1
Department of Neurochemistry, Max-Planck Institute for Brain Research, Frankfurt am Main, Germany
2
Hertie Research Group, Max-Planck Institute for Brain Research, Frankfurt am Main, Germany
3
AG Molekulare und zelluläre Neurophysiologie, Technische Universität Darmstadt, Darmstadt, Germany
Glycine has diverse functions within the mammalian central nervous system. It inhibits postsynaptic neurons via strychnine-sensitive glycine receptors (GlyRs) and enhances neuronal excitation through co-activation of N-methyl-D-aspartate (NMDA) receptors. Classical Ca2+-permeable NMDA receptors are composed of glycine-binding NR1 and glutamate-binding NR2 subunits, and hence require both glutamate and glycine for efficient activation. In contrast, recombinant receptors composed of NR1 and the glycine binding NR3A and/or NR3B subunits lack glutamate binding sites and can be activated by glycine alone. Therefore these receptors are also named “Excitatory glycine receptors”. Co-application of antagonists of the NR1 glycine-binding site or of the divalent cation Zn2+ markedly enhances the glycine responses of these receptors. To gain further insight into the properties of these glycine-gated NMDA receptors, we investigated their current-voltage (I–V) dependence. Whole-cell current-voltage relations of glycine currents recorded from NR1/NR3B and NR1/NR3A/NR3B expressing oocytes were found to be linear under our recording conditions. In contrast, NR1/NR3A receptors displayed a strong outwardly rectifying I–V relation. Interestingly, the voltage-dependent inward current block was abolished in the presence of NR1 antagonists, Zn2+ or a combination of both. Further analysis revealed that Ca2+ (1.8 mM) present in our recording solutions was responsible for the voltage-dependent inhibition of ion flux through NR1/NR3A receptors. Since physiological concentrations of the divalent cation Mg2+ did not affect the I–V dependence, our data suggest that relief of the voltage-dependent Ca2+ block of NR1/NR3A receptors by Zn2+ may be important for the regulation of excitatory glycinergic transmission, according to the Mg2+-block of conventional NR1/NR2 NMDA receptors.
The simplest of all amino acids, glycine, has diverse functions within the mammalian central nervous system (CNS). Glycine mediates synaptic inhibition through hyperpolarizing glycine receptors (GlyRs) and contributes to neuronal excitation by acting as a co-agonist at glutamate-type N-methyl-D-aspartate (NMDA) receptors (see overview in Dingledine et al., 1999
; Betz and Laube, 2006
). The GlyR is a pentameric protein composed of two types (α and β) of membrane-spanning subunits (Grudzinska et al., 2005
). In contrast, the NMDA receptor is a hetero-tetrameric membrane protein (Laube et al., 1998
) composed of glycine-binding NR1 and glutamate-binding NR2 subunits (Laube et al., 1997
; overview in Cull-Candy et al., 2001
) and/or the recently discovered glycine-binding NR3A and NR3B subunits (Sucher et al., 1995
; Nishi et al., 2001
; Yao and Mayer, 2006
). The ligand-binding domains (LBDs) of the NMDA receptor subunits are formed by two extracellular segments S1 and S2 and are thought to be arranged in a dimer-of-heterodimer orientation (Furukawa et al., 2005
; Schüler et al., 2008
). Binding of the agonist occurs between the two extracellular segments and results in a closure of the LBD and subsequent opening of the ion channel (Armstrong and Gouaux, 2000
; overview in Mayer, 2006
).
Conventional NMDA receptors composed of two NR1 and two NR2 subunits require the simultaneous binding of glutamate and glycine for efficient channel opening (Johnson and Ascher, 1987
; Kuryatov et al., 1994
). In contrast, NMDA receptors composed of NR1 and NR3 subunits result in nonselective cation-channels exclusively activated by glycine, termed “excitatory glycine receptors“, which are unaffected by glutamate (Chatterton et al., 2002
). The physiological role of NR1/NR3 NMDA receptors is however still discussed controversially (Chatterton et al., 2002
; Tong et al., 2008
). Recombinantly expressed NR1/NR3A and NR1/NR3B receptors generate only small excitatory currents upon activation by glycine (Awobuluyi et al., 2007
; Madry et al., 2007a
; Smothers and Woodward, 2009
). This low functionality cannot be attributed to impaired receptor assembly or decreased surface insertion but derives from opposite roles of glycine which acts agonistically at NR3 subunits and inhibitory through binding to the NR1 subunit (Awobuluyi et al., 2007
; Madry et al., 2007a
). NR1/NR3A receptor activation by glycine is strongly potentiated in the presence of either the divalent cation Zn2+ (ten-fold) or NR1 glycine-site antagonists such as MDL-29951 (MDL; >20-fold). Coapplication of both Zn2+ and MDL generates a > 120-fold “supralinear” potentiation of glycine-induced currents (Madry et al., 2008
). In addition, Zn2+ alone elicits receptor currents with similar efficacy as glycine at concentrations > 100 μM (Madry et al., 2008
).
In the present study we investigated the current-voltage (I–V) dependence and permeation properties of glycine-gated NR1/NR3 NMDA receptors. We found that NR1/NR3A receptors display a strong outwardly rectifying I–V relationship, whereas NR3B subunit-containing receptors (NR1/NR3B and NR1/NR3A/NR3B) do not show a voltage-dependent inward current block at physiological ion concentrations. Further analyses revealed that the voltage-dependent inhibition of ion flux seen with NR1/NR3A receptors is (i) due to Ca2+ present in our extracellular recording solution, and is (ii) relieved by potentiating ligands like Zn2+ or/and MDL acting at the NR1 subunit. We conclude from these data that voltage-dependent inhibition of NR1/NR3A receptors by physiological concentrations of extracellular Ca2+ may be important for regulating excitatory glycinergic transmission, as described for the voltage-dependent Mg2+ block of conventional NR1/NR2 NMDA receptors important for glutamatergic signalling.
MDL-29951 was purchased from Tocris (Biotrend, Cologne, Germany). All other chemicals used were obtained from Sigma (Taufkirchen, Germany). All experimental procedures were done according to German law (Animal licence no. V 54–19c20/15-F126/13; Regierungspräsidium Darmstadt).
cDNA Constructs, Oocyte Expression and Electrophysiology
The NR1-1a, NR1-1aF466A, NR3A and NR3B expression constructs have been described previously (Kuryatov et al., 1994
; Madry et al., 2007a
). In vitro synthesis of cRNA (mCAP mRNA Capping Kit, Ambion, Austin, TX, USA) was performed as described (Laube et al., 2004
; Madry et al., 2007a
) in the presence of 115 mM NaCl, 1 mM KCl, 1.8 mM CaCl2 and 10 mM Hepes (pH 7.2). For heterologous expression of NR1/NR3 receptors, 25 ng of cRNA was injected at a NR1:NR3 ratio of 1:3 into Xenopus laevis oocytes. Oocytes were isolated and maintained as described previously (Laube et al., 1995
). Two-electrode voltage-clamp recording of whole-cell currents was performed according to Madry et al. (2007b)
. N-methyl-D-Glucamine chloride (NMDG-Cl) was used in external solutions in which NaCl was replaced for analysis of divalent permeability. To monitor the voltage dependence of NR1, NR3A and NR3B receptor combinations, whole-cell current-voltage relationships of saturating glycine-induced currents were recorded in 20 mV-intervals ranging from −90 mV to +30 mV and normalized to the current value obtained at +30 mV. Data points were aligned by using a 3rd-order polynomial fit according to Geiger et al. (1995)
. To quantify the extent of rectification, the current ratios at 40 mV above and 80 mV below the individual reversal potentials (Erev between −10 and 0 mV) were determined as rectification indices (Ri). The relative divalent to monovalent permeability (Pdiv/Pmono) was calculated by the Goldman-Hodgkin-Katz constant field (GHK) voltage equation assuming no anion permeability as described previously (Geiger et al., 1995
). The internal concentrations of Na+ and K+ used in the calculations were 20 mM and 150 mM, respectively (Katz et al., 2000
; Weisstaub et al., 2002
). Permeability ratios were calculated for each oocyte and then averaged. In order to avoid the activation of the oocytes’ native Ca2+-sensitive chloride currents, all experiments using Ca2+ containing extracellular solutions were carried out in oocytes incubated for 30 min at room temperature with the membrane-permeant Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA-AM, 100 μM) prior to electrophysiological recordings (Weisstaub et al., 2002
).
Statistical Analyses
Values given represent means ± SEM. Statistical significance was determined at the p < 0.05 (*), p < 0.01(**) and p < 0.001 (***) levels using a Student’s two-tailed, unpaired t-test.
Glycine-Gated NR1/NR3A and NR1/NR3B Receptors Differ in their Current-Voltage (I–V) Relationships
In a previous study, we described a potentiating effect of the NR1 glycine-binding site antagonist MDL-29951 (MDL) on NR1/NR3A and NR1/NR3B receptors expressed in oocytes (Madry et al., 2007a
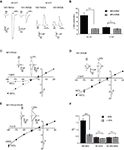
). When further analyzing the effect of MDL on NR1/NR3A and NR1/NR3B receptor currents elicited by glycine (1 mM), we found that at a negative holding potential (−90 mV) NR1/NR3A receptors were significantly more potentiated by 200 nM MDL than NR1/NR3B receptors, with a potentiation of 8.8 ± 1.2-fold and 2.5 ± 0.1-fold, respectively (p < 0.001; Figures 1
A,B). However, at a positive holding potential (+30 mV), potentiation of the glycine-induced currents by MDL was not different between the two receptor combinations (3.1 ± 0.5-fold and 2.4 ± 0.4-fold, respectively; p > 0.05; Figures 1
A,B). We therefore analyzed whole-cell current-voltage relationships (I–V curves) of glycine-induced currents from NR1/NR3A and NR1/NR3B receptors over a voltage range of −90 mV to +30 mV (Figures 1
C,D). I–V curves of NR1/NR3B receptors were found to be linear with a reversal potential of around −10 mV (Figure 1
D), whereas those obtained from NR1/NR3A receptors showed an identical reversal potential but a strong outwardly rectifying behavior, with an inward current block emerging at a holding potential <−30 mV (Figure 1
C). Interestingly, co-application of 200 nM MDL caused a linearization of the I–V curve for glycine-activated NR1/NR3A receptors (Figure 1
C), whereas the linear I–V relation of receptors containing the NR3B subunit was not altered in the presence of MDL (Figure 1
D).
Figure 1. I–V relationships of glycine-gated NR1/NR3 receptors. (A,B) Effect of holding-potential on MDL-29951 (MDL) potentiated glycine currents of NR1/NR3A and NR1/NR3B receptors. (A) Sample traces at −90mV (left) and +30 mV (right) activated with a saturating glycine concentration (1 mM) in the absence or presence of 200 nM MDL. (B) Relative potentiation by MDL of NR1/NR3A (black bars) and NR1/NR3B (gray bars) receptor currents at −90 and +30 mV. Note that MDL-potentiation was at −90 mV about 3-fold larger for NR1/NR3A receptors compared to NR1/NR3B (p < 0.01; n = 5). (C–E) Normalized I–V plots of NR1/NR3A (C), NR1/NR3B (D) and NR1/NR3A/NR3B (E) receptor currents recorded from −90 to +30 mV in 20-mV intervals activated by a saturated glycine concentration in the absence (triangle) and presence (square) of 200 nM MDL. Respective sample traces are shown above. Note that NR1/NR3A receptors display an ourwardly rectifying I–V curve in the presence of glycine alone, which becomes linear upon MDL-potentiation. (F) Quantification of I–V relationships of NR1/NR3 receptors in the absence (black bars) and presence (gray bars) of 200 nM MDL by determining the rectification index (Ri) of the currents measured at 40 mV above (Δ+40 mV) and 80 mV below (Δ–80 mV) the respective reversal potential.
In order to quantify the extent of rectification of NR1/NR3 receptor currents, we determined current ratios at +30 mV and −90 mV and calculated rectification indices (Ri). Based on a reversal potential of –10 mV, linear I–V relationships result in a Ri value of about 0.5 whereas outwardly rectifying I–V curves display Ri values > 1.5. Consistent with the data shown above, MDL potentiation caused a significant change of the Ri for NR1/NR3A receptors (−MDL: 1.65 ± 0.15, +MDL: 0.62 ± 0.08; p < 0.001), whereas no difference was seen for NR1/NR3B receptors in the absence and presence of the antagonist (−MDL: 0.43 ± 0.08, +MDL: 0.34 ± 0.02; p > 0.05; Figure 1
F). Finally, we analyzed the I–V curve of triheteromeric receptors composed of NR1, NR3A and NR3B subunits. Maximal inducible currents of these tri-heteromeric NR1/NR3A/NR3B receptors were more than 10-fold larger than those obtained from di-heteromeric NR1/NR3A and NR1/NR3B receptors (data not shown), which is consistent with an efficient tri-heteromeric NR1/NR3A/NR3B receptor assembly (Ulbrich and Isacoff, 2008
; Smothers and Woodward, 2009
). I–V curves from NR1/NR3A/NR3B expressing oocytes were found to be linear in both, the absence and presence of MDL (Figure 1
E). Analyses of the Ri revealed values of 0.42 ± 0.06 vs. 0.44 ± 0.04 in the absence and presence of 200 nM MDL for NR1/NR3A/NR3B-receptors, respectively (p > 0.05; Figure 1
F). Thus, MDL caused a linearization of the outwardly rectifying I–V curve of NR1/NR3A receptors by a relief of the voltage-dependent inward current block, whereas NR3B containing combinations showed a linear I–V-relationship irrespective whether MDL was present or not.
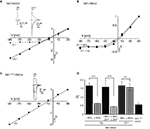
Differential Effects of Zn2+ on NR1/NR3A Receptor I–V Relations
The divalent cation Zn2+ exerts complex and opposing effects at NR1/NR3 receptors. At NR1/NR3A receptors it acts in the lower micromolar concentration range as a positive modulator of glycine-currents and as a full principal agonist at Zn2+ concentrations > 100 μM (Madry et al., 2008
). In contrast, NR1/NR3B receptors neither become potentiated nor activated by Zn2+. Therefore we wondered whether Zn2+-potentiation of NR1/NR3A receptor currents would display a linear I–V relationship similar to that found for MDL-potentiated receptor currents. Indeed, co-application of glycine and 50 μM Zn2+ fully linearized the outwardly rectifying I–V curve seen in the absence of Zn2+ to Ri values resembling those found in the presence of MDL (Figures 2
A,D). Since maximal potentiation of NR1/NR3A receptors is observed upon co-application of the modulators Zn2+ and MDL (Madry et al., 2008
), we also analyzed the I–V curves of these supralinearly potentiated NR1/NR3A receptors. In line with a relief of the glycine-mediated inward current block by MDL and Zn2+ alone, we found a linear I–V relationship of the glycine-induced currents when both 200 nM MDL and 100 μM Zn2+ were co-applied (Figure 2
D, and data not shown). In conclusion, potentiation of glycine-induced NR1/NR3A receptor currents by Zn2+, NR1 antagonists or a combination of both abrogated the inward current block seen upon application of glycine alone.
Figure 2. Zn2+ mediated effects at NR1/NR3A receptors. (A) Zn2+ potentiation of glycine-induced currents at NR1/NR3A receptors. Normalized I–V plot of NR1/NR3A receptor currents activated by a saturating glycine concentration in the absence (triangle) and presence (square) of 50 μM Zn2+. Similar to MDL, also co-application of 50 μM Zn2+ with 100 μM glycine causes a linearization of NR1/NR3A receptor I–V relationship. (B) MDL potentiation of Zn2+-induced currents at NR1/NR3A receptors. Normalized I–V plot of NR1/NR3A receptor currents activated by a saturating Zn2+ concentration in the absence (triangle) and presence (square) of 200 nM MDL. Note that NR1/NR3A receptors display an outwardly rectifying I–V curve upon activation by Zn2+ irrespectively of whether MDL is present or not. (C) Normalized I–V plot of the LBD-mutant NR1F466A/NR3A receptor currents activated by a saturating glycine concentration. Note that NR1F466A/NR3A receptors display a linear I–V relation. (D) Rectification indices (Ri) of I–V relationships of wt and mutant NR1/NR3 receptors in the absence (black bars) and presence (gray bars) of potentiators.
Higher concentrations of Zn2+ (>100 μM) activate NR1/NR3A receptors with a comparable efficacy as glycine (Madry et al., 2008
). Hence, we wanted to know whether both agonists display similar I–V relationships. We found that Zn2+-gated NR1/NR3A receptors displayed a similar inward current block than seen with glycine (Figure 2
B). Strikingly, MDL potentiation of Zn2+-induced currents was not accompanied by a linearization of the I–V curve, and thus did not cause a decrease of the rectification index (Figures 2
B,D). Apparently, the voltage-dependent blocks seen with both glycine and Zn2+ are differentially affected by MDL. Since both Zn2+ and MDL are thought to act via the NR1-LBD, we analyzed the effect of a mutation within the glycine-binding site of the NR1 subunit (phenylalanine 466 to alanine) on glycine-gated I–V relations of NR1/NR3A receptors (Figure 2
C). Previous mutational analyses have shown that the affinity of both glycine and MDL to the NR1 subunit is reduced by the NR1F466A substitution (Kuryatov et al., 1994
; Madry et al., 2007a
), and that the potentiating and activating effects of Zn2+ at NR1/NR3A receptors are abolished (Madry et al., 2008
) by this mutation. Here we found a linear I–V relationship of glycine-induced currents recorded from NR1F466A/NR3A receptors (Ri: 0.49 ± 0.02; Figures 2
C,D). This indicates that the NR1 LBD is crucial for the rectification behavior of NR1/NR3A receptors.
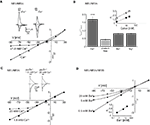
Physiological Concentrations of Ca2+ Cause Voltage-Dependent Inhibition of NR1/NR3A Receptor-Mediated Ion Flux
Mg2+ ions are known to block NR1/NR2 NMDA receptor channels at negative membrane potentials (overview in Cull-Candy et al., 2001
). We therefore analyzed whether divalent cations are responsible for the voltage-dependent inward current block seen with NR1/NR3A receptors. First we measured glycine-mediated currents of NR1/NR3A receptors in the absence of divalent cations. I–V relationships obtained under divalent-free conditions were found to be linear, with a rectification index in the range of those seen with glycine-gated NR1/NR3B and NR1/NR3A/NR3B, or potentiated NR1/NR3A receptors (Ri: 0.43 ± 0.04; Figures 3
A,B). To further test whether the voltage-dependent inhibition of NR1/NR3A receptor-mediated ion flux is due to a specific divalent cation, we analyzed I–V relations of glycine-induced currents in the presence of Ca2+, Ba2+ and Mg2+ (1.8 mM each). The presence of either 1.8 mM Ba2+ or Mg2+ resulted only in a minor inhibition of inward current flow with Ri-values of 0.84 ± 0.05 and 0.84 ± 0.03, respectively (Figure 3
B). In contrast, I–V relations in the presence of 1.8 mM Ca2+ revealed a strong inward rectification with a highly significant larger RiCa of 1.68 ± 0.09 (p < 0.001; Figure 3
B). To estimate the efficacy of Ca2+ and Mg2+ to block inward currents, I–V relationships with increasing concentrations (1, 10 and 20 mM) of the two divalent cations were measured. Only higher Mg2+ concentrations (>10 mM) resulted in a pronounced inward rectification with Ri-values > 1 similar to those found with low Ca2+ concentrations, whereas I–V curves in the presence of 1 mM Mg2+ were linear (Figure 3
B, inset). This is consistent with different affinities of the two cations tested for ion channel block and shows that under physiological divalent cation concentrations Ca2+ and not Mg2+ determines the I–V relationship of NR1/NR3A receptors.
Figure 3. Dependency of I–V relationship on divalent cations for glycine-activated NR1/NR3A receptors. (A) Normalized I–V plot of NR1/NR3A receptor currents activated by a saturating glycine concentration in the absence (triangle) and presence (square) of 1.8 mM Ca2+. Note, that application of a saturating glycine concentration (100 μM) in the absence of any divalent cations results in a linear I–V relationship, whereas 1.8 mM Ca2+ causes an inward current block (see also Figure 1
C). Sample traces are shown above the I–V plot. (B) Quantification of divalent-dependent inward current block of NR1/NR3A receptors. Rectification indices (Ri) of I–V relationships of NR1/NR3A receptors in the absence of divalent cations and in the presence of 1.8 mM Ca2+, Ba2+ and Mg2+ are shown. Inset shows a plot of the three different rectification indices (Ri) fitted against the respective log of (Ca2+) (open symbol) and (Mg2+) (closed symbol). (C) Effect of different Ca2+ concentrations on Zn2+ potentiated glycine-activated currents of NR1/NR3A receptors. Normalized I–V plot of potentiated NR1/NR3A receptor currents activated by a saturating glycine concentration and 50 μM Zn2+ in the presence of 1.8 mM (square) and 20 mM (triangle) Ca2+. Note that an increase of the extracellular Ca2+ concentration from 1.8 to 20 mM led to an outwardly rectifying I-V-relationship similar to those found under non-potentiated conditions in the presence of low Ca2+. Sample traces are shown above the I–V plot. (D) Increasing divalent cation concentrations lead to outwardly rectifying I-V relationships in NR3B containing NR1/NR3 receptors. Normalized I–V plot of NR1/NR3A/NR3B receptor currents activated by a saturating glycine concentration in the presence of 0.5 mM (square), 5 mM (circle) and 20 mM Ba2+ (triangle). Inset shows a plot of the three different rectification indices (Ri) fitted against the respective log [Ba2+].
To test whether potentiated NR1/NR3A glycine currents might be affected at non-physiological elevated Ca2+ concentrations, we analyzed the I–V relationship of Zn2+-potentiated (50 μM) glycine-induced currents in the presence of 20 mM Ca2+. This increased Ca2+ concentration produced an inward current block at holding potentials <–30 mV (Figure 3
C) as seen in the absence of Zn2+ at low Ca2+ (1.8 mM). Based on this result, we re-investigated the divalent cation dependency of the I–V curves of NR1/NR3B and NR1/NR3A/NR3B receptors, which both exhibit linear I–V relationships under physiological salt concentrations. Similarly, increasing the extracellular Ca2+ or Ba2+ concentration to 20 mM led to the emergence of an outwardly rectifying I–V curve at both NR1/NR3B and NR1/NR3A/NR3B receptors (Figure 3
D). This implies that in the presence of elevated divalent cation concentrations NR3B subunit containing receptor combinations display an outwardly rectifying I–V relationship as found for NR1/NR3A receptors in the presence of physiological Ca2+ concentrations.
In summary, physiological Ca2+ conditions are responsible for the outward rectification of glycine-gated NR1/NR3A receptors, whereas potentiated NR1/NR3A and NR3B containing receptors are blocked only at higher Ca2+ concentrations. Thus, differences in the affinity of the Ca2+ block seem to underlie the differential rectification behavior of NR3A and NR3B subunit containing receptors.
Permeability for Divalent Cations is not Altered in Supralinearly Potentiated NR1/NR3A Receptors
Removal of a cation-dependent open channel block has been shown in both transient receptor potential (TRP) channels and conventional NMDA receptors by an increase in the passage-rate of the blocking ion through the channel pore (Parnas et al., 2009
). Therefore the relief of the Ca2+-mediated block seen with MDL and Zn2+-potentiated NR1/NR3A receptors may derive from an increased Ca2+ permeability. To test this hypothesis, we substituted Na+ with the ion channel pore impermeable compound N-methyl-D-glucamine chloride (NMDG-Cl) and determined I–V curves in the presence of different Ca2+ concentrations in order to obtain an approximate estimate of the relative divalent to monovalent cation permeability PCa/PNa. Figure 4
A shows that the reversal potentials pooled from three different oocytes become more depolarized as a function of the concentration of Ca2+ in the extracellular medium. However, the ratio of PCa/PNa was <0.25 in all measurements as determined by the Goldman-Hodgkin-Katz voltage equation (see Geiger et al., 1995
), confirming a low Ca2+ permeability of the NR1/NR3A receptor under non-potentiated conditions (Chatterton et al., 2002
). In a next step we compared the I–V relationships of glycine-induced currents with that of supralinearly potentiated currents in the presence of divalent cations. Neither the analysis of I–V curves in the presence of 10 mM Ba2+ nor using 10 mM Ca2+ in the extracellular solution with BAPTA-AM pre-incubated cells revealed any shifts in the reversal potentials (Erev −67 mV; Figure 4
B). We also tested the accessibility to MK-801, a classical ion channel pore blocker of NR1/NR2 NMDA receptors, which has no effect on glycine-gated NR1/NR3A receptors (Wong et al., 1986
; Chatterton et al., 2002
). However, even when activated with glycine in the presence of Zn2+ and MDL, MK-801 (100 μM) remained ineffective at NR1/NR3A receptors (data not shown). Together these data indicate that the loss of the voltage-dependent Ca2+-block seen with NR1/NR3A receptors in the presence of MDL and Zn2+ is not accompanied by an increased Ca2+ permeability of the ion channel.
Figure 4. Effect on divalent cation permeability upon supralinear potentiation of glycine-gated NR1/NR3A receptors. (A) Relative divalent to monovalent permeability of NR1/NR3A receptors. Representative I–V recordings around the Erev obtained in Na+-free ringer containing 1 and 10 mM Ca2+. Arrows indicate the reversal potential (Erev) of each I–V curve. (B) I–V recordings of saturated glycine-induced currents (triangles) versus supralinear potentiated currents with 0.2 μM MDL and 50 μM Zn2+ (squares) in Na+-free extracellular solution substituted with 115 mM NMDG+ and 10 mM divalent cation (Ba2+) present. Enlargement illustrates no changes in the respective reversal potentials (−67 mV) for the two conditions.
In this article, we show that glycine-gated NR1/NR3A and NR1/NR3B NMDA receptors display a differential sensitivity for Ca2+ upon heterologous expression in Xenopus oocytes. At negative holding potentials, physiological concentrations of Ca2+ (1.8 mM) caused a pronounced inward current block of NR1/NR3A receptors, whereas receptors containing the NR3B subunit were only inhibited at elevated Ca2+ concentrations (>10 mM). Interestingly, the voltage-dependent inhibition of NR1/NR3A receptor currents by external Ca2+ was abrogated upon co-application of Zn2+, glycine-site antagonist or mutations within the glycine-binding site of the NR1 subunit; all these conditions resulted in a linear I–V relationship. Ion substitution experiments revealed that neither MDL or Zn2+ potentiation nor the relief of the Ca2+-block was accompanied by changes in Ca2+ permeability. Notably, when NR1/NR3A receptor mediated currents were elicited by Zn2+, MDL co-application did not result in a linear I–V, consistent with different mechanisms underlying glycine and Zn2+ agonism.
An interesting finding of this study is that the difference in maximal MDL potentiation of glycine currents seen between NR1/NR3A and NR1/NR3B receptors (Madry et al., 2007a
) is due to a pronounced Ca2+-dependent outward rectification of NR1/NR3A receptors. Thus, at physiological Ca2+ concentrations NR1/NR3A receptor channels are blocked at negative holding potentials whereas NR1/NR3 receptors containing the NR3B subunit are not affected. Notably, a similar outward rectification of the here described voltage-dependent Ca2+ block of the NR1/NR3A receptor exists in conventional NMDA receptors composed of NR1/NR2 subunits. Their voltage-dependent block at resting membrane potentials is mediated by extracellular Mg2+ (overview in Cull-Candy et al., 2001
). Molecular structures responsible for the Mg2+ block have been partially identified and comprise sites in the middle and at the entrance of the channel forming segments of NMDA receptor subunits (overview in Dingledine et al., 1999
). For example, asparagine residues of the QRN site in the M2 segment of NR1 and NR2 subunits have been shown to determine the block by Mg2+ (Kuner et al., 1996
). In addition, a DRPEER motif in NR1 (Watanabe et al., 2002
), a tryptophan residue in the M2 regions of NR2 subunits (Williams et al., 1998
) and the common SYTANLAAF motif in TM3 (Yuan et al., 2005
; Wada et al., 2006
) affect the Mg2+ block. Comparing the sequences of NR1, NR2 and NR3 subunits reveals a remarkable conservation of these regions, although especially within the QRN site and the SYTANLAAF motif several exchanges between NR1, NR2 and NR3 subunits are found. For example, the corresponding NR3 residue of the QRN site is a glycine. Although all residues mentioned above are highly conserved in NR2 subunits, channels containing NR2A or NR2B subunits are more sensitive to Mg2+ block compared with NR2C or NR2D-containing channels, suggesting that additional elements exist that determine subunit specificity to divalent cations. However, the well known physiological function of conventional NMDA receptors in the mammalian brain is to serve as coincidence detectors of presynaptic and postsynaptic activity. This function is achieved through removal of the Mg2+ block upon postsynaptic membrane depolarization (Cull-Candy et al., 2001
). Likewise, a similar mechanism can be envisaged for NR1/NR3A receptors where release of both, the principal agonist glycine and a second so far unknown ligand may result in a pronounced potentiation of glycine-currents and relief of the voltage-dependent Ca2+ block (this study). A previous report has disclosed that the neuromodulator Zn2+ (overview in Frederickson et al., 2005
) is essential for proper functioning of glycinergic inhibitory neurotransmission (Hirzel et al., 2006
). Thus, Zn2+ may be similarly essential for efficient activation of NR1/NR3A receptors (Madry et al., 2008
).
A second important result of this study is that at least two ligands have to bind simultaneously for abrogating Ca2+-dependent outward rectification of NR1/NR3A receptors. Accordingly, efficient channel gating of NR1/NR3 receptors requires simultaneous occupancy of the NR1 and NR3 LBDs (Awobuluyi et al., 2007
; Madry et al., 2007a
). Here we show that only ligand-binding to both, the NR3A and NR1 LBD resulted in a linearization of the I–V curve, whereas co-application of the full agonist Zn2+ and the NR1 antagonist MDL, both binding within the NR1 LBD, did not abrogate the inward-rectifying Ca2+ block. This suggests a remarkable mechanistic similarity in ion channel activation between NR1/NR3A and conventional NR1/NR2 NMDA receptors. Both conventional and glycine-gated NMDA receptors require binding of two ligands within the LBDs of both subunits for efficient channel opening. Thus, only highly cooperative interactions between fully liganded subunits enable the conformational transition of NMDA receptors to the fully open state. Notably, although a remarkable potentiation is found upon binding of both the full agonist Zn2+ and MDL, the voltage-dependent Ca2+ block is not diminished due to the lack of NR3 LBD occupation. A similar contribution of multiply liganded subunits to efficient channel activation has been proposed for the AMPA receptor, a related member of the iGluR family (Rosenmund et al., 1998
). According to this view, occupancy of the NR1 or NR3 binding sites is in principle sufficient to drive channel opening, but additional occupancy of the respective other subunits increases receptor current which might be mediated due to changes in the open probability and/or sub-conductance states of the ion channel. Whatever the precise mechanism(s) of glycine vs. Zn2+ action may be, our results show that ion permeability is not changed upon supralinear potentiation of the glycine current. Thus, removal of the Ca2+-dependent channel block is not accompanied by an increase in the passage-rate of Ca2+ through the channel pore.
Taken together our data demonstrate fundamental differences in the I–V dependence of di-heteromeric NR1/NR3 receptors that might provide a versatile electrophysiological tool to discriminate NR1/NR3A and NR1/NR3B receptors in vivo.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was supported by the Max-Planck-Gesellschaft (Heinrich Betz), Gemeinnützige Hertie-Stiftung (Bodo Laube, Jörg R. P. Geiger), Deutsche Forschungsgemeinschaft (LA 1086/5-1, Bodo Laube) and Fonds der Chemischen Industrie (Heinrich Betz).
Tong, G., Takahashi, H., Tu, S., Shin, Y., Talantova, M., Zago, W., Xia, P., Nie, Z., Goetz, T., Zhang, D., Lipton, S. A., and Nakanishi, N. (2008). Modulation of NMDA receptor properties and synaptic transmission by the NR3A subunit in mouse hippocampal and cerebrocortical neurons. J. Neurophysiol. 99, 122–132.