Response inhibition and interference control in obsessive–compulsive spectrum disorders
- 1GGZ InGeest, Amsterdam, Netherlands
- 2Neuroscience Campus Amsterdam (NCA), Amsterdam, Netherlands
- 3Department of Psychiatry, VU University Medical Center, Amsterdam, Netherlands
- 4Department of Anatomy and Neurosciences, VU University Medical Center, Amsterdam, Netherlands
Over the past 20 years, motor response inhibition and interference control have received considerable scientific effort and attention, due to their important role in behavior and the development of neuropsychiatric disorders. Results of neuroimaging studies indicate that motor response inhibition and interference control are dependent on cortical–striatal–thalamic–cortical (CSTC) circuits. Structural and functional abnormalities within the CSTC circuits have been reported for many neuropsychiatric disorders, including obsessive–compulsive disorder (OCD) and related disorders, such as attention-deficit hyperactivity disorder, Tourette’s syndrome, and trichotillomania. These disorders also share impairments in motor response inhibition and interference control, which may underlie some of their behavioral and cognitive symptoms. Results of task-related neuroimaging studies on inhibitory functions in these disorders show that impaired task performance is related to altered recruitment of the CSTC circuits. Previous research has shown that inhibitory performance is dependent upon dopamine, noradrenaline, and serotonin signaling, neurotransmitters that have been implicated in the pathophysiology of these disorders. In this narrative review, we discuss the common and disorder-specific pathophysiological mechanisms of inhibition-related dysfunction in OCD and related disorders.
Introduction
Response inhibition, the ability to suppress pre-potent behavior that is inappropriate or no longer required, is critical for goal-directed behavior in everyday life (Chambers et al., 2009). Over the past decades, researchers have shown increased interest in response inhibition. Response inhibition is considered an operationalization of certain aspects of impulsivity and compulsivity (Bari and Robbins, 2013). Impulsivity is commonly defined as a tendency to act on impulses, acts performed immediately and without voluntary control, whereas compulsivity is the tendency to repeat specific behavior and to be unable to inhibit the behavior even when it is no longer appropriate (Bari and Robbins, 2013). Due to the importance of response inhibition in everyday life, many neuropsychological paradigms have been developed to probe inhibitory performance. In these paradigms, subjects are asked to respond to a target stimulus, but withhold this response to irrelevant or distracting stimuli, or distracting stimulus characteristics (Nigg, 2000).
Response inhibition is not a unitary construct and consists of motor response inhibition and interference control. Motor response inhibition involves the inhibition of pre-potent and automatic motor responses, and can be further differentiated into action restraint (or action suppression) and action cancelation (Schachar et al., 2007). The Go/No Go task (Donders, 1969) is considered to probe action restraint, whereas the Stop-signal task (Logan, 1994) measures action cancelation (see Figure 1 for a description of these tasks). Interference control on the other hand, refers to the cognitive control needed to prevent interference due to competition of relevant and irrelevant stimuli or stimulus characteristics (Nigg, 2000). Several tasks including the Stroop task, the Flanker task and the Simon task are measures of interference control (see Figure 1). It has been proposed that the inhibitory load is highest in the Stop-signal task, as the response that needs to be suppressed has already been initiated (Schachar et al., 2007). Contrary to the motor response inhibition tasks, interference control tasks may also rely on response selection processes (Nee et al., 2007). It has been suggested that interference control, action restraint, and action cancelation represent early, intermediate, and late processes of response inhibition, respectively (Sebastian et al., 2013a).
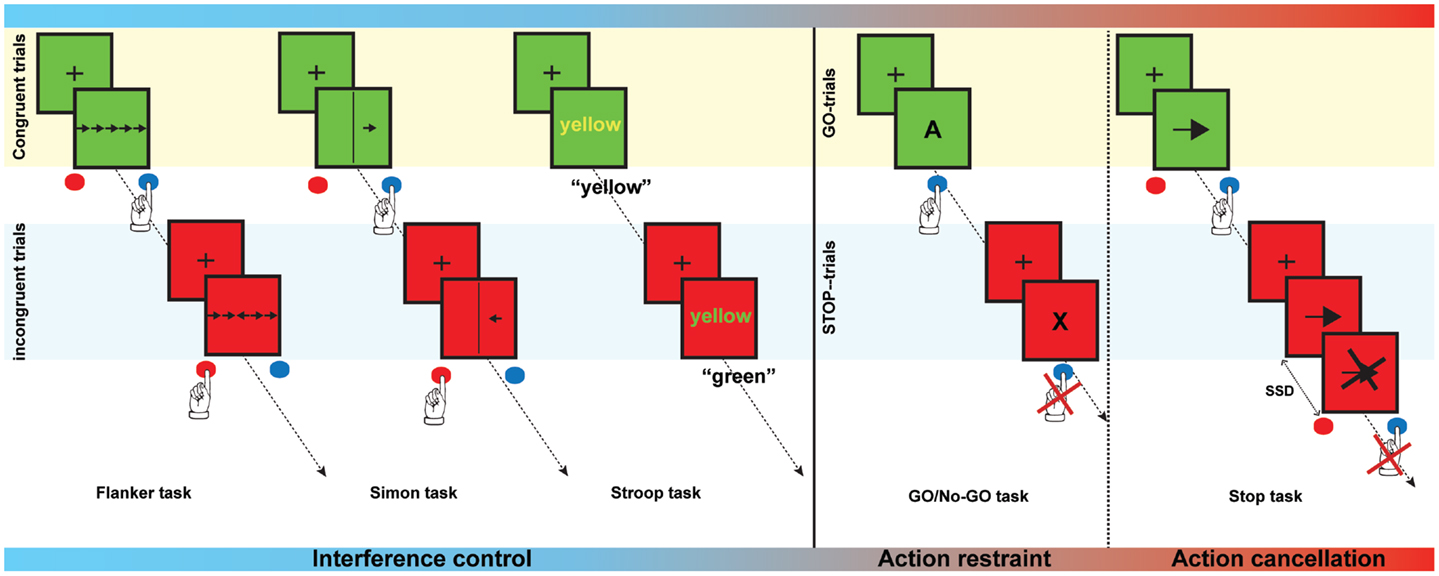
Figure 1. Examples of interference control and motor response inhibition tasks. The Flanker task is a test in which subjects are asked to respond to a target stimulus by pressing a button to indicate the direction of the target stimulus. The target, however, is flanked by non-target distracter stimuli, which are presented in the same or in the opposite direction as the target (congruent and incongruent trials, respectively). During a Simon task, participants are asked to press a button depending on the orientation of the arrow, irrespective of the location of the arrow. Orientation and location can either be congruent of incongruent. In the Stroop task names of colors are presented in either the same (congruent) or a different color (incongruent). Subjects are instructed to name to color of the word but not the word itself. In the Go/No-go task, subjects need to respond as fast as possible when letters are presented (Go-trials), but must withhold the response when a certain letter (e.g., “X”) is presented (Stop-trials). In a Stop-signal task, the participant is asked to respond as fast as possible by pressing a button to a stimulus (Go-trials) that is presented. On a minority of trials, a stop-signal is presented and the subject is asked to suppress the response when the stop-signal occurs. Task demands gradually increase from interference control to action cancelation.
The symptoms of obsessive–compulsive and related disorders within the impulsive–compulsive spectrum are characterized by a failure to inhibit certain behaviors, e.g., washing hands, pulling hair, motor tics, or impulsive actions. Response inhibition might therefore be a suitable measure to investigate the neural substrates of these shared symptoms. In this narrative review, we will provide an overview of studies that have examined the neuroanatomical and functional underpinnings of response inhibition impairment in healthy subjects and patients with these disorders but this review by no means constitutes an exhaustive account of the current literature. We will focus on the shared mechanisms that may underlie the inhibitory dysfunction and symptoms of these disorders. Pharmacological and genetic alterations are also addressed and focus on the dopamine, serotonin, and noradrenalin system. We acknowledge that other neurotransmitters, such as glutamate and gamma-Aminobutyric acid, are also important for response inhibition and the pathophysiology obsessive–compulsive and related disorders (MacMaster et al., 2003; Turner et al., 2003; van Minnen et al., 2003; DeVito et al., 2005; Starck et al., 2008; Silveri et al., 2013), but discussion of all these neurotransmitters would considerably lengthen this review.
Neural Correlates of Response Inhibition in Healthy Controls
Neuroimaging of Response Inhibition
Neuroimaging studies in healthy controls have revealed the neural substrates of response inhibition [for excellent reviews, see Robbins (2007), Chambers et al. (2009), and Aron (2011)]. While major contributions to our understanding of response inhibition come from electrophysiological studies, in this review, we will focus on neuroimaging studies. Readers interested in the electrophysiology of response inhibition are directed to Huster et al. (2013).
In brief, response inhibition activates a network of mainly right lateralized frontal brain areas. The inferior frontal gyrus (IFG) and pre-supplementary motor area (pre-SMA) are key components (Aron et al., 2003b; Chambers et al., 2006; Floden and Stuss, 2006; Cai et al., 2012), and the neural stop-signal is then sent from these frontal areas to the motor cortex through cortico-striatal–thalamic–cortical (CSTC) projections (Chambers et al., 2009).
The subcomponents of response inhibition are found to depend on overlapping, yet distinct, brain areas. Interference inhibition, action restraint and action cancelation are all associated with activation of the IFG, anterior insula, anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), pre-SMA, and parietal regions (Wager et al., 2005; Nee et al., 2007; Sebastian et al., 2013b). When inhibitory task load increases, activation of frontal–striatal regions increases and additional inhibition-related brain areas are recruited (Blasi et al., 2006; Swick et al., 2011; Sebastian et al., 2013b). However, each task recruits distinct brain areas as well, depending on the unique cognitive processes that they represent. Regions involved in response selection, including the parietal cortex, for instance, are activated to a greater extent during interference control tasks and action restraint (Rubia et al., 2001; Sebastian et al., 2013b).
Neurotransmitters in Response Inhibition
In addition to differences in neural activation, differences in the neurotransmitter systems underlying interference control, action restraint, and action cancelation have also been observed. Current studies suggest that interference control is dependent on serotonin and dopamine neurotransmission. Depletion of serotonin and dopamine has been shown to decrease interference effects on incongruent trials, and thus improve performance, during the Stroop task (Schmitt et al., 2000; Scholes et al., 2007). Decreases in serotonin may improve performance by increasing arousal and attention (Scholes et al., 2007). Increased activation of the DLPFC and ACC was observed during performance of a Stroop task after serotonin depletion (Horacek et al., 2005). Stroop performance was positively correlated with serotonin transporter (SERT) binding in the right DLPFC as well (Madsen et al., 2011). However, contradicting results have also been reported, as neuroimaging studies showed that decreased dopamine transporter (DaT) binding in the striatum in women (Mozley et al., 2001) and decreased postsynaptic striatal D2-receptor availability was associated with poor performance on a Stroop task (Volkow et al., 1998). Administration of a dopamine D2 agonist decreased interference, and thereby improved performance on the Stroop task (Roesch-Ely et al., 2005). Based on the available literature in healthy subjects it seems that both serotonin and dopamine are important for interference control.
Action restraint (Go/No Go task) seems to be primarily mediated by serotonin [for a review of evidence, see Eagle et al. (2008)]. Serotonin depletion has been shown to decrease activation of the IFG during inhibition and decreases activation of the medial prefrontal lobe during error monitoring in the Go/No Go task (Rubia et al., 2005a; Evers et al., 2006). Administration of a serotonin 2C receptor agonist (Anderson et al., 2002) or mirtazapine (Vollm et al., 2006), which acts on both the noradrenalin and serotonin system, increased inhibition-related activation of the right IFG. Nevertheless, dopamine may play a role in action restraint as well since methylphenidate, a dopamine re-uptake inhibitor, improved performance on the Go/No Go task and led to decreased task-related striatal activation (Vaidya et al., 1998).
Several lines of evidence support an important role for dopamine in action cancelation. Increased Dopamine D2/3-receptor availability in the striatum is associated with better Stop-signal task performance, i.e., shorter stop-signal reaction time (SSRT), and correlates positively with inhibition-related activation of the dorsal caudate and putamen (Ghahremani et al., 2012). Also, administration of a D2-receptor agonist improved action cancelation (Nandam et al., 2013). Administration of methylphenidate and atomoxetine, which both target the dopamine and noradrenalin system, by inhibiting the re-uptake from the synaptic cleft, decreased SSRT in humans and in animals, raising the possibility that noradrenalin is involved in motor response inhibition as well (Chamberlain et al., 2006b; Eagle et al., 2007; Bari et al., 2009; Nandam et al., 2011). Serotonin does not seem to mediate performance of the Stop-signal task, as use of selective serotonin re-uptake inhibitors (SSRIs) and serotonin depletion did not affect action cancelation in humans or in animals (Clark et al., 2005; Chamberlain et al., 2006b; Bari et al., 2009; Eagle et al., 2009; Drueke et al., 2010).
To summarize, interference control seems to be mediated by both serotonin and dopamine. Action restraint seems to be predominantly mediated by serotonin, whereas action cancelation seems to be mediated by dopamine and noradrenalin. More detailed information on the neuropharmacology of response inhibition is provided by Dalley and Roiser (2012) and Bari and Robbins (2013).
Genes in Response Inhibition
Several gene-association studies have examined the relationship between genes involved in the dopaminergic and serotonergic systems and response inhibition. Genetic polymorphisms in the dopamine D4-receptor gene (DRD4), associated with reduced functional activity (Asghari et al., 1995), have been related to decreased performance on the Stop-signal task (Congdon et al., 2008), although conflicting results have also been reported (Kramer et al., 2009).
The DRD2 gene codes for the dopamine receptor D2. The presence of a TaqIA allele, which has been linked to decreased availability of striatal D2-receptors (Thompson et al., 1997), was associated with poor response inhibition in the Stop-signal task (White et al., 2008). A second polymorphism, which has been associated with decreased cortical and thalamic D2-receptor availability (Hirvonen et al., 2009), was also related to poor action cancelation (Colzato et al., 2010).
Genetic polymorphisms that increased expression of the DaT have been associated with impaired performance on an interference control task (Cornish et al., 2005) and decreased brain activation in the STN and pre-SMA during action cancelation (Congdon et al., 2009). Furthermore, two novel single nucleotide polymorphisms in the DaT gene predicted individual SSRT (Cummins et al., 2012) and genotype of one of these polymorphisms predicted activation of frontal areas and the caudate nucleus during task performance.
Polymorphisms in catechol-O-methyltransferase (COMT) and monoamine oxidase A (MAO-A) genes, coding for enzymes playing a role in neurotransmitter metabolism, have been associated with normal variations in inhibition-related activity as well. COMT is involved in the degradation of dopamine and noradrenalin and MAO-A is involved in degradation of dopamine, noradrenalin, and serotonin. A polymorphism of COMT with decreased function (Chen et al., 2004), was associated with increased activation of the IFG during action cancelation (Congdon et al., 2009) and decreased interference inhibition (Solis-Ortiz et al., 2010), although conflicting results have also been reported (Kramer et al., 2007). Polymorphisms of the MAO-A gene, which increase MAO-A activity, were associated with increased activity in the right IFG and ACC and decreased activity in the superior parietal cortex during action restraint (Passamonti et al., 2006).
Gene-association studies have focused on genes involved in serotonergic transmission as well. Serotonin synthesis in the brain is regulated by tryptophan-hydroxylase-2 (TPH-2) (Walther and Bader, 2003). Individuals homozygous for the T-allele of a polymorphism in the TPH-2 gene showed increased SSRT in the Stop-signal task (Stoltenberg et al., 2006). A second study found that two other polymorphisms in the TPH-2 gene were associated with reduced brain activity during action restraint in an EEG study (Baehne et al., 2009). Lastly, Osinsky et al. (2009) found that a polymorphism located in the promotor region of the TPH-2 gene, affected reaction time during performance of a Stroop task. Interpretation of these findings is, however, challenging as it is uncertain how these polymorphisms affect serotonin levels.
Polymorphisms in the SERT gene (SLC6A4) that decrease the rate of re-uptake from the synaptic cleft, were associated with decreased interference inhibition (Holmes et al., 2010), but not to action cancelation (Clark et al., 2005). Participants with a decreased function polymorphism in SERT also showed increased rostral ACC activation in response to errors and decreased activation of the dorsal ACC in response to conflict during the Flanker task (Holmes et al., 2010).
Intermediate Summary
In summary, current evidence suggests that response inhibition is dependent on brain areas in the CSTC circuits and activation in these circuits increases with increasing inhibitory load. Proper function of these CSTC circuits depends on a complex interplay between dopamine, serotonin, and noradrenalin, although the weight of their importance may differ between the subcomponents of response inhibition. While reducing levels of serotonin and dopamine appears to ameliorate interference control, increasing dopamine levels appears to ameliorate action restraint and action cancelation. Gene-association studies have primarily reported that polymorphisms associated with decreased dopamine signaling are also associated with decreased motor response inhibition performance.
Structural and functional alterations in CSTC circuits and altered serotonin, noradrenalin, and dopamine transmission may underlie response inhibition deficits in obsessive–compulsive disorder (OCD) patients and in patients with related disorders.
Obsessive–Compulsive Disorder and Inhibition
Obsessive–compulsive disorder is an anxiety disorder that affects 2–3% of the population and causes severe impairment in social and occupational functioning (Ruscio et al., 2010). The disorder is characterized by distress- and anxiety provoking obsessions (repetitive intrusive thoughts) and compulsions (repetitive ritualistic behavior), which are performed to diminish anxiety (American Psychiatric Association, 2013). These symptoms are common, as more than 25% of the population experiences sub-clinical obsessions or compulsions in their lives (Ruscio et al., 2010). Pharmacotherapy for OCD consists mainly of SSRIs, which suggests involvement of the serotonin system in the pathophysiology of the disorder. Nevertheless, an estimated 40–60% of patients does not respond to this treatment and require additional treatment with atypical antipsychotics, which affects both the serotonergic and dopaminergic system (Denys et al., 2004a; Fineberg et al., 2005). Neuroimaging studies have strengthened the notion of serotonergic dysfunction in OCD by providing evidence for reduced availability of SERTs in the midbrain, thalamus, and brainstem and reduced availability of serotonin 2A receptors in prefrontal, parietal, and temporal brain regions (Hesse et al., 2005; Perani et al., 2008). Abnormalities in the dopamine system have also been observed in OCD patients, such as increased DaT levels in the striatum and reduced availability of the D1- and D2-receptors in the striatum (Kim et al., 2003; Denys et al., 2004b; van der Wee et al., 2004; Olver et al., 2009).
In the past several years, research interest has focused on response inhibition as a model of OCD symptoms (Chamberlain et al., 2005). In support of this, deficits in interference control, e.g., increased reaction times during incongruent trials, have been described in OCD (Bannon et al., 2002; Penades et al., 2007; Nabeyama et al., 2008; Nakao et al., 2009; Schlosser et al., 2010). A number of studies have used interference control paradigms in OCD research during functional neuroimaging (see Table 1; Fitzgerald et al., 2005; Nakao et al., 2005a, 2009; van den Heuvel et al., 2005; Viard et al., 2005; Nabeyama et al., 2008; Woolley et al., 2008; Page et al., 2009; Schlosser et al., 2010; Huyser et al., 2011; Rubia et al., 2011a; Marsh et al., 2013). Some studies reported hyperactivation of the ACC in adults and children with OCD following errors and interference control (Fitzgerald et al., 2005; Huyser et al., 2011), while others reported hypoactivation of the ACC (Nakao et al., 2005a; Rubia et al., 2011a). Altered inhibition-related brain activation has also been observed in the pre-SMA (Fitzgerald et al., 2005; Rubia et al., 2011a) and insular cortex (Huyser et al., 2011). Increased activation in frontal–striatal regions, including the IFG and putamen, was seen in OCD patients during performance of a Simon task (Marsh et al., 2013).
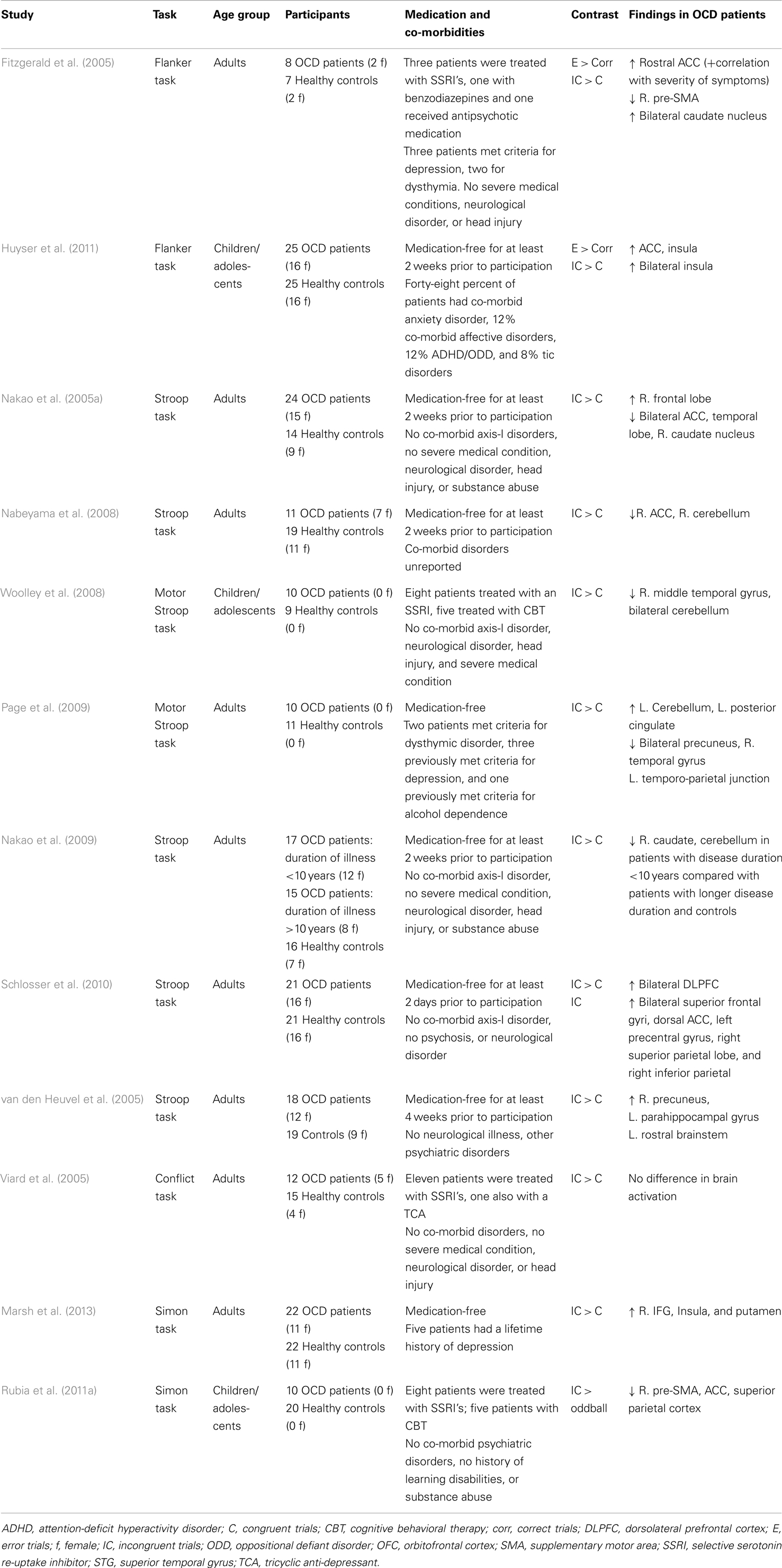
Table 1. Overview of fMRI studies that have used interference control tasks in obsessive–compulsive disorder.
Abnormalities in activation during interference control tasks have also been observed at a network level. Schlosser et al. (2010) used dynamic causal modeling (DCM) to examine functional connectivity in a fronto-cingulate network during performance on a Stroop task, and found increased connectivity between the DLPFC and ACC in OCD patients compared with healthy controls. Increased functional connectivity between the putamen and the inferior parietal cortex, caudate, thalamus, and frontal areas was observed in patients during performance of a Simon task (Marsh et al., 2013).
Impaired action cancelation and action restraint has been described for OCD (Chamberlain et al., 2007b; Penades et al., 2007); patients showed increased SSRT (i.e., slower inhibition) in the Stop-signal task and higher error rates on the Go/No Go task compared with healthy control subjects. Deficits in motor response inhibition were also observed in unaffected first-degree relatives of OCD patients (Chamberlain et al., 2007b; Menzies et al., 2007), suggesting that motor response inhibition may be considered an endophenotype [a trait that is heritable and co-segregates with the illness in families (Gottesman and Gould, 2003)] of OCD patients.
Structural neural correlates of impaired motor response inhibition in OCD patients have been identified. Deficits in action cancelation in OCD patients and first-degree relatives were associated with increased gray matter volume in the ACC, putamen, caudate, amygdala, parietal areas, and the cerebellum, and decreased gray matter volume in the OFC, IFG, ACC, premotor cortex, and regions in the temporal cortex (Menzies et al., 2007).
Functional neural correlates of motor response inhibition impairments in OCD have also been identified (see Table 2; Maltby et al., 2005; Roth et al., 2007; Woolley et al., 2008; Page et al., 2009; Rubia et al., 2010; de Wit et al., 2012; Kang et al., 2012). Decreased task-related activation is seen in the CSTC circuits during inhibition in OCD patients (Roth et al., 2007; Woolley et al., 2008; Page et al., 2009; Rubia et al., 2010; de Wit et al., 2012; Kang et al., 2012), although, one study reported increased activation of these regions (Maltby et al., 2005). In the largest study to date, de Wit et al. (2012) found decreased activation of the IFG and inferior parietal cortex during inhibition in unmedicated OCD patients and increased activation of the left pre-SMA. This pre-SMA hyperactivation was present in their unaffected siblings as well. Activation of the pre-SMA correlated negatively with SSRT in patients and siblings, indicating that hyperactivation of the pre-SMA may be considered a compensatory mechanism. Overall, the most consistent finding is decreased activation of the DLPFC, IFG, striatum, and thalamus in OCD patients during inhibition (Roth et al., 2007; Woolley et al., 2008; Page et al., 2009; Rubia et al., 2010; de Wit et al., 2012).
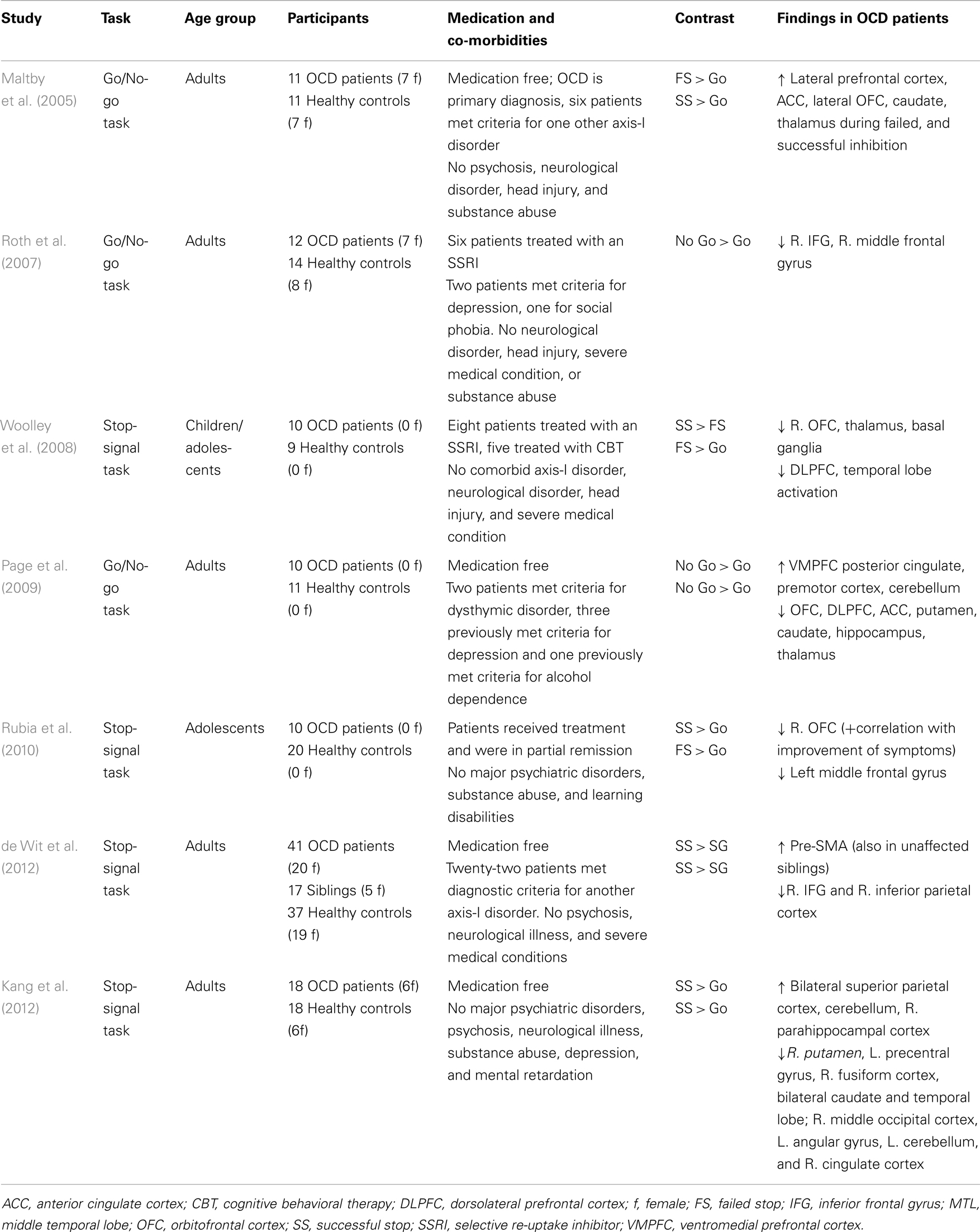
Table 2. Overview of fMRI studies that have used response inhibition paradigms in obsessive–compulsive disorder.
In a recent study, we examined functional connectivity during performance of the Stop-signal task in unmedicated adult OCD patients, their unaffected siblings, and healthy controls (van Velzen et al., under review). We performed psychophysiological interaction (PPI) analyses and DCM and found abnormal connectivity between the IFG and amygdala in patients and their siblings, suggesting that this pattern of connectivity is an endophenotype. Limbic activation may interfere with CSTC circuit activation in OCD. We did not find evidence for altered connectivity between the IFG, pre-SMA, and striatum during inhibition. These results warrant replication in other samples.
Two studies have investigated the effects of pharmacological treatment for OCD on response inhibition. Treatment with SSRI’s increased task-relevant brain activation during performance of an interference control task along with symptom improvement (Nakao et al., 2005b). However, due to the study design, it remains unknown if this change in activation occurs secondary to symptom improvement or due to the pharmacological treatment. A second study reported increased activation of multiple cortical and subcortical brain areas during a Go/No Go task in OCD patients treated with SSRIs compared to OCD patients who were not treated with SSRIs (Roth et al., 2007). However, this study was crossectional, included small patient groups and did not study the relationship with disease severity.
In summary, OCD patients show impairment in both interference control and motor response inhibition. Prefrontal and other brain areas within the CSTC circuits appear to be hyperactive during interference control, although results have been inconsistent. As task load increases during action restraint and action cancelation, CSTC areas generally become hypoactive compared with controls, although some compensation may occur. Decreased serotonin and increased dopamine transmission in CSTC circuits may underlie the response inhibition deficits. The presence of, and functional correlates of response inhibition deficits have also been investigated in disorders related to OCD, such as Tourette’s syndrome (TS), trichotillomania (TTM), and attention-deficit hyperactivity disorder (ADHD), enabling the disorder specificity of these cognitive dysfunctions and enabling comparison of these inhibition deficits across these disorders.
Inhibition in Other Frontal–Striatal Disorders
Tourette’s Syndrome
Gilles de la Tourette’s syndrome, also known as Tourette’s syndrome, is a neurodevelopmental disorder characterized by motor tics and vocal tics (American Psychiatric Association, 2013). TS affects between 0.4 and 1% of the population (Swain et al., 2007; Robertson, 2008).
Like in OCD, dysfunction of the serotonergic and dopaminergic systems is implicated in the pathophysiology of TS [for a review, see Steeves and Fox (2008)]. Several clinical trials have shown that administration of dopamine antagonists, such as risperidone and haloperidol, are effective in suppressing tics in most patients (Bloch et al., 2011; Roessner et al., 2011). Neuroimaging studies have reported decreased availability of the D2 and D3-receptors in cortical (OFC, ACC, insula, temporal, and occipital cortex) and subcortical areas (thalamus and hippocampus) (Gilbert et al., 2006; Steeves et al., 2010) and increased striatal DaT availability (Malison et al., 1995; Muller-Vahl et al., 2000; Cheon et al., 2004; Serra-Mestres et al., 2004; Liu et al., 2010), although conflicting results have also been reported (Singer et al., 2002; Hwang et al., 2008). Neuroimaging of the serotonergic system in TS has shown increased binding of the serotonin 2A-receptor in many cortical (OFC, ACC, insula, temporal lobe, parietal lobe, and occipital lobe) and subcortical areas (thalamus, caudate, and hippocampus) (Haugbol et al., 2007) and increased SERT availability in the striatum and midbrain (Wong et al., 2008).
There is increasing evidence for frontal–striatal dysfunction in TS [for reviews, see Albin and Mink (2006) and Felling and Singer (2011)]. For instance, symptom severity correlated negatively with the degree of activation of CSTC circuits during tic suppression (Peterson et al., 1998) and prefrontal cortical thickness (Draganski et al., 2010) and volume of prefrontal CSTC areas was decreased in TS patients compared with healthy controls (Draganski et al., 2010).
More than 90% of all patients with TS also have co-morbid psychiatric disorders, most often OCD or ADHD (Robertson, 2011). It has been estimated that between 45 and 60% of TS patients suffer from OCD as well (Ghanizadeh and Mosallaei, 2009). As in OCD, many studies have investigated whether the involuntary motor symptoms in TS are related to motor response inhibition and interference control. Evidence for this, however, has been mixed; as some studies report impaired performance (Baron-Cohen et al., 1994; Crawford et al., 2005; Rankins et al., 2006; Channon et al., 2009; Eichele et al., 2010), especially with increasing task demands, while others do not (Ozonoff et al., 1994; Ozonoff and Jensen, 1999; Hershey et al., 2004; Verte et al., 2005; Watkins et al., 2005; Channon et al., 2006; Ray Li et al., 2006; Marsh et al., 2007; Raz et al., 2009; Sukhodolsky et al., 2010). These studies often included TS patients with co-morbid disorders and patients often used psychotropic medication. A meta-analysis of four studies using the Stop-signal task in TS found mild inhibitory deficits (Lipszyc and Schachar, 2010).
While evidence for behavioral impairment is not straightforward, inhibition-related brain activity seems to be altered in TS (see Table 3). With increasing age, patients with TS, compared with healthy controls, show increased recruitment of CSTC regions during interference control (Raz et al., 2009). Greater activation of CSTC areas, which was observed in TS patients during interference inhibition, might be considered a compensatory mechanism (Marsh et al., 2007). During motor response inhibition, patients with TS showed increased inhibition-related frontal brain activity in an event-related potential (ERP) study (Johannes et al., 2001). The authors noted that compensatory brain activation may explain why studies have not consistently observed response inhibition deficits in TS patients. No difference was found in brain activation between patients and controls during performance of a Go/No Go task, although the sample size was limited (Hershey et al., 2004). No study has yet investigated the direct effects of pharmacological treatment on behavioral or functional measures of response inhibition in TS, although, see Wylie et al. (2013).
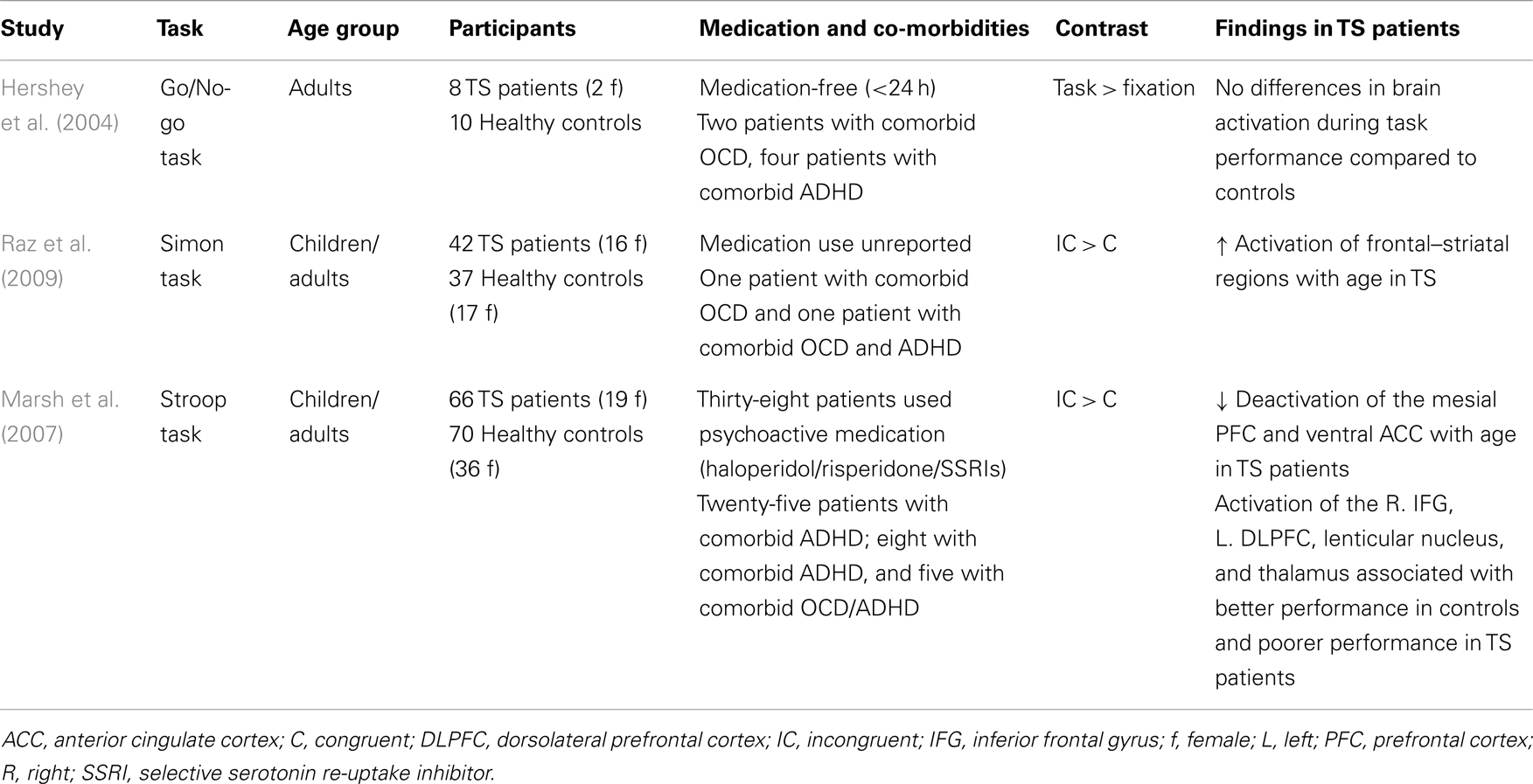
Table 3. Overview of fMRI studies that have used interference control tasks and response inhibition tasks in patients with Tourette’s syndrome.
In summary, behavioral inhibitory deficits may be limited to a subgroup of Tourette’s patients, as compensatory brain activation during inhibition may conceal behavioral deficits in response inhibition in some patients. Increased dopamine transmission in CSTC circuits may underlie the deficits in response inhibition.
Trichotillomania
Trichotillomania is an obsessive–compulsive related disorder (American Psychiatric Association, 2013). Patients with this disorder experience an urge to pull out their hair, which causes distress and functional impairment. Due to similarities between TTM and OCD, TTM was historically treated with SSRIs. Although initially considered effective in TTM (Stein et al., 1997), more recent studies report that SSRIs are ineffective in TTM (Streichenwein and Thornby, 1995; van Minnen et al., 2003) or only effective in a specific subgroup of TTM patients (Stanley et al., 1997a; Gadde et al., 2007). More recent clinical trials showed that treatment with atypical antipsychotics, such as olanzapine and aripiprazole, which exert their effects on among others, the serotonergic and dopaminergic system, are more promising (Van Ameringen et al., 2010; White and Koran, 2011).
It has been suggested that TTM symptoms originate from CSTC circuit dysfunction (Mataix-Cols and van den Heuvel, 2006). In support of this hypothesis, structural abnormalities in frontal areas and regions of the striatum have been observed in TTM (Chamberlain et al., 2008). As patients have difficulty suppressing a motor response, i.e., pulling out their hair, deficits in response inhibition may underlie the symptoms of this disorder (Chamberlain et al., 2006a).
Research on response inhibition in TTM is limited and conflicting. While performance of a related cognitive control task was unaltered, TTM patients showed deficits in interference control in the Stroop task (Stanley et al., 1997b; Bohne et al., 2005). Deficits in action cancelation have been reported, and the degree of impairment correlated with disease severity (Chamberlain et al., 2006a). Impairment in action restraint was limited to a distinct subgroup of patients with an early onset of the disorder (Bohne et al., 2008).
The neural or pharmacological substrates of response inhibition deficits in TTM have not yet been fully elucidated, as no inhibition-related neuroimaging studies have been performed in this patient group. Nor have there been any studies on the effects of pharmacological treatment on response inhibition. TTM patients do, however, exhibit structural abnormalities in CSTC circuit regions associated with inhibition, for instance in the striatum, IFG, SMA, and prefrontal areas (Grachev, 1997; O’Sullivan et al., 1997; Chamberlain et al., 2008), which may underlie response inhibition impairment in TTM.
Attention-Deficit Hyperactivity Disorder
Attention-deficit hyperactivity disorder is a neuropsychiatric disorder characterized by hyperactivity, inattentiveness, and impulsiveness (American Psychiatric Association, 2013). It is a common disorder, as it is thought to affect almost 10% of school-aged children (Froehlich et al., 2007). The neuropharmacology of ADHD is complex and still not well-understood. Current evidence suggest that ADHD is characterized by deficits in the noradrenalin and dopamine systems [for a review, see McAlonan et al. (2009)], although some studies show additional involvement of the serotonergic (Oades et al., 2002) system. Pharmacotherapeutic treatment of ADHD with methylphenidate, amphetamines, or atomoxetine is effective in treating symptoms, presumably through increasing extracellular levels of dopamine and noradrenalin [see Prince (2008) for a review].
Patients with ADHD show behavioral impairments on a number of interference control tasks, including the Simon task and the Flanker task (Rubia et al., 2011a; Sebastian et al., 2012). Activation of the ACC, IFG, thalamus, SMA, striatum, and inferior parietal cortex is decreased in ADHD patients during interference control (see Table 4). A recent meta-analysis revealed decreased activation of CSTC areas, including the right IFG, insular cortex, right caudate nucleus, left inferior parietal cortex, and left ACC in ADHD patients during performance of the Stroop and the Simon task (Hart et al., 2012).
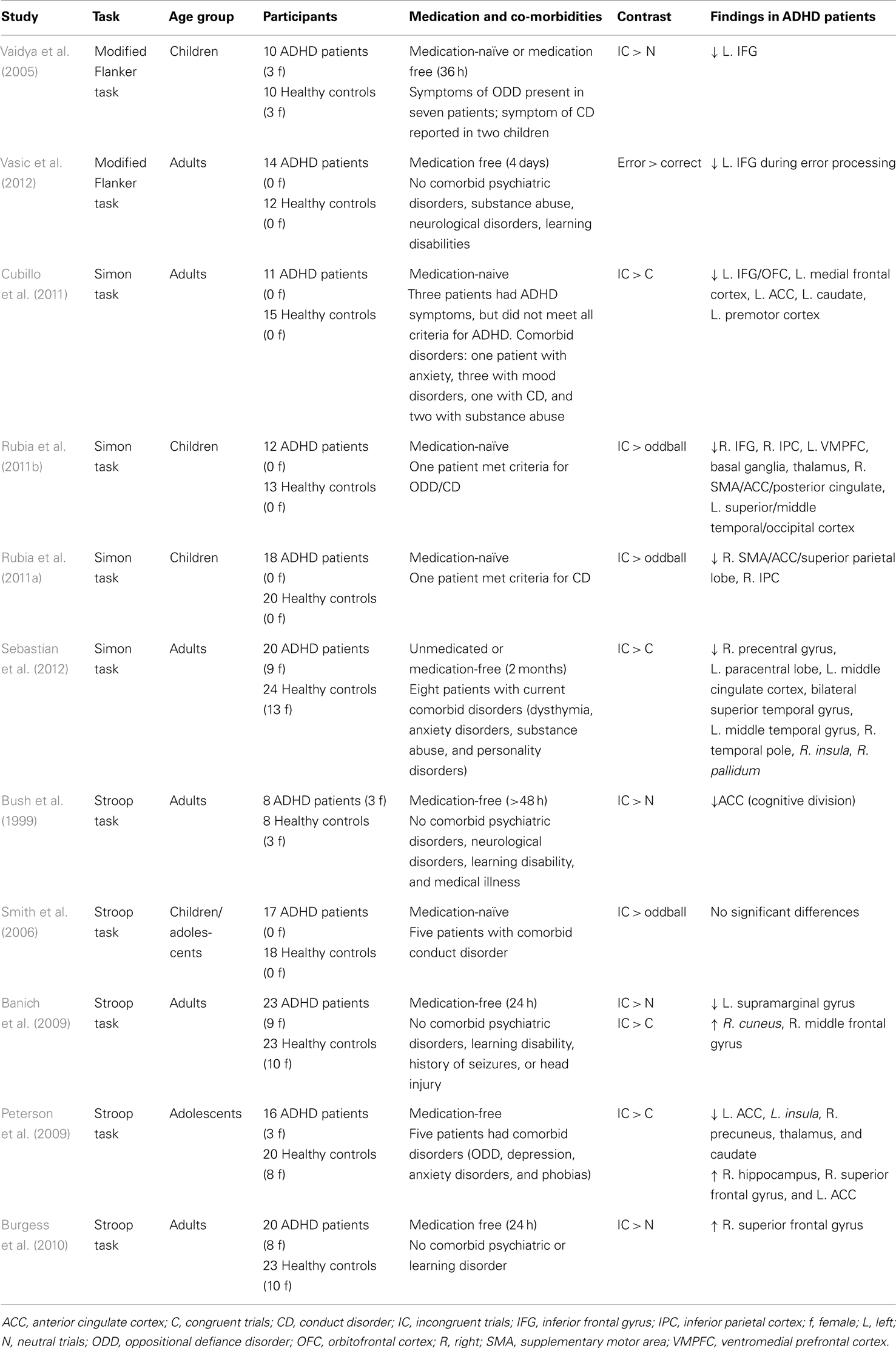
Table 4. Overview of fMRI studies that have used interference control tasks in patients with attention-deficit hyperactivity disorder.
Motor response inhibition is also affected in ADHD. A meta-analysis of 24 Stop-signal paradigm studies showed increased SSRT and mean GO reaction times in ADHD patients (Alderson et al., 2007). Structural abnormalities have been observed in CSTC circuit areas, including the IFG, caudate, and globus pallidus (Durston, 2003; Sowell et al., 2003; Batty et al., 2010; Depue et al., 2010; Frodl and Skokauskas, 2012), leading some to argue that altered brain structure of these areas may underlie the impairments in response inhibition (Chambers et al., 2009). Gray matter volume of the right IFG, ACC, caudate nucleus, medial temporal lobe, and globus pallidus correlated negatively with task performance in patients (Casey et al., 1997; McAlonan et al., 2009). Functional neuroimaging studies show reduced inhibition-related activation of the caudate nucleus, IFG, and SMA, and increased activation of areas in the temporal and parietal lobe in children and adults with ADHD (see Table 5; Schulz et al., 2004; Tamm et al., 2004; Booth et al., 2005; Rubia et al., 2005b; Suskauer et al., 2008; Dibbets et al., 2009; Dillo et al., 2010; Kooistra et al., 2010; Mulligan et al., 2011; Spinelli et al., 2011). A recent meta-analysis of 21 response inhibition studies revealed hypoactivation of the right ACC, right IFG, right insular cortex, right thalamus, left caudate nucleus, and right fusiform gyrus (Hart et al., 2012). In addition to decreased frontal–striatal connectivity, altered frontal–parietal connectivity may also play a role in the response inhibition impairment of ADHD (Cubillo et al., 2010).
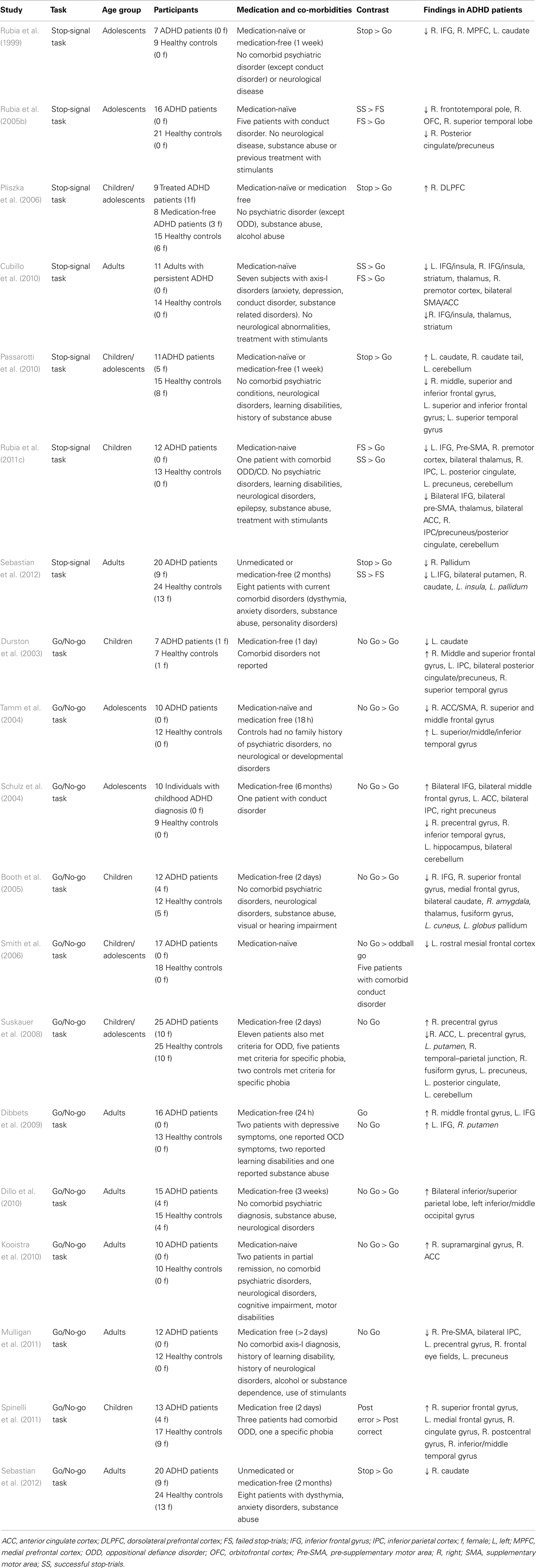
Table 5. Overview of fMRI studies that have used response inhibition tasks in patients with attention-deficit hyperactivity disorder.
Pharmacological studies show that administration of methylphenidate and atomoxetine improve action cancelation (Aron et al., 2003a; Chamberlain et al., 2007a; DeVito et al., 2009; Coghill et al., 2013) and action withholding (Vaidya et al., 1998) in ADHD patients, thereby suggesting that deficits in dopamine and noradrenalin underlie motor response inhibition deficits. Furthermore, use of methylphenidate increased prefrontal and striatal activation during performance of a Go/No Go task in ADHD patients (Vaidya et al., 1998). Methylphenidate also normalizes activation deficits in prefrontal, parietal, temporal, and cerebellar regions during performance of the Stop-signal task (Rubia et al., 2011b). When effects of atomoxetine and methylphenidate were directly compared, both medications normalized left prefrontal underactivation during performance of the stop-signal task, while normalization of the right prefrontal activation was specific to use of methylphenidate (Cubillo et al., 2014).
Several candidate gene studies report on an association between genotype and response inhibition deficits in ADHD. In patients with ADHD, a polymorphism of the DRD4 gene, which is associated with decreased functional activity of the dopamine D4-receptor (Asghari et al., 1995), was related to altered performance on tasks with an inhibitory component (Langley et al., 2004; Bellgrove et al., 2005), impaired performance on the Stroop task (Loo et al., 2008), and reduced prefrontal cortical thickness (Shaw et al., 2007).
Attention-deficit hyperactivity disorder patients homozygous for a polymorphism of the DaT gene associated with increased transporter expression (Brookes et al., 2007), showed increased frontal and parietal brain activation during a modulated Go/no-go task (Braet et al., 2011), and showed increased activation in the striatum, premotor, and parietal cortices during inhibition in the Go/No-go task (Bedard et al., 2010). In contrast, a second study found increased striatal activity during inhibition in polymorphisms that result in decreased function (Durston et al., 2008).
In individuals with a specific polymorphism of the MAO-A gene, associated with lower levels of MAO-A, ADHD symptoms were related to decreased IFG activation during the Stop-signal task (Nymberg et al., 2013). MAO-A genotype of ADHD patients was not related to interference inhibition (Liu et al., 2011).
In summary, behavioral deficits in interference control and motor response inhibition are prominent in ADHD and associated with decreased volume and hypoactivation of CSTC areas. Results of gene-association studies suggest that reduced inhibitory performance may be related to decreased dopamine transmission in CSTC circuits.
Comparison between and Integration Across Disorders
All discussed disorders exhibit symptoms that signify a failure to inhibit certain impulses or responses. Response inhibition tasks therefore provide a very good operationalization to study the neural correlates of some dysfunctions contributing to the symptomatology of these disorders. From the above reviewed literature we can conclude that, overall, patients with obsessive–compulsive or related disorders exhibit deficits in response inhibition concomitant with alterations in the task-related brain activity. Whether these brain areas are hypo- or hyperactivated compared with matched healthy controls depends largely on the complexity of the task. In general, we can state that patients compensate behavior by recruiting additional inhibition-related brain areas, explaining why behavioral performance is often normal, but only during less complex tasks (e.g., the Flanker task and the Simon task). With increasing task demand (e.g., the Go/no-go and the Stop-signal task), these compensational mechanisms fail and patients start to show behavioral impairments and decreased inhibition-related neural circuit activity. This phenomenon has also been observed in healthy subjects (Sebastian et al., 2013a), although they can “endure” tasks with higher demands before overstressing the inhibition network and concomitant decrements in performance. In other words, patients exhibit performance impairments and failure of compensatory activation at a lower task load than healthy controls. This has also been observed in OCD patients and their siblings and adult patients with ADHD while performing a working memory task such as the N-Back (de Vries et al., 2013; Ko et al., 2013). Figure 2 illustrates this as a shift to the left of an inverse U-shape relation between task load and inhibition-related activity. This shift in compensatory abilities does not have to be specific for the discussed disorders but may also apply to others, such Parkinson’s disease (Vriend et al., under review) or even natural aging (Sebastian et al., 2013a).
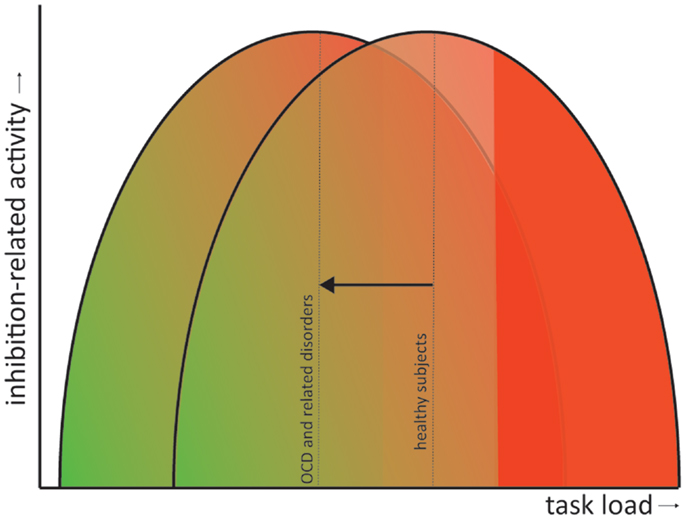
Figure 2. Shift in the inverted U-shaped relation between task load and inhibition-related activity. Inhibition-related neural circuit activity gradually increases with task load (green to red gradient). However, when task demands become too high the compensatory activity starts to fail and behavioral performance becomes impaired (solid red). In obsessive– compulsive and related disorders performance impairments and failure of compensatory neural activation occur at a lower task load than in healthy controls (visualized as a shift of the inverted U-shaped curve to the left).
The actual neurobiological mechanism for this shift is, unfortunately, less apparent and may involve (interactions between) electrophysiological anomalies, neurotransmitter dysfunction, genetic variance, etc. A prime candidate for the cause of the shift might by dysfunction of dopamine signaling. Dopamine is the major neuromodulator in the CSTC circuits and can either facilitate or inhibit their activation depending on the activation of their different receptor subtypes and dopamine concentrations (Alexander et al., 1986; Vriend et al., 2014).
As reviewed above, the CSTC circuits seem to be important for response inhibition (Aron, 2011) and are also involved in the pathophysiology underlying the dysfunctions related to the symptomatology of the obsessive–compulsive and related disorders. Whether or not dopamine is primarily involved in the pathophysiology of these disorders is still under debate, with some of the above reviewed studies showing clear associations, while others do not. Nevertheless, current evidence suggests that ADHD can be seen as a hypodopaminergic disorder, whereas OCD, TTM, and TS can be regarded as hyperdopaminergic disorders (Buse et al., 2013). This is also consistent with the currently available pharmacological treatments, whose neurobiological mechanism is thought to rely on restoring dopamine to physiological levels (Abi-Dargham and Laruelle, 2005; Gerlach et al., 2013). Even SSRI’s and tricyclic antidepressants, the first line pharmacological treatment of OCD, may normalize dopamine levels by upregulation of serotonin signaling, that has an inhibitory effect on dopamine (Boureau and Dayan, 2010). Figure 3 provides a schematic representation of the proposed relation between dopamine levels and inhibitory control. This relation is similar to the inverse U-shaped relation proposed for dopamine levels and working memory function (Cools and D’Esposito, 2011).
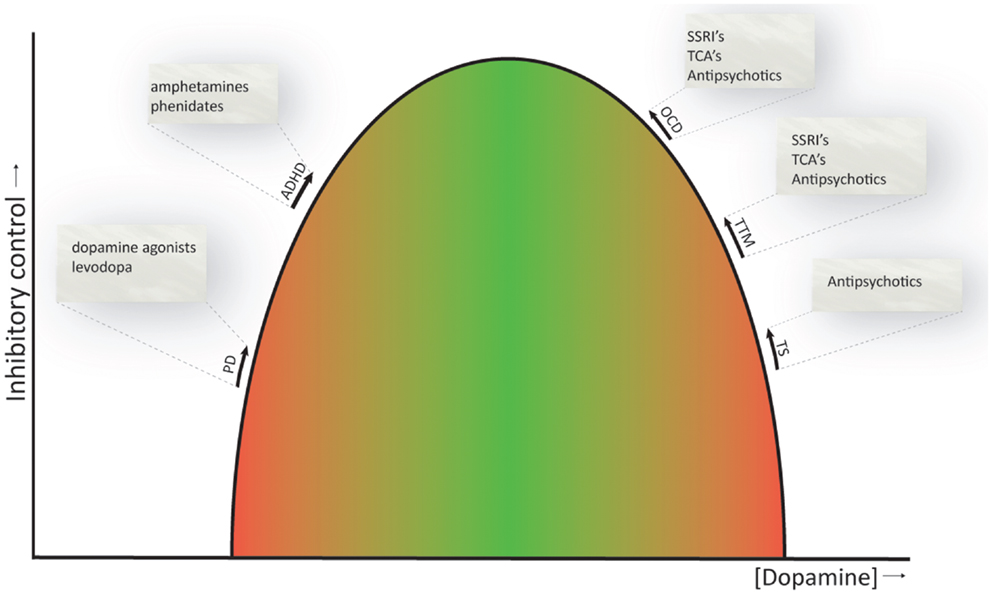
Figure 3. Inverted U-shaped relation between dopamine levels and inhibitory control. The ability to control behaviors, impulses, and urges is influenced by dopamine, and both reduced and increased dopamine levels (green to red gradient) have a detrimental effect on inhibitory control. Current evidence suggests that ADHD is a hypodopaminergic disorder, while OCD, TTM, and TS are considered hyperdopaminergic disorders. Inhibitory deficits are also evident in patients with Parkinson’s disease, a prototypical hypodopaminergic disease. Pharmacotherapeutics used to treat the symptoms of these disorders are listed and are thought to normalize dopamine levels and thereby ameliorate response inhibition (indicated by the arrows). PD, Parkinson’s disease; ADHD, attention-deficit hyperactivity disorder; OCD, obsessive–compulsive disorder; TTM, trichotillomania; TS, Tourette’s syndrome. NB. Since comparison studies across OCD, TTM, and TS are in short supply, the spacing between these disorders on the U-shaped curve is arbitrary and does not necessarily represent actual differences in dopamine levels between these disorders.
The proposed relation obviously does not provide the full story and is merely intended as a framework to understand some of the findings discussed in this review. Dopamine has differential effects in the prefrontal cortex and striatum, different firing modes (i.e., tonic and phasic) and highly complex interactions with other neurotransmitter systems, including the serotonin, noradrenalin, and glutamate system, and even hormones, such as estrogens (Boureau and Dayan, 2010; Cools and D’Esposito, 2011; de Bartolomeis et al., 2013), which prohibits a clear understanding of the influence of these neurotransmitters on brain activity and behavior.
In short, the functional and behavioral deficits in response inhibition in obsessive–compulsive and related disorders can be conceptualized as a shift in the relation between task demands and inhibition-related neural circuit activity. What causes this shift and what could thereby underlie the symptoms of these disorders is currently unknown, although we postulate that dopamine plays a critical role.
Conclusion and Future Directions
The aim of this review was to provide an overview of the studies that examined the neural, pharmacological, and genetic substrates of inhibitory impairment of disorders within the impulsive–compulsive spectrum, with a focus on OCD, ADHD, TS, and TTM. We have shown that functionally and behaviorally impaired response inhibition is a shared characteristic among these disorders and may underlie at least some of the dysfunctions related to the symptomatology of the disorders. Neuroimaging studies suggest that inhibition-related brain areas are mostly hypoactivated in ADHD and OCD (although dependent on the task load), while studies in TS have provided mixed results. To our knowledge, no study has yet been published on the neural correlates of response inhibition in TTM. Dopamine and serotonin signaling seems to be important for response inhibition and dysfunction of these neurotransmitters has been frequently observed in the obsessive–compulsive and related disorders. Nevertheless, almost all imaging studies on neurotransmitters have been performed in patients that received (chronic) pharmacotherapy, which may have influenced the scan directly, due to competition of the drug with a radioligand for a specific binding site, or indirectly because the brain adapts to the pharmacological effects (Wang et al., 2013). For a better understanding of the pathophysiology of the disease itself and the identification of novel treatment targets, more studies are needed in medication-naïve patients. Prospective follow-up of these patients after commencing treatment can subsequently provide insights into the effect of treatment on response inhibition impairments and its relation to disorder-specific symptoms. Lastly, there is a relative lack of studies that compare the pathophysiology of inhibitory deficits across related mental disorders. Such studies allow the identification of common as well as specific disease biomarkers of impulsivity and compulsivity symptoms.
Author Contributions
All authors were involved in design of this review; Laura S. van Velzen and Chris Vriend drafted the first version of the manuscript; all authors contributed to the interpretation of the literature, revised earlier drafts of this article and have approved the final version of the manuscript and its submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This article was supported by a NARSAD young investigator award of the Brain and Behavior Research Foundation granted to Dr. Odile A. van den Heuvel and a grant from the Netherlands Organization for Scientific Research (NWO-ZonMw VENI grant 916.86.036, Dr. Odile A. van den Heuvel).
References
Abi-Dargham, A., and Laruelle, M. (2005). Mechanisms of action of second generation antipsychotic drugs in schizophrenia: insights from brain imaging studies. Eur. Psychiatry 20, 15–27. doi: 10.1016/j.eurpsy.2004.11.003
Albin, R. L., and Mink, J. W. (2006). Recent advances in Tourette syndrome research. Trends Neurosci. 29, 175–182. doi:10.1016/j.tins.2006.01.001
Alderson, R. M., Rapport, M. D., and Kofler, M. J. (2007). Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. J. Abnorm. Child Psychol. 35, 745–758. doi:10.1007/s10802-007-9131-6
Alexander, G. E., DeLong, M. R., and Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381. doi:10.1146/annurev.ne.09.030186.002041
American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders. 5th Edn, Arlington: American Psychiatric Publishing.
Anderson, I. M., Clark, L., Elliott, R., Kulkarni, B., Williams, S. R., and Deakin, J. F. (2002). 5-HT(2C) receptor activation by m-chlorophenylpiperazine detected in humans with fMRI. Neuroreport 13, 1547–1551. doi:10.1097/00001756-200208270-00012
Aron, A. R. (2011). From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol. Psychiatry 69, e55–e68. doi:10.1016/j.biopsych.2010.07.024
Aron, A. R., Dowson, J. H., Sahakian, B. J., and Robbins, T. W. (2003a). Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 54, 1465–1468. doi:10.1016/S0006-3223(03)00609-7
Aron, A. R., Fletcher, P. C., Bullmore, E. T., Sahakian, B. J., and Robbins, T. W. (2003b). Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 6, 115–116. doi:10.1038/nn1003
Asghari, V., Sanyal, S., Buchwaldt, S., Paterson, A., Jovanovic, V., and Van Tol, H. H. (1995). Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J. Neurochem. 65, 1157–1165. doi:10.1046/j.1471-4159.1995.65031157.x
Baehne, C. G., Ehlis, A. C., Plichta, M. M., Conzelmann, A., Pauli, P., Jacob, C., et al. (2009). TPH2 gene variants modulate response control processes in adult ADHD patients and healthy individuals. Mol. Psychiatry 14, 1032–1039. doi:10.1038/mp.2008.39
Banich, M. T., Burgess, G. C., Depue, B. E., Ruzic, L., Bidwell, L. C., Hitt-Laustsen, S., et al. (2009). The neural basis of sustained and transient attentional control in young adults with ADHD. Neuropsychologia 47, 3095–3104. doi:10.1016/j.neuropsychologia.2009.07.005
Bannon, S., Gonsalvez, C. J., Croft, R. J., and Boyce, P. M. (2002). Response inhibition deficits in obsessive-compulsive disorder. Psychiatry Res. 110, 165–174. doi:10.1016/S0165-1781(02)00104-X
Bari, A., Eagle, D. M., Mar, A. C., Robinson, E. S., and Robbins, T. W. (2009). Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl.) 205, 273–283. doi:10.1007/s00213-009-1537-0
Bari, A., and Robbins, T. W. (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 108, 44–79. doi:10.1016/j.pneurobio.2013.06.005
Baron-Cohen, S., Cross, P., Crowson, M., and Robertson, M. (1994). Can children with Gilles de la Tourette syndrome edit their intentions? Psychol. Med. 24, 29–40. doi:10.1017/S0033291700026805
Batty, M. J., Liddle, E. B., Pitiot, A., Toro, R., Groom, M. J., Scerif, G., et al. (2010). Cortical gray matter in attention-deficit/hyperactivity disorder: a structural magnetic resonance imaging study. J. Am. Acad. Child Adolesc. Psychiatry 49, 229–238. doi:10.1097/00004583-201003000-00006
Bedard, A. C., Schulz, K. P., Cook, E. H. Jr., Fan, J., Clerkin, S. M., Ivanov, I., et al. (2010). Dopamine transporter gene variation modulates activation of striatum in youth with ADHD. Neuroimage 53, 935–942. doi:10.1016/j.neuroimage.2009.12.041
Bellgrove, M. A., Hawi, Z., Lowe, N., Kirley, A., Robertson, I. H., and Gill, M. (2005). DRD4 gene variants and sustained attention in attention deficit hyperactivity disorder (ADHD): effects of associated alleles at the VNTR and -521 SNP. Am. J. Med. Genet. B Neuropsychiatr. Genet. 136B, 81–86. doi:10.1002/ajmg.b.30193
Blasi, G., Goldberg, T. E., Weickert, T., Das, S., Kohn, P., Zoltick, B., et al. (2006). Brain regions underlying response inhibition and interference monitoring and suppression. Eur. J. Neurosci. 23, 1658–1664. doi:10.1111/j.1460-9568.2006.04680.x
Bloch, M., State, M., and Pittenger, C. (2011). Recent advances in Tourette syndrome. Curr. Opin. Neurol. 24, 119–125. doi:10.1097/WCO.0b013e328344648c
Bohne, A., Keuthen, N. J., Tuschen-Caffier, B., and Wilhelm, S. (2005). Cognitive inhibition in trichotillomania and obsessive-compulsive disorder. Behav. Res. Ther. 43, 923–942. doi:10.1016/j.brat.2004.06.014
Bohne, A., Savage, C. R., Deckersbach, T., Keuthen, N. J., and Wilhelm, S. (2008). Motor inhibition in trichotillomania and obsessive-compulsive disorder. J. Psychiatr. Res. 42, 141–150. doi:10.1016/j.jpsychires.2006.11.008
Booth, J. R., Burman, D. D., Meyer, J. R., Lei, Z., Trommer, B. L., Davenport, N. D., et al. (2005). Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD). J. Child Psychol. Psychiatry 46, 94–111. doi:10.1111/j.1469-7610.2004.00337.x
Boureau, Y.-L., and Dayan, P. (2010). Opponency revisited: competition and cooperation between dopamine and serotonin. Neuropsychopharmacology 36, 74–97. doi:10.1038/npp.2010.151
Braet, W., Johnson, K. A., Tobin, C. T., Acheson, R., McDonnell, C., Hawi, Z., et al. (2011). fMRI activation during response inhibition and error processing: the role of the DAT1 gene in typically developing adolescents and those diagnosed with ADHD. Neuropsychologia 49, 1641–1650. doi:10.1016/j.neuropsychologia.2011.01.001
Brookes, K. J., Neale, B. M., Sugden, K., Khan, N., Asherson, P., and D’Souza, U. M. (2007). Relationship between VNTR polymorphisms of the human dopamine transporter gene and expression in post-mortem midbrain tissue. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B, 1070–1078. doi:10.1002/ajmg.b.30572
Burgess, G. C., Depue, B. E., Ruzic, L., Willcutt, E. G., Du, Y. P., and Banich, M. T. (2010). Attentional control activation relates to working memory in attention-deficit/hyperactivity disorder. Biol. Psychiatry 67, 632–640. doi:10.1016/j.biopsych.2009.10.036
Buse, J., Schoenefeld, K., Munchau, A., and Roessner, V. (2013). Neuromodulation in Tourette syndrome: dopamine and beyond. Neurosci. Biobehav. Rev. 37, 1069–1084. doi:10.1016/j.neubiorev.2012.10.004
Bush, G., Frazier, J. A., Rauch, S. L., Seidman, L. J., Whalen, P. J., Jenike, M. A., et al. (1999). Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting Stroop. Biol. Psychiatry 45, 1542–1552. doi:10.1016/S0006-3223(99)00083-9
Cai, W., George, J. S., Verbruggen, F., Chambers, C. D., and Aron, A. R. (2012). The role of the right presupplementary motor area in stopping action: two studies with event-related transcranial magnetic stimulation. J. Neurophysiol. 108, 380–389. doi:10.1152/jn.00132.2012
Casey, B. J., Castellanos, F. X., Giedd, J. N., Marsh, W. L., Hamburger, S. D., Schubert, A. B., et al. (1997). Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 36, 374–383. doi:10.1097/00004583-199703000-00016
Chamberlain, S. R., Blackwell, A. D., Fineberg, N. A., Robbins, T. W., and Sahakian, B. J. (2005). The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci. Biobehav. Rev. 29, 399–419. doi:10.1016/j.neubiorev.2004.11.006
Chamberlain, S. R., Del Campo, N., Dowson, J., Muller, U., Clark, L., Robbins, T. W., et al. (2007a). Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol. Psychiatry 62, 977–984. doi:10.1016/j.biopsych.2007.03.003
Chamberlain, S. R., Fineberg, N. A., Blackwell, A. D., Robbins, T. W., and Sahakian, B. J. (2006a). Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am. J. Psychiatry 163, 1282–1284. doi:10.1176/appi.ajp.163.7.1282
Chamberlain, S. R., Fineberg, N. A., Menzies, L. A., Blackwell, A. D., Bullmore, E. T., Robbins, T. W., et al. (2007b). Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am. J. Psychiatry 164, 335–338. doi:10.1176/appi.ajp.164.2.335
Chamberlain, S. R., Menzies, L. A., Fineberg, N. A., Del Campo, N., Suckling, J., Craig, K., et al. (2008). Grey matter abnormalities in trichotillomania: morphometric magnetic resonance imaging study. Br. J. Psychiatry 193, 216–221. doi:10.1192/bjp.bp.107.048314
Chamberlain, S. R., Muller, U., Blackwell, A. D., Clark, L., Robbins, T. W., and Sahakian, B. J. (2006b). Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311, 861–863. doi:10.1126/science.1121218
Chambers, C. D., Bellgrove, M. A., Stokes, M. G., Henderson, T. R., Garavan, H., Robertson, I. H., et al. (2006). Executive “brake failure” following deactivation of human frontal lobe. J. Cogn. Neurosci. 18, 444–455. doi:10.1162/089892906775990606
Chambers, C. D., Garavan, H., and Bellgrove, M. A. (2009). Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 33, 631–646. doi:10.1016/j.neubiorev.2008.08.016
Channon, S., Drury, H., Martinos, M., Robertson, M. M., Orth, M., and Crawford, S. (2009). Tourette’s syndrome (TS): inhibitory performance in adults with uncomplicated TS. Neuropsychology 23, 359–366. doi:10.1037/a0014552
Channon, S., Gunning, A., Frankl, J., and Robertson, M. M. (2006). Tourette’s syndrome (TS): cognitive performance in adults with uncomplicated TS. Neuropsychology 20, 58–65. doi:10.1037/0894-4105.20.1.58
Chen, J., Lipska, B. K., Halim, N., Ma, Q. D., Matsumoto, M., Melhem, S., et al. (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 75, 807–821. doi:10.1086/425589
Cheon, K. A., Ryu, Y. H., Namkoong, K., Kim, C. H., Kim, J. J., and Lee, J. D. (2004). Dopamine transporter density of the basal ganglia assessed with [123I]IPT SPECT in drug-naive children with Tourette’s disorder. Psychiatry Res. 130, 85–95. doi:10.1016/j.pscychresns.2003.06.001
Clark, L., Roiser, J. P., Cools, R., Rubinsztein, D. C., Sahakian, B. J., and Robbins, T. W. (2005). Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: implications for the 5-HT theory of impulsivity. Psychopharmacology (Berl.) 182, 570–578. doi:10.1007/s00213-005-0104-6
Coghill, D. R., Seth, S., Pedroso, S., Usala, T., Currie, J., and Gagliano, A. (2013). Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biol. Psychiatry. doi:10.1016/j.biopsych.2013.10.005
Colzato, L. S., van den Wildenberg, W. P., Van der Does, A. J., and Hommel, B. (2010). Genetic markers of striatal dopamine predict individual differences in dysfunctional, but not functional impulsivity. Neuroscience 170, 782–788. doi:10.1016/j.neuroscience.2010.07.050
Congdon, E., Constable, R. T., Lesch, K. P., and Canli, T. (2009). Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biol. Psychol. 81, 144–152. doi:10.1016/j.biopsycho.2009.03.005
Congdon, E., Lesch, K. P., and Canli, T. (2008). Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 27–32. doi:10.1002/ajmg.b.30557
Cools, R., and D’Esposito, M. (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 69, e113–e125. doi:10.1016/j.biopsych.2011.03.028
Cornish, K. M., Manly, T., Savage, R., Swanson, J., Morisano, D., Butler, N., et al. (2005). Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol. Psychiatry 10, 686–698. doi:10.1038/sj.mp.4001641
Crawford, S., Channon, S., and Robertson, M. M. (2005). Tourette’s syndrome: performance on tests of behavioural inhibition, working memory and gambling. J. Child Psychol. Psychiatry 46, 1327–1336. doi:10.1111/j.1469-7610.2005.01419.x
Cubillo, A., Halari, R., Ecker, C., Giampietro, V., Taylor, E., and Rubia, K. (2010). Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J. Psychiatr. Res. 44, 629–639. doi:10.1016/j.jpsychires.2009.11.016
Cubillo, A., Halari, R., Giampietro, V., Taylor, E., and Rubia, K. (2011). Fronto-striatal underactivation during interference inhibition and attention allocation in grown up children with attention deficit/hyperactivity disorder and persistent symptoms. Psychiatry Res. 193, 17–27. doi:10.1016/j.pscychresns.2010.12.014
Cubillo, A., Smith, A. B., Barrett, N., Giampietro, V., Brammer, M. J., Simmons, A., et al. (2014). Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys. Cereb. Cortex 24, 174–185. doi:10.1093/cercor/bhs296
Cummins, T. D., Hawi, Z., Hocking, J., Strudwick, M., Hester, R., Garavan, H., et al. (2012). Dopamine transporter genotype predicts behavioural and neural measures of response inhibition. Mol. Psychiatry 17, 1086–1092. doi:10.1038/mp.2011.104
Dalley, J. W., and Roiser, J. P. (2012). Dopamine, serotonin and impulsivity. Neuroscience 215, 42–58. doi:10.1016/j.neuroscience.2012.03.065
de Bartolomeis, A., Buonaguro, E., and Iasevoli, F. (2013). Serotonin-glutamate and serotonin-dopamine reciprocal interactions as putative molecular targets for novel antipsychotic treatments: from receptor heterodimers to postsynaptic scaffolding and effector proteins. Psychopharmacology 225, 1–19. doi:10.1007/s00213-012-2921-8
de Vries, F. E., de Wit, S. J., Cath, D. C., van der Werf, Y. D., van der Borden, V., van Rossum, T. B., et al. (2013). Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol. Psychiatry. doi:10.1016/j.biopsych.2013.11.021
de Wit, S. J., de Vries, F. E., van der Werf, Y. D., Cath, D. C., Heslenfeld, D. J., Veltman, E. M., et al. (2012). Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am. J. Psychiatry 169, 1100–1108. doi:10.1176/appi.ajp.2012.12010073
Denys, D., de Geus, F., van Megen, H. J., and Westenberg, H. G. (2004a). A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors. J. Clin. Psychiatry 65, 1040–1048. doi:10.4088/JCP.v65n0803
Denys, D., van der Wee, N., Janssen, J., De Geus, F., and Westenberg, H. G. (2004b). Low level of dopaminergic D2 receptor binding in obsessive-compulsive disorder. Biol. Psychiatry 55, 1041–1045. doi:10.1016/j.biopsych.2004.01.023
Depue, B. E., Burgess, G. C., Bidwell, L. C., Willcutt, E. G., and Banich, M. T. (2010). Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Res. 182, 231–237. doi:10.1016/j.pscychresns.2010.01.012
DeVito, E. E., Blackwell, A. D., Clark, L., Kent, L., Dezsery, A. M., Turner, D. C., et al. (2009). Methylphenidate improves response inhibition but not reflection-impulsivity in children with attention deficit hyperactivity disorder (ADHD). Psychopharmacology (Berl.) 202, 531–539. doi:10.1007/s00213-008-1337-y
DeVito, T. J., Drost, D. J., Pavlosky, W., Neufeld, R. W., Rajakumar, N., McKinlay, B. D., et al. (2005). Brain magnetic resonance spectroscopy in Tourette’s disorder. J. Am. Acad. Child Adolesc. Psychiatry 44, 1301–1308. doi:10.1097/01.chi.0000181046.52078.f4
Dibbets, P., Evers, L., Hurks, P., Marchetta, N., and Jolles, J. (2009). Differences in feedback- and inhibition-related neural activity in adult ADHD. Brain Cogn. 70, 73–83. doi:10.1016/j.bandc.2009.01.001
Dillo, W., Goke, A., Prox-Vagedes, V., Szycik, G. R., Roy, M., Donnerstag, F., et al. (2010). Neuronal correlates of ADHD in adults with evidence for compensation strategies – a functional MRI study with a Go/No-Go paradigm. Ger. Med. Sci. 8, Doc09. doi:10.3205/000098
Donders, F. C. (1969). On the speed of mental processes. Acta Psychol. 30, 412–431. [Originally published in Dutch, translated by W. G. Koster]
Draganski, B., Martino, D., Cavanna, A. E., Hutton, C., Orth, M., Robertson, M. M., et al. (2010). Multispectral brain morphometry in Tourette syndrome persisting into adulthood. Brain 133(Pt 12), 3661–3675. doi:10.1093/brain/awq300
Drueke, B., Boecker, M., Schlaegel, S., Moeller, O., Hiemke, C., Grunder, G., et al. (2010). Serotonergic modulation of response inhibition and re-engagement? Results of a study in healthy human volunteers. Hum. Psychopharmacol. 25, 472–480. doi:10.1002/hup.1141
Durston, S. (2003). A review of the biological bases of ADHD: what have we learned from imaging studies? Ment. Retard. Dev. Disabil. Res. Rev. 9, 184–195. doi:10.1002/mrdd.10079
Durston, S., Fossella, J. A., Mulder, M. J., Casey, B. J., Ziermans, T. B., Vessaz, M. N., et al. (2008). Dopamine transporter genotype conveys familial risk of attention-deficit/hyperactivity disorder through striatal activation. J. Am. Acad. Child Adolesc. Psychiatry 47, 61–67. doi:10.1097/chi.0b013e31815a5f17
Durston, S., Tottenham, N. T., Thomas, K. M., Davidson, M. C., Eigsti, I. M., Yang, Y., et al. (2003). Differential patterns of striatal activation in young children with and without ADHD. Biol. Psychiatry 53, 871–878. doi:10.1016/S0006-3223(02)01904-2
Eagle, D. M., Bari, A., and Robbins, T. W. (2008). The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl.) 199, 439–456. doi:10.1007/s00213-008-1127-6
Eagle, D. M., Lehmann, O., Theobald, D. E., Pena, Y., Zakaria, R., Ghosh, R., et al. (2009). Serotonin depletion impairs waiting but not stop-signal reaction time in rats: implications for theories of the role of 5-HT in behavioral inhibition. Neuropsychopharmacology 34, 1311–1321. doi:10.1038/npp.2008.202
Eagle, D. M., Tufft, M. R., Goodchild, H. L., and Robbins, T. W. (2007). Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology (Berl.) 192, 193–206. doi:10.1007/s00213-007-0701-7
Eichele, H., Eichele, T., Hammar, A., Freyberger, H. J., Hugdahl, K., and Plessen, K. J. (2010). Go/NoGo performance in boys with Tourette syndrome. Child Neuropsychol. 16, 162–168. doi:10.1080/09297040903150182
Evers, E. A., van der Veen, F. M., van Deursen, J. A., Schmitt, J. A., Deutz, N. E., and Jolles, J. (2006). The effect of acute tryptophan depletion on the BOLD response during performance monitoring and response inhibition in healthy male volunteers. Psychopharmacology (Berl.) 187, 200–208. doi:10.1007/s00213-006-0411-6
Felling, R. J., and Singer, H. S. (2011). Neurobiology of Tourette syndrome: current status and need for further investigation. J. Neurosci. 31, 12387–12395. doi:10.1523/JNEUROSCI.0150-11.2011
Fineberg, N. A., Sivakumaran, T., Roberts, A., and Gale, T. (2005). Adding quetiapine to SRI in treatment-resistant obsessive-compulsive disorder: a randomized controlled treatment study. Int. Clin. Psychopharmacol. 20, 223–226. doi:10.1097/00004850-200507000-00005
Fitzgerald, K. D., Welsh, R. C., Gehring, W. J., Abelson, J. L., Himle, J. A., Liberzon, I., et al. (2005). Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol. Psychiatry 57, 287–294. doi:10.1016/j.biopsych.2004.10.038
Floden, D., and Stuss, D. T. (2006). Inhibitory control is slowed in patients with right superior medial frontal damage. J. Cogn. Neurosci. 18, 1843–1849. doi:10.1162/jocn.2006.18.11.1843
Frodl, T., and Skokauskas, N. (2012). Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr. Scand. 125, 114–126. doi:10.1111/j.1600-0447.2011.01786.x
Froehlich, T. E., Lanphear, B. P., Epstein, J. N., Barbaresi, W. J., Katusic, S. K., and Kahn, R. S. (2007). Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch. Pediatr. Adolesc. Med. 161, 857–864. doi:10.1001/archpedi.161.9.857
Gadde, K. M., Ryan Wagner, H. II, Connor, K. M., and Foust, M. S. (2007). Escitalopram treatment of trichotillomania. Int. Clin. Psychopharmacol. 22, 39–42. doi:10.1097/01.yic.0000224799.59524.50
Gerlach, M., Grunblatt, E., and Lange, K. W. (2013). Is the treatment with psychostimulants in children and adolescents with attention deficit hyperactivity disorder harmful for the dopaminergic system? Atten. Defic. Hyperact. Disord. 5, 71–81. doi:10.1007/s12402-013-0105-y
Ghahremani, D. G., Lee, B., Robertson, C. L., Tabibnia, G., Morgan, A. T., De Shetler, N., et al. (2012). Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J. Neurosci. 32, 7316–7324. doi:10.1523/JNEUROSCI.4284-11.2012
Ghanizadeh, A., and Mosallaei, S. (2009). Psychiatric disorders and behavioral problems in children and adolescents with Tourette syndrome. Brain Dev. 31, 15–19. doi:10.1016/j.braindev.2008.03.010
Gilbert, D. L., Christian, B. T., Gelfand, M. J., Shi, B., Mantil, J., and Sallee, F. R. (2006). Altered mesolimbocortical and thalamic dopamine in Tourette syndrome. Neurology 67, 1695–1697. doi:10.1212/01.wnl.0000242733.18534.2c
Gottesman, I. I., and Gould, T. D. (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160, 636–645. doi:10.1176/appi.ajp.160.4.636
Grachev, I. D. (1997). MRI-based morphometric topographic parcellation of human neocortex in trichotillomania. Psychiatry Clin. Neurosci. 51, 315–321. doi:10.1111/j.1440-1819.1997.tb03205.x
Hart, H., Radua, J., Nakao, T., Mataix-Cols, D., and Rubia, K. (2012). Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. Arch. Gen. Psychiatry 70, 1–14. doi:10.1001/jamapsychiatry.2013.277
Haugbol, S., Pinborg, L. H., Regeur, L., Hansen, E. S., Bolwig, T. G., Nielsen, F. A., et al. (2007). Cerebral 5-HT2A receptor binding is increased in patients with Tourette’s syndrome. Int. J. Neuropsychopharmacol. 10, 245–252. doi:10.1017/S1461145706006559
Hershey, T., Black, K. J., Hartlein, J., Braver, T. S., Barch, D. M., Carl, J. L., et al. (2004). Dopaminergic modulation of response inhibition: an fMRI study. Brain Res. Cogn. Brain Res. 20, 438–448. doi:10.1016/j.cogbrainres.2004.03.018
Hesse, S., Muller, U., Lincke, T., Barthel, H., Villmann, T., Angermeyer, M. C., et al. (2005). Serotonin and dopamine transporter imaging in patients with obsessive-compulsive disorder. Psychiatry Res. 140, 63–72. doi:10.1016/j.pscychresns.2005.07.002
Hirvonen, M. M., Laakso, A., Nagren, K., Rinne, J. O., Pohjalainen, T., and Hietala, J. (2009). C957T polymorphism of dopamine D2 receptor gene affects striatal DRD2 in vivo availability by changing the receptor affinity. Synapse 63, 907–912. doi:10.1002/syn.20672
Holmes, A. J., Bogdan, R., and Pizzagalli, D. A. (2010). Serotonin transporter genotype and action monitoring dysfunction: a possible substrate underlying increased vulnerability to depression. Neuropsychopharmacology 35, 1186–1197. doi:10.1038/npp.2009.223
Horacek, J., Zavesicka, L., Tintera, J., Dockery, C., Platilova, V., Kopecek, M., et al. (2005). The effect of tryptophan depletion on brain activation measured by functional magnetic resonance imaging during the Stroop test in healthy subjects. Physiol. Res. 54, 235–244.
Huster, R. J., Enriquez-Geppert, S., Lavallee, C. F., Falkenstein, M., and Herrmann, C. S. (2013). Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. Int. J. Psychophysiol. 87, 217–233. doi:10.1016/j.ijpsycho.2012.08.001
Huyser, C., Veltman, D. J., Wolters, L. H., de Haan, E., and Boer, F. (2011). Developmental aspects of error and high-conflict-related brain activity in pediatric obsessive-compulsive disorder: a fMRI study with a Flanker task before and after CBT. J. Child Psychol. Psychiatry 52, 1251–1260. doi:10.1111/j.1469-7610.2011.02439.x
Hwang, W. J., Yao, W. J., Fu, Y. K., and Yang, A. S. (2008). [99mTc]TRODAT-1/[123I]IBZM SPECT studies of the dopaminergic system in Tourette syndrome. Psychiatry Res. 162, 159–166. doi:10.1016/j.pscychresns.2007.04.006
Johannes, S., Wieringa, B. M., Mantey, M., Nager, W., Rada, D., Muller-Vahl, K. R., et al. (2001). Altered inhibition of motor responses in Tourette syndrome and obsessive-compulsive disorder. Acta Neurol. Scand. 104, 36–43. doi:10.1034/j.1600-0404.2001.00308.x
Kang, D. H., Jang, J. H., Han, J. Y., Kim, J. H., Jung, W. H., Choi, J. S., et al. (2012). Neural correlates of altered response inhibition and dysfunctional connectivity at rest in obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 40, 340–346. doi:10.1016/j.pnpbp.2012.11.001
Kim, C. H., Koo, M. S., Cheon, K. A., Ryu, Y. H., Lee, J. D., and Lee, H. S. (2003). Dopamine transporter density of basal ganglia assessed with [123I]IPT SPET in obsessive-compulsive disorder. Eur. J. Nucl. Med. Mol. Imaging 30, 1637–1643. doi:10.1007/s00259-003-1245-7
Ko, C.-H., Yen, J.-Y., Yen, C.-F., Chen, C.-S., Lin, W.-C., Wang, P.-W., et al. (2013). Brain activation deficit in increased-load working memory tasks among adults with ADHD using fMRI. Eur. Arch. Psychiatry Clin. Neurosci. 263, 1–13. doi:10.1007/s00406-013-0407-2
Kooistra, L., van der Meere, J. J., Edwards, J. D., Kaplan, B. J., Crawford, S., and Goodyear, B. G. (2010). Preliminary fMRI findings on the effects of event rate in adults with ADHD. J. Neural Transm. 117, 655–662. doi:10.1007/s00702-010-0374-y
Kramer, U. M., Cunillera, T., Camara, E., Marco-Pallares, J., Cucurell, D., Nager, W., et al. (2007). The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. J. Neurosci. 27, 14190–14198. doi:10.1523/JNEUROSCI.4229-07.2007
Kramer, U. M., Rojo, N., Schule, R., Cunillera, T., Schols, L., Marco-Pallares, J., et al. (2009). ADHD candidate gene (DRD4 exon III) affects inhibitory control in a healthy sample. BMC Neurosci. 10:150. doi:10.1186/1471-2202-10-150
Langley, K., Marshall, L., van den Bree, M., Thomas, H., Owen, M., O’Donovan, M., et al. (2004). Association of the dopamine D4 receptor gene 7-repeat allele with neuropsychological test performance of children with ADHD. Am. J. Psychiatry 161, 133–138. doi:10.1176/appi.ajp.161.1.133
Lipszyc, J., and Schachar, R. (2010). Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J. Int. Neuropsychol. Soc. 16, 1064–1076. doi:10.1017/S1355617710000895
Liu, H., Dong, F., Meng, Z., Zhang, B., Tan, J., and Wang, Y. (2010). Evaluation of Tourette’s syndrome by (99m)Tc-TRODAT-1 SPECT/CT imaging. Ann. Nucl. Med. 24, 515–521. doi:10.1007/s12149-010-0389-3
Liu, L., Guan, L. L., Chen, Y., Ji, N., Li, H. M., Li, Z. H., et al. (2011). Association analyses of MAOA in Chinese Han subjects with attention-deficit/hyperactivity disorder: family-based association test, case-control study, and quantitative traits of impulsivity. Am. J. Med. Genet. B Neuropsychiatr. Genet. 156B, 737–748. doi:10.1002/ajmg.b.31217
Logan, G. D. (1994). “On the ability to inhibit thought and action: a user’s guide to the stop signal paradigm,” in Inhibitory Processes in Attention, Memory and Language, eds D. Dagenbach and T. H. Carr (San Diego, CA: Academic Press), 189–239.
Loo, S. K., Rich, E. C., Ishii, J., McGough, J., McCracken, J., Nelson, S., et al. (2008). Cognitive functioning in affected sibling pairs with ADHD: familial clustering and dopamine genes. J. Child Psychol. Psychiatry 49, 950–957. doi:10.1111/j.1469-7610.2008.01928.x
MacMaster, F. P., Carrey, N., Sparkes, S., and Kusumakar, V. (2003). Proton spectroscopy in medication-free pediatric attention-deficit/hyperactivity disorder. Biol. Psychiatry 53, 184–187. doi:10.1016/S0006-3223(02)01401-4
Madsen, K., Erritzoe, D., Mortensen, E. L., Gade, A., Madsen, J., Baare, W., et al. (2011). Cognitive function is related to fronto-striatal serotonin transporter levels – a brain PET study in young healthy subjects. Psychopharmacology (Berl.) 213, 573–581. doi:10.1007/s00213-010-1926-4
Malison, R. T., McDougle, C. J., van Dyck, C. H., Scahill, L., Baldwin, R. M., Seibyl, J. P., et al. (1995). [123I]beta-CIT SPECT imaging of striatal dopamine transporter binding in Tourette’s disorder. Am. J. Psychiatry 152, 1359–1361.
Maltby, N., Tolin, D. F., Worhunsky, P., O’Keefe, T. M., and Kiehl, K. A. (2005). Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage 24, 495–503. doi:10.1016/j.neuroimage.2004.08.041
Marsh, R., Horga, G., Parashar, N., Wang, Z., Peterson, B. S., and Simpson, H. B. (2013). Altered activation in fronto-striatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biol. Psychiatry 75, 615–622. doi:10.1016/j.biopsych.2013.02.004
Marsh, R., Zhu, H., Wang, Z., Skudlarski, P., and Peterson, B. S. (2007). A developmental fMRI study of self-regulatory control in Tourette’s syndrome. Am. J. Psychiatry 164, 955–966. doi:10.1176/appi.ajp.164.6.955
Mataix-Cols, D., and van den Heuvel, O. A. (2006). Common and distinct neural correlates of obsessive-compulsive and related disorders. Psychiatr. Clin. North Am. 29, 391–410. doi:10.1016/j.psc.2006.02.006
McAlonan, G. M., Cheung, V., Chua, S. E., Oosterlaan, J., Hung, S. F., Tang, C. P., et al. (2009). Age-related grey matter volume correlates of response inhibition and shifting in attention-deficit hyperactivity disorder. Br. J. Psychiatry 194, 123–129. doi:10.1192/bjp.bp.108.051359
Menzies, L., Achard, S., Chamberlain, S. R., Fineberg, N., Chen, C. H., del Campo, N., et al. (2007). Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain 130(Pt 12), 3223–3236. doi:10.1093/brain/awm205
Mozley, L. H., Gur, R. C., Mozley, P. D., and Gur, R. E. (2001). Striatal dopamine transporters and cognitive functioning in healthy men and women. Am. J. Psychiatry 158, 1492–1499. doi:10.1176/appi.ajp.158.9.1492
Muller-Vahl, K. R., Berding, G., Brucke, T., Kolbe, H., Meyer, G. J., Hundeshagen, H., et al. (2000). Dopamine transporter binding in Gilles de la Tourette syndrome. J. Neurol. 247, 514–520. doi:10.1007/PL00007806
Mulligan, R. C., Knopik, V. S., Sweet, L. H., Fischer, M., Seidenberg, M., and Rao, S. M. (2011). Neural correlates of inhibitory control in adult attention deficit/hyperactivity disorder: evidence from the Milwaukee longitudinal sample. Psychiatry Res. 194, 119–129. doi:10.1016/j.pscychresns.2011.02.003
Nabeyama, M., Nakagawa, A., Yoshiura, T., Nakao, T., Nakatani, E., Togao, O., et al. (2008). Functional MRI study of brain activation alterations in patients with obsessive-compulsive disorder after symptom improvement. Psychiatry Res. 163, 236–247. doi:10.1016/j.pscychresns.2007.11.001
Nakao, T., Nakagawa, A., Yoshiura, T., Nakatani, E., Nabeyama, M., Sanematsu, H., et al. (2009). Duration effect of obsessive-compulsive disorder on cognitive function: a functional MRI study. Depress. Anxiety 26, 814–823. doi:10.1002/da.20484
Nakao, T., Nakagawa, A., Yoshiura, T., Nakatani, E., Nabeyama, M., Yoshizato, C., et al. (2005a). A functional MRI comparison of patients with obsessive-compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Res. 139, 101–114. doi:10.1016/j.pscychresns.2004.12.004
Nakao, T., Nakagawa, A., Yoshiura, T., Nakatani, E., Nabeyama, M., Yoshizato, C., et al. (2005b). Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol. Psychiatry 57, 901–910. doi:10.1016/j.biopsych.2004.12.039
Nandam, L. S., Hester, R., Wagner, J., Cummins, T. D., Garner, K., Dean, A. J., et al. (2011). Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol. Psychiatry 69, 902–904. doi:10.1016/j.biopsych.2010.11.014
Nandam, L. S., Hester, R., Wagner, J., Dean, A. J., Messer, C., Honeysett, A., et al. (2013). Dopamine D(2) receptor modulation of human response inhibition and error awareness. J. Cogn. Neurosci. 25, 649–656. doi:10.1162/jocn_a_00327
Nee, D. E., Wager, T. D., and Jonides, J. (2007). Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn. Affect. Behav. Neurosci. 7, 1–17. doi:10.3758/CABN.7.1.1
Nigg, J. T. (2000). On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol. Bull. 126, 220–246. doi:10.1037/0033-2909.126.2.220
Nymberg, C., Jia, T., Lubbe, S., Ruggeri, B., Desrivieres, S., Barker, G., et al. (2013). Neural mechanisms of attention-deficit/hyperactivity disorder symptoms are stratified by MAOA genotype. Biol. Psychiatry 74, 607–614. doi:10.1016/j.biopsych.2013.03.027
Oades, R. D., Slusarek, M., Velling, S., and Bondy, B. (2002). Serotonin platelet-transporter measures in childhood attention-deficit/hyperactivity disorder (ADHD): clinical versus experimental measures of impulsivity. World J. Biol. Psychiatry 3, 96–100. doi:10.3109/15622970209150607
Olver, J. S., O’Keefe, G., Jones, G. R., Burrows, G. D., Tochon-Danguy, H. J., Ackermann, U., et al. (2009). Dopamine D1 receptor binding in the striatum of patients with obsessive-compulsive disorder. J. Affect. Disord. 114, 321–326. doi:10.1016/j.jad.2008.06.020
Osinsky, R., Schmitz, A., Alexander, N., Kuepper, Y., Kozyra, E., and Hennig, J. (2009). TPH2 gene variation and conflict processing in a cognitive and an emotional Stroop task. Behav. Brain Res. 198, 404–410. doi:10.1016/j.bbr.2008.11.022
O’Sullivan, R. L., Rauch, S. L., Breiter, H. C., Grachev, I. D., Baer, L., Kennedy, D. N., et al. (1997). Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biol. Psychiatry 42, 39–45. doi:10.1016/S0006-3223(96)00297-1
Ozonoff, S., and Jensen, J. (1999). Brief report: specific executive function profiles in three neurodevelopmental disorders. J. Autism Dev. Disord. 29, 171–177. doi:10.1023/A:1023052913110
Ozonoff, S., Strayer, D. L., McMahon, W. M., and Filloux, F. (1994). Executive function abilities in autism and Tourette syndrome: an information processing approach. J. Child Psychol. Psychiatry 35, 1015–1032. doi:10.1111/j.1469-7610.1994.tb01807.x
Page, L. A., Rubia, K., Deeley, Q., Daly, E., Toal, F., Mataix-Cols, D., et al. (2009). A functional magnetic resonance imaging study of inhibitory control in obsessive-compulsive disorder. Psychiatry Res. 174, 202–209. doi:10.1016/j.pscychresns.2009.05.002
Passamonti, L., Fera, F., Magariello, A., Cerasa, A., Gioia, M. C., Muglia, M., et al. (2006). Monoamine oxidase-A genetic variations influence brain activity associated with inhibitory control: new insight into the neural correlates of impulsivity. Biol. Psychiatry 59, 334–340. doi:10.1016/j.biopsych.2005.07.027
Passarotti, A. M., Sweeney, J. A., and Pavuluri, M. N. (2010). Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res. 181, 36–43. doi:10.1016/j.pscychresns.2009.07.002
Penades, R., Catalan, R., Rubia, K., Andres, S., Salamero, M., and Gasto, C. (2007). Impaired response inhibition in obsessive compulsive disorder. Eur. Psychiatry 22, 404–410. doi:10.1016/j.eurpsy.2006.05.001
Perani, D., Garibotto, V., Gorini, A., Moresco, R. M., Henin, M., Panzacchi, A., et al. (2008). In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage 42, 306–314. doi:10.1016/j.neuroimage.2008.04.233
Peterson, B. S., Potenza, M. N., Wang, Z., Zhu, H., Martin, A., Marsh, R., et al. (2009). An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am. J. Psychiatry 166, 1286–1294. doi:10.1176/appi.ajp.2009.08050724
Peterson, B. S., Skudlarski, P., Anderson, A. W., Zhang, H., Gatenby, J. C., Lacadie, C. M., et al. (1998). A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch. Gen. Psychiatry 55, 326–333. doi:10.1001/archpsyc.55.4.326
Pliszka, S. R., Glahn, D. C., Semrud-Clikeman, M., Franklin, C., Perez, R. III, Xiong, J., et al. (2006). Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am. J. Psychiatry 163, 1052–1060. doi:10.1176/appi.ajp.163.6.1052
Prince, J. (2008). Catecholamine dysfunction in attention-deficit/hyperactivity disorder: an update. J. Clin. Psychopharmacol. 28(3 Suppl. 2), S39–S45. doi:10.1097/JCP.0b013e318174f92a
Rankins, D., Bradshaw, J. L., and Georgiou-Karistianis, N. (2006). The semantic Simon effect in Tourette’s syndrome and obsessive-compulsive disorder. Brain Cogn. 61, 225–234. doi:10.1016/j.bandc.2006.01.002
Ray Li, C. S., Chang, H. L., Hsu, Y. P., Wang, H. S., and Ko, N. C. (2006). Motor response inhibition in children with Tourette’s disorder. J. Neuropsychiatry Clin. Neurosci. 18, 417–419. doi:10.1176/appi.neuropsych.18.3.417
Raz, A., Zhu, H., Yu, S., Bansal, R., Wang, Z., Alexander, G. M., et al. (2009). Neural substrates of self-regulatory control in children and adults with Tourette syndrome. Can. J. Psychiatry 54, 579–588.
Robbins, T. W. (2007). Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 917–932. doi:10.1098/rstb.2007.2097
Robertson, M. M. (2008). The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: the epidemiological and prevalence studies. J. Psychosom. Res. 65, 461–472. doi:10.1016/j.jpsychores.2008.03.006
Robertson, M. M. (2011). Gilles de la Tourette syndrome: the complexities of phenotype and treatment. Br. J. Hosp. Med. (Lond) 72, 100–107.
Roesch-Ely, D., Scheffel, H., Weiland, S., Schwaninger, M., Hundemer, H. P., Kolter, T., et al. (2005). Differential dopaminergic modulation of executive control in healthy subjects. Psychopharmacology (Berl.) 178, 420–430. doi:10.1007/s00213-004-2027-z
Roessner, V., Plessen, K. J., Rothenberger, A., Ludolph, A. G., Rizzo, R., Skov, L., et al. (2011). European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur. Child Adolesc. Psychiatry 20, 173–196. doi:10.1007/s00787-011-0163-7
Roth, R. M., Saykin, A. J., Flashman, L. A., Pixley, H. S., West, J. D., and Mamourian, A. C. (2007). Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biol. Psychiatry 62, 901–909. doi:10.1016/j.biopsych.2006.12.007
Rubia, K., Cubillo, A., Smith, A. B., Woolley, J., Heyman, I., and Brammer, M. J. (2010). Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum. Brain Mapp. 31, 287–299. doi:10.1002/hbm.20864
Rubia, K., Cubillo, A., Woolley, J., Brammer, M. J., and Smith, A. (2011a). Disorder-specific dysfunctions in patients with attention-deficit/hyperactivity disorder compared to patients with obsessive-compulsive disorder during interference inhibition and attention allocation. Hum. Brain Mapp. 32, 601–611. doi:10.1002/hbm.21048
Rubia, K., Halari, R., Cubillo, A., Smith, A. B., Mohammad, A. M., Brammer, M., et al. (2011b). Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naive boys with attention-deficit hyperactivity disorder. Neuropsychopharmacology 36, 1575–1586. doi:10.1038/npp.2011.30
Rubia, K., Halari, R., Mohammad, A. M., Taylor, E., and Brammer, M. (2011c). Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol. Psychiatry 70, 255–262. doi:10.1016/j.biopsych.2011.04.018
Rubia, K., Lee, F., Cleare, A. J., Tunstall, N., Fu, C. H., Brammer, M., et al. (2005a). Tryptophan depletion reduces right inferior prefrontal activation during response inhibition in fast, event-related fMRI. Psychopharmacology (Berl.) 179, 791–803. doi:10.1007/s00213-004-2116-z
Rubia, K., Overmeyer, S., Taylor, E., Brammer, M., Williams, S. C., Simmons, A., et al. (1999). Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am. J. Psychiatry 156, 891–896.
Rubia, K., Russell, T., Overmeyer, S., Brammer, M. J., Bullmore, E. T., Sharma, T., et al. (2001). Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage 13, 250–261. doi:10.1006/nimg.2000.0685
Rubia, K., Smith, A. B., Brammer, M. J., Toone, B., and Taylor, E. (2005b). Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am. J. Psychiatry 162, 1067–1075. doi:10.1176/appi.ajp.162.6.1067
Ruscio, A. M., Stein, D. J., Chiu, W. T., and Kessler, R. C. (2010). The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Mol. Psychiatry 15, 53–63. doi:10.1038/mp.2008.94
Schachar, R., Logan, G. D., Robaey, P., Chen, S., Ickowicz, A., and Barr, C. (2007). Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. J. Abnorm. Child Psychol. 35, 229–238. doi:10.1007/s10802-006-9075-2
Schlosser, R. G., Wagner, G., Schachtzabel, C., Peikert, G., Koch, K., Reichenbach, J. R., et al. (2010). Fronto-cingulate effective connectivity in obsessive compulsive disorder: a study with fMRI and dynamic causal modeling. Hum. Brain Mapp. 31, 1834–1850. doi:10.1002/hbm.20980
Schmitt, J. A., Jorissen, B. L., Sobczak, S., van Boxtel, M. P., Hogervorst, E., Deutz, N. E., et al. (2000). Tryptophan depletion impairs memory consolidation but improves focussed attention in healthy young volunteers. J. Psychopharmacol. 14, 21–29. doi:10.1177/026988110001400102
Scholes, K. E., Harrison, B. J., O’Neill, B. V., Leung, S., Croft, R. J., Pipingas, A., et al. (2007). Acute serotonin and dopamine depletion improves attentional control: findings from the Stroop task. Neuropsychopharmacology 32, 1600–1610. doi:10.1038/sj.npp.1301262
Schulz, K. P., Fan, J., Tang, C. Y., Newcorn, J. H., Buchsbaum, M. S., Cheung, A. M., et al. (2004). Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event-related FMRI study. Am. J. Psychiatry 161, 1650–1657. doi:10.1176/appi.ajp.161.9.1650
Sebastian, A., Baldermann, C., Feige, B., Katzev, M., Scheller, E., Hellwig, B., et al. (2013a). Differential effects of age on subcomponents of response inhibition. Neurobiol. Aging 34, 2183–2193. doi:10.1016/j.neurobiolaging.2013.03.013
Sebastian, A., Gerdes, B., Feige, B., Kloppel, S., Lange, T., Philipsen, A., et al. (2012). Neural correlates of interference inhibition, action withholding and action cancelation in adult ADHD. Psychiatry Res. 202, 132–141. doi:10.1016/j.pscychresns.2012.02.010
Sebastian, A., Pohl, M. F., Kloppel, S., Feige, B., Lange, T., Stahl, C., et al. (2013b). Disentangling common and specific neural subprocesses of response inhibition. Neuroimage 64, 601–615. doi:10.1016/j.neuroimage.2012.09.020
Serra-Mestres, J., Ring, H. A., Costa, D. C., Gacinovic, S., Walker, Z., Lees, A. J., et al. (2004). Dopamine transporter binding in Gilles de la Tourette syndrome: a [123I]FP-CIT/SPECT study. Acta Psychiatr. Scand. 109, 140–146. doi:10.1111/j.0001-690X.2004.00214.x
Shaw, P., Gornick, M., Lerch, J., Addington, A., Seal, J., Greenstein, D., et al. (2007). Polymorphisms of the dopamine D4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 64, 921–931. doi:10.1001/archpsyc.64.8.921
Silveri, M. M., Sneider, J. T., Crowley, D. J., Covell, M. J., Acharya, D., Rosso, I. M., et al. (2013). Frontal lobe gamma-aminobutyric acid levels during adolescence: associations with impulsivity and response inhibition. Biol. Psychiatry 74, 296–304. doi:10.1016/j.biopsych.2013.01.033
Singer, H. S., Szymanski, S., Giuliano, J., Yokoi, F., Dogan, A. S., Brasic, J. R., et al. (2002). Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am. J. Psychiatry 159, 1329–1336. doi:10.1176/appi.ajp.159.8.1329
Smith, A. B., Taylor, E., Brammer, M., Toone, B., and Rubia, K. (2006). Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am. J. Psychiatry 163, 1044–1051. doi:10.1176/appi.ajp.163.6.1044
Solis-Ortiz, S., Perez-Luque, E., Morado-Crespo, L., and Gutierrez-Munoz, M. (2010). Executive functions and selective attention are favored in middle-aged healthy women carriers of the Val/Val genotype of the catechol-O-methyltransferase gene: a behavioral genetic study. Behav. Brain Funct. 6, 67. doi:10.1186/1744-9081-6-67
Sowell, E. R., Thompson, P. M., Welcome, S. E., Henkenius, A. L., Toga, A. W., and Peterson, B. S. (2003). Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet 362, 1699–1707. doi:10.1016/S0140-6736(03)14842-8
Spinelli, S., Joel, S., Nelson, T. E., Vasa, R. A., Pekar, J. J., and Mostofsky, S. H. (2011). Different neural patterns are associated with trials preceding inhibitory errors in children with and without attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 50, 705.e–715.e. doi:10.1016/j.jaac.2011.03.014
Stanley, M. A., Breckenridge, J. K., Swann, A. C., Freeman, E. B., and Reich, L. (1997a). Fluvoxamine treatment of trichotillomania. J. Clin. Psychopharmacol. 17, 278–283. doi:10.1097/00004714-199708000-00007
Stanley, M. A., Hannay, H. J., and Breckenridge, J. K. (1997b). The neuropsychology of trichotillomania. J. Anxiety Disord. 11, 473–488. doi:10.1016/S0887-6185(97)00024-8
Starck, G., Ljungberg, M., Nilsson, M., Jonsson, L., Lundberg, S., Ivarsson, T., et al. (2008). A 1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: relationship between metabolite concentrations and symptom severity. J. Neural Transm. 115, 1051–1062. doi:10.1007/s00702-008-0045-4
Steeves, T. D., and Fox, S. H. (2008). Neurobiological basis of serotonin-dopamine antagonists in the treatment of Gilles de la Tourette syndrome. Prog. Brain Res. 172, 495–513. doi:10.1016/S0079-6123(08)00924-2
Steeves, T. D., Ko, J. H., Kideckel, D. M., Rusjan, P., Houle, S., Sandor, P., et al. (2010). Extrastriatal dopaminergic dysfunction in Tourette syndrome. Ann. Neurol. 67, 170–181. doi:10.1002/ana.21809
Stein, D. J., Bouwer, C., and Maud, C. M. (1997). Use of the selective serotonin reuptake inhibitor citalopram in treatment of trichotillomania. Eur. Arch. Psychiatry Clin. Neurosci. 247, 234–236. doi:10.1007/BF02900220
Stoltenberg, S. F., Glass, J. M., Chermack, S. T., Flynn, H. A., Li, S., Weston, M. E., et al. (2006). Possible association between response inhibition and a variant in the brain-expressed tryptophan hydroxylase-2 gene. Psychiatr. Genet. 16, 35–38. doi:10.1097/01.ypg.0000176528.30362.34
Streichenwein, S. M., and Thornby, J. I. (1995). A long-term, double-blind, placebo-controlled crossover trial of the efficacy of fluoxetine for trichotillomania. Am. J. Psychiatry 152, 1192–1196.
Sukhodolsky, D. G., Landeros-Weisenberger, A., Scahill, L., Leckman, J. F., and Schultz, R. T. (2010). Neuropsychological functioning in children with Tourette syndrome with and without attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 49, 1155–1164. doi:10.1016/j.jaac.2010.08.008
Suskauer, S. J., Simmonds, D. J., Fotedar, S., Blankner, J. G., Pekar, J. J., Denckla, M. B., et al. (2008). Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J. Cogn. Neurosci. 20, 478–493. doi:10.1162/jocn.2008.20032
Swain, J. E., Scahill, L., Lombroso, P. J., King, R. A., and Leckman, J. F. (2007). Tourette syndrome and tic disorders: a decade of progress. J. Am. Acad. Child Adolesc. Psychiatry 46, 947–968. doi:10.1097/chi.0b013e318068fbcc
Swick, D., Ashley, V., and Turken, U. (2011). Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 56, 1655–1665. doi:10.1016/j.neuroimage.2011.02.070
Tamm, L., Menon, V., Ringel, J., and Reiss, A. L. (2004). Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 43, 1430–1440. doi:10.1097/01.chi.0000140452.51205.8d
Thompson, J., Thomas, N., Singleton, A., Piggott, M., Lloyd, S., Perry, E. K., et al. (1997). D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 7, 479–484. doi:10.1097/00008571-199712000-00006
Turner, D. C., Robbins, T. W., Clark, L., Aron, A. R., Dowson, J., and Sahakian, B. J. (2003). Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl.) 165, 260–269. doi:10.1007/s00213-002-1250-8
Vaidya, C. J., Austin, G., Kirkorian, G., Ridlehuber, H. W., Desmond, J. E., Glover, G. H., et al. (1998). Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc. Natl. Acad. Sci. U.S.A. 95, 14494–14499. doi:10.1073/pnas.95.24.14494
Vaidya, C. J., Bunge, S. A., Dudukovic, N. M., Zalecki, C. A., Elliott, G. R., and Gabrieli, J. D. (2005). Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am. J. Psychiatry 162, 1605–1613. doi:10.1176/appi.ajp.162.9.1605
Van Ameringen, M., Mancini, C., Patterson, B., Bennett, M., and Oakman, J. (2010). A randomized, double-blind, placebo-controlled trial of olanzapine in the treatment of trichotillomania. J. Clin. Psychiatry 71, 1336–1343. doi:10.4088/JCP.09m05114gre
van den Heuvel, O. A., Veltman, D. J., Groenewegen, H. J., Witter, M. P., Merkelbach, J., Cath, D. C., et al. (2005). Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch. Gen. Psychiatry 62, 922–933. doi:10.1001/archpsyc.62.8.922
van der Wee, N. J., Stevens, H., Hardeman, J. A., Mandl, R. C., Denys, D. A., van Megen, H. J., et al. (2004). Enhanced dopamine transporter density in psychotropic-naive patients with obsessive-compulsive disorder shown by [123I]{beta}-CIT SPECT. Am. J. Psychiatry 161, 2201–2206. doi:10.1176/appi.ajp.161.12.2201
van Minnen, A., Hoogduin, K. A., Keijsers, G. P., Hellenbrand, I., and Hendriks, G. J. (2003). Treatment of trichotillomania with behavioral therapy or fluoxetine: a randomized, waiting-list controlled study. Arch. Gen. Psychiatry 60, 517–522. doi:10.1001/archpsyc.60.5.517
Vasic, N., Plichta, M. M., Wolf, R. C., Fallgatter, A. J., Sosic-Vasic, Z., and Gron, G. (2012). Reduced neural error signaling in left inferior prefrontal cortex in young adults with ADHD. J. Atten. Disord. doi:10.1177/1087054712446172
Verte, S., Geurts, H. M., Roeyers, H., Oosterlaan, J., and Sergeant, J. A. (2005). Executive functioning in children with autism and Tourette syndrome. Dev. Psychopathol. 17, 415–445. doi:10.1017/S0954579405050200
Viard, A., Flament, M. F., Artiges, E., Dehaene, S., Naccache, L., Cohen, D., et al. (2005). Cognitive control in childhood-onset obsessive-compulsive disorder: a functional MRI study. Psychol. Med. 35, 1007–1017. doi:10.1017/S0033291704004295
Volkow, N. D., Gur, R. C., Wang, G. J., Fowler, J. S., Moberg, P. J., Ding, Y. S., et al. (1998). Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am. J. Psychiatry 155, 344–349.
Vollm, B., Richardson, P., McKie, S., Elliott, R., Deakin, J. F., and Anderson, I. M. (2006). Serotonergic modulation of neuronal responses to behavioural inhibition and reinforcing stimuli: an fMRI study in healthy volunteers. Eur. J. Neurosci. 23, 552–560. doi:10.1111/j.1460-9568.2005.04571.x
Vriend, C., Pattij, T., van der Werf, Y. D., Voorn, P., Booij, J., Rutten, S., et al. (2014). Depression and impulse control disorders in Parkinson’s disease: two sides of the same coin? Neurosci. Biobehav. Rev. 38, 60–71. doi:10.1016/j.neubiorev.2013.11.001
Wager, T. D., Sylvester, C. Y., Lacey, S. C., Nee, D. E., Franklin, M., and Jonides, J. (2005). Common and unique components of response inhibition revealed by fMRI. Neuroimage 27, 323–340. doi:10.1016/j.neuroimage.2005.01.054
Walther, D. J., and Bader, M. (2003). A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 66, 1673–1680. doi:10.1016/S0006-2952(03)00556-2
Wang, G.-J., Volkow, N. D., Wigal, T., Kollins, S. H., Newcorn, J. H., Telang, F., et al. (2013). Long-term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PLoS ONE 8:e63023. doi:10.1371/journal.pone.0063023
Watkins, L. H., Sahakian, B. J., Robertson, M. M., Veale, D. M., Rogers, R. D., Pickard, K. M., et al. (2005). Executive function in Tourette’s syndrome and obsessive-compulsive disorder. Psychol. Med. 35, 571–582. doi:10.1017/S0033291704003691
White, M. J., Morris, C. P., Lawford, B. R., and Young, R. M. (2008). Behavioral phenotypes of impulsivity related to the ANKK1 gene are independent of an acute stressor. Behav. Brain Funct. 4, 54. doi:10.1186/1744-9081-4-54
White, M. P., and Koran, L. M. (2011). Open-label trial of aripiprazole in the treatment of trichotillomania. J. Clin. Psychopharmacol. 31, 503–506. doi:10.1097/JCP.0b013e318221b1ba
Wong, D. F., Brasic, J. R., Singer, H. S., Schretlen, D. J., Kuwabara, H., Zhou, Y., et al. (2008). Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology 33, 1239–1251. doi:10.1038/sj.npp.1301528
Woolley, J., Heyman, I., Brammer, M., Frampton, I., McGuire, P. K., and Rubia, K. (2008). Brain activation in paediatric obsessive compulsive disorder during tasks of inhibitory control. Br. J. Psychiatry 192, 25–31. doi:10.1192/bjp.bp.107.036558
Keywords: response inhibition, obsessive–compulsive disorder, Tourette’s syndrome, trichotillomania, attention-deficit hyperactivity disorder, interference control
Citation: van Velzen LS, Vriend C, de Wit SJ and van den Heuvel OA (2014) Response inhibition and interference control in obsessive–compulsive spectrum disorders. Front. Hum. Neurosci. 8:419. doi: 10.3389/fnhum.2014.00419
Received: 07 March 2014; Accepted: 24 May 2014;
Published online: 11 June 2014.
Edited by:
Guido Van Wingen, Academic Medical Center Amsterdam, NetherlandsReviewed by:
Robert Hester, University of Melbourne, AustraliaJens Kuhn, University of Cologne, Germany
Copyright: © 2014 van Velzen, Vriend, de Wit and van den Heuvel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Odile A. van den Heuvel, Department of Psychiatry, VU University Medical Center, PO Box 7057, Amsterdam 1007 MB, Netherlands e-mail: oa.vandenheuvel@vumc.nl
†Laura S. van Velzen and Chris Vriend have contributed equally to this work.
 Laura S. van Velzen
Laura S. van Velzen Chris Vriend
Chris Vriend Stella J. de Wit
Stella J. de Wit Odile A. van den Heuvel
Odile A. van den Heuvel