Cognitive demands during quiet standing elicit truncal tremor in two frequency bands: differential relations to tissue integrity of corticospinal tracts and cortical targets
- 1Alcohol Translational Neuroscience Program, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA, USA
- 2Neuroscience Program, SRI International, Menlo Park, CA, USA
The ability to stand quietly is disturbed by degradation of cerebellar systems. Given the complexity of sensorimotor integration invoked to maintain upright posture, the integrity of supratentorial brain structures may also contribute to quiet standing and consequently be vulnerable to interference from cognitive challenges. As cerebellar system disruption is a common concomitant of alcoholism, we examined 46 alcoholics and 43 controls with a force platform to derive physiological indices of quiet standing during cognitive (solving simple, mental arithmetic problems) and visual (eyes closed) challenges. Also tested were relations between tremor velocity and regional gray matter and white matter tissue quality measured with the diffusion tensor imaging (DTI) metric of mean diffusivity (MD), indexing disorganized microstructure. Spectral analysis of sway revealed greater tremor in alcoholic men than alcoholic women or controls. Cognitive dual-tasking elicited excessive tremor in two frequency bands, each related to DTI signs of degradation in separate brain systems: tremor velocity at a low frequency (2–5 Hz/0–2 Hz) correlated with higher MD in the cerebellar hemispheres and superior cingulate bundles, whereas tremor velocity at a higher frequency (5–7 Hz) correlated with higher MD in the motor cortex and internal capsule. These brain sites may represent “tremorgenic networks” that, when disturbed by disease and exacerbated by cognitive dual-tasking, contribute to postural instability, putting affected individuals at heightened risk for falling.
Introduction
Tremor in alcohol-dependent individuals observed clinically is typically the result of acute withdrawal from alcohol and most obviously exhibited as “the shakes” (e.g., Neiman et al., 1990). Beyond delirium tremens, tremor can persist with extended sobriety (Koller et al., 1985). Postural tremor elicited during quiet standing, initially described by Holmes (1917), has been attributed to damage of the cerebellum and its efferent circuitry and was described physiologically by involuntary movement in the 3–10 Hz frequency band in case studies of patients with known or presumed cerebellar lesions (e.g., Brown et al., 1997). Among such patients are chronic alcoholics, who as a group, can exhibit cellular damage of cerebellar vermis postmortem (e.g., Victor et al., 1989; Pentney, 1993; Baker et al., 1999; Harper et al., 2003) and vermian and cerebellar hemisphere shrinkage in vivo (e.g., Sullivan et al., 2000, 2006; Makris et al., 2008; Le Berre et al., 2014).
Consistent with those case studies, truncal tremor during quiet standing, measured by analyzing the temporal frequency of sway paths derived from a force platform, was detected in the 2–5 Hz frequency band and the 5–7 Hz band in alcoholic men (Sullivan et al., 2006) and women (Sullivan et al., 2010) tested well beyond acute withdrawal. Both sway and tremor could be quelled by introducing sensory and motor-stabilizing factors of vision, touch, and broad-based stance, even though the magnitude of sway and 5–7 Hz tremor were related to local CNS integrity, i.e., volume shrinkage of the anterior vermis (Sullivan et al., 2006, 2010). Although some factors can quell sway and tremor, it remains untested whether engaging in a cognitively challenging task known to impair stability (Pellecchia, 2003) can promote instability or tremor during quiet standing in recovering alcoholics and whether the extent of such disruption would be associated with tissue integrity of cerebellar structures or cortical regions.
Maintenance of upright posture requires complex sensorimotor integration (Diener and Dichgans, 1992; Baloh et al., 1998). Thus, the ability to stand quietly can potentially be impaired by engagement in competing cognitive tasks that share attentional resources with motor stability systems (Shumway-Cook et al., 1997; Pellecchia and Turvey, 2001). Specifically, engagement in difficult compared with simple working memory tasks resulted in greater difficulty in controlling postural sway (Pellecchia, 2003) and, in certain situations, with increasing rigidity while attempting to reduce sway (Dault et al., 2003). That cognitive tasks can interact with postural stability suggests that neural sources of these actions and interactions involve both cerebellar and supratentorial brain motor and cognitive systems, especially ones that share connections. This possibility was borne out in a recent experiment using functional near infrared spectroscopy (fNIRS) acquired while participants were standing still or walking and while engaged in a simple cognitive task (counting forward) or a complex one (serially subtracting 7 s) (Mirelman et al., 2014). Frontal cortical brain activation showed a graded effect, being greatest under the most challenging condition of walking while doing serial 7 s, less so when standing while doing serial 7 s, and negligible when standing or walking while counting forward. Thus, articulation alone was inadequate to invoke frontal activation when engaged in motor-cognitive dual tasking.
Relations between abnormally hyperintense magnetic resonance imaging (MRI) signal of nonselective supratentorial white matter and falling in the elderly are well established (for review Zheng et al., 2011). Evidence for the role of extensive circuitry in tremor production derives from observations of relief of essential tremor by application of repetitive transmagnetic stimulation over the motor cortex (Rogasch and Todd, 2013). Particularly convincing are results from a study combining deep brain stimulation (DBS) of the ventral thalamus for relief of intractable tremor and fiber tracking of diffusion tensor imaging (DTI) data. That study used the locus inducing successful relief as the seed point for fiber tracking of DTI data acquired in the patients. In addition to confirming the relevance of connectivity between the ventral thalamus and primary motor cortex to successful DBS outcome, structural connectivity sites included precentral gyrus, supplementary motor area, frontal gyri, anterior cingulum, and the cerebellar hemispheres at the level of the superior cerebellar peduncles (Klein et al., 2012).
Here, we sought to examine relations among quantitative assessment of quiet standing on a force platform in alcoholic men and women with extended sobriety compared with age- and sex-matched controls. Path length and frequency data were acquired while participants engaged in cognitive challenges of increasing difficulty related to mental arithmetic and visual input and were evaluated for their relation with measures of brain white matter fiber tract and gray matter tissue integrity using mean diffusivity (MD) measures from DTI potentially contributing to postural stability. The white matter systems examined were the internal capsule, pontocerebellar tract, genu and splenium of the corpus callosum, three segments of the cingulate bundle; the gray matter nodes examined were the precentral, superior, middle, and inferior frontal cortical gray matter, and thalamus, Crus I and II, and anterior, posterior, and inferior sectors of the vermis. MD was chosen over other DTI metrics, including anisotropy, because of its sensitivity to detection of alcoholism-related brain deficits and demonstration of brain-behavior relations (Pfefferbaum et al., 2006, 2009) and because MD is more readily interpretable than anisotropy as an index of gray matter tissue integrity.
Materials and Methods
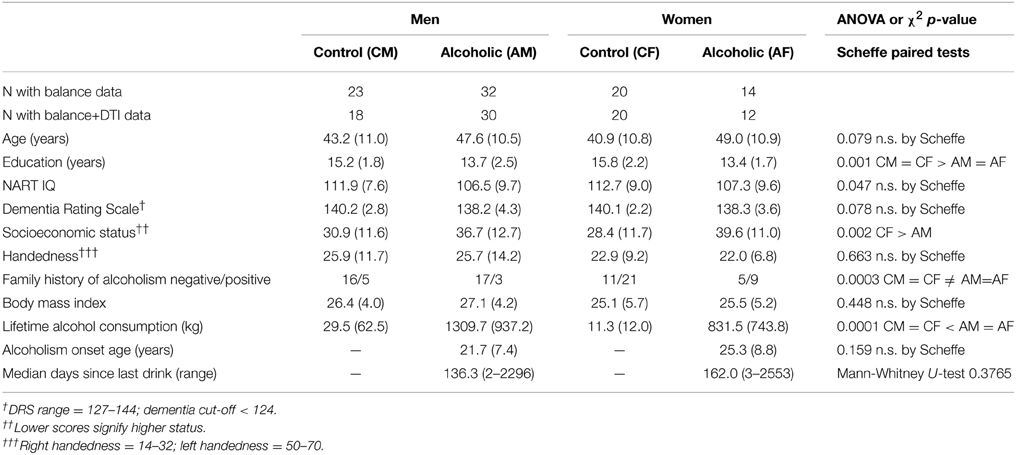
Participants
The groups comprised 46 alcoholics and 43 controls age-range matched to the alcoholics. All participants gave written informed consent for participation in this study, which was approved by the Internal Review Boards of Stanford University School of Medicine and SRI International; these boards assured that our study was conducted in accordance with the Declaration of Helsinki. Although the alcoholics had fewer years of education and were of lower socioeconomic status (Hollingshead, 1975) than the controls, the groups did not differ substantially in intelligence estimated with the National Adult Reading Test (NART) (Nelson, 1982), Dementia Rating Scale (Mattis, 2004), handedness (Crovitz and Zener, 1962), or body mass index (Table 1). The alcoholic men and women had a higher incidence of family history of alcoholism than control men and women. Although the alcoholic men had greater lifetime consumption of alcohol than alcoholic women, neither age of alcoholism onset nor median number of days since their last drink differed significantly between the sexes. Three alcoholic men and one alcoholic woman reported drinking within a week of testing; none demonstrated withdrawal symptoms.
Quiet Standing Quantified with Force Platform Analysis
Test Conditions
Subjects wore standard rubber-soled socks and stood still on a force plate with feet together with arms relaxed at their sides. Each of three conditions, comprising one easy and one hard arithmetic condition and a no-task control, was performed with eyes open and with eyes closed, producing six test conditions. A camera monitored compliance with eyes-closed instruction. In the two arithmetic conditions, subjects listened to sets of 9, pre-recorded digit strings, broadcast through speakers mounted on each side of the force platform. Number strings were spoken in 4.5 s epochs, followed by 0.5 s intervals for responding (50 s = 2 s before a trial+45 s of math+3 s intertrial interval). In the two easy conditions, subjects heard three digits and two operands followed by a correct (66%) or incorrect (33%) answer (e.g., 2 + 5−3 = 4; 3−1 + 8 = 11) to which they said “yes” if correct or “no” if incorrect. In the two hard conditions, subjects additionally responded “odd” or “even” to classify the given answer. Before testing, subjects completed practice trials of the arithmetic problems while seated.
Sway Path Analysis
Balance was assessed with a microcomputer-controlled force plate (model 9284; Kistler, Amherst, NY) with multiple transducers and analog-digital converters. Data were sampled at 1000 Hz; raw data were 45 s continuous trials of center-of-pressure displacements (x-y pairs). Data were subjected to a 10 Hz lowpass filter (99 terms, −50 db Gibbs); sway path length (P) was expressed as the line integral (cm):
Tremor Analysis
Frequency analyses used fast Fourier transform on the anterior-posterior and lateral-medial sway path velocity (2-point differential of the filtered sway path). To characterize the frequency (Hz) of maximal sway velocity, data were divided into three frequency components: 0–2, 2–5, and 5–7 Hz. Following the method of Baloh et al. (1998) to characterize the frequency (Hz) of maximal sway velocity selective to patients with cerebellar damage, we derived a frequency quotient, which was the power of the spectral frequencies between 2 and 5 Hz divided by those between 0 and <2 Hz, with the expectation that the alcoholics would have a higher ratio than control subjects. Further, only velocity was analyzed because prior work had shown that frequency analysis of center-of-pressure sway-path velocity (distance/time) to be more sensitive than amplitude (distance) (Baloh et al., 1998).
Sensory Testing
Most participants underwent sensory testing of the lower extremities to assess the contribution of cutaneous and other peripheral sensory impairment on metrics of quiet standing. Deep Tendon Reflex, Great Toe Vibration, and 2-point Discrimination are standard neurological tests of the lower limbs (Bigley, 1990).
Subjective Assessment of Peripheral Neuropathy
Subjects were asked to rate on a 0–10 point scale discomfort (pain, aching, burning; pins and needles; numbness) in feet or legs that might by symptomatic of peripheral neuropathy.
Deep Tendon Reflex
The subject sat on an examination table. The examiner used one hand to press upward on ball of foot (dominant side first) dorsiflexing the subject's ankle to 90° and then striking the Achilles tendon with a reflex hammer. Reflexes were felt by examiner's hand as plantar flexions. Deep Tendon Reflex can discern hyporeflexia, an absent or diminished response to tapping, typically indicating disease involving one or more of the components of the reflex arc or hyperreflexia, hyperactive or repeating (clonic) reflexes, indicating an interruption of corticospinal and other descending pathways that influence the reflex arc.
Perception of Great Toe Vibration
The subject lay supine on the examination table. The examiner then struck a tuning fork and placed it on distal interphalangeal joint of the great toe on the dominant side and asked when the vibration stopped. The time from touch to response was timed with a stopwatch. The procedure was repeated on the other great toe. The timed vibratory test is the most sensitive noninvasive method of detecting mild to moderate impairments in vibratory sensation and used to detect sensory neuropathy (Cherry et al., 2005).
2-Point Discrimination
Using a 3-point aesthesiometer, the examiner touched the sole of one foot with 1 or 2 points, to which the subject (with eyes closed) responded “one” or “two.” Testing avoided calloused skin and proceeded with descending limits (starting at 50 mm distance between points); the threshold was the shortest distance on which fewer than 3 errors were made (Corkin et al., 1970). The procedure was repeated with the other foot.
MRI and DTI Protocol and Analysis
Neuroimaging and balance data of the alcoholics (30 men and 12 women) and most controls (18 men and 20 women) were collected either on the same day or within 1 week of each other. Exceptions were three control men with multiple MRIs, for whom we chose the MRI data set closest to the balance date (1–2.5 years difference).
DTI and MRI Acquisition
Imaging data were acquired on a General Electric 3T clinical human MR system with an 8-channel head coil after higher-order (nonlinear) shimming (Kim et al., 2002). DTI and fast spin-echo (FSE) structural data were collected with the same slice locations: DTI (2D echo-planar, axial plane, TR = 7300 ms, TE = 86.6 ms, thickness = 2.5 mm, skip = 0 mm, locations = 62, b = 0 (5 NEX) + 15 noncollinear diffusion directions b = 860 s/mm2 (2 NEX)+15 opposite-polarity noncollinear diffusion directions b = 860 s/mm2 (2 NEX), FOV = 240 mm, x-dim = 96, y-dim = 96, reconstructed to 128 × 128, 4030 total images); FSE (2D axial, TR = 7850 ms, TE = 17/102 ms, thickness = 2.5 mm, skip = 0 mm, locations = 62). An aligned T1-weighted SPGR (3D axial IR-prep, TR = 6.5 ms, TE = 1.6 ms, thick = 1.25 mm, skip = 0 mm, locations = 124) was collected, such that two 1.25 mm SPGR slices subtended each 2.5 mm-thick FSE/DTI slice. A field-map was generated from a gradient recalled echo sequence pair (TR = 460 ms, TE = 3/5 ms, thickness = 2.5 mm, skip = 0 mm, locations = 62).
DTI Analysis
DTI quantification was preceded by eddy-current correction on a slice-by-slice basis using within-slice registration. B0-field inhomogeneity-induced geometric distortion in the eddy current-corrected images was corrected with PRELUDE [Phase Region Expanding Labeller for Unwrapping Discrete Estimates (Jenkinson, 2003)] and FUGUE [FMRIB's Utility for Geometrically Unwarping EPIs (Jenkinson and Smith, 2001)].
Fiber Bundle Identification and Tracking
Fiber tracking was performed with software by Gerig et al. (2005) based on the “target” and “source” method of Mori and Van Zijl (2002). In short, streamlines originate from each pixel in a “source” region and follow the directions of the principal eigenvectors of the diffusion tensors until either the curvature exceeds a maximum angle or the local fractional anisotropy (FA) falls below a threshold. Tracking parameters included white matter extraction threshold (minimum FA) of 0.17, fiber tracking threshold of 0.125, and maximum voxel-to-voxel coherence minimum transition smoothness threshold of 0.80 (~37° maximum deviation between voxels), with essentially no limit on the number of fibers. Only streamlines that also traverse at least one pixel of a “target” region are kept; all other streamlines are discarded. Targets and sources were identified on the FA image of the SRI24 atlas (Rohlfing et al., 2010) (http://nitrc.org/projects/sri24) and mapped to the corresponding locations on the native-space DTI images for each subject using coordinate transformations computed by nonrigid image registration (Rohlfing and Maurer, 2003) (http://nitrc.org/projects/cmtk/). The output of the fiber tracking for each subject and each source-target pair was a 3D geometric model of the fiber paths comprising a table of all point locations along each fiber with local DTI metrics; mean diffusivity (MD) for each fiber bundle was the unit of analysis.
Gray Matter and Tissue Mean Diffusivity (MD) Analysis
The T1-weighted SPGR images were registered nonrigidly to the T1 image of the SRI24 atlas, and tissue probability maps transferred from the atlas to each SPGR image. Using these probabilities to both initialize and guide tissue classification, the SPGR images were then segmented into CSF, gray matter, white matter, or tissue (gray+white matter) using FSL's FAST tool (Zhang et al., 2001). Furthermore, by transferring label maps from the SRI24 atlas to each SPGR image, the latter was parcellated into cortical and subcortical regions of interest. Selected regions were also mapped, again via a nonrigid registration to the corresponding locations on the native-space MD images providing an index of tissue quality, with higher gray matter MD indicative of greater presence of interstitial fluid.
Statistical Analysis
Group and condition effects for the primary balance measures of sway path length and tremor frequency (2–5/0–2 Hz and 5–7 Hz) were tested with repeated-measures analysis of variance (ANOVA) for 4 groups (control and alcoholic men and women), two vision conditions (eyes open, eyes closed), two sway or tremor directions (anterior-posterior, lateral-medial), and three task conditions (no, easy, hard), with Geiser-Greenhouse (GG) correction where appropriate. Bivariate and multiple regression analyses examined relations between and among variables and follow up with calculations of confidence intervals based on bootstrapping computations based on 2000 permutations. To reduce the number of comparisons, correlations were based on anterior/posterior (AP)—medial/lateral (ML) difference measures (i.e., AP–ML) of sway path length and tremor velocity.
Results
Measures of Quiet Standing
Sway Path Length
ANOVA (4 groups × 2 eyes open/closed conditions × 3 dual tasks conditions of no, easy, and hard arithmetic × 2 sway directions [AP, ML]) identified three significant effects and two interactions but no significant group differences or interactions involving group (Figure 1). The significant effects indicated that, regardless of group, sway paths were longer under the following conditions: with eyes closed than open [F(1, 85) = 268.144, p = 0.0001 GG], with hard relative to easy arithmetic dual task [F(2, 170) = 5.039, p = 0.0121 GG], and in the AP relative to the ML direction [F(1, 85) = 4.026, p = 0.0480 GG]. The significant interactions involved vision × task [F(2, 170) = 4.037, p = 0.0212 GG] and vision x task x direction [F(2, 170) = 9.239, p = 0.0002 GG].
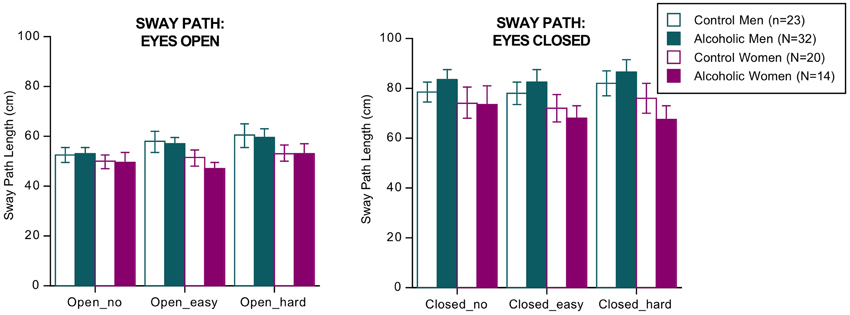
Figure 1. Bar graphs = mean ± SE of the sway path length in each group. None of the group differences was significant.
The groups did not differ in number of errors committed on the arithmetic task, and in no condition did sway path length (based on the AP–ML difference) correlate with number of arithmetic errors by the alcoholic men nor with body mass index. The results did not change when recalculated without the four alcoholics who had drunk within a week of testing.
Tremor
Separate ANOVAs were conducted for the two tremor bands (Figure 2). For 2–5/0–2 Hz, the effects of group [F(3, 85) = 3.188, p = 0.0278], vision [F(1, 85) = 15.198, p = 0.0002], direction [F(1, 85) = 18.826, p = 0.0001], group × direction interaction (3, 85) = 6.359, p = 0.0006 GG), and dual task × direction interaction [F(2, 170) = 5.800, p = 0.0038 GG] were significant.
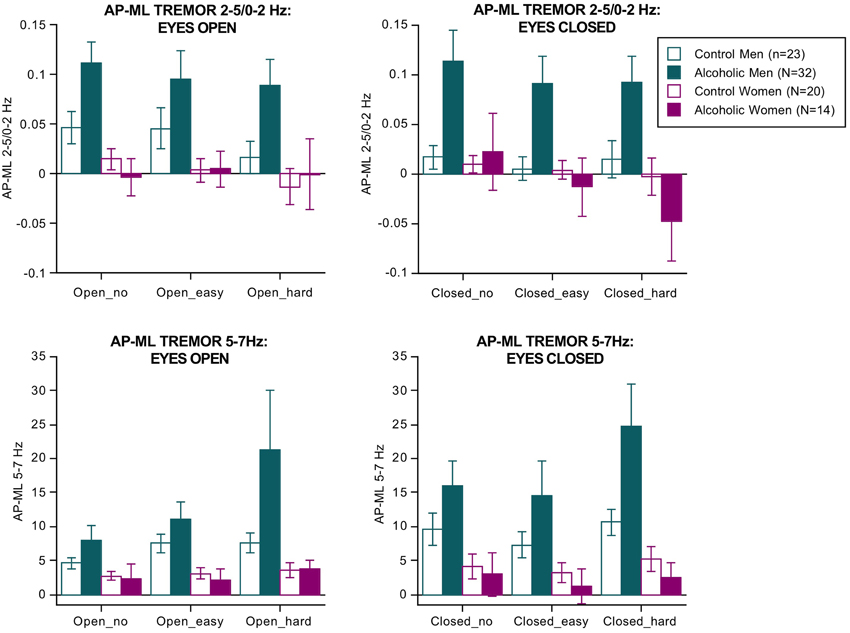
Figure 2. Bar graphs = mean ± SE of the 2–5/0–2 Hz (top) and 5–7 Hz frequency bands expressed as the difference between the anterior-posterior tremor velocity minus the medial-lateral tremor velocity in each group. Tremor was greatest in the alcoholic men; 5–7 Hz tremor velocity showed a step-wise increase with increasing difficulty of the dual task.
For the 5–7 Hz band, the effects of group [F(3, 85) = 3.472, p = 0.0196], vision [F(1, 85) = 63.288, p = 0.0001], and dual task [F(2, 170) = 12.890, p = 0.0001 GG] were significant. Also significant were the group × direction [F(3,85) = 5.142, p = 0.0026 GG] and the group × direction-dual task [F(6, 170) = 2.895, p = 0193 GG] interactions. These effects indicate that the alcoholic men exhibited by far the greatest tremor in both frequency bands of any group, especially in the AP direction, and showed a modest increase in tremor with increasing task difficulty regardless of visual condition (Figure 2). These findings endured when the 3 alcoholic men who had drunk alcohol within a week of testing were excluded from analysis.
In the alcoholic men, greater AP-ML tremor velocity occurred with fewer arithmetic errors and was significant for the 2–5/0–2 Hz ratio with eyes open (easy Rho= −0.43, p = 0.0245; hard Rho = −0.421, p = 0.0285) and for the 5–7 Hz band with eyes closed (easy Rho = −0.47, p = 0.0143; hard Rho = −0.385, p = 0.0455). Path length (AP–ML, eyes open, hard arithmetic) correlated with tremor velocity only in the 5–7 Hz band (Rho = 0.43, p = 0.0215). In no condition did tremor velocity in either frequency band correlate with body mass index or length of sobriety.
Sensory Testing
Subjective lower limb discomfort was rated none to mild: rating = 1 by one alcoholic man and 3 by one alcoholic woman. The only objective measures on which the groups differed were vibration perception of the left great toe (Kruskal-Wallis H = 10.16, p = 0.0173) and two-point discrimination (Kruskal-Wallis H = 16.30, left foot p = 0.001, H = 12.34 right foot p = 0.0063; 1 subject's values >11 SD and were Windsorized to the next highest value; thus left foot value moved from 45 to 25, and right foot value moved from 40 to 24). For both objective tests, only the alcoholic men had abnormally high scores. Correlations conducted between the mean of the left+right foot 2-point discrimination scores and the balance measures identified one modest correlation, which was with tremor velocity in the 2–5/0–2 Hz frequency band in the eyes-open condition (r = 0.40, p = 0.0415; Rho = 0.41, p = 0.0402).
Fiber Tracking and Gray Matter MD Correlates of Sway Path Length and Tremor
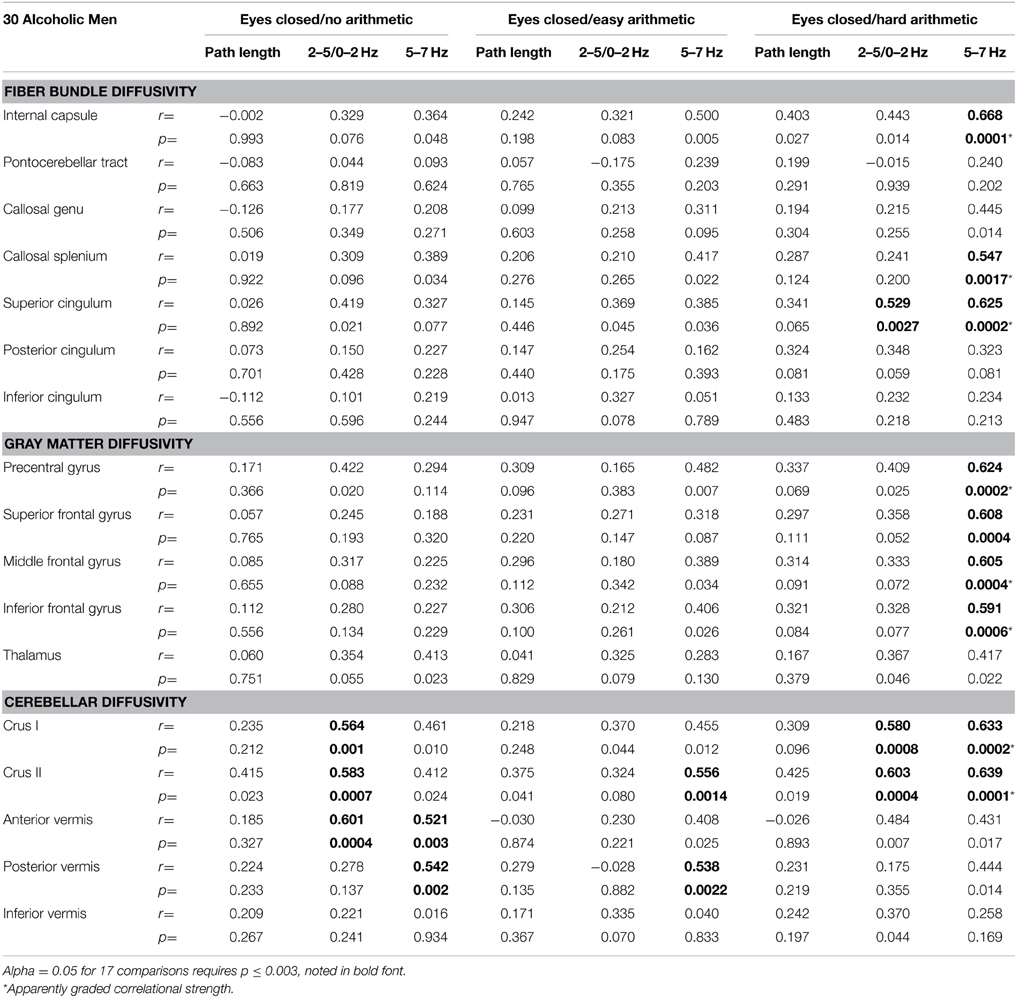
White matter fiber bundle correlates of balance measures evaluated were the internal capsule, pontocerebellar tract, genu and splenium of the corpus callosum, and three segments of the cingulate bundle. Diffusivity correlates were the precentral, superior, middle, and inferior frontal cortical gray matter and tissue (gray matter+white matter) of the thalamus, cerebellar Crus I and II, and anterior, posterior, and inferior sectors of the vermis. Although the groups did not differ in MD in these regions or fiber tracts, greater tremor correlated with higher MD in a number of regions in the alcoholic men (Table 2).
Eight MD measures (internal capsule, splenium, superior cingulum, precentral, middle, and inferior frontal gyri, and Crus I and II) showed a step-wise increase in the magnitude of the correlations with 5–7 Hz tremor velocity (eyes closed) from no task to easy arithmetic to hard arithmetic (Table 2). Exploratory multiple regressions indicated that regional MD measures were consistently better predictors of AP-ML tremor with eyes closed than eyes open, for the 5–7 Hz over lower frequency tremor, and for the harder than easier arithmetic condition. Consequently, to minimize the number of comparisons, our primary DTI-tremor relations focused on path length and tremor measured while engaged in the hard arithmetic task with eyes closed (Figure 3).
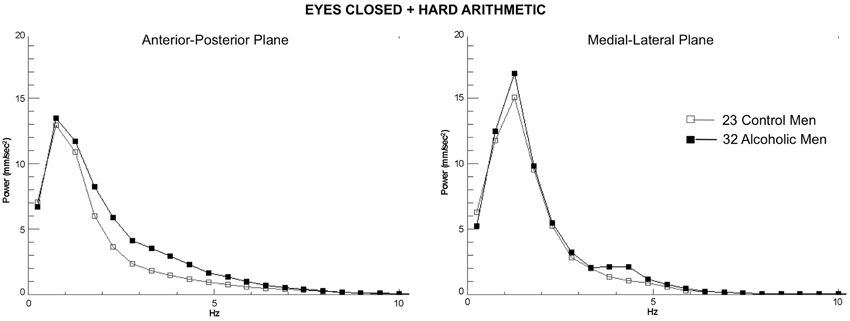
Figure 3. Group average spectra from the frequency analysis of center-of-pressure sway path velocity (distance/time), presented in ~0.5 Hz frequency bins for the most difficult test condition (eyes closed, hard arithmetic) for the 32 alcoholic men (black lines and filled circles) and 23 control men (gray lines and squares). Spectra from the anterior-posterior plane and the medial-lateral plane are plotted separately.
Sway Path Length
Neither the control men or women nor the alcoholic women showed significant correlations between sway path length and regional MD. For the alcoholic men, a few modest correlations emerged between regional diffusivity and balance metrics (Table 2), but none was significant after correcting for multiple comparisons (family-wise Bonferroni correction for 17 comparison with a = 0.05 required p ≤.003).
2–5/0–2 Hz Tremor
Greater AP-ML tremor velocity correlated significantly with higher diffusivity in the superior cingulum, Crus I, and Crus II (Table 2). Multiple regression analysis revealed that MD as a predictor of tremor velocity accounted for 38.9% of the adjusted variance (p = 0.0012), where superior cingulate MD made a greater independent contribution to the variance (p = 0.08) than MD in either cerebellar region (p > 0.26) (Figure 4). Entering mean 2-point discrimination scores together with superior cingulate MD into a multiple regression accounted for 30.5% of the variance (p = 0.0058), with greater unique contribution from MD (p = 0.0094) than 2-point discrimination performance (p = 0.09).
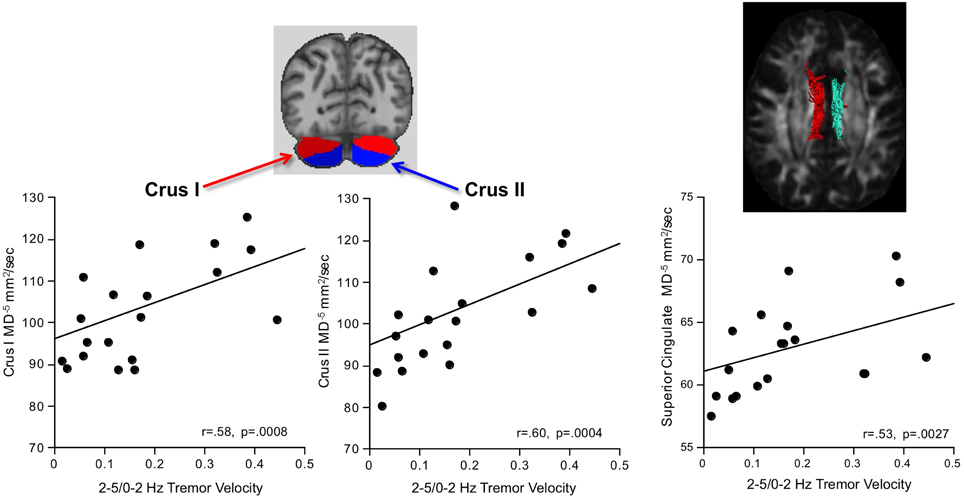
Figure 4. In alcoholic men, greater 2–5/0–2 Hz tremor velocity correlated with higher MD in the cerebellar Crus I and II.
5-7 Hz Tremor
Greater AP-ML tremor velocity in this frequency band correlated significantly with higher MD in 9 of the 17 regions examined (Table 2): internal capsule, splenium, superior cingulum, four frontal gyri, Crus I, and Crus II. Although entering all 9 MD measures as predictors of tremor accounted for 50.9% (p = 0.003) of the variance, a follow-up multiple regression identified major contributions simply from MD in motor cortex (p = 0.032) and internal capsule (p = 0.0075), together accounting for 49.9% (p = 0.0001) of the adjusted variance. Further analysis included 2-point discrimination scores along with motor cortex and internal capsule MD, together accounting for 63.5% (p = 0.0001) of the 5–7 Hz tremor variance, and each contributed uniquely to the overall variance (motor cortex MD p = 0.0022; internal capsule MD p = 0.0934; 2-point discrimination score p = 0.0206) (Figure 5).
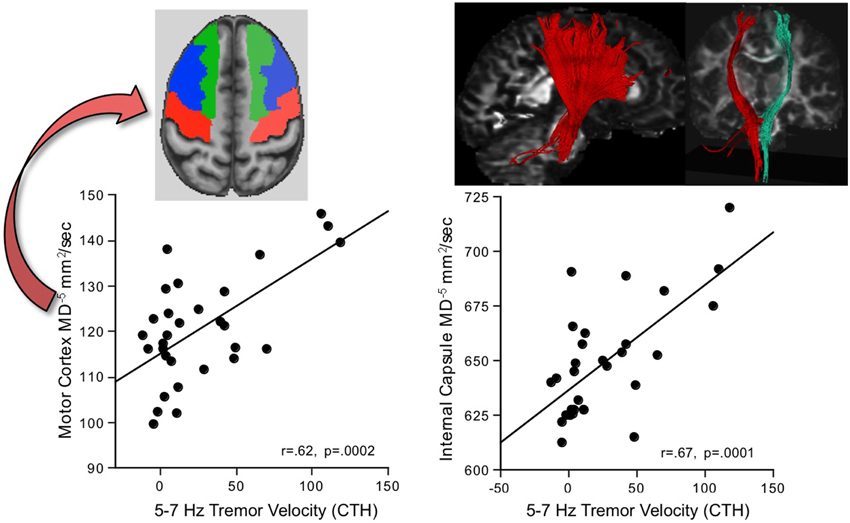
Figure 5. In alcoholic men, greater 5–7 Hz tremor velocity correlated with higher MD in the motor cortex and internal capsule.
Follow-up analyses used a bootstrapping computation conducted in R using 2000 permutations to create confidence intervals for the correlations. The results of bootstrapping supported the Pearson correlations (Figure 6).
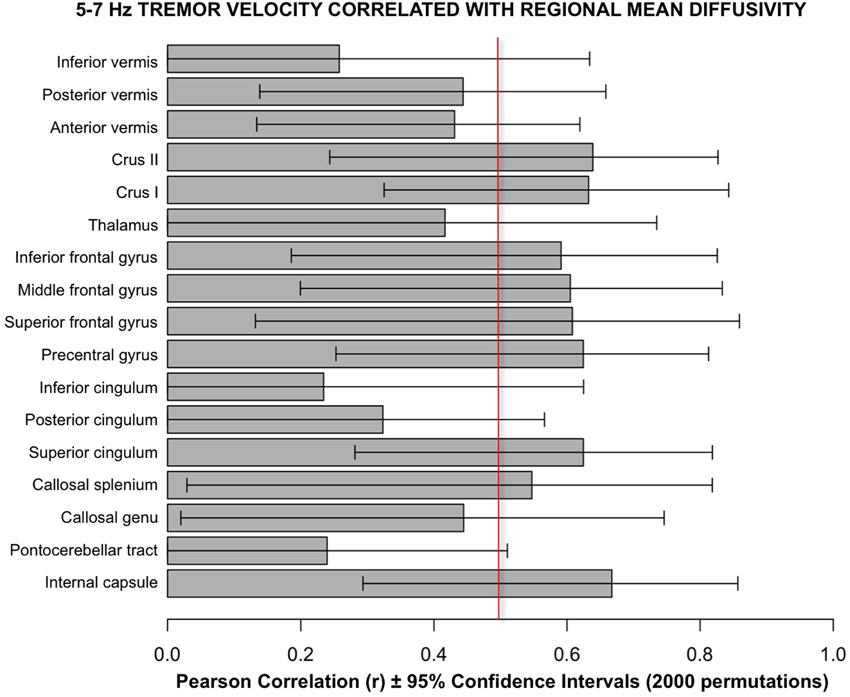
Figure 6. Bars depict Pearson correlation r for the 5–7 Hz tremor velocity with each of the 17 regional MD measures. The error bars are the 95% confidence intervals derived from the bootstrapping method using 2000 permutations. The vertical red line marks the approximate level of correlation required to achieve statistical significance after correction for multiple correlations.
Discussion
Sway path lengths were longer with eyes closed than eyes open and while engaged in the arithmetic, dual task but were not different among the groups. By contrast, truncal tremor, marked by prominences in the 2–5 Hz (normalized to 0–2 Hz) and 5–7 Hz frequency bands, was substantial in the alcoholic men but not in alcoholic women and exacerbated with dual tasking because the fewer the arithmetic errors (indicative of greater attention paid to the arithmetic task), the greater the tremor. With few exceptions, the alcoholics were tested after prolonged sobriety, and no measure of sway or tremor correlated with length of sobriety. Thus, alcoholism-related effects on balance could not be attributed to acute withdrawal. Tremor, greater in the anterior-posterior (AP) than medial-lateral (ML) direction, was related to tissue integrity of an extensive neural system, extending from the cerebellum to frontal cortex and dissociable on the basis of tremor power.
Relation to Tissue Integrity of the CNS Motor System
The DTI diffusivity metric, MD, a measure of freely diffusing water, assesses brain tissue quality. Abnormally high MD can reflect higher than expected interstitial tissue water or edema, as reported in stroke (Holdsworth et al., 2014), neurodegenerative diseases, and alcoholism (for review Zahr, 2014). Another source of high MD in tissue is partial voluming, that is, inclusion of nontarget signal (typically from sulcal or ventricular cerebrospinal fluid) in the tissue region measured. Although the alcoholics herein did not exhibit abnormally high MD relative to controls, greater tremor in the lower and higher frequency bands did correlate selectively with higher regional MD in alcoholic men. These relations have foundation in lesion studies and disease models, described next.
Monkeys treated with MPTP to model Parkinson's disease exhibited 5–7 Hz arm tremor, which was associated with lesions in the “cerebellar” thalamus and was distinguishable from higher-frequency (>7 Hz) tremor arising from the “pallidal” thalamus (Guehl et al., 2003). To the extent that tremor frequency characterizes compromise of a selective, “tremorgenic” neural system (cf., Brittain and Brown, 2013; Pedrosa et al., 2013) regardless of its peripheral origin, presence of enduring 5–7 Hz postural tremor in long-sober alcoholics may also arise from damage in cerebellar-pontine-thalamic structures (Makris et al., 2008; Sullivan and Pfefferbaum, 2009), frontocerebellar circuitry (Le Berre et al., 2014), or both. Supporting this possibility, greater tremor in the 5–7 Hz frequency band in our alcoholic men correlated selectively with signs of greater tissue compromise in brain regions contributing to motor abilities, including the motor cortex, and corticospinal fiber tracts of the internal capsule, which connect regions enabling sensorimotor integration and information exchange across motor, prefrontal, thalamic, and cerebellar sites. Further, that truncal tremor was greater in the anterior-posterior than medial-lateral axis is similar to symptoms of “frontal lobe ataxia” that can occur with lesions of the cerebellar vermis, thalamus, or frontal cortex (Thompson, 2012).
Differences in tremor characteristics have brain structural underpinnings; indeed, tremors can be classified according to their frequency, amplitude, and anatomical location. For example, Parkinson's Disease is associated with tremor in the 4–6 Hz range, whereas essential tremor typically occurs between 4 and 8 Hz (Anouti and Koller, 1995). Patients with anterior cerebellar lobe lesions show a 3 Hz postural tremor in the AP direction, whereas those with cerebellar hemisphere lesions cannot be separated from controls (Mauritz et al., 1979; Diener et al., 1984), but tremor in the 3–10 Hz range has been associated with cerebellar pathology of various etiologies (Brown et al., 1997).
We used the ratio of 2–5/0–2 Hz to enable comparison of our results in chronic alcoholics, whose cerebellar damage would likely be modest, with results in the patients of the Bahol et al. studies (i.e., Baloh et al., 1994, 1998), who had clinically diagnosed olivopontocerebellar atrophy or isolated cerebellar atrophy and severe gait and balance impairment without visual-vestibular deficits. In the Baloh study, the 2–5/0–2 Hz ratio tremor was observed in patients with cerebellar atrophy but not in those with bilateral vestibular impairment under test conditions of static balance with eyes open (Baloh et al., 1998). The ratio was even greater in the cerebellar group with eyes closed, although 3 of 10 patients in the vestibular group showed such tremor. Alcoholics in our study had no clinical evidence of tremor other than that detected with quantitative measures afforded by force plate analysis; nonetheless, our alcoholic men exhibited enhanced 2–5/0–2 Hz ratio tremor, which was similar to that reported in the severely affected patients with cerebellar lesions reported by Baloh et al.
The constellation of neural sites observed as correlates of alcoholism-related tremor in both power prominences identified herein is consistent with results from the DTI study of Klein et al. (2012), who tracked a network of structures from the ventral thalamic sites that relieved tremor through deep brain stimulation. That network included the precentral gyrus, primary and supplementary motor areas, frontal gyri, anterior cingulum, and the cerebellar hemispheres. These findings also comport with a DTI study of essential tremor that reported abnormally high diffusivity in far-reaching sites, including frontoparietal white matter, anterior internal capsule, thalamus, brain stem, and cerebellar hemispheres and peduncles (Saini et al., 2012).
Interaction of Postural Stability with Cognitive Challenge
Engagement in a cognitively challenging task elicited a subtle abnormality, physiological truncal tremor, while alcoholic men maintained upright posture. Although path length was little affected, tremor was exacerbated while engaged in solving multi-step mental arithmetic. Another study (Pellecchia, 2003) found that engaging in a cognitive task concurrent with quiet standing resulted in increased sway, which was even greater when the concurrent task required information reduction, similar to that required in the difficult arithmetic task used herein. Further, performance by elderly men and women on the Walk While Talking test was poorer with a difficult task (saying every other letter of the alphabet) compared with a simpler task (saying the alphabet) (Verghese et al., 2002). Most relevantly, our results comport with the blood flow study conducted in healthy young adults who exhibited graded frontal brain activation, observed with fNIRS, depending on level of difficulty of the cognitive task engaged in while standing still or walking (Mirelman et al., 2014). Our findings extend the fNIRS study by identifying a physiological outcome in tremor elicited while standing and performing a difficult cognitive task and by identifying extensive brain networks from cerebellum to frontal cortex as underlying the tremor prominences observed with cognitive-motor dual tasking.
An early position held that engagement in higher-order cognition could not intrude on motor performance required for quiet standing, which was considered to be under automatic motor control (for review, Woollacott and Shumway-Cook, 2002). Nonetheless, we found that cognitive tasks can interact with postural stability and that neural sources of these actions and interactions involved both cerebellar and supratentorial brain motor and cognitive systems, including ones that share connections. A functional MRI study revealed a similar network of regions involving the inferior frontal gyrus, middle cerebellar cortex, and thalamus that were invoked while young healthy adults engaged in mental arithmetic (Menon et al., 2000). All nodes of this circuitry have been implicated in motor control [e.g., postural stability (Victor et al., 1989; Diener and Dichgans, 1992; Sullivan et al., 2006), motor preparation (Diener et al., 1989), and articulation (Spencer and Slocomb, 2007; Timmann et al., 2008)] and cognition, including working memory (e.g., Desmond et al., 1997; Schmahmann, 1997; Chanraud et al., 2010), and are commonly disturbed in long-term chronic alcoholism (for reviews, Sullivan and Pfefferbaum, 2005; Oscar-Berman and Marinkovic, 2007). One caveat considers the possible role of articulation in exacerbating tremor. Recognizing the “dose” effect of greater tremor with the more complex, mental arithmetic task, it could be argued that articulation compounded the difficult cognitive challenge and resulted in greater tremor velocity than with the easier cognitive challenge (cf., Dault et al., 2003).
Sensory Status and Tremor
Sensory testing was essentially normal in the alcoholic men and women and showed at most a modest relation to tremor. When considered in the context of the DTI brain measures, 2-point discrimination performance did contribute to the overall variance of the 5–7 Hz velocity of truncal tremor, suggesting an interaction of peripheral sensory system condition with a centrally modulated, physiological response of tremor.
Conclusion
Enhanced physiological resonance (i.e., tremor) in quiet standing is measurable in alcoholic men and endures beyond acute alcohol withdrawal. Elevated tremor, notable in the anterior-posterior axis in two frequency bands, can be detectable during quiet standing even in the absence of excessive sway path length. Excessive 2–5 Hz tremor velocity was related to higher diffusivity in tissue of the cerebellar hemispheres and fibers of the superior cingulate bundle. The 5–7 Hz tremor velocity was correlated with tissue integrity of a network of frontocerebellar sites and internal capsule, a principal connection for these sites. These far-reaching systems associated with enhanced truncal tremor intersect with neural systems underlying cognition, thereby providing avenues for interference observed as exacerbated tremor by engaging in a concurrent, cognitively challenging, task. We speculate that these different tremor prominences identified in chronic alcoholism, where the lower frequency is associated with compromise of brainstem systems and the higher frequency is associated with compromise of brainstem plus supratentorial systems, is consistent with a model of tremor generation based on investigations of Wilson's Disease (Sudmeyer et al., 2006). In that study, tremor in the 4–6 Hz range was associated with oscillatory coherence determined from EEG scalp recordings between cerebellum-thalamus (but not motor cortex), whereas 15–33 Hz tremor was associated with coherence between thalamus and cortical areas, including primary sensory and motor cortices, premotor cortex, and posterior parietal cortex. Taken together, these observations suggest a progression of tremor power generated by (or at least associated with) activity in lower to higher brainstem to cortical circuitry.
Within the context of alcohol dependence, neuroimaging studies report widespread brain tissue volume deficits, but with a predilection for the prefrontal cortex and the cerebellum (e.g., Makris et al., 2008; Le Berre et al., 2014), thalamus (De Bellis et al., 2005; Sullivan and Pfefferbaum, 2009), pons (Pfefferbaum et al., 2002; Sullivan and Pfefferbaum, 2009), and cerebellar peduncles (Chanraud et al., 2007; Mechtcheriakov et al., 2007). These sites have the potential of representing “tremorgenic networks” (Brittain and Brown, 2013; Pedrosa et al., 2013) that when disturbed contribute to postural instability in the recovering alcoholic and potentially put them at heightened risk for falling.
Author Contributions
All authors participated in the analysis and interpretation of the data and the writing of the manuscript. All authors also approved the final version submitted for publication and are responsible for the integrity of the data and analysis herein.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by grants U.S. National Institute on Alcohol Abuse and Alcoholism (AA010723, AA012388, AA017168).
References
Anouti, A., and Koller, W. C. (1995). Tremor disorders. Diagnosis and management. West. J. Med. 162, 510–513.
Baker, K., Harding, A., Halliday, G., Kril, J., and Harper, C. (1999). Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke's encephalopathy. Neuroscience 91, 429–438. doi: 10.1016/S0306-4522(98)90664-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baloh, R. W., Fife, T. D., Zwerling, L., Socotch, T., Jacobson, K., Bell, T., et al. (1994). Comparison of static and dynamic posturography in young and older normal people. J. Am. Geriatr. Soc. 42, 405–412.
Baloh, R. W., Jacobson, K. M., Beykirch, K., and Honrubia, V. (1998). Static and dynamic posturography in patients with vestibular and cerebellar lesions. Arch. Neurol. 55, 649–654. doi: 10.1001/archneur.55.5.649
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bigley, G. K. (1990). “Sensation,” in Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd Edn., eds H. K. Walker, W. D. Hall, and J. W. Hurst (Boston, MA: Butterworths), 343–350.
Brittain, J. S., and Brown, P. (2013). The many roads to tremor. Exp. Neurol. 250, 104–107. doi: 10.1016/j.expneurol.2013.09.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brown, P., Rothwell, J. C., Stevens, J. M., Lees, A. J., and Marsden, C. D. (1997). Cerebellar axial postural tremor. Mov. Disord. 12, 977–984. doi: 10.1002/mds.870120622
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chanraud, S., Martelli, C., Delain, F., Kostogianni, N., Douaud, G., Aubin, H. J., et al. (2007). Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology 32, 429–438. doi: 10.1038/sj.npp.1301219
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chanraud, S., Pitel, A.-L., Rohlfing, T., Pfefferbaum, A., and Sullivan, E. V. (2010). Dual tasking and working memory in alcoholism: relation to frontocerebellar circuitry. Neuropsychopharmacology 35, 1868–1878. doi: 10.1038/npp.2010.56
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cherry, C. L., Wesselingh, S. L., Lal, L., and McArthur, J. C. (2005). Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology 65, 1778–1781. doi: 10.1212/01.wnl.0000187119.33075.41
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Corkin, S., Milner, B., and Rasmussen, T. (1970). Somatosensory thresholds: contrasting effects of postcentral-gyrus and posterior parietal lobe excisions. Arch. Neurol. 23, 41–58. doi: 10.1001/archneur.1970.00480250045007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Crovitz, H. F., and Zener, K. A. (1962). Group test for assessing hand and eye dominance. Am. J. Psychol. 75, 271–276. doi: 10.2307/1419611
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dault, M. C., Yardley, L., and Frank, J. S. (2003). Does articulation contribute to modifications of postural control during dual-task paradigms? Brain Res. Cogn. Brain Res. 16, 434–440. doi: 10.1016/S0926-6410(03)00058-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
De Bellis, M. D., Narasimhan, A., Thatcher, D. L., Keshavan, M. S., Soloff, P., and Clark, D. B. (2005). Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol. Clin. Exp. Res. 29, 1590–1600. doi: 10.1097/01.alc.0000179368.87886.76
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Desmond, J. E., Gabrieli, J. D. E., Wagner, A. D., Ginier, B. L., and Glover, G. H. (1997). Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J. Neurosci. 17, 9675–9685.
Diener, H.-C., and Dichgans, J. (1992). “Pathophysiology of ataxia in humans,” in Cerebellar Degenerations: Clinical Neurobiology, ed A. Plaitakis (Boston, MA: Kluwer Academic Publishers), 261–280.
Diener, H. C., Dichgans, J., Bacher, M., and Gompf, B. (1984). Quantification of postural sway in normals and patients with cerebellar diseases. Electroencephalogr. Clin. Neurophysiol. 57, 134–142. doi: 10.1016/0013-4694(84)90172-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Diener, H. C., Dichgans, J., Guschlbauer, B., Bacher, M., and Langenbach, P. (1989). “Disturbances of motor preparation in basal ganglia and cerebellar disorders,” in Progress in Brain Research, Vol 80, Afferent Control of Posture and Locomotion, eds J. H. H. Allun and M. Hulliger (Amsterdam: Elsevier Scientific Publishers B.V.), 481–488.
Gerig, G., Corouge, I., Vachet, C., Krishnan, K. R., and Macfall, J. R. (2005). “Quantitative analysis of diffusion properties of white matter fiber tracts: a validation study,” in 13th Proceedings of the International Society for Magnetic Resonance in Medicine, (Miami, FL), Abstract no. 1337.
Guehl, D., Pessiglione, M., Francois, C., Yelnik, J., Hirsch, E. C., Feger, J., et al. (2003). Tremor-related activity of neurons in the ‘motor’ thalamus: changes in firing rate and pattern in the MPTP vervet model of parkinsonism. Eur. J. Neurosci. 17, 2388–2400. doi: 10.1046/j.1460-9568.2003.02685.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Harper, C., Dixon, G., Sheedy, D., and Garrick, T. (2003). Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 951–961. doi: 10.1016/S0278-5846(03)00155-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Holdsworth, S. J., Yeom, K. W., Antonucci, M. U., Andre, J. B., Rosenberg, J., Aksoy, M., et al. (2014). Diffusion-weighted imaging with dual-echo echo-planar imaging for better sensitivity to acute stroke. Am. J. Neuroradiol. 35, 1293–1302. doi: 10.3174/ajnr.A3921
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hollingshead, A. (1975). Four-Factor Index of Social Status. New Haven, CT: Yale University, Department of Sociology.
Holmes, G. (1917). The symptoms of acute cerebellar injuries due to gunshot injuries. Brain 40, 461–535. doi: 10.1093/brain/40.4.461
Jenkinson, M. (2003). A fast, automated, N-dimensional phase unwrapping algorithm. Magn. Reson. Med. 49, 193–197. doi: 10.1002/mrm.10354
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jenkinson, M., and Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156. doi: 10.1016/S1361-8415(01)00036-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, D. H., Adalsteinsson, E., Glover, G. H., and Spielman, D. M. (2002). Regularized higher-order in vivo shimming. Magn. Reson. Med. 48, 715–722. doi: 10.1002/mrm.10267
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Klein, J. C., Barbe, M. T., Seifried, C., Baudrexel, S., Runge, M., Maarouf, M., et al. (2012). The tremor network targeted by successful VIM deep brain stimulation in humans. Neurology 78, 787–795. doi: 10.1212/WNL.0b013e318249f702
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Koller, W., O'Hara, R., Dorus, W., and Bauer, J. (1985). Tremor in chronic alcoholism. Neurology 35, 1660–1662. doi: 10.1212/WNL.35.11.1660
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Le Berre, A. P., Pitel, A. L., Chanraud, S., Beaunieux, H., Eustache, F., Marinot, J. L., et al. (2014). Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: comparison of effects in France and the United States. Hum. Brain Mapp. 35, 4635–4653. doi: 10.1002/hbm.22500
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Makris, N., Oscar-Berman, M., Jaffin, S. K., Hodge, S. M., Kennedy, D. N., Caviness, V. S., et al. (2008). Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry 64, 192–202. doi: 10.1016/j.biopsych.2008.01.018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mattis, S. (2004). Dementia Rating Scale (DRS) Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc.
Mauritz, K. H., Dichgans, J., and Hufschmidt, A. (1979). Quantitative analysis of stance in late cortical cerebellar atrophy of the anterior lobe and other forms of cerebellar ataxia. Brain 102, 461–482. doi: 10.1093/brain/102.3.461
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mechtcheriakov, S., Brenneis, C., Egger, K., Koppelstaetter, F., Schocke, M., and Marksteiner, J. (2007). A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J. Neurol. Neurosurg. Psychiatr. 78, 610–614. doi: 10.1136/jnnp.2006.095869
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Menon, V., Rivera, S. M., White, C. D., Glover, G. H., and Reiss, A. L. (2000). Dissociating prefrontal and parietal cortex activation during arithmetic processing. Neuroimage 12, 357–365. doi: 10.1006/nimg.2000.0613
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mirelman, A., Maidan, I., Bernad-Elazari, H., Nieuwhof, F., Reelick, M., Giladi, N., et al. (2014). Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J. Neuroeng. Rehabil. 11:85. doi: 10.1186/1743-0003-11-85
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mori, S., and Van Zijl, P. C. (2002). Fiber tracking: principles and strategies—a technical review. NMR Biomed. 15, 468–480. doi: 10.1002/nbm.781
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Neiman, J., Lang, A. E., Fornazzari, L., and Carlen, P. L. (1990). Movement disorders in alcoholism: a review. Neurology 40, 741–746. doi: 10.1212/WNL.40.5.741
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Oscar-Berman, M., and Marinkovic, K. (2007). Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol. Rev. 17, 239–257. doi: 10.1007/s11065-007-9038-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pedrosa, D. J., Auth, M., Eggers, C., and Timmermann, L. (2013). Effects of low-frequency thalamic deep brain stimulation in essential tremor patients. Exp. Neurol. 248, 205–212. doi: 10.1016/j.expneurol.2013.06.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pellecchia, G. L. (2003). Postural sway increases with attentional demands of concurrent cognitive task. Gait Posture 18, 29–34. doi: 10.1016/S0966-6362(02)00138-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pellecchia, G. L., and Turvey, M. T. (2001). Cognitive activity shifts the attractors of bimanual rhythmic coordination. J. Mot. Behav. 33, 9–15. doi: 10.1080/00222890109601898
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pentney, R. J. (1993). “Alterations in the structure of the cerebellum after long-term ethanol consumption,” in Alcohol-Induced Brain Damage: NIAAA Research Monograph No. 22, eds W. A. Hunt and S. J. Nixon (Rockville, MD: National Institute of Health), 249–276.
Pfefferbaum, A., Adalsteinsson, E., and Sullivan, E. V. (2006). Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol. Aging 27, 994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pfefferbaum, A., Rosenbloom, M. J., Rohlfing, T., and Sullivan, E. V. (2009). Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol. Psychiatry 65, 680–690. doi: 10.1016/j.biopsych.2008.10.039
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pfefferbaum, A., Rosenbloom, M. J., Serventi, K., and Sullivan, E. V. (2002). Corpus callosum, pons and cortical white matter in alcoholic women. Alcohol. Clin. Exp. Res. 26, 400–405. doi: 10.1111/j.1530-0277.2002.tb02552.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rogasch, N. C., and Todd, G. (2013). rTMS over human motor cortex can modulate tremor during movement. Eur. J. Neurosci. 37, 323–329. doi: 10.1111/ejn.12023
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rohlfing, T., and Maurer, C. R. (2003). Nonrigid image registration in shared-memory multiprocessor environments with application to brains, breasts, and bees. IEEE Trans. Inf. Technol. Biomed. 7, 16–25. doi: 10.1109/TITB.2003.808506
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rohlfing, T., Zahr, N. M., Sullivan, E. V., and Pfefferbaum, A. (2010). The SRI24 multi-channel atlas of normal adult human brain structure. Hum. Brain Mapp. 31, 798–819. doi: 10.1002/hbm.20906
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Saini, J., Bagepally, B. S., Bhatt, M. D., Chandran, V., Bharath, R. D., Prasad, C., et al. (2012). Diffusion tensor imaging: tract based spatial statistics study in essential tremor. Parkinsonism Relat. Disord. 18, 477–482. doi: 10.1016/j.parkreldis.2012.01.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shumway-Cook, A., Woollacott, M., Kerns, K. A., and Baldwin, M. (1997). The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. J. Gerontol. 52, M232–M240. doi: 10.1093/gerona/52A.4.M232
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Spencer, K. A., and Slocomb, D. L. (2007). The neural basis of ataxic dysarthria. Cerebellum 6, 58–65. doi: 10.1080/14734220601145459
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sudmeyer, M., Pollok, B., Hefter, H., Gross, J., Butz, M., Wojtecki, L., et al. (2006). Synchronized brain network underlying postural tremor in Wilson's disease. Mov. Disord. 21, 1935–1940. doi: 10.1002/mds.21104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sullivan, E. V., Deshmukh, A., Desmond, J. E., Lim, K. O., and Pfefferbaum, A. (2000). Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: relation to ataxia. Neuropsychology 14, 341–352. doi: 10.1037/0894-4105.14.3.341
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sullivan, E. V., and Pfefferbaum, A. (2005). Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl). 180, 583–594. doi: 10.1007/s00213-005-2267-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sullivan, E. V., and Pfefferbaum, A. (2009). Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 44, 155–165. doi: 10.1093/alcalc/agn103
Sullivan, E. V., Rose, J., and Pfefferbaum, A. (2006). Effect of vision, touch, and stance on cerebellar vermian-related sway and tremor: a quantitative MRI and physiological study. Cereb. Cortex 16, 1077–1086. doi: 10.1093/cercor/bhj048
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sullivan, E. V., Rose, J., and Pfefferbaum, A. (2010). Physiological and focal cerebellar substrates of abnormal postural sway and tremor in alcoholic women. Biol. Psychiatry 67, 44–51. doi: 10.1016/j.biopsych.2009.08.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thompson, P. D. (2012). Frontal lobe ataxia. Handb. Clin. Neurol. 103, 619–622. doi: 10.1016/B978-0-444-51892-7.00044-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Timmann, D., Brandauer, B., Hermsdorfer, J., Ilg, W., Konczak, J., Gerwig, M., et al. (2008). Lesion-symptom mapping of the human cerebellum. Cerebellum 7, 602–606. doi: 10.1007/s12311-008-0066-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Verghese, J., Buschke, H., Viola, L., Katz, M., Hall, C., Kuslansky, G., et al. (2002). Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J. Am. Geriatr. Soc. 50, 1572–1576. doi: 10.1046/j.1532-5415.2002.50415.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Victor, M., Adams, R. D., and Collins, G. H. (1989). The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition, 2nd Edn. Philadelphia: F.A. Davis Co.
Woollacott, M., and Shumway-Cook, A. (2002). Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 16, 1–14. doi: 10.1016/S0966-6362(01)00156-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zahr, N. M. (2014). Structural and microstructral imaging of the brain in alcohol use disorders. Handb. Clin. Neurol. 125, 275–290. doi: 10.1016/B978-0-444-62619-6.00017-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, Y., Brady, M., and Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med. Imag. 20, 45–57.
Zheng, J. J., Delbaere, K., Close, J. C., Sachdev, P. S., and Lord, S. R. (2011). Impact of white matter lesions on physical functioning and fall risk in older people: a systematic review. Stroke 42, 2086–2090. doi: 10.1161/STROKEAHA.110.610360
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: diffusion tensor imaging, cerebellum, motor cortex, tremor, quiet standing, cognitive demands, corticospinal tract
Citation: Sullivan EV, Zahr NM, Rohlfing T and Pfefferbaum A (2015) Cognitive demands during quiet standing elicit truncal tremor in two frequency bands: differential relations to tissue integrity of corticospinal tracts and cortical targets. Front. Hum. Neurosci. 9:175. doi: 10.3389/fnhum.2015.00175
Received: 16 December 2014; Accepted: 13 March 2015;
Published: 07 April 2015.
Edited by:
Pierfilippo De Sanctis, Albert Einstein College of Medicine, USAReviewed by:
Stephan Bickel, Albert Einstein College of Medicine, USAArnaud Yves Falchier, Nathan Kline Institute, USA
Copyright © 2015 Sullivan, Zahr, Rohlfing and Pfefferbaum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edith V. Sullivan, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine (MC5723), 401 Quarry Road, Stanford, CA 94305-5723, USA edie@stanford.edu
†Torsten Rohlfing's current affiliation is Google Inc.
 Edith V. Sullivan
Edith V. Sullivan Natalie M. Zahr
Natalie M. Zahr Torsten Rohlfing
Torsten Rohlfing Adolf Pfefferbaum
Adolf Pfefferbaum