- 1Department of Physiology, University of Melbourne, Parkville, VIC, Australia
- 2Departments of Anatomy and Cell Biology, University of Melbourne, Parkville, VIC, Australia
The roles of 5-HT3 and 5-HT4 receptors in the modulation of intestinal propulsion by luminal application of 5-HT and augmentation of endogenous 5-HT effects were studied in segments of guinea-pig ileum in vitro. Persistent propulsive contractions evoked by saline distension were examined using a modified Trendelenburg method. When 5-HT (30 nM), fluoxetine (selective serotonin reuptake inhibitor; 1 nM), 2-methyl-5-HT (5-HT3 receptor agonist; 1 mM), or RS 67506 (5-HT4 receptor agonist, 1 μM) was infused into the lumen, the pressure needed to initiate persistent propulsive activity fell significantly. A specific 5-HT4 receptor antagonist, SB 207266 (10 nM in lumen), abolished the effects of 5-HT, fluoxetine, and RS 67506, but not those of 2-methyl-5-HT. Granisetron (5-HT3 receptor antagonist; 1 μM in lumen) abolished the effect of 5-HT, fluoxetine, RS 67506, and 2-methyl-5-HT. The NK3 receptor antagonist SR 142801 (100 nM in lumen) blocked the effects of 5-HT, fluoxetine, and 2-methyl-5-HT. SB 207266, granisetron, and SR 142801 had no effect by themselves. Higher concentrations of fluoxetine (100 and 300 nM) and RS 67506 (3 and 10 μM) had no effect on the distension threshold for propulsive contractions. These results indicate that luminal application of exogenous 5-HT, or increased release of endogenous mucosal 5-HT above basal levels, acts to lower the threshold for propulsive contractions in the guinea-pig ileum via activation of 5-HT3 and 5-HT4 receptors and the release of tachykinins. The results further indicate that basal release of 5-HT is insufficient to alter the threshold for propulsive motor activity.
Introduction
The intestinal mucosa is the major site of synthesis of serotonin (5-HT) in the body, but the role of mucosal 5-HT in controlling intestinal motility remains controversial. Gastric emptying, jejunal transit and transit through the entire gastrointestinal tract in vivo are normal in mice lacking tryptophan hydroxylase 1, the rate limiting enzyme for 5-HT synthesis in enterochromaffin (EC) cells (Li et al., 2011). However, in vitro analysis of propulsive motility in the colon from the same mice indicates that 5-HT released by EC cells facilitates normal propulsion; in knock out animals, reflex responses to distension were reduced and only larger fecal pellets were propelled (Heredia et al., 2013). These studies are consistent with other studies showing that complete removal of the mucosa and blockade of mucosal 5-HT release does not prevent initiation of colonic propulsion (Spencer et al., 2011), colonic contractile complexes (Keating and Spencer, 2010) or polarized reflex responses of the muscle (Costa and Furness, 1976). This may reflect the finding that distension does not produce 5-HT release from the colonic mucosa (Grider et al., 1996). However, luminal application of 5-HT antagonists can slow, but not prevent, propulsion in guinea-pig colon (Jin et al., 1999). Thus, it appears that colonic propulsion does not require mucosal 5-HT, but may be enhanced when mucosal 5-HT is released.
A similar picture is seen in the small intestine where mucosal 5-HT does not initiate propulsive motor activity evoked by distension, but may facilitate this activity (Tuladhar et al., 1997). Moreover, the mechanisms by which mucosally released 5-HT activates different motor patterns in the small intestine of the guinea-pig appear to be complex. Mucosal 5-HT plays a key role in nutrient (decanoic acid) induced segmentation in guinea-pig small intestine as blocking either 5-HT3 or 5-HT4 receptors, at the level of the mucosa, virtually abolishes this motor pattern (Ellis et al., 2013). However, while luminal decanoic acid causes an increase in both segmenting and propulsive motor activity, luminal fluoxetine (100–300 nM) which releases mucosal 5-HT only triggers segmenting activity, again via 5-HT3 and 5-HT4 receptors (Ellis et al., 2013). On the other hand, cholera toxin, which would be expected to release mucosal 5-HT, increases propulsive motor activity at resting intraluminal pressures and enhances propulsive motor patterns evoked by distension, each via mechanism(s) independent of 5-HT3 receptors (Fung et al., 2010).
It has been reported that propulsive motor activity evoked by saline distension is enhanced by luminal 5-HT via 5-HT3 receptors close to, if not in, the mucosal epithelium and that 5-HT4 receptors are not required (Tuladhar et al., 1997). In contrast, local inhibitory reflexes evoked by amino acids transiently applied to the mucosa are depressed by blockade of both 5-HT3 and 5-HT4 receptors, but not blockade of either subtype on its own (Gwynne and Bornstein, 2007). Furthermore, 5-HT applied via the serosa can enhance saline evoked propulsive motor patterns via 5-HT4 receptors, while EC cells have been shown to express 5-HT4 receptors (Hoffman et al., 2012). Whether 5-HT3 and 5-HT4 mediated effects of exogenous 5-HT are independent of each other or are part of the same pathway is unclear, as is their relevance to the roles of endogenous 5-HT. This study was designed to address these issues.
Electrophysiological studies of guinea-pig ileum indicate that 5-HT applied to the mucosa excites the mucosal terminals of AH neurons with cell bodies in the myenteric plexus via 5-HT3 receptors (Bertrand et al., 1997, 2000; Bertrand and Bornstein, 2002). Mucosally applied 5-HT also evokes slow excitatory synaptic potentials (EPSPs) in myenteric AH neurons (Bertrand et al., 1997, 2000) presumably via synapses from directly activated AH neurons. These slow EPSPs would be expected to increase the firing of distension-sensitive AH neurons, thus enhancing reflexes evoked by distension. Most slow EPSPs in myenteric AH neurons are blocked by the specific NK3 tachykinin receptor antagonist SR 142801 (Alex et al., 2001; Johnson and Bornstein, 2004) which suggests that luminally applied 5-HT may facilitate propulsive reflexes in the guinea-pig ileum via the release of a tachykinin. Accordingly, we also investigated this possibility.
Propulsive motor activity was studied using a modified Trendelenberg preparation in which the stimulus was a saline distension; agonists and antagonists were added to the luminal perfusion solution. The concentration of 5-HT used was 30 nM as preliminary experiments indicated that this was sufficient to facilitate propulsive reflexes. The time of exposure was over 1 h to mimic the effects of a nutrient stimulus that might release 5-HT (Gwynne et al., 2004; Ellis et al., 2013). The effects of exogenous 5-HT were compared with those of endogenous 5-HT whose effects were revealed by a selective serotonin reuptake inhibitor, fluoxetine.
Materials and Methods
Tissue Preparation
Guinea-pigs (150–350 g) of either sex were killed by being stunned and having their carotid arteries severed. This procedure was approved by the University of Melbourne Animal Experimentation Ethics Committee. The composition (in mM) of physiological saline used in all experiments was: NaCl 118, KCl 4.8, NaH2PO4 1, NaHCO3 25, MgSO4 1.2, d-glucose 11, CaCl2 2.5, bubbled with 95% O2, 5% CO2. Segments of ileum 8–10 cm in length, were taken 10–20 cm proximal to the ileo-caecal junction, flushed clean, cannulated, and placed in an organ bath containing physiological saline at 37°C. Intraluminal pressure was measured via a T-piece connected to the anal cannula and recorded using a BIOPAC Systems MP100 recording unit and Acknowledge v. 3.25 software (SDR Clinical technology, NSW). In some experiments, video images of the intestinal segment were recorded and later processed using edge-detection software to produce spatio-temporal maps. These methods have previously been described in Gwynne et al. (2004) and confirmed that the recorded pressure increases corresponded to a propulsive motor pattern.
Experimental Protocols
Once the ileal segments had been dissected and cannulated, 10 mL of the physiological saline was flushed through the lumen and the preparation left to equilibrate for 1 h. During the equilibration period, the height of the saline in the reservoir was level with the segment of ileum, so the inflow pressure head was held to zero, below the threshold for initiation of propulsive contractions. Following equilibration, the pressure threshold for initiation of persistent propulsive contractions (measured as repeated transient increases in intraluminal pressure, see Gwynne et al., 2004 for definition) was determined by raising the inflow pressure in steps of 1 cm H2O at intervals of approximately 30 s, until this motor pattern was initiated. The inflow pressure was then returned to control levels. The pressure which triggered the persistent propulsive contractions was consistent for several hours in any one preparation and was taken as the threshold for propulsive motor activity (or peristalsis) (Gwynne et al., 2004). After each trial, the height of the inflow reservoir was returned to the starting level and the preparation was allowed to rest for 10 min. After 3–4 control measurements, an agonist (or fluoxetine) was added to the inflow reservoir and the drug was flushed into the lumen. The preparation was allowed to equilibrate for 1 h before a second set of 3 threshold measurements was recorded. When the effects of antagonists were studied after the initial exposure to an agonist, approximately 30 mL of fresh saline was flushed through the lumen and left to equilibrate for 20 min before a combination of agonist and antagonist was applied luminally. After a further 1 h equilibration period, a final set of 3 threshold measurements was made. Each antagonist was also tested by itself using the protocol for agonists. Where video recordings were made, images were acquired at a frame rate of 10 frames per second for the duration of the threshold measurement.
Data in the text is given as mean ± s.e.m. except where otherwise stated.
Statistical comparisons were made using paired t-tests or One Way analysis of variance (ANOVA); P-values < 0.05 were considered significant.
Drugs
Drugs used were 5-HT, fluoxetine, 2-methyl-5-hydroxytryptamine (2-me-5-HT) (all from Sigma Aldrich, NSW), RS 67506 (Tocris Cookson Ltd. Bristol, UK), tetrodotoxin (Alomone Labs Ltd. Jerusalem, Israel), granisetron, SB 207266 (both supplied by Glaxo SmithKline, Harlow, UK), and SR 142801 (supplied courtesy of Dr. X. Emonds-Alt, Sanofi Recherche, France). All were initially made up in distilled water to make stock solutions. To avoid oxidation, new 5-HT solutions were made up each week.
Results
Figure 1 shows an example of the propulsive contractions evoked by increasing the intraluminal pressure to threshold. Step-wise increases in intraluminal pressure to levels below the threshold for persistent propulsive contractions sometimes evoked one or more propagating contractions of the circular muscle (not shown here). We have previously described these as “transient propulsive contractions” (Gwynne et al., 2004). Threshold pressures that evoked consistent propulsive contractions ranged from 4 to 8 cm H2O (median 6.5 cm H2O) in 102 preparations. When the threshold was reached, the propulsive contractions were regular and persisted throughout the period of increased luminal pressure, ceasing immediately the pressure was reduced below the threshold (Figure 1). Luminal 5-HT (30 nM) and luminal fluoxetine (1 nM) reduced the threshold for the persistent propulsive contractions by 25 and 40%, respectively (Figures 2A,B; 5-HT −P < 0.01, n = 6, fluoxetine −P < 0.001, n = 6). In each case, the effects reversed on washout of the agonist. The changes in threshold were accompanied by a statistically significant increase in the rate of propagation of the propulsive contractions measured via spatiotemporal maps (control 21.6 ± 1.1 mm/s, 5-HT 30.4 ± 1.4 mm/s, n = 6, P < 0.001; control 14.5 ± 1.0 mm/s, fluoxetine 20.2 ± 1.3 mm/s, n = 4, P < 0.01) and a significant reduction in the diameter of the ileum at the threshold pressure (control 3.2 ± 0.02 mm, 5-HT 2.8 ± 0.01 mm, P < 0.05, n = 4; control 3.0 ± 0.02 mm, fluoxetine 2.4 ± 0.01 mm, P < 0.05, n = 4).
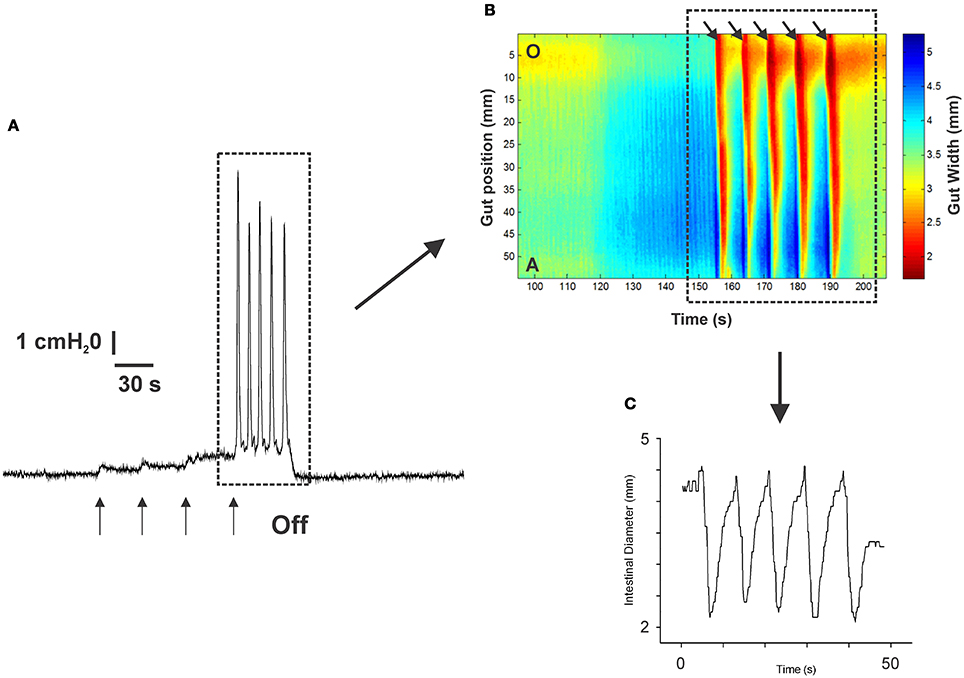
Figure 1. The propulsive motor pattern (peristalsis) in an isolated segment of guinea-pig ileum. The segment was distended in cumulative 1 cm H2O steps by raising the lumenal perfusion reservoir, which caused phasic increases in intraluminal pressure (A). (B) The corresponding spatio-temporal map of the propulsive contractions (black arrows) recorded at threshold. Contraction is shown in red and dilation in blue. (C) The diameter changes occurring at one point along the intestinal segment during the propulsive contractions (see dotted black line in B) at threshold. The pressure at which this motor pattern was initiated was highly reproducible in any one preparation.
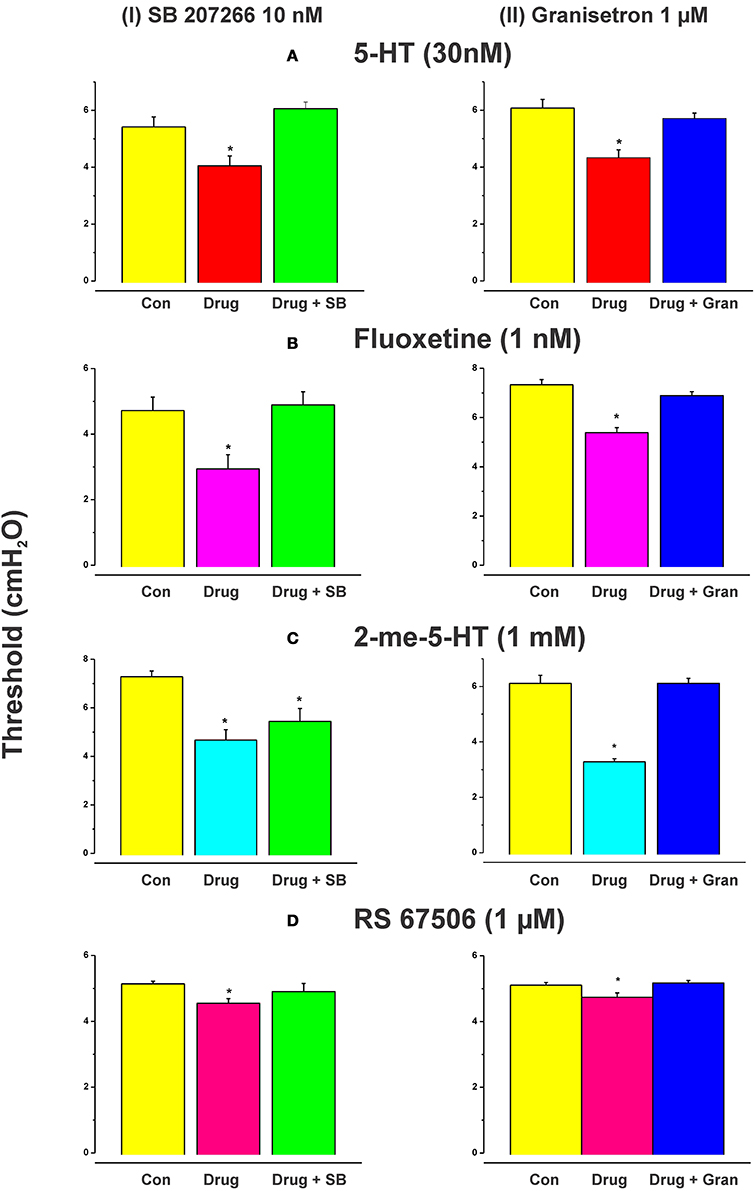
Figure 2. Effects of exogenous 5-HT, endogenous 5-HT potentiated by fluoxetine, 2-me-5-HT, and RS 67506 on the threshold pressure for initiation of propulsive contractions. Histograms showing the mean (±s.e.m.) thresholds in cm H2O for initiation of peristalsis in control, with the drug present or with drug and antagonist present. 5-HT (30 nM), fluoxetine (1 nM), 2-me-5-HT (1 mM), and RS 67506 (1 μM) each reversibly reduced the threshold for initiation of propulsive motor patterns in the ileum when applied to the lumen. The reduction in threshold was significant in each case [(A) 5-HT, P < 0.01; (B) fluoxetine, P < 0.01, 2-me-5-HT, P < 0.0001, RS 67506, P < 0.01]. The histograms in the left column show the effects of the 5-HT4 receptor antagonist SB 207266 (10 nM) in the lumenal perfusate on the changes in threshold for initiation of propulsive motor patterns in the ileum induced by 5-HT (A), fluoxetine (B), 2-me-5-HT (C), or RS 67506 (D). The right column shows the effects of the 5-HT3 receptor antagonist granisetron (1 μM) on the changes induced by the same compounds. SB 207266 blocked the facilitation caused by luminal 5-HT, fluoxetine, and RS 67506 [panels A(I),B(I),D(I)] but did not abolish the effects of 2-me-5-HT [panel C(I)]. In contrast, granisetron blocked the facilitation of peristalsis produced by all of the agonists tested [A(II),B(II),C(II),D(II)]. *indicates significantly different from control with P < 0.05 in all cases.
Higher concentrations of fluoxetine (100 and 300 nM) in the lumen had no significant effect on the threshold pressure for the persistent propulsive contractions (control 4.8 ± 0.1 100 nM fluoxetine 4.8 ± 0.1 cm H2O; control 4.6 ± 0.1, 300 nM fluoxetine 4.7 ± 0.1 cm H2O) although they did produce an increase in contractile activity at basal pressures. The effects of luminal 5-HT were mimicked by the specific 5-HT3 receptor agonist 2-me-5-HT (1 mM, n = 6), and by a specific 5-HT4 receptor agonist RS 67506 (1 μM, n = 6), which reduced the pressure threshold for propulsive contractions by 35% (P < 0.0001) and 12% (P < 0.01), respectively (Figures 2C,D). As with fluoxetine, higher concentrations of RS 67506 (3 or 10 μM) produced no apparent change in threshold for activation of persistent propulsive contractions, but increased the overall contractile activity at basal pressures. In each case, the increased contractile activity at basal pressures involved predominantly segmenting contractions (see Figure 1 of Ellis et al., 2013).
When added to the luminal perfusion solution, a highly specific 5-HT4 receptor antagonist SB 207266 (10 nM) (Wardle et al., 1996) blocked the facilitation caused by luminal 5-HT, fluoxetine, and RS 67506 [Figures 2A(I),B(I),D(I), all n = 6] but did not alter the effects of 2-me-5-HT [Figure 2C(I), n = 6]. In contrast, a specific 5-HT3 receptor antagonist granisetron (1 μM) (29) blocked the facilitation of persistent propulsive contractions produced by all agonists tested. (5-HT n = 8, fluoxetine n = 6, RS 67506 n = 6 and 2-me-5-HT n = 6) [Figures 2A(II),B(II),C(II),D(II)].
SB 207266 (10 nM) and granisetron (1 μM) when added to the luminal perfusate on their own, or in combination, had no effect on the threshold pressure for initiation of persistent propulsive contractions (Figure 4, n = 6 in each case).
Role of NK3 Tachykinin Receptors
The effects of luminal 5-HT, fluoxetine, and 2-me-5HT were all prevented by simultaneous addition of the specific NK3 tachykinin receptor antagonist SR 142801 (100 nM) to the luminal perfusate (Figure 3, each n = 6, RS 67506 not tested). SR 142801 had no effect on the threshold pressure when added to the luminal perfusate on its own (Figure 4D, n = 6) or when added to the superfusing solution (not illustrated).
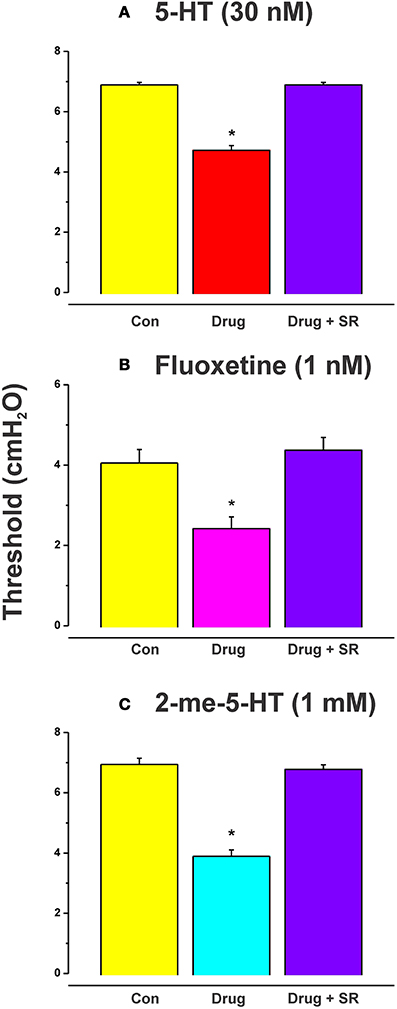
Figure 3. Effects of blockade of NK3 tachykinin receptors on the reduction in threshold for initiation of propulsion evoked by serotonin agonists. The specific NK3 tachykinin receptor antagonist SR 142801 (SR; 100 nM) blocked the reduction in threshold for initiation of propulsive contractions in the ileum induced by 5-HT (A), fluoxetine (B), and 2-me-5-HT (C). *Indicates significantly different from control with P < 0.05 in all cases.
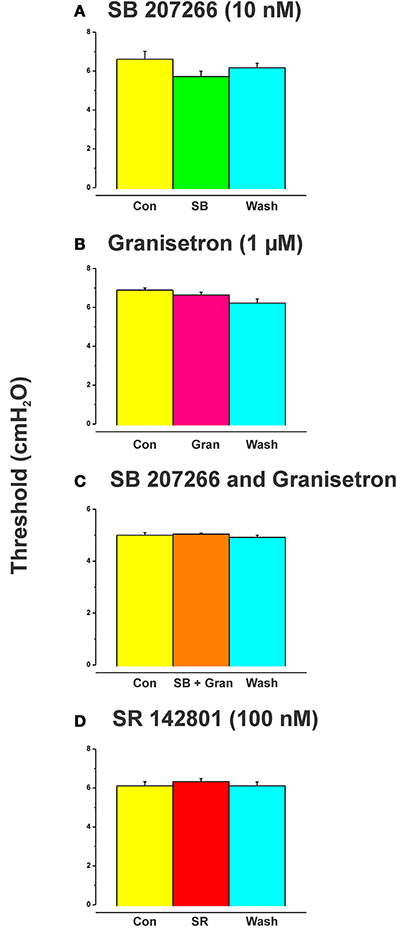
Figure 4. Effects of blockade of 5-HT4, 5-HT3, and NK3 receptors on the threshold for initiation of propulsion in the absence of other drugs. SB 207266 and granisetron when added to the luminal perfusate on their own, or in combination, had no effect on the threshold pressure for initiation of propulsive contractions (A–C). The specific NK3 tackykinin receptor antagonist SR 142801 (100 nM) also had no effect on the threshold pressure for propulsive contractions (D).
Discussion
This study indicates that perfusion of exogenous 5-HT through the lumen, or increases in release of endogenous mucosal 5-HT above basal levels, facilitates intestinal propulsion evoked by saline distension of the ileum via activation of 5-HT4 and 5-HT3 receptors and the release of tachykinins acting at NK3 receptors. We found no evidence that basal release of mucosal 5-HT was sufficient to activate these receptors.
Site of Action of 5-HT
Figure 5 illustrates the sites of action and locations of receptors that are deduced from this study. Facilitation of propulsion by 5-HT or fluoxetine was reversed by either the 5-HT4 receptor antagonist, SB 207266 or the 5-HT3 receptor antagonist, granisetron. Furthermore, luminal application of the specific 5-HT3 receptor agonist 2-me-5-HT or 5-HT4 receptor agonist RS 67506 mimicked the effects of 5-HT and fluoxetine. This indicates that the mechanism underlying the facilitation occurs via activation of both 5-HT3 and 5-HT4 receptors in this system. These data contrast with previous research by Tuladhar et al. (1997) who found that mucosally applied 5-HT facilitates propulsive reflexes via activation of 5-HT3 receptors, but they found no evidence for 5-HT4 receptor involvement. One reason for this difference is likely to be the marked differences in concentration of 5-HT used in the two studies. We observed facilitation with 30 nM 5-HT, three orders of magnitude lower than the lowest effective concentration used by Tuladhar et al. (1997). Previous studies have indicated that 5-HT3 receptors in guinea-pig ileum are unlikely to be directly activated by 30 nM 5-HT. For example, Lucchelli et al. (1995) found that at least 300 nM 5-HT is needed to produce minimal activation of 5-HT3 receptors on neurons in this tissue. Thus, the concentrations used by Tuladhar et al. would directly excite 5-HT3 receptors in a manner analogous to 2-me-5-HT in the present study, but 30 nM 5-HT might only activate the receptors indirectly, for example by releasing mucosal 5-HT. The initial action of luminal 5-HT is probably at a receptor with a much higher affinity for 5-HT. The EC50 for 5-HT4 receptors in this tissue is 15 nM (Buchheit et al., 1985), which is consistent with these receptors being the targets of low concentrations of 5-HT applied in the lumen. This was demonstrated directly in the present study, since SB 207266 blocked the effects of both 5-HT and RS 67506. Interestingly, granisetron reversed the facilitation induced by both RS 67506 and 2-me-5-HT, whereas SB 207266 had no effect on the facilitation induced by 2-me-5-HT. That is, 5-HT3 receptors are required when 5-HT4 receptors facilitate peristalsis, but not the reverse. This implies that the two types of receptor are arranged in series with 5-HT4 receptors preceding 5-HT3 receptors in the pathway (Figure 5). Both classes of 5-HT receptors are widely expressed within the enteric neural circuitry, but the efficacy of the luminally applied antagonists suggests that the receptors for facilitation of propulsion are in, or close, to the mucosa. Evidence for this conclusion comes from the finding that these antagonists have differing effects on motility when they are applied to the lumen or to the serosa in modified Trendelenberg preparations (Tuladhar et al., 1997; Ellis et al., 2013). The most likely mechanism is that low concentrations of 5-HT in the lumen act via 5-HT4 receptors on EC cells to release enough 5-HT to raise the mucosal concentration to a level capable of exciting 5-HT3 receptors (Figure 5). Guinea-pig EC cells are immunoreactive for 5-HT4 receptors and activation of these receptors leads to release of 5-HT (Hoffman et al., 2012). The 5-HT3 receptors involved in the facilitation of propulsion by luminal 5-HT are probably those on mucosal nerve terminals of myenteric AH neurons that have been found to be excited by mucosal application of 5-HT (Bertrand et al., 2000).
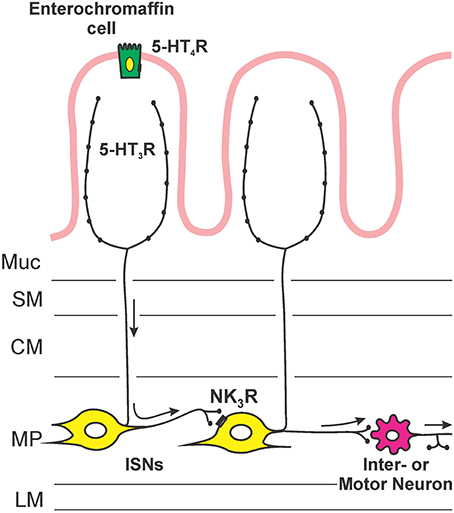
Figure 5. An hypothesis about the sequence of mechanisms by which exogenous or endogenous 5-HT in the lumen enhances propulsion in the guinea-pig ileum. 5-HT acts on 5-HT4 receptors on enterochromaffin cells to release more 5-HT, this higher concentration of 5-HT then excites mucosal terminals of myenteric AH neurons via 5-HT3 receptors. The myenteric AH neurons then release tachykinins to enhance the excitability of distension-sensitive myenteric AH neurons via NK3 receptors.
Role of NK3 Tachykinin Receptors
The facilitation of peristalsis induced by 5-HT, fluoxetine, and 2-me-5-HT was blocked by the specific NK3 tachykinin antagonist, SR 142801. This indicates that the release of a tachykinin, and its subsequent interaction with NK3 receptors, plays a critical role in the facilitatory pathway. NK3 tachykinin receptors are found on many neurons in the guinea-pig enteric nervous system, including myenteric AH neurons (Jenkinson et al., 1999) and are implicated in both ascending and descending reflex pathways in the guinea-pig ileum (Johnson et al., 1996, 1998). However, their role in reflexes in dissected flat sheet preparations is largely confined to responses to mucosal distortion, while reflex responses to distension are unaffected by blockade of these receptors (Johnson et al., 1998). This suggests that NK3 receptors are involved in the circuitry underlying the facilitation of distension by luminal 5-HT, but not in reflex pathways activated by distension. Slow EPSPs mediated by NK3 tachykinin receptors are prominent in myenteric AH neurons (Alex et al., 2001; Johnson and Bornstein, 2004). Furthermore, computer simulations show that slow synaptic transmission within the circumferentially projecting network of connections between myenteric AH neurons is important for determining the magnitudes of responses to ongoing physiological stimuli (Thomas et al., 2000, 2004). This raises the possibility that excitation of myenteric AH neurons by luminal 5-HT leads to activation of a recurrent feedback circuit involving slow transmission (via NK3 receptors) between myenteric AH neurons. This could in turn generate enhanced responsiveness to sensory stimuli such as distension, i.e., facilitation of propulsion.
This leads us to postulate that low concentrations of 5-HT act on 5-HT4 receptors on EC cells in the mucosa, releasing 5-HT that then activates 5-HT3 receptors on mucosal terminals of myenteric AH neurons. Higher concentrations of 5-HT in the lumen (Tuladhar et al., 1997) or 5-HT3 agonists like 2-me-5-HT act directly on the mucosal nerve endings of myenteric AH neurons, bypassing the requirement for activation of 5-HT4 receptors. The activation of myenteric AH neurons leads to the release of tachykinins acting at NK3 receptors on other myenteric AH neurons, thereby enhancing their overall excitability and making them more sensitive to the saline distensions used to excite peristalsis (Figure 4).
Mechanism of Action of Fluoxetine
The similar sensitivity of responses to 5-HT4 antagonists, and the fact that fluoxetine is a specific inhibitor of 5-HT reuptake, suggests that 1 nM fluoxetine acts to increase the extracellular concentration of 5-HT in the intestinal mucosa enough to activate 5-HT4 receptors in a similar way to 30 nM 5-HT. This could also account for the enhancement by 1 nM fluoxetine of propulsion of artificial fecal pellets in the guinea-pig distal colon (Wade et al., 1996). However, higher concentrations of fluoxetine (100 and 300 nM) had no effect on the initiation of propulsive motor patterns, and Wade et al. (1996) found that such concentrations of fluoxetine inhibited colonic transit (a result we have confirmed in an unpublished study). Thus, while low concentrations of fluoxetine facilitate propulsive motor patterns in both ileum and colon, higher concentrations do not.
The concentration dependence may result from the properties of the 5-HT transporter itself. Chen et al. (1998) found that the Ki for fluoxetine on a cloned guinea-pig serotonin transporter was about 48 nM. That is, fluoxetine depresses reuptake of 5-HT at concentrations comparable to those that inhibit propulsive motor patterns in the colon and has very little effect at concentrations that facilitate ileal or colonic transit. Thus, increases in extracellular 5-HT produced by low concentrations of fluoxetine may be highly localized, with uninhibited transporters limiting spread of 5-HT beyond the vicinity of the mucosal terminals of enteric neurons. At higher concentrations, transporter capacity might be reduced sufficiently to allow diffusion of 5-HT to more distant sites where it can interact with inhibitory 5-HT1A receptors (Galligan et al., 1988), thereby suppressing the facilitation of peristalsis or even reversing it.
Role of Mucosal 5-HT
There is no doubt that release of mucosal 5-HT plays a major role in the nausea and vomiting produced by ingestion of various toxins (Sanger and Andrews, 2006) and the hypersecretion leading to diarrhea produced by luminal exposure to either the bacterium Vibrio cholera or its exotoxin, cholera toxin (Farthing, 2002; Lundgren, 2002). However, other physiological roles of mucosal 5-HT are significantly less clear. The present data strongly indicate that mucosal 5-HT is not necessary for distension to evoke propulsive motor activity. Both the absence of an effect of luminal 5-HT3 or 5-HT4 antagonists on their own on the threshold pressure for distension-evoked propulsion and previous results showing that such reflexes can be evoked in the absence of the mucosa (Costa and Furness, 1976; Yokoyama and Ozaki, 1980; Tsuji et al., 1992; Spencer et al., 2011) or mucosal 5-HT (Heredia et al., 2013) support this conclusion. This contrasts with the finding that nutrient-induced segmentation is largely suppressed by 5-HT3 or 5-HT4 antagonists in the lumen of the small intestine (Ellis et al., 2013). Both physiological data and computer simulations suggest that the initial sensory transduction step in nutrient-induced segmentation is mediated by both cholecystokinin (CCK) and 5-HT, with mucosal 5-HT also playing a role in providing feedback to enter neural motor pattern generators about the overall contractile state of the muscle (Chambers et al., 2011; Ellis et al., 2013). It has recently been demonstrated that a specific subset of enteroendocrine cells contains both CCK and 5-HT, while there are others that contain only one of these mediators (Cho et al., 2014). Thus, the physiological role of mucosal 5-HT may vary according to the stimulus that evokes its release. Chemical stimuli, different nutrients, and other food components, might be expected to produce moderate levels of release in various combinations with other mucosal mediators, while contractile activity appears to release 5-HT via mechanical deformation of the mucosa (Bertrand, 2006).
Conclusions
This study proposes a way in which 5-HT facilitates propulsive contractions in the guinea-pig small intestine. Increased availability of 5-HT at low concentrations in the mucosa of the intestine activates high affinity 5-HT4 receptors, which triggers the release of more 5-HT. This then acts on 5-HT3 receptors to excite enteric neural circuits, likely to involve release of tachykinins that facilitate propulsive reflexes.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by SmithKline Beecham, Australia and grants from the National Health and Medical Research Council of Australia (114103, 56642).
References
Alex, G., Kunze, W. A., Furness, J. B., and Clerc, N. (2001). Comparison of the effects of neurokinin-3 receptor blockade on two forms of slow synaptic transmission in myenteric AH neurons. Neuroscience 104, 263–269. doi: 10.1016/S0306-4522(01)00064-1
Bertrand, P. P. (2006). Real-time measurement of serotonin release and motility in guinea pig ileum. J. Physiol. 577, 689–704. doi: 10.1113/jphysiol.2006.117804
Bertrand, P. P., and Bornstein, J. C. (2002). ATP as a putative sensory mediator: activation of intrinsic sensory neurons of the myenteric plexus via P2X receptors. J. Neurosci. 22, 4767–4775.
Bertrand, P. P., Kunze, W. A., Bornstein, J. C., Furness, J. B., and Smith, M. L. (1997). Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am. J. Physiol. 273, G422–G435.
Bertrand, P. P., Kunze, W. A., Furness, J. B., and Bornstein, J. C. (2000). The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 101, 459–469. doi: 10.1016/S0306-4522(00)00363-8
Buchheit, K. H., Engel, G., Mutschler, E., and Richardson, B. (1985). Study of the contractile effect of 5-hydroxytryptamine (5-HT) in the isolated longitudinal muscle strip from guinea-pig ileum. Evidence for two distinct release mechanisms. Naunyn Schmiedebergs Arch. Pharmacol. 329, 36–41. doi: 10.1007/BF00695189
Chambers, J. D., Bornstein, J. C., and Thomas, E. A. (2011). Multiple neural oscillators and muscle feedback are required for the intestinal fed state motor program. PLoS ONE 6:e19597. doi: 10.1371/journal.pone.0019597
Chen, J. X., Pan, H., Rothman, T. P., Wade, P. R., and Gershon, M. D. (1998). Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am. J. Physiol. 275, G433–G448.
Cho, H. J., Callaghan, B., Bron, R., Bravo, D. M., and Furness, J. B. (2014). Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res. 356, 77–82. doi: 10.1007/s00441-013-1780-x
Costa, M., and Furness, J. B. (1976). The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch. Pharmacol. 294, 47–60. doi: 10.1007/BF00692784
Ellis, M., Chambers, J. D., Gwynne, R. M., and Bornstein, J. C. (2013). Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G749–G761. doi: 10.1152/ajpgi.00358.2012
Farthing, M. J. (2002). Novel targets for the control of secretory diarrhoea. Gut 50(Suppl. 3), III15–III18. doi: 10.1136/gut.50.suppl_3.iii15
Fung, C., Ellis, M., and Bornstein, J. C. (2010). Luminal cholera toxin alters motility in isolated guinea-pig jejunum via a pathway independent of 5-HT(3) receptors. Front. Neurosci. 4:162. doi: 10.3389/fnins.2010.00162
Galligan, J. J., Surprenant, A., Tonini, M., and North, R. A. (1988). Differential localization of 5-HT1 receptors on myenteric and submucosal neurons. Am. J. Physiol. 255, G603–G611.
Grider, J. R., Kuemmerle, J. F., and Jin, J. G. (1996). 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am. J. Physiol. 270, G778–G782.
Gwynne, R. M., and Bornstein, J. C. (2007). Local inhibitory reflexes excited by mucosal application of nutrient amino acids in guinea pig jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1660–G1670. doi: 10.1152/ajpgi.00580.2006
Gwynne, R. M., Thomas, E. A., Goh, S. M., Sjovall, H., and Bornstein, J. C. (2004). Segmentation induced by intraluminal fatty acid in isolated guinea-pig duodenum and jejunum. J. Physiol. 556, 557–569. doi: 10.1113/jphysiol.2003.057182
Heredia, D. J., Gershon, M. D., Koh, S. D., Corrigan, R. D., Okamoto, T., and Smith, T. K. (2013). Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J. Physiol. 591, 5939–5957. doi: 10.1113/jphysiol.2013.256230
Hoffman, J. M., Tyler, K., Maceachern, S. J., Balemba, O. B., Johnson, A. C., Brooks, E. M., et al. (2012). Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142, 844.e4–854.e4. doi: 10.1053/j.gastro.2011.12.041
Jenkinson, K. M., Morgan, J. M., Furness, J. B., and Southwell, B. R. (1999). Neurons bearing NK(3) tachykinin receptors in the guinea-pig ileum revealed by specific binding of fluorescently labelled agonists. Histochem. Cell Biol. 112, 233–246. doi: 10.1007/s004180050411
Jin, J. G., Foxx-Orenstein, A. E., and Grider, J. R. (1999). Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J. Pharmacol. Exp. Ther. 288, 93–97.
Johnson, P. J., and Bornstein, J. C. (2004). Neurokinin-1 and -3 receptor blockade inhibits slow excitatory synaptic transmission in myenteric neurons and reveals slow inhibitory input. Neuroscience 126, 137–147. doi: 10.1016/j.neuroscience.2004.03.021
Johnson, P. J., Bornstein, J. C., and Burcher, E. (1998). Roles of neuronal NK1 and NK3 receptors in synaptic transmission during motility reflexes in the guinea-pig ileum. Br. J. Pharmacol. 124, 1375–1384. doi: 10.1038/sj.bjp.0701967
Johnson, P. J., Bornstein, J. C., Yuan, S. Y., and Furness, J. B. (1996). Analysis of contributions of acetylcholine and tachykinins to neuro-neuronal transmission in motility reflexes in the guinea-pig ileum. Br. J. Pharmacol. 118, 973–983. doi: 10.1111/j.1476-5381.1996.tb15495.x
Keating, D. J., and Spencer, N. J. (2010). Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 138, 659–670; 670.e1–670.e2. doi: 10.1053/j.gastro.2009.09.020
Li, Z., Chalazonitis, A., Huang, Y. Y., Mann, J. J., Margolis, K. G., Yang, Q. M., et al. (2011). Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 31, 8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011
Lucchelli, A., Santagostino-Barbone, M. G., Barbieri, A., Candura, S. M., and Tonini, M. (1995). The interaction of antidepressant drugs with central and peripheral (enteric) 5-HT3 and 5-HT4 receptors. Br. J. Pharmacol. 114, 1017–1025. doi: 10.1111/j.1476-5381.1995.tb13307.x
Lundgren, O. (2002). Enteric nerves and diarrhoea. Pharmacol. Toxicol. 90, 109–120. doi: 10.1034/j.1600-0773.2002.900301.x
Sanger, G. J., and Andrews, P. L. (2006). Treatment of nausea and vomiting: gaps in our knowledge. Auton. Neurosci. 129, 3–16. doi: 10.1016/j.autneu.2006.07.009
Spencer, N. J., Nicholas, S. J., Robinson, L., Kyloh, M., Flack, N., Brookes, S. J., et al. (2011). Mechanisms underlying distension-evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells? Am. J. Physiol. Gastrointest. Liver Physiol. 301, G519–G527. doi: 10.1152/ajpgi.00101.2011
Thomas, E. A., Bertrand, P. P., and Bornstein, J. C. (2000). A computer simulation of recurrent, excitatory networks of sensory neurons of the gut in guinea-pig. Neurosci. Lett. 287, 137–140. doi: 10.1016/S0304-3940(00)01182-4
Thomas, E. A., Sjovall, H., and Bornstein, J. C. (2004). Computational model of the migrating motor complex of the small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G564–G572. doi: 10.1152/ajpgi.00369.2003
Tsuji, S., Anglade, P., Ozaki, T., Sazi, T., and Yokoyama, S. (1992). Peristaltic movement evoked in intestinal tube devoid of mucosa and submucosa. Jpn. J. Physiol. 42, 363–375. doi: 10.2170/jjphysiol.42.363
Tuladhar, B. R., Kaisar, M., and Naylor, R. J. (1997). Evidence for a 5-HT3 receptor involvement in the facilitation of peristalsis on mucosal application of 5-HT in the guinea pig isolated ileum. Br. J. Pharmacol. 122, 1174–1178. doi: 10.1038/sj.bjp.0701503
Wade, P. R., Chen, J., Jaffe, B., Kassem, I. S., Blakely, R. D., and Gershon, M. D. (1996). Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J. Neurosci. 16, 2352–2364.
Wardle, K. A., Bingham, S., Ellis, E. S., Gaster, L. M., Rushant, B., Smith, M. I., et al. (1996). Selective and functional 5-hydroxytryptamine4 receptor antagonism by SB 207266. Br. J. Pharmacol. 118, 665–670. doi: 10.1111/j.1476-5381.1996.tb15452.x
Keywords: serotonin, 5-HT3 receptors, 5-HT4 receptors, NK3 tachykinin receptors, intestinal motility
Citation: Gwynne RM, Clarke AJ, Furness JB and Bornstein JC (2014) Both exogenous 5-HT and endogenous 5-HT, released by fluoxetine, enhance distension evoked propulsion in guinea-pig ileum in vitro. Front. Neurosci. 8:301. doi: 10.3389/fnins.2014.00301
Received: 02 July 2014; Paper pending published: 01 August 2014;
Accepted: 03 September 2014; Published online: 19 September 2014.
Edited by:
Jack Grider, Virginia Commonwealth University, USAReviewed by:
Nicholas Spencer, Flinders University, AustraliaXiaochun Bian, Michigan State University, USA
Copyright © 2014 Gwynne, Clarke, Furness and Bornstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel M. Gwynne, Department of Physiology, University of Melbourne, Medical Building, Cnr Grattan St. and Royal Pde., Parkville, VIC 3010, Australia e-mail: rgwynne@unimelb.edu.au
 Rachel M. Gwynne
Rachel M. Gwynne Amanda J. Clarke1
Amanda J. Clarke1 John B. Furness
John B. Furness Joel C. Bornstein
Joel C. Bornstein