COMT Val158Met genotypes differentially influence subgenual cingulate functional connectivity in healthy females
- 1Department of Psychiatry and Medical Psychology, Ghent University, Ghent, Belgium
- 2Department of Psychiatry, University Hospital (UZBrussel), Brussels, Belgium
- 3Ghent Experimental Psychiatry (GHEP) Lab, Ghent University, Ghent, Belgium
- 4Department of Data Analysis, Ghent University, Ghent, Belgium
- 5Department of Psychiatry, University Hospital (UZLeuven), Leuven, Belgium
- 6Key Laboratory of Cognition and Personality, Faculty of Psychology, Southwest University, Chongqing, China
- 7Department of Radiology and Medical Imaging, University Hospital (UZBrussel), Brussels, Belgium
- 8Department of Experimental Clinical and Health Psychology, Ghent University, Ghent, Belgium
Brain imaging studies have consistently shown subgenual Anterior Cingulate Cortical (sgACC) involvement in emotion processing. catechol-O-methyltransferase (COMT) Val158 and Met158 polymorphisms may influence such emotional brain processes in specific ways. Given that resting-state fMRI (rsfMRI) may increase our understanding on brain functioning, we integrated genetic and rsfMRI data and focused on sgACC functional connections. No studies have yet investigated the influence of the COMT Val158Met polymorphism (rs4680) on sgACC resting-state functional connectivity (rsFC) in healthy individuals. A homogeneous group of 61 Caucasian right-handed healthy female university students, all within the same age range, underwent rsfMRI. Compared to Met158 homozygotes, Val158 allele carriers displayed significantly stronger rsFC between the sgACC and the left parahippocampal gyrus, ventromedial parts of the inferior frontal gyrus (IFG), and the nucleus accumbens (NAc). On the other hand, compared to Val158 homozygotes, we found in Met158 allele carriers stronger sgACC rsFC with the medial frontal gyrus (MFG), more in particular the anterior parts of the medial orbitofrontal cortex. Although we did not use emotional or cognitive tasks, our sgACC rsFC results point to possible distinct differences in emotional and cognitive processes between Val158 and Met158 allele carriers. However, the exact nature of these directions remains to be determined.
Introduction
Brain imaging studies point to functional differences between catechol-O-methyltransferase (COMT) Val158Met carriers in the prefrontal and limbic areas related to emotional processing (Rasch et al., 2010; Shehzad et al., 2012). Whereas Met158 allele carriers may have higher risk of developing mood and anxiety disorders (Hosák, 2007; Kocabas et al., 2010; Witte and Flöel, 2012), individuals carrying the Val158 variant are thought to display more cognitive difficulties (Swart et al., 2011). However, these assumptions are not yet fully established and reverse findings have been reported (Amstadter et al., 2012). Indeed, some studies reported that the Met158 allele is associated with increased limbic responsiveness to negative stimuli (Smolka et al., 2005, 2007), whereas others reported the opposite (Kempton et al., 2009; Domschke et al., 2012) or described null-results (Drabant et al., 2006). Further, the meta-analysis of Mier et al. (2010) provided evidence for a neural prefrontal cortical substrate of the pleiotropic behavioral effects of COMT Val158Met (rs4680) genetic variation. This means that a single gene has an effect on the expression of two or more phenotypic traits; in this case the COMT Val158Met gene that aligns with a differential impact on cognitive and emotional function. These opposing effects were found for executive cognition paradigms (favoring Met158 allele carriers) and emotional paradigms (favoring the Val158 variant). To summarize, at the brain level it remains poorly understood how these Val158Met single nucleotide polymorphisms (SNP) interfere with cognitive and/or emotion associated neurocircuits.
Resting state fMRI (rsfMRI)—used to measure neuronal connections between distinct regions, referred to as resting-state functional connectivity (rsFC; Biswal et al., 1995)–may have the potential to gain more insight in the genetic influence on these neuronal processes (Fox et al., 2012). More in particular, rsFC has several practical and theoretical advantages over task based fMRI, including improved signal to noise, a reduced need for participant compliance, and the avoidance of task performance confounds (Fox and Greicius, 2010). However, until now, only two brain imaging studies applied rsFC together with the COMT Val158Met gene typing. Tunbridge et al. (2013) showed that Val158 homozygotes displayed greater rsFC than the Met158 allele carriers between an executive control network and the ventrolateral parts of the prefrontal cortex during cognitive task performance (working memory). These findings concur with the observations of Liu et al. (2010) on default network connectivity where the Val158 homozygotes compared to COMT Val158 and Met158 heterozygous individuals also showed poor cognitive performance, possibly through differential effects on prefrontal dopamine levels. Indeed, homozygote Val158 carriers display higher enzymatic activity resulting in less prefrontal dopamine, whereas for the Met158 variant carriers this is the reverse (Heinz and Smolka, 2006). Further, stress-related influences may affect COMT Val158 and Met158 allele carriers differently (Antypa et al., 2013). Under stress, working memory performance of Met158 homozygotes was significantly worse compared to Val158 homozygotes (Buckert et al., 2012). Carriers of the Val158 allele of COMT (rs4680) were also more likely to quit a task measuring the level of distress intolerance than those without a Val158 allele (Amstadter et al., 2012). This is in line with the assumption that the Val158 allele is associated with cognitive inefficiency during tasks involving cognitive control. Bishop et al. (2006) found that, during the performance of a house-matching task under emotional distraction, the Val158 load correlated positively with activity in parahippocampal regions.
Importantly, dopaminergic activity within the ventromedial prefrontal cortex (vmPFC), including the subgenual anterior cingulate cortex (sgACC), has been associated with subjective levels of psychosocial stress (Lataster et al., 2011). This ACC region is part of distributed corticolimbic neurocircuits implicated in “visceromotor” functions and in modulating affect, such as sadness and ruminative thought patterns (Disner et al., 2011; Smith et al., 2011; Davey et al., 2012). The sgACC also participates in cognitive functions that create sad moods and lead to pessimism (Meyer, 2012).
Because the sgACC is a key region in modulating emotional behavior (Drevets et al., 2008), our main research objective was to examine whether in the Val158Met SNP (rs4680) differently influences sgACC rsFC with areas related to emotion and/or cognitive functioning. Because age (Ferreira and Busatto, 2013) and gender (Wang et al., 2014), may confound rsFC results, and given that age and gender influences have also been reported for the COMT Val158Met gene (Harrison and Tunbridge, 2008; Witte and Flöel, 2012), all female participants were selected within a narrow age range and were documented never to have suffered from neuropsychiatric illnesses. The chosen sgACC seed Montreal Neurological Institute (MNI) coordinates were based on brain anatomical coordinates provided by a neuroimaging study of emotion processing and emotion regulation in women resilient or susceptible to the depressogenic effects of early life stress (Cisler et al., 2013).
In Val158 carriers we hypothesized predominantly sgACC rsFC correlations with anatomical areas related with cognitive functioning. In Met158 carriers we expected sgACC rsFC associations with neurocircuits implicated in emotional processing. Of note, at this stage we cannot draw hypotheses about the direction of these correlations.
Materials and Methods
Participants
The study was approved by the ethics committee of the University Hospital (UZBrussel) and all subjects gave written informed consent. It was part of a larger project for investigating different neuro-cognitive markers in affective disorders, in which a total of 80 healthy females were recruited. All participated in another study which evaluated the influence of the COMT Val158Met gene on personality dimensions. These results are published elsewhere (Baeken et al., in press).
Sixty-one right-handed Caucasian female participants, all university students (mean age = 21.8 years, sd = 2.5 years), participated in the rsfMRI study. Right-handedness was assessed with the van Strien questionnaire (van Strien and Van Beek, 2000). No subjects had ever used major psychotropic medications and at the time of the experiments all were free of any drug or medication, other than birth-control pills. To exclude psychiatric or neurological diseases all volunteers were screened by the first author (Chris Baeken). Psychiatric disorders were assessed by the Dutch version of the (MINI; Sheehan et al., 1998). Subjects with a psychiatric disorder and/or a score higher than eight on the Beck Depression Inventory (BDI-II; Beck et al., 1996) were excluded.
Genetics
In a first step, after the rsfMRI scan EDTA acid anti-coagulated blood samples were drawn from each participant and DNA was isolated. In a second step, genotyping of COMT rs4680 SNP was performed using the MassARRAY platform (SEQUENOM, San Diego, CA).
Scanning Procedure
All participants were instructed to stay awake with their eyes closed and to think of nothing in particular during the resting state measurements, involving exactly 5 min of scanning. To reduce sensory confounds as much as possible, the light in the room was dimmed during scanning. After the scan, the subjects were asked to confirm that they had been awake throughout the scan and had complied with the instructions. All rsfMRI scans were performed on Monday afternoons, between 15:00 and 18:00.
To obtain individual anatomical information, all subjects underwent a T1-weighted MRI of the brain (3D-TFE, voxel size 1 × 1 × 1 mm) using a 3T Achieva MR scanner with an eight-channel SENSE head coil (Philips, Best, The Netherlands). The rsfMRI used a SE-EPI sequence (TR/TE = 3000/70 ms; flip angle = 90°; FOV = 230 × 230 mm2; resolution = 1.80 × 1.80 mm2; slice thickness/gap = 4.00/1.0 mm; number of slices = 24; number of dynamics = 100; time resolution = 3000 ms). After the rsfMRI scan an additional 3D anatomical scan using a 3D T1 TFE sequence (TR/TE = 12.00/3.71 ms; flip angle = 10°; FOV = 240 × 240 × 200 mm3; resolution = 1.00 × 1.00 × 2.0 mm3; number of slices = 100) was performed, yielding an anatomical underlay for the fMRI results.
The fMRI data were analyzed with the SPM8 software (Wellcome Department of Cognitive Neurology, London, UK). Slice-time correction was performed to correct for small differences in the time offset of consecutively measured slices. Hereafter, the images were realigned to the first volume of the time series in order to correct for head movements. Subsequently, all fMRI brain volumes were normalized to the EPI MNI template; resampled to 3-mm isotropic voxels and spatially smoothed using an 8-mm full-width half-maximum Gaussian kernel. The anatomical scans were normalized to the T1 MNI template.
Functional imaging data were linearly detrended and band-pass filtered (0.01–0.8 Hz). Spurious or nonspecific sources of variance were removed from the data through linear regression of: (1) the six head-motion parameters obtained in the realigning step; (2) the signal from a region in the cerebrospinal fluid; (3) the signal from a region centered in the white matter; and (4) the whole-brain signal. Correlation maps were obtained by extracting the BOLD time course from a seed region, then computing the correlation coefficients characterizing the correlations between that time course and the time courses from all other brain voxels. The seed region was a 6-mm-diameter sphere designed to encompass the (sgACC: x = 1, y = 25, z = −11), as provided by Cisler et al. (2013). We already used these seed sgACC coordinates in depressed patients (Baeken et al., 2014b), as well as the amygdalae seed coordinates in stress sensitive healthy females (Baeken et al., 2014a). Further, in a second step to increase the chance to get signal from functionally overlapping regions, we doubled the diameter of the sgACC sphere to 12 mm.
The correlation maps were submitted to a random-effect analysis in SPM8 following a one-way ANCOVA containing COMT (Val158–Val/Met158–Met158) as factor and age as covariate. These analyses were thresholded with Alphasim correction (residuals) as implemented in the SPM REST toolbox1 at p < 0.05. The result was a cluster extent threshold (K) of 46 voxels and a voxel significance threshold of 0.01. The anatomical labels and MNI coordinates were obtained by the xjView MATLAB toolbox.2 In a following step, to investigate whether the significant clusters corresponded to increased or decreased functional connectivity, post-hoc paired t-tests were performed in Marsbar (Brett et al., 2002). Paired variables were the contrast values for the three different groups (Val158–Val/Met158–Met158) separately. These paired t-tests were corrected for the number of ROIs. The significance level was set at p ≤ 0.05 (corrected), two-tailed.
Results
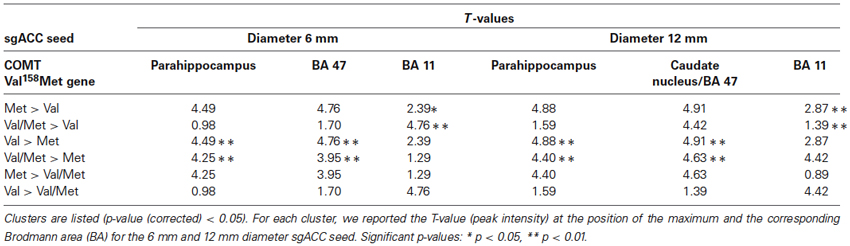
From the 61 participants, 14 were Val158 carriers, 37 Val/Met158 heterozygotes, and 10 were Met158 homozygotes. No volunteer stated to have fallen asleep during scanning. The calculation of the Hardy-Weinberg Equilibrium for two alleles showed no deviation of this assumption (X2(1, N = 61) = 2.89, p = 0.09). A one-way ANOVA did not show age differences between the three groups (F(2,58) = 0.62, p = 0.54). Significant one-way ANOVA sgACC rsFC clusters are displayed in Figure 1 for the 6 and 12 mm diameter seed sphere separately. For an overview of all significant clusters see also Table 1.
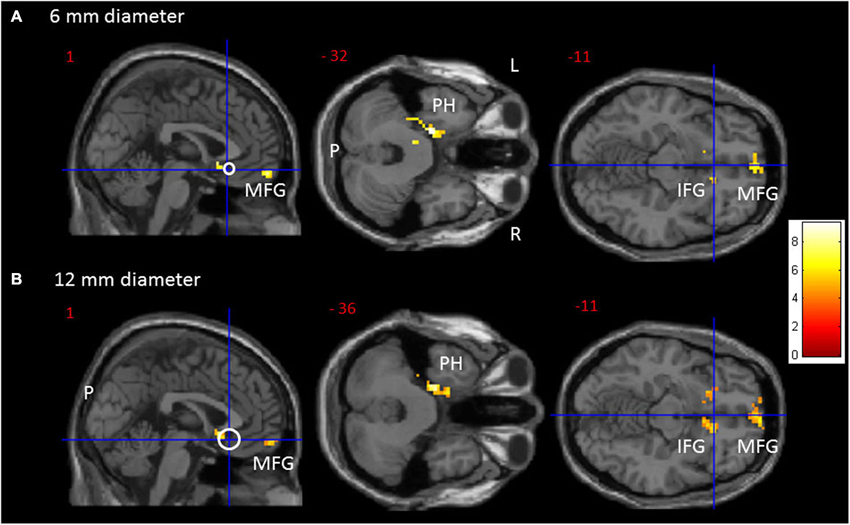
Figure 1. Transversal and sagittal slides exhibiting significant rsFC clusters (red to yellow) for the sgACC seed (MNI: x = 1, y = 25, z = −11). (A) For the 6 mm and (B) for the 12 mm diameter sgACC seed. The seed is displayed as a crosshair on an open white circle. CAVE: these white circles represent an estimate and not by definition a 1/1 scale. For an overview of all significant interaction clusters see Table 2. P = posterior, L = left, R = right. IFG = Inferior Frontal Gyrus, MFG = Medial Frontal Gyrus, PH = parahippocampus.
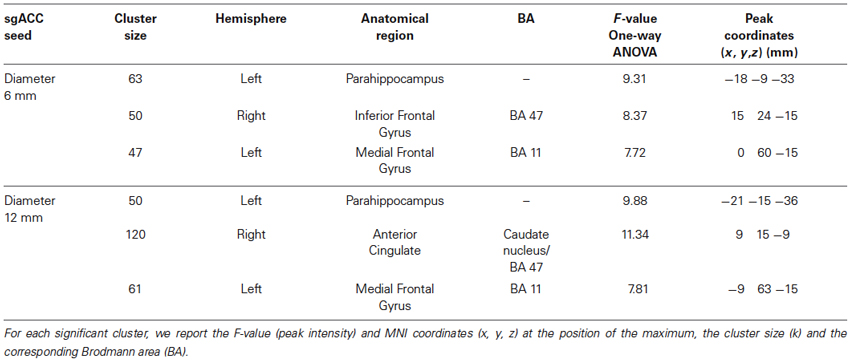
Table 1. Results of the one-way ANCOVA for the sgACC rsFC containing COMT (Val158–Met158–Val/Met158) as factor and age as covariate for the 6 mm and radius 12 mm diameter sgACC seed.
Results Confined to the 6 mm sgACC Seed
The one-way ANOVA revealed three significant clusters with the first located in the left parahippocampal gyrus (MNI coordinates: x = −18, y = −9, z = −33) and the second in the right inferior frontal gyrus (IFG) (BA 47: x = 15, y = 24, z = −15). Here post-hoc testing showed significantly stronger rsFC between the sgACC and these clusters in Val158 and Val/Met158 carriers compared to Met158 carriers. The third interaction cluster was located in the left medial frontal gyrus (MFG) (BA 11: x = 0, y = 60, z = −15) and here the post-hoc test indicated a stronger sgACC rsFC with the left BA 11 in Met158 and Val/Met158 carriers compared to Val158 carriers. See Table 2.
Results Confined to the 12 mm sgACC Seed
Here, the one-way ANOVA also revealed three significant clusters located in similar areas as before: the left parahippocampal gyrus (MNI coordinates: x = −21, y = −15, z = −36), the right IFG extending to the caudate nucleus (x = 9, y = 15, z = −9), and the left MFG (BA 11: x = −9, y = 63, z = −15). Post-hoc testing showed similar directions as found for the 6 mm sgACC seed for the Val158–Val/Met158–Met158 carriers. See also Table 2.
Discussion
As hypothesized, we found different sgACC rsFC patterns in Val158 and Met158 carriers. Compared to the Met158 homozygotes, COMT Val158 and Val/Met158 carriers were found to exhibit stronger sgACC rsFC associations with the left parahippocampus (PH), the ventromedial parts of the inferior frontal cortex, and the nucleus accumbens (NAc) and caudate nucleus. The involvement of hippocampal areas has been demonstrated earlier in different brain imaging studies examining the effect of the COMT Val158Met polymorphism on cognitive processes, usually reporting that Val158 carriers perform worse on memory tasks than Met158 homozygotes (Bertolino et al., 2008; Heim et al., 2013); in particular in stressful situations (Buckert et al., 2012). As mentioned before, Val158 carriers recruited the parahippocampal regions while performing a house-matching task during emotional distraction, a typical attentional control related task (Bishop et al., 2006). Although we did not observe differences between homo-and heterozygote Val158 carriers and we did not include stress-related tasks (other than lying in the scanner with eyes closed), our findings support the hypothesis that COMT Val158 and Met158 carriers engage different neurocircuits while performing cognitive loaded tasks (Dennis et al., 2010). Indeed, because the sgACC serves as a facilitator of visceral responses during emotional processing–more in particular in stress-related conditions (Mayberg et al., 1999; Baeken et al., 2010)–the observed FC between the sgACC and the (left) PH in Val158 carriers could imply a stronger “emotional” participation associated with memory and recollection processes which may interfere with task achievement outcomes (Eichenbaum et al., 2007, 2012). Val158 carriers also displayed a stronger sgACC rsFC with the ventromedial IFG, which extended bilaterally to the NAc (which is part of the ventral striatum), and to the caudate nucleus (12 mm diameter sgACC seed), both areas comprising mostly dopaminergic neurons. This could be in line with the neurobiological action of the COMT Val158Met gene where Val158 carriers display higher enzymatic activity resulting in less prefrontal dopamine (Witte and Flöel, 2012). Again, as we did not include cognitive tasks and no direct dopaminergic measurements, these assumptions should be interpreted cautiously at this point. Because this is a post-hoc data driven interpretation on FC, additional data on the dopaminergic system will be crucial to verify that a stronger sgACC rsFC in Val158 carriers is related to dopamine enzymatic activity. Indeed, in spite that functional connectivity is a unique powerful tool able to increase our knowledge on human brain organization, rsFC is based on an inherently ambiguous measure reflecting constraints both from static anatomical connectivity and from poorly understood functional coupling changes that are dynamic; physiological data from other sources may be required to confirm and interpret FC findings (Buckner et al., 2013).
Post-hoc t-tests showed that compared to Val158 homozygotes, Met158 and Val/Met158 carriers displayed stronger sgACC rsFC with the MFG, more in particular the (left) orbitofrontal cortex (OFC: Brodmann area (BA) 11). Again, no differences between Met158 homo- and heterozygote allele carriers were observed. Other researchers also found OFC involvement in Met158 carriers related to emotional or arousal processing (Drabant et al., 2006; Montag et al., 2008; Dreher et al., 2009). Here, in contrast to the more lateral parts of the OFC, the medial OFC regions showed stronger connectivity with the default mode network, the limbic system, and areas related to autonomic processes. For a recent overview see Zald et al. (2014). Interestingly, it has recently been hypothesized by Falk et al. (2012) that Met158 allele carriers may be more sensitive to cues that signal social reward or punishment and that carrying the Met158 allele may be associated with greater neural activity to reward-related stimuli (Lancaster et al., 2012). This might predispose them to seek approval and thus exhibit more social conformity (Deuker et al., 2013). In line with these findings, a recent meta-analysis showed that medial OFC/vmPFC may subserve different roles in processing of reward magnitude (Diekhof et al., 2012). These dopaminergic neurons coming from the ventral tegmental area (VTA) are crucial for the recognition of rewards and their consumption or lack of consumption (Russo and Nestler, 2013). Although reverse findings have been reported (Hopkins et al., 2013), carrying the Met158 allele is associated with increased activity in limbic areas and prefrontal cortex. Of note, Met158homozygotes also exhibit greater anxiety (Montag et al., 2008; Baumann et al., 2013).
However, as our sample is relatively small, the interpretation of these results should be done cautiously. Due to the nature of this study, we can only draw conclusions regarding right-handed healthy young women. Although the selection of psychopathology-free female subjects within a narrow age-range can be considered as a major advantage of the study, including only healthy women within a certain age range does mean that we cannot generalize our findings to men, older women or subjects with any form of psychiatric illness. Because we did not a priori select our participants based on their genetic COMT Val158Met profile, the three groups were unbalanced, which might have influenced our imaging results. To some extent this may explain the lack of sgACC rsFC differences between COMT Val158Met homo- and heterozygotes. Another limitation of our study is the fact that no cardiac and respiratory data were collected during rsfMRI. Besides head movement, cardiac pulsation during scanning results in brain tissue movement and inflow effects leading to correlated signal fluctuations. Further, chest movement during breathing results in magnetic field changes that distort the MR image acquired of the brain (For an overview see Birn, 2012). Further, our hypothesis on sgACC involvement in the COMT Val158Met polymorphism was determined a priori. By not examining other dedicated seeds important information may have been missed. However, our main research objective was to examine the COMT Val158 Met gene in relation a specific node documented in women to be involved in emotion (dys)regulation in brain networks (Cisler et al., 2013), which makes the choice of the sgACC seed particularly relevant. Further, this seed based correlation analysis technique has been successfully applied by many research groups and has been proven useful in revealing the connectivity properties of similar seed areas (Cole et al., 2010). In addition, the test-retest reliability of such seed based correlation analysis has indicated moderate to high reliability to examine resting-state networks (Shehzad et al., 2009).
To summarize, in line with our hypotheses, we found distinct sgACC rsFC differences between Val158 and Met158 allele carriers. In spite that we did not use emotional or cognitive tasks, our sgACC rsFC results could represent specific neural substrates for the pleiotropic behavioral effects of COMT genetic variation between Val158 and Met158 allele carriers. Although our results corroborate an emotional influence on the COMT Val158Met polymorphism, future imaging research examining this SNP, also with dopaminergic brain measurements, may do well to include a larger number of seeds implicated in emotional and cognitive functioning.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by a grant from the Scientific Fund W. Gepts UZBrussel and supported by the Ghent University Multidisciplinary Research Partnership “The integrative neuroscience of behavioral control”.
Footnotes
References
Amstadter, A. B., Daughters, S. B., Macpherson, L., Reynolds, E. K., Danielson, C. K., Wang, F., et al. (2012). Genetic associations with performance on a behavioral measure of distress intolerance. J. Psychiatr. Res. 46, 87–94. doi: 10.1016/j.jpsychires.2011.09.017
Antypa, N., Drago, A., and Serretti, A. (2013). The role of COMT gene variants in depression: bridging neuropsychological, behavioral and clinical phenotypes. Neurosci. Biobehav. Rev. 37, 1597–1610. doi: 10.1016/j.neubiorev.2013.06.006
Baeken, C., Claes, S., and De Raedt, R. (in press). The influence of COMT Val 158 Met genotype on the character dimension cooperativeness in healthy females. Brain Behav. doi: 10.1002/brb3.233
Baeken, C., Marinazzo, D., Van Schuerbeek, P., Wu, G. R., De Mey, J., Luypaert, R., et al. (2014a). Left and right amygdala—mediofrontal cortical functional connectivity is differentially modulated by harm avoidance. PLoS One 9:e95740. doi: 10.1371/journal.pone.0095740
Baeken, C., Marinazzo, D., Wu, G. R., Van Schuerbeek, P., DeMey, J., Marchetti, I., et al. (2014b). Accelerated HF-rTMS in treatment-resistant unipolar depression: insights from subgenual anterior cingulate functional connectivity. World J. Biol. Psychiatry 15, 286–297. doi: 10.3109/15622975.2013.872295
Baeken, C., Van Schuerbeek, P., De Raedt, R., Ramsey, N. F., Bossuyt, A., De Mey, J., et al. (2010). Reduced left subgenual anterior cingulate cortical activity during withdrawal-related emotions in melancholic depressed female patients. J. Affect. Disord. 127, 326–331. doi: 10.1016/j.jad.2010.05.013
Baumann, C., Klauke, B., Weber, H., Domschke, K., Zwanzger, P., Pauli, P., et al. (2013). The interaction of early life experiences with COMT val158met affects anxiety sensitivity. Genes Brain Behav. 12, 821–829. doi: 10.1111/gbb.12090
Beck, A. T., Steer, R. A., and Brown, G. K. (1996). Beck Depression Inventory Manual. 2nd Edn. San Antonio: The Psychological Corporation.
Bertolino, A., Di Giorgio, A., Blasi, G., Sambataro, F., Caforio, G., Sinibaldi, L., et al. (2008). Epistasis between dopamine regulating genes identifies a nonlinear response of the human hippocampus during memory tasks. Biol. Psychiatry 64, 226–234. doi: 10.1016/j.biopsych.2008.02.001
Birn, R. M. (2012). The role of physiological noise in resting-state functional connectivity. Neuroimage 62, 864–870. doi: 10.1016/j.neuroimage.2012.01.016
Bishop, S. J., Cohen, J. D., Fossella, J., Casey, B. J., and Farah, M. J. (2006). COMT genotype influences prefrontal response to emotional distraction. Cogn. Affect. Behav. Neurosci. 6, 62–70. doi: 10.3758/cabn.6.1.62
Biswal, B. B., Yetkin, F. Z., Haughton, V. M., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echoplanar MRI. Magn. Reson. Med. 34, 537–541. doi: 10.1002/mrm.1910340409
Brett, M., Anton, J. L., and Valabregue, R. (2002). “Region of interest analysis using an SPM toolbox [abstract],” Presented at the Eighth International Conference of Functional Mapping of the Human Brain (Sendai, Japan). Available on CD-ROM in NeuroImage, Vol. 16, No. 2.
Buckert, M., Kudielka, B. M., Reuter, M., and Fiebach, C. J. (2012). The COMT Val158Met polymorphism modulates working memory performance under acute stress. Psychoneuroendocrinology 37, 1810–1821. doi: 10.1016/j.psyneuen.2012.03.014
Buckner, R. L., Krienen, F. M., and Yeo, B. T. (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 16, 832–837. doi: 10.1038/nn.3423
Cisler, J. M., James, G. A., Tripathi, S., Mletzko, T., Heim, C., Hu, X. P., et al. (2013). Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol. Med. 43, 507–518. doi: 10.1017/s0033291712001390
Cole, D. M., Smith, S. M., and Beckmann, C. F. (2010). Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 4:8. doi: 10.3389/fnsys.2010.00008
Davey, C. G., Harrison, B. J., Yücel, M., and Allen, N. B. (2012). Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol. Med. 42, 2071–2081. doi: 10.1017/s0033291712000323
Dennis, N. A., Need, A. C., LaBar, K. S., Waters-Metenier, S., Cirulli, E. T., Kragel, J., et al. (2010). COMT val108/158 met genotype affects neural but not cognitive processing in healthy individuals. Cereb. Cortex 20, 672–683. doi: 10.1093/cercor/bhp132
Deuker, L., Müller, A. R., Montag, C., Markett, S., Reuter, M., Fell, J., et al. (2013). Playing nice: a multi-methodological study on the effects of social conformity on memory. Front. Hum. Neurosci. 7:79. doi: 10.3389/fnhum.2013.00079
Diekhof, E. K., Kaps, L., Falkai, P., and Gruber, O. (2012). The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 50, 1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007
Disner, S. G., Beevers, C. G., Haigh, E. A., and Beck, A. T. (2011). Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 12, 467–477. doi: 10.1038/nrn3027
Domschke, K., Baune, B. T., Havlik, L., Stuhrmann, A., Suslow, T., Kugel, H., et al. (2012). Catechol-O-methyltransferase gene variation: impact on amygdala response to aversive stimuli. Neuroimage 60, 2222–2229. doi: 10.1016/j.neuroimage.2012.02.039
Drabant, E. M., Hariri, A. R., Meyer-Lindenberg, A., Munoz, K. E., Mattay, V. S., Kolachana, B. S., et al. (2006). Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch. Gen. Psychiatry 63, 1396–1406.
Dreher, J. C., Kohn, P., Kolachana, B., Weinberger, D. R., and Berman, K. F. (2009). Variation in dopamine genes influences responsivity of the human reward system. Proc. Natl. Acad. Sci. U S A 106, 617–622. doi: 10.1073/pnas.0805517106
Drevets, W. C., Savitz, J., and Trimble, M. (2008). The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 13, 663–681.
Eichenbaum, H., Sauvage, M., Fortin, N., Komorowski, R., and Lipton, P. (2012). Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci. Biobehav. Rev. 36, 1597–1608. doi: 10.1016/j.neubiorev.2011.07.006
Eichenbaum, H., Yonelinas, A. P., and Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 30, 123–152. doi: 10.1146/annurev.neuro.30.051606.094328
Falk, E. B., Way, B. M., and Jasinska, A. J. (2012). An imaging genetics approach to understanding social influence. Front. Hum. Neurosci. 6:168. doi: 10.3389/fnhum.2012.00168
Ferreira, L. K., and Busatto, G. F. (2013). Resting-state functional connectivity in normal brain aging. Neurosci. Biobehav. Rev. 37, 384–400. doi: 10.1016/j.neubiorev.2013.01.017
Fox, M. D., and Greicius, M. (2010). Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 4:19. doi: 10.3389/fnsys.2010.00019
Fox, M. D., Halko, M. A., Eldaief, M. C., and Pascual-Leone, A. (2012). Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 62, 2232–2243. doi: 10.1016/j.neuroimage.2012.03.035
Harrison, P. J., and Tunbridge, E. M. (2008). Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology 33, 3037–3045. doi: 10.1038/sj.npp.1301543
Heim, A. F., Coyne, M. J., Kamboh, M. I., Ryan, C., and Jennings, J. R. (2013). The catechol-o-methyltransferase Val(158)Met polymorphism modulates organization of regional cerebral blood flow response to working memory in adults. Int. J. Psychophysiol. 90, 149–156. doi: 10.1016/j.ijpsycho.2013.06.023
Heinz, A., and Smolka, M. N. (2006). The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev. Neurosci. 17, 359–367. doi: 10.1515/revneuro.2006.17.3.359
Hopkins, S. C., Reasner, D. S., and Koblan, K. S. (2013). Catechol-O-methyltransferase genotype as modifier of superior responses to venlafaxine treatment in major depressive disorder. Psychiatry Res. 208, 285–287. doi: 10.1016/j.psychres.2013.04.021
Hosák, L. (2007). Role of the COMT gene Val158Met polymorphism in mental disorders: a review. Eur. Psychiatry 22, 276–281. doi: 10.1016/j.eurpsy.2007.02.002
Kempton, M. J., Haldane, M., Jogia, J., Christodoulou, T., Powell, J., Collier, D., et al. (2009). The effects of gender and COMT Val158Met polymorphism on fearful facial affect recognition: a fMRI study. Int. J. Neuropsychopharmacol. 12, 371–381. doi: 10.1017/S1461145708009395
Kocabas, N. A., Faghel, C., Barreto, M., Kasper, S., Linotte, S., Mendlewicz, J., et al. (2010). The impact of catechol-O-methyltransferase SNPs and haplotypes on treatment response phenotypes in major depressive disorder: a case-control association study. Int. Clin. Psychopharmacol. 25, 218–227. doi: 10.1097/YIC.0b013e328338b884
Lancaster, T. M., Linden, D. E., and Heerey, E. A. (2012). COMT val158met predicts reward responsiveness in humans. Genes Brain Behav. doi: 10.1111/j.1601-183x.2012.00838.x. [Epub ahead of print].
Lataster, J., Collip, D., Ceccarini, J., Haas, D., Booij, L., van Os, J., et al. (2011). Psychosocial stress is associated with in vivo dopamine release in human ventromedial prefrontal cortex: a positron emission tomography study using [18F]fallypride. Neuroimage 58, 1081–1089. doi: 10.1016/j.neuroimage.2011.07.030
Liu, B., Song, M., Li, J., Liu, Y., Li, K., Yu, C., et al. (2010). Prefrontal-related functional connectivities within the default network are modulated by COMT val158met in healthy young adults. J. Neurosci. 30, 64–69. doi: 10.1523/JNEUROSCI.3941-09.2010
Mayberg, H. S., Liotti, M., Brannan, S. K., McGinnis, S., Mahurin, R. K., Jerabek, P. A., et al. (1999). Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry 156, 675–682.
Meyer, J. H. (2012). Neuroimaging markers of cellular function in major depressive disorder: implications for therapeutics, personalized medicine and prevention. Clin. Pharmacol. Ther. 91, 201–214. doi: 10.1038/clpt.2011.285
Mier, D., Kirsch, P., and Meyer-Lindenberg, A. (2010). Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol. Psychiatry 15, 918–927. doi: 10.1038/mp.2009.36
Montag, C., Buckholtz, J. W., Hartmann, P., Merz, M., Burk, C., Hennig, J., et al. (2008). COMT genetic variation affects fear processing: psychophysiological evidence. Behav. Neurosci. 122, 901–909. doi: 10.1037/0735-7044.122.4.901
Rasch, B., Spalek, K., Buholzer, S., Luechinger, R., Boesiger, P., de Quervain, D. J., et al. (2010). Aversive stimuli lead to differential amygdala activation and connectivity patterns depending on catechol-O-methyltransferase Val158Met genotype. Neuroimage 52, 1712–1719. doi: 10.1016/j.neuroimage.2010.05.054
Russo, S. J., and Nestler, E. J. (2013). The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14, 609–925. doi: 10.1038/nrn3381
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 34–57.
Shehzad, Z., DeYoung, C. G., Kang, Y., Grigorenko, E. L., and Gray, J. R. (2012). Interaction of COMT val158met and externalizing behavior: relation to prefrontal brain activity and behavioral performance. Neuroimage 60, 2158–2168. doi: 10.1016/j.neuroimage.2012.01.097
Shehzad, Z., Kelly, A. M., Reiss, P. T., Gee, D. G., Gotimer, K., Uddin, L. Q., et al. (2009). The resting brain: unconstrained yet reliable. Cereb. Cortex 19, 2209–2229. doi: 10.1093/cercor/bhn256
Smith, R., Fadok, R. A., Purcell, M., Liu, S., Stonnington, C., Spetzler, R. F., et al. (2011). Localizing sadness activation within the subgenual cingulate in individuals: a novel functional MRI paradigm for detecting individual differences in the neural circuitry underlying depression. Brain Imaging Behav. 5, 229–239. doi: 10.1007/s11682-011-9127-2
Smolka, M. N., Bühler, M., Schumann, G., Klein, S., Hu, X. Z., Moayer, M., et al. (2007). Gene-gene effects on central processing of aversive stimuli. Mol. Psychiatry 12, 307–317. doi: 10.1038/sj.mp.4001946
Smolka, M. N., Schumann, G., Wrase, J., Grüsser, S. M., Flor, H., Mann, K., et al. (2005). Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J. Neurosci. 25, 836–842. doi: 10.1523/jneurosci.1792-04.2005
Swart, M., Bruggeman, R., Larøi, F., Alizadeh, B. Z., Kema, I., Kortekaas, R., et al. (2011). COMT Val158Met polymorphism, verbalizing of emotion and activation of affective brain systems. Neuroimage 55, 338–344. doi: 10.1016/j.neuroimage.2010.12.017
Tunbridge, E. M., Farrell, S. M., Harrison, P. J., and Mackay, C. E. (2013). Catechol-O-methyltransferase (COMT) influences the connectivity of the prefrontal cortex at rest. Neuroimage 68, 49–54. doi: 10.1016/j.neuroimage.2012.11.059
van Strien, J. W., and Van Beek, S. (2000). Ratings of emotion in laterally presented faces: sex and handedness effects. Brain Cogn. 44, 645–652. doi: 10.1006/brcg.1999.1137
Wang, G., Erpelding, N., and Davis, K. D. (2014). Sex differences in connectivity of the subgenual anterior cingulate cortex. Pain 155, 755–763. doi: 10.1016/j.pain.2014.01.005
Witte, A. V., and Flöel, A. (2012). Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain Res. Bull. 88, 418–428. doi: 10.1016/j.brainresbull.2011.11.012
Keywords: sgACC, functional connectivity, COMT, females, genotype differences
Citation: Baeken C, Marinazzo D, Claes S, Wu G-R, Van Schuerbeek P, De Mey J, Luypaert R and De Raedt R (2014) COMT Val158Met genotypes differentially influence subgenual cingulate functional connectivity in healthy females. Front. Hum. Neurosci. 8:481. doi: 10.3389/fnhum.2014.00481
Received: 20 March 2014; Accepted: 13 June 2014;
Published online: 30 June 2014.
Edited by:
Shuhei Yamaguchi, Shimane University, JapanReviewed by:
Peter Kirsch, Zentralinstitut für Seelische Gesundheit, GermanyUdo Dannlowski, University of Münster, Germany
Copyright © 2014 Baeken, Marinazzo, Claes, Wu, Van Schuerbeek, De Mey, Luypaert and De Raedt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chris Baeken, Department of Psychiatry and Medical Psychology, Ghent University, De Pintelaan 185, 9000 Ghent, Belgium e-mail: chris.baeken@UGent.be
 Chris Baeken
Chris Baeken Daniele Marinazzo
Daniele Marinazzo Stephan Claes5
Stephan Claes5  Guo-Rong Wu
Guo-Rong Wu Rudi De Raedt
Rudi De Raedt