Adolescent resting state networks and their associations with schizotypal trait expression
- 1 Office Médico-Pédagogique Research Unit, Department of Psychiatry, University of Geneva, Geneva, Switzerland
- 2 Department of Radiology and Medical Informatics, University of Geneva, Geneva, Switzerland
- 3 Lemanic Neuroscience Doctoral School, Faculty of Psychology and Educational Sciences, University of Geneva, Geneva, Switzerland
- 4 Institute of Bioengineering, école Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
- 5 Adolescence Clinical Psychology Research Unit, Faculty of Psychology and Educational Sciences, University of Geneva, Geneva, Switzerland
- 6 University Hospital Geneva, Geneva, Switzerland
- 7 Department of Genetic Medicine and Development, School of Medicine, University of Geneva, Geneva, Switzerland
The rising interest in temporally coherent brain networks during baseline adult cerebral activity finds convergent evidence for an identifiable set of resting state networks (RSNs). To date, little is know concerning the earlier developmental stages of functional connectivity in RSNs. This study’s main objective is to characterize the RSNs in a sample of adolescents. We further examine our data from a developmental psychopathology perspective of psychosis-proneness, by testing the hypothesis that early schizotypal symptoms are linked to disconnection in RSNs. In this perspective, this study examines the expression of adolescent schizotypal traits and their potential associations to dysfunctional RSNs. Thirty-nine adolescents aged between 12 and 20 years old underwent an 8-min functional magnetic resonance imaging (fMRI) “resting state” session. In order to explore schizotypal trait manifestations, the entire population was assessed by the Schizotypal Personality Questionnaire (SPQ). After conventional processing of the fMRI data, we applied group-level independent component analysis (ICA). Twenty ICA maps and associated time courses were obtained, among which there were RSNs that are consistent with findings in the literature. We applied a regression analysis at group level between the energy of RSN-associated time courses in different temporal frequency bins and the clinical measures (3 in total). Our results highlight the engagement of six relevant RSNs; (1) a default-mode network (DMN); (2) a dorso-lateral attention network; (3) a visual network (VN); (4) an auditory network (AN); (5) a sensory motor network (SMN); (6) a self-referential network (SRN). The regression analysis reveals a statistically significant correlation between the clinical measures and some of the RSNs, specifically the visual and the AN. In particular, a positive correlation is obtained for the VN in the low frequency range (0.05 Hz) with SPQ measures, while the AN correlates negatively in the high frequency range (0.16–0.19 Hz). Trend-like significance for the SRN may hint to its implication in disorganized thoughts and behaviors during adolescence. Unlike DMN activity in schizophrenic patients, adolescent DMN was unrelated to schizotypal trait expression. This suggests that relationships between the DMN and schizotypy may be modified in later developmental stages of both functional connectivity and psychotic expression. These results are discussed in light of RSNs literature involving children, adults, and individuals with schizophrenia.
Introduction
Over the past 15 years, functional magnetic resonance imaging (fMRI) studies have devoted sustained interest in the intrinsic baseline activity of the brain, also referred to as the brain’s resting state or its default-mode activity. During this resting state, the brain’s blood oxygen level-dependent (BOLD) signal displays spontaneous fluctuations in its low or high frequencies showing a high degree of temporal correlation across separated cortical areas. These temporal correlations underline intrinsic functional connectivity between functional networks which are crucial for processes such as vision, auditory processing, language, (Hampson et al., 2002; Beckmann et al., 2005); in particular the default-mode network (DMN) has constituted the main interest of past studies that implemented seed-voxel techniques (Biswal et al., 1995; Raichle et al., 2001) as well as independent component analysis (ICA) (Greicius et al., 2004; van de Ven et al., 2004; Beckmann et al., 2005). Through analyses such as an ICA of the functional connectivity between widespread brain areas, several plausible functional resting state networks (RSN) can be inferred (Gusnard et al., 2001; Greicius et al., 2003; Van den Heuvel et al., 2009). One of the principal networks observable during the brain’s resting state is the DMN, generally thought to implicate the posterior cingulate cortex (PCC), the posterior lateral parietal cortices and the medial prefrontal cortex (MPFC) (Broyd et al., 2009). Other networks have also been identified, including a self-referential system engaging the medial prefrontal regions, a posterior network involved in visual processing, an attention network engaging superior frontal and parietal cortex, a superior temporal system and a network engaging precentral and postcentral cortex (Gusnard et al., 2001; Greicius et al., 2003; Fox and Raichle, 2007; Mantini et al., 2007).
More recently, neuroscientific investigations of RSNs examined the developmental characteristics of baseline cerebral activity by comparing functional connectivity in children and adults (Fair et al., 2008; Supekar et al., 2009, 2010). Although children show relatively immature connectivity in the DMN, cross-sectional data illustrates that specialized functional networks operate as early as age 7 following a local distribution of anatomically proximal clusters of activity (Fair et al., 2008). Brain maturation, most notably cerebral pruning and myelinization during adolescence (Sowell et al., 2004), sustains the development of large-scale networks that cast longer range functional connectivity and greatly enhance cortico-cortical networks (Supekar et al., 2009, 2010). In parallel to these observations, studies on RSNs in children and adolescents have started to address the issue of functional connectivity maturation in relation to the development of high-level cognitive abilities occurring during adolescence. The main dimensions of investigation concern within-network functional connectivity and the degree to which networks interfere/facilitate reciprocal interactions (Stevens et al., 2009). In a developmental study conducted by Kelly et al. (2009) targeting cingulate based intrinsic components networks, the authors report a significant age related shift in functional connectivity patterns by comparing children, adolescents and adults. While children showed a more dispersed pattern of correlation with seed region of interest (ROI), young adults exhibited a better constrained correlation pattern in line with that observed in adult participants. These finding are consistent with age related developmental trajectories of functional connectivity (Fair et al., 2008). In adolescent samples, studies have examined task-related modifications in neural networks (Stevens et al., 2007, 2009). Recently, Stevens et al. (2009) compared a group of 48 adolescents to a group of 52 adults and reported 13 common RSNs using ICA (Stevens et al., 2009). They further suggested that age is associated with greater within-network connectivity and increased efficiency between network influences. Together, these preliminary findings suggest that adolescence constitutes a crucial period for the deployment of functional connectivity networks that enhance information processing efficiency, and thus prompt further characterization of the RSNs during adolescence.
RSN investigations during adolescence can also lead to a deeper understanding in psychopathology research, especially because many serious psychiatric disorders emerge during late adolescence transitioning into early adulthood (Paus et al., 2008). Schizophrenia is one of the psychiatric disorders that typically unfolds with increased manifestations of schizotypal traits during adolescence (such as transient hallucination and delusion-like symptoms), which can potentially progress into clinically meaningful manifestations of psychosis. While a number of RSN investigations have been performed with adults meeting the diagnostic criteria of schizophrenia (Garrity et al., 2007), information is lacking concerning the early expression of schizotypal traits and its underlying RSN correlates. The results already reported in schizophrenia research can guide such examinations designed to assess the relationships between baseline cerebral activity and early schizotypal trait manifestations. To date, the functional connectivity data on RSNs in schizophrenia show some disparity, perhaps due to methodological differences and extensive heterogeneity in the schizophrenic samples. Nevertheless, three observations can be drawn from this quickly evolving literature; (1) studies showing greater DMN connectivity in their schizophrenia samples suggest exaggerated allocation of attention to introspective activity together with increased preoccupation for unexpected or novel external events; (2) in line with the first observations, studies finding increased anti-correlation between RSN networks interpret their results as representing the characteristic rivalry between introspective and extrospective mental activity in schizophrenia; (3) Finally, studies find specific deactivations for central nodes of the DMN such as the middle frontal gyrus and precuneus, suggesting that they are associated with the positive symptom dimension of schizophrenia (Broyd et al., 2009). Indeed, in a recent study (Rotarska-Jagiela et al., 2010) investigating aberrant DMN in schizophrenic patients, a significant correlation was found between positive symptoms and deactivation of the right frontal parietal network. It remains to be determined whether increased schizotypal traits during adolescence are associated with any one or many of the DMN alterations previously observed in adults with schizophrenia.
In this context, the present study carries two principal aims. Firstly, we wish to describe the functional organization of endogenous activation in adolescent baseline cerebral activity by examining functional connectivity of the putative DMN as well as other significant RSNs. Secondly, we wish to investigate the potential relationships between schizotypal trait expression and functional connectivity of the different RSNs in our adolescent sample. In order to conduct this examination, we employ an ICA to identify the functional connectivity of RSNs among widespread brain regions.
Materials and Methods
Participants
The study included 39 adolescents, 12–20 years old, who spoke French as their mother tongue (22 females, 17 males), with normal or corrected to normal vision. Participants were recruited through the Child and Adolescent outpatient Public Service (Office Médico Pédagogique) of Geneva member of the University of Geneva’s Psychiatry Department and of the Canton of Geneva Education Department. The motive for recruiting adolescents from the community (n = 16) in combination with those seeking psychological help (n = 23) was to obtain a distribution representing the wide range of schizotypy trait expression in our final sample. The sample mean age was 16.19, SD 2.201. All participants underwent an intellectual assessment, using the Wechsler Intelligence Scale for Children-III block design subtest (mean = 11.32, SD 3.5). In order to investigate psychotic symptoms and their dimensions, subjects completed the Schizotypal Personality Questionnaire (SPQ), translated into French and validated by Dumas et al. (2000). A clinical psychologist observed the questionnaire progression to guarantee that subjects understood all questionnaire items. The questionnaire looks at three main factor scores (Cognitive-Perceptual, Interpersonal, and Disorganization) and nine subscale scores (Ideas of Reference, Social Anxiety, Odd Beliefs/Magical Thinking, Unusual Perceptual Experiences, Eccentric/Odd Behavior and Appearance, No Close Friends, Odd Speech, Constricted Affect, Suspiciousness/Paranoid Ideation). It has been applied to explore the multiple dimensional analyses of schizotypy (Rossi and Daneluzzo, 2002), and it is valid with adolescents (Axelrod et al., 2001). In order to insure that our adolescent’s group distribution was normal, we tested for normality of distribution regarding the SPQ total score and the three subscale scores. The results indicate that full scale and subscale measures had normal distributions (SPQ total score: Kolmogorov–Smirnov Z = 0.56, p = 0.91; SPQ positive score: Kolmogorov–Smirnov Z = 0.97, p = 0.31; SPQ negative score: Kolmogorov–Smirnov Z = 0.55, p = 0.80; SPQ disorganization score: Kolmogorov–Smirnov Z = 0.66, p = 0.78).
Furthermore, we looked in the clinical files of the patients to find additional psychiatric diagnoses: four participants met CIM-10 diagnosis (European equivalent of DSM-IV) at time of participation (one attention deficit hyperactivity disorder, one generalized anxiety disorder, two schizotypal personality disorder). Only the last two participants had higher than group average schizotypy scores, which would be expected from their clinical diagnosis.
Written informed consent was obtained from participants and their parents under protocols approved by the Institutional Review Board of the Department of Psychiatry of the University of Geneva Medical School.
Imaging Acquisition and Data Analysis
fMRI data acquisition
Scanning was performed using a 3 Tesla Trio Tim Siemens at the Hôpitaux Universitaires of Geneva. High resolution 3D anatomical images were acquired [repetition time (TR) = 2500 ms, echo time (TE) = 30 ms, 192 coronal slices, slice thickness = 1.1 mm, slice gap = 0.5 mm, flip angle = 8°, field of view (FOV) = 220 mm2], followed by a resting state functional scan of 8 min, containing 200 BOLD images (TR = 2400 ms, TE = 30 ms, 38 axial slices, slice thickness = 3.20 mm, no gap, flip angle = 85°, FOV = 235 mm2). Subject’s head was stabilized with a vacuum cushion to minimize motion.
fMRI data analysis
Data were processed and analyzed using Statistical Parametric Mapping (SPM) 5, (Welcome Department of Neuroscience, London, UK). Functional images were at first corrected for motion by realigning every image with respect to the first one. Next, slice timing correction was performed using the middle slice as a reference, under descending acquisition. Structural images of each participant were coregistered to the mean of the realigned functional images. Gray matter separation was established by segmentation of the anatomical image. Realigned and slice-timed images were then spatially normalized into the Montreal Neurological Institute (MNI) template using 3 mm × 3 mm × 3 mm isotropic voxels, followed by spatial smoothing with an isotropic Gaussian smoothing Kernel of 10 mm full width half maximum (FWHM). Relevant RSNs were identified by ICA. ICA is a technique able to separate spatio-temporal BOLD signal into spatially statistically independent components (Beckmann and Smith, 2004). Each of the determined components is the expression of temporal waveform associated to specific brain network activity extracted from all regions. ICA allows separating out physiological artifacts and at the same time to identify functional active networks. Recently ICA has been employed for the identification of RSNs (Greicius et al., 2004; van de Ven et al., 2004; Mantini et al., 2007).
Group-level spatial ICA was conducted for all 39 participants, using the GIFT implementation (Calhoun et al., 2001; Calhoun and Adali, 2006; available at http://icatb.sourceforge.net/, version 1.3b)). that relies on the infomax algorithm (Bell and Sejnowski, 1995; Spatial ICA decomposes the data into spatial components and associated time courses based on a criterion that favors spatial independence. The group-level analysis is applied on the preprocessed data. The spatial components were converted into z-values (Beckmann et al., 2005) while time courses were calibrated as signal percentage change. The number of components was chosen as 20, which is consistent with the minimum description length estimate. Each of the spatial components was manually inspected for the presence of obvious artifacts that get effectively separated out by ICA (e.g., motion, ventricles) (Stevens et al., 2006). We retained six RSNs components that were consistent with the literature (Mantini et al., 2007).
ICA correlation with clinical measures
Regression analysis of the ICA findings against the clinical measures was conducted using custom scripts in Matlab 7.9 (R2009b) that relied upon the functionality of the GIFT implementation. In particular, a linear regression analysis was applied at the group level between the energy of the RSN-associated time courses, separated into 6 frequency bins (range 0.02–0.19 Hz: 0.02, 0.05, 0.09, 0.12, 0.16, and 0.19 Hz), and 3 clinical measures (SPQ positive factor, SPQ negative factor, SPQ disorganized factor). The design matrices of the regression analyses contained the respective clinical measure together with a constant regressor, for which we ran a t-test for the clinical measure of interest with 36 degrees of freedom. We dealt with multiple comparisons (3 clinical measures × 6 frequency bins) using Bonferroni correction. Frequency-dependent behavior of correlation between clinical measures and RSN temporal behavior is an important feature since past studies have effectively demonstrated the presence of low frequency oscillations in typical RSNs (0.01–0.1 Hz) (Mantini et al., 2007), while modulation of high frequency components has been found in schizophrenic patients (Keri and Janka, 2000; Garrity et al., 2007).
In what follows, we report both corrected and uncorrected p-values. We consider p < 0.05 corrected as being strongly significant (t > 2.95). For some correlations, we also report less significant values, but that could be of interest for future studies.
Results
Descriptive and Clinical Measures
Table 1 shows the mean scores obtained during the neuropsychological evaluation.
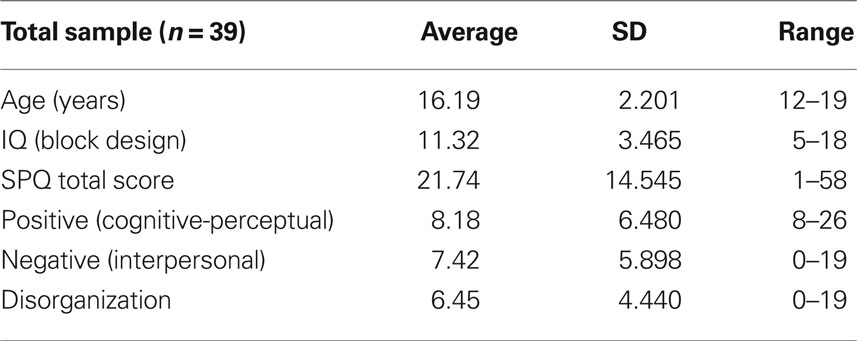
Table 1. Descriptive and clinical measures of age, intelligence and results of the self-response questionnaires (total and subscale scores).
Consistent Resting State Networks Found Across Adolescents
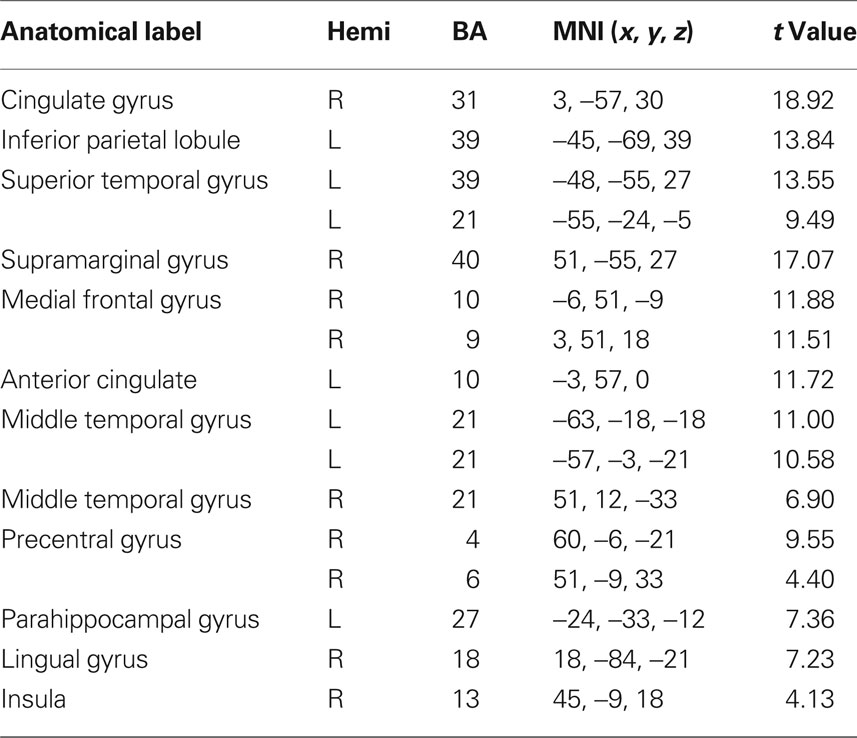
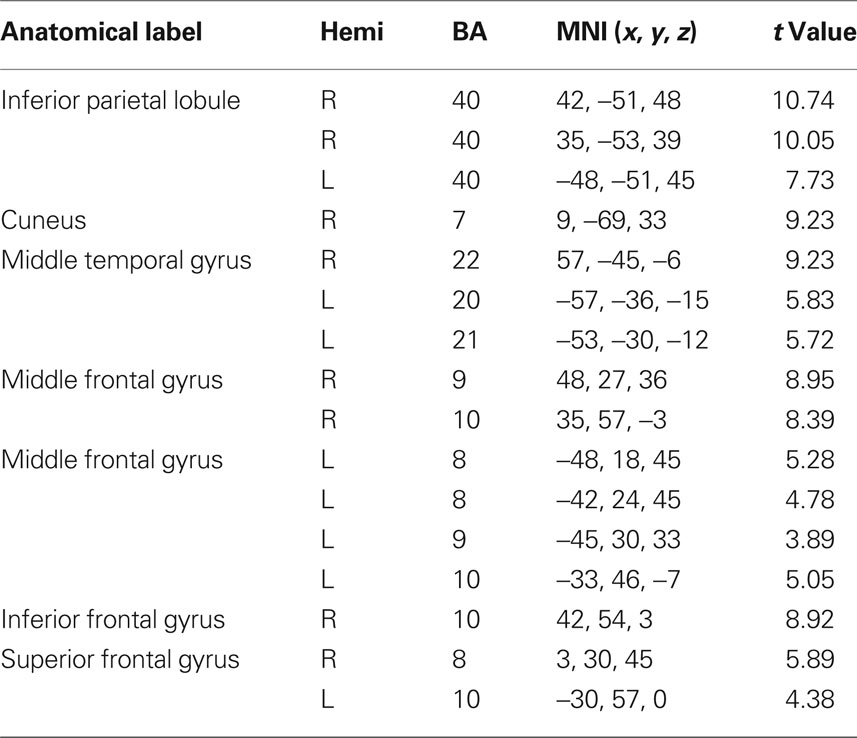
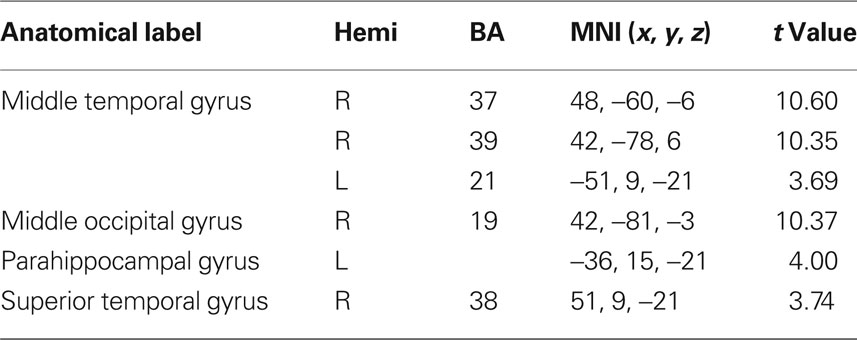
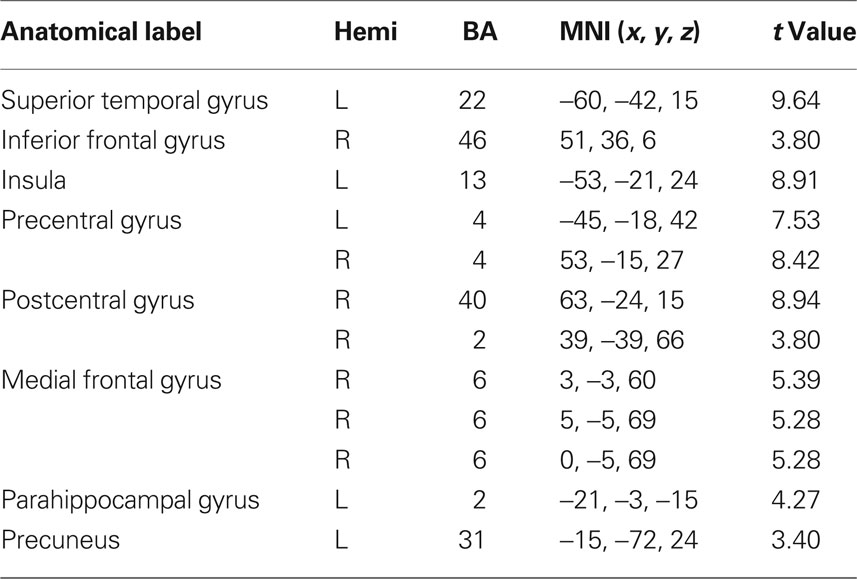
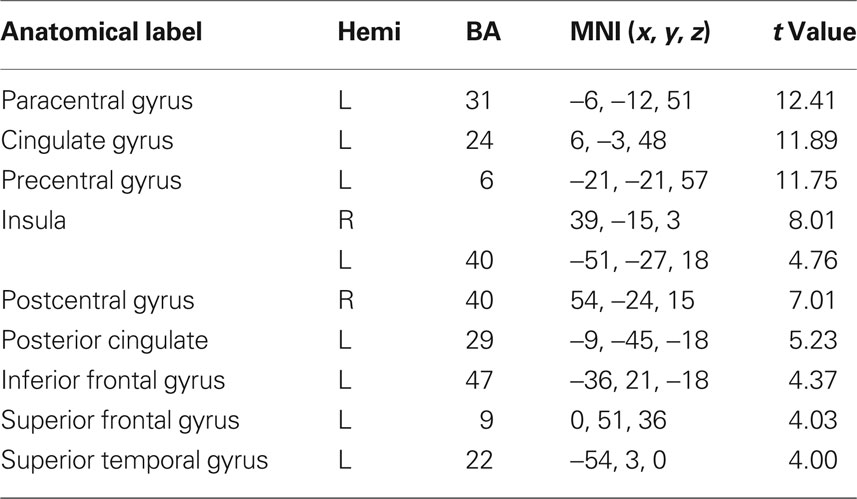
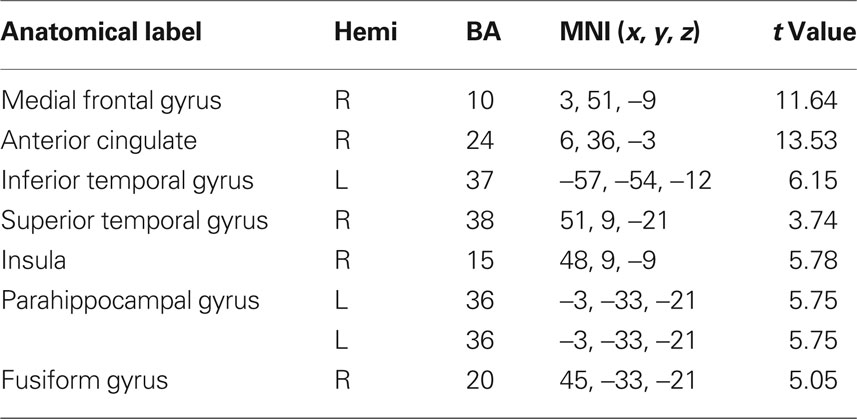
Peak activity were obtained by the implement of SPM 5 one sample t-test run for each relevant RSN. The spatial maps in Figures 1A–F show the six identified RSNs. Tables 2–7 summarize the active regions and the MNI peak coordinates, as well as Brodmann areas (BA) in which activation occurs. The results are explained on the base of past evidence of RSNs and they are described by highlighting the functional system they sustain (Beckmann et al., 2005; De Luca et al., 2006; Mantini et al., 2007; Jafri et al., 2008).
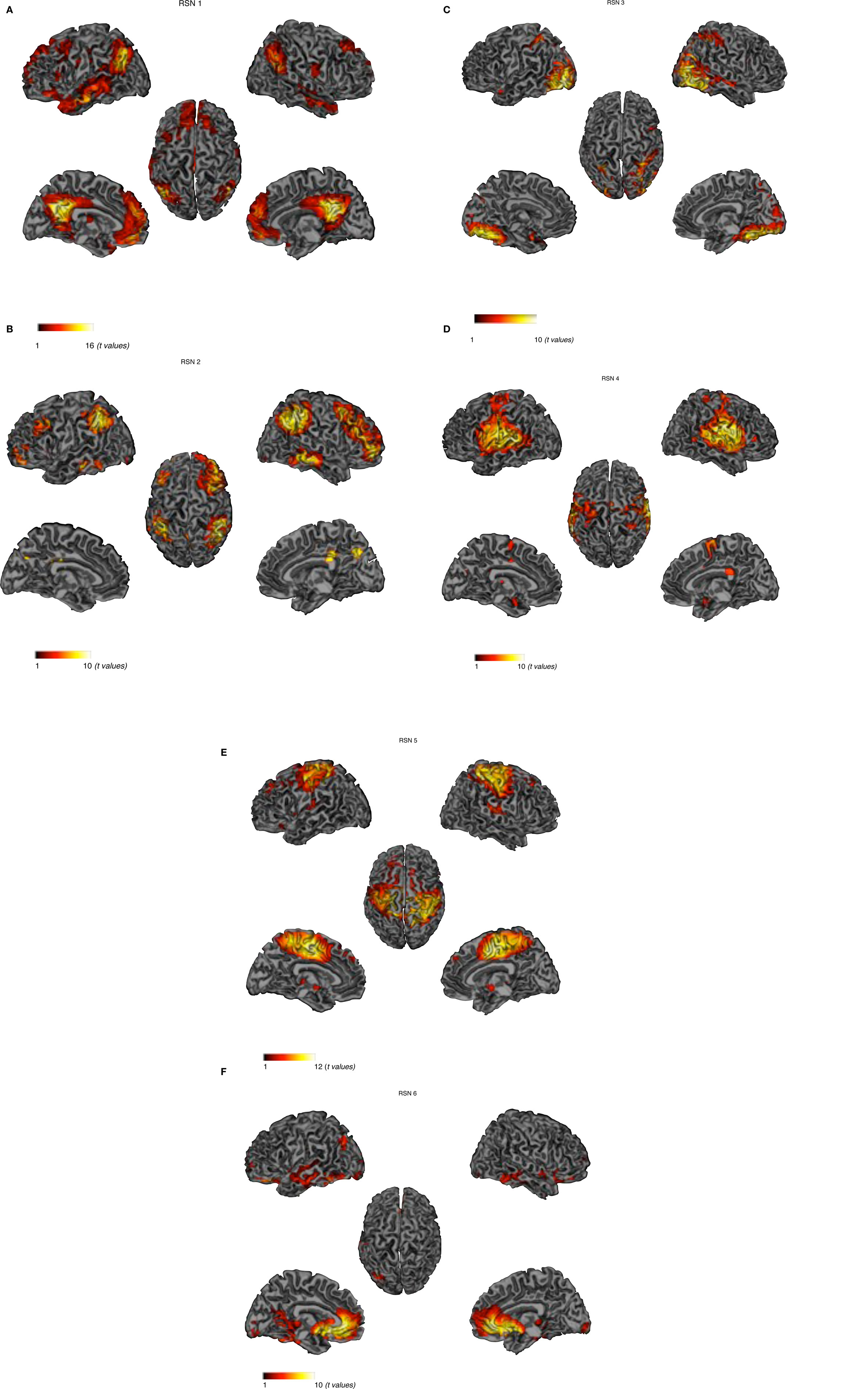
Figure 1. Cortical representation of the activity in the six RSNs. For each RSN (Left) Lateral and medial views of left hemisphere. (Center) Dorsal view. (Right) Lateral and medial views of right hemisphere. (A) Default-mode network – DMN, (B) dorso-lateral attention network – DAN. (C) visual network – VN, (D) auditory network – AN. (E) sensory motor network – SMN, (F) self-referential network – SRN.
RSN 1
This network is represented by the DMN, which includes the bilateral superior gyrus, anterior cingulate, the MPFC and the parietal cortex. Activity was also underlined in the left temporal gyrus. This network as been conceptualized as a “stand-alone” function or system (Raichle et al., 2001; Garrity et al., 2007). Briefly, this system is thought to hold recognition, self-projection and cognitive demands.
RSN 2
The dorso-lateral attention network (DAN) including the bilateral inferior parietal gyrus and the bilateral superior frontal sulcus; this system appears to be related to goal directed responses (Mantini et al., 2007).
RSN 3
Visual network (VN) involving the occipital and bilateral temporal regions; this functional system has been linked to the visual processing network and mental imagery (Ganis et al., 2004; Mantini et al., 2007). Brain areas active in RSN 3 are thought to include the retinotopic system. Core regions of this network have been found to be abnormal in schizophrenic patients (Levy et al., 2000).
RSN 4
Auditory network (AN) including the superior temporal and inferior frontal gyrus: these regions are known for being responsible in auditory processing and language comprehension. Studies on positive psychotic symptoms have found the temporal gyrus to be implied in auditory hallucinations (Gaser et al., 2004).
RSN 5
Sensory motor network (SMN), involving the precentral, postcentral gyrus and portion of the frontal gyrus corresponding to the primary sensory motor cortex (Biswal et al., 1995; Fox et al., 2006) and the supplementary motor areas.
RSN 6
Self-referential network (SRN) including the medial prefrontal, the anterior cingulate cortex and the hypothalamus: this network it is thought to involve the self-referential system (Mantini et al., 2007). It has been established that these areas are engaged in executive functions and they have been described as being abnormal in schizophrenic patients (Chan et al., 2006).
Correlations Between SPQ Measures and RSNs
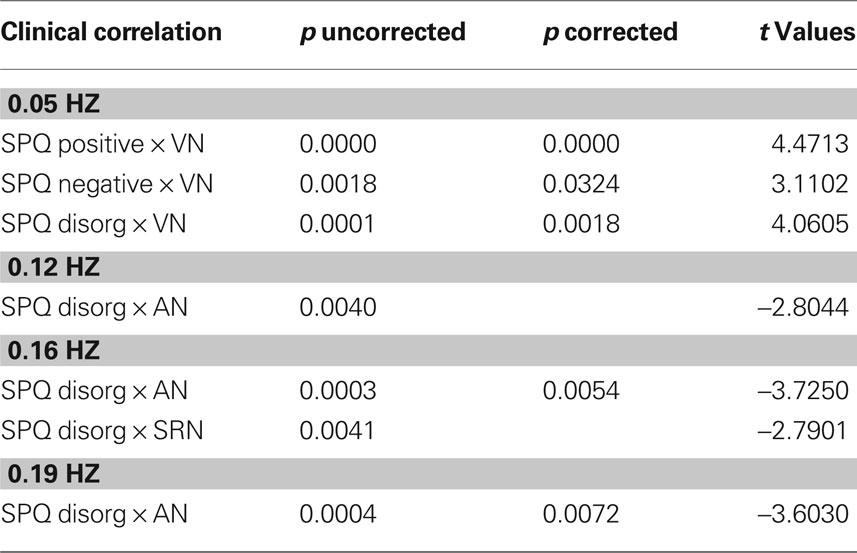
In Figures 2A–C we show the results of the regression analyses between the IC time courses and the clinical measures. In particular, strong significant positive correlations were found between the low frequency bin (0.05 Hz) of the VN and the positive SPQ factor, p = 0.0000 corrected. Significant positive correlations were also revealed for the VN low frequency range (0.05 Hz) and the SPQ negative factor p < 0.0324 corrected. Significant positive correlation was also underlined with the disorganized factor, p < 0.0018 corrected, in the same frequency (0.05 Hz). In the high frequency bins (0.16 and 0.19 Hz) a strong negative correlation was uncovered between the AN and disorganized factor, p < 0.0054 corrected, and p < 0.0072 corrected respectively. A weaker correlation was also uncovered between the disorganized subscale and SRN, p = 0.0041 uncorrected in the high frequency bin (0.16 Hz). Table 8 shows corrected, uncorrected p-values and t-values for each correlation.
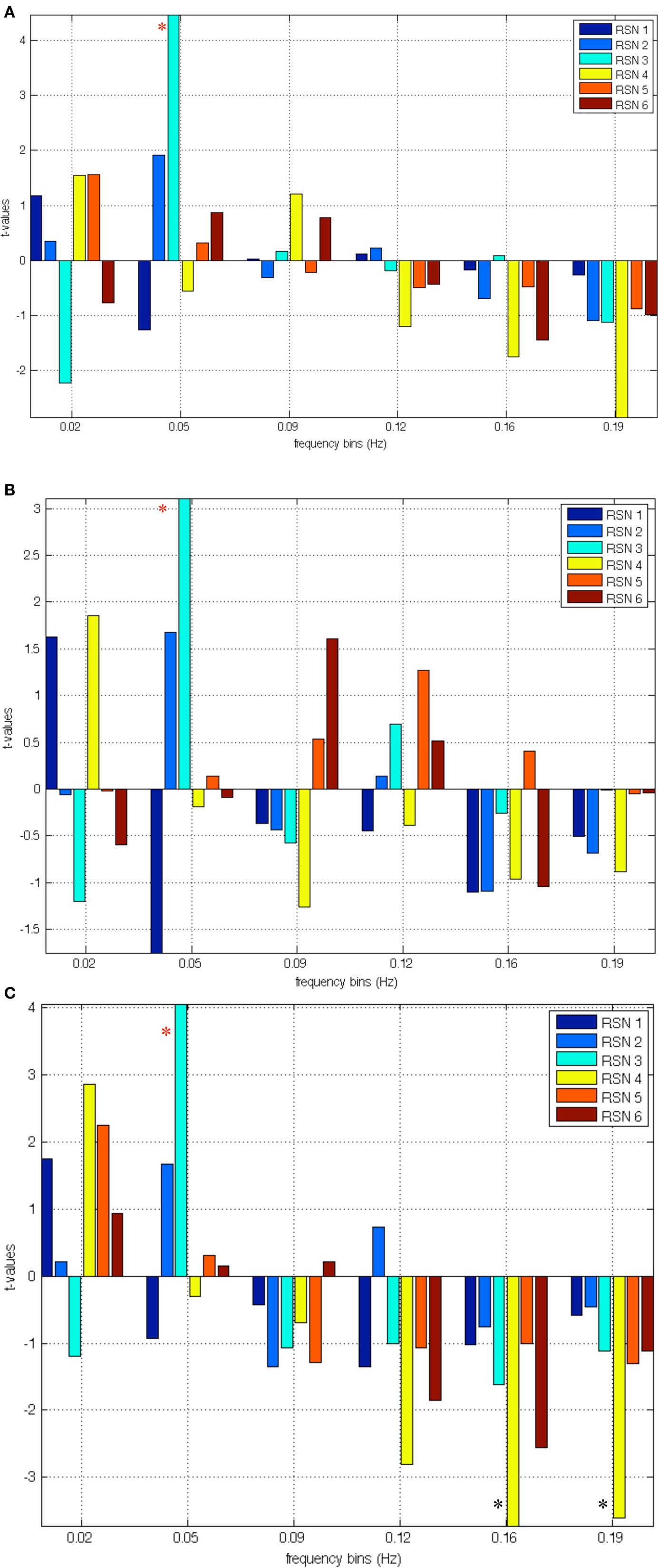
Figure 2. (A) Correlation between relevant RSNs frequency bins and scores at the positive factor; the red asterisk indicates significant correlations with RSN 3 (VN). (B) Correlation between relevant RSNs frequency bins and scores at the negative factor; the red asterisk indicates correlations with RSN 3 (VN). (C) Correlation between relevant RSNs frequency bins and scores at the disorganized factor; the red asterisk indicates significant correlations with RSN 3 (VN); the black asterisks indicate negative correlations with disorganized factor and hypo-connectivity of RSN 4 (AN).
Discussion
The first objective of this study was to characterize the putative DMN as well as other potential RSNs in a sample of adolescents. The ICA analyses performed upon the resting state data acquisition obtained through fMRI revealed a DMN network together with five reliable RSNs in our adolescent sample. These first results will be discussed in light of previous literature regarding RSNs in adult populations. Our second objective was to assess the relationship between schizotypal trait expression and the functional connectivity of reliable RSNs in our sample. Our results suggest that discrete dimensions of schizotypal trait expression may be selectively associated with three different RSNs, namely the SRN, the AN, and the VN. These results will be discussed in relation to the literature on RSN studies involving individuals with schizophrenia.
Resting State Networks in Adolescence
The identification of six reliable RSNs in our adolescent sample further characterizes functional connectivity during this important developmental period. A recent study by Stevens and collaborators described three networks composed of traditional DMN components in their sample of adolescents aged between 12 and 20 years old (Stevens et al., 2009). One of these networks, Component 11 described by these authors, is perhaps the closest match to the DMN network found in our sample. The fact that we only found one main DMN network may be due to methodological issues such as the length of the fMRI acquisition sequence and the sample composition that differ from the previous study. When visually comparing our findings to analogous results reported in an adult sample (Mantini et al., 2007), we may observe a more definite engagement of the left superior and middle temporal gyri during baseline cerebral activity in our sample, although any interpretation would deserve caution and further empirical evidence. We may consider that this temporal region covers BA 21, which plays an important part in auditory processing and also underlies language processing. Interestingly, parts of BA 21 appear to be robustly implicated with expression of psychotic symptoms such as hallucinations and delusions in schizophrenic patients as well as in subjects at risk of developing psychosis (McGuire et al., 1995; Allen et al., 2004). However our data do not indicate a clear linear correlation between activity in adolescent DMN and their expression of perceptual aberrations as measured by the SPQ. It may be that during adolescent brain maturation, functional connectivity in the DMN is not fully constituted as to show definite links to symptomatic makers as in adults with schizophrenia. Another explanation may be that important dysfunctions in DMN functional connectivity sustain more serious manifestations of psychosis, which themselves commonly unfold during adulthood. Our finding requires further research in children with childhood-onset schizophrenia and adolescents with a very high-risk for developing schizophrenia to further elucidate the relationship between DMN activity and clinical levels of schizophrenia.
Adolescent RSNs and Schizotypal Trait Expression
Conversely, the expression of adolescent schizotypal traits correlates with the VN’s functional connectivity in our sample of adolescents. We note that low frequency oscillations deficits for visual stimuli are well documented in schizophrenia (Spencer, 2008). The possible interpretations linking VN to early schizotypal trait expression merit caution, as mixed reports have implicated a variety of psychotic symptoms in schizophrenia (Broyd et al., 2009; Uhlhaas and Singer, 2010). We suggest that the positive correlation between VN low frequency bins and adolescent schizotypy scores could involve processes such as mental imagery, and more generally, social cognitive information processing. In the current study, both the positive, negative, and disorganization factors were positively correlated with the VN. This network included the visual cortex areas together with occipito-temporal areas, which comprise neuronal links shared by perceptual processing and mental imagery (Ganis et al., 2004). Indeed, the inferior temporal cortices can be activated by both mental imagery and visual perception (Ganis et al., 2004). Convergent evidence from schizophrenia research suggests that schizophrenic patients display increased vividness when engaged in mental imagery (Sack et al., 2005; Oertel et al., 2009). More generally, the occipito-temporal regions involved in this network also play an active role in social perception, in particular the interpretation of social intentions and actions using biological motion, facial expression and gaze information (Pelphrey et al., 2003). Therefore, the significant correlation of schizotypal trait dimensions in adolescence with the posterior RSN network involving in mental imagery and social perception may lead us to consider how social cognitive processes contribute to the disruption of social functioning in schizotypic youths. It is noteworthy to mention that social isolation is one of the best predictive factors for psychosis onset in youths expressing high levels of schizotypy. Our results suggest that increased activity in the VN may underlie mental representation processes that promote schizotypal trait expression.
We may also consider that the VN and the AN identified in this study, both correlating with the schizotypy measure, are composed of anatomically proximal regions. As mentioned in the introduction, the development of functional connectivity from childhood to adolescence proceeds from local to more widely distributed networks (Supekar et al., 2009). It is thought that the segregated local networks in childhood tend, with maturational change promoting long range functional connectivity, to distend and reorganize themselves within an integrated arrangement of interconnected cerebral networks (Fair et al., 2008; Supekar et al., 2010). The adolescent period represents a key transitional epoch where functional connectivity maturation takes place. In light of these developmental processes, we may hypothesize that our results reflect disturbed maturational processes of local networks (visual, auditory) that still operate in segregated fashion. If complex information such as that involved in social exchange requires sophisticated social cognitive processes involving multi-network integrated functional connectivity, then poorly integrated local networks may set the stage for faulty information processing in adolescents. In this view, faulty local network connectivity or altered functional connectivity maturation could hypothetically constitute the developmental antecedents of schizotypal trait expression.
Possible Implications of Self-Referential Processing
It is possible to gain further insight into schizotypal trait expression during adolescence by looking at the SRN observed in our sample. The SRN involves the coordination of cerebral structures responsible for reality monitoring, which is the capacity to accurately discriminate mental events originating from oneself from mental events originating from an external agent (Johnson et al., 1993). Reality monitoring deficits have been observed in several studies involving schizophrenic patients (Vinogradov et al., 1997, 2008) as well as in adolescents showing schizotypal traits (Debbané et al., 2009). Reality monitoring deficits observed along the continuum of schizotypal expression, from benign to clinically meaningful manifestations, lead several theorists to argue that such reality monitoring deficits contribute to the formation of psychotic symptoms (Frith and Frith, 2003). In our study, we observe a significant correlation (uncorrected) between dysfunctional connectivity of SRN and schizotypy trait expression. More precisely, the SRN functional connectivity negatively correlates with the disorganization dimension, which is comprised of both odd speech and odd behaviors. The RSN activation in this study includes both parts of BA 21 discussed above as well as the involvement of the MPFC, which we know sustains a variety of self-referential processes (D’Argembeau et al., 2005; Simons et al., 2008; Vinogradov et al., 2008). We may hypothesize that a disorganization in speech may involve inefficient connectivity between areas sustaining self- referential processes and areas sustaining language functions, which can already be observed in the expression of the disorganization trait in adolescence. This may sustain the hypothesis suggesting that impaired language and thought functions in schizophrenia are a result of disruptions in the processes of higher order construction of self-relevant meaning (Kuperberg et al., 2009). The finding that the AN correlates with the disorganization dimension in our adolescent sample further contributes to the hypothesis that cerebral areas sustaining language processing can contribute to odd/disorganized speech manifestations and altered language processing. Furthermore, high frequency (0.20 Hz) oscillations have been found in schizophrenic patients when compared to normal controls (Garrity et al., 2007). Relevant RSNs have shown typical frequency oscillations below 0.1 Hz (Cordes et al., 2001). However, significantly stronger power in higher frequencies has been seen in the DMN of a sample schizophrenic patients, specifically between the 0.1 and 0.24 Hz (Garrity et al., 2007). This finding suggested less temporal synchronicity between the brain regions involved. In addition, Cordes and colleagues have highlighted that harmonic respiratory cycles do not arise under 0.25 Hz (to 0.5 Hz), and cardiac rate arises around 0.9 Hz, therefore none of the physiological noise should arise into the low frequency bins (0.1 Hz). These oscillations may be representative of less effective connections between brain regions which enable information processing (Garrity et al., 2007).
To conclude, in agreement with previous studies on adult RSNs (Gusnard and Raichle, 2001; Gusnard et al., 2001; Raichle et al., 2001; Mantini et al., 2007), we report on six reliable RSNs identified in baseline cerebral activity in an adolescent population. Significant correlation with schizotypal trait expression could be observed with the auditory and VNs. This study provides an illustration of the influences of baseline cerebral activity on personality characteristics during adolescence. More specifically, altered connectivity in the self-referential and ANs may intensify the expression of disorganized speech and behavior, while increased functional connectivity in the VN may constitute a putative mechanism which perpetuates increased vividness in mental imagery and undermine social evaluation. Further research on adolescent samples are required to better understand the influence of resting state activity on personality trait expression.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the volunteer participants. Additional thanks go to the LAVIM neuroimaging plateform, as well as the Adolescent Outpatient Psychiatry Services, Drs Dario Balanzin, Serges Djapo-Yogwa and Danny Dukes for their collaboration. This work has been supported in part by the centre for bio medical imaging of the University of Geneva, Lausanne and EPFL. Our gratitude also goes to Pascal Challande and Frank Henry for the help with data acquisition. This work was funded by the Gertrude Von Meissner Foundation (ME 7871) grant to S. Eliez and M. Debbané, and by the Swiss National Science Foundation (PP00B-102864) grants to S. Eliez and by the grant PP00P2-123438 to Dimitri Van De Ville.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/neuroscience/systemsneuroscience/paper/10.3389/fnsys.2010.00035/
References
Allen, P. P., Johns, L. C., Fu, C. H., Broome, M. R., Vythelingum, G. N., and McGuire, P. K. (2004). Misattribution of external speech in patients with hallucinations and delusions. Schizophr. Res. 69, 277–287.
Axelrod, S. R., Grilo, C. M., Sanislow, C., and McGlashan, T. H. (2001). Schizotypal personality questionnaire brief: factor structure and convergent validity in inpatient adolescents. J. Pers. Disord. 15, 168–179.
Beckmann, C. F., De Luca, M., Devlin, J. T., and Smith, S. M. (2005). Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 360, 1001–1013.
Beckmann, C. F., and Smith, S. M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 23, 137–152.
Bell, A. J., and Sejnowski, T. J. (1995). An information maximisation approach to blind separation and blind deconvolution. Neural Comput. 7, 1129–1159.
Biswal, B., Yetkin, F. Z., Haughton, V. M., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541.
Broyd, S. J., Charmaine, D., Debener, S., Helps, S. K., James, C. J., and Sonuga-Barke, E. J. S. (2009). Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 33, 279–296.
Calhoun, V., and Adali, T. (2006). Unmixing fMRI with independent component analysis. IEEE Eng. Med. Biol. Mag. 25, 79–90.
Calhoun, V., Adali, T., Pearlson, G., and Pekar, J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 14, 140–151.
Chan, R. C. K., Chen, E. Y. H., and Law, C. W. (2006). Specific executive dysfunction in patients with first-episode medication-naïve schizophrenia. Schizophr. Res. 82, 51–64.
Cordes, D., Haughton, V. M., Arfanakis, K., Carew, J. D., Turski, P. A., Moritz, C. H., Quigley, M. A., and Meyerand, M. E. (2001). Frequencies contributing to functional connectivity in the cerebral cortex in “Resting-state” Data. AJNR Am. J. Neuroradiol. 22, 1326–1333.
D’Argembeau, A., Collette, F., Van der Linden, M., Laureys, S., Del Fiore, G., Degueldre, C., Luxen, A., and Salmon, E. (2005). Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage 25, 616–624.
Debbané, M., Van der Linden, M., Gex-Fabry, M., and Eliez, S. (2009). Cognitive and emotional associations to positive schizotypy during adolescence. J. Child. Psychol. Psychiatry 50, 326–334.
De Luca, M., Beckmann, C. F., De Stefano, N., Matthews, P. M., and Smith, S. M. (2006). fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage 29, 1359–1367.
Dumas, P., Bouafia, S., Gutknecht, C., Saoud, M., Dalery, J., and d’Amato, T. (2000). Validation of the French version of the Raine Schizotypal Personality Disorder Questionnaire – categorical and dimensional approach to schizotypal personality traits in a normal student population. Encephale 26, 23–29.
Fair, D., Cohen, A. L., Dosenbach, N. U. F., Church, J. A., Miezin, F. M., Barch, D. M., Raichle, M. E., Petersen, S. E., and Schlaggar, B. L. (2008). The maturing architecture of the brain’s default network. Proc. Natl. Acad. Sci. U.S.A. 105, 1028–1032.
Fox, M. D., and Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711.
Fox, M. D., Snyder, A. Z., Zacks, J. M., and Raichle, M. E. (2006). Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat. Neurosci. 9, 23–25.
Frith U., and Frith, C. D. (2003). Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 358, 459–473.
Ganis, G., Thompson, W. L., and Kosslyn, M. S. (2004). Brain areas underlying visual mental imagery and visual perception: an fMRI study. Brain Res. Cogn. Brain Res. 20, 226–241.
Garrity, A. G., Pearlson, G. D., McKiernan, K., Lloyd, D., Kiehl, K. A., and Calhoun, V. D. (2007). Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry 164, 450–457.
Gaser, C., Nenadic, I., Volz, H. P., Büchel, C., and Sauer, H. (2004). Neuroanatomy of “hearing voices”: a frontotemporal brain structural abnormality associated with auditory hallucinations in schizophrenia. Cereb. Cortex 14, 91–96.
Greicius, M. D., Krasnow, B., Reiss, A. L., and Menon, V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 100, 253–258.
Greicius, M. D., Srivastava, G., Reiss, A. L., and Menon, V. (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. U.S.A. 101, 4637–4642.
Gusnard, D. A., Akbudak, E., Shulman, G. L., and Raichle, M. E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 4259–4264.
Gusnard, D. A., and Raichle, M. E. (2001). Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2, 685–694.
Hampson, M., Peterson, B. S., Skudlarski, P., Gatenby, J. C., and Gore, J. C. (2002). Detection of functional connectivity using temporal correlations in MR images. Hum. Brain Mapp. 15, 247–262.
Jafri, M. J., Pearlson, G. D., Stevens, M., and Calhoun, V. D. (2008). A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage 39, 1666–1681.
Johnson, M. K., Hashtroudi, S., and Lindsay, D. S. (1993). Source monitoring. Psychol. Bull. 114, 28.
Kelly, C. A. M., Di Martino, A., Uddin, L. Q., Shehzad, Z., Gee, D. G., Reiss, P. T., Margulies, S., Castellanos, F. X., and Milham, M. P. (2009). Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb. Cortex 19, 640–657.
Keri, S., and Janka, Z. (2000). Cognitive dysmetria in schizophrenia. Am. J. Psychiatry 157, 662–663.
Kuperberg, G. R., Kreher, D. A., and Ditman, T. (2009). What can event-related potentials tell us about language, and perhaps even thought, in schizophrenia? Int. J. Psychophysiol. 75, 66–76.
Levy, D. L., Lajonchere, C. M., Dorogusker, B., Min, D., Lee, S., Tartaglini, A., Lieberman, J. A., and Mendell, N. R. (2000). Quantitative characterization of eye tracking dysfunction in schizophrenia. Schizophr. Res. 42, 171–185.
Mantini, D., Perrucci, M. G., Del Gratta, D., Romani, G. L., and Corbetta, M. (2007). Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U.S.A. 104, 13170–13175.
McGuire, P. K., Silbersweig, D. A., Wright, I., Murray, R. M., David, A. S., Frackowiak, R. S., and Frith, C. D. (1995). Abnormal monitoring of inner speech: a physiological basis for auditory hallucinations. Lancet 346, 596–600.
Oertel, V., Rotarska-Jagiela, A., van de Ven, V., Haenschel, C., Grube, M., Stangier, U., Maurer, k., and Linden, D. E. J. (2009). Mental imagery vividness as a trait marker across the schizophrenia spectrum. Psychiatry Res. 167, 1–11.
Paus, T., Keshavan, M., and Giedd, J. N. (2008). Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 9, 947–957.
Pelphrey, K. A., Mitchell, T. V., McKeown, M. J., Goldstein, J., Allison, T., and McCarthy, G. (2003). Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. J. Neurosci. 23, 6819–6825.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., and Shulman, G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682.
Rossi, A., and Daneluzzo, E. (2002). Schizotypal dimensions in normals and schizophrenic patients: a comparison with other clinical samples. Schizophr. Res. 54, 67–75.
Rotarska-Jagiela, A., van de Ven, V., Oertel Knöchel, V., Uhlhaas, P., Vogeley, K., and Linden, D. E. J. (2010). Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 17, 21–31.
Sack, A. T., Camprodon, J. A., Pascual-Leone, A., and Goebel, R. (2005). The dynamics of interhemispheric compensatory processes in mental imagery. Science 308, 702–704.
Simons, J. S., Henson, R. N. A., Gilbert, S. J., and Fletcher, P. C. (2008). Separable forms of reality monitoring supported by the anterior prefrontal cortex. J. Cogn. Neurosci. 20, 447–457.
Sowell, E. R., Thompson, P. M., and Toga, A. W. (2004). Mapping changes in the human cortex throughout the span of life. Neuroscientist 10, 372–392.
Spencer, K. M. (2008). Visual gamma oscillations in schizophrenia: implications for understanding neural circuitry abnormalities. Clin. EEG Neurosci. 39, 65–68.
Stevens, M. C., Kiehl, K. A., Pearlson, G. D., and Calhoun, V. D. (2007). Functional neural networks underlying response inhibition in adolescents and adults. Behav. Brain Res. 181, 12–22.
Stevens, M. C., Pearlson, G. D., and Calhoun, V. D. (2006). Functional neural circuits for mental time keeping. Hum. Brain Mapp. 28, 394–408.
Stevens, M. C., Pearlson, G. D., and Calhoun, V. D. (2009). Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum. Brain Mapp. 30, 2356–2366.
Supekar, K., Musen, M., and Menon, V. (2009). Development of large-scale functional brain networks in children. PLoS Biol. 7, e1000157. doi: 10.1371/journal.pbio.1000157.
Supekar, K., Uddin, L. Q., Prater, K., Amin, H., Greicius, M. D., and Menon, V. (2010). Development of functional and structural connectivity within the default mode network in young children. Neuroimage 52, 290–301.
Uhlhaas, P. J., and Singer, W. (2010). Abnormal neural oscillation and synchrony in schizophrenia. Nat. Rev. Neurosci. 11, 100–113.
van de Ven, V. G., Formisano, E., Prvulovic, D., Roeder, C. H., and Linden, D. E. J. (2004). Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum. Brain Mapp. 22, 165–178.
Van den Heuvel, M. P., Mandl, R. C. W., Kahn, R. S., and Hulshoff Pol, E. H. (2009). Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum. Brain Mapp. 3, 3127–3141.
Vinogradov, S., Luks, T. L., Schulman, B. J., and Simpson, G. V. (2008). Deficit in a neural correlate of reality monitoring in schizophrenia patients. Cereb. Cortex 18, 2532–2539.
Keywords: fMRI, default mode network, ICA, adolescence, psychosis, personality, SPQ, schizotypy
Citation: Lagioia A, Van De Ville D, Debbané M, Lazeyras F and Eliez S (2010) Adolescent resting state networks and their associations with schizotypal trait expression. Front. Syst. Neurosci. 4:35. doi: 10.3389/fnsys.2010.00035
Received: 05 February 2010;
Paper pending published: 25 March 2010;
Accepted: 02 July 2010;
Published online: 05 August 2010
Edited by:
Lucina Q. Uddin, Stanford University, USAReviewed by:
Matthew Hoptman, Nathan S. Kline Institute, USAKatie Karlsgodt, University of California, Los Angeles, USA
Copyright: © 2010 Lagioia, Van De Ville, Debbané, Lazeyras and Eliez. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Dimitri Van De Ville, Department of Radiology and Medical Informatics, University of Geneva Rue Gabrielle-Perret-Gentil, 4, 1211 Geneva 14. e-mail: dimitri.vandeville@epfl.ch