Unwinding the molecular basis of interval and circadian timing
- 1 Laboratorio de Cronobiología, Departamento de Ciencia y Tecnología, Universidad Nacional de Quilmes, Buenos Aires, Argentina
- 2 Department of Psychology and Neuroscience, Duke University, Durham, NC, USA
Neural timing mechanisms range from the millisecond to diurnal, and possibly annual, frequencies. Two of the main processes under study are the interval timer (seconds-to-minute range) and the circadian clock. The molecular basis of these two mechanisms is the subject of intense research, as well as their possible relationship. This article summarizes data from studies investigating a possible interaction between interval and circadian timing and reviews the molecular basis of both mechanisms, including the discussion of the contribution from studies of genetically modified animal models. While there is currently no common neurochemical substrate for timing mechanisms in the brain, circadian modulation of interval timing suggests an interaction of different frequencies in cerebral temporal processes.
Introduction
Timing is crucial to all aspects of our lives. Indeed, biological timing includes diverse time-related mechanisms that encompass several orders of magnitude (Hinton and Meck, 1997; Buhusi and Meck, 2005, 2009b; Buonomano and Laje, 2010). Besides interval timing (in the seconds-to-minutes range), most – if not all – organisms exhibit daily and circadian rhythms with periods of ca. 24 h, which also serve as the basis for seasonal-encoding mechanisms and might be related to lifespan-related processes. In particular, timing oscillators in the fast (seconds–minutes) and medium (circadian) frequencies might share some properties, including common steps in molecular pathways that lead to the neurochemical basis of such mechanisms. There is evidence suggesting that circadian pacemakers may influence the rate of the interval timer; however, these relationships have not been elucidated, neither at the behavioral nor the molecular level. The major terms relevant to this discussion are defined in the glossary provided in Table 1.
Circadian Timing
The circadian clock is a self-sustained biological oscillator with a period close to 24 h in constant conditions. Circadian clocks in nature are, however, rarely subjected to the constant conditions that allow a free-running oscillation. On the contrary, they are normally exposed to a rhythmic environment, so that appropriate signals (called Zeitgebers, from German Zeit, “time”; geben, “to give”), such as light, temperature, or food, synchronize its oscillation (Golombek and Rosenstein, 2010). Thus, the circadian system consists of three main components: (i) an input pathway integrating external signals to adjust circadian phase and period, (ii) a central oscillator that generates the circadian signal, and (iii) an output pathway driving circadian periodicity of biological processes as illustrated in Figure 1A. Nevertheless, entrainment of the endogenous clock is not the only mechanism controlling the output rhythm. Most Zeitgebers not only entrain circadian rhythms by controlling the phase and period of the pacemaker, but also affect them directly; as a result, they “mask” the behavior of the pacemaker. Masking signals are able to bypass the central oscillator and to directly affect physiology and behavior (Mrosovsky, 1999). There could also be an adjustment of the rate of cycling by neural or endocrine output signals, which define a feedback pathway from rhythms to the clock. This behavioral feedback occurs, for example, with spontaneous locomotor activity (Mistlberger and Holmes, 2000).
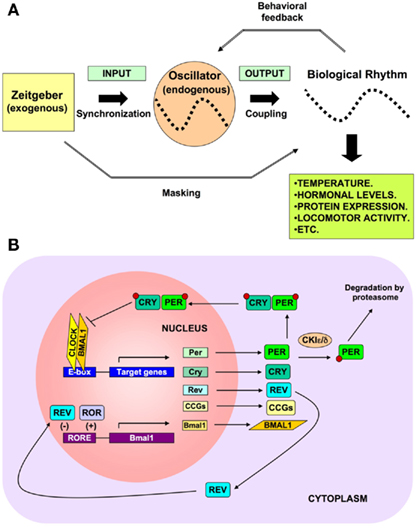
Figure 1. (A) Main components of the circadian timing system. Circadian rhythms consists of three main components: (i) an input pathway integrating exogenous signals to synchronize circadian phase and period, (ii) a central oscillator that generates the endogenous circadian signal, and (iii) an output pathway driving circadian periodicity and coupling of biological processes. (B) Molecular mechanisms of circadian timing. The molecular mechanisms of circadian rhythms can be illustrated by the transcription of the Period (Per1and Per2) and Cryptochrome (Cry1, Cry2) genes that are activated by heteromeric complexes containing CLOCK and BMAL1 proteins that act through the E-box regulatory sequences of their target genes. The newly synthesized PER and CRY proteins are translocated into the nucleus, where they inhibit BMAL1–CLOCK activity, and therefore, their own transcription. Clock and Bmal1 both contain basic helix–loop–helix (bHLH) motifs for DNA binding at their N-terminus and Per–Arnt–Sim (PAS) domains. The controlled degradation of PER and CRY proteins by the ubiquitin pathway decreases their protein levels and results in an oscillation of their mRNA and protein levels. During this negative transcriptional feedback loop many of the clock proteins become posttranslationally modified by phosphorylation and ubiquitination (Reppert and Weaver, 2002). This core oscillation is augmented and stabilized by a secondary loop involving two orphan nuclear receptor proteins, REV-ERBα and RORA. Both are activated in phase with the Per and Cry genes by CLOCK and BMAL1, but in turn they affect Bmal1 expression (Preitner et al., 2002). While RORA has a positive role, REV-ERBα is a suppressor of Bmal1, and they coordinate action through RORE regulatory sequences. A positive feedback loop is built by the stimulated transcription of BMAL1 by PER2. Protein phosphorylation events are essential contributors to these feedback loops. Two members of the casein kinase I family (CKIε and CKIδ) phosphorylate PER proteins in order to (i) target them for ubiquitin-mediated proteasomal degradation, and (ii) modulate their nuclear import. A mutation of CKIε shortens rhythm in hamsters (Lowrey et al., 2000) and a mutation of CKIδ shortens rhythm in humans (Xu et al., 2005). The result of these complex regulatory pathways is that the mRNA and protein levels of most circadian genes – except Clock and CKIε – oscillate with a 24-h period. Importantly, the CLOCK–BMAL1 heterodimer regulates the transcription of many clock-controlled genes (CCGs), which in turn influence a wide array of physiological functions external to the oscillatory mechanism. This mediates the output function of the clock, thereby controlling food intake, hormonal synthesis and release, body temperature, metabolism, and many other functions.
Molecular Mechanisms of Circadian Oscillation
The molecular mechanism of the endogenous circadian clock is comprised of interlocking feedback loops composed of cycling gene products that control transcription by means of negative and positive regulation of clock genes and proteins (Reppert and Weaver, 2002; Takahashi et al., 2008). Post-transcriptional regulation of clock proteins plays an important role in rhythm generation and entrainment; mutations in key protein kinases have been shown to affect the circadian machinery (Lowrey et al., 2000; Gallego and Virshup, 2007). This cycling molecular framework can also control the transcription of other genes by acting upon specific elements in their promoter regions, such as E-boxes.
In mammals, the transcription factors CLOCK and BMAL1 have been described as positive regulators whereas PERIOD (PER1 and 2) as well as CRYPTOCHROME (CRY1 and 2) proteins provide negative regulatory functions (Reppert and Weaver, 2002). The transcription of PER and CRY is stimulated by the CLOCK–BMAL1 heterodimer bound to the E-box enhancer as illustrated in Figure 1B. In turn, PER and CRY proteins are translocated into the nucleus, bind to the BMAL1–CLOCK heterodimer thereby inhibiting their own transcription. The controlled degradation of PER and CRY proteins by the ubiquitin pathway (signaled by phosphorylation through casein kinase Iε/δ) decreases their protein levels and contributes to the oscillation of their mRNA and protein levels. Other posttranslational regulations (e.g., acetylation) also undergo circadian changes (Hirayama et al., 2007). The consequences of protein modification include alterations in activity, subcellular localization, protein–protein interactions, and protein stability. Moreover, additional stabilizing feedback loops, including inhibition of Bmal1 transcription by REV-ERBα (Preitner et al., 2002) further contribute to the timing and robustness of the cycle.
The output of circadian rhythms is coordinated by the expression of another set of genes called clock-controlled genes (CCGs). The pathways that control circadian rhythmicity in mammals have been closely studied using genetically modified animals (see Table 2 for a description of the behavioral phenotypes of different mutant mice).
The Light-Entrainable Oscillator
In mammals, many daily physiological and behavioral rhythms are generated by a master pacemaker located in the suprachiasmatic nuclei (SCN) of the hypothalamus. The most powerful synchronizer or Zeitgeber known is the daily light/dark cycle which entrains and modulates the light-entrainable oscillator (LEO). Light stimulates a group of photosensitive retinal ganglion cells that contain the photopigment melanopsin (Panda et al., 2002) and project to the SCN through the retinohypothalamic tract. Glutamate and pituitary adenylate cyclase activating polypeptide (PACAP) are the primary neurotransmitters responsible for mediating the synchronizing properties of light, and act upon NMDA, AMPA/kainate receptors for glutamate, and the PACAP-specific receptor (PAC1). This leads to an increase of the intracellular concentrations of Ca2+, which initiates a signal transduction cascade in SCN neurons that ultimately results in a phase shift of the circadian system (Golombek et al., 2003, 2004; Morin and Allen, 2006; Golombek and Rosenstein, 2010). Moreover, the mGluR5 and mGluR2/3 metabotropic glutamate receptors have been shown to exert both positive and negative modulation of circadian activity rhythms as a function of the phase of the light/dark cycle (Gannon and Millan, 2011).
Exposure to light pulses at night synchronizes the LEO by inducing phase delays during the early night and phase advances during the late subjective night (i.e., when under constant conditions the animal behaves as if it were the night), led by diverse signal transduction pathways which ultimately rely on the activation of transcription factors such as CREB and clock genes (Lowrey and Takahashi, 2000). During the late night, when light induces phase advances of behavioral rhythms, photic stimulation specifically activates the guanylyl cyclase (GC)/cGMP/cGMP-dependent kinase (PKG) pathway (Golombek et al., 2004; Agostino et al., 2007). Therefore, the accessibility of specific signaling pathways is fundamental for regulation of circadian timing.
Food-Entrainable Oscillators
The discovery of clock gene expression in brain regions outside of the SCN has suggested the temporal control of motivated behaviors independent of such nuclei. In nocturnal rodents, for example, natural feeding occurs principally during the night. In experimental conditions, when access to food is restricted to a few hours during the day, animals become active in anticipation of mealtime. In response to food stimulation, there are also phase advances of the circadian rhythms of gene expression in the liver, kidney, heart, pancreas, and other tissues, as well as in some brain structures, uncoupling them from the control by the SCN whose entrainment to light remains intact (Mendoza, 2007). All these data suggest that peripheral clocks within and outside of the brain are affected by restricted feeding schedules (Feillet et al., 2006a; Balsam et al., 2009).
It has been shown that food-anticipatory activity (FAA) is still present in SCN-ablated animals (Stephan, 2002). FAA is expressed in wheel running, general activity, feeder approaches, and unreinforced bar pressing in an operant chamber. Moreover, some physiological parameters entrained to restricted feeding are still present after SCN lesions, suggesting the presence of an additional circadian oscillator. The food-entrainable oscillator (FEO) displays clear circadian characteristics. One of the most important of these is that its behavioral output (FAA) persists in the absence of food, suggesting that the FEO is able to generate a sustained free-running rhythm (Stephan, 2002).
The circadian mechanism of the FEO at the molecular level is not clear. Moreover, mice with mutations of clock genes are able to entrain activity rhythms to restricted feeding, suggesting there are alternative molecular pathways related to this kind of non-photic entrainment (Mendoza, 2007; see Table 2). On the other hand, the reward value of food and its motivational properties are important in entrainment. Mendoza et al. (2005) have observed entrainment of the rat SCN by a palatable meal (chocolate) without food deprivation. This entrainment effect was evident in the circadian rhythm of locomotor activity, a relevant output of the SCN. Their results indicate that the SCN can be entrained by palatable food without undergoing a chronic energy deprivation, probably due to the high level of arousal produced in such conditions.
A crucial role of the dorsomedial hypothalamic (DMH) nucleus has been reported for the FAA expression. In mice, the DMH exhibits little or no mPer1 or mPer2 expression when food is freely available, but strong circadian expression when food is restricted to a limited time of day (Mieda et al., 2006). In rats, neurotoxic lesions destroying 75% to 90% of DMH neurons strongly attenuate food-anticipatory rhythms of locomotion and EEG-defined waking, as well as eliminate the pre-meal rise in core body temperature evident in intact animals (Gooley et al., 2006). However, it was found that rats sustaining complete ablation of the DMH were capable of essentially normal FAA rhythms (Landry et al., 2007). Therefore, it remains to be elucidated which brain structures are necessary for the generation and persistence of food-anticipatory circadian behavioral rhythms. Interestingly, it was recently suggested that the functional model for the FEO is a network of interconnected brain structures entrained by fluctuation of different humoral factors (Carneiro and Araujo, 2009; Aguilar-Roblero and Diaz-Muñoz, 2010). In this sense, a distributed system arranged in a non-hierarchical manner to control FAA has been proposed. Moreover, it has also been reported that regulators of G protein signaling are involved in both the LEO and FEO circadian systems, suggesting a common mechanism of interaction (Hayasaka et al., 2011).
The Circadian Influence on Reward-Related Behavior
Results from Roybal et al. (2007) indicate that the central transcriptional activator of molecular rhythms, CLOCK, has an important role in the ventral tegmental area (VTA) in regulating dopaminergic activity, locomotor activity, and anxiety. Moreover, several genes involved in dopaminergic signaling are differentially regulated in the VTA of the Clock mutant mice, suggesting that CLOCK affects the transcription of these genes through its actions in this brain region. Several findings support a role for the SCN in controlling distal reward circuitry, perhaps via its influence on rhythmic dopaminergic neurotransmission within mesolimbic structures. Indeed, dopamine (DA) and its related metabolites and receptors exhibit daily fluctuations in their levels in different brain regions (Kafka et al., 1986). Furthermore, most elements of dopaminergic transmission have a diurnal rhythm in striatal regions, including the expression of the DA transporter (DAT), DA receptors, and the rate-limiting enzyme in DA synthesis, tyrosine hydroxylase (TH; McClung, 2007). Administration of haloperidol has been found to increase expression levels of clock genes involved in the transcriptional feedback loop responsible for circadian rhythms, both in vivo and in cultured SCN cells (Viyoch et al., 2005). McClung et al. (2005) reported that Clock mutant mice reveal increased dopaminergic function, suggesting that the CLOCK protein plays a part in regulating the transmission of DA in the brain.
The role of the SCN as a synchronizer or driver of oscillators outside the hypothalamus is well established, and many brain regions implicated in cocaine-seeking behavior also contain molecular clocks. Circadian fluctuations in extracellular DA levels in the striatum and nucleus accumbens have been described (Castaneda et al., 2004). Furthermore, identification of specific clock binding elements (E-boxes) within the promoter regions of the DAT, D1A receptor, and TH genes supports the existence of an interaction between circadian clocks and dopaminergic neurotransmission. Indeed, it was recently discovered that the SCN is at least partially responsible for the presence of normal day/night differences in DAT and TH protein expression in the nucleus accumbens, mPFC, and caudate (Sleipness et al., 2007a), as well as for the day/night variation in cocaine-seeking behavior in rats (Sleipness et al., 2007b).
Interval Timing
The perception of time in the seconds-to-minutes range, referred to as interval timing, is involved in foraging, decision making and multiple-step arithmetic, and has been demonstrated in birds, fish, rodents, primates, and human infants and adults. The psychophysics of interval timing in humans and other animals has been studied extensively (Gibbon, 1977; Gibbon et al., 1984a, 1997; Allen and Gibbon, 1991; Penney et al., 2008). One consistent feature of the behavioral data is that the variability in timed responses increases in direct proportion to the duration of the interval timed, such that the coefficient of variation (the ratio of the SD to the mean response) is a constant, i.e., variability exhibits a scalar property (Gibbon et al., 1997; Buhusi and Meck, 2005). Much closer examinations of timing data across a broad range of closely spaced intervals however, reveal occasional yet systematic departures from scalar variability. These findings have led some to argue that interval timing depends not on a linear accumulator, but rather on a series of biological oscillators with different periods (Crystal, 2003; Crystal and Baramidze, 2007). If it is the case that multiple biological oscillators are responsible for interval timing, then the molecular mechanisms underlying these oscillators may share components with the circadian oscillator. In fact, a Multiple-Oscillator model of interval timing in which entrainment and selection of an appropriate range of oscillators from a series with periods potentially spanning milliseconds to years has been proposed. In this case, time is represented by the phase of the selected oscillators and non-linearities will occur to the extent that these oscillators are non-overlapping (Church and Broadbent, 1990).
Recent neurophysiological modeling of interval timing proposes that temporally coding neural inputs arise from the electrical activity of large areas of the cortex (Buhusi and Meck, 2005; Coull et al., 2011; Oprisan and Buhusi, 2011). The frontal cortex in particular contains neurons that oscillate at different rates (5–15 Hz) and striatal spiny neurons that receive their synaptic input from the cortex can monitor the oscillatory patterns of cortical neural activity. According to the striatal beat frequency (SBF) model of interval timing (Matell and Meck, 2004; Lustig et al., 2005; Allman and Meck, 2011; Coull et al., 2011), coincidence detection in the striatum results in the identification of a pattern of oscillatory firings or beats (i.e., similar to a musical chord) among other beats that represent noise or unrelated information. The probability that a particular “chord” will be identified as a signal increases as the number of detectors that simultaneously respond to such beats increases. In the SBF model, signal durations are translated into a particular cortical pattern or “chord” formed by the firing of multiple neurons with different rates of oscillations. Such a coding scheme ensures that a large number of specific supra-second intervals can be produced by the integration of a limited number of primitives represented by different sub-second oscillation frequencies in the cortex. The relevant anatomical connections, neurotransmitters systems, and signal transduction pathways specified by the SBF model of interval timing are illustrated in Figure 2A. In comparison with traditional pacemaker/accumulator models of interval timing (Meck, 1996; Matell and Meck, 2000) where DA is assumed to be the neurobiological substrate of the pacemaker pulses, in the SBF model the role of DA is assumed to act as a “start gun” by indicating the onset of a relevant signal – leading to the synchronization of cortical oscillations and the resetting of the membrane properties of the striatal spiny neurons. Consequently, this initial DA pulse coincides with the “closing of the switch” to begin timing and later, at the end of the interval, a second DA pulse co-occurring with the delivery of reward serves to strengthen synaptic connections that are active within the striatum at the time of feedback – thereby building a “coincidence detector” for a specific signal duration (Matell et al., 2003; Matell and Meck, 2004).
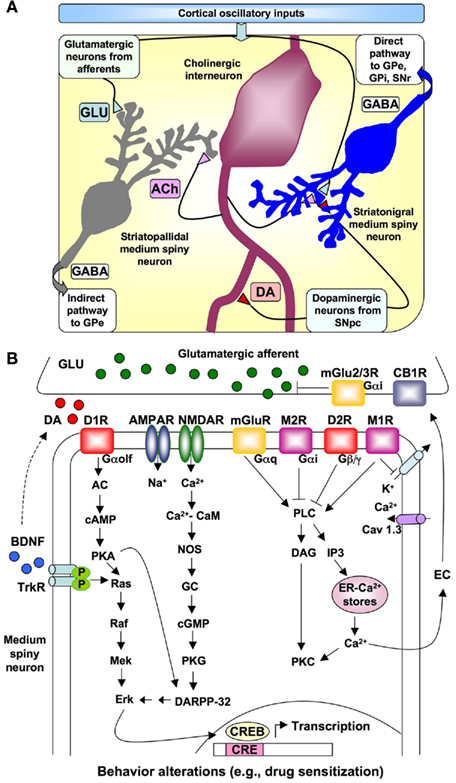
Figure 2. Relationships of different neurons in the striatum and neurotransmitter signaling involved in interval timing. (A) Schematic representation of the relationship among oscillatory cortical inputs, medium spiny neurons, cholinergic interneurons, glutamatergic afferents, and dopaminergic axons projecting from the substantia nigra pars compacta (SNpc) to the striatum as specified by the Striatal Beat Frequency model of interval timing. The direct output pathway to the globus pallidus – external (GPe) and internal (GPi) segments, and substantia nigra reticulata (SNr) as well as the indirect pathway to the GPe are indicated. Relevant neurotransmitters = acetylcholine (ACh), dopamine (DA), γ-aminobutyric acid (GABA), glutamate (GLU). (B) Detail of dopaminergic, glutamatergic, and cholinergic input to a striatonigral medium spiny neuron as well as the principal signal transduction pathways modulating the contribution of striatal spiny neurons to interval timing. Abbreviations: AC, adenyl cyclase; ACh, acetylcholine; AMPAR, AMPA receptor; CB1, cannabinoid receptor type 1; CRE, cyclic-AMP-response element; CREB, CRE binding protein; DA, dopamine; DAG, 1,2-diacylglycerol; DARPP-32, camp-regulated phosphoprotein of 32 kDa; D1R, dopamine D1 receptor; D2R, dopamine D2 receptor; EC, endocannabinoids; GABA, γ-aminobutyric acid; Glu, glutamate; GP, globus pallidus; IP3, inositol 1,4,5 trisphosphate; M1R, muscrinic acetylcholine receptor 1; M2R, muscarinic acetylcholine receptor 2; mGluR, metabotropic glutamate receptor; NMDAR, N-methyl-D-aspartic acid receptor; NOS, nitric oxide synthase; PKA, protein kinase A; PKC, protein kinase C; SNpc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; TrKR, tyrosine kinase receptor.
Molecular Basis of Interval Timing
The molecular mechanisms supporting the various ways in which humans and other animals time intervals measured in seconds-to-minutes remain poorly understood (Buonomano, 2007).
Some of the mechanisms believed to be involved in interval timing, including neurotransmitter receptors and signal transduction pathways, are outlined in Figure 2B. Signaling by DA, which activates both D1- and D2-like receptors, is involved in the regulation of the timing speed, since DA receptor agonists or antagonists are able to shift the perception of the signal duration (Meck, 1996; Williamson et al., 2008; Coull et al., 2011). Strong activation of cortical glutamate-releasing afferent axons results in release of glutamate in the striatum, postsynaptic depolarization, and elevation of intracellular Ca2+ levels in the medium spiny neurons. Activation of NMDA-type glutamate receptors (NMDARs) is also important for interval timing mechanisms (Cheng et al., 2006, 2007a; Coull et al., 2011; Hata, 2011). These signaling pathways might lead to the activation of the cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) and the cyclic-AMP-response element binding protein (CREB), which in turn interact with specific substrates to regulate temporal control of behavior. It has been proposed that a shift from subcortical-DA-dependent mechanisms to cortical-Glu-dependent mechanisms occurs as a function of the amount of training and mGluR2/3 activation (Cheng et al., 2006, 2007a,b; Bhave et al., 2008). Moreover, a postsynaptically released endocannabinoid (EC) could act as a retrograde messenger, and lead CB1 cannabinoid receptor inhibition of synaptic release of glutamate in the dorsolateral striatum (Gerdeman and Lovinger, 2001; Hilário et al., 2007).
In addition, recent studies of molecular genetics have demonstrated the importance of specific DA regulators on cognitive functioning. Among them, promising candidates are the DRD2/ANKK1-Taq1a, which is a D2 receptor polymorphism associated with decreased D2 density in the striatum, and the genes regulating the Catechol-O-methyltransferase (COMT) enzyme, – which degrades catecholamines in the frontal cortex (reviewed in Savitz et al., 2006). The most frequently studied of these COMT-related genes is COMT Val158Met, due to its natural allelic variation in humans. The Val158Met polymorphism is a valine-to-methionine conversion that occurs within the COMT gene, affecting the enzymatic activity of the COMT enzyme. Importantly, these polymorphisms – DRD2/ANKK1-Taq1a and COMT Val158Met – have been shown to be correlated with the variability for the timing of specific durations (e.g., 500 and 2,000 ms standards) as well as the determination of preferred tempos (Wiener et al., 2011). In another study related to the COMT Val158Met polymorphism and timing, it was found that subjects carrying the VAL allele (VAL/VAL, VAL/MET) showed a significant speed up of the internal clock in comparison to carriers without the VAL allele (MET/MET) in a second production task (Reuter et al., 2005). Moreover, a study conducted on synchronous swimmers showed that individual differences in the COMT polymorphism were associated with the reproduction of short time intervals (<2 s). Thus, the carriers of MET/MET polymorphism over-reproduced 1–2 s durations in a duration reproduction task (Portnova et al., 2007). Furthermore, polymorphisms in genes coding for serotonin (5-HT) availability in the cell (5HTT, MOAO, and 5HT2a) showed association with the “loss rate” of duration representations (Sysoeva et al., 2010), which can be related to the properties of interval timing, such as clock-speed and/or rate of decay of the clock reading (Buhusi and Meck, 2009a; Coull et al., 2011).
What Types of Circadian Influence are There on Interval Timing?
There are a variety of similarities between interval and circadian timing at the behavioral level to suggest a possible shared molecular basis. As described above, animals use both interval and circadian timing in complementary ways to anticipate the temporal regularity of daily feedings (Terman et al., 1984); as a particular example, such mechanisms are needed to estimate the amount of time that a female ringdove spends sitting on its nest and when it is time for the male ringdove to take over (Gibbon et al., 1984b). Time-of-day effects have been observed for the timing of auditory and visual signals in the seconds-to-minutes range (Aschoff, 1985; Chandrashekaran et al., 1991; Meck, 1991; Pati and Gupta, 1994; Kuriyama et al., 2005). For example, the accuracy for the reproduction of short durations varies with the circadian cycle, such that reproductions are longer at night and in the morning than in the middle of the day (Aschoff, 1998b), while the differential allocation of attention to auditory and visual signal durations covaries as a function, among other variables, of circadian phase (Lustig and Meck, 2001). When humans live in isolation with no external time cues, their perception of the duration of an hour is highly correlated with τ (tau), their mean circadian period (Aschoff, 1984). In contrast, the production of short intervals within the range of 10- to 20-s is neither correlated with the subject’s 1-h time estimates or with the duration of wake time (Aschoff, 1985, 1998a). Nevertheless, the remembered time of reinforcement in the peak-interval procedure using target durations in the seconds-to-minutes range has been shown to exhibit photoperiodic variation in a manner similar to that previously observed for reproductive function in rodents (MacDonald et al., 2007). Consistent with this finding, a circadian rhythm in time estimates was documented in control subjects, but was found to be disrupted in shift workers (Pati and Gupta, 1994). It has also been reported that sleep deprivation influences diurnal variation of time estimation in humans (Soshi et al., 2010). In Drosophila melanogaster, for example, the timing of short intervals is disrupted in circadian mutants (Kyriacou and Hall, 1980). Moreover, rats exhibit circadian variations in time perception similar to those that have been demonstrated in humans (Shurtleff et al., 1990; Meck, 1991). Recently, significant differences in the estimation of 24-s intervals at different times of day were reported in mice (Agostino et al., 2011). These differences were maintained under constant dark (DD) conditions. Interval timing was also impaired in mice under constant light (LL) conditions, which abolish circadian rhythmicity. Taken together, these results suggest that time estimation in the seconds-to-minutes range may be modulated by the circadian clock (Meck, 1991; Hinton and Meck, 1997). It is important to note that circadian effects on interval timing might also be mediated not directly through the endogenous clock, but also by changes in external stimulation [such as the light–dark (LD) cycle, access to food, temperature, etc.]. In particular, alterations of time perception in shift workers (as well as what could happen in other conditions of circadian disruption) might also be related to changes in anxiety and stress, as well as the relative sleep deprivation state that accompanies these types of work schedules (Åkerstedt, 2003).
An obvious question is whether the orchestration of interval timing with circadian rhythms shares at least part of their molecular machinery. The accurate timing in seconds, minutes, hours, and days allows foraging animals not only to calculate their rate of return and gage a safe length of time before competitors or predators appear, but also to set a temporal horizon before going to sleep or making decisions about future events (Bateson, 2003; Rosati et al., 2007). Two recent studies using mutant and knockout mice, however, indicate that interval and circadian timing are relatively independent at the molecular level (Cordes and Gallistel, 2008; Papachristos et al., 2011). Cordes and Gallistel (2008) have reported intact interval timing in Clock mutant mice, which have previously been shown to have a point mutation in the Clock gene leading to inactive CLOCK proteins and impaired circadian timing. When housed in a 12:12-h LD cycle, Clock mutant mice entrain to the light cycle and maintain rhythmicity like their wild-type littermates. In complete darkness, however, heterozygotes have a longer rhythm than wild types (∼24.4 h, as compared with ∼23.3 h) while homozygotes maintain an even longer period (∼27.3 h), before losing rhythmicity within the first 5–15 cycles (Vitaterna et al., 1994). Consequently, Cordes and Gallistel (2008) trained Clock mutant mice and controls in a peak-interval timing procedure using 10 and 20-s visual signal durations in order to determine if expression of the Clock gene was necessary for normal interval timing. The results indicated no impairments in the timing of the 10- and 20-s signal durations across the three Clock genotypes. If anything, the data suggest that homozygous Clock mice are both more accurate and precise in timing short intervals as compared with their wild-type littermates – possibly due to an increased clock-speed resulting from enhanced dopaminergic function (McClung et al., 2005). It should be noted, however, that under the experimental conditions utilized by Cordes and Gallistel (2008), Clock mutant mice were constantly entrained to the LD cycle and therefore maintained normal rhythmicity much like their wild-type littermates. Because of this LD entrainment, it would be important to study the effects of a Clock mutation on interval timing either under DD or LL conditions during which the circadian clocks in heterozygous and homozygous mice could “free run” differentially as a function of the Clock genotype (see Vitaterna et al., 1994). Recently, Papachristos et al. (2011) trained Cry1/Cry2 double knockout mice on an interval timing task with durations that ranged between 3 and 27 s. Homozygous knockouts displayed an accurate and precise temporal memory similar to that of the control mice, suggesting that the Cry1 and Cry2 genes are not an important component of the interval timer. However, it should be noted that in this study interval timing was assessed in a different group of mice than the one used for the evaluation of circadian rhythmicity and, in addition, mice were fed once a day at the same time of day, therefore providing a potential temporal cue that might mask circadian rhythmicity and influence time perception in the seconds-to-minutes range (see Challet et al., 2003; Feillet et al., 2006a; Challet, 2007; Balsam et al., 2009; Steinman et al., 2011).
In general, these results suggest that expression of the Clock or Cry genes is not necessary for normal interval timing in the mouse. Although these findings suggest that interval and circadian timing are independent at the molecular level, other genes need to be explored in this regard, (e.g., Period). Moreover, more strict circadian paradigms need to be applied in order to clearly dissect the behaviors under study (including experiments under constant light or constant dark situations, as well as testing for additional memory tasks).
Rather than relying on common oscillatory mechanisms, the behavioral correlations observed between interval and circadian timing may be indicative of a different sort of relationship. Diverse lines of evidence suggest functional links among mesolimbic, nigrostriatal, and mesocortical dopaminergic systems (Meck, 1983, 1996, 2006a,b; Gu et al., 2011). For example, pharmacological manipulations indicate that cortico-striatal DA levels regulate the speed of the interval timer, as administration of indirect DA agonists such as cocaine and methamphetamine produce a proportional leftward shift of timing functions (i.e., speeds up the interval timer; Meck, 1983, 1996; Matell et al., 2004, 2006), while DA receptor blockers such as haloperidol and raclopride produce the opposite effect (Meck, 1983, 1986, 1996; Drew et al., 2003; MacDonald and Meck, 2004, 2005, 2006). The D2 receptor has been identified as being critical to the mediation of these pharmacological effects (Meck, 1986; MacDonald and Meck, 2006) and transient overexpression of striatal D2 receptors impairs the acquisition of temporal control in a 24-s peak-interval procedure (Drew et al., 2007). In addition, deletion of the DAT gene, but not the norepinephrine transporter (NET) gene, abolishes the ability to discriminate supra-second durations in homozygous mice and leads to a decreased sensitivity to the clock-speed enhancing effects of methamphetamine in the heterozygous mice, indicating that excess levels of DA “flood” the temporal integration process and impair interval timing (Meck et al., 2011). Likewise, lesions of the DA/DAT rich areas such as the substantia nigra pars compacta and dorsal striatum lead to decreased levels of DA and impairments in supra-second timing in both humans and rats (Malapani et al., 1998; Meck, 2006b; Coull et al., 2011). Moreover, electrophysiological recordings from striatal spiny neurons that receive both dopaminergic and glutaminergic inputs show them to be involved in the coding of durations in the seconds-to-minutes range (Matell et al., 2003; Cheng et al., 2007a; Chiba et al., 2008; Meck et al., 2008). The dopaminergic–glutamatergic pathways that modulate interval timing in mammals are outlined in Figure 2A, whereas studies using genetically modified mice to explore the molecular basis of circadian and interval timing are outlined in Table 2.
Open Questions about Timing Mechanisms
Behaviorally, interval timing and reward prediction have been demonstrated across various vertebrate models of learning, including humans, primates, rodents, birds, and fish, as well as invertebrate models, such as Drosophila melanogaster and Caenorhabditis elegans (Lejeune and Wearden, 1991; Hills, 2003; Penney et al., 2008). One structure of particular interest with regard to interval timing and reward prediction in vertebrates is the habenula, a well-conserved component of the epithalamus and a prominent structure in a model system such as zebrafish (Lee et al., 2010; Cheng et al., 2011). Importantly, zebrafish have an interesting asymmetry in habenula input, i.e., only the right habenula receives input from the forebrain (Hendricks and Jesuthasan, 2007). This asymmetry may provide an ideal situation for localizing timing and reward prediction mechanisms (Bromberg-Martin et al., 2010a,b). Investigation of the role of the habenula in neural circuits for the anticipation of reward has yet to be extended to zebrafish, and should prove worthwhile considering the emerging recognition of the importance of the habenula to cognition and behavior. Moreover, memory of time intervals in the order of seconds, for durations up to 20-s, has been observed in zebrafish larvae (Sumbre et al., 2008). Given that robust circadian rhythms in the locomotor activity of larval (10- to 15-day-old) zebrafish have been observed in constant lighting conditions, this model is likely to prove useful for mutational analyses of both vertebrate interval and circadian timing. In this and other animal models (certainly including mammals and, in particular, rodents), there are still some of outstanding questions to be addressed. For example, the exact molecular mechanisms underlying interval timing remain to be established. Moreover, the circadian modulation of interval timing is lacking a mechanistic explanation and a neuroanatomical substrate (or substrates). Finally, the neurochemical common nature of both processes and their interaction is also matter of controversy.
Perspectives on Future Directions
While circadian modulation of interval timing may involve a variety of brain regions including the SCN, recent evidence suggests that this structure alone does not directly mediate the timing of short durations (Lewis et al., 2003). However, the SCN may nevertheless modulate circadian changes in interval timing. This modulation can be interpreted in terms of adaptation requirements, given that the same accuracy of time estimation might not be needed at all times throughout the daily cycle. Consistent with this account are the observations that time judgments in humans co-vary with normal circadian rhythms (Kuriyama et al., 2005) and are disrupted in shift workers (Pati and Gupta, 1994). Moreover, rats and mice exhibit circadian variations in time perception similar to those that have been demonstrated in humans (Shurtleff et al., 1990; Meck, 2001; Agostino et al., 2011).
In addition, both timing mechanisms might share a common link in terms of the regulation of arousal or motivational states. Indeed, acquisition of operant cycles of reinforcement, frequently used for the evaluation of interval timing, requires the activation of reward pathways in the brain, usually driven by food stimulation in partially deprived animals (Church and Lacourse, 2001). It is worth noting that at least some features of circadian entrainment (such as non-photic synchronization induced by forced locomotion, feeding or neurochemical stimulation by methamphetamine, and other agents) also depend upon reward-related mechanisms, including dopaminergic activation. Consequently, a common molecular basis related to dopaminergic function in cortico-striatal pathways appears to be the most promising link between interval and circadian timing.
In summary, it is clear that timing and time perception have been instrumental for adaptation to a cyclic and somewhat predictable environment. Endogenous timing mechanisms cover several orders of magnitude of event frequencies and could be interpreted as a continuum that extends from duration estimation in the seconds range to developmental and lifespan experiences on the order of years. Unwinding the molecular basis for these relationships should lead to a better understanding of the intricate labyrinths of cognitive and neural timing systems.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Agostino, P. V., do Nascimento, M., Bussi, I. L., Eguía, M. C., and Golombek, D. A. (2011). Circadian modulation of interval timing in mice. Brain Res. 1370, 154–163.
Agostino, P. V., Plano, S. A., and Golombek, D. A. (2007). Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules. Proc. Natl. Acad. Sci. U.S.A. 104, 9834–9839.
Aguilar-Roblero, R., and Diaz-Muñoz, M. (2010). Chronostatic adaptation in the liver to restricted feeding: the FEO as an emergent oscillator. Sleep Biol. Rhythms 8, 9–17.
Allen, L. G., and Gibbon, J. (1991). Human bisection at the geometric mean. Learn. Motiv. 22, 39–58.
Allman, M. J., and Meck, W. H. (2011). Pathophysiological distortions in time perception and timed performance. Brain doi: 10.1093/brain/awr21
Aschoff, J. (1985). On the perception of time during prolonged temporal isolation. Hum. Neurobiol. 4, 41–52.
Aschoff, J. (1998a). Carcadian parameters as individual characteristics. J. Biol. Rhythms 13, 123–131.
Aschoff, J. (1998b). Human perception of short and long time intervals: its correlation with body temperature and the duration of wake time. J. Biol. Rhythms 13, 437–442.
Balci, F., Ludvig, E. A., Abner, R., Zhuang, X., Poon, P., and Brunner, D. (2010). Motivational effects on interval timing in dopamine transporter (DAT) knockdown mice. Brain Res. 1325, 89–99.
Balci, F., Meck, W. H., Moore, H., and Brunner, D. (2009). “Timing deficits in aging and neuropathology,” in Animal Models of Human Cognitive Aging, eds J. L. Bizon, and A. Wood (Totowa, NJ: Humana Press), 161–201.
Balci, F., Papachristos, E. B., Gallistel, C. R., Brunner, D., Gibson, J., and Shumyatsky, G. P. (2008). Interval timing in genetically modified mice: a simple paradigm. Genes Brain Behav. 7, 373–384.
Balsam, P., Sanchez-Castillo, H., Taylor, K., van Volkinburg, H., and Ward, R. D. (2009). Timing and anticipation: conceptual and methodological approaches. Eur. J. Neurosci. 30, 1749–1755.
Bateson, M. (2003). “Interval timing and optimal foraging,” in Functional and Neural Mechanisms of Interval Timing, ed. W. H. Meck (Boca Raton, FL: CRC Press), 113–141.
Bhave, S. R., Cheng, R. K., and Meck, W. H. (2008). “The metabotropic glutamate 2/3 receptor agonist LY379268 counteracts the clock-speed enhancing effects of cocaine,” in Society for Neuroscience, Washington, 877.20.
Bromberg-Martin, E. S., Matsumoto, M., and Hikosaka, O. (2010a). Distinct tonic and phasic anticipatory activity in lateral habenula and dopamine neurons. Neuron 67, 114–155.
Bromberg-Martin, E. S., Matsumoto, M., Nakahara, H., and Hikosaka, O. (2010b). Multiple timescales of memory in lateral habenula and dopamine neurons. Neuron 67, 499–510.
Buhusi, C. V., Aziz, D., Winslow, D., Carter, R. E., Swearington, J. E., and Buhusi, M. C. (2009). Interval timing accuracy and scalar timing in c57BL/6 mice. Behav. Neurosci. 123, 1102–1113.
Buhusi, C. V., and Meck, W. H. (2005). What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 6, 755–765.
Buhusi, C. V., and Meck, W. H. (2009a). Relative time sharing: New findings and an extension of the resource allocation model of temporal processing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1875–1885.
Buhusi, C. V., and Meck, W. H. (2009b). Relativity theory and time perception: single or multiple clocks? PLoS ONE 4, e6268. doi: 10.1371/journal.pone.0006268
Bunger, M. K., Wilsbacher, L. D., Moran, S. M., Clendenin, C., Radcliffe, L. A., Hogenesch, J. B., Simon, M. C., Takahashi, J. S., and Bradfield, C. A. (2000). Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017.
Buonomano, D. V., and Laje, R. (2010). Population clocks: motor timing with neural dynamics. Trends Cogn. Sci. (Regul. Ed.) 14, 520–527.
Carneiro, B. T., and Araujo, J. F. (2009). The foo-entrainable oscillator: a network of interconnected brain structures entrained by humoral signal? Chronobiol. Int. 26, 1273–1289.
Castaneda, T. R., de Prado, B. M., Prieto, D., and Mora, F. (2004). Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J. Pineal Res. 36, 177–185.
Cevik, M. O. (2003). “Neurogenetics of interval timing,” in Functional and Neural Mechanisms of Interval Timing, ed. W. H. Meck (CRC Press), 297–316.
Challet, E., Caldelas, I., Graff, C., and Pévet, P. (2003). Synchronization of the molecular clockwork by light- and food-related cues in mammals. Biol. Chem. 384, 711–719.
Chandrashekaran, M. K., Marimuthu, G., Subbaraj, R., Kumarasamy, P., Ramkumar, M. S., and Sripathi, K. (1991). Direct correlation between the circadian sleep-wakefulness rhythm and time estimation in humans under social and temporal isolation. J. Biosci. 16, 97–101.
Cheng, R., Jesuthasan, S., and Penney, T. B. (2011). Time for Zebrafish. Front. Integr. Neurosci. 5:40. doi: 10.3389/fnint.2011.00040
Cheng, R. K., Ali, Y. M., and Meck, W. H. (2007a). Ketamine “unlocks” the reduced clock-speed effects of cocaine following extended training: evidence for dopamine-glutamate interactions in timing and time perception. Neurobiol. Learn. Mem. 88, 149–159.
Cheng, R. K., Hakak, O. L., and Meck, W. H. (2007b). Habit formation and the loss of control of an internal clock: inverse relationship between the level of baseline training and the clock-speed enhancing effects of methamphetamine. Psychopharmacology (Berl.) 193, 351–362.
Cheng, R. K., MacDonald, C. J., and Meck, W. H. (2006). Differential effects of cocaine and ketamine on time estimation: implications for neurobiological models of interval timing. Pharmacol. Biochem. Behav. 85, 114–122.
Cheng, R. K., and Meck, W. H. (2007). Prenatal choline supplementation increases sensitivity to time by reducing non-scalar sources of variance in adult temporal processing. Brain Res. 1186, 242–254.
Chiba, A., Oshio, K., and Inase, M. (2008). Striatal neurons encoded temporal information in duration discrimination task. Exp. Brain Res. 186, 671–676.
Church, R. M., and Broadbent, H. A. (1990). Alternative representations of time, number, and rate. Cognition 37, 55–81.
Church, R. M., and Lacourse, D. M. (2001). Temporal memory of interfood interval distributions with the same mean and variance. Learn. Motiv. 32, 2–21.
Cordes, S., and Gallistel, C. R. (2008). Interval timing in circadian CLOCK mutants. Brain Res. 1227, 120–127.
Coull, J. T., Cheng, R. K., and Meck, W. H. (2011). Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 36, 3–25.
Crystal, J. D. (2003). “Nonlinearities in sensitivity to time: implications for oscillator-based representations of interval circadian clocks,” in Functional and Neural Mechanisms of Interval Timing, ed. W. H. Meck (Boca Raton, FL: CRC Press), 61–76.
Crystal, J. D., and Baramidze, G. T. (2007). Endogenous oscillations in short-interval timing. Behav. Processes 74, 152–158.
Drew, M. R., Fairhurst, S., Malapani, C., Horvitz, J. C., and Balsam, P. D. (2003). Effects of dopamine antagonists on the timing of two intervals. Pharmacol. Biochem. Behav. 75, 9–15.
Drew, M. R., Simpson, E. H., Kellendonk, C., Herzberg, W. G., Lipatova, O., Fairhurst, S., Kandel, E. R., Malapani, C., and Balsam, P. D. (2007). Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J. Neurosci. 27, 7731–7739.
Dudley, C. A., Erbel-Sieler, C., Estill, S. J., Reick, M., Franken, P., Pitts, S., and McKnight, S. L. (2003). Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 301, 379–383.
Feillet, C. A., Albrecht, U., and Challet, E. (2006a). “Feeding time” for the brain: a matter of clocks. J. Physiol. Paris 100, 252–260.
Feillet, C. A., Ripperger, J. A., Magnone, M. C., Dulloo, A., Albrecht, U., and Challet, E. (2006b). Lack of food anticipation in Per2 mutant mice. Curr. Biol. 16, 2016–2022.
Gallego, M., and Virshup, D. M. (2007). Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol. 8, 139–148.
Gannon, R. L., and Millan, M. J. (2011). Positive and negative modulation of circadian activity rhythms by mGluR5 and mGluR2/3 metabotropic glutamate receptors. Neuropharmacology 60, 209–215.
Gerdeman, G., and Lovinger, D. M. (2001). CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J. Neurophysiol. 85, 468–471.
Gibbon, J., Church, R. M., and Meck, W. H. (1984a). Scalar timing in memory. Ann. N. Y. Acad. Sci. 423, 52–77.
Gibbon, J., Morrell, M., and Silver, R. (1984b). Two kinds of timing in circadian incubation rhythm of ring doves. Am. J. Physiol. 247, R1083–R1087.
Gibbon, J., Malapani, C., Dale, C. L., and Gallistel, C. (1997). Toward a neurobiology of temporal cognition: advances and challenges. Curr. Opin. Neurobiol. 7, 170–184.
Golombek, D. A., Agostino, P. V., Plano, S. A., and Ferreyra, G. A. (2004). Signaling in the mammalian circadian clock: the NO/cGMP pathway. Neurochem. Int. 45, 929–936.
Golombek, D. A., Ferreyra, G. A., Agostino, P. V., Murad, A. D., Rubio, M. F., Pizzio, G. A., Katz, M. E., Marpegan, L., and Bekinschtein, T. A. (2003). From light to genes: moving the hands of the circadian clock. Front. Biosci. 8, s285–s293.
Golombek, D. A., and Rosenstein, R. E. (2010). The physiology of circadian entrainment. Physiol. Rev. 90, 1063–1102.
Gooley, J. J., Schomer, A., and Saper, C. B. (2006). The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat. Neurosci. 9, 398–407.
Gu, B. M., Yin, B., Cheng, R. K., and Meck, W. H. (2011). Quinpirole-induced sensitization to noisy/sparse periodic input: temporal synchronization as a component of obsessive-compulsive disorder. Neuroscience 179, 143–150.
Hata, T. (2011). Glutamate – a forgotten target for interval timing. Front. Integr. Neurosci. 5:27. doi: 10.3389/fnint.2011.00027
Hayasaka, N., Aoki, K., Kinoshita, S., Yamaguchi, S., Wakefield, J. K., Tsuji-Kawahara, S., Horikawa, K., Ikegami, H., Wakana, S., Murakami, T., Ramabhadran, R., Miyazawa, M., and Shibata, S. (2011). Attenuated food anticipatory activity and abnormal circadian locomotor rhythms in Rgs16 knockdown mice. PLoS ONE 6, e17655. doi: 10.1371/journal.pone.0017655
Hendricks, M., and Jesuthasan, S. (2007). Asymmetric innervation of the habenula in zebrafish. J. Comp. Neurol. 502, 611–619.
Hilário, M. R. F., Clouse, E., Yin, H. H., and Costa, R. M. (2007). Endocannabiniod signaling is critical for habit formation. Front. Integr. Neurosci. 1:6 doi: 10.3389/neuro.07.006.2007
Hills, T. T. (2003). “Toward a unified theory of animal event timing,” in Functional and Neural Mechanisms of Interval Timing, ed. W. H. Meck (Boca Raton, FL: CRC Press), 77–111.
Hinton, S. C., and Meck, W. H. (1997). The ‘internal clocks’ of circadian and interval timing. Endeavour 21, 82–87.
Hirayama, J., Sahar, S., Grimaldi, B., Tamaru, T., Takamatsu, K., Nakahata, Y., and Sassone-Corsi, P. (2007). CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 450, 1086–1090.
Iijima, M., Yamaguchi, S., van der Horst, G. T., Bonnefont, X., Okamura, H., and Shibata, S. (2005). Altered food-anticipatory activity rhythm in cryptochrome-deficient mice. Neurosci. Res. 52, 166–173.
Kafka, M. S., Benedito, M. A., Roth, R. H., Steele, L. K., Wolfe, W. W., and Catravas, G. N. (1986). Circadian rhythms in catecholamine metabolites and cyclic nucleotide production. Chronobiol. Int. 3, 101–115.
Kaur, S., Thankachan, S., Begum, S., Blanco-Centurion, C., Sakurai, T., Yanagisawa, M., and Shiromani, P. J. (2008). Entrainment of temperature and activity rhythms to restricted feeding in orexin knock out mice. Brain Res. 1205, 47–54.
Kuriyama, K., Uchiyama, M., Suzuki, H., Tagaya, H., Ozaki, A., Aritake, S., Shibui, K., Xin, T., Lan, L., Kamei, Y., and Takahashi, K. (2005). Diurnal fluctuation of time perception under 30-h sustained wakefulness. Neurosci. Res. 53, 123–128.
Kyriacou, C. P., and Hall, J. (1980). Circadian rhythm mutations in Drosophila melanogaster affect short-term fluctuations in the male’s courtship song. Proc. Natl. Acad. Sci. U.S.A. 77, 6729–6733.
Landry, G. J., Yamakawa, G. R., Webb, I. C., Mear, R. J., and Mistlberger, R. E. (2007). The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J. Biol. Rhythms 22, 467–478.
Lee, A., Mathuru, A. S., The, C., Kibat, C., Korzh, V., Penney, T. B., and Jesuthasan, S. (2010). The habenula prevents helpless behavior in larval zebrafish. Curr. Biol. 20, 2211–2216.
Lejeune, H., and Wearden, J. H. (1991). The comparative psychology of fixed-interval responding: some quantitative analyses. Learn. Motiv. 22, 84–111.
Lewis, P. A., Miall, R. C., Daan, S., and Kacelnik, A. (2003). Interval timing in mice does not rely upon the circadian pacemaker. Neurosci. Lett. 348, 131–134.
Lowrey, P. L., Shimomura, K., Antoch, M. P., Yamazaki, S., Zemenides, P. D., Ralph, M. R., Menaker, M., and Takahashi, J. S. (2000). Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288, 483–492.
Lowrey, P. L., and Takahashi, J. S. (2000). Genetics of the mammalian circadian system: photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu. Rev. Genet. 34, 533–562.
Lustig, C., Matell, M. S., and Meck, W. H. (2005). Not “just” a coincidence: frontal-striatal interactions in working memory and interval timing. Memory 13, 441–448.
Lustig, C., and Meck, W. H. (2001). Paying attention to time as one gets older. Psychol. Sci. 12, 478–484.
MacDonald, C. J., Cheng, R. K., Williams, C. L., and Meck, W. H. (2007). Combined organizational and activational effects of short and long photoperiods on spatial and temporal memory in rats. Behav. Processes 74, 226–233.
MacDonald, C. J., and Meck, W. H. (2004). Systems-level integration of interval timing and reaction time. Neurosci. Biobehav. Rev. 28, 747–769.
MacDonald, C. J., and Meck, W. H. (2005). Differential effects of clozapine and haloperidol on interval timing in the supra seconds range. Psychopharmacology (Berl.) 182, 232–244.
MacDonald, C. J., and Meck, W. H. (2006). Interaction of raclopride and preparatory-interval effects on simple reaction-time performance. Behav. Brain Res. 175, 62–74.
Malapani, C., Rakitin, B., Levy, R., Meck, W. H., Deweer, B., Dubois, B., and Gibbon, J. (1998). Coupled temporal memories in Parkinson’s disease: a dopamine-related dysfunction. J. Cogn. Neurosci. 10, 316–331.
Matell, M. S., Bateson, M., and Meck, W. H. (2006). Single-trials analyses demonstrate that increases in clock speed contribute to the methamphetamine-induced horizontal shifts in peak-interval timing functions. Psychopharmacology (Berl.) 188, 201–212.
Matell, M. S., King, G. R., and Meck, W. H. (2004). Differential adjustment of interval timing by the chronic administration of intermittent or continuous cocaine. Behav. Neurosci. 118, 150–156.
Matell, M. S., and Meck, W. H. (2000). Neuropsychological mechanisms of interval timing behavior. Bioessays 22, 94–103.
Matell, M. S., and Meck, W. H. (2004). Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain. Res. Cogn. Brain Res. 21, 139–170.
Matell, M. S., Meck, W. H., and Nicolelis, M. A. (2003). Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav. Neurosci. 117, 760–773.
McClung, C. A. (2007). Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. ScientificWorldJournal 7, 194–202.
McClung, C. A., Sidiropoulou, K., Vitaterna, M., Takahashi, J. S., White, F. J., Cooper, D. C., and Nestler, E. J. (2005). Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc. Natl. Acad. Sci. U.S.A. 102, 9377–9381.
Meck, W. H. (1983). Selective adjustment of the speed of internal clock and memory processes. J. Exp. Psychol. Anim. Behav. Process. 9, 171–201.
Meck, W. H. (1986). Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacol. Biochem. Behav. 25, 1185–1189.
Meck, W. H. (1991). Modality-specific circadian rhythmicities influence mechanisms of attention and memory for interval timing. Learn. Motiv. 22, 153–179.
Meck, W. H. (1996). Neuropharmacology of timing and time perception. Brain. Res. Cogn. Brain Res. 3, 227–242.
Meck, W. H. (2001). Interval timing and genomics: what makes mutant mice tick? Int. J. Comp. Psychol. 14, 211–231.
Meck, W. H. (2006a). Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Res. 1108, 157–167.
Meck, W. H. (2006b). Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res. 1109, 93–107.
Meck, W. H., Cheng, R. K., Macdonald, C. J., Gainetdinov, R. R., Caron, M. G., and Cevik, M. O. (2011). Gene-dose dependent effects of methamphetamine on interval timing in dopamine-transporter knockout mice. Neuropharmacology. doi: 10.1016/j.neuropharm.2011.01.042. [Epub ahead of print].
Meck, W. H., Penney, T. B., and Pouthas, V. (2008). Cortico-striatal representation of time in animals and humans. Curr. Opin. Neurobiol. 18, 145–152.
Mendoza, J., Angeles-Castellanos, M., and Escobar, C. (2005). A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur. J. Neurosci. 22, 2855–2862.
Mieda, M., Williams, S. C., Richardson, J. A., Tanaka, K., and Yanagisawa, M. (2006). The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc. Natl. Acad. Sci. U.S.A. 103, 12150–12155.
Mistlberger, R. E., and Holmes, M. M. (2000). Behavioral feedback regulation of circadian rhythm phase angle in light-dark entrained mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R813–R821.
Oprisan, S. A., and Buhusi, C. V. (2011). Modeling pharmacological clock and memory patterns of interval timing in a striatal beat-frequency model of realistic, noisy neurons. Front. Integr. Neurosci. 5:52. doi: 10.3389/fnint.2011.00052
Panda, S., Sato, T. K., Castrucci, A. M., Rollag, M. D., DeGrip, W. J., Hogenesch, J. B., Provencio, I., and Kay, S. A. (2002). Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298, 2213–2216.
Papachristos, E. B., Jacobs, E. H., and Elgersma, Y. (2011). Interval timing is intact in arrhythmic cry1/cry2-deficient mice. J. Biol. Rhythms 26, 305–313.
Pati, A. K., and Gupta, S. (1994). Time estimation circadian rhythm in shift workers and diurnally active humans. J. Biosci. 19, 325–330.
Penney, T. B., Gibbon, J., and Meck, W. H. (2008). Categorical scaling of duration bisection in pigeons (Columba livia), mice (Mus musculus), and humans (Homo sapiens). Psychol. Sci. 19, 1103–1109.
Pitts, S., Perone, E., and Silver, R. (2003). Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R57–67.
Portnova, G. V., Sysoeva, O. V., Maliuchenko, N. V., Timofeeva, M. A., Kulikova, M. A., Tonevitskii, A. G., Kirpichnikov, M. P., and Ivanitskii, A. M. (2007). Genetic basis of time perception in athletes. Zh. Vyssh. Nerv. Deiat. Im. I P Pavlova. 57, 450–460.
Preitner, N., Damiola, F., Lopez-Molina, L., Zakany, J., Duboule, D., Albrecht, U., and Schibler, U. (2002). The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260.
Reppert, S. M., and Weaver, D. R. (2002). Coordination of circadian timing in mammals. Nature 418, 935–941.
Reuter, M., Peters, K., Schroeter, K., Koebke, W., Lenardon, D., Bloch, B., and Hennig, J. (2005). The influence of the dopaminergic system on cognitive functioning: a molecular genetic approach. Behav. Brain Res. 164, 93–99.
Rosati, A. G., Stevens, J. R., Hare, B., and Hauser, M. D. (2007). The evolutionary origins of human patience: temporal preferences in chimpanzees, bonobos, and human adults. Curr. Biol. 17, 1663–1668.
Roybal, K., Theobold, D., Graham, A., DiNieri, J. A., Russo, S. J., Krishnan, V., Chakravarty, S., Peevey, J., Oehrlein, N., Birnbaum, S., Vitaterna, M. H., Orsulak, P., Takahashi, J. S., Nestler, E. J., Carlezon, W. A. Jr., and McClung, C. A. (2007). Mania-like behavior induced by disruption of CLOCK. Proc. Natl. Acad. Sci. U.S.A. 104, 6406–6411.
Savitz, J., Solms, M., and Ramesar, R. (2006). The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 5, 311–328.
Sheward, W. J., Maywood, E. S., French, K. L., Horn, J. M., Hastings, M. H., Seckl, J. R., Holmes, M. C., and Harmar, A. J. (2007). Entrainment to feeding but not to light: circadian phenotype of VPAC2 receptor-null mice. J. Neurosci. 27, 4351–4358.
Shumyatsky, G. P., Tsvetkov, E., Malleret, G., Vronskaya, S., Hatton, M., Hampton, L., Battey, J. F., Dulac, C., Kandel, E. R., and Bolshakov, V. Y. (2002). Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell 111, 905–918.
Shurtleff, D., Raslear, T. G., and Simmons, L. (1990). Circadian variations in time perception in rats. Physiol. Behav. 47, 931–939.
Sleipness, E. P., Sorg, B. A., and Jansen, H. T. (2007a). Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Res. 1129, 34–42.
Sleipness, E. P., Sorg, B. A., and Jansen, H. T. (2007b). Contribution of the suprachiasmatic nucleus to day:night variation in cocaine-seeking behavior. Physiol. Behav. 91, 523–530.
Soshi, T., Kuriyama, K., Aritake, S., Enomoto, M., Hida, A., Tamura, M., Kim, Y., and Mishima, K. (2010). Sleep deprivation influences diurnal variation of human time perception with prefrontal activity change: a functional near-infrared spectroscopy study. PLoS ONE 5, e8395. doi: 10.1371/journal.pone.0008395
Steinman, M. Q., Crean, K. K., and Trainor, B. C. (2011). Photoperiod interacts with food restriction in performance in the Barnes maze in female California mice. Eur. J. Neurosci. 33, 361–370.
Stephan, F. K. (2002). The “other” circadian system: food as a Zeitgeber. J. Biol. Rhythms 17, 284–292.
Sumbre, G., Muto, A., Baier, H., and Poo, M. M. (2008). Entrained rhythmic activities of neuronal ensembles as perceptual memory of time interval. Nature 456, 102–106.
Sysoeva, O. V., Tonevitsky, A. G., and Wackermann, J. (2010). Genetic determinants of time perception mediated by the serotonergic system. PLoS ONE 5, e12650. doi: 10.1371/journal.pone.0012650
Takahashi, J. S., Hong, H. K., Ko, C. H., and McDearmon, E. L. (2008). The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 9, 764–775.
Terman, M., Gibbon, J., Fairhurst, S., and Waring, A. (1984). Daily meal anticipation: interaction of circadian and interval timing. Ann. N. Y. Acad. Sci. 423, 470–487.
van der Horst, G. T., Muijtjens, M., Kobayashi, K., Takano, R., Kanno, S., Takao, M., de Wit, J., Verkerk, A., Eker, A. P., van Leenen, D., Buijs, R., Bootsma, D., Hoeijmakers, J. H., and Yasui, A. (1999). Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630.
Vincent, S. G., Waddell, A. E., Caron, M. G., Walker, J. K., and Fisher, J. T. (2007). A murine model of hyperdopaminergic state displays altered respiratory control. FASEB J. 21, 1463–1471.
Vitaterna, M. H., King, D. P., Chang, A. M., Kornhauser, J. M., Lowrey, P. L., McDonald, J. D., Dove, W. F., Pinto, L. H., Turek, F. W., and Takahashi, J. S. (1994). Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264, 719–725.
Viyoch, J., Matsunaga, N., Yoshida, M., To, H., Higuchi, S., and Ohdo, S. (2005). Effect of haloperidol on mPer1 gene expression in mouse suprachiasmatic nuclei. J. Biol. Chem. 280, 6309–6315.
Wiener, M., Lohoff, F. W., and Coslett, H. B. (2011). Double dissociation of dopamine genes and timing in humans. J. Cogn. Neurosci. 23, 2811–2821.
Williamson, L. L., Cheng, R. K., Etchegaray, M., and Meck, W. H. (2008). “Speed” warps time: methamphetamine’s interactive roles in drug abuse, habit formation, and the biological clocks of circadian and interval timing. Curr. Drug Abuse Rev. 1, 203–212.
Xu, Y., Padiath, Q. S., Shapiro, R. E., Jones, C. R., Wu, S. C., Saigoh, N., Saigoh, K., Ptácek, L. J., and Fu, Y. H. (2005). Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature 434, 640–644.
Keywords: circadian system, interval timing, cortico-striatal circuits, suprachiasmatic nuclei, dopamine, glutamate, serotonin
Citation: Agostino PV, Golombek DA and Meck WH (2011) Unwinding the molecular basis of interval and circadian timing. Front. Integr. Neurosci. 5:64. doi: 10.3389/fnint.2011.00064
Received: 06 August 2011; Accepted: 30 September 2011;
Published online: 18 October 2011.
Edited by:
Agnes Gruart, University Pablo de Olavide, SpainReviewed by:
John F. Araujo, Federal University of Rio Grande do Norte, BrazilOlga V. Sysoeva, Washington University in St Louis School of Medicine, USA
Copyright: © 2011 Agostino, Golombek and Meck. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Warren H. Meck, Department of Psychology and Neuroscience, Duke University – Box 91050, 572 Research Drive, Durham, NC 27708-0086, USA. e-mail: meck@psych.duke.edu