- Department of Physiology, University of Pretoria, Pretoria, Gauteng, South Africa
Caffeine is the most widely used psychoactive stimulant with prevalent use across all age groups. It is a naturally occurring substance found in the coffee bean, tea leaf, the kola nut, cocoa bean. Recently there has been an increase in energy drink consumption leading to caffeine abuse, with aggressive marketing and poor awareness on the consequences of high caffeine use. With caffeine consumption being so common, it is vital to know the impact caffeine has on the body, as its effects can influence cardio-respiratory, endocrine, and perhaps most importantly neurological systems. Detrimental effects have being described especially since an over consumption of caffeine has being noted. This review focuses on the neurophysiological impact of caffeine and its biochemical pathways in the human body.
Introduction
In today’s fast-paced lives people need vigor to keep up with their demanding schedules and lifestyles. Often, they need some assistance in doing so. Caffeine is a naturally occurring chemical and is referred to as an “ancient wonder drug” (McCarthy et al., 2008) for its potential to revive weary workaholics. It was discovered in the coffee bean (Coffea arabica) in Arabia, the tea leaf (Thea sinensis) in China, the kola nut (Cola nitida) in West Africa, and the cocoa bean (Theobroma cacao) in Mexico (Chou, 1992). Caffeine products are so widely distributed these days that abuse of the substance may be unnoticed. In fact, caffeine is the world’s most widely consumed stimulant, with 54% of adults in America consuming on average three cups of coffee a day (Chen and Parrish, 2008). Aside from occurring organically in tea and coffee, caffeine is now an additive in soft drinks, energy drinks, chocolates, bottled water, chewing gum, and medication (Mednick et al., 2008). The aim of this paper is to elicit an awareness of the neurophysiological effects of caffeine. This article emphasizes caffeine’s potential effects on the nervous system within the context of increased caffeinated energy drink consumption around the world.
Caffeine Consumption
Aside from being added to beverages, caffeine is now being added to food products such as potato chips, chocolates, and bottled water, which confirms its growing popularity (Temple, 2009). Since the introduction of Red Bull in 1987, the energy drink market has grown extensively, with hundreds of different brands of varying caffeine content now available (Reissig et al., 2009).
There has been an increase in reports of caffeine-intoxication since 1982, with 41 cases of caffeine abuse reported in the United States from 2002 to 2004 (Reissig et al., 2009). This could be an indicator of an increase in caffeine dependence and withdrawal symptoms (Reissig et al., 2009).
European and North American statistics report that 90% of adults consume caffeine on a daily basis, with an average intake of 227 mg (Reissig et al., 2009; Temple, 2009). The South African Food Based Dietary guidelines recommend that adults limit their daily intake of caffeine drinks to no more than four cups of coffee per day or eight cups of tea per day, which is in line with the US Food and Drug Administration reporting a moderate caffeine use as safe (a moderate daily dose being 300 mg and below and high daily doses being 500–2000 mg1; Temple, 2009). Unfortunately, the statistics on South African consumptions are not readily available2, so European and North American statistics are described instead: The top three sources of caffeine are coffee (70%), cold drinks (16%), and tea (12%) in the United States. Table 1 shows the caffeine content in some popular dietary sources.
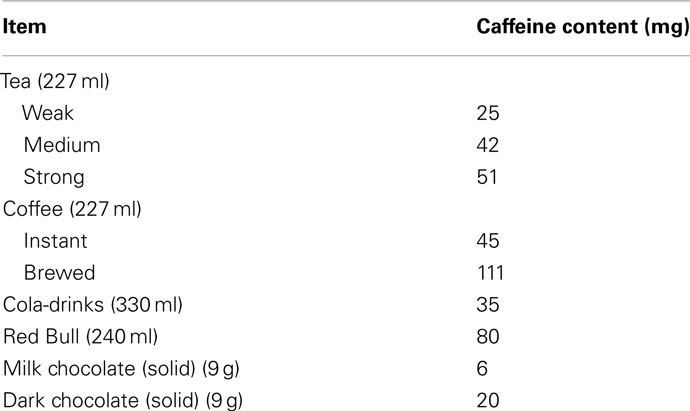
Table 1. Types of food and drink caffeine content (Jones and Fernyhough, 2008).
From Table 1 it is clear that brewed coffee has almost three times the amount of caffeine as instant. Energy drinks have twice the amount of caffeine as regular cold drinks and with ever increasing consumption, this constrains us to be informed about the neurological consequences this could have.
Biochemical Characteristics
Caffeine has a chemical structure of is 1,3,7-trimethylxanthine (Figure 1). Methylxanthine has a similar structure to purines, adenosine, xanthine, and uric acid (Chou, 1992).
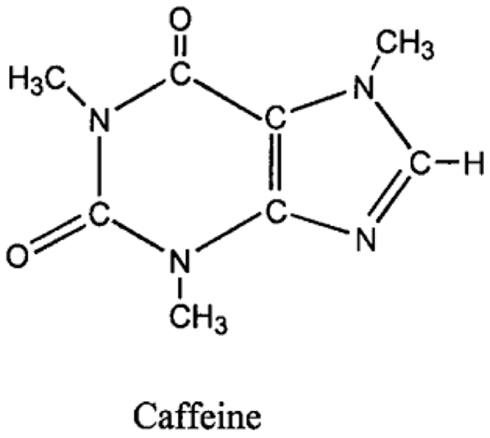
Figure 1. Chemical structure of caffeine (Deng et al., 2008).
In nature, caffeine is found in a diversity of plants, kola nuts, cherries, and cocoa beans and is presumed to offer protection to plants by acting as an anti-herbivory and allelopathic agent (Chen et al., 2008). In humans, caffeine is quickly absorbed by the gastrointestinal tract. Caffeine from coffee is absorbed faster than caffeine from cold drinks. Some reasons for this could be: the lower temperature of the beverage may decrease the rate of blood flow within the intestines; phosphoric acid in cold drinks could decrease gastric emptying; absorption rate could increase with caffeine dose; sugar in cold drinks could inhibit gastric emptying of caffeine and delay absorption (Ligouri et al., 1997). In fact, 99% of the orally ingested chemical is taken up within 45 min (Chou, 1992).
Caffeine disperses throughout the body and penetrates the biological membranes, the blood brain barrier and placenta, however it does not accumulate in the tissues or organs (Chou, 1992; Temple, 2009). Just 15–20 min after oral ingestion, peak plasma concentration is reached. The half-life in adult males is decreased by 30–50% in smokers and is doubled in women taking oral contraceptives and extended further in the last trimester of pregnancy and in patients with chronic liver disease (Chou, 1992). This means that there is increased caffeine metabolism in conjunction with cigarets while oral contraceptives, pregnancy, and chronic liver disease delay metabolism of caffeine in the body. This can be accounted for by examining caffeine’s metabolism which is species-specific. In Camellia (tea) species caffeine is degraded via theophylline into primary metabolites. In coffea (coffee) species caffeine is also degraded via theophylline but through a different route (Ashihara et al., 2008). Caffeine is converted into dimethylxanthines, dimethyl and monomethyl uric acids, trimethyl and dimethyl-allantoin and uracil derivatives in the liver. Only 2–3% of caffeine is excreted in urine unchanged (Chou, 1992; Nehlig, 1998). While caffeine itself is eliminated overnight from the body, some primary metabolites such as theobromine and theophylline have longer half-lives.
Caffeine and its primary metabolites, theobromine, paraxanthine, and theophylline are identified in all body fluids (Grosso and Bracken, 2005). Paraxanthine levels decrease less rapidly than caffeine and are further metabolized via two independent reactions. These paraxanthine metabolites are found in urine (Grosso and Bracken, 2005). Theobromine makes up the largest part of caffeine metabolites, with only 50% excreted in urine. Some of the effects of caffeine in systems other than the nervous system are described.
Cardiovascular and Respiratory Effects
Caffeine induces various acute cardiovascular effects such as an up regulation of circulating catecholamines. Arterial stiffness and endothelium dependent vasodilatation also result, leading to increases in systolic and diastolic blood pressure (DBP; Riksen et al., 2009). An increase in the respiration rate (RR) is the prime effect dependent on the plasma caffeine value (Chou, 1992).
Endocrine and Metabolic Effects
Caffeine enhances circulating catecholamine levels. Owing to this mechanism there is an increase in the basal metabolic rate – this includes lipolysis which releases free fatty acids (Chou, 1992).
Gastrointestinal and Urinary Effects
Caffeine excites the small intestine, causing secretion of water and sodium (Chou, 1992). Its pharmacological effects include diuresis.
From a medical view, caffeine has been seen to promote apoptosis in UVB-damaged cells, to antagonize adenosine receptors for regulating contraction of blood vessels and even serves as a psychoactive drug in the treatment of Parkinson’s disease (Chen et al., 2008). With its potential utilization in medicine, the safety and effects of caffeine are important issues.
Benefits of Caffeine to the Human Body
Unfortunately, a review of the literature shows two important limitations in caffeine research. Firstly, research on animals uses doses that are hundreds to thousands of times higher than those seen in human consumption. Therefore, relating the results to humans becomes difficult (Chou, 1992). Secondly, some studies investigate pure caffeine, while others pose research questions pertaining to coffee, not pointing out the other components in coffee and their potential confounding effects (Chou, 1992).
Despite these limitations, extensive explorations of caffeine have been carried out and have provided a great deal of information regarding the effects of caffeine. Under the next few headings the major neurophysiologic effects of caffeine are discussed as the main focus of this article.
Arousal and Fatigue
Arousal is considered to be a variable state reflecting the present energetic factors and task-related activation, the degree of “awareness” an individual has and in neuropsychology context an increase in arousal relates to a better ability to carry out a task (Barry et al., 2005). There is an apparent link between caffeine effects and dopamine functions. This evidence does not preclude the involvement of other neuromodulator systems, though. In fact, it has been reported that caffeine increases the firing rates in mesopontine cholinergic neurons, which participate in the production of arousal (Lorist and Tops, 2003). These cholinergic neurons are inhibited by adenosine, providing a coupling mechanism that links arousal and caffeine, yielding proof for the role of caffeine in the behavioral state of arousal (Lorist and Tops, 2003).
A study conducted by Barry et al. (2008) proves that caffeine does increase arousal by increasing skin conductance level (SCL), while decreasing heart rate (HR) and decreasing levels of DBP. Arousal effects from caffeine were noted in a 30-min period approximately 25 min after ingestion. An increase in RR was noted to peak at 33 min and then decrease with time. The subjects carried out two tasks – an auditory task composed of an active auditory oddball task and a visual task requiring the subjects to focus their vision on a specific spot without excessive blinking. Barry et al. (2008) suggest that caffeine produced a reduction in reaction time in the tasks carried out, supporting the idea that blood pressure effects reflect effortful task-related activity rather than arousal changes. Caffeine produced an increase in SCL levels and a reduction in alpha power. Alpha generators are unchanged by caffeine and therefore caffeine causes arousal without manipulating task requirements, consistent with caffeine’s antagonistic effects on adenosine receptors reducing inhibition of cholinergic neurons. Post-task effects of caffeine included changes in blood pressure activity and alpha and beta power implying that caffeine may have effects on task performance above arousal effects (Barry et al., 2008). Caffeine also has beneficial effects on choice reaction time, especially in the elderly with a daily dosage of 200–400 mg (Smith, 2002).
It is noted that caffeine can affect the attention system. Attention can alter the neural activity in cortical areas that may intensify the responsivity of cells to specific stimulus features (Lorist and Tops, 2003). A substantial number of studies show that caffeine consumption increases alertness and decreases fatigue (Barry et al., 2008; Smith, 2002; Biggs et al., 2007; Kennedy et al., 2008) in large and moderate doses.
To evaluate the effect of caffeine on sleep deprivation and driving Biggs et al. (2007) conducted a study using 12 regular drivers that are non-smokers, with healthy BMIs, between the ages of 20 and 30 years. Coffee and placebo were administered, with every subject acting as his/her own control. Caffeine was shown to have a beneficial effect on all driving tests and the data suggests that caffeine returned driving performance to baseline (pre-sleep deprivation) levels (Kennedy et al., 2008); thereby proving that caffeine does increase alertness and can be beneficial in driving tasks.
Perceptual Processing
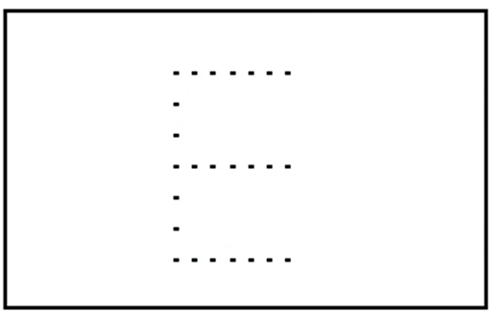
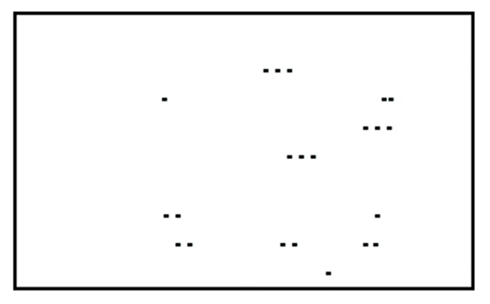
Perception is a process of gaining some form of knowledge through thought, experience, and the senses (Wang, 2009). Lorist and Tops (2003) used a task consisting of a stimulus quality which was manipulated. The non-degraded stimulus consisted of a dot pattern surrounded by a rectangular frame of dots (see Figure 2). In the degraded condition dots were placed from the frame into variable positions. This new arrangement impaired the identification of the stimulus. Caffeine increased the ability to process degraded stimuli (see Figure 3; Lorist and Tops, 2003). By contrast, Smith (2002) conducted a perceptual task requiring participants to discriminate between two targets per trial. The group that received caffeine showed no significant difference in the perceptual task compared to those that did not receive caffeine. Another study conducted by Ruijter et al. (2000) tested the effect of caffeine on sustained attention required by subjects to work continuously for 10 min in a self-paced task. The task consisted of a color selection task, a spatial selection task, and a concentration task. Subjects were administered a moderate dose of 250 mg of caffeine. The results showed an increase in arousal but no change in perceptual behavior. From this we can see that the effect of caffeine on perception is inconsistent.
Motor Behavior
Motor skill learning is one of the main functions of the central nervous system. It is a process of increasing the spatial and temporal accuracy of movements with practice. Motor skill has two prominent learning phases: an initial fast learning phase and later, slow learning phase (Xiong et al., 2009). In the early stage of learning a great deal of improvement in performance is attainable within a few minutes. Precise knowledge of the movement is used to promote the control and co-ordination of specific body action. During the later phase, there is slow learning progress and less attention is needed to perform the task. The prefrontal cortex is responsible for movement in the parts of the body, it also guides eye and head co-ordination (Xiong et al., 2009).
Dopamine is a neurotransmitter with excitatory and inhibitory effects. Neurons of the substantia nigra have nerve endings in the caudate nucleus and putamen of the cerebrum, where they release dopamine. Dopamine acts as an inhibitor in the basal ganglia and is excitatory in other areas of the brain (Xiong et al., 2009). U-shaped dose–response curves in humans show that either too much or too little dopamine results in diminished prefrontal cortex functioning.
The mesocorticolimbic dopaminergic system mediates approach motivation. Dopamine receptors D2 regulate neural networks that are involved in selective and involuntary attention (Lorist and Tops, 2003). Caffeine increases behavior related to dopamine by inhibiting adenosine A2A receptors and increasing transmission via dopamine D2 receptors (Lorist and Tops, 2003). Lorist and Tops (2003) used an echoencephalograph (EEG) to highlight the alpha brain wavelength (alpha power). They found that caffeine intake increased left frontal activation compared to the right, suggesting that dopamine function could be linked to fatigue, with caffeine reducing fatigue.
Mednick et al. (2008), conducted a study was composed of 61 adults 18–39 years old that were given a motor task requiring them to finger tap a 4-1-3-2-4 sequence on a keyboard with the non-dominant hand. The caffeine group showed significantly impaired motor learning.
Learning and Memory
Learning is the acquisition of new information by the nervous system, resulting in changes in behavior or “analytical-specific perceptual skills” (Mangina and Sokolov, 2006). Memory is the ability to store, process, and recall learnt information. Modality-non-specific memory is associated with the limbic system, especially the hippocampus (Mangina and Sokolov, 2006). Neurons in the hippocampus contribute to the formations of declarative memory units. These neurons can be trained to memorize perceptual images and these “trained” neurons are then arranged in a column in accordance to their learning sequence (Mangina and Sokolov, 2006).
Long-term potentiation (LTP) is a major candidate for the neurophysiological basis of learning and memory. The LTP mechanism seems to be dependent on activity of glutamatergic receptors and N-methyl D-aspartate (NMDA) receptors are required for induction of LTP while expression of LTP involves α-amino-3-hydorxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Gamma-aminobutyric acid (GABA) receptors also seem to be involved in the mechanisms of LTP, along with other neurotransmitters such as acetylcholine, dopamine, serotonin, and norepinephrine (Myhrer, 2003) Dopamine has an impact on performances, motivational processes, and procedural memory, while acetylcholine interacts with dopamine in cognitive functions and with serotonin acts in cognitive behavior, to a lesser extent serotonin and norepinephrine have a weaker influence.
The results from a perceptual learning task and a motor task according to Smith (2002) may be explained by the relative level of explicit information involved in learning. The perceptual learning task requires the least explicit material, while the motor task shows a strong explicit component. The information shows that caffeine may help in some tasks but impair others. This may be because caffeine increases alertness and decreases fatigue, causing a better performance in some tasks (Smith, 2002).
There is concern regarding strategies that can improve the elderly quality of life regarding the diminished cognitive and motor functions that occur with aging (Costa et al., 2008). In a study conducted by Costa et al. (2008) it is stated that the caffeine administered in adult mice prevented age-associated decline in recognition memory when evaluated 90 min after training (corresponding to short term memory). There is however, the possibility of anxiety being elicited, due to high caffeine doses.
Stress and Addiction
Stress can be defined to when the human body is not able to cope suitably to physical or emotional threats. The brain is the major component of interpreting and responding to potentially stressful events and determines what is stressful. It is also the central organ of the behavioral and physiological response to stressors and is also a target for the actions of stress hormones such as glucocorticoids (Ferreira et al., 2004).
Studies show that during periods of increased stress, caffeine consumption increases (Yeomans et al., 2007). Caffeine increases cortisol secretion by stimulating the central nervous system so it is advisable to individuals with hypertension to avoid caffeine during periods of stress as this further increases blood pressure (Yeomans et al., 2007; Dack and Reed, 2009). Chronic caffeine consumption causes sensitization of a specific subset of cannabinoid receptors in the striatum, consistent with the psychoactive properties of the compound (Sheperd et al., 2000; Herrick et al., 2009). This may explain why enhanced relaxation and a sense of well being are some of the reported effects of caffeine use during stressful events. Caffeine mechanism action can be explained as follows.
With regular average doses of caffeine in humans, caffeine acts as an antagonist of the adenosine receptors and exhibits an equal affinity for A1 and A2A receptors. However when acutely administered caffeine acts dominantly on A1 receptors (as ambient adenosine activates it). Chronic caffeine consumption causes tolerances of the A1 receptors, caffeine then has negligible effects on A1 receptor and dominant effects on A2A receptors (Rossi et al., 2010).
The endocannabinoid, endogenous ligands of the cannabinoids receptors are synthesized when needed, in response to the increased neuronal excitation and activates the presynaptic CB1 receptor, decreases the levels of cyclic AMP (cAMP) released and decreases neurotransmitter release (Rossi et al., 2010). Caffeine increases neurotransmitter release by removing inhibitory control for acetylcholine in the hippocampus and prefrontal cortex, regulating the opening of potassium channels which is mediated by A1 receptors, increasing the firing rate of neurons (Rossi et al., 2010).
A2A receptor striatum dendrite spines, here they inhibit Glutamatergic-thalamo-cortical neurons by inducing cell activation and stimulating adenylate cyclase pathway. Caffeine blocks A2A receptors and decreases stimulatory actions on cAMP induced by adenosine (Rossi et al., 2010).
The striatum is the main receiving area of the basal ganglia, consisting mainly of GABAergic neurons that receive excitatory input from the cortex, inhibitory input from the axon collaterals and striatal interneurons, and modulatory input from the midbrain dopaminergic neurons. Impacts of these inputs control the outputs to the substantia nigra and globus pallidus and play a role on the effect caffeine has on the striatal neurotransmission (Rossi et al., 2010).
Caffeine can reduce the inhibition on striatal dopamine transmission reducing the activity of striatal neurons and causing thalamo-cortical projections neurons to disinhibit. The activation of A2A receptors results in cAMP production, activation of D2 receptors decreases the production of cAMP causes a reverse regulation of the activity of cAMP-dependant protein kinase (PKA; Rossi et al., 2010). Since caffeine mimics the dopamine action on the striatopallidal neurons, it causes a progressive sensitization of cannabinoid CB1 receptors controlling GABergic inhibitory postsynaptic currents (IPSCs; Herrick et al., 2009). Caffeine blocking the A2A receptors reduces the activation of cAMP–PKA pathways causing an increase in glutamate release, activation of metabotropic mGlu5 receptors, and endocannabinoid release (Rossi et al., 2010). The blockade of adenosine A2A receptors in the striatum, has been associated with the psychoactive properties of caffeine. Also, evidence shows that a specific genetic polymorphism of the adenosine A2A receptor influences the habitual caffeine consumption in humans (Herrick et al., 2009).
Evidence has also shown that caffeine induces striatal synaptic adaptations and does not alter the sensitivity of glutamate synapses to CB1 receptor stimulation in mice, displaying the existence of differential regulation mechanisms of distinct cannabinoid receptors in the striatum (Rossi et al., 2010). The caffeine induced adaptation of the endocannabinoid in mice was reversible and after drug withdrawal, symptoms were reversed with a decline in 15 days and totally reversed in 30 days.
Caffeine induced alteration of cannabinoid transmission may have synaptic consequences during the physiological activity of the striatum since chronic caffeine has been shown to enhance the sensitivity of the GABergic synapses to synthetic cannabinoid CB1 receptors agonist and the endocannabinoids, mobilized to respond to the stimulation of metabotropic receptors (Rossi et al., 2010). A study on mice shows at toxic levels, caffeine causes calcium release from intracellular space, inhibition of phosphodiesterase, GABBA receptor antagonism, protein kinase C activity (Rossi et al., 2010). It is likely that caffeine effects in humans are more complicated than it is in animal studies.
Evidence exists that cannabinoid receptors are implicated in the mechanism of action of psychoactive drugs and stress, so enhanced activity of the cannabinoid CB1 receptors plays a role in the rewarding effects of morphine, heroin, cocaine, ethanol, amphetamine, and nicotine (Herrick et al., 2009). Caffeine induced alterations of cannabinoid transmission may have relevant outcomes during the physiological activity of the striatum. Caffeine effects on the striatal cannabinoid system were similar to those of cocaine (such as the enhanced synaptic defects prompted by stress). It also has been reported that caffeine and cocaine have additive properties and caffeine reinforces cocaine-seeking behavior following elimination of cocaine self-administration (Herrick et al., 2009).
The Disadvantages of Caffeine to the Human Body
As previously stated, caffeine could have detrimental effects on a hypertensive that is stressed and consumes caffeine as ultimately caffeine is a stimulant and as with as all stimulants and substance’s abuse or overuse has negative effects. This review looks at some of the detriments of caffeine on the nervous system.
Deficits in Learning
Multiple doses of caffeine are consumed in individuals suffering from insomnia to reduce fatigue and increase alertness (Mednick et al., 2008). However caffeine may have negative effects on cognition in general and perceptual memory and learning in particular (Mednick et al., 2008). The study by Mednick et al. (2008) shows a comparison of a nap and caffeine on verbal, motor, and perceptual memory. Caffeine causes an increase in hippocampal acetylcholine. This may block consolidation by congesting replay of memories. A moderate dosage of caffeine impairs motor skill and may not be an adequate substitute for memory enhancements or daytime sleep (Mednick et al., 2008).
Neurogenesis is the growth and development of the nervous tissue. Neurogenesis occurs in the hippocampal and olfactory bulbs until adulthood (Guyton and Hall, 2006; Wentz and Magavi, 2009). A study conducted by Wentz and Magavi (2009) showed that administering high doses of caffeine (20–30 mg/ml) to adult mice influenced the proliferation of hippocampal neural precursors in a duration-dependent dose. This negatively influenced the neural circuits into which adult-born neurons are integrated (Wentz and Magavi, 2009). Caffeine can therefore depress hippocampal neurogenesis.
Anxiety and Panic Attacks
When a single dose of 300 mg is administered, caffeine can increase anxiety and tension. Meanwhile a 400-mg dose of caffeine increases anxiety when paired with a stressful task (Smith, 2002). In general, high doses of caffeine may increase anxiety, but this is rarely seen in normal consumption (Smith, 2002).
The study by Nardi et al. (2007) analyzed in two ways how panic from the caffeine challenge test manifested in subjects that suffer from anxiety. The aim of the study was to determine whether patients with PD experience more caffeine-related symptoms or whether they perceive their symptoms more severely than others.
Nardi et al. (2007) suggest that patients with panic disorder (PD), when compared to depressive patients, showed increased sensitivity to the effects of low doses of caffeine. Patients with PD have an increase in subject-related anxiety, nervousness, fear, nausea, palpitations, and tremors after administration of caffeine. The precise mechanism underlying caffeine panicogenic potentials is uncertain, with the antagonism of adenosine receptors being the most likely pathway (Nardi et al., 2007). Imaging studies of cerebral blood flow using position emission topography indicates a decrease in panic attacks with caffeine and increase in glucose utilization (Nardi et al., 2007). However, not all individuals with PD display increased panic attack frequency with caffeine ingestion, suggesting that there might be subgroups of patients with PD with caffeine-provoked panic being linked to long-lasting anxiety symptoms lasting hours (Nardi et al., 2007).
Hallucinations
Caffeine users that consume caffeine approximate to seven cups of instant coffee (>300 mg caffeine) a day are more likely to report hallucinatory experiences such as seeing things that are not there and hearing voices, when compared to low-level caffeine users that consume caffeine equivalent to one to three cups of coffee a day (Jones and Fernyhough, 2009). Researchers indicate that the hallucinatory experiences may be due to caffeine intensifying the physiological effects of stress, as cortisol is released during stressful periods when people have recently taken in caffeine (Lovallo et al., 2006), this additional upsurge of cortisol may link caffeine consumption with a higher tendency to hallucinate (Jones and Fernyhough, 2009). Caffeine use can lead to caffeine-intoxication, symptoms of which are nervousness, irritability, anxiety, muscle-twitching, insomnia, headaches, palpitations (Jones and Fernyhough, 2009).
Caffeine Dependence
Ninety-eight percent of North America consumes some form of caffeine, making it the most widely used drug on that continent (Jones and Fernyhough, 2009). Many caffeine consumers proclaim that they are addicted to the substance, however the evidence is inconsistent. Table 2 lists the DSM-IV Criteria for evaluating substance dependency (American Psychiatric Association, 2000; Dews et al., 2002). Users must meet a minimum of three criteria to be considered dependent on a substance. However, with caffeine, complications arise in the grading of the criteria as the effects of caffeine are highly variable across consumers and because the use of caffeine is socially acceptable. Entire afternoons are planned around coffee dates and many social rituals revolve around the drink.

Table 2. The DSM-IV criteria for evaluating substance dependence (American Psychiatric Association, 2000; Dews et al., 2002).
Using the DSM criteria it was reported that 11% of 6778 daily caffeine users evaluated proclaimed to experience withdrawal symptoms (American Psychiatric Association, 2000; Dews et al., 2002). Controversy arose when the withdrawal symptoms were reported to be mild to moderately bearable and diminishing over a short period of time, therefore reducing the intensity of withdrawal symptoms (Dews et al., 2002; Jones and Fernyhough, 2008). According to Keast and Riddell (2007) only the minority of the caffeine users are actually dependent on caffeine. The debate about the possible addictive strength of caffeine remains unsettled, but caffeine withdrawal has been linked with feelings of fatigue, increased depression, and anxiety (Smith, 2002).
Discussion
In summary, the most salient effects of caffeine can be summarized as follows.
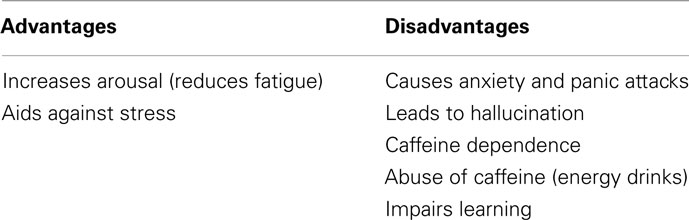
Most people consume caffeine to compensate for a lack of sleep, to complete tiring tasks, or to ease stress. Caffeine products are ubiquitously used for these reasons and more yet, Table 3 indicates that the disadvantages of caffeine are more clearly documented than the advantages. The health benefits from caffeine are increased arousal and facilitating against stress in the human body. These benefits are important in maintaining safety and efficacy in the workplace and other environments. To derive an increase in arousal could lead to individuals over consuming caffeine drinks, this over consumption could then bring about some of the disadvantages such as anxiety and panic attacks (when doses more than 300 mg caffeine are ingested) or cause individuals to hallucinate (again when doses more than 300 mg are ingested). It was noted that when individuals are stressed, their caffeine intake increases and caffeine lead to a sensitization of the cannabinoid receptors to help alleviate stress. Despite this benefit, it could create a larger predicament by causing individuals to become dependent on the substance or exemplifying this by becoming dependent on other drugs. Morphine, heroin, cocaine, and ethanol also cause enhancement of the cannabinoid receptors and caffeine has additive properties (like cocaine). Furthermore it reinforces the cocaine-seeking behavior strengthening the dependence potential of caffeine. While caffeine may be used to increase arousal, in contrast it causes impairment in learning by congesting replay of memories and impairing motor skills with a moderate dose of caffeine.
It appears that the most significant benefits derived from caffeine involve increased alertness. Further benefits are generally only derived from high dosages of caffeine. However, such doses cause harmful effects on neurophysiological health, with the exception of the effects on cardiac conditions which are experienced even at low to moderate doses of caffeine.
One of the most important current caffeine concerns involves energy drinks. There has been a vast increase in energy drink consumption in young adults aged 18–24 years (Côté, 2009). These energy drinks are not to be confused with sports drinks as they contain high amounts of caffeine and taurine and do not hydrate the body. In 2006 Thailand had the leading energy drink consumption per person but the United States reported the highest sales of energy drinks (Reissig et al., 2009). The energy drink industry has grown exponentially with almost 500 brands launched internationally in 2006.
The drinks differ rather dramatically in caffeine content (Reissig et al., 2009). From Table 4 it is clear that levels of caffeine in these drinks are very high. These drinks are sold without age restrictions and the majority of these drinks do not have a warning label advising the consumer on the caffeine content and the potential health risks (Reissig et al., 2009).
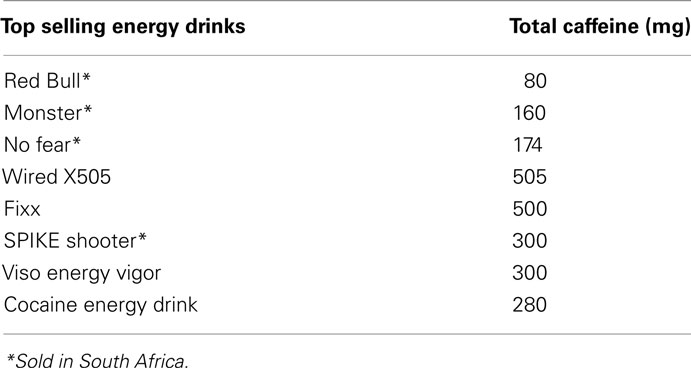
Table 4. Energy drinks in the United States (Reissig et al., 2009).
Aside from the possible addictive potential of caffeine, caffeine intoxication is a recognized syndrome (Reissig et al., 2009). The high caffeine content in energy drinks increases the risk for caffeine overdose, so awareness of this is required (Reissig et al., 2009). Unfortunately though, there is no regulation of the marketing of energy drinks targeted at the young adults. This is surprising, given that pharmacological and epidemiological studies show an association between caffeine use, dependence on alcohol, nicotine, and drugs such as cocaine, morphine, and heroin because caffeine shares features with these commonly studied drugs (Reissig et al., 2009; Dack and Reed, 2009).
There is also increased popularization of combined use of alcoholic beverages and energy drinks. This may seem harmless, given that some reports suggest that energy drinks could decrease the intensity of the depressant effects of ethanol (Ferreira et al., 2004). In the study by Ferreira et al. (2004) ethanol in doses of 0.5, 1.0, 1.5, and 2.5 g/kg was combined with a well-known energy drink and administered to mice. The results are found in Table 5.

Table 5. Effects of the energy drink combined with ethanol administered (Ferreira et al., 2004).
The data obtained suggests that energy drinks did antagonize the depressant effect of ethanol in the locomotor activity of mice but only at high does of ethanol. Considering that mice have a much faster metabolism than humans, the alterations of the levels of locomotor activity in mice cannot simply be interpreted as a reversion of the symptoms of acute effects of alcohol (Ferreira et al., 2004). Furthermore, the combination of energy drinks and alcohol reduces participants’ perceptions of impairment of motor co-ordination but does not decrease objective measures of alcohol-induced impairment of motor co-ordination, reaction time, or breathe alcohol concentration. This may increase the possibility of alcohol-related injury and motor accidents as the individuals may feel that the energy drink has antagonized the effects of alcohol while their co-ordination and judgment are still impaired (Reissig et al., 2009).
Conclusion
The massive popularity of caffeine has created a need to discover the possible inflictions on the human body. By delving into the biochemical characteristics of caffeine, findings on its structure and chemical properties have led to findings on its function, absorption in the body and metabolism. The neurophysiological benefits of caffeine are brief and ironically could lead to health disadvantages. Therefore in order to obtain the benefits consumption should be limited to moderate doses. The neurophysiological health disadvantages of caffeine include anxiety and panic attacks and hallucinations brought about by above moderate doses of caffeine. In addition to this caffeine may impair learning and memory. However, most alarming is the similarity of caffeine to other drugs such as morphine, heroin, ethanol, and most importantly to cocaine. Caffeine shows the most similarity to cocaine and reinforces cocaine-seeking behavior after elimination of the drug. This finding strengthens the argument that the potential of caffeine dependence is high and awareness of this should be created.
Regarding caffeine in energy drinks, a number of questions arise out of this review. For example, should such aggressive marketing be allowed for a substance that serves as a portal to other forms of drug dependence? With energy drinks decreasing the perceived depressant effects of alcohol individuals may consume more alcohol and therefore jeopardize their perception and hence safety. Given the neurophysiological implication of caffeine use, advertising and marketing of energy drinks and caffeinated soft drinks should be considered. Research on caffeine in South Africa is very limited and considering the potential negative health impacts of this drug further research to focusing on the negative health impacts of moderate caffeine consumption is needed. In the mean time, awareness on its potential health consequences, caffeine intoxication, withdrawal, and dependence should be mandatory.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
- ^http://www.fda.gov/Food/FoodIngredientsPackaging/FoodAdditives/FoodAdditiveListings/ucm091048.htm [accessed August 31, 2011]
- ^http://sun025.sun.ac.za/portal/page/portal/Health_Sciences/English/Centres%20and%20Institutions/Nicus/NutritionFactssheets/Beverage%20Consumption.pdf-beverageconsumption [accessed August 31, 2011]
References
American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR, 4th Edn. Washington, DC: American Psychiatric Association.
Ashihara, H., Sano, H., and Crozier, A. (2008). Caffeine and related purine alkaloids: biosynthesis, catabolism, function and genetic engineering. Phytochemistry 69, 841–856.
Barry, R. J., Clarke, A. R., Johnstone, S. J., and Rushby, J. A. (2008). Timing of caffeine’s impact on automatic and central nervous system measures: clarification of arousal effects. Biol. Psychol. 77, 304–316.
Barry, R. J., Rushby, J. A., Wallace, M. L., Clarke, A. R., Johnstone, S. J., and Zlojutro, I. (2005). Caffeine effects on resting-state arousal. Clin. Neurophysiol. 116, 2693–2700.
Biggs, S. N., Smith, A., Dorrian, J., Reid, K., Dawson, D., Van den Heuvel, C., and Baulk, S. (2007). Perception of stimulated driving performance after sleep restriction and caffeine. J. Psychosom. Res. 63, 573–577.
Chen, Y., Huang, Y., Wen, C., Wang, Y., Chen, W., Chen, L., and Tsay, H. (2008). Movement disorder and neuromuscular change in zebrafish embryos after exposure to caffeine. Neurotoxicol. Teratol. 30, 440–447.
Chen, Y., and Parrish, D. B. (2008). Caffeine’s effects on cerebrovascular reactivity and coupling between cerebral blood flow and oxygen metabolism. Neuroimage 44, 647–652.
Chou, T. (1992). Wake up and smell the coffee. Caffeine, coffee and the medical consequences. West. J. Med. 157, 544–553.
Costa, M. S., Botton, P. H., Mioranzza, S., Souza, D. O., and Porciuncula, L. O. (2008). Caffeine prevents age-associated recognition memory decline and changes brain derived neurotrophic factor and tyrosine kinase receptor (TkrB) content in mice. Neuroscience 153, 1071–1078.
Côté, S. (2009). Energy Drinks: The Coffee of a New Generation? University of Montreal, Montreal, QC.
Dack, C., and Reed, P. (2009). Caffeine reinforces flavour preferences and behaviour in moderate not users but not in low users. Learn. Motiv. 40, 35–45.
Deng, W., Li, Y., Ogita, S., and Ashihara, H. (2008). Fine control of caffeine biosynthesis in tissue cultures of Camellia sinensis. Phytochem. Lett. 1, 195–198.
Dews, P. B., O’ Brien, C. P., and Bergman, J. (2002). Caffeine: behavioural effects of withdrawal and related issues. Food Chem. Toxicol. 40, 1257–1261.
Ferreira, S. E., Quadros, I. M. H., Tridnade, A. A., Takashi, S., Koyama, R. G., and Souza-Formigioni, M. L. O. (2004). Can energy drinks reduce the depressor effect of ethanol? An experimental study in mice. Physiol. Behav. 82, 841–847.
Grosso, L. M., and Bracken, M. B. (2005). Caffeine metabolism, genetics and perinatal outcomes: a review of exposure assessment considerations during pregnancy. Ann. Epidemiol. 15, 460–466.
Guyton, A. C., and Hall, J. E. (2006). Textbook of Medical Physiology. Pennsylvania: Elsevier Saunders.
Herrick, J., Shecterie, L. M., and Cyr, J. A. (2009). D-ribose – an additive with caffeine. Med. Hypotheses 72, 499–500.
Jones, S., and Fernyhough, C. (2009). High caffeine intake lead to hallucination proneness. Pers. Individ. Dif.
Jones, S. R., and Fernyhough, C. (2008). Caffeine, stress and proneness to psychosis-like experiences: a preliminary investigation. Pers. Individ. Dif. 46, 562–564.
Keast, R. S. L., and Riddell, L. J. (2007). Caffeine as a flavour additive in soft-drinks. Appetite 49, 255–259.
Kennedy, M. D., Galloway, A. V., Dickau, L. J., and Hudson, M. K. (2008). The cumulative effect of coffee and a mental stress task on heart rate, blood pressure, and mental alertness is similar in caffeine-naive and caffeine-habituated females. Nutr. Res. 28, 609–614.
Ligouri, A., Hughes, J. R., and Grass, J. A. (1997). Absorption and subjective effects of caffeine from coffee, cola and capsules. Pharmacol. Biochem. Behav. 58, 721–726.
Lovallo, W. R., Farag, N. H., Vincent, A. S., Thomas, T. L., and Wilson, M. F. (2006). Cortisol responses to mental stress, exercise and meals following caffeine intake in men and women. Pharmacol. Biochem. Behav. 83, 441–447.
Mangina, C. A., and Sokolov, E. N. (2006). Neuronal plasticity in memory and learning abilities: theoretical position and selective review. Int. J. Psychophysiol. 60, 203–214.
McCarthy, D. M., Mycyk, M. B., and DesLauriers, C. A. (2008). Hospitalization for caffeine abuse is associated with abuse of other pharmaceutical substances. Am. J. Emerg. Med. 26, 799–802.
Mednick, S. C., Cai, D. J., Kanady, J., and Drummond, S. P. A. (2008). Comparing the benefits of caffeine, naps and placebo on verbal, motor and perceptual memory. Behav. Brain Res. 193, 79–86.
Myhrer, T. (2003). Neurotransmitter systems involved in learning and memory on the rat: a meta-analysis based on studies of four behavioural tasks. Brain Res. Rev. 41, 268–287.
Nardi, A. E., Lopes, F. L., Valenca, A. M., Freire, R. C., Veras, A. B., de-Melo-Neto, L., Nascimento, I., King, A. L., Mezzasalma, M. A., Soares-Filho, G. L., and Zin, W. A. (2007). Caffeine challenge test in panic disorder and depression with panic attacks. Compr. Psychiatry 48, 257–263.
Nehlig, A. (1998). Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci. Biobehav. Rev. 23, 563–576.
Reissig, C. J., Strain, E. C., and Griffiths, R. R. (2009). Caffeinated energy drinks – a growing problem. Drug Alcohol Depend. 99, 1–10.
Riksen, N. P., Rongen, G. A., and Smits, P. (2009). Acute and long-term cardiovascular effects of coffee: implications for coronary heart disease. Pharmacol. Ther. 121, 185–191.
Rossi, S., De Chiara, V., Musella, A., Mataluni, G., Sacchetti, L., Siracusano, A., Bernadi, G., Usiello, A., and Centonze, D. (2010). Effects of caffeine on striatal neurotransmission: focus on cannabinoid CB1 receptors. Mol. Nutr. Food Res. 54, 525–531.
Ruijter, J., Lorist, M. M., Snel, J., and De Ruiter, M. B. (2000). The influence of caffeine on sustained attention: an EPR study. Pharmacol. Biochem. Behav. 66, 29–37.
Sheperd, J. D., al’Abasi, M., Whitsett, T. L., Passey, R. B., and Lovallo, W. R. (2000). Additive pressor effects of caffeine and stress in male medical students at risk for hypertension. Am. J. Hypertens. 13, 475–481.
Temple, J. L. (2009). Caffeine use in children: what we know, what we have to learn and why we should worry. Neurosci. Biobehav. Rev. 33, 793–806.
Wang, Q. (2009). Are Asians forgetful? Perception, retention and recall in episodic remembering. Cognition 111, 123–131.
Wentz, C. T., and Magavi, S. S. P. (2009). Caffeine alters proliferation of neuronal precursors in the adults hippocampus. Neuropharmacology 56, 994–1000.
Xiong, J., Ma, L., Wang, B., Narayana, S., Duff, E. P., Egan, G. F., and Fox, P. T. (2009). Long-term motor training induced changes in regional cerebral blood flow in both tasks and resting states. Neuroimage 45, 75–82.
Keywords: caffeine, neurophysiology, adenosine receptors, energy drinks
Citation: Persad LAB (2011) Energy drinks and the neurophysiological impact of caffeine. Front. Neurosci. 5:116. doi: 10.3389/fnins.2011.00116
Received: 06 June 2011; Paper pending published: 05 July 2011;
Accepted: 12 September 2011; Published online: 21 October 2011.
Edited by:
Nicholas Moore, Albany Molecular Research, Inc., USAReviewed by:
Nicholas Moore, Albany Molecular Research, Inc., USAEnrico Sanna, University of Cagliari, Italy
Copyright: © 2011 Persad. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Leeana Aarthi Bagwath Persad, Department of Physiology, University of Pretoria, Pretoria, Gauteng, South Africa. e-mail: mwpersad@mweb.co.za