Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding
- 1 National Center for PTSD, VA Boston Healthcare System, Boston, MA, USA
- 2 Department of Psychiatry, Boston University School of Medicine, Boston, MA, USA
- 3 Duke-UNC Brain Imaging and Analysis Center, Duke University, Durham, NC, USA
- 4 Mental Illness Research Education and Clinical Center, Durham VA Medical Center, Durham, NC, USA
- 5 Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham, NC, USA
- 6 Center for Cognitive Neuroscience, Duke University, Durham, NC, USA
- 7 Department of Psychology, Yale University, New Haven, CT, USA
During times of emotional stress, individuals often engage in emotion regulation to reduce the experiential and physiological impact of negative emotions. Interestingly, emotion regulation strategies also influence memory encoding of the event. Cognitive reappraisal is associated with enhanced memory while expressive suppression is associated with impaired explicit memory of the emotional event. However, the mechanism by which these emotion regulation strategies affect memory is unclear. We used event-related fMRI to investigate the neural mechanisms that give rise to memory formation during emotion regulation. Twenty-five participants viewed negative pictures while alternately engaging in cognitive reappraisal, expressive suppression, or passive viewing. As part of the subsequent memory design, participants returned to the laboratory two weeks later for a surprise memory test. Behavioral results showed a reduction in negative affect and a retention advantage for reappraised stimuli relative to the other conditions. Imaging results showed that successful encoding during reappraisal was uniquely associated with greater co-activation of the left inferior frontal gyrus, amygdala, and hippocampus, suggesting a possible role for elaborative encoding of negative memories. This study provides neurobehavioral evidence that engaging in cognitive reappraisal is advantageous to both affective and mnemonic processes.
Introduction
When faced with a distressing situation, individuals often attempt to assess and then regulate their resulting negative emotions. By doing so, individuals not only change their emotional reaction to the negative event, but also alter how the event is encoded in memory. The processes involved in negative memory encoding and retrieval are thought to play a major role in the development and maintenance of certain psychiatric disorders, such as depression and posttraumatic stress disorder (Watkins et al., 1992; Brewin, 2001; Hamilton and Gotlib, 2008). Consequently, investigating how regulatory strategies alter information encoding has the potential to inform our understanding of the etiology and clinical treatment of these disorders.
Recent behavioral studies have shown that regulation techniques have differential consequences for long-term memory. For instance, engaging in cognitive reappraisal enhances explicit memory performance (Richards and Gross, 2000; Richards et al., 2003; Dillon et al., 2007). By employing cognitive reappraisal, one can either decrease or increase the negative associations of a stimulus by changing its meaning or self-relevance. As an example, negative affect for a picture of a horrific car crash may be reduced by imagining that the passengers were not seriously injured. By contrast, several studies have shown that expressive suppression impairs explicit memory (Richards and Gross, 1999, 2000, 2006; Richards et al., 2003; Bonanno et al., 2004; Dillon et al., 2007). During suppression, individuals refrain from displaying emotional expressions so that an outside observer would be unable to discern their internal emotional state. In addition to being associated with diminished memory performance, suppression is generally inferior to reappraisal in reducing negative affect after viewing negative film clips (Goldin et al., 2008) and negative pictures (Richards and Gross, 2000), and prior to broaching a relationship conflict with a romantic partner (Richards et al., 2003). Taken together, the available evidence suggests that engaging in cognitive reappraisal may actually be beneficial for memory even in the throes of emotional distress, whereas suppression may lead to the formation of incomplete representations of the negative event.
The observation that cognitive reappraisal is associated with enhanced memory and reduced arousal presents a dilemma for neurobiological theories of emotional memory. The memory modulation hypothesis (McGaugh, 2004) states that the amygdala is critical for modulating hippocampal-dependent consolidation processes as a function of arousal, a finding that has received substantial support across species and neuroscientific methods (Cahill and McGaugh, 1998; LaBar and Cabeza, 2006). However, human experience of emotion is unequivocally complex. Individuals typically do not simply watch negative experiences unfold without attempting to manage their negative emotions. Studies have shown that when employing cognitive reappraisal to decrease negative affect, amygdala activity and self-reported affect are reduced relative to passive viewing conditions (Ochsner et al., 2002; Ochsner, 2004; Dillon et al., 2007). Therefore, it is currently unknown how neural activity in the medial temporal lobe (MTL) and other regions could predict encoding success despite a reduction in negative arousal.
We have previously proposed that the memory retention advantage during cognitive reappraisal is related to deep stimulus elaboration (Dillon et al., 2007). In our view, reappraisal encourages meaningful analysis of the negative stimulus, promoting deep and elaborative semantic encoding. We further suggest that during suppression, the focus on inhibition of emotional expression directs attention away from stimulus elaboration, resulting in poorer memory performance. The idea that memory encoding is enhanced for items that are meaningful is known as the levels-of-processing effect (Craik and Lockhart, 1972). Although previous neuroimaging studies of cognitive reappraisal have not explicitly investigated memory effects, results have consistently shown recruitment of a network of prefrontal regions thought to subserve elaborative memory, including the left inferior frontal gyrus (LIFG) and medial prefrontal cortex (Ochsner and Gross, 2005). The LIFG might be a likely candidate to support the memory enhancement effect during reappraisal because it is reliably activated during deep encoding of subsequently remembered stimuli (Otten et al., 2001; Fletcher et al., 2003) and is involved in the cognitive control of memory, including lexical–semantic processing (Gabrieli et al. 1998) and working memory (D’Esposito et al., 1999).
The purpose of the present study was to investigate the neural correlates of memory encoding under different regulation strategies. To elucidate the role of the prefrontal cortex and MTL regions in successful memory encoding during emotion regulation attempts, we compared co-activation patterns of the LIFG, amygdala, and hippocampus in an experimental design that combined emotion regulation instructions with a subsequent memory paradigm (Paller and Wagner, 2002). Participants underwent event-related fMRI while viewing negative scenes and engaging in one of three regulation strategies: “view,” “reappraise,” or “suppress” (Figure 1). At the end of each trial, participants made a valence and confidence judgment regarding their success in engaging in each strategy. Participants returned two weeks later for a surprise recognition memory test. Study items were partitioned into events that were later remembered or forgotten. Greater brain activity for subsequently remembered than forgotten items is known as the difference due to memory effect (Dm effect) and is thought to reflect successful encoding processes (Paller and Wagner, 2002). In support of the levels-of-processing hypothesis, we predicted that subsequent memory during reappraisal would be associated with greater LIFG–hippocampal coupling relative to the other conditions. To test this hypothesis, we performed a correlation analysis that examined memory success-related co-activation of these regions for each condition (cognitive reappraisal, suppression, negative view). Previously, we used this approach to show that the memory advantage for emotional information over neutral information is subserved by greater amygdala–MTL correlations for emotional Dm (Dolcos et al., 2004). Therefore, we predicted that the LIFG and hippocampus should be more positively correlated when individuals engaged in cognitive reappraisal than during suppression or negative viewing that reflects a deeper level of encoding.
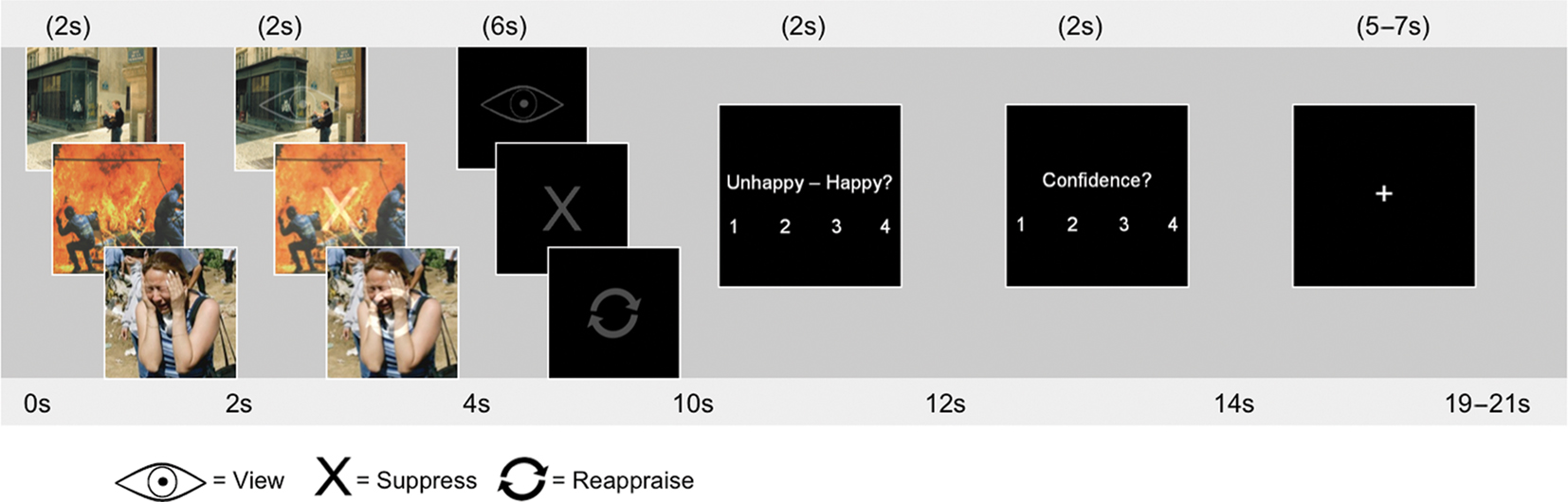
Figure 1. Study protocol. Subjects viewed pictures for 2 s, after which the regulation instruction was superimposed on the picture with 50% transparency for 2 s. Participants viewed the regulation strategy only for an additional 6 s. They were instructed to make a valence and confidence judgment regarding their success in implementing the strategy correctly. The symbols used were an eye for “view,” a refresh sign for “reappraise,” and an X for “suppress.”
Materials and Methods
Participants
Twenty-five participants (11 female) between the ages of 18 and 27 (average age ± SD: 21.6 ± 2.5; 15 Caucasian, 5 Asian, 3 Black, 2 Other/Multiracial, 23 right-handed, 2 left-handed) were recruited from a research recruitment database of college and community participants. All participants provided written informed consent for procedures approved by the Institutional Review Board of Duke University Medical Center.
Materials
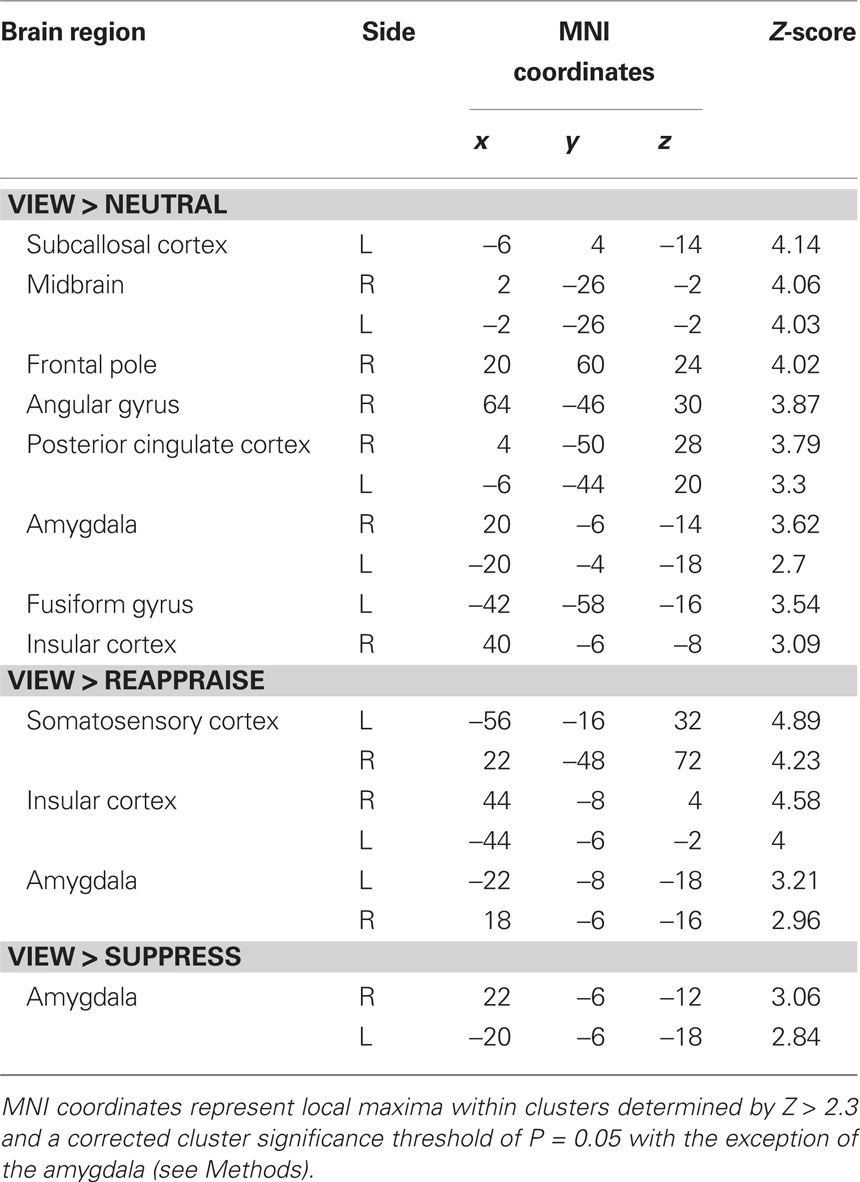
Stimuli consisted of 120 high-arousing negatively valenced and 40 neutral pictures selected from the International Affective Picture Set (IAPS; Lang et al., 1997) and supplemented with in-house pictures that had normative data from a separate group of healthy individuals. The negative pictures were partitioned into 40 each for the negative view, reappraise, and suppress conditions while the 40 neutral pictures were selected for the neutral condition. Negative pictures across the three conditions (view, reappraise, suppress) were equated for valence [F(2,78) = 0.31, P > 0.73] and arousal [F(2,78) = 0.610, P > 0.54]. Negative pictures were more negatively valenced and higher in arousal than neutral pictures (based on ratings on a 1–9 point scale). The average (±SD) valence ratings were: neutral = 5.08 ± 0.36; view = 2.5 ± 0.65; reappraise 2.5 ± 0.67; suppress = 2.6 ± 0.64. Average arousal ratings were: neutral = 3.6 ± 0.52; view = 5.88 ± 0.81; reappraise = 5.93 ± 0.80; suppress = 5.81 ± 0.70. Pictures were also equated for social content (i.e., presence of human faces) and the amount of red color, as differences in pictures in these dimensions may influence emotional experience and therefore neural signal (Kaya and Epps, 2004; Hill and Barton, 2005; Elliot and Maier, 2007).
Procedure
Prior to engaging in the fMRI task, participants were trained in each regulation strategy. For the view and neutral trials, participants were instructed to “simply look at the picture and let any emotions you’re feeling unfold naturally.” For suppress trials, participants were instructed to “not let any emotion you are feeling show on your face.” For reappraise trials, participants were instructed to “place yourself as an observer in the scene, but change the way you think about it by making it not relevant to you or your loved ones.” These instructions, which were previously used in our laboratory (Dillon et al., 2007), were explained in greater detail, and several examples were provided. When participants reported understanding the difference between the strategies, they performed a practice task in which they viewed negative and neutral pictures on a desktop computer and rehearsed each strategy while articulating its implementation aloud. The experimenter answered questions and corrected participants if necessary. Participants then practiced the task as they would in the fMRI environment, without verbalizing their strategy and while making valence and confidence ratings.
The stimulus set of 160 pictures was divided into 10 encoding runs. To avoid the induction of long-lasting mood states, the pictures within each run were pseudo-randomized so that no more than three negative pictures were consecutively presented. Instruction cues were randomized. Each trial began with a 2-s presentation of a picture, which was followed by an instruction symbol overlaid on the picture with 50% transparency for 2 s (see Figure 1). The picture then disappeared while the instruction symbol remained for an additional 6 s. This overlapping sequence was used to unconfound instructional cue interpretation from the regulation period. Subjects made a valence rating on a four-point rating scale (1 = unhappy, 4 = happy), indicating their own emotional response to the picture, and a confidence rating regarding how well they were able to engage in the strategy (1 = not confident, 4 = very confident). Participants had 2 s to make each of these judgments. A fixation cross (jittered 5–7 s) followed for a total trial length of 19–21 s.
Participants returned 2 weeks after the encoding session for a surprise memory test (incidental encoding). Participants viewed the original 160 pictures along with 100 novel foils (60 negative, 40 neutral). The pictures were presented in black and white to increase task difficulty (as based on our previous pilot data). Participants made an “old” or “new” judgment and a confidence rating on a 4-point scale.
Magnetic Resonance Imaging Acquisition
Functional images were acquired on a 4-T GE scanner with an inverse spiral pulse sequence to reduce susceptibility artifact (Guo and Song, 2003). A series of 34 interleaved axial functional slices were acquired for full-brain coverage with the following parameters: TR = 2000 ms; TE = 31 ms; FOV = 240 mm2; matrix size = 642; slice thickness = 3.75 mm × 3.75 mm × 3.8 mm voxels. High-resolution three-dimensional spin-echo co-planar structural images were acquired in 68 axial slices (TR = 12 ms; TE = 5.4 ms; FOV = 240 mm2; matrix size = 2562; slice thickness = 1 mm × 1 mm × 1.9 mm voxels).
fMRI Data Analysis
Functional data sets were analyzed using FSL version 4.1.0. Preprocessing was applied to individual subjects’ data in the following steps: (i) brain extraction for non-brain removal (Smith, 2002), (ii) motion correction using MCFLIRT (Jenkinson et al., 2002), (iii) spatial smoothing using a Gaussian kernel of FWHM 5 mm, and (iv) high-pass filtering (Jenkinson et al., 2002; Smith et al., 2004). Functional images of each subject were co-registered to structural images in native space, and structural images were normalized to MNI space. The same transformation matrices were then used for functional-to-standard space transformations. Pre-whitening was estimated and corrected using FMRIB’s Improved Linear Model (FILM; Woolrich et al., 2001). Onset times of events, which corresponded to the onset of each regulation instruction, were used to model a signal response containing a regressor for each condition (i.e., hits and misses for each condition of neutral, view, reappraise, and suppress) and convolved with a gamma function. Parameter estimates from individual fMRI runs were fed into a second-level statistical analysis. Average maps representative of the general population were calculated in a mixed effects higher level analysis using Bayesian estimation techniques, FMRIB Local Analysis of Mixed Effects (FLAME; Beckmann et al., 2003; Woolrich et al., 2004) with cluster mean threshold of Z > 2.3 and a cluster-corrected significance threshold of P < 0.05 to protect against false positive detection of activation clusters (Worsley, 2001). Due to our a priori interest in amygdala activity, we applied an amygdala mask to extract parameter estimates from each individual in normalized space. Monte Carlo permutation (5000 permutations) provided a distribution for an (near) exact test statistic to compute a significance threshold of P = 0.05. Thus no assumptions are made about the distribution (e.g., Guassianity) under the null hypothesis. The outcome of this test statistic was subjected to threshold free cluster enhancement to control the family wise error (Smith and Nichols, 2009).
To identify co-activation between regions-of-interest (ROIs), functional ROIs were defined from the main effect of memory in the omnibus ANOVA which compared hits to misses across all trials presented in the experiment. Note that this criterion is statistically independent of the emotion regulation × memory × ROI interactions used in the connectivity analyses. Hits were defined as items that were subsequently remembered with high confidence (rating of 3 or 4 on a 4-point confidence scale). Only high confidence hits were used because previous work in our laboratory suggests that memory for low confidence items is as chance, and therefore, may not reflect true successful memory. All misses were included in the analysis, across confidence ratings. Across the negative conditions, there were on average 17 hit trials (SD = 1) and 14 miss trials (SD = 1). For the neutral condition, there were on average 14 hit trials (SD = 14) and 17 miss trials (SD = 17). Given our strong a priori interest in examining specific PFC and MTL regions related to our hypotheses, ROIs were identified that survived a voxelwise threshold of P < 0.01 and a cluster extent threshold corrected for multiple comparisons to P < 0.05 identified by Monte Carlo simulations (Slotnick and Schacter, 2004). This procedure relies on the notion that the probability of observing clusters of activity due to noise decreases with increasing cluster size. The simulation consisted of 1000 independent iterations where brain volume was modeled using a 64 × 64 × 34 matrix and assuming a type 1 error voxel activation probability of 0.01. The simulations resulted in a cluster extent threshold of 14 resampled voxels (112 mm3). The prefrontal and MTL regions surviving this threshold were selected for the ROI analyses and included the amygdala, hippocampus, an anterior, and posterior region of the left IFG, superior frontal gyrus, frontal pole, and medial cortex. Regions were not spatially overlapping or contiguous. Percent signal change was extracted from each ROI using custom scripts. For each individual, fMRI signal was averaged as a function of condition type (i.e., reappraise hits, reappraise misses, suppress hits, suppress misses, view hits, and view misses) and 1 pre-stimulus and 10 post-stimulus time points. Percent signal change at peak time points 8–10 s were averaged for hits and misses separately for each ROI and experimental condition, and subtracted to obtain the Dm estimate. Correlation analyses utilized the Dm estimate for each condition in ROIs. For example, to calculate the correlation between memory success activity in the amygdala and hippocampus during cognitive reappraisal, we extracted percent signal change for reappraise hits and reappraise misses in the amygdala and hippocampus separately for each individual subject. Percent signal change for reappraise misses was subtracted from the signal for reappraise hits to obtain the Dm estimate for each individual. The reappraisal Dm estimate in the amygdala for each individual was then correlated with their reappraisal Dm estimate in the hippocampus. This approach provides information regarding regions that covary as a function of memory success.
Results
Effect of Emotion and its Regulation
Behavioral valence and regulation success ratings
We first examined the effect of emotion regulation on valence ratings (Figure 2A). Repeated-measures ANOVA for condition type (reappraisal, suppression, view, and neutral) revealed a highly significant effect of condition [F(3,72) = 104.16, P < 0.0001]. Post hoc tests confirmed that participants had lower valence ratings during each of the negative picture conditions than the neutral condition (P’s < 0.0001). As expected, participants reported higher valence ratings when instructed to reappraise a negative picture than when instructed to suppress or view a negative picture (P’s < 0.02). Furthermore, participants had higher valence ratings when instructed to suppress a negative picture than simply view a negative picture (P < 0.001). These results indicate that emotional distress for negative picture viewing was reduced when participants engaged in emotion regulation, with greater reduction during reappraisal than suppression.
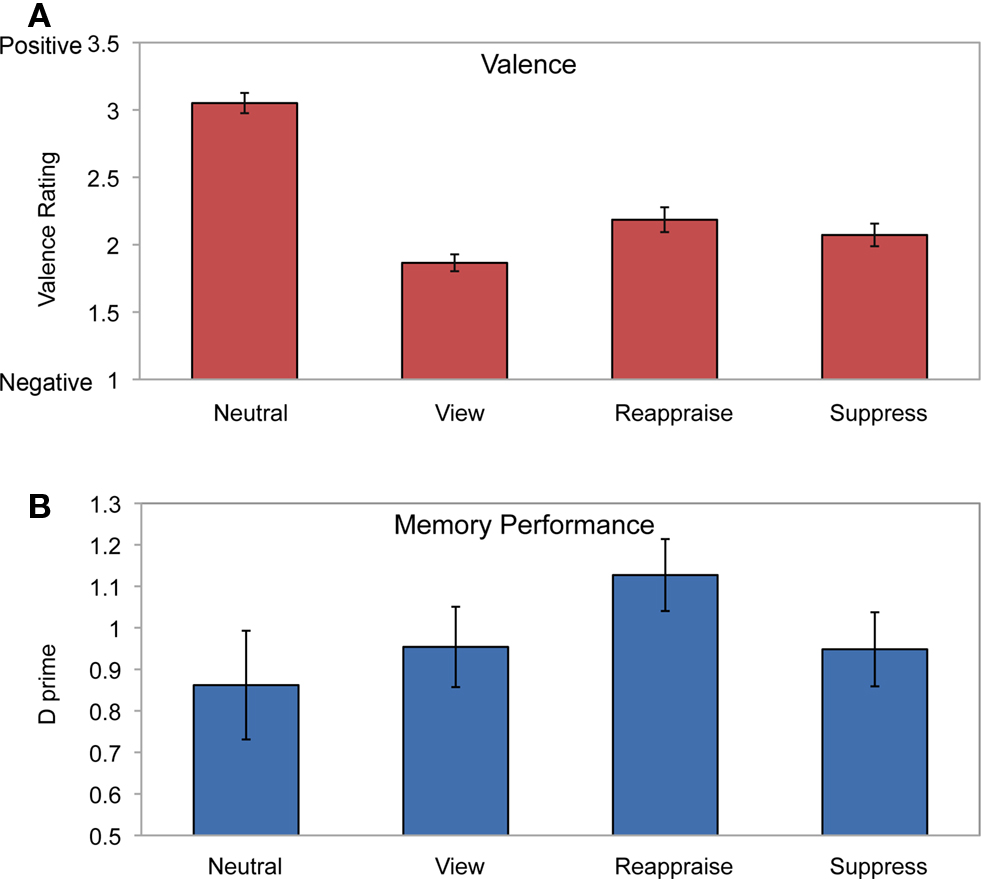
Figure 2. Behavioral results. (A) Valence ratings for each condition. Higher numbers on the scale indicate more positive valence ratings. Participants had the highest valence ratings for the neutral condition. Within negative picture viewing, participants had significantly higher valence ratings after reappraising pictures relative to suppressing or viewing. (B) Subsequent memory performance showed greatest retention for reappraised pictures than the other conditions. Error bars represent standard error of the mean.
To examine whether participants showed a reduction in negative valence as a function of successful use of each strategy, we compared valence ratings for unsuccessful attempts in executing the strategy (combined across confidence ratings 1 and 2) to successful attempts (combined across confidence ratings 3 and 4). Valence significantly improved when participants were successful in reappraising a picture [t(23) = 2.35, P < 0.03] but not after successfully engaging in suppression [t(23) = 1.53, P > 0.13] or after successfully viewing negative pictures [t(21) = 0.73, P > 0.47]. These results support prior research indicating that cognitive reappraisal is a more effective emotion regulation strategy than expressive suppression (Richards and Gross, 2000; Goldin et al., 2008).
Neural systems underlying negative emotion and its regulation
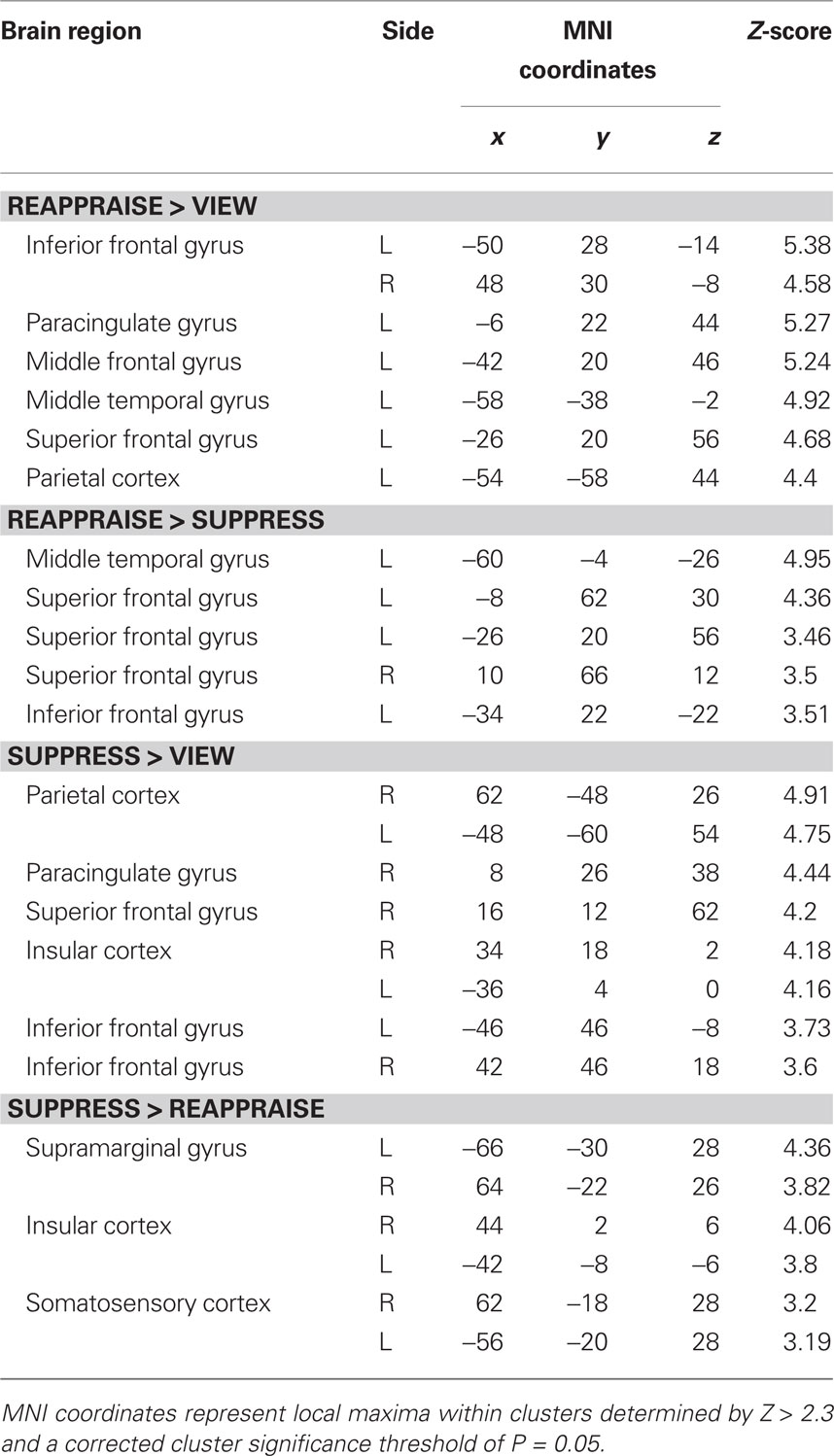
Whole brain voxel analyses showed that viewing negative pictures without engaging in a regulation strategy (versus neutral pictures) activated several regions including bilateral amygdala, insular cortex, subcallosal cortex, and bilateral posterior cingulate cortex (Table 1). The contrast of view > reappraise revealed activity in bilateral insula and amygdala (Figure 3A; Table 1). The contrast of view > suppress also revealed bilateral activation in the amygdala (Figure 3B; Table 1). Taken together, these results suggest that viewing negative pictures activated a network of regions implicated in emotion, and that engaging in emotion regulation was effective in reducing amygdala activity.
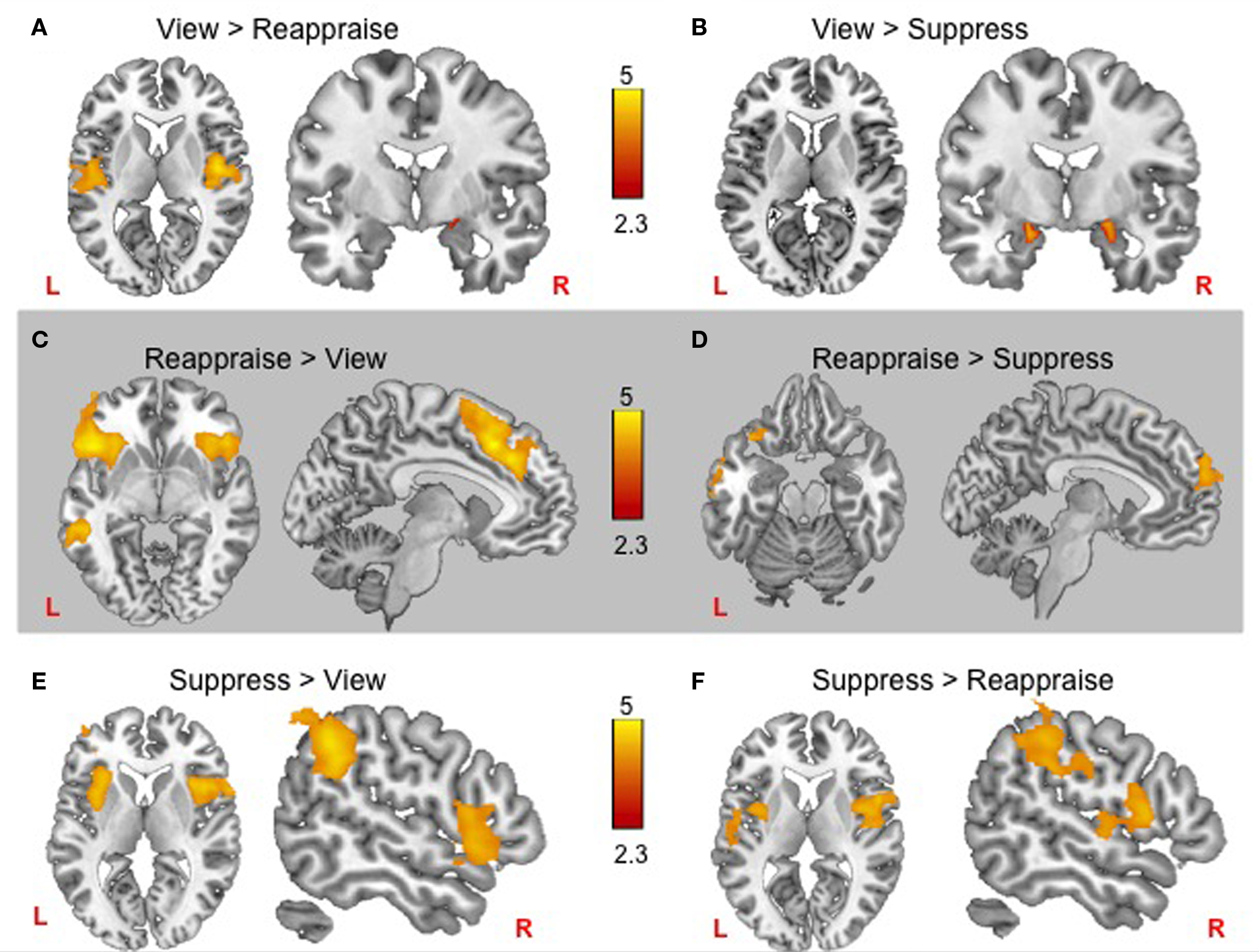
Figure 3. fMRI results for each negative emotion regulation condition. Top row: Passively viewing negative pictures yielded more bilateral amygdala activity than either reappraise (A) or suppress (B), and greater bilateral insula activity than reappraise (A). Middle row: Frontal cortex increases during reappraisal included (C) greater bilateral inferior frontal gyrus and paracingulate gyrus activity than passive viewing, and (D) greater left inferior frontal gyrus and frontal polar responses than during suppression. Bottom row: Suppression engaged bilateral insula relative to both passive viewing and reappraisal, with additional supramarginal gyrus recruitment relative to passive viewing (E) and somatosensory cortex recruitment relative to reappraisal (F).
We next compared regions involved in cognitive reappraisal and suppression. In comparison to the view condition, reappraisal activated a network of prefrontal cortex activity including bilateral inferior prefrontal cortex and paracingulate gyrus (Figure 3C; Table 2). The comparison of reappraise > suppress revealed activity in several prefrontal cortex regions including the LIFG and frontal pole (Figure 3D; Table 2). By contrast, suppressing facial expressions (relative to passive viewing) engaged bilateral insular cortex, supramarginal gyrus, and middle frontal gyrus (Figure 3E; Table 2). The contrast of suppress > reappraise revealed activation in bilateral insula and supramarginal gyrus (Figure 3F; Table 2). There was no amygdala activity in the reappraise or suppress contrasts. These results demonstrate greater activity in prefrontal cortex during reappraisal than suppression. Although both reappraisal and suppression were effective in reducing amygdala activity, reappraisal also reduced insular activity, which is associated with representation of internal bodily states including those related to negative affect (Craig, 2009).
Effect of Emotion Regulation on Memory Systems
Memory performance
D prime (d′) was computed as a measure of memory performance for each condition (Figure 2B). Repeated-measures ANOVA revealed a significant main effect of emotion regulation on memory [F(3,72) = 3.09, P < 0.05]. As expected, memory performance was greater for the reappraise condition than the suppress (P < 0.009), view (P < 0.02), and neutral (P < 0.04) conditions. These results are consistent with previous behavioral findings of explicit memory enhancement for reappraisal relative to suppression (Dillon et al., 2007).
Emotion regulation effect on memory
The Dm effect was calculated for each condition and ROI (see Materials and Methods). Correlational analyses were then performed across subjects to examine co-activation patterns between the hippocampus and other areas, including the amygdala and prefrontal cortical regions that index successful memory encoding. In support of previous studies of emotional memory (Dolcos et al., 2004), a strong correlation between the amygdala Dm and hippocampus Dm was observed for the view condition [r(22) = 0.75, P < 0.0001]. A strong correlation between the Dm of these regions was also observed for reappraise [r(23) = 0.89, P < 0.00001], but not for suppress [r(22) = 0.26, P > 0.2]. Fisher Z-tests confirmed that the amygdala–hippocampal Dm correlation values were significantly higher for view than suppress (P < 0.03) and reappraise than suppress (P < 0.001), but not for reappraise and view (P = 0.16; Figure 4A).
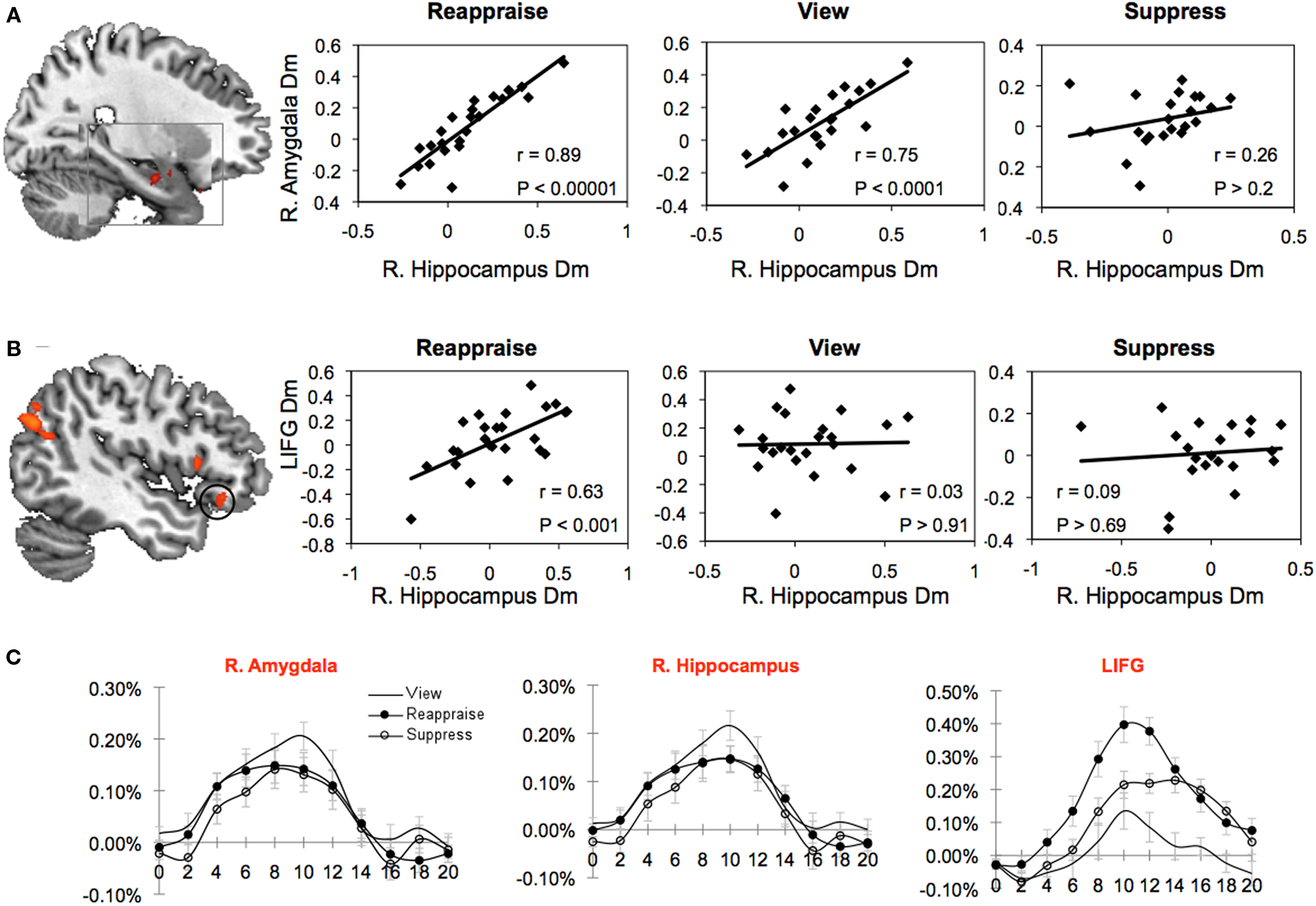
Figure 4. Subsequent memory due to memory (Dm) correlations between prefrontal and medial temporal lobe regions-of-interest (ROI) as a function of regulation strategy. (A) Stronger right amygdala–hippocampal correlation for the reappraise and view conditions than for suppress. (B) Stronger LIFG–right hippocampal correlation for the reappraise condition than for view and suppress. (C) Percent signal changes for each condition and ROI, collapsed across memory. R = right hemisphere, Dm = difference due to memory, LIFG = left inferior frontal gyrus.
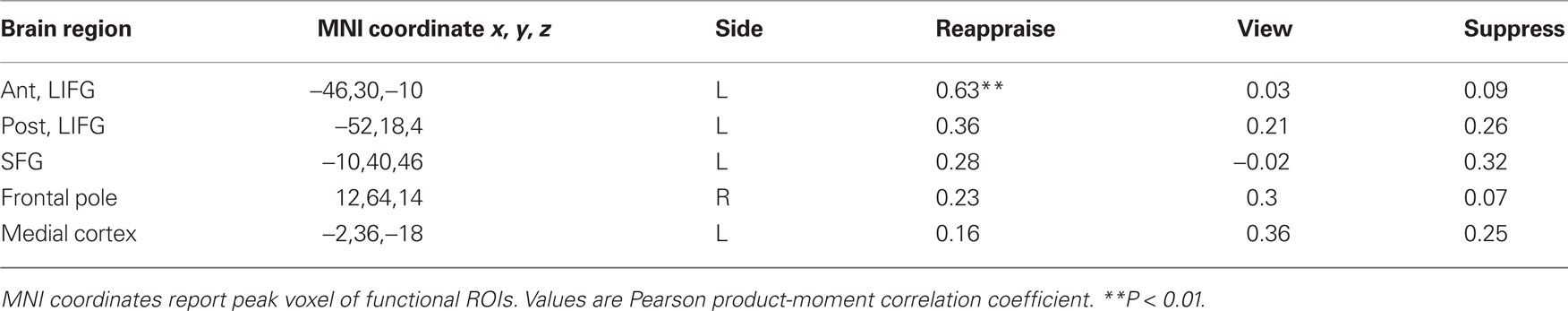
We observed a significant Dm correlation between the hippocampus and LIFG for reappraisal [r(24) = 0.63, P < 0.001], but not for view [r(23) = 0.03, P > 0.91] or suppress [r(21) = 0.09, P > 0.69] (Figure 4B). Fisher Z-tests confirmed that the reappraise Dm correlation was significantly higher than view (P < 0.03) and suppress (P < 0.05).
To examine the possibility that additional prefrontal cortical regions may influence memory encoding during emotion regulation, we computed Dm correlations between the hippocampus and four additional prefrontal ROIs that were activated in the main effect of memory: left posterior inferior frontal gyrus, left superior frontal gyrus, frontal pole, and left medial prefrontal cortex. As shown in Table 3, there were no significant Dm correlations between the hippocampus and any of the additional prefrontal ROIs. These results suggest that LIFG may serve a specific role in the memory enhancement effect during cognitive reappraisal.
Discussion
The present study examined the neural circuitry of memory formation while participants actively attempted to regulate their negative emotion. There were three main results. First, participants showed a reduction in negative affect and better memory performance after engaging in cognitive reappraisal than when instructed to suppress or passively view negative images. Second, the hippocampus, amygdala, and LIFG were highly and selectively correlated during cognitive reappraisal, whereas the hippocampus and amygdala were correlated during the passive view condition only. Finally, examination of the neural main effects of emotion regulation revealed that engaging in either cognitive reappraisal or expressive suppression resulted in the down-regulation of putative affective circuits relative to passive viewing, with cognitive reappraisal resulting in even greater activity reductions than expressive suppression.
The finding that cognitive reappraisal resulted in enhanced behavioral memory performance is consistent with the prior literature. We have previously noted that the memory advantage for cognitive reappraisal may be subserved by the levels-of-processing effect (Dillon et al., 2007), which is characterized by deeper cognitive analysis of stimuli (Craik and Lockhart, 1972). Reappraisal involves modification of emotional experience, which requires the individual to identify negative thoughts that initially arise from stimulus viewing and actively change the meaning and intensity of the stimulus. Recruitment of the LIFG and hippocampus simultaneously during cognitive reappraisal may support the observed memory enhancement. Previous work has shown that the LIFG is responsive to an interaction between deep encoding and subsequent memory (Wagner et al., 1998; Otten et al., 2001; Fletcher et al., 2003). Furthermore, the LIFG has been implicated in tasks in language tasks requiring decisions about word meaning, suggesting that this region is involved in the analysis of meaning during episodic encoding (Gabrieli et al., 1998). Across several studies, the locus of LIFG activity in predicting encoding success varies from more dorsal activity along the inferior frontal gyrus to more ventral activity along the lateral orbitofrontal cortex, encompassing Brodmann’s areas 6, 44, 45, and 47 (Buckner et al., 1999). We observed LIFG activity in a more ventral anterior region (Figure 4). This region overlaps almost exactly with that observed during a prior deep encoding task (Fletcher et al., 2003). By contrast, the LIFG was not correlated with the hippocampus during the suppression or negative view conditions. Thus, it stands to reason that the LIFG may underlie meaning change and deep encoding during cognitive reappraisal.
By contrast, expressive suppression encourages shallow encoding of the stimulus by re-directing attention from the stimulus to monitoring one’s external expression of emotion. Therefore a comparison of cognitive reappraisal and suppression constitutes a levels-of-processing manipulation in the affective domain. Our view is that during the act of reappraising, individuals elaborate upon the stimulus to the extent that encoding is facilitated and the formation of long-term representations is enhanced. It follows that reappraisal requires ongoing, effortful processing to reduce negative affect and boost memory performance once a negative stimulus is encountered. Interestingly, others have posited that the memorial benefits of cognitive reappraisal may be subserved by a reduction in cognitive resources (Richards and Gross, 2000). Specifically, this hypothesis suggests that the resources required to cognitively reappraise are expended very early in the regulation process, freeing availability of cognitive resources for encoding. These two hypotheses (elaborative encoding view and reduced resource view) highlight the differences in the temporal features of cognitive reappraisal. Whereas our paradigm examines the processes involved after individuals are presented with a negative situation, Gross (1998) suggests that cognitive reappraisal is an antecedent strategy, occurring prior to a negative response (Gross, 1998). Although it is likely that different cognitive processes may be involved as a function of timing (Kalisch, 2009), nevertheless, it appears that memory is enhanced during cognitive reappraisal whether individuals engage in reappraisal prior to encountering a negative stimulus or after the negative situation is presented.
Interestingly, the significant co-activation of right amygdala and hippocampus observed during reappraisal suggests that the amygdala may influence memory encoding even when its activity is diminished overall compared to passive viewing of negative pictures. Although speculative, it is possible that in order to change one’s cognitions, individuals must engage with the negative information, leading to amygdala modulation of hippocampal-dependent memory processes. Whereas this negative engagement continues under the passive viewing condition, negative cognitions are altered under the reappraisal condition, thereby reducing negative affect. By contrast, individuals attempt to avoid negative emotions during the suppress condition, uncoupling amygdala modulation of the hippocampus during this condition. We cannot fully test this idea in the present study given that we did not observe a detriment in expressive suppression memory performance relative to passive viewing as often observed in the behavioral literature; therefore, greater study of amygdala and hippocampal activity during regulation strategies is clearly warranted.
Taken together, the data from the present study provide some intriguing answers to questions regarding memory enhancement during cognitive reappraisal. The memory benefit derived from reappraisal may reflect both amygdala and LIFG modulation of the hippocampus during encoding, reflecting elaboration of emotionally laden content. Both of these functional interactions are absent during suppression, and the suppression condition yielded poorer memory relative to reappraisal. This cognitive cost may be due to greater internal monitoring of negative emotion and its display, in lieu of deep cognitive analysis of the stimuli. We observed the memory benefit for cognitively reappraised items after a 2-week delay, suggesting that the effect of cognitive reappraisal is evident for at least 2 weeks. However, a recent study suggests that there may not be a memory benefit for cognitive reappraisal after 1 year (Erk et al., 2010). A natural future direction of this work will be to examine how long-lasting the positive effects are, and factors that strengthen or weaken the observed positive outcomes of reappraisal.
In addition to the novel findings discussed above, the present study replicated and extended the current knowledge of neural circuitry underlying cognitive reappraisal and expressive suppression. The act of reappraising one’s emotions likely invokes several cognitive processes, including working memory, lexical analysis, selective attention, and response inhibition. Consistent with our findings, in which we observed activity in several PFC regions during reappraisal, reviews of the neuroimaging literature have shown that medial and lateral PFC regions are engaged during reappraisal that may constitute a cognitive control system for regulating emotion (Ochsner and Gross, 2005, 2008). While it is yet unclear what the precise subcomponents of cognitive reappraisal are, our findings provide evidence for the LIFG’s involvement in cognitive processes leading to meaning change.
The present results provide evidence for an internal monitoring process as a putative neural mechanism for expressive suppression. The suppression condition was associated with greater activity in somatosensory and insular cortices. The insular cortex has been associated with attention to internal bodily states (Critchley et al., 2004), body movement (Farrer et al., 2003), and subjective feelings of anger, disgust, and aversion, among others (Craig, 2009). The increased insular activity observed during suppression dovetails with the notion that suppression leads to heightened self-focus, as individuals direct their attention inward to monitor their expressions (Richards and Gross, 2000). The improvement in valence ratings and reduction in amygdala activity observed during suppression may suggest that the internal monitoring of one’s expressions was somewhat helpful in managing one’s negative affect. Another recent study also observed that individuals reported more positive valence ratings after suppressing than when viewing negative pictures, despite greater activity in the insula and somatosensory cortices (Goldin et al., 2008). Engaging in suppression may still be beneficial toward managing one’s self perceptions as well as outward appearance, which could explain why some individuals continue to utilize suppression despite its relative inferior performance in reducing negative affect. While interpreting the valence results, it could be argued that the improvement in valence ratings during emotion regulation reflects individuals responding how they think they should feel in the study rather than how they actually felt. However, this possibility is made less likely by the fact that participants had to engage in two different strategies, and would not know which strategy they were supposed to feel better doing. We found that although individuals had improved valence ratings for both reappraisal and suppression, they had even higher valence ratings for reappraisal, suggesting that there is a true benefit for reappraisal. Additionally, we included a confidence measure in our design, asking individuals to rate how well they were able to engage in the given strategy (Figure 1). Analysis of these ratings suggested that individuals rated improved valence when they were successful in reappraising, but not suppressing or viewing the picture.
Examination of the main effects of emotion regulation revealed that engaging in either cognitive reappraisal or expressive suppression resulted in the down-regulation of subjective ratings of negative affect, reduction of amygdala activity, and increased prefrontal cortex activity. However, when reappraisal and suppression were compared, reappraisal resulted in an even greater down-regulation of negative experience, increase in prefrontal cortex activity, and reduction of activity in the insular cortex. These results are largely consistent with previous work suggesting that cognitive reappraisal is a superior strategy to expressive suppression in regulating negative affect (Richards and Gross, 2000; Goldin et al., 2008). Our study uniquely adds to this literature by characterizing how brain activity relates to downstream cognitive consequences of engaging in these regulatory strategies.
Conclusion
The present study combined emotion regulation and subsequent memory paradigms to examine the neural underpinnings of explicit memory formation while participants altered their affective experience. We observed robust prefrontal cortex activity and co-activation of the LIFG and MTL structures, including the amygdala and hippocampus, for successful encoding of reappraised stimuli. The data are also consistent with prior behavioral evidence that the process of thought change boosts long-term memory while simultaneously reducing negative affect. In contrast to reappraisal, expressive suppression yielded poorer memory performance, milder valence benefits, no LIFG–MTL coupling, and greater activity in somatic monitoring regions. These results advance neurobiological models of emotional memory and show how task-dependent functional connectivity analyses complement univariate statistical approaches to understand complex emotion–memory interactions. Because cognitive reappraisal is a cornerstone of cognitive–behavioral therapy, these findings have important clinical ramifications for understanding how to help individuals “stay cool when things get hot.”
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to extend thanks to Jessica Nasser and Elizabeth Selgrade for their help with data collection and analysis, and Dan Dillon, Florin Dolcos, and Scott Hayes for helpful discussions regarding the project. This work was supported by the Department of Veterans Affairs, Mental Illness Research Education and Clinical Center Grant for Post-Deployment Mental Health, and US National Institutes of Health (grant numbers K23 MH073091, K23 MH084013, and 2 P01 NS041328.
References
Beckmann, C. F., Jenkinson, M., and Smith, S. M. (2003). General multilevel linear modeling for group analysis in FMRI. Neuroimage 20, 1052–1063.
Bonanno, G. A., Papa, A., Lalande, K., Westphal, M., and Coifman, K. (2004). The importance of being flexible – The ability to both enhance and suppress emotional expression predicts long-term adjustment. Psychol. Sci. 15, 482–487.
Brewin, C. R. (2001). Memory processes in post-traumatic stress disorder. Int. Rev. Psychiatry 13, 159–163.
Buckner, R. L., Kelley, W. M., and Petersen, S. E. (1999). Frontal cortex contributes to human memory formation. Nat. Neurosci. 2, 311–314.
Cahill, L., and McGaugh, J. L. (1998). Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 21, 294–299.
Craig, A. D. (2009). How do you feel now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70.
Craik, F. I. M., and Lockhart, R. S. (1972). Levels of processing – framework for memory research. J. Verbal Learn. Verbal Behav. 11, 671–684.
Critchley, H. D., Wiens, S., Rotshtein, P., Ohman, A., and Dolan, R. J. (2004). Neural systems supporting interoceptive awareness. Nat. Neurosci. 7, 189–195.
D’Esposito, M., Postle, B., Jonides, J., and Smith, E. (1999). The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proc. Natl. Acad. Sci. U.S.A. 96, 7514.
Dillon, D. G., Ritchey, M., Johnson, B. D., and LaBar, K. S. (2007). Dissociable effects of conscious emotion regulation strategies on explicit and implicit memory. Emotion 7, 354–365.
Dolcos, F., LaBar, K. S., and Cabeza, R. (2004). Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron 42, 855–863.
Elliot, A., and Maier, M. (2007). Color and psychological functioning. Curr. Dir. Psychol. Sci. 16, 250.
Erk, S., von Kalckreuth, A., and Walter, H. (2010). Neural long-term effects of emotion regulation on episodic memory processes. Neuropsychologia 48, 989–996.
Farrer, C., Franck, N., Georgieff, N., Frith, C. D., Decety, J., and Jeannerod, M. (2003). Modulating the experience of agency: a positron emission tomography study. Neuroimage 18, 324–333.
Fletcher, P. C., Stephenson, C. M. E., Carpenter, T. A., Donovan, T., and Bullmore, E. T. (2003). Regional brain activations predicting subsequent memory success: an event-related FMRI study of the influence of encoding tasks. Cortex 39, 1009–1026.
Gabrieli, J., Poldrack, R., and Desmond, J. (1998). The role of left prefrontal cortex in language and memory. Proc. Natl. Acad. Sci. U.S.A. 95, 906.
Goldin, P. R., McRae, K., Ramel, W., and Gross, J. J. (2008). The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry 63, 577–586.
Gross, J. J. (1998). Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J. Pers. Soc. Psychol. 74, 224–237.
Guo, H., and Song, A. W. (2003). Spiral-in-and-out functional image acquisition with embedded z-shimming for susceptibility signal recovery. J. Magn. Reson. Imaging 18, 389–395.
Hamilton, J., and Gotlib, I. (2008). Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol. Psychiatry 63, 1155–1162.
Jenkinson, M., Bannister, P., Brady, M., and Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841.
Kalisch, R. (2009). The functional neuroanatomy of reappraisal: time matters. Neurosci. Biobehav. Rev. 33, 1215–1226.
Kaya, N., and Epps, H. (2004). Relationship between color and emotion: a study of college students. Coll. Stud. J. 38, 396–406.
LaBar, K. S., and Cabeza, R. (2006). Cognitive neuroscience of emotional memory. Nat. Rev. Neurosci. 7, 54–64.
Lang, P. J., Bradley, M. M., and Cuthbert, B. N. (1997). International Affective Picture System. Gainesville: NIMH Center for the Study of Emotion and Attention.
McGaugh, J. L. (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 27, 1–28.
Ochsner, K. N. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of emotion. Neuroimage 23, 483–499.
Ochsner, K. N., Bunge, S. A., Gross, J. J., and Gabrieli, J. D. E. (2002). Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 14, 1215–1229.
Ochsner, K. N., and Gross, J. J. (2005). The cognitive control of emotion. Trends Cogn. Sci. 9, 242–249.
Otten, L. J., Henson, R. N. A., and Rugg, M. D. (2001). Depth of processing effects on neural correlates of memory encoding – relationship between findings from across and within-task comparisons. Brain 124, 399–412.
Paller, K. A., and Wagner, A. D. (2002). Observing the transformation of experience into memory. Trends Cogn. Sci. 6, 93–102.
Richards, J. M., Butler, E. A., and Gross, J. J. (2003). Emotion regulation in romantic relationships: the cognitive consequences of concealing feelings. J. Soc. Pers. Relat. 20, 599–620.
Richards, J. M., and Gross, J. J. (1999). Composure at any cost? The cognitive consequences of emotion suppression. Pers. Soc. Psychol. Bull. 25, 1033–1044.
Richards, J. M., and Gross, J. J. (2000). Emotion regulation and memory: the cognitive costs of keeping one’s cool. J. Pers. Soc. Psychol. 79, 410–424.
Richards, J. M., and Gross, J. J. (2006). Personality and emotional memory: how regulating emotion impairs memory for emotional events. J. Res. Pers. 40, 631–651.
Slotnick, S., and Schacter, D. (2004). A sensory signature that distinguishes true from false memories. Nat. Neurosci. 7, 664–672.
Smith, S., and Nichols, T. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98.
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E. J., Johansen-Berg, H., Bannister, P. R., De Luca, M., Drobnjak, I., Flitney, D. E., Niazy, R. K., Saunders, J., Vickers, J., Zhang, Y., De Stefano, N., Brady, J. M., and Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl. 1), S208–S219.
Wagner, A. D., Schacter, D. L., Rotte, M., Koutstaal, W., Maril, A., Dale, A. M., Rosen, B. R., and Buckner, R. L. (1998). Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 281, 1188–1191.
Watkins, P., Mathews, A., Williamson, D., and Fuller, R. (1992). Mood-congruent memory in depression: emotional priming or elaboration? J. Abnorm. Psychol 101, 581–586.
Woolrich, M. W., Behrens, T. E. J., Beckmann, C. F., Jenkinson, M., and Smith, S. M. (2004). Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21, 1732–1747.
Keywords: arousal, cognitive reappraisal, declarative memory, expressive suppression, subsequent memory paradigm, hippocampus, amygdala, left inferior frontal gyrus
Citation: Hayes JP, Morey RA, Petty CM, Seth S, Smoski MJ, McCarthy G and LaBar KS (2010) Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Front. Hum. Neurosci. 4:230. doi: 10.3389/fnhum.2010.00230
Received: 28 July 2010;
Accepted: 08 December 2010;
Published online: 22 December 2010.
Edited by:
Maryse Lassonde, Université de Montréal, CanadaCopyright: © 2010 Hayes, Morey, Petty, Seth, Smoski, McCarthy and LaBar. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Jasmeet Pannu Hayes, National Center for PTSD (116B-2), VA Boston Healthcare System, 150 S. Huntington Ave, Boston, MA 02130, USA. e-mail: jphayes@bu.edu