Antithetical modes of and the Ca2+ sensors targeting in ANF-RGC and ROS-GC1 membrane guanylate cyclases
- 1Research Divisions of Biochemistry and Molecular Biology, The Unit of Regulatory and Molecular Biology, Salus University, Elkins Park, PA, USA
- 2Department of Biology and Environmental Sciences, Biochemistry Group, University of Oldenburg, Oldenburg, Germany
The membrane guanylate cyclase family has been branched into three subfamilies: natriuretic peptide hormone surface receptors, Ca2+-modulated neuronal ROS-GC, and Ca2+-modulated odorant surface receptor ONE-GC. The first subfamily is solely modulated by the extracellularly generated hormonal signals; the second, by the intracellularly generated sensory and sensory-linked signals; and the third, by combination of these two. The present study defines a new paradigm and a new mechanism of Ca2+ signaling. (1) It demonstrates for the first time that ANF-RGC, the prototype member of the surface receptor subfamily, is stimulated by free [Ca2+]i. The stimulation occurs via myristoylated form of neurocalcin δ, and both the guanylate cyclase and the calcium sensor neurocalcin δ are present in the glomerulosa region of the adrenal gland. (2) The EF-2, EF-3 and EF-4 hands of GCAP1 sense the progressive increment of [Ca2+]i and with a K1/2 of 100 nM turn ROS-GC1 “OFF.” In total reversal, the same EF hands upon sensing the progressive increment of [Ca2+]i with K1/2 turn ONE-GC “ON.” The findings suggest a universal Ca2+-modulated signal transduction theme of the membrane guanylate cyclase family; demonstrate that signaling of ANF-RGC occurs by the peptide hormones and also by [Ca2+]i signals; that for the Ca2+ signal transduction, ANF-RGC functions as a two-component transduction system consisting of the Ca2+ sensor neurocalcin δ and the transducer ANF-RGC; and that the neurocalcin δ in this case expands beyond its NCS family. Furthermore, the study shows a novel mechanism of the [Ca2+]i sensor GCAP1 where it acts as an antithetical NCS for the signaling mechanisms of ROS-GC1 and ONE-GC.
Introduction
Ca2+ sensor proteins form a group of Ca2+ binding proteins that, in defined concentrations of free intracellular Ca2+, function as modulators of the activities of specific target proteins. They acquire these modulatory abilities by binding Ca2+ through specific helix-loop-helix structural motifs called EF hands. Binding of Ca2+ to the EF hand motif triggers conformational changes in the respective Ca2+ sensor protein that enable it to perform Ca2+-dependent functions ranging from regulation of ion channels permeability to gene expression, cellular survival and apoptosis (reviewed in Bhattacharya et al., 2004; Braunewell, 2005). Neuronal Ca2+ sensor (NCS) proteins constitute a subfamily of Ca2+ sensor proteins and were initially considered to be expressed exclusively in neurons, but now are found in other tissues as well. NCS proteins are subclassified into five groups based on their sequence similarities. In mammals, 14 conserved NCS proteins exist (reviewed in Burgoyne, 2007) and all of them have 4 EF hand Ca2+ binding motifs but inactivating amino acid substitutions make the first EF hand non-functional for Ca2+ binding. In addition to the first inactive EF hand, recoverin and K+-channel interacting protein type1 (KChIP1) harbor another non-functional EF hand. Except for KChIP2, 3 and 4 all mammalian NCS proteins contain a consensus sequence for N-terminal myristoylation (reviewed in Burgoyne and Weiss, 2001; Burgoyne et al., 2004). Some isoforms of KChIP2 and 3 are possibly palmitoylated (Takimoto et al., 2002). Both myristoylation and palmitoylation of NCS proteins allows for some of them membrane association, either permanently or transiently in response to changes in the intracellular Ca2+ concentration. For GCAP1 and NCS-1, however, Ca2+ plays no role in membrane attachment of the protein (Hwang and Koch, 2002; O'Callaghan et al., 2002; Orban et al., 2010).
Neurocalcin δ together with visinin-like proteins (VILIPs) and hippocalcin form a distinct subfamily of NCS proteins. It is acylated at the N-terminus by myristic acid and undergoes a classical calcium-myristoyl switch (Ladant, 1995) e.g. it buries the myristoyl group in a hydrophobic pocket in a Ca2+-free form and expose it in Ca2+-bound form as it is observed for recoverin (Zozulya and Stryer, 1992). However, once it binds in a Ca2+-dependent fashion to the membrane phospholipids part of it remains membrane bound even after removing Ca2+ by the addition of EGTA (Krishnan et al., 2004). Although the highest level of neurocalcin δ has been detected in neuronal tissues, its expression level in peripheral tissues is also significant. Functionally, neurocalcin δ has been linked to receptor endocytosis through interaction with α- and β-clathrin and β-adaptin (Ivings et al., 2002), trafficking and membrane delivery of glutamate receptors of the kainate type (Coussen and Mulle, 2006), and due to its Ca2+-dependent affinity for S100B protein and tubulin β-chain (Okazaki et al., 1995), with microtubule assembly (Iino et al., 1995). In the sensory and sensory-linked neurons, the presence of neurocalcin δ has been found in the inner plexiform layer of the retina, e.g. in the amacrine and ganglion cells (Krishnan et al., 2004), olfactory sensory neurons (Duda et al., 2001, 2004) and very recently, it has been identified in type II cells of mouse circumvallate taste papillae, indicating its possible role in the gustatory transduction (Rebello et al., 2011).
Further, neurocalcin δ can act as Ca2+-dependent modulator of membrane guanylate cyclase ROS-GC1 in the retina and ONE-GC, in the olfactory neuroepithelium. There, it co-localizes with its respective target guanylate cyclases (Duda et al., 2001, 2004; Krishnan et al., 2004). The exact physiological significance of the ROS-GC1-neurocalcin δ signaling system in the retinal neurons is not known yet, it has, however, been proposed that the system may be involved in synaptic processes (Krishnan et al., 2004). In the olfactory neuroepithelium neurocalcin δ serves as a Ca2+ sensor component of the two-step odorant uroguanylin signaling machinery (Duda and Sharma, 2009).
Guanylate cyclase activating protein type 1 (GCAP1) is a well characterized member of the NCS proteins subfamily (reviewed in Palczewski et al., 2004; Sharma et al., 2004; Behnen et al., 2010; Koch et al., 2010). Like other homologs and orthologs of the subfamily, it harbors four EF hand Ca2+ binding motifs, of which the first one is inactive. GCAP1 is acylated at the N-terminus by myristic acid that is buried in a hydrophobic pocket (Stephen et al., 2007) and changing Ca2+ concentrations do not trigger exposure of the myristoyl group (Orban et al., 2010). Instead the myristoyl group remains buried in a hydrophobic cavity. Thus, GCAP1 does not interact with the membranes in a Ca2+-dependent fashion (Hwang and Koch, 2002; Haynes and Burgoyne, 2008) and does not undergo a classical calcium myristoyl switch. Identified first in the retinal photoreceptors (Gorczyca et al., 1994; Frins et al., 1996), GCAP1 transmits Ca2+ signals to and controls the activity of rod outer segment guanylate cyclase, ROS-GC. It activates ROS-GC in the absence of Ca2+, when immediately after illumination the cytoplasmic Ca2+ drops in a photoreceptor cell. GCAPs are thought to switch to a Ca2+-free but Mg2+-bound state, which represents the activating form (Peshenko and Dizhoor, 2006). Increasing concentrations of free Ca2+ diminish the activation process leading even to an inhibition below the basal cyclase activity level (Duda et al., 1996). This regulatory process of ROS-GC is essential for the photo-response recovery of visual cells (Mendez et al., 2001; Howes et al., 2002).
Here, new observations on Ca2+-dependent modes of neurocalcin δ and GCAP1 in modulating membrane guanylate cyclase signaling are presented. The results disclose a new model of Ca2+-neurocalcin δ signaling of ANF-RGC and a new signaling mechanism of GCAP1 in which it serves as an antithetical Ca2+ sensor in the phototransduction and the olfactory sensory neurons. Thus, they indicate an increasing complexity in the regulatory modes of membrane guanylate cyclases and this enables them to perform multiple cellular functions.
Materials and Methods
Mutagenesis. Point mutations in ONE-GC and GCAP1 cDNA were introduced using Quick Change mutagenesis kit (Stratagene) and appropriate mutagenic primers. ONE-GC F585S mutation- Forward primer 5′-TGGCTGAAGAAGTCTGAGGCAGGC ACG-3′; Reverse primer 5′-CGTGCCTGCCTCAGACTTCTTCAG CCA-3′ (the mutated sequence is underlined). Construction of the GCAP1(D100E) mutant is described in detail in (Kitiratschky et al., 2009). The mutants were verified by sequencing. To construct the ANF-RGC Ext− mutant two Hpa1 restriction sites were introduced at nucleotide positions 437–442 (Forward primer 5′-GTGGTGCTGCCGCTGGTAAACAACACCTCGTACCCG-3′, Reverse primer 5′-CGGGTACGAGGTGTTTACCAGCGGCAGCACCAC-3′; Hpa1 recognition sequence is underlined) and at nucleotide positions 1697–1702 (Forward primer 5′-AATGAGGACCCAGCCGTAAACCAAGACCACTTT-3′; Reverse primer 5′-AAAGTGGTCTTGGTTTACGGCTGGGTCCTCATT-3′; Hpa1 recognition sequence is underlined). The 1242 bp fragment was excised and the remaining part was religated.
Expression in COS cells. COS7 cells (simian virus 40-transformed African green monkey kidney cells) were maintained in DMEM medium supplemented with 10% fetal bovine serum and penicillin, streptomycin antibiotics on 10 cm diameter cell culture dishes in humidified atmosphere of 95% O2/5% CO2. At approximately 65% confluency the cells were transfected with 25 μg of appropriate plasmid cDNA using calcium phosphate co-precipitation technique (Sambrook et al., 1989). In control experiments the cells were transfected with 25 μg of empty expression vector. 64 h after transfection cells were washed with 50 mM Tris-HCl pH 7.4/10 mM Mg2+ buffer, homogenized and the particulate fraction pelleted by centrifugation.
Guanylate cyclase activity assay. The membranes were incubated on ice-bath with or without GCAP1 or neurocalcin δ in the assay system containing 10 mM theophylline, 15 mM phosphocreatine, 20 μg creatine kinase and 50 mM Tris-HCl, pH 7.5. Appropriate Ca2+ concentrations were adjusted with pre-calibrated Ca2+/EGTA solutions (Molecular Probes). The total assay volume was 25 μl. The reaction was initiated by addition of the substrate solution (4 mM MgCl2 and 1 mM GTP, final concentration) and maintained by incubation at 37°C for 10 min. The reaction was terminated by the addition of 225 μl of 50 mM sodium acetate buffer, pH 6.2 followed by heating on a boiling water bath for 3 min. The amount of cyclic GMP formed was determined by radioimmunoassay (Nambi et al., 1982).
Expression and purification of GCAP1, GCAP1(D100E) and neurocalcin δ. GCAP1 was expressed and purified as in (Duda et al., 1999), GCAP1(D100E) as in (Kitiratschky et al., 2009), and neurocalcin δ as in (Duda et al., 2004).
Antibodies. The specificity of antibody against neurocalcin δ has been described previously (Duda et al., 2004). Antibody against ANF-RGC was raised against the kinase homology domain in rabbits. Specificity of the antibody was tested through Western blot using membranes of COS cells expressing all three receptor guanylate cyclases, ANF-RGC, CNP-receptor guanylate cyclase (CNP-RGC) and enterotoxin receptor guanylate cyclase (STa-RGC). The antibody recognized only ANF-RGC (data not shown). The antibodies were affinity purified. Secondary antibodies conjugated to a fluorescent dye (DyLight 488 and DyLight 549) were purchased from Jackson ImmunoResearch Laboratories, Inc., West Grove, PA.
Immunohistochemistry. Mice were sacrificed by lethal injection of ketamine/xylazine (the protocol approved by the Salus University IUCAC) and perfused through the heart, first with a standard Tris-buffered saline (TBS) and then with freshly prepared 4% paraformaldehyde in TBS. The adrenal glands were removed and fixed for 1–4 h in 4% paraformaldehyde with TBS at 4°C, cryoprotected in 30% sucrose overnight at 4°C and cut into 20 μm sections using Hacker-Bright OTF5000 microtome cryostat (HACKER Instruments and Industries Inc., Winnsboro, SC). The sections were washed with TBS, blocked in 10% normal serum in TBS/0.5% Triton X-100 (TTBS) for 1 h at room temperature, washed with TTBS, incubated with respective antibody in blocking solution overnight at 4°C, washed with TTBS for and then incubated with DyLight conjugated donkey anti-rabbit antibody (200:1) for 1 h, washed with TTBS. Images were acquired using an inverted Olympus IX81 microscope/FV1000 Spectral laser confocal system, and analyzed using Olympus FluoView FV10-ASW software. Digital images were processed using Adobe Photoshop software.
Western blot. After boiling in a gel-loading buffer [62.5 mM Tris-HCl, (pH 7.5), 2% SDS, 5% glycerol, 1 mM β-mercaptoethanol (βME), and 0.005% bromophenol blue] the proteins (membranes of transfected COS cells or mouse adrenal gland homogenate) were subjected to SDS-polyacrylamide gel electrophoresis in a buffer (pH 8.3) containing 0.025 M Tris, 0.192 M glycine, and 0.1% SDS. The proteins were transferred to immobilon membranes (Millipore) in the same buffer but containing 5% methanol. The blot was incubated in (TBS pH 7.5) containing 100 mM Tris-HCl, 0.9% NaCl, and 0.05% Tween-20 (TBS-T) with 5% powdered non-fat Carnation milk (blocking buffer) overnight at 4°C and rinsed with TBS-T. The antibodies were added to the solution and the incubation continued for 1 h at room temperature. After the blot was rinsed with with TBS-T, the incubation was continued with the secondary antibody conjugated to horseradish peroxidase in blocking buffer for another hour. Finally, the blot was treated with SuperSignalR West Pico chemiluminescent substrate (Thermo Scienitifc; according to the manufacturer's protocol). The immunoreactive band was visualized by exposing the blot to Kodak X-ray film.
Results and Discussion
ANF Receptor Guanylate Cyclase, ANF-RGC, is Modulated by Ca2+ Signals
Neurocalcin δ transmits Ca2+ signal to ANF-RGC
Based on almost three decades of research the family of mammalian membrane guanylate cyclases has been firmly divided into two subfamilies, receptor guanylate cyclases and intracellular Ca2+ regulated guanylate cyclases. The first group included the receptor for natriuretic factor type A (ANF) and type B (BNP) guanylate cyclase ANF-RGC, the receptor for type C natriuretic peptide guanylate cyclase CNP-RGC, and heat-stable enterotoxin (and also guanylin and uroguanylin) receptor guanylate cyclase STa-RGC; the second group is comprised of the photoreceptor guanylate cyclases ROS-GC1 and ROS-GC2 and the olfactory neuroepithelium guanylate cyclase ONE-GC.
To determine whether a receptor guanylate cyclase could also respond to Ca2+ signals transmitted to it through a calcium sensor protein, the prototype receptor cyclase ANF-RGC and NCS protein neurocalcin δ were chosen. A clue for selecting neurocalcin δ was the observation that it targets the conserved membrane guanylate cyclase catalytic domain of ROS-GC1.
Membranes of COS cells expressing recombinant ANF-RGC were incubated with series of increasing concentrations of purified myristoylated neurocalcin δ at a fixed 10 μM Ca2+. No extracellular ligand of ANF-RGC, ANF or BNP, was added to the reaction mixture. ANF-RGC activity was stimulated in the neurocalcin δ concentration-dependent manner; half-maximal activation of the cyclase occurred at ∼0.5 μM and the maximal activation of 4.8-fold above the basal value was observed at 2 μM myristoylated neurocalcin δ (Figure 1: closed circles). The calculated Hill's coefficient for the stimulatory effect was 2.1 ± 0.5. In the membranes of cells transfected with the vector alone the cyclase activity was negligible, 0.2 pmol cyclic GMP min−1(mg protein)−1 (Figure 1: closed diamonds) and was unaffected by neurocalcin δ in the presence of Ca2+. To verify that the observed effect of Ca2+-neurocalcin δ is specific the cyclase activity was measured in the presence of neurocalcin δ but in the absence of Ca2+ (1 mM EGTA was added to the reaction mixture). The absence of Ca2+ did not affect the basal ANF-RGC activity; it was 12 ± 2 pmol cyclic GMP min−1(mg protein)−1 in the presence of 10 μM Ca2+ and 11.9 ± 1.8 pmol cyclic GMP min−1(mg protein)−1 in the presence of 1 mM EGTA. In the absence of Ca2+ neurocalcin δ, however, did not stimulate ANF-RGC activity (Figure 1: open circles). Thus, ANF-RGC activity is not only regulated by ANF or BNP; in vitro it is also regulated by myristoylated neurocalcin δ in the presence of Ca2+.
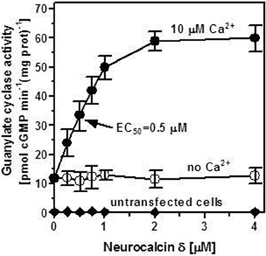
Figure 1. Ca2+-bound neurocalcin δ stimulates ANF-RGC activity. COS cells were transfected with ANF-RGC cDNA and their membrane fraction was analyzed for neurocalcin δ-dependent cyclase activity in the absence (open circles) and presence of 10 μM Ca2+ (closed circles). COS cells transfected with an empty vector were analyzed identically (closed diamonds). The extracellular hormone ligand of ANF-RGC, the atrial natriuretic factor (ANF) was absent from the reaction mixture. The experiment was done in triplicate and repeated four times. The results shown are average ± SD from these experiments. The EC50 value was determined graphically. Neurocalcin δ used was myristoylated. The myristoylated form of neurocalcin δ was expressed and purified as described in Krishnan et al. (2004).
Neurocalcin δ targets the intracellular domain of ANF-RGC
It is well established that ANF and BNP, the hormone-ligands of ANF-RGC, signal through the cyclase's extracellular domain (Duda et al., 1991; Ogawa et al., 2004; reviewed in Sharma, 2002, 2010). Neurocalcin δ, on the other hand, is an intracellular protein, therefore the respective target sites of these two types of ligand, ANF/BNP and neurocalcin δ, reside on the opposite sites of the transmembrane domain of ANF-RGC. To determine the biochemical requirements for neurocalcin δ effect on ANF-RGC activity namely, whether the isolated intracellular portion of ANF-RGC is sufficient for neurocalcin δ to exhibit its stimulatory effect or whether the intact ANF-RGC protein is necessary, an ANF-RGC deletion mutant was prepared in which the extracellular receptor domain (aa 12–433) was deleted. The mutant, however, had retained the leader sequence to ensure its proper membrane targeting. This mutant was transiently expressed in COS cells and their membranes were appropriately treated with ANF or myristoylated neurocalcin δ and 10 μM Ca2+. Both proteins had comparable basal guanylate cyclase activities, 13 and 12.2 pmol cyclic GMP min−1(mg protein)−1 for the full-length ANF-RGC and the deletion mutant, respectively. As expected, the mutant was unresponsive to ANF (Figure 2: open circles), however, neurocalcin δ stimulated its activity in a dose-dependent fashion (Figure 2: closed diamonds). The stimulatory profile was indistinguishable from that of the full-length ANF-RGC (Figure 2: closed circles). Also the Hill's coefficients for the neurocalcin δ effect on both cyclases were identical 2.1 ± 0.5 and 2.05 ± 0.4 for the full-length ANF-RGC and for the deletion mutant, respectively. It is, therefore, concluded that the extracellular domain has no structural role in ANF-RGC ability to respond to and be stimulated by myristoylated neurocalcin δ.
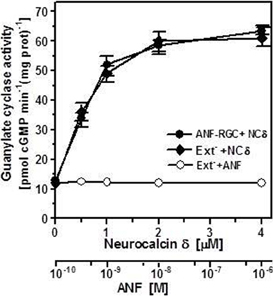
Figure 2. The intracellular portion of ANF-RGC is sufficient for neurocalcin δ and Ca2+ to stimulate ANF-RGC activity. The ANF-RGC deletion mutant lacking the extracellular receptor domain aa12–433, was constructed and expressed in COS cells. The particulate fraction of these cells was assayed for guanylate cyclase activity in the presence of increasing concentrations of myristoylated neurocalcin δ and 10 μM Ca2+ or 0.5 mM ATP and increasing concentrations of ANF. Membranes of COS cells expressing full-length ANF-RGC were processed in parallel as positive control. The experiment was performed in triplicate and repeated two times. The results shown are mean ± SD from these experiments.
ANF-RGC and neurocalcin δ co-exist in the glomerulosa cells of the adrenal gland
Are these in vitro biochemical findings on ANF-RGC activity modulation by Ca2+via neurocalcin δ of physiological relevance? A first hint could be the co-expression of both proteins in the same tissue, organ or cell type. Guided by intuition the mouse adrenal gland was analyzed for the presence of ANF-RGC and neurocalcin δ, first by Western blot and then through immunocytochemistry.
The gland was homogenized and the homogenate after SDS-polyacrylamide gel electrophoresis was analyzed for the presence of ANF-RGC or neurocalcin δ by Western blot. As shown in Figure 3, with antibody against ANF-RGC as a probe (panel “ANF-RGC”) an intense immunoreactive band was observed at expected molecular weight of ∼130 kDa. With neurocalcin δ antibody as a probe (Figure 3: panel “NCδ”) the presence of an immunoreactive protein with mobility of ∼23 kDa corresponding to the molecular weight of neurocalcin δ was observed. These results show that both ANF-RGC and neurocalcin δ are expressed in the mouse adrenal, they, however, do not provide information whether these proteins are expressed in the same type of adrenal gland cells. To determine whether these proteins co-localize in the adrenal gland immunocytochemical analyzes were carried out. Sections of the adrenal gland were immunostained with specific antibodies against ANF-RGC and neurocalcin δ. Because both antibodies used were raised in rabbits, co-immunostaining was not feasible, therefore, staining of consecutive sections was performed. The results are shown in Figure 4A. Intense staining with anti neurocalcin δ antibody was observed in the adrenal zona glomerulosa (Figure 4A: panel “NCδ” Z.G.). Also, strong immunoreactivity with ANF-RGC antibodies was observed in zona glomerulosa (Figure 4A: panel “ANF-RGC” Z.G.). This localization of ANF-RGC within the mouse adrenal gland is consistent with the protein's localization in the bovine adrenal gland (Meloche et al., 1988). To verify the specificity of the staining, in control reactions the primary antibodies were omitted but secondary antibody was added. Without the primary antibodies there was no specific staining in the sections analyzed (Figure 4B: control). It was therefore concluded that both ANF-RGC and neurocalcin δ co-exist in the adrenal glomerulosa cells. Although some faint staining in both sections was observed for the fasciculate-reticularis cells (Z.F-R. region in both “NCδ” and “ANF-RGC” panels), without detailed co-localization experiments it is not possible to conclude on ANF-RGC and neurocalcin δ co-existence there.
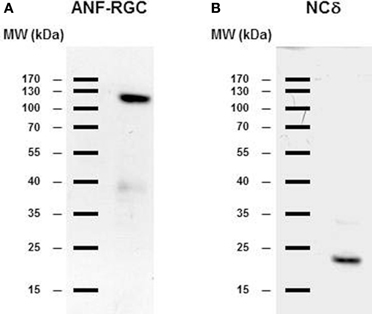
Figure 3. ANF-RGC and neurocalcin δ are expressed in the mouse adrenal gland. Mouse adrenal gland was homogenized in 50 mM Tri-HCl/10 mM MgCl2 buffer (pH 7.5) containing protease inhibitor cocktail (Sigma). The proteins (∼40 μg/lane) were subject to SDS-polyacrylamide gel electrophoresis and analyzed by Western blot using antibody against ANF-RGC or neurocalcin δ as described in the “Materials and Methods” section. (A) immunoreactivity with ANF-RGC antibody. (B) immunoreactivity with neurocalcin δ antibody.
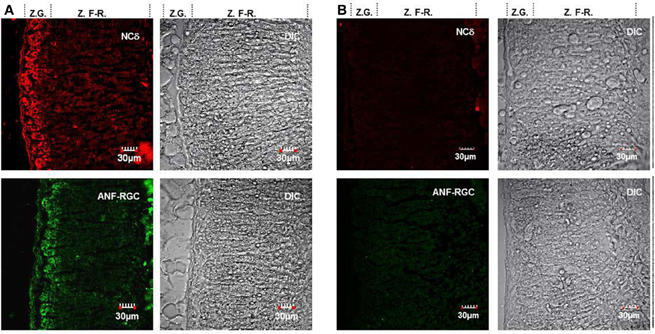
Figure 4. ANF-RGC and neurocalcin δ are co-expressed in the glomerulosa cells of mouse adrenal gland. (A) Serial cryosections of the mouse adrenal gland were immunostained with neurocalcin δ (panel “NCδ”) or ANF-RGC (panel “ANF-RGC”) antibodies. The DIC image showing the integrity of the adrenal gland sections are presented at the left (“DIC”). “Z.G.” and “Z.F-R.” denote zona glomerulosa and zona fasciculata-reticularis, respectively. Intense staining with either antibody was observed in the zona glomerulosa. (B) The immunostaining with the primary antibodies is specific. The mouse adrenal gland cryosections were processed identically as in (A) except that the primary antibody for ANF-RGC or neurocalcin δ was omitted in the respective incubation mixture but incubation with fluorescently labeled secondary antibody was carried out as in (A). The DIC image showing the integrity of the sections are presented (“DIC”).
The second question asked was: are ANF-RGC and neurocalcin δ co-existing in the adrenal gland functionally linked? Mouse adrenal gland was homogenized in the presence of 1 mM EGTA or 10 μM Ca2+, the particulate fraction was prepared and analyzed for guanylate cyclase activity. In the absence of Ca2+ the activity was 70 ± 9 pmol cyclic GMP min−1 (mg protein)−1 and in the presence of Ca2+, 245 ± 28 pmol cyclic GMP min−1(mg protein)−1 (Figure 5). At this stage it is not possible to conclude with certainty, however, if native neurocalcin δ in the adrenal gland is pre-bound to ANF-RGC or if it is undergoing a Ca2+ mirystoyl switch and interacts with ANF-RGC in a reversible manner, the guanylate cyclase activity measured at 10 μM Ca2+ reflects the neurocalcin δ-stimulated ANF-RGC activity.
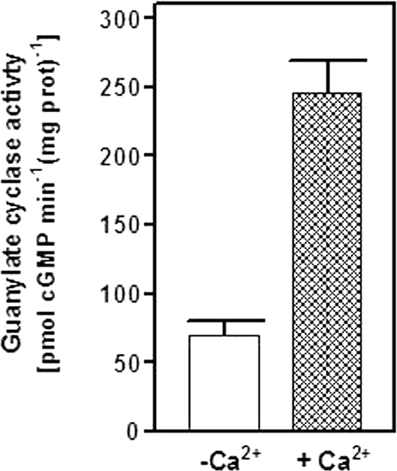
Figure 5. Neurocalcin δ stimulates ANF-RGC in membranes of mouse adrenal gland. Mouse adrenal gland was homogenized in Tris-Mg2+ buffer pH 7.4 with or without 10 μM Ca2+. The particulate fractions were prepared from each homogenate and assayed for guanylate cyclase activity. The experiment was done in triplicate and repeated two times. The results shown are average ± SD from these experiments.
What is the physiological significance of neurocalcin δ modulation of ANF-RGC activity in the adrenal zona glomerulosa? At this moment there is no definite answer to this question. A clue, however, may be provided by the facts that the adrenal glomerulosa cells are the site of aldosterone synthesis, that aldosterone synthesis is triggered by the increase in cytosolic Ca2+ concentration, and that the ANF-RGC activity offsets the renin-angiotensin-aldosterone system and inhibits aldosterone synthesis (Burnett et al., 1984; Brenner et al., 1990; Aoki et al., 2000; Shi et al., 2001). Because a measurable time is necessary for hormonal (ANF) turning “ON” the ANF-RGC signal transduction system resulting in the inhibition of aldosterone synthesis, it is tempting to hypothesize that before the hormonal ANF signal is activated, Ca2+-bound neurocalcin δ stimulates ANF-RGC. In this situation ANF-RGC response will be very rapid and the cyclic GMP produced will start to inhibit aldosterone synthesis almost immediately. Cyclic GMP synthesized by ANF-RGC affects number of effectors of aldosterone synthesis. They include cyclic GMP-gated channels, cyclic GMP-dependent protein kinases, and cyclic GMP-regulated phosphodiesterases (reviewed in Lohmann et al., 1997; Pfeifer et al., 1999). Several reports indicate that cyclic GMP-driven inhibition of aldosterone synthesis is, at least in part, mediated by cyclic GMP-stimulated phosphodiesterase (PDE 2) that is expressed at high levels in adrenal glomerulosa cells (MacFarland et al., 1991; Côté et al., 1999). This hypothesis needs now experimental validation.
GCAP1 - Antithetical Calcium Sensor
Since its discovery, GCAP1 has been exclusively regarded as the component of the phototransduction machinery sensing the fall in Ca2+ concentration after illumination, transmitting this information to photoreceptor guanylate cyclase ROS-GC and stimulating it to synthesize cyclic GMP at a faster rate (reviewed in Pugh et al., 1997; Koch et al., 2010). With the ensuing raise of Ca2+ concentration, Ca2+-bound GCAP1 inhibits ROS-GC activity bringing it to the basal level. This activator/inhibitor mode of GCAP1 operation is well established both in vivo and in vitro.
Recent work, however, indicated that GCAP1 could target to another sensory membrane guanylate cyclase, an odorant receptor ONE-GC [alternatively termed GC-D (Fülle et al., 1995)] expressed in a subpopulation of olfactory sensory neurons (Duda et al., 2006; Pertzev et al., 2010).
GCAP1 transmits the Ca2+-stimulatory signal to the odorant receptor guanylate cyclase, ONE-GC
Recombinant ONE-GC expressed in COS cell was exposed to increasing Ca2+ concentrations and constant, 4 μM, GCAP1 concentration. As a positive control, recombinant ROS-GC1 was treated identically. Both cyclases were expressed in COS to approximately the same level as verified by Western blot (Figure 6 inset). As expected, based on previous reports (Duda et al., 1996), at or below 10 nM Ca2+, GCAP1 maximally stimulated ROS-GC1 (Figure 6: closed circles). The stimulation decreased with increasing free Ca2+ concentration and the half-maximal inhibition was at about 100 nM Ca2+. Contrary to that, at about 100 nM Ca2+ there was practically no effect on GCAP1-dependent ONE-GC activity (Figure 6: open circles). However, with the Ca2+ concentrations increasing beyond 100 nM there was a dose-dependent increase in ONE-GC activity. The half-maximal activation of the cyclase occurred at 0.7 μM Ca2+ and the maximal activation at about 2.5 μM. At Ca2+ concentrations above 2.5 μM there was no statistically significant increase in the cyclase activity. The expression levels of ROS-GC1 and ONE-GC in COS cells was comparable (Figure 6: inset). These results essentially confirm the previous published in Duda et al. (2006). The main conclusion is that GCAP1 can function as calcium-dependent regulator of guanylate cyclase activity, but depending on the target it can activate the cyclase in a Ca2+-dependent manner with reverse premise making it an antithetical modulator.
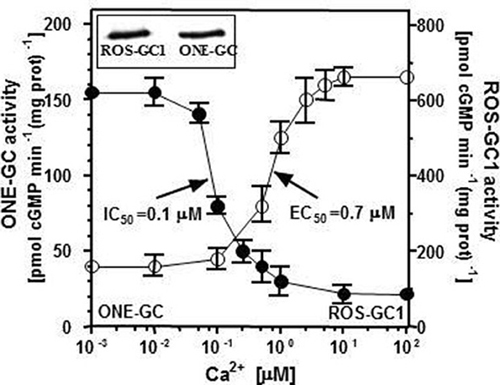
Figure 6. Antithetical Ca2+ modulated GCAP1 effect on ONE-GC and ROS-GC1 activities. Membranes of COS cells expressing ONE-GC or ROS-GC1 (control) were individually assayed for guanylate cyclase activity in the presence of 4 μM GCAP1 and indicated concentrations of Ca2+. The experiment was carried out in triplicate and repeated three times. The results shown are shown are average ± SD from these experiments. The IC50 and EC50 values were determined graphically. Inset: Expression levels of ROS-GC1 and ONE-GC in COS cells were monitored by Western blot using antibodies against ROS-GC1 or ONE-GC.
How can GCAP1 exhibit these opposite modulatory effects? To answer this question GCAP1 and ONE-GC mutants were employed.
Several mutations in the GCAP1 gene have been found in patients suffering from autosomal dominant cone-rod dystrophies (Behnen et al., 2010). All these mutations except one are located within the regions coding for EF hands 3 and 4. The D100E mutation is within EF hand 3; it causes perturbation in Ca2+ coordination, and leads to a dramatic decrease in affinity for Ca2+. As a consequence, the D100E mutant remains in an active conformation at 10 μM Ca2+. The activation is half-maximal at 20 μM Ca2+ and the cyclase activity returns to the basal level only at approx. 60 μM free Ca2+ (Behnen et al., 2010; Dell'Orco et al., 2010). This mutant was tested for its effect on ONE-GC activity in the presence of two Ca2+ concentrations, 10 nM and 10 μM. For comparison, in parallel experiment, the effect of the D100E mutant on ROS-GC1 activity at the same Ca2+ concentrations was determined. The results are shown in Figure 7. The mutant stimulated ROS-GC1 activity at both tested Ca2+ concentrations. It, however, had no effect on ONE-GC activity at 10 nM Ca2+ but stimulated its activity at 10 μM Ca2+. Thus, dysfunctional EF hand 3 does not have any effect on Ca2+-dependent GCAP1 stimulation of ONE-GC. Because the third EF hand has the highest affinity for Ca2+ (Lim et al., 2009) these results indicated that low affinity Ca2+ binding to GCAP1 is sufficient for ONE-GC stimulation. These results further showed that the D100E mutation in GCAP1 leads to a protein conformation that can act as a constitutive activator with Ca2+-sensing properties that are significantly shifted to higher free Ca2+. One can deduce from these results that the interaction sites of GCAP1 in ROS-GC1 and ONE-GC are different, i.e., are not located in corresponding homologous regions. This hypothesis was tested by creating a mutant of ONE-GC with a point mutation in a region that is conserved among sensory guanylate cyclases and that is critical for GCAP1-dependent regulation of ROS-GC1.
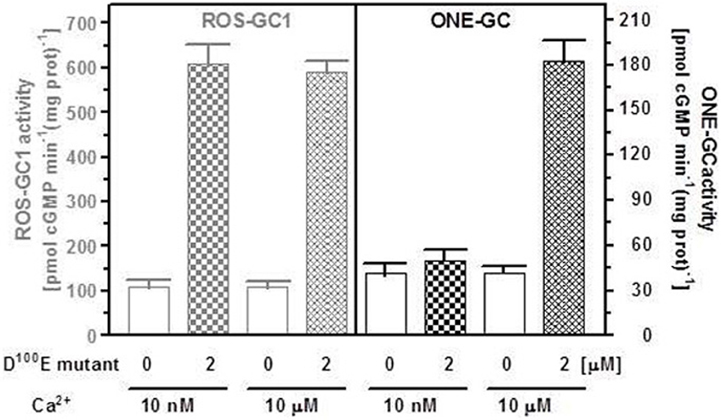
Figure 7. The D100E GCAP1 mutant stimulates both ONE-GC and ROS-GC1 at high Ca2+ concentration but only ROS-GC1 in the absence of Ca2+. The membrane fraction of COS cells expressing ONE-GC or ROS-GC1 (control) were assayed for guanylate cyclase activity in the presence (2 μM) or absence of D100E GCAP1 mutant and 1 mM EGTA (10 μM Ca2+) or 10 μM Ca2+. The experiment was done in triplicate and repeated two times for reproducibility. The results shown are average ± SD from these experiments.
GCAP1 signaling modes in its Ca2+-free and Ca2+-bound states are different
The first ROS-GC1 gene mutation linked with visual disorder was the F514S mutation identified in cases of Leber's congenital amaurosis type 1 (LCA1) (Perrault et al., 1996). Studies aimed at explaining the molecular basis of LCA1 demonstrated that this mutation totally disables GCAP1 modulation of ROS-GC1 activity (Duda et al., 1999). Because phenylalanine in this position is conserved in all membrane guanylate cyclases, the obvious question to ask was: would a similar mutation in ONE-GC disable GCAP1-mediated Ca2+ signaling of its activity? ONE-GC F585S mutant (corresponding to the F514S in ROS-GC1) was constructed and its activity was determined in the presence of 10 μM Ca2+ and increasing concentrations of GCAP1 (Figure 8A: closed circles). The results show that contrary to ROS-GC1 and its F514S mutant (Figure 8A: open and closed triangles, respectively; the activity was measured in the absence of Ca2+), the F→S mutation in ONE-GC does not affect the Ca2+-GCAP1-dependent activation of the cyclase (Figure 8A: compare the profiles with closed and open circles). The Hill's coefficients calculated for the GCAP1 stimulation of wt ONE-GC and of its F585S mutant were virtually identical, 1.83 ± 0.3. The mutation also does not affect the Ca2+ sensitivity of the mutant (Figure 8B: compare the closed and open circles). It is, therefore, concluded that Ca2+-free and Ca2+-bound GCAP1 exert different signals for activation of membrane guanylate cyclase. They probably do so by acting on different target regions, since the mutation F514S is located in a previously identified interaction and/or regulatory region of ROS-GC1 (Lange et al., 1999). Finally, while the F514S mutation in ROS-GC1 results in blindness at birth or soon thereafter (Perrault et al., 1996), the corresponding F585S mutation in ONE-GC would not result in anosmia.
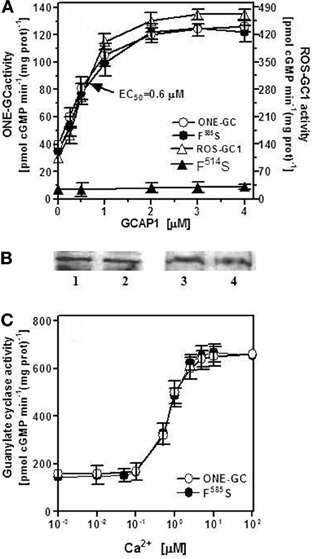
Figure 8. F585S mutation does not affect Ca2+-GCAP1 signaling of ONE-GC activity. (A) Membranes of COS cells expressing ONE-GC or its F585S mutant were assayed for guanylate cyclase activity in the presence of increasing concentrations of GCAP1 and constant 10 μM Ca2+; Membranes of COS cells expressing ROS-GC1 or its F514S mutant were analyzed for GCAP1 effect in the absence of Ca2+ (1 mM EGTA was present in the assay mixture). (B) Membranes of COS cells expressing ONE-GC and its F585S mutant or ROS-GC1 and its F514S mutant were analyzed by Western blot using antibodies against ROS-GC1 and ONE-GC. Approx 20 μg of membrane protein was applied on each lane of the gel. Lane 1, ONE-GC; Lane 2, ONE-GC F585S; Lane 3, ROS-GC1; Lane 4, ROS-GC1 F514S. (C) Membranes of COS cells expressing ONE-GC or its F585S mutant were assayed for guanylate cyclase activity in the presence of increasing concentrations of Ca2+ and constant, 4 μM GCAP1. The experiments were done in triplicate and repeated for reproducibility with different preparations of transfected COS cells. The results presented (mean ± SD) are from one representative experiment.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the National Heart Blood and Lung Institute: HL084584 and S82701 and by the Pennsylvania Lions Sight Conservation and Eye Research Foundation (Teresa Duda) and by the Deutsche Forschungsgemeinschaft (KO948/10-1) (Karl.-W. Koch).
References
Aoki, H., Richmond, M., Izumo, S., and Sadoshima, J. (2000). Specific role of the extracellular signal-regulated kinase pathway in angiotensin II-induced cardiac hypertrophy in vitro. Biochem. J. 347, 275–284.
Behnen, P., Dell'Orco, D., and Koch, K. W. (2010). Involvement of the calcium sensor GCAP1 in hereditary cone dystrophies. Biol. Chem. 391, 631–637.
Bhattacharya, S., Bunick, C. G., and Chazin, W. J. (2004). Target selectivity in EF-hand calcium binding proteins. Biochim. Biophys. Acta 1742, 69–79.
Braunewell, K. H. (2005). The darker side of Ca2+ signaling by neuronal Ca2+-sensor proteins: from Alzheimer's disease to cancer. Trends Pharmacol. Sci. 26, 345–351.
Brenner, B. M., Ballermann, B. J., Gunning, M. E., and Zeidel, M. L. (1990). Diverse biological actions of atrial natriuretic peptide. Physiol. Rev. 70, 665–699.
Burgoyne, R. D. (2007). Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat. Rev. Neurosci. 8, 182–193.
Burgoyne, R. D., O'Callaghan, D. W., Hasdemir, B., Haynes, L. P., and Tepikin, A. V. (2004). Neuronal Ca2+-sensor proteins: multitalented regulators of neuronal function. Trends Neurosci. 27, 203–209.
Burgoyne, R. D., and Weiss, J. L. (2001). The neuronal calcium sensor family of Ca2+-binding proteins. Biochem. J. 353, 1–12.
Burnett, J. C. Jr., Granger, J. P., and Opgenorth, T. J. (1984). Effect of synthetic atrial natriuretic factor on renal function and rennin release. Am. J. Physiol. 247, F863–F866.
Côté, M., Payet, M. D., Rousseau, E., Guillon, G., and Gallo-Payet, N. (1999). Comparative involvement of cyclic nucleotide phosphodiesterases and adenylyl cyclase on adrenocorticotropin-induced increase of cyclic adenosine monophosphate in rat and human glomerulosa cells. Endocrinology 140, 3594–3601.
Coussen, F., and Mulle, C. (2006). Kainate receptor-interacting proteins and membrane trafficking. Biochem. Soc. Trans. 34, 927–930.
Dell'Orco, D., Behnen, P., Linse, S., and Koch, K. W. (2010). Calcium binding, structural stability and guanylate cyclase activation in GCAP1 variants associated with human cone dystrophy. Cell. Mol. Life Sci. 67, 973–984.
Duda, T., Fik-Rymarkiewicz, E., Venkataraman, V., Krishnan, A., and Sharma, R. K. (2004). Calcium-modulated ciliary membrane guanylate cyclase transduction machinery: constitution and operational principles. Mol. Cell. Biochem. 267, 107–122.
Duda, T., Goraczniak, R., Surgucheva, I., Rudnicka-Nawrot, M., Gorczyca, W. A., Palczewski, K., Sitaramayya, A., Baehr, W., and Sharma, R. K. (1996). Calcium modulation of bovine photoreceptor guanylate cyclase. Biochemistry 35, 8478–8482.
Duda, T., Goraczniak, R. M., and Sharma, R. K. (1991). Site-directed mutational analysis of a membrane guanylate cyclase cDNA reveals the atrial natriuretic factor signaling site. Proc. Natl. Acad. Sci. U.S.A. 88, 7882–7886.
Duda, T., Jankowska, A., Venkataraman, V., Nagele, R. G., and Sharma, R. K. (2001). A novel calcium-regulated membrane guanylate cyclase transduction system in the olfactory neuroepithelium. Biochemistry 40, 12067–12077.
Duda, T., Krishnan, R., and Sharma, R. K. (2006). GCAP1-Antithetical calcium sensor of ROS-GC transduction machinery. Calcium Bind. Proteins 1, 102–107.
Duda, T., and Sharma, R. K. (2009). Ca2+-modulated ONE-GC odorant signal transduction. FEBS Lett. 583, 1327–1330.
Duda, T., Venkataraman, V., Goraczniak, R., Lange, C., Koch, K. W., and Sharma, R. K. (1999). Functional consequences of a rod outer segment membrane guanylate cyclase (ROS-GC1) gene mutation linked with Leber's congenital amaurosis. Biochemistry 38, 509–515.
Frins, S., Bönigk, W., Müller, F., Kellner, R., and Koch, K. W. (1996). Functional characterization of a guanylyl cyclase-activating protein from vertebrate rods. Cloning, heterologous expression, and localization. J. Biol. Chem. 271, 8022–8027.
Fülle, H. J., Vassar, R., Foster, D. C., Yang, R. B., Axel, R., and Garbers, D. L. (1995). A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc. Natl. Acad. Sci. U.S.A. 92, 3571–3575.
Gorczyca, W. A., Gray-Keller, M. P., Detwiler, P. B., and Palczewski, K. (1994). Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc. Natl. Acad. Sci. U.S.A. 91, 4014–4018.
Haynes, L. P., and Burgoyne, R. D. (2008). Unexpected tails of a Ca2+ sensor. Nat. Chem. Biol. 4, 90–91.
Howes, K. A., Pennesi, M. E., Sokal, I., Church-Kopish, J., Schmidt, B., Margolis, D., Frederick, J. M., Rieke, F., Palczewski, K., Wu, S. M., Detwiler, P. B., and Baehr, W. (2002). GCAP1 rescues rod photoreceptor response in GCAP1/GCAP2 knockout mice. EMBO J. 21, 1545–1554.
Hwang, J. Y., and Koch, K. W. (2002). Calcium- and myristoyl-dependent properties of guanylate cyclase-activating protein-1 and protein-2. Biochemistry 41, 13021–13028.
Iino, S., Kobayashi, S., Okazaki, K., and Hidaka, H. (1995). Immunohistochemical localization of neurocalcin in the rat inner ear. Brain Res. 680, 128–134.
Ivings, L., Pennington, S. R., Jenkins, R., Weiss, J. L., and Burgoyne, R. D. (2002). Identification of Ca2+-dependent binding partners for the neuronal calcium sensor protein neurocalcin delta: interaction with actin, clathrin and tubulin. Biochem. J. 363, 599–608.
Kitiratschky, V. B., Behnen, P., Kellner, U., Heckenlively, J. R., Zrenner, E., Jägle, H., Kohl, S., Wissinger, B., and Koch, K. W. (2009). Mutations in the GUCA1A gene involved in hereditary cone dystrophies impair calcium-mediated regulation of guanylate cyclase. Hum. Mutat. 30, E782–E796.
Koch, K. W., Duda, T., and Sharma, R. K. (2010). Ca(2+)-modulated vision-linked ROS-GC guanylate cyclase transduction machinery. Mol. Cell. Biochem. 334, 105–115.
Krishnan, A., Venkataraman, V., Fik-Rymarkiewicz, E., Duda, T., and Sharma, R. K. (2004). Structural, biochemical, and functional characterization of the calcium sensor neurocalcin delta in the inner retinal neurons and its linkage with the rod outer segment membrane guanylate cyclase transduction system. Biochemistry 43, 2708–2723.
Ladant, D. (1995). Calcium and membrane binding properties of bovine neurocalcin delta expressed in Escherichia coli. J. Biol. Chem. 270, 3179–3185.
Lange, C., Duda, T., Beyermann, M., Sharma, R. K., and Koch, K. W. (1999). Regions in vertebrate photoreceptor guanylyl cyclase ROS-GC1 involved in Ca(2+)-dependent regulation by guanylyl cyclase-activating protein GCAP-1. FEBS Lett. 460, 27–31.
Lim, S., Peshenko, I., Dizhoor, A., and Ames, J. B. (2009). Effects of Ca2+, Mg2+, and myristoylation on guanylyl cyclase activating protein 1 structure and stability. Biochemistry 48, 850–862.
Lohmann, S. M., Vaandrager, A. B., Smolenski, A., Walter, U., and de Jonge, H. R. (1997). Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem. Sci. 22, 307–312.
MacFarland, R. T., Zelus, B. D., and Beavo, J. A. (1991). High concentrations of a cGMP-stimulated phosphodiesterase mediate ANP-induced decreases in cAMP and steroidogenesis in adrenal glomerulosa cells. J. Biol. Chem. 266, 136–142.
Meloche, S., McNicoll, N., Liu, B., Ong, H., and de Léan, A. (1988). Atrial natriuretic factor R1 receptor from bovine adrenal zona glomerulosa: purification, characterization, and modulation by amiloride. Biochemistry 27, 8151–8158.
Mendez, A., Burns, M. E., Sokal, I., Dizhoor, A. M., Baehr, W., Palczewski, K., Baylor, D. A., and Chen, J. (2001). Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc. Natl. Acad. Sci. U.S.A. 98, 9948–9953.
Nambi, P., Aiyar, N. V., and Sharma, R. K. (1982). Adrenocorticotropin-dependent particulate guanylate cyclase in rat adrenal and adrenocortical carcinoma: comparison of its properties with soluble guanylate cyclase and its relationship with ACTH-induced steroidogenesis. Arch. Biochem. Biophys. 217, 638–646.
O'Callaghan, D. W., Ivings, L., Weiss, J. L., Ashby, M. C., Tepikin, A. V., and Burgoyne, R. D. (2002). Differential use of myristoyl groups on neuronal calcium sensor proteins as a determinant of spatio-temporal aspects of Ca2+ signal transduction. J. Biol. Chem. 277, 14227–14237.
Ogawa, H., Qiu, Y., Ogata, C. M., and Misono, K. S. (2004). Crystal structure of hormone-bound atrial natriuretic peptide receptor extracellular domain: rotation mechanism for transmembrane signal transduction. J. Biol. Chem. 279, 28625–28631.
Okazaki, K., Obata, N. H., Inoue, S., and Hidaka, H. (1995). S100 beta is a target protein of neurocalcin delta, an abundant isoform in glial cells. Biochem. J. 306, 551–555.
Orban, T., Bereta, G., Miyagi, M., Wang, B., Chance, M. R., Sousa, M. C., and Palczewski, K. (2010). Conformational changes in guanylate cyclase-activating protein 1 induced by Ca2+ and N-terminal fatty acid acylation. Structure 18, 116–126.
Palczewski, K., Sokal, I., and Baehr, W. (2004). Guanylate cyclase-activating proteins: structure, function, and diversity. Biochem. Biophys. Res. Commun. 322, 1123–1130.
Perrault, I., Rozet, J. M., Calvas, P., Gerber, S., Camuzat, A., Dollfus, H., Châtelin, S., Souied, E., Ghazi, I., Leowski, C., Bonnemaison, M., Le Paslier, D., Frézal, J., Dufier, J. L., Pittler, S., Munnich, A., and Kaplan, J. (1996). Retinal-specific guanylate cyclase gene mutations in Leber's congenital amaurosis. Nat. Genet. 14, 461–464.
Pertzev, A., Duda, T., and Sharma, R. K. (2010). Ca(2+) sensor GCAP1: a constitutive element of the ONE-GC-modulated odorant signal transduction pathway. Biochemistry 49, 7303–7313.
Peshenko, I. V., and Dizhoor, A. M. (2006). Ca2+ and Mg2+ binding properties of GCAP-1. Evidence that Mg2+-bound form is the physiological activator of photoreceptor guanylyl cyclase. J. Biol. Chem. 281, 23830–23841.
Pfeifer, A., Ruth, P., Dostmann, W., Sausbier, M., Klatt, P., and Hofmann, F. (1999). Structure and function of cGMP-dependent protein kinases. Rev. Physiol. Biochem. Pharmacol. 135, 105–149.
Pugh, E. N. Jr, Duda, T., Sitaramayya, A., and Sharma, R. K. (1997). Photoreceptor guanylate cyclases: a review. Biosci. Rep. 17, 429–473.
Rebello, M. R., Atkas, A., and Medle, K. F. (2011). Expression of calcium binding proteins in mouse type II taste cells. J. Histochem. Cytochem. 59, 530–539.
Sambrook, M. J., Fritsch, E. F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
Sharma, R. K. (2002). Evolution of the membrane guanylate cyclase transduction system. Mol. Cell. Biochem. 230, 3–30.
Sharma, R. K. (2010). Membrane guanylate cyclase is a beautiful signal transduction machine: overview. Mol. Cell. Biochem. 334, 3–36.
Sharma, R. K., Duda, T., Venkataraman, V., and Koch, K. W. (2004). Calcium modulated mammalian membrane guanylate cyclase ROS-GC transduction machinery in sensory neurons. Curr. Top. Biochem. Res. 322, 111–144.
Shi, S.-J., Nguyen, H. T., Sharma, G. D., Navar, G., and Pandey, K. N. (2001). Genetic disruption of atrial natriuretic peptide receptor-a alters rennin and angiotensinII levels. Am. J. Physiol. Renal Physiol. 281, F665–F673.
Stephen, R., Bereta, G., Golczak, M., Palczewski, K., and Sousa, M. C. (2007). Stabilizing function for myristoyl group revealed by the crystal structure of a neuronal calcium sensor, guanylate cyclase-activating protein 1. Structure 15, 1392–1402.
Takimoto, K., Yang, E. K., and Conforti, L. (2002). Palmitoylation of KChIP splicing variants is required for efficient cell surface expression of Kv4.3 channels. J. Biol. Chem. 277, 26904–26911.
Keywords: calcium, GCAP1, neurocalcin δ, neuronal calcium sensors, membrane guanylate cyclase, cyclic GMP, atrial natriuretic factor receptor membrane guanylate cyclase, olfactory neuroepithelium membrane guanylate cyclase
Citation: Duda T, Pertzev A, Koch K-W and Sharma RK (2012) Antithetical modes of and the Ca2+ sensors targeting in ANF-RGC and ROS-GC1 membrane guanylate cyclases. Front. Mol. Neurosci. 5:44. doi: 10.3389/fnmol.2012.00044
Received: 07 March 2012; Accepted: 21 March 2012;
Published online: 09 April 2012.
Edited by:
Michael R. Kreutz, Leibniz-Institute for Neurobiology, GermanyReviewed by:
Frank Schmitz, Saarland University, GermanyLee Haynes, The University of Liverpool, UK
Copyright: © 2012 Duda, Pertzev, Koch and Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Teresa Duda, Research Divisions of Biochemistry and Molecular Biology, The Unit of Regulatory and Molecular Biology, Salus University, 8360 Old York Road, Elkins Park, PA 19027, USA. e-mail: tduda@salus.edu