Hippocampal volume varies with educational attainment across the life-span
- Department of Pediatrics, Gertrude H. Sergievsky Center, Columbia University, New York, NY, USA
Recent advances in neuroimaging methods have made accessible new ways of disentangling the complex interplay between genetic and environmental factors that influence structural brain development. In recent years, research investigating associations between socioeconomic status (SES) and brain development have found significant links between SES and changes in brain structure, especially in areas related to memory, executive control, and emotion. This review focuses on studies examining links between structural brain development and SES disparities of the magnitude typically found in developing countries. We highlight how highly correlated measures of SES are differentially related to structural changes within the brain.
Introduction
Human development does not occur within a vacuum. The environmental contexts and social connections a person experiences throughout his or her lifetime significantly impact the development of both cognitive and social skills. The incorporation of neuroscience into topics more commonly associated with the social sciences, such as culture or socioeconomic status (SES), has led to an increased understanding of the mechanisms that underlie development across the lifespan. However, more research is necessary to disentangle the complexities surrounding early environmental variation and neural development. This review highlights studies examining links between structural brain development and SES disparities of the magnitude typically found in developing countries. We do not include studies examining children who have experienced extreme forms of early adversity, such as institutionalization or severe abuse. We also limit this review to findings concerning socioeconomic disparities in brain structure, as opposed to brain function.
KEY CONCEPT 1. Socioeconomic status (SES)
Refers to an individual's access to economic and social resources, as well as the benefits and social standing that come from these resources. Most often measured by educational attainment, income, or occupation.
SES is a multidimensional construct, combining objective factors such as an individual's (or parent's) education, occupation, and income (McLoyd, 1998). Neighborhood SES is also often considered (Leventhal and Brooks-Gunn, 2000), as are subjective measures of social status (Adler et al., 2000). In 2012, 46.5 million people in the United States (15%) lived below the official poverty line (United States Census Bureau, 2012) and numerous studies have reported socioeconomic disparities profoundly affecting physical health, mental well-being, and cognitive development (Anderson and Armstead, 1995; Brooks-Gunn and Duncan, 1997; McLoyd, 1998; Evans, 2006). In turn, SES accounts for approximately 20% of the variance in childhood IQ (Gottfried et al., 2003) and it has been estimated that by age five, chronic poverty is associated with a 6- to 13-point IQ reduction (Brooks-Gunn and Duncan, 1997; Smith et al., 1997). Disparities in cognitive development outweigh disparities in physical health, possibly contributing to the propagation of poverty across generations (Duncan et al., 1998).
KEY CONCEPT 2. Poverty
Comparison of a household's income with a threshold level of income that varies with family size and inflation. Households below the poverty threshold are considered “poor.” Households above this threshold are considered “not poor” even if the amount of money between “poor” and “not poor” is diminutive. Poverty guideline for a family of four in 2014 is $23,850.
Evidence suggests multiple possible, and non-mutually-exclusive, explanations for these findings. Socioeconomically disadvantaged children tend to experience less linguistic, social, and cognitive stimulation from their caregivers and home environments than children from higher SES homes (Hart and Risley, 1995; Bradley et al., 2001; Bradley and Corwyn, 2002; Rowe and Goldin-Meadow, 2009). Additionally, individuals from lower SES homes report more stressful events during their lifetime, and the biological response to stressors has been hypothesized as one of the underlying mechanisms for health and cognitive disparities in relation to SES (Anderson and Armstead, 1995; Hackman and Farah, 2009; Noble et al., 2012a).
In turn, these experiential differences are likely to have relatively specific downstream effects on particular brain structures (see Figure 1 for one theoretical model). For example, disparities in the quantity and quality of linguistic stimulation in the home have been associated with developmental differences in language-supporting cortical regions in the left hemisphere (Kuhl et al., 2003; Conboy and Kuhl, 2007; Kuhl, 2007). In contrast, the experience of stress has important negative effects on the hippocampus (Buss et al., 2007; McEwen and Gianaros, 2010; Tottenham and Sheridan, 2010), the amygdala (McEwen and Gianaros, 2010; Tottenham and Sheridan, 2010), and areas of the prefrontal cortex (Liston et al., 2009; McEwen and Gianaros, 2010)—structures which are linked together anatomically and functionally (McEwen and Gianaros, 2010). As discussed below, different components of SES may differentially relate to these varying experiences, and thus may have varying associations with particular structures across the brain.
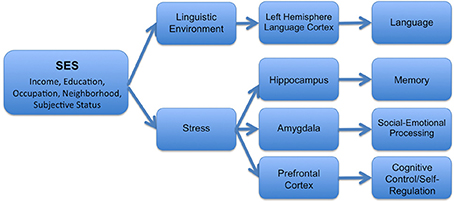
Figure 1. Hypothesized mechanisms by which SES operates to influence structural and functional brain development.
Measures of parental SES are often used as indicators of children's family or home conditions, but these distal measures may not fully account for children's experiences. For example, while a parent may be highly educated, unforeseen circumstances, such as a recession, may cause short- or long-term unemployment and inadequate income, leading to reduced resources and increased family stress experienced by the child. Studies examining an individual's own SES may more accurately represent the individual's current experience during adulthood, but may possibly discount the environmental experiences that shaped neural development as a child. Some studies have included measures of both childhood and adult SES (see Table 1), attempting to obtain a complete measure of SES development, but retrospective SES relies on the individual's memory of past events, and therefore may be biased. Overall, accurate and complete measures of SES are often difficult to obtain and these complications render it difficult to disentangle precise associations between specific socioeconomic indicators and outcomes of interest. Despite this, even approximate assessments of SES have, across multiple independent laboratories, been shown to predict clinically and statistically significant differences in brain structure and function, signifying the prominent association between environmental factors and brain development.
SES Variables Reported in Structural Imaging Studies
Although many studies have reported a high degree of correlation between various components of SES, different socioeconomic factors reflect different aspects of experience and should not be used interchangeably (Duncan and Magnuson, 2012). For example, families with greater economic resources may be better able to purchase more nutritious foods, provide more enriched home learning environments, or afford higher-quality child care settings or safer neighborhoods. In contrast, parental education may influence children's development by shaping the quality of parent–child interactions (Duncan and Magnuson, 2012). The notion that these SES components might differentially influence development is supported by the neuroscience literature, in which whole-brain structural analyses (Lange et al., 2010; Jednoróg et al., 2012) and studies with a priori testing of regions of interest (Hanson et al., 2011; Noble et al., 2012a; Luby et al., 2013) have indicated that different SES components may be associated with different brain structural attributes. Additionally, SES disparities tend not to be global, but rather, are disproportionately associated with differences in the structures of the hippocampus, amygdala, and the prefrontal cortex (see Table 1).
Income
Household or family income is usually calculated as the sum of total income, typically measured monthly or annually. Although income can be considered a continuous variable, many studies ask participants to select what category of income they fall into. For example, a participant may indicate that they earn between $30,000 and $60,000 dollars per year, and researchers often take the midpoint of the participant's estimate (i.e., $45,000), thereby reducing variability between participants. Income is one of the more volatile of the SES markers, as family circumstances frequently fluctuate across time, resulting in varying levels of income throughout childhood and adolescence (Duncan, 1988; Duncan and Magnuson, 2012). Income-to-Needs (ITN) is a similar marker of SES, in which total family income is divided by the official poverty threshold for a family of that size. Hanson et al. (2011); Noble et al. (2012a) and Luby et al. (2013) all find significant positive correlations between income/ITN and hippocampal size, with children and adolescents from lower SES families having smaller hippocampal volumes. Examining income-related differences in amygdala volumes, we find some discrepancies across studies. While both Hanson et al. (2011) and Noble et al. (2012a) find no association between income/ITN and amygdala volume, Luby et al. (2013) report a significant positive correlation, where children from lower income homes also have smaller amygdala volumes. The families in the latter study reported lower family income than the families in the other two studies; thus it may be possible that, unlike the hippocampus, substantial income insufficiency is necessary to observe structural differences in amygdala volumes.
KEY CONCEPT 3. Income-to-Needs
The ratio of total family income divided by the federal poverty level for a family of that size, in the year data were collected. A family living at the poverty line would have an income-to-needs of ratio of 1. In 2012, 20.4 million people reported an income below 50% of their poverty threshold, including 7.1 million children under the age of 18.
Education
Parental education or educational attainment is usually measured by participants reporting their highest level (or their parents' highest levels) of education (e.g., college degree). While family income has been associated with resources available to the family and levels of environmental stress (Evans and English, 2002), parental education has been more closely linked to cognitive stimulation in the home (Hoff-Ginsberg and Tardif, 1995). Compared to parents with lower levels of education, parents with higher levels of education tend to spend more time with their children (Guryan et al., 2008), use more varied and complex language (Hart and Risley, 1995; Hoff, 2003), and engage in parenting practices that promote socioemotional development (Duncan et al., 1994; McLoyd, 1997; Bradley and Corwyn, 2002). Again, like income/ITN, we find some inconsistencies across studies when examining links between parental education and children's brain structure. Luby et al. (2013) and Noble et al. (2012a) find no significant correlations between parental education (measured as the average or highest level of education of any parents or guardians living in the home) and hippocampal volumes. Hanson et al. (2011) report a significant association between right hippocampal volumes and paternal, but not maternal, education levels. There are differences across studies in reported amygdala volumes as well. Whereas Noble et al. (2012a) find a negative correlation between parental education and amygdala volumes, Luby et al. (2013) and Hanson et al. (2011) find no association. These differences may be due in part to how parental education was measured (average parental education vs. separate indicators for mothers and fathers) and/or how parental education was coded (continuously vs. categorically).
Examining the relation between brain structure and one's own educational attainment in adulthood (as opposed to parental education), both Gianaros et al. (2012) and Piras et al. (2011) found positive associations between educational attainment and increases in white matter integrity using diffusion tensor imaging (indexed by increases in fractional anisotropy and decreases in mean diffusivity, respectively). Whereas Gianaros and colleagues found widespread associations, Piras and colleagues found that, once controlling for age, only microstructural changes in the hippocampi significantly correlated with educational attainment. Noble et al. (2012b) also found no simple correlation between reported educational attainment and either hippocampal or amygdala volumes in adulthood. Educational attainment did, however, moderate the association between age and hippocampal volume. Specifically, as has been reported previously, age was quadratically related to hippocampal volume, with the volume of this structure tending to increase until approximately the age of 30, at which point volume starts to decline (Grieve et al., 2011). Although this quadratic relation between hippocampal volume and age was present across the entire sample, the volumetric reduction seen at older ages was more pronounced among less educated individuals, and was buffered among more highly educated individuals. Differences in hippocampal structure between higher and lower educated individuals may therefore be most apparent in the later stages of the lifespan.
Occupation
Occupations generally reflect education, earnings, and prestige (Jencks et al., 1988), and have been extensively studied as an important aspect of SES as they are directly related to both education and income. Chiang et al. (2011) found that occupational status, measured using the Australian Socioeconomic Index (SEI), a 0–100 scale based on an individual's occupational category, was not related to white matter integrity. However, the authors did find an interaction between occupational status and white matter integrity, controlling for subjects' age and sex. Specifically, higher SEI was associated with higher heritability white matter integrity in the thalamus, left middle temporal gyrus, and callosal splenium.
SES Composite Measures
Some studies have combined different SES markers to create average or composite measures. Cavanagh et al. (2013) used indicators of early life SES (number of siblings, number of people per room, paternal social class, parental housing tenure, and use of car by family) and current SES (current income, current social class, and current housing tenure) to predict cerebellar gray matter volume. Both composite measures positively predicted cerebellar structure, where current SES explained significant additional variance to early life SES, but not vice-versa. Staff et al. (2012) also measured both childhood SES (indexed by paternal education and childhood home conditions) as well as adult SES (indexed by the individual's educational attainment, occupational status, and neighborhood deprivation). These authors reported a significant association between hippocampal volume and childhood SES, after adjusting for the individual's SES as an adult more than 50 years later. These results may suggest that early life conditions may have an effect on structural brain development over and above conditions later in life.
The Hollingshead scale (Hollingshead, 1975) is a commonly used measure of SES, which combines occupation and education (Two-Factor Index) or occupation, education, marital status, and employment status (Four-Factor Index). Duncan and Magnuson (2003) have argued that aggregating these SES measures is faulty as fluctuations within each measure of SES differentially affect parenting and child developmental outcomes. Imaging studies using these composite measures of SES have found significant correlations between composite scores and regions in the medial temporal lobe and frontal lobe (Raizada et al., 2008; Jednoróg et al., 2012), but without knowing associations to specific SES markers, it is difficult to compare these studies with other structural imaging studies.
Neighborhood SES
Of note, SES can describe a single participant, the participant's family or even the participant's neighborhood. The neighborhood context is associated with various health outcomes (Pickett and Pearl, 2001) as it is another source of potential exposure to stressors (e.g., violence) or protection from them (e.g., community resources, social support). Some studies have found correlations between neighborhood disadvantage and cognitive outcomes independent of individual level SES (Wight et al., 2006; Sampson et al., 2008), whereas others have not (Hackman et al., 2014). Studies examining neighborhood SES and brain structure have also had mixed findings. Gianaros et al. (2007, 2012) have used census tract level data (median household income, percentage of adults with college degrees or higher, proportion of households below federal poverty line, and single mother households) to create composite indicators of community SES. Although community SES was not associated with total brain volume or gray matter volumes in regions of interest (Gianaros et al., 2007), community SES was positively associated with white matter integrity independent of self-reported levels of stress and depressive symptoms (Gianaros et al., 2012). Similarly, Krishnadas et al. (2013) found that neighborhood SES, indexed using the Scottish Index of Multiple Deprivation, was related to cortical thickness, with men living in more disadvantaged areas demonstrating more cortical thinning in areas that support language function (bilateral perisylvian cortices) than men living in more advantaged areas.
KEY CONCEPT 4. Cortical thickness
Defined in neuroimaging studies as the shortest distance between the white matter surface and pial gray matter surface.
Subjective Social Status
Finally, subjective social status is another marker of SES used in some research. In these studies, participants are typically asked to indicate on a drawing of a ladder where they believe they rank in terms of social standing among a particular group. In past studies, lower social ladder standings have been correlated with negative physical and mental health outcomes (Adler et al., 2000; Kopp et al., 2004; Hu et al., 2005), even after accounting for objective measures of education, income, and potential reporting biases (Adler et al., 1994). Gianaros et al. (2007) found that subjective social status was not correlated with hippocampal or amygdala volumes, but was significantly associated with reduced gray matter volume in the perigenual area of the anterior cingulate cortex (pACC). This finding may be understood by recognizing that the pACC is a region in the brain involved in experiencing emotions and regulating behavioral and physiological reactivity to stress. Measures of subjective social status may not take into account objective measures of SES, but relate more to the individual's experience of disadvantage.
Words of Caution in Selecting SES Variables
Collecting and utilizing multiple independent measures of SES is necessary to accurately assess structural brain changes throughout development. SES is too complex to be captured by a single indicator or even a composite measure. Each measure of SES is its own distinct construct with varying associations with experience and cognitive development. However, while SES variables are not interchangeable, they are nonetheless highly correlated. It is therefore essential to avoid model multicollinearity in statistical analyses. This may be accomplished by first carefully considering which variables are most appropriate for testing particular hypotheses, and then confirming low variance inflation factors (VIF) within the model. Increasing sample size, centering variables, and utilizing residuals are additional methods to avoid inappropriate analysis and interpretation.
As a final word of caution, many of the SES indicators referenced above are based on studies completed in Western countries. Further work will be necessary to explore the generalizability of findings across different countries and cultures (Minujin et al., 2006; Lipina et al., 2011).
Covariates, Mediators, and Moderators
When examining SES disparities in brain structural development, additional demographic factors must be considered as well. First and foremost, the age of the participant must be taken into account, as brain structural volumes change significantly across childhood and adolescence (Paus et al., 1999; Lenroot and Giedd, 2006). Further, the timing of volumetric growth and reductions vary across different brain structures (Grieve et al., 2011). Inconsistencies in results across studies highlighted above may therefore be due to variability in the age ranges of the samples studied. Caution is advised when generalizing results reported within a narrow-age-range sample, as SES disparities in brain structure may vary substantially as a function of age.
Several studies include relatively wide age ranges, recruiting, for example, both children and adolescents in their imaging samples (Lange et al., 2010; Hanson et al., 2011; Noble et al., 2012a; Lawson et al., 2013). Two additional studies have taken a lifespan approach to examining SES and structural brain development (Piras et al., 2011; Noble et al., 2012b). Incorporating wide age ranges into a study allows researchers to consider whether results vary as a function of participant age. For example, both Noble et al. (2012b) and Piras et al. (2011) examine associations between subcortical structures and educational attainment in a wide age range of participants. Piras et al. (2011) found that microstructural changes in the hippocampus, but not changes in gross volume in this structure, were significantly predicted by education levels. However, due to a large negative correlation between education and age, the decreases in microstructure may have been more closely related to older age than greater education. As discussed above, Noble et al. (2012b) reported that higher levels of educational attainment buffered against age-related reductions in hippocampal volume, signifying that the association between age and hippocampal volume is not constant across all levels of education. Of course, distinctions between development and decline are, in some respects, arbitrary, and may be more appropriately classified according to functional rather than structural measures.
Sex is another important demographic characteristic to consider. Volumetric variation in brain structures increase within and between males and females during puberty (Sowell et al., 2003). Sex differences have been reported for cortical thickness. Using a longitudinal sample of participants ages 9–22 years, Raznahan et al. (2010) observed differences in cortical maturation, with males demonstrating a thicker cortex in frontopolar regions at younger ages and subsequent greater cortical thinning than females during adolescence. It has also been reported that females demonstrate more rapid cortical thinning than males in specific cortical areas (right temporal, left temporoparietal junction, and left orbitofrontal cortex) corresponding to the “social brain” (Mutlu et al., 2013). It will be important in future work to better understand how the links between SES variables and structural brain development may vary by sex, and/or a combination of sex and age.
In addition, studies have reported that families living in chronic poverty have differential outcomes based on when and for how long poverty was experienced (National Institute of Child Health and Human Development Early Child Care Research Network, 2005). While the brain is most malleable in early childhood, it nonetheless retains a substantial degree of plasticity throughout the lifespan, and the extent to which the timing and duration of socioeconomic disadvantage are associated with brain structural differences is virtually unexplored in the neuroscience literature to date.
Finally, it is important to consider environmental exposures and experiences that may account for links between distal socioeconomic factors and brain structural differences. For example, Luby et al. (2013) recently reported that links between income and hippocampal volume were mediated by caregiving support/hostility and stressful life events. Of course, there are many potential experiential correlates of SES that have not been well studied in the context of SES disparities in brain development, including nutrition, exposure to environmental toxins, safety of the play environment, or quality of the child's linguistic environment. In order to develop interventions that effectively target the SES gap in achievement, it will be essential to try to understand the particular component(s) of the environment that are most influential in explaining disparities.
Volume vs. Cortical Thickness/Surface Area
Differences in findings across studies may also be accounted for by the techniques used to measure morphometry. Most studies examining SES differences in brain structure have reported cortical volumes as their outcome of interest (but see Jednoróg et al., 2012; Liu et al., 2012; Krishnadas et al., 2013; Lawson et al., 2013). However, cortical volume is a composite measure that is determined by the product of surface area and cortical thickness, two genetically and phenotypically independent structures (Panizzon et al., 2009; Raznahan et al., 2011). Though the cellular mechanisms are not fully understood, it has been hypothesized that symmetrical cell division in the neural stem cell pool contribute to exponential increase in the number of radial columns that result in surface area, without changes to cortical thickness. In contrast, asymmetrical cell division in founder cells is independently responsible for a linear increase in the number of neurons in the radial column, leading to changes in cortical thickness but not surface area (Rakic, 2009). As such, these two properties of the cortical sheet develop differentially; cortical surface area tends to expand through childhood and early adolescence and decrease in adulthood, whereas cortical thickness tends to decrease rapidly in childhood and early adolescence, followed by a more gradual thinning and ultimately plateauing (Schnack et al., 2014). Cortical thinning is related to both synaptic pruning and increases in white matter myelination, resulting in a reduction of gray matter as measured on MRI (Sowell et al., 2003). These maturational changes occur concurrently and together contribute to the development of the mature human brain.
KEY CONCEPT 5. Cortical volumes
The most commonly used outcome in studies of socioeconomic disparities in brain structure. Cortical volume is actually a composite of cortical thickness and surface area, two genetically and phenotypically distinct morphometric properties of the brain.
KEY CONCEPT 6. Surface area
The area of exposed cortical surface or convex hull area (CHA) and the area of cortex hidden in sulci.
Thus, studies in which the dependent measure is cortical volume may not adequately reflect the complexities of morphometric brain development. Indeed, cross-sectional comparisons of cortical volume are poor indicators of brain maturation (Giedd and Rapoport, 2010), whereas cortical thickness has been shown to be a more meaningful index of brain development (Sowell et al., 2004; Paus, 2005) and has been associated with both cognitive ability (Porter et al., 2011) and behavior (Shaw et al., 2011). For example, IQ has been correlated with the trajectory of cortical thickness, such that, during childhood, more intelligent children have thinner cortices than children with lower IQ, with this association strengthening through adolescence. In contrast, by middle adulthood, a thicker cortex is related to higher IQ (Schnack et al., 2014). Importantly, IQ has also been independently correlated with the trajectory of surface area development, such that more intelligent children exhibit greater surface area during childhood, though surface area expansion is completed earlier and then decreases more quickly in more intelligent adults (Schnack et al., 2014). Together, these findings suggest that both surface area and cortical thickness may be critical in accounting for individual differences in cognitive abilities, and that these factors must be considered independently rather than lumping them into a single composite measure of cortical volume.
In summary, when considering associations between experience and brain morphometry, cortical thickness and surface area should be assessed separately, rather than reporting on the composite metric of cortical volume (Winkler et al., 2010; Raznahan et al., 2011). Research investigating cortical complexity and its association with SES variables will be vital to further understanding how environmental influences over the life course influence structural brain development.
Conclusions
Children living in socioeconomic disadvantage are more likely to experience cognitive delays and emotional problems (Brooks-Gunn and Duncan, 1997), but the underlying causal pathways between disadvantage and developmental outcomes are not clear. The nascent field of socioeconomic disparities in brain structure is an exciting one, which holds promise in helping to understand this question. However, while progress has been made in understanding how socioeconomic disparities may affect brain development, there are many avenues for further research. Careful social science approaches to assessing individual socioeconomic factors must be combined with cutting-edge neuroscientific approaches to measuring precise aspects of brain morphometry. Consideration of how results interact with demographic factors such as age and sex are critical. Differences in exposures and experiences that may mediate socioeconomic disparities in brain development must be rigorously assessed to help identify or confirm underlying mechanisms.
Although this review has focused on SES disparities in brain structure as opposed to function, it is readily acknowledged that the two approaches are complementary. While a structural approach lends itself to greater spatial resolution as well as, arguably, more precision in understanding proximal experience-dependent mechanisms, it is limited in terms of functional interpretations. Ultimately, linking both structural and functional imaging to cognitive outcomes is essential for examining associations between anatomy, physiology, and behavior. Brain structural measures can be viewed as mediators between SES and cognition, or as outcome variables in their own right; having clear theoretical pathways ensures accurate interpretation of results and implications, and will help inform the design of effective policies, emphasizing early and targeted interventions.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
The authors are grateful for funding from the Robert Wood Johnson Foundation Health and Society Scholars program and the GH Sergievsky Center.
Author Biography


References
Adler, N. E., Boyce, T., Chesney, M. A., Cohen, S., Folkman, S., Kahn, R. L., et al. (1994). Socioeconomic status and health: the challenge of the gradient. Am. Psychol. 49:15. doi: 10.1037/0003-066X.49.1.15
Adler, N. E., Epel, E. S., Castellazzo, G., and Ickovics, J. R. (2000). Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 19, 586–592. doi: 10.1037/0278-6133.19.6.586
Anderson, N. B., and Armstead, C. A. (1995). Toward understanding the association of socioeconomic status and health: a new challenge for the biopsychosocial approach. Psychosom. Med. 57, 213–225. doi: 10.1097/00006842-199505000-00003
Bradley, R. H., and Corwyn, R. F. (2002). Socioeconomic status and child development. Annu. Rev. Psychol. 53, 371–399. doi: 10.1146/annurev.psych.53.100901.135233
Bradley, R. H., Corwyn, R. F., Burchinal, M., McAdoo, H. P., and García Coll, C. (2001). The home environments of children in the United States Part II: relations with behavioral development through age thirteen. Child Dev. 72, 1868–1886. doi: 10.1111/1467-8624.t01-1-00383
Brain Development Cooperative Group. (2012). Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI study of normal brain development. Cereb. Cortex 22, 1–12. doi: 10.1093/cercor/bhr018
Brooks-Gunn, J., and Duncan, G. J. (1997). The effects of poverty on children. Future Child. 7, 55–71. doi: 10.2307/1602387
Buss, C., Lord, C., Wadiwalla, M., Hellhammer, D. H., Lupien, S. J., Meaney, M. J., et al. (2007). Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J. Neurosci. 27, 2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007
Butterworth, P., Cherbuin, N., Sachdev, P., and Anstey, K. J. (2012). The association between financial hardship and amygdala and hippocampal volumes: results from the PATH through life project. Soc. Cogn. Affect. Neurosci. 7, 548–556. doi: 10.1093/scan/nsr027
Cavanagh, J., Krishnadas, R., Batty, G. D., Burns, H., Deans, K. A., Ford, I., et al. (2013). Socioeconomic status and the cerebellar grey matter volume. Data from a well-characterized population sample. Cerebellum 12, 882–891. doi: 10.1007/s12311-013-0497-4
Chiang, M. C., McMahon, K. L., de Zubicaray, G. I., Martin, N. G., Hickie, I., Toga, A. W., et al. (2011). Genetics of white matter development: a DTI study of 705 twins and their siblings aged 12 to 29. Neuroimage 54, 2308–2317. doi: 10.1016/j.neuroimage.2010.10.015
Conboy, B. T., and Kuhl, P. K. (2007). “Early speech perception: developing a culturally specific way of listening through social interaction,” in On Being Moved: From Mirror Neurons to Empathy, ed S. Braten (Amsterdam: John Benjamins), 175–199. doi: 10.1075/aicr.68.15con
Duncan, G., Brooks-Gunn, J., Yeung, J., and Smith, J. (1998). How much does childhood poverty affect the life chances of children? Am. Sociol. Rev. 63, 406–423.
Duncan, G. J. (1988). “The volatility of family income over the life course,” in Life-Span Development and Behavior, eds P. Baltes, D. Featherman, and R. M. Lerner (Hillsdale, NJ: Lawrence Erlbaum Associates), 317–358.
Duncan, G. J., Brooks-Gunn, J., and Klebanov, P. K. (1994). Economic deprivation and early childhood development. Child Dev. 65, 296–318. doi: 10.2307/1131385
Duncan, G. J., and Magnuson, K. (2012). Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdiscip. Rev. Cogn. Sci. 3, 377–386. doi: 10.1002/wcs.1176
Duncan, G. J., and Magnuson, K. A. (2003). “Off with Hollingshead: socioeconomic resources, parenting, and child development,” in Socioeconomic Status, Parenting, and Child Development, eds M. H. Bornstein and R. H. Bradley (Mahwah, NJ: Lawrence Erlbaum), 83–106.
Eckert, M. A., Lombardino, L. J., and Leonard, C. M. (2001). Planar asymmetry tips the phonological playground and environment raises the bar. Child Dev. 72, 988–1002. doi: 10.1111/1467-8624.00330
Evans, G. W. (2006). Child development and the physical environment. Annu. Rev. Psychol. 57, 423–451. doi: 10.1146/annurev.psych.57.102904.190057
Evans, G. W., and English, K. (2002). The environment of poverty: multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev. 73, 1238–1248. doi: 10.1111/1467-8624.00469
Gianaros, P. J., Horenstein, J. A., Cohen, S., Matthews, K. A., Brown, S. M., Flory, J. D., et al. (2007). Perigenual anterior cingulate morphology covaries with perceived social standing. Soc. Cogn. Affect. Neurosci. 2, 161–173. doi: 10.1093/scan/nsm013
Gianaros, P. J., Marsland, A. L., Sheu, L. K., Erickson, K. I., and Verstynen, T. D. (2012). Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb. Cortex 23, 2058–2071. doi: 10.1093/cercor/bhs191
Giedd, J. N., and Rapoport, J. L. (2010). Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 67, 728–734. doi: 10.1016/j.neuron.2010.08.040
Gottfried, A. W., Gottfried, A. E., Bathurst, K., Guerin, D. W., and Parramore, M. M. (2003). “Socioeconomic status in children's development and family environment: infancy through adolescence,” in Socioeconomic Status, Parenting and Child Development, eds M. H. Bornstein and R. H. Bradley (Mahwah, NJ: Lawrence Erlbaum), 189–207.
Grieve, S. M., Korgaonkar, M. S., Clark, C. R., and Williams, L. M. (2011). Regional heterogeneity in limbic maturational changes: evidence from integrating cortical thickness, volumetric and diffusion tensor imaging measures. Neuroimage 55, 868–879. doi: 10.1016/j.neuroimage.2010.12.087
Guryan, J., Hurst, E., and Kearney, M. (2008). Parental education and parental time with children. J. Econ. Perspect. 22, 23–46. doi: 10.1257/jep.22.3.23
Hackman, D. A., Betancourt, L. M., Gallop, R., Romer, D., Brodsky, N. L., Hurt, H., et al. (2014). Mapping the trajectory of socioeconomic disparity in working memory: parental and neighborhood factors. Child Dev. 85, 1433–1445. doi: 10.1111/cdev.12242
Hackman, D. A., and Farah, M. J. (2009). Socioeconomic status and the developing brain. Trends Cogn. Sci. 13, 65–73. doi: 10.1016/j.tics.2008.11.003
Hanson, J. L., Chandra, A., Wolfe, B. L., and Pollak, S. D. (2011). Association between income and the hippocampus. PLoS ONE 6:e18712. doi: 10.1371/journal.pone.0018712
Hanson, J. L., Hair, N., Shen, D. G., Shi, F., Gilmore, J. H., Wolfe, B. L., et al. (2013). Family poverty affects the rate of human infant brain growth. PLoS ONE 8:e80954. doi: 10.1371/journal.pone.0080954
Hart, B., and Risley, T. R. (1995). Meaningful Differences in the Everyday Experience of Young American Children. Baltimore, MD: Paul H Brookes Publishing.
Hoff, E. (2003). The specificity of environmental influence: socioeconomic status affects early vocabulary development via maternal speech. Child Dev. 74, 1368–1378. doi: 10.1111/1467-8624.00612
Hoff-Ginsberg, E., and Tardif, T. (1995). “Socioeconomic status and parenting,” in Handbook of Parenting, Volume 2: Biology and Ecology of parenting, ed M. H. Bornstein (Hillsdale, NJ: Lawrence Erlbaum Associates), 161–188.
Hu, P., Adler, N. E., Goldman, N., Weinstein, M., and Seeman, T. E. (2005). Relationship between subjective social status and measures of health in older Taiwanese persons. J. Am. Geriatr. Soc. 53, 483–488. doi: 10.1111/j.1532-5415.2005.53169.x
Jednoróg, K., Altarelli, I., Monzalvo, K., Fluss, J., Dubois, J., Billard, C., et al. (2012). The influence of socioeconomic status on children's brain structure. PLoS ONE 7:e42486. doi: 10.1371/journal.pone.0042486
Jencks, C., Perman, L., and Rainwater, L. (1988). What is a good job? A new measure of labor-market success. Am. J. Sociol. 93, 1322–1357. doi: 10.1086/228903
Kopp, M., Skrabski, Á., Réthelyi, J., Kawachi, I., and Adler, N. E. (2004). Self-rated health, subjective social status, and middle-aged mortality in a changing society. Behav. Med. 30, 65–72. doi: 10.3200/BMED.30.2.65-72
Krishnadas, R., Kim, J., McLean, J., Batty, G. D., McLean, J. S., Millar, K., et al. (2013). The environment and the connectome: exploring the structural noise in the human brain associated with socioeconomic deprivation. Front. Hum. Neurosci. 7:722. doi: 10.3389/fnhum.2013.00722
Kuhl, P. K. (2007). Is speech learning “gated” by the social brain? Dev. Sci. 10, 110–120. doi: 10.1111/j.1467-7687.2007.00572.x
Kuhl, P. K., Tsao, F.-M., and Liu, H.-M. (2003). Foreign-language experience in infancy: effects of short-term exposure and social interaction on phonetic learning. Proc. Natl. Acad. Sci. U.S.A. 100, 9096–9101. doi: 10.1073/pnas.1532872100
Lange, N., Froimowitz, M. P., Bigler, E. D., Lainhart, J. E., and Brain Development Cooperative Group. (2010). Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Dev. Neuropsychol. 35, 296–317. doi: 10.1080/87565641003696833
Lawson, G. M., Duda, J. T., Avants, B. B., Wu, J., and Farah, M. J. (2013). Associations between children's socioeconomic status and prefrontal cortical thickness. Dev. Sci. 16, 641–652. doi: 10.1111/desc.12096
Lenroot, R. K., and Giedd, J. N. (2006). Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 30, 718–729. doi: 10.1016/j.neubiorev.2006.06.001
Leventhal, T., and Brooks-Gunn, J. (2000). The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol. Bull. 126, 309–337. doi: 10.1037/0033-2909.126.2.309
Lipina, S. J., Simonds, J., and Segretin, M. S. (2011). Recognizing the child in child poverty. Vulnerable Child. Youth Stud. 6, 8–17. doi: 10.1080/17450128.2010.521598
Liston, C., McEwen, B. S., and Casey, B. J. (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl. Acad. Sci. U.S.A. 106, 912–917. doi: 10.1073/pnas.0807041106
Liu, Y., Julkunen, V., Paajanen, T., Westman, E., Wahlund, L. O., Aitken, A., et al. (2012). Education increases reserve against Alzheimer's disease—evidence from structural MRI analysis. Neuroradiology 54, 929–938. doi: 10.1007/s00234-012-1005-0
Luby, J., Belden, A., Botteron, K., Marrus, N., Harms, M. P., Babb, C., et al. (2013). The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 167, 1135–1142. doi: 10.1001/jamapediatrics.2013.3139
McEwen, B. S., and Gianaros, P. J. (2010). Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N.Y. Acad. Sci. 1186, 190–222. doi: 10.1111/j.1749-6632.2009.05331.x
McLoyd, V. C. (1997). “The impact of poverty and low socioeconomic status on the socioemotional functioning of African-American children and adolescents: mediating effects,” in Social and Emotional Adjustment and Family Relations in Ethnic Minority Families, eds R. D. Taylor and M. Wang (Mahwah, NJ: Erlbaum), 7–34.
McLoyd, V. C. (1998). Socioeconomic disadvantage and child development. Am. Psychol. 53:185. doi: 10.1037/0003-066X.53.2.185
Minujin, A., Delamonica, E., Davidziuk, A., and Gonzalez, E. D. (2006). The definition of child poverty: a discussion of concepts and measurement. Environ. Urban. 18, 481–500. doi: 10.1177/0956247806069627
Mutlu, A. K., Schneider, M., Debbané, M., Badoud, D., Eliez, S., and Schaer, M. (2013). Sex differences in thickness, and folding developments throughout the cortex. Neuroimage 82, 200–207. doi: 10.1016/j.neuroimage.2013.05.076
National Institute of Child Health Human Development Early Child Care Research Network (2005). Duration and developmental timing of poverty and children's cognitive and social development from birth through third grade. Child Dev. 76, 795–810. doi: 10.1111/j.1467-8624.2005.00878.x
Noble, K. G., Grieve, S. M., Korgaonkar, M. S., Engelhardt, L. E., Griffith, E. Y., Williams, L. M., et al. (2012b). Hippocampal volume varies with educational attainment across the life-span. Front. Hum. Neurosci. 6:307. doi: 10.3389/fnhum.2012.00307
Noble, K. G., Houston, S. M., Kan, E., and Sowell, E. R. (2012a). Neural correlates of socioeconomic status in the developing human brain. Dev. Sci. 15, 516–527. doi: 10.1111/j.1467-7687.2012.01147.x
Noble, K. G., Korgaonkar, M. S., Grieve, S. M., and Brickman, A. M. (2013). Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Dev. Sci. 16, 653–664. doi: 10.1111/desc.12077
Panizzon, M. S., Fennema-Notestine, C., Eyler, L. T., Jernigan, T. L., Prom-Wormley, E., Neale, M., et al. (2009). Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex 19, 2728–2735. doi: 10.1093/cercor/bhp026
Paus, T. (2005). Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 9, 60–68. doi: 10.1016/j.tics.2004.12.008
Paus, T., Zijdenbos, A., Worsley, K., Collins, D. L., Blumenthal, J., Giedd, J. N., et al. (1999). Structural maturation of neural pathways in children and adolescents: in vivo study. Science 283, 1908–1911.
Pickett, K. E., and Pearl, M. (2001). Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J. Epidemiol. Community Health 55, 111–122. doi: 10.1136/jech.55.2.111
Piras, F., Cherubini, A., Caltagirone, C., and Spalletta, G. (2011). Education mediates microstructural changes in bilateral hippocampus. Hum. Brain Mapp. 32, 282–289. doi: 10.1002/hbm.21018
Porter, J. N., Collins, P. F., Muetzel, R. L., Lim, K. O., and Luciana, M. (2011). Associations between cortical thickness and verbal fluency in childhood, adolescence, and young adulthood. Neuroimage 55, 1865–1877. doi: 10.1016/j.neuroimage.2011.01.018
Raizada, R. D., Richards, T. L., Meltzoff, A., and Kuhl, P. K. (2008). Socioeconomic status predicts hemispheric specialization of the left inferior frontal gyrus in young children. Neuroimage 40, 1392–1401. doi: 10.1016/j.neuroimage.2008.01.021
Rakic, P. (2009). Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. 10, 724–735. doi: 10.1038/nrn2719
Raznahan, A., Lee, Y., Stidd, R., Long, R., Greenstein, D., Clasen, L., et al. (2010). Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc. Natl. Acad. Sci. U.S.A. 107, 16988–16993. doi: 10.1073/pnas.1006025107
Raznahan, A., Shaw, P., Lalonde, F., Stockman, M., Wallace, G. L., Greenstein, D., et al. (2011). How does your cortex grow? J. Neurosci. 31, 7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011
Rowe, M. L., and Goldin-Meadow, S. (2009). Early gesture selectively predicts later language learning. Dev. Sci. 12, 182–187. doi: 10.1111/j.1467-7687.2008.00764.x
Sampson, R. J., Sharkey, P., and Raudenbush, S. W. (2008). Durable effects of concentrated disadvantage on verbal ability among African-American children. Proc. Natl. Acad. Sci. 105, 845–852. doi: 10.1073/pnas.0710189104
Schnack, H. G., van Haren, N. E., Brouwer, R. M., Evans, A., Durston, S., Boomsma, D. I., et al. (2014). Changes in Thickness and Surface Area of the Human Cortex and Their Relationship with Intelligence. Cereb. Cortex. doi: 10.1093/cercor/bht357. [Epub ahead of print].
Shaw, P., Gilliam, M., Liverpool, M., Weddle, C., Malek, M., Sharp, W., et al. (2011). Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. American Journal of Psychiatry, 168,143–151. doi: 10.1176/appi.ajp.2010.10030385
Smith, J. R., Brooks-Gunn, J., and Klebanov, P. (1997). “The consequences of living in poverty for young children's cognitive and verbal ability and early school achievement,” in Consequences of Growing Up Poor, eds G. J. Duncan and J. Brooks-Gunn (New York, NY: Russell Sage Foundation), 132–189.
Sowell, E. R., Peterson, B. S., Thompson, P. M., Welcome, S. E., Henkenius, A. L., and Toga, A. W. (2003). Mapping cortical change across the human life span. Nat. Neurosci. 6, 309–315. doi: 10.1038/nn1008
Sowell, E. R., Thompson, P. M., Leonard, C. M., Welcome, S. E., Kan, E., and Toga, A. W. (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 24, 8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004
Staff, R. T., Murray, A. D., Ahearn, T. S., Mustafa, N., Fox, H. C., and Whalley, L. J. (2012). Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Ann. Neurol. 71, 653–660. doi: 10.1002/ana.22631
Tottenham, N., and Sheridan, M. (2010). A review of adversity, the amygdala, and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 3:68. doi: 10.3389/neuro.09.068.2009
United States Census Bureau. (2012). Income, Poverty and Health Insurance in the United States: 2012 – Highlights. Available online at: http://www.census.gov/hhes/www/poverty/data/incpovhlth/2012/highlights.html
Wight, R. G., Aneshensel, C. S., Miller-Martinez, D., Botti- cello, A. L., Cummings, J. R., Karlamangla, A. S., et al. (2006). Urban neighborhood context, educational attainment, and cognitive function among older adults. Am. J. Epidemiol. 163, 1071–1078. doi: 10.1093/aje/kwj176
Keywords: socioeconomic status, brain development, structural imaging, environmental variation
Citation: Brito NH and Noble KG (2014) Socioeconomic status and structural brain development. Front. Neurosci. 8:276. doi: 10.3389/fnins.2014.00276
Received: 02 June 2014; Accepted: 17 August 2014;
Published online: 04 September 2014.
Edited by:
Hauke R. Heekeren, Freie Universität Berlin, GermanyReviewed by:
Sebastian J. Lipina, Unidad de Neurobiología Aplicada (UNA, CEMIC-CONICET), ArgentinaRajeev Krishnadas, University of Glasgow, UK
Martha Farah, University of Pennsylvania, USA
Copyright © 2014 Brito and Noble. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: nhb2111@columbia.edu
*Correspondence: kgn2106@columbia.edu
 Natalie H. Brito
Natalie H. Brito Kimberly G. Noble
Kimberly G. Noble