Self-esteem modulates automatic attentional responses to self-relevant stimuli: evidence from event-related brain potentials
- 1Cognition and Human Behavior Key Laboratory of Hunan Province and Department of Psychology, Hunan Normal University, Changsha, China
- 2Department of Foreign Languages, College of Mobile Telecommunications, Chongqing University of Posts and Telecommunications, Chongqing, China
Previous studies have widely shown that self-esteem modulates the attention bias towards social rejection or emotion-related information. However, little is known about the influences of self-esteem on attention bias towards self-relevant stimuli. We aimed to investigate neural correlates that underlie the modulation effect of self-esteem on self-relevant processing. Event-related potentials (ERP) were recorded for subjects’ own names and close others’ names (the names of their friends) while subjects performed a three-stimulus oddball task. The results showed larger P2 amplitudes for one’s own name than for close-other’s name in the low self-esteem group, whereas this P2 effect were not observed in the high self-esteem group. In addition, one’s own name elicited equivalent N250 amplitudes and larger P3 amplitudes compared with close-other’s name in both high and low self-esteem groups. However, no interaction effects were observed between self-esteem and self-relevant processing in the N250 and P3 components. Thus, we found that the modulation effects of self-esteem on self-relevant processing occurred at the early P2 stage, but not at the later N250 and P3 stages. These findings reflect that individuals with low self-esteem demonstrate automatic attention towards their own names.
Introduction
Self-esteem is a personality variable that refers to the degree to which one values and accepts himself or herself, and reflects one’s attitude or overall affective bias towards his or her own value (Rosenberg, 1965; Pruessner et al., 2005). Self-esteem has a pervasive and powerful effect on human cognition. Specifically, considerable studies have reported that self-esteem could modulate attentional bias for social rejection or emotional information. For example, a behavioral study has shown that people with low self-esteem exhibit significantly more interference in rejection words than in acceptance words during a rejection stroop task, whereas no such difference was observed in high self-esteem individuals. People with low self-esteem show an obvious attentional bias towards rejection-related information, which disturbed the process of naming the color of words (Dandeneau and Baldwin, 2004). During an attention shifting task, Li et al. (2012) examined the neurophysiological response to rejection cues in individuals with low and high self-esteem and found that P2 amplitudes elicited by social rejection were larger for individuals with low self-esteem than those with high self-esteem (Li et al., 2012). Using a visual-probe task, Li and Yang (2013) found that individuals with low self-esteem demonstrated attentional bias toward happy and angry faces indexed by enhanced P1 and N1 activity, whereas such attentional bias was not observed in high self-esteem individuals (Li and Yang, 2013).
As described above, social rejection or emotional information are preferentially attended by low self-esteem individuals compared with high self-esteem individuals. Individuals with low self-esteem frequently encounter social rejection or negative evaluation during their lives (Harter, 1983, 1993). Thus, they become more sensitive to rejection-related information and care excessively about the evaluation of others. This enhanced sensitivity to negative information in low self-esteem individuals may further strengthen their feelings of low self-worth. However, studies that investigate the relationship between self-esteem and attentional processing focus mostly on the attentional bias towards feedback from others or external stimuli, such as evaluation of others, social rejection, and external emotional events.
It is known that individuals with low self-esteem valued themselves quite differently from those with high self-esteem. For example, when individuals with low self-esteem experience failure, they would more likely blame themselves, negatively evaluate their abilities, and establish association between negative events and themselves. Thus, low self-esteem individuals may also demonstrate attentional bias towards self-relevant information. More recently, some studies have shown an interaction among self-esteem, self-relevance, and emotion valence (Zhang et al., 2013; Yang et al., 2014). For example, Zhang et al. (2013) found that individuals with low self-esteem demonstrated more pronounced P2 latencies in processing negative-low self-relevant words compared with positive-low self-relevant words, whereas such effect was not observed in individuals with high self-esteem (Zhang et al., 2013). Yang et al. (2014) also found a significantly negative correlation between self-esteem scores and P2 latencies in processing negative-high self-relevant words, which suggests that individuals with a lower level of self-esteem would divert more attentional resources to highly negative self-relevant words (Yang et al., 2014). However, these studies mainly focused on the emotional (positive vs. negative) aspect of self-processing (Zhang et al., 2013; Yang et al., 2014). The cognitive and emotional aspects of self-referential processing are two essential components of self-reflection (Moran et al., 2006). Thus, we focused on the cognitive (self-relevance vs. non-self-relevance) aspect of self-processing, using the event-related potential (ERP) technique to investigate whether self-esteem could modulate attentional bias towards self-relevant information.
Given that one’s own name captures very important personal significance in everyday life, the present study selected the names of the participants as the self-relevant stimuli, and the names of theirs friends as familiar but non-self-relevant stimuli. To set up an experimental situation similar to real-life settings, where the occurrence of one’s own name is often unexpected (e.g., detecting one’s own name at a cocktail party), we used a three-stimulus oddball task, in which participants were asked to detect a rare target; the experimental stimuli (participant’s own name, the name of the participant’s friend) were interspersed unexpectedly in the stream of standard and target trials as distractor stimuli (Polich, 2007). Research regarding self-relevant processing has widely reported that one’s own name can attract attentional resources more preferentially and automatically compared with other names. For example, early studies on the “cocktail party” phenomenon have shown that people could sometimes detect their own names even in the absence of attention, whereas other messages were not detected in this manner (Moray, 1959; Wood and Cowan, 1995).
ERP studies have shown that larger P2 amplitudes were elicited by the names owned by the individuals than by other names, which reflects the autonomic and fast recruitment of attentional resource towards self-names (Chen et al., 2011; Hu et al., 2011). Moreover, larger P3 amplitudes were also elicited by self-names than by other names, which shows the voluntary, top-down controlled attentional processing and the cognitive evaluation of self-relevant information (Berlad and Pratt, 1995; Perrin et al., 2005; Zhao et al., 2009; Tacikowski and Nowicka, 2010; Chen et al., 2011, 2013). Taken together, these ERP findings suggest that attention bias for one’s own name occurs at each step of the information processing stream, from early autonomic attention to voluntarily controlled attention at a later stage.
Thus, we further aimed to determine whether the modulating effect of self-esteem on self-name processing occurred at the early autonomic attention stage, or at the later stage of controlled attention, or both. Previous studies suggest that individuals with low self-esteem demonstrated greater attentional bias for social rejection, emotional stimuli, or self-relevant emotional stimuli compared with individuals with high self-esteem (Dandeneau and Baldwin, 2004; Li et al., 2012; Li and Yang, 2013; Zhang et al., 2013; Yang et al., 2014). Based on these studies, we hypothesized that individuals with low self-esteem would also demonstrate greater attentional bias for their own names at the early automatic stage of information processing indicated by enhanced P2 amplitudes, or at the late controlled stage indicated by enhanced P3 amplitudes or both.
Materials and Methods
Participants
A total of 252 undergraduate students were recruited to fill out the Rosenberg self-esteem (RSE) scale (Rosenberg, 1965). The RSE is a 4-point scale (1 = “strongly disagree” to 4 = “strongly agree”) with 10 items. The previous study found that the eighth item of the scale (“I wish I could respect myself more”) show a low correlation with other items for Chinese participants (Zhou and Wang, 2005). Thus, this item was excluded when computing the scores. The Cronbach’s α in the present study is 0.83. Based on their scores, individuals who scored in the upper 15th percentile of the distribution were categorized as the high self-esteem group, and who scored in the lower 15th percentile of the distribution were categorized as the low self-esteem group. From these groups, we invited 15 participants with low-esteem (five men, 10 women; 18–21 years, mean age = 19.33, SD = 0.82) and 15 participants with high-esteem (six men, nine women; 18–22 years, mean age = 19.6, SD = 1.06) to attend the electrophysiological study. The mean score for the low self-esteem group was 21.73 (SD = 1.44, from 18 to 25), and that for the high self-esteem group was 33.73 (SD = 1.58 from 30 to 36). All participants were healthy, right-handed, with normal or corrected-to-normal vision, and free from any neurological dysfunctions. The local review board for human participant research approved the experimental protocol. All participants joined the study with informed consents and compensation.
Stimuli
During the three-stimulus oddball task, we used a small circle (1.43° × 1.43°) as the target stimulus, and a big circle (2.29° × 2.29°) as the standard stimulus. The names of the participants and the names of their friends were used as distracters. The close others are participants’ friends, who are participants’ classmates studying in the same university as them. All participants and their paired friends were matched in age and gender, and knowing each other for about 2 years. The names were presented visually as two-character (1.15° × 2.29°) or three-character (1.15° × 3.44°) Chinese words, with the length also matched between these two name-categories.
Experimental Procedure
About 3 weeks after the self-esteem assessment, participants attended the ERP experiment. Participants conducted 25 practice trials before the formal experiment. During the formal experiment, the big circle was presented 420 times (approximately 70%), the small circle 60 times (approximately 10%), and each set of name stimuli 60 times (10%). Each trial was initiated using a 300-ms presentation of a small black cross on the gray computer screen. Afterwards, a gray screen was presented with a duration ranging from 500–800 ms. Subsequently, one of the four categories of stimuli was presented for 300 ms. The task of the participants was to detect the small circle and to press the J key on the keyboard with their right index finger if the small circle was presented. No response was required for other stimuli. Each experimental stimulus was followed by a gray screen for 1200 ms. There were totally six blocks, and the sequence of stimuli was randomized across conditions in each block. Subjects were allowed to rest for several minutes after each block.
Electroencephalography (EEG) Recordings
Electroencephalography (EEG) was recorded from 64 scalp sites using tin electrodes mounted in an elastic cap (Brain Products), with the references on the left and right mastoids (average mastoid reference; Luck, 2005) and a ground electrode on the medial frontal aspect. Horizontal electrooculograms (EOGs) were recorded at the right and left orbital rim. Vertical EOGs were recorded supra-orbitally and infra-orbitally at the left eye. Electrode impedance was maintained below 5 kΩ. EEG and EOG activity was amplified with a dc ~100 Hz bandpass and continuously sampled at 500 Hz/channel. EEG data, time-locked to the onset of the experimental stimuli, were epoched from −200 to 1000 ms, and baseline corrected using the prestimulus 200 ms time interval. ERP trials with EOG artifacts (mean EOG voltage exceeding ±80 V), or peak-to-peak deflection exceeding ±80 V was excluded from averaging. Artifact-free ERP trials were averaged separately for each experimental condition.
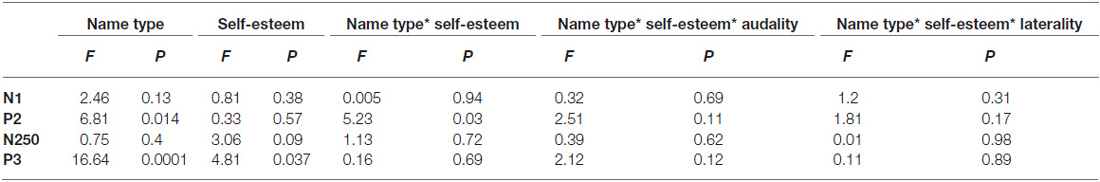
Because the previous studies have suggested a lateralization of visual self-recognition (Turk et al., 2002; Uddin et al., 2005; Ma and Han, 2010; Heinisch et al., 2011), we examined the caudality and laterality effects, by selecting the following 15 electrode sites for statistical analysis: F3, FC3, C3, CP3, P3 (five left sites); Fz, FCz, Cz, CPz, Pz (five midline sites); and F4, FC4, C4, CP4, P4 (five right sites). As shown in Figure 1, prominent N1 (80–120 ms), P2 (150–250 ms), N250 (260–330 ms) and P3 (350–500 ms) components were elicited during all three conditions. The peak amplitudes and latencies of the N1, P2, N250 and P3 components were measured and analyzed at their corresponding time intervals. A four-way repeated measures analysis of variance (ANOVA) was performed on all measured amplitudes and latencies for each component. ANOVA factors were the self-esteem group (two levels: low and high self-esteem groups), the name type (two levels: one’s own name and close-other’s name), laterality (three levels: the left, midline and right sites) and caudality (three levels for the N1, P2 and N250 components: frontal, frontocentral and central; five levels for the P3 component: frontal, frontocentral, central, centroparietal, and parietal). The ERP data were analyzed by the software of Brain Products Analyzer, and the statistical analysis was conducted by the SPSS 16.0. The degrees of freedom of the F-ratio were corrected according to the Greenhouse-Geisser method.
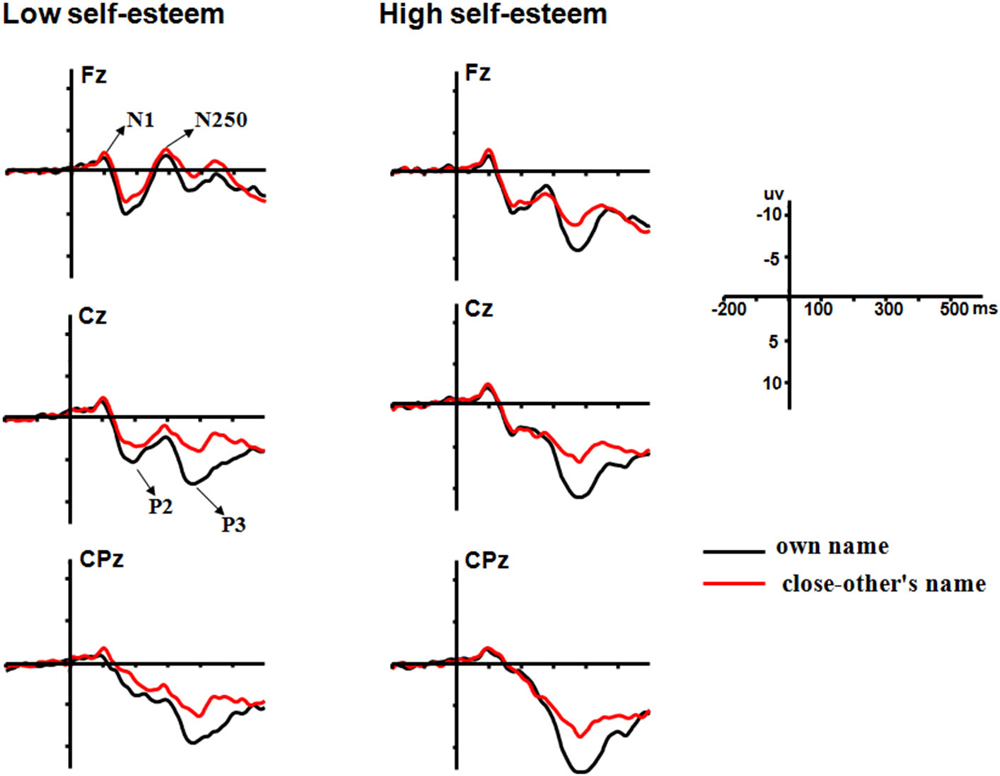
Figure 1. Averaged ERPs at Fz, Cz and CPz to one’s own (black lines) and friend’s (red lines) names for participants with low self-esteem (left panels) and high self-esteem (right panels).
Results
N1
The ANOVA for amplitudes of N1 showed that neither the main effect for name type (F(1,28) = 2.46, p = 0.13, = 0.08) nor its interaction with self-esteem group reached significance (F(1,28) = 0.005, p = 0.94, = 0.00; see Tables 1, 2). Furthermore, the main effect of laterality was significant (F(2,56) = 11.24, p < 0.001, = 0.29). Post hoc comparisons with bonferroni correction (the same afterwards) showed that the scalp midline regions (−2.8 μV) showed larger N1 amplitudes than the right lateralized (−2.18 μV, p < 0.001) and left lateralized (−2.42 μV, p = 0.05) regions. In addition, neither the main effect for name type nor its interaction with self-esteem group was significant on N1 latencies (ps > 0.1).
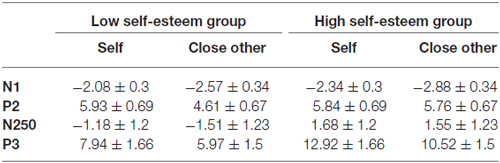
Table 1. The amplitudes of N1, P2, N250 and P3 components in the self and close other conditions during low and high self-esteem groups (μV, M ± SE).
P2
The ANOVA for amplitudes of P2 demonstrated significant main effects of name type (F(1,28) = 6.81, p = 0.014, = 0.2) and laterality (F(2,56) = 25.53, p < 0.0001, = 0.48; see Figure 1). One’s own name (5.89 μV) elicited larger P2 amplitudes than close-other’s name (5.18 μV). Post hoc comparisons showed that the scalp midline regions (6.56 μV) showed larger P2 amplitudes than the right lateralized (5.18 μV, p < 0.0001) and left lateralized (4.86 μV, p < 0.0001) regions. Moreover, the interaction between name type and self-esteem group was significant (F(1,28) = 5.23, p = 0.03, = 0.16). One’s own name elicited larger P2 amplitudes than close-other’s name (F(1,28) = 13.68, p = 0.002, = 0.49) in the low self-esteem group, whereas no significant difference was observed between one’s own name and close-other’s name (F(1,28) = 0.05, p = 0.83, = 0.003) in the high self-esteem group (see Table 2; Figure 2). These results indicated that self-esteem modulated the self-relevant effect at the early P2 stage. In addition, neither the main effect for name type nor its interaction with self-esteem group was significant on P2 latencies (ps > 0.05).
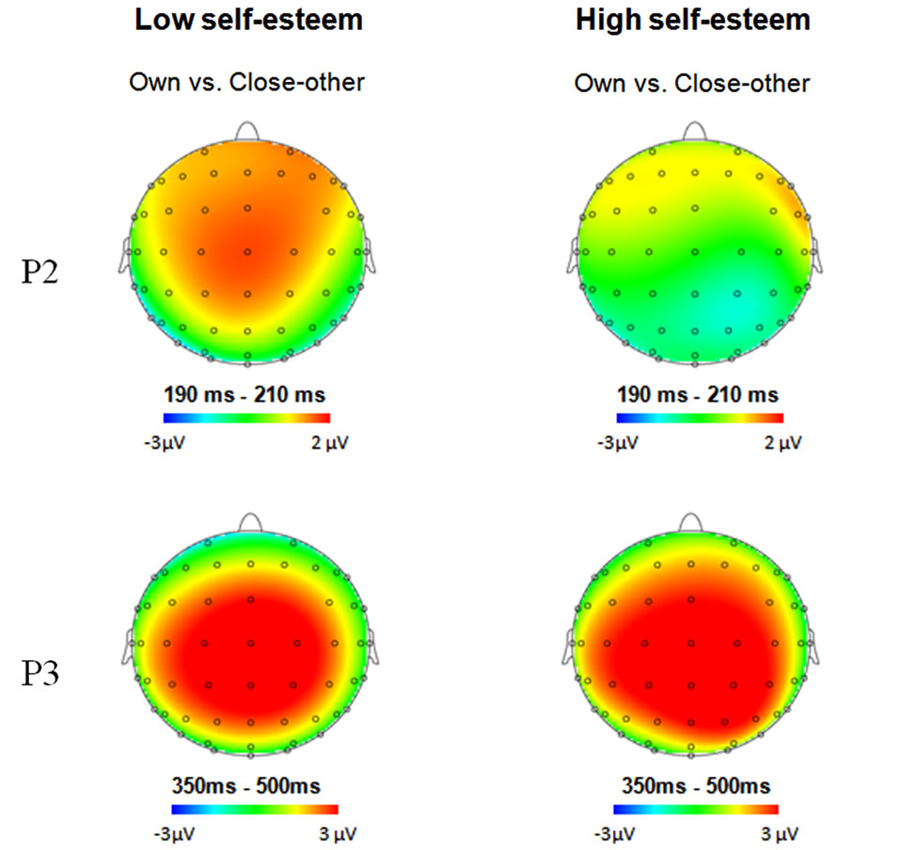
Figure 2. Topographical maps of voltage amplitudes for one’s own name minus close-other’s name difference ERPs at the P2 and P3 components for participants with low self-esteem (left panels) and high self-esteem (right panels).
N250
The ANOVA for N250 amplitudes showed that neither the main effect for name type (F(1,28) = 0.75, p = 0.4, = 0.03) nor its interaction with the self-esteem group was significant (F(1,28) = 0.13, p = 0.72, = 0.005). Furthermore, the main effect of frontality was significant (F(2,56) = 65.43, p < 0.0001, = 0.7). The scalp frontal regions (−1.48 μV) showed more negative N250 waves than the frontal-central (0.34 μV, p < 0.001) and central (1.55 μV, p < 0.001) regions. In addition, neither the main effect for name type nor its interaction with self-esteem group was significant on N250 latencies (ps > 0.05).
P3
The ANOVA for amplitudes of P3 demonstrated the significant main effects of name type (F(1,28) = 16.64, p < 0.0001, = 0.37) and frontality (F(4,112) = 10.71, p = 0.001, = 0.28; see Figure 1). The names of the subjects (10.43 μV) elicited larger P3 amplitudes than the names of their friends (8.24 μV). Post hoc tests showed that the P3 amplitudes were largest at the central-parietal (11.11 μV) regions and smallest at the frontal regions (6.83 μV). In addition, the main effect of name type was also significant on P3 latencies (F(1,28) = 4.25, p = 0.049, = 0.13), with shorter P3 latencies for one’s own name (402.58 ms) than for the close-other’s name (414.82 ms). However, the interaction between self-esteem group and name type was not significant for P3 amplitudes (F(1,28) = 0.16, p = 0.69, = 0.006; Figure 2) and latencies (F(1,28) = 0.000, p = 0.98, = 0.000).
Additional Analyses
To further test whether the P2 component was a valid index for the effect of self-esteem on self-relevant processing, we ran the correlation analysis between the self-esteem scores and the differential P2 or P3 amplitudes to the one’s own and close-other’s names (P2 or P3 amplitudes to one’s own name minus P2 or P3 amplitudes to close-other’s name). The results showed a significant negative correlation between the self-esteem scores and the differential P2 amplitudes to one’s own and close-other’s names (r = −0.41, p = 0.023, df = 28). However, we failed to observe significant correlation between the self-esteem scores and the differential P3 amplitudes to one’s own and close-other’s names (r = 0.12, p = 0.54, df = 28).
Moreover, the P2 and P3 amplitudes were entered into a three-way repeated-measures ANOVAs with the Name type (one’s own name and close-other’s name) and Component (P2, P3) as within-subjects factors, and the self-esteem score as the continuous between-subjects variable.The results showed a significant three-way interaction effect among name type, component and self-esteem score (F(1,28) = 4.89, p = 0.035, = 0.15). The subsequent analysis showed a significant interaction between name type and self-esteem score for P2 component (F(1,28) = 5.79, p = 0.023, = 0.17). However, this two-way interaction was not significant for the P3 component (F(1,28) = 0.4, p = 0.54, = 0.01).
Thus, these results taken together indicated that the P2 component was sensitive to the modulation effect of self-esteem on self-relevant processing rather than the P3 component.
Discussion
The present study examines the modulating effect of self-esteem on self-name processing using ERP measures and further determines whether the modulating effect occurs at the early autonomic attention stage, later stage of controlled attention, or both. The findings showed that individuals with low self-esteem demonstrated larger P2 amplitudes in response to their own names compared with the names of their friends. No such P2 difference was observed in individuals with high self-esteem. However, no self-esteem by name type interaction effect was observed for P3 amplitudes, although individuals with both low and high self-esteem demonstrated larger P3 amplitudes in response to their own names than to the names of their friends. These findings showed that the modulating effect of self-esteem on self-name processing occurred at the early P2 stage, but not on the later P3 stage.
The N1 component represents the early visual processing of stimuli (Luck and Hillyard, 2000; Vogel and Luck, 2000). In the present study, no different N1 amplitudes and latencies were observed between one’s own and close-other names in individuals with low and high self-esteem perhaps because the name stimuli are in Chinese characters, which are equal in size, word length, and complexity. Thus, the early visual processing was similar during these name conditions (Chen et al., 2011, 2013).
The P2 component is considered a neural index of automatic attention responses to highly salient stimuli and larger P2 amplitudes reflect enhanced recruitment of attentional resources (Karayanidis and Michie, 1996; Carretié et al., 2001, 2004, 2011; Meixner and Rosenfeld, 2010). Previous ERP studies have also found that individuals with low self-esteem tend to easily direct their attention to emotional stimuli, such as social rejection and negatively self-relevant stimuli, and demonstrated enhanced P2 amplitudes or prolonged P2 latencies for processing these emotion-related stimuli (Li et al., 2012; Yang et al., 2012, 2014; Li and Yang, 2013; Zhang et al., 2013). Consistent with these studies, the present study found that individuals with low self-esteem demonstrated larger P2 amplitudes in response to their own names than to close others’ names, but individuals with high self-esteem did not show such effect. The occurrence of our own name in everyday life may indicate that some significant events (such as criticism, praise, or a warning) will happen to us (Tacikowski et al., 2014). Thus, our findings showed that low self-esteem individuals demonstrated greater mobilization of attentional resources toward their own names, which may be attributed to the important significance conveyed by the occurrence of their own names.
In addition, some inconsistences existed in previous studies regarding whether the early P2 component could be modulated by self-relevance. Some studies showed that self-relevant stimuli (e.g., the name of the subject) elicited larger P2 amplitudes than self-irrelevant stimuli (e.g., the names of their father, famous people, or strangers) (Chen et al., 2011, 2013; Hu et al., 2011; Fan et al., 2013). However, Tacikowski et al. (2014) found that no significant differences were present on P2 amplitudes between participants’ own names and the names of their friends (Tacikowski et al., 2014). Su et al. (2010) also found that the hand of the participant and the hand of a stranger elicited equivalent P2 amplitudes and latencies (Su et al., 2010). The inconsistent results observed in these studies may be attributed to the different experimental stimuli and paradigm used; the effects of stimulus familiarity and task-relevance should be considered in future studies regarding self-processing (Su et al., 2010; Tacikowski et al., 2014). We observed that individuals with low self-esteem demonstrated larger P2 amplitudes for their own names than for close others’ names, whereas no significant difference was observed in individuals with high self-esteem. Thus, self-esteem as a personality variable should also be considered when investigating the self-relevant effect.
Previous studies suggested that N250 appears at latencies of approximately 200–300 ms; this value varied with familiarity and was larger in familiar faces and names (Schweinberger et al., 1995; Herzmann et al., 2004; Zhao et al., 2011). In the present study, one’s own and close-other’s names elicited equivalent N250 amplitudes and latencies in high and low self-esteem groups. This finding may suggest that the familiarity is similar for both types of names.
Moreover, we found that individuals with high and low self-esteem demonstrated larger P3 amplitudes for their own names than for close others’ names. It has been widely reported that the P3 component could be modulated by one’s own name (Gray et al., 2004; Zhao et al., 2009; Tacikowski and Nowicka, 2010; Chen et al., 2011, 2013; Tacikowski et al., 2011; Fan et al., 2013). P3, a positive component that appears approximately 300 ms after the stimulus onset, was related to multiple cognitive functions, including top-down controlled attentional processes, cognitive evaluation, and the updating of representations in working memory (Donchin, 1981; Donchin and Coles, 1988; Polich, 2007). Thus, our findings regarding lager P3 amplitudes for one’s own name could be explained by the fact that one’s own name garners a larger amount of attentional and cognitive resources and evokes enhanced motivational responses than close-other’s name. However, the size of self-relevant effect, which is indicated by the amplitude difference between one’s own and close-other names, was equivalent for high and low self-esteem individuals. Thus, the modulation effect of self-esteem on self-relevant processing did not occur at the late P3 stage.
It should be noted that the present study only measured the explicit self-esteem, and failed to take into account the factor of implicit self-esteem. Considerable studies have used various methodologies such as the implicit association test (IAT), Go/Nogo association (GNAT) and name letter evaluations to explore the implicit self-esteem (Greenwald and Banaji, 1995; Greenwald and Farnham, 2000; Greenwald et al., 2002; Wu et al., 2014; Grundy et al., 2015). Moreover, the implicit and explicit self-esteem have been considered to be different constructs, and they might have different influences on cognitive processing or social behavior (Greenwald and Banaji, 1995; Bosson et al., 2000; Greenwald and Farnham, 2000). Thus, it is necessary for future studies to determine the influences of implicit self-esteem on self-relevant processing.
Moreover, it has been suggested that self-esteem is a self-conception with the characteristics of culture, and should be related to culture-related variables such as self-construal (Markus and Kitayama, 1991a). Specifically, both theoretical and empirical accounts revealed that East Asians with a dominant interdependent self-construal tended to show weaker self-enhancement or lower self-esteem than Westerners with a dominant independent self-construal (Markus and Kitayama, 1991b; Feather and McKee, 1993; Heine et al., 1999; Singelis et al., 1999; Heine and Renshaw, 2002). Previous studies investigating the influences of self-esteem on cognitive processing usually didn’t take account of the factor of self-construal (Dandeneau and Baldwin, 2004; Li et al., 2012; Li and Yang, 2013; Zhang et al., 2013; Yang et al., 2014). Moreover, our correlation analysis showed that the self-relevant effect, indicated by the P2 difference between self-relevant and non-self-relevant conditions, was significantly correlated with the level of self-esteem. However, it would be interesting and necessary to introduce the culture-related variables such as self-construal into future studies of self-esteem, especially explore the effects of self-esteem on cognition within a cultural neuroscience framework (Han et al., 2013).
Taken together, in addition to the modulation effect of self-esteem on attention bias towards social rejection cues, emotional events, and emotional aspect of self-relevant stimuli reported in the previous studies, the present study further showed that self-esteem could modulate attention bias towards one’s own name. This modulation effect occurred at the early automatic attention stage as indexed by the P2 component, but not at the late stage of top-down controlled processing as indexed by the P3 component. These findings may suggest that self-esteem can affect our self-perception in an implicit or unconscious manner. Future studies should adopt other self-relevant stimuli and experimental tasks to investigate the modulation effect of self-esteem on self-processing, particularly using high-spatial-resolution fMRI to unravel neural substrates that mediate this modulation effect, and how these neural activities relate to their psychological well-being. It still should be noted that the factor of intimate relationship should be considered and is necessary to make a quantified measurement of intimate relationship when investigating the cognitive and neural representations of self and close others (Ma and Han, 2010, 2012; Wang et al., 2012; Sui et al., 2013).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (NSFC31300866), the Specialized Research Fund for the Doctoral Program of Higher Education (20132136120002) and the Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning (CNLYB1318).
References
Berlad, I., and Pratt, H. (1995). P300 in response to the subject’s own name. Electroencephalogr. Clin. Neurophysiol. 96, 472–474. doi: 10.1016/0168-5597(95)00116-a
Bosson, J. K., Swann, W. B., and Pennebaker, J. W. (2000). Stalking the perfect measure of implicit self-esteem: the blind men and the elephant revisited? J. Pers. Soc. Psychol. 79, 631–643. doi: 10.1037/0022-3514.79.4.631
Carretié, L., Hinojosa, J., Martín-Loeches, M., Mercado, F., and Tapia, M. (2004). Automatic attention to emotional stimuli: neural correlates. Hum. Brain Mapp. 22, 290–299. doi: 10.1002/hbm.20037
Carretié, L., Mercado, F., Tapia, M., and Hinojosa, J. A. (2001). Emotion, attention and the ‘negativity bias’, studied through event-related potential. Int. J. Psychophysiol. 41, 75–85. doi: 10.1016/s0167-8760(00)00195-1
Carretié, L., Ruiz-Padial, E., López-Martín, S., and Albert, J. (2011). Decomposing unpleasantness: differential exogenous attention to disgusting and fearful stimuli. Biol. Psychol. 86, 247–253. doi: 10.1016/j.biopsycho.2010.12.005
Chen, J., Yuan, J. J., Feng, T. Y., Chen, A. T., Gu, B. B., and Li, H. (2011). Temporal features of the degree effect in self relevance: neural correlates. Biol. Psychol. 87, 290–295. doi: 10.1016/j.biopsycho.2011.03.012
Chen, J., Zhang, Y. X., Zhong, J., Hu, L., and Li, H. (2013). The primacy of the individual versus the collective self: evidence from an event-related potential study. Neurosci. Lett. 535, 30–34. doi: 10.1016/j.neulet.2012.11.061
Dandeneau, S. D., and Baldwin, M. W. (2004). The inhibition of socially rejecting information among people with high versus low self-esteem: the role of attentional bias and the effects of bias reduction training. J. Soc. Clin. Psychol. 23, 584–602. doi: 10.1521/jscp.23.4.584.40306
Donchin, E. (1981). Surprise!…Surprise? Psychophysiology 18, 493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x
Donchin, E., and Coles, M. G. H. (1988). Is the P300 component a manifestation of context updating? Behav. Brain Sci. 11, 357–374. doi: 10.1017/s0140525x00058027
Fan, W., Chen, J., Wang, X.-Y., Cai, R., Tan, Q., Chen, Y., et al. (2013). Electrophysiological correlation of the degree of self-reference effect. PLoS One 8:e80289. doi: 10.1371/journal.pone.0080289
Feather, N. T., and McKee, I. R. (1993). Global self-esteem and attitudes toward the high achiever for Australian and Japanese students. Soc. Psychol. Q. 56, 65–76. doi: 10.2307/2786646
Gray, H. M., Ambady, N., Lowenthal, W. T., and Deldin, P. (2004). P300 as an index of attention to self-relevant stimuli. J. Exp. Soc. Psychol. 40, 216–224. doi: 10.1016/s0022-1031(03)00092-1
Greenwald, A. G., and Banaji, M. R. (1995). Implicit social cognition: attitudes, self-esteem and stereotypes. Psychol. Rev. 102, 4–27. doi: 10.1037/0033-295x.102.1.4
Greenwald, A. G., Banaji, M. R., Rudman, L. A., Farnham, S. D., Nosek, B. A., and Mellott, D. S. (2002). A unified theory of implicit attitudes, stereotypes, self-esteem and self-concept. Psychol. Rev. 109, 3–25. doi: 10.1037/0033-295x.109.1.3
Greenwald, A. G., and Farnham, S. D. (2000). Using the implicit association test to measure self-esteem and selfconcept. J. Pers. Soc. Psychol. 79, 1022–1038. doi: 10.1037/0022-3514.79.6.1022
Grundy, J. G., Benarroch, M. F., Lebarr, A. N., and Shedden, J. M. (2015). Electrophysiological correlates of implicit valenced self-processing in high vs. low self-esteem individuals. Soc. Neurosci. 10, 100–112. doi: 10.1080/17470919.2014.965339
Han, S., Northoff, G., Vogeley, K., Wexler, B. E., Kitayama, S., and Varnum, M. E. W. (2013). A cultural neuroscience approach to the biosocial nature of the human brain. Annu. Rev. Psychol. 64, 335–359. doi: 10.1146/annurev-psych-071112-054629
Harter, S. (1993). “Causes and consequences of low self-esteem in children and adolescents,” in Self-Esteem: The Puzzle of Low Self-Regard, ed. R. F. Baumeister (New York: Plenum Press), 87–116.
Heine, S. J., Lehman, D. R., Markus, H. R., and Kitayama, S. (1999). Is there a universal need for positive self-regard? Psychol. Rev. 106, 766–794. doi: 10.1037/0033-295x.106.4.766
Heine, S. J., and Renshaw, K. (2002). Interjudge agreement, self-enhancement and liking: cross-cultural divergences. Pers. Soc. Psychol. Bull. 28, 578–587. doi: 10.1177/0146167202288002
Heinisch, C., Dinse, H. R., Tegenthoff, M., Juckel, G., and Brüne, M. (2011). An rTMS study into self-face recognition using video-morphing technique. Soc. Cogn. Affect. Neurosci. 6, 442–449. doi: 10.1093/scan/nsq062
Herzmann, G., Schweinberger, S. R., Sommer, W., and Jentzsch, I. (2004). What’s special about personally familiar faces? A multimodal approach. Psychophysiology 41, 688–701. doi: 10.1111/j.1469-8986.2004.00196.x
Hu, X., Wu, H., and Fu, G. (2011). Temporal course of executive control when lying about self- and other-referential information: an ERP study. Brain Res. 1369, 149–157. doi: 10.1016/j.brainres.2010.10.106
Karayanidis, F., and Michie, P. T. (1996). Frontal processing negativity in a visual selective attention task. Electroencephalogr. Clin. Neurophysiol. 99, 38–56. doi: 10.1016/0921-884x(96)95116-4
Li, H., and Yang, J. (2013). Low self-esteem elicits greater mobilization of attentional resources toward emotional stimuli. Neurosci. Lett. 548, 286–290. doi: 10.1016/j.neulet.2013.05.071
Li, H., Zeigler-Hill, V., Luo, J., Yang, J., and Zhang, Q. (2012). Self-esteem modulates attentional responses to rejection: evidence from event-related brain potentials. J. Res. Pers. 46, 459–464. doi: 10.1016/j.jrp.2012.02.010
Luck, S. J. (2005). “An introduction to event-related potentials and their neural origins,” in An Introduction to the Event-Related Potential Technique, ed. S. J. Luch (Cambridge, MA: MIT), 22–107.
Luck, S. J., and Hillyard, S. A. (2000). “The operation of selective attention at multiple stages of processing: evidence from human and monkey electrophysiology,” in The New Cognitive Neurosciences, ed. M. S. Gazzaniga (Cambridge MA: MIT Press), 687–700.
Ma, Y., and Han, S. (2010). Why we respond faster to the self than to others? An implicit positive association theory of self-advantage during implicit face recognition. J. Exp. Psychol. Hum. Percept. Perform. 36, 619–633. doi: 10.1037/a0015797
Ma, Y., and Han, S. (2012). Is the self always better than a friend? Self-face recognition in Christians and atheists. PLoS One 7:e37824. doi: 10.1371/journal.pone.0037824
Markus, H. R., and Kitayama, S. (1991a). Culture and the self: implications for cognition, emotion and motivation. Psychol. Rev. 98, 224–253. doi: 10.1037/0033-295x.98.2.224
Markus, H. R., and Kitayama, S. (1991b). “Cultural variation in the self-concept,” in Multidisciplinary Perspectives on the Self, eds G. R. Goethals and J. Strauss (New York/Berlin: Springer-Verlag), 18–48.
Meixner, J. B., and Rosenfeld, J. P. (2010). Countermeasure mechanisms in a P300-based concealed information test. Psychophysiology 47, 57–65. doi: 10.1111/j.1469-8986.2009.00883.x
Moran, J. M., Macrae, C. N., Heatherton, T. F., Wyland, C. L., and Kelley, W. M. (2006). Neuroanatomical evidence for distinct cognitive and affective components of self. J. Cogn. Neurosci. 18, 1586–1594. doi: 10.1162/jocn.2006.18.9.1586
Moray, N. (1959). Attention in dichotic listening: affective cues and the influence of instruction. Q. J. Exp. Psychol. 11, 56–60. doi: 10.1080/17470215908416289
Perrin, F., Maquet, P., Peigneux, P., Ruby, P., Degueldre, C., Balteau, E., et al. (2005). Neural mechanism involved in the detection of our first name: a combined ERPs and PET study. Neuropsychologia 43, 12–19. doi: 10.1016/j.neuropsychologia.2004.07.002
Polich, J. (2007). Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 118, 2128–2148. doi: 10.1016/j.clinph.2007.04.019
Pruessner, J. C., Baldwin, M., Dedovic, K., Renwick, R., Mahani, N., Lord, C., et al. (2005). Self-esteem, locus of control, hippocampal volume and cortisol regulation in young and old adulthood. Neuroimage 28, 815–826. doi: 10.1016/j.neuroimage.2005.06.014
Rosenberg, M. (1965). Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press.
Schweinberger, S. R., Pfütze, E. M., and Sommer, W. (1995). Repetition priming and associative priming of face recognition: evidence from event-related potentials. J. Exp. Psychol. Learn. Mem. Cogn. 21, 722–736. doi: 10.1037/0278-7393.21.3.722
Singelis, T. M., Bond, M. H., Sharkey, W. F., and Lai, C. S. Y. (1999). Unpackaging culture’s influence on self-esteem and embarrassability: the role of self-construals. J. Cross. Cult. Psychol. 30, 315–341. doi: 10.1177/0022022199030003003
Su, Y. H., Chen, A., Yin, H., Qiu, J., Lv, J., Wei, D. T., et al. (2010). Spatiotemporal cortical activation underlying self-referencial processing evoked by self-hand. Biol. Psychol. 85, 219–225. doi: 10.1016/j.biopsycho.2010.07.004
Sui, J., Hong, Y., Liu, C. H., Humphreys, G. W., and Han, S. (2013). Dynamic cultural modulation of neural responses to one’s own and friend’s faces. Soc. Cogn. Affect. Neurosci. 8, 326–332. doi: 10.1093/scan/nss001
Tacikowski, P., Brechmann, A., Marchewka, A., Jednoróg, K., Dobrowolny, M., and Nowicka, A. (2011). Is it about the self or the significance? An fMRI study of self-name recognition. Soc. Neurosci. 6, 98–107. doi: 10.1080/17470919.2010.490665
Tacikowski, P., Cygan, H. B., and Nowicka, A. (2014). Neural correlates of own and close-other’s name recognition: ERP evidence. Front. Hum. Neurosci. 8:194. doi: 10.3389/fnhum.2014.00194
Tacikowski, P., and Nowicka, A. (2010). Allocation of attention to self-name and self-face: an ERP study. Biol. Psychol. 84, 318–324. doi: 10.1016/j.biopsycho.2010.03.009
Turk, D. J., Heartherton, T. F., Kelley, W. M., Funnell, M. G., Gazzaniga, M. S., and Macrae, C. N. (2002). Mike or me? Self-recognition in a split-brain patient. Nat. Neurosci. 5, 841–842. doi: 10.1038/nn907
Uddin, L. Q., Rayman, J., and Zaidel, E. (2005). Split-brain reveals separate but equal selfrecognition in the two cerebral hemispheres. Conscious. Cogn. 14, 633–640. doi: 10.1016/j.concog.2005.01.008
Vogel, E. K., and Luck, S. J. (2000). The visual N1 component as an index of a discrimination process. Psychophysiology 37, 190–203. doi: 10.1111/1469-8986.3720190
Wang, G., Mao, L., Ma, Y., Yang, X., Cao, J., Liu, X., et al. (2012). Neural representations of close others in collectivistic brains. Soc. Cogn. Affect. Neurosci. 7, 222–229. doi: 10.1093/scan/nsr002
Wood, N., and Cowan, N. (1995). The cocktail party phenomenon revisited: how frequent are attention shifts to one’s name in an irrelevant auditory channel? J. Exp. Psychol. Learn. Mem. Cogn. 21, 255–260. doi: 10.1037/0278-7393.21.1.255
Wu, L., Cai, H., Gu, R., Luo, Y. L., Zhang, J., Yang, J., et al. (2014). Neural manifestations of implicit self-esteem: an ERP study. PLoS One 9:e101837. doi: 10.1371/journal.pone.0101837
Yang, J., Dedovic, K., Chen, W., and Zhang, Q. (2012). Self-esteem modulates dorsal anterior cingulated cortical response in self-referential processing. Neuropsychologia 50, 1267–1270. doi: 10.1016/j.neuropsychologia.2012.02.010
Yang, J., Qi, M. M., and Guan, L. L. (2014). Self-estee modulates the latency of P2 component in implicit self-relevant processing. Biol. Psychol. 97, 22–26. doi: 10.1016/j.biopsycho.2014.01.004
Zhang, H., Guan, L. L., Qi, M. M., and Yang, J. (2013). Self-esteem modulates the time course of self-positivity bias in explicit self-evaluation. PLoS One 8:e81169. doi: 10.1371/journal.pone.0081169
Zhao, K., Wu, Q., Zimmer, H. D., and Fu, X. (2011). Electrophysiological correlates of visually processing subject’s own name. Neurosci. Lett. 491, 143–147. doi: 10.1016/j.neulet.2011.01.025
Zhao, K., Yuan, J. J., Zhong, Y. P., Peng, Y. S., Chen, J., Zhou, L. P., et al. (2009). Event-related potential correlates of the collective self-relevant effect. Neurosci. Lett. 464, 57–61. doi: 10.1016/j.neulet.2009.07.017
Keywords: low self-esteem, high self-esteem, subject’s own name, ERP, P2, P3
Citation: Chen J, Shui Q and Zhong Y (2015) Self-esteem modulates automatic attentional responses to self-relevant stimuli: evidence from event-related brain potentials. Front. Hum. Neurosci. 9:376. doi: 10.3389/fnhum.2015.00376
Received: 18 December 2014; Accepted: 15 June 2015;
Published: 29 June 2015.
Edited by:
Hauke R. Heekeren, Freie Universität Berlin, GermanyReviewed by:
Peter Beim Graben, Humboldt-Universität zu Berlin, GermanyChristoph W. Korn, University of Zurich, Switzerland
Copyright © 2015 Chen, Shui and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Chen and Yiping Zhong, Cognition and Human Behavior Key Laboratory of Hunan Province and Department of Psychology, Hunan Normal University, 36 Lushan Road, Changsha 410081, China, xlxchen@163.com;
ypzhong@163.com
 Jie Chen
Jie Chen Qing Shui2
Qing Shui2