Modulation of firing and synaptic transmission of serotonergic neurons by intrinsic G protein-coupled receptors and ion channels
- Department of Zoology and Neurobiology, Ruhr-University Bochum, Bochum, Germany
Serotonergic neurons project to virtually all regions of the central nervous system and are consequently involved in many critical physiological functions such as mood, sexual behavior, feeding, sleep/wake cycle, memory, cognition, blood pressure regulation, breathing, and reproductive success. Therefore, serotonin release and serotonergic neuronal activity have to be precisely controlled and modulated by interacting brain circuits to adapt to specific emotional and environmental states. We will review the current knowledge about G protein-coupled receptors and ion channels involved in the regulation of serotonergic system, how their regulation is modulating the intrinsic activity of serotonergic neurons and its transmitter release and will discuss the latest methods for controlling the modulation of serotonin release and intracellular signaling in serotonergic neurons in vitro and in vivo.
Introduction
The serotonergic system consists of a small number of neurons that are born in the ventral regions of the hindbrain (Deneris and Wyler, 2012). In the adult nervous system, serotonergic neurons [5-HT (5-hydroxytryptamine) neurons] are located in the nine raphe nuclei that are restricted to the basal plate of the midbrain, pons, and medulla (Dahlstrom and Fuxe, 1964). 5-HT neurons located in the rostral raphe nuclei, such as the dorsal raphe nucleus (DRN) and the median raphe nucleus (MRN), give rise to the majority of the serotonergic ascending fibers into the forebrain including cerebral cortex, limbic system, and basal ganglia (Jacobs and Azmitia, 1992). The activity of the serotonergic system is regulated via transmitter release from local interneurons and/or afferents to the raphe nuclei (hetero-regulation), via mechanisms arising from 5-HT neurons themselves (auto-regulation), and potentially via alterations in the extracellular milieu (e.g., increase in CO2; Pineyro and Blier, 1999; Richerson, 2004). In this review, we will discuss G protein-coupled receptors (GPCRs) and ion channels located at somatodendritic and presynaptic regions of 5-HT neurons in the DRN and MRN that contribute to the modulation of 5-HT neuronal activity and 5-HT release (Figure 1).
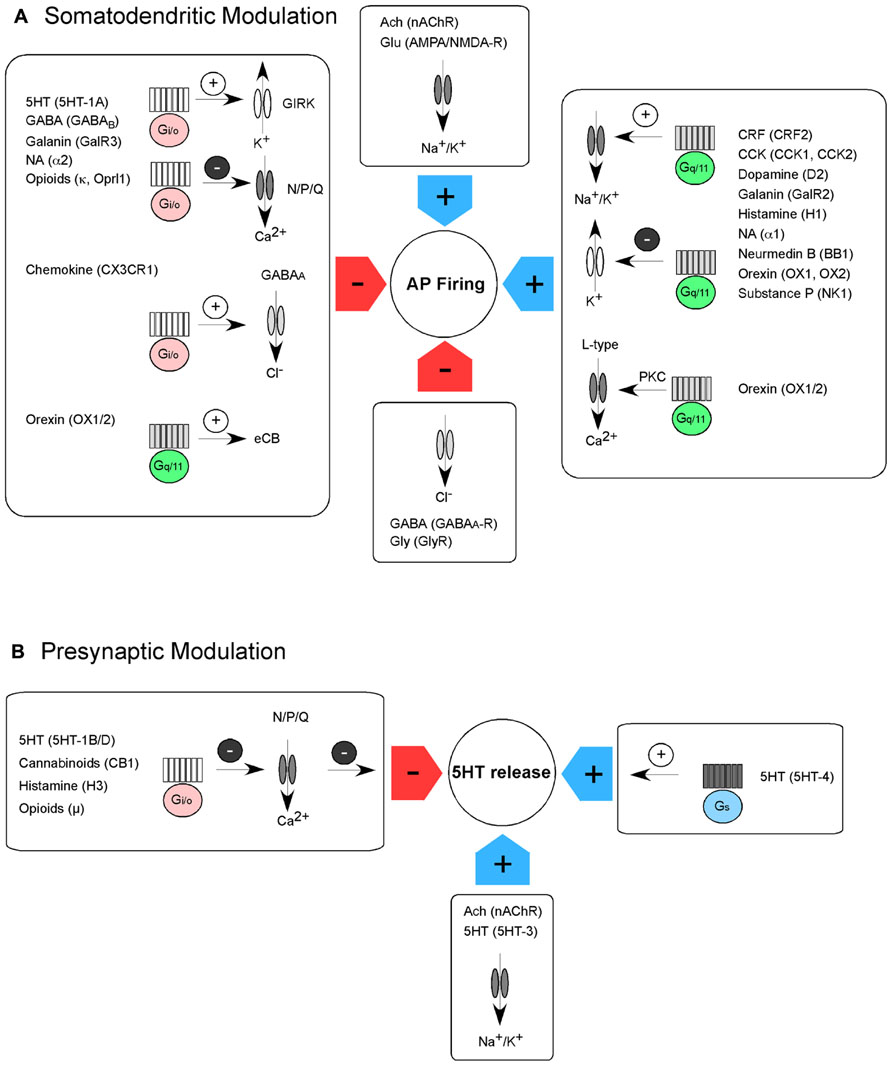
FIGURE 1. Intrinsic somatodendritic and presynaptic modulation of action potential (AP) firing and transmitter release in 5-HT neurons. (A) AP firing of 5-HT neurons is modulated by GPCRs and ion channels. AP firing is reduced (red arrows) via activation of GPCRs coupling to the Gi/o pathway or activation of inhibitory ligand-gated ion channels. Gi/o pathway activation can lead to the activation of GIRK channels, inhibition of voltage-gated Ca2+ channels or increase in GABAA receptor currents. In addition, Gq/11 activation can lead to synthesis of endocannabinoids, which inhibit transmitter release onto 5-HT neurons. AP firing is increased (blue arrows) via activation of GPCRs coupling to the Gq/11 pathway or activation of excitatory ligand-gated ion channels. Gq/11 pathway activation can lead to the activation of non-selective cation channels or inhibition of K+ conductance. In addition, the activation of L-type Ca2+ channels via protein kinase C (PKC) can most likely increase membrane depolarization and transcription. (B) 5-HT release of 5-HT neurons is also modulated by GPCRs and ion channels. 5-HT release is reduced (red arrow) via activation of GPCRs coupling to the Gi/o pathway. Gi/o pathway activation leads to the inhibition of presynaptic Ca2+ channels, reduction in Ca2+ influx and therefore reduction in transmitter release. 5-HT release is increased (blue arrows) via activation of GPCRs coupling to the Gs pathway and via opening of excitatory, ligand-gated ion channels.
The DRN and MRN are the primary nuclei of 5-HT projections to forebrain and provide the neural substrate to communicate between global forebrain and other neuromodulatory systems by sending a wide range of 5-HT projections and receiving a wide variety of afferents (Jacobs and Azmitia, 1992). The DRN is located right beneath the posterior part of cerebella aqueduct and contains about half of all 5-HT neurons in the central nervous system (CNS), which can be further divided into six regions: rostral, caudal, dorsomedial, ventromedial, interfascicular, and lateral parts. The MRN is located at the ventral expansion of the DRN or the midline of the pontine tegmentum where many 5-HT neurons are densely packed in the midline and some 5-HT neurons are scattered in the periphery. Within the DRN and MRN 5-HT neurons project to defined target area in brain (Adell et al., 2002; Lechin et al., 2006). For example, DRN 5-HT neurons innervate the prefrontal cortex, lateral septum, and ventral hippocampus, while MRN 5-HT neurons innervate the temporal cortex, medial septum, and dorsal hippocampus. Afferent projections to the raphe nuclei are diverse and include acetylcholine (ACh) from the laterodorsal tegmental nucleus, dopamine from the substantia nigra and ventral tegmentum area, histamine from tuberomammillary hypothalamic nucleus, noradrenaline (NA) from the locus coeruleus, serotonin itself from the raphe nuclei and several neuropeptides as well as excitatory glutamatergic and inhibitory GABAergic inputs. Glutamatergic inputs come from several nuclei including medial prefrontal cortex and lateral habenula nucleus (Aghajanian and Wang, 1977; Celada et al., 2001; Adell et al., 2002; Bernard and Veh, 2012). Some inputs make direct contacts with 5-HT neurons, while others project onto local GABAergic interneurons that provide feedforward inhibitory input to 5-HT neurons (Sharp et al., 2007). In addition to the local GABAergic interneurons located in the raphe nuclei and in the neighboring periaqueductal gray area, extrinsic GABAergic projections have been suggested (Gervasoni et al., 2000). The responsiveness of 5-HT neurons to each of the inputs differs between DRN and MRN, and also within the subnuclei of the DRN (Adell et al., 2002; Lechin et al., 2006). The differences in responsiveness most likely depend on differences in the strength of the afferent inputs for each of the nuclei and subnuclei and also on the expression and types of the ionotropic and metabotropic receptors in 5-HT neurons. Furthermore, the intrinsic membrane excitability of 5-HT neurons has been reported to differ in the distinct raphe nuclei (Beck et al., 2004; Crawford et al., 2010). Importantly, beyond the anatomical and physiological differences, it has been reported that subpopulation of 5-HT neurons have distinct implications in specific physiological function and behavior (Abrams et al., 2004; Lechin et al., 2006; Hale and Lowry, 2010).
Auto-Regulation
The midbrain 5-HT neurons elicit spontaneous action potentials (APs), with a regular, slow firing pattern (1–5 APs/s; Aghajanian and Vandermaelen, 1982; Vandermaelen and Aghajanian, 1983). 5-HT released from 5-HT neurons act either on the 5-HT neuron itself or on the target circuits. There are several ways how 5-HT neurons may receive 5-HT. First, dendrodendritic synapses releasing 5-HT have been described in raphe nuclei between 5-HT neurons. Second, recurrent axonal collaterals have been suggested to back-propagate to the raphe nucleus itself to release 5-HT. Finally, 5-HT neurons between different raphe nuclei such as DRN and MRN communicate with each other via 5-HT (for review, see Adell et al., 2002; Harsing, 2006; Lechin et al., 2006). Indeed electrical stimulation in DRN slice preparations induces 5-HT1A receptor-mediated slow inhibitory postsynaptic potentials (IPSPs) in 5-HT neurons (Pan et al., 1989; Morikawa et al., 2000), demonstrating 5-HT release in the proximity of 5-HT neurons.
Once 5-HT is released, 5-HT receptors will be activated. Seven subgroups of 5-HT receptors encoding ionotropic as well as metabotropic receptors have been described (5-HT1–5-HT7), with 15 total variants identified to date (Barnes and Sharp, 1999; Hoyer et al., 2002; Kroeze et al., 2002). The 5-HT GPCRs can be divided into three major subgroups depending on which G protein signaling pathway they activate. 5-HT1 receptors couple mainly to the Gi/o pathway; 5-HT4, 5-HT5, 5-HT6, and 5-HT7 receptors couple to the Gs pathway; and 5-HT2 receptors activate the Gq/11 pathway. The 5-HT3 receptors are ligand-gated ion channels.
5-HT1A, 5-HT1B, and 5-HT1D receptors are found on somatodendritic and axonal region of 5-HT neurons (McDevitt and Neumaier, 2011). All three receptors act as negative feedback effectors for 5-HT neuronal firing and 5-HT release (Andrade, 1998). Somatodendritically located 5-HT1A receptors down-regulate the firing rate of 5-HT neurons via activation of G protein-coupled inwardly rectifying potassium channels (GIRK) leading to membrane hyperpolarization, and reduction or complete block of AP firing (Colino and Halliwell, 1987; Sprouse and Aghajanian, 1987; Blier et al., 1989; Hjorth and Sharp, 1991; Penington et al., 1993; Stamford et al., 2000). In addition, 5-HT1A receptors inhibit voltage-gated Ca2+ channels of the N- and P/Q-type in 5-HT neurons, as application of a selective 5-HT1A agonist diminishes somatic Ca2+ channel currents (Penington and Kelly, 1990; Penington et al., 1992; Bayliss et al., 1997b). The functional consequence of Ca2+ channel inhibition is an increase in the firing rate due to reduction in the afterhyperpolarization, which may involve Ca2+ activated-K+ channel (Bayliss et al., 1997b). The physiological role of the differential effects of 5-HT1A receptors on the AP firing has not been addressed so far, but may involve input specificity due to 5-HT1A/GIRK and 5-HT1A/Ca2+ channel colocalization in specific subcellular domains and/or differences in the regulatory properties of heterogeneous 5-HT neurons within and among different raphe nuclei (Calizo et al., 2011).
The predominant 5-HT receptors at the presynaptic terminal are the 5-HT1B/1D receptors. 5-HT1B/1D receptors have been shown to inhibit 5-HT release from the axonal varicosities as demonstrated with electrophysiological experiments (Sprouse and Aghajanian, 1987; Boeijinga and Boddeke, 1993; Morikawa et al., 2000), probably due to inhibition of Ca2+ influx through voltage-gated Ca2+ channels such as P/Q-type and N-type Ca2+ channel (Kimura et al., 1995; Harvey et al., 1996). The 5-HT1B receptors have been shown to underlie presynaptic autoinhibition of 5-HT release in which 5-HT1A-mediated slow IPSPs are reduced by previous released 5-HT activating presynaptic 5-HT1B receptors (Morikawa et al., 2000). In addition, 5-HT1B receptors have been suggested to up-regulate 5-HT reuptake by serotonin transporters (Xie et al., 2008; Hagan et al., 2012) and 5-HT synthesis by itself might be under the control of 5-HT1B (Hjorth et al., 1995). Thus, 5-HT1B autoreceptors may have the ability to control 5-HT release independently from the actual firing rate.
5-HT1F receptor mRNA has also been detected in the raphe nuclei (Bruinvels et al., 1994). Since 5-HT1F receptors have a high affinity for sumatriptan, a 5-HT1B/1D agonist, and the sumatriptan-induced reduction in 5-HT release (monitored by voltammetry in brain slices) could not be blocked by 5-HT1A/1B/1D receptor antagonists, 5-HT1F had been suggested as a possible candidate of a serotonergic autoreceptor in the MRN (Hopwood and Stamford, 2001).
While the expression and function of 5-HT1 receptors has been directly demonstrated in 5-HT neurons, involvement of other 5-HT receptors such as 5-HT2, 5-HT3, and 5-HT4-7 is less clear and may differ among species, the developmental stage of the animal and 5-HT neuron subtypes.
5-HT2 receptors are functionally expressed in particular on GABAergic interneurons in the DRN, since activation of 5-HT2A/2C receptors increase fast inhibitory postsynaptic current (IPSC) frequency in 5-HT neurons and reduce 5-HT neuronal firing as electrophysiologically measured in brain slices (Liu et al., 2000; Leysen, 2004). 5-HT2 receptor mRNA and proteins have been identified in the DRN and embryonic 5-HT neurons (Wright et al., 1995; Clemett et al., 2000; Wylie et al., 2010). Additionally, 5-HT2 receptors have been postulated to increase 5-HT1A-mediated responses in 5-HT neurons (Kidd et al., 1991). However, a direct modulatory effect of 5-HT2 in 5-HT neurons has not been demonstrated.
The 5-HT3 receptors have also been suggested to act as presynaptic autoreceptors in serotonergic nerve terminals. Although 5-HT3 receptors have been shown to enhance 5-HT release in various brain areas including the raphe nuclei as monitored by [3H]5-HT assays (Bagdy et al., 1998), there is no direct immunohistochemical and electrophysiological evidence of the presence of 5-HT3 receptors in 5-HT neurons (van Hooft and Vijverberg, 2000).
For the Gs protein-coupled 5-HT4-7 receptors, mainly indirect evidence exists for an autoregulatory role of these GPCRs in 5-HT neurons.
5-HT4 receptors seem to be located somatodendritically and presynaptically. A presynaptic potentiating effect of 5-HT4 receptor on glutamate release, which can be counteracted by 5-HT1A receptor-mediated inhibitory action, has been described in hippocampal neurons (Kobayashi et al., 2008). Since neurotransmitter release of various transmitters including 5-HT is modulated by 5-HT4 agonists, a presynaptic localization of 5-HT4 receptors on 5-HT neurons seems possible (Mengod et al., 2010).
While the function of 5-HT5 receptors in the CNS has not been thoroughly studied, two studies suggest a role of 5-HT5 receptors for modulating 5-HT neurons. First, 5-HT5B receptor mRNA, a receptor which is expressed in rodents but not in humans, is colocalized with the mRNA of 5-HT transporter in the DRN (Serrats et al., 2004). Second, block of 5-HT5A receptors in the DRN attenuates the 5-carboxamidotryptamine (5-CT; non-selective agonist in particular for 5-HT1A/1B/1D receptors) induced reduction of 5-HT neuronal firing but fail to affect 5-HT release measured using fast cyclic voltammetry in vitro (Thomas et al., 2006). The data suggest an autoreceptor modulation of 5-HT neurons via 5-HT5A receptors.
5-HT6 and 5-HT7 receptor protein and mRNA have been detected in cells in the raphe nuclei including the DRN (Ruat et al., 1993; To et al., 1995; Gustafson et al., 1996; Woolley et al., 2004; see also Gerard et al., 1997; Hamon et al., 1999), but a functional role as autoreceptors for modulating 5-HT neurons could not be demonstrated so far (Bourson et al., 1998; Roberts et al., 2001).
Thus, the auto-regulation of 5-HT neuronal firing is in particular regulated by 5-HT1A receptors via activation of the Gi/o pathway and opening K+ and closing Ca2+ conductance. At the presynaptic terminal, 5-HT1B/1D receptor activation reduces 5-HT release most likely via Gi/o protein-mediated inhibition of presynaptic Ca2+ channels. In addition, potentiation of 5-HT release by activating 5-HT4 receptors via the Gs pathway seems possible. Since other 5-HT receptor mRNAs have been detected in 5-HT neurons, other autoregulatory mechanisms may exist in subgroups of 5-HT neurons or during different developmental stages of the serotonergic transmitter system. In particular, animal models for the selective activation of these GPCRs during development will further elucidate the modulatory role of other 5-HT receptors in the auto-regulation of 5-HT neuronal firing and 5-HT release.
Hetero-Regulation
5-HT modulates various complex behaviors and therefore the serotonergic transmitter system receives feedback and feedforward information from other brain areas and networks involved in regulating the different behaviors (Adell et al., 2002; Lechin et al., 2006; Sharp et al., 2007). Thus, the hetero-regulation of the 5-HT neurons involves various transmitter systems.
Forty-nine different GPCRs belonging to all four GPCR subfamilies were identified in postmitotic embryonic 5-HT neurons using microarray expression profiling (Wylie et al., 2010). These GPCRs include adrenergic, calcitonin, cannabinoid, GABA, histamine, opioid, and serotonin receptors. For these transmitter systems, a postnatal modulatory role for 5-HT neurons has been described (see below). In addition, other GPCRs such as thrombin, chemokine, prostaglandin E, melanin-concentrating hormone, cadherin, and parathyroid hormone receptors as well as frizzled (FZD) and smoothened (SMO) homolog and the orphan receptors (GPR 19, 56, 85, 98, 125 135, 162, and 173) were also identified but a physiological role for their modulation of 5-HT neurons still needs to be defined and investigated (for a complete list, see Wylie et al., 2010). We will summarize recent findings on the intrinsic hetero-regulation of the serotonergic transmitter system.
GABA, Glycine, and Glutamate
5-HT neurons within the raphe nuclei receive in particular GABAergic but also glutamatergic input. As expected, the GABAergic input onto 5-HT neurons reduces the neuronal firing, while glutamatergic input increases the firing activity (Pan and Williams, 1989; Levine and Jacobs, 1992; Becquet et al., 1993a,b; for review, see Adell et al., 2002; Harsing, 2006). These effects have been mainly attributed to the expression of ionotropic GABA and glutamate receptors (GluRs) in 5-HT neurons (Tao and Auerbach, 2000; Gartside et al., 2007), which is in agreement with the expression of 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (AMPA), N-methyl-D-aspartate (NMDA), kainate receptors [GluR 1,2; NMDA-R-2B, kainate receptor 5 (Grik5)] as well as GABAA receptor subunits (GABAA β1–3 and γ2) in embryonic 5-HT neurons (Wylie et al., 2010). Interestingly, the glycine receptor α1 and β subunits have also been identified in human brain and mice embryonic 5-HT neurons (Baer et al., 2003; Wylie et al., 2010). Indeed the DRN receives input from glycinergic fibers (Rampon et al., 1996, 1999) to inhibit 5-HT neuronal firing and 5-HT release (Gallager and Aghajanian, 1976; Wang and Aghajanian, 1977; Becquet et al., 1993a).
The modulation of 5-HT neurons by GABAB receptors has been addressed in various studies. In general, activation of GABAB receptor by selective agonists decreases 5-HT release (Becquet et al., 1993b). The decrease of 5-HT release by GABAB receptors located within 5-HT neurons is most likely mediated via activation of GIRK channels leading to a reduction in AP firing (Innis and Aghajanian, 1987; Williams et al., 1988; Bayliss et al., 1997a; Cornelisse et al., 2007). Within 5-HT neurons, GABAB receptors are located extrasynaptically, suggesting that spillover of GABA during high activity of GABAergic neurons would modulate 5-HT neuronal activity within raphe nuclei (Varga et al., 2002). On the other hand, there is little information about modulatory effects of metabotropic GluRs (mGluRs) in 5-HT neurons so far. Although administration of group II mGluR (mGluR2/3) antagonist has been reported to increase 5-HT neuronal activity, an indirect effect on presynaptic excitatory neurons seems to be involved (Kawashima et al., 2005).
Corelease of Glutamate or GABA from 5-HT Neurons
Previous reports have suggested the possibility of glutamate release from 5-HT neurons based on the presence of vesicular glutamate transporter type 3 in a subset of 5-HT neurons (Gras et al., 2002; Amilhon et al., 2010). Using optogenetic techniques, the corelease of glutamate and 5-HT from serotonergic terminals could be demonstrated in a serotonergic projection from the MRN to hippocampal GABAergic interneurons (Varga et al., 2009). The serotonergic fibers make direct synaptic contacts to the GABAergic neurons and exert fast synaptic transmission mediated by ionotropic GluRs and 5-HT3 receptors. In addition to the glutamate transporters, GABA and its synthesizing enzyme, glutamic acid decarboxylase (GAD) have also been reported in subsets of 5-HT neurons, suggesting the corelease of GABA and 5-HT (Nanopoulos et al., 1982; Belin et al., 1983; Fu et al., 2010; Hioki et al., 2010; Shikanai et al., 2012). However, vesicular inhibitory amino acid transporter which is necessary for filling synaptic vesicles with GABA was absent in 5-HT/GAD67 positive neurons and their projections, suggesting that GABA may be released by non-vesicular mechanisms such as a reverse transport through GABA transporters (Shikanai et al., 2012). Thus, 5-HT axonal projections have a potential to modulate the neuronal activity in target areas using at least three different transmitters, i.e., 5-HT, glutamate, and GABA. It is intriguing to speculate that the auto-regulation of 5-HT neurons itself might be modulated by corelease of glutamate and GABA.
Acetylcholine
The DRN also receives cholinergic input from the laterodorsal tegmental nucleus (Wang et al., 2000). Modulation of 5-HT neurons by ACh mainly involves nicotinic ACh receptors (nAChRs) and can increase 5-HT neuronal firing (Mihailescu et al., 2002) for example, via presynaptic modulation of glutamate release (Garduno et al., 2012) or via opening of nAChR expressed in 5-HT neurons (Galindo-Charles et al., 2008; Chang et al., 2011). Very limited information is available for the expression and function of muscarinic ACh receptors (mAChRs) in 5-HT neurons. mAChR-M1 receptors (Gq/11) might be expressed on serotonergic projections into the hippocampus (Rouse and Levey, 1996) and application of mAChR antagonist, atropine into the DRN enhances antidepressant-like 5-HT1A agonist effects (Haddjeri et al., 2004), which may involve M2 (Gi/o) but not M1 (Gq/11) receptors (Haddjeri et al., 2000). Nevertheless a detailed and direct demonstration of the involvement of mAChRs in 5-HT neurons is essential.
Dopamine
The 5-HT neurons of the DRN reciprocally interact with the dopaminergic mesencephalic transmitter system involving dopamine receptors. D1-like receptors (D1 and D5) couple to the Gs pathway, while dopamine D2-like receptors (D2-4) have been described to couple to the Gi/o pathway. In the DRN, D2 and D3 receptor expression has been detected so far, with very little or no expression of D1-like receptors (Bouthenet et al., 1987; Dawson et al., 1988; Cortes et al., 1989; Wamsley et al., 1989; Yokoyama et al., 1994; Suzuki et al., 1998). Dopamine increases 5-HT neuronal firing in DRN in vivo and in slice electrophysiological recording, and also increases 5-HT release detected by in vivo microdialysis in DRN and other brain areas (Ferre and Artigas, 1993; Ferre et al., 1994; Matsumoto et al., 1996; Mendlin et al., 1998; Haj-Dahmane, 2001; Martin-Ruiz et al., 2001). The modulatory effects on 5-HT neurons have been shown to be mediated by D1- and D2-like receptors located outside the DRN (Martin-Ruiz et al., 2001) or by direct activation of D2 receptors expressed in 5-HT neurons (Haj-Dahmane, 2001). Activation of D2-like receptors in 5-HT neurons leads to membrane depolarization, involving activation of G proteins, phospholipase C, and a non-selective cation current, most likely mediated by a transient receptor potential (TRP) channel (Aman et al., 2007). The D2-like receptor effects in 5-HT neurons suggest that D2-like receptors may activate the Gq/11 rather than the Gi/o signaling pathway. The Gq/11 protein coupling might be explained by the heterodimerization between D1- and D2-like receptors (Rashid et al., 2007; Hasbi et al., 2010). It has to be noted that the experiments suggesting the modulation of 5-HT neurons by intrinsic D2-like receptors have been performed with relatively high concentrations of quinpirole and sulpiride in in vitro preparations. Therefore, the experiments have to be interpreted carefully.
Noradrenaline
5-HT neurons in the raphe nuclei receive noradrenergic input in particular from the locus coeruleus (Adell et al., 2002; Lechin et al., 2006). α1 and α2 adrenergic receptor mRNA and protein have been detected in the DRN and MRN (Unnerstall et al., 1985; Rosin et al., 1993; Scheinin et al., 1994; Talley et al., 1996; Day et al., 1997; Strazielle et al., 1999). α1 and α2 adrenergic receptors couple to the Gq/11 and Gi/o pathway, respectively. Therefore depending on pathway activation, an increase or decrease in 5-HT neuronal activity and 5-HT release can be postulated if adrenergic receptors are expressed in 5-HT neurons. Early studies revealed that NA causes an increase in 5-HT neuronal firing in DRN. This effect has been suggested to be mediated via activation of α1 adrenoceptors (presumably α1B adrenoceptor subtype) located on 5-HT neurons (Vandermaelen and Aghajanian, 1983; Day et al., 1997) and may involve the suppression of a 4-aminopyridine sensitive K+ conductance (IA; Aghajanian, 1985). In vivo experiments suggest that α1 adrenoceptors are tonically activated by endogenous NA (Adell and Artigas, 1999; Pudovkina et al., 2003). In contrast, 5-HT release detected by voltammetry or [3H]5-HT assay in the DRN slice preparation is inhibited by NA, an effect which has been attributed to α2 adrenoceptors (involving α2A adrenoceptor subtype) and also perhaps indirectly to α1 adrenoceptors (Frankhuijzen et al., 1988; Hopwood and Stamford, 2001). Since α2 receptors couple to the Gi/o pathway, 5-HT release and 5-HT neuronal firing could be reduced via α2 adrenoceptors located at the soma or presynaptic terminal of 5-HT neurons itself (Hopwood and Stamford, 2001), or via inhibition of NA release at noradrenergic synaptic terminals lowering the effective NA concentration for α1 adrenoceptors/Gq signaling pathway activation. The direct effect of α2 adrenoceptors in 5-HT neurons is supported by the fact that embryonic 5-HT neurons express α2A adrenoceptors (Wylie et al., 2010).
Histamine
There are four histamine receptors (H1-4), which couple to different G protein pathways, i.e., H1 (Gq/11), H2 (Gs), H3, and H4 (Gi/o). Early studies suggested that histamine reduces the firing of 5-HT neurons in the DRN (Lakoski and Aghajanian, 1983) via H2 receptors (Lakoski et al., 1984). Since H2 couples to the Gq/11 pathway, the results suggest that H2 receptors are localized on GABAergic terminals. Later findings showed that histamine increased 5-HT neuronal firing in the DRN via activation of H1 receptors and the opening of a non-selective cation conductance through Gq/11 signaling pathways (Barbara et al., 2002; Brown et al., 2002). Expression profiling in mouse embryos suggested the expression of H3 receptors in 5-HT neurons, which is consistent with high mRNA levels in the DRN (Lovenberg et al., 1999; Drutel et al., 2001; Pillot et al., 2002). However, the low binding of a H3 receptor selective radioligand in the DRN suggests that H3 receptors are mainly functional at the presynaptic terminal of 5-HT projections (Pillot et al., 2002). Indeed increasing levels of histamine decrease 5-HT release detected by an in vivo electrochemical technique (Hashemi et al., 2011).
Endocannabinoids
The cannabinoid receptor family consists of two subtypes, CB1 and CB2. Modulation of neuronal activity is mainly exerted via CB1, which couples to the Gi/o protein (Howlett et al., 1986) and probably also to the Gs pathway (Glass and Felder, 1997). CB1 receptors are localized in particular at presynaptic terminals, where they inhibit presynaptic Ca2+ influx and reduce transmitter release via endocannabinoid (eCB)-mediated retrograde signaling (Maejima et al., 2001). CB1 receptors are expressed in serotonergic fibers (Haring et al., 2007; Ferreira et al., 2012) and their mRNA is found early in development (Wylie et al., 2010). 5-HT release in projection areas from the DRN is reduced by activation of CB1 as monitored by microdialysis (Egashira et al., 2002) and [3H]5-HT assay (Nakazi et al., 2000; Egashira et al., 2002), and increased by inhibition of CB1 in vivo and in vitro (Darmani et al., 2003; Tzavara et al., 2003; Aso et al., 2009). Studies in CB1 knock-out animals and chronic activation of CB1 receptors in vivo suggest that CB1 may regulate the function and expression of 5-HT1A receptors (Aso et al., 2009; Moranta et al., 2009; Zavitsanou et al., 2010). Interestingly, 5-HT neurons itself synthesize eCBs in an activity-dependent manner (Haj-Dahmane and Shen, 2009, 2011). eCB release from 5-HT neurons can be induced by orexin-B leading to the activation of orexin (OX) receptors via the Gq/11 pathway (Liu et al., 2002b; Haj-Dahmane and Shen, 2005). It has been therefore speculated that the activity-dependent activation of the Gq/11 pathway in general may lead to the production of eCBs in 5-HT neurons (Haj-Dahmane and Shen, 2011). Within the DRN eCBs mainly act on glutamatergic terminals and probably also on GABAergic terminals (Liu et al., 2002b; Haj-Dahmane and Shen, 2009; Mendiguren and Pineda, 2009; Tao and Ma, 2012), leading to a reduction in glutamate and GABA release onto 5-HT neurons and therefore changes the activity of the 5-HT neurons itself.
Frizzled Receptors
Four frizzled receptors (FZD1–3 and SMO) have been detected in postmitotic embryonic 5-HT neurons (Wylie et al., 2010). These receptors mainly couple to the Wnt signaling cascade and are most likely involved in the development and maturation of 5-HT neurons (Simon et al., 2005; Song et al., 2012). However, the role of frizzled receptors in 5-HT neurons remains to be determined.
Neuropeptides
Dr. Hökfelt’s laboratory demonstrated that various peptide transmitters are expressed in the DRN with species-specific differences between mice and rats (Fu et al., 2010). The identified neuropeptides are cholecystokinin (CCK), calcitonin gene-related peptide (CGRP), vasoactive intestinal peptide (VIP), somatostatin, substance P, dynorphin, neurotensin, thyrotropin-releasing hormone (TRH), enkephalin, galanin, neuropeptide Y (NPY), and corticotropin-releasing factor (CRF). Among these, various peptide receptors have been identified and functionally described in 5-HT neurons.
Bombesin
To date three types of bombesin receptors have been described, i.e., the neuromedin B (NMB) receptor (BB1 receptor), the gastrin-releasing peptide (GRP) receptor (BB2 receptor) and the bombesin receptor subtype 3 (BRS-3 or BB3 receptor; Jensen et al., 2008). The NMB receptors are functionally expressed in 5-HT neurons in the DRN (Pinnock et al., 1994; Woodruff et al., 1996). BB1 receptor activation leads to an increase in 5-HT neuronal firing via suppression of K+ current, involving most likely the activation of the Gq/11 pathway (Benya et al., 1992; Woodruff et al., 1996) and as a consequence, an increase in 5-HT release to projection site such as the hippocampus, which is monitored by in vivo microdialysis (Merali et al., 2006).
Calcitonin
Calcitonin receptor (CalcR) mRNA has been localized in 5-HT neurons in the DRN (Nakamoto et al., 2000), which is in agreement with the expression profiling studies of postmitotic embryonic 5-HT neurons (Wylie et al., 2010). The CalcR couples to the Gs pathway (Hay et al., 2005) and Gq/11 pathway (Offermanns et al., 1996). Since high levels of amylin binding sites are detected in the DRN (Sexton et al., 1994) and mRNA for CGRP has been localized in 5-HT positive axon terminals in monkeys (Arvidsson et al., 1990), it is most likely that CalcR assemble with receptor activity-modifying proteins (RAMPs) in 5-HT neurons to respond to the various peptide transmitters (i.e., CGRP, adrenomedullin, and amylin; Tilakaratne et al., 2000). A direct function of CalcR in 5-HT neurons has not yet been demonstrated. However, injection of CGRP into rats induces anxiety-like behaviors and increases c-Fos expression in the DRN (Sink et al., 2011).
Chemokine Receptors
Two subtypes of the 18 identified chemokine receptors have been detected in microarray analyses from embryonic 5-HT neurons, i.e., Duffy antigen/chemokine (C-X-C motif) receptor 4 (CXCR4; Wylie et al., 2010). CXCR4 is expressed in the majority of 5-HT neuron outer membranes in the DRN (Heinisch and Kirby, 2010). So far only an indirect action of CXCR4 for modulation of 5-HT neuron has been demonstrated, since application of the CXCR4 ligands and antagonists modulate GABA and glutamate release onto 5-HT neurons (Heinisch and Kirby, 2010), which is in agreement with the described Gi/o protein-mediated inhibition of Ca2+ channels via CXCR4 (Oh et al., 2002). In addition, CX3CR1 is also expressed in 5-HT neurons in the DRN and MRN (Heinisch and Kirby, 2009). Here the CX3CR1 specific ligand, fractalkine/CX3CL1 increased evoked IPSC amplitude on 5-HT neurons (Heinisch and Kirby, 2009), an effect which is most likely mediated postsynaptically and not presynaptically. The effect is surprising, since CX3CR1 has been described to couple to the Gi/o pathway (Oh et al., 2002), which would inhibit synaptic transmitter release and induce paired-pulse facilitation (PPF) if activated on GABAergic terminals. Therefore, the CX3CL1 could increase/modulate GABAA receptor trafficking and GABAA receptor currents in 5-HT neurons via activation of CX3CR1. A signaling function for Duffy antigen remains to be determined.
Cholecystokinin
The expression of CCK receptors in 5-HT neurons in the DRN has also been suggested. Application of CCK increases 5-HT neuronal firing, which is blocked by the CCK1 antagonist L-364,718 (Boden et al., 1991). In addition, 5-HT release measured as outflow of [3H]5-HT in cortical slices is increased by CCK-4, which involves CCK2 receptors (Siniscalchi et al., 2001). Both CCK1 and CCK2 receptors mainly couple to the Gq/11 and Gs pathway (de Weerth et al., 1993; Lee et al., 1993; Ulrich et al., 1993).
Corticotropin-Releasing Factor
Two CRF (CRF1 and CRF2) receptor subtypes have been described in brain and both seem to be localized in GABAergic neurons in the DRN as well as in 5-HT and non-5-HT neurons with differential subcellular localizations (for review, see Valentino et al., 2010). CRF1 and CRF2 couple to the Gs pathway (Chang et al., 1993; Perrin et al., 1993; Vita et al., 1993; Lovenberg et al., 1995; Liaw et al., 1996) and also the Gq/11 pathway in heterologous expression systems (Dautzenberg et al., 2004), leading to the assumption that stimulation of CRF1 or CRF2 will increase neuronal firing. Indeed, activation of CRF1 on GABAergic neurons increases GABA release onto 5-HT neurons, while activation of CRF2 elicits an inward current in 5-HT neurons (Kirby et al., 2008). Based on the differential localization of the CRF receptors within the DRN and its receptor type-specific action on 5-HT neurons, it has been suggested that at low concentrations of CRF, 5-HT neuronal activity is decreased, while at high concentrations, 5-HT neuronal activity is increased (Kirby et al., 2008; Valentino et al., 2010).
Galanin
The neuropeptide galanin activates three types of galanin receptors (GalR1-3). GalR1 and GalR2 are highly expressed in the DRN (Melander et al., 1988; Larm et al., 2003; Lu et al., 2005; Sharkey et al., 2008). Moreover, GalR1 expression has also been described in 5-HT neurons from rats but not in mice (Xu et al., 1998; Larm et al., 2003). GalR activation in the DRN causes a K+ conductance-mediated hyperpolarization in rat brain slices (Xu et al., 1998), most likely via GalR3-mediated Gi/o activation of GIRK channels (Swanson et al., 2005). These effects are in agreement with in vivo microdialysis studies showing that injection of galanin into the DRN reduces 5-HT release via GalR activation in the hippocampus (Kehr et al., 2002). In contrast to the inhibitory action of galanin on 5-HT neuronal activity, a reduction in inhibitory input onto 5-HT neurons has also been described (Sharkey et al., 2008). Here, the pan GalR1-3 agonist reduced GABA-mediated fast synaptic transmission accompanied by increase of PPF, suggesting that GalRs are expressed on GABAergic terminals and inhibit presynaptic Ca2+ channels via the Gi/o pathway. On the other hand, GalR2 agonist, galanin (2–11) reduced IPSP amplitude but did not cause PPF, suggesting a postsynaptic action (Branchek et al., 2000; Sharkey et al., 2008). Additionally, galanin (2–11) was demonstrated to increase 5-HT release in hippocampal tissue by immunofluorescence and high-performance liquid chromatography (HPLC) measurement (Mazarati et al., 2005). Therefore GalR2 receptors may activate 5-HT neurons via reduction in GABAergic input onto 5-HT neurons and/or via Gq/11-mediated increase in 5-HT neuronal firing. Galanin also modulates 5-HT1A autoreceptor responses in vivo. A possible mechanism of this modulation could be the heterodimerization of GalR with 5-HT1A which has been observed in heterologous expression systems (for review, see Kuteeva et al., 2008; Borroto-Escuela et al., 2010).
Hypocretin–Orexin
The two hypocretin/orexin (OX1 and OX2) receptors are expressed in tryptophan hydroxylase-positive neurons in the DRN (Brown et al., 2002). Their intracellular signaling targets are rather complex involving activation of Gi/o, Gq/11, Gs, and other G proteins (Scammell and Winrow, 2010). Orexin positive fibers project onto GABAergic as well as 5-HT neurons in the DRN (Peyron et al., 1998). Application of the neuropeptides orexin-A and orexin-B causes a Na+/K+ non-selective cation current in 5-HT neurons (Brown et al., 2002; Liu et al., 2002b; Kohlmeier et al., 2008), suggesting that activation of OX1 and OX2 leads to the increase of 5-HT neuronal firing. The neuropeptides also induce GABA release onto 5-HT neurons at higher peptide concentrations (Liu et al., 2002b). In addition, orexin increases the somatic L-type Ca2+ current in 5-HT neurons in a protein kinase C-dependent manner (Kohlmeier et al., 2008). It has therefore been suggested that modulation of Ca2+ transients by orexin may be involved in the transcriptional regulation of long-term processes (Kohlmeier et al., 2008).
Opioids
Raphe nuclei receive dynorphinergic, enkephalinergic, and β-endophinergic innervation (Adell et al., 2002). These transmitters activate μ, κ and δ opioid receptors, which primarily couple to the Gi/o pathway. Injection of morphine into the DRN causes an increase in 5-HT release detected in forebrain microdialysis (Tao and Auerbach, 1994). The increase in 5-HT release is most likely mediated via Gi/o protein-mediated inhibition of GABAergic interneurons in the DRN, involving μ opioid receptors located on GABAergic neurons (Jolas and Aghajanian, 1997). The modulation of 5-HT release in the DRN by κ and δ opioid receptors has also been described (Tao and Auerbach, 2002). Activation of δ receptors increased, while activation of κ receptors decreased 5-HT release measured with in vivo microdialysis. The κ receptors effects do not involve the modulation of GABAergic or glutamatergic inputs in the DRN (Tao and Auerbach, 2002), suggesting that κ receptors are expressed in 5-HT neurons. Likewise, opioid receptor-like1 (Oprl1) are most likely located and expressed in 5-HT neurons as follows. Oprl1 or nociceptin (NOP) receptors belong to the opioid receptor family but are activated by NOP (orphanin FQ), a neuropeptide derived from prepronociceptin protein. High levels of NOP receptor binding sites have been detected in the DRN (Florin et al., 2000) and Oprl1 receptor expression could be detected in embryonic 5-HT neurons (Wylie et al., 2010). NOP/orphanin FQ inhibits 5-HT release in the DRN via Oprl1 (Tao et al., 2007), suggesting a functional role of Oprl1 in 5-HT neurons early in development and in the adult brain.
Substance P
Substance P belongs to the tachykinin family and has a high affinity for the three different neurokinin receptors (NK1-3), in particular to NK1 (Hokfelt et al., 2001). These GPCRs couple mainly to the Gq/11 pathway (Stratowa et al., 1995), but Gs pathway activation has also been reported for NK1 in cell culture systems (Martini et al., 2002). Various histological studies have revealed extensive expression of NK1 receptors in the DRN (Maeno et al., 1993; Saffroy et al., 1994; Vigna et al., 1994; Charara and Parent, 1998; Sergeyev et al., 1999; Froger et al., 2001). Most studies suggest that NK1 receptors are not localized on 5-HT neurons (Froger et al., 2001; Santarelli et al., 2001), while others revealed NK1 receptor expression in a subpopulation of 5-HT neurons (Santarelli et al., 2001; Lacoste et al., 2006, 2009). Interestingly, NK1 receptors are found in the cytoplasm of the 5-HT neurons and in dendritic membranes of GABAergic neurons. After administration of NK1 antagonist or deafferentation of substance P releasing projections, the density of membrane bound NK1 receptors is increased in the somatodendritic region of 5-HT neurons, suggesting that membrane trafficking of NK1 receptors may be regulated by Substance P input. This mechanism may contribute to the modulation of 5-HT neuronal firing under certain physiological conditions (Lacoste et al., 2009). In addition, controversial results exist for the effect of NK1 on 5-HT release and firing of 5-HT neurons. Inhibition of NK1 in the DRN using antagonists or knock-out strategies leads to an increase in firing activity of 5-HT neurons in vivo (Haddjeri and Blier, 2001; Santarelli et al., 2001). In contrast, activation of NK1 and NK3 increases spontaneous excitatory postsynaptic currents (EPSCs) in DRN 5-HT neurons resulting in an increased firing of the 5-HT neurons as observed in brain slice recording (Liu et al., 2002a). These effects could be blocked by NK1 and NK3 antagonists (Liu et al., 2002a). Also, activation of NK1 via intra-raphe injection of substance P in the DRN increases 5-HT release within the DRN, but decreases 5-HT release in frontal cortex as measured with in vivo microdialysis (Guiard et al., 2007). These effects and also the described increase in 5-HT firing in NK1 knock-out mice involve changes in 5-HT1A autoreceptor levels, suggesting at least a functional coupling between NK1 and 5-HT1A receptors. Further investigations to verify these interactions in 5-HT neurons are required.
In summary, the various heteroreceptors integrate incoming information via two main pathways, i.e., Gi/o and Gq/11 leading to inhibition or activation of 5-HT neuronal firing and 5-HT release, respectively. Besides the “classical” Gi/o protein-mediated, membrane-delimited modulation of GIRK and presynaptic Ca2+ channels, other ion channel targets have been identified in 5-HT neurons. For example, two-pore-domain K+ channels [TWIK-related acid-sensitive K-1 (TASK-1) and TASK-3] have been described in dorsal and caudal raphe 5-HT neurons (Washburn et al., 2002). TASK channels are inhibited by GPCRs coupling to the Gq/11 pathway most likely in a membrane-delimited manner involving the direct binding of Gαq subunits (Chen et al., 2006). The existence of voltage-sensitive but not ATP-dependent K+ channels in DRN neurons including 5-HT neurons have been proposed based on drug application studies (Harsing, 2006). In addition, TRP channels have been described to be modulated by D2-like receptors (Aman et al., 2007). According to the microarray expression profiling studies, various ion channel targets of GPCRs are expressed in embryonic 5-HT neurons including TRP (Trpm4 and Trpm7), two-pore channels (TPCN1), cyclic nucleotide gate channels (Hcn3), and KCNQ (Kcnq2; Wylie et al., 2010). Therefore, more detailed studies have to be performed to determine the role of other ion channel targets and in particular long-term effects of GPCR modulation for the serotonergic system.
Integration and Signal Processing of Modulatory Information by Serotonergic Neurons: Why So Many GPCRs?
The serotonergic transmitter system modulates many physiological functions such as mood, sexual behavior, feeding, sleep/wake cycle, memory, cognition, blood pressure regulation, breathing, and reproductive success (Mooney et al., 1998; Abrams et al., 2004; Lechin et al., 2006; Lerch-Haner et al., 2008; McDevitt and Neumaier, 2011). Because of the complexity and variety of the different behaviors modulated by serotonin, it is expected that modulatory signals from other brain areas including sensory information is integrated by GPCR signals in nuclei containing 5-HT neurons using a high diversity of GPCRs. While GABA and glutamatergic input into the raphe nuclei will adjust 5-HT neurons to the current inhibitory/excitatory state of the brain, other transmitter systems will inform 5-HT neurons more directly about the serotonin-associated behavior. For example, dopamine is involved in reward-driven learning; ACh modulates arousal and reward; NA and CRF are involved in stress responses; histamine is involved in sleep regulation and sexual function; bombesin and CCK regulate eating behavior; eCBs modulate memory, appetite, stress, social behavior, anxiety, and sleep; galanin has been implicated in the regulation of sleep–wake cycle, cognition, emotion, and blood pressure; hypocretin–orexin modulate arousal, wakefulness, and appetite; and opioids and substance P are involved in pain perception and mood (White and Rumbold, 1988; Woodruff et al., 1996; Greenough et al., 1998; Bear et al., 2001; Merali et al., 2006; Monti, 2010; Haj-Dahmane and Shen, 2011). Since all different behavioral responses can be integrated in nuclei containing 5-HT neurons, regulation of serotonin release will affect similar behaviors as stated above. Thus there is a tight interaction and signaling exchange between the different transmitter systems to precisely modulate behavioral output. Consequently, long-term changes in serotonin release can involve changes in the auto-regulation involving 5-HT receptor or hetero-regulation involving the above mentioned GPCRs and can cause neuropsychiatric disorders, most notably depression, anxiety, schizophrenia, and dementia (Lucki, 1998; Davidson et al., 2000; Mann et al., 2001; Nelson and Chiavegatto, 2001). The modulation of the different behaviors is even more complex since other GPCRs, such as orphan GPCRs, with so far unknown function are also expressed in 5-HT neurons. Therefore, new strategies and techniques have to be applied and developed to understand complex behaviors related to the serotonergic system.
New Approaches to Control and Understand Serotonergic G Protein-Coupled Receptor Signaling Pathways
Recently, several new approaches to manipulate the activity of 5-HT neurons in a cell type-specific manner have been developed. Various promoter/enhancer sequences have been isolated and characterized, which allow for the expression of proteins of choice within at least subsets of 5-HT neurons. These DNA sequences include the promoter or enhancer sequences of Pet-1/Fev transcription factor, the serotonin transporter (SLC6A4), and the tryptophan hydroxylase 2 (TPH2; Scott et al., 2005). Using these different promoter/enhancer sequences, different mouse lines and virus approaches have been developed to activate and/or silence/delete reporter genes such as green fluorescent protein (GFP) or tdTomato, Cre or Flip recombinases, tetracycline inducible systems, tetanus toxin light chain, and genes of interest (Scott et al., 2005; Kim et al., 2009; Madisen et al., 2010, 2012; Richardson-Jones et al., 2010; Zhao et al., 2011). Using the tetracycline inducible system, for example, the genetic ablation of 5-HT1A from the majority of 5-HT neurons could be achieved (Richardson-Jones et al., 2010, 2011).
For the investigation of the modulation and function of neuronal circuits in general and for the serotonergic system in particular, chemical and optogenetic techniques have been developed in recent years (Herlitze and Landmesser, 2007; Masseck et al., 2010). For control of neuronal activity in various neuronal circuits including the 5-HT system, the light-gated non-selective cation channel, ChR2 has been used and allows for the dissection of 5-HT-mediated behavioral effects in different raphe nuclei (Li et al., 2005; Varga et al., 2009; Zhao et al., 2011; Madisen et al., 2012; see also Kim et al., 2009). For the investigation of GPCR signals, various chemically and light-activated GPCRs have been developed (Masseck et al., 2010). For example vertebrate rhodopsin (vRh) has been used to regulate Gi/o signaling pathways in neurons by light (Li et al., 2005). Exogenously expressed vRh inhibits neuronal firing and neurotransmitter release in vitro and in vivo most likely via activation of GIRK and inhibition of presynaptic Ca2+ channels (Li et al., 2005; Oh et al., 2010; Gutierrez et al., 2011). Since vRh belongs to class A or rhodopsin like group of GPCRs, like serotonergic autoreceptors 5-HT1A/1B/1D, the idea arose to generate light-activated chimeric receptors which couple to intracellular signaling pathways in 5-HT1 receptor domains. The feasibility of receptor domain swapping has been demonstrated by Dr. Khorana’s group (Kim et al., 2005). They replaced the intracellular loops of vRh with that of the β2-adrenergic receptor and turned vRh into a light-activated Gs protein-coupled receptor, Opto-β2AR. The approach however was not suitable for the exchange of vRh intracellular peptide loops by the 5-HT1A intracellular receptor domains, since this chimeric receptor revealed altered activation and deactivation kinetics in respect to GIRK channel modulation (Oh et al., 2010). However, it could be shown that the C-terminus (CT) of the 5-HT1A receptor was sufficient to target vRh into expression domains of 5-HT1A receptor and functionally substitute for Gi/o pathway activation. The CTs of GPCRs contain peptide signal domains for subcellular targeting and G protein interaction. The CT of 5-HT1A had been shown to be necessary for trafficking of the receptor to dendritic domains via interaction with trafficking protein Yif1B (Carrel et al., 2008). Fusion of the 5-HT1A receptor CT onto vRh was therefore sufficient to target the chimeric construct Rh-CT5-HT1A into somatodendritic regions of hippocampal neurons and 5-HT neurons in the DRN and exclude the expression of the chimeric receptor from the axons. The Rh-CT5-HT1A receptors were able to functionally substitute for intracellular 5-HT1A signals in DRN neurons of 5-HT1A knock-out mice, i.e., light illumination induced K+ conductance (most likely GIRK) reduced the firing rate of spontaneously active 5-HT neurons. Thus, the adjustment of for example light-activated or chemically activated GPCRs and their coupling and anchoring to and in intracellular signaling domains will allow for the dissection of multiple GPCR pathways within the serotonergic system and their interaction in vitro and in vivo.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abrams, J. K., Johnson, P. L., Hollis, J. H., and Lowry, C. A. (2004). Anatomic and functional topography of the dorsal raphe nucleus. Ann. N. Y. Acad. Sci. 1018, 46–57.
Adell, A., and Artigas, F. (1999). Regulation of the release of 5-hydroxytryptamine in the median raphe nucleus of the rat by catecholaminergic afferents. Eur. J. Neurosci. 11, 2305–2311.
Adell, A., Celada, P., Abellan, M. T., and Artigas, F. (2002). Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res. Brain Res. Rev. 39, 154–180.
Aghajanian, G. K. (1985). Modulation of a transient outward current in serotonergic neurones by alpha 1-adrenoceptors. Nature 315, 501–503.
Aghajanian, G. K., and Vandermaelen, C. P. (1982). Intracellular recordings from serotonergic dorsal raphe neurons: pacemaker potentials and the effect of LSD. Brain Res. 238, 463–469.
Aghajanian, G. K., and Wang, R. Y. (1977). Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 122, 229–242.
Aman, T. K., Shen, R. Y., and Haj-Dahmane, S. (2007). D2-like dopamine receptors depolarize dorsal raphe serotonin neurons through the activation of nonselective cationic conductance. J. Pharmacol. Exp. Ther. 320, 376–385.
Amilhon, B., Lepicard, E., Renoir, T., Mongeau, R., Popa, D., Poirel, O., et al. (2010). VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J. Neurosci. 30, 2198–2210.
Andrade, R. (1998). Regulation of membrane excitability in the central nervous system by serotonin receptor subtypes. Ann. N. Y. Acad. Sci. 861, 190–203.
Arvidsson, U., Schalling, M., Cullheim, S., Ulfhake, B., Terenius, L., Verhofstad, A., et al. (1990). Evidence for coexistence between calcitonin gene-related peptide and serotonin in the bulbospinal pathway in the monkey. Brain Res. 532, 47–57.
Aso, E., Renoir, T., Mengod, G., Ledent, C., Hamon, M., Maldonado, R., et al. (2009). Lack of CB1 receptor activity impairs serotonergic negative feedback. J. Neurochem. 109, 935–944.
Baer, K., Waldvogel, H. J., During, M. J., Snell, R. G., Faull, R. L., and Rees, M. I. (2003). Association of gephyrin and glycine receptors in the human brainstem and spinal cord: an immunohistochemical analysis. Neuroscience 122, 773–784.
Bagdy, E., Solyom, S., and Harsing, L. G. Jr. (1998). Feedback stimulation of somatodendritic serotonin release: a 5-HT3 receptor-mediated effect in the raphe nuclei of the rat. Brain Res. Bull. 45, 203–208.
Barbara, A., Aceves, J., and Arias-Montano, J. A. (2002). Histamine H1 receptors in rat dorsal raphe nucleus: pharmacological characterisation and linking to increased neuronal activity. Brain Res. 954, 247–255.
Barnes, N. M., and Sharp, T. (1999). A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152.
Bayliss, D. A., Li, Y. W., and Talley, E. M. (1997a). Effects of serotonin on caudal raphe neurons: activation of an inwardly rectifying potassium conductance. J. Neurophysiol. 77, 1349–1361.
Bayliss, D. A., Li, Y. W., and Talley, E. M. (1997b). Effects of serotonin on caudal raphe neurons: inhibition of N- and P/Q-type calcium channels and the afterhyperpolarization. J. Neurophysiol. 77, 1362–1374.
Bear, M. F., Connors, B. W., and Paradiso, M. A. (2001). Neuroscience: Exploring the Brain. Baltimore, MD: Lippincott Williams & Wilkins.
Beck, S. G., Pan, Y. Z., Akanwa, A. C., and Kirby, L. G. (2004). Median and dorsal raphe neurons are not electrophysiologically identical. J. Neurophysiol. 91, 994–1005.
Becquet, D., Hery, M., Deprez, P., Faudon, M., Fache, M. P., Giraud, P., et al. (1993a). N-methyl-D-aspartic acid/glycine interactions on the control of 5-hydroxytryptamine release in raphe primary cultures. J. Neurochem. 61, 1692–1697.
Becquet, D., Hery, M., Francois-Bellan, A. M., Giraud, P., Deprez, P., Faudon, M., et al. (1993b). Glutamate, GABA, glycine and taurine modulate serotonin synthesis and release in rostral and caudal rhombencephalic raphe cells in primary cultures. Neurochem. Int. 23, 269–283.
Belin, M. F., Nanopoulos, D., Didier, M., Aguera, M., Steinbusch, H., Verhofstad, A., et al. (1983). Immunohistochemical evidence for the presence of gamma-aminobutyric acid and serotonin in one nerve cell. A study on the raphe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res. 275, 329–339.
Benya, R. V., Wada, E., Battey, J. F., Fathi, Z., Wang, L. H., Mantey, S. A., et al. (1992). Neuromedin B receptors retain functional expression when transfected into BALB 3T3 fibroblasts: analysis of binding, kinetics, stoichiometry, modulation by guanine nucleotide-binding proteins, and signal transduction and comparison with natively expressed receptors. Mol. Pharmacol. 42, 1058–1068.
Bernard, R., and Veh, R. W. (2012). Individual neurons in the rat lateral habenular complex project mostly to the dopaminergic ventral tegmental area or to the serotonergic raphe nuclei. J. Comp. Neurol. 520, 2545–2558.
Blier, P., Steinberg, S., Chaput, Y., and de Montigny, C. (1989). Electrophysiological assessment of putative antagonists of 5-hydroxytryptamine receptors: a single-cell study in the rat dorsal raphe nucleus. Can. J. Physiol. Pharmacol. 67, 98–105.
Boden, P. R., Woodruff, G. N., and Pinnock, R. D. (1991). Pharmacology of a cholecystokinin receptor on 5-hydroxytryptamine neurones in the dorsal raphe of the rat brain. Br. J. Pharmacol. 102, 635–638.
Boeijinga, P. H., and Boddeke, H. W. (1993). Serotonergic modulation of neurotransmission in the rat subicular cortex in vitro: a role for 5-HT1B receptors. Naunyn Schmiedebergs Arch. Pharmacol. 348, 553–557.
Borroto-Escuela, D. O., Narvaez, M., Marcellino, D., Parrado, C., Narvaez, J. A., Tarakanov, A. O., et al. (2010). Galanin receptor-1 modulates 5-hydroxtryptamine-1A signaling via heterodimerization. Biochem. Biophys. Res. Commun. 393, 767–772.
Bourson, A., Boess, F. G., Bos, M., and Sleight, A. J. (1998). Involvement of 5-HT6 receptors in nigro-striatal function in rodents. Br. J. Pharmacol. 125, 1562–1566.
Bouthenet, M. L., Martres, M. P., Sales, N., and Schwartz, J. C. (1987). A detailed mapping of dopamine D-2 receptors in rat central nervous system by autoradiography with [125I]iodosulpride. Neuroscience 20, 117–155.
Branchek, T. A., Smith, K. E., Gerald, C., and Walker, M. W. (2000). Galanin receptor subtypes. Trends Pharmacol. Sci. 21, 109–117.
Brown, R. E., Sergeeva, O. A., Eriksson, K. S., and Haas, H. L. (2002). Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J. Neurosci. 22, 8850–8859.
Bruinvels, A. T., Landwehrmeyer, B., Gustafson, E. L., Durkin, M. M., Mengod, G., Branchek, T. A., et al. (1994). Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology 33, 367–386.
Calizo, L. H., Akanwa, A., Ma, X., Pan, Y. Z., Lemos, J. C., Craige, C., et al. (2011). Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology 61, 524–543.
Carrel, D., Masson, J., Al Awabdh, S., Capra, C. B., Lenkei, Z., Hamon, M., et al. (2008). Targeting of the 5-HT1A serotonin receptor to neuronal dendrites is mediated by Yif1B. J. Neurosci. 28, 8063–8073.
Celada, P., Puig, M. V., Casanovas, J. M., Guillazo, G., and Artigas, F. (2001). Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J. Neurosci. 21, 9917–9929.
Chang, B., Daniele, C. A., Gallagher, K., Madonia, M., Mitchum, R. D., Barrett, L., et al. (2011). Nicotinic excitation of serotonergic projections from dorsal raphe to the nucleus accumbens. J. Neurophysiol. 106, 801–808.
Chang, C. P., Pearse, R. V. II, O’Connell, S., and Rosenfeld, M. G. (1993). Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron 11, 1187–1195.
Charara, A., and Parent, A. (1998). Chemoarchitecture of the primate dorsal raphe nucleus. J. Chem. Neuroanat. 15, 111–127.
Chen, X., Talley, E. M., Patel, N., Gomis, A., McIntire, W. E., Dong, B., et al. (2006). Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc. Natl. Acad. Sci. U.S.A. 103, 3422–3427.
Clemett, D. A., Punhani, T., Duxon, M. S., Blackburn, T. P., and Fone, K. C. (2000). Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology 39, 123–132.
Colino, A., and Halliwell, J. V. (1987). Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature 328, 73–77.
Cornelisse, L. N., Van der Harst, J. E., Lodder, J. C., Baarendse, P. J., Timmerman, A. J., Mansvelder, H. D., et al. (2007). Reduced 5-HT1A- and GABAB receptor function in dorsal raphe neurons upon chronic fluoxetine treatment of socially stressed rats. J. Neurophysiol. 98, 196–204.
Cortes, R., Gueye, B., Pazos, A., Probst, A., and Palacios, J. M. (1989). Dopamine receptors in human brain: autoradiographic distribution of D1 sites. Neuroscience 28, 263–273.
Crawford, L. K., Craige, C. P., and Beck, S. G. (2010). Increased intrinsic excitability of lateral wing serotonin neurons of the dorsal raphe: a mechanism for selective activation in stress circuits. J. Neurophysiol. 103, 2652–2663.
Dahlstrom, A., and Fuxe, K. (1964). Localization of monoamines in the lower brain stem. Experientia 20, 398–399.
Darmani, N. A., Janoyan, J. J., Kumar, N., and Crim, J. L. (2003). Behaviorally active doses of the CB1 receptor antagonist SR 141716A increase brain serotonin and dopamine levels and turnover. Pharmacol. Biochem. Behav. 75, 777–787.
Dautzenberg, F. M., Gutknecht, E., Van der Linden, I., Olivares-Reyes, J. A., Durrenberger, F., and Hauger, R. L. (2004). Cell-type specific calcium signaling by corticotropin-releasing factor type 1 (CRF1) and 2a (CRF2(a)) receptors: phospholipase C-mediated responses in human embryonic kidney 293 but not SK-N-MC neuroblastoma cells. Biochem. Pharmacol. 68, 1833–1844.
Davidson, R. J., Putnam, K. M., and Larson, C. L. (2000). Dysfunction in the neural circuitry of emotion regulation – a possible prelude to violence. Science 289, 591–594.
Dawson, T. M., Barone, P., Sidhu, A., Wamsley, J. K., and Chase, T. N. (1988). The D1 dopamine receptor in the rat brain: quantitative autoradiographic localization using an iodinated ligand. Neuroscience 26, 83–100.
Day, H. E., Campeau, S., Watson, S. J. Jr., and Akil, H. (1997). Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J. Chem. Neuroanat. 13, 115–139.
Deneris, E. S., and Wyler, S. C. (2012). Serotonergic transcriptional networks and potential importance to mental health. Nat. Neurosci. 15, 519–527.
de Weerth, A., Pisegna, J. R., Huppi, K., and Wank, S. A. (1993). Molecular cloning, functional expression and chromosomal localization of the human cholecystokinin type A receptor. Biochem. Biophys. Res. Commun. 194, 811–818.
Drutel, G., Peitsaro, N., Karlstedt, K., Wieland, K., Smit, M. J., Timmerman, H., et al. (2001). Identification of rat H3 receptor isoforms with different brain expression and signaling properties. Mol. Pharmacol. 59, 1–8.
Egashira, N., Mishima, K., Katsurabayashi, S., Yoshitake, T., Matsumoto, Y., Ishida, J., et al. (2002). Involvement of 5-hydroxytryptamine neuronal system in delta(9)-tetrahydrocannabinol-induced impairment of spatial memory. Eur. J. Pharmacol. 445, 221–229.
Ferre, S., and Artigas, F. (1993). Dopamine D2 receptor-mediated regulation of serotonin extracellular concentration in the dorsal raphe nucleus of freely moving rats. J. Neurochem. 61, 772–775.
Ferre, S., Cortes, R., and Artigas, F. (1994). Dopaminergic regulation of the serotonergic raphe-striatal pathway: microdialysis studies in freely moving rats. J. Neurosci. 14, 4839–4846.
Ferreira, S. G., Teixeira, F. M., Garcao, P., Agostinho, P., Ledent, C., Cortes, L., et al. (2012). Presynaptic CB(1) cannabinoid receptors control frontocortical serotonin and glutamate release – species differences. Neurochem. Int. 61, 219–226.
Florin, S., Meunier, J., and Costentin, J. (2000). Autoradiographic localization of [3H]nociceptin binding sites in the rat brain. Brain Res. 880, 11–16.
Frankhuijzen, A. L., Wardeh, G., Hogenboom, F., and Mulder, A. H. (1988). Alpha 2-adrenoceptor mediated inhibition of the release of radiolabelled 5-hydroxytryptamine and noradrenaline from slices of the dorsal region of the rat brain. Naunyn Schmiedebergs Arch. Pharmacol. 337, 255–260.
Froger, N., Gardier, A. M., Moratalla, R., Alberti, I., Lena, I., Boni, C., et al. (2001). 5-hydroxytryptamine (5-HT)1A autoreceptor adaptive changes in substance P (neurokinin 1) receptor knock-out mice mimic antidepressant-induced desensitization. J. Neurosci. 21, 8188–8197.
Fu, W., Le Maitre, E., Fabre, V., Bernard, J. F., David Xu, Z. Q., and Hokfelt, T. (2010). Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J. Comp. Neurol. 518, 3464–3494.
Galindo-Charles, L., Hernandez-Lopez, S., Galarraga, E., Tapia, D., Bargas, J., Garduno, J., et al. (2008). Serotoninergic dorsal raphe neurons possess functional postsynaptic nicotinic acetylcholine receptors. Synapse 62, 601–615.
Gallager, D. W., and Aghajanian, G. K. (1976). Effect of antipsychotic drugs on the firing of dorsal raphe cells. II. Reversal by picrotoxin. Eur. J. Pharmacol. 39, 357–364.
Garduno, J., Galindo-Charles, L., Jimenez-Rodriguez, J., Galarraga, E., Tapia, D., Mihailescu, S., et al. (2012). Presynaptic alpha4beta2 nicotinic acetylcholine receptors increase glutamate release and serotonin neuron excitability in the dorsal raphe nucleus. J. Neurosci. 32, 15148–15157.
Gartside, S. E., Cole, A. J., Williams, A. P., McQuade, R., and Judge, S. J. (2007). AMPA and NMDA receptor regulation of firing activity in 5-HT neurons of the dorsal and median raphe nuclei. Eur. J. Neurosci. 25, 3001–3008.
Gerard, C., Martres, M. P., Lefevre, K., Miquel, M. C., Verge, D., Lanfumey, L., et al. (1997). Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res. 746, 207–219.
Gervasoni, D., Peyron, C., Rampon, C., Barbagli, B., Chouvet, G., Urbain, N., et al. (2000). Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J. Neurosci. 20, 4217–4225.
Glass, M., and Felder, C. C. (1997). Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 17, 5327–5333.
Gras, C., Herzog, E., Bellenchi, G. C., Bernard, V., Ravassard, P., Pohl, M., et al. (2002). A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J. Neurosci. 22, 5442–5451.
Greenough, A., Cole, G., Lewis, J., Lockton, A., and Blundell, J. (1998). Untangling the effects of hunger, anxiety, and nausea on energy intake during intravenous cholecystokinin octapeptide (CCK-8) infusion. Physiol. Behav. 65, 303–310.
Guiard, B. P., Guilloux, J. P., Reperant, C., Hunt, S. P., Toth, M., and Gardier, A. M. (2007). Substance P neurokinin 1 receptor activation within the dorsal raphe nucleus controls serotonin release in the mouse frontal cortex. Mol. Pharmacol. 72, 1411–1418.
Gustafson, E. L., Durkin, M. M., Bard, J. A., Zgombick, J., and Branchek, T. A. (1996). A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br. J. Pharmacol. 117, 657–666.
Gutierrez, D. V., Mark, M. D., Masseck, O., Maejima, T., Kuckelsberg, D., Hyde, R. A., et al. (2011). Optogenetic control of motor coordination by Gi/o protein-coupled vertebrate rhodopsin in cerebellar Purkinje cells. J. Biol. Chem. 286, 25848–25858.
Haddjeri, N., and Blier, P. (2001). Sustained blockade of neurokinin-1 receptors enhances serotonin neurotransmission. Biol. Psychiatry 50, 191–199.
Haddjeri, N., Faure, C., Lucas, G., Mnie-Filali, O., Chouvet, G., Astier, B., et al. (2004). In-vivo modulation of central 5-hydroxytryptamine (5-HT1A) receptor-mediated responses by the cholinergic system. Int. J. Neuropsychopharmacol. 7, 391–399.
Haddjeri, N., Lucas, G., and Blier, P. (2000). Role of cholinergic and GABAergic systems in the feedback inhibition of dorsal raphe 5-HT neurons. Neuroreport 11, 3397–3401.
Hagan, C. E., McDevitt, R. A., Liu, Y., Furay, A. R., and Neumaier, J. F. (2012). 5-HT(1B) autoreceptor regulation of serotonin transporter activity in synaptosomes. Synapse 66, 1024–1034.
Haj-Dahmane, S. (2001). D2-like dopamine receptor activation excites rat dorsal raphe 5-HT neurons in vitro. Eur. J. Neurosci. 14, 125–134.
Haj-Dahmane, S., and Shen, R. Y. (2005). The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J. Neurosci. 25, 896–905.
Haj-Dahmane, S., and Shen, R. Y. (2009). Endocannabinoids suppress excitatory synaptic transmission to dorsal raphe serotonin neurons through the activation of presynaptic CB1 receptors. J. Pharmacol. Exp. Ther. 331, 186–196.
Haj-Dahmane, S., and Shen, R. Y. (2011). Modulation of the serotonin system by endocannabinoid signaling. Neuropharmacology 61, 414–420.
Hale, M. W., and Lowry, C. A. (2010). Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl.) 213, 243–264.
Hamon, M., Doucet, E., Lefevre, K., Miquel, M. C., Lanfumey, L., Insausti, R., et al. (1999). Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology 21, 68S–76S.
Haring, M., Marsicano, G., Lutz, B., and Monory, K. (2007). Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience 146, 1212–1219.
Harsing, L. G. Jr. (2006). The pharmacology of the neurochemical transmission in the midbrain raphe nuclei of the rat. Curr. Neuropharmacol. 4, 313–339.
Harvey, J., Wedley, S., Findlay, J. D., Sidell, M. R., and Pullar, I. A. (1996). omega-Agatoxin IVA identifies a single calcium channel subtype which contributes to the potassium-induced release of acetylcholine, 5-hydroxytryptamine, dopamine, gamma-aminobutyric acid and glutamate from rat brain slices. Neuropharmacology 35, 385–392.
Hasbi, A., O’Dowd, B. F., and George, S. R. (2010). Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Curr. Opin. Pharmacol. 10, 93–99.
Hashemi, P., Dankoski, E. C., Wood, K. M., Ambrose, R. E., and Wightman, R. M. (2011). In vivo electrochemical evidence for simultaneous 5-HT and histamine release in the rat substantia nigra pars reticulata following medial forebrain bundle stimulation. J. Neurochem. 118, 749–759.
Hay, D. L., Christopoulos, G., Christopoulos, A., Poyner, D. R., and Sexton, P. M. (2005). Pharmacological discrimination of calcitonin receptor: receptor activity-modifying protein complexes. Mol. Pharmacol. 67, 1655–1665.
Heinisch, S., and Kirby, L. G. (2009). Fractalkine/CX3CL1 enhances GABA synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuroscience 164, 1210–1223.
Heinisch, S., and Kirby, L. G. (2010). SDF-1alpha/CXCL12 enhances GABA and glutamate synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuropharmacology 58, 501–514.
Herlitze, S., and Landmesser, L. T. (2007). New optical tools for controlling neuronal activity. Curr. Opin. Neurobiol. 17, 87–94.
Hioki, H., Nakamura, H., Ma, Y. F., Konno, M., Hayakawa, T., Nakamura, K. C., et al. (2010). Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J. Comp. Neurol. 518, 668–686.
Hjorth, S., and Sharp, T. (1991). Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sci. 48, 1779–1786.
Hjorth, S., Suchowski, C. S., and Galloway, M. P. (1995). Evidence for 5-HT autoreceptor-mediated, nerve impulse-independent, control of 5-HT synthesis in the rat brain. Synapse 19, 170–176.
Hokfelt, T., Pernow, B., and Wahren, J. (2001). Substance P: a pioneer amongst neuropeptides. J. Intern. Med. 249, 27–40.
Hopwood, S. E., and Stamford, J. A. (2001). Multiple 5-HT(1) autoreceptor subtypes govern serotonin release in dorsal and median raphe nuclei. Neuropharmacology 40, 508–519.
Howlett, A. C., Qualy, J. M., and Khachatrian, L. L. (1986). Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol. Pharmacol. 29, 307–313.
Hoyer, D., Hannon, J. P., and Martin, G. R. (2002). Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 71, 533–554.
Innis, R. B., and Aghajanian, G. K. (1987). Pertussis toxin blocks 5-HT1A and GABAB receptor-mediated inhibition of serotonergic neurons. Eur. J. Pharmacol. 143, 195–204.
Jacobs, B. L., and Azmitia, E. C. (1992). Structure and function of the brain serotonin system. Physiol. Rev. 72, 165–229.
Jensen, R. T., Battey, J. F., Spindel, E. R., and Benya, R. V. (2008). International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 60, 1–42.
Jolas, T., and Aghajanian, G. K. (1997). Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro. Brain Res. 755, 229–245.
Kawashima, N., Karasawa, J., Shimazaki, T., Chaki, S., Okuyama, S., Yasuhara, A., et al. (2005). Neuropharmacological profiles of antagonists of group II metabotropic glutamate receptors. Neurosci. Lett. 378, 131–134.
Kehr, J., Yoshitake, T., Wang, F. H., Razani, H., Gimenez-Llort, L., Jansson, A., et al. (2002). Galanin is a potent in vivo modulator of mesencephalic serotonergic neurotransmission. Neuropsychopharmacology 27, 341–356.
Kidd, E. J., Garratt, J. C., and Marsden, C. A. (1991). Effects of repeated treatment with 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) on the autoregulatory control of dorsal raphe 5-HT neuronal firing and cortical 5-HT release. Eur. J. Pharmacol. 200, 131–139.
Kim, J. C., Cook, M. N., Carey, M. R., Shen, C., Regehr, W. G., and Dymecki, S. M. (2009). Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron 63, 305–315.
Kim, J. M., Hwa, J., Garriga, P., Reeves, P. J., RajBhandary, U. L., and Khorana, H. G. (2005). Light-driven activation of beta 2-adrenergic receptor signaling by a chimeric rhodopsin containing the beta 2-adrenergic receptor cytoplasmic loops. Biochemistry 44, 2284–2292.
Kimura, M., Yamanishi, Y., Hanada, T., Kagaya, T., Kuwada, M., Watanabe, T., et al. (1995). Involvement of P-type calcium channels in high potassium-elicited release of neurotransmitters from rat brain slices. Neuroscience 66, 609–615.
Kirby, L. G., Freeman-Daniels, E., Lemos, J. C., Nunan, J. D., Lamy, C., Akanwa, A., et al. (2008). Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J. Neurosci. 28, 12927–12937.
Kobayashi, K., Ikeda, Y., Haneda, E., and Suzuki, H. (2008). Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. J. Neurosci. 28, 6272–6280.
Kohlmeier, K. A., Watanabe, S., Tyler, C. J., Burlet, S., and Leonard, C. S. (2008). Dual orexin actions on dorsal raphe and laterodorsal tegmentum neurons: noisy cation current activation and selective enhancement of Ca2+ transients mediated by L-type calcium channels. J. Neurophysiol. 100, 2265–2281.
Kroeze, W. K., Kristiansen, K., and Roth, B. L. (2002). Molecular biology of serotonin receptors structure and function at the molecular level. Curr. Top. Med. Chem. 2, 507–528.
Kuteeva, E., Hokfelt, T., Wardi, T., and Ogren, S. O. (2008). Galanin, galanin receptor subtypes and depression-like behaviour. Cell. Mol. Life Sci. 65, 1854–1863.
Lacoste, B., Riad, M., and Descarries, L. (2006). Immunocytochemical evidence for the existence of substance P receptor (NK1) in serotonin neurons of rat and mouse dorsal raphe nucleus. Eur. J. Neurosci. 23, 2947–2958.
Lacoste, B., Riad, M., Ratte, M. O., Boye, S. M., Levesque, D., and Descarries, L. (2009). Trafficking of neurokinin-1 receptors in serotonin neurons is controlled by substance P within the rat dorsal raphe nucleus. Eur. J. Neurosci. 29, 2303–2314.
Lakoski, J. M., and Aghajanian, G. K. (1983). Effects of histamine, H1- and H2-receptor antagonists on the activity of serotonergic neurons in the dorsal raphe nucleus. J. Pharmacol. Exp. Ther. 227, 517–523.
Lakoski, J. M., Gallager, D. W., and Aghajanian, G. K. (1984). Histamine-induced depression of serotoninergic dorsal raphe neurons: antagonism by cimetidine, a reevaluation. Eur. J. Pharmacol. 103, 153–156.
Larm, J. A., Shen, P. J., and Gundlach, A. L. (2003). Differential galanin receptor-1 and galanin expression by 5-HT neurons in dorsal raphe nucleus of rat and mouse: evidence for species-dependent modulation of serotonin transmission. Eur. J. Neurosci. 17, 481–493.
Lechin, F., van der Dijs, B., and Hernandez-Adrian, G. (2006). Dorsal raphe vs. median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: relevance for neuropharmacological therapy. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 565–585.
Lee, Y. M., Beinborn, M., McBride, E. W., Lu, M., Kolakowski, L. F. Jr., and Kopin, A. S. (1993). The human brain cholecystokinin-B/gastrin receptor. Cloning and characterization. J. Biol. Chem. 268, 8164–8169.
Lerch-Haner, J. K., Frierson, D., Crawford, L. K., Beck, S. G., and Deneris, E. S. (2008). Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat. Neurosci. 11, 1001–1003.
Levine, E. S., and Jacobs, B. L. (1992). Neurochemical afferents controlling the activity of serotonergic neurons in the dorsal raphe nucleus: microiontophoretic studies in the awake cat. J. Neurosci. 12, 4037–4044.
Li, X., Gutierrez, D. V., Hanson, M. G., Han, J., Mark, M. D., Chiel, H., et al. (2005). Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. U.S.A. 102, 17816–17821.
Liaw, C. W., Lovenberg, T. W., Barry, G., Oltersdorf, T., Grigoriadis, D. E., and de Souza, E. B. (1996). Cloning and characterization of the human corticotropin-releasing factor-2 receptor complementary deoxyribonucleic acid. Endocrinology 137, 72–77.
Liu, R., Ding, Y., and Aghajanian, G. K. (2002a). Neurokinins activate local glutamatergic inputs to serotonergic neurons of the dorsal raphe nucleus. Neuropsychopharmacology 27, 329–340.
Liu, R. J., van den Pol, A. N., and Aghajanian, G. K. (2002b). Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J. Neurosci. 22, 9453–9464.
Liu, R., Jolas, T., and Aghajanian, G. (2000). Serotonin 5-HT(2) receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 873, 34–45.
Lovenberg, T. W., Liaw, C. W., Grigoriadis, D. E., Clevenger, W., Chalmers, D. T., De Souza, E. B., et al. (1995). Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. U.S.A. 92, 836–840.
Lovenberg, T. W., Roland, B. L., Wilson, S. J., Jiang, X., Pyati, J., Huvar, A., et al. (1999). Cloning and functional expression of the human histamine H3 receptor. Mol. Pharmacol. 55, 1101–1107.
Lu, X., Barr, A. M., Kinney, J. W., Sanna, P., Conti, B., Behrens, M. M., et al. (2005). A role for galanin in antidepressant actions with a focus on the dorsal raphe nucleus. Proc. Natl. Acad. Sci. U.S.A. 102, 874–879.
Madisen, L., Mao, T., Koch, H., Zhuo, J. M., Berenyi, A., Fujisawa, S., et al. (2012). A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802.
Madisen, L., Zwingman, T. A., Sunkin, S. M., Oh, S. W., Zariwala, H. A., Gu, H., et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140.
Maejima, T., Hashimoto, K., Yoshida, T., Aiba, A., and Kano, M. (2001). Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 31, 463–475.
Maeno, H., Kiyama, H., and Tohyama, M. (1993). Distribution of the substance P receptor (NK-1 receptor) in the central nervous system. Brain Res. Mol. Brain Res. 18, 43–58.
Mann, J. J., Brent, D. A., and Arango, V. (2001). The neurobiology and genetics of suicide and attempted suicide: a focus on the serotonergic system. Neuropsychopharmacology 24, 467–477.
Martini, L., Hastrup, H., Holst, B., Fraile-Ramos, A., Marsh, M., and Schwartz, T. W. (2002). NK1 receptor fused to beta-arrestin displays a single-component, high-affinity molecular phenotype. Mol. Pharmacol. 62, 30–37.
Martin-Ruiz, R., Ugedo, L., Honrubia, M. A., Mengod, G., and Artigas, F. (2001). Control of serotonergic neurons in rat brain by dopaminergic receptors outside the dorsal raphe nucleus. J. Neurochem. 77, 762–775.
Masseck, O. A., Rubelowski, J. M., Spoida, K., and Herlitze, S. (2010). Light- and drug-activated G-protein-coupled receptors to control intracellular signalling. Exp. Physiol. 96, 51–56.
Matsumoto, M., Yoshioka, M., Togashi, H., Ikeda, T., and Saito, H. (1996). Functional regulation by dopamine receptors of serotonin release from the rat hippocampus: in vivo microdialysis study. Naunyn Schmiedebergs Arch. Pharmacol. 353, 621–629.
Mazarati, A. M., Baldwin, R. A., Shinmei, S., and Sankar, R. (2005). In vivo interaction between serotonin and galanin receptors types 1 and 2 in the dorsal raphe: implication for limbic seizures. J. Neurochem. 95, 1495–503.
McDevitt, R. A., and Neumaier, J. F. (2011). Regulation of dorsal raphe nucleus function by serotonin autoreceptors: a behavioral perspective. J. Chem. Neuroanat. 41, 234–246.
Melander, T., Kohler, C., Nilsson, S., Hokfelt, T., Brodin, E., Theodorsson, E., et al. (1988). Autoradiographic quantitation and anatomical mapping of 125I-galanin binding sites in the rat central nervous system. J. Chem. Neuroanat. 1, 213–233.
Mendiguren, A., and Pineda, J. (2009). Effect of the CB(1) receptor antagonists rimonabant and AM251 on the firing rate of dorsal raphe nucleus neurons in rat brain slices. Br. J. Pharmacol. 158, 1579–1587.
Mendlin, A., Martin, F. J., and Jacobs, B. L. (1998). Involvement of dopamine D2 receptors in apomorphine-induced facilitation of forebrain serotonin output. Eur. J. Pharmacol. 351, 291–298.
Mengod, G., Cortes, R., Vilaro, M. T., and Hoyer, D. (2010). “Distribution of 5-HT receptors in the central nervous system,” in Handbook of Behavioral Neurobiology of Serotonin, eds C. P. Muller and B. Jacobs (London: Elsevier B.V.), 123–138.
Merali, Z., Bedard, T., Andrews, N., Davis, B., McKnight, A. T., Gonzalez, M. I., et al. (2006). Bombesin receptors as a novel anti-anxiety therapeutic target: BB1 receptor actions on anxiety through alterations of serotonin activity. J. Neurosci. 26, 10387–10396.
Mihailescu, S., Guzman-Marin, R., Dominguez Mdel, C., and Drucker-Colin, R. (2002). Mechanisms of nicotine actions on dorsal raphe serotoninergic neurons. Eur. J. Pharmacol. 452, 77–82.
Monti, J. M. (2010). The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med. Rev. 14, 319–327.
Mooney, R. D., Crnko-Hoppenjans, T. A., Ke, M., Bennett-Clarke, C. A., Lane, R. D., Chiaia, N. L., et al. (1998). Augmentation of serotonin in the developing superior colliculus alters the normal development of the uncrossed retinotectal projection. J. Comp. Neurol. 393, 84–92.
Moranta, D., Esteban, S., and Garcia-Sevilla, J. A. (2009). Chronic treatment and withdrawal of the cannabinoid agonist WIN 55,212-2 modulate the sensitivity of presynaptic receptors involved in the regulation of monoamine syntheses in rat brain. Naunyn Schmiedebergs Arch. Pharmacol. 379, 61–72.
Morikawa, H., Manzoni, O. J., Crabbe, J. C., and Williams, J. T. (2000). Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol. Pharmacol. 58, 1271–1278.
Nakamoto, H., Soeda, Y., Takami, S., Minami, M., and Satoh, M. (2000). Localization of calcitonin receptor mRNA in the mouse brain: coexistence with serotonin transporter mRNA. Brain Res. Mol. Brain Res. 76, 93–102.
Nakazi, M., Bauer, U., Nickel, T., Kathmann, M., and Schlicker, E. (2000). Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch. Pharmacol. 361, 19–24.
Nanopoulos, D., Belin, M. F., Maitre, M., Vincendon, G., and Pujol, J. F. (1982). Immunocytochemical evidence for the existence of GABAergic neurons in the nucleus raphe dorsalis. Possible existence of neurons containing serotonin and GABA. Brain Res. 232, 375–389.
Nelson, R. J., and Chiavegatto, S. (2001). Molecular basis of aggression. Trends Neurosci. 24, 713–719.
Offermanns, S., Iida-Klein, A., Segre, G. V., and Simon, M. I. (1996). G alpha q family members couple parathyroid hormone (PTH)/PTH-related peptide and calcitonin receptors to phospholipase C in COS-7 cells. Mol. Endocrinol. 10, 566–574.
Oh, E., Maejima, T., Liu, C., Deneris, E., and Herlitze, S. (2010). Substitution of 5-HT1A receptor signaling by a light-activated G protein-coupled receptor. J. Biol. Chem. 285, 30825–30836.
Oh, S. B., Endoh, T., Simen, A. A., Ren, D., and Miller, R. J. (2002). Regulation of calcium currents by chemokines and their receptors. J. Neuroimmunol. 123, 66–75.
Pan, Z. Z., Colmers, W. F., and Williams, J. T. (1989). 5-HT-mediated synaptic potentials in the dorsal raphe nucleus: interactions with excitatory amino acid and GABA neurotransmission. J. Neurophysiol. 62, 481–486.
Pan, Z. Z., and Williams, J. T. (1989). GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro. J. Neurophysiol. 61, 719–726.
Penington, N. J., and Kelly, J. S. (1990). Serotonin receptor activation reduces calcium current in an acutely dissociated adult central neuron. Neuron 4, 751–758.
Penington, N. J., Kelly, J. S., and Fox, A. P. (1992). Action potential waveforms reveal simultaneous changes in ICa and IK produced by 5-HT in rat dorsal raphe neurons. Proc. Biol. Sci. 248, 171–179.
Penington, N. J., Kelly, J. S., and Fox, A. P. (1993). Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. J. Physiol. 469, 387–405.
Perrin, M. H., Donaldson, C. J., Chen, R., Lewis, K. A., and Vale, W. W. (1993). Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology 133, 3058–3061.
Peyron, C., Tighe, D. K., van den Pol, A. N., de Lecea, L., Heller, H. C., Sutcliffe, J. G., et al. (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10015.
Pillot, C., Heron, A., Cochois, V., Tardivel-Lacombe, J., Ligneau, X., Schwartz, J. C., et al. (2002). A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience 114, 173–193.
Pineyro, G., and Blier, P. (1999). Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol. Rev. 51, 533–591.
Pinnock, R. D., Reynolds, T., and Woodruff, G. N. (1994). Different types of bombesin receptors on neurons in the dorsal raphe nucleus and the rostral hypothalamus in rat brain slices in vitro. Brain Res. 653, 119–124.
Pudovkina, O. L., Cremers, T. I., and Westerink, B. H. (2003). Regulation of the release of serotonin in the dorsal raphe nucleus by alpha1 and alpha2 adrenoceptors. Synapse 50, 77–82.
Rampon, C., Luppi, P. H., Fort, P., Peyron, C., and Jouvet, M. (1996). Distribution of glycine-immunoreactive cell bodies and fibers in the rat brain. Neuroscience 75, 737–755.
Rampon, C., Peyron, C., Gervasoni, D., Pow, D. V., Luppi, P. H., and Fort, P. (1999). Origins of the glycinergic inputs to the rat locus coeruleus and dorsal raphe nuclei: a study combining retrograde tracing with glycine immunohistochemistry. Eur. J. Neurosci. 11, 1058–1066.
Rashid, A. J., So, C. H., Kong, M. M., Furtak, T., El-Ghundi, M., Cheng, R., et al. (2007). D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. U.S.A. 104, 654–659.
Richardson-Jones, J. W., Craige, C. P., Guiard, B. P., Stephen, A., Metzger, K. L., Kung, H. F., et al. (2010). 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65, 40–52.
Richardson-Jones, J. W., Craige, C. P., Nguyen, T. H., Kung, H. F., Gardier, A. M., Dranovsky, A., et al. (2011). Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J. Neurosci. 31, 6008–6018.
Richerson, G. B. (2004). Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat. Rev. Neurosci. 5, 449–461.
Roberts, C., Allen, L., Langmead, C. J., Hagan, J. J., Middlemiss, D. N., and Price, G. W. (2001). The effect of SB-269970, a 5-HT(7) receptor antagonist, on 5-HT release from serotonergic terminals and cell bodies. Br. J. Pharmacol. 132, 1574–1580.
Rosin, D. L., Zeng, D., Stornetta, R. L., Norton, F. R., Riley, T., Okusa, M. D., et al. (1993). Immunohistochemical localization of alpha 2A-adrenergic receptors in catecholaminergic and other brainstem neurons in the rat. Neuroscience 56, 139–155.
Rouse, S. T., and Levey, A. I. (1996). Expression of m1-m4 muscarinic acetylcholine receptor immunoreactivity in septohippocampal neurons and other identified hippocampal afferents. J. Comp. Neurol. 375, 406–416.
Ruat, M., Traiffort, E., Leurs, R., Tardivel-Lacombe, J., Diaz, J., Arrang, J. M., et al. (1993). Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. U.S.A. 90, 8547–8551.
Saffroy, M., Beaujouan, J. C., Petitet, F., Torrens, Y., and Glowinski, J. (1994). Differential localization of 3H-[Pro9]SP binding sites in the guinea pig and rat brain. Brain Res. 633, 317–325.
Santarelli, L., Gobbi, G., Debs, P. C., Sibille, E. T., Blier, P., Hen, R., and Heath, M. J. (2001). Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function. Proc. Natl. Acad. Sci. U.S.A. 98, 1912–1917.
Scammell, T. E., and Winrow, C. J. (2010). Orexin receptors: pharmacology and therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 51, 243–266.
Scheinin, M., Lomasney, J. W., Hayden-Hixson, D. M., Schambra, U. B., Caron, M. G., Lefkowitz, R. J., et al. (1994). Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res. Mol. Brain Res. 21, 133–149.
Scott, M. M., Wylie, C. J., Lerch, J. K., Murphy, R., Lobur, K., Herlitze, S., et al. (2005). A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc. Natl. Acad. Sci. U.S.A. 102, 16472–16477.
Sergeyev, V., Hokfelt, T., and Hurd, Y. (1999). Serotonin and substance P co-exist in dorsal raphe neurons of the human brain. Neuroreport 10, 3967–3970.
Serrats, J., Raurich, A., Vilaro, M. T., Mengod, G., and Cortes, R. (2004). 5-ht5B receptor mRNA in the raphe nuclei: coexpression with serotonin transporter. Synapse 51, 102–111.
Sexton, P. M., Paxinos, G., Kenney, M. A., Wookey, P. J., and Beaumont, K. (1994). In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience 62, 553–567.
Sharkey, L. M., Madamba, S. G., Siggins, G. R., and Bartfai, T. (2008). Galanin alters GABAergic neurotransmission in the dorsal raphe nucleus. Neurochem. Res. 33, 285–291.
Sharp, T., Boothman, L., Raley, J., and Queree, P. (2007). Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol. Sci. 28, 629–636.
Shikanai, H., Yoshida, T., Konno, K., Yamasaki, M., Izumi, T., Ohmura, Y., et al. (2012). Distinct neurochemical and functional properties of GAD67-containing 5-HT neurons in the rat dorsal raphe nucleus. J. Neurosci. 32, 14415–14426.
Simon, H. H., Scholz, C., and O’Leary, D. D. (2005). Engrailed genes control developmental fate of serotonergic and noradrenergic neurons in mid- and hindbrain in a gene dose-dependent manner. Mol. Cell. Neurosci. 28, 96–105.
Siniscalchi, A., Rodi, D., Cavallini, S., Marino, S., Beani, L., and Bianchi, C. (2001). Effects of cholecystokinin tetrapeptide (CCK(4)) and anxiolytic drugs on the electrically evoked [(3)H]5-hydroxytryptamine outflow from rat cortical slices. Brain Res. 922, 104–111.
Sink, K. S., Walker, D. L., Yang, Y., and Davis, M. (2011). Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J. Neurosci. 31, 1802–1810.
Song, L., Liu, Y., Yu, Y., Duan, X., and Qi, S. (2012). Shh signaling guides spatial pathfinding of raphespinal tract axons by multidirectional repulsion. Cell Res. 22, 697–716.
Sprouse, J. S., and Aghajanian, G. K. (1987). Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse 1, 3–9.
Stamford, J. A., Davidson, C., McLaughlin, D. P., and Hopwood, S. E. (2000). Control of dorsal raphe 5-HT function by multiple 5-HT(1) autoreceptors: parallel purposes or pointless plurality? Trends Neurosci. 23, 459–465.
Stratowa, C., Machat, H., Burger, E., Himmler, A., Schafer, R., Spevak, W., et al. (1995). Functional characterization of the human neurokinin receptors NK1, NK2, and NK3 based on a cellular assay system. J. Recept. Signal Transduct. Res. 15, 617–630.
Strazielle, C., Lalonde, R., Hebert, C., and Reader, T. A. (1999). Regional brain distribution of noradrenaline uptake sites, and of alpha1-alpha2- and beta-adrenergic receptors in PCD mutant mice: a quantitative autoradiographic study. Neuroscience 94, 287–304.
Suzuki, M., Hurd, Y. L., Sokoloff, P., Schwartz, J. C., and Sedvall, G. (1998). D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res. 779, 58–74.
Swanson, C. J., Blackburn, T. P., Zhang, X., Zheng, K., Xu, Z. Q., Hokfelt, T., et al. (2005). Anxiolytic- and antidepressant-like profiles of the galanin-3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299. Proc. Natl. Acad. Sci. U.S.A. 102, 17489–17494.
Talley, E. M., Rosin, D. L., Lee, A., Guyenet, P. G., and Lynch, K. R. (1996). Distribution of alpha 2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. J. Comp. Neurol. 372, 111–134.
Tao, R., and Auerbach, S. B. (1994). Increased extracellular serotonin in rat brain after systemic or intraraphe administration of morphine. J. Neurochem. 63, 517–524.
Tao, R., and Auerbach, S. B. (2000). Regulation of serotonin release by GABA and excitatory amino acids. J. Psychopharmacol. 14, 100–113.
Tao, R., and Auerbach, S. B. (2002). Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J. Pharmacol. Exp. Ther. 303, 549–556.
Tao, R., and Ma, Z. (2012). Neural circuit in the dorsal raphe nucleus responsible for cannabinoid-mediated increases in 5-HT efflux in the nucleus accumbens of the rat brain. ISRN Pharmacol. 2012, 276902.
Tao, R., Ma, Z., Thakkar, M. M., McCarley, R. W., and Auerbach, S. B. (2007). Nociceptin/orphanin FQ decreases serotonin efflux in the rat brain but in contrast to a kappa-opioid has no antagonistic effect on mu-opioid-induced increases in serotonin efflux. Neuroscience 147, 106–116.
Thomas, D. R., Soffin, E. M., Roberts, C., Kew, J. N., de la Flor, R. M., Dawson, L. A., et al. (2006). SB-699551-A (3-cyclopentyl-N-[2-(dimethylamino)ethyl]-N-[(4′-{[(2-phenylethyl)amino]methyl}-4-biphenylyl)methyl]propanamide dihydrochloride), a novel 5-ht5A receptor-selective antagonist, enhances 5-HT neuronal function: Evidence for an autoreceptor role for the 5-ht5A receptor in guinea pig brain. Neuropharmacology 51, 566–577.
Tilakaratne, N., Christopoulos, G., Zumpe, E. T., Foord, S. M., and Sexton, P. M. (2000). Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J. Pharmacol. Exp. Ther. 294, 61–72.
To, Z. P., Bonhaus, D. W., Eglen, R. M., and Jakeman, L. B. (1995). Characterization and distribution of putative 5-ht7 receptors in guinea-pig brain. Br. J. Pharmacol. 115, 107–116.
Tzavara, E. T., Davis, R. J., Perry, K. W., Li, X., Salhoff, C., Bymaster, F. P., et al. (2003). The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br. J. Pharmacol. 138, 544–553.
Ulrich, C. D., Ferber, I., Holicky, E., Hadac, E., Buell, G., and Miller, L. J. (1993). Molecular cloning and functional expression of the human gallbladder cholecystokinin A receptor. Biochem. Biophys. Res. Commun. 193, 204–211.
Unnerstall, J. R., Fernandez, I., and Orensanz, L. M. (1985). The alpha-adrenergic receptor: radiohistochemical analysis of functional characteristics and biochemical differences. Pharmacol. Biochem. Behav. 22, 859–874.
Valentino, R. J., Lucki, I., and Van Bockstaele, E. (2010). Corticotropin-releasing factor in the dorsal raphe nucleus: linking stress coping and addiction. Brain Res. 1314, 29–37.
Vandermaelen, C. P., and Aghajanian, G. K. (1983). Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 289, 109–119.
van Hooft, J. A., and Vijverberg, H. P. (2000). 5-HT(3) receptors and neurotransmitter release in the CNS: a nerve ending story? Trends Neurosci. 23, 605–610.
Varga, V., Losonczy, A., Zemelman, B. V., Borhegyi, Z., Nyiri, G., Domonkos, A., et al. (2009). Fast synaptic subcortical control of hippocampal circuits. Science 326, 449–453.
Varga, V., Sik, A., Freund, T. F., and Kocsis, B. (2002). GABA(B) receptors in the median raphe nucleus: distribution and role in the serotonergic control of hippocampal activity. Neuroscience 109, 119–132.
Vigna, S. R., Bowden, J. J., McDonald, D. M., Fisher, J., Okamoto, A., McVey, D. C., et al. (1994). Characterization of antibodies to the rat substance P (NK-1) receptor and to a chimeric substance P receptor expressed in mammalian cells. J. Neurosci. 14, 834–845.
Vita, N., Laurent, P., Lefort, S., Chalon, P., Lelias, J. M., Kaghad, M., et al. (1993). Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 335, 1–5.
Wamsley, J. K., Gehlert, D. R., Filloux, F. M., and Dawson, T. M. (1989). Comparison of the distribution of D-1 and D-2 dopamine receptors in the rat brain. J. Chem. Neuroanat. 2, 119–137.
Wang, Q. P., Guan, J. L., and Shioda, S. (2000). Synaptic contacts between serotonergic and cholinergic neurons in the rat dorsal raphe nucleus and laterodorsal tegmental nucleus. Neuroscience 97, 553–563.
Wang, R. Y., and Aghajanian, G. K. (1977). Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science 197, 89–91.
Washburn, C. P., Sirois, J. E., Talley, E. M., Guyenet, P. G., and Bayliss, D. A. (2002). Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J. Neurosci. 22, 1256–1265.
White, J. M., and Rumbold, G. R. (1988). Behavioural effects of histamine and its antagonists: a review. Psychopharmacology (Berl.) 95, 1–14.
Williams, J. T., Colmers, W. F., and Pan, Z. Z. (1988). Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J. Neurosci. 8, 3499–3506.
Woodruff, G. N., Hall, M. D., Reynolds, T., and Pinnock, R. D. (1996). Bombesin receptors in the brain. Ann. N. Y. Acad. Sci. 780, 223–243.
Woolley, M. L., Marsden, C. A., and Fone, K. C. (2004). 5-ht6 receptors. Curr. Drug Targets CNS Neurol. Disord. 3, 59–79.
Wright, D. E., Seroogy, K. B., Lundgren, K. H., Davis, B. M., and Jennes, L. (1995). Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 351, 357–373.
Wylie, C. J., Hendricks, T. J., Zhang, B., Wang, L., Lu, P., Leahy, P., et al. (2010). Distinct transcriptomes define rostral and caudal serotonin neurons. J. Neurosci. 30, 670–684.
Xie, Z., Westmoreland, S. V., and Miller, G. M. (2008). Modulation of monoamine transporters by common biogenic amines via trace amine-associated receptor 1 and monoamine autoreceptors in human embryonic kidney 293 cells and brain synaptosomes. J. Pharmacol. Exp. Ther. 325, 629–640.
Xu, Z. Q., Zhang, X., Pieribone, V. A., Grillner, S., and Hokfelt, T. (1998). Galanin-5-hydroxytryptamine interactions: electrophysiological, immunohistochemical and in situ hybridization studies on rat dorsal raphe neurons with a note on galanin R1 and R2 receptors. Neuroscience 87, 79–94.
Yokoyama, C., Okamura, H., Nakajima, T., Taguchi, J., and Ibata, Y. (1994). Autoradiographic distribution of [3H]YM-09151-2, a high-affinity and selective antagonist ligand for the dopamine D2 receptor group, in the rat brain and spinal cord. J. Comp. Neurol. 344, 121–136.
Zavitsanou, K., Wang, H., Dalton, V. S., and Nguyen, V. (2010). Cannabinoid administration increases 5HT1A receptor binding and mRNA expression in the hippocampus of adult but not adolescent rats. Neuroscience 169, 315–324.
Keywords: 5-HT system, GPCRs, auto-regulation, hetero-regulation, optogenetics
Citation: Maejima T, Masseck OA, Mark MD and Herlitze S (2013) Modulation of firing and synaptic transmission of serotonergic neurons by intrinsic G protein-coupled receptors and ion channels. Front. Integr. Neurosci. 7:40. doi: 10.3389/fnint.2013.00040
Received: 11 February 2013; Accepted: 03 May 2013;
Published online: 23 May 2013.
Edited by:
Kae Nakamura, Kansai Medical University, JapanReviewed by:
David M. Lovinger, National Institutes of Health, USAEvan Deneris, Case Western Reserve University, USA
Copyright: © 2013 Maejima, Masseck, Mark and Herlitze. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Stefan Herlitze, Department of Zoology and Neurobiology, Ruhr-University Bochum, Universitätsstr. 150, ND 7/31, D-44780 Bochum, Germany. e-mail: stefan.herlitze@rub.de