Studying Autism Spectrum Disorder with Structural and Diffusion Magnetic Resonance Imaging: A Survey
- 1BioImaging Laboratory, Department of Bioengineering, University of Louisville, Louisville, KY, USA
- 2Departments of Pediatrics and Biomedical Sciences, University of South Carolina, Columbia, SC, USA
- 3Department of Computer Science, University of Auckland, Auckland, New Zealand
Magnetic resonance imaging (MRI) modalities have emerged as powerful means that facilitate non-invasive clinical diagnostics of various diseases and abnormalities since their inception in the 1980s. Multiple MRI modalities, such as different types of the sMRI and DTI, have been employed to investigate facets of ASD in order to better understand this complex syndrome. This paper reviews recent applications of structural magnetic resonance imaging (sMRI) and diffusion tensor imaging (DTI), to study autism spectrum disorder (ASD). Main reported findings are sometimes contradictory due to different age ranges, hardware protocols, population types, numbers of participants, and image analysis parameters. The primary anatomical structures, such as amygdalae, cerebrum, and cerebellum, associated with clinical-pathological correlates of ASD are highlighted through successive life stages, from infancy to adulthood. This survey demonstrates the absence of consistent pathology in the brains of autistic children and lack of research investigations in patients under 2 years of age in the literature. The known publications also emphasize advances in data acquisition and analysis, as well as significance of multimodal approaches that combine resting-state, task-evoked, and sMRI measures. Initial results obtained with the sMRI and DTI show good promise toward the early and non-invasive ASD diagnostics.
1. Introduction
The term “autism spectrum disorder” (ASD) refers to a collection of neuro-developmental disorders that affect linguistic, behavioral, and social skills. Autism has many symptoms, most prominently, social impairment and repetitive behaviors. The most severe form of the ASDs is autistic disorder (AD), while milder forms include Asperger syndrome (ASP), childhood disintegrative disorder, and not-otherwise-specified pervasive developmental disorder (NPDD). By some estimates, the ASD affects 1 out of 68 eight years of age, with males being four times more likely to develop it than females. About 30% of children with ASD have epilepsy at later stages NIMH (2015). Autism is typically diagnosed at the age of 3; however, some characteristics can sometimes be observed at around 12 months of age. What causes autism is yet unknown; however, it is mainly believed that genetic and environmental factors in complex combinations are responsible NIMH (2015).
After the advent in the nineteen eighties, MRI soon became one of the most promising non-invasive modalities for visualization and diagnostics of ASD-related abnormalities. Along with its main advantage of no exposure to radiation, high contrast and spatial resolution, the recent advances to MRI modalities have notably increased diagnostic certainty. The modalities, being most helpful for studying ASD, include structural MRI (sMRI; Damasio and Maurer, 1978; Bauman and Kemper, 1985; Gaffney et al., 1987a,b; Courchesne et al., 1988; Gaffney et al., 1988; Horwitz et al., 1988; Minshew and Payton, 1988; Ritvo and Garber, 1988; Gaffney et al., 1989; Garber et al., 1989; Murakami et al., 1989), and diffusion tensor imaging (DTI; Gropman et al., 2010; Mori and Tournier, 2013).
In applications to ASD, sMRI helps in investigating structural brain changes in autistic subjects. Many scan sequences of the sMRI are volumetric, i.e., allow for measuring specific brain structures to calculate tissue volumes. In spite of diagnostic abilities found for the sMRI, the earlier published results obtained for a limited number of subjects were often contradictory. Specific pulse sequences employed in the sMRI help to reveal different properties of normal and abnormal brain tissues. Modifying the pulse sequence parameters, such as repetition time (TR) and echo time (TE), may emphasize the contrast between gray matter (GM) and white matter (WM), e.g., in the T1-weighted sMRI with short TR and TE, or brain tissue and cerebrospinal fluid (CSF), e.g., in the T2-weighted sMRI with long TR and TE.
The recent DTI characterizes three-dimensional (3D) diffusion of water molecules in a biological tissue Basser et al. (1994a,b). The DTI has a wide range of clinical applications. In particular, it is used to examine normative white matter (WM) development, neurodevelopmental disorders, and neurodegenerative disorders, e.g., autism, and amyotrophic lateral sclerosis Dong et al. (2004).
In neurological studies, where each patient's status can be assessed, the chosen imaging modality should be able to clearly demonstrate the abnormalities. The conventional MRI lacks sensitivity in distinguishing the abnormalities on an individual-subject basis Mori and Tournier (2013), whereas the DTI has the potential to reveal such abnormalities. This new information comes from better image contrast, more detailed WM morphology, refined anatomical locations, and more accurate connectivity analysis Mori and Tournier (2013). Additional DTI offers many contrast-related measurements, including the widely used fractional anisotropy (FA) and estimates of shapes and sizes of specific WM tracts, i.e., WM morphology Mori and Tournier (2013). Moreover, DTI provides superior anatomical information for clearer identification of areas with WM abnormalities Gropman et al. (2010). It also provides unique brain connectivity measurements via 3D fiber reconstruction, e.g., tractography, Basser et al. (2000).
This survey presents applications of the sMRI and DTI to study the ASD (mostly, for the last two decades) in order to outline the most important findings with these modalities across all life stages. This provides a comprehensive study unlike the recent surveys that either focus on one modality, Palmen and van Engeland (2004); Blackmon (2015); Conti et al. (2015); Rane et al. (2015), or address a specific life stage, Conti et al. (2015); Zeglam et al. (2015). Moreover, this survey highlights the methodologies conducted in each study and categorizes them with respect to the approach (e.g., whether they are volumetric-based, or surface-based in sMRI), and user's intervention (manual, automated, semi-automated).
The survey is structured as follows: Sections 2, 3 below address the use of the sMRI and DTI, respectively. Findings in the reviewed publications emphasize benefits for studies of the ASD, as well as other medical abnormalities, by acquiring and analyzing complementary multi-modality data.
2. Studying ASD with sMRI
The earlier findings of the 1980s–1990s Damasio and Maurer (1978); Bauman and Kemper (1985); Gaffney et al. (1987a,b); Courchesne et al. (1988); Gaffney et al. (1988); Horwitz et al. (1988); Minshew and Payton (1988); Ritvo and Garber (1988); Gaffney et al. (1989); Garber et al. (1989); Murakami et al. (1989); Nowell et al. (1990); Hsu et al. (1991); Zola-Morgan et al. (1991); Garber and Ritvo (1992); George et al. (1992); Hashimoto et al. (1992, 1993); Holttum et al. (1992); Kleiman et al. (1992); Piven et al. (1992); Bailey et al. (1993); Courchesne et al. (1993, 1994a,b); Adolphs et al. (1994); Bachevalier (1994); Bauman and Kemper (1994); Minshew and Dombrowski (1994); Egaas et al. (1995); Hashimoto et al. (1995); Piven et al. (1995); Saitoh et al. (1995); Zilbovicius et al. (1995); Schaefer et al. (1996); Giedd et al. (1996); Piven et al. (1996); Siegel et al. (1996); Ciesielski et al. (1997); Elia et al. (1997); Lainhart et al. (1997); Piven et al. (1997); Bailey et al. (1998); Dawson et al. (1998); Piven et al. (1998); Abell et al. (1999); Aylward et al. (1999); Courchesne et al. (1999); Fombonne et al. (1999); Gillberg (1999); Levitt et al. (1999); Manes et al. (1999); Sears et al. (1999); Townsend et al. (1999) have many contradictions. However, the much better spatial resolution and contrast of the recent advanced MRI technology made the recent findings of the 2000s–2010s more consistent Brambilla et al. (2003); Stanfield et al. (2008); Chen et al. (2011). This survey focuses on the latter studies and attempts to classify the reported abnormalities at different life stages for each of the autism-related anatomical structures.
Cross-sectional or longitudinal sMRI scans, collected at different time instants for the same individual, are studied by either region-of-interest (ROI) based volumetry, or surface-based morphometry (abbreviated as RBV, and SBM respectively). The RBV usually focuses on the total volume or area measures for a chosen region, but requires manual intervention by experts to delineate it. This age-sensitive and time consuming process depends on level of automation, yet is powerful in a statistical sense. A method that is correlated to RBV and is known as voxel-based morphometry, VBM, targets tissue density, e.g., relative GM concentration, or volume, e.g., regional volume differences of a certain tissue. The SBM addresses topological shape features, like surface curvature and folding degree, that cannot be obtained directly using the RBV on a brain sMRI. The SBM is applied mostly to the cerebral cortex, along with its lobes and gyrification patterns.
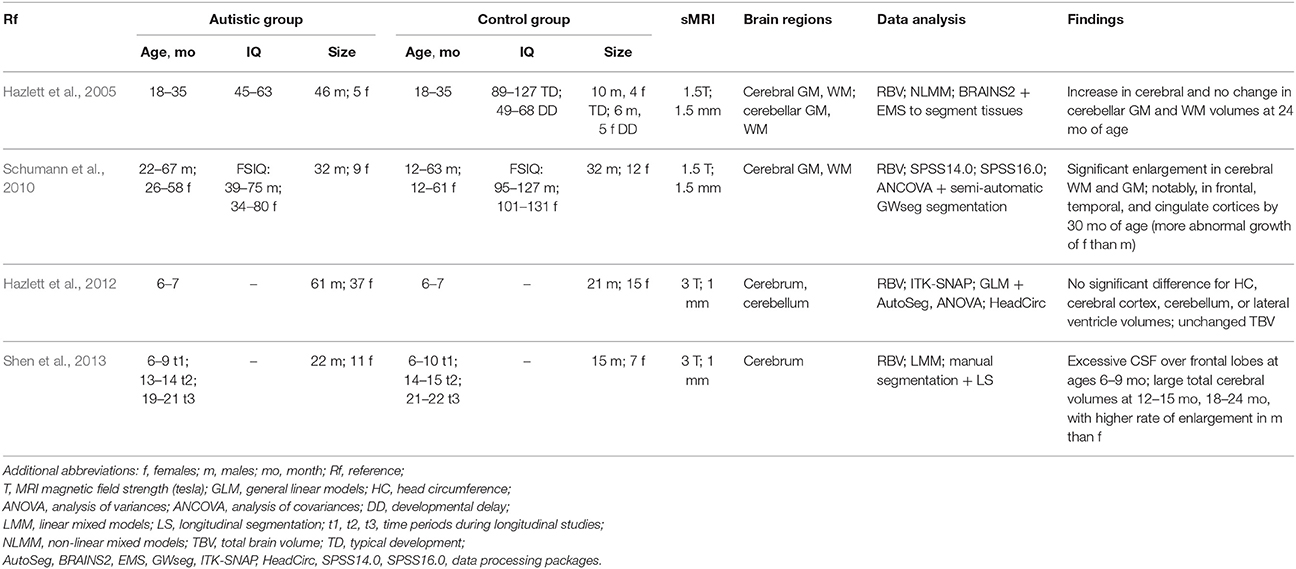
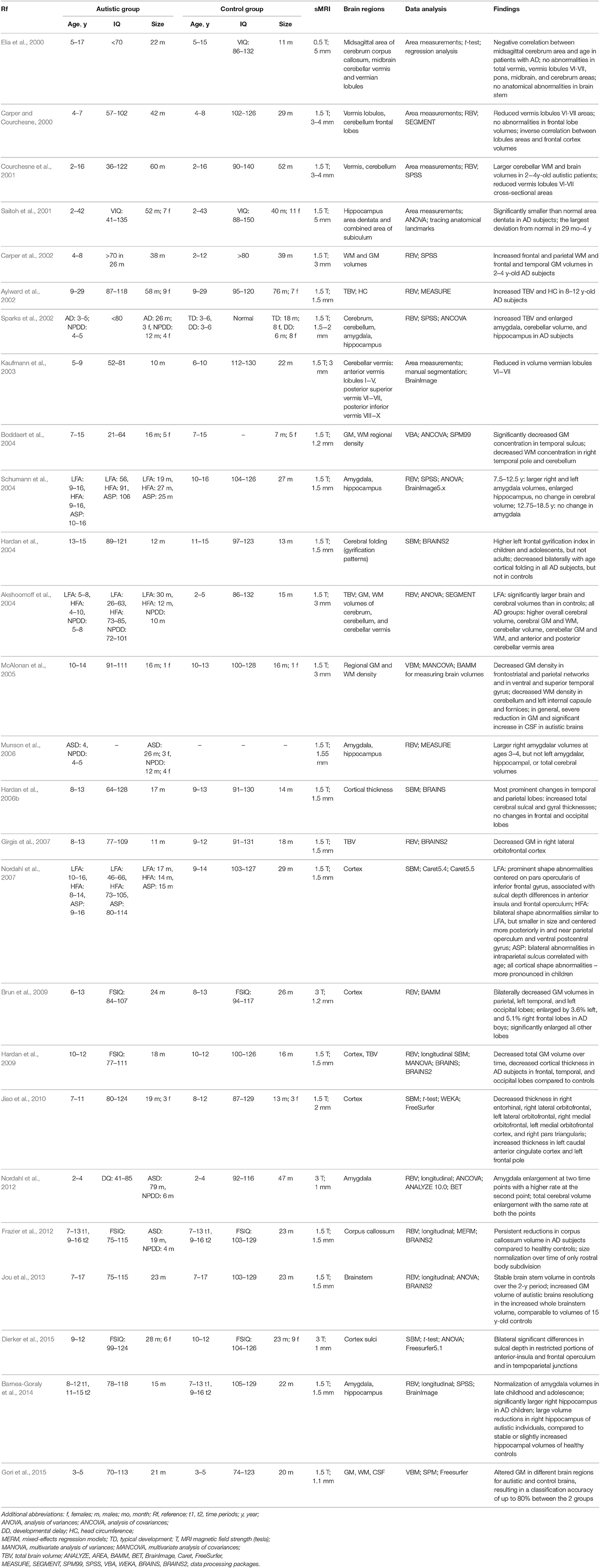
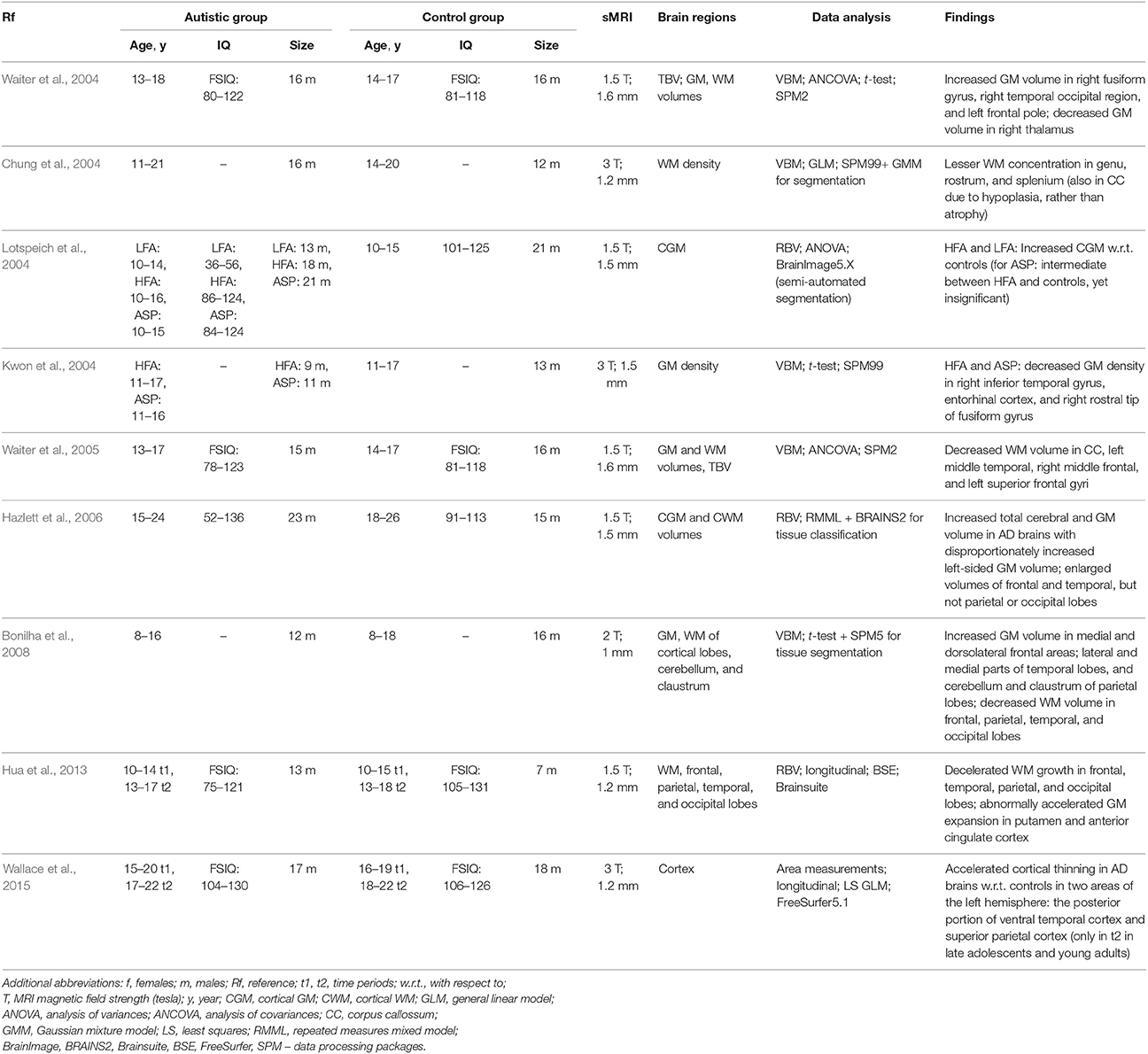
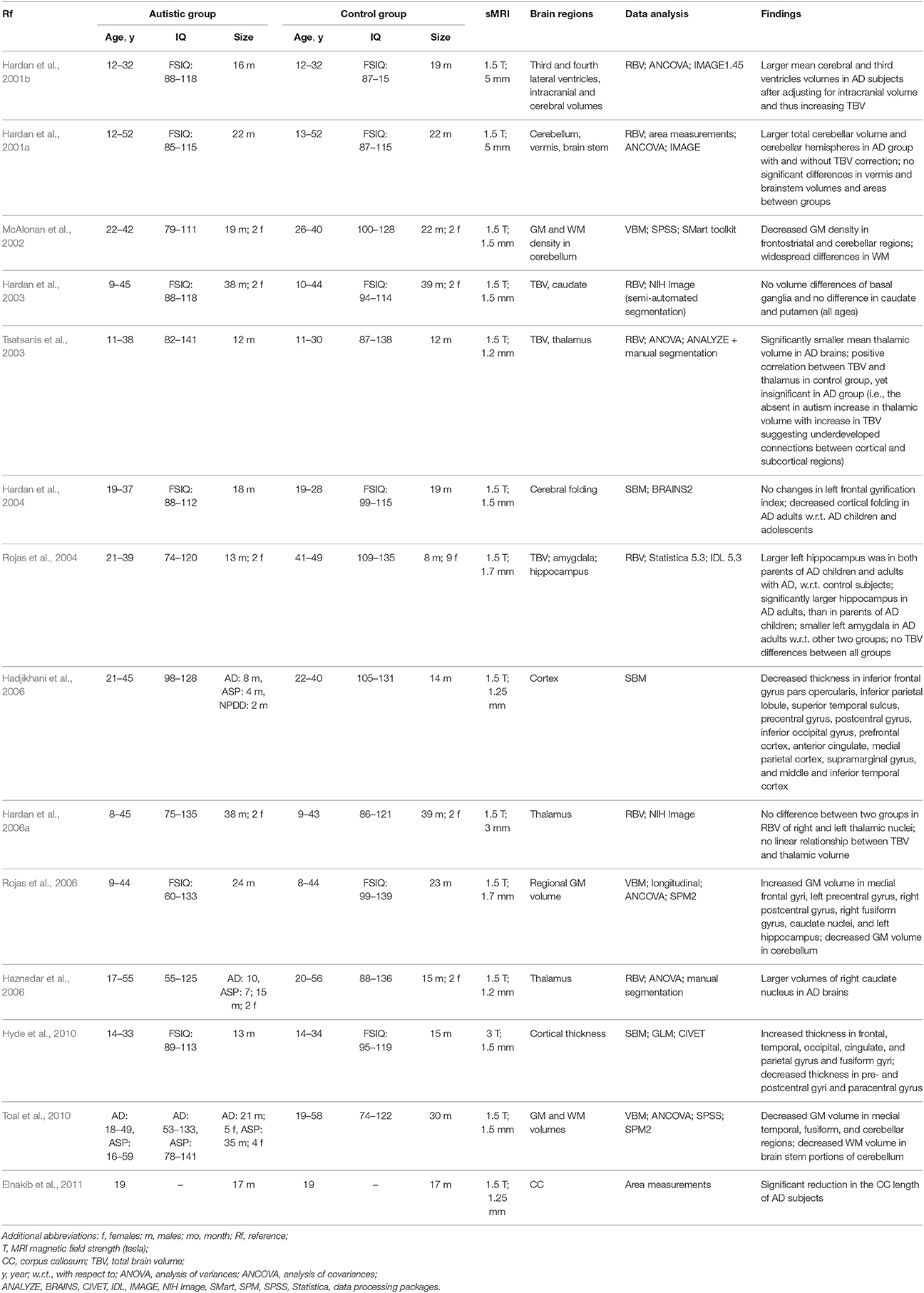
The ASD studies with the sMRI, including data processing tools and main abnormalities found in brain structures are considered below. Abnormalities found in the cerebral cortex; posterior fossa (vermis, brain stem, and cerebellum); corpus callossum; amygdalae; hippocampus, and thalamus, are addressed at four main life stages: infancy (0–2 years); childhood (3–11 years); adolescence (12–18 years), and adulthood (above 18 years). The measurements include also the total brain volume and head circumference.
2.1. Studying ASD Impacts on Anatomical Structures with sMRI
2.1.1. Cerebral Cortex
This uppermost brain layer that plays a leading role in human intelligence and perception has been the focus of plenty of sMRI studies on ASD. Changes of cortical thickness and gyrification patterns were analyzed with the SBM, whereas the RBV and VBM measured regional differences in volumes and densities of the cerebral GM and WM.
2.1.1.1. Infancy
According to the longitudinal VBM Hazlett et al. (2005) on 51 autistic children and 25 controls (aged 18–35 months), both the cerebral GM and WM volumes increase in autistic brains. The longitudinal RBV on children aged 1.5 to 5 years Schumann et al. (2010) revealed no changes in the occipital lobe. On the other hand, the cerebral GM volumes were significantly enlarged in the frontal, temporal, and parietal lobes, and in the cingulate gyrus (located in the limbic lobe) of autistic toddlers (aged about 2.5 years). Additionally, there were more abnormal growth profiles for females. The RBV in Hazlett et al. (2012) monitored the total brain volume changes for children aged 6 months to compare high risk infants to low risk ones with no autistic family members. The fact that cerebrum or lateral ventricle volumes had no significant difference for both groups confirmed earlier findings that the brain enlargement is a postnatal event, occurring around 12 months of age. Another extended longitudinal study Shen et al. (2013) pursued the goal of identifying 6–9-month-old infants who might later develop the ASD. It was revealed that the ASD causes an extra-axial fluid and is characterized by excessive CSF over the frontal lobes at 6–9 months of age, which persists at 12–15 and 18–24 months. This leads to large total cerebral volumes that tend to increase with age at a higher rate in males, than females. This finding contradicts the earlier one Schumann et al. (2010), which showed more enlargement in females. This inconsistency might be caused by manual segmentation, along with the younger ages and the 20% smaller number of participants in Shen et al. (2013).
2.1.1.2. Childhood
Volumetric, voxel-wise, and thickness changes in the cerebral cortex in children with autism have been extensively studied in the literature. In particular, the autistic children aged 2–3 years had 18% and 12% larger cerebral cortical WM and GM volumes, respectively, than the control ones, while the older children did not demonstrate such enlargement Courchesne et al. (2001). The increased frontal and temporal GM and frontal and parietal WM volumes at such a young age (2–4 years) was confirmed in Carper et al. (2002). The increased cerebral volumes in autistic children aged around 4 years and 6 years were found also in Sparks et al. (2002) and Akshoomoff et al. (2004), respectively. The latter study reported the increased total cerebral volume, as well as the increased WM and GM volumes for children with both low-functioning autism (LFA) and high-functioning autism (HFA), the total cerebral volume being significantly larger in the LFA group.
The VBM of the GM and WM densities on autistic children around 9 years of age Boddaert et al. (2004) has found a significant decrease in the GM density in the superior temporal sulcus and a decrease in the WM density in the right temporal pole. This finding was supported by McAlonan et al. (2005) where the GM density was found to significantly decrease in the frontostriatal and parietal networks, as well as in the ventral and superior temporal gyrus in autistic children aged around 12 years. The RBV revealed the decreased GM volume in the right lateral orbitofrontal cortex in 10-year-old boys in Girgis et al. (2007) and the decreased GM volumes in the parietal, left temporal, and left occipital lobes bilaterally in Brun et al. (2009). The left and right frontal lobes of the autistic boys had enlarged 3.6 and 5.1%, respectively, while all the other lobes grew more significantly.
The cerebral cortices of children with autism were often studied with the SBM as well. The atlas-based SBM in Hardan et al. (2006b) monitored the cortical thickness changes in autistic brains of children aged 10 years. The frontal or occipital lobes did not change, whereas the temporal and parietal lobes had most prominent increases of the total cerebral sulcal and gyral thicknesses. According to the subsequent longitudinal SBM Hardan et al. (2009) on 10-year old autistic children, with a follow-up scan 2 years later, the cortical thickness in autistic subjects has decreased in the frontal, temporal, and occipital lobes, comparing to controls. Also, the SBM Jiao et al. (2010) on 9-year-old children with autism showed the decreased thickness in the right entorhinal, right lateral orbitofrontal, left lateral orbitofrontal, right medial orbitofrontal, left medial orbitofrontal cortex, and right pars triangularis. However, the thickness also increased in the left caudal anterior cingulate cortex and left frontal pole. Significant bilateral differences in sulcal depth in restricted portions of the anterior-insula, frontal-operculum, and in tempoparietal junction, were found in Dierker et al. (2015). The study by Gori et al. (2015) on 4-year-old males was based on extracting features from GM, WM, and CSF to classify autistic and control brains. Only GM features in different subregions showed a classification performance that reached up to 80%.
2.1.1.3. Adolescence
Volumetric and voxel-wise cerebral changes have been investigated in a number of publications. The VBM in Waiter et al. (2004), for example, revealed the increased GM volume in the cortical lobes of 15-year-old autistic males, namely, in the right fusiform gyrus, right temporal and occipital region, and left frontal pole. This work was extended in Waiter et al. (2005) to investigate changes of the WM volume in 15-year-old autistic males and found that the WM volume decreases at the left middle temporal, right middle frontal, and left superior frontal gyri. The VBM in Kwon et al. (2004) conducted on subjects with the HFA and ASP showed the lower cortical GM density in the right inferior temporal gyrus, entorhinal cortex, and right rostral tip of fusiform gyrus. The RBV in Lotspeich et al. (2004) reported a growth of the cerebral GM in both the HFA and LFA subjects, compared to the controls, but only non-significant changes for people with the ASP. The VBM in Chung et al. (2004) on 16-year-old males revealed the lesser WM concentration in the genu, rostrum, and splenium regions in autistic brains, whereas the RBV in Hazlett et al. (2006) on a group of young adolescents has found a larger total cerebral volume of the autistic brains. The lobe volume growth due to the GM volume enlargement was also noticed in the frontal and temporal, but not parietal or occipital lobes. The VBM on autistic and control males around the age of 13 in Bonilha et al. (2008) has found an increase in the GM volume in the parietal lobes, medial and dorsolateral frontal areas, and lateral and medial parts of temporal lobes, as well as a decrease in the WM volume in the frontal, parietal, temporal, and occipital lobes. The longitudinal RBV on 13-year-old adolescents in Hua et al. (2013) has found a decelerated WM growth in the frontal, temporal, parietal, and occipital lobes, together with an abnormally accelerated GM expansion in the putamen and anterior cingulate cortex.
To monitor cortical changes in the adolescent groups due to autism, the SBM on a group of 12-year-old autistic and control adolescents was used in Hardan et al. (2004) to measure changes in the cerebral folding and better investigate the gyrification patterns. As was found, the adolescents had the higher left frontal gyrification index, than the adults, as well as the cortical folding had decreased bilaterally with age in all the autistic subjects, but not in the controls. The SBM on a wide variety of patients including the LFA, HFA, and ASP subjects aged 11–13 years in Nordahl et al. (2007) has found for the LFA subjects prominent shape abnormalities centered on pars opercularis of the inferior frontal gyrus, associated with sulcal depth differences in the anterior insula and frontal operculum. The bilateral shape abnormalities in the HFA group were similar to those of the LFA group, but smaller in size and centered more posteriorly in and near the parietal operculum and ventral postcentral gyrus. The ASP group had the correlated with age bilateral abnormalities in the intraparietal sulcus. All these cortical shape abnormalities were more pronounced in the children than in the adolescents. Two successive longitudinal studies on the same group of subjects when their mean age was 17.4 and 19 years, respectively, in Wallace et al. (2015) demonstrated an accelerated cortical thinning in the autistic brains with respect to the controls in two areas in the left hemisphere, namely, in the posterior portion of the ventral temporal cortex and the superior parietal cortex. This acceleration has happened only for the older adolescents and young adults of the second study.
2.1.1.4. Adulthood
The RBV on 16 autistic males of the average age of 22 years in Hardan et al. (2001b) revealed the larger mean cerebral and third ventricle volumes in the autistic subjects rather than in the controls. The VBM in Rojas et al. (2006) gave the larger GM volume in the medial frontal gyri, left precentral gyrus, right postcentral gyrus, and right fusiform gyrus. The more recent VBM Toal et al. (2010) showed the decreased GM volume in the medial, temporal, and fusiform regions. The SBM in Hardan et al. (2004) explored changes of the cerebral folding in 27-year-old males. While the left frontal gyrification index had no changes, the cortical folding decreased in autistic adults, compared to children and adolescents. The SBM in Hadjikhani et al. (2006) on the autistic, ASP, and NPDD subjects aged around 33 years has shown the decreased thickness in the inferior frontal gyrus, pars opercularis, inferior parietal lobule, superior temporal sulcus, precentral and postcentral gyrus, inferior occipital gyrus, prefrontal cortex, anterior cingulate, medial parietal cortex, supramarginal gyrus, and middle and inferior temporal cortex. The SBM in Hyde et al. (2010) on 15 autistic 22.7-year-old (on average) males has shown that the thickness increases in the frontal, temporal, occipital, cingulate, and parietal gyrus, as well as in the fusiform gyri, but decreases in the pre- and post-central gyri and para-central gyrus.
Conclusions
According to the above studies, the cerebral cortex starts changing in the autistic brains at the age of around 12 months, and no significant differences could be monitored earlier, except of the recent findings in Shen et al. (2013). The cerebrum changes of an autistic brain at such an early age include the age-proportional enlargements of the lobes and increases of the cortical WM and GM. The growing cerebral volume, including the GM and WM, in early childhood indicates simultaneously the decreasing GM and WM density. Also, children with autism demonstrate significant changes in the cortical thickness. These volumetric and thickness differences continue to increase at the later stages of life.
2.1.2. Posterior Cranial Fossa (Cerebellum, Vermis, and Brain Stem)
Posterior cranial fossa has also been investigated in the literature for any correlates with ASD at different life stages.
2.1.2.1. Childhood
Area measurements on 22 autistic children in Elia et al. (2000) showed no abnormalities in the total vermis, vermis lobules VI-VII, pons, and midbrain, which could be related to autism. However, the decreased WM density in the cerebellum of children with autism was reported in McAlonan et al. (2005) and Boddaert et al. (2004), and the larger by 39% cerebellar WM volume was found in Courchesne et al. (2001) for 2–3-year-old children with autism compared to controls. Autistic boys had the lesser GM and smaller GM-to-WM ratios and vermian lobules than the normal ones. The reduced cross-sectional areas of the vermis lobules VI-VII in autistic children were also reported in Courchesne et al. (2001), Kaufmann et al. (2003), and Carper and Courchesne (2000). The larger cerebellar WM and GM volumes and increased area of the anterior and posterior cerebellar vermis were found in the 6-year-old children with the LFA, HFA, and NPDD in Akshoomoff et al. (2004).
Multiple brain stem volume studies gave contradicting conclusions. The early areal measurements in Gaffney et al. (1988) and Ciesielski et al. (1997) claimed reduced brain stem size, whereas the subsequent works, such as Garber and Ritvo (1992), Piven et al. (1992), Kleiman et al. (1992), Hsu et al. (1991), and Elia et al. (2000) found no significant differences between the autistic and control groups. No differences in the brain stem volumes for these groups were found also in Hardan et al. (2001a) and Herbert et al. (2003). However, the recent study Jou et al. (2013) of 10-year-old children, with a follow-up after 2 years, came up with different findings: the brain stem volume was stable in the controls over the 2-year period, but the increased GM volume of an autistic brain implied the larger entire brain stem volume, so that the autistic brain volumes eventually became comparable to those of the 15-year old controls.
2.1.2.2. Adolescence and adulthood
An increase in the GM volume in the cerebellum in Bonilha et al. (2008) is consistent with the studies of children. The cerebellar volumes of autistic children and adolescents are also consistent with those of adults. As shown in Hardan et al. (2001a) on 22-year-old subjects, the total cerebellar volume and cerebellar hemispheres are larger in the autistic group both with and without correction by the total brain volume. But the volumetric and area measurements of the vermis and brain stem did not differ significantly between the autistic and control groups. Also, the lower GM density in the frontostriatal and cerebellar regions, along with the widespread WM differences, had been reported in McAlonan et al. (2002) and the lower brain stem and total brain stem GM volumes in the autistic adults have been found in Jou et al. (2009).
Conclusions
The posterior fossa structures are significantly affected with the ASD from an early age of 2 years and during all the subsequent life stages. Some inconsistencies in the brain stem abnormalities found might be caused by wide age and gender differences of the participants.
2.1.3. Amygdalae
Amygdalae have also been investigated in efforts to find any correlates with the ASD.
2.1.3.1. Infancy
To our knowledge, the amygdalar changes in the infancy period are not studied yet, and the youngest subjects in such studies are around 3 years old.
2.1.3.2. Childhood
The RBV on 29 autistic and 26 control subjects (the average age of 3.9 years) in Sparks et al. (2002) has shown that in the autistic subjects the amygdalae are enlarged proportionally to the overall increase of the entire cerebral volume. The more recent volumetry Munson et al. (2006) on a larger group of the 45 autistic subjects suggested the enlargement of only the right amygdalar volume at the ages of 3–4 years. The longitudinal RBV in Nordahl et al. (2012), which was performed in order to precisely define the age at which the amygdalae begin to enlarge, started with the 85 autistic subjects aged 37 months on average, and had the follow-up scan 1 year later on 45 of these autistic subjects. That enlarging the amygdalar volume was found in both the cases, although with a higher rate for the latter group, confirms that this enlargement is present at such an early age of around 3 years. The volumetry on an older population (7.5–12.5 years) in Schumann et al. (2004) demonstrated that both the right and left amygdalae volumes were enlarged. However, the longitudinal RBV in Barnea-Goraly et al. (2014), which involved 15 autistic subjects aged 10.6 years on average and their follow-up scan after reaching adolescence, has found no significant difference between the left, right, or total amygdalar volumes for the autistic and control subjects. This contradiction to all other studies might be caused by the relatively small population used.
2.1.3.3. Adolescence
No significant difference in left, right, or total amygdalae volumes in the adolescents was found in various studies, e.g., Schumann et al. (2004) and Barnea-Goraly et al. (2014).
2.1.3.4. Adulthood
The RBV in Rojas et al. (2004) explored the hypothesis that parents of autistic children would show similar structural changes. This study was conducted in part on the amygdalar volumes of 15 autistic subjects (the average age of 30.3 years), 17 controls (the average age of 43.6 years), and 17 parents of autistic children. The amygdalae were smaller in the autistic group than in the other two groups.
Conclusions
The amygdalar volumes generally increase in autistic brains at early stage of life, with the rate of increase being proportional to the age (the known studies started from the 3-year-old subjects). By adolescence, no significant differences in this structure could be found. However, at the subsequent life stages its volume decreases in autistic brains.
2.1.4. Hippocampus
Hippocampus also proved to have some correlates with the ASD from a young age.
2.1.4.1. Infancy
Similarly to Section 2.1.3 above, no hippocampal changes have been explored at this period, with the youngest subjects age having been around 2.5–3 years.
2.1.4.2. Childhood
The enlarged hippocampus was found in autistic children at the ages of both 3–4 Sparks et al. (2002) and 7.5–12.5 years Schumann et al. (2004), especially at the HFA group in the latter study. The enlargement only in the right hippocampus in children aged around 10 years was reported in Barnea-Goraly et al. (2014). The earlier study Saitoh et al. (2001) suggested that the area dentata in autistic subjects was significantly smaller than in the normal ones, with the largest deviation at the ages from 29 months to 4 years.
2.1.4.3. Adolescence
The hippocampus was found enlarged in autistic subjects in Schumann et al. (2004); however, this finding differs from the more recent results in Barnea-Goraly et al. (2014), where the right hippocampal volume has increased in the autistic children, but not in the adolescents. In the latter case, it was found that differences between hippocampal volumes in autistic and control brains became insignificant with time.
2.1.4.4. Adulthood
The hippocampus of autistic adults was confirmed to be significantly enlarged Rojas et al. (2004), compared to controls and also to parents of autistic children. The left hippocampus was larger in both the parents of autistic children and the adults with autism, compared to the controls. The VBM in Rojas et al. (2006) has shown an increase in the GM volume of the left hippocampus.
Conclusion
In autism, the hippocampus is enlarged at all ages.
2.1.5. Corpus Callosum
Corpus callosum is found to be correlated with the ASD. In particular, the two successive longitudinal RBV studies Frazier et al. (2012) on 19 subjects with autism and 4 subjects with NPDD (first, at the age of 10.6 years and then 13.1 years on average) have found persistent reductions in the total corpus callosum volumes in the autistic subjects compared to the healthy controls. Only the size of rostral body subdivision has normalized over time. The centerline length of the corpus callosum was significantly reduced in young adults with autism compared to controls Elnakib et al. (2011).
2.1.5.1. Conclusion
The corpus callosum is reduced in size in the ASD.
2.1.6. Thalamus, Caudate, and Putamen
The GM volume decreases in the right thalamus of adolescent males with autism and the thalamic volume decreases in adults with autism compared to controls, as was found, respectively, in Waiter et al. (2004) and Tsatsanis et al. (2003). However, no volume differences of the basal ganglia, caudate, or putamen at all ages have been found in Hardan et al. (2003). The more recent RBV by the same research group Hardan et al. (2006a) found no differences in both the right and left thalamic nuclei between the 19-year-old, on average, control and autistic groups. Studying the older adults (aged 28 years on average) showed that the right caudate nucleus has a larger volume in the autistic brain Haznedar et al. (2006).
2.1.7. Total Brain Volume and Head Circumference
Changes in the total brain volume and head circumference have been associated with the ASD in several publications.
2.1.7.1. Infancy
As shown in Hazlett et al. (2005) for 18–35 month-old infants, the total brain volume increases in the group with autism and their normal at birth head circumference becomes significantly larger around 12 months of age compared to the healthy group. This finding was confirmed in Hazlett et al. (2012), showing no significant difference in the head circumference at 6 months of age in infants with high risk of autism.
2.1.7.2. Childhood
The head circumference grows also in children with autism. The increased brain volume and head circumference in a large group of 10-year-old children with autism have been confirmed in Aylward et al. (2002) and supported in other studies, e.g., in Herbert et al. (2003) on 9-year-old autistic males and in Carper et al. (2002). However, a more recent publication Hardan et al. (2006b) reported insignificant increases in the total brain volumes for children with autism (this contradiction to the above findings might be due to a small number of participants). The later stages of life show no difference between the autistic and neurotypical groups in the total brain volumes, probably because the growth rate of the normal brains increases at these stages.
2.2. Summary
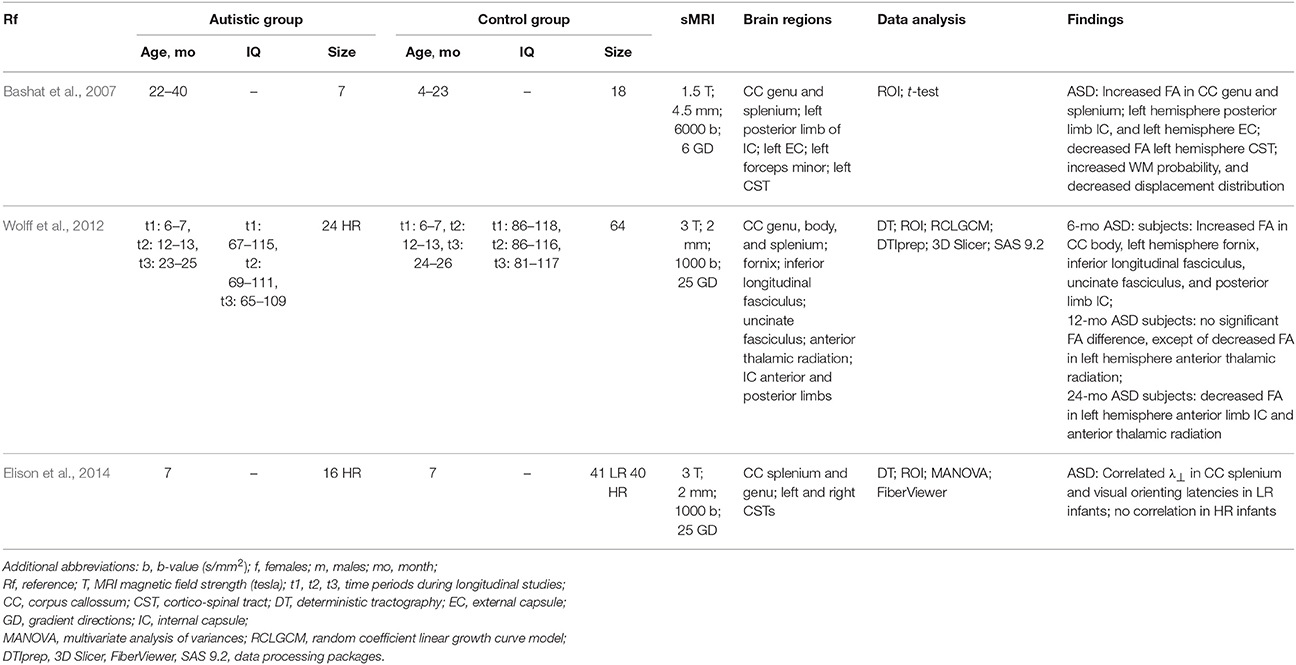
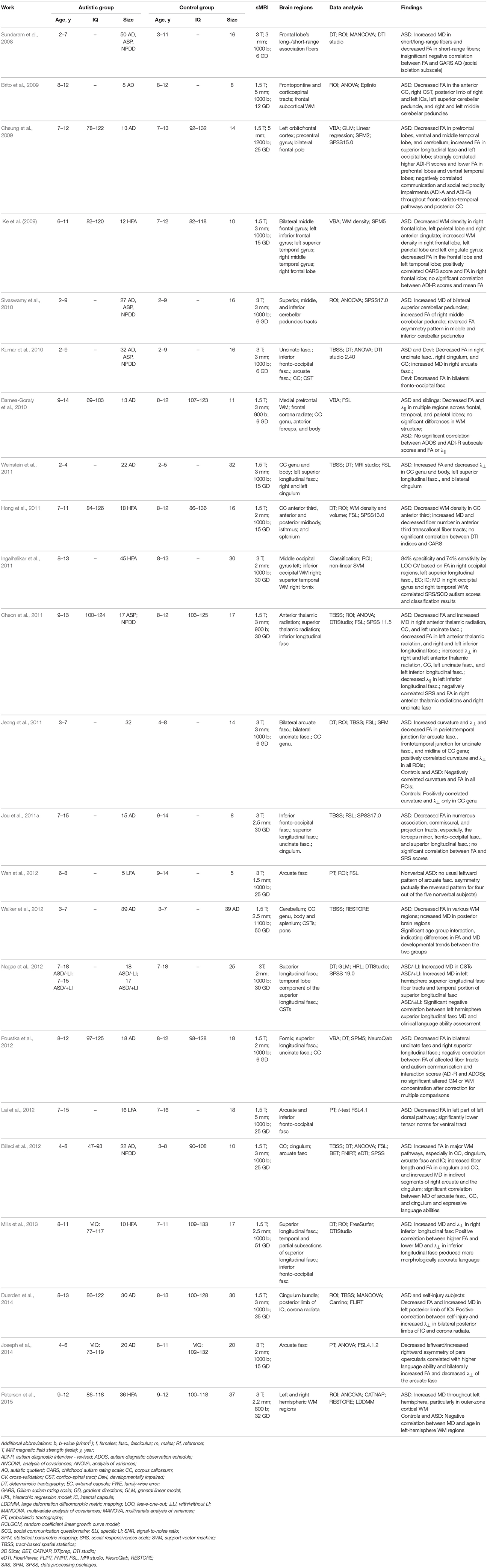
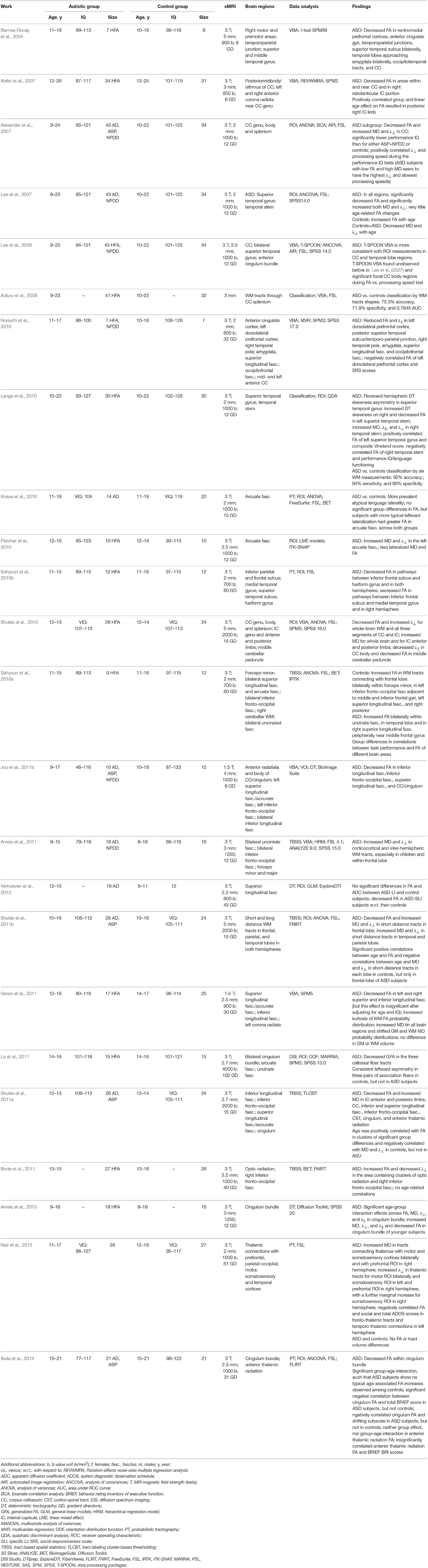
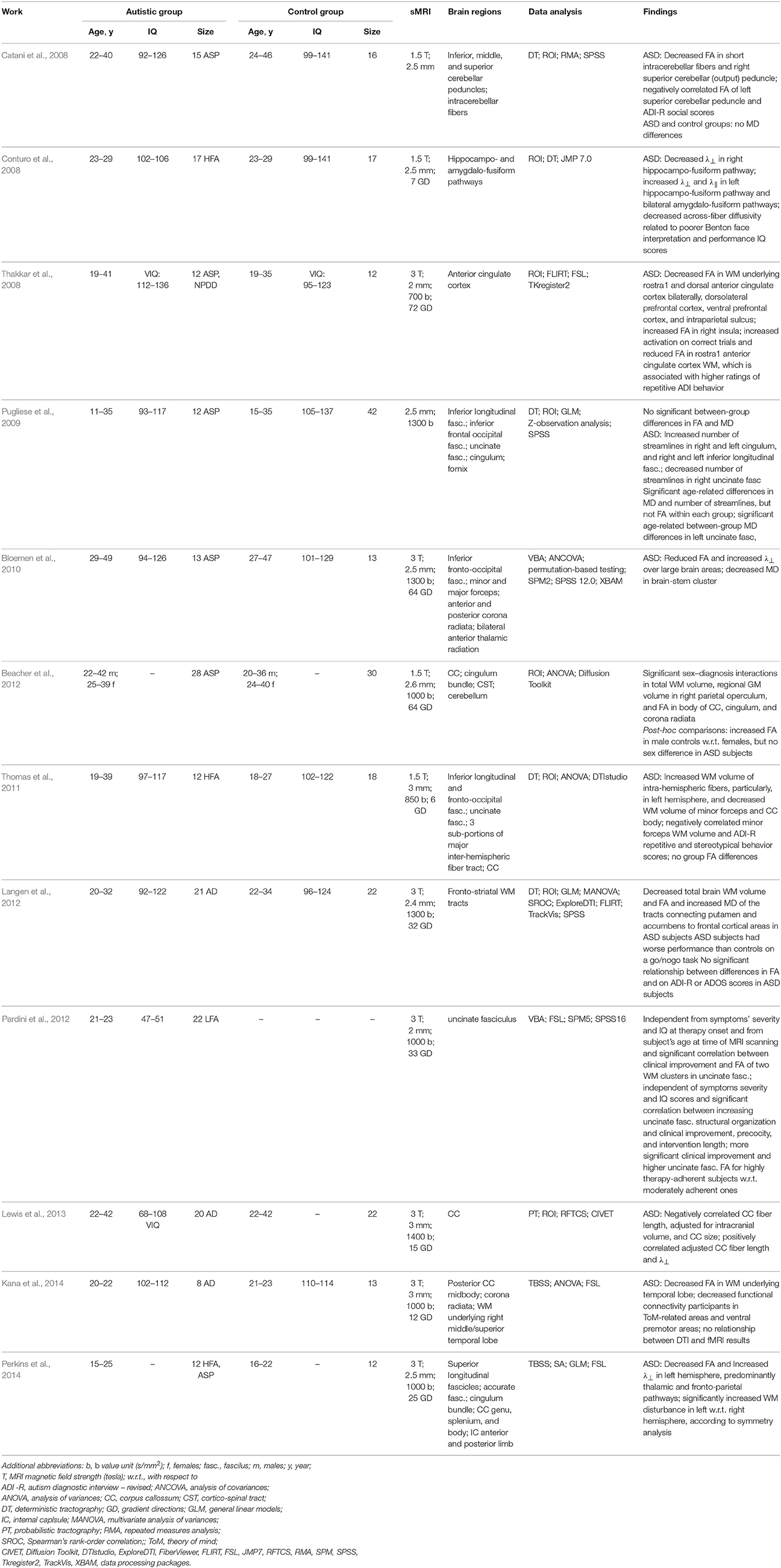
Tables 1–4 summarize, in accordance with the life stage, basic current results of studying the ASD with the sMRI.
3. Studying ASD with DTI
DTI characterizes 3D diffusion of water molecules in a biological tissue with DT Basser et al. (1994a,b). The DTI is widely used in clinical applications, e.g., to examine a normative WM development, neurodevelopmental disorders, like ASD, and neurodegenerative disorders, such as amyotrophic lateral sclerosis Dong et al. (2004). For completeness, basic principles of the DTI are reviewed first below.
3.1. DTI: Basic Concepts
An unconstrained medium, such as the CSF, ensures the isotropic diffusion where the water molecules move in a similar manner in all directions. The isotropic diffusion is typical, e.g., for the brain ventricles, whereas the molecular diffusion in the WM is constrained by spatial orientations of the WM tracts. The molecules can diffuse along a fiber track more freely than across it, so that the diffusion becomes anisotropic with a privileged direction. As a result, water diffusion patterns for the brain tissues provide information about the underlying anatomical structures Mori and Tournier (2013).
Generally, a fiber is oriented arbitrarily and has different diffusion coefficients along different directions.
DT is usually specified with certain parameters at each voxel, that are usually converted into maps of scalar diffusion measurements to facilitate interpreting the voxel-wise DTI data. The most common anisotropy and microstructural measurements include fractional (FA) and relative (RA) anisotropy together with mean (MD), axial (λ||), and radial (λ⊥) diffusivity.
The orientation-independent mean diffusivity, also called the trace, measures an overall diffusion in a voxel or region. A slightly different MD definition has been used to measure the diffusion descent in brain ischemia van Gelderen et al. (1994). Since the MD values in the CSF are higher than it is in other types of brain tissues, the MD is recommended for the CSF-related disease studies Narr et al. (2009).
The fractional anisotropy, described first in Koay et al. (2006), is the most popular rotationally invariant (i.e., orientation-independent) measure of how isotropic is the voxel- or region-wise diffusion. The FA of a physically realizable diffusion with non-negative eigenvalues ranges from 0 to 1 in the opposite extreme complete isotropic and linear anisotropic cases, respectively. For example, the WM appears whiter due to its higher FA. The reduced FA values usually indicate changes in myelination or degraded axonal structures of the WM Lerner et al. (2014).
The relative anisotropy is similar to the FA and takes the range between 0 (the complete isotropy) to (the complete, i.e., linear anisotropy). The RA is also defined as the ratio of anisotropic and isotropic parts of the diffusion Le Bihan et al. (2001).
The axial, or parallel diffusivity, λ||, measures the diffusion along the principal axis (parallel to axons), whereas the radial, or perpendicular diffusivity, λ⊥, averages the diffusion along the two minor axes to measure the degree of restriction due to membranes and other effects.
These two measurements are closely connected to the WM pathology Alexander et al. (2007) and have been used to observe developmental and pathological fiber alterations, e.g., to study dysmyelinating disorders Song et al. (2005).
Main fiber trajectories are extracted from the DTI and visualized using 2D color-coded fiber orientation maps. These maps provide unique information, which cannot be obtained with other MRI techniques. In particular, the fiber orientations could classify and stratify specific WM tracts in a host of medical applications that need more anatomical details Alexander et al. (2007).
These 2D color-coded maps present the voxel-wise fiber orientations related to the WM tracts, but cannot reveal 3D WM trajectories and connection patterns Mori and Tournier (2013). The latter are obtained with the computer-aided 3D WM fiber tractography, recognizing and tracing WM tracts and their connections with other WM tracts or GM structures. The tractography is either deterministic, or probabilistic Basser et al. (2000); Nucifora et al. (2007), and constructs, respectively, only one trajectory for each start voxel, or the most probable, or minimum-energy path between two selected voxels or regions Mori and Tournier (2013).
The deterministic (or tract propagation) fiber tractography is built by extracting fiber orientation and propagating pathway until termination criteria are met Mori and Tournier (2013). Generally, the local voxel-wise fiber orientations are estimated directly from planar diffusion profiles. These estimates fail if the ellipsoid is isotropic or the diffusion profile is planar.
Image noise, patient movements, and other imaging artifacts cause uncertainty in the fiber orientations obtained by the deterministic fiber tractography. The probabilistic fiber tractography attempts to increase the confidence Basser and Jones (2002) by estimating probability distributions of all fiber orientations and selecting the most probable orientations. Tracing many different pathways with marginally altered orientations allows for measuring the connection probability and assessing fiber connectivity between different brain regions by a voxel-wise connectivity index Behrens et al. (2003). The main advantage of the probabilistic fiber tractography is its ability to stratify the entire WM tracts. However, its accuracy is limited and depends on the accuracy of the DT and estimated pathway probability distributions. Moreover, the probabilistic tractography cannot differentiate between ante- and retrograde along the fiber's path Basser and Jones (2002).
3.2. Studying ASD Impacts on Anatomical Structures with DTI
Recent molecular and functional ASD studies confirmed the vital importance of localizing atypical development and examining neural networks and connectivity of different brain areas Travers et al. (2012). At the molecular level, the postmortem studies of brains of children with ASD have shown more reduced and less compact minicolumns Casanova et al. (2002a,b, 2006). At the functional level, examining brain connectivity with the fMRI revealed how activities of various brain areas are organized. Most of the ASD patients have demonstrated reduced connectivity between the frontal and posterior brain parts during various cognitive tasks and in a resting state Schipul et al. (2011). Therefore, according to both molecular and functional studies, the brain connectivity and the underlying WM tracts might be impaired in these patients.
However, earlier studies had limited abilities to provide sufficient morphological information about these WM tracts and their development in a living human Travers et al. (2012). To overcome this drawback, multiple noninvasive DTI-based studies of both the macro- and microstructure (e.g., axons) of the brain WM tracts were conducted for the last decade to investigate the ASD Travers et al. (2012). In particular, the DTI facilitated examining microstructural properties of the WM circuitry and detecting abnormalities of the WM fiber tract integrity Wolff et al. (2012). Below, the most important current methods of studying the ASD with the DTI (in total, from 60 publications) are summarized by stratification into five categories: (i) the whole-brain voxel-based analysis (VBA); (ii) the analysis of tract-based spatial statistics (TBSS); (iii) the ROI analysis; (iv) tractography; and (iv) the classification-based analysis. Since the DTI findings in the literature are mainly concerned with WM connectivity across multiple structures, classification here is not based on the structures, such as in Section 2. It is rather based on the methodology the WM connectivity and the fiber tracts through those structures are investigated with. These methods differ in how group differences are investigated by measuring DTI heterogeneity, e.g., the FA. The same four age stages, as in Section 2, i.e., infancy, childhood, adolescence, and adulthood, are considered separately for more in-depth presentation of the ASD findings.
3.2.1. Whole-Brain VBA
The brain images across subjects are spatially co-aligned in order to ensure that each individual voxel has the same anatomic location in all the subjects. Then the voxel-wise statistics are used to find areas of significant difference between the ASD and control patients. Due to its comprehensive examination, the whole-brain VBA overcomes problems of possible user bias in and insufficient prior knowledge for selecting the ROI to be analyzed. However, the VBA has drawbacks that are absent in the ROI analysis: high sensitivity to accuracy of aligning the images and low reliability of voxel-wise statistical decisions. In spite of these drawbacks, the VBA is still widely used due to its simplicity and ability to explore the whole brain.
3.2.1.1. Childhood
Relationships between communication, social interaction, and repetitive behaviorial impairments have been investigated in Cheung et al. (2009) by the VBA of FA data for each group of subjects. For the ASD group, the bilateral prefrontal and temporal regions had reduced FA values, as well as lower FA along the frontal striatio-temporal pathways or more posterior brain pathways were associated with communication and social reciprocity impairments or repetitive behaviors, respectively. In a multi-modality study Ke et al. (2009), the WM abnormalities were investigated in HFA Chinese children, and the VBA showed that the WM density decreases in the right frontal lobe, left parietal lobe and right anterior cingulate. In addition, the FA values were lower in the frontal lobe and left temporal lobe. Combining the VBA and RBV of the DTI has showed consistent WM abnormalities in the HFA patients. Children with ASD and their unaffected siblings have been compared with the control group in Barnea-Goraly et al. (2010). The ASD and sibling groups had the prevalent reduced FA and λ|| values in the frontal, parietal, and temporal lobes, especially, in the regions related to social cognition. However, no significant relationships between the WM measurements in the ASD and sibling groups have been reported. To what extent disconnectivity of networks, which are important for social communication, relates to behavioral impairments in children with ASD was investigated in Poustka et al. (2012). The VBA indicated the decreased FA values in the uncinate fasciculus and right superior longitudinal fasciculus. The additional analysis revealed a negative correlation between the FA values of the affected fiber tracts and the ASD symptoms.
3.2.1.2. Adolescence
An early study Barnea-Goraly et al. (2004) of the WM abnormalities performed the VBA on a small group of male HFA children and adolescents. It has found low FA values in various brain regions, mainly, in the WM adjacent to the ventromedial prefrontal cortices, in the anterior cingulate gyri, and in the temporoparietal junctions. A similar, but larger study Keller et al. (2007), has investigated the WM abnormalities in a large male HFA group aged from 10 to 35 years. The HFA subjects had lower FA values near the corpus callosum and in the right retrolenticular portion of the internal capsule. A new tissue-specific, smoothing-compensated (T-SPOON) VBA introduced in Lee et al. (2009) minimizes effects of partial volume averaging and image smoothing by applying a regional mask with the same smoothing parameters. Compared to the conventional VBA, results of the T-SPOON on a large group of the ASD subjects and corresponding controls were more consistent with the FA obtained by analyzing the corpus callosum and temporal lobe ROIs. The VBA of the WM of a small group of HFA and matched controls in Noriuchi et al. (2010) has shown the reduced FA and λ⊥ in various ROIs in the brain, including the left dorsolateral prefrontal cortex, cingulum bundle, arcuate fasciculus, and superior longitudinal fasciculus. Social impairment scores correlated negatively with the FA of the left dorsolateral prefrontal cortex, suggesting that the WM in cortical regions is vital for the ASD patients development. The WM integrity of the major fiber tracts connecting the amygdala, fusiform face area, and superior temporal sulcus in the ASD subjects was explored in Jou et al. (2011b) by the FA-based VBA. The ASD subjects had reduced FA values in the investigated WM tracts, including inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, corpus callosum, and cingulum bundle. A multi-modality study Groen et al. (2011) used the T1 MRI to study the brain volumetrics and the DTI to investigate both the WM and GM integrity in a limited group of HFA adolescents. There were no significant volumetric GM or WM differences between the two groups.
3.2.1.3. Adulthood
The ASP adults, examined in Bloemen et al. (2010) for the WM integrity of the whole brain, have shown lower FA of 13 WM clusters in the internal capsule, frontal, temporal, parietal, and occipital lobes, as well as the cingulum and corpus callosum. Studying the WM abnormalities on a small group of young LFA men Pardini et al. (2012) indicated a positive correlation between the FA in the uncinate fasciculus and clinical improvement, precocity, and intervention duration.
3.2.2. Tract-Based Spatial Statistics (TBSS)
To overcome some shortcomings of the traditional VBA, the recent DTI-optimized analysis of TBSS Smith et al. (2006) uses a non-linear registration to a common target to co-align the subjects' FA maps. Following the alignment, a mean FA skeleton is built and thresholded to exclude areas of high inter-subject variability. Based on the corresponding FA values, each aligned subject's FA map is projected onto the skeleton to facilitate collecting standard voxel-wise FA statistics across all the subjects. Generally, such tract-based analysis is more accurate than the VBA, but requires a sophisticated data projection method for better results. It also does not handle partial volume effects, is sensitive to motion distortions, has high computational complexity, and may fail if the tracks change much at junctions or due to apparent pathologies.
3.2.2.1. Childhood
The deterministic tractography and TBSS were combined in Kumar et al. (2010) to investigate the corpus callosum region, including the uncinate fasciculus, in young ASD children aged 5 years on average. The ASD manifests itself in lower FA, higher MD, larger number of streamlines and voxels, and longer streamlines. There were also ASD-related macrostructural changes in the uncinate fasciculus correlate with the popular symptomatic scores of the GARS (Gilliam autism rating scale). The TBSS, VOI, and tractography have been used also in Weinstein et al. (2011) to investigate the WM abnormalities of very young ASD children in several clusters within the genu and body of the corpus callosum, left superior longitudinal fasciculus, and right and left cingulum. The FA increased in these regions as a consequence of the decreased radial diffusivity, λ⊥. The tractography revealed that increased FA was concentrated in the mid-body of the corpus callosum and in the left cingulum.
To study the integrity of the thalamic radiation of older ASD children in Cheon et al. (2011), four DTI measurements, namely, FA, MD, λ⊥, and λ||, were examined in the anterior thalamic radiation, superior thalamic radiation, posterior thalamic radiation, corpus callosum, uncinate fasciculus, and inferior longitudinal fasciculus by combining the whole brain VBA analysis with the TBSS and ROI analyses. Anticipated WM abnormalities in the thalamo-frontal connections in the ASD children are indicated by the reduced FA and λ|| and Increased MD and λ⊥ across various brain regions. The TBSS, combined with a VBA were applied in Jou et al. (2011a) to a small group of older ASD and control children. The FA was reduced, especially, in the forceps minor, inferior fronto-occipital fasciculus, and superior longitudinal fasciculus. Regional distributions of differences between young ASD and control children were examined in Walker et al. (2012) using the TBSS and the whole-brain VBA. While the FA values were reduced in various brain regions, the increased MD was found only in the posterior ones. These small (1–2%) regional differences between both groups were accompanied by distinct regional differences in imaging artifacts. They also demonstrated vulnerability of the between-group differences to such artifacts, which may cause errors in biological inferences. The TBSS and deterministic tractography were also used in Billeci et al. (2012) to analyze young ASD children with and without mental retardation. The statistics have detected a widespread FA increase in major WM pathways, and the tractography showed increased FA and fiber length in the cingulum and corpus callosum. Moreover, the MD increase was correlated with expressive language functioning in the indirect segments of the right arcuate and left cingulum.
3.2.2.2. Adolescence
Examining differences in the WM integrity between the HFA and control subjects and its association with pictorial reasoning under various linguistic levels in Sahyoun et al. (2010a) indicated that visuospatial reasoning performance relates to the FA of the peripheral parietal and superior precentral WM in the HFA subjects, but the superior longitudinal fasciculus, callosal, and frontal WM in the controls. The whole-brain VBA followed by analyzing the TBSS in Ameis et al. (2011) evaluated the WM in a group of ASD and control children and adolescents. The VBA has shown the increased MD and radial diffusivity (λ⊥) in the ASD children, but not the adolescents in the frontal WM, and the statistics analysis has revealed alterations in the right uncinate fasciculus. The right inferior longitudinal fasciculus in the ASD case may indicate a disrupted fronto-temporal-occipital circuit playing a significant role in social and emotional processing. The TBSS was analyzed in Shukla et al. (2011b) to examine short- and long-distance WM tracts in the frontal, parietal, and temporal lobes of the ASD subjects and matched controls. The short-distance tracts had reduced FA in the ASD group, increased MD and λ⊥ in the frontal, temporal, and parietal lobes. The age and DTI measurements were correlated in the control, but not ASD group. As was suggested, these typical age-related correlations were absent due to altered maturation of the short-distance tracts in the ASD group.
The whole-brain VBA with TBSS in Shukla et al. (2011a) assessed the WM tracts in the ASD children and adolescents. The reduced FA and the higher MD and radial diffusivity have been found for the ASD subjects in the corpus callosum, anterior and posterior limbs of the internal capsule, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, cingulum, anterior thalamic radiation, and corticospinal tract. Moreover, the age-dependent analysis has shown no maturational changes in the ASD subjects. The WM tracts of a relatively larger group of the adolescents were studied in Bode et al. (2011) by examining the TBSS after correcting the entire image, rather than a ROI, which is more typical. As was found in the ASD group, the FA has increased, specially in the right inferior fronto-occipital fasciculus and affected visual perception. It suggests an abnormal information flow between the insular salience processing areas and occipital visual areas of the ASD patients.
3.2.2.3. Adulthood
A recent fMRI and DTI study Kana et al. (2014) examined causal attribution in the ASD and confirmed the relationship for the temporo-parietal junction that exists in the theory of mind. The response to intentional causality showed lower activation of the temporo-parietal junction in the fMRI of the ASD adults, and the analysis of the tract-based spatial DTI statistics revealed reduced FA in the temporal lobe. The tract-based statistics were used also in a more recent investigation Perkins et al. (2014) of the WM integrity in a small group of the ASD adults and their matched controls. The ASD subjects showed significantly decreased FA and high radial diffusivity, λ⊥, values in the left hemisphere, mainly, in the thalamic and fronto-parietal pathways. Moreover, the WM disturbance was higher in the left hemisphere.
3.2.3. ROI Analysis
These analyses depend on prior assumptions about certain brain regions that might be impaired in the ASD subjects. The regions are extracted manually, semi-automatically (using delineation protocols), or automatically. Manual extraction is time-consuming and suffers from user variability, whereas automatic and semi-automatic region segmentation are affected by registration errors, as in the VBA. The fact that the VOI are grouped in a predefined way is the main advantage of analyzing the ROI, as the total number of multiple comparisons is much smaller than in the VBA, statistical decisions become more accurate. However, the ROI analysis cannot infer conclusions about microstructural properties of the WM tracts. It is impractical for examining every brain region, especially for large groups, and has limited accuracy because of low resolution and relatively thick slices of the DTI.
3.2.3.1. Infancy
Microstructural WM differentiation between the ASD and control groups of infants and very young children was examined in Bashat et al. (2007). The fact that the left hemisphere's frontal lobe of the ASD subjects had predominately increased FA and probability, as well as reduced displacement supports the previous findings indicating an abnormal brain overgrowth in very young children.
3.2.3.2. Childhood
The reduced FA of various cerebral WM tracts, which was found in Brito et al. (2009), suggests the ASD correlates with reduced connectivity in the corpus callosum, internal capsule, and superior and middle cerebellar peduncles. The ROI analysis of the cerebellar outflow and inflow pathways in Sivaswamy et al. (2010) has shown the bilaterally decreased MD in the superior cerebellar peduncles, together with the asymmetric FA of the middle cerebellar peduncle and the inferior cerebellar peduncle in the ASD patients. The effect of self-injurious behavior on cortical development of the ASD children was studied in Duerden et al. (2014) by using both T1 MRI and DTI scans. According to the sMRI analysis, both the thickness of the right superior parietal lobule and bilateral primary somatosensory cortices and the volume of the left ventroposterior nucleus of the thalamus correlate with the self-injury scores. The atlas-based ROI analysis has revealed that children engaged in self-injury had significantly decreased FA and increased MD in the the left posterior limb of the internal capsule, as well as increased radial diffusivity, λ⊥, in the bilateral posterior limbs of the internal capsule and corona radiate. Recently, an atlas-based ROI analysis Peterson et al. (2015) was used to investigate abnormalities in various left and right hemispheric WM regions of the HFA children. Their significantly increased MD of the outer-zone cortical left hemisphere WM suggested hypomyelination and increased short-range cortico-cortical connections caused by the early WM overgrowth.
3.2.3.3. Adolescence
The entire corpus callosum and its subregions (genu, body and splenium) were explored for a large group of the HFA subjects and matched controls in Alexander et al. (2007) using the DTI (FA, MD, λ⊥, and λ⊥) and RBV. The low-IQ HFA subgroup differed by small corpus callosum volumes, increased MD, decreased FA, and increased λ⊥. The decreased FA and increased MD and λ⊥ in the superior temporal gyrus and temporal stem in the HFA subjects of the same group have been reported in Lee et al. (2007). Analyzing the WM over the whole brain and in several ROIs in Shukla et al. (2010) has detected decreased FA and increased λ⊥ in both the whole brain and corpus callosum; increased MD for the whole brain and the anterior and posterior limbs of the internal capsule; decreased λ|| in the corpus callossum body, and decreased FA in the middle cerebellar peduncle of the HFA subjects.
3.2.3.4. Adulthood
The fMRI and DTI have been used in Thakkar et al. (2008) to determine whether the structure and function of the anterior cingulate cortex relate to a repetitive behavior in the ASD. The ASD subjects showed an increased rostral anterior cingulate cortex activation to both correct and erroneous responses and had reduced FA in the WM underlying the anterior cingulate cortex. These results correlate also with ratings of the rigid, repetitive behavior. A multi-modality study Beacher et al. (2012) used the VBM and ROI analysis to estimate the GM and WM volumes from the sMRI and evaluate the main WM tracts in the DTI, respectively, for the ASP adults. The total WM volume, regional GM volume in the right parietal operculum, and FA in the body of the corpus callosum, cingulum, and cerebellum suggested a correlation between the diagnosis and subject's gender. These findings confirmed the importance of understanding the sex-specific brain differentiation in the ASD.
3.2.4. Tractography
Tractography reconstructs virtual 3D trajectories of the WM tracts to define the ROIs required for examining several such tracts simultaneously and characterizes macrostructural tract properties with additional DTI measurements. The deterministic tractography reconstructs the WM tracts and measures their lengths, densities, or volumes from a number of streamlines propagated between voxels with similar diffusion properties. But it provides no uncertainty of the reconstructed tracks due to noise or insufficient spatial resolution, whereas the probabilistic tractography not only estimates the fiber tracts, but also measures their uncertainty. However, branching and false-positive tracts affect the tractography accuracy in defining the ROIs, specifically at the ends of the reconstructed tracts. Also, the DTI tractography fails if the DT is inadequate to describe a region. For example, the FA is significantly lower for complex WM fiber crossings. To meet these challenges, new diffusion models, scanning paradigms, and analysis methods are constantly being developed.
3.2.4.1. Infancy
Data from the IBIS (infant brain imaging study) group have been used for a recent longitudinal DTI analysis Wolff et al. (2012) of how the WM fiber tract is organized from 6 to 24 months in high-risk siblings' infants, who developed the ASD by 24 months. The FA and axial (λ||) and radial (λ⊥) diffusivity measured for the DTI were investigated to characterize microstructural properties of the WM fiber tracts. The FA for the infants who developed the ASD differed significantly from those who did not develop it in 12 out of the 15 fiber tracts investigated, including the fornix, uncinate fasciculus, and inferior longitudinal fasciculus. For most of the investigated ASD infants, the FA of the developing fiber tracts was higher at 6 months, had no differences at 12 months, and decreased at 24 months of age. This study provided evidence for the altered brain growth of the WM pathways related to manifesting autistic symptoms for the first year of life, thus confirming the critical importance of the longitudinal studies in revealing the age-related brain and behavior changes underlying the neurodevelopmental disorders. The DTI tractography in Elison et al. (2014) used the same IBIS group, but focused on determining whether specific oculomotor functioning and visual orienting patterns characterize 7-month-old infants diagnosed with the ASD at 24 months and detecting neural associates of their behaviors. The measurements included an average saccadic reaction time in a visually guided saccade procedure and the radial diffusivity, λ⊥, of corticospinal pathways and the splenium and genu of the corpus callosum fiber tracts. Strong association between the λ⊥ of the splenium of the corpus callosum and visual orienting latencies in low-risk infants has been found, but this correlation was missing in the infants having the ASD. These results confirm the potential of acquiring infant imaging groups in identifying early ASD markers, which is critical for early clinical intervention.
3.2.4.2. Childhood
Whether the deterministic tractography of the DTI can detect the WM abnormalities in the frontal lobe and check for short range connectivity changes in the ASD children was investigated in Sundaram et al. (2008). The higher MD in the whole frontal lobe, as well as in long and short range association fibers in the ASD group have been reported. The FA was reduced in the ASD group for the short range, but not long range fibers, and the necessity of advanced DTI technology to re-examine the short range connectivity in ASD was indicated. The brain connectivity in the corpus callosum of the HFA patients was examined in Hong et al. (2011) by measuring both the DTI and T1 MRI. The corpus callosum volume and density extracted from the MRI were compared to the FA, MD, average fiber length, and fiber number obtained from the DTI by deterministic tractography. The decreased WM density in the anterior third of the corpus callosum, and the higher MD and lower fiber number in the anterior third transcallosal fiber tracts of the HFA subjects have been found. The frontal lobe association pathways in the ASD children were studied in Jeong et al. (2011) by analyzing the tract curvature, FA, λ||, and λ⊥. This study suggested that higher curvatures and λ⊥ in the parietotemporal junction of accurate fasciculus, frontotemporal junction of uncinate fasciculus, and the midline of the genu of the corpus callosum could be caused by larger attenuation of the thinner axons in the frontal lobe tracts of the ASD children.
The lack of the nonverbal ASD studies was addressed in Wan et al. (2012) by employing the probabilistic tractography to find language-related WM tracts (arcuate fasciculus). A small group of five nonverbal ASD children demonstrated the reversed arcuate fasciculus asymmetry. To what extent the ASD language ability is associated with the DTI measurements (FA and MD) of the language-related WM tracts (superior longitudinal fasciculus) and non-language-related WM tracts (corticospinal tracts) was investigated in Nagae et al. (2012) with the deterministic tractography. The obtained results have revealed the higher MD in the left hemisphere temporal portion of the superior longitudinal fasciculus fiber tracts of the ASD children with language impairment, as well as a significant negative correlation between the MD and language ability scores. The fMRI and probabilistic tractography of the DTI in Lai et al. (2012) examined the functional and structural organization of neural systems overlapping for language and music in the ASD children. The ASD children have demonstrated decreased functional responses to speech stimulation in the left inferior frontal gyrus and secondary auditory cortices in the left temporal lobe, as well as decreased FA of the left dorsal pathway and the decreased tensor norms in the ventral tract. All the findings indicated that speech and song processing functional systems are more responsive for the song than the speech of the ASD children.
Relations between the WM microstructure and developing the morphosyntax in a spoken narrative were examined in Mills et al. (2013) on a small group of the older HFA children and their matched controls. The HFA children showed abnormally increased MD in the right inferior longitudinal fasciculus. A positive correlation between the morphological accuracy and WM integrity in the right inferior longitudinal fasciculus was found within the HFA group. This study has shown that the HFA children rely on the ventral, rather than typical pathways in their daily use of real world language. Both the sMRI and probabilistic DTI tractography were used in Joseph et al. (2014) to investigate the GM and WM related to language ability in the young ASD children. Compared to the control group, the ASD children had decreased leftward asymmetry of volume and λ⊥ of the arcuate fasciculus, but no difference in the GM asymmetries.
3.2.4.3. Adolescence
Examining the arcuate fasciculus in a group of ASD and control adolescent subjects with the probabilistic tractography Knaus et al. (2010) showed no group differences in FA. But atypical language laterality was more predominant in the ASD, than the control group. Advantages of volumetric DTI segmentation over tractography (its higher robustness to imaging noise, better compatibility with statistical analysis, and no region-to-region analysis) were exemplified in Fletcher et al. (2010) on extracting the WM tracts to study the same arcuate fasciculus on a group of the adolescent HFA and control subjects. The ASD group had increased MD and λ⊥, and decreased FA. The overlap between specific LI and ASD LI in a group of the adolescents and children has been studied in Verhoeven et al. (2012). The deterministic tractography was applied to extract the superior longitudinal fascicle, and no significant differences in the FA and MD have been revealed between the ASD LI participants and controls. However, the FA was significantly reduced in children with the specific LI compared to their controls. The diffusion tractography was used in Lo et al. (2011) to investigate three association fibers, such as the bilateral cingulum bundle, bilateral arcuate fasciculus, and bilateral uncinate fasciculus, as well as the callosal fiber tracts. High-resolution diffusion spectrum imaging (DSI) was applied to a small group of the HFA subjects and controls in order to assess generalized fractional anisotropy (FA) and asymmetry patterns in the targeted fiber tracts. The HFA adolescents showed no leftward asymmetry found in the controls, but had the significantly reduced generalized FA in the three callosal fibers. The deterministic tractography in Ameis et al. (2013) extracted the cingulum bundle from a larger group of children and adolescent HFA subjects and controls. Significant age group interaction for the FA and diffusivity (MD, λ⊥, and λ||), which is driven by reduced FA and increased diffusivity in the ASD groups, but not in the adolescent groups, has been revealed. The fMRI and DTI probabilistic tractography were used in Nair et al. (2013) to examine the integrity of thalamo-cortical connectivity in children and adolescents with the ASD. Increased MD and λ⊥ in the thalamo-cortical connections, and decreased functional connectivity in the thalamo-cortical circuitry, as well as a negative correlation between the fronto-thalamic FA and the social and total autism diagnostic observation schedule (ADOS) scores were reported. The cingulum bundle of a group of the ASD and control adolescents and young adults was investigated in Ikuta et al. (2014). By applying the probabilistic tractography, the low FA of the bilateral anterior cingulum bundle and negative correlation between this FA and the behavior rating inventory of executive function (BRIEF) scores were also identified.
3.2.4.4. Adulthood
The deterministic tractography was used in Catani et al. (2008) to examine microstructural integrity of the intracerebellar pathways in the ASP adults. The study revealed reduced FA of the short intracerebellar fibers and right superior cerebellar output peduncle. It also showed negative correlation with the autism diagnostic interview (ADI) scores. The deterministic tractography in Conturo et al. (2008) reconstructed the WM tracts from the amygdala to the fusiform cortex, and the hippocampus to the fusiform cortex in the ASD and control adults. The ASD group has shown increased λ⊥ in the bilateral amygdala fusiform connections and left hemisphere hippocampus fusiform connections, as well as decreased λ⊥ in the right hippocampus-to-fusiform connections, being associated with the lower face recognition scores and performance IQ. The very first study Pugliese et al. (2009), which examined connections between socio-emotional structures in the adults with ASD using the deterministic tractography, reported an increased number of streamlines (tract volume) in the right cingulum bundle and inferior longitudinal fasciculus of the ASP adults in contrast to their reduced number of streamlines in the right uncinated fasciculus. Significant age-related differences between the autistic and control groups were found in the MD of the left uncinate fasciculus. The deterministic tractography was used also in Thomas et al. (2011) to extract the intra-hemispheric visual-association WM tracts for the HFA adults and detect their high numbers of streamlines in the intra-hemispheric fibers, mainly, in the left hemisphere, and a low number of streamlines in the minor forceps and the body of the corpus callossum. A multi-modality (sMRI and DTI) approach in Langen et al. (2012) has examined differences in the bulk striatum volume and fronto-striatal WM integrity and their relationship with repetitive behavior and inhibitory control of the ASD adults. Analyzing the MRI has shown a smaller WM volume, and the DTI tractography revealed reduced FA in the WM connecting putamen to the frontal cortical areas and increased MD of the WM tracts connecting the accumbens to the frontal cortex. The relationship between the inter-hemispheric connectivity and the brain overgrowth in the ASD adults has been tested in Lewis et al. (2013) with the probabilistic tractography. The estimated callosal fiber length measured the maximum brain size achieved during the development, and compared to the size and structure of the corpus callosum extracted from the T1-weighted MRI. In the ASD adults, the callosal fiber length correlated inversely with the corpus callosum size and positively with the radial diffusivity, λ⊥.
3.2.5. Classification-Based Analysis
Since the advent of their basic concept in the mid-1980s, computer-aided diagnostic (CAD) systems remain in great demand among neuroradiologists Arimura et al. (2009). Most of the present CAD systems perform data preprocessing, feature extraction, and classification. Initial efforts to use features extracted from DTI to classify and diagnose ASD are detailed below.
3.2.5.1. Childhood
Different DT coefficients from many areas across the brain were used in Ingalhalikar et al. (2011) to distinguish autistic children from controls. A high-dimensional nonlinear SVM has learned an underlying ASD pattern of numerous atlas-based regional DTI features, e.g., FA and MD, extracted from the various brain ROIs. According to the leave-one-out (LOO) cross validation, 84% specificity and 74% sensitivity were achieved in separating the autistic patients from the control ones.
3.2.5.2. Adolescence
Shape representations of the WM tracts extracted from the DTI were utilized in Adluru et al. (2009) to distinguish between the autistic and control patients. These fiber bundles were seeded in the splenium of the corpus callosum, and the classification features were built using 3D shape context Belongie et al. (2002). The LOO cross-validation has resulted in an accuracy of 75%; both the specificity and sensitivity of 71%, and an average AUC of 0.765 (the area under the receiver operating characteristic curve). The DTI features of the ROI from the WM of superior temporal gyrus and temporal stem brain regions, which were assumed to have an important role in language, emotion, and social cognition development, were used in Lange et al. (2010) to study a large group of the HFA subjects and their matched controls. The extracted features included the FA, MD, λ⊥, λ||, normalized DT skewness, and hemispheric asymmetry index. Using the selected aforementioned six WM measurements, the system was able to separate the ASD subjects from the controls with 92% accuracy; 94% sensitivity, and 90% specificity.
3.3. Summary
Tables 5–8 summarize, in accordance with the life stage, basic current results of studying the ASD with the DTI.
4. Discussion
The overviewed results of the widespread investigation of the sMRI and DTI modalities in ASD diagnostics outline promising directions of the future work.
Diagnosing ASD with sMRI
Obviously, from the sMRI investigations completed to date, it is clear that the brain of an autistic child is growing abnormally through early childhood. However, a consistent pathology amongst the autistic children is yet to be identified, and few, if any, studies include patients below 2 years of age. Nonetheless, early brain overgrowth does appear to be a consistent and repetitive ASD feature, but this feature is heterogeneous and not all autistic children have an enlarged cerebral volume. Moreover, due to a common misconception of gender bias in the ASD, most of the studies primarily or exclusively focused on males. Also, the statistical significance of the reported findings are hindered due to the small population of subjects considered.
Within the literature, the effects of age on various anatomical features such as, the dynamics of the amygdalae and cerebral volumes, tend to be contradictory. Furthermore, multiple cross-sectional studies examining brain stem size in ASD patients have shown inconsistent results, and no study to date specifically assesses how the brain stem develops with age. Also, studying the brain stem should become volumetric and focus on selecting proper planes of cut, because most of the current studies have transected the brain stem and provided only area measurements. As a result, additional longitudinal studies are needed before any conclusions can be drawn on the role of brainstem development in autism.
Deciphering the role of different brain regions and networks in autism may benefit significantly from using multimodality approaches, which combine various resting state, task-evoked, and structural MRI measurements and rely on advanced data acquisition and analysis.
The utilization of MRI-based methods to study brain development in larger populations will likely begin to address heterogeneity in ASD patients and identify distinct autism subtypes, each with a specific associated neuroanatomical phenotype. However, it is anticipated that the maximum scientific benefit will be achieved from conducting longitudinal studies in younger populations with additional characterization of the underlying genetic abnormalities, along with other potential biomarkers, driving the overgrowth and onset of autistic symptoms at a critical time in the developmental process. Hence, it is of great significance to establish more effective biomarkers in order to facilitate diagnostics in patients before their conventional ASD symptoms become evident.
Future studies that include more than two time points of MRI data would be particularly helpful in the construction and comparison of ASD and normal brain development patterns. Yet, another promising method for understanding the relationship between brain development and ASD is to statistically analyze local 3D shapes and surfaces of the primary components of the brain and correlate these results to the common behavioral scores, such as the social responsiveness scale (SBS). However, correlating the results will be challenging, since such scales were developed as screening tools to qualitatively describe ASD severity, rather than to quantify the health deficits.
Diagnosing ASD with DTI
The VBA, applied mostly to small and predominantly adolescent groups, has shown decreased FA in the major WM tracts of patients with ASD. The usefulness of VBA of DTI remains in doubt because this method depends on the size of the smoothing kernel and hinders confident conclusions. The TBSS, optimizing the VBA of DTI and thus outperforming the traditional VBA, have been used in the majority of cases to examine the whole brain in older children and adolescents. Most of the larger studies have reported reduced FA and increased diffusivity in the ASD groups. Also, the ROI-based DTI studies of the older population (older children, adolescents, and adults) vs. their controls confirm the finding of decreased FA in various brain areas including the anterior cingulate, the superior temporal gyrus, and the temporal stem WM. However, since the ROIs target only specific components of particular WM tracts, conclusions cannot be drawn on the entire WM tract or other infrequently examined tracts. Nonetheless, studying the DTI of older children, adolescents, and adults using the VBA, TBSS, and ROI methods suggest that the ASD-related microstructural WM alterations differ depending on the age of the individuals studied.
Mostly, the deterministic, rather than the advanced probabilistic tractography is employed to study the DTI, and the bulk of these studies have focused on the WM abnormalities in the uncinate fasciculus, the arcuate fasciculus, the cingulum bundle, the inferior longitudinal fasciculus, and the inferior fronto-occipital fasciculus. The abnormalities include lower FA and higher diffusivity (primarily, in children and adolescents) and altered macrostructure (mainly, in adults). The only ASD-related longitudinal DTI study to date Wolff et al. (2012) confirmed that at least during the first 2 years of life, a number of WM tracts seem to be affected by a dynamic process occurring in the brains of children who develop the ASD.
General conclusions
Although a variety of MRI-based studies consisting of small groups of individuals, ranging widely in age, present diverse clinical ASD symptoms compared to controls, these studies have yielded valuable, yet varying results. Multi-site studies with large patient groups that comprehensively characterize the clinical, behavioral, and cognitive symptoms may be one of the most important strategies for increasing the probability for the discovery of new, effective biomarkers. Future studies of particular importance will track brain maturation from infancy to adulthood in individuals at high risk of developing ASD by using multi-modality macro-/microstructural and functional brain images. By studying a larger, non-gender biased population of patients, it is anticipated that the “false positive” rate of ASD diagnosis will decrease.
Author Contributions
MI: wrote the structural MRI survey part, and helped in DTI part. Reviewed the entire paper. RK: reviewed the paper, reshaped the outline and structure. MM: wrote the DTI part. AELT: reviewed the paper, helped collecting literature. MC: reviewed, emphasized the clinical aspect of the paper, edited the future directions section. GG: reviewed the engineering aspect of the paper, reshaped the paper, and extensively edited it. AELB: directed the paper, helped with the outline and the general structure.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
References
Abell, F., Krams, M., Ashburner, J., Passingham, R., Friston, K., Frackowiak, R., et al. (1999). The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport 10, 1647–1651. doi: 10.1097/00001756-199906030-00005
Adluru, N., Hinrichs, C., Chung, M. K., Lee, J.-E., Singh, V., Bigler, E. D., et al. (2009). “Classification in dti using shapes of white matter tracts,” in Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE (Minneapolis, MN: IEEE), 2719–2722. doi: 10.1109/iembs.2009.5333386
Adolphs, R., Tranel, D., Damasio, H., and Damasio, A. (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372, 669–672. doi: 10.1038/372669a0
Akshoomoff, N., Lord, C., Lincoln, A. J., Courchesne, R. Y., Carper, R. A., Townsend, J., et al. (2004). Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. J. Am. Acad. Child Adolesc. Psychiatry 43, 349–357. doi: 10.1097/00004583-200403000-00018
Alexander, A. L., Lee, J. E., Lazar, M., Boudos, R., DuBray, M. B., Oakes, T. R., et al. (2007). Diffusion tensor imaging of the corpus callosum in autism. Neuroimage 34, 61–73. doi: 10.1016/j.neuroimage.2006.08.032
Ameis, S. H., Fan, J., Rockel, C., Soorya, L., Wang, A. T., and Anagnostou, E. (2013). Altered cingulum bundle microstructure in autism spectrum disorder. Acta Neuropsychiatrica 25, 275–282. doi: 10.1017/neu.2013.2
Ameis, S. H., Fan, J., Rockel, C., Voineskos, A. N., Lobaugh, N. J., Soorya, L., et al. (2011). Impaired structural connectivity of socio-emotional circuits in autism spectrum disorders: a diffusion tensor imaging study. PLoS ONE 6:e28044. doi: 10.1371/journal.pone.0028044
Arimura, H., Magome, T., Yamashita, Y., and Yamamoto, D. (2009a). Computer-aided diagnosis systems for brain diseases in magnetic resonance images. Algorithms 2, 925–952. doi: 10.3390/a2030925
Aylward, E., Minshew, N., Goldstein, G., Honeycutt, N., Augustine, A., Yates, K., et al. (1999). MRI volumes of amygdala and hippocampus in non–mentally retarded autistic adolescents and adults. Neurology 53, 2145–2145. doi: 10.1212/WNL.53.9.2145
Aylward, E. H., Minshew, N., Field, K., Sparks, B., and Singh, N. (2002). Effects of age on brain volume and head circumference in autism. Neurology 59, 175–183. doi: 10.1212/WNL.59.2.175
Bachevalier, J. (1994). Medial temporal lobe structures and autism: a review of clinical and experimental findings. Neuropsychologia 32, 627–648. doi: 10.1016/0028-3932(94)90025-6
Bailey, A., Luthert, P., Bolton, P., Le Couteur, A., Rutter, M., and Harding, B. (1993). Autism and megalencephaly. Lancet 341, 1225–1226. doi: 10.1016/0140-6736(93)91065-T
Bailey, A., Luthert, P., Dean, A., Harding, B., Janota, I., Montgomery, M., et al. (1998). A clinicopathological study of autism. Brain 121, 889–905. doi: 10.1093/brain/121.5.889
Barnea-Goraly, N., Frazier, T. W., Piacenza, L., Minshew, N. J., Keshavan, M. S., Reiss, A. L., et al. (2014). A preliminary longitudinal volumetric mri study of amygdala and hippocampal volumes in autism. Progress Neuro-Psychopharmacol. Biol. Psychiatry 48, 124–128. doi: 10.1016/j.pnpbp.2013.09.010
Barnea-Goraly, N., Kwon, H., Menon, V., Eliez, S., Lotspeich, L., and Reiss, A. L. (2004). White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol. Psychiatry 55, 323–326. doi: 10.1016/j.biopsych.2003.10.022
Barnea-Goraly, N., Lotspeich, L. J., and Reiss, A. L. (2010). Similar white matter aberrations in children with autism and their unaffected siblings: a diffusion tensor imaging study using tract-based spatial statistics. Arch. Gen. Psychiatry 67, 1052–1060. doi: 10.1001/archgenpsychiatry.2010.123
Bashat, D. B., Kronfeld-Duenias, V., Zachor, D. A., Ekstein, P. M., Hendler, T., Tarrasch, R., et al. (2007). Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage 37, 40–47. doi: 10.1016/j.neuroimage.2007.04.060
Basser, P. J., and Jones, D. K. (2002). Diffusion-tensor MRI: theory, experimental design and data analysis–a technical review. NMR Biomed. 15, 456–467. doi: 10.1002/nbm.783
Basser, P. J., Mattiello, J., and LeBihan, D. (1994a). Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B 103, 247–254. doi: 10.1006/jmrb.1994.1037
Basser, P. J., Mattiello, J., and LeBihan, D. (1994b). MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267. doi: 10.1016/S0006-3495(94)80775-1
Basser, P. J., Pajevic, S., Pierpaoli, C., Duda, J., and Aldroubi, A. (2000). In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 44, 625–632. doi: 10.1002/1522-2594(200010)44:4<625::AID-MRM17>3.0.CO;2-O
Bauman, M., and Kemper, T. L. (1985). Histoanatomic observations of the brain in early infantile autism. Neurology 35, 866–866. doi: 10.1212/WNL.35.6.866
Bauman, M. L., and Kemper, T. L. (1994). Neuroanatomic observations of the brain in autism. Neurobiol. Autism 612, 119–145.
Beacher, F., Minati, L., Baron-Cohen, S., Lombardo, M., Lai, M.-C., Gray, M., et al. (2012). Autism attenuates sex differences in brain structure: a combined voxel-based morphometry and diffusion tensor imaging study. Am. J. Neuroradiol. 33, 83–89. doi: 10.3174/ajnr.A2880
Behrens, T., Woolrich, M., Jenkinson, M., Johansen-Berg, H., Nunes, R., Clare, S., et al. (2003). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 50, 1077–1088. doi: 10.1002/mrm.10609
Belongie, S., Malik, J., and Puzicha, J. (2002). Shape matching and object recognition using shape contexts. IEEE Trans. Patt. Anal. Mach. Intell. 24, 509–522. doi: 10.1109/34.993558
Billeci, L., Calderoni, S., Tosetti, M., Catani, M., and Muratori, F. (2012). White matter connectivity in children with autism spectrum disorders: a tract-based spatial statistics study. BMC Neurol. 12:148. doi: 10.1186/1471-2377-12-148
Blackmon, K. (2015). Structural MRI biomarkers of shared pathogenesis in autism spectrum disorder and epilepsy. Epilepsy Behav. 47, 172–182. doi: 10.1016/j.yebeh.2015.02.017
Bloemen, O. J., Deeley, Q., Sundram, F., Daly, E. M., Barker, G. J., Jones, D. K., et al. (2010). White matter integrity in asperger syndrome: a preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Res. 3, 203–213. doi: 10.1002/aur.146
Boddaert, N., Chabane, N., Gervais, H., Good, C., Bourgeois, M., Plumet, M., et al. (2004). Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage 23, 364–369. doi: 10.1016/j.neuroimage.2004.06.016
Bode, M. K., Mattila, M.-L., Kiviniemi, V., Rahko, J., Moilanen, I., Ebeling, H., et al. (2011). White matter in autism spectrum disorders–evidence of impaired fiber formation. Acta Radiol. 52, 1169–1174. doi: 10.1258/ar.2011.110197
Bonilha, L., Cendes, F., Rorden, C., Eckert, M., Dalgalarrondo, P., Li, L. M., et al. (2008). Gray and white matter imbalance–typical structural abnormality underlying classic autism? Brain Dev. 30, 396–401. doi: 10.1016/j.braindev.2007.11.006
Brambilla, P., Hardan, A., di Nemi, S. U., Perez, J., Soares, J. C., and Barale, F. (2003). Brain anatomy and development in autism: review of structural MRI studies. Brain Res. Bull. 61, 557–569. doi: 10.1016/j.brainresbull.2003.06.001
Brito, A. R., Vasconcelos, M. M., Domingues, R. C., da Cruz, H. Jr., Celso, L., Rodrigues, L. D. S., et al. (2009). Diffusion tensor imaging findings in school-aged autistic children. J. Neuroimaging 19, 337–343. doi: 10.1111/j.1552-6569.2009.00366.x
Brun, C. C., Nicolson, R., Leporé, N., Chou, Y.-Y., Vidal, C. N., DeVito, T. J., et al. (2009). Mapping brain abnormalities in boys with autism. Hum. Brain Mapp. 30, 3887–3900. doi: 10.1002/hbm.20814
Carper, R. A., and Courchesne, E. (2000). Inverse correlation between frontal lobe and cerebellum sizes in children with autism. Brain 123, 836–844. doi: 10.1093/brain/123.4.836
Carper, R. A., Moses, P., Tigue, Z. D., and Courchesne, E. (2002). Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage 16, 1038–1051. doi: 10.1006/nimg.2002.1099
Casanova, M. F., Buxhoeveden, D. P., and Brown, C. (2002a). Clinical and macroscopic correlates of minicolumnar pathology in autism. J. Child Neurol. 17, 692–695. doi: 10.1177/088307380201700908
Casanova, M. F., Buxhoeveden, D. P., Switala, A. E., and Roy, E. (2002b). Neuronal density and architecture (gray level index) in the brains of autistic patients. J. Child Neurol. 17, 515–521. doi: 10.1177/088307380201700708
Casanova, M. F., van Kooten, I. A., Switala, A. E., van Engeland, H., Heinsen, H., Steinbusch, H. W., et al. (2006). Minicolumnar abnormalities in autism. Acta Neuropathol. 112, 287–303. doi: 10.1007/s00401-006-0085-5
Catani, M., Jones, D. K., Daly, E., Embiricos, N., Deeley, Q., Pugliese, L., et al. (2008). Altered cerebellar feedback projections in asperger syndrome. Neuroimage 41, 1184–1191. doi: 10.1016/j.neuroimage.2008.03.041
Chen, R., Jiao, Y., and Herskovits, E. H. (2011). Structural MRI in autism spectrum disorder. Pediatr. Res. 69, 63R–68R. doi: 10.1203/pdr.0b013e318212c2b3
Cheon, K.-A., Kim, Y.-S., Oh, S.-H., Park, S.-Y., Yoon, H.-W., Herrington, J., et al. (2011). Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: a diffusion tensor imaging study. Brain Res. 1417, 77–86. doi: 10.1016/j.brainres.2011.08.020
Cheung, C., Chua, S., Cheung, V., Khong, P., Tai, K., Wong, T., et al. (2009). White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J. Child Psychol. Psychiatry 50, 1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x
Chung, M. K., Dalton, K. M., Alexander, A. L., and Davidson, R. J. (2004). Less white matter concentration in autism: 2d voxel-based morphometry. Neuroimage 23, 242–251. doi: 10.1016/j.neuroimage.2004.04.037
Ciesielski, K. T., Harris, R. J., Hart, B. L., and Pabst, H. F. (1997). Cerebellar hypoplasia and frontal lobe cognitive deficits in disorders of early childhood. Neuropsychologia 35, 643–655. doi: 10.1016/S0028-3932(96)00119-4
Conti, E., Calderoni, S., Marchi, V., Muratori, F., Cioni, G., and Guzzetta, A. (2015). The first 1000 days of the autistic brain: a systematic review of diffusion imaging studies. Front. Hum. Neurosci. 9:159. doi: 10.3389/fnhum.2015.00159
Conturo, T. E., Williams, D. L., Smith, C. D., Gultepe, E., Akbudak, E., and Minshew, N. J. (2008). Neuronal fiber pathway abnormalities in autism: an initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. J. Int. Neuropsychol. Soc. 14, 933–946. doi: 10.1017/S1355617708081381
Courchesne, E., Karns, C., Davis, H., Ziccardi, R., Carper, R., Tigue, Z., et al. (2001). Unusual brain growth patterns in early life in patients with autistic disorder an MRI study. Neurology 57, 245–254. doi: 10.1212/WNL.57.2.245
Courchesne, E., Müller, R.-A., and Saitoh, O. (1999). Brain weight in autism: normal in the majority of cases, megalencephalic in rare cases. Neurology 52, 1057–1057. doi: 10.1212/WNL.52.5.1057
Courchesne, E., Press, G., and Yeung-Courchesne, R. (1993). Parietal lobe abnormalities detected with MR in patients with infantile autism. Am. J. Roentgenol. 160, 387–393. doi: 10.2214/ajr.160.2.8424359
Courchesne, E., Saitoh, O., Yeung-Courchesne, R., Press, G., Lincoln, A., Haas, R., et al. (1994a). Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. Am. J. Roentgenol. 162, 123–130. doi: 10.2214/ajr.162.1.8273650
Courchesne, E., Townsend, J., and Saitoh, O. (1994b). The brain in infantile autism posterior fossa structures are abnormal. Neurology 44, 214. doi: 10.1212/WNL.44.2.214
Courchesne, E., Yeung-Courchesne, R., Hesselink, J., and Jernigan, T. (1988). Hypoplasia of cerebellar vermal lobules VI and VII in autism. N. Engl. J. Med. 318, 1349–1354. doi: 10.1056/NEJM198805263182102
Damasio, A. R., and Maurer, R. G. (1978). A neurological model for childhood autism. Arch. Neurol. 35, 777–786. doi: 10.1001/archneur.1978.00500360001001
Dawson, G., Meltzoff, A. N., Osterling, J., and Rinaldi, J. (1998). Neuropsychological correlates of early symptoms of autism. Child Dev. 69, 1276–1285. doi: 10.2307/1132265
Dierker, D. L., Feczko, E., Pruett, J. R. Jr., Petersen, S. E., Schlaggar, B. L., Constantino, J. N., et al. (2015). Analysis of cortical shape in children with simplex autism. Cereb. Cortex 25, 1042–1051. doi: 10.1093/cercor/bht294
Dong, Q., Welsh, R. C., Chenevert, T. L., Carlos, R. C., Maly-Sundgren, P., Gomez-Hassan, D. M., et al. (2004). Clinical applications of diffusion tensor imaging. J. Magn. Reson. Imaging 19, 6–18. doi: 10.1002/jmri.10424
Duerden, E. G., Card, D., Roberts, S. W., Mak-Fan, K. M., Chakravarty, M. M., Lerch, J. P., et al. (2014). Self-injurious behaviours are associated with alterations in the somatosensory system in children with autism spectrum disorder. Brain Struct. Funct. 219, 1251–1261. doi: 10.1007/s00429-013-0562-2
Egaas, B., Courchesne, E., and Saitoh, O. (1995). Reduced size of corpus callosum in autism. Arch. Neurol. 52, 794–801. doi: 10.1001/archneur.1995.00540320070014
Elia, M., Ferri, R., Musumeci, S. A., Panerai, S., Bottitta, M., and Scuderi, C. (2000). Clinical correlates of brain morphometric features of subjects with low-functioning autistic disorder. J. Child Neurol. 15, 504–508. doi: 10.1177/088307380001500802
Elia, M., Manfre, L., Ferri, R., Musumeci, S., Panerai, S., Bottitta, M., et al. (1997). Brain morphometry and psychobehavioural measures in autistic low-functioning subjects. Rivista Neuroradiologia 10, 431–436. doi: 10.1177/197140099701000406
Elison, J. T., Paterson, S. J., Wolff, J. J., Reznick, J. S., Sasson, N. J., Gu, H., et al. (2014). White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am. J. Psychiatry 170, 899–908. doi: 10.1176/appi.ajp.2012.12091150
Elnakib, A., Casanova, M., Gimel'farb, G., Switala, A. E., and El-Baz, A. (2011). “Autism diagnostics by centerline-based shape analysis of the corpus callosum,” in Biomedical Imaging: From Nano to Macro, 2011 IEEE International Symposium on (Chicago, IL: IEEE), 1843–1846. doi: 10.1109/isbi.2011.5872766
Fletcher, P. T., Whitaker, R. T., Tao, R., DuBray, M. B., Froehlich, A., Ravichandran, C., et al. (2010). Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage 51, 1117–1125. doi: 10.1016/j.neuroimage.2010.01.083
Fombonne, E., Rogé, B., Claverie, J., Courty, S., and Fremolle, J. (1999). Microcephaly and macrocephaly in autism. J. Autism Dev. Disord. 29, 113–119. doi: 10.1023/A:1023036509476
Frazier, T. W., Keshavan, M. S., Minshew, N. J., and Hardan, A. Y. (2012). A two-year longitudinal MRI study of the corpus callosum in autism. J. Autism Dev. Disord. 42, 2312–2322. doi: 10.1007/s10803-012-1478-z
Gaffney, G. R., Kuperman, S., Tsai, L. Y., and Minchin, S. (1988). Morphological evidence for brainstem involvement in infantile autism. Biol. Psychiatry 24, 578–586. doi: 10.1016/0006-3223(88)90168-0
Gaffney, G. R., Kuperman, S., Tsai, L. Y., and Minchin, S. (1989). Forebrain structure in infantile autism. J. Am. Acad. Child Adolesc. Psychiatry 28, 534–537. doi: 10.1097/00004583-198907000-00011
Gaffney, G. R., Kuperman, S., Tsai, L. Y., Minchin, S., and Hassanein, K. M. (1987a). Midsagittal magnetic resonance imaging of autism. Br. J. Psychiatry 151, 831–833. doi: 10.1192/bjp.151.6.831
Gaffney, G. R., Tsai, L. Y., Kuperman, S., and Minchin, S. (1987b). Cerebellar structure in autism. Am. J. Dis. Child. 141, 1330–1332. doi: 10.1001/archpedi.1987.04460120096044
Garber, H. J., and Ritvo, E. R. (1992). Magnetic resonance imaging of the posterior fossa in autistic adults. Am. J. Psychiatry 149, 245–247.
Garber, H. J., Ritvo, E. R., Chiu, L. C., Griswold, V. J., Kashanian, A., Freeman, B. J., et al. (1989). A magnetic resonance imaging study of autism: normal fourth ventricle size and absence of pathology. Am. J. Psychiatry 146, 532–534.
George, M. S., Costa, D. C., Kouris, K., Ring, H. A., and Ell, P. J. (1992). Cerebral blood flow abnormalities in adults with infantile autism. J. Nervous Ment. Dis. 180, 413–417. doi: 10.1097/00005053-199207000-00002
Giedd, J. N., Snell, J. W., Lange, N., Rajapakse, J. C., Casey, B., Kozuch, P. L., et al. (1996). Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb. Cortex 6, 551–559. doi: 10.1093/cercor/6.4.551
Gillberg, C. (1999). Neurodevelopmental processes and psychological functioning in autism. Dev. Psychopathol. 11, 567–587. doi: 10.1017/S0954579499002217
Girgis, R. R., Minshew, N. J., Melhem, N. M., Nutche, J. J., Keshavan, M. S., and Hardan, A. Y. (2007). Volumetric alterations of the orbitofrontal cortex in autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 31, 41–45. doi: 10.1016/j.pnpbp.2006.06.007
Gori, I., Giuliano, A., Muratori, F., Saviozzi, I., Oliva, P., Tancredi, R., et al. (2015). Gray matter alterations in young children with autism spectrum disorders: comparing morphometry at the voxel and regional level. J. Neuroimaging 25, 866–874. doi: 10.1111/jon.12280
Groen, W. B., Buitelaar, J. K., Van Der Gaag, R. J., and Zwiers, M. P. (2011). Pervasive microstructural abnormalities in autism: a DTI study. J. Psychiatry Neurosci. 36, 32. doi: 10.1503/jpn.090100
Gropman, A., Gertz, B., Shattuck, K., Kahn, I., Seltzer, R., Krivitsky, L., et al. (2010). Diffusion tensor imaging detects areas of abnormal white matter microstructure in patients with partial ornithine transcarbamylase deficiency. Am. J. Neuroradiol. 31, 1719–1723. doi: 10.3174/ajnr.A2122
Hadjikhani, N., Joseph, R. M., Snyder, J., and Tager-Flusberg, H. (2006). Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb. Cortex 16, 1276–1282. doi: 10.1093/cercor/bhj069
Hardan, A. Y., Girgis, R. R., Adams, J., Gilbert, A. R., Keshavan, M. S., and Minshew, N. J. (2006a). Abnormal brain size effect on the thalamus in autism. Psychiatry Res. 147, 145–151. doi: 10.1016/j.pscychresns.2005.12.009
Hardan, A. Y., Jou, R. J., Keshavan, M. S., Varma, R., and Minshew, N. J. (2004). Increased frontal cortical folding in autism: a preliminary MRI study. Psychiatry Res. 131, 263–268. doi: 10.1016/j.pscychresns.2004.06.001
Hardan, A. Y., Kilpatrick, M., Keshavan, M. S., and Minshew, N. J. (2003). Motor performance and anatomic magnetic resonance imaging (MRI) of the basal ganglia in autism. J. Child Neurol. 18, 317–324. doi: 10.1177/08830738030180050801
Hardan, A. Y., Libove, R. A., Keshavan, M. S., Melhem, N. M., and Minshew, N. J. (2009). A preliminary longitudinal magnetic resonance imaging study of brain volume and cortical thickness in autism. Biol. Psychiatry 66, 320–326. doi: 10.1016/j.biopsych.2009.04.024
Hardan, A. Y., Minshew, N. J., Harenski, K., and Keshavan, M. S. (2001a). Posterior fossa magnetic resonance imaging in autism. J. Am. Acad. Child Adolesc. Psychiatry 40, 666–672. doi: 10.1097/00004583-200106000-00011
Hardan, A. Y., Minshew, N. J., Mallikarjuhn, M., and Keshavan, M. S. (2001b). Brain volume in autism. J. Child Neurol. 16, 421–424. doi: 10.1177/088307380101600607
Hardan, A. Y., Muddasani, S., Vemulapalli, M., Keshavan, M. S., and Minshew, N. J. (2006b). An MRI study of increased cortical thickness in autism. Am. J. Psychiatry 163, 1290–1292. doi: 10.1176/ajp.2006.163.7.1290
Hashimoto, T., Murakawa, K., Miyazaki, M., Tayama, M., and Kuroda, Y. (1992). Magnetic resonance imaging of the brain structures in the posterior fossa in retarded autistic children. Acta Paediatrica 81, 1030–1034. doi: 10.1111/j.1651-2227.1992.tb12169.x
Hashimoto, T., Tayama, M., Miyazaki, M., Murakawa, K., and Kuroda, Y. (1993). Brainstem and cerebellar vermis involvement in autistic children. J. Child Neurol. 8, 149–153. doi: 10.1177/088307389300800207
Hashimoto, T., Tayama, M., Murakawa, K., Yoshimoto, T., Miyazaki, M., Harada, M., et al. (1995). Development of the brainstem and cerebellum in autistic patients. J. Autism Dev. Disord. 25, 1–18. doi: 10.1007/BF02178163
Hazlett, H. C., Gu, H., McKinstry, R. C., Shaw, D. W., Botteron, K. N., Dager, S. R., et al. (2012). Brain volume findings in 6-month-old infants at high familial risk for autism. Am. J. Psychiatry 169, 601–608. doi: 10.1176/appi.ajp.2012.11091425
Hazlett, H. C., Poe, M., Gerig, G., Smith, R. G., Provenzale, J., Ross, A., et al. (2005). Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch. Gen. Psychiatry 62, 1366–1376. doi: 10.1001/archpsyc.62.12.1366
Hazlett, H. C., Poe, M. D., Gerig, G., Smith, R. G., and Piven, J. (2006). Cortical gray and white brain tissue volume in adolescents and adults with autism. Biol. Psychiatry 59, 1–6. doi: 10.1016/j.biopsych.2005.06.015
Haznedar, M. M., Buchsbaum, M. S., Hazlett, E. A., LiCalzi, E. M., Cartwright, C., and Hollander, E. (2006). Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. Am. J. Psychiatry 163, 1252–1263. doi: 10.1176/ajp.2006.163.7.1252
Herbert, M., Ziegler, D., Deutsch, C., Obrien, L., Lange, N., Bakardjiev, A., et al. (2003). Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain 126, 1182–1192. doi: 10.1093/brain/awg110
Holttum, J. R., Minshew, N. J., Sanders, R. S., and Phillips, N. E. (1992). Magnetic resonance imaging of the posterior fossa in autism. Biol. Psychiatry 32, 1091–1101. doi: 10.1016/0006-3223(92)90189-7
Hong, S., Ke, X., Tang, T., Hang, Y., Chu, K., Huang, H., et al. (2011). Detecting abnormalities of corpus callosum connectivity in autism using magnetic resonance imaging and diffusion tensor tractography. Psychiatry Res. 194, 333–339. doi: 10.1016/j.pscychresns.2011.03.009
Horwitz, B., Rumsey, J. M., Grady, C. L., and Rapoport, S. I. (1988). The cerebral metabolic landscape in autism: intercorrelations of regional glucose utilization. Arch. Neurol. 45, 749–755. doi: 10.1001/archneur.1988.00520310055018
Hsu, M., Yeung-Courchesne, R., Courchesne, E., and Press, G. A. (1991). Absence of magnetic resonance imaging evidence of pontine abnormality in infantile autism. Arch. Neurol. 48, 1160–1163. doi: 10.1001/archneur.1991.00530230068024
Hua, X., Thompson, P. M., Leow, A. D., Madsen, S. K., Caplan, R., Alger, J. R., et al. (2013). Brain growth rate abnormalities visualized in adolescents with autism. Hum. Brain Mapp. 34, 425–436. doi: 10.1002/hbm.21441
Hyde, K. L., Samson, F., Evans, A. C., and Mottron, L. (2010). Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum. Brain Mapp. 31, 556–566. doi: 10.1002/hbm.20887
Ikuta, T., Shafritz, K. M., Bregman, J., Peters, B. D., Gruner, P., Malhotra, A. K., et al. (2014). Abnormal cingulum bundle development in autism: a probabilistic tractography study. Psychiatry Res. 221, 63–68. doi: 10.1016/j.pscychresns.2013.08.002
Ingalhalikar, M., Parker, D., Bloy, L., Roberts, T. P., and Verma, R. (2011). Diffusion based abnormality markers of pathology: toward learned diagnostic prediction of ASD. Neuroimage 57, 918–927. doi: 10.1016/j.neuroimage.2011.05.023
Jeong, J.-W., Kumar, A., Sundaram, S. K., Chugani, H. T., and Chugani, D. C. (2011). Sharp curvature of frontal lobe white matter pathways in children with autism spectrum disorders: tract-based morphometry analysis. Am. J. Neuroradiol. 32, 1600–1606. doi: 10.3174/ajnr.A2557
Jiao, Y., Chen, R., Ke, X., Chu, K., Lu, Z., and Herskovits, E. H. (2010). Predictive models of autism spectrum disorder based on brain regional cortical thickness. Neuroimage 50, 589–599. doi: 10.1016/j.neuroimage.2009.12.047
Joseph, R. M., Fricker, Z., Fenoglio, A., Lindgren, K. A., Knaus, T. A., and Tager-Flusberg, H. (2014). Structural asymmetries of language-related gray and white matter and their relationship to language function in young children with ASD. Brain Imaging Behav. 8, 60–72. doi: 10.1007/s11682-013-9245-0
Jou, R., Mateljevic, N., Kaiser, M., Sugrue, D., Volkmar, F., and Pelphrey, K. (2011a). Structural neural phenotype of autism: preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. Am. J. Neuroradiol. 32, 1607–1613. doi: 10.3174/ajnr.A2558
Jou, R. J., Frazier, T. W., Keshavan, M. S., Minshew, N. J., and Hardan, A. Y. (2013). A two-year longitudinal pilot MRI study of the brainstem in autism. Behav. Brain Res. 251, 163–167. doi: 10.1016/j.bbr.2013.04.021
Jou, R. J., Jackowski, A. P., Papademetris, X., Rajeevan, N., Staib, L. H., and Volkmar, F. R. (2011b). Diffusion tensor imaging in autism spectrum disorders: preliminary evidence of abnormal neural connectivity. Aust. New Zealand J. Psychiatry 45, 153–162. doi: 10.3109/00048674.2010.534069
Jou, R. J., Minshew, N. J., Melhem, N. M., Keshavan, M. S., and Hardan, A. Y. (2009). Brainstem volumetric alterations in children with autism. Psychol. Med. 39, 1347–1354. doi: 10.1017/S0033291708004376
Kana, R. K., Libero, L. E., Hu, C. P., Deshpande, H. D., and Colburn, J. S. (2014). Functional brain networks and white matter underlying theory-of-mind in autism. Soc. Cogn. Affect. Neurosci. 9, 98–105. doi: 10.1093/scan/nss106
Kaufmann, W. E., Cooper, K. L., Mostofsky, S. H., Capone, G. T., Kates, W. R., Newschaffer, C. J., et al. (2003). Specificity of cerebellar vermian abnormalities in autism: a quantitative magnetic resonance imaging study. J. Child Neurol. 18, 463–470. doi: 10.1177/08830738030180070501
Ke, X., Tang, T., Hong, S., Hang, Y., Zou, B., Li, H., et al. (2009). White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res. 1265, 171–177. doi: 10.1016/j.brainres.2009.02.013
Keller, T. A., Kana, R. K., and Just, M. A. (2007). A developmental study of the structural integrity of white matter in autism. Neuroreport 18, 23–27. doi: 10.1097/01.wnr.0000239965.21685.99
Kleiman, M. D., Neff, S., and Rosman, N. P. (1992). The brain in infantile autism are posterior fossa structures abnormal? Neurology 42, 753–753. doi: 10.1212/WNL.42.4.753
Knaus, T. A., Silver, A. M., Kennedy, M., Lindgren, K. A., Dominick, K. C., Siegel, J., et al. (2010). Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain Lang. 112, 113–120. doi: 10.1016/j.bandl.2009.11.005
Koay, C. G., Chang, L.-C., Carew, J. D., Pierpaoli, C., and Basser, P. J. (2006). A unifying theoretical and algorithmic framework for least squares methods of estimation in diffusion tensor imaging. J. Magn. Reson. 182, 115–125. doi: 10.1016/j.jmr.2006.06.020
Kumar, A., Sundaram, S. K., Sivaswamy, L., Behen, M. E., Makki, M. I., Ager, J., et al. (2010). Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cereb. Cortex 20, 2103–2113. doi: 10.1093/cercor/bhp278
Kwon, H., Ow, A. W., Pedatella, K. E., Lotspeich, L. J., and Reiss, A. L. (2004). Voxel-based morphometry elucidates structural neuroanatomy of high-functioning autism and asperger syndrome. Dev. Med. Child Neurol. 46, 760–764. doi: 10.1111/j.1469-8749.2004.tb00996.x
Lai, G., Pantazatos, S. P., Schneider, H., and Hirsch, J. (2012). Neural systems for speech and song in autism. Brain 135, 961–975. doi: 10.1093/brain/awr335
Lainhart, J. E., Piven, J., Wzorek, M., Landa, R., Santangelo, S. L., Coon, H., et al. (1997). Macrocephaly in children and adults with autism. J. Am. Acad. Child Adolesc. Psychiatry 36, 282–290. doi: 10.1097/00004583-199702000-00019
Lange, N., DuBray, M. B., Lee, J. E., Froimowitz, M. P., Froehlich, A., Adluru, N., et al. (2010). Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. 3, 350–358. doi: 10.1002/aur.162
Langen, M., Leemans, A., Johnston, P., Ecker, C., Daly, E., Murphy, C. M., et al. (2012). Fronto-striatal circuitry and inhibitory control in autism: findings from diffusion tensor imaging tractography. Cortex 48, 183–193. doi: 10.1016/j.cortex.2011.05.018
Le Bihan, D., Mangin, J.-F., Poupon, C., Clark, C. A., Pappata, S., Molko, N., et al. (2001). Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging 13, 534–546. doi: 10.1002/jmri.1076
Lee, J. E., Bigler, E. D., Alexander, A. L., Lazar, M., DuBray, M. B., Chung, M. K., et al. (2007). Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci. Lett. 424, 127–132. doi: 10.1016/j.neulet.2007.07.042
Lee, J. E., Chung, M. K., Lazar, M., DuBray, M. B., Kim, J., Bigler, E. D., et al. (2009). A study of diffusion tensor imaging by tissue-specific, smoothing-compensated voxel-based analysis. Neuroimage 44, 870–883. doi: 10.1016/j.neuroimage.2008.09.041
Lerner, A., Mogensen, M. A., Kim, P. E., Shiroishi, M. S., Hwang, D. H., and Law, M. (2014). Clinical applications of diffusion tensor imaging. World Neurosurg. 82, 96–109. doi: 10.1016/j.wneu.2013.07.083
Levitt, J. G., Blanton, R., Capetillo-Cunliffe, L., Guthrie, D., Toga, A., and McCracken, J. T. (1999). Cerebellar vermis lobules viiix in autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 23, 625–633. doi: 10.1016/S0278-5846(99)00021-4
Lewis, J. D., Theilmann, R. J., Fonov, V., Bellec, P., Lincoln, A., Evans, A. C., et al. (2013). Callosal fiber length and interhemispheric connectivity in adults with autism: brain overgrowth and underconnectivity. Hum. Brain Mapp. 34, 1685–1695. doi: 10.1002/hbm.22018
Lo, Y.-C., Soong, W.-T., Gau, S. S.-F., Wu, Y.-Y., Lai, M.-C., Yeh, F.-C., et al. (2011). The loss of asymmetry and reduced interhemispheric connectivity in adolescents with autism: a study using diffusion spectrum imaging tractography. Psychiatry Res. 192, 60–66. doi: 10.1016/j.pscychresns.2010.09.008
Lotspeich, L. J., Kwon, H., Schumann, C. M., Fryer, S. L., Goodlin-Jones, B. L., Buonocore, M. H., et al. (2004). Investigation of neuroanatomical differences between autism and aspergersyndrome. Arch. Gen. Psychiatry 61, 291–298. doi: 10.1001/archpsyc.61.3.291
Manes, F., Piven, J., Vrancic, D., Nanclares, V., Plebst, C., and Starkstein, S. E. (1999). An MRI study of the corpus callosum and cerebellum in mentally retarded autistic individuals. J. Neuropsychiatry Clin. Neurosci. 11, 470–474. doi: 10.1176/jnp.11.4.470
McAlonan, G. M., Cheung, V., Cheung, C., Suckling, J., Lam, G. Y., Tai, K., et al. (2005). Mapping the brain in autism. a voxel-based mri study of volumetric differences and intercorrelations in autism. Brain 128, 268–276. doi: 10.1093/brain/awh332
McAlonan, G. M., Daly, E., Kumari, V., Critchley, H. D., van Amelsvoort, T., Suckling, J., et al. (2002). Brain anatomy and sensorimotor gating in aspergers syndrome. Brain 125, 1594–1606. doi: 10.1093/brain/awf150
Mills, B. D., Lai, J., Brown, T. T., Erhart, M., Halgren, E., Reilly, J., et al. (2013). White matter microstructure correlates of narrative production in typically developing children and children with high functioning autism. Neuropsychologia 51, 1933–1941. doi: 10.1016/j.neuropsychologia.2013.06.012
Minshew, N. J., and Dombrowski, S. M. (1994). “In vivo neuroanatomy of autism: neuroimaging studies,” in The Neurobiology of Autism, eds M. L. Bauman and T. L. Kemper (Baltimore, MD: The Johns Hopkins University Press), 66–85.
Minshew, N. J., and Payton, J. B. (1988). New perspectives in autism, part I: the clinical spectrum of autism. Curr. Prob. Pediatr. 18, 567–610. doi: 10.1016/0045-9380(88)90021-7
Mori, S., and Tournier, J.-D. (2013). Introduction to Diffusion Tensor Imaging and Higher Order Models (Oxford, UK: Academic Press).
Munson, J., Dawson, G., Abbott, R., Faja, S., Webb, S. J., Friedman, S. D., et al. (2006). Amygdalar volume and behavioral development in autism. Arch. Gen. Psychiatry 63, 686–693. doi: 10.1001/archpsyc.63.6.686
Murakami, J. W., Courchesne, E., Press, G. A., Yeung-Courchesne, R., and Hesselink, J. R. (1989). Reduced cerebellar hemisphere size and its relationship to vermal hypoplasia in autism. Arch. Neurol. 46, 689–694. doi: 10.1001/archneur.1989.00520420111032
Nagae, L., Zarnow, D., Blaskey, L., Dell, J., Khan, S., Qasmieh, S., et al. (2012). Elevated mean diffusivity in the left hemisphere superior longitudinal fasciculus in autism spectrum disorders increases with more profound language impairment. Am. J. Neuroradiol. 33, 1720–1725. doi: 10.3174/ajnr.A3037
Nair, A., Treiber, J. M., Shukla, D. K., Shih, P., and Müller, R.-A. (2013). Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain 136, 1942–1955. doi: 10.1093/brain/awt079
Narr, K. L., Hageman, N., Woods, R. P., Hamilton, L. S., Clark, K., Phillips, O., et al. (2009). Mean diffusivity: a biomarker for csf-related disease and genetic liability effects in schizophrenia. Psychiatry Res. 171, 20–32. doi: 10.1016/j.pscychresns.2008.03.008
Nordahl, C. W., Dierker, D., Mostafavi, I., Schumann, C. M., Rivera, S. M., Amaral, D. G., et al. (2007). Cortical folding abnormalities in autism revealed by surface-based morphometry. J. Neurosci. 27, 11725–11735. doi: 10.1523/JNEUROSCI.0777-07.2007
Nordahl, C. W., Scholz, R., Yang, X., Buonocore, M. H., Simon, T., Rogers, S., et al. (2012). Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Arch. Gen. Psychiatry 69, 53–61. doi: 10.1001/archgenpsychiatry.2011.145
Noriuchi, M., Kikuchi, Y., Yoshiura, T., Kira, R., Shigeto, H., Hara, T., et al. (2010). Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res. 1362, 141–149. doi: 10.1016/j.brainres.2010.09.051
Nowell, M. A., Hackney, D. B., Muraki, A. S., and Coleman, M. (1990). Varied mr appearance of autism: fifty-three pediatric patients having the full autistic syndrome. Magn. Reson. Imaging 8, 811–816. doi: 10.1016/0730-725X(90)90018-W
Nucifora, P. G., Verma, R., Lee, S.-K., and Melhem, E. R. (2007). Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology 245, 367. doi: 10.1148/radiol.2452060445
Palmen, S. J., and van Engeland, H. (2004). Review on structural neuroimaging findings in autism. J. Neural Transm. 111, 903–929. doi: 10.1007/s00702-003-0068-9
Pardini, M., Elia, M., Garaci, F. G., Guida, S., Coniglione, F., Krueger, F., et al. (2012). Long-term cognitive and behavioral therapies, combined with augmentative communication, are related to uncinate fasciculus integrity in autism. J. Autism Dev. Disord. 42, 585–592. doi: 10.1007/s10803-011-1281-2
Perkins, T. J., Stokes, M. A., McGillivray, J. A., Mussap, A. J., Cox, I. A., Maller, J. J., et al. (2014). Increased left hemisphere impairment in high-functioning autism: a tract based spatial statistics study. Psychiatry Res. 224, 119–123. doi: 10.1016/j.pscychresns.2014.08.003
Peterson, D., Mahajan, R., Crocetti, D., Mejia, A., and Mostofsky, S. (2015). Left-hemispheric microstructural abnormalities in children with high-functioning autism spectrum disorder. Autism Res. 8, 61–72. doi: 10.1002/aur.1413
Piven, J., Arndt, S., Bailey, J., and Andreasen, N. (1996). Regional brain enlargement in autism: a magnetic resonance imaging study. J. Am. Acad. Child Adolesc. Psychiatry 35, 530–536. doi: 10.1097/00004583-199604000-00020
Piven, J., Arndt, S., Bailey, J., Havercamp, S., Andreasen, N. C., and Palmer, P. (1995). An MRI study of brain size in autism. Am. J. Psychiatry 152, 1145–1149. doi: 10.1176/ajp.152.8.1145
Piven, J., Bailey, J., Ranson, B. J., and Arndt, S. (1997). An MRI study of the corpus callosum in autism. Am. J. Psychiatry 154, 1051–1056. doi: 10.1176/ajp.154.8.1051
Piven, J., Bailey, J., Ranson, B. J., and Arndt, S. (1998). No difference in hippocampus volume detected on magnetic resonance imaging in autistic individuals. J. Autism Dev. Disord. 28, 105–110. doi: 10.1023/A:1026084430649
Piven, J., Nehme, E., Simon, J., Barta, P., Pearlson, G., and Folstein, S. E. (1992). Magnetic resonance imaging in autism: measurement of the cerebellum, pons, and fourth ventricle. Biol. Psychiatry 31, 491–504. doi: 10.1016/0006-3223(92)90260-7
Poustka, L., Jennen-Steinmetz, C., Henze, R., Vomstein, K., Haffner, J., and Sieltjes, B. (2012). Fronto-temporal disconnectivity and symptom severity in children with autism spectrum disorder. World J. Biol. Psychiatry 13, 269–280. doi: 10.3109/15622975.2011.591824
Pugliese, L., Catani, M., Ameis, S., Dell'Acqua, F., de Schotten, M. T., Murphy, C., et al. (2009). The anatomy of extended limbic pathways in asperger syndrome: a preliminary diffusion tensor imaging tractography study. Neuroimage 47, 427–434. doi: 10.1016/j.neuroimage.2009.05.014
Rane, P., Cochran, D., Hodge, S. M., Haselgrove, C., Kennedy, D. N., and Frazier, J. A. (2015). Connectivity in autism: a review of mri connectivity studies. Harvard Rev. Psychiatry 23, 223–244. doi: 10.1097/HRP.0000000000000072
Ritvo, E. R., and Garber, H. (1988). Cerebellar hypoplasia and autism. New Engl. J. Med. 319, 1152–1154. doi: 10.1056/NEJM198810273191709
Rojas, D. C., Peterson, E., Winterrowd, E., Reite, M. L., Rogers, S. J., and Tregellas, J. R. (2006). Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry 6:56. doi: 10.1186/1471-244X-6-56
Rojas, D. C., Smith, J. A., Benkers, T. L., Camou, S. L., Reite, M. L., and Rogers, S. J. (2004). Hippocampus and amygdala volumes in parents of children with autistic disorder. Am. J. Psychiatry 161, 2038–2044. doi: 10.1176/appi.ajp.161.11.2038
Sahyoun, C. P., Belliveau, J. W., and Mody, M. (2010a). White matter integrity and pictorial reasoning in high-functioning children with autism. Brain Cogn. 73, 180–188. doi: 10.1016/j.bandc.2010.05.002
Sahyoun, C. P., Belliveau, J. W., Soulières, I., Schwartz, S., and Mody, M. (2010b). Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia 48, 86–95. doi: 10.1016/j.neuropsychologia.2009.08.013
Saitoh, O., Courchesne, E., Egaas, B., Lincoln, A., and Schreibman, L. (1995). Cross-sectional area of the posterior hippocampus in autistic patients with cerebellar and corpus callosum abnormalitigs. Neurology 45, 317–324. doi: 10.1212/WNL.45.2.317
Saitoh, O., Karns, C. M., and Courchesne, E. (2001). Development of the hippocampal formation from 2 to 42 years. Brain 124, 1317–1324. doi: 10.1093/brain/124.7.1317
Schaefer, G. B., Thompson, J. N., Bodensteiner, J. B., McConnell, J. M., Kimberling, W. J., Gay, C. T., et al. (1996). Hypoplasia of the cerebellar vermis in neurogenetic syndromes. Ann. Neurol. 39, 382–385. doi: 10.1002/ana.410390316
Schipul, S. E., Keller, T. A., and Just, M. A. (2011). Inter-regional brain communication and its disturbance in autism. Front. Syst. Neurosci. 5:7. doi: 10.3389/fnsys.2011.00010
Schumann, C. M., Bloss, C. S., Barnes, C. C., Wideman, G. M., Carper, R. A., Akshoomoff, N., et al. (2010). Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci. 30, 4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010
Schumann, C. M., Hamstra, J., Goodlin-Jones, B. L., Lotspeich, L. J., Kwon, H., Buonocore, M. H., et al. (2004). The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J. Neurosci. 24, 6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004
Sears, L. L., Vest, C., Mohamed, S., Bailey, J., Ranson, B. J., and Piven, J. (1999). An MRI study of the basal ganglia in autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 23, 613–624. doi: 10.1016/S0278-5846(99)00020-2
Shen, M. D., Nordahl, C. W., Young, G. S., Wootton-Gorges, S. L., Lee, A., Liston, S. E., et al. (2013). Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain 136, 2825–2835. doi: 10.1093/brain/awt166
Shukla, D. K., Keehn, B., Lincoln, A. J., and Müller, R.-A. (2010). White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J. Am. Acad. Child Adolesc. Psychiatry 49, 1269–1278.e2. doi: 10.1016/j.jaac.2010.08.018
Shukla, D. K., Keehn, B., and Müller, R.-A. (2011a). Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J. Child Psychol. Psychiatry 52, 286–295. doi: 10.1111/j.1469-7610.2010.02342.x
Shukla, D. K., Keehn, B., Smylie, D. M., and Müller, R.-A. (2011b). Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia 49, 1378–1382. doi: 10.1016/j.neuropsychologia.2011.02.022
Siegel, D. J., Minshew, N. J., and Goldstein, G. (1996). Wechsler iq profiles in diagnosis of high-functioning autism. J. Autism Dev. Disord. 26, 389–406. doi: 10.1007/BF02172825
Sivaswamy, L., Kumar, A., Rajan, D., Behen, M., Muzik, O., Chugani, D., et al. (2010). A diffusion tensor imaging study of the cerebellar pathways in children with autism spectrum disorder. J. Child Neurol. 25, 1223–1231. doi: 10.1177/0883073809358765
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505. doi: 10.1016/j.neuroimage.2006.02.024
Song, S.-K., Yoshino, J., Le, T. Q., Lin, S.-J., Sun, S.-W., Cross, A. H., et al. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26, 132–140. doi: 10.1016/j.neuroimage.2005.01.028
Sparks, B., Friedman, S., Shaw, D., Aylward, E., Echelard, D., Artru, A., et al. (2002). Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59, 184–192. doi: 10.1212/WNL.59.2.184
Stanfield, A. C., McIntosh, A. M., Spencer, M. D., Philip, R., Gaur, S., and Lawrie, S. M. (2008). Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur. Psychiatry 23, 289–299. doi: 10.1016/j.eurpsy.2007.05.006
Sundaram, S. K., Kumar, A., Makki, M. I., Behen, M. E., Chugani, H. T., and Chugani, D. C. (2008). Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb. Cortex 18, 2659–2665. doi: 10.1093/cercor/bhn031
Thakkar, K. N., Polli, F. E., Joseph, R. M., Tuch, D. S., Hadjikhani, N., Barton, J. J., et al. (2008). Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD). Brain 131, 2464–2478. doi: 10.1093/brain/awn099
Thomas, C., Humphreys, K., Jung, K.-J., Minshew, N., and Behrmann, M. (2011). The anatomy of the callosal and visual-association pathways in high-functioning autism: a DTI tractography study. Cortex 47, 863–873. doi: 10.1016/j.cortex.2010.07.006
Toal, F., Daly, E., Page, L., Deeley, Q., Hallahan, B., Bloemen, O., et al. (2010). Clinical and anatomical heterogeneity in autistic spectrum disorder: a structural MRI study. Psychol. Med. 40, 1171–1181. doi: 10.1017/S0033291709991541
Townsend, J., Courchesne, E., Covington, J., Westerfield, M., Harris, N. S., Lyden, P., et al. (1999). Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. J. Neurosci. 19, 5632–5643.
Travers, B. G., Adluru, N., Ennis, C., Tromp, D. P., Destiche, D., Doran, S., et al. (2012). Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res. 5, 289–313. doi: 10.1002/aur.1243
Tsatsanis, K. D., Rourke, B. P., Klin, A., Volkmar, F. R., Cicchetti, D., and Schultz, R. T. (2003). Reduced thalamic volume in high-functioning individuals with autism. Biol. Psychiatry 53, 121–129. doi: 10.1016/S0006-3223(02)01530-5
van Gelderen, P., de Vleeschouwer, M. H., DesPres, D., Pekar, J., van Zijl, P., and Moonen, C. T. (1994). Water diffusion and acute stroke. Magn. Reson. Med. 31, 154–163. doi: 10.1002/mrm.1910310209
Verhoeven, J. S., Rommel, N., Prodi, E., Leemans, A., Zink, I., Vandewalle, E., et al. (2012). Is there a common neuroanatomical substrate of language deficit between autism spectrum disorder and specific language impairment? Cereb. Cortex 22, 2263–2271. doi: 10.1093/cercor/bhr292
Waiter, G. D., Williams, J. H., Murray, A. D., Gilchrist, A., and Perrett, D. I. (2005). Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: a voxel-based investigation. Neuroimage 24, 455–461. doi: 10.1016/j.neuroimage.2004.08.049
Waiter, G. D., Williams, J. H., Murray, A. D., Gilchrist, A., Perrett, D. I., and Whiten, A. (2004). A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage 22, 619–625. doi: 10.1016/j.neuroimage.2004.02.029
Walker, L., Gozzi, M., Lenroot, R., Thurm, A., Behseta, B., Swedo, S., et al. (2012). Diffusion tensor imaging in young children with autism: biological effects and potential confounds. Biol. Psychiatry 72, 1043–1051. doi: 10.1016/j.biopsych.2012.08.001
Wallace, G. L., Eisenberg, I. W., Robustelli, B., Dankner, N., Kenworthy, L., Giedd, J. N., et al. (2015). Longitudinal cortical development during adolescence and young adulthood in autism spectrum disorder: increased cortical thinning but comparable surface area changes. J. Am. Acad. Child Adolesc. Psychiatry 54, 464–469. doi: 10.1016/j.jaac.2015.03.007
Wan, C. Y., Marchina, S., Norton, A., and Schlaug, G. (2012). Atypical hemispheric asymmetry in the arcuate fasciculus of completely nonverbal children with autism. Ann. N.Y. Acad. Sci. 1252, 332–337. doi: 10.1111/j.1749-6632.2012.06446.x
Weinstein, M., Ben-Sira, L., Levy, Y., Zachor, D. A., Itzhak, E. B., Artzi, M., et al. (2011). Abnormal white matter integrity in young children with autism. Hum. Brain Mapp. 32, 534–543. doi: 10.1002/hbm.21042
Wolff, J. J., Gu, H., Gerig, G., Elison, J. T., Styner, M., Gouttard, S., et al. (2012). Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry 169, 589–600. doi: 10.1176/appi.ajp.2011.11091447
Zeglam, A. M., Al-Ogab, M. F., and Al-Shaftery, T. (2015). MRI or not to MRI! should brain MRI be a routine investigation in children with autistic spectrum disorders? Acta Neurol. Belgica 115, 351–354. doi: 10.1007/s13760-014-0384-x
Zilbovicius, M., Garreau, B., Samson, Y., Remy, P., Barthelemy, C., Syrota, A., et al. (1995). Delayed maturation of the frontal cortex in childhood autism. Am. J. Psychiatry 152, 248–252. doi: 10.1176/ajp.152.2.248
Keywords: autism, structural MRI, diffusion tensor MRI, multi-modal approaches, life stages
Citation: Ismail MMT, Keynton RS, Mostapha MMMO, ElTanboly AH, Casanova MF, Gimel'farb GL and El-Baz A (2016) Studying Autism Spectrum Disorder with Structural and Diffusion Magnetic Resonance Imaging: A Survey. Front. Hum. Neurosci. 10:211. doi: 10.3389/fnhum.2016.00211
Received: 05 January 2016; Accepted: 25 April 2016;
Published: 11 May 2016.
Edited by:
Yong He, Beijing Normal University, ChinaReviewed by:
Dong-Hoon Lee, Johns Hopkins University School of Medicine, USAMiao Cao, Beijing Normal University, China
Copyright © 2016 Ismail, Keynton, Mostapha, ElTanboly, Casanova, Gimel'farb and El-Baz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayman El-Baz, aselba01@louisville.edu
 Marwa M. T. Ismail
Marwa M. T. Ismail Robert S. Keynton
Robert S. Keynton Mahmoud M. M. O. Mostapha
Mahmoud M. M. O. Mostapha Ahmed H. ElTanboly
Ahmed H. ElTanboly Manuel F. Casanova
Manuel F. Casanova Georgy L. Gimel'farb
Georgy L. Gimel'farb Ayman El-Baz
Ayman El-Baz