Brain networks of perceptual decision-making: an fMRI ALE meta-analysis
- 1Faculty of Social and Behavioural Science, Cognitive Science Center Amsterdam, University of Amsterdam, Amsterdam, Netherlands
- 2Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
- 3Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University Düsseldorf, Düsseldorf, Germany
- 4Research Centre Jülich, Institute of Neuroscience and Medicine (INM-1), Jülich, Germany
- 5Leipzig University Medical Center, IFB Adiposity Diseases, Leipzig, Germany
In the recent perceptual decision-making literature, a fronto-parietal network is typically reported to primarily represent the neural substrate of human perceptual decision-making. However, the view that only cortical areas are involved in perceptual decision-making has been challenged by several neurocomputational models which all argue that the basal ganglia play an essential role in perceptual decisions. To consolidate these different views, we conducted an Activation Likelihood Estimation (ALE) meta-analysis on the existing neuroimaging literature. The results argue in favor of the involvement of a frontal-parietal network in general perceptual decision-making that is possibly complemented by the basal ganglia, and modulated in substantial parts by task difficulty. In contrast, expectation of reward, an important aspect of many decision-making processes, shows almost no overlap with the general perceptual decision-making network.
Introduction
Many of our decisions in everyday life rely on our senses and how quickly and accurately we extract information from our environment. Consider, for example, driving down the highway on a motorcycle while it starts to rain. Soon, your visibility is significantly reduced due to accumulating raindrops on your helmet visor and it becomes harder to see if the car in front of you is slowing down and whether you need to slow down as well.
Which brain areas are involved in these kinds of perceptual decision-making processes is a key question in cognitive neuroscience (Schall, 2001; Krawczyk, 2002; Platt, 2002; Romo and Salinas, 2003; Gold and Shadlen, 2007; Heekeren et al., 2008; Ding and Gold, 2013). While most of the current insights stem from single-unit recordings in monkeys, an increasing number of functional magnetic resonance imaging (fMRI) studies have addressed the neural correlates of perceptual decision-making in humans (Kim and Shadlen, 1999; Schall, 2001; Shadlen and Newsome, 2001; Krawczyk, 2002; Platt, 2002; Glimcher, 2003; Romo and Salinas, 2003; Romo et al., 2003; Gold and Shadlen, 2007; Churchland et al., 2008; Heekeren et al., 2008). These studies frequently employ simple perceptual discrimination tasks, which typically feature two or more forced-choice alternatives at varying levels of difficulty (Gold and Shadlen, 2007). A straightforward way of manipulating the difficulty is to change the amount of sensory evidence provided by the experimental stimuli. For example, the number of coherently moving dots in the often-used “random dot motion paradigm” may be reduced in order to make the judgment on the direction of motion considerably more difficult (Britten et al., 1992; Palmer et al., 2005).
Evidently, a key question in this line of research is which brain areas or networks are involved in choices that are based on varying degrees of sensory evidence. Neurophysiological evidence in monkeys suggests that the decision-forming process for such simple perceptual decision-making tasks starts off with the integration of sensory evidence for each choice by lower-level sensory neurons (Heekeren et al., 2008). The decision is then thought to be computed in higher-order cortical regions by comparing the difference in amount of sensory information for each choice. Once enough evidence in favor of a certain choice has been accumulated, the information is passed on to the motor system, thereby enabling the execution of an action associated with that specific decision (Gold and Shadlen, 2001; Heekeren et al., 2008). Previous studies have argued for a fronto-parietal network to subserve this functionality and hence to enable simple perceptual decision-making (Ho et al., 2009; Kable and Glimcher, 2009; Li et al., 2009; Mulder et al., 2012).
The presumption that perceptual decision-making is primarily implemented by a fronto-parietal network, however, has been challenged by recent neuro-computational models. These models state that the basal ganglia (BG) are likewise essential for the computation of perceptual decisions and should not be neglected in theorizing (Bogacz, 2007; Ding and Gold, 2013). The BG is a collection of subcortical nuclei that anatomically consist of the striatum and pallidum. Additionally, the subthalamic nucleus and the substantia nigra are functionally considered to be part of the BG (Federative Committee on Anatomical Terminology, 1998). It has been argued that the BG as a whole implement a central gating mechanism by evaluating the evidence of each choice alternative facilitating the appropriate behavioral response for the alternative with the most supporting evidence (Lo and Wang, 2006; Bogacz and Gurney, 2007; Frank et al., 2007). More specifically, the model by Bogacz and Gurney (2007) proposes that the striatum is involved in encoding certain actions whereas the subthalamic nucleus inhibits the output to the thalamus until enough information is accumulated. In line with this reasoning, several fMRI studies have shown an involvement of the striatum in flexibly adapting the response regime in such simple perceptual decision-making tasks (Forstmann et al., 2008a, 2010a,b; van Maanen et al., 2011). Other studies argue for the involvement of the subthalamic nucleus in task-switching or in mediating the decision-threshold under stimulus conflict (Cavanagh et al., 2011; Mansfield et al., 2011). In addition, there is a large body of literature on the involvement of the BG in reward-based decision-making (Kawagoe et al., 1998; Tanaka et al., 2004; Liu et al., 2011; Mulder et al., 2013). While these results point toward an involvement of subcortical brain structures in several aspects of perceptual decision-making, it remains unclear if the BG are also involved in decision-making aspects such as task difficulty.
Finally, several recent reviews hypothesize that the perceptual decision-making network serves as a core network that can be recruited for other forms of decision-making such as reward-based decision-making (Gold and Shadlen, 2007; Heekeren et al., 2008). However, whether or not this theory holds, has yet to be shown. By combining the literatures on perceptual and reward-based decision-making, it becomes possible to test whether reward-based decision-making recruits a similar network as does perceptual decision-making.
The present study set out to address the following questions:
(1) Which cortical and subcortical brain areas are consistently involved in simple perceptual decision-making?
(2) To what extent is this perceptual decision-making network modulated by task difficulty?
(3) To what extent does the task-general network for simple perceptual decision-making overlap with a reward-based decision-making network?
In order to answer these questions a number of Activation Likelihood Estimation (ALE) meta-analyses of fMRI studies were conducted on simple perceptual decision-making tasks as well as reward-based decision-making. Such meta-analyses go beyond qualitatively pooling results from diverse neuroimaging experiments by quantitatively modeling reported brain coordinates and statistically testing their convergence across studies in standard brain space (Turkeltaub et al., 2002; Neumann et al., 2008; Eickhoff et al., 2009, 2012).
Methods
A comprehensive search for relevant neuroimaging studies in the field of perceptual decision-making was carried out using the PubMed database (www.pubmed.org). The three main keywords utilized were “fMRI,” “neural,” and “brain.” Each of these keywords was entered in combination with general keywords (e.g., “perceptual decision-making”) as well as more specific keywords (e.g., “random dot motion” see Table 1 for all keywords). Based on the information contained in the abstracts of all papers returned, empirical studies were selected to meet the following inclusion criteria: (1) Studies were published in peer-review English language journals between January 2000 and March 2012; (2) they employed fMRI in healthy adults; (3) participants engaged in simple decision-making tasks with at least two alternatives that did not explicitly require higher-order cognitive functions such as language or memory; (4) studies reported a Task > Control contrast or a Hard > Easy contrast of the experimental task; and (5) they reported whole-brain activations as 3D coordinates in stereotactic space of Talairach or the Montreal Neurological Institute (MNI). Subsequently, the full texts of all applicable studies were read to confirm the valid inclusion in the meta-analysis. Finally, the included empirical studies were cross-referenced, and the whole selection process was repeated for the newly obtained empirical papers.
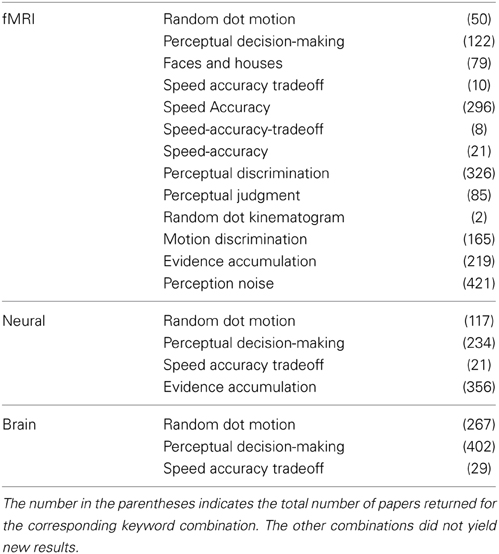
Table 1. All the keyword combinations used to search the PubMed database where the first column was combined with the second column.
Additionally, to increase the number of possible relevant empirical studies, all abstracts returned by PubMed were scanned for reviews. This was done by searching the initial list of abstracts returned by PubMed for the keywords “review,” “summary,” and “summarize.” Based on the abstracts of the obtained reviews, only reviews that covered the topic of decision-making were then cross-referenced, and the whole selection process was repeated for the newly obtained empirical papers.
Two independent raters completed the entire article inclusion procedure, and only articles that both raters agreed on were included in the final sample. See Figure 1 for an overview of the selection and inclusion process and Table 2 for the included studies. Note that several studies reported both Task > Control and Hard > Easy contrasts.
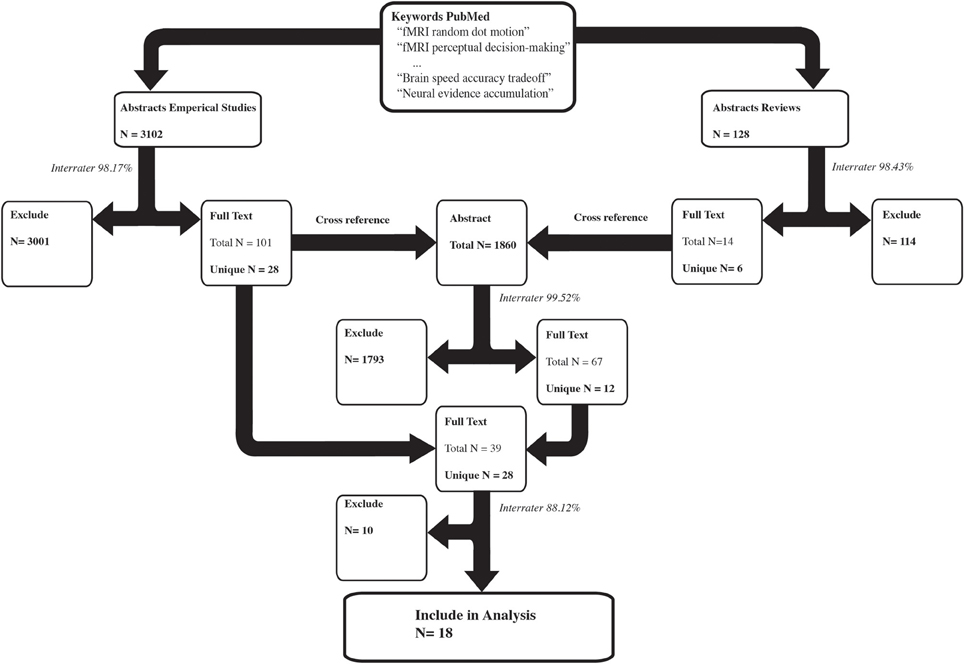
Figure 1. The selection procedure for the inclusion of empirical studies. The left arm shows the selection process of the empirical studies based on the abstracts. The right arm shows the selection process of the review papers based on the abstracts. The number of results per selection stage is reported in bold. Several keywords resulted in the inclusion of the same study, which is reflected by the total N. Subsequently only the unique papers were used in the next selection step. The interrater congruency between the two independent raters is reported in italics. For instance, of the 3102 empirical abstracts, both raters independently agreed on 98.17% of the abstracts to either exclude them or to read the full text. The remaining abstracts were discussed and a consensus was reached on whether to exclude the abstract or to read the full text.
For answering the third question of this paper (i.e., the specificity of the task-general network for simple perceptual decision-making tasks), a separate meta-analysis was conducted on reward-based decision-making to assess the overlap as well as the difference between perceptual and reward-based decision-making. Selection of relevant studies was based on a recently published ALE meta-analysis on reward processing in the brain conducted by Liu et al. (2011). For this meta-analysis, the authors identified 142 neuroimaging studies that examined brain activation in reward-related decision-making tasks in healthy adults. In order to ensure direct comparability with the perceptual decision-making meta-analysis, only a subset of these studies was chosen here. Specifically, only studies reporting Reward > Control contrasts were considered. In these studies, the reward condition contained a cue informing the participant that one of the choice alternatives would result in a larger reward, whereas in the control condition the participant received a neutral cue. In addition, only studies that assessed the BOLD response in a time window comparable to the perceptual decision-making tasks (i.e., the actual decision process rather than later components such as the period between making a response and receiving feedback) were selected.
See Table 3 for the studies included in the meta-analysis on reward-based decision-making.
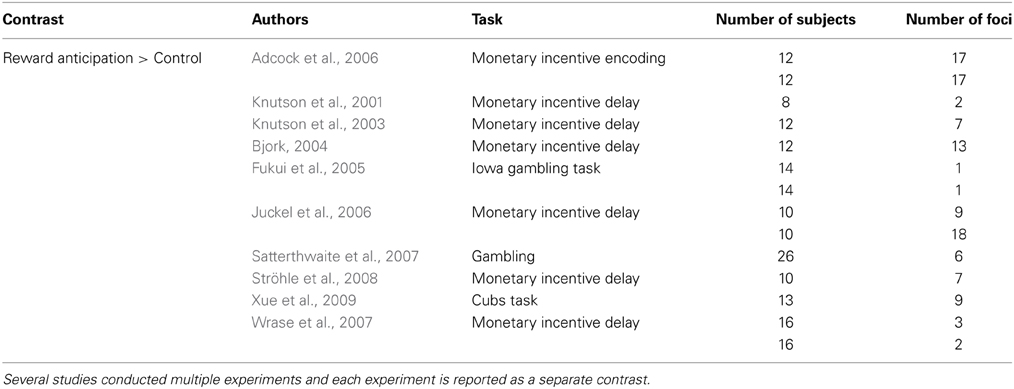
Table 3. The studies selected from the meta-analysis of Liu et al. (2011).
Activation Likelihood Estimation
ALE analyses were performed using the BrainMap application GingerALE, version 2.3 (http://brainmap.org/ale/). All activation foci of the included studies that were originally reported in Talairach space were converted to the MNI stereotactic space using the Lancaster et al. (2007) transformation algorithm. Within ALE, these activation foci are modeled as the center of a three-dimensional Gaussian probability distribution reflecting the spatial uncertainly associated with the respective neuroimaging findings. Combining these distributions within and across experiments, a statistical whole-brain map is created that yields an estimate of the activation likelihood (i.e., ALE value) for each voxel, based on all reported activation foci (Eickhoff et al., 2009). In order to confine the number of inflated ALE values arising from experiments reporting many proximate activation foci, a non-additive ALE method was chosen (Turkeltaub et al., 2012). Utilizing this approach, significant ALE values are less likely to be caused by simple within-experiment effects, but reflect the actual concordance in activation patterns between the different experiments. Furthermore, the FWHM of the 3D probability distribution was estimated per individual study, resulting in a higher specificity of the actual overlap between studies (Eickhoff et al., 2009).
Subsequently, to test against the null hypothesis of spatial independence of activation foci, the analytical approach based on a non-linear histogram integration as described by Eickhoff et al. (2012) was employed. To correct for multiple comparisons, a cluster-level approach with a cluster-forming threshold of p = 0.05 was used. To estimate a null-distribution of cluster sizes, a random set of experiments was created with the same characteristic as the actual data but with random coordinates. For this random set, the same ALE analysis was performed and this process was repeated 10,000 times to create a null-distribution of cluster sizes. In the following, all clusters that exceeded the critical threshold to control for the cluster-level family wise error rate at p < 0.05 are reported.
Testing for Overlap Between Different Task Aspects
The overlap between the Task > Control contrast, the Hard > Easy contrast, and the Reward > Control contrast was analyzed by computing the pairwise minimum conjunction of the respective ALE maps (Nichols et al., 2005), whereas unique clusters for each contrast were identified by pairwise subtraction analyses (Eickhoff et al., 2011). The subtraction analysis entailed that all experiments that contributed to the initial contrast were pooled and randomly divided into two equally sized groups. The ALE values for these two randomly divided groups were then calculated, and the difference between these ALE values was recorded per voxel. This process was repeated 10,000 times and resulted in a null-distribution for the difference in ALE values. The actual observed difference between the two contrasts was then compared to the null-distribution and resulted in a p-value map. This map was statistically thresholded at a level of p < 0.05, resulting in a set of areas that were reliably associated with one of the two networks but not the other.
Anatomical labels for the final activation cluster locations were determined using the Anatomy Toolbox and the Harvard-Oxford atlas as implemented in FSL, version 5.0.2 (Eickhoff et al., 2005, 2006, 2007; Choi et al., 2006; Desikan et al., 2006; Makris et al., 2006; Caspers et al., 2008).
Results
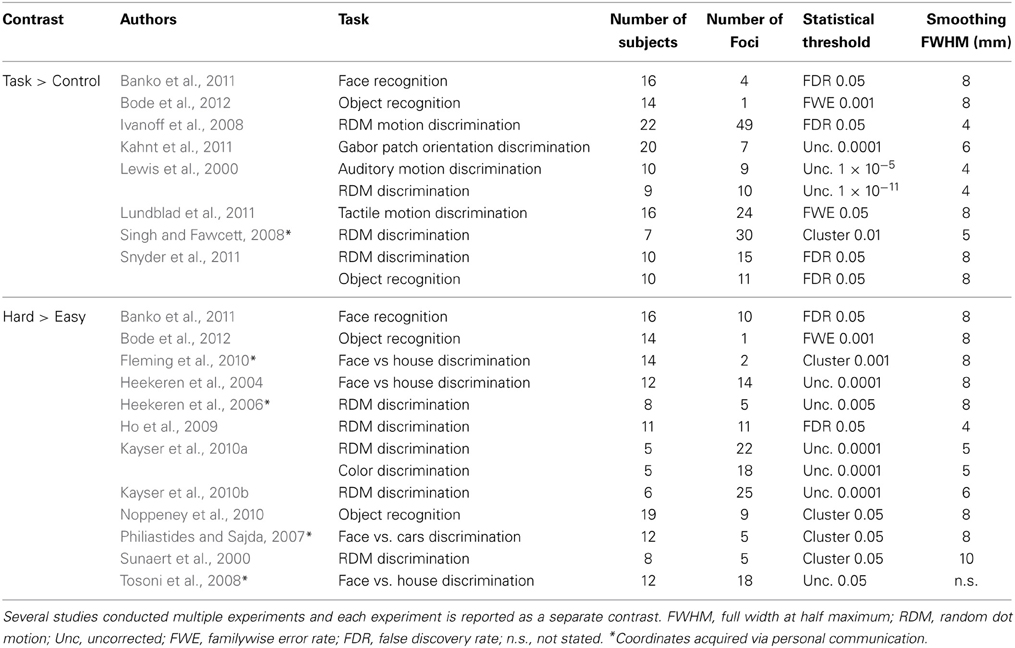
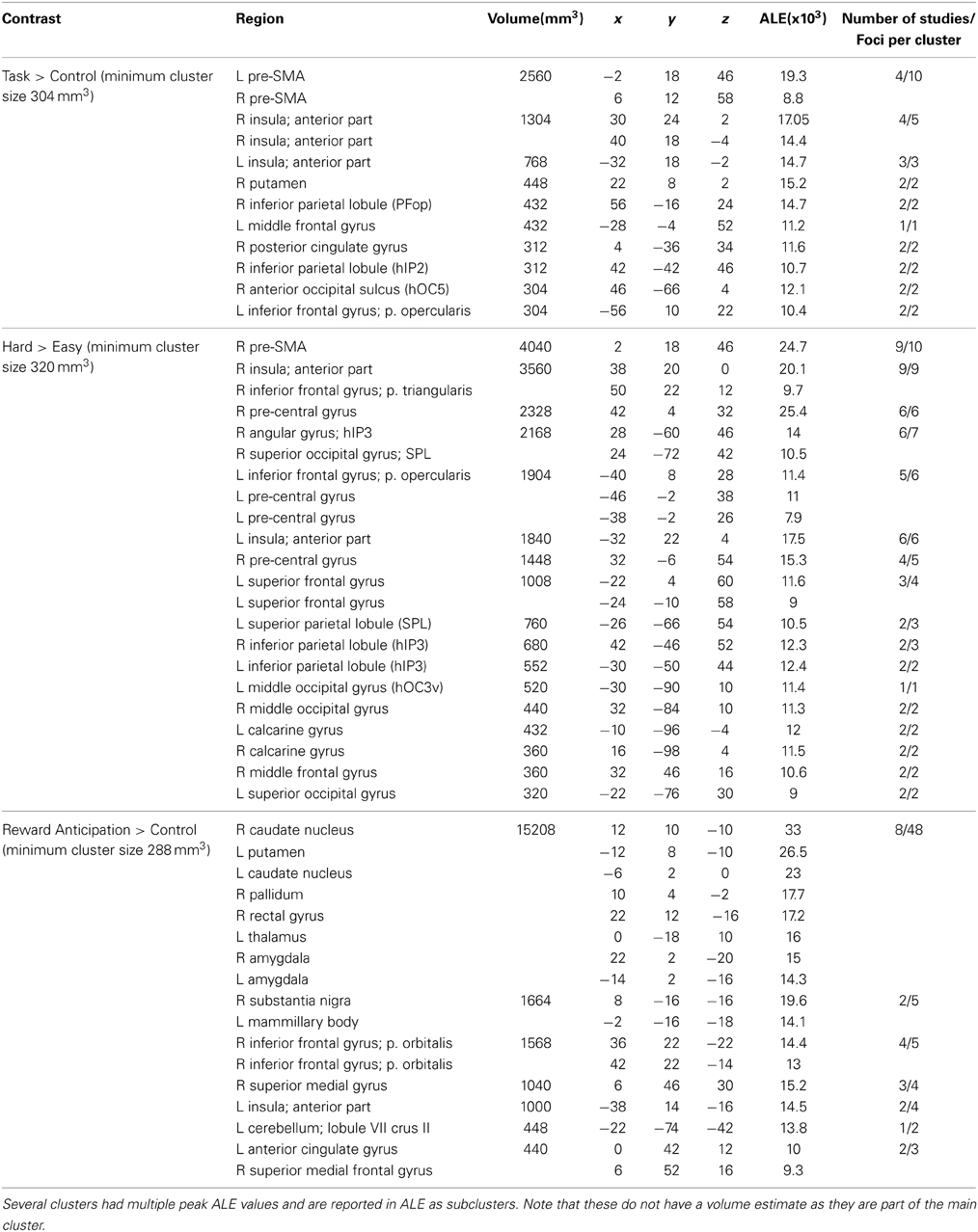
Figure 2 shows the location of the activation clusters revealed by the three individual meta-analyses. The ALE meta-analysis for Task > Control, based on 10 contrasts and 160 foci, revealed 12 significant clusters. The largest clusters were located in the bilateral pre-supplementary motor area (pre-SMA), bilateral anterior insula, the right putamen, the right opercular supramarginal area (PFop, Triarhou, 2007; Caspers et al., 2008) located in the supramarginal gyrus, and the left middle frontal gyrus (MFG). See Table 4 for the coordinates of all the 12 clusters that form this perceptual decision-making network.
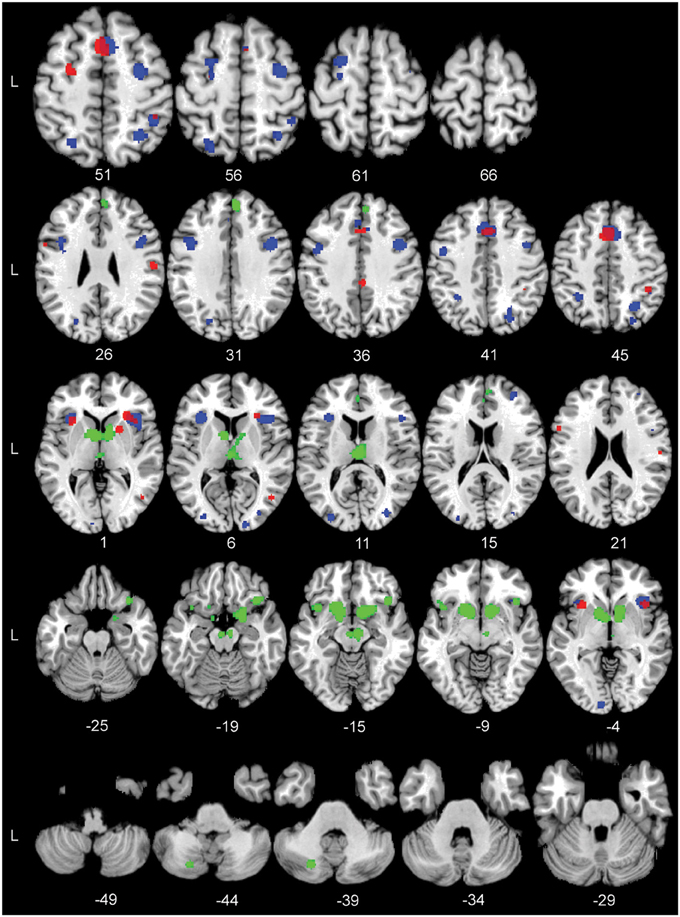
Figure 2. The significant Activation Likelihood Estimate (ALE) clusters for the three separate ALE analyses in standard Montreal Neurological Institute space. Red: Task > Control; blue: Hard > Easy; green: Reward > Control. Numbers indicate Z coordinates in MNI space.
The ALE analysis for Hard > Easy was based on 13 contrasts and 145 foci and revealed 17 separate clusters of activation. The largest clusters where found in the right pre-SMA, bilateral anterior insula, bilateral pre-central gyrus, bilateral inferior frontal gyrus (IFG), and the left superior frontal gyrus (SFG). See Table 4 for the coordinates of the 17 clusters that are part of the task-difficulty-related network.
The ALE analysis for the Reward > Control contrast, based on 14 contrasts and 112 foci, showed a network that was distributed over frontal and subcortical areas. The largest clusters were located in the bilateral striatum, right substantia nigra (SN), right IFG, left insula, and right superior medial gyrus (SMG). Notably, the Reward > Control ALE analysis did not show any parietal activation. See Table 4 for the coordinates of the local maximum ALE values.
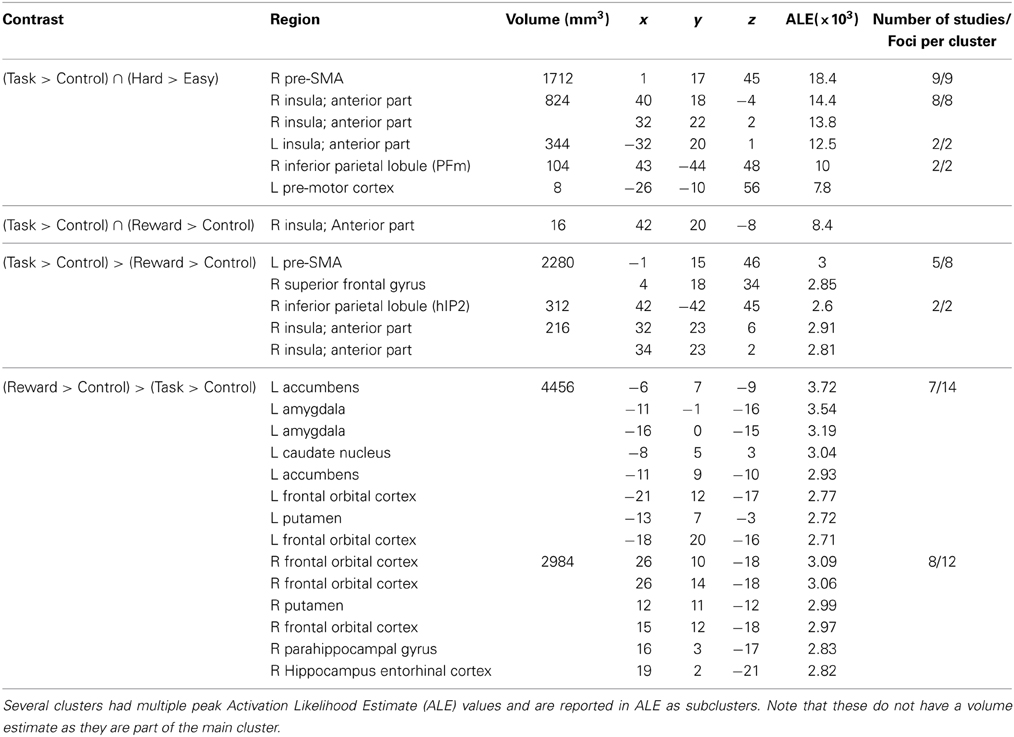
Subsequently, a conjunction analysis across the Hard > Easy and Task > Control contrasts was performed in order to assess to what extent the perceptual decision-making network was modulated by task difficulty. Results of this analysis revealed a substantial overlap in the conjunction map including a cluster in the right anterior insula, right pre-SMA, left pre-motor cortex and right magnocellular supramarginal area (PFm, Triarhou, 2007; Caspers et al., 2008) in the inferior parietal lobule. Thus, considerable parts of the perceptual decision-making network were modulated by task difficulty. See Figure 3 for the location of the significant conjunction clusters. Finally, the subtraction analysis did not show any significant differences in the likelihood of activation for the Hard > Easy contrast compared to the Task > Control contrast or vice versa.
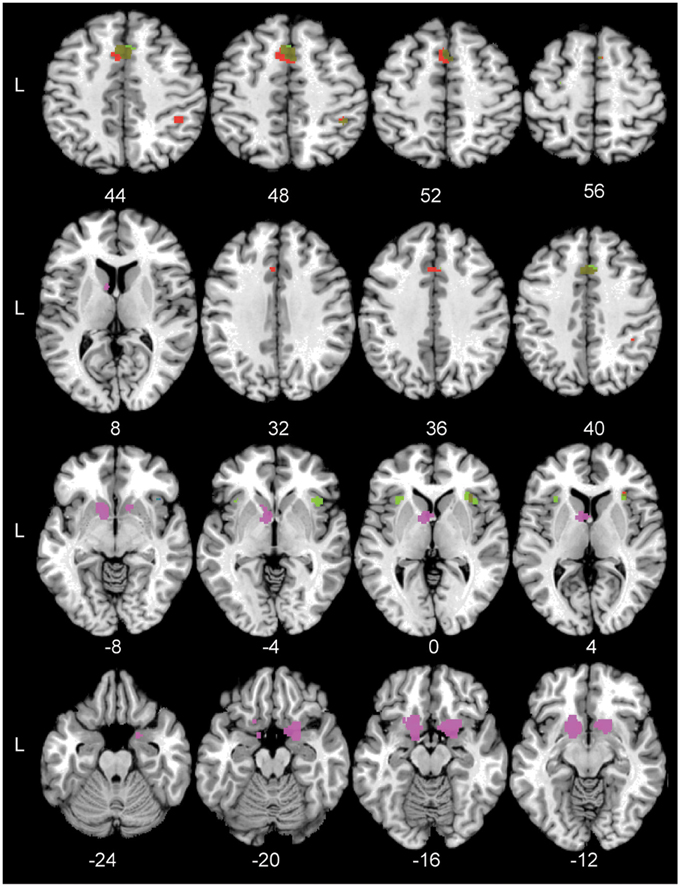
Figure 3. The significant conjunction and subtraction clusters in standard Montreal Neurological Institute space. Green: The significant conjunction clusters for the task-general network and the task difficulty network are located in the right pre-SMA, left pre-motor cortex, bilateral anterior insula, and right PFm. Blue: The significant conjunction cluster for the task-general and reward networks is located in right anterior insula. Red: The unique areas for the task-general network compared to the reward-based network are located in left pre-SMA cortex, right hIP2, and right anterior insula. Violet: The unique areas for the reward-based network are located in left nucleus accumbens and right frontal orbital cortex. Numbers indicate Z coordinates in MNI space.
To test the specificity of the task-general network for simple perceptual decision-making, it was compared to the reward-based decision-making network. The conjunction analysis across the Task > Control and Reward > Control contrasts showed one small area of overlap in the right anterior insula. See Figure 3 for the significant conjunction cluster. The subtraction analysis revealed that the perceptual decision-making network had unique activation in the left pre-SMA, the right second human intraparietal sulcus (hIP2, Choi et al., 2006), and again in the right anterior insula, the latter cluster being located more inferior than the anterior insula cluster resulting from the conjunction. Conversely, the reward-based decision-making network showed unique involvement of the left nucleus accumbens and right orbito-frontal cortex (see Figure 3). See Table 5 for the coordinates of the local maximum ALE values revealed by the conjunction and differences between the two networks.
Discussion
This study set out to identify the consistent brain network underlying perceptual decision-making tasks. Such network can be obtained from quantitative meta-analyses techniques for functional imaging studies such as ALE, multi-kernel density analysis (MKDA), model-based clustering, or similar approaches that received considerable attention in the neuroimaging community in recent years (e.g., Turkeltaub et al., 2002; Neumann et al., 2005, 2008, 2011; Wager et al., 2009; Yarkoni et al., 2011; Eickhoff et al., 2012). We thus conducted an ALE meta-analysis of fMRI findings in simple perceptual decision-making experiments. In addition to identifying the task-general network for perceptual decision-making, we assessed its possible modulation by task difficulty. Finally, we tested the specificity of the perceptual decision-making network by comparing the results to a meta-analysis of reward-based decision-making experiments.
Perceptual Decision-Making Network
In accordance with several reviews (Schall, 2001; Gold and Shadlen, 2007; Heekeren et al., 2008), the task general network for perceptual decision-making revealed by our analysis comprised several distinct cortical areas. Frontal areas included the pre-SMA, involved in setting response thresholds (Forstmann et al., 2008b; Mansfield et al., 2011; van Maanen et al., 2011); and the left IFG pars opercularis. The latter is an area that is conventionally thought to be involved in linguistic processes, but there are several reports on its involvement in motor planning and response inhibition that could explain why a cluster of activation was found in this region (Heiser et al., 2003; Johnson and Grafton, 2003; Gough et al., 2005; Pobric, 2006; Swick et al., 2008). Finally, a recent meta-analysis further revealed that the posterior part of Brodmann area 44, which would overlap with the IFG pars opercularis, is involved in action processes (Clos et al., 2013).
In addition to these frontal areas, the bilateral anterior insula was found to be involved in the perceptual decision-making network. This area is believed to play an integrative role in perception-action coupling and is shown to be consistently involved in a wide range of paradigms (Kurth et al., 2010; Sterzer and Kleinschmidt, 2010; Chang et al., 2013; Langner and Eickhoff, 2013).
The parietal areas of the perceptual decision-making network included the PFop; an area implicated in processing spatial orientation (Mochizuki et al., 2002) and the hIP2; an area involved in processing spatial attention and numerical cognition (Wu et al., 2009; Uddin et al., 2010). Further smaller clusters were found in the posterior cingulate cortex (PCC), an area thought to be involved in task engagement or decision salience (Heilbronner et al., 2011). Additionally, a small cluster was found in the fifth human occipital area (hOC5), an area reported to be involved in the coding for visual form, motion, and the representation of objects (Vaina et al., 2001; Malikovic et al., 2006).
Importantly, as predicted by several neuro-computational models, this cortical network was complemented by subcortical activation, specifically, in the right putamen. The putamen together with the caudate forms the striatum and functions as a major input structure for the BG, as it receives a wide range of cortical inputs and is thought to be essential for action selection, learning, and reward prediction (Bogacz, 2007; Bogacz and Gurney, 2007; Chakravarthy et al., 2010; Ding and Gold, 2013). According to the model by Bogacz and Gurney (2007), the activity in the striatum reflects the encoding of certain actions. While the neuro-computational models of Bogacz and Gurney do not make explicit differential predictions for the two striatal subparts, the finding of convergence in the putamen was not surprising. Previous work has shown that the putamen is more involved in limb movements whereas the caudate might be more involved in oculomotor responses (Alexander and Crutcher, 1990; Ding and Gold, 2013). Furthermore, the putamen is known to be connected to several of the aforementioned cortical areas (Leh et al., 2007; Helmich et al., 2010).
However, it should be noted that the two studies contributing to the putamen cluster both used a passive control task where no response was necessary. In both studies subjects had to either respond with both hands or only with the left hand (Ivanoff et al., 2008; Lundblad et al., 2011). Therefore, we cannot rule out the possibility that the putamen cluster solely reflects a difference in motor-related task demands. However, based on evidence complementing our analysis, we would propose that the observed putamen likely implements more than just the motor response. Previous model-based fMRI studies have attributed activation in the putamen not solely to the motor implementation of the decision, but also to the processing of prior information regarding the stimuli (Forstmann et al., 2010b; Nagano-Saito et al., 2012). Using linear accumulation models to analyse the functional data, both studies were able to separate the actual motor response from the decision-making process and found evidence for the putamen being involved in encoding response bias (Ding and Gold, 2013). However, in our results this interpretation of the putamen cluster should be taken with caution, as the contributing coordinates for the putamen were derived without using such mathematical models and warrant further investigation.
While the involvement of the BG cannot be resolved conclusively based on the data currently available for meta-analyses, results regarding our first research question speak in favor of a perceptual decision-making network that comprises of both cortical and possibly subcortical regions.
Moreover, the analysis revealed that this network consisted of a set of nodes involved in task engagement, information encoding, response caution setting, and finally action implementation, resembling most stages necessary for making a decision (Shadlen and Newsome, 2001; Platt, 2002; Glimcher, 2003; Gold and Shadlen, 2007).
Task Difficulty Effects on the Perceptual Decision-Making Network
The individual meta-analysis on task difficulty revealed that the right pre-SMA, involved in setting response thresholds (Forstmann et al., 2008b; van Maanen et al., 2011) and the left SFG an area that is reported to be involved in selective attention (Cutini et al., 2008), were part of the task difficulty network. In the parietal lobule, a significant cluster was found in the bilateral area hIP3 an area that is the possible human homolog of the macaque ventral portion of the lateral intraparietal cortex (LIP, Gillebert et al., 2013). LIP activity has been repeatedly shown to reflect the amount of information accumulated for each choice alternative (e.g., Shadlen and Newsome, 2001; Churchland et al., 2008). Based on these findings one would predict a lower BOLD response in hIP3 for hard trials compared to easy trials as the amount of available information is lower. An explanation for why the current meta-analysis found an overall higher BOLD response in hIP3 could be an increased top-down modulation of attention (Bisley, 2003; Heekeren et al., 2004; Hebart et al., 2012). Finally, bilateral early visual cortices were found to be involved in task difficulty and this activation might again be due to increased top-down modulation of attention (Spitzer et al., 1988; Sunaert et al., 2000).
The conjunction analysis showed that task difficulty modulated a subset of the perceptual decision-making network including right pre-SMA, right anterior insula, left pre-motor cortex, and right PFm. If task difficulty increases, it is expected that more attentional resources are necessary so that task performance does not suffer (Posner, 1980). The PFm might facilitate this attentional modulation as it is thought to be essential in the orientation of attention (Wu et al., 2009; Jakobs et al., 2012). With respect to our second research question, the conjunction analysis revealed that several cortical areas involved in perceptual decision making are effected by task difficulty.
Differences Between Perceptual and Reward-Based Decision-Making
The final question of the present study was to test the specificity of the perceptual decision-making network. We conducted an additional ALE meta-analysis focusing on reward-related decision-making tasks. The Reward > Control contrast showed several regions including right SMG, right IFG pars orbitalis, left insula, and anterior cingulate gyrus (ACC). Several subcortical structures were found to be active including the striatum, the thalamus, and the SN, all areas that are deemed essential to the processing of reward-related information (Helfinstein et al., 2013). No clusters were found in the parietal cortex, which may seem surprising when taking the perceptual decision-making and task difficulty meta-analysis results into account. The main paradigm employed in the included reward-based decision-making studies was a monetary incentive delay (MID) task. In the MID task, the participant is instructed to respond as quickly as possible after receiving a cue that indicates the possible reward (Knutson et al., 2001). In such a task, one might argue that continuous stimulus information does not need to be accumulated, whereas in tasks such as the random dot motion paradigm where this is clearly the case. This fundamental difference in paradigms would explain why areas such as hIP3 are not consistently found in the current analysis on reward-based decision making. Comparing the perceptual and the reward-based decision-making networks only showed partial similarities as the conjunction analysis showed an overlap in the right anterior insula. The subtraction analysis revealed that the perceptual decision-making network recruited a number of unique areas compared to the reward-based decision-making network and vice versa.
In conclusion, these results indicate that the core network involved in perceptual decision-making has almost no overlap with the network involved in reward-based decision-making. The anterior insula was the only area that was found to be active in all three networks (including activity associated with perceptual task difficulty). This finding is in line with previous work that argues that the anterior insula is a key node involved in the integration of information from different sources and modalities (Ho et al., 2009; Sterzer and Kleinschmidt, 2010; Chang et al., 2013). The fact that the anterior insula is active during simple perceptual decision-making, dissociates between low and high task difficulty, and is involved in reward-based decision-making supports the hypothesis of the integrative nature of this area (Kurth et al., 2010; Menon and Uddin, 2010).
Limitations of the Current Study
The present study entails several limitations. First, only a limited number of studies could be included in the current ALE meta-analyses on perceptual decision-making. After a rigorous literature search, only 18 out of 3230 potential articles were deemed suitable to be included. But even with the strict inclusion criteria, the suitable studies still varied on a large number of variables such as statistical thresholds, smoothing kernels, registration procedures to standard space, and MRI scanning parameters. While current coordinate-based meta-analysis methods cannot account for all these differences separately, ALE does estimate a spatial uncertainty per individual study thereby alleviating some of the between-studies variability arising from varying study specific parameters such as the number of subjects or the use of different brain templates (Eickhoff et al., 2009). The second limitation is the anatomical specificity of the results. The included studies only report the peak coordinates of what is most likely a larger activation area and are based on statistical procedures that include smoothing kernels ranging from 4 to 8 mm FWHM. This inherently limits the anatomical specificity of the results of both the included studies as well as the current meta-analysis. A third limitation is the lack of methodological or procedural information in some of the original studies, which limits the assessment of their similarity to other data included in the meta-analysis (Poldrack et al., 2008). For instance information regarding the use of a linear or non-linear registration normalization procedure, whether incorrect responses were included or whether the subjects responded with one or both hands was not always indicated. Fourth, the included data did not allow us to determine the precise role of the BG in perceptual decision making as, based solely on activation coordinates, we cannot distinguish different possible underlying processes that might have given rise to the BG activation in the original publications. This would require model-based imaging methods that use parametric analyses to directly link functional activation to cognitive mechanisms. While such approaches exist (Forstmann et al., 2010b; Nagano-Saito et al., 2012) the currently available meta-analysis techniques cannot combine results from parametric analyses with coordinates from purely contrast-based analyses. The final more general, limitation is that not all conducted studies are reported in the literature, as studies that fail to find significant results are typically not published. This phenomenon is often referred to as the “bias against null results” or “the file drawer problem” (Rosenthal, 1979). While this problem is common to all meta-analyses and cannot be resolved at present, initiatives such as the Open Science Framework (http://openscienceframework.org/) and pre-registered reports in journals (Chambers, 2013) will help to overcome this limitation in the future.
Conclusions
The results of the current meta-analysis argue in favor of a frontal-parietal network involved in perceptual decision-making that is possibly complemented by the basal ganglia. While several cortical parts of this network, i.e., pre-SMA, anterior insula, pre-motor cortex and the PFm, are modulated by task difficulty, our conjunction analysis yielded only a small functional overlap between perceptual and reward-based decision-making that is restricted to the anterior insula. In contrast, the subtraction analysis revealed that a considerable number of areas that were uniquely involved in perceptual decision-making compared to reward-based decision-making and vice versa.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work was supported by a Vidi grant by the Dutch Organization for Scientific Research (NWO) to Birte U. Forstmann; a starter grant from the European Research Council (ERC) to Birte U. Forstmann; the German Federal Ministry of Education and Research (FKZ:01EO1001); and the German Research Foundation (DFG; SFB 1052 Obesity mechanisms to Jane Neumann; EI 816/4-1 to Simon B. Eickhoff; LA 3071/3-1 to Robert Langner and Simon B. Eickhoff).
References
Adcock, R., Thangavel, A., Whitfield-Gabrieli, S., Knutson, B., and Gabrieli, J. (2006). Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron 50, 507–517. doi: 10.1016/j.neuron.2006.03.036
Alexander, G., and Crutcher, M. (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271. doi: 10.1016/0166-2236(90)90107-L
Banko, E. M., Gal, V., Kortvelyes, J., Kovacs, G., and Vidnyanszky, Z. (2011). Dissociating the effect of noise on sensory processing and overall decision difficulty. J. Neurosci. 31, 2663–2674. doi: 10.1523/JNEUROSCI.2725-10.2011
Bisley, J. W. (2003). Neuronal activity in the lateral intraparietal area and spatial attention. Science 299, 81–86. doi: 10.1126/science.1077395
Bjork, J. M. (2004). Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 24, 1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004
Bode, S., Bogler, C., Soon, C. S., and Haynes, J.-D. (2012). The neural encoding of guesses in the human brain. Neuroimage 59, 1924–1931. doi: 10.1016/j.neuroimage.2011.08.106
Bogacz, R. (2007). Optimal decision-making theories: linking neurobiology with behaviour. Trends Cogn. Sci. 11, 118–125. doi: 10.1016/j.tics.2006.12.006
Bogacz, R., and Gurney, K. (2007). The basal ganglia and cortex implement optimal decision making between alternative actions. Neural Comput. 19, 442–477. doi: 10.1162/neco.2007.19.2.442
Britten, K. H., Shadlen, M. N., Newsome, W. T., and Movshon, J. A. (1992). The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci. 12, 4745–4765.
Caspers, S., Eickhoff, S. B., Geyer, S., Scheperjans, F., Mohlberg, H., Zilles, K., et al. (2008). The human inferior parietal lobule in stereotaxic space. Brain Struct. Funct. 212, 481–495. doi: 10.1007/s00429-008-0195-z
Cavanagh, J. F., Wiecki, T. V., Cohen, M. X., Figueroa, C. M., Samanta, J., Sherman, S. J., et al. (2011). Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat. Neurosci. 14, 1462–1467. doi: 10.1038/nn.2925
Chakravarthy, V., Joseph, D., and Bapi, R. (2010). What do the basal ganglia do? A modeling perspective. Biol. Cybern. 103, 237–253. doi: 10.1007/s00422-010-0401-y
Chang, L. J., Yarkoni, T., Khaw, M. W., and Sanfey, A. G. (2013). Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb. Cortex 23, 739–749. doi: 10.1093/cercor/bhs065
Choi, H.-J., Zilles, K., Mohlberg, H., Schleicher, A., Fink, G. R., Armstrong, E., et al. (2006). Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J. Comp. Neurol. 495, 53–69. doi: 10.1002/cne.20849
Churchland, A., Kiani, R., and Shadlen, M. (2008). Decision-making with multiple alternatives. Nat. Neurosci. 11, 693. doi: 10.1038/nn.2123
Clos, M., Amunts, K., Laird, A. R., Fox, P. T., and Eickhoff, S. B. (2013). Tackling the multifunctional nature of Broca's region meta-analytically: co-activation-based parcellation of area 44. Neuroimage 83, 174–188. doi: 10.1016/j.neuroimage.2013.06.041
Cutini, S., Scatturin, P., Menon, E., Bisiacchi, P. S., Gamberini, L., Zorzi, M., et al. (2008). Selective activation of the superior frontal gyrus in task-switching: an event-related fNIRS study. Neuroimage 42, 945–955. doi: 10.1016/j.neuroimage.2008.05.013
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Ding, L., and Gold, J. I. (2013). The basal ganglia's contributions to perceptual decision making. Neuron 79, 640–649. doi: 10.1016/j.neuron.2013.07.042
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F., and Fox, P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361. doi: 10.1016/j.neuroimage.2011.09.017
Eickhoff, S. B., Bzdok, D., Laird, A. R., Roski, C., Caspers, S., Zilles, K., et al. (2011). Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57, 938–949. doi: 10.1016/j.neuroimage.2011.05.021
Eickhoff, S. B., Heim, S., Zilles, K., and Amunts, K. (2006). Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage 32, 570–582. doi: 10.1016/j.neuroimage.2006.04.204
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., and Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926. doi: 10.1002/hbm.20718
Eickhoff, S. B., Paus, T., Caspers, S., Grosbras, M.-H., Evans, A. C., Zilles, K., et al. (2007). Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36, 511–521. doi: 10.1016/j.neuroimage.2007.03.060
Eickhoff, S. B., Stephan, K. E., Mohlberg, H., Grefkes, C., Fink, G. R., Amunts, K., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25, 1325–1335. doi: 10.1016/j.neuroimage.2004.12.034
Federative Committee on Anatomical Terminology. (ed.). (1998). Terminologia Anatomica. New York, NY: Thieme Stuttgart, 1–292.
Fleming, S. M., Whiteley, L., Hulme, O. J., Sahani, M., and Dolan, R. J. (2010). Effects of category-specific costs on neural systems for perceptual decision-making. J. Neurophysiol. 103, 3238–3247. doi: 10.1152/jn.01084.2009
Forstmann, B., Jahfari, S., Scholte, H., Wolfensteller, U., van den Wildenberg, W., and Ridderinkhof, K. (2008b). Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J. Neurosci. 28:9790. doi: 10.1523/JNEUROSCI.1465-08.2008
Forstmann, B. U., Anwander, A., Schafer, A., Neumann, J., Brown, S., Wagenmakers, E.-J., et al. (2010a). Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc. Natl. Acad. Sci. U.S.A. 107, 15916–15920. doi: 10.1073/pnas.1004932107
Forstmann, B. U., Brown, S., Dutilh, G., Neumann, J., and Wagenmakers, E.-J. (2010b). The neural substrate of prior information in perceptual decision making: a model-based analysis. Front. Hum. Neurosci. 4:40. doi: 10.3389/fnhum.2010.00040
Forstmann, B. U., Dutilh, G., Brown, S., Neumann, J., von Cramon, D. Y., Ridderinkhof, K. R., et al. (2008a). Striatum and pre-SMA facilitate decision-making under time pressure. Proc. Natl. Acad. Sci. U.S.A. 105, 17538–17542. doi: 10.1073/pnas.0805903105
Frank, M. J., Samanta, J., Moustafa, A. A., and Sherman, S. J. (2007). Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318, 1309–1312. doi: 10.1126/science.1146157
Fukui, H., Murai, T., Fukuyama, H., Hayashi, T., and Hanakawa, T. (2005). Functional activity related to risk anticipation during performance of the Iowa gambling task. Neuroimage 24, 253–259. doi: 10.1016/j.neuroimage.2004.08.028
Gillebert, C. R., Mantini, D., Peeters, R., Dupont, P., and Vandenberghe, R. (2013). Cytoarchitectonic mapping of attentional selection and reorienting in parietal cortex. Neuroimage 67, 257–272. doi: 10.1016/j.neuroimage.2012.11.026
Glimcher, P. W. (2003). The neurobiology of visual-saccadic decision making. Ann. Rev. Neurosci. 26, 133–179. doi: 10.1146/annurev.neuro.26.010302.081134
Gold, J., and Shadlen, M. (2001). Neural computations that underlie decisions about sensory stimuli. Trends Cogn. Sci. 5, 10–16. doi: 10.1016/S1364-6613(00)01567-9
Gold, J. I., and Shadlen, M. N. (2007). The neural basis of decision making. Ann. Rev. Neurosci. 30, 535–574. doi: 10.1146/annurev.neuro.29.051605.113038
Gough, P., Nobre, A., and Devlin, J. (2005). Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J. Neurosci. 25, 8010. doi: 10.1523/JNEUROSCI.2307-05.2005
Hebart, M. N., Donner, T. H., and Haynes, J.-D. (2012). Human visual and parietal cortex encode visual choices independent of motor plans. Neuroimage 63, 1–11. doi: 10.1016/j.neuroimage.2012.08.027
Heekeren, H., Marrett, S., Ruff, D., Bandettini, P., and Ungerleider, L. (2006). Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proc. Natl. Acad. Sci. U.S.A. 103, 10023. doi: 10.1073/pnas.0603949103
Heekeren, H. R., Marrett, S., Bandettini, P. A., and Ungerleider, L. G. (2004). A general mechanism for perceptual decision-making in the human brain. Nature 431, 859–862. doi: 10.1038/nature02966
Heekeren, H. R., Marrett, S., and Ungerleider, L. G. (2008). The neural systems that mediate human perceptual decision making. Nat. Rev. Neurosci. 9, 467–479. doi: 10.1038/nrn2374
Heilbronner, S. R., Hayden, B. Y., and Platt, M. L. (2011). Decision salience signals in posterior cingulate cortex. Front. Neurosci. 5:55. doi: 10.3389/fnins.2011.00055
Heiser, M., Iacoboni, M., Maeda, F., Marcus, J., and Mazziotta, J. C. (2003). The essential role of Broca's area in imitation. Eur. J. Neurosci. 17, 1123–1128. doi: 10.1046/j.1460-9568.2003.02530.x
Helfinstein, S. M., Kirwan, M. L., Benson, B. E., Hardin, M. G., Pine, D. S., Ernst, M., et al. (2013). Validation of a child-friendly version of the monetary incentive delay task. Soc. Cogn. Affect. Neurosci. 8, 720–726. doi: 10.1093/scan/nss057
Helmich, R. C., Derikx, L. C., Bakker, M., Scheeringa, R., Bloem, B. R., and Toni, I. (2010). Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb. Cortex 20, 1175–1186. doi: 10.1093/cercor/bhp178
Ho, T. C., Brown, S., and Serences, J. T. (2009). Domain general mechanisms of perceptual decision making in human cortex. J. Neurosci. 29, 8675–8687. doi: 10.1523/JNEUROSCI.5984-08.2009
Ivanoff, J., Branning, P., and Marois, R. (2008). fMRI evidence for a dual process account of the speed-accuracy tradeoff in decision-making. PLoS ONE 3:e2635. doi: 10.1371/journal.pone.0002635
Jakobs, O., Langner, R., Caspers, S., Roski, C., Cieslik, E. C., Zilles, K., et al. (2012). Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus–context integration. Neuroimage 60, 2389–2398. doi: 10.1016/j.neuroimage.2012.02.037
Johnson, S. H., and Grafton, S. T. (2003). From “acting on” to “acting with”: the functional anatomy of object-oriented action schemata. Prog. Brain Res. 142, 127–139. doi: 10.1016/S0079-6123(03)42010-4
Juckel, G., Schlagenhauf, F., Koslowski, M., Wüstenberg, T., Villringer, A., Knutson, B., et al. (2006). Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage 29, 409–416. doi: 10.1016/j.neuroimage.2005.07.051
Kable, J. W., and Glimcher, P. W. (2009). The neurobiology of decision: consensus and controversy. Neuron 63, 733–745. doi: 10.1016/j.neuron.2009.09.003
Kahnt, T., Grueschow, M., Speck, O., and Haynes, J.-D. (2011). Perceptual learning and decision-making in human medial frontal cortex. Neuron 70, 549–559. doi: 10.1016/j.neuron.2011.02.054
Kawagoe, R., Takikawa, Y., and Hikosaka, O. (1998). Expectation of reward modulates cognitive signals in the basal ganglia. Nat. Neurosci. 1, 411–416. doi: 10.1038/1625
Kayser, A. S., Buchsbaum, B. R., Erickson, D. T., and D'Esposito, M. (2010a). The functional anatomy of a perceptual decision in the human brain. J. Neurophysiol. 103, 1179–1194. doi: 10.1152/jn.00364.2009
Kayser, A. S., Erickson, D. T., Buchsbaum, B. R., and D'Esposito, M. (2010b). Neural representations of relevant and irrelevant features in perceptual decision making. J. Neurosci. 30, 15778–15789. doi: 10.1523/JNEUROSCI.3163-10.2010
Kim, J., and Shadlen, M. (1999). Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat. Neurosci. 2, 176–185. doi: 10.1038/5739
Knutson, B., Adams, C. M., Fong, G. W., and Hommer, D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 21, RC159.
Knutson, B., Fong, G. W., Bennett, S. M., Adams, C. M., and Hommer, D. (2003). A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 18, 263–272. doi: 10.1016/S1053-8119(02)00057-5
Krawczyk, D. C. (2002). Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci. Biobehav. Rev. 26, 631–664. doi: 10.1016/S0149-7634(02)00021-0
Kurth, F., Zilles, K., Fox, P. T., Laird, A. R., and Eickhoff, S. B. (2010). A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 214, 519–534. doi: 10.1007/s00429-010-0255-z
Lancaster, J. L., Tordesillas-Gutiérrez, D., Martinez, M., Salinas, F., Evans, A., Zilles, K., et al. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 28, 1194–1205. doi: 10.1002/hbm.20345
Langner, R., and Eickhoff, S. B. (2013). Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol. Bull. 134, 870–900. doi: 10.1037/a0030694
Leh, S. E., Ptito, A., Chakravarty, M. M., and Strafella, A. P. (2007). Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci. Lett. 419, 113–118. doi: 10.1016/j.neulet.2007.04.049
Lewis, J. W., Beauchamp, M. S., and DeYoe, E. A. (2000). A comparison of visual and auditory motion processing in human cerebral cortex. Cereb. Cortex 10, 873–888. doi: 10.1093/cercor/10.9.873
Li, S., Mayhew, S. D., and Kourtzi, Z. (2009). Learning shapes the representation of behavioral choice in the human brain. Neuron 62, 441–452. doi: 10.1016/j.neuron.2009.03.016
Liu, X., Hairston, J., Schrier, M., and Fan, J. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 35, 1219–1236. doi: 10.1016/j.neubiorev.2010.12.012
Lo, C. C., and Wang, X. J. (2006). Cortico–basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat. Neurosci. 9, 956–963. doi: 10.1038/nn1722
Lundblad, L. C., Olausson, H. W., Hermansson, A.-K., and Wasling, H. B. (2011). Cortical processing of tactile direction discrimination based on spatiotemporal cues in man. Neurosci. Lett. 501, 45–49. doi: 10.1016/j.neulet.2011.06.040
Makris, N., Goldstein, J. M., Kennedy, D., Hodge, S. M., Caviness, V. S., Faraone, S. V., et al. (2006). Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr. Res. 83, 155–171. doi: 10.1016/j.schres.2005.11.020
Malikovic, A., Amunts, K., Schleicher, A., Mohlberg, H., Eickhoff, S. B., Wilms, M., et al. (2006). Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cereb. Cortex 17, 562–574. doi: 10.1093/cercor/bhj181
Mansfield, E. L., Karayanidis, F., Jamadar, S., Heathcote, A., and Forstmann, B. U. (2011). Adjustments of response threshold during task switching: a model-based functional magnetic resonance imaging study. J. Neurosci. 31, 14688–14692. doi: 10.1523/JNEUROSCI.2390-11.2011
Menon, V., and Uddin, L. Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667. doi: 10.1007/s00429-010-0262-0
Mochizuki, H., Tashiro, M., Tagawa, M., Kano, M., Itoh, M., Okamura, N., et al. (2002). The effects of a sedative antihistamine, d-chlorpheniramine, on visuomotor spatial discrimination and regional brain activity as measured by positron emission tomography (PET). Hum. Psychopharmacol. Clin. Exp. 17, 413–418. doi: 10.1002/hup.430
Mulder, M. J., Boekel, W., Ratcliff, R., and Forstmann, B. U. (2013). Cortico-subthalamic connection predicts individual differences in value-driven choice bias. Brain Struct. Funct. doi: 10.1007/s00429-013-0561-3. [Epub ahead of print].
Mulder, M. J., Wagenmakers, E.-J., Ratcliff, R., Boekel, W., and Forstmann, B. U. (2012). Bias in the brain: a diffusion model analysis of prior probability and potential payoff. J. Neurosci. 32, 2335–2343. doi: 10.1523/JNEUROSCI.4156-11.2012
Nagano-Saito, A., Cisek, P., Perna, A. S., Shirdel, F. Z., Benkelfat, C., Leyton, M., et al. (2012). From anticipation to action, the role of dopamine in perceptual decision making: an fMRI-tyrosine depletion study. J. Neurophysiol. 108, 501–512. doi: 10.1152/jn.00592.2011
Neumann, J., Cramon, D. Y. V., and Lohmann, G. (2008). Model-based clustering of meta-analytic functional imaging data. Hum. Brain Mapp. 29, 177–192. doi: 10.1002/hbm.20380
Neumann, J., Lohmann, G., Derrfuss, J., and von Cramon, D. Y. (2005). Meta-analysis of functional imaging data using replicator dynamics. Hum. Brain Mapp. 25, 165–173. doi: 10.1002/hbm.20133
Neumann, J., Turner, R., Fox, P. T., and Lohmann, G. (2011). Exploring functional relations between brain regions from fMRI meta-analysis data: comments on Ramsey, Spirtes, and Glymour. Neuroimage 57, 331–333. doi: 10.1016/j.neuroimage.2010.11.012
Nichols, T., Brett, M., Andersson, J., Wager, T., and Poline, J.-B. (2005). Valid conjunction inference with the minimum statistic. Neuroimage, 25, 653–660. doi: 10.1016/j.neuroimage.2004.12.005
Noppeney, U., Ostwald, D., and Werner, S. (2010). Perceptual decisions formed by accumulation of audiovisual evidence in prefrontal cortex. J. Neurosci. 30, 7434–7446. doi: 10.1523/JNEUROSCI.0455-10.2010
Palmer, J., Huk, A., and Shadlen, M. (2005). The effect of stimulus strength on the speed and accuracy of a perceptual decision. J.Vis. 5, 376–404. doi: 10.1167/5.5.1
Philiastides, M. G., and Sajda, P. (2007). EEG-informed fMRI reveals spatiotemporal characteristics of perceptual decision making. J. Neurosci. 27, 13082–13091. doi: 10.1523/JNEUROSCI.3540-07.2007
Platt, M. L. (2002). Neural correlates of decisions. Curr. Opin. Neurobiol. 12, 141–148. doi: 10.1016/S0959-4388(02)00302-1
Pobric, G. (2006). Action understanding requires the left inferior frontal cortex. Curr. Biol. 16, 524–529. doi: 10.1016/j.cub.2006.01.033
Poldrack, R., Fletcher, P., Henson, R., Worsley, K., Brett, M., and Nichols, T. (2008). Guidelines for reporting an fMRI study. Neuroimage 40, 409–414. doi: 10.1016/j.neuroimage.2007.11.048
Posner, M. I. (1980). Orienting of attention. Q. J. Exp. Psychol. 32, 3–25. doi: 10.1080/00335558008248231
Romo, R., Hernández, A., and Zainos, A. (2003). Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron 41, 165–173. doi: 10.1016/S0896-6273(03)00817-1
Romo, R., and Salinas, E. (2003). Flutter Discrimination: neural codes, perception, memory and decision making. Nat. Rev. Neurosci. 4, 203–218. doi: 10.1038/nrn1058
Rosenthal, R. (1979). The file drawer problem and tolerance for null results. Psychol. Bull. 86, 638–641. doi: 10.1037/0033-2909.86.3.638
Satterthwaite, T. D., Green, L., Myerson, J., Parker, J., Ramaratnam, M., and Buckner, R. L. (2007). Dissociable but inter-related systems of cognitive control and reward during decision making: evidence from pupillometry and event-related fMRI. Neuroimage 37, 1017–1031. doi: 10.1016/j.neuroimage.2007.04.066
Schall, J. D. (2001). Neural basis of deciding, choosing and acting. Nat. Rev. Neurosci. 2, 33–42. doi: 10.1038/35049054
Shadlen, M., and Newsome, W. (2001). Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J. Neurophysiol. 86, 1916.
Singh, K. D., and Fawcett, I. P. (2008). Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage 41, 100–112. doi: 10.1016/j.neuroimage.2008.01.051
Snyder, A. N., Bockbrader, M. A., Hoffa, A. M., Dzemidzic, M. A., Talavage, T. M., Wong, D., et al. (2011). Psychometrically matched tasks evaluating differential fMRI activation during form and motion processing. Neuropsychology 25, 622–633. doi: 10.1037/a0022984
Spitzer, H., Desimone, R., and Moran, J. (1988). Increased attention enhances both behavioral and neuronal performance. Science 240, 338–340. doi: 10.1126/science.3353728
Sterzer, P., and Kleinschmidt, A. (2010). Anterior insula activations in perceptual paradigms: often observed but barely understood. Brain Struct. Funct. 214, 611–622. doi: 10.1007/s00429-010-0252-2
Ströhle, A., Stoy, M., Wrase, J., Schwarzer, S., Schlagenhauf, F., Huss, M., et al. (2008). Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage 39, 966–972. doi: 10.1016/j.neuroimage.2007.09.044
Sunaert, S., Van Hecke, P., Marchal, G., and Orban, G. A. (2000). Attention to speed of motion, speed discrimination, and task difficulty: an fmri study. Neuroimage 11, 612–623. doi: 10.1006/nimg.2000.0587
Swick, D., Ashley, V., and Turken, A. U. (2008). Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 9:102. doi: 10.1186/1471-2202-9-102
Tanaka, S. C., Doya, K., Okada, G., Ueda, K., Okamoto, Y., and Yamawaki, S. (2004). Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat. Neurosci. 7, 887–893. doi: 10.1038/nn1279
Tosoni, A., Galati, G., Romani, G. L., and Corbetta, M. (2008). Sensory-motor mechanisms in human parietal cortex underlie arbitrary visual decisions. Nat. Neurosci. 11, 1446–1453. doi: 10.1038/nn.2221
Triarhou, L. C. (2007). A proposed number system for the 107 cortical areas of economo and koskinas, and brodmann area correlations. Stereotact. Funct. Neurosurg. 85, 204–215. doi: 10.1159/000103259
Turkeltaub, P. E., Eden, G. F., Jones, K. M., and Zeffiro, T. A. (2002). Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16, 765–780. doi: 10.1006/nimg.2002.1131
Turkeltaub, P. E., Eickhoff, S. B., Laird, A. R., Fox, M., Wiener, M., and Fox, P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 33, 1–13. doi: 10.1002/hbm.21186
Uddin, L. Q., Supekar, K., Amin, H., Rykhlevskaia, E., Nguyen, D. A., Greicius, M. D., et al. (2010). Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb. Cortex 20, 2636–2646. doi: 10.1093/cercor/bhq011
Vaina, L. M., Solomon, J., Chowdhury, S., Sinha, P., and Belliveau, J. W. (2001). Functional neuroanatomy of biological motion perception in humans. Proc. Natl. Acad. Sci. U.S.A. 98, 11656–11661. doi: 10.1073/pnas.191374198
van Maanen, L., Brown, S. D., Eichele, T., Wagenmakers, E.-J., Ho, T., Serences, J., et al. (2011). Neural correlates of trial-to-trial fluctuations in response caution. J. Neurosci. 31, 17488–17495. doi: 10.1523/JNEUROSCI.2924-11.2011
Wager, T. D., Lindquist, M. A., Nichols, T. E., Kober, H., and Van Snellenberg, J. X. (2009). Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage 45, S210–S221. doi: 10.1016/j.neuroimage.2008.10.061
Wrase, J., Schlagenhauf, F., Kienast, T., Wüstenberg, T., Bermpohl, F., Kahnt, T., et al. (2007). Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 35, 787–794. doi: 10.1016/j.neuroimage.2006.11.043
Wu, S. S., Chang, T. T., Majid, A., Caspers, S., Eickhoff, S. B., and Menon, V. (2009). Functional heterogeneity of inferior parietal cortex during mathematical cognition assessed with cytoarchitectonic probability maps. Cereb. Cortex 19, 2930–2945. doi: 10.1093/cercor/bhp063
Xue, G., Lu, Z., Levin, I. P., Weller, J. A., Li, X., and Bechara, A. (2009). Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb. Cortex 19, 1019–1027. doi: 10.1093/cercor/bhn147
Keywords: decision-making, meta-analysis, fronto-parietal-basal ganglia
Citation: Keuken MC, Müller-Axt C, Langner R, Eickhoff SB, Forstmann BU and Neumann J (2014) Brain networks of perceptual decision-making: an fMRI ALE meta-analysis. Front. Hum. Neurosci. 8:445. doi: 10.3389/fnhum.2014.00445
Received: 04 November 2013; Accepted: 02 June 2014;
Published online: 19 June 2014.
Edited by:
Aron K. Barbey, University of Illinois at Urbana-Champaign, USAReviewed by:
Erick Joseph Paul, University of Illinois Urbana Champaign, USAAndrew S. Kayser, University of California at San Francisco, USA
Copyright © 2014 Keuken, Müller-Axt, Langner, Eickhoff, Forstmann and Neumann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Max C. Keuken, Cognitive Science Center Amsterdam, University of Amsterdam, Nieuwe Prinsengracht 130, 1018 VZ Amsterdam, Netherlands e-mail: mckeuken@gmail.com
 Max C. Keuken
Max C. Keuken Christa Müller-Axt
Christa Müller-Axt Robert Langner
Robert Langner Simon B. Eickhoff
Simon B. Eickhoff Birte U. Forstmann
Birte U. Forstmann Jane Neumann
Jane Neumann