- 1 Department of Neuroscience, University of Minnesota, Minneapolis, MN, USA
- 2 Center for Neuroscience, Swammerdam Institute for Life Sciences, University of Amsterdam, Amsterdam, Netherlands
- 3 University of Maryland School of Medicine, Baltimore, MD, USA
- 4 Department of Psychology and Neuroscience Program, University of Michigan, Ann Arbor, MI, USA
A vast literature implicates the ventral striatum in the processing of reward-related information and in mediating the impact of such information on behavior. It is characterized by heterogeneity at the local circuit, connectivity, and functional levels. A tool for dissecting this complex structure that has received relatively little attention until recently is the analysis of ventral striatal local field potential oscillations, which are more prominent in the gamma band compared to the dorsal striatum. Here we review recent results on gamma oscillations recorded from freely moving rats. Ventral striatal gamma separates into distinct frequency bands (gamma-50 and gamma-80) with distinct behavioral correlates, relationships to different inputs, and separate populations of phase-locked putative fast-spiking interneurons. Fast switching between gamma-50 and gamma-80 occurs spontaneously but is influenced by reward delivery as well as the application of dopaminergic drugs. These results provide novel insights into ventral striatal processing and highlight the importance of considering fast-timescale dynamics of ventral striatal activity.
The ventral striatum is one of the major subdivisions of the vertebrate striatum, sharing with more dorsal striatal regions features such as its dominant cell class (the medium-sized spiny neuron or MSN), dopaminergic inputs from the midbrain, and participation in topographically organized cortico-striatal projection loops (Mogenson et al., 1980; Alexander and Crutcher, 1990; Pennartz et al., 1994, 2009; Houk et al., 1995). In the absence of a single feature that defines a sharp anatomical boundary between the dorsal and ventral striatum, the distinction between the two is usually framed as one of gradients, in which multiple overlapping distributions of afferent and efferent projections, cell types, immunohistochemical and cytological properties combine to form distinct domains (Graybiel, 1990; Gerfen, 1992; Voorn et al., 2004; Humphries and Prescott, 2010)1. Accordingly, there are numerous examples of dissociations at the behavioral level between lesions or inactivations of the ventral and dorsal striatum (Robbins et al., 1989; Balleine, 2005; Atallah et al., 2007) supported by differences in recording and imaging studies during behavior (O’Doherty et al., 2004; Tanaka et al., 2004; Kimchi and Laubach, 2009; van der Meer et al., 2009). Overall, such dissociations support the idea of “parallel processing domains” in dorsal and ventral striatum with distinct functions; ventral striatum plays a central role in processing aspects of reward (affective and motivational processing), sometimes articulated as “mediating the effects of reward-predictive cues and contexts” (Mogenson et al., 1980; Pennartz et al., 1994; Robbins and Everitt, 1996; Cardinal et al., 2002; Schoenbaum and Setlow, 2003). Yet, its precise role in such motivated behavior continues to be challenging to pin down, not in the least because under this overall characterization lies a complex, heterogeneous structure with dissociable subregions or processing channels within ventral striatum, the organizational principles of which are far from understood.
A potential tool to aid in the dissection of the structure and function of ventral striatum is the analysis of local field potentials (LFPs). In general, when recording a wideband signal from within the brain, the higher frequencies (∼600–10000 Hz) may be used to detect spiking activity in a relatively small number of individual neurons, while the lower “LFP” frequencies (∼1–300 Hz) reflect slower membrane potential (voltage) changes in a larger population of neurons. The main contributor to the LFP signal is generally thought to be synaptic input potentials (EPSPs and IPSPs), but other slow membrane changes, such as those arising from afterhyperpolarizations, can also play a part (Mitzdorf, 1987; Kamondi et al., 1998; Logothetis, 2003; Buzsáki, 2006). Because the LFP signal is an average of a relatively large area, the presence of rhythmic oscillations in the LFP implies some degree of systematic, coordinated activity, which can be related to external stimuli and behavior, as well as to spiking activity and LFPs in other areas or frequency bands. Commonly used basic analysis methods for LFPs include power, an estimate of the strength of a given frequency component, and coherence, a measure of the degree to which LFP power in two signals (LFPs or spike trains; see also phase locking) co-occurs.
In this review, we focus on ventral striatal LFP oscillations in the gamma band (a frequency range of roughly 40–100 Hz). Recording from freely moving rats, Berke et al. (2004) found a gradient of gamma power across the striatum, appearing smallest in dorsolateral and largest in ventromedial striatum. Similarly, Masimore et al. (2005) found movement-related gamma oscillations in the striatum, also noting a ventral–dorsal gradient of diminishing power (Masimore, 2008). These observations suggest that gamma oscillations may be relevant to the structure and function of the ventral striatum in particular. Three recent studies (Berke, 2009; van der Meer and Redish, 2009b; Kalenscher et al., 2010) sought to characterize the relationship of gamma oscillations in ventral striatum to behavior, spiking activity, LFPs in other areas, and rewards. As some of the first studies to document these properties, the data do not yet converge on all points; in this review, we indicate both the commonalities and discrepancies, before considering some implications of the properties of ventral striatal gamma oscillations for the organization of ventral striatum. We close by pointing to open questions for future work.
Ventral Striatal Gamma Oscillations Consist of Distinct, Switching Rhythms
In line with previous studies recording from ventral striatum (Leung and Yim, 1993; Kasanetz et al., 2002; Berke et al., 2004; Hunt et al., 2006), Berke (2009), van der Meer and Redish (2009b), and Kalenscher et al. (2010) found prominent LFP power in the gamma range. However, they noted distinct power bands, with one centered at around 50 Hz (“gamma-50”), and another, broader band from ∼70–100 Hz (“gamma-80”). Gamma oscillations in both bands tended to occur in “bursts” of ∼150–250 ms (Figure 1A); this pattern is consistent with power modulation by the phase of slower rhythms such as theta/alpha (in dorsal striatum, Tort et al., 2008; hippocampus, Colgin et al., 2009; human ventral striatum, Cohen et al., 2009)2. Oscillations in the two bands had a tendency to alternate spontaneously, rather than co-occur (Figure 1A), suggesting a degree of mutually exclusive coordination between the two. Berke (2009) showed further that switching between gamma-50 and gamma-80 was influenced by systemic application of dopaminergic drugs3, shifting away from gamma-50 toward gamma-80 (Figure 2C). As ventral striatum receives a strong dopaminergic projection from the midbrain (Mogenson et al., 1980; Swanson, 1982) and synaptic and intrinsic membrane properties of ventral striatal neurons are modulated by dopamine (DA) receptor activity (Bracci et al., 2003; Kreitzer and Malenka, 2008) it is possible that this effect is mediated by processes in ventral striatum4. Switching between ventral striatal gamma-50 and gamma-80 is also present in anesthetized animals (Sharott et al., 2009) and appears similar to gamma-band switching found in a number of other areas (hippocampal CA1, Colgin et al., 2009; piriform cortex, Kay et al., 2009), although the effects of DA in those cases is not known. In any case, the structure of ventral striatal gamma activity, with its distinct gamma-50 and gamma-80 power bands, provides a basis for investigating their behavioral and neural correlates separately.
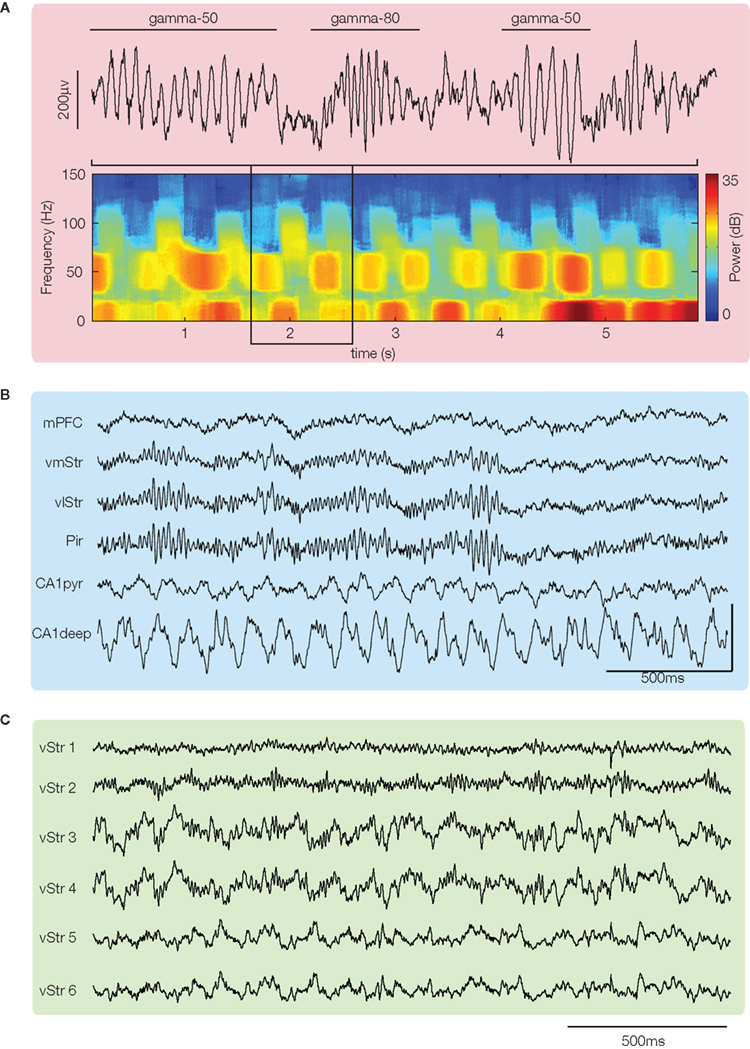
Figure 1. Local field potentials recorded from ventral striatum show prominent gamma oscillations. (A) Example trace and spectrogram highlighting spontaneously alternating bursts of “gamma-50” (∼45–55 Hz) and “gamma-80” (∼70–100 Hz). Hot colors indicate high power; figure from van der Meer and Redish (2009b). (B) Simultaneous recording from ventral striatum and piriform cortex (Pir) shows that LFP gamma oscillations can be nearly identical in both. Figure adapted from Berke (2005, 2009; vmStr is ventromedial striatum, vlStr ventrolateral). (C) Simultaneously recorded local field potential (LFP) traces from sites within ventral striatum suggest that gamma power can show heterogeneity between different recording sites. Traces taken from the data set in Kalenscher et al. (2010).
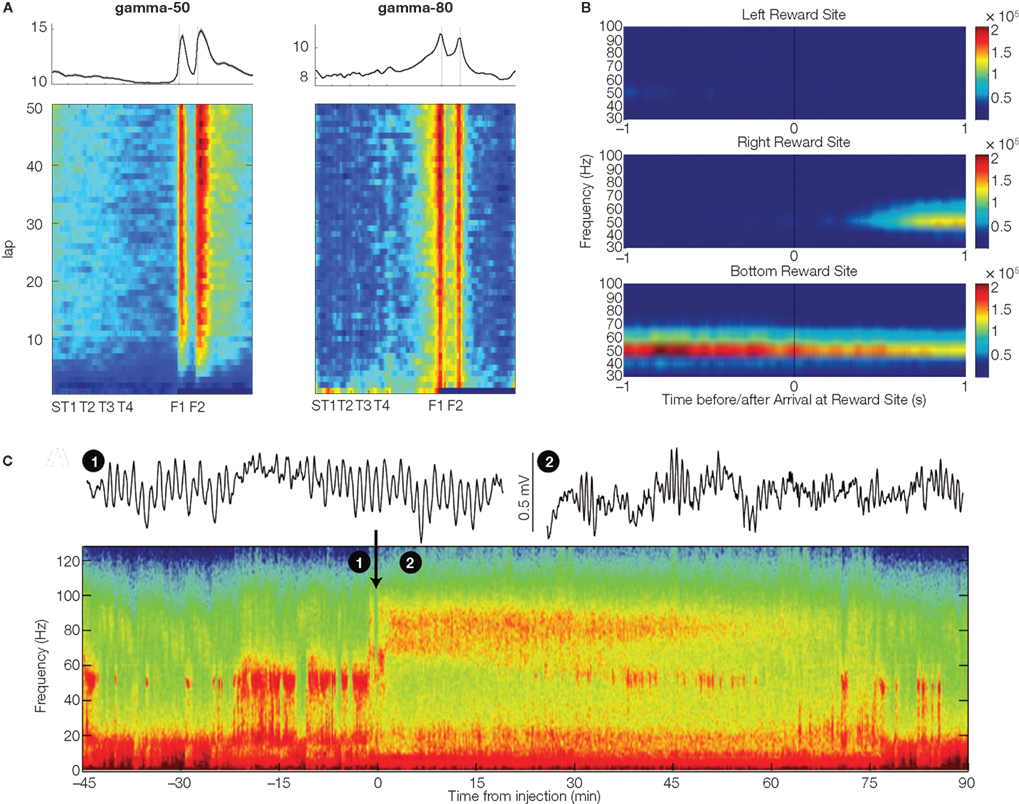
Figure 2. Three salient properties of ventral striatal gamma oscillations. (A) Gamma-50 (left) and gamma-80 power (right) were distributed differently over a track where rats ran for food reward (provided at reward sites F1 and F2). Gamma-80 ramped up to the reward sites, while gamma-50 increased abruptly. Plot shows average across four subjects; because rats ran a lap-based task, data were linearized for display (see Figure 4 for task details). Data from van der Meer and Redish (2009b). (B) Example session in which gamma power differentiated between distinct reward sites (with different appetitive rewards) on a triangular track in Kalenscher et al. (2010). This particular LFP trace had almost no gamma power at the left reward site, but strong power at the bottom site. (C) Systemic application of the dopaminergic agonist apomorphine (at time 0; black arrow) caused a switch from gamma-50 to gamma-80. Example traces (1) and (2) were taken from the times indicated in the spectrogram, before and after drug administration respectively. Data from Berke (2009).
It is important to establish whether the recorded LFP oscillations are related to neural activity at the recording site, and not simply volume-conducted from nearby but distinct structures without any relationship to ventral striatal processing. For instance, theta-band oscillations (∼6–10 Hz) generated in the hippocampus can be detected several millimeters from their generation site, with power decreasing only about 20% per millimeter depending on the direction of conduction (Sirota et al., 2008). Thus, Berke (2009), van der Meer and Redish (2009b), and Kalenscher et al. (2010) asked whether the firing of single neurons in ventral striatum, recorded simultaneously with LFP oscillations, was related to gamma phase and power. Indeed, both putative fast-spiking interneurons (FSIs; about 90%, Kalenscher et al., 2010; over 50%, van der Meer and Redish, 2009b; about 15%, Berke, 20095), and MSNs (about 3%; Kalenscher et al., 2010) had significant phase relationships (phase locking) to the gamma LFP, as indicated by spike-triggered averages, phase distributions, and coherence analyses. Interestingly, many FSIs cohered with either gamma-50 or gamma-80, but not both, indicating that the different gamma bands are associated with distinct FSI populations (Figure 3). While in vitro studies have shown that ventral striatal FSIs show intrinsic resonance in the gamma range (Bracci et al., 2003; Taverna et al., 2007), those studies did not report FSI groups specifically tuned to gamma-50 or gamma-80; it is possible that the observed FSI subclasses result from differences in the source of their inputs, or different responses to neuromodulators. The observation that FSIs were preferentially entrained to gamma oscillations is consistent with their integral role in the generation of gamma oscillations in other brain regions, as assessed by dynamic, in vivo control using optogenetic tools (Fuchs et al., 2007; Cardin et al., 2009; Sohal et al., 2009), though usually as part of recurrent local excitatory–inhibitory loops (Bartos et al., 2007; Fries et al., 2007). Striatal FSIs can show task-modulated entrainment to gamma oscillations even without changes in firing rate (Berke, 2009), part of a set of recent findings suggesting that FSI spike timing, and FSI spike rate, may be controlled by distinct aspects of their connectivity (Berke, 2008; Gage et al., 2010; Lau et al., 2010; Wiltschko et al., 2010). Thus, not only do such spiking relationships indicate that gamma oscillations are relevant to local processing, they also provide novel insights into the organization of ventral striatal circuitry.
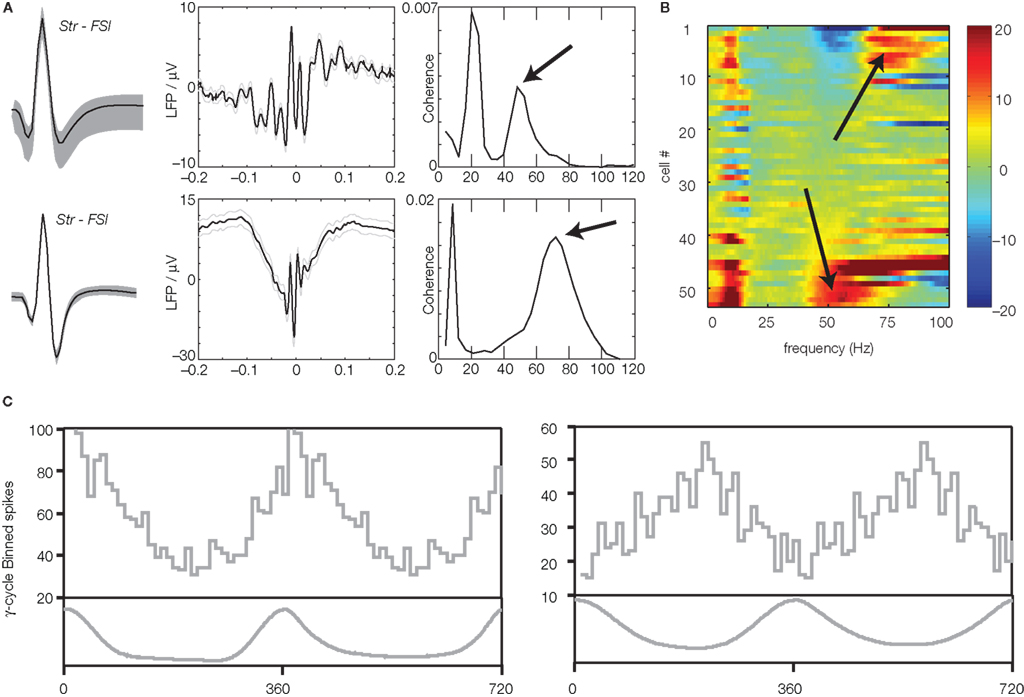
Figure 3. Neurons in ventral striatum show firing relationships to gamma band local field potentials. (A) Putative fast-spiking interneurons (FSIs) tended to show strong relationships to either gamma-50 or gamma-80. Shown is the average spike waveform (left), spike-triggered LFP average (middle) and spike-field coherence (right) of two example putative FSIs in Berke (2009). Note how the top neuron is coherent with gamma-50 but not gamma-80, while the bottom neuron is more coherent at gamma-80 (arrows). van der Meer and Redish (2009b) similarly examined the coherence of a population of putative FSIs (B) finding subgroups of gamma-50 and gamma-80 coherent neurons. Plot shows the z-scored difference of the observed spike-field coherence relative to a set of interspike-interval-shuffled controls; high z-scores (hot colors) indicate high coherence. Note the two distinct populations at the top (gamma-80) and bottom (gamma-50; arrows). (C) Phase histograms for two example putative medium-sized spiny neurons (MSNs) in Kalenscher et al. (2010), showing a tendency to fire at a particular phase of the gamma cycle.

Figure 4. Schematic representation of the tasks used (top) in Berke (2009, left), van der Meer and Redish (2009b, middle), and Kalenscher et al. (2010, right), and summary of the main findings in each (bottom). In each study, rats ran to obtain appetitive rewards at reward sites (filled circles). In Berke (2009) the rewarded arm was indicated by cue lights visible from the central choice point. In van der Meer and Redish (2009b) only one side of the maze was rewarded, but the rewarded side was changed between recording sessions. In Kalenscher et al. (2010) each reward site provided a different reward. Note that, in the summary of the findings presented here (as in the main text), a distinction is made between “reward delivery”, which is assessed by comparing rewarded and unrewarded trials, and “reward sites” which may include components (such as an anticipatory ramp) independent of actual reward delivery. ECoG, electrocorticogram; DA, dopamine; FSI, fast-spiking interneuron; MSN, medium-sized spiny neuron.
Task- and Reward-Related Modulation of Gamma-50 and Gamma-80
All three studies (Berke, 2009; van der Meer and Redish, 2009b; Kalenscher et al., 2010) found changes in gamma oscillations associated with reward sites and delivery. In one manifestation of such reward relationships, van der Meer and Redish (2009b) found strikingly different distributions of gamma-50 and gamma-80 power across the running track, with gamma-50 increasing abruptly at the reward sites before trailing off gradually, whereas gamma-80 ramped up gradually to the reward sites (Figure 2A). Zooming in on the time of reward delivery, gamma-80 power showed a complex pattern, ramping up gradually before falling sharply at the time of reward receipt, but then rising again before returning to baseline (note that this refers to fast-timescale changes beyond those seen in Figure 2A; see Figure 9 in van der Meer and Redish 2009b). Interestingly, the initial ramping effect was independent of whether reward was actually received or not, while the post-arrival increase was only seen when reward was actually delivered. In apparent contrast, Berke (2009) found that gamma-50 power was virtually abolished following reward receipt, while gamma-80 power showed a transient increase. Thus, both Berke (2009) and van der Meer and Redish (2009b) found an increase in gamma-80 specific to reward delivery, but in van der Meer and Redish (2009b) gamma-80 additionally ramped up to the reward sites, reminiscent of firing patterns found in ventral striatal neurons, which have been widely shown to anticipate reward delivery (Schultz et al., 1992; Lavoie and Mizumori, 1994; Miyazaki et al., 1998; Khamassi et al., 2008; van der Meer and Redish, 2009a). In further contrast to Berke (2009), van der Meer and Redish (2009b) reported that gamma-50 power, like gamma-80, showed a multiphasic relationship to reward delivery, increasing abruptly immediately following reward receipt, before decreasing and then rebounding, persisting well past initiation of the following lap6. The more immediate post-arrival increase in gamma-50 power was dependent on actual reward delivery, while the later one was not7. Kalenscher et al. (2010) similarly found that gamma-50 power tended to be higher on rewarded (compared to unrewarded) trials, and in line with van der Meer and Redish (2009b), that gamma-80 power was increased prior to reward delivery.
In these analyses, it should be noted that behavioral differences (such as grooming, consumption, frustration, initiation of movement to the next reward site) could be different between rewarded and non-rewarded trials. However, in van der Meer and Redish (2009b) the early components of the gamma-50 and gamma-80 differentiated between rewarded and non-rewarded trials even though movement speeds were similar. Furthermore, Kalenscher et al. (2010) showed that especially gamma-50 (and to a lesser degree gamma-80 power) differentiated between visits to different reward sites with distinct appetitive rewards (Figure 2B). Since behavioral differences such as grooming and consumption are not site-specific and should hence influence gamma power at all reward locations equally, differential effects of site on gamma power suggest that gamma power modulation is at least in part related to reward-specific processing. Thus, while the three studies differ in the precise pattern of gamma-50 and gamma-80 power changes relative to reward, taken together they support the notion that ventral striatal gamma oscillations are affected by reward anticipation and receipt.
Beyond relationships to the reward sites and reward delivery, van der Meer and Redish (2009b) noted a number of further differences in the task- and behavioral correlates of gamma-50 and gamma-80. Gamma-50 and gamma-80 differed in their development as animals learned to choose the correct maze arm, with gamma-80 power being highest early in a session, while gamma-50 power was almost absent early and developed later (within the first ∼5 min or 10 laps, even when rats were already very familiar with the environment). Gamma-80 power was increased specifically at the maze’s choice point early in sessions, similar to the time course and location of both forward representations (“sweeps”) in the hippocampus, and covert activation of ventral striatal reward-responsive neurons (Johnson and Redish, 2007; van der Meer and Redish, 2009a, 2010). van der Meer and Redish (2009b) also found, consistent with an earlier report (Masimore et al., 2005), that gamma-50 but not gamma-80 power was transiently increased before movement initiation (starting to run from having been previously stationary), even when they only considered movements away from the reward sites to exclude any potential contribution of reward delivery. In apparent contrast, Kalenscher et al. (2010) found only very weak correlations between gamma power and movement parameters, even when the analysis was restricted to test the effect of positive acceleration on gamma power. However, in addition to the fact that these diverging results may be attributed to differences in the analyses used, this set of observations may not in fact be incompatible, but rather result from gamma-50 power being increased before – but not at – the time of, positive acceleration accompanying movement initiation.
In sum, it appears that the full complement of task- and behavioral correlates of ventral striatal gamma-band activity, which includes not only the complex multiphasic pattern at reward sites, but also movement and other correlates, is not easily captured by a unitary explanation – whether in the case of gamma-50 or gamma-80. This observation raises the question to what extent these different correlates are generated by the same neural process or circuit, or whether they are generated by different (topographically segregated) circuits, but mixed together by volume conduction. For instance, in the case of gamma-80 (in van der Meer and Redish 2009b at least), is the ramping up to reward (which is independent of reward delivery) and the transient response to actual reward delivery, linked at the neural level within ventral striatum? Or, alternatively, are there separate circuits, one generating the ramp and another generating the transient reward response, which merely appear linked because both components appear in LFPs that reflect a wide area? We consider these possibilities in the next section.
Origins of Ventral Striatal Gamma
As discussed above, several lines of evidence suggest that at least part of ventral striatal gamma activity is locally generated. These include relationships between spiking activity and gamma oscillation phase, reports of intrinsic membrane oscillations in the gamma range in striatal FSIs, and the role of similar interneurons in generating gamma oscillations in other structures. What are the possible sources for the locally generated component of ventral striatal gamma? These could include synaptic inputs from afferent areas, such as hippocampus, amygdala, prefrontal cortex and thalamus (which are known to exhibit gamma oscillations: e.g., Colgin et al., 2009; Popescu et al., 2009), synaptic currents resulting from local projections within the striatum (both FSIs and MSNs make local connections), local intrinsic membrane potentials, and multi-unit spiking activity8. These possibilities thus raise the question: how much of ventral striatal gamma is due to structure already present in its inputs, and what (if any) is the contribution of local ventral striatal processing? To address this, Berke (2009) recorded ventral striatal LFPs simultaneously with piriform and frontal cortical (skull screw electrocorticogram) LFPs, finding that ventral striatal gamma-50 appeared similar to piriform gamma (Figure 1B). While piriform gamma oscillations can include both gamma-50 and a higher frequency, ∼70 Hz component (Kay et al., 2009), Berke (2009) found that gamma-80 in ventral striatum could be coherent with frontal cortical gamma-80. These findings suggest that gamma-50 recorded from rat ventral striatum contains a contribution from piriform gamma; however, because ventral striatal neuronal firing can cohere with gamma-50 (Figure 3), this is unlikely to be due to volume conduction alone. Ventral striatal gamma-80 may reflect increased inputs from frontal cortical areas, again relevant to processing within ventral striatum as indicated by FSI firing relationships.
The above results suggest a possible conception of ventral striatal gamma as resulting from synaptic currents from excitatory afferents that already possess oscillatory structure: the major sources of afferent input into ventral striatum (amygdala, hippocampal formation, prefrontal cortex, piriform cortex, thalamus) are all known to contain gamma oscillations (Chrobak and Buzsáki, 1998; Lee et al., 2003; Colgin et al., 2009; Kay et al., 2009)9,10. In this scenario, ventral striatal gamma oscillations simply result from synaptic currents generated by afferent inputs to FSIs and MSNs. On the one hand, this would be consistent with the observation that ventral striatum lacks the recurrent inhibitory–excitatory loops thought to be responsible for de novo generation (that is, not present in inputs) of gamma oscillations (Bartos et al., 2007; Fries et al., 2007; Tiesinga and Sejnowski, 2009) and central to computational models of gamma synchronization (e.g., Lytton and Sejnowski, 1991; Vreeswijk et al., 1994; Traub et al., 1997). On the other hand, in such a scenario it is not clear how coordinated switching between gamma-50 and gamma-80 (as in Figure 1A) can arise from distinct inputs; these would already have to be coordinated in order to generate a consistent switching pattern. An alternative scenario is that the intrinsic membrane properties of FSIs allow them to convert excitatory inputs without oscillatory structure into local gamma frequency inhibition. In particular, the subthreshold gamma oscillations observed in FSIs in response to non-oscillatory depolarization in vitro (Bracci et al., 2003; Taverna et al., 2007) may contribute to LFP gamma oscillations directly, but also generate rhythmic, potentially widespread inhibition of MSNs, even for non-oscillatory input barrages. While FSIs are thought to be relatively rare in ventral striatum, and may not act in a coordinated manner (Berke, 2008), they do have a high probability of being connected with surrounding MSNs via powerful inhibitory synapses (Koós and Tepper, 1999; Taverna et al., 2007). Further experiments may be able to decide between these two proposals for how ventral striatal gamma is generated. For instance, nasal occlusions are known to abolish piriform gamma (Vanderwolf, 2000), an effect that might be exploited to exclude contributions of piriform gamma to ventral striatum. A different, powerful approach would be to excite or inhibit ventral striatal FSIs directly, as has been done with optogenetic techniques in different brain areas (Cardin et al., 2009; Sohal et al., 2009). This could test whether ventral striatal FSIs are causally involved in the generation of ventral striatal gamma, as well as open the door to studies of their functional significance (Tiesinga and Sejnowski, 2009).
Whatever the precise source of ventral striatal gamma – inherited from afferents or generated by local circuitry, with potentially different sources for gamma-50 and gamma-80 – it is possible that even oscillations within these separate bands are themselves not unitary phenomena. In particular, different components within the bands, such as the ramp and reward receipt response for gamma-80, the reward site selectivity and movement initiation correlates for gamma-50, may well arise from distinct circuits or inputs within ventral striatum. An important point for future research would be to investigate to what extent single neurons (especially MSNs, which make up the vast majority of neurons in ventral striatum) actually participate in these different components; are these multiple correlates also linked at the single neuron level, or do they become mixed together at the LFP level? There are many known subdomains of ventral striatum, such as the distinction between core/shell, patch/matrix, and the variety of interacting input gradients (for cortical and hippocampal formation inputs) and spots (in the case of amygdala) which could in principle support such a separation.
In support of the idea that different ventral striatal gamma correlates may arise from spatially distinct sources, Kalenscher et al. (2010) found that the gamma power time series in a substantial number of simultaneously recorded LFPs were remarkably poorly correlated (Figure 1C). This was the case even when all estimated recording locations were in the ventral striatum core, and poor correlations could not be attributed to differences in net gamma power, or generally low gamma power, suggesting that distinct gamma patterns stemmed from spatially and functionally distinct compartments within ventral striatum. In contrast, Berke (2005, 2009) found that the time course of gamma oscillations appeared extremely similar across widely separated areas of striatum. Similarly, in Masimore et al. (2005; see also Masimore, 2008) gamma-50 oscillatory events tended to occur at similar times across recording sites. A possible explanation for this divergence may lie in a subtle difference in the analysis methods used: while Kalenscher et al. (2010) generated a single power time series for a relatively broad frequency band (30–100 Hz) to correlate between recording sites, Berke (2009) correlated power between recording sites for a range of narrow frequency bands (i.e., without “mixing” frequencies across the gamma band). Thus, it may be that while the time course of single (narrow) gamma frequencies shows relatively little regional differentiation (Berke, 2009), more complex relationships across different frequencies could give rise to heterogeneity only detectable at wider frequency ranges (Kalenscher et al., 2010). Another possibility is that particular, spatially restricted micro-domains within ventral striatum exhibit different gamma oscillations. Further studies and analyses, such as those that can reveal cross-frequency correlations between sites (Masimore et al., 2004), as well as higher-density recordings made possible by silicon recording probes, will be important in investigating these possibilities.
Where Next? Functional Relevance of Ventral Striatal Gamma Oscillations
The anatomical location of the ventral striatum as a site of convergence with inputs from frontal cortex, hippocampus, and amygdala, modulated by DA and providing outputs with motor influence inspired the classical notion of “a pathway from motivation to action” (Mogenson et al., 1980) and more recently, that of a “switchboard” (Redgrave et al., 1999; Goto and Grace, 2008; Gruber et al., 2009). These proposals imply that rather than simply being a passive recipient of incoming signals, ventral striatum contains a mechanism for switching between them. In such a switch scenario, ventral striatal activity is not dependent on inputs alone, but instead emphasizes (selects) a dominant input depending on the state of the “switch” – realized in some internal property of ventral striatum, which may itself be controlled by a distinct “switching” input. Such a mechanism is consistent with a popular proposal about the function of gamma oscillations in the brain: that they serve to “bind” together aspects of a common representation by functionally linking neural ensembles or brain areas when they synchronize at the same (gamma) frequency (Fries et al., 2007; Uhlhaas et al., 2009). Applied to ventral striatum, this suggests a possible implementation of the switchboard idea in that during ventral striatal gamma-50, areas exhibiting 50 Hz activity such as the piriform cortex or amygdala are functionally linked to ventral striatum and in control of its influence on behavior, while during gamma-80, frontal cortical areas control ventral striatal output. While it is currently unknown to what extent ventral striatal gamma oscillations are locally generated (as discussed above), the ability of ventral striatal neurons to dynamically phase-lock to gamma oscillations in a task-dependent manner (Berke, 2009) provides one possible switching mechanism. The observation that dopaminergic drugs induce gamma-80 (Berke, 2009) suggests that switching can also occur on a more coordinated scale, consistent with in vitro findings on the effects of DA agonists on local inhibitory mechanisms within ventral striatum (Bracci et al., 2002; Taverna et al., 2005).
The switchboard concept does not imply that ventral striatum is a “dumb” switch whose only function is to pass on the selected input verbatim; in particular, it does not exclude the possibility of further computation taking place. For instance, ventral striatal neurons encode predicted outcomes (Setlow et al., 2003; Roitman et al., 2005), specific action values (Ito and Doya, 2009; Roesch et al., 2009), and entrain to hippocampal activity patterns (Berke et al., 2004, 2009; Lansink et al., 2009). When these findings are taken together with the effects of ventral striatum lesions on behaviors dependent on associative learning (Pennartz et al., 1994; Schoenbaum and Setlow, 2003; Humphries and Prescott, 2010) they suggest ventral striatum is not simply a (switching) waystation but contains sufficient intrinsic circuitry for operations such as the association of spatial information (where can a reward be found) with information about a rewards’ motivating properties (how good is the reward; Lansink et al., 2009) or output selection by recurrent inhibition amongst MSNs (Czubayko and Plenz, 2002; Gurney et al., 2004). From this perspective, future work might address the relationship between ventral striatal processing during gamma-50 and gamma-80: is ventral striatum performing the same, or different, operations in those two states? More generally, the results reviewed here are part of a broad recognition that basal ganglia operations involve specific patterns of synchrony and rhythmicity, rather than firing rates changes alone (e.g., Bevan et al., 2002). The study of LFPs can be a valuable tool for the exploration of how such dynamics contribute to information processing underlying choices, reinforcement learning, and rewards.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the referees for their constructive comments on an earlier version of the manuscript. This work was supported by a Human Frontier Science Program grant (RGP0127/2001) and a SenterNovem grant (BSIK-03053) to Cyriel M. A. Pennartz; grants from the Tourette Syndrome Association, the Whitehall Foundation, and the National Institute on Drug Abuse (R01-DA14318) to Joshua D. Berke; a grant from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO-VENI 016.081.144) to Tobias Kalenscher; and R01-MH080318 to A. David Redish.
Key Concepts
Medium-sized spiny neuron (MSN)
The striatum’s projection neuron, GABAergic to both striatal efferents and to other MSNs. Ninety to ninety-five percent of striatal neurons are MSNs.
Low-frequency (< ∼300 Hz) electrical signal recorded from within the brain, reflecting slower voltage changes of a large population of neurons in the vicinity (up to several millimeters) of the recording electrode. Dendritic input currents and other subthreshold membrane fluctuations, rather than action potentials, are thought to be the main contributor.
A measure of how similar, over time, the spectral content (how much power at different frequencies) is between two signals, such as one LFP to another, or neuronal firing and a LFP.
LFP oscillations in the ∼40–100 Hz range, present in a wide range of brain areas across species, including rodents and humans. Generally implicated in functions such as attention, binding of perceptual components, and cognitive processing.
Fast-spiking interneuron (FSI)
A class of aspiny, GABAergic interneuron which in the striatum sends projections to a local field of MSNs. About 1% of striatal neurons are thought to be FSIs.
In the context of LFPs, phase locking is said to occur when the spiking activity of a neuron is systematically related to the phase of a LFP (within some frequency range, such as theta or gamma). See also coherence.
Footnotes
- ^Not all ventral striatal heterogeneity can be described by gradients, however; for instance, the “shell” subregion of ventral striatum has a distinct set of connections such as its unique output to the lateral hypothalamus (Berendse et al., 1992; Zahm and Brog, 1992; Groenewegen et al., 1996).
- ^Note, however, that longer, more sustained gamma bursts were also observed; see Figure 1B for an example.
- ^The psychomotor stimulant amphetamine, which increases the concentration of dopamine at dopaminergic release sites, and apomorphine, a D1/D2 dopamine receptor agonist.
- ^More specific, local applications of DA could establish to what extent the ventral striatal gamma frequency shift seen by Berke (2009) is due to other (e.g., frontal cortical) afferent areas contributing to striatal gamma modulation; see also the discussion in “Origins of ventral striatal gamma”, below.
- ^While van der Meer and Redish (2009b) only included FSIs recorded from ventral striatum, Berke (2009) included putative FSIs recorded throughout the striatum, including more dorsal areas where FSIs are more frequent but gamma oscillations are less prominent, in this analysis.
- ^As before, please note that these fast-timescale changes are not visible in Figure 2A; see Figure 9 in van der Meer and Redish (2009b).
- ^One possibility is that differences between the behavioral tasks used in the three studies contributed to discrepancies in reward-related gamma modulation. The delivery of rewards in van der Meer and Redish (2009b) and Kalenscher et al. (2010), at fixed locations, may have been more predictable than in Berke (2009) where reward delivery was dependent on correct responding to a visual cue; see Figure 4 for task schematics.
- ^Although the contribution of spiking activity to the LFP is generally thought to be small in cortical areas (Mitzdorf, 1985; Berke, 2005; Berens et al., 2008), there is evidence from in vitro studies that striatal cells can produce population spikes of considerable magnitude (Misgeld et al., 1979; Pennartz et al., 1990).
- ^Although Berke (2009) found that the ventral striatal LFP showed relatively low gamma coherence with dorsal hippocampus, this does not exclude the possibility that other parts of the hippocampal formation such as the entorhinal cortex, or the ventral hippocampus, may cohere with ventral striatal gamma.
References
Alexander, G. E., and Crutcher, M. D. (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271.
Atallah, H. E., Lopez-Paniagua, D., Rudy, J. W., and O’Reilly, R. C. (2007). Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat. Neurosci. 10, 126–131.
Balleine, B. W. (2005). Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Physiol. Behav. 86, 717–730.
Bartos, M., Vida, I., and Jonas, P. (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 8, 45–56.
Berendse, H. W., de Graaf, Y. G., and Groenewegen, H. J. (1992). Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 316, 314–347.
Berens, P., Keliris, G. A., Ecker, A. S., Logothetis, N. K., and Tolias, A. S. (2008). Feature selectivity of the gamma-band of the local field potential in primate primary visual cortex. Front. Neurosci. 2, 199–207. doi: 10.3389/neuro.01.037.2008.
Berke, J. D. (2005). “Participation of striatal neurons in large-scale oscillatory networks,” in The Basal Ganglia VIII, Vol. 56, Advances in Behavioral Biology, eds J. P. Bolam, C. A. Ingham, and P. J. Magill (New York, NY: Springer) 25–35.
Berke, J. D. (2008). Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. J. Neurosci. 28, 10075–10080.
Berke, J. D. (2009). Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur. J. Neurosci. 30, 848–859.
Berke, J. D., Breck, J. T., and Eichenbaum, H. (2009). Striatal versus hippocampal representations during win-stay maze performance. J. Neurophysiol. 101, 1575–1587.
Berke, J. D., Okatan, M., Skurski, J., and Eichenbaum, H. B. (2004). Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43, 883–896.
Bevan, M. D., Magill, P. J., Terman, D., Bolam, J. P., and Wilson, C. J. (2002). Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 25, 525–531.
Bracci, E., Centonze, D., Bernardi, G., and Calabresi, P. (2002). Dopamine excites fast-spiking interneurons in the striatum. J. Neurophysiol. 87, 2190–2194.
Bracci, E., Centonze, D., Bernardi, G., and Calabresi, P. (2003). Voltage-dependent membrane potential oscillations of rat striatal fast-spiking interneurons. J. Physiol. 549(Pt 1), 121–130.
Cardin, J. A., Carlén, M., Meletis, K., Knoblich, U., Zhang, F., Deisseroth, K., Tsai, L.-H., and Moore, C. I. (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667.
Cardinal, R. N., Parkinson, J. A., Hall, J., and Everitt, B. J. (2002). Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 26, 321–352.
Chrobak, J. J., and Buzsáki, G. (1998). Gamma oscillations in the entorhinal cortex of the freely behaving rat. J. Neurosci. 18, 388–398.
Cohen, M. X., Axmacher, N., Lenartz, D., Elger, C. E., Sturm, V., and Schlaepfer, T. E. (2009). Good vibrations: cross-frequency coupling in the human nucleus accumbens during reward processing. J. Cogn. Neurosci. 21, 875–889.
Colgin, L. L., Denninger, T., Fyhn, M., Hafting, T., Bonnevie, T., Jensen, O., Moser, M.-B., and Moser, E. I. (2009). Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357.
Czubayko, U., and Plenz, D. (2002). Fast synaptic transmission between striatal spiny projection neurons. Proc. Natl. Acad. Sci. U.S.A. 99, 15764–15769.
Fuchs, E. C., Zivkovic, A. R., Cunningham, M. O., Middleton, S., Lebeau, F. E. N., Bannerman, D. M., Rozov, A., Whittington, M. A., Traub, R. D., Rawlins, J. N. P., and Monyer, H. (2007). Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron 53, 591–604.
Gage, G. J., Stoetzner, C. R., Wiltschko, A. B. and Berke, J. D. (2010). Selective activation of striatal fast spiking interneurons during choice execution. Neuron (in press).
Gerfen, C. (1992). The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu. Rev. Neurosci. 15, 285–320.
Goto, Y., and Grace, A. A. (2008). Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 31, 552–558.
Graybiel, A. (1990). Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 13, 244–254.
Groenewegen, H. J., Wright, C. I., and Beijer, A. V. (1996). The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog. Brain Res. 107, 485–511.
Gruber, A. J., Hussain, R. J., and O’Donnell, P. (2009). The nucleus accumbens: a switchboard for goal-directed behaviors. PLoS ONE 4, e5062. doi: 10.1371/journal.pone.0005062.
Gurney, K., Prescott, T., Wickens, J., and Redgrave, P. (2004). Computational models of the basal ganglia: from robots to membranes. Trends Neurosci. 27, 453–459.
Houk, J. C., Davis, J. L., and Beiser, D. G. (eds). (1995). Models of Information Processing in the Basal Ganglia. Cambridge MA: MIT Press.
Humphries, M. D., and Prescott, T. J. (2010). The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog. Neurobiol. 90, 385–417.
Hunt, M. J., Raynaud, B., and Garcia, R. (2006). Ketamine dose-dependently induces high-frequency oscillations in the nucleus accumbens in freely moving rats. Biol. Psychiatry 60, 1206–1214.
Ito, M., and Doya, K. (2009). Validation of decision-making models and analysis of decision variables in the rat basal ganglia. J. Neurosci. 29, 9861–9874.
Johnson, A., and Redish, A. D. (2007). Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J. Neurosci. 27, 12176–12189.
Kalenscher, T., Lansink, C. S., Lankelma, J. V., and Pennartz, C. M. A. (2010). Reward-associated gamma oscillations in ventral striatum are regionally differentiated and modulate local firing activity. J. Neurophysiol. 103, 1658–1672.
Kamondi, A., Acsády, L., Wang, X. J., and Buzsáki, G. (1998). Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus 8, 244–261.
Kasanetz, F., Riquelme, L. A., and Murer, M. G. (2002). Disruption of the two-state membrane potential of striatal neurones during cortical desynchronisation in anaesthetised rats. J. Physiol. 543(Pt 2):577–589.
Kay, L. M., Beshel, J., Brea, J., Martin, C., Rojas-Líbano, D., and Kopell, N. (2009). Olfactory oscillations: the what, how and what for. Trends Neurosci. 32, 207–214.
Khamassi, M., Mulder, A. B., Tabuchi, E., Douchamps, V., and Wiener, S. I. (2008). Anticipatory reward signals in ventral striatal neurons of behaving rats. Eur. J. Neurosci. 28, 1849–1866.
Kimchi, E. Y., and Laubach, M. (2009). Dynamic encoding of action selection by the medial striatum. J. Neurosci. 29, 3148–3159.
Koós, T., and Tepper, J. M. (1999). Inhibitory control of neostriatal projection neurons by gabaergic interneurons. Nat. Neurosci. 2, 467–472.
Kreitzer, A. C., and Malenka, R. C. (2008). Striatal plasticity and basal ganglia circuit function. Neuron 60, 543–554.
Lansink, C. S., Goltstein, P. M., Lankelma, J. V., McNaughton, B. L., and Pennartz, C. M. A. (2009). Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 7, e1000173. doi: 10.1371/journal.pbio.1000173.
Lau, T., Gage, G. J., Berke, J. D., and Zochowski, M. (2010). Local dynamics of gap-junction-coupled interneuron networks. Phys. Biol. 7, 016015. doi: 10.1088/1478-3975/7/1/016015.
Lavoie, A. M., and Mizumori, S. J. (1994). Spatial, movement- and reward-sensitive discharge by medial ventral striatum neurons of rats. Brain Res. 638, 157–168.
Lee, K.-H., Williams, L. M., Breakspear, M., and Gordon, E. (2003). Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res. Brain Res. Rev. 41, 57–78.
Leung, L. S., and Yim, C. Y. (1993). Rhythmic delta-frequency activities in the nucleus accumbens of anesthetized and freely moving rats. Can. J. Physiol. Pharmacol. 71, 311–320.
Logothetis, N. K. (2003). The underpinnings of the bold functional magnetic resonance imaging signal. J. Neurosci. 23, 3963–3971.
Lytton, W. W., and Sejnowski, T. J. (1991). Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. J. Neurophysiol. 66, 1059–1079.
Masimore, B. (2008). Fluctuation Phenomena in Neurological Local Field Potentials. PhD thesis, University of Minnesota, Minneapolis.
Masimore, B., Kakalios, J., and Redish, A. D. (2004). Measuring fundamental frequencies in local field potentials. J. Neurosci. Methods 138, 97–105.
Masimore, B., Schmitzer-Torbert, N. C., Kakalios, J., and Redish, A. D. (2005). Transient striatal gamma local field potentials signal movement initiation in rats. Neuroreport 16, 2021–2024.
Misgeld, U., Okada, Y., and Hassler, R. (1979). Locally evoked potentials in slices of rat neostriatum: a tool for the investigation of intrinsic excitatory processes. Exp. Brain Res. 34, 575–590.
Mitzdorf, U. (1985). Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and eeg phenomena. Physiol. Rev. 65, 37–100.
Mitzdorf, U. (1987). Properties of the evoked potential generators: current source-density analysis of visually evoked potentials in the cat cortex. Int. J. Neurosci. 33, 33–59.
Miyazaki, K., Mogi, E., Araki, N., and Matsumoto, G. (1998). Reward-quality dependent anticipation in rat nucleus accumbens. Neuroreport 9, 3943–3948.
Mogenson, G. J., Jones, D. L., and Yim, C. Y. (1980). From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 14, 69–97.
O’Doherty, J., Dayan, P., Schultz, J., Deichmann, R., Friston, K., and Dolan, R. J. (2004). Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304, 452–454.
Pennartz, C. M., Boeijinga, P. H., and da Silva, F. H. L. (1990). Locally evoked potentials in slices of the rat nucleus accumbens: NMDA and non-NMDA receptor mediated components and modulation by GABA. Brain Res. 529, 30–41.
Pennartz, C. M., Groenewegen, H. J., and da Silva, F. H. L. (1994). The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 42, 719–761.
Pennartz, C. M. A., Berke, J. D., Graybiel, A. M., Ito, R., Lansink, C. S., van der Meer, M., Redish, A. D., Smith, K. S., and Voorn, P. (2009). Corticostriatal interactions during learning, memory processing, and decision making. J. Neurosci. 29, 12831–12838.
Popescu, A. T., Popa, D., and Paré, D. (2009). Coherent gamma oscillations couple the amygdala and striatum during learning. Nat. Neurosci. 12, 801–807.
Redgrave, P., Prescott, T. J., and Gurney, K. (1999). The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 89, 1009–1023.
Robbins, T. W., Cador, M., Taylor, J. R., and Everitt, B. J. (1989). Limbic-striatal interactions in reward-related processes. Neurosci. Biobehav. Rev. 13, 155–162.
Robbins, T. W., and Everitt, B. J. (1996). Neurobehavioural mechanisms of reward and motivation. Curr. Opin. Neurobiol. 6, 228–236.
Roesch, M. R., Singh, T., Brown, P. L., Mullins, S. E., and Schoenbaum, G. (2009). Ventral striatal neurons encode the value of the chosen action in rats deciding between differently delayed or sized rewards. J. Neurosci. 29, 13365–13376.
Roitman, M. F., Wheeler, R. A., and Carelli, R. M. (2005). Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron 45, 587–597.
Schoenbaum, G., and Setlow, B. (2003). Lesions of nucleus accumbens disrupt learning about aversive outcomes. J. Neurosci. 23, 9833–9841.
Schultz, W., Apicella, P., Scarnati, E., and Ljungberg, T. (1992). Neuronal activity in monkey ventral striatum related to the expectation of reward. J. Neurosci. 12, 4595–4610.
Sederberg, P. B., Schulze-Bonhage, A., Madsen, J. R., Bromfield, E. B., McCarthy, D. C., Brandt, A., Tully, M. S., and Kahana, M. J. (2007). Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb. Cortex 17, 1190–1196.
Setlow, B., Schoenbaum, G., and Gallagher, M. (2003). Neural encoding in ventral striatum during olfactory discrimination learning. Neuron 38, 625–636.
Sharott, A., Moll, C. K. E., Engler, G., Denker, M., Grün, S., and Engel, A. K. (2009). Different subtypes of striatal neurons are selectively modulated by cortical oscillations. J. Neurosci. 29, 4571–4585.
Sirota, A., Montgomery, S., Fujisawa, S., Isomura, Y., Zugaro, M., and Buzsáki, G. (2008). Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60, 683–697.
Sohal, V. S., Zhang, F., Yizhar, O., and Deisseroth, K. (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702.
Swanson, L. W. (1982). The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 9, 321–353.
Tanaka, S. C., Doya, K., Okada, G., Ueda, K., Okamoto, Y., and Yamawaki, S. (2004). Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat. Neurosci. 7, 887–893.
Taverna, S., Canciani, B., and Pennartz, C. M. A. (2005). Dopamine D1-receptors modulate lateral inhibition between principal cells of the nucleus accumbens. J. Neurophysiol. 93, 1816–1819.
Taverna, S., Canciani, B., and Pennartz, C. M. A. (2007). Membrane properties and synaptic connectivity of fast-spiking interneurons in rat ventral striatum. Brain Res. 1152, 49–56.
Tiesinga, P., and Sejnowski, T. J. (2009). Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron 63, 727–732.
Tort, A. B. L., Kramer, M. A., Thorn, C., Gibson, D. J., Kubota, Y., Graybiel, A. M., and Kopell, N. J. (2008). Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc. Natl. Acad. Sci. U.S.A. 105, 20517–20522.
Traub, R. D., Jefferys, J. G., and Whittington, M. A. (1997). Simulation of gamma rhythms in networks of interneurons and pyramidal cells. J. Comput. Neurosci. 4, 141–150.
Uhlhaas, P. J., Pipa, G., Lima, B., Melloni, L., Neuenschwander, S., Nikolic?, D., and Singer, W. (2009). Neural synchrony in cortical networks: history, concept and current status. Front. Integr. Neurosci. 3:17. doi: 10.3389/neuro.07.017.2009.
van der Meer, M. A. A., Johnson, A., Schmitzer-Torbert, N. C., and Redish, A. D. (2009). “Dissociations in ensemble dynamics between rat dorsal striatum, ventral striatum, and hippocampus,” in Frontiers in Systems Neuroscience Conference Abstract: Computational and Systems Neuroscience 2009. doi: 10.3389/conf.neuro.06.2009.03.353.
van der Meer, M. A. A., and Redish, A. D. (2009a). Covert expectation-of-reward in rat ventral striatum at decision points. Front. Integr. Neurosci. 3, 1. doi: 10.3389/neuro.07.001.2009.
van der Meer, M. A. A., and Redish, A. D. (2009b). Low and high gamma oscillations in rat ventral striatum have distinct relationships to behavior, reward, and spiking activity on a learned spatial decision task. Front. Integr. Neurosci. 3:9. doi: 10.3389/neuro.07.009.2009.
van der Meer, M. A. A., and Redish, A. D. (2010). Expectancies in decision making, reinforcement learning, and ventral striatum. Front. Neurosci. 4, 29–37. doi: 10.3389/neuro.01.006.2010.
Vanderwolf, C. H. (2000). What is the significance of gamma wave activity in the pyriform cortex? Brain Res. 877, 125–133.
Voorn, P., Vanderschuren, L. J. M. J., Groenewegen, H. J., Robbins, T. W., and Pennartz, C. M. A. (2004). Putting a spin on the dorsal–ventral divide of the striatum. Trends Neurosci. 27, 468–474.
Vreeswijk, C. V., Abbott, L. F., and Ermentrout, G. B. (1994). When inhibition not excitation synchronizes neural firing. J. Comput. Neurosci. 1, 313–321.
Wiltschko, A. B., Pettibone, J. R., and Berke, J. D. (2010). Opposite effects of stimulant and antipsychotic drugs on striatal fast-spiking interneurons. Neuropsychopharmacology. 35, 1261–1270.
Keywords: nucleus accumbens, local field potential, phase locking, fast-spiking interneuron, tetrode recording
Citation: van der Meer MAA, Kalenscher T, Lansink CS, Pennartz CMA, Berke JD and Redish AD (2010) Integrating early results on ventral striatal gamma oscillations in the rat. Front. Neurosci. 4:300. doi: 10.3389/fnins.2010.00300
Received: 23 February 2010;
Paper pending published: 06 April 2010;
Accepted: 28 April 2010;
Published online: 15 September 2010
Edited by:
Rui M. Costa, Instituto Gulbenkian de Ciência, PortugalReviewed by:
Henry H. Yin, Duke University, USAShih-Chieh Lin, National Institutes of Health, USA
Copyright: © 2010 van der Meer, Kalenscher, Lansink, Pennartz, Berke and Redish. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Matthijs van der Meer and A. David Redish, Department of Neuroscience, University of Minnesota, 6-145 Jackson Hall, 321 Church St. SE, Minneapolis, MN 55455. mvdm@umn.edu and redish@umn.edu
