer R, Lemmens J, Limburg M, de Wit R. Bedside screening tests vs. videofluoroscopy or fibreoptic endoscopic evaluation of swallowing to detect dysphagia in patients with neurological disorders: systematic review. J Adv Nurs (2009) 65(3):477–93. doi:10.1111/j.1365-2648.2008.04915.x
5. Kober SE, Wood G. Changes in hemodynamic signals accompanying motor imagery and motor execution of swallowing: a near-infrared spectroscopy study. Neuroimage (2014) 93:1–10. doi:10.1016/j.neuroimage.2014.02.019
6. Faralli A, Bigoni M, Mauro A, Rossi F, Carulli D. Noninvasive strategies to promote functional recovery after stroke. Neural Plast (2013) 2013(12):1–16. doi:10.1155/2013/854597
7. Guillot A, Di Rienzo F, MacIntyre T, Moran A, Collet C. Imagining is not doing but involves specific motor commands: a review of experimental data related to motor inhibition. Front Hum Neurosci (2012) 6:247. doi:10.3389/fnhum.2012.00247
8. Kober S, Wood G, Kurzmann J, Friedrich E, Stangl M, Wippel T. Near-infrared spectroscopy based neurofeedback training increases specific motor imagery related cortical activation compared to sham feedback. Biol Psychol (2014) 95:21–30. doi:10.1016/j.biopsycho.2013.05.005
9. Decety J. Do imagined and executed actions share the same neural substrate? Brain Res (1996) 3(2):87–93.
10. Hanakawa T, Dimyan MA, Hallett M. Motor planning, imagery, and execution in the distributed motor network: a time-course study with functional MRI. Cereb Cortex (2008) 18(12):2775–88. doi:10.1093/cercor/bhn036
11. Jeannerod M. Mental imagery in the motor context. Neuropsychologia (1995) 33(11):1419–32. doi:10.1016/0028-3932(95)00073-C
12. Jeannerod M, Decety J. Mental motor imagery: a window into the representational stages of action. Curr Opin Neurobiol (1995) 5(6):727–32. doi:10.1016/0959-4388(95)80099-9
13. Lacourse MG, Orr EL, Cramer SC, Cohen MJ. Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage (2005) 27(3):505–19. doi:10.1016/j.neuroimage.2005.04.025
14. Neuper C, Scherer R, Reiner M, Pfurtscheller G. Imagery of motor actions: differential effects of kinesthetic and visual–motor mode of imagery in single-trial EEG. Cogn Brain Res (2005) 25(3):668–77. doi:10.1016/j.cogbrainres.2005.08.014
15. Pfurtscheller G, Neuper C. Motor imagery activates primary sensorimotor area in humans. Neurosci Lett (1997) 239(2–3):65–8. doi:10.1016/S0304-3940(97)00889-6
16. Wriessnegger SC, Kurzmann J, Neuper C. Spatio-temporal differences in brain oxygenation between movement execution and imagery: a multichannel near-infrared spectroscopy study. Int J Psychophysiol (2008) 67(1):54–63. doi:10.1016/j.ijpsycho.2007.10.004
17. Hamdy S, Aziz Q, Rothwell J, Singh K, Barlow J, Hughes D. The cortical topography of human swallowing musculature in health and disease. Nat Med (1996) 2:1217–24. doi:10.1038/nm1196-1217
18. Hamdy S, Mikulis D, Crawley A, Xue S, Law H, Henry S, et al. Cortical activation during human volitional swallowing: an event related fMRI study. Am J Physiol Gastrointest Liver Physiol (1999) 277:219–25.
19. Hamdy S, Rothwell J, Brook D, Bailey D, Aziz Q, Thompson D. Identification of the cerebral loci processing human swallowing with H2 15O PET activation. J Neurophysiol (1999) 81:1917–26.
20. Hamdy S, Rothwell JC, Aziz Q, Thompson DG. Organization and reorganization of human swallowing motor cortex: implications for recovery after stroke. Clin Sci (2000) 98:151–7. doi:10.1042/CS19990300
21. Martin RE, Goodyear GB, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol (2001) 85:938–50.
22. Furlong P, Hobson A, Aziz Q, Barnes G, Singh K, Hillebrand A. Dissociating the spatio-temporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. Neuroimage (2004) 22(4):1447–55. doi:10.1016/j.neuroimage.2004.02.041
23. Martin R, Barr A, MacIntosh B, Smith R, Stevens T, Taves D. Cerebral cortical processing of swallowing in older adults. Exp Brain Res (2006) 176(1):12–22. doi:10.1007/s00221-006-0592-6
24. Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: a systematic review. Dysphagia (2007) 22(3):266–75. doi:10.1007/s00455-007-9080-9
25. Sörös P, Inamoto Y, Martin RE. Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum Brain Mapp (2009) 30(8):2426–39. doi:10.1002/hbm.20680
26. Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp (2009) 30(10):3209–26. doi:10.1002/hbm.20743
27. Hartnick C, Rudolph C, Willging J, Holland S. Functional magnetic resonance imaging of the pediatric swallow: imaging the cortex and the brainstem. Laryngoscope (2001) 111:1183–91. doi:10.1097/00005537-200107000-00010
28. Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol (2001) 280:G354–60.
29. Toogood JA, Barr AM, Stevens TK, Gati JS, Menon RS, Martin RE. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: a functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Experimental Brain Research (2005) 161(1):81–90. doi:10.1007/s00221-004-2048-1
30. Miller A. Neurophysiological basis of swallowing. Dysphagia (1986) 1(2):91–100. doi:10.1007/BF02407121
31. Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res (2001) 140(3):280–9. doi:10.1007/s002210100813
32. Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia (2003) 18(2):71–7. doi:10.1007/s00455-002-0088-x
33. Satow T, Ikeda A, Yamamoto J-I, Begum T, Thuy DHD, Matsuhashi M. Role of primary sensorimotor cortex and supplementary motor area in volitional swallowing: a movement-related cortical potential study. Am J Physiol Gastrointest Liver Physiol (2004) 287(2):G459. doi:10.1152/ajpgi.00323.2003
34. Suntrup S, Teismann I, Wollbrink A, Winkels M, Warnecke T, Flöel A. Magnetoencephalographic evidence for the modulation of cortical swallowing processing by transcranial direct current stimulation. Neuroimage (2013) 83:346–54. doi:10.1016/j.neuroimage.2013.06.055
35. Teismann IK, Steinstraeter O, Schwindt W, Ringelstein EB, Pantev C, Dziewas R. Age-related changes in cortical swallowing processing. Neurobiol Aging (2010) 31(6):1044–50. doi:10.1016/j.neurobiolaging.2008.07.001
36. Luan B, Sörös P, Sejdić E, He Y. A study of brain networks associated with swallowing using graph-theoretical approaches. PLoS One (2013) 8(8):e73577. doi:10.1371/journal.pone.0073577
37. Lowell SY, Reynolds RC, Chen G, Horwitz B, Ludlow CL. Functional connectivity and laterality of the motor and sensory components in the volitional swallowing network. Exp Brain Res (2012) 219(1):85–96. doi:10.1007/s00221-012-3069-9
38. Ertekin C. Voluntary versus spontaneous swallowing in man. Dysphagia (2011) 26(2):183–92. doi:10.1007/s00455-010-9319-8
39. Mistry S, Verin E, Singh S, Jefferson S, Rothwell JC, Thompson DG, et al. Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals functional asymmetry in the hemispheric projections to human swallowing. J Physiol (2007) 585(2):525–38. doi:10.1113/jphysiol.2007.144592
40. Ertekin C, Aydogdu I. Neurophysiology of swallowing. Neurophysiol Clin (2003) 114(12):2226–44. doi:10.1016/S1388-2457(03)00237-2
41. Daniels SK, Brailey K, Foundas AL. Lingual discoordination and dysphagia following acute stroke: analyses of lesion localization. Dysphagia (1999) 14(2):85–92. doi:10.1007/PL00009592
42. Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. Br Med J (1987) 295(6595):411–4. doi:10.1136/bmj.295.6595.411
43. Ushioda T, Watanabe Y, Sanjo Y, Yamane G-Y, Abe S, Tsuji Y, et al. Visual and auditory stimuli associated with swallowing activate mirror neurons: a magnetoencephalography study. Dysphagia (2012) 27(4):504–13. doi:10.1007/s00455-012-9399-8
44. Weiskopf N. Real-time fMRI and its application to neurofeedback. Neuroimage (2012) 62(2):682–92. doi:10.1016/j.neuroimage.2011.10.009
45. Hoshi Y, Kobayashi N, Tamura M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol (2001) 90:1657–62.
46. Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage (2006) 29(2):368–82. doi:10.1016/j.neuroimage.2005.08.065
47. Sitaram R, Caria A, Birbaumer N. Hemodynamic brain–computer interfaces for communication and rehabilitation: brain-machine interface. Neural Netw (2009) 22(9):1320–8. doi:10.1016/j.neunet.2009.05.009
48. Muehlemann T, Haensse D, Wolf M. Wireless miniaturized in-vivo near infrared imaging. Opt Express (2008) 16(14):10323–30. doi:10.1364/OE.16.010323
49. Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci Biobehav Rev (2010) 34(3):269–84. doi:10.1016/j.neubiorev.2009.07.008
50. Nambu I, Osu R, Sato M-A, Ando S, Kawato M, Naito E. Single-trial reconstruction of finger-pinch forces from human motor-cortical activation measured by near-infrared spectroscopy (NIRS). Neuroimage (2009) 47(2):628–37. doi:10.1016/j.neuroimage.2009.04.050
51. Obrig H. NIRS in clinical neurology – a ‘promising’ tool? Neuroimage (2014) 8(1):535–46. doi:10.1016/j.neuroimage.2013.03.045
53. Baxter PE, Jack SM. Qualitative case study methodology: study design and implementation for novice researchers. Qual Rep (2008) 13(4):544–59.
54. Bartolome G, Schröter-Morasch H, editors. Schluckstörungen – Diagnostik und Rehabilitation. 5th ed. München: Elsevier (2014).
55. Bartolome G, Schröter-Morasch H. Bogenhausener Untersuchungsprotokoll für die Klinische Schluckuntersuchung (KSU). 5th ed. In: Bartolome G, Schröter-Morasch H, editors. Schluckstörungen – Diagnostik und Rehabilitation. München: Elsevier (2014). p. 155–68.
56. Starrost U, Bartolome G, Schröter-Morasch H, Ziegler W, Fussenegger C, Marano C, et al. Der Bogenhausener Dysphagiescore – BODS: Inhaltsvalidität und Reliabilität. In: Stanschus S, Rosenbek J, Wilmskötter J, editors. Dysphagieforum 2 (2012). p. 2–12.
57. Singh AK, Okamoto M, Dan H, Jurcak V, Dan I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage (2005) 27(4):842–51. doi:10.1016/j.neuroimage.2005.05.019
58. Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp (1997) 5(4):238–42. doi:10.1002/(SICI)1097-0193(1997)5:4<238:AID-HBM6>3.0.CO;2-4
59. Talairach JTP. Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Dimensional Proportional System: An Approach to Cerebral Imaging. New York, NY: Thieme Medical Publishers (1988).
60. Singh AK, Dan I. Exploring the false discovery rate in multichannel NIRS. Neuroimage (2006) 33(2):542–9. doi:10.1016/j.neuroimage.2006.06.047
61. Sato H, Obata A, Moda I, Ozaki K, Yasuhara T, Yamamoto Y. Application of near-infrared spectroscopy to measurement of hemodynamic signals accompanying stimulated saliva secretion. J Biomed Opt (2011) 16(4):1–8. doi:10.1117/1.3565048
62. Penfield W, Faulk ME. The insula; further observations on its function. Brain (1955) 78(4):445–70. doi:10.1093/brain/78.4.445
63. Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res (1996) 22(3):229–44. doi:10.1016/S0165-0173(96)00011-2
64. Kobayakawa T, Endo H, Ayabe-Kanamura S, Kumagai T, Yamaguchi Y, Kikuchi Y. The primary gustatory area in human cerebral cortex studied by magnetoencephalography. Neurosci Lett (1996) 212(3):155–8. doi:10.1016/0304-3940(96)12798-1
65. Schoenfeld M, Neuer G, Tempelmann C, Schüßler K, Noesselt T, Hopf J-M, et al. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience (2004) 127(2):347–53. doi:10.1016/j.neuroscience.2004.05.024
66. Cerf-Ducastel B, van de Moortele P, MacLeod P, Le Bihan D, Faurion A. Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: a functional magnetic resonance imaging study. Chem Senses (2001) 26(4):371–83. doi:10.1093/chemse/26.4.371
67. Humbert IA, Fitzgerald ME, McLaren DG, Johnson S, Porcaro E, Kosmatka K. Neurophysiology of swallowing: effects of age and bolus type. Neuroimage (2009) 44(3):982–91. doi:10.1016/j.neuroimage.2008.10.012
68. Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia (1997) 12(3):146–56. doi:10.1007/PL00009529
69. Malandraki GA, Sutton BP, Perlman AL, Karampinos DC. Age-related differences in laterality of cortical activations in swallowing. Dysphagia (2010) 25(3):238–49. doi:10.1007/s00455-009-9250-z
70. Khedr EM, Abo-Elfetoh N, Rothwell JC. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol Scand (2009) 119(3):155–61. doi:10.1111/j.1600-0404.2008.01093.x
71. Singh S, Hamdy S. Dysphagia in stroke patients. Postgrad Med J (2006) 82(968):383–91. doi:10.1136/pgmj.2005.043281
72. Martin RE. Neuroplasticity and swallowing. Dysphagia (2009) 24(2):218–29. doi:10.1007/s00455-008-9193-9
73. Gentili RJ, Shewokis PA, Ayaz H, Contreras-Vidal JL. Functional near-infrared spectroscopy-based correlates of prefrontal cortical dynamics during a cognitive-motor executive adaptation task. Front Hum Neurosci (2013) 7:277. doi:10.3389/fnhum.2013.00277
74. Kasess CH, Windischberger C, Cunnington R, Lanzenberger R, Pezawas L, Moser E. The suppressive influence of SMA on M1 in motor imagery revealed by fMRI and dynamic causal modeling. Neuroimage (2008) 40(2):828–37. doi:10.1016/j.neuroimage.2007.11.040
75. Terzi N, Orlikowski D, Prigent H, Denise P, Normand H, Lofaso F. Noninvasive monitoring of breathing and swallowing interaction. In: Schwartz M, ed. EMG Methods for Evaluating Muscle and Nerve Function. InTech (2011). p. 413–24.
76. Garrison KA, Winstein CJ, Aziz-Zadeh L. The mirror neuron system: a neural substrate for methods in stroke rehabilitation. Neurorehabil Neural Repair (2010) 24(5):404–12. doi:10.1177/1545968309354536
77. Bray S, Shimojo S, O’Doherty JP. Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. J Neuroscience (2007) 27(28):7498–507.
78. Mihara M, Hattori N, Hatakenaka M, Yagura H, Kawano T, Hino T, et al. Near-infrared spectroscopy-mediated neurofeedback enhances efficacy of motor imagery-based training in poststroke victims: a pilot study. Stroke (2013) 44(4):1091–8. doi:10.1161/STROKEAHA.111.674507
79. Teo W-P, Chew E. Is motor-imagery brain-computer interface feasible in stroke rehabilitation? PM R (2014) 6(8):723–8. doi:10.1016/j.pmrj.2014.01.006
80. Zich C, Debener S, Kranczioch C, Bleichner MG, Gutberlet I, de Vos M. Real-time EEG feedback during simultaneous EEG–fMRI identifies the cortical signature of motor imagery. Neuroimage (2015) 114(0):438–47. doi:10.1016/j.neuroimage.2015.04.020
81. Laffont I, Bakhti K, Coroian F, van Dokkum L, Mottet D, Schweighofer N, et al. Innovative technologies applied to sensorimotor rehabilitation after stroke. Ann Phys Rehabil Med (2014) 57(8):543–51. doi:10.1016/j.rehab.2014.08.007
82. Yang H, Guan C, Ang K, Wang C, Phua K, Yu J. Dynamic initiation and dual-tree complex wavelet feature-based classification of motor imagery of swallow EEG signals. WCCI 2012 IEEE World Congress on Computational Intelligence. Brisbane: IEEE (2012). p. 1710–5.
Keywords: near-infrared spectroscopy, motor imagery, motor execution, swallowing, dysphagia, stroke
Citation: Kober SE, Bauernfeind G, Woller C, Sampl M, Grieshofer P, Neuper C and Wood G (2015) Hemodynamic signal changes accompanying execution and imagery of swallowing in patients with dysphagia: a multiple single-case near-infrared spectroscopy study. Front. Neurol. 6:151. doi: 10.3389/fneur.2015.00151
Received: 20 February 2015; Accepted: 22 June 2015;
Published: 06 July 2015
Edited by:
Scott E. Kasner, University of Pennsylvania, USAReviewed by:
Velandai Srikanth, Monash University, AustraliaKaren Wheeler-Hegland, University of Florida, USA
Copyright: © 2015 Kober, Bauernfeind, Woller, Sampl, Grieshofer, Neuper and Wood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Erika Kober, Department of Psychology, University of Graz, Universitaetsplatz 2/III, Graz 8010, Austria, silvia.kober@uni-graz.at
 Silvia Erika Kober
Silvia Erika Kober Günther Bauernfeind
Günther Bauernfeind Carina Woller4
Carina Woller4 Christa Neuper
Christa Neuper Guilherme Wood
Guilherme Wood