The clinical assessment and remote administration tablet
- 1 Mind Research Network, Albuquerque, NM, USA
- 2 Department of Psychiatry, University of New Mexico, Albuquerque, NM, USA
- 3 Huntington’s Disease Center of Excellence, University of Iowa, Iowa City, IA, USA
- 4 Advanced Biomedical Informatics Group, Iowa City, IA, USA
- 5 Department of Electrical and Computer Engineering, University of New Mexico, Albuquerque, NM, USA
Electronic data capture of case report forms, demographic, neuropsychiatric, or clinical assessments, can vary from scanning hand-written forms into databases to fully electronic systems. Web-based forms can be extremely useful for self-assessment; however, in the case of neuropsychiatric assessments, self-assessment is often not an option. The clinician often must be the person either summarizing or making their best judgment about the subject’s response in order to complete an assessment, and having the clinician turn away to type into a web browser may be disruptive to the flow of the interview. The Mind Research Network has developed a prototype for a software tool for the real-time acquisition and validation of clinical assessments in remote environments. We have developed the clinical assessment and remote administration tablet on a Microsoft Windows PC tablet system, which has been adapted to interact with various data models already in use in several large-scale databases of neuroimaging studies in clinical populations. The tablet has been used successfully to collect and administer clinical assessments in several large-scale studies, so that the correct clinical measures are integrated with the correct imaging and other data. It has proven to be incredibly valuable in confirming that data collection across multiple research groups is performed similarly, quickly, and with accountability for incomplete datasets. We present the overall architecture and an evaluation of its use.
Introduction
Mental health research conducted on human subjects involves a rich set of clinical, neuropsychological, and sociodemographic assessments. Traditionally, these assessments which result from face to face interactions between the researcher and the study participant are recorded on paper. The paper forms are then passed at a later date to separate data-entry operators, who either scan the documents as graphics or use a combination of automatic text recognition and manual methods to enter the needed values into a database. This time-consuming transcription process can be a source of both delays and random error in large studies (Welker, 2007; Babre, 2011).
The process of transferring paper forms to electronic data can be cumbersome, confusing, and error-prone. Errors may slip past data-entry operators, who are not trained as raters and may not recognize that data is in fact illogical or missing, and the problematic data may only be discovered by data-entry operators months or longer after the assessment is performed. Clinical trials organizations implement continuing oversight processes with regular audits of the paper forms, to ensure that data are being collected correctly; effective, source data verification (SDV) creates an incredible cost in personnel time both on the part of the local research team and the study as a whole. Large-scale research studies without that level of personnel hours to invest in auditing or SDV can find that subjects may be excluded from analysis for an extended period of time due to incomplete data. In extreme cases, some subjects’ data, so costly to collect, may be entirely lost to analysis. In studies where it is difficult to find a sufficient number of qualified subjects, exclusion of subjects may compromise the research. These problems can become even more pronounced in multi-site research protocols that use paper-based assessments and centralized data-entry operations (Vessey et al., 2003). Automated methods are needed to ensure that data are complete.
Electronic data capture (EDC) promises a solution: The data are collected electronically and can be validated at the time of collection, confirming logical errors or notational problems, and ensuring that the required forms are complete. EDC can alleviate the need for time-consuming audits, since each subject’s data can be electronically audited, in a sense, at the time of collection (Kahn et al., 2007; Carvalho et al., 2011). The overall progress of a study can be monitored more closely during its actual execution and incomplete data identified promptly, through the integration of EDC methods with data management systems which track subject enrollment and other progress.
Web-based forms for EDC such as those developed by REDCap (Harris et al., 2009) are extremely useful for either clinician- or self-assessment, allowing the subjects to answer the questions on their own time in the comfort of their own home over the internet, and the answers can be collated without the introduction of an extra transcription step. However, self-assessment may not be an option in some situations. In the case of psychiatric assessments, the clinician researcher often must be the person either summarizing or making their best judgment about the subject’s response in order to complete an assessment, as in conducting the Structured Clinical Interview for Diagnosis (Spitzer et al., 1992) or reporting the level of consciousness in a neurological assessment of a near-comatose patient, for example. Interviewers cannot maintain eye contact or a comfortable interaction when there is a computer monitor between them, which is problematic in various studies; having the clinician turn away to type into a web browser may be disruptive to assessing body language cues or the flow of the interview.
Electronic data capture methods for research that occurs in a medical institution to integrate health records with research records (El Fadly et al., 2011) can depend on internet access during data collection and integration. EDC methods are also needed for studies that need to be performed at locations without internet access, remote rural areas, prisons, or mental health facilities that do not provide computing resources and may even block internet access. These problems make traditional web and desktop software impractical for many studies, which in turn keeps these more distributed clinical studies tethered to paper forms. REDCap is one of the few to address this, with a “REDCap Mobile” version which allows researchers in these situations to have encrypted laptops with a push–pull relationship to the centralized REDCap database to allow data collection while off-line (Borlawsky et al., 2011).
A list of current EDC packages are listed in Table 1, with their published characteristics. Current web-based EDC solutions suffer from two primary disadvantages, as can be seen: The first is the need to have internet access in order to function, which makes them unusable in remote situations such as those noted above, with the exception of REDCap. The second is that many of them are integrated into select data management systems. To use a specific EDC system one must also use a specific database and presumably port any pre-existing data into it. This is less attractive when the research group has a mature data management system in place for their neuroimaging data, and is looking to add EDC for clinical assessments.

Table 1. Electronic data capture systems available for clinical data capture and transmission to a database.
The solution is a system that combines the automated data capture of web-based self-assessment with the dynamics of a clinician’s expertise and needs, and the abilities to work off-line until interface with an arbitrary database is needed. The Mind Research Network (MRN) has developed a software tool for the real-time acquisition and validation of clinical assessments in remote environments: The clinical assessment and remote administration tablet (CARAT). We have developed this tool on a Microsoft Windows PC tablet system, and it has been used successfully to collect and administer clinical assessments in several large-scale studies. It has proven to be incredibly valuable in confirming that data collection across multiple research groups is performed similarly, quickly, and with accountability for incomplete datasets. Here we present its specifications and current availability, focusing on its use in conjunction with the Functional Biomedical Informatics Research Network (FBIRN) multi-site neuroimaging studies in schizophrenia, and the FBIRN Human Imaging Databases (Keator et al., 2008); and in conjunction with the MRN data management system (Bockholt et al., 2010; Scott et al., 2011), a centralized institutional level system managing multiple federated studies simultaneously. It has also been applied within an OpenClinica1 data management system (Bockholt et al., in preparation).
CARAT Specifications
The current application approached the problem of real-time clinical assessment entry and quality control with the following specifications:
1. Tablet hardware with convertible keyboard: Interviewers had to be able to hold a tablet on their laps, maintain eye contact with the subject, and enter data or make notes using the touch screen. Tablets offer a form factor uniquely suitable to an assessment interview. They are slim, easily carried, and shaped similarly to a sheet of paper or a notepad, allowing them to be held without attracting attention during an assessment. Touch screens allow for a rater to quickly select answers to questions with multiple choice options, and a touch keyboard is available for free-form questions and annotations.
2. Internet-independence for most functions: Users needed to be able to work remotely, entering case report forms (CRF) data and saving it to a central repository when the internet became available.
3. Potential for interaction with arbitrary database platforms and schemas: From the beginning, the software needed to be able to both submit to and receive data from several different databases as used by several investigators.
4. Real-Time Data Quality Validation: Data had to be checked as it was entered into each CRF for each interview or assessment.
5. Generic engine useful for any assessment and arbitrary visit-based groupings of assessments: The CRF metadata, including the questions, possible answers, data validation and required field information, and identifiers for upload to the database, had to be easily understood and expandable. CRFs may be changed after the study begins, or modified from one study to another. CRFs must be able to be grouped for initial screening visits and follow-up visits to follow the flow of a study protocol.
CARAT Implementation
The overall flow of information when using CARAT is shown in Figure 1. Clinical study setup (upper half of the figure) requires CRF form layout on the tablet, as well as arranging the CRFs into visits for each study; the data collection steps (lower half) use the XML representations to send the subject’s responses via the web service back to the database. The data collection steps from Figure 1 are expanded in the workflow shown in Figure 2. These are discussed below.
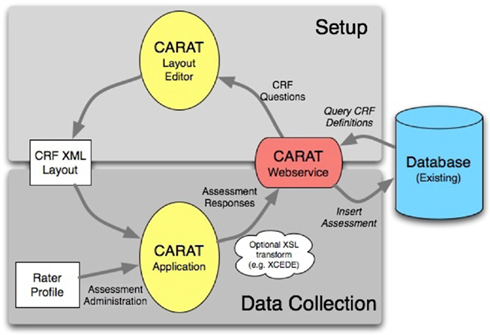
Figure 1. The flow of information for study setup and data collection using CARAT. The currently usable databases are the FBIRN human imaging database (HID) and the MRN medical imaging computer information system (MICIS), but others can be modeled in the web service. The study definition portion determines which CRFs are required in what layout for which visits and subject types within the study. The CRF XML layout captures how the questions should be presented on the tablet and the validation steps required. Following the administration of the assessments, the subject’s responses are sent via the web service back to the available database.
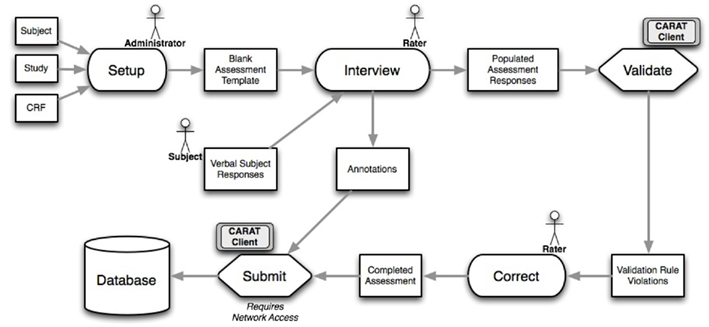
Figure 2. The workflow for CARAT, expanding on the data collection parts of Figure 1. Following study setup, data collection can proceed through the needed steps of interviews, validation, correction, addition of other notes or annotations, and final submission.
Tablet hardware. The current version of CARAT is built in C#. Net and designed to run on Windows XP Tablet Edition. It also runs successfully on Windows 7 desktops, laptops, and slate tablets.
Internet-independence. CARAT does not require an Internet or database connection for the entry of new clinical data. In the workflow shown in Figure 2, once the CRF information has been created during study setup, the data collection steps that follow, the subject interviews and data validation, data correction, and inclusion of any additional annotations, can be completed without any database connectivity. When network access is available, the submission process can be completed: The completed assessments that have passed data quality validation are exported, and can be inserted into a central database via the web service module shown in Figure 1.
Linking to other databases. To ensure database platform and schema independence, this step is performed by a SOAP web service that is individually customized by a software engineer associated with the required database platform. This web service need be written only once per database schema and then applied to all studies that are stored using the same database schema. Modification for communication with FBIRN Human Imaging Database (FBIRN HID)2 led to the addition of the ability to export assessments in different XML formats. As shown in Figure 1, CARAT can export the subject’s data using the XML-based Clinical Experiment Data Exchange (XCEDE) schema, which the FBIRN HID uses to import data3.
Real-time data validation. The validation steps can be set per question per assessment, and can be changed for different visits within a study if needed. They include checking required fields for completion, data type (e.g., numeric, character string, or date), bounds-checking (e.g., systolic blood pressure is a number between 0 and 300), and question dependencies (e.g., Question 2 “How many cigarets do you smoke a day?” does not need to be answered if the answer to Question 1 “Do you smoke?” is NO). CARAT provides some complex controls like tree selects, and repeating groups of fields, as one might use for a medication or drug history. During an interview, the rater is notified immediately when a required field is skipped or data entered does not meet quality criteria, but the software does not require the rater to fix the data immediately. This allows the rater to complete the interview smoothly and fix data issues at a later time if necessary. CRFs that do not pass data quality validation may be stored on the rater’s tablet and edited at any time, but they may not be submitted to the database until all issues are resolved. Examples are shown in Figures 4 and 6.
Generic CRF specifications. The CRF questions, possible answers, data validation and required field information, and identifiers for upload to the database, are stored in XML specification files, created from the database specifications of the CRF questions. The XML file also specifies traditional GUI elements, such as radio buttons and drop-down list boxes, and their location on the screen. Data-entry screens may then be created dynamically for any assessment the rater needs to use. The general data model is shown in Figure 3: A subject has an ID and a type or group assignment; a study may have multiple visits with varying assessments, and may be a multi-site study. The CRF is linked to many questions, each of which contains the question text and the validation rules for the answers. The CRF, in combination with information regarding the visit and study, forms an assessment for that study and visit. The subject’s responses for that assessment are tagged with the information about which question it came from on the CRF, as well as the subject, study, and visit information.
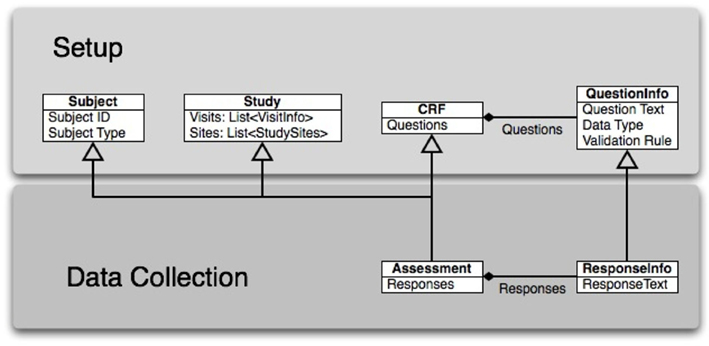
Figure 3. The data model for CARAT is lightweight, combining an individual’s responses with their study-specific information and response validation prior to inserting the information in the database. The upper row indicates those parts of the model that draw from study setup, while the lower row indicates the parts that are populated during data collection. The solid bulleted connections indicate a many-to-one relationship: a single CRF pulls from many questions, and a completed assessment includes several responses.
Both Figures 4 and 5 show data-entry screens dynamically generated from XML layout specification files. This provides flexibility and frees the researchers from the management of separate software packages to handle different clinical assessments.
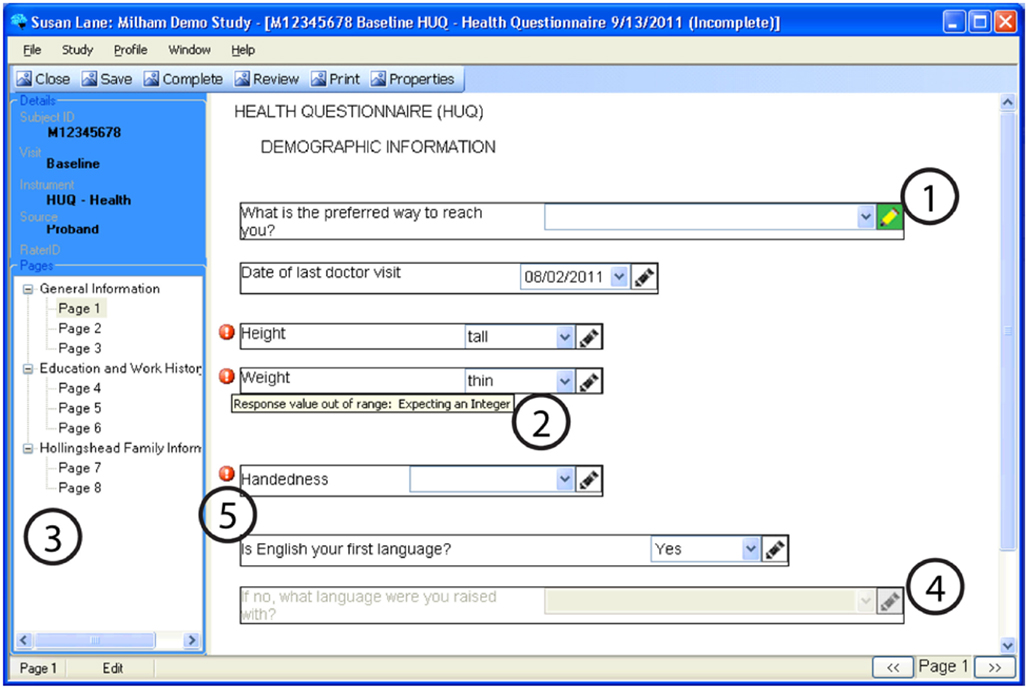
Figure 4. An example of a questionnaire on CARAT, demonstrating: (1) Annotations may be stored for any question by clicking the pencil icon (the pencil icon then displays in green). (2) Data types and reasonable responses are enforced, with error messages available to clarify. (3) Navigation through a form can be done using either the navigation tree or the buttons on the lower right corner. (4) Conditional skipping of questions: Here, since the participant answered “Yes” about English being their primary language, the follow-up question is not required. (5) Required fields are marked with a red icon.
A user-friendly method of creating electronic forms is required, to allow for rapid prototyping of new assessments for a study, and flexibility in re-use of forms. Clinicians may find that they habitually rearrange the order of questions on an assessment from the paper form during an interview, for example; electronic forms can allow this kind of rearrangement to be done to allow for a more natural interviewing environment. Questions that are not required based on the current interviewee’s answers may be automatically and dynamically hidden. Medication categories are included so that drug names can be selected rather than re-typed each time they are used.
In CARAT, XML layout specifications for CRFs are created using a graphic tool that uses the SOAP web service to pull the question metadata from the study protocols already in the database. The layout tool is designed for raters or other study staff to use to create detailed layouts, including GUI elements and data validation, with a minimum of technical expertise. Questions may be dragged and dropped into a GUI form and then modified as necessary. If metadata about a particular CRF question does not exist in a database, the metadata can be provided directly in the layout tool. The layout is then exported as an XML CRF specification to be included in CARAT.
CARAT User Interface
The CARAT software has spent the last almost 3 years in long-term piloting with multiple studies. During this time, it has been through three rounds of enhancement in response to user feedback. User needs led to extensive expansion of the options for assessment appearance, keyboard shortcuts for studies that made more use of the keyboard, expansion of user preferences so that default behavior better matches specific study needs, allowing sorting and re-ordering of assessments on the fly, creating missing data explanation fields, turning pen use on and off as needed, and various other usability changes.
Figure 4 demonstrates some of the user interface features of the prototype. The prototype provides a wide variety of data-entry methods from simple free text entry to methods of multiple choice that may be selected by a mouse click or a pen tap. Supported forms of data-entry include: Radio Buttons (displayed in Figure 4), Checkboxes, Date Selector, Extended Text Drop-Down Combo Box (displayed in Figure 5), Free Text Entry, and Tree Menus. Free text annotations may be stored for any question by clicking the pencil icon or tapping it with the tablet stylus.
Navigation of lengthy CRFs on paper is often not linear; clinicians may skip back and forth as the flow of the interview require. To recreate this in CARAT, users may navigate through the CRF pages using the arrow buttons in the lower right-hand corner, or they may go to a specific page using the navigation tree.
Questions may be skipped based on the answers to other questions. For example, one question may record if the subject ever received a specific diagnosis. If the answer is no, then the subsequent question about the diagnosis is non-applicable and is skipped.
The validation of data types and reasonable responses are enforced using regular expressions. If, for example, text is entered in a field expecting an integer or an out-of-range response is entered, or if a required response is missing, the user is immediately notified with a red exclamation icon. If the cursor hovers over the icon, then a specific error message is displayed.
When Complete or Review buttons at the top of the form are clicked, the prototype validates all questions in the CRF and displays a list of errors for the user to address, as shown in Figure 6. Clicking or tapping the error listing takes the user to the question that needs to be addressed. CRFs may be edited and saved with known errors, but they may not be submitted to the database until all errors are addressed. The CARAT interface includes the ability to create an exception, explaining how the data were not able to be collected, if this is the case. This method ensures that an error does not obstruct an interview, but that all issues are addressed before the data appears in the database.
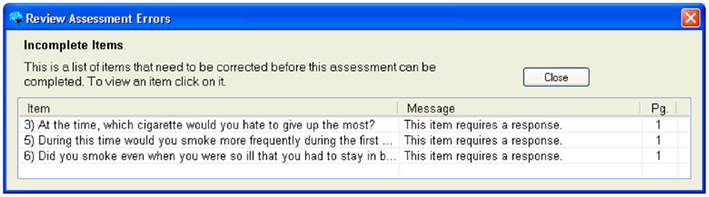
Figure 6. Example of error messages to identify which questions the error occurred on, what the error was, and what page of the assessment it occurred on.
Evaluation of CARAT
This prototype has been in long-term pilot with multiple groups, of which we will describe two examples: Psychopathy Research under Dr. Kent Kiehl located at the MRN and FBIRN (Keator et al., 2008; Potkin et al., 2009), a collaboration featuring more than 10 distributed sites. These groups use different database schemas and have different data collection needs. These projects have been instrumental in gathering user feedback as well as demonstrating the usefulness of the software in real assessment gathering conditions. We have recently added a self-assessment mode to allow the same CARAT system to be used in computer labs for self-assessments.
The psychopathy research team currently conducts five related studies. The team has been using the tablet application for longitudinal acquisition of clinical and sociodemographic assessments on an incarcerated adult psychopathy population since January 2009 (Ermer and Kiehl, 2010; Harenski and Kiehl, 2010; Harenski et al., 2010). The tablet product is ideal in a prison setting, since network desktop PC clients are typically disallowed and internet access is not available. Among the assessments in use are several standard questionnaires, personality checklists, and symptom severity indices, which use a broad range of question types and answers. Some of them have been modified for specific study needs; they are implemented with type-checking (dates, strings, numbers) and range-checking where relevant. Assessment data is entered in the MRN database, as part of the COINS system (Scott et al., this issue). The vast majority of CRFs for the psychopathy research studies are now entered via tablet.
Because the Kiehl psychopathy team phased the tablet entry into ongoing studies that had been using manual transcription and dual-entry, it is possible to compare the old method to the new method to evaluate the effects. For example, as of this writing, 15,393 assessments were entered manually from paper forms into the MRN’s database. The average time between the assessment interview and the time the data were verified as complete in the database was 232 days. In contrast, 5,567 assessments have been entered using the tablet prototype, and the average time between interview and complete data in the database is 3 days. This trend indicates that assessment data can be made available for analysis very quickly after it is gathered, and results may be available more than 7 months earlier than they would be otherwise.
The FBIRN community has used the tablet application as its primary method of assessment gathering for its Phase III schizophrenia study since January 2009. These assessments are conducted with schizophrenic and normal control participants across eight different data collection sites. For this project, we added functionality to the current prototype application to support data exchange using the XCEDE standard (Keator et al., 2006, 2009). Completed CRFs for FBIRN are uploaded to distributed instances of the Human Imaging Database, one at each data collection site.
Similarly in FBIRN as in the psychopathy research, two large-scale studies have been performed that can be compared. The Phase II dataset (Potkin and Ford, 2009; Potkin et al., 2009) was collected using the dual-entry method, including requiring manual double entry of the transcribed data to minimize errors. As project manager of the FBIRN at the time, Dr. Turner was very aware of the delays and errors in clinical data availability due to the dual-entry method, and the final data aggregation across assessments ranged from 100% available (for simple measures such as age and gender) to only 70% available or less (for more detailed questions regarding duration of illness or the full list and dosing information for current medications, for example). This was highly problematic in the full analysis of the final study. In the next multi-site study, the currently ongoing Phase III study, the clinical tablet is being used in all eight data collection sites, of which MRN is one. In conjunction with the multi-database querying allowed by the HID infrastructure, this allows regular confirmation of which subjects have been enrolled, screened, scanned, and have their clinical data complete – rather than simply asking the data collection teams for their assurances, electronic audits are being performed automatically and reviewed by the whole FBIRN regularly. As of this writing, over 300 subjects have been enrolled, with some sites showing 100% complete clinical assessments and others very close to that. This allows immediate accountability for incomplete data, and the ability to correct it rapidly to increase the proportion of usable datasets.
Caveats
With the exception of the upload of completed CRFs to a database, all major functions on CARAT are available without internet or network access. Asynchronous updates, however, can lead to duplicate or existing data in the centralized data management system, which must not be overwritten. REDCap has developed an elegant system to manage these issues (Borlawsky et al., 2011); CARAT handles those problems to some extent, though not as completely. At this point, the MICIS and BIRN web services will check against the subject ID, assessment, study, and visit to see if duplicate data already exists in the database. We are working to make the generic web service more flexible and secure for these issues while maintaining its agnostic approach to database schema
Clinical assessment and remote administration tablet was designed and implemented on a Windows system. Convertible tablets, for which Windows was the dominant operating system, are being overwhelmed by the new popularity of slate tablets. Slates tend to run Android or iOS, as well as Windows; CARAT is being re-factored to be platform-independent, to take advantage of these new options.
A challenge specific to multi-site studies is the need to have all users have the same implementation of CARAT, the same forms and layouts. For studies which decide on their protocol and never vary from it, it is not a challenge to set up one instantiation of CARAT and disseminate it to all the participating sites. Many clinical and research studies, however, find that they need new or revised CRFs over the course of a study; confirming all sites are using the same version has been a challenge for CARAT in multi-site studies. Future versions of CARAT will implement automatic update detection, so that all sites are running with the latest version for a given study whenever they have internet access.
Discussion
Electronic data capture of the assessments and interviews associated with clinical neuroimaging studies have been used in many studies, and the CARAT system which we present has been tested in a wide range of study situations. The needs which EDC systems commonly address include using a web interface to transmit data directly into a database, usually over the internet so that only a browser is needed for the user. The ability to handle complex forms, multiple types of questions, conditional branching, and constraint checking is also needed for an adequate system (Harris et al., 2009). Unique needs for many research programs include the ability to use a tablet system rather than a desktop or laptop, and to work where the internet is not available, without giving up the ability to capture the data and transmit it directly to a study database. The demands of neuroimaging studies includes the ability to identify the subject’s imaging data with their clinical and other data, preferably within a centralized multi-modal data repository.
The first advantage of CARAT is that it was designed to work on a tablet, mimicking natural interviewing style. The use of the stylus on the tablet screen has been popular with researchers who prefer the pen and paper approach. CARAT also includes the ability to handle constraint checking and complex conditional branching, similar to what REDCap provides. Data validation supports regular expressions, so error detection can become extremely detailed. Responses can be validated not just by data type (date, number, character string, etc.) but by complex ranges of these in almost any combination. CARAT at the moment does not implement the ability to reference multiple questions in a single validation rule, however; it can require the response field be filled in according to highly complex string-formation rules combining letters and numbers, but we can’t say that the response must be less the answer to the previous question plus 100, for example. That has not been a common request from users, though it could be implemented if needed.
An appropriate EDC solution must also provide a high degree of CRF customization to accommodate the almost constant stream of new assessments, and modifications to existing assessments, which comes with many research programs. The CRFs and other assessment forms must be extremely customizable, allowing for entry of common, rare, and unique study-specific assessments. It should be modifiable by the research team that is collecting and/or managing the data, rather than depending on the original software developers – ease of use in the CARAT layout system is currently a focus of ongoing development. Ideally, for non-copyright material, a repository of assessments should be available, built from studies already using CARAT, so that the forms for a new study can be set up quickly and re-use existing forms automatically whenever possible, similar to the system implemented in (El Fadly et al., 2011).
Data-gathering software appropriate to the specific needs of clinical research interviews is unlikely to be widely adopted if it cannot conform to the data storage solutions already employed at mature research sites. It may or may not be possible to integrate data from independent software packages that employ their own distinct database schemas. The software must be able to plug into the existing frameworks already in use by mature research programs. It must have a mechanism to communicate with existing databases and schemas, be they off-the-shelf like OpenClinica or custom solutions such as REDCap. CARAT has the flexibility to interact with arbitrary databases, rather than a single proprietary database specialized for clinical assessments, thus allowing the ability of combining clinical and imaging data within a single repository.
Franklin et al. (2011) reviewed several common EDC systems within their needs for small-scale clinical trials; their list of valued features overlapped with the CARAT specifications to some extent. Their list included availability of training materials, the presence of site and user roles and permissions, ease of designing CRFs including error detection, the ability to create a visit schedule for data-entry, and the ease in importing or exporting data in a standards-compliant way. CARAT provides training materials for the user interface, and the developers’ manual for the web interface is in development. The fact that CARAT is built to work with both MICIS and HID and to be expandable to other database system means many of the security issues and permissions are considered the purview of the database, however, rather than the data collection system. CARAT can validate that the rater’s username and password is a valid rater for the study, and that the subject ID being used is for a subject who is enrolled in the study. Other levels of permission that are not related to collecting assessment data are not within the scope of CARAT at this time.
Clinical assessment and remote administration tablet is organized around the idea of visit schedules, which are part of the fundamentals of the tablet interface. The grouping of assessments that need to be done at baseline screening and subsequent visits throughout a study protocol are defined in the primary configuration file and are required. Each assessment or group of assessments for an individual subject, can be exported as XML as described above; data import and export from the database repository, whether in text or comma separated values or other formats, is again, a choice for that data management system.
Availability
The CARAT software is available from www.nitric.org, under multiple licenses. For the research community with free re-use, it is available under the GNU General Public License (GPL). If the software is to be used with a commercial interest, other licenses can be negotiated.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by funding from the National Institutes of Health as part of the FBIRN project (U24 RR021992, S. G. Potkin PI), by 1R01EB006841 (Vince D. Calhoun), and from the Department of Energy, DE-FG02-08ER64581.
Footnotes
References
Babre, D. (2011). Electronic data capture – narrowing the gap between clinical and data management. Perspect. Clin. Res. 2, 1–3.
Bockholt, H. J., Scully, M., Courtney, W., Rachakonda, S., Scott, A., Caprihan, A., Fries, J., Kalyanam, R., Segall, J. M., de la Garza, R., Lane, S., and Calhoun, V. D. (2010). Mining the mind research network: a novel framework for exploring large scale, heterogeneous translational neuroscience research data sources. Front. Neuroinform. 3:36. doi: 10.3389/neuro.11.036.2009
Borlawsky, T. B., Lele, O., Jensen, D., Hood, N. E., and Wewers, M. E. (2011). Enabling distributed electronic research data collection for a rural Appalachian tobacco cessation study. J. Am. Med. Inform. Assoc. doi: 10.1136/amiajnl-2011-000354. [Epub ahead of print].
Carvalho, J. C., Bottenberg, P., Declerck, D., van Nieuwenhuysen, J. P., Vanobbergen, J., and Nyssen, M. (2011). Validity of an information and communication technology system for data capture in epidemiological studies. Caries Res. 45, 287–293.
El Fadly, A., Rance, B., Lucas, N., Mead, C., Chatellier, G., Lastic, P. Y., Jaulent, M. C., and Daniel, C. (2011). Integrating clinical research with the healthcare enterprise: from the RE-USE project to the EHR4CR platform. J. Biomed. Inform. doi: 10.1016/j.jbi.2011.07.007. [Epub ahead of print].
Ermer, E., and Kiehl, K. A. (2010). Psychopaths are impaired in social exchange and precautionary reasoning. Psychol. Sci. 21, 1399–1405.
Franklin, J. D., Guidry, A., and Brinkley, J. F. (2011). A partnership approach for electronic data capture in small-scale clinical trials. J. Biomed. Inform. PMID: 21651992. [Epub ahead of print].
Harenski, C. L., Harenski, K. A., Shane, M. S., and Kiehl, K. A. (2010). Aberrant neural processing of moral violations in criminal psychopaths. J. Abnorm. Psychol. 119, 863–874.
Harenski, C. L., and Kiehl, K. A. (2010). Reactive aggression in psychopathy and the role of frustration: susceptibility, experience, and control. Br. J. Psychol. 101(Pt 3), 401–406.
Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., and Conde, J. G. (2009). Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381. [Research Support, N.I.H., Extramural].
Kahn, M. G., Kaplan, D., Sokol, R. J., and DiLaura, R. P. (2007). Configuration challenges: implementing translational research policies in electronic medical records. Acad. Med. 82, 661–669.
Keator, D. B., Gadde, S., Grethe, J. S., Taylor, D. V., Potkin, S. G., and First, B. (2006). A general XML schema and SPM toolbox for storage of neuro-imaging results and anatomical labels. Neuroinformatics 4, 199–212.
Keator, D. B., Grethe, J. S., Marcus, D., Ozyurt, B., Gadde, S., Murphy, S., Pieper, S., Greve, D., Notestine, R., Bockholt, H. J., Papadopoulos, P., Function, B., Morphometry, B., and Coordinating, B. (2008). A national human neuroimaging collaboratory enabled by the Biomedical Informatics Research Network (BIRN). IEEE Trans. Inf. Technol. Biomed. 12, 162–172.
Keator, D. B., Wei, D., Gadde, S., Bockholt, J., Grethe, J. S., Marcus, D., Aucoin, N., and Ozyurt, I. B. (2009). Derived data storage and exchange workflow for large-scale neuroimaging analyses on the BIRN grid. Front. Neuroinform. 3:30. doi: 10.3389/neuro.11.030.2009
Potkin, S. G., and Ford, J. M. (2009). Widespread cortical dysfunction in schizophrenia: the FBIRN imaging consortium. Schizophr. Bull. 35, 15–18.
Potkin, S. G., Turner, J. A., Brown, G. G., McCarthy, G., Greve, D. N., Glover, G. H., Manoach, D. S., Belger, A., Diaz, M., Wible, C. G., Ford, J. M., Mathalon, D. H., Gollub, R., Lauriello, J., O’Leary, D., van Erp, T. G., Toga, A. W., Preda, A., Lim, K. O., and FBIRN. (2009). Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr. Bull. 35, 19–31.
Scott, A., Courtney, W., Wood, D., De la Garza, R., Lane, S., Turner, J. A., and Calhoun, V. D. (2011). COINS: an innovative informatics and neuroimaging tool suite built for large heterogeneous datasets. Front. Neuroinform. 5:33. doi: 10.3389/fninf.2011.00033
Spitzer, R. L., Williams, J. B., Gibbon, M., and First, M. B. (1992). The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch. Gen. Psychiatry 49, 624–629.
Vessey, J. A., Broome, M. E., and Carlson, K. (2003). Conduct of multisite clinical studies by professional organizations. J. Spec. Pediatr. Nurs. 8, 13–21.
Keywords: electronic data capture, case report forms, clinical assessments
Citation: Turner JA, Lane SR, Bockholt HJ and Calhoun VD (2011) The clinical assessment and remote administration tablet. Front. Neuroinform. 5:31. doi: 10.3389/fninf.2011.00031
Received: 14 September 2011; Paper pending published: 08 October 2011;
Accepted: 21 November 2011; Published online: 27 December 2011.
Edited by:
John Van Horn, University of California at Los Angeles, USAReviewed by:
Yann Le Franc, University of Antwerp, BelgiumFernando Perez, University of California at Berkeley, USA
Copyright: © 2011 Turner, Lane, Bockholt and Calhoun. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Jessica A. Turner, Mind Research Network, 1101 Yale Boulevard, NE, Albuquerque, NM 87106, USA. e-mail: jturner@mrn.org
