What have we learned from “perturbing” the human cortical motor system with transcranial magnetic stimulation?
- 1 Department of Psychology, Centre for Brain and Mind, University of Western Ontario, London, ON, Canada
- 2 Department of Psychology, The Rotman Research Institute, University of Toronto, Toronto, ON, Canada
- 3 Department of Psychiatry, The Rotman Research Institute, University of Toronto, Toronto, ON, Canada
The purpose of this paper is twofold. First, we will review different approaches that one can use with transcranial magnetic stimulation (TMS) to study both its effects on motor behavior and on neural connections in the human brain. Second, we will present evidence obtained in TMS-based studies showing that the dorsal premotor area (PMd), the ventral premotor area (PMv), the supplementary motor area (SMA), and the pre-supplementary motor area (pre-SMA) each have different roles to play in motor behavior. We highlight the importance of the PMd in response selection based on arbitrary cues and in the control of arm movements, the PMv in grasping and in the discrimination of bodily actions, the SMA in movement sequencing and in bimanual coordination, and the pre-SMA in cognitive control. We will also discuss ways in which TMS can be used to chart “true” cerebral reorganization in clinical populations and how TMS might be used as a therapeutic tool to facilitate motor recovery after stroke. We will end our review by discussing some of the methodological challenges and future directions for using this tool in basic and clinical neuroscience.
Introduction
Hughlings Jackson proposed that the central nervous system was composed of a number of hierarchical levels: each level containing a complete set of representations of the next lower level that enables it to exert influence on motor behavior (see Hughlings Jackson, 1958). This hierarchical organization of the motor system was challenged in the 1990s with the emergence of anatomical studies in the monkey that demonstrated a number of cortical areas other than the primary motor cortex (M1) with direct projections to the spinal cord (Dum and Strick, 1991; He et al., 1993, 1995). We now know that several areas in the frontal lobe have the anatomical substrate to influence motor output both through connections with M1 and through direct projections to the spinal cord. These non-primary motor areas include the premotor, the supplementary motor, and the cingulate motor areas. These areas can be further divided into caudal and rostral subdivisions based on the degree to which they can influence motor output (Picard and Strick, 1996, 2001). Caudal subdivisions for each of these areas exert a much stronger influence on motor output than their rostral subdivisions (Barbas and Pandya, 1987). The latter exert little or no direct influence on motor output. The presence of analogous areas in the human brain has been proposed based on a series of meta-analyses carried out to characterize functional activation during motor tasks (Picard and Strick, 1996, 2001). These areas are shown in Figure 1. They include the dorsal premotor area (PMd), the ventral premotor area (PMv), the supplementary motor area (SMA), the pre-SMA, and the cingulate motor areas (CMAs). The purpose of this review is twofold. First, we will describe the different approaches that one can use with transcranial magnetic stimulation (TMS) to study both its effects on motor behavior and neural connections in the human brain. Second, we will present the evidence obtained in TMS-based studies showing that PMd, PMv, SMA, and pre-SMA each have different roles to play in motor behavior. We will also discuss ways in which TMS can be used to chart “true” cerebral reorganization in clinical populations and how TMS might be used as a therapeutic tool to help motor recovery after stroke. We will end our review by discussing some of the future avenues for using this tool in basic and clinical neuroscience.
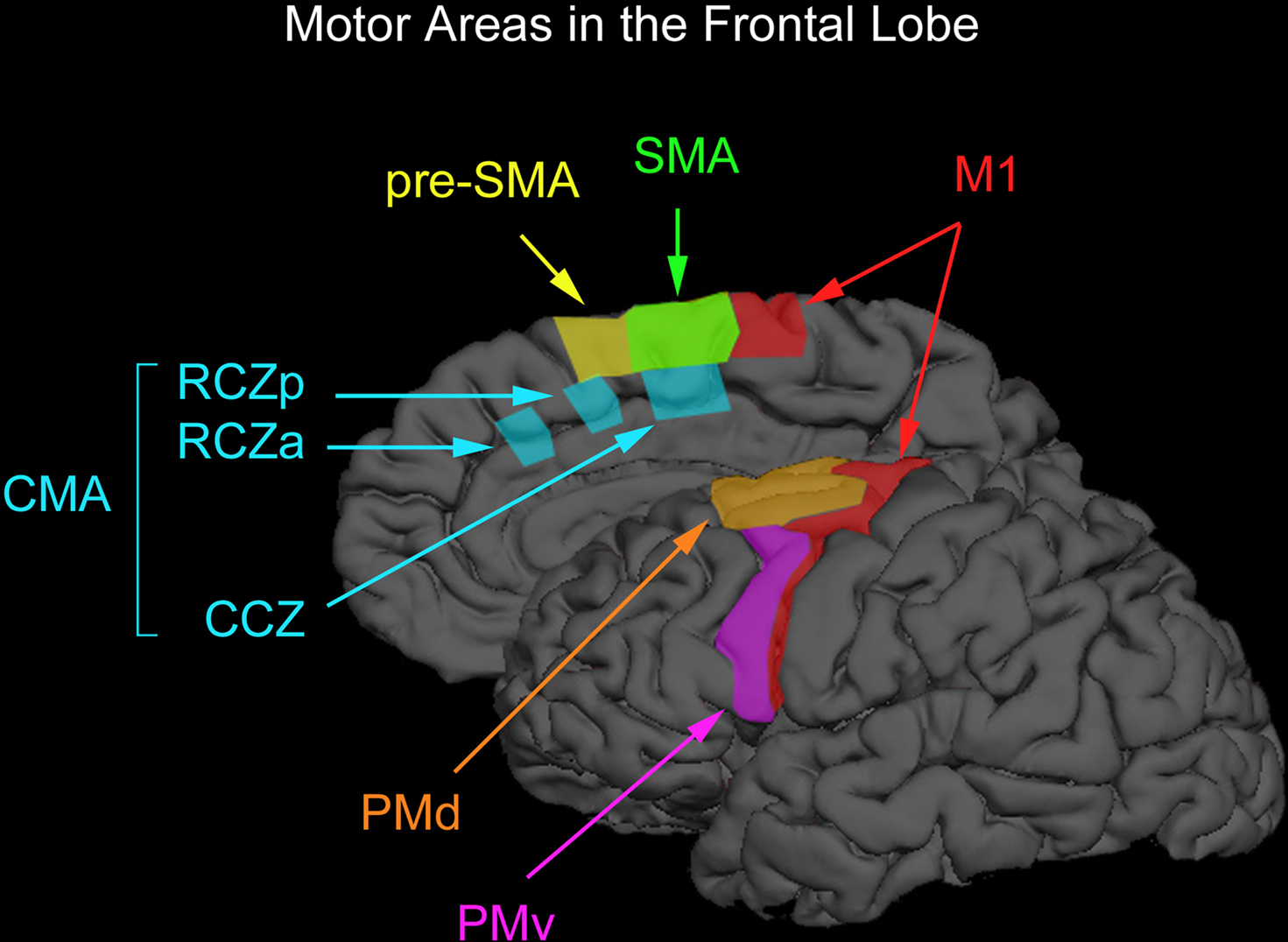
Figure 1. Motor areas in the frontal lobe. The premotor cortex on the lateral surface of the brain can be divided into the dorsal and ventral premotor areas (PMd and PMv) and the supplementary motor cortex on the medial wall of the brain can be divided into the supplementary motor and pre-supplementary motor areas (SMA and pre-SMA). Premotor cortex below the superior frontal sulcus is typically considered PMv whereas premotor cortex above this anatomical landmark is typically considered PMd. The vertical anterior-commissural line is often used to denote the boundary between SMA and pre-SMA. One can further divide PMd according to a rostral subdivision located along the superior frontal gyrus and a caudal subdivision located along the precentral gyrus. However, one cannot dissociate these two subdivisions with TMS easily and we therefore do not discuss them separately. There also exists two cingulate motor areas (RCZa and RZp) anterior to the vertical anterior-commissural line and one cingulate motor area (CCZ) posterior to the vertical anterior-commissural line. This parcellation of non-primary motor areas in the human was proposed by Picard and Strick (1996, 2001). We have arbitrarily drawn boundaries on a surface-rendered cortical surface loosely based on definitions proposed by Picard and Strick (1996, 2001).
What is TMS?
Transcranial magnetic stimulation is a “perturbation” technique. It perturbs neural activity in space and time by inducing brief electrical currents in a restricted region of the cerebral cortex. A brief current passes through a stimulating coil, which is placed over the person’s scalp, that then induces a rapid rise of magnetic field, and this transient field in turn induces electrical current in the underlying brain tissue. Barker et al. (1985) performed the first TMS experiment and the technique has since acquired importance as a non-invasive method for examining motor, perceptual, and cognitive processes in the human brain. Transcranial direct current stimulation (tDCS) is another perturbation technique that is used to study brain processes. Unlike TMS, tDCS is used to modulate the activity of neurons by applying weak electrical currents through an electrode placed on the scalp (for review, see Been et al., 2007). The underlying principle of both techniques is simple. Using a “perturb-and-measure” approach, one can perturb one brain region and measure the consequences that this manipulation has on either behavior or brain activity (Paus, 2005). As perturbation techniques, TMS and tDCS are designed to study consequences of modulating brain activity and, as such, to inject a certain level of causality in investigations of brain-behavior relationships. In this manner, they are fundamentally different than functional neuroimaging. Both functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) are measurement techniques that are designed to answer the following question: what regions in the brain are engaged during a behavior? We will focus our review on TMS studies.
Although TMS enables one to make conclusions about the necessity of a particular brain region for a given behavior (i.e., stimulated region ‘X’ is necessary), it does not enable one to conclude that the region is “sufficient” for this behavior (i.e., whether or not other brain regions may also be necessary). To illustrate this point, consider the following analogy that is sometimes cited in the literature (Huettel et al., 2009). Damage to one part of a radio, such as the speakers, the tuner, or the power switch, will result in an inability to play music. Because damage to any of these parts will cause the radio to stop playing music, one should not go about damaging one of these parts and then claim that this manipulation knocked out the “music-playing” region in the radio. The same logic also applies to TMS. Nonetheless, because performance on a task often relies on multiple brain regions, TMS can provide opportunities to examine how different brain regions might interact with each other to accomplish a behavior – this is known as functional connectivity. In this paper, we will review the various ways in which TMS can be used to measure its consequences on behavior and how the technique can also be used to examine both functional and effective (i.e., the influence that one brain region exerts over another) connectivity.
How is TMS Applied?
There are a number of ways that one can perturb neural activity with TMS. One can use a small number of pulses applied at a high frequency (5 Hz or more) to disrupt transiently neural activity in a brain region “on-line” during task performance. Using this same approach, one can further reduce the number of pulses delivered and/or increase the frequency with which the pulses are delivered to examine when in time a stimulated brain region is engaged during a given behavior. Similar “event-related” TMS can also be performed using single-pulse stimulation. One important disadvantage of the on-line approach, however, is that the TMS creates acoustic artifacts and tactile sensations on the scalp that can interfere with task performance by distracting the participant. Although these effects are usually controlled for with additional experiments, some researchers will opt to use “off-line” TMS. For off-line TMS, one can use certain stimulation protocols to test the effects of stimulation on task performance after the stimulation has been applied. This can be accomplished with either one continuous train of low-frequency (∼1 Hz) stimulation or with multiple bursts of high-frequency (∼50 Hz) stimulation that are spaced in time. The latter is known as theta-burst stimulation (TBS; Huang et al., 2005, 2008). The precise physiological mechanisms that underlie TMS-induced perturbations for each of these different applications of TMS are not completely understood (for review on putative mechanisms, see Ridding and Rothwell, 2007; Bestmann, 2008; Miniussi et al., 2009; Siebner et al., 2009). We should point out that TBS is relatively new. At the present time, the safety of TBS is not completely understood and there are no recommended guidelines on how to administer this type of stimulation safely (for the latest guidelines on TMS safety, see Rossi et al., 2009). Furthermore, we are not aware of any studies that have compared directly, in the same group of participants, the efficacy of TBS in disrupting task performance against other forms of brain stimulation.
Targeting specific cortical regions with TMS can be achieved with a variety of approaches – some of which work better than others. Using power analysis, Sack et al. (2009) revealed that out of the four most commonly used methods for targeting cortical regions with TMS, functional localization was the method that resulted in the most powerful effects. This approach consists of localizing targets with fMRI and then using an MRI-guided navigation system to guide the TMS coil over the functionally defined regions. Alternative approaches consist of guiding the TMS coil to a location defined by anatomical landmarks on an individual’s MRI (the second most effective approach tested by Sack et al., 2009), guiding the TMS coil to a location defined in Talairach coordinates (the third most effective approach tested by Sack et al., 2009, which has less spatial precision compared with the previous two methods given variability in brain anatomy), and guiding the TMS coil relative to positions in the 10-20 EEG system (not surprisingly, the least effective approach tested by Sack et al., 2009, which has little spatial precision). On the topic of spatial precision, one should also consider the spread of current induced by TMS. Spread of current can be minimized using a figure-of-eight coil, which has become standard practice, and reducing the intensity of TMS. All work that we will cover here have used a figure-of-eight coil and stimulated non-primary motor areas at reduced levels of stimulation so as to avoid encroaching on M1 and other adjacent cortical structures. Figure-of-eight coils are commercially available in different sizes so it might be worthwhile to invest in a smaller figure-of-eight coil to allow for more focal stimulation.
Despite these recommendations, TMS does not rival fMRI (or PET) in its spatial resolution. In fact, the gap between the two is widening. High-resolution fMRI (i.e., voxels smaller than 2 mm in isotropic size) is becoming increasingly more common. In comparison, the spread of current induced by TMS with a standard figure-of-eight coil is ∼1.5 cm at motor-threshold intensities (Thielscher and Kammer, 2004). Nonetheless, the two techniques can be used in a complementary manner to make up for the shortcomings of the other. For example, the temporal response of the blood supply underlying fMRI (∼5 s) is much slower than the electrical signals that define neuronal communication. In contrast, TMS offers far better temporal resolution (∼1 ms). Also, fMRI is a measurement technique and, therefore, it can only measure correlates of behavior and does not allow one to make inferences about causality. In contrast, TMS is a perturbation technique and it can therefore be used to study causal relationships (Paus, 2005). The two approaches are therefore useful for providing converging evidence to argue for or against any functional attributions inferred by the other.
To illustrate the complementary roles of the perturbation approach using TMS and the activation approach using functional neuroimaging, let us consider some of the first author’s fMRI (Chouinard et al., 2008) and TMS (Chouinard et al., 2009a) work on object identification. FMRI usually reveals that the presentation of objects in central vision engages extrastriate visual areas in the two hemispheres – “activation” is typically seen bilaterally in a ventral-stream area known as the lateral-occipital complex (LOC), which is thought to be important for analyzing the form of objects (Malach et al., 1995; Kanwisher et al., 1996). But it has been this author’s experience that TMS applied to LOC in either hemisphere (as defined by fMRI) has little effect in disrupting object identification when these objects are presented in central vision (unpublished data). Yet, when the same stimulation is applied when objects are presented in the contralateral but not in the ipsilateral hemifield, TMS applied over LOC in either hemisphere can produce deficits in identifying objects (Chouinard et al., 2009a). This finding is hardly surprising if one considers that extrastriate visual areas, such as LOC, are retinotopically organized (Op De Beeck and Vogels, 2000). It also suggests, however, that the unstimulated LOC could perhaps stand in for the stimulated LOC when objects are presented in central vision. This idea fits well with the notion that ventral-stream damage in the two hemispheres must occur to produce visual-form agnosia (Farah, 2004).
The Non-Primary Motor Areas in Behavior
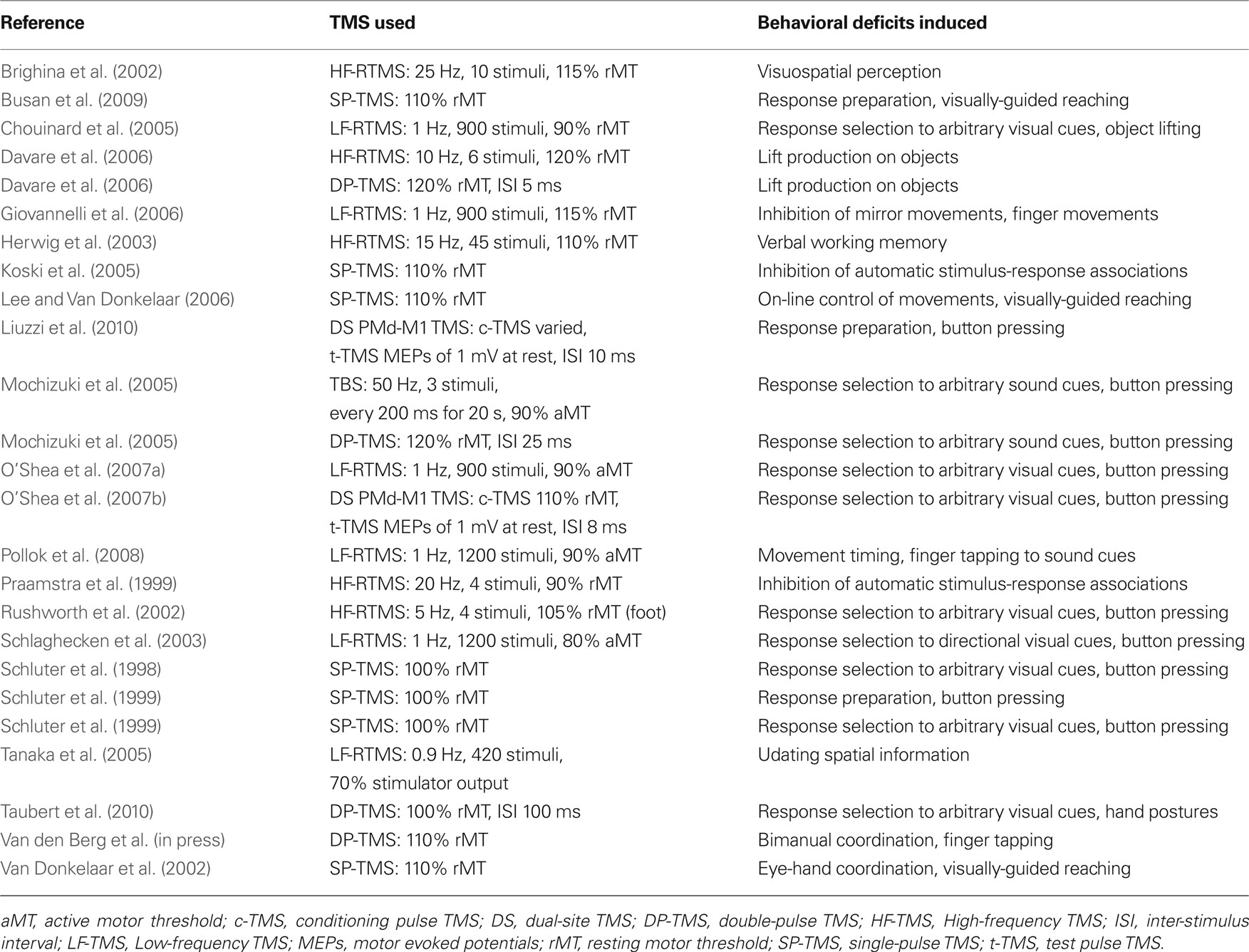
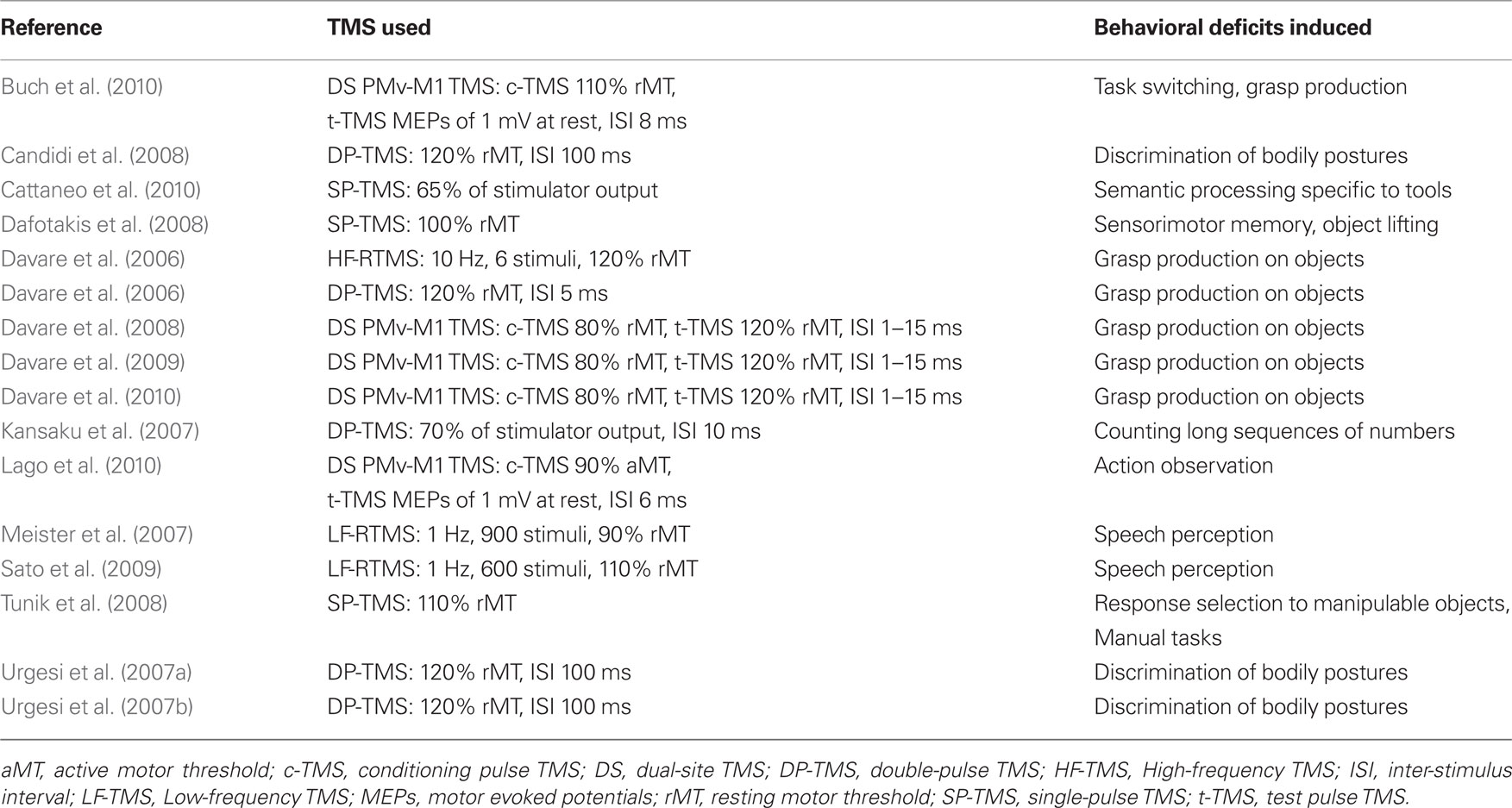
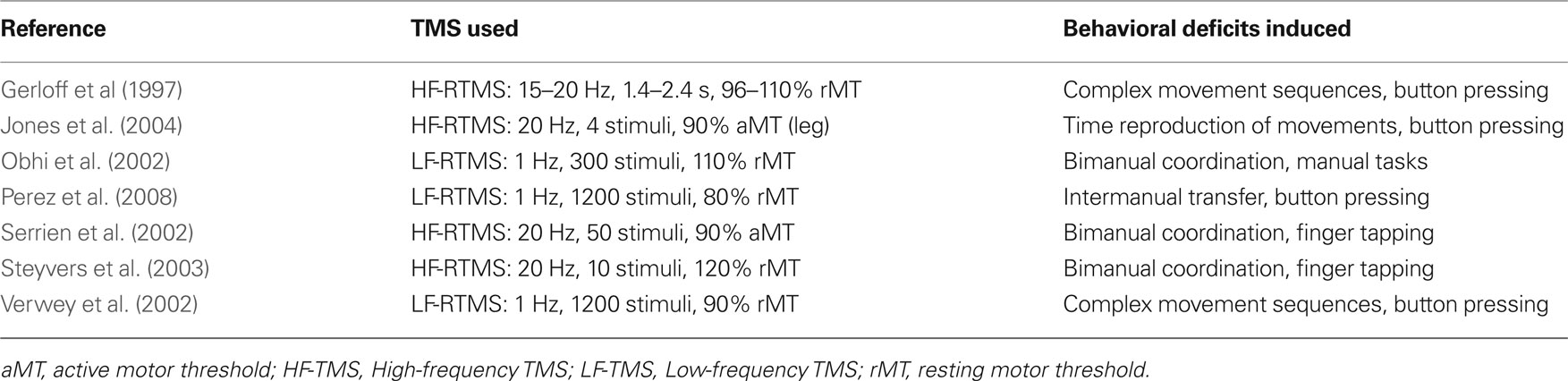
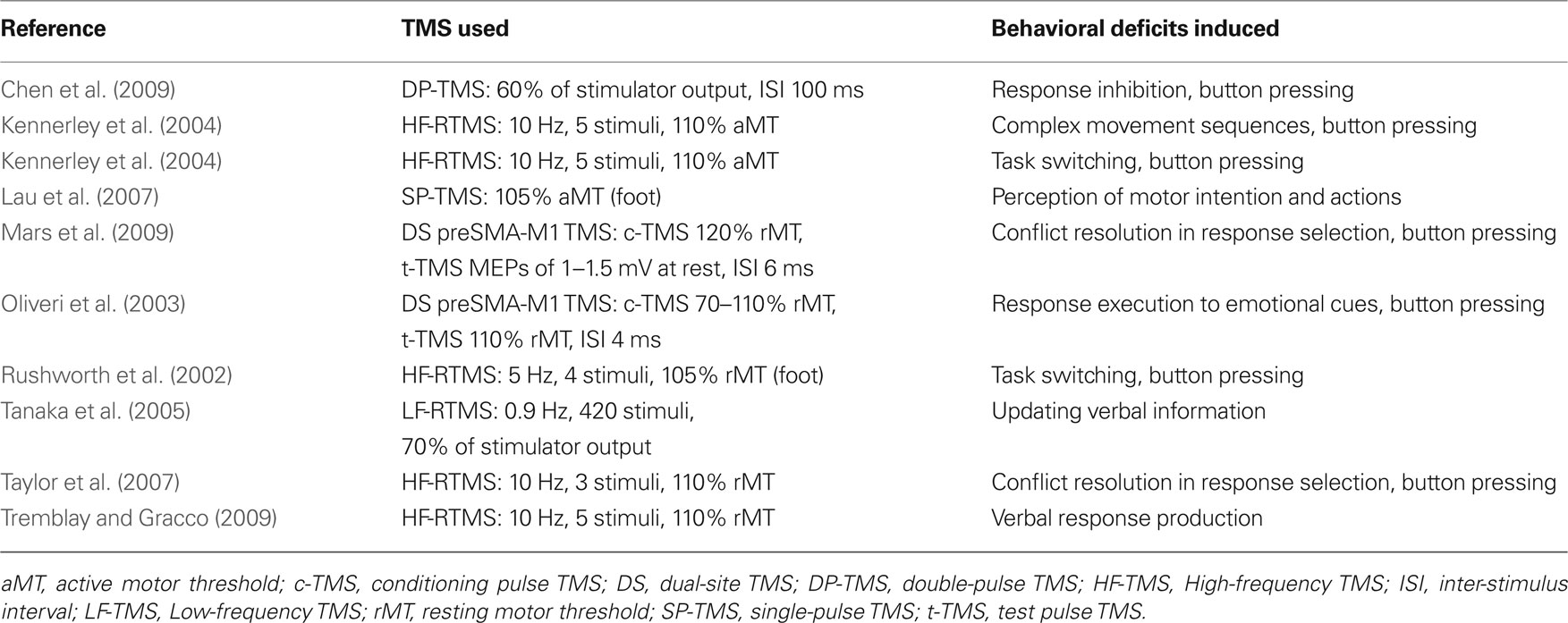
Tables 1–4 provide a summary of all TMS experiments that examined the behavioral consequences of stimulating a non-primary motor area (N = 50). Here, we will focus our discussion on the most consistent findings. Our review highlights the importance of PMd in response selection based on arbitrary cues and in the control of arm movements, the importance of PMv in grasping and in the discrimination of bodily actions, the importance of SMA in movement sequencing and in bimanual coordination, and the importance of pre-SMA in cognitive control processes.
PMd in Motor Behavior
People often select motor responses according to arbitrary rules. For example, our movements while driving a car can be instructed by color cues that we see on traffic lights. These associations are learned and are thought to involve neural processes that differ from the ones that are used in standard visuomotor transformations (Chouinard and Goodale, 2009). As it turns out, a number of studies show that response selection to arbitrary visual cues can be disrupted when TMS is applied to the left PMd irrespectively of whether participants have to make selections using button pressing (Schluter et al., 1998, 1999; Rushworth et al., 2002; O’Shea et al., 2007a,b), hand postures (Taubert et al., 2010), or fingertip forces during object lifting (Chouinard et al., 2005). Response selection to auditory-presented arbitrary cues can also be disrupted with TMS to the left PMd (Mochizuki et al., 2005). Taken together, the left PMd seems to be necessary for selecting responses to arbitrary cues irrespectively of whether these cues are visual or auditory and the type of actions that are made in response to these cues.
The TMS literature also highlights the importance of PMd in arm movements during pointing tasks (van Donkelaar et al., 2002; Busan et al., 2009) including a role in the visual online control of these movements (Lee and van Donkelaar, 2006). This is not surprising given that PMd in the macaque monkey is reciprocally connected to areas MIP and V6A in the parietal lobe, which together form a frontal-parietal circuit important for the visual guidance of arm movement trajectories (Colby and Duhamel, 1991; Galletti et al., 1996; Matelli and Luppino, 2000). Note that arm movements are important not only for reaching but also for lifting objects; this is because we tend to lift objects using proximal muscles of the arm. Davare et al. (2006) demonstrated that TMS to PMd disrupts the generation of proximal arm movements when people lift objects. Namely, electromyography of the arm revealed that participants took a longer time to move the arm when TMS was applied to PMd during the lifting phase of the movement as compared with the same stimulation applied earlier in time. Taken together, these studies are consistent with the notion that PMd is important for both the online control and the execution of arm movements (Kalaska et al., 1997).
PMv in Motor Behavior
Research in the monkey shows that PMv makes an important contribution in object grasping (Jeannerod et al., 1995). The first TMS study to confirm this in humans was performed by Davare et al. (2006); they demonstrated that participants took a longer time to position their fingers on a task object when TMS was applied to PMv. Measurements acquired with force transducers mounted inside the task object revealed that this same stimulation also caused participants to place their index finger and thumb less accurately on the object. As it turns out, many PMv neurons that discharge while monkeys grasp objects also discharge while monkeys see either another monkey or human perform the same action. This class of neurons is called mirror neurons (Rizzolatti and Luppino, 2001) and are thought by some to be important for understanding the meaning of actions (Umilta et al., 2001). In support of this idea, TMS studies show that PMv plays an important role in the discrimination of bodily actions (Urgesi et al., 2007a,b; Candidi et al., 2008). For example, Candidi et al. (2008) stimulated PMv while participants had to decide which of two images presented in either possible or impossible hand configurations corresponded to the same action that was presented to them in an earlier probe image. Stimulation of PMv impaired performance on this discrimination task but only when the possible hand postures were presented. Thus, PMv’s involvement in the discrimination of bodily actions seems to be specific to meaningful actions.
SMA in Motor Behavior
In the early “activation” studies carried out with PET, it was reported that SMA is strongly activated when people imagine themselves performing complex sequences of finger movements (Roland et al., 1980). This observation, along with other lines of evidence, lead to theories that motor areas in the medial wall of the cortex are important for the internal generation of complex movements (Goldberg, 1985). But more recent work, including that carried out with TMS, highlights the importance of SMA in movement sequencing and in bimanual coordination. Gerloff et al. (1997) showed that TMS applied over SMA interferes with the generation of complex sequences of finger movements but not with the generation of simple repetitive finger movements. In a different TMS study, Verwey et al. (2002) examined whether or not SMA played a role in initiating “chunks” of movements in a complex sequence of finger movements. Behavioral research shows that people bin complex movement sequences into motor chunks (Verwey, 1996, 1999) in the same way that people bin phone numbers into shorter series. Contrary to the prediction, however, stimulating SMA did not disrupt the initiation of chunks but instead disrupted the execution of each finger movement. As we will see later, this finding differs from what was obtained in a different study that stimulated the pre-SMA (Kennerley et al., 2004). Other studies show that TMS applied over SMA can also disrupt performance on bimanual tasks as a function of task complexity (Obhi et al., 2002; Serrien et al., 2002; Steyvers et al., 2003). For example, both Serrien et al. (2002) and Steyvers et al. (2003) showed that TMS applied over SMA disrupts bimanual movements of the index finger when these movements are performed in the opposite anti-phase direction but not when these movements are performed in the same in-phase direction. TMS over SMA can also disrupt inter-manual transfer (Perez et al., 2008), which relates to a phenomenon whereby training one hand on a manual task can lead to improvements in the untrained hand.
Pre-SMA in Motor Behavior
The vertical anterior-commissural line is sometimes used to denote the boundary between SMA and pre-SMA. This division was proposed by Picard and Strick (1996, 2001) based on a meta-analysis carried out to characterize functional activation on the medial wall of the cerebral cortex. They showed that “more complex” tasks tended to engage cortex anterior to this boundary while “less complex” tasks tended to engage more posterior cortex. In support of this view, TMS applied over pre-SMA tends to disrupt processes that are “higher-order” than those that are disrupted from stimulating SMA. These include processes of “cognitive control” such as task switching, response inhibition, and conflict resolution. With respect to task switching, Rushworth et al. (2002) examined the role of pre-SMA in a response selection task in which the stimulus-response rules were sometimes switched during the course of the experiment. For example, participants could begin the experiment with a triangle instructing them to respond with their right hand and a square instructing them to respond with their left hand. After several consecutive trials, they were then instructed to do the reverse. By doing this, Rushworth et al. (2002) revealed that TMS applied over pre-SMA affected response selection but only after a switch was introduced. Similarly, Kennerley et al. (2004) revealed that TMS over pre-SMA can disrupt the initiation of a complex sequence of finger movements but only after participants were instructed to switch between two learned sequences. Interestingly, Kennerley et al. (2004) also revealed that TMS over pre-SMA applied at “chunk” points in these sequences could disrupt task performance, which is different than what Verwey et al. (2002) had shown from stimulating SMA. Chen et al. (2009) further revealed that the pre-SMA has a role to play in response inhibition. They found that stimulating pre-SMA impaired people’s performance to react to a stop signal (presented on 25% of the trials) that instructed them to refrain from responding to a cue that they had just seen. Other studies have shown that TMS applied over pre-SMA also plays a role in conflict resolution (Taylor et al., 2007; Mars et al., 2009).
Paired-Pulse TMS and Dual-Site TMS
Paired-pulse TMS consists of applying two TMS pulses separated closely in time. This type of TMS has been widely used to examine intra-cortical circuits in M1 (Kujirai et al., 1993). Typically, a sub-threshold “conditioning” stimulus is applied over M1 at different time periods before applying a supra-threshold “test” stimulus through the same coil. Given that the intensity of the conditioning pulse is too small to produce motor output from M1, it is generally accepted that any influence that the conditioning pulse has on motor excitability (as assessed by the amplitude of muscle responses invoked by the test pulse) is at the level of neural circuits in M1 but not at the level of neural circuits in the spinal cord. The influence of the conditioning pulse on motor excitability can be either inhibitory, if the inter-stimulus interval (ISI) between the two pulses ranges between 1 and 4 ms, or facilitatory, if the ISI ranges between 8 and 20 ms.
One can also use paired-pulse TMS to examine the time course of interactions in a “two-node” neural circuit using two different coils (Figure 2); this approach is known as “dual-site” TMS. The first demonstration of this approach was carried out by Ferbert et al. (1992). Ferbert et al. (1992) showed that applying a conditioning pulse over M1 in one hemisphere suppressed the excitability of M1 in the opposite hemisphere. Importantly, Ferbert et al. (1992) also demonstrated that this suppression was mediated by cortical but not by spinal mechanisms. Namely, the conditioning pulse applied over M1 in one hemisphere had no effect on motor output induced by electrical stimulation of the opposite M1, which is thought to activate corticospinal projections directly, nor did it have any effect on the H-reflex of ipsilateral hand muscles. Subsequent studies have revealed that a conditioning pulse applied over either PMv or pre-SMA can suppress or facilitate the excitability of M1 in the same hemisphere (Oliveri et al., 2003; Davare et al., 2008; Mars et al., 2009). Likewise, a conditioning pulse applied to PMd can suppress or facilitate the excitability of M1 in the opposite hemisphere (Mochizuki et al., 2004; Baumer et al., 2006). Dual-site TMS can also be used to test whether or not these connections can be modulated by task demands. In the next three subsections, we provide examples of this type of modulation. We should point out that all these studies used figure-of-eight coils to achieve more focal stimulation.
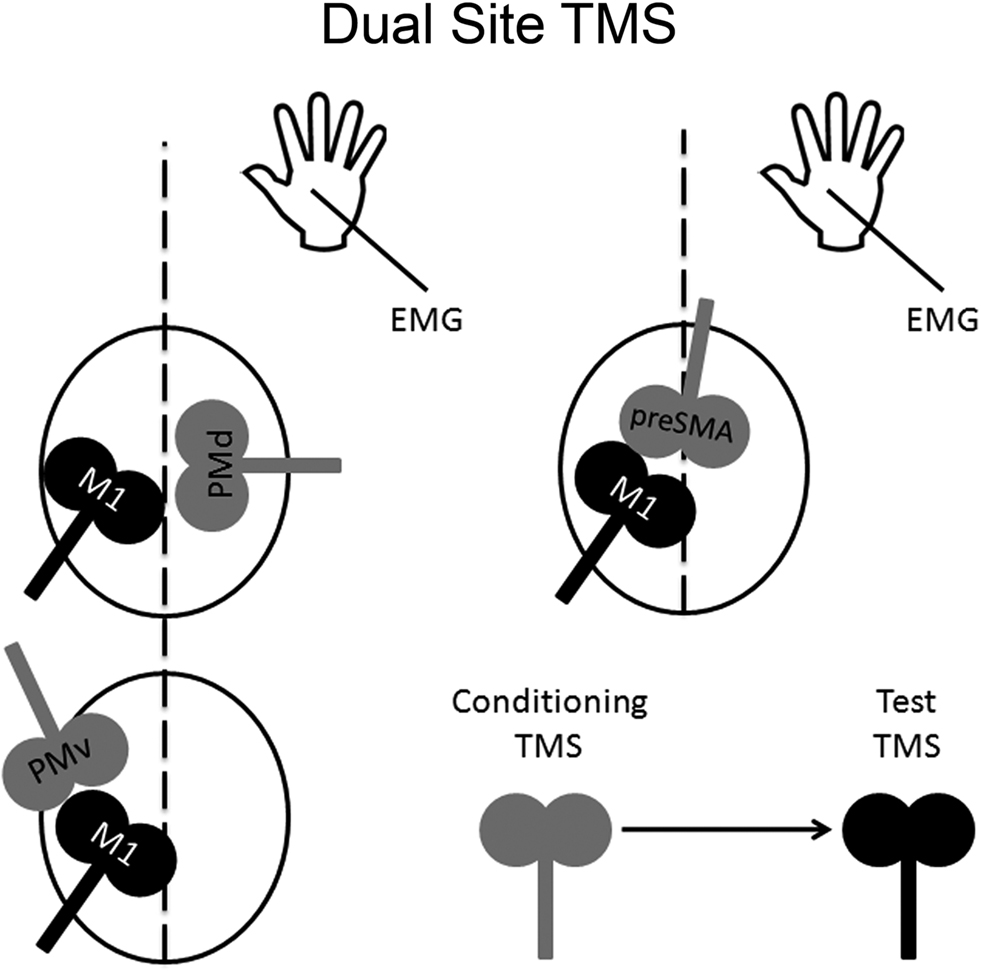
Figure 2. Dual-site TMS approach. Dual-site TMS approach can be used to examine the time course of interactions in a particular neural circuit containing a non-primary motor area and M1. The idea is to stimulate a non-primary motor area with a conditioning pulse to examine its effect on a subsequent supra-threshold test pulse to M1. Changes in M1 can be inferred by measuring any possible changes in its motor excitability on a hand muscle using electromyography.
PMd – M1 Connectivity
O’Shea et al. (2007b) examined whether or not functional connectivity between PMd and M1 can be modulated while participants performed a task that required them to choose between different responses based on arbitrary visual cues. The authors revealed that dual-site TMS (conditioning pulse over PMd and test pulse over M1 in the opposite hemisphere) disrupted behavior when this stimulation was applied 100 ms after cue presentation. The authors also revealed that motor excitability of M1 was facilitated during task performance when dual-site TMS was applied 75 ms after cue presentation. Taken together, the authors concluded that selecting responses on the basis of arbitrary visual cues engages PMd 75–100 ms after cue presentation and that during this time period PMd exerts an influence on M1. It is worthwhile to point out that, at present, examination of PMd-M1 connectivity within the same hemisphere is not feasible with dual-site TMS. This is because the physical size of figure-of-eight coils precludes one to target PMd with one coil and M1 with the other coil. Although Civardi et al. (2001) did claim to have examined PMd-M1 connectivity within the same hemisphere using dual-site TMS, it would appear from their figures that a more rostral area in the prefrontal cortex was primarily targeted with the conditioning pulse instead. The same issue also applies for targeting SMA (proper) and M1 with dual-site TMS.
PMv – M1 Connectivity
In three different studies, Davare et al. (2008, 2009, 2010) examined whether or not functional connectivity between PMv and M1 can be modulated differentially when people grasp objects using either a precision grip or a whole-hand grasp. In all three studies, TMS was applied over the left M1 either in isolation or after a conditioning stimulus was applied over the left PMv. In the first study, Davare et al. (2008) showed that a conditioning stimulus to PMv during no movement suppressed motor excitability of M1 whereas a conditioning stimulus to PMv during a whole-hand grasp on a tennis ball caused this inhibition to disappear. They also showed that a conditioning stimulus to PMv during precision grip on a small cube facilitated motor excitability of M1. Given these differential effects exerted by the conditioning stimulus (to PMv) on motor excitability (of M1), the authors concluded that PMv-M1 circuits are differentially modulated under different types of grasps. In the second study, Davare et al. (2009) repeated a similar experiment in which they had participants grasp either a pen using a precision grip or a circular disk using a whole-hand grasp. They also recorded EMG activity in different hand muscles. In doing so, what they had found was that a conditioning stimulus to PMv facilitated motor excitability of M1 but only in the specific hand muscles that were employed in the types of grasp that were carried out. In the third study, Davare et al. (2010) repeated the same experiment before and after applying TBS over the left anterior intra-parietal sulcus (aIPS); TBS to aIPS reduced all effects of the conditioning stimulus to PMv. Based on these results, the authors suggested that the modulations of the PMv-M1 circuit observed before TBS (and in their earlier studies) might be driven by sensory information about the objects’ geometrical properties it receives from aIPS.
Pre-SMA-M1 Connectivity
Oliveri et al. (2003) examined whether or not functional connectivity between pre-SMA and the left M1 is differentially modulated by simple button responses to photographs that were either neutral or unpleasant in emotional content. They reasoned that pre-SMA might play a greater role in the execution of movements in response to visual stimuli with stronger emotional content. Consistent with their hypothesis, their results revealed that a conditioning pulse of TMS applied over pre-SMA facilitated motor excitability of M1 only when participants saw the emotionally unpleasant photographs as compared with either the presentation of these same photographs during TMS applied over M1 alone or when dual-site TMS was applied during the presentation of the emotionally neutral photographs. In a very different study, Mars et al. (2009) examined the timing of the pre-SMA effects on M1 in a response selection task during conflict resolution. Two different colored-flanker stimuli were presented in each hemifield (e.g., green on the left and red on the right) and participants were required to respond to a central cue that became either green or red, which instructed them to respond with the index finger of the hand that was on the same side as the flanker of the same color. The critical manipulation was that the central cue took the same color for several consecutive trials before switching to the next color. Their results revealed that a conditioning stimulus to pre-SMA facilitated motor excitability of the left M1 during task performance but only during the switch trials. Based on these findings, the authors concluded that the pre-SMA influences the left M1 during a response selection task in cases when re-programming is necessary.
Combining TMS and Functional Neuroimaging in Normal Individuals
The second half of the 1990s saw the emergence of studies that combined TMS and functional neuroimaging concurrently (Fox et al., 1997; Paus et al., 1997; Bohning et al., 1998; Siebner et al., 1998). The underlying logic is simple. Using a perturb-and-measure approach, one can alter neural activity in one brain region to evaluate the effects that this manipulation has on neural activity elsewhere in the brain (Paus, 2005). Changes in neural activity in regions in the brain other than the one that was stimulated can be inferred as being connected to the region that was stimulated. In other words, one can examine connections in the brain by stimulating a target region of the cortex with TMS and measuring changes in neural activity elsewhere in the brain with either PET or fMRI. In this section, we will review some studies that used this combined approach for examining PMd connectivity.
In a combined TMS/PET study, we examined the effects of applying off-line 1-Hz repetitive TMS to either the left PMd or the left M1 on both the motor excitability of the left M1 and regional cerebral blood flow (CBF) throughout the brain (Chouinard et al., 2003). Specifically, we mapped networks of brain regions in which changes in CBF correlated with changes in the motor excitability of the left M1 after applying repetitive TMS over either the left PMd or the left M1. We interpreted these correlations as an index of neural modulation induced by the repetitive TMS. Although repetitive stimulation at the two adjacent cortical sites produced the same effects on motor excitability, statistical maps of correlations between the magnitude of MEP (motor evoked potential) suppression and changes in CBF revealed two distinct patterns of distal neural modulation (Figure 3). Neural modulation occurred in a small number of brain regions after 1-Hz repetitive TMS to the left M1, many of these confined to the non-primary motor areas and sub-cortical motor structures. In contrast, neural modulation occurred in multiple regions after 1-Hz repetitive TMS to the left PMd; these included motor areas in the frontal cortex as well as more associational regions in the parietal and prefrontal cortices. We concluded that these findings were consistent with known differences between PMd and M1 in the extent of their anatomical connectivity in the macaque monkey. Lee et al. (2003) and Siebner et al. (2003) have also performed similar TMS/PET studies that examined the effects of applying off-line 1-Hz repetitive TMS over either the left PMd or the left M1. In addition, Bestmann et al. (2005, 2008) have done some interesting work stimulating the left PMd during fMRI. Although this procedure is technically challenging, activation patterns induced by stimulating the left PMd concurrently during fMRI acquisition appears to be in agreement with those obtained in the TMS/PET studies.
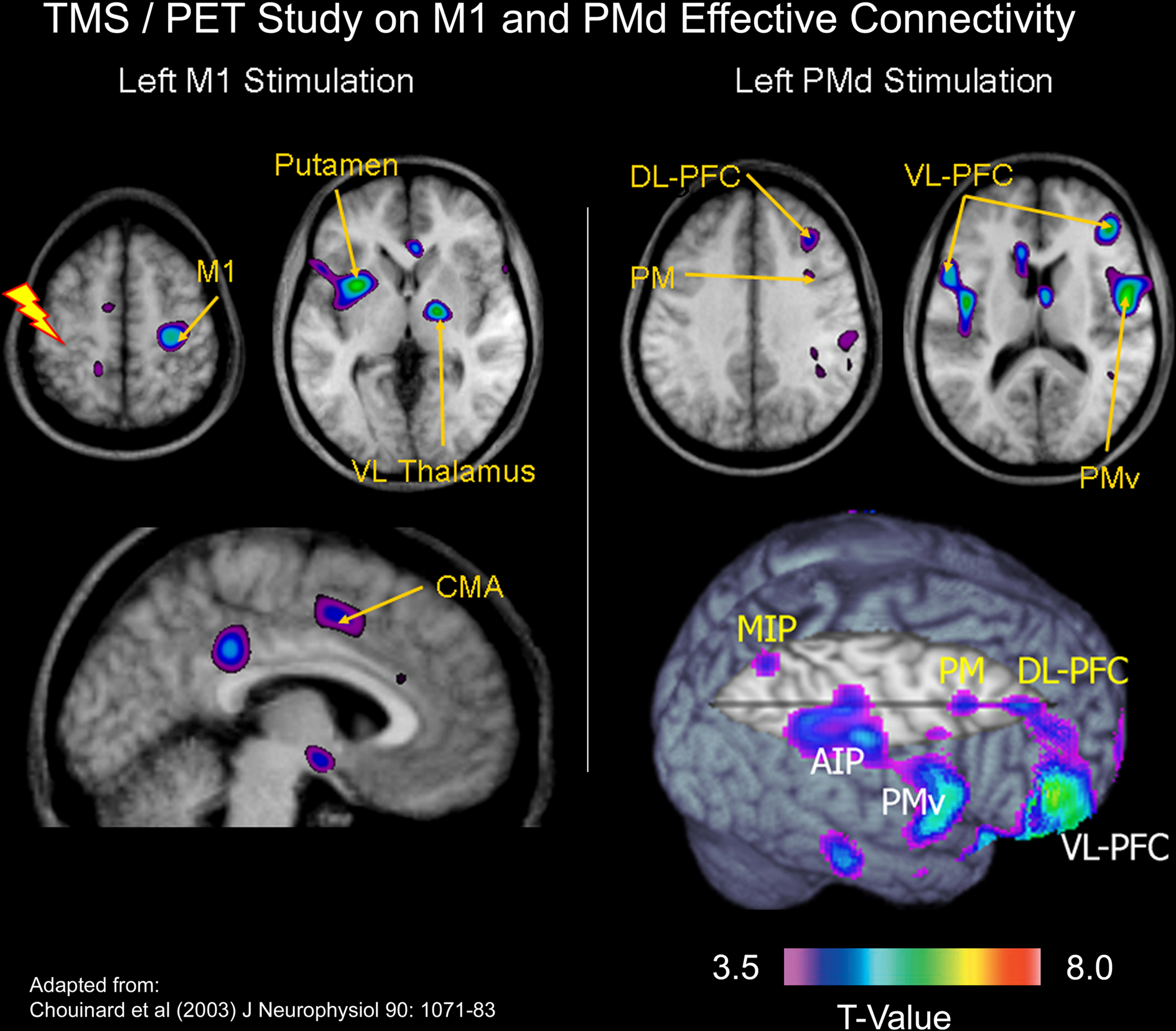
Figure 3. TMS/PET study on M1 and PMd effective connectivity. In a combined TMS/PET study, we mapped networks of brain regions in which changes in cerebral blood flow correlated with changes in the motor excitability of the left M1 after applying repetitive TMS over either the left PMd or the left M1 (Chouinard et al., 2003). We interpreted these correlations as an index of neural modulation induced by the repetitive TMS. Although repetitive stimulation at the two adjacent cortical sites produced the same effects on motor excitability, statistical maps of correlations between the magnitude of MEP suppression and changes in cerebral blood flow revealed two distinct patterns of distal neural modulation. Abbreviations: MIP = medial intraparietal area; DL-PFC = dorsolateral prefrontal cortex; AIP = anterior intra-parietal area; VL-PFC = ventrolateral prefrontal cortex; VL Thalamus = ventrolateral thalamus.
In a different study, O’Shea et al. (2007a) demonstrated that a network of cortical regions can compensate for function when the left PMd is disrupted by TMS. In one experiment, without fMRI, the authors showed that after applying 1-Hz repetitive TMS over the left PMd, performance on a response-selection task was disrupted temporarily – but recovered after only 4 min. Equally important, this disruption in behavior did not coincide with reductions in motor excitability of the left M1, which remained suppressed for a considerable amount of time after performance had recovered. Taken together, this suggested to the authors that some sort of adaptive compensation had taken place. Namely, a different region might be taking over function of the left PMd, which they believed was still disrupted as indexed by the suppression in the motor excitability of the left M1. In a different experiment, the authors then used fMRI to measure changes in BOLD after performance had recovered from this TMS-induced disruption. They found changes in BOLD in the right PMd (as well as in other distal brain regions) during the performance of the response-selection task. In a final experiment, without fMRI, O’Shea et al. (2007a) found that delivering TMS over the right PMd by itself did not disrupt response selection but doing exactly the same thing after first disrupting the left PMd did result in deficits. Taken together, the authors concluded from these series of experiments that the observed compensation in performance following TMS-induced disruption of neural processing in the left PMd depended critically on intact neural processing in the right PMd.
Combining TMS and Functional Neuroimaging in Stroke Patients
One of the biggest problems that researchers face in charting recovery in the brain after cerebral damage is how to disentangle compensatory mechanisms from “true” cerebral reorganization (Krakauer, 2007). Patients with brain damage are impaired in behavior and they will perform a task in a way that is, by necessity, different from that performed by neurologically intact controls. This difference in how a task is carried out can lead to differences in brain activation between these two groups of participants that might reflect different neural operations to complete the task – also known as compensatory mechanisms. Not only is this a problem for cross-sectional studies but it is also a problem for longitudinal studies. As patients make a meaningful recovery, by definition, their performance improves and they will perform a task differently after their recovery. It is often unclear whether any resulting differences in brain activation might reflect compensatory mechanisms or cerebral reorganization or both. The question then arises as to how one can examine cerebral reorganization without examining changes in compensation? One solution is to use TMS to examine the integrity of neural circuits independently of behavior. In this section, we present a study that we conducted a few years ago that illustrates how one can combine TMS and functional neuroimaging concurrently to provide a behavior-independent assay of effective connectivity to chart motor recovery after stroke. The point that we wish to highlight is that TMS can offer the opportunity to chart changes in effective connectivity without any task confounds by examining its effects on hemodynamic measurements while patients are not engaged in a task.
In a TMS/PET study, we examined changes in the effective connectivity of M1 in seven patients with capsular strokes who underwent constraint-induced movement therapy (CI therapy; Taub et al., 2002) one year after their stroke (Chouinard et al., 2006). We adopted a TMS/PET paradigm that had previously been used by our research group. In normal volunteers, Paus et al. (1998) applied sub-threshold 10-Hz repetitive TMS over M1 and varied the number of TMS trains delivered during each block of PET scanning. In doing so, the CBF response correlated negatively with the number of stimulus trains delivered both at the site of stimulation and in several distal brain regions known to be connected trans-synaptically in the monkey (Figure 4A). Based on these findings, Paus et al. (1998) speculated that the trains of stimulation resulted in an activation of local inhibitory mechanisms and a subsequent reduction of excitatory synaptic activity in the stimulated region and in an interconnected network. Given the success of this protocol in normal volunteers, we carried out the same procedures before and after the stroke patients had their CI therapy.
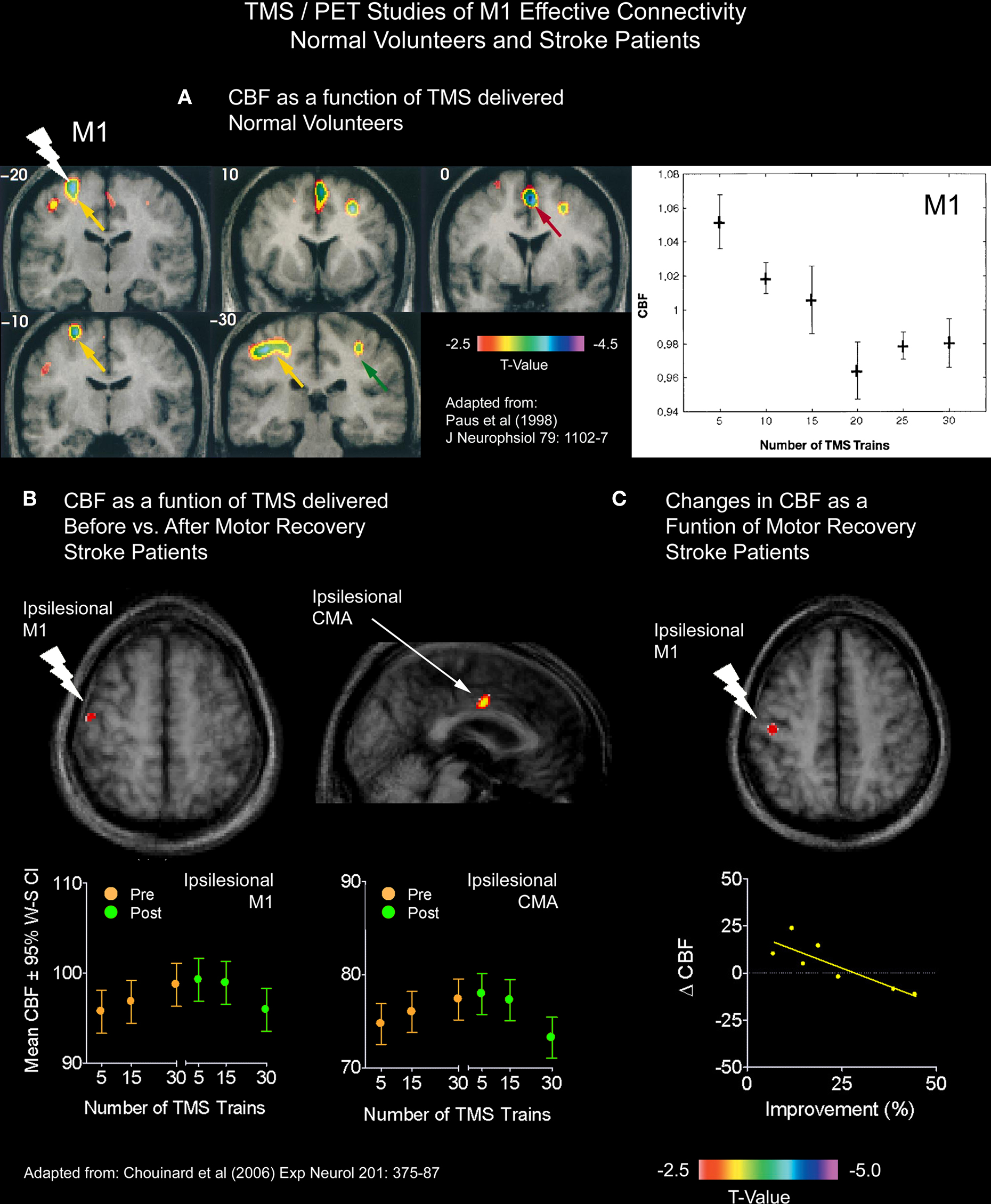
Figure 4. TMS/PET studies of M1 effective connectivity on normal volunteers and stroke patients. (A) Paus et al. (1998) applied sub-threshold 10-Hz repetitive TMS over M1 and varied the number of TMS trains delivered during each block of PET scanning. In doing so, the CBF response correlated negatively with the number of stimulus trains delivered both at the site of stimulation and in several distal brain regions. Given the success of this protocol in normal volunteers, we carried out the same procedures before and after the stroke patients had their CI therapy. (B) In one analysis, we asked whether or not correlations between CBF and TMS trains differed for any brain regions after CI therapy from those seen before CI therapy. This analysis revealed that both the stimulated ipsilesional M1 and a more distal ipsilesional CMA reverted back to the more normal inverse relationship between CBF and TMS trains. (C) In another analysis, we examined the relationship between motor improvement and changes in the CBF response to TMS between the two PET sessions. This analysis revealed an inverse relationship locally in the stimulated ipsilesional M1, which suggests that the observed changes in M1 were adaptive.
We analyzed the CBF data in the patients in two different ways. In the first analysis, we asked whether or not correlations between CBF and TMS trains differed for any brain regions after CI therapy from those seen before CI therapy. This analysis (Figure 4B) revealed that both the stimulated ipsilesional M1 (i.e., M1 in the damaged hemisphere) and a more distal ipsilesional CMA reverted back to the more normal inverse relationship between CBF and TMS trains previously seen in healthy participants (Paus et al., 1998). We speculated that that these findings reflected a strengthening of local inhibitory neurons in the ipsilesional M1, which have been shown to be important for the fractionation or the isolation of proximal and distal muscles (Keller, 1993), as well as a strengthening of connections between the ipsilesional M1 and CMA in the same hemisphere. In the second analysis, we examined the relationship between motor improvement and changes in the CBF response to TMS between the two PET sessions. This analysis revealed an inverse relationship locally in the stimulated ipsilesional M1 (Figure 4C) which suggested to us that the observed changes in M1 were adaptive. This relationship, however, did not reach significance in the ipsilesional CMA – which may relate to a lack of power from having only seven patients.
Also, we should mention that there is an emerging interest in the use of TMS as a therapeutic tool to drive motor recovery after stroke. Not all motor deficits after stroke relate directly to the damaged brain tissue. Abnormal interactions with cortical regions remote from the site of damage can also contribute to motor deficits (for review, see Nowak et al., 2009). There have been reports that capsular strokes can lead to greater trans-callosal inhibition in the ipsilesional M1 originating from the contralesional M1 as revealed by dual-site TMS (Murase et al., 2004). This observation has led some researchers to inquire whether or not TMS-induced down regulation of the contralesional M1 might aid motor recovery after capsular stroke. Nowak et al. (2008) revealed that 10 min of off-line 1-Hz repetitive TMS to the contralesional M1 can lead to motor improvements. The study also found that when the same patients performed a grip task with the affected hand, task performance after the TMS was applied invoked smaller blood oxygenation-level dependent (BOLD) responses in the contralesional motor areas and greater BOLD responses in ipsilesional motor areas as compared with task performance after a sham stimulation was delivered. In a different paper, Grefkes et al. (2010) reanalyzed these data using dynamic causal modeling. The reanalysis revealed a reduction in neuronal “coupling” between the two primary motor areas and an increase in neuronal coupling between the ipsilesional M1 and the ipsilesional non-primary motor areas. In another study, Ameli et al. (2009) demonstrated that an off-line TMS protocol consisting of short bursts of 10-Hz TMS applied to the ipsilesional M1 over a period of several minutes can also lead to motor improvements. The study also revealed that when the same patients performed a grip task with the affected hand, task performance after the TMS was applied invoked smaller BOLD responses in the contralesional motor areas as compared with task performance after a sham stimulation. These studies concluded that TMS can drive motor improvements in patients with capsular strokes via a TMS-induced down regulation of the contralesional M1 and a TMS-induced up regulation of the ipsilesional M1.
Methodological Challenges and Future Directions for Using TMS
More work is needed to demonstrate functional specificity between non-primary motor areas through double dissociations. Although there have been some notable demonstrations (e.g., Davare et al., 2006 showed that PMd plays a role in arm reaching but not in grasping while PMv plays a role in grasping but not in reaching; Chouinard et al., 2005 showed with TMS that when people lift objects, M1 but not PMd plays a role in scaling lifting forces based on information acquired during a previous lift and that PMd but not M1 plays a role in scaling forces based on arbitrary visual cues), many of the TMS studies that we reviewed only stimulated one cortical site. Designing a TMS study aimed at testing for double dissociations is a good idea for a number of other reasons. One of the reasons is that stimulating two different cortical areas can serve as a better control than sham stimulation. Sham stimulation does not control for all peripheral effects associated with the real stimulation. It releases magnetic fields around the head (not in the brain) and it feels noticeably different to participants than real TMS. Another issue to consider is that non-primary motor areas along the medial wall of the brain (SMA and pre-SMA) require higher levels of stimulation than those located on the lateral surface of the brain (PMd and PMv). This is due to the fact that these medial areas are further away from the TMS coil. Thus, it is conceivable that targeting a non-primary motor area on the medial wall could result in concurrent disruption of neural processing in the medial aspect of PMd close to the interhemispheric fissure. One solution to this problem would be to use PMd as a control site (e.g., Rushworth et al., 2002; Kennerley et al., 2004; Mars et al., 2009).
As we mentioned earlier, an important limitation of the dual-site approach is that the physical size of the figure-of-eight coils precludes one to target either the ipsilateral PMd or SMA (proper) with one coil and M1 with the other coil. Moreover, as we discussed in the Introduction, the more caudal portions of the non-primary motor areas exert a much stronger influence on M1 than the more rostral portions of the non-primary motor areas – at least in the monkey (Barbas and Pandya, 1987). It is therefore unclear as to whether or not conditioning TMS in dual-site TMS studies might encroach on more caudal regions of non-primary motor areas, which cannot be targeted directly, or whether or not the influences reported in studies reflect indirect connections between the cortical area that is being stimulated with the conditioning TMS pulse and M1. One way to resolve this issue would be to carry out dual-site TMS concurrently with functional neuroimaging. Also, the dual-site approach only enables one to examine the influence that one cortical area exerts over M1. In contrast, the combined TMS and functional neuroimaging approach enables one to examine the influence that one cortical area exerts on more than one area in the brain.
The sensitivity of TMS could improve. One important avenue for future research would be to compare variability and effect sizes induced by different stimulation protocols, which is somewhat lacking in the TMS literature, and to develop new approaches to TMS that might tap into more subtle forms of neuronal processing. For example, some researchers have started to incorporate principles similar to those underlying fMRI-adaptation as a way to tap into the processing of a small subset of neurons in a stimulated cortical region (Silvanto et al., 2007; Cattaneo et al., 2010). FMRI-adaptation is used in both vision (for review, see Grill-Spector et al., 2006) and motor (Chouinard and Goodale, 2009; Chouinard et al., 2009b) research as a way to tap into the processing of a small subset of neurons. In the case of fMRI, when a brain area contains neurons that code for a particular stimulus or action, the hemodynamic response is higher during conditions in which the stimulus or action changes across trials as compared to conditions in which the stimulus or action remains the same. In a similar way, TMS can be used to disrupt behavioral priming effects to test whether or not a particular brain area contains a subset of neurons that code for a particular stimulus or action. Cattaneo et al. (2010) recently used this approach to demonstrate category-specific neuronal representations in the left PMv in semantic processing for tools but not for animals.
Transcranial magnetic stimulation has two important advantages over functional neuroimaging: TMS offers better temporal resolution and allows one to examine causality. These two strengths can make TMS more suitable for examining effective connectivity than functional neuroimaging alone. The temporal response of the blood supply, which is the basis of fMRI, is much slower than the electrical signals that underlie neuronal communication (Kim et al., 1997). As a result, it is difficult to infer with fMRI how brain areas are interconnected. Although a number of mathematical methods have been put forth to make inferences about connections in fMRI data (e.g., structural equation modeling: McIntosh and Gonzalez-Lima, 1991; dynamic causal modeling: Friston et al., 2003; granger causality: Goebel et al., 2003; graph theory: Reijneveld et al., 2007; Bullmore and Sporns, 2009), results from these types of analyses, because they are based on computing correlations, may reflect relationships between different task components (and/or “third” parties) rather than true connections. Moreover, TBS offers potential to make the combined TMS and functional neuroimaging approach more accessible to laboratories that either do not have access to PET or do not wish to employ TMS inside an MRI scanner. This is because TBS protocols take less time to apply outside of the magnet than other off-line protocols such as in prolonged periods of stimulation at 1-Hz. For these reasons, we foresee an increase in the use of TMS for examining effective connectivity in the brain.
Last, we discussed the use of TMS for charting and driving motor recovery processes after stroke. One of the biggest problems that researchers face in charting recovery in the brain after cerebral damage is how to disentangle compensatory mechanisms from “true” cerebral reorganization (Krakauer, 2007). As we mentioned earlier, one solution would be to use TMS independently of behavior. The same principles that we discussed earlier can be equally applied for charting the progression of a number of brain disorders and the mechanisms of recovery that underlie their treatments. In recent years, we have seen a considerable amount of research devoted to developing TMS as a viable treatment option for stroke (Ridding and Rothwell, 2007; Nowak et al., 2009), movement disorders (Edwards et al., 2008), epilepsy (Kimiskidis, 2010), depression (Ridding and Rothwell, 2007; Kim et al., 2009), schizophrenia (Kim et al., 2009) and a number of other brain disorders (Ridding and Rothwell, 2007). This research has considered a number of ethical and safety issues: how to identify patients that might benefit from TMS treatment, how TMS might interact with concurrent treatments, what stimulation protocols are most effective in treating a particular disorder, what are the cost and benefits to the patients, and a better understanding of the physiological mechanisms that underlie changes in symptoms. Thus, we foresee a rise in the use of TMS in clinical practice.
Closing Remarks
We reviewed the different approaches that one can use with TMS to study both its effects on motor behavior and neural connections in the brain. We also presented the evidence obtained from TMS showing that PMd, PMv, SMA, and pre-SMA each have different roles to play in motor behavior. Namely, we highlighted the importance of PMd in response selection based on arbitrary cues and in the control of arm movements, PMv in grasping and in the discrimination of bodily actions, SMA in movement sequencing and in bimanual coordination, and pre-SMA in cognitive control. We also presented the evidence from dual-site TMS that each of these areas can influence M1. We then went on to show that the combination of TMS and functional neuroimaging can provide opportunities to examine how a non-primary motor area (or any other region that can be stimulated with TMS for that matter) is connected to regions other than M1. We discussed some of the challenges that imagers face when charting “true” cerebral reorganization in the brain and proposed that the use of TMS can help eliminate these problems when used independently of task. We also discussed how TMS can be used as a therapeutic tool to aid motor recovery after stroke. We then ended our review by discussing some of the methodological challenges and future directions for using this tool in basic and clinical neuroscience.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Philippe Chouinard is supported by a post-doctoral award from the Ontario Mental Health Foundation. Tomás? Paus’ work is supported by the Canadian Institutes of Health Research, the National Institutes of Health (United States), and the Sixth Framework Program of the European Union.
References
Ameli, M., Grefkes, C., Kemper, F., Riegg, F. P., Rehme, A. K., Karbe, H., Fink, G. R., and Nowak, D. A. (2009). Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann. Neurol. 66, 298–309. 2.
Barbas, H., and Pandya, D. N. (1987). Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J. Comp. Neurol. 256, 211–228.
Barker, A. T., Jalinous, R., and Freeston, I. L. (1985). Non-invasive magnetic stimulation of human motor cortex. Lancet 1, 1106–1107.
Baumer, T., Bock, F., Koch, G., Lange, R., Rothwell, J. C., Siebner, H. R., and Munchau, A. (2006). Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. J. Physiol. 572, 857–868.
Been, G., Ngo, T. T., Miller, S. M., and Fitzgerald, P. B. (2007). The use of tDCS and CVS as methods of non-invasive brain stimulation. Brain Res. Rev. 56, 346–361.
Bestmann, S. (2008). The physiological basis of transcranial magnetic stimulation. Trends Cogn. Sci. 12, 81–83.
Bestmann, S., Baudewig, J., Siebner, H. R., Rothwell, J. C., and Frahm, J. (2005). BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage 28, 22–29.
Bestmann, S., Swayne, O., Blankenburg, F., Ruff, C. C., Haggard, P., Weiskopf, N., Josephs, O., Driver, J., Rothwell, J. C., and Ward, N. S. (2008). Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb. Cortex 18, 1281–1291.
Bohning, D. E., Shastri, A., Nahas, Z., Lorberbaum, J. P., Andersen, S. W., Dannels, W. R., Haxthausen, E. U., Vincent, D. J., and George, M. S. (1998). Echoplanar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation. Invest. Radiol. 33, 336–340.
Brighina, F., Bisiach, E., Piazza, A., Oliveri, M., La Bua, V., Daniele, O., and Fierro, B. (2002). Perceptual and response bias in visuospatial neglect due to frontal and parietal repetitive transcranial magnetic stimulation in normal subjects. Neuroreport 13, 2571–2575.
Buch, E. R., Mars, R. B., Boorman, E. D., and Rushworth, M. F. (2010). A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J. Neurosci. 30, 1395–1401.
Bullmore, E., and Sporns, O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198.
Busan, P., Barbera, C., Semenic, M., Monti, F., Pizzolato, G., Pelamatti, G., and Battaglini, P. P. (2009). Effect of transcranial magnetic stimulation (TMS) on parietal and premotor cortex during planning of reaching movements. PLoS ONE 4, e4621. doi:10.1371/journal.pone.0004621.
Candidi, M., Urgesi, C., Ionta, S., and Aglioti, S. M. (2008). Virtual lesion of ventral premotor cortex impairs visual perception of biomechanically possible but not impossible actions. Soc. Neurosci. 3, 388–400. 28.
Cattaneo, Z., Devlin, J. T., Salvini, F., Vecchi, T., and Silvanto, J. (2010). The causal role of category-specific neuronal representations in the left ventral premotor cortex (PMv) in semantic processing. Neuroimage 49, 2728–2734.
Chen, C. Y., Muggleton, N. G., Tzeng, O. J., Hung, D. L., and Juan, C. H. (2009). Control of prepotent responses by the superior medial frontal cortex. Neuroimage 44, 537–545.
Chouinard, P. A., and Goodale, M. A. (2009). FMRI adaptation during performance of learned arbitrary visuomotor conditional associations. Neuroimage 48, 696–706.
Chouinard, P. A., Whitwell, R. L., and Goodale, M. A. (2009a). The lateral-occipital and the inferior-frontal cortex play different roles during the naming of visually presented objects. Hum. Brain Mapp. 30, 3851–3864.
Chouinard, P. A., Large, M. E., Chang, E. C., and Goodale, M. A. (2009b). Dissociable neural mechanisms for determining the perceived heaviness of objects and the predicted weight of objects during lifting: an fMRI investigation of the size-weight illusion. Neuroimage 44, 200–212.
Chouinard, P. A., Leonard, G., and Paus, T. (2005). Role of the primary motor and dorsal premotor cortices in the anticipation of forces during object lifting. J. Neurosci. 25, 2277–2284.
Chouinard, P. A., Leonard, G., and Paus, T. (2006). Changes in effective connectivity of the primary motor cortex in stroke patients after rehabilitative therapy. Exp. Neurol. 201, 375–387.
Chouinard, P. A., Morrissey, B. F., Kohler, S., and Goodale, M. A. (2008). Repetition suppression in occipital-temporal visual areas is modulated by physical rather than semantic features of objects. Neuroimage 41, 130–144.
Chouinard, P. A., Van Der Werf, Y. D., Leonard, G., and Paus, T. (2003). Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J. Neurophysiol. 90, 1071–1083.
Civardi, C., Cantello, R., Asselman, P., and Rothwell, J. C. (2001). Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage 14, 1444–1453.
Colby, C. L., and Duhamel, J. R. (1991). Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia 29, 517–537.
Dafotakis, M., Sparing, R., Eickhoff, S. B., Fink, G. R., and Nowak, D. A. (2008). On the role of the ventral premotor cortex and anterior intraparietal area for predictive and reactive scaling of grip force. Brain Res. 1228, 73–80.
Davare, M., Andres, M., Cosnard, G., Thonnard, J. L., and Olivier, E. (2006). Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J. Neurosci. 26, 2260–2268.
Davare, M., Lemon, R., and Olivier, E. (2008). Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J. Physiol. 586, 2735–2742.
Davare, M., Montague, K., Olivier, E., Rothwell, J. C., and Lemon, R. N. (2009). Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex 45, 1050–1057.
Davare, M., Rothwell, J. C., and Lemon, R. N. (2010). Causal connectivity between the human anterior intraparietal area and premotor cortex during grasp. Curr. Biol. 20, 176–181.
Dum, R. P., and Strick, P. L. (1991). The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 11, 667–689.
Edwards, M. J., Talelli, P., and Rothwell, J. C. (2008). Clinical applications of transcranial magnetic stimulation in patients with movement disorders. Lancet Neurol. 7, 827–840.
Ferbert, A., Priori, A., Rothwell, J. C., Day, B. L., Colebatch, J. G., and Marsden, C. D. (1992). Interhemispheric inhibition of the human motor cortex. J. Physiol. 453, 525–546.
Fox, P., Ingham, R., George, M. S., Mayberg, H., Ingham, J., Roby, J., Martin, C., and Jerabek, P. (1997). Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport 8, 2787–2791.
Friston, K. J., Harrison, L., and Penny, W. (2003). Dynamic causal modelling. Neuroimage 19, 1273–1302.
Galletti, C., Fattori, P., Battaglini, P. P., Shipp, S., and Zeki, S. (1996). Functional demarcation of a border between areas V6 and V6A in the superior parietal gyrus of the macaque monkey. Eur. J. Neurosci. 8, 30–52.
Gerloff, C., Corwell, B., Chen, R., Hallett, M., and Cohen, L. G. (1997). Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain 120, 1587–1602.
Giovannelli, F., Borgheresi, A., Balestrieri, F., Ragazzoni, A., Zaccara, G., Cincotta, M., and Ziemann, U. (2006). Role of the right dorsal premotor cortex in “physiological” mirror EMG activity. Exp. Brain Res. 175, 633–640.
Goebel, R., Roebroeck, A., Kim, D. S., and Formisano, E. (2003). Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn. Reson. Imaging 21, 1251–1261.
Goldberg, G. (1985). Supplementary motor area structure and function: review and hypotheses. Behav. Brain. Sci. 8, 567–616.
Grefkes, C., Nowak, D. A., Wang, L. E., Dafotakis, M., Eickhoff, S. B., and Fink, G. R. (2010). Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage 50, 233–242.
Grill-Spector, K., Henson, R., and Martin, A. (2006). Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 10, 14–23.
He, S. Q., Dum, R. P., and Strick, P. L. (1993). Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J. Neurosci. 13, 952–980.
He, S. Q., Dum, R. P., and Strick, P. L. (1995). Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J. Neurosci. 15, 3284–3306.
Herwig, U., Abler, B., Schonfeldt-Lecuona, C., Wunderlich, A., Grothe, J., Spitzer, M., and Walter, H. (2003). Verbal storage in a premotor-parietal network: evidence from fMRI-guided magnetic stimulation. Neuroimage 20, 1032–1041.
Huang, Y. Z., Edwards, M. J., Rounis, E., Bhatia, K. P., and Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron 45, 201–206.
Huang, Y. Z., Rothwell, J. C., Edwards, M. J., and Chen, R. S. (2008). Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb. Cortex 18, 563–570.
Huettel, S., Song, A. W., and McCarthy, G. (2009). Functional Magnetic Resonance Imaging (Sunderland, Mass: Sinauer Associates).
Hughlings Jackson, J. (1958). “On the anatomical and physiological localization of movements in the brain,” in Selected Writings of John Hughlings Jackson, ed. J. Taylor, (New York: Basic Books), 37–76.
Jeannerod, M., Arbib, M. A., Rizzolatti, G., and Sakata, H. (1995). Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 18, 314–320.
Jones, C. R., Rosenkranz, K., Rothwell, J. C., and Jahanshahi, M. (2004). The right dorsolateral prefrontal cortex is essential in time reproduction: an investigation with repetitive transcranial magnetic stimulation. Exp. Brain Res. 158, 366–372.
Kalaska, J. F., Scott, S. H., Cisek, P., and Sergio, L. E. (1997). Cortical control of reaching movements. Curr. Opin. Neurobiol. 7, 849–859.
Kansaku, K., Carver, B., Johnson, A., Matsuda, K., Sadato, N., and Hallett, M. (2007). The role of the human ventral premotor cortex in counting successive stimuli. Exp. Brain Res. 178, 339–350.
Kanwisher, N., Chun, M. M., McDermott, J., and Ledden, P. J. (1996). Functional imaging of human visual recognition. Brain Res. Cogn. Brain Res. 5, 55–67.
Kennerley, S. W., Sakai, K., and Rushworth, M. F. (2004). Organization of action sequences and the role of the pre-SMA. J. Neurophysiol. 91, 978–993.
Kim, D. R., Pesiridou, A., and O’Reardon, J. P. (2009). Transcranial magnetic stimulation in the treatment of psychiatric disorders. Curr. Psychiatry Rep. 11, 447–452.
Kim, S. G., Richter, W., and Ugurbil, K. (1997). Limitations of temporal resolution in functional MRI. Magn. Reson. Med. 37, 631–636.
Kimiskidis, V. K. (2010). Transcranial magnetic stimulation for drug-resistant epilepsies: rationale and clinical experience. Eur. Neurol. 63, 205–210.
Koski, L., Molnar-Szakacs, I., and Iacoboni, M. (2005). Exploring the contributions of premotor and parietal cortex to spatial compatibility using image-guided TMS. Neuroimage 24, 296–305.
Krakauer, J. W. (2007). Avoiding performance and task confounds: multimodal investigation of brain reorganization after stroke rehabilitation. Exp. Neurol. 204, 491–495.
Kujirai, T., Caramia, M. D., Rothwell, J. C., Day, B. L., Thompson, P. D., Ferbert, A., Wroe, S., Asselman, P., and Marsden, C. D. (1993). Corticocortical inhibition in human motor cortex. J. Physiol. 471, 501–519.
Lago, A., Koch, G., Cheeran, B., Marquez, G., Sanchez, J. A., Ezquerro, M., Giraldez, M., and Fernandez-Del-Olmo, M. (2010). Ventral premotor to primary motor cortical interactions during noxious and naturalistic action observation. Neuropsychologia 48, 1802–1806.
Lau, H. C., Rogers, R. D., and Passingham, R. E. (2007). Manipulating the experienced onset of intention after action execution. J. Cogn. Neurosci. 19, 81–90.
Lee, J. H., and van Donkelaar, P. (2006). The human dorsal premotor cortex generates on-line error corrections during sensorimotor adaptation. J. Neurosci. 26, 3330–3334.
Lee, L., Siebner, H. R., Rowe, J. B., Rizzo, V., Rothwell, J. C., Frackowiak, R. S., and Friston, K. J. (2003). Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J. Neurosci. 23, 5308–5318.
Liuzzi, G., Hörniß, V., Hoppe, J., Heise, K., Zimerman, M., Gerloff, C., and Hummel, F. C. (2010). Distinct temporospatial interhemispheric interactions in the human primary and premotor cortex during movement preparation. Cereb. Cortex 20, 1323–1331.
Malach, R., Reppas, J. B., Benson, R. R., Kwong, K. K., Jiang, H., Kennedy, W. A., Ledden, P. J., Brady, T. J., Rosen, B. R., and Tootell, R. B. (1995). Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc. Natl. Acad. Sci. U.S.A. 92, 8135–8139.
Mars, R. B., Klein, M. C., Neubert, F. X., Olivier, E., Buch, E. R., Boorman, E. D., and Rushworth, M. F. (2009). Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict. J. Neurosci. 29, 6926–6931.
Matelli, M., and Luppino, G. (2000). Parietofrontal circuits: parallel channels for sensory-motor integrations. Adv. Neurol. 84, 51–61.
McIntosh, A. R., and Gonzalez-Lima, F. (1991). Structural modeling of functional neural pathways mapped with 2-deoxyglucose: effects of acoustic startle habituation on the auditory system. Brain Res. 547, 295–302.
Meister, I. G., Wilson, S. M., Deblieck, C., Wu, A. D., and Iacoboni, M. (2007). The essential role of premotor cortex in speech perception. Curr. Biol. 17, 1692–1696.
Miniussi, C., Ruzzoli, M., and Walsh, V. (2010). The mechanism of transcranial magnetic stimulation in cognition. Cortex 46, 128–130.
Mochizuki, H., Huang, Y. Z., and Rothwell, J. C. (2004). Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J. Physiol. 561, 331–338.
Mochizuki, H., Franca, M., Huang, Y. Z., and Rothwell, J. C. (2005). The role of dorsal premotor area in reaction task: comparing the “virtual lesion” effect of paired pulse or theta burst transcranial magnetic stimulation. Exp. Brain Res. 167, 414–421.
Murase, N., Duque, J., Mazzocchio, R., and Cohen, L. G. (2004). Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 55, 400–409.
Nowak, D. A., Grefkes, C., Ameli, M., and Fink, G. R. (2009). Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil. Neural. Repair 23, 641–656.
Nowak, D. A., Grefkes, C., Dafotakis, M., Eickhoff, S., Kust, J., Karbe, H., and Fink, G. R. (2008). Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch. Neurol. 65, 741–747.
Obhi, S. S., Haggard, P., Taylor, J., and Pascual-Leone, A. (2002). rTMS to the supplementary motor area disrupts bimanual coordination. Motor. Control. 6, 319–332.
Oliveri, M., Babiloni, C., Filippi, M. M., Caltagirone, C., Babiloni, F., Cicinelli, P., Traversa, R., Palmieri, M. G., and Rossini, P. M. (2003). Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Exp. Brain Res. 149, 214–221.
Op De Beeck, H., and Vogels, R. (2000). Spatial sensitivity of macaque inferior temporal neurons. J. Comp. Neurol. 426, 505–518.
O’Shea, J., Johansen-Berg, H., Trief, D., Gobel, S., and Rushworth, M. F. (2007a). Functionally specific reorganization in human premotor cortex. Neuron 54, 479–490.
O’Shea, J., Sebastian, C., Boorman, E. D., Johansen-Berg, H., and Rushworth, M. F. (2007b). Functional specificity of human premotor-motor cortical interactions during action selection. Eur. J. Neurosci. 26, 2085–2095.
Paus, T. (2005). Inferring causality in brain images: a perturbation approach. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 360, 1109–1114.
Paus, T., Jech, R., Thompson, C. J., Comeau, R., Peters, T., and Evans, A. C. (1997). Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J. Neurosci. 17, 3178–3184.
Paus, T., Jech, R., Thompson, C. J., Comeau, R., Peters, T., and Evans, A. C. (1998). Dose-dependent reduction of cerebral blood flow during rapid-rate transcranial magnetic stimulation of the human sensorimotor cortex. J. Neurophysiol. 79, 1102–1107.
Perez, M. A., Tanaka, S., Wise, S. P., Willingham, D. T., and Cohen, L. G. (2008). Time-specific contribution of the supplementary motor area to intermanual transfer of procedural knowledge. J. Neurosci. 28, 9664–9669.
Picard, N., and Strick, P. L. (1996). Motor areas of the medial wall: a review of their location and functional activation. Cereb. Cortex 6, 342–353.
Picard, N., and Strick, P. L. (2001). Imaging the premotor areas. Curr. Opin. Neurobiol. 11, 663–672.
Pollok, B., Rothkegel, H., Schnitzler, A., Paulus, W., and Lang, N. (2008). The effect of rTMS over left and right dorsolateral premotor cortex on movement timing of either hand. Eur. J. Neurosci. 27, 757–764.
Praamstra, P., Kleine, B. U., and Schnitzler, A. (1999). Magnetic stimulation of the dorsal premotor cortex modulates the Simon effect. Neuroreport 10, 3671–3674.
Reijneveld, J. C., Ponten, S. C., Berendse, H. W., and Stam, C. J. (2007). The application of graph theoretical analysis to complex networks in the brain. Clin Neurophysiol 118, 2317–2331.
Ridding, M. C., and Rothwell, J. C. (2007). Therapeutic use of rTMS. Nat. Rev. Neurosci. 8, 559–567.
Roland, P. E., Larsen, B., Lassen, N. A., and Skinhoj, E. (1980). Supplementary motor area and other cortical areas in organization of voluntary movements in man. J. Neurophysiol. 43, 118–136.
Rossi, S., Hallett, M., Rossini, P. M., and Pascual-Leone, A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120, 2008–2039.
Rushworth, M. F., Hadland, K. A., Paus, T., and Sipila, P. K. (2002). Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J. Neurophysiol. 87, 2577–2592.
Sack, A. T., Cohen Kadosh, R., Schuhmann, T., Moerel, M., Walsh, V., and Goebel, R. (2009). Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J. Cogn. Neurosci. 21, 207–221.
Sato, M., Tremblay, P., and Gracco, V. L. (2009). A mediating role of the premotor cortex in phoneme segmentation. Brain Lang. 111, 1–7.
Schlaghecken, F., Munchau, A., Bloem, B. R., Rothwell, J., and Eimer, M. (2003). Slow frequency repetitive transcranial magnetic stimulation affects reaction times, but not priming effects, in a masked prime task. Clin. Neurophysiol. 114, 1272–1277.
Schluter, N. D., Rushworth, M. F., Mills, K. R., and Passingham, R. E. (1999). Signal-, set-, and movement-related activity in the human premotor cortex. Neuropsychologia 37, 233–243.
Schluter, N. D., Rushworth, M. F., Passingham, R. E., and Mills, K. R. (1998). Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain 121, 785–799.
Serrien, D. J., Strens, L. H., Oliviero, A., and Brown, P. (2002). Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) degrades bimanual movement control in humans. Neurosci. Lett. 328, 89–92.
Siebner, H. R., Filipovic, S. R., Rowe, J. B., Cordivari, C., Gerschlager, W., Rothwell, J. C., Frackowiak, R. S., and Bhatia, K. P. (2003). Patients with focal arm dystonia have increased sensitivity to slow-frequency repetitive TMS of the dorsal premotor cortex. Brain 126, 2710–2725.
Siebner, H. R., Hartwigsen, G., Kassuba, T., and Rothwell, J. C. (2009). How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex 45, 1035–1042.
Siebner, H. R., Willoch, F., Peller, M., Auer, C., Boecker, H., Conrad, B., and Bartenstein, P. (1998). Imaging brain activation induced by long trains of repetitive transcranial magnetic stimulation. Neuroreport 9, 943–948.
Silvanto, J., Muggleton, N. G., Cowey, A., and Walsh, V. (2007). Neural adaptation reveals state-dependent effects of transcranial magnetic stimulation. Eur. J. Neurosci. 25, 1874–1881.
Steyvers, M., Etoh, S., Sauner, D., Levin, O., Siebner, H. R., Swinnen, S. P., and Rothwell, J. C. (2003). High-frequency transcranial magnetic stimulation of the supplementary motor area reduces bimanual coupling during anti-phase but not in-phase movements. Exp. Brain Res. 151, 309–317.
Tanaka, S., Honda, M., and Sadato, N. (2005). Modality-specific cognitive function of medial and lateral human Brodmann area 6. J. Neurosci. 25, 496–501.
Taub, E., Uswatte, G., and Elbert, T. (2002). New treatments in neurorehabilitation founded on basic research. Nat. Rev. Neurosci. 3, 228–236.
Taubert, M., Dafotakis, M., Sparing, R., Eickhoff, S., Leuchte, S., Fink, G. R., and Nowak, D. A. (2010). Inhibition of the anterior intraparietal area and the dorsal premotor cortex interfere with arbitrary visuo-motor mapping. Clin. Neurophysiol. 121, 408–413.
Taylor, P. C., Nobre, A. C., and Rushworth, M. F. (2007). Subsecond changes in top down control exerted by human medial frontal cortex during conflict and action selection: a combined transcranial magnetic stimulation electroencephalography study. J. Neurosci. 27, 11343–11353.
Thielscher, A., and Kammer, T. (2004). Electric field properties of two commercial figure-8 coils in TMS: calculation of focality and efficiency. Clin. Neurophysiol. 115, 1697–1708.
Tremblay, P., and Gracco, V. L. (2009). Contribution of the pre-SMA to the production of words and non-speech oral motor gestures, as revealed by repetitive transcranial magnetic stimulation (rTMS). Brain Res. 1268, 112–124.
Tunik, E., Lo, O. Y., and Adamovich, S. V. (2008). Transcranial magnetic stimulation to the frontal operculum and supramarginal gyrus disrupts planning of outcome-based hand-object interactions. J. Neurosci. 28, 14422–14427.
Umilta, M.A., Kohler, E., Gallese, V., Fogassi, L., Fadiga, L., Keysers, C., and Rizzolatti, G. (2001). I know what you are doing a neurophysiological study. Neuron. 31, 155–165.
Urgesi, C., Calvo-Merino, B., Haggard, P., and Aglioti, S. M. (2007a). Transcranial magnetic stimulation reveals two cortical pathways for visual body processing. J. Neurosci. 27, 8023–8030.
Urgesi, C., Candidi, M., Ionta, S., and Aglioti, S. M. (2007b). Representation of body identity and body actions in extrastriate body area and ventral premotor cortex. Nat. Neurosci. 10, 30–31.
van den Berg, F. E., Swinnen, S. P., and Wenderoth, N. (in press). Hemispheric asymmetries of the premotor cortex are task specific as revealed by disruptive TMS during bimanual versus unimanual movements. Cereb. Cortex. doi:10.1093/cercor/bhq034.
van Donkelaar, P., Lee, J. H., and Drew, A. S. (2002). Eye-hand interactions differ in the human premotor and parietal cortices. Hum. Mov. Sci. 21, 377–386.
Verwey, W. B. (1996). Buffer loading and chunking in sequential keypressing. J. Exp. Psychol. Hum. Percept. Perform. 22, 544–562.
Verwey, W. B. (1999). Evidence for a multistage model of practice in a sequential movement task. J. Exp. Psychol. Hum. Percept Perform 25, 1–16.
Keywords: transcranial magnetic stimulation, motor system, functional connectivity, effective connectivity, premotor area, supplementary motor area, stroke recovery, functional neuroimaging
Citation: Chouinard PA and Paus T (2010) What have we learned from “perturbing” the human cortical motor system with transcranial magnetic stimulation? Front. Hum. Neurosci. 4:173. doi:10.3389/fnhum.2010.00173.
Received: 25 May 2010;
Paper pending published: 14 June 2010;
Accepted: 13 August 2010;
Published online: 19 October 2010.
Edited by:
Michael X. Cohen, University of Amsterdam, NetherlandsReviewed by:
Hartwige Siebner, Danish Research Centre for Magnetic Resonance, DenmarkKatsuyuki Sakai, The University of Tokyo, Japan
Copyright: © 2010 Chouinard and Paus. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Philippe A. Chouinard, Centre for Brain and Mind, Department of Psychology, University of Western Ontario, 1151 Richmond Street, London, ON N6A 5C2, Canada. e-mail: pchouin@uwo.ca