A Voxel-Based Morphometry Study Reveals Local Brain Structural Alterations Associated with Ambient Fine Particles in Older Women
- 1Department of Biostatistical Sciences, Wake Forest School of Medicine, Winston-Salem, NC, USA
- 2Department of Preventive Medicine, University of Southern California, Los Angeles, CA, USA
- 3University of North Carolina, Chapel Hill, NC, USA
- 4Department of Neurology, University of Southern California, Los Angeles, CA, USA
- 5Department of Psychology, University of Wisconsin-Milwaukee, Milwaukee, WI, USA
- 6Laboratory of Behavioral Neuroscience, Intramural Research Program, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA
Objective: Exposure to ambient fine particulate matter (PM2.5: PM with aerodynamic diameters < 2.5 μm) has been linked with cognitive deficits in older adults. Using fine-grained voxel-wise analyses, we examined whether PM2.5 exposure also affects brain structure.
Methods: Brain MRI data were obtained from 1365 women (aged 71–89) in the Women's Health Initiative Memory Study and local brain volumes were estimated using RAVENS (regional analysis of volumes in normalized space). Based on geocoded residential locations and air monitoring data from the U.S. Environmental Protection Agency, we employed a spatiotemporal model to estimate long-term (3-year average) exposure to ambient PM2.5 preceding MRI scans. Voxel-wise linear regression models were fit separately to gray matter (GM) and white matter (WM) maps to analyze associations between brain structure and PM2.5 exposure, with adjustment for potential confounders.
Results: Increased PM2.5 exposure was associated with smaller volumes in both cortical GM and subcortical WM areas. For GM, associations were clustered in the bilateral superior, middle, and medial frontal gyri. For WM, the largest clusters were in the frontal lobe, with smaller clusters in the temporal, parietal, and occipital lobes. No statistically significant associations were observed between PM2.5 exposure and hippocampal volumes.
Conclusions: Long-term PM2.5 exposures may accelerate loss of both GM and WM in older women. While our previous work linked smaller WM volumes to PM2.5, this is the first neuroimaging study reporting associations between air pollution exposure and smaller volumes of cortical GM. Our data support the hypothesized synaptic neurotoxicity of airborne particles.
Introduction
Growing evidence suggests that exposure to ambient air pollutants, especially particulate matter (PM), is a novel environmental risk factor of brain aging (Block et al., 2012). Cross-sectional studies have indicated that residing in places with higher levels of fine particulate matter (i.e., PM2.5) is associated with poorer cognitive functioning in older adults (Ailshire and Crimmins, 2014; Gatto et al., 2014). Further support comes from longitudinal studies showing that greater ambient PM2.5 exposure is associated with accelerated cognitive aging (Weuve et al., 2012; Tonne et al., 2014). In addition, neurotoxic effects of exposure to particulate air pollutants on the brain have been reported in animal models (Fonken et al., 2011; Davis et al., 2013).
Despite increasing epidemiologic evidence linking late-life exposure to ambient air pollution with accelerated cognitive aging (Block et al., 2012), only a few studies have examined associations with brain structure in humans using neuroimaging data. Wilker et al. recently reported that greater residential exposure to PM2.5 was associated with smaller cerebral volumes in the Framingham Offspring Study (Wilker et al., 2015). We recently reported that participants in the Women's Health Initiative Memory Study (WHIMS) who lived for at least 6–7 years in places with greater levels of PM2.5 had smaller overall brain and white matter (WM) volumes compared to women with less exposure (Chen et al., 2015).
Both of the aforementioned studies used ROI-based analyses, which aggregate volumetric measures within pre-defined neuroanatomical regions and assume homogenous associations across all voxels within each ROI. While ROI-based analyses reduce the dimensionality of imaging data, regions of interest have to be defined in advance and the quality of the analyses depends on the precision of the segmentation approaches (Lee et al., 2015). Detecting patterns that extend continuously across multiple regions may be challenging for these approaches.
Voxel-based morphometry (VBM) is a complementary technique that measures local brain volumes in a normalized space and thus does not suffer from these limitations (Goldszal et al., 1998; Good et al., 2001). Our analyses are based on the Regional Analysis of Volumes Examined in Normalized Space (RAVENS) which is a well-validated form of voxel-based morphometry that preserves local tissue volumes after transformation to stereotaxic space (Davatzikos et al., 2001). The RAVENS approach has been extensively used in the last 15 years in large-scale neuroimaging studies such as Alzheimer's Disease Neuroimaging Initiative (Misra et al., 2009), Baltimore Longitudinal Aging Study (Davatzikos et al., 2009; Driscoll et al., 2012), WHIMS-MRI (Zhang et al., 2016), etc. We hypothesized that conducting more detailed analyses of the associations between air pollution neurotoxicity and local brain structure using RAVENS approaches would generate further insights about the impact of air pollution on brain structure.
Methods
Participants
The Women's Health Initiative Memory Study (WHIMS) investigated the effects of postmenopausal hormone therapy on the risk of dementia and changes in cognitive function in women aged 65–80 at enrollment (1996–1998) into the WHI randomized placebo-controlled clinical trials (Shumaker et al., 1998; Espeland et al., 2004). The WHIMS Magnetic Resonance Imaging study (WHIMS-MRI) study enrolled WHIMS participants from 14 of 39 sites, (Jaramillo et al., 2007; Resnick et al., 2009) from January 2005 through April 2006. Here we analyzed images from 1365 participants who met WHIMS-MRI reading criteria. These criteria were described previously (Coker et al., 2014). This study was also conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent. This research was approved by the Wake Forest School of Medicine IRB.
Image Acquisition and Pre-processing
MRI scans were performed using a standardized protocol developed by the MRI Quality Control Center in the Department of Radiology of the University of Pennsylvania. Details on procedures for acquisition and processing were published previously (Coker et al., 2009; Resnick et al., 2009). Briefly, the scans were obtained with a field of view = 22 cm and a matrix of 256 × 256. Included were oblique axial spin density/T2-weighted spin echo (TR:3200 ms, TE = 30/120 ms, slice thickness = 3 mm), fluid-attenuated inversion recovery (FLAIR) T2-weighted spin echo (TR = 8000 ms, TI = 2000 ms, TE = 100 ms, slice thickness = 3 mm), and oblique axial three-dimensional T1-weighted gradient echo (flip angle = 30 degrees, TR = 21 ms, TE = 8 ms, slice thickness = 1.5 mm) images from the vertex to the skull base parallel to the anterior commissure–posterior commissure (AC-PC) plane.
For voxel-based analyses, the T1-weighted images were preprocessed using the following steps: (1) alignment of the brain with the AC-PC plane; (2) removal of extracranial material; (3) tissue segmentation into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF), using a method described elsewhere (Zhang et al., 2016); (4) high-dimensional image warping to a standard MNI space through an elastic registration method (Shen and Davatzikos, 2002); (5) applying the deformation field that resulted from the spatial registration to the segmented images, thereby generating mass-preserved volumetric maps (or tissue density maps), named Regional Analysis of Volumes Examined in Normalized Space (RAVENS) maps (Davatzikos et al., 2001); (6) the RAVENS maps are normalized by the intracranial volumes to control for inter-subject differences in head size; (7) resampling the RAVENS maps to have 2 × 2 × 2 mm voxel size; and (8) smoothing of the GM and WM RAVENS maps using an 8 mm isotropic Gaussian kernel.
Ambient Air Pollution Data
We estimated residential exposures to PM2.5 from ambient sources, using a Bayesian Maximum Entropy (BME)-based spatiotemporal modeling approach. BME is a powerful stochastic modeling and mapping method for characterizing environmental exposure and human-ecosystem interactions (Christakos et al., 2001), which has been used in several large epidemiological cohort studies (Jerrett et al., 2013; Chen et al., 2015). In order to minimize the scaling error resulting from temporal misalignment in both the exposure source data and the subsequent estimates, a BME spatiotemporal model was constructed to produce daily ambient PM2.5 concentration at each geocoded location where WHIMS participants resided. To evaluate the validity of resulting exposure estimates, we conducted cross-validation analyses on the estimation accuracy, using US Environmental Protection Agency (EPA) air monitoring data. We first randomly divided the data into 10 distinctive sets of monitoring stations. For each “held-out” 10% of these data, we obtained daily BME estimates using only data from the remaining 90% of monitoring stations. We then pooled the cross-validation statistics across 10 distinctive sets and found moderate correlations between the “held-out” data and their BME estimates (cross-validation R2 = 0.74 for daily PM2.5). These daily BME estimates were then aggregated and combined with the residential histories, including relocations to calculate the 3-year average exposures preceding each brain MRI scan. These 3-year average exposures were highly correlated (Pearson's R = 0.93) with the cumulative exposure estimates of yearly PM2.5 used in previous work (Chen et al., 2015).
Measurement of Covariates
At the WHIMS enrollment, participants completed structured questionnaires to provide information on demographics (age, race/ethnicity), socioeconomic status (including education, family income, employment status), lifestyle factors (smoking, alcohol consumption), clinical characteristics (cardiovascular disease [CVD] and related risk factors), and prior hormone therapy use. History of CVD included previous coronary heart disease (myocardial infarction, coronary angioplasty, or coronary artery bypass graft), stroke, or transient ischemic attack. Body mass index (kg/m2) was calculated. Hypertension was defined as use of antihypertensive medication or elevated blood pressure (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg). Treated diabetes mellitus (DM) was defined as a physician diagnosis plus oral medications or insulin therapy. Good reliability and validity of both the self-reported medical histories and the physical measures have been documented (Heckbert et al., 2004).
Statistical Analysis
Voxel-wise linear regression models (Good et al., 2001) were fit to GM and WM RAVENS maps using Statistical Parametric Mapping (SPM) software (version 8) to examine the associations that brain structures had with PM2.5 exposure after adjusting for intracranial volume and potential confounders including age, race, BMI, geographic region (Northeast, South, Midwest, and West; Chen et al., 2015), education, family income, employment status, smoking, alcohol consumption, CVD history, hypertension, treated diabetes, and prior hormone therapy use. We investigated both negative and positive associations of PM2.5 with tissue volumes. All results were corrected for multiple comparisons using a false discovery rate (FDR) < 0.05 (Benjamini and Hochberg, 1995). Clusters with fewer than 50 voxels were removed from the results.
Results
Demographic, lifestyle, and clinical characteristics of participants are listed in Table 1. Greater PM2.5 exposure was associated with spatial patterns of smaller brain volumes in cortical GM and subcortical WM areas (Figures 1, 2). For GM, higher PM2.5 was associated with smaller volumes clustered in the bilateral superior, middle, and medial frontal gyri. Other clusters of negative associations were in the left inferior frontal gyrus and bilateral superior parietal lobule and occipital poles. For WM, the largest clusters of negative associations were in the anterior and posterior extreme/external capsule and the calcarine gyri. No correlation was found between corpus callosum and PM2.5 exposure. In addition, we found no evidence for smaller hippocampal or temporal lobe volumes with PM2.5 exposure. Statistically significant clusters of associations were found in deep gray matter nuclei (q < 0.05 FDR corrected): larger volumes were associated with increased PM2.5 exposures (Figure 3). These local GM regions, pinpointed by colors in Figure 3, included the thalamus, putamen, and globus pallidus bilaterally, as well as the posterior insula. There were no WM areas with significant positive associations with increased PM2.5 exposure.
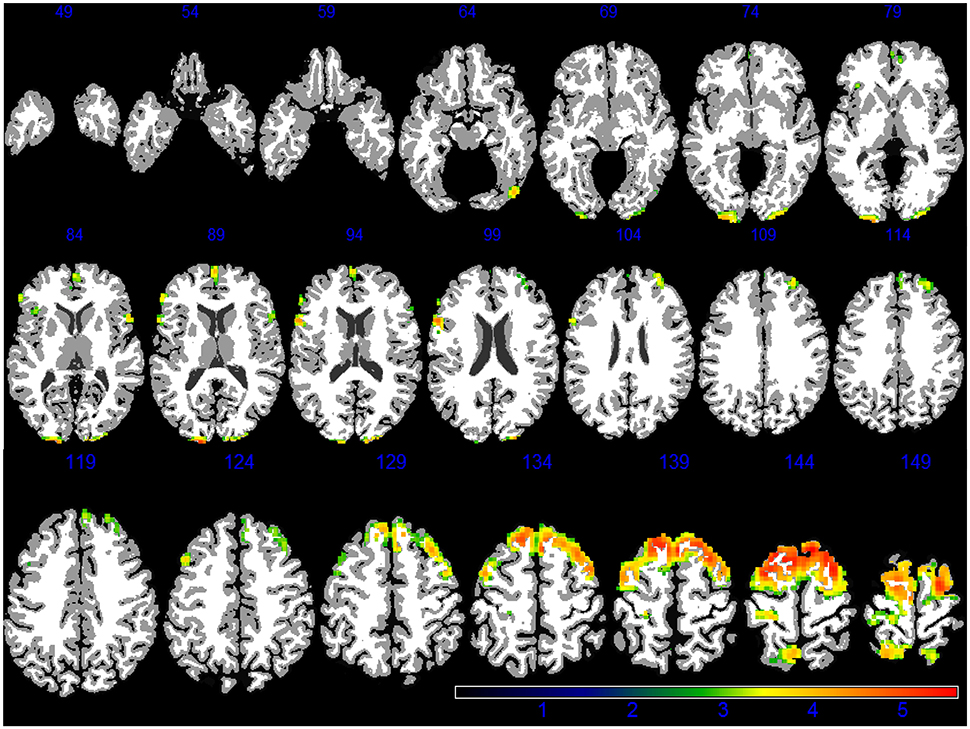
Figure 1. GM areas negatively associated to PM2.5 exposure (q < 0.05 FDR corrected) in the VBM linear regression models are presented in color. Images are oriented according to the neurological convention.
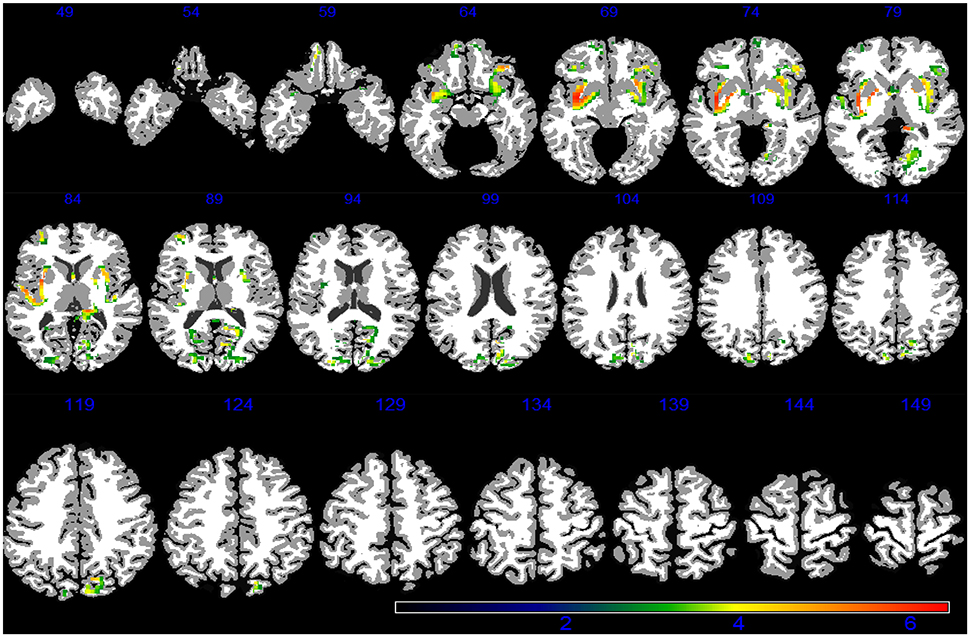
Figure 2. WM areas with decreased volumes associated to increased PM2.5 exposure (q < 0.05 FDR corrected) in the VBM linear regression models are presented in color. Images are oriented according to the neurological convention.
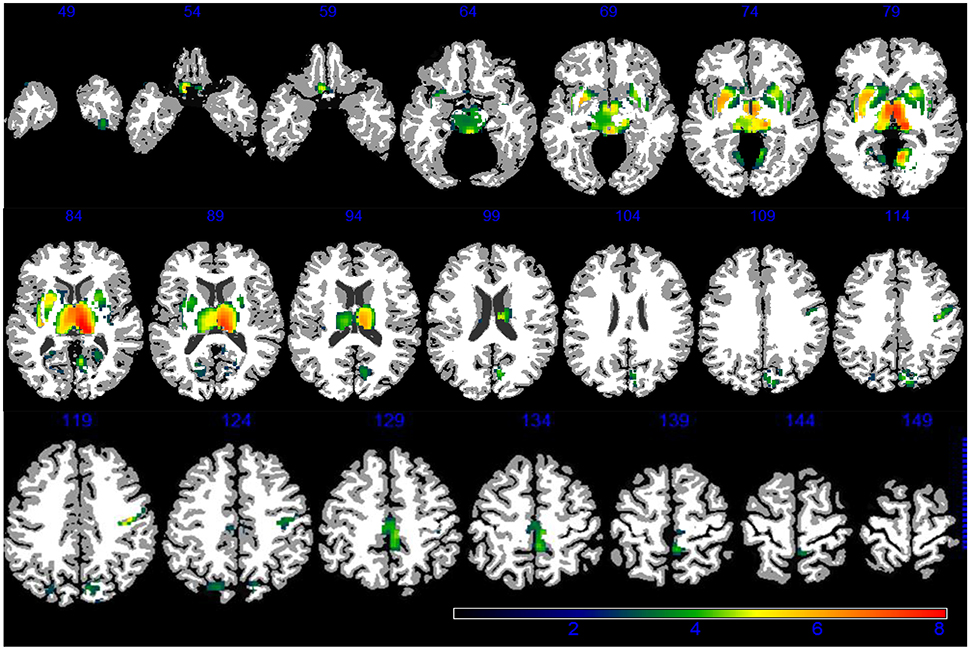
Figure 3. GM areas positively associated to PM2.5 (q < 0.05 FDR corrected) according to VBM linear regression are presented in color. Images are oriented according to the neurological convention.
Discussion
Our detailed analyses identified specific subcortical areas in which smaller WM volumes were associated with greater PM2.5 exposure, namely the external and extreme capsule and the calcarine cortices. This observation suggests that regions involved in important functional networks, such as the salience and visual networks, appear to be affected by ambient PM2.5. We also found that ambient PM2.5 exposure was associated with local GM brain structures. Our findings provide the first epidemiologic evidence that PM2.5-induced neurotoxic effects may involve structural damage to cortical GM. In cohorts like WHIMS-MRI participants, lower GM volumes may reflect shrinkage of neurons, reductions of synaptic spines, and dendritic arborization, and lower numbers of synapses (Fjell and Walhovd, 2010) rather than neuronal loss. To date, there is limited data from animal studies showing evidence for PM-induced neuronal toxicity, including the reduction of dopaminergic neurons in the striatum of genetically-modified mice (Veronesi et al., 2005) exposed to concentrated PM2.5 representing the ambient background and cortical neuronal loss in rats with oral ingestion of PM from vehicular emissions with unspecified particle sizes (Ejaz et al., 2014). However, there is growing evidence that synaptic neurotoxicity results from exposure to ambient particles. In the mouse hippocampus, impaired synaptic function is induced by short-term in vitro exposure to particulate matter from urban traffic (Davis et al., 2013). Reduced synaptic plasticity (decreased dendritic spine density and branching) may result from long-term inhaled exposure to ambient PM2.5 (Fonken et al., 2011).
The associations between PM2.5 exposure and patterns of smaller GM volumes that we identified were primarily in the dorsolateral and medial prefrontal cortex, regions associated with higher cognitive function such as working memory, episodic memory retrieval, and executive function. Age-related deficits in retrieval of episodic memory have been associated with volume reductions and functional changes in the middle frontal gyrus (Buckner et al., 2000; Raz et al., 2005). Weuve et al. reported memory function declined in older women (70–81 years) living in locations with higher PM2.5 exposures (Weuve et al., 2012). Two other studies also reported associations between PM2.5 exposure and low performance of episodic memory (Ailshire and Crimmins, 2014; Tonne et al., 2014).
We found little evidence that PM2.5 exposure was related to hippocampal volume. This is consistent with two previous studies employing ROI-based analyses (Chen et al., 2015; Wilker et al., 2015). This null finding may be influenced by the nature of the cohort and characteristics of the exposure. Longitudinal brain MRI studies have shown that loss of hippocampal volume starts in young adulthood, with age-related accelerated shrinkage in the mid-50s (Raz et al., 2005). Long-term (10-month) exposure to concentrated ambient PM2.5 decreased dendritic spine density in hippocampal CA1 neurons of 4-week-old wild-type mice (C57BL/6; Fonken et al., 2011). It is therefore possible that PM2.5 exposure affects hippocampal volume in early- or mid-life. Also, because our exposure estimation relied exclusively on EPA's ambient monitoring data, we cannot exclude the possibility that reduced hippocampal volume might be found in older adults exposed to other particulate matter with different profiles of neurotoxicity (e.g., the ultrafine particles from vehicular exhausts; Davis et al., 2013).
The positive associations we observed between PM2.5 exposure and GM volumes in basal ganglia were unexpected, and the potential underlying mechanisms are unclear. In our previous ROI-based analyses, we found no positive associations between PM2.5 exposure and basal ganglia volume (Chen et al., 2015). Experimentally, exposure to small particles may result in a loss of dopaminergic neurons in striatum, as shown with in vitro (Gillespie et al., 2013) or inhalation exposure (Veronesi et al., 2005) to concentrated ambient particles. These results would predict an association between PM2.5 and smaller volumes of basal ganglia. On the other hand, environmental exposures to paramagnetic substances (e.g., magnesium and iron) may distort T1-weighted images and interfere with volumetric estimation (Goto et al., 2013; Lorio et al., 2014). One recent neuropathological study identified (Maher et al., 2016) the magnetite nanoparticle from environmental sources in human brains, and others have shown that airborne particles with magnetic properties are abundant in polluted cities (Gargiulo et al., 2016). Larger basal ganglia volumes have been linked to some pathological processes in the brain (e.g., schizophrenia; Mamah et al., 2007) and use of antipsychotic medications(Scherk and Falkai, 2006). However, we are unaware of prior studies showing increased psychiatric disease/use of antipsychotics or changes in paramagnetic properties resulting from long-term PM2.5 exposure. It is also unclear why higher PM2.5 exposure would be associated with larger volumes in the thalamus and lenticular nucleus, but have a less pronounced effect on caudate. Our sample size was unusually large for VBM analyses, which could mean that we detected subtle differences not readily identified in previous VBM studies.
Our findings strengthen the evidence that WM architecture may represent a novel target of particle-induced neurotoxicity. While associations with GM volumes are largely restricted to frontal gyri (Figure 1), the impact of PM2.5 on WM volumes appears to be more regionally-distributed (Figure 2) and involves the same regions (frontal, parietal, and temporal lobes) as previously reported in our ROI-based study (Chen et al., 2015). These observed differences in affected brain regions raise the interesting possibility that the smaller WM volumes reflect adverse effects on oligodendrocytes and/or myelin damage, while smaller GM volumes may imply synaptic neurotoxicity, both possibly resulting from long-term PM2.5 exposure. Investigation on the neurobiological mechanisms (e.g., neuroinflammation, oxidative stress) linking PM exposure to central neurotoxicity is an active area of research in environmental neurosciences, likely involving multi-level pathways perturbed at the molecular levels (e.g., activation of TNF-alpha; Levesque et al., 2011; Cheng et al., 2016), selected target tissues (e.g., remodeling glutamatergic synapses; Morgan et al., 2011), and interactions among different neural cells (e.g., neuron-glial interaction; Block and Calderón-Garcidueñas, 2009) and across systems (e.g., via the neurohormonal stress response to air pollution; Kodavanti, 2016). Subclinical cerebrovascular injuries may also result in loss of brain tissues, although published neuroimaging studies with late-life exposure to PM2.5 so far (Chen et al., 2015; Wilker et al., 2015, 2016) had not produced strong evidence for this neurovascular pathway linking air pollution to brain aging.
One recent cross-sectional study also showed that early-life PM2.5 exposure may affect age-related WM maturation (Peterson et al., 2015). In a sample of 40 minority urban-dwelling school-age children, prenatal exposures to polycyclic aromatic hydrocarbons (measured from personal air samples of PM2.5 during pregnancy) was associated with a smaller local WM volume, as indicated by the reduction of surface areas (Peterson et al., 2015). PM-induced WM damage, as reflected by hypomyelination and aberrant white matter structural integrity were recently demonstrated in mouse models with early-life exposure to concentrated ambient ultrafine particles (Allen et al., 2015). Beyond volumetric measures, future studies should consider diffusion tensor tractography (Madden et al., 2009) and MR spectroscopy (Bray and Mullins, 2014) to better understand the WM connectomes and molecular profilles potentially disrupted by PM exposure. To elucidate the neuropathology and mechanisms underlying the observed neurotoxicity on WM, we also need to understand whether PM2.5 exposure results in myelination disturbance (Kohama et al., 2012) and age-related decrease of the oligodendrocytes in subcortical WM (Chen et al., 2011). VBM and ROI methods operate based on different assumptions. Thus, while often they show some degree of coincidence, they also can lead to different findings. It is interesting to note that the present VBM analyses did not reveal a statistically significant association between PM2.5 and corpus callosum, in contrast to our findings using ROI based methods (Chen et al., 2015). In one study of schizophrenic patients (Giuliani et al., 2005), despite some similarities in results, there also were brain areas uncovered differentially by each of the methods. In general, VBM and ROI approaches are complementary; their relative effectiveness is likely related to the specific shape of the spatial patterns of brain tissue atrophy and the image warping and segmentation methods used to preprocess the MRI data. Finally, we used in our analyses RAVENS maps that were ICV adjusted as part of the image preprocessing. The ICV-adjustment strategies (e.g., proportional, residuals, nuisance covariate, etc.) have been the subject of debate in the past (Arndt et al., 1991; Barnes et al., 2010) and more recently (Voevodskaya et al., 2014; Nordenskjöld et al., 2015). While other analyses are possible, they would be beyond the scope of this particular paper.
Our study has some limitations. First, our analyses were based on cross-sectional measures of brain volume. Longitudinal studies with repeated brain MRI scans are needed to characterize associations with rates of changes in brain volumes. We only studied older women, so our findings may not generalize to men. This cohort was composed of relatively well-educated and mostly Caucasian women, which may not be representative of the general population. We only studied PM2.5, and have not assessed emission sources, particle constituents, or interactions with other pollutant mixtures. The lack of nationwide monitoring data before 1999 prevented us from assessing the impact of earlier exposures. Finally, long-term chronic exposure, especially if accumulated since mid- or earlier life, might have different—and potentially greater—adverse effects than what we observed.
Conclusions
This first neuroepidemiologic VBM analysis of brain regions associated with air pollution provides further evidence for the adverse effect of particulate air pollutants on brain structure in older women. Long-term PM2.5 exposures are linked to potential loss of brain volume in both GM and WM tissues, but in different brain networks. Longitudinal studies are needed to clarify the sequence of pathogenetic events associated with long term exposure to fine particles.
Investigators Participating in the WHIMS-MRI
WHIMS-MRI Clinical Centers: Albert Einstein College of Medicine, Bronx, NY: Sylvia Wassertheil-Smoller, Mimi Goodwin, Richard DeNise, Michael Lipton, James Hannigan, Anthony Carpini, David Noble, Wilton Guzman; Medical College of Wisconsin, Milwaukee: Jane Morley Kotchen, Joseph Goveas, Diana Kerwin, John Ulmer, Steve Censky, Troy Flinton, Tracy Matusewic, Robert Prost; Stanford Center for Research in Disease Prevention, Stanford University, CA: Marcia L. Stefanick, Sue Swope, Anne Marie Sawyer-Glover, Susan Hartley; The Ohio State University, Columbus: Rebecca Jackson, Rose Hallarn, Bonnie Kennedy, Jill Bolognone, Lindsay Casimir, Amanda Kochis; University of California at Davis, Sacramento: John Robbins, Sophia Zaragoza, Cameron Carter, John Ryan, Denise Macias, Jerry Sonico; University of California at Los Angeles: Lauren Nathan, Barbara Voigt, Pablo Villablanca, Glen Nyborg, Sergio Godinez, Adele Perrymann; University of Florida, Gainesville/Jacksonville: Marian Limacher, Sheila Anderson, Mary Ellen Toombs, Jeffrey Bennett, Kevin Jones, Sandy Brum, Shane Chatfield, Kevin Vantrees; University of Iowa, Davenport: Jennifer Robinson, Candy Wilson, Kevin Koch, Suzette Hart, Jennifer Carroll, Mary Cherrico; University of Massachusetts, Worcester: Judith Ockene, Linda Churchill, Douglas Fellows, Anthony Serio, Sharon Jackson, Deidre Spavich; University of Minnesota, Minneapolis: Karen Margolis, Cindy Bjerk, Chip Truwitt, Margaret Peitso, Alexa Camcrena, Richard Grim, Julie Levin, Mary Perron; University of Nevada, Reno: Robert Brunner, Ross Golding, Leslie Pansky, Sandie Arguello, Jane Hammons, Nikki Peterson; University of North Carolina, Chapel Hill: Carol Murphy, Maggie Morgan, Mauricio Castillo, Thomas Beckman, Benjamin Huang; University of Pittsburgh, PA: Lewis Kuller, Pat McHugh, Carolyn Meltzer, Denise Davis, Joyce Davis, Piera Kost, Kim Lucas, Tom Potter, Lee Tarr.
WHIMS-MRI Clinical Coordinating Center: Wake Forest School of Medicine, Winston-Salem, NC: Sally Shumaker, Mark Espeland, Laura Coker, Jeff Williamson, Debbie Felton, LeeAnn Gleiser, Steve Rapp, Claudine Legault, Maggie Dailey, Ramon Casanova, Julia Robertson, Patricia Hogan, Sarah Gaussoin, Pam Nance, Cheryl Summerville, Ricardo Peral, Josh Tan.
WHIMS-MRI Quality Control Center: University of Pennsylvania, Philadelphia: Nick Bryan, Christos Davatzikos, Lisa Desiderio.
U.S. National Institutes of Health: National Institute on Aging, Bethesda, MD: Neil Buckholtz, Susan Molchan, Susan Resnick; National Heart, Lung, and Blood Institute, Bethesda, MD, Jacques Rossouw, Linda Pottern.
Author Contributions
Design/Conceptualization of the study (RC, ME, JC); Acquisition of data (RC, ME, JC, MS, WV, JR, YA); Analysis of the data (RC, XW, ME, JC, MS, WV, HC, SR); Interpretation of the data (RC, ME, JC, HC, SR); Drafting the manuscript (RC, ME, JC); Critical revision of the manuscript for important intellectual content (All listed authors); Final approval of the current version of this manuscript (All listed authors).
Funding
The Women's Health Initiative is funded by the National Heart, Lung, and Blood Institute. The Women's Health Initiative Memory Study was funded in part by Wyeth Pharmaceuticals, Inc., St. Davids, PA. The research work was supported by R21AG051113-01 (PIs: RC and JC) R01AG033078 (PI: JC). HC is supported by P50-AG05142. SR is supported by the Intramural Research Program, NIA, NIH.
Conflict of Interest Statement
ME has served on an advisory panel for Takeda Global Research and Development. He currently serves on a steering committee for Boehringer-Ingelheim Pharmaceuticals. He serves on the editorial board for the Journal of Gerontology Medical Sciences.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The investigators appreciate Dr. Eric Whitsel for assisting in post-estimation data processing and delivering of pollution exposure data used in this study. We acknowledge the editorial assistance of Karen Klein, MA, ELS, through the Wake Forest Clinical and Translational Science Institute (UL1TR001420; PI: McClain).
References
Ailshire, J. A., and Crimmins, E. M. (2014). Fine particulate matter air pollution and cognitive function among older US adults. Am. J. Epidemiol. 180, 359–366. doi: 10.1093/aje/kwu155
Allen, J. L., Oberdorster, G., Morris-Schaffer, K., Wong, C., Klocke, C., Sobolewski, M., et al. (2015). Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. doi: 10.1016/j.neuro.2015.12.014. [Epub ahead of print].
Arndt, S., Cohen, G., Alliger, R. J., Swayze, V. W. II., and Andreasen, N. C. (1991). Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Res. 40, 79–89. doi: 10.1016/0925-4927(91)90031-K
Barnes, J., Ridgway, G. R., Bartlett, J., Henley, S. M., Lehmann, M., Hobbs, N., et al. (2010). Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage 53, 1244–1255. doi: 10.1016/j.neuroimage.2010.06.025
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. JRSS Ser. B 57, 289–300.
Block, M. L., and Calderón-Garcidueñas, L. (2009). Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 32, 506–516. doi: 10.1016/j.tins.2009.05.009
Block, M. L., Elder, A., Auten, R. L., Bilbo, S. D., Chen, H., Chen, J. C., et al. (2012). The outdoor air pollution and brain health workshop. Neurotoxicology 33, 972–984. doi: 10.1016/j.neuro.2012.08.014
Bray, M. D., and Mullins, M. E. (2014). Metabolic white matter diseases and the utility of MR spectroscopy. Radiol. Clin. North Am. 52, 403–411. doi: 10.1016/j.rcl.2013.11.012
Buckner, R. L., Snyder, A. Z., Sanders, A. L., Raichle, M. E., and Morris, J. C. (2000). Functional brain imaging of young, nondemented, and demented older adults. J. Cogn. Neurosci. 12(Suppl. 2), 24–34. doi: 10.1162/089892900564046
Chen, J. C., Wang, X., Wellenius, G. A., Serre, M. L., Driscoll, I., Casanova, R., et al. (2015). Ambient air pollution and neurotoxicity on brain structure: evidence from women's health initiative memory study. Ann. Neurol. 78, 466–476. doi: 10.1002/ana.24460
Chen, L., Lu, W., Yang, Z., Yang, S., Li, C., Shi, X., et al. (2011). Age-related changes of the oligodendrocytes in rat subcortical white matter. Anat. Rec. 294, 487–493. doi: 10.1002/ar.21332
Cheng, H., Davis, D. A., Hasheminassab, S., Sioutas, C., Morgan, T. E., and Finch, C. E. (2016). Urban traffic-derived nanoparticulate matter reduces neurite outgrowth via TNFalpha in vitro. J. Neuroinflammation 13:19. doi: 10.1186/s12974-016-0480-3
Christakos, G., Bogaert, P., and Serre, M. L. (2001). Temporal Gis: Advanced Functions for Field-Based Applications. Berlin; New York, NY: Springer.
Coker, L. H., Espeland, M. A., Hogan, P. E., Resnick, S. M., Bryan, R. N., Robinson, J. G., et al. (2014). Change in brain and lesion volumes after CEE therapies: the WHIMS-MRI studies. Neurology 82, 427–434. doi: 10.1212/WNL.0000000000000079
Coker, L. H., Hogan, P. E., Bryan, N. R., Kuller, L. H., Margolis, K. L., Bettermann, K., et al. (2009). Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology 72, 125–134. doi: 10.1212/01.wnl.0000339036.88842.9e
Davatzikos, C., Genc, A., Xu, D., and Resnick, S. M. (2001). Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage 14, 1361–1369. doi: 10.1006/nimg.2001.0937
Davatzikos, C., Xu, F., An, Y., Fan, Y., and Resnick, S. M. (2009). Longitudinal progression of Alzheimer's-like patterns of atrophy in normal older adults: the SPARE-AD index. Brain 132, 2026–2035. doi: 10.1093/brain/awp091
Davis, D. A., Akopian, G., Walsh, J. P., Sioutas, C., Morgan, T. E., and Finch, C. E. (2013). Urban air pollutants reduce synaptic function of CA1 neurons via an NMDA/NO pathway in vitro. J. Neurochem. 127, 509–519. doi: 10.1111/jnc.12395
Driscoll, I., Beydoun, M. A., An, Y., Davatzikos, C., Ferrucci, L., Zonderman, A. B., et al. (2012). Midlife obesity and trajectories of brain volume changes in older adults. Hum. Brain Mapp. 33, 2204–2210. doi: 10.1002/hbm.21353
Ejaz, S., Anwar, K., and Ashraf, M. (2014). MRI and neuropathological validations of the involvement of air pollutants in cortical selective neuronal loss. Environ. Sci. Pollut. Res. Int. 21, 3351–3362. doi: 10.1007/s11356-013-2294-5
Espeland, M. A., Rapp, S. R., Shumaker, S. A., Brunner, R., Manson, J. E., Sherwin, B. B., et al. (2004). Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA 291, 2959–2968. doi: 10.1001/jama.291.24.2959
Fjell, A. M., and Walhovd, K. B. (2010). Structural brain changes in aging: courses, causes and cognitive consequences. Rev. Neurosci. 21, 187–221. doi: 10.1515/REVNEURO.2010.21.3.187
Fonken, L. K., Xu, X., Weil, Z. M., Chen, G., Sun, Q., Rajagopalan, S., et al. (2011). Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol. Psychiatry 16, 987–995. doi: 10.1038/mp.2011.76
Gargiulo, J. D., Kumar, R. S., Chaparro, M. A. E., Natal, M., and Rajkumar, P. (2016). Magnetic properties of air suspended particles in thirty eight cities from south India. Atmos. Pollut. Res. 7, 626–637. doi: 10.1016/j.apr.2016.02.008
Gatto, N. M., Henderson, V. W., Hodis, H. N., St John, J. A., Lurmann, F., Chen, J. C., et al. (2014). Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology 40, 1–7. doi: 10.1016/j.neuro.2013.09.004
Gillespie, P., Tajuba, J., Lippmann, M., Chen, L. C., and Veronesi, B. (2013). Particulate matter neurotoxicity in culture is size-dependent. Neurotoxicology 36, 112–117. doi: 10.1016/j.neuro.2011.10.006
Giuliani, N. R., Calhoun, V. D., Pearlson, G. D., Francis, A., and Buchanan, R. W. (2005). Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr. Res. 74, 135–147. doi: 10.1016/j.schres.2004.08.019
Goldszal, A. F., Davatzikos, C., Pham, D. L., Yan, M. X., Bryan, R. N., and Resnick, S. M. (1998). An image-processing system for qualitative and quantitative volumetric analysis of brain images. J. Comput. Assist. Tomogr. 22, 827–837. doi: 10.1097/00004728-199809000-00030
Good, C. D., Johnsrude, I. S., Ashburner, J., Henson, R. N., Friston, K. J., and Frackowiak, R. S. (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14, 21–36. doi: 10.1006/nimg.2001.0786
Goto, M., Abe, O., Miyati, T., Aoki, S., Takao, H., Hayashi, N., et al. (2013). Association between iron content and gray matter missegmentation with voxel-based morphometry in basal ganglia. J. Magn. Reson. Imaging 38, 958–962. doi: 10.1002/jmri.23916
Heckbert, S. R., Kooperberg, C., Safford, M. M., Psaty, B. M., Hsia, J., McTiernan, A., et al. (2004). Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. Am. J. Epidemiol. 160, 1152–1158. doi: 10.1093/aje/kwh314
Jaramillo, S. A., Felton, D., Andrews, L., Desiderio, L., Hallarn, R. K., Jackson, S. D., et al. (2007). Enrollment in a brain magnetic resonance study: results from the Women's Health Initiative Memory Study Magnetic Resonance Imaging Study (WHIMS-MRI). Acad. Radiol. 14, 603–612. doi: 10.1016/j.acra.2007.02.001
Jerrett, M., Burnett, R. T., Beckerman, B. S., Turner, M. C., Krewski, D., Thurston, G., et al. (2013). Spatial analysis of air pollution and mortality in California. Am. J. Respir. Crit. Care Med. 188, 593–599. doi: 10.1164/rccm.201303-0609OC
Kodavanti, U. P. (2016). Stretching the stress boundary: Linking air pollution health effects to a neurohormonal stress response. Biochim Biophys Acta. 12, 2880–2890. doi: 10.1016/j.bbagen.2016.05.010
Kohama, S. G., Rosene, D. L., and Sherman, L. S. (2012). Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. Age 34, 1093–1110. doi: 10.1007/s11357-011-9357-7
Lee, S., Zipunnikov, V., Reich, D. S., and Pham, D. L. (2015). Statistical image analysis of longitudinal RAVENS images. Front. Neurosci. 9:368. doi: 10.3389/fnins.2015.00368
Levesque, S., Surace, M. J., McDonald, J., and Block, M. L. (2011). Air pollution & the brain: subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J. Neuroinflammation 8:105. doi: 10.1186/1742-2094-8-105
Lorio, S., Lutti, A., Kherif, F., Ruef, A., Dukart, J., Chowdhury, R., et al. (2014). Disentangling in vivo the effects of iron content and atrophy on the ageing human brain. Neuroimage 103, 280–289. doi: 10.1016/j.neuroimage.2014.09.044
Madden, D. J., Bennett, I. J., and Song, A. W. (2009). Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol. Rev. 19, 415–435. doi: 10.1007/s11065-009-9113-2
Maher, B. A., Ahmed, I. A. M., Karloukovski, V., MacLaren, D. A., Foulds, P. G., Allsop, D., et al. (2016). Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. U.S.A. 113, 10797–10801. doi: 10.1073/pnas.1605941113
Mamah, D., Wang, L., Barch, D., de Erausquin, G. A., Gado, M., and Csernansky, J. G. (2007). Structural analysis of the basal ganglia in schizophrenia. Schizophr. Res. 89, 59–71. doi: 10.1016/j.schres.2006.08.031
Misra, C., Fan, Y., and Davatzikos, C. (2009). Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: results from ADNI. Neuroimage 44, 1415–1422. doi: 10.1016/j.neuroimage.2008.10.031
Morgan, T. E., Davis, D. A., Iwata, N., Tanner, J. A., Snyder, D., Ning, Z., et al. (2011). Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ. Health Perspect. 119, 1003–1009. doi: 10.1289/ehp.1002973
Nordenskjöld, R., Malmberg, F., Larssön, E. M., Simmons, A., Ahlstrom, H., Johansson, L., et al. (2015). Intracranial volume normalization methods: considerations when investigating gender differences in regional brain volume. Psychiatry Res. 231, 227–235. doi: 10.1016/j.pscychresns.2014.11.011
Peterson, B. S., Rauh, V. A., Bansal, R., Hao, X., Toth, Z., Nati, G., et al. (2015). Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 72, 531–540. doi: 10.1001/jamapsychiatry.2015.57
Raz, N., Lindenberger, U., Rodrigue, K. M., Kennedy, K. M., Head, D., Williamson, A., et al. (2005). Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb. Cortex 15, 1676–1689. doi: 10.1093/cercor/bhi044
Resnick, S. M., Espeland, M. A., Jaramillo, S. A., Hirsch, C., Stefanick, M. L., Murray, A. M., et al. (2009). Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology 72, 135–142. doi: 10.1212/01.wnl.0000339037.76336.cf
Scherk, H., and Falkai, P. (2006). Effects of antipsychotics on brain structure. Curr. Opin. Psychiatry 19, 145–150. doi: 10.1097/01.yco.0000214339.06507.d8
Shen, D., and Davatzikos, C. (2002). HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans. Med. Imaging 21, 1421–1439. doi: 10.1109/TMI.2002.803111
Shumaker, S. A., Reboussin, B. A., Espeland, M. A., Rapp, S. R., McBee, W. L., Dailey, M., et al. (1998). The Women's Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control. Clin. Trials 19, 604–621. doi: 10.1016/S0197-2456(98)00038-5
Tonne, C., Elbaz, A., Beevers, S., and Singh-Manoux, A. (2014). Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology 25, 674–681. doi: 10.1097/EDE.0000000000000144
Veronesi, B., Makwana, O., Pooler, M., and Chen, L. C. (2005). Effects of subchronic exposures to concentrated ambient particles. VII. Degeneration of dopaminergic neurons in Apo E-/- mice. Inhal. Toxicol. 17, 235–241. doi: 10.1080/08958370590912888
Voevodskaya, O., Simmons, A., Nordenskjöld, R., Kullberg, J., Ahlström, H., Lind, L., et al. (2014). The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer's disease. Front. Aging Neurosci. 6:264. doi: 10.3389/fnagi.2014.00264
Weuve, J., Puett, R. C., Schwartz, J., Yanosky, J. D., Laden, F., and Grodstein, F. (2012). Exposure to particulate air pollution and cognitive decline in older women. Arch. Intern. Med. 172, 219–227. doi: 10.1001/archinternmed.2011.683
Wilker, E. H., Martinez-Ramirez, S., Kloog, I., Schwartz, J., Mostofsky, E., Koutrakis, P., et al. (2016). Fine particulate matter, residential proximity to major roads, and markers of small vessel disease in a memory study population. J. Alzheimers Dis. 53, 1315–1323. doi: 10.3233/JAD-151143
Wilker, E. H., Preis, S. R., Beiser, A. S., Wolf, P. A., Au, R., Kloog, I., et al. (2015). Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke 46, 1161–1166. doi: 10.1161/STROKEAHA.114.008348
Keywords: air pollution, brain, MRI, PM2.5, VBM
Citation: Casanova R, Wang X, Reyes J, Akita Y, Serre ML, Vizuete W, Chui HC, Driscoll I, Resnick SM, Espeland MA, Chen J-C for the WHIMS-MRI Study Group (2016) A Voxel-Based Morphometry Study Reveals Local Brain Structural Alterations Associated with Ambient Fine Particles in Older Women. Front. Hum. Neurosci. 10:495. doi: 10.3389/fnhum.2016.00495
Received: 29 March 2016; Accepted: 20 September 2016;
Published: 13 October 2016.
Edited by:
Tetsuo Kida, National Institute for Physiological Sciences (NIPS), JapanReviewed by:
Anelyssa D'Abreu, University of Campinas, BrazilGianfranco Spalletta, Fondazione Santa Lucia (IRCCS), Italy
Copyright © 2016 Casanova, Wang, Reyes, Akita, Serre, Vizuete, Chui, Driscoll, Resnick, Espeland, Chen for the WHIMS-MRI Study Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramon Casanova, casanova@wakehealth.edu
 Ramon Casanova
Ramon Casanova Xinhui Wang
Xinhui Wang Jeanette Reyes3
Jeanette Reyes3  Marc L. Serre
Marc L. Serre Ira Driscoll
Ira Driscoll Mark A. Espeland
Mark A. Espeland Jiu-Chiuan Chen
Jiu-Chiuan Chen