Proprioceptive dysfunction in focal dystonia: from experimental evidence to rehabilitation strategies
- 1Section of Human Physiology, Department of Experimental Medicine, Centro Polifunzionale di Scienze Motorie, University of Genoa, Genoa, Italy
- 2Department of Neurological and Movement Sciences, University of Verona, Verona, Italy
Dystonia has historically been considered a disorder of the basal ganglia, mainly affecting planning and execution of voluntary movements. This notion comes from the observation that most lesions responsible for secondary dystonia involve the basal ganglia. However, what emerges from recent research is that dystonia is linked to the dysfunction of a complex neural network that comprises basal ganglia–thalamic–frontal cortex, but also the inferior parietal cortex and the cerebellum. While dystonia is clearly a motor problem, it turned out that sensory aspects are also fundamental, especially those related to proprioception. We outline experimental evidence for proprioceptive dysfunction in focal dystonia from intrinsic sensory abnormalities to impaired sensorimotor integration, which is the process by which sensory information is used to plan and execute volitional movements. Particularly, we will focus on proprioceptive aspects of dystonia, including: (i) processing of vibratory input, (ii) temporal discrimination of two passive movements, (iii) multimodal integration of visual-tactile and proprioceptive inputs, and (iv) motor control in the absence of visual feedback. We suggest that these investigations contribute not only to a better understanding of dystonia pathophysiology, but also to develop rehabilitation strategies aimed at facilitating the processing of proprioceptive input.
Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both (Fahn, 1988). Dystonia can be classified along two axes: clinical characteristics – including body distribution – and etiology, which includes nervous system pathology and inheritance (Albanese et al., 2013). Classification by body regions identifies: focal dystonia, segmental dystonia, multifocal dystonia, generalized dystonia, and hemi-dystonia.
Focal dystonias are usually adult-onset and affect only one body region. Many cases of focal dystonia with onset in adulthood are idiopathic (meaning that dystonia is the only neurological symptom, presumably with a genetic component), though secondary, acquired cases are documented. Typical examples of focal forms are blepharospasm, oromandibular dystonia, cervical dystonia, laryngeal dystonia, and focal hand dystonia.
Sensory Aspects of Focal Dystonia
Dystonia has been considered a “basal ganglia disorder” and attributed to a functional disturbance of the cortico-striato-thalamic-cortical circuits. This notion comes from the observation that most lesions responsible for unilateral secondary dystonia are usually confined to the putamen, caudate, globus pallidus, and thalamus (Bhatia and Marsden, 1994). The common idea is that a dysfunction of the basal ganglia and/or their connections to motor cortex plays a major role in the pathogenesis of dystonia, by influencing the final organization and execution of movement (Berardelli et al., 1998). This idea is supported by the clinical picture of dystonia, mainly characterized by motor symptoms (Currá et al., 2000; Gregori et al., 2008). However, the pathophysiology of dystonia has been largely re-discussed in the last years (Kanovský and Rosales, 2011; Avanzino and Abbruzzese, 2012; Quartarone and Hallett, 2013). Certainly, it is now widely accepted that somatosensory inputs play a substantial role in dystonia (Stamelou et al., 2012; Patel et al., 2014).
Somatosensory inputs include touch, pain, temperature, and proprioception. The contribution of the somatosensory system to the mechanism of the dystonia is supported by the following clinical aspects: (1) alleviation of dystonia with “sensory tricks” (Wissel et al., 1999; Müller et al., 2001); (2) photosensitivity and other ocular discomforts in patients with blepharospasm (Stamelou et al., 2012); (3) neck pain that often precedes cervical dystonia (Ghika et al., 1993; Stamelou et al., 2012); (4) improvement of dystonic movements after administration of local anesthetic (Kaji et al., 1995).
Apart from these symptoms and signs, patients with dystonia present mild sensory abnormalities to special testing like heat-evoked potentials (Suttrup et al., 2011) and cutaneous spatial and temporal discrimination tests (Sanger et al., 2001; Fiorio et al., 2008).
Further, in the following sections, we will focus more deeply on the neurophysiological aspects of proprioception and on the experimental evidence in support of proprioceptive dysfunction in dystonia.
Proprioception Serves for Motor Control and Higher Order Sensation
Proprioception refers to the ability to sense the position and movements of our limbs and trunk (kinesthesia). The principal receptor involved in proprioception is the muscle spindle, which includes the primary and secondary endings of spindles. Primary endings respond to the size and speed of muscle length changes (Matthews, 1972). They are sub-served by Ia afferents and may contribute both to the sense of limb position and movement (Goodwin et al., 1972). Secondary endings do not have pronounced velocity sensitivity and signal only the length change, they are sub-served by group II afferents and may contribute to the sense of position (Matthews, 1972). After the signals from proprioceptors enter the central nervous system, a series of higher order neurons, in the cerebellum and the cerebral cortex, process the stream of proprioceptive information (for a review, see Proske and Gandevia, 2012).
It has been suggested that the input to the cerebellum is used for computations of predictive information (Wolpert et al., 1998), while that to the cerebral cortex is responsible for generating proprioceptive sensations. Neuroimaging studies showed that human kinesthesia is associated with a network of active brain areas that consists of motor areas, cerebellum, and the right fronto-parietal areas, including high-order somatosensory areas (Naito et al., 2002, 2005; Hagura et al., 2009). The neuro-anatomical correlates of kinesthesia well fit with the emerging idea that, apart from the well-established role in motor control, proprioception is largely involved in higher order functions, such as the construction of the body schema and the sense of body ownership (Proske and Gandevia, 2012).
Experimental Evidence of Proprioceptive Dysfunction in Focal Dystonia
Among the different senses of the somatosensory system, proprioception is surely the one that is more linked to motor control. Thus, for a long time, proprioceptive dysfunction has been indicated as a good candidate for somatosensory dysfunction in dystonia.
Proprioceptive function in dystonia was studied with different approaches: muscle vibration of the arm and neck, temporal discrimination of two passive movements, reaching movements in absence of visual input, and the rubber hand illusion (RHI).
Muscle vibration is a suitable method to investigate proprioception. Vibration of the muscle belly or tendon at 50–120 Hz causes a tonic vibration reflex (TVR) that is the result of the activation of muscle spindles and γ-motoneurons. Perception of the TVR is tested by asking participants to match position and movement of the vibrated arm with the opposite arm. While the TVR per se is normal in different forms of focal dystonia, the perception of arm movement during the TVR is abnormal (Kaji et al., 1995; Grünewald et al., 1997; Yoneda et al., 2000). Abnormal perception occurs even for illusory movements induced by vibration. More precisely, when the vibrated arm is immobilized, an illusion of movement is produced. Since sensory information from the joints and the skin is reduced, a main contribution of Ia fibers can be suggested to account for the illusion (Rome and Grünewald, 1999; Frima and Grünewald, 2005; Frima et al., 2008). Abnormal perception of Ia afferent information with a preserved TVR suggests a central rather than a peripheral origin of the disorder. Accordingly, Bove et al. (2004) demonstrated that Ia afferent information from the neck is misinterpreted in patients with cervical dystonia (Bove et al., 2004).
A psychophysical method to investigate proprioception is the temporal discrimination of two passive movements. In this case, stimulation with needle electrodes of the first dorsal interosseus or the flexor carpi radialis muscles causes finger abduction or wrist flexion, respectively (Tinazzi et al., 2005a). Pairs of stimuli separated by short time intervals are delivered and the blindfolded subjects are asked to refer whether they perceived one or two movements (Tinazzi et al., 2005a). The temporal discrimination movement threshold is the shortest interval between two stimuli at which subjects perceived two separate movements (Tinazzi et al., 2005a). This function is preserved in patients with focal hand dystonia (Tinazzi et al., 2006a). It should be noted that this task does not necessarily require an estimation of the amount or the speed of movement, but rather the perception of the time at which the movement occurred. Hence, it could be assumed that while perception of limb velocity (sub-served by Ia afferents) is abnormal, as evidenced by the abovementioned studies on muscle vibration, perception of limb position (sub-served by group II afferents) is normal (Tinazzi et al., 2006a).
Another way to study proprioception is to ask participants to perform reaching movements with the upper limb toward a target. In the absence of visual information, this task relies on proprioception to be optimally performed. Impairments in reaching movements were shown not only in patients with dystonia of the upper limb (Inzelberg et al., 1995), but also with cervical dystonia (Pelosin et al., 2009), suggesting that the proprioceptive function can be impaired also in body parts remote from the affected district. It was hypothesized that this deficit could be due to an error in the spatial representation of the hand location or to a failure in integrating proprioceptive information with the motor output (Marinelli et al., 2011).
An original way to indirectly investigate the proprioceptive function is the RHI paradigm. The RHI is the illusion of owing an artificial hand and occurs after synchronous stroking (with paintbrushes) of the subject’s own hidden hand and a fake visible hand (Botvinick and Cohen, 1998). Typically, after synchronous stroking participants perceive their own hand as located nearer to the artificial hand – proprioceptive drift (Tsakiris and Haggard, 2005). In patients with focal hand dystonia, a dissociation was found on the affected hand between the proprioceptive drift (reduced) and the illusory feeling of ownership (preserved), whereas patients with cervical dystonia had a RHI similar to healthy subjects (Fiorio et al., 2011). The selective impairment of the proprioceptive drift in focal hand dystonia could suggest a failure in integrating the synchronous visual-tactile input with the proprioceptive location sense, because of an underlying kinesthetic deficit (Fiorio et al., 2011).
Proprioceptive Dysfunction in Focal Dystonia: A Matter of Central Misprocessing
The abovementioned experimental evidence on proprioceptive dysfunction in focal dystonia points to an abnormality of central processing of sensory information, rather than to a peripheral problem.
Abnormal somatotopy at the cortical level was demonstrated with somatosensory-evoked potential mapping with EEG (Bara-Jimenez et al., 1998), MEG (Meunier et al., 2001), and fMRI (Butterworth et al., 2003; Nelson et al., 2009). The representations of the fingers at the cortical level are not only closer together in patients with dystonia than in healthy controls, but also in the wrong order. These abnormalities are present, and even more evident, on the unaffected side of patients with unilateral hand dystonia (Meunier et al., 2001). Interestingly, all these results in humans mimic results obtained in a primate model of dystonia, in which a plastic reorganization of the hand area in S1 was observed after months of repetitive hand movements (Byl et al., 1996). The monkeys also presented abnormal hand control and performed poorly on motor tasks, suggesting that learning-induced dedifferentiation of the sensory cortex may contribute to the genesis of dystonia.
One possible explanation for the altered cortical representation of body parts in dystonia is related to an abnormal functioning of the inhibitory interneurons that act on the sensory cortex. Tamura et al. (2008), by means of paired pulse stimulation technique, showed an impaired intracortical inhibition in S1 in focal hand dystonia. In both normal animals and humans, a conditioning (preceding) stimulus induces suppression of somatosensory-evoked potential (SEP) amplitudes evoked by a following test stimulus (Shagass and Schwartz, 1964; Angel, 1967; Wiederholt, 1978). The attenuation of the sensory evoked responses observed in healthy subjects is not observed in patients with focal hand dystonia, indicating an impaired process of “sensory gating” (Tamura et al., 2008). In addition, patients with dystonia present abnormalities in surround inhibition processes within the somatosensory system (Tinazzi et al., 2000). Surround inhibition helps to sharpen the borders of sensory afferent information, as to optimize object perception. Deficits of surround inhibition in focal dystonia were demonstrated in a SEPs study. If the median and ulnar nerves SEPs are produced together, the combined SEP should be less than the sum of the two individual ones because of mutual inhibition (Okajima et al., 1991; Huttunen et al., 1992). This is true in normal subjects, but not in patients with focal hand dystonia (Tinazzi et al., 2000).
Link between Sensory Deficits and Motor Symptoms: Abnormal Sensorimotor Integration
Abnormal processing of somatosensory information in focal dystonia may play a crucial role in the development of motor symptoms. In this regard, it was hypothesized that in dystonia an altered somatosensory representation at the cortical level could lead to an abnormal process of sensorimotor integration in sensory, premotor, and motor cortices and in the cerebellum, which at the end results in a noisy output from the motor cortex (Konczak and Abbruzzese, 2013). In humans, sensorimotor integration can be studied at a cortical level by means of transcranial magnetic stimulation (TMS). By applying a conditioning electrical stimulus to a mixed nerve followed by a TMS stimulus on the motor cortex, inhibition of motor cortex excitability can be observed. These effects, more evident at interstimulus intervals of 20 and 200 ms, are described as short-latency (SAI) and long-latency (LAI) afferent inhibition, respectively (Tokimura et al., 2000). For SAI, it is not clear yet if the effect is mediated directly through somatosensory projections to the primary motor cortex (M1) or indirectly through S1. LAI probably involves other pathways, such as the basal ganglia or cortical association areas. LAI is defective in patients with focal hand dystonia (Abbruzzese et al., 2001), while SAI is normal (Avanzino et al., 2008), indicating abnormal central processing of sensory inputs. Another way of studying sensorimotor integration is to combine TMS with low amplitude muscle vibration. If the TMS pulse is delivered over M1 after 1 s of hand muscle vibration, M1 excitability is increased in the vibrated muscle and decreased in adjacent muscles (Rosenkranz and Rothwell, 2003). Further, the activity of the inhibitory interneurons targeting the vibrated muscle is reduced, and the opposite changes occur in surrounding muscles (Rosenkranz and Rothwell, 2003). This pattern of sensorimotor interaction is abnormal in patients with focal hand dystonia, with a little effect of vibration on cortical excitability (Rosenkranz et al., 2005).
In this scenario, it was also hypothesized an involvement of the cerebellum (Avanzino and Abbruzzese, 2012). It is well established that the cerebellum plays a primary role in predictive (feed-forward) motor control (Bastian, 2006). In the “forward” model, current body state and motor commands are combined to estimate body state in the future (Miall et al., 1993; Wolpert et al., 1995; Paulin, 2005). In this model, proprioception is the main source of information that the cerebellum processes in order to depict the current sensory state. Recent studies showed alteration in forward model prediction of sensory outcome of self produced (Lee et al., 2013) and observed (Avanzino et al., 2013) motor actions in patients with focal dystonia. Particularly, it was shown that patients with focal hand dystonia presented an abnormal performance on the temporal expectation of visually perceived handwriting movements, likely due to an abnormality in the integrative role of the cerebellum over sensory and motor cortical areas (Avanzino et al., 2013). This hypothesis finds support in the modern view of dystonia pathophysiology, which suggests that focal dystonia is linked to the dysfunction of a complex neural network comprising not only the basal ganglia–thalamic–frontal cortex circuit, but also the inferior parietal cortex and the cerebellum (Poston and Eidelberg, 2012; Hutchinson et al., 2014).
Finally, neuromodulation studies supported the idea that abnormal premotor–motor interactions may also play a role in the pathophysiology of focal dystonia (Murase et al., 2005; Huang et al., 2012; Furuya et al., 2014).
Hence, an aberrant activity in every node of the sensorimotor network (the sensory cortex, the premotor–motor cortex, and cerebellum) may play a role in inducing dystonic symptoms.
Rehabilitation Strategies Based on Proprioception
The hypothesis that focal dystonia could be a sensorimotor disorder lead to the suggestion that rehabilitation strategies aimed at facilitating the processing of proprioceptive input could be beneficial (Figure 1). These approaches modulate sensory processing by means of sensory retraining and learning-based sensorimotor re-education.
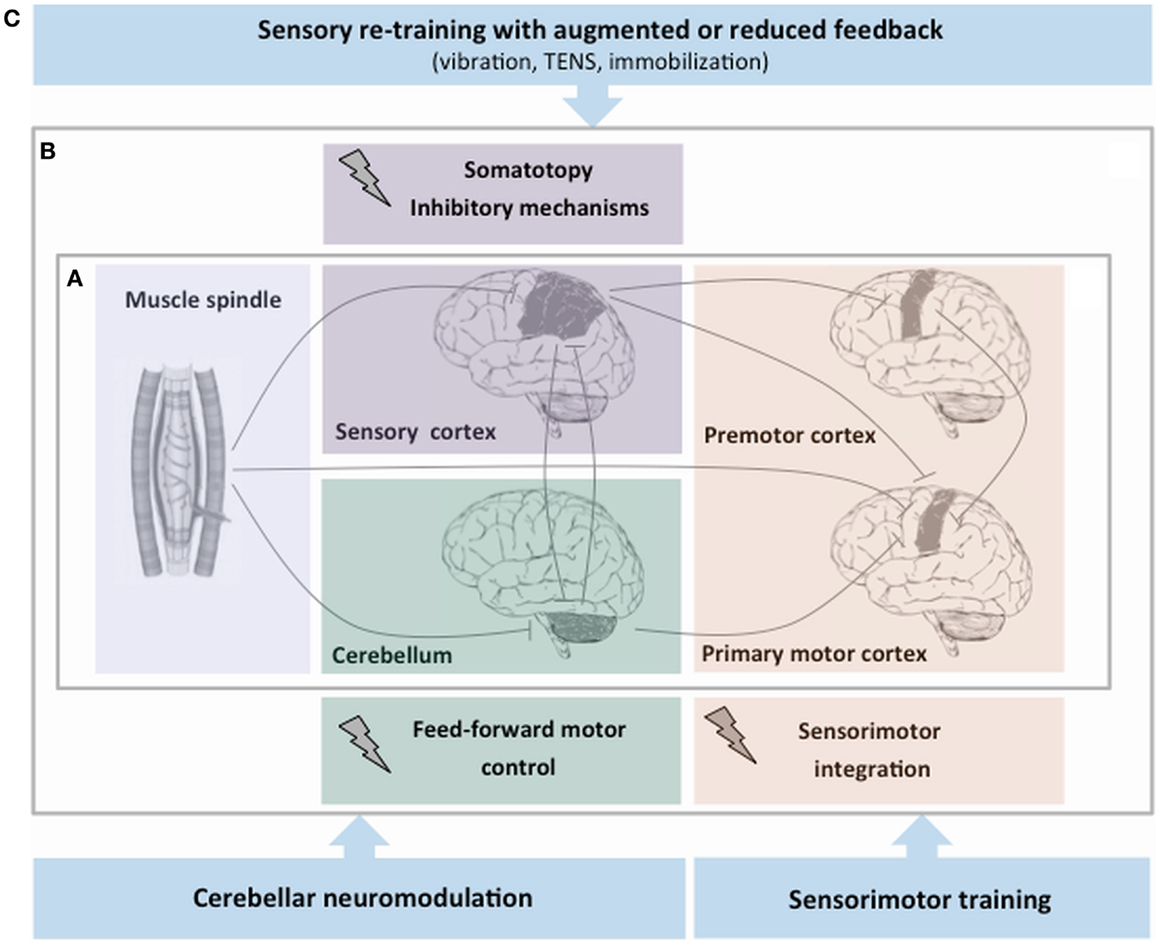
Figure 1. Simplified schema of proprioceptive dysfunction and rehabilitation strategies aimed at facilitating the proprioceptive processing in focal dystonia. (A) After the proprioceptive signals from muscle spindles enter the central nervous system, a series of higher order neurons located in the cortex and in subcortical structures process this information. (B) Experimental evidence on proprioceptive dysfunction in focal dystonia points to an abnormality of central processing of sensory information at different levels of the central nervous system: the sensory cortex (abnormal somatotopy and inhibitory mechanisms), the premotor–motor cortex (malfunctioning sensorimotor integration process), and the cerebellum (altered feed-forward motor control). (C) The hypothesis that dystonia could be primarily a sensorimotor disorder has led the suggestion that rehabilitation strategies may target the abnormal sensory processing of proprioceptive information.
There is emerging evidence that vibration induces sensory reorganization at a central level (Avanzino et al., 2014) and may help to reduce involuntary muscle activity. Rosenkranz et al. (2008) adopted a proprioceptive training consisting in the vibration of the abductor pollicis brevis muscle at a frequency of 80 Hz for 15 min. This procedure reversed the abnormal sensorimotor organization of the hand area in patients with focal dystonia (Rosenkranz et al., 2008). Most importantly, this intervention had a beneficial impact on the patients’ hand motor functions (Rosenkranz et al., 2009). It is important to mention that also in a single case of cervical dystonia long-term neck muscle vibration was associated with improvements in head and trunk position (Karnath et al., 2000).
Also transcutaneous electrical nerve stimulation (TENS) and kinesio-taping were used for sensory retraining. Improvement of dystonic symptoms in patients with focal hand dystonia was observed after 2 weeks of TENS of the forearm flexor muscle and lasted for 3 weeks after intervention (Tinazzi et al., 2005b, 2006b). Likely, TENS re-established a balanced activation between agonist and antagonist muscles (Tinazzi et al., 2005b). In a recent pilot study, kinesio-taping was used as a means of inducing muscle-stretching and promoting better sensory processing in patients with focal hand and cervical dystonia (Pelosin et al., 2013).
An opposite approach is sensory deprivation by means of immobilization. In patients with focal hand dystonia, immobilization of the upper limb with orthesis re-established the cortical map topography (Lissek et al., 2009; Roll et al., 2012). Selective immobilization can be applied together with motor training (Candia et al., 2005; Zeuner et al., 2005). A study in 10 patients with focal hand dystonia applied motor exercise of one finger while the other four were immobilized by a splint, for a period of 4–12 weeks (Zeuner et al., 2005). A highly variable subjective improvement, assessed by a self-rating scale, was observed.
Learning-based sensorimotor re-education can be achieved in cervical dystonia with visual or auditory EMG biofeedback techniques (Cleeland, 1973; Korein et al., 1976; Leplow, 1990). The underlying principle is to gain more volitional control over the abnormally active muscles. In patients with focal hand dystonia, instead, sensorimotor re-education has been based on a relearning process where the goal is to learn a new way of writing. In a relatively large and controlled study of 50 patients, Schenk et al. (2004) found an improvement of various writing performance components by applying individually tailored writing exercises one session per week for 4 months.
Finally, a recent study exploited neuromodulation in cervical dystonia with the aim of targeting the abnormal cerebellar function (Koch et al., 2014). Cerebellar continuous theta burst stimulation for 2 weeks induced a small but significant clinical improvement and a modification of the connectivity between the cerebellum and M1, suggesting that the cerebello-thalamo-cortical circuit could be a potential target to partially reduce some dystonic symptoms and deserves further in-depth studies.
Concluding Remarks
The study of proprioceptive function in focal dystonia could help not only to clarify the pathophysiology, but also to highlight new rehabilitation strategies that positively impact on the motor symptoms.
Author Contributions
Both Laura Avanzino and Mirta Fiorio contributed to the conception, drafting, and revision of the work. Further, Laura Avanzino and Mirta Fiorio made the final approval of the version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abbruzzese, G., Marchese, R., Buccolieri, A., Gasparetto, B., and Trompetto, C. (2001). Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain 124, 537–545. doi: 10.1093/brain/124.3.537
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Albanese, A., Bhatia, K., Bressman, S. B., Delong, M. R., Fahn, S., Fung, V. S., et al. (2013). Phenomenology and classification of dystonia: a consensus update. Mov. Disord. 28, 863–873. doi:10.1002/mds.25475
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Angel, A. (1967). Cortical responses to paired stimuli applied peripherally and at sites along the somato-sensory pathway. J. Physiol. 191, 427–448.
Avanzino, L., and Abbruzzese, G. (2012). How does the cerebellum contribute to the pathophysiology of dystonia? Basal Ganglia 2, 231–235. doi:10.1016/j.baga.2012.05.003
Avanzino, L., Martino, D., Martino, I., Pelosin, E., Vicario, C. M., Bove, M., et al. (2013). Temporal expectation in focal hand dystonia. Brain 136, 444–454. doi:10.1093/brain/aws328
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Avanzino, L., Martino, D., van de Warrenburg, B. P., Schneider, S. A., Abbruzzese, G., Defazio, G., et al. (2008). Cortical excitability is abnormal in patients with the “fixed dystonia” syndrome. Mov. Disord. 23, 646–652. doi:10.1002/mds.21801
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Avanzino, L., Pelosin, E., Abbruzzese, G., Bassolino, M., Pozzo, T., and Bove, M. (2014). Shaping motor cortex plasticity through proprioception. Cereb. Cortex 24, 2807–2814. doi:10.1093/cercor/bht139
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bara-Jimenez, W., Catalan, M. J., Hallett, M., and Gerloff, C. (1998). Abnormal somatosensory homunculus in dystonia of the hand. Ann. Neurol. 44, 828–831. doi:10.1002/ana.410440520
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bastian, A. J. (2006). Learning to predict the future: the cerebellum adapts feedforward movement control. Curr. Opin. Neurobiol. 16, 645–649. doi:10.1016/j.conb.2006.08.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Berardelli, A., Rothwell, J. C., Hallett, M., Thompson, P. D., Manfredi, M., and Marsden, C. D. (1998). The pathophysiology of primary dystonia. Brain 121, 1195–1212. doi:10.1093/brain/121.7.1195
Bhatia, K. P., and Marsden, C. D. (1994). The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 117, 859–876. doi:10.1093/brain/117.4.859
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Botvinick, M., and Cohen, J. (1998). Rubber hands ‘feel’ touch that eyes see. Nature 391, 756. doi:10.1038/35784
Bove, M., Brichetto, G., Abbruzzese, G., Marchese, R., and Schieppati, M. (2004). Neck proprioception and spatial orientation in cervical dystonia. Brain 127, 2764–2778. doi:10.1093/brain/awh291
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Butterworth, S., Francis, S., Kelly, E., McGlone, F., Bowtell, R., and Sawle, G. V. (2003). Abnormal cortical sensory activation in dystonia: an fMRI study. Mov. Disord. 18, 673–682. doi:10.1002/mds.10416
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Byl, N. N., Merzenich, M. M., and Jenkins, W. M. (1996). A primate genesis model of focal dystonia and repetitive strain injury: I. Learning-induced dedifferentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Neurology 47, 508–520. doi:10.1212/WNL.47.2.508
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Candia, V., Rosset-Llobet, J., Elbert, T., and Pascual-Leone, A. (2005). Changing the brain through therapy for musicians’ hand dystonia. Ann. N. Y. Acad. Sci. 1060, 335–342. doi:10.1196/annals.1360.028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cleeland, C. (1973). Behavioral technics in the modification of spasmodic torticollis. Neurology 23, 1241–1247. doi:10.1212/WNL.23.11.1241
Currá, A., Berardelli, A., Agostino, R., Giovannelli, M., Koch, G., and Manfredi, M. (2000). Movement cueing and motor execution in patients with dystonia: a kinematic study. Mov. Disord. 15, 103–112. doi:10.1002/1531-8257(200001)15:1<103::AID-MDS1016>3.0.CO;2-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fahn, S. (1988). Concept and classification of dystonia. Adv. Neurol. 50, 1–8. doi:10.1212/WNL.50.5_Suppl_5.S1
Fiorio, M., Tinazzi, M., Scontrini, A., Stanzani, C., Gambarin, M., Fiaschi, A., et al. (2008). Tactile temporal discrimination in patients with blepharospasm. J. Neurol. Neurosurg. Psychiatr. 79, 796–798. doi:10.1136/jnnp.2007.131524
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fiorio, M., Weise, D., Önal-Hartmann, C., Zeller, D., Tinazzi, M., and Classen, J. (2011). Impairment of the rubber hand illusion in focal hand dystonia. Brain 134, 1428–1437. doi:10.1093/brain/awr026
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Frima, N., and Grünewald, R. A. (2005). Abnormal vibration induced illusion of movement in essential tremor: evidence for abnormal muscle spindle afferent function. J. Neurol. Neurosurg. Psychiatr. 76, 55–57. doi:10.1136/jnnp.2004.036640
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Frima, N., Nasir, J., and Grünewald, R. A. (2008). Abnormal vibration-induced illusion of movement in idiopathic focal dystonia: an endophenotypic marker? Mov. Disord. 23, 373–377. doi:10.1002/mds.21838
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Furuya, S., Nitsche, M. A., Paulus, W., and Altenmüller, E. (2014). Surmounting retraining limits in musicians’ dystonia by transcranial stimulation. Ann. Neurol. 75, 700–707. doi:10.1002/ana.24151
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ghika, J., Regli, F., and Growdon, J. H. (1993). Sensory symptoms in cranial dystonia: a potential role in the etiology? J. Neurol. Sci. 116, 142–147. doi:10.1016/0022-510X(93)90318-S
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Goodwin, G. M., McCloskey, D. I., and Matthews, P. B. (1972). The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain 95, 705–748. doi:10.1093/brain/95.4.705
Gregori, B., Agostino, R., Bologna, M., Dinapoli, L., Colosimo, C., Accornero, N., et al. (2008). Fast voluntary neck movements in patients with cervical dystonia: a kinematic study before and after therapy with botulinum toxin type A. Clin. Neurophysiol. 119, 273–280. doi:10.1016/j.clinph.2007.10.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Grünewald, R. A., Yoneda, Y., Shipman, J. M., and Sagar, H. J. (1997). Idiopathic focal dystonia: a disorder of muscle spindle afferent processing? Brain 120, 2179–2185. doi:10.1093/brain/120.12.2179
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hagura, N., Oouchida, Y., Aramaki, Y., Okada, T., Matsumura, M., Sadato, N., et al. (2009). Visuokinesthetic perception of hand movement is mediated by cerebro-cerebellar interaction between the left cerebellum and right parietal cortex. Cereb. Cortex 19, 176–186. doi:10.1093/cercor/bhn068
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Huang, Y. Z., Lu, C. S., Rothwell, J. C., Lo, C. C., Chuang, W. L., Weng, Y. H., et al. (2012). Modulation of the disturbed motor network in dystonia by multisession suppression of premotor cortex. PLoS ONE 7:e47574. doi:10.1371/journal.pone.0047574
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hutchinson, M., Isa, T., Molloy, A., Kimmich, O., Williams, L., Molloy, F., et al. (2014). Cervical dystonia: a disorder of the midbrain network for covert attentional orienting. Front Neurol. 5:54. doi:10.3389/fneur.2014.00054
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Huttunen, J., Ahlfors, S., and Hari, R. (1992). Interaction of afferent impulses in the human primary sensorimotor cortex. Electroencephalogr. Clin. Neurophysiol. 82, 176–181. doi:10.1016/0013-4694(92)90165-E
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Inzelberg, R., Flash, T., Schechtman, E., and Korczyn, A. D. (1995). Kinematic properties of upper limb trajectories in idiopathic torsion dystonia. J. Neurol. Neurosurg. Psychiatr. 58, 312–319. doi:10.1136/jnnp.58.3.312
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kaji, R., Rothwell, J. C., Katayama, M., Ikeda, T., Kubori, T., Kohara, N., et al. (1995). Tonic vibration reflex and muscle afferent block in writer’s cramp. Ann. Neurol. 38, 155–162. doi:10.1002/ana.410380206
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kanovský, P., and Rosales, R. L. (2011). Debunking the pathophysiological puzzle of dystonia – with special reference to botulinum toxin therapy. Parkinsonism Relat. Disord. 17, S11–S14. doi:10.1016/j.parkreldis.2011.06.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Karnath, H. O., Konczak, J., and Dichgans, J. (2000). Effect of prolonged neck muscle vibration on lateral head tilt in severe spasmodic torticollis. J. Neurol. Neurosurg. Psychiatr. 69, 658–660. doi:10.1136/jnnp.69.5.658
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Koch, G., Porcacchia, P., Ponzo, V., Carrillo, F., Cáceres-Redondo, M. T., Brusa, L., et al. (2014). Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul. 7, 564–572. doi:10.1016/j.brs.2014.05.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Konczak, J., and Abbruzzese, G. (2013). Focal dystonia in musicians: linking motor symptoms to somatosensory dysfunction. Front. Hum. Neurosci. 7:297. doi:10.3389/fnhum.2013.00297
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Korein, J., Brudny, J., Grynbaum, B., Sachs-Frankel, G., Weisinger, M., and Levidow, L. (1976). Sensory feedback therapy of spasmodic torticollis and dystonia: results in treatment of 55 patients. Adv. Neurol. 14, 375–402.
Lee, A., Furuya, S., Karst, M., and Altenmüller, E. (2013). Alteration in forward model prediction of sensory outcome of motor action in focal hand dystonia. Front. Hum. Neurosci. 7:172. doi:10.3389/fnhum.2013.00172
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Leplow, B. (1990). Heterogeneity of biofeedback training effects in spasmodic torticollis: a single-case approach. Behav. Res. Ther. 28, 359–365. doi:10.1016/0005-7967(90)90091-V
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lissek, S., Wilimzig, C., Stude, P., Pleger, B., Kalisch, T., Maier, C., et al. (2009). Immobilization impairs tactile perception and shrinks somatosensory cortical maps. Curr. Biol. 19, 837–842. doi:10.1016/j.cub.2009.03.065
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Marinelli, L., Pelosin, E., Trompetto, C., Avanzino, L., Ghilardi, M. F., Abbruzzese, G., et al. (2011). In idiopathic cervical dystonia movement direction is inaccurate when reaching in unusual workspaces. Parkinsonism Relat. Disord. 17, 470–472. doi:10.1016/j.parkreldis.2011.01.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Meunier, S., Garnero, L., Ducorps, A., Mazières, L., Lehéricy, S., du Montcel, S. T., et al. (2001). Human brain mapping in dystonia reveals both endophenotypic traits and adaptive reorganization. Ann. Neurol. 50, 521–527. doi:10.1002/ana.1234
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Miall, R. C., Weir, D. J., Wolpert, D. M., and Stein, J. F. (1993). Is the cerebellum a Smith predictor? J. Mot. Behav. 25, 203–216. doi:10.1080/00222895.1993.9942050
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Müller, J., Wissel, J., Masuhr, F., Ebersbach, G., Wenning, G. K., and Poewe, W. (2001). Clinical characteristics of the geste antagoniste in cervical dystonia. J. Neurol. 248, 478–482. doi:10.1007/s004150170156
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Murase, N., Rothwell, J. C., Kaji, R., Urushihara, R., Nakamura, K., Murayama, N., et al. (2005). Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer’s cramp. Brain 128, 104–115. doi:10.1093/brain/awh315
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Naito, E., Roland, P. E., and Ehrsson, H. H. (2002). I feel my hand moving: a new role of the primary motor cortex in somatic perception of limb movement. Neuron 36, 979–988. doi:10.1016/S0896-6273(02)00980-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Naito, E., Roland, P. E., Grefkes, C., Choi, H. J., Eickhoff, S., Geyer, S., et al. (2005). Dominance of the right hemisphere and role of area 2 in human kinesthesia. J. Neurophysiol. 93, 1020–1034. doi:10.1152/jn.00637.2004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nelson, A. J., Blake, D. T., and Chen, R. (2009). Digit-specific aberrations in the primary somatosensory cortex in Writer’s cramp. Ann. Neurol. 66, 146–154. doi:10.1002/ana.21626
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Okajima, Y., Chino, N., Saitoh, E., and Kimura, A. (1991). Interactions of somatosensory evoked potentials: simultaneous stimulation of two nerves. Electroencephalogr. Clin. Neurophysiol. 80, 26–31. doi:10.1016/0168-5597(91)90039-Z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Patel, N., Jankovic, J., and Hallett, M. (2014). Sensory aspects of movement disorders. Lancet Neurol. 13, 100–112. doi:10.1016/S1474-4422(13)70213-8
Paulin, M. G. (2005). Evolution of the cerebellum as a neuronal machine for Bayesian state estimation. J. Neural Eng. 2, S219–S234. doi:10.1088/1741-2560/2/3/S06
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pelosin, E., Avanzino, L., Marchese, R., Stramesi, P., Bilanci, M., Trompetto, C., et al. (2013). Kinesiotaping reduces pain and modulates sensory function in patients with focal dystonia: a randomized crossover pilot study. Neurorehabil. Neural Repair 27, 722–731. doi:10.1177/1545968313491010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pelosin, E., Bove, M., Marinelli, L., Abbruzzese, G., and Ghilardi, M. F. (2009). Cervical dystonia affects aimed movements of nondystonic segments. Mov. Disord. 24, 1955–1961. doi:10.1002/mds.22693
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Poston, K. L., and Eidelberg, D. (2012). Functional brain networks and abnormal connectivity in the movement disorders. Neuroimage 62, 2261–2270. doi:10.1016/j.neuroimage.2011.12.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Proske, U., and Gandevia, S. C. (2012). The proprioceptive senses: their roles in signalling body shape, body position and movement, and muscle force. Physiol. Rev. 92, 1651–1697. doi:10.1152/physrev.00048.2011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Quartarone, A., and Hallett, M. (2013). Emerging concepts in the physiological basis of dystonia. Mov. Disord. 28, 958–967. doi:10.1002/mds.25532
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Roll, R., Kavounoudias, A., Albert, F., Legré, R., Gay, A., Fabre, B., et al. (2012). Illusory movements prevent cortical disruption caused by immobilization. Neuroimage 62, 510–519. doi:10.1016/j.neuroimage.2012.05.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rome, S., and Grünewald, R. A. (1999). Abnormal perception of vibration-induced illusion of movement in dystonia. Neurology 53, 1794–1800. doi:10.1212/WNL.53.8.1794
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rosenkranz, K., Butler, K., Williamon, A., Cordivari, C., Lees, A. J., and Rothwell, J. C. (2008). Sensorimotor reorganization by proprioceptive training in musician’s dystonia and writer’s cramp. Neurology 70, 304–315. doi:10.1212/01.wnl.0000296829.66406.14
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rosenkranz, K., Butler, K., Williamon, A., and Rothwell, J. C. (2009). Regaining motor control in musician’s dystonia by restoring sensorimotor organization. J. Neurosci. 29, 14627–14636. doi:10.1523/JNEUROSCI.2094-09.2009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rosenkranz, K., and Rothwell, J. C. (2003). Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J. Physiol. 551, 649–660. doi:10.1113/jphysiol.2003.043752
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rosenkranz, K., Williamon, A., Butler, K., Cordivari, C., Lees, A. J., and Rothwell, J. C. (2005). Pathophysiological differences between musician’s dystonia and writer’s cramp. Brain 128, 918–931. doi:10.1093/brain/awh402
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sanger, T. D., Tarsy, D., and Pascual-Leone, A. (2001). Abnormalities of spatial and temporal sensory discrimination in writer’s cramp. Mov. Disord. 16, 94–99. doi:10.1002/1531-8257(200101)16:1<94::AID-MDS1020>3.0.CO;2-O
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schenk, T., Bauer, B., Steidle, B., and Marquardt, C. (2004). Does training improve writer’s cramp? An evaluation of a behavioral treatment approach using kinematic analysis. J. Hand Ther. 17, 349–363. doi:10.1197/j.jht.2004.04.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shagass, C., and Schwartz, M. (1964). Recovery functions of somatosensory peripheral nerve and cerebral evoked responses in man. Electroencephalogr. Clin. Neurophysiol. 17, 126–135. doi:10.1016/0013-4694(64)90144-0
Stamelou, M., Edwards, M. J., Hallett, M., and Bhatia, K. P. (2012). The non-motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain 135, 1668–1681. doi:10.1093/brain/awr224
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Suttrup, I., Oberdiek, D., Suttrup, J., Osada, N., Evers, S., and Marziniak, M. (2011). Loss of sensory function in patients with idiopathic hand dystonia. Mov. Disord. 26, 107–113. doi:10.1002/mds.23425
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tamura, Y., Matsuhashi, M., Lin, P., Ou, B., Vorbach, S., Kakigi, R., et al. (2008). Impaired intracortical inhibition in the primary somatosensory cortex in focal hand dystonia. Mov. Disord. 23, 558–565. doi:10.1002/mds.21870
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tinazzi, M., Fiorio, M., Stanzani, C., Moretto, G., Smania, N., Fiaschi, A., et al. (2006a). Temporal discrimination of two passive movements in writer’s cramp. Mov. Disord. 21, 1131–1135. doi:10.1002/mds.20892
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tinazzi, M., Zarattini, S., Valeriani, M., Stanzani, C., Moretto, G., Smania, N., et al. (2006b). Effects of transcutaneous electrical nerve stimulation on motor cortex excitability in writer’s cramp: neurophysiological and clinical correlations. Mov. Disord. 21, 1908–1913. doi:10.1002/mds.21081
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tinazzi, M., Priori, A., Bertolasi, L., Frasson, E., Mauguière, F., and Fiaschi, A. (2000). Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow. Brain 123, 42–50. doi:10.1093/brain/123.1.42
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tinazzi, M., Stanzani, C., Fiorio, M., Smania, N., Moretto, G., Fiaschi, A., et al. (2005a). Temporal discrimination of two passive movements in humans: a new psychophysical approach to assessing kinaesthesia. Exp. Brain Res. 166, 184–189. doi:10.1007/s00221-005-2353-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tinazzi, M., Farina, S., Bhatia, K., Fiaschi, A., Moretto, G., Bertolasi, L., et al. (2005b). TENS for the treatment of writer’s cramp dystonia: a randomized, placebo-controlled study. Neurology 64, 1946–1948. doi:10.1212/01.WNL.0000163851.70927.7E
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tokimura, H., Di Lazzaro, V., Tokimura, Y., Oliviero, A., Profice, P., Insola, A., et al. (2000). Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J. Physiol. 523, 503–513. doi:10.1111/j.1469-7793.2000.t01-1-00503.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tsakiris, M., and Haggard, P. (2005). The rubber hand illusion revisited: visuotactile integration and self-attribution. J. Exp. Psychol. Hum. Percept. Perform. 31, 80–91. doi:10.1037/0096-1523.31.1.80
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wiederholt, W. C. (1978). Recovery function of short latency components of surface and depth recorded somatosensory evoked potentials in the cat. Electroencephalogr. Clin. Neurophysiol. 45, 259–267. doi:10.1016/0013-4694(78)90009-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wissel, J., Müller, J., Ebersbach, G., and Poewe, W. (1999). Trick maneuvers in cervical dystonia: investigation of movement- and touch-related changes in polymyographic activity. Mov. Disord. 14, 994–999. doi:10.1002/1531-8257(199911)14:6<994::AID-MDS1013>3.0.CO;2-K
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wolpert, D. M., Ghahramani, Z., and Jordan, M. I. (1995). An internal model for sensorimotor integration. Science 29, 1880–1882. doi:10.1126/science.7569931
Wolpert, D. M., Miall, R. C., and Kawato, M. (1998). Internal models in the cerebellum. Trends Cogn. Sci. 2, 338–347. doi:10.1016/S1364-6613(98)01221-2
Yoneda, Y., Rome, S., Sagar, H. J., and Grünewald, R. A. (2000). Abnormal perception of the tonic vibration reflex in idiopathic focal dystonia. Eur. J. Neurol. 7, 529–533. doi:10.1046/j.1468-1331.2000.t01-1-00102.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zeuner, K. E., Shill, H. A., Sohn, Y. H., Molloy, F. M., Thornton, B. C., Dambrosia, J. M., et al. (2005). Motor training as treatment in focal hand dystonia. Mov. Disord. 20, 335–341. doi:10.1002/mds.20314
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: dystonia, proprioception, sensory system, pathophysiology, rehabilitation
Citation: Avanzino L and Fiorio M (2014) Proprioceptive dysfunction in focal dystonia: from experimental evidence to rehabilitation strategies. Front. Hum. Neurosci. 8:1000. doi: 10.3389/fnhum.2014.01000
Received: 04 August 2014; Accepted: 25 November 2014;
Published online: 09 December 2014.
Edited by:
Lorenzo Masia, Nanyang Technological University, SingaporeReviewed by:
Shinichi Furuya, Sophia University, JapanJames T. H. Teo, Kings College Hospital London, UK
Copyright: © 2014 Avanzino and Fiorio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Avanzino, Section of Human Physiology, Department of Experimental Medicine, Viale Benedetto XV 3, Genoa 16132, Italy e-mail: lavanzino76@gmail.com
 Laura Avanzino
Laura Avanzino Mirta Fiorio
Mirta Fiorio