The impact of stimulation induced short-term synaptic plasticity on firing patterns in the globus pallidus of the rat
- 1 The Leslie and Susan Gonda Multidisciplinary Brain Research Center, Bar-Ilan University, Ramat Gan, Israel
- 2 The Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat Gan, Israel
Electrical stimulation in the globus pallidus (GP) leads to complex modulations of neuronal activity in the stimulated nucleus. Multiple in vivo studies have demonstrated the modulation of both firing rates and patterns during and immediately following the GP stimulation. Previous in vitro studies, together with computational studies, have suggested the involvement of short-term synaptic plasticity (STP) during the stimulation. The aim of the current study was to explore in vitro the effects of STP on neuronal activity of GP neurons during local repetitive stimulation. We recorded synaptic potentials and assessed the modulations of spontaneous firing in a postsynaptic neuron in acute brain slices via a whole-cell pipette. Low-frequency repetitive stimulation locked the firing of the neuron to the stimulus. However, high-frequency repetitive stimulation in the GP generated a biphasic modulation of the firing frequency consisting of inhibitory and excitatory phases. Using blockers of synaptic transmission, we show that GABAergic synapses mediated the inhibitory and glutamatergic synapses the excitatory part of the response. Furthermore, we report that at high stimulation frequencies both types of synapses undergo short-term depression leading to a time dependent modulation of the neuronal firing. These findings indicate that STP modulates the dynamic responses of pallidal activity during electrical stimulation, and may contribute to a better understanding of the mechanism underlying deep brain stimulation like protocols.
Introduction
High-frequency deep brain stimulation (HF–DBS) in the globus pallidus internus (GPi) and subthalamic nucleus (STN) is a widely employed method for treating Parkinson’s disease (PD; Benabid, 2003) and other disorders associated with the basal ganglia (BG). However, despite its proven therapeutic success, the physiological effects of DBS in the BG are still unclear. Some electrophysiological recordings performed on humans intra-operatively (Dostrovsky et al., 2000; Wu et al., 2001; Filali et al., 2004) have reinforced the view of full inhibition of neuronal firing in the stimulated nucleus. However, other experimental (Hashimoto et al., 2003; Bar-Gad et al., 2004; Meissner et al., 2005; Montgomery and Gale, 2008; McCairn and Turner, 2009) and theoretical (McIntyre et al., 2004a; Johnson and McIntyre, 2008) studies have reported that stimulation leads to complex activation of the stimulated nucleus or its targets. Furthermore, HF–DBS has been shown to affect other firing pattern properties including oscillatory and bursty activity (Meissner et al., 2005; Montgomery, 2006; Hahn et al., 2008; Xu et al., 2008; McCairn and Turner, 2009). Thus, the accumulating evidence highlights the importance of both firing rate and pattern changes during stimulation.
While studies of therapeutic stimulation have focused on the static effects of DBS, other dynamic aspects have been studied in response to stimulation in vitro. Specifically, the firing dynamics of the BG has been shown to be highly responsive to short-term synaptic plasticity (STP; Hammond et al., 2008; Gubellini et al., 2009). For example, the globus pallidus [GP – the rodent homolog of the primate globus pallidus externus (GPe)] function is controlled by GABAergic input from the striatum (Francois et al., 1984; Percheron et al., 1984; Yelnik et al., 1984; Kita and Kitai, 1994), collaterals of neighboring GP neurons (Parent et al., 2000), and additional glutamatergic inputs from the STN (Smith and Parent, 1988). Several studies have shown that STP can affect firing dynamics in GP neurons in vitro (Rav-Acha et al., 2005; Sims et al., 2008). It has been suggested that GABA may induce GP synchronization by affecting the timing of GP spiking, and that the GP GABAergic synapse displays short-term depression (Rav-Acha et al., 2005; Sims et al., 2008). Moreover, excitatory inputs reaching the GP from the STN are also shaped by STP (Hanson and Jaeger, 2002). In addition to these short-term dynamics, both excitatory and inhibitory synapses in the GP have been reported to express metabotropic receptors that further modulate the plasticity of the circuit and its response to prolonged stimulation (Kaneda and Kita, 2005; Kaneda et al., 2005, 2007; Hashimoto and Kita, 2008). Other examples demonstrating the effect of STP on neuronal firing have been observed in the STN, and the entopeduncular nucleus (EP – the rodent homolog of the GPi; Hammond et al., 2008; Gubellini et al., 2009). However, while STP has thus been found to affect the firing of BG neurons, the role of these STP rules during prolonged, DBS-like stimulation has yet to be investigated.
Recent computational and experimental studies have suggested the existence of STP during HF–DBS (McIntyre et al., 2004b; McCairn and Turner, 2009). These findings led us to hypothesize that STP is involved in altering the information transmission which underlies the effects of HF–DBS on PD. We have recently shown that high-frequency microstimulation in either segment of the GP in the Parkinsonian primate results in short-term modulations (Erez et al., 2009). In particular, we suggested that the efficacy of the synapses is reduced over a time course of a few seconds and is restored within a short time frame after the cessation of the stimulation. This efficacy determines both the changes in the response timing (firing rate) and response pattern (timing). Defining the nature of these dynamic processes and the characterization of their cellular and network properties can shed light on the use of DBS in PD and other BG-related disorders. Here we used the whole-cell configuration of the patch-clamp technique to follow the synaptic and firing dynamics of GP neurons in response to repetitive stimulation.
Materials and Methods
In vitro Slice Preparation and Solutions
Thick sagittal slices of 300 μm were obtained from rat somatosensory cortex, striatum, and GP using previously described techniques (Stuart et al., 1993; Bugaysen et al., 2010). Wistar rats, 16–22 days old, were killed by rapid decapitation according to the guidelines of the Bar-Ilan University animal welfare committee. This procedure was approved by the national committee for experiments on laboratory animals at the Israeli Ministry of Health (permit number BIU281206). The brain was quickly removed and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 125 NaCl, 4 KCl, 25 NaHCO3, 1.25 Na2HPO4, 2 CaCl2, 2 MgCl2, 25 glucose, and 0.5 Na-ascorbate (pH 7.4 with 95% O2/5%CO2). Slices were cut using an HR2 Slicer (Sigman Electronic, Germany) and transferred to a submersion-type chamber, where they were maintained for the remainder of the day. In several experiments the following drugs were added to ACSF: bicuculline (BCC) methiodide to block GABAa receptors (final concentration 50 μM), APV (50 μM), and CNQX (15 μM) to block NMDA and AMPA receptors, respectively.
The experiments reported here were carried out at 34°C. The GP nucleus and individual GP neurons were visualized using infrared differential interference contrast (IR-DIC) microscopy. The recording chamber was constantly perfused with oxygenated ACSF. The standard pipette solution contained (in mM): 140 K-gluconate, 10 NaCl, 10 HEPES, 4 MgATP, 0.05 SPERMIN, 5 L-Glutathione, 0.2 EGTA, and 0.4 GTP (Sigma, pH 7.2 with KOH). The reference electrode was an Ag–AgCl pellet placed in the bath. The 10-mV liquid junction potential measured under the ionic conditions reported here was not corrected for.
In vitro Electrophysiology
Cell-attached and whole-cell recordings were obtained from the soma of GP neurons with an Axopatch-200B amplifier (Axon Instruments). Voltage was filtered at 10 kHz and sampled at 20 kHz, unless stated otherwise, using patch pipettes (4–8 MΩ) pulled from thick-walled borosilicate glass capillaries (2.0 mm outer diameter, 0.5 mm wall thickness, Hilgenberg, Malsfeld, Germany). Electrical stimulation was performed using monopolar 0.2–0.7 MΩ glass-coated tungsten microelectrodes. The stimulation pulses consisted of 50 μA biphasic currents (200 μs cathodal followed by 200 μs anodal phase). The anode was an Ag–AgCl pellet placed in the bath. The stimulating electrode was positioned at the dorsal side of the GP. The recording electrodes were positioned 0.5 mm toward the center of the GP away from the stimulating electrode. The interval between consecutive pulses was 100, 50, and 25 ms, over 30 s leading to stimulation frequencies of 10 Hz (300 stimuli), 20 Hz (600 stimuli), and 40 Hz (1200 stimuli), respectively.
Data Analysis
All off-line analyses were carried out using Offline Sorter 2.8.6 (Plexon), NeuroExplorer 4.007 (Nex Technologies), Matlab R2007b (Mathworks), and IgorPro 6.0 (WaveMetrics) on a personal computer. The pre-stimulus average extracellular and intracellular firing rate was calculated from spikes extracted from 30 s of continuous recording. Throughout the manuscript the firing rate is the time dependent average firing rate aligned to the stimulation onset. The maximal inhibition of firing rate was calculated as the difference between the minimal firing rate during the stimulus and the pre-stimulus average firing rate. The latency to maximal inhibition was calculated as the time from the onset of the stimulus to the minimal firing rate. Experimental results were consistently obtained from cells from at least seven rats. All the results for a particular experiment were pooled and displayed as mean ± SD, unless otherwise stated. Significant changes in measured parameters before and after application of the drugs during stimulation were tested using a t-test (* < 0.05 and ** < 0.01).
Results
We applied the whole-cell configuration of the patch-clamp technique to evaluate the impact of short-term STP on the firing pattern of GP neurons during repetitive stimulation. A possible drawback of the whole-cell configuration is the washout of important cellular components by the pipette solution. We have previously shown that the whole-cell configuration did not alter significantly the spontaneous firing rate of GP neurons (Bugaysen et al., 2010). To evaluate the impact of this configuration during repetitive stimulation we first performed a control experiment in the cell-attached mode of the patch-clamp technique. Following the onset of a 40-Hz stimulation sequence, the cells responded with a period in which no APs were observed (Figure 1A, top). This inhibitory effect lasted 3 s after which APs were once again observed. A similar response to stimulation was observed when the recordings were performed on the whole-cell configuration (Figure 1A, bottom). We quantified the pre-stimulus firing rate, the maximal inhibition of the average firing rate during stimulation, and the latency to maximal inhibition (Figure 1B). All these parameters did not differ significantly between cell-attached recordings and whole-cell recordings (p > 0.05, n = 12).
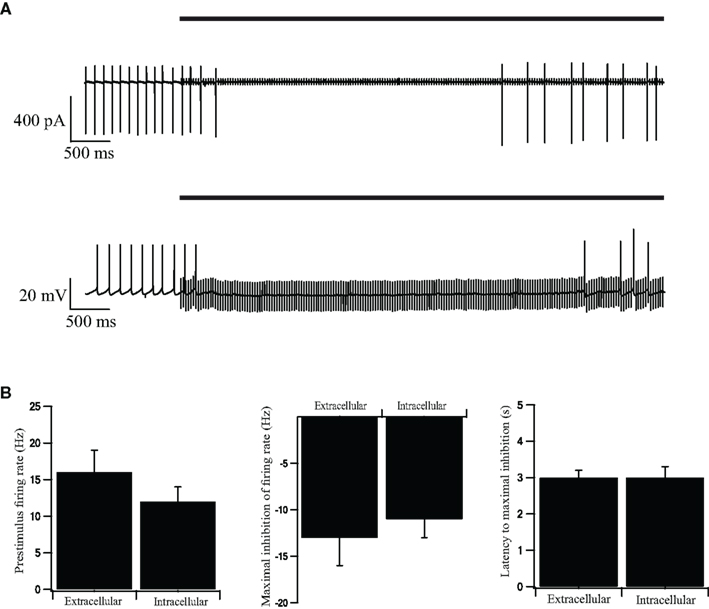
Figure 1. Intracellular and extracellular recordings from stimulated GP neurons in acute rat brain slices. (A) Continuous recording of the extracellular potential (top trace) recorded in the cell-attached mode of the patch-clamp technique and the membrane potential (bottom trace) recorded in the whole-cell mode, during an extracellular stimulation in the GP. The stimulation artifact of the cell-attached recording was scaled down by a factor of 20 to enable a clearer view of the extracellular spikes. The bold horizontal lines indicate the period of repetitive stimulation. (B) Extracellular and intracellular average pre-stimulus firing rate, inhibition of firing rate, and latency to maximal inhibition, recorded in the cell-attached, and whole-cell modes across the population of neurons in GP (n = 12). Error bars are expressed as mean ± SEM.
It has been previously reported that GABAergic synapses in the GP display short-term depression (Rav-Acha et al., 2005). That study showed that a short 10 Hz stimulation burst generates a 50% depression of the GABAergic synapse while stimulation bursts above 20 Hz cause almost complete depression of the synapse. We have previously reported that in acute brain slices the average spontaneous firing rate was 13 Hz (Bugaysen et al., 2010). These findings prompted us to select two stimulation frequencies for the current study. To investigate the effect of low-frequency stimulation we used a repetitive stimulation at 10 Hz. To investigate the effect of high-frequency stimulation we applied a repetitive stimulation of 40 Hz.
Visual inspection of the recordings during a 10-Hz stimulation protocol did not reveal any change in the spontaneous firing frequency, although apparent IPSPs could be clearly discerned following each stimulus (Figure 2A, top). To verify that the stimulation pulses excited GABAergic synapses we bath applied the GABAA blocker BCC. Following application of BCC the spontaneous firing frequency appeared to increase (Figure 2A, bottom). To quantify the effect of BCC we calculated the stimulus-triggered average (STA) of the membrane potential (Figure 2B). A clear IPSP could be observed following the stimulus (Figure 2B, top trace). This average IPSP was abolished following the application of BCC (Figure 2B, bottom trace). In some of the cases, a small depolarization could be discerned in the STA following BCC application. This suggested that the stimulus additionally activated excitatory fibers. To test this suggestion we repeated the 10-Hz stimulation experiment while blocking glutamatergic synaptic transmission. Following the bath application of APV and CNQX the firing frequency appeared to decrease (Figure 2C). In the example (Figure 2D), the STA under control conditions displayed a shallow IPSP that increased following the application of APV and CNQX. Thus, microstimulation of the GP in acute brain slices from the rat excited both GABAergic and glutamatergic synapses.
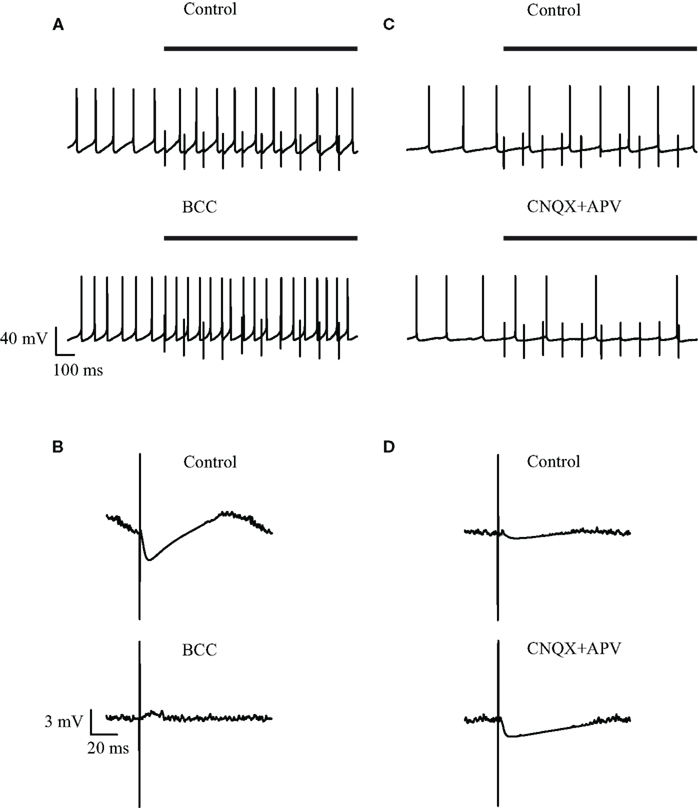
Figure 2. Effect of GABAergic and glutamatergic antagonists on the membrane potential during low-frequency (10 Hz) stimulation. (A,C) Continuous recording of the membrane potential before (top trace) and after (bottom trace) application of (A) 50 μM bicuculline or (C) 15 μM CNQX and 50 μM APV, during the stimulation. The bold horizontal lines indicate the period of repetitive stimulation. (B,D) Stimulus-triggered average of membrane potential before (top) and after (bottom) application of (B) 50 μM bicuculline or (D) 15 μM CNQX and 50 μM APV, during the stimulation.
To quantify the effects that were observed visually we calculated the population average firing rate (n = 41 cells) and normalized it to the pre-stimulus population average firing rate. During a 30-s stimulation at 10 Hz the firing rate decreased slightly (Figure 3A). Following the application of BCC the pre-stimulus population average firing rate increased almost 3-fold (Figure 3B). During the 10-Hz stimuli train the population average firing rate increased slightly (Figure 3B). In a different experiment, bath application of APV and CNQX reduced the pre-stimulus population average firing rate (Figure 3C). No effect on the population average firing rate was observed during the 10-Hz stimuli train (Figure 3C). Next we calculated the population average post-stimulus time histograms (PSTHs) for the experiments in which a 10-Hz stimuli train was applied (Figure 4). Under control conditions, the firing rate increased slightly and then decreased substantially immediately following the stimulus (Figure 4A). This post-stimulus inhibition was eliminated by the application of BCC leaving only the immediate excitation (Figure 4B). Inversely, application of APV and CNQX left the inhibition almost intact while abolishing the initial post-stimulus excitation (Figure 4C). Thus, as predicted by short burst stimulation studies (Rav-Acha et al., 2005), repetitive stimulation at 10 Hz appeared to synchronize the firing of GP neurons by generating common delays from the stimulation pulses.
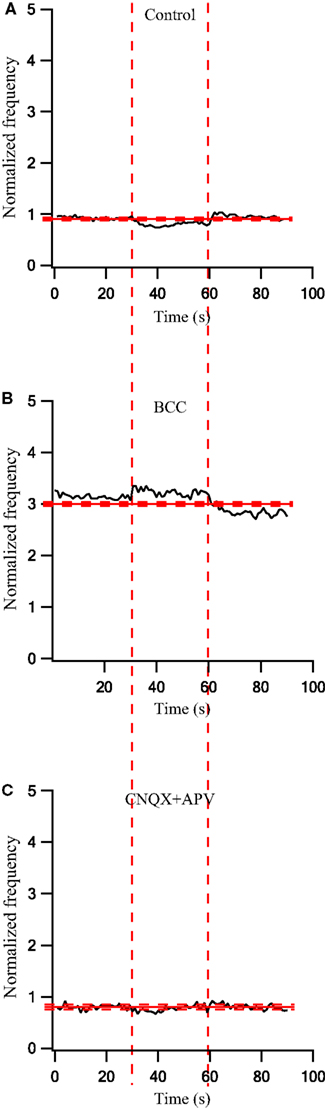
Figure 3. Firing rate changes during stimulation at 10 Hz before and after application of GABAergic and glutamatergic antagonists. (A) Population firing rate of GP neurons in normal extracellular solution (n = 41). (B) Population frequency of GP neurons after applying 50 μM bicuculline to the extracellular solution (n = 17). (C) Population frequency of GP neurons after applying 15 μM CNQX and 50 μM APV to the extracellular solution (n = 14). The thick and dashed horizontal lines indicate the average and SD of the pre-stimulus frequency, respectively. The dashed vertical lines mark the beginning and the end of the stimulus.
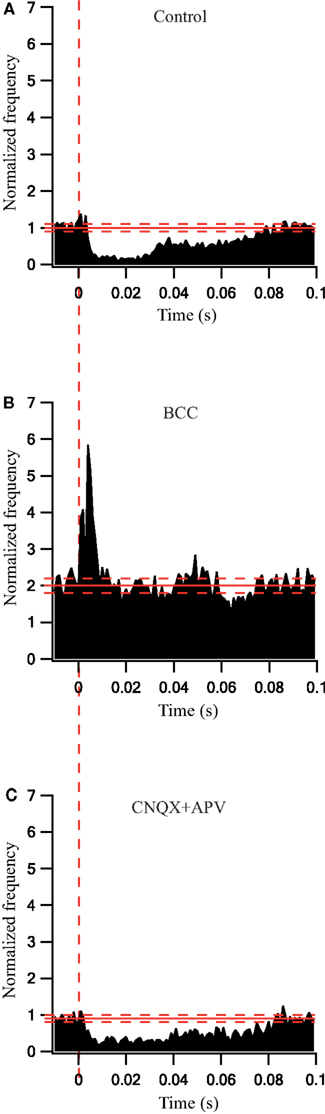
Figure 4. Synaptically locked response changes during stimulation at 10 Hz before and after application of GABAergic and glutamatergic antagonists. (A) Population PSTH of GP neurons in normal extracellular solution (n = 38). (B) Population PSTH of GP neurons after applying 50 μM bicuculline to the extracellular solution (n = 17). (C) Population PSTH of GP neurons after applying 15 μM CNQX and 50 μM APV to the extracellular solution (n = 14). The thick and dashed horizontal lines indicate the average and SD of pre-stimulus frequency, respectively. The dashed vertical line marks the beginning of the stimulation pulse.
Next, we investigated the influence of 40 Hz repetitive stimulation on the firing of GP neurons. Following the onset of stimulation the cells responded with a period in which no APs were observed (Figures 5A,C, top traces). Application of BCC increased the pre-stimulus firing rate and eliminated the inhibitory period (Figure 5A, bottom trace). Conversely, application of APV and CNQX did not appear to alter the inhibitory period but reduced the firing rate following this period (Figure 5C). Since the 10-Hz stimulation did not change the firing frequency we were able to calculate a STA and get an average synaptic response (Figure 2). At higher stimulation frequencies the firing frequency was not stationary. Therefore it was not possible to calculate the STA. To offer a qualitative display of the synaptic potentials during the stimulation we added to Figure 5 membrane potential responses to a single stimulus. Figures 5B,D display a single, apparently inhibitory, synaptic response 100 ms following the onset of the 40-Hz stimulation. As predicted by voltage-clamp experiments (Rav-Acha et al., 2005; Sims et al., 2008) the amplitude of the synaptic potential was considerably reduced 1 s into the stimulus train (Figures 5B,D, gray line). The IPSP observed immediately following the onset of the stimulation was abolished following the application of BCC (Figure 5B, bottom trace) but not by the application of APV and CNQX (Figure 5D, bottom trace).
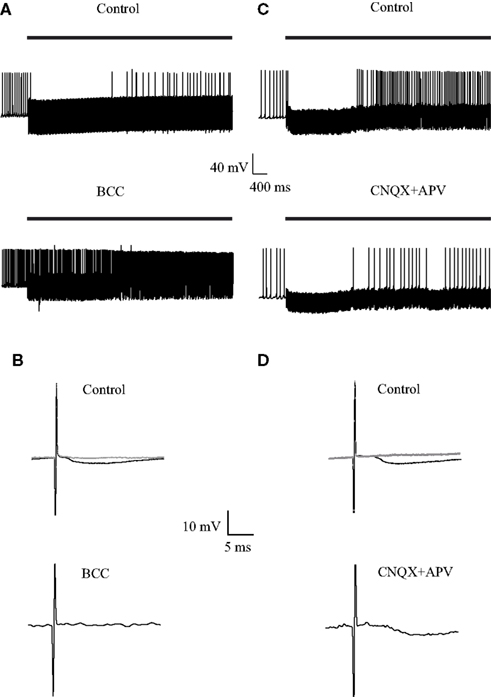
Figure 5. Effect of GABAergic and glutamatergic antagonists on the membrane potential during stimulation at 40 Hz. (A,C) Continuous recording of the membrane potential before (top trace) and after (bottom trace) application of (A) 50 μM bicuculline or (C) 15 μM CNQX and 50 μM APV, during the stimulation. The bold horizontal lines indicate the period of repetitive stimulation. (B,D) Representative responses of the membrane potential to a single before (top) and after (bottom) application of (B) 50 μM bicuculline or (D) 15 μM CNQX and 50 μM APV, during the stimulation. The black traces were from 100 ms following the onset of the stimulus train. The gray traces were from 1 s following the onset of the stimulus train.
To quantify the effects that were observed visually we calculated the population average firing rate (n = 43 cells) and normalized it to the pre-stimulus population average firing rate. During a 30-s stimulation at 40 Hz the firing rate first decreased and then increased (Figure 6A). Following the application of BCC the pre-stimulus population average firing rate increased 2.5-fold (Figure 6B). During the 40-Hz stimuli train the population average firing rate increased to almost 4-fold of the control values and then started to decrease (Figure 6B). Bath application of APV and CNQX eliminated the excitatory phase of the cellular response and decreased the inhibitory response (Figure 6C). Since these effects were not stationary in time we did not calculate the PSTHs under these conditions.
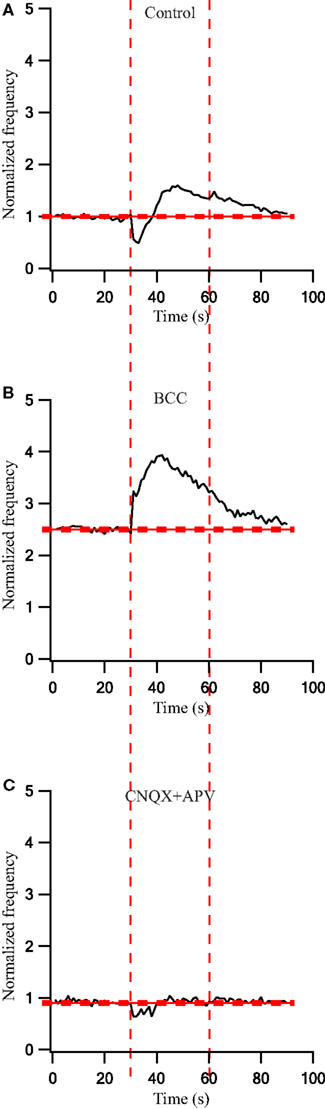
Figure 6. Firing rate changes during stimulation at 40 Hz before and after application of GABAergic and glutamatergic antagonists. (A) Population frequency of GP neurons in normal extracellular solution (n = 43). (B) Population frequency of GP neurons after applying 50 μM bicuculline to the extracellular solution (n = 25). (C) Population frequency of GP neurons after applying 15 μM CNQX and 50 μM APV to the extracellular solution (n = 13). The thick and dashed horizontal lines indicate the average and SD of pre-stimulus frequency, respectively. The dashed vertical lines mark the beginning and the end of the stimulus.
Discussion
In the current work we described the dynamic changes to the firing rate and pattern of GP neurons during repetitive stimulation. We showed that at a low rate of stimulation the firing of the neurons is locked to the stimulus due to the combined activation of excitatory and inhibitory synapses. At higher stimulation frequencies, the firing rate of the neurons displayed a biphasic response consisting of an initial inhibition followed by excitation. The dynamics of the response suggest that both GABAergic and glutamatergic synapses in the GP undergo short-term depression during the stimulation.
Several elegant studies have shown that both GABAergic and glutamatergic synapses in the GP undergo short-term depression (Hanson and Jaeger, 2002; Rav-Acha et al., 2005; Sims et al., 2008; Erez et al., 2009). It has been shown that when blocking inhibition with picrotoxin, the excitatory synapses from the STN display both short-term facilitation and depression (Hanson and Jaeger, 2002). This complex short-term plasticity most likely accounts for the initial increase and later decrease in the firing of GP neurons during HFS (Figure 6B). In addition, GABAergic synapses in the GP have been shown to display short-tem depression (Rav-Acha et al., 2005; Sims et al., 2008). Activation of these synapses and their subsequent depression provides an explanation for the initial inhibition of the firing rate during HFS (Figures 1, 5, and 6). As HFS increases the depression of the GABAergic synapse in the GP the IPSPs will decrease. This leads to a reduction in the locking to the stimulation induced by the inhibitory input. Thus, prolonged HFS in the GP will lead to a drastic depression of both inhibitory and excitatory synapses. This in turn will reduce the coupling of the GP to its input and the synchronization within the GP. Moreover, depression of the GABAergic synapses may reduce the overall inhibitory drive in the GP leading to an increase in the spontaneous firing rate of GP neurons. Application of BCC demonstrated that reduction in this drive may result in a substantial increase in the spontaneous firing rate (Figures 3 and 6).
Contrary to HFS, it is tempting to speculate that low-frequency stimulation may increase synchrony in the GP. It has been shown (Rav-Acha et al., 2005) that a 10-Hz stimulation will depress GABAergic synapses in the GP to a steady state level of ∼50%. Thus, low-frequency stimulation stimulates a network with active inhibitory and excitatory synapses. This leads to the locking observed in this study (Figures 3 and 4). The firing rate of GP neurons in vivo ranges from 5 to 80 Hz (Gardiner and Kitai, 1992; Kelland et al., 1995). This may suggest that in the rat GP synaptic depression is constantly modulated by network activity.
It is interesting to compare the current in vitro findings in acute brain slices in the rat to the extracellular recordings obtained from behaving primates. A dynamic process affecting the synaptic transmission has been reported in the Parkinsonian (MPTP treated) primate (Erez et al., 2009). The stimulation rate in the primate was higher (135 Hz) than the one tested in this study (40 Hz). This is in line with previous studies that have shown slower processes in the rat compared to the primate. The baseline firing rate in the normal primate is 50–75 spikes/s (DeLong, 1971) which is higher than the average frequency found in in vivo rat studies (Gardiner and Kitai, 1992; Kelland et al., 1995). In the primate study, a dynamic process was observed during both the excitatory and inhibitory responses to the stimulation. This may have been due to the dominance of the fibers stimulated: excitatory axons from the STN in one case versus the inhibitory axons from the striatum and GP in the other. This difference in dominance in different recording sessions may have arisen from the inhomogeneous distribution of the incoming fibers and the change in the stimulating electrode and recording electrode locations. Thus, it is tempting to speculate, based on the results presented here, that short-term plasticity in the GP contributes differentially to the activity of the network depending on the stimulation frequency.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the Legacy Heritage Bio-Medical Program of the Israeli Science Foundation to Alon Korngreen and Izhar Bar-Gad (Grant no. 981/10).
References
Bar-Gad, I., Elias, S., Vaadia, E., and Bergman, H. (2004). Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J. Neurosci. 24, 7410–7419.
Benabid, A. L. (2003). Deep brain stimulation for Parkinson’s disease. Curr. Opin. Neurobiol. 13, 696–706.
Bugaysen, J., Bronfeld, M., Tischler, H., Bar-Gad, I., and Korngreen, A. (2010). Electrophysiological characteristics of globus pallidus neurons. PLoS ONE 5, e12001. doi: 10.1371/journal.pone.0012001
Dostrovsky, J. O., Levy, R., Wu, J. P., Hutchison, W. D., Tasker, R. R., and Lozano, A. M. (2000). Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J. Neurophysiol. 84, 570–574.
Erez, Y., Czitron, H., McCairn, K., Belelovsky, K., and Bar-Gad, I. (2009). Short-term depression of synaptic transmission during stimulation in the globus pallidus of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J. Neurosci. 29, 7797–7802.
Filali, M., Hutchison, W. D., Palter, V. N., Lozano, A. M., and Dostrovsky, J. O. (2004). Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp. Brain Res. 156, 274–281.
Francois, C., Percheron, G., Yelnik, J., and Heyner, S. (1984). A Golgi analysis of the primate globus pallidus. I. Inconstant processes of large neurons, other neuronal types, and afferent axons. J. Comp. Neurol. 227, 182–199.
Gardiner, T. W., and Kitai, S. T. (1992). Single-unit activity in the globus pallidus and neostriatum of the rat during performance of a trained head movement. Exp. Brain Res. 88, 517–530.
Gubellini, P., Salin, P., Kerkerian-Le Goff, L., and Baunez, C. (2009). Deep brain stimulation in neurological diseases and experimental models: from molecule to complex behavior. Prog. Neurobiol. 89, 79–123.
Hahn, P. J., Russo, G. S., Hashimoto, T., Miocinovic, S., Xu, W., McIntyre, C. C., and Vitek, J. L. (2008). Pallidal burst activity during therapeutic deep brain stimulation. Exp. Neurol. 211, 243–251.
Hammond, C., Ammari, R., Bioulac, B., and Garcia, L. (2008). Latest view on the mechanism of action of deep brain stimulation. Mov. Disord. 23, 2111–2121.
Hanson, J. E., and Jaeger, D. (2002). Short-term plasticity shapes the response to simulated normal and Parkinsonian input patterns in the globus pallidus. J. Neurosci. 22, 5164–5172.
Hashimoto, K., and Kita, H. (2008). Serotonin activates presynaptic and postsynaptic receptors in rat globus pallidus. J. Neurophysiol. 99, 1723–1732.
Hashimoto, T., Elder, C. M., Okun, M. S., Patrick, S. K., and Vitek, J. L. (2003). Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J. Neurosci. 23, 1916–1923.
Johnson, M. D., and McIntyre, C. C. (2008). Quantifying the neural elements activated and inhibited by globus pallidus deep brain stimulation. J. Neurophysiol. 100, 2549–2563.
Kaneda, K., and Kita, H. (2005). Synaptically released GABA activates both pre- and postsynaptic GABA(B) receptors in the rat globus pallidus. J. Neurophysiol. 94, 1104–1114.
Kaneda, K., Kita, T., and Kita, H. (2007). Repetitive activation of glutamatergic inputs evokes a long-lasting excitation in rat globus pallidus neurons in vitro. J. Neurophysiol. 97, 121–133.
Kaneda, K., Tachibana, Y., Imanishi, M., Kita, H., Shigemoto, R., Nambu, A., and Takada, M. (2005). Down-regulation of metabotropic glutamate receptor 1 alpha in globus pallidus and substantia nigra of Parkinsonian monkeys. Eur. J. Neurosci. 22, 3241–3254.
Kelland, M. D., Soltis, R. P., Anderson, L. A., Bergstrom, D. A., and Walters, J. R. (1995). In vivo characterization of two cell types in the rat globus pallidus which have opposite responses to dopamine receptor stimulation: comparison of electrophysiological properties and responses to apomorphine, dizocilpine, and ketamine anesthesia. Synapse 20, 338–350.
Kita, H., and Kitai, S. T. (1994). The morphology of globus pallidus projection neurons in the rat: an intracellular staining study. Brain Res. 636, 308–319.
McCairn, K. W., and Turner, R. S. (2009). Deep brain stimulation of the globus pallidus internus in the Parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J. Neurophysiol. 101, 1941–1960.
McIntyre, C. C., Grill, W. M., Sherman, D. L., and Thakor, N. V. (2004a). Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J. Neurophysiol. 91, 1457–1469.
McIntyre, C. C., Savasta, M., Kerkerian-Le Goff, L., and Vitek, J. L. (2004b). Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin. Neurophysiol. 115, 1239–1248.
Meissner, W., Leblois, A., Hansel, D., Bioulac, B., Gross, C. E., Benazzouz, A., and Boraud, T. (2005). Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain 128, 2372–2382.
Montgomery, E. B. Jr. (2006). Effects of GPi stimulation on human thalamic neuronal activity. Clin. Neurophysiol. 117, 2691–2702.
Montgomery, E. B. Jr., and Gale, J. T. (2008). Mechanisms of action of deep brain stimulation(DBS). Neurosci. Biobehav. Rev. 32, 388–407.
Parent, A., Sato, F., Wu, Y., Gauthier, J., Levesque, M., and Parent, M. (2000). Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci. 23, S20–S27.
Percheron, G., Yelnik, J., and Francois, C. (1984). A Golgi analysis of the primate globus pallidus. III. Spatial organization of the striato-pallidal complex. J. Comp. Neurol. 227, 214–227.
Rav-Acha, M., Sagiv, N., Segev, I., Bergman, H., and Yarom, Y. (2005). Dynamic and spatial features of the inhibitory pallidal GABAergic synapses. Neuroscience 135, 791–802.
Sims, R. E., Woodhall, G. L., Wilson, C. L., and Stanford, I. M. (2008). Functional characterization of GABAergic pallidopallidal and striatopallidal synapses in the rat globus pallidus in vitro. Eur. J. Neurosci. 28, 2401–2408.
Smith, Y., and Parent, A. (1988). Neurons of the subthalamic nucleus in primates display glutamate but not GABA immunoreactivity. Brain Res. 453, 353–356.
Stuart, G. J., Dodt, H. U., and Sakmann, B. (1993). Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 423, 511–518.
Wu, Y. R., Levy, R., Ashby, P., Tasker, R. R., and Dostrovsky, J. O. (2001). Does stimulation of the GPi control dyskinesia by activating inhibitory axons? Mov. Disord. 16, 208–216.
Xu, W., Russo, G. S., Hashimoto, T., Zhang, J., and Vitek, J. L. (2008). Subthalamic nucleus stimulation modulates thalamic neuronal activity. J. Neurosci. 28, 11916–11924.
Keywords: globus pallidus, rat, extracellular potential, action potential, patch-clamp, short-term plasticity, synaptic plasticity, extracellular stimulation
Citation: Bugaysen J, Bar-Gad I and Korngreen A (2011) The impact of stimulation induced short-term synaptic plasticity on firing patterns in the globus pallidus of the rat. Front. Syst. Neurosci. 5:16. doi: 10.3389/fnsys.2011.00016
Received: 29 December 2010;
Paper pending published: 17 February 2011;
Accepted: 21 March 2011;
Published online: 30 March 2011.
Edited by:
James M. Tepper, Rutgers, The State University of New Jersey, USAReviewed by:
Atsushi Nambu, National Institute for Physiological Sciences, JapanHitoshi Kita, The University of Tennessee Health Science Center, USA
Copyright: © 2011 Bugaysen, Bar-Gad and Korngreen. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Alon Korngreen, Mina and Everard Goodman Faculty of Life Sciences, Leslie and Susan Gonda Multidisciplinary Brain Research Center, Bar-Ilan University, Ramat Gan 52900, Israel. e-mail: korngra@mail.biu.ac.il