The corticostriatal system in dissociated cell culture
- 1 Brain Mechanisms for Behaviour Unit, Okinawa Institute of Science and Technology, Okinawa, Japan
- 2 Department of Cellular and Molecular Medicine, University of Ottawa, Ottawa, ON, Canada
The sparse connectivity within the striatum in vivo makes the investigation of individual corticostriatal synapses very difficult. Most studies of the corticostriatal input have been done using electrical stimulation under conditions where it is hard to identify the precise origin of the cortical input. We have employed an in vitro dissociated cell culture system that allows the identification of individual corticostriatal pairs and have been developing methods to study individual neuron inputs to striatal neurons. In mixed corticostriatal cultures, neurons had resting activity similar to the system in vivo. Up/down states were obvious and seemed to encompass the entire culture. Mixed cultures of cortical neurons from transgenic mice expressing green fluorescent protein with striatal neurons from wild-type mice of the same developmental stage allowed visual identification of individual candidate corticostriatal pairs. Recordings were performed between 12 and 37 days in vitro (DIV). To investigate synaptic connections we recorded from 69 corticostriatal pairs of which 44 were connected in one direction and 25 reciprocally. Of these connections 41 were corticostriatal (nine inhibitory) and 53 striatocortical (all inhibitory). The observed excitatory responses were of variable amplitude (−10 to −370 pA, n = 32). We found the connections very secure – with negligible failures on repeated stimulation (approximately 1 Hz) of the cortical neuron. Inhibitory corticostriatal responses were also observed (−13 to −314 pA, n = 9). Possibly due to the mixed type of culture we found an inhibitory striatocortical response (−14 to −598 pA, n = 53). We are now recording from neurons in separate compartments to more closely emulate neuroanatomical conditions but still with the possibility of the easier identification of the connectivity.
Introduction
The striatum is the primary gateway for cortical inputs to the basal ganglia, in order to understand how inputs from the cortex to the basal ganglia influence behavior the characteristics of the corticostriatal projections must be understood.
Anatomical considerations suggest massive cortical convergence on striatal neurons. Both Kincaid et al. (1998) and ourselves (Wickens and Arbuthnott, 2010) estimate that about 5,000 cortical neurons might have synaptic contact with a single striatal neuron. Surprisingly, each cortical neuron of the 5,000 might only make one synapse on the striatal target neuron. Even more surprising is the statistic that the likelihood of overlap between cortical inputs to adjacent striatal neurons is less than 10%. Of course, if 10% of the inputs are enough to fire the neurons, the overlap will allow the formation of adjacent groups of striatal neurons relaying common information from the cortex. However, the estimates of the number of cortical neurons likely to be needed to drive one striatal neuron suggest each input is so weak that almost 1,000 synapses must be co-activated to cause firing of a medium spiny neuron. That estimate derives from calculations on corticostriatal organotypic cultures (Blackwell et al., 2003) but up to one-half of the depolarization might derive from GABA inputs rather than from cortical synapses.
There are other ways to approach this question. We know from our own (Wright et al., 2001) and Wilson’s (Sachdev et al., 2004) work that a single striatal neuron can fire in response to the movement of one whisker. That implies that the cortical neurons of one whisker barrel can fire a single striatal neuron; and cortical barrels have well known numerical anatomy (Helmstaedter et al., 2007). Of the 1,500 neurons in layer V of a single barrel about 225 might be inhibitory interneurons and of the rest, about 1/3 are likely to project to the striatum. So a barrel might have about 425 layer V corticostriatal neurons of which about 20% fire on any individual whisker deflection (Higley and Contreras, 2005). Therefore, it might take only 85 neurons to fire a single striatal neuron.
Others have estimated the size of one cortical input to a striatal neuron from stimuli small enough to cause failures of transmission (so called “minimal stimulation”) in slices of the corticostriatal system. That estimate ranges from 12 to 26 pA (Mori, 1994a,b,c; Ding et al., 2008). Assuming an input resistance of about 100 MΩ and the need of 30–40 mV depolarization to fire the neuron from a −80 mV resting potential, about 15–30 simultaneous inputs would be required to reach threshold. Of course the duration of the synaptic responses and the non-linearity of the membrane responses will modify that estimate. One objective of our study of corticostriatal cultures was to get a direct estimate of the strength of the excitatory connection between a single cortical neuron and a single striatal neuron.
We have established an in vitro dissociated cell culture method that allows identification of anatomically connected cortical and striatal neurons. Electrophysiological recording of connected neurons is a versatile tool allowing the study of many basic properties of neuronal communication in brain circuits (Debanne et al., 2008). Here we describe the electrophysiological characteristics of cortical and striatal neurons in the dissociated culture, as well as the functional synapses between pairs of neurons. We show that dissociated neurons of the cortex and striatum in culture have comparable electrophysiological characteristics to neurons recorded both in slices and in vivo. We also show that functional synaptic connections form in culture.
This experimental system provides a useful platform for in-depth investigation of the corticostriatal system in an in vitro preparation. In particular the spontaneous activity in striatal neurons has been unavailable to study in corticostriatal slices without pharmacological manipulation (Wilson and Groves, 1980, 1981; Wilson, 1993; Wilson and Kawaguchi, 1996; Vergara et al., 2003). However, spontaneous firing characteristics have been studied in organotypic cultures (Plenz and Kitai, 1998) and they are visible in mixed cultures of dissociated primary cortical and striatal cells (Arbuthnott et al., 2005). We took advantage of recordings in this culture system to study some of the properties of this spontaneous activity, and to look for the connectivity between cortical and striatal neurons. Cortical and striatal neurons were readily distinguishable in culture as one type was always prepared from a green fluorescent protein (GFP) transgenic mouse (Tsirigotis et al., 2001).
Striatal neurons probably receive input from thousands of cortical neurons in vivo (Kincaid et al., 1998; Wickens and Arbuthnott, 2010) and therefore studying individual pairs of connected cortical and striatal neurons is almost impossible. This multiplicity of connections does not occur in mixed cultures and patching neighboring neurons has often allowed us to identify corticostriatal synaptic pairs for study.
Materials and Methods
Cryopreserved mouse neurons were obtained from QBM Cell Science Ottawa and defrosted and cultured on 35 mm IBIDI plates (Martinsried, Germany) with a marked grid on the base. The dishes were pre-coated with poly-D-lysine. Cells were restricted to the area in the center of the plates with a silicon flexiPERM ring (Greiner Bio-One). The cultures were plated at a cell density of ≈700 cells/μl with mixtures of cortical cells from a UBC driven 6 his-ubiquitin/GFP mouse (Tsirigotis et al., 2001) and striatal cells from wild-type mouse of the same age and strain. For the first 18 h in culture, the medium (Brain Bits LLC, Springfield) was supplemented with 5% heat-inactivated horse serum (Invitrogen, Carlsbad, CA, USA). The cultures were maintained in a humidified incubator (Forma Steri-cycle, Thermo Scientific) at 37°C, 5% CO2/95% O2. Half of the culture medium was exchanged twice-a-week with new medium including 1% penicillin/streptomycin (Invitrogen).
Neurons were typically recorded at 15–20 DIV (range 12–37 DIV). On the day of recording the dish was placed on the stage of an inverted microscope (Olympus IX81) with two Sutter micromanipulators on the stage. The dish was perfused with artificial CSF (NaCl 136; KCl 5; MgCl 1; CaCl 2.5; Hepes buffer 10; glucose 10 – all mM) at a rate of about 2 ml/min. Neurons were patched with microelectrodes (6–12 MΩ) filled with potassium gluconate internal solution (NaCl 8; potassium gluconate 132; MgATP 2; NaGTP 0.4; KCl 6; HEPES buffer 10 – all mM). Alexa 594 (2 μg/ml) was added to the striatal electrode and Alexa 488 (2 μg/ml, Invitrogen) to the cortical one. In later experiments both pipettes had 0.2% biocytin (Invitrogen) also in the internal solution. The electrodes were placed close to the relevant neuron and whole cell patch clamp recordings were made with a two-channel amplifier (Axon 700b) and pCLAMP software (Molecular Devices Inc.). Because of the arrangement of the extended stage of the microscope the neurons needed to be close together – within the field of view of the 20× objective that was used to visualize the cells (<300 μm). Initially, the properties of each neuron were characterized in voltage clamp. Neuron membrane potential was manipulated from −90 to −20 mV using current steps to look at the membrane properties of the neurons. For pairs of neurons in voltage clamp, we stimulated one with depolarizing voltage pulses sufficient to elicit one action potential every 1.2 s and recorded the response in the other neuron while manipulating the resting potential of the postsynaptic neuron from −80 to −30 mV. Further protocols to study spontaneous activity and the responses of the neurons to trains of stimuli were recorded as time permitted. In earlier experiments we simply studied the spontaneous activity in striatal or cortical neurons one at a time and in those experiments we applied 8 μM DNQX (6,7-dinitroquinoxaline-2,3-dione, Sigma-Aldrich) for 2 min before returning to artificial CSF flow.
Filled pairs were fixed with 4% paraformaldehyde, washed and stored in 10% phosphate buffered saline. They were imaged using a fluorescent microscope. Following imaging, neurons were treated with VECTASTAIN Elite ABC kit (Vector Labs) and then stained with 3,3′-diaminobenzidine (DAB) substrate kit (Vector labs) to reveal the biocytin staining.
Results
Identification of Corticostriatal Pairs in Culture
Cortical and striatal neurons in a mixed culture are very difficult to discern based on their cytological differences alone. For example, neurons with what looked like apical dendrites were visible in both cortical and striatal cells. The use of GFP-expressing cells from a transgenic mouse allowed for clear differential identification of cortical neurons in mixed cultures, since striatal cells were from a wild-type mouse (Figure 1). We designated any two neurons (one GFP-positive and one GFP-negative) recorded at the same time as a pair. Slightly more than 50% of recorded pairs showed synaptic activity between them (69). Of those 44 pairs were connected only in one direction (16 corticostriatal and 28 striatocortical) the rest (25) were reciprocally connected. We analyzed all the connections independently which gave a total of 41 corticostriatal and 53 striatocortical connections.
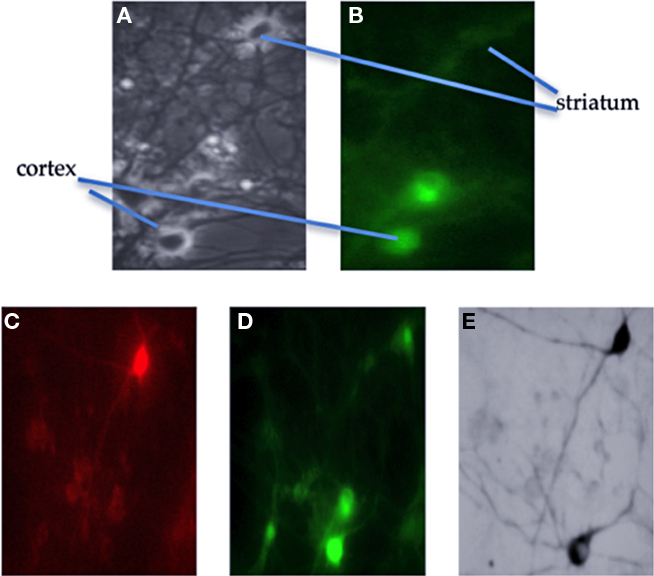
Figure 1. Identification of anatomically connected neurons. Cortical neurons were prepared from a transgenic mouse labeled with green fluorescent protein (GFP). (A) Phase contrast image of the field from which neurons were selected. The recorded neurons are indicated in this photograph and in (B) which is a fluorescence image of the same field. After the recording; (C) shows the same field as B but this time with filters to show the red fluorescent dye in the striatal neuron. (D) Now the lower cortical neuron is filled with green dye. (E) Shows the neurons after fixation and re-staining for the biocytin also present in the recording electrodes; now both neurons are black.
Stimulation of Cortical Neurons
The expected cortical EPSCs in striatal neurons evoked by stimulation of a single cortical neuron in the culture were seen (Figure 2) in 32/41 corticostriatal connections (though of course some spread of activity among the cortical cells in the dish was expected – and seen sometimes in synaptic responses with multiple phases). The excitatory responses were of variable amplitude (−10 to −370 pA, n = 32).
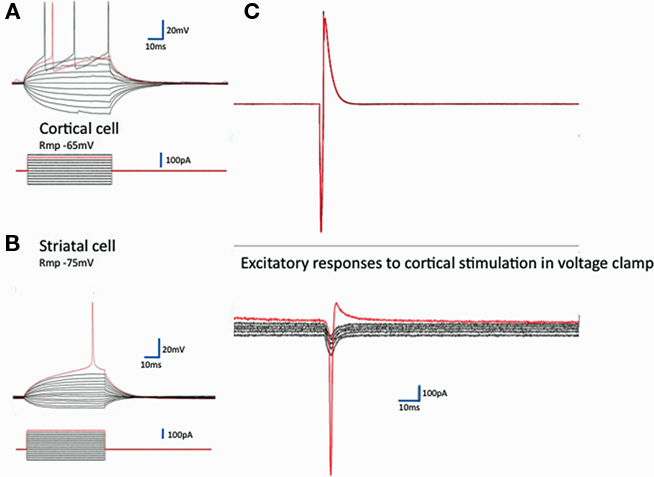
Figure 2. Synaptic communication in corticostriatal pairs. On the left are typical records from identified cortical (A) and striatal neurons (B) recorded in current clamp. Regular current steps drive typical voltage changes in these two classes of neurons (Rmp, resting membrane potential). The red traces are at threshold for action potential generation. To the right, in (C) records from a pair of neurons – the excitatory currents evoked in a striatal neuron by the firing of the cortical neuron. The synaptic currents are the average of five repeats of the stimulation with the receiving neuron clamped at each of six different resting potentials −80 to −30 mV (10 mV steps). The response to the stimulation in this striatal neuron is close to the mean response in all 32 cases (−100 pA at −80 mV). The red traces again show the threshold for activation of an action current in the striatal neuron. The (unclamped) action current in the cortical neuron illustrated above in response to 150 mV intracellular depolarizing pulse 2 ms long allows estimation of the latency of the response – here 1.8 ms.
Other cortical neurons identified by the GFP (9) produced an inhibitory response on striatal neurons as shown in Figure 3B. Since the GFP tag is driven by the ubiquitin promoter, it is present in all cells and in separate experiments we have looked for and not found any GFP-negative cells in cultures from these mice.
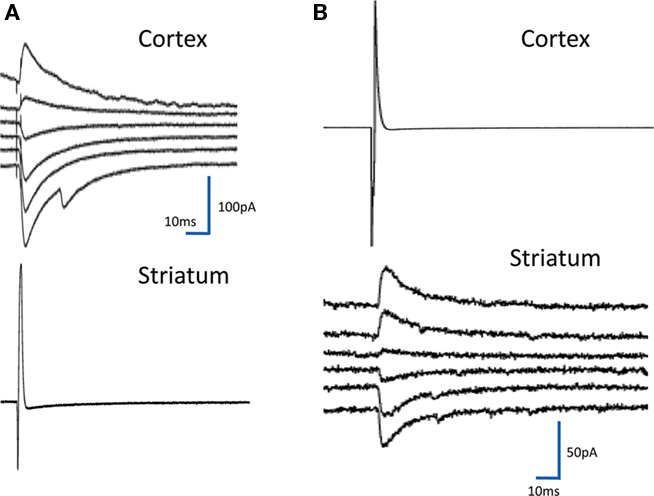
Figure 3. Inhibitory synaptic events. The figure shows on the left (A) a response in a cortical neuron to stimulation of the striatal neuron recorded below. The response reverses between −50 and −40 mV in the range of the equilibrium potential for chloride in our system. There were many of these responses in the cultures and in 25 cases they were reciprocal with connections from cortical neurons to striatal neurons and from striatal neurons to cortical ones. In (B) is the less common event of a cortical input to a striatal neuron, which is inhibitory.
Inhibitory responses are assumed to arise from activation of an inhibitory neuron by the stimulus. All but three of the inhibitory responses had latencies of less than 2 ms. In two cases the response was mixed with a reversing and non-reversing component. Given the number of reciprocally connected neurons this small number is surprising but not all the striatal neurons fired action potentials in response to the cortical neuron input.
In 53 connections the activity was in the opposite direction to what was expected; stimulation of a striatal neuron induced an IPSC in the cortical neuron (Figure 3A). Inhibition was defined by looking at the reversal potential of the response. Since chloride reversal potential in our neurons is about −45 mV we assumed that these reversing potentials were driven by GABA that is known to be the major transmitter in striatal neurons. Most of the responses had latencies compatible with monosynaptic connections (mean 1.92 ± 0.92 ms, N = 94) but there were some longer ones that may have been polysynaptic. There was no obvious relationship between size and latency in the current set therefore we cannot assume that the larger post synaptic currents are polysynaptic.
Resting Spontaneous Conditions
The resting spontaneous firing is illustrated in Figure 4. The firing is typically in bursts of action potentials (Figure 4) sitting on long depolarizations typical of “UP” states as seen in striatal neurons in vivo. These “UP” states (Figure 5) are variable between cultures but have several properties in common. They are markedly synchronous. The rate of responding is modified by membrane polarization. Neurons fire less often from more hyperpolarized potentials although subthreshold depolarizations are larger in the more hyperpolarized states.
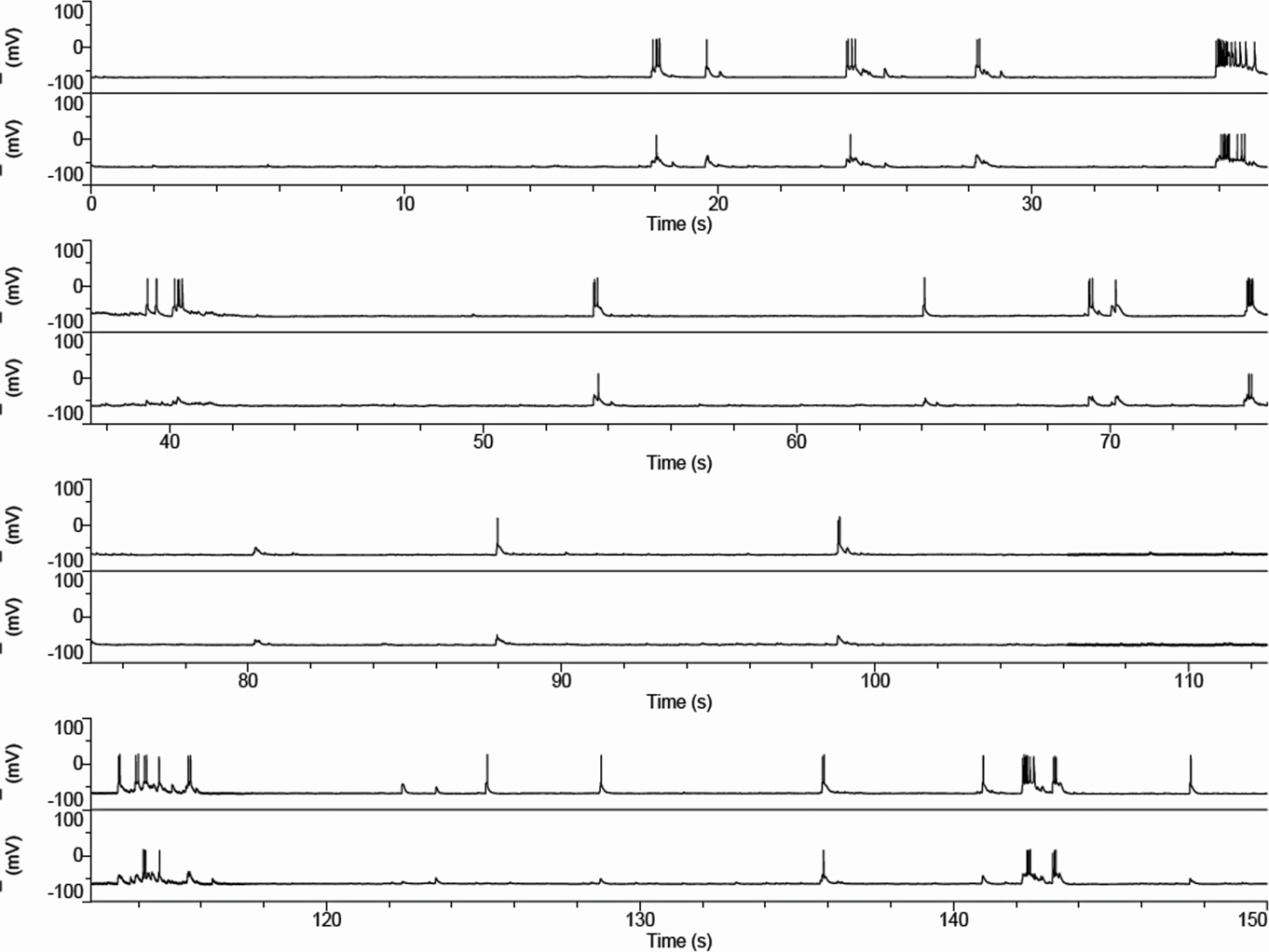
Figure 4. Spontaneous activity in corticostriatal cultures. Current clamp records of a pair of cortical (upper trace) and striatal neuron firing patterns in culture show occasional bursts at about 0.1 Hz with “UP” states that last approximately 0.5 s. Although slower than the typical pattern in vivo the pattern is very reminiscent of that seen in the anesthetized rat (Wilson and Groves, 1981; Wilson et al., 1983). The records shown are continuously recorded in time.
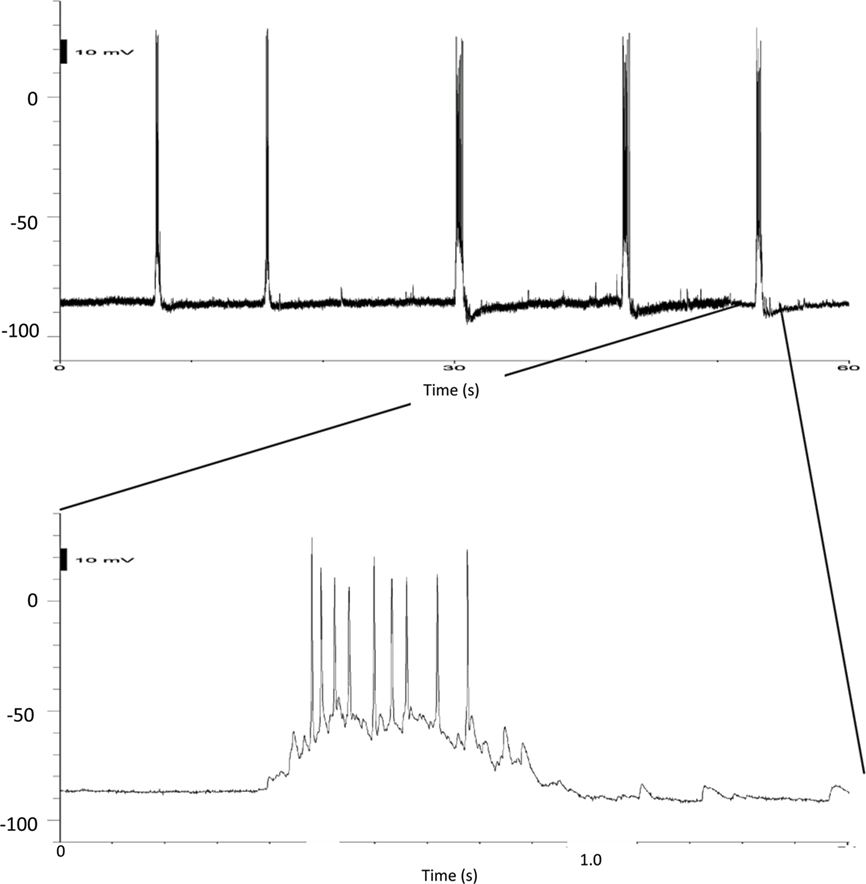
Figure 5. Details of UP states. Current clamp recordings of a striatal neuron in a mixed culture. The upper record on a slower timescale shows the rate of the bursts, while the final burst on the record is shown at a faster timescale below.
Cortical neurons also show similar “UP/DOWN” states (Cowan and Wilson, 1994) and they do so in cultures even in the absence of striatal neurons.
Pharmacological blockade
Previous work on cortical cultures (Corner et al., 2002) exploring the response of burst firing to blockade of glutamate receptors showed that bursts were extremely sensitive to DNQX applied to the bath. Our cortical neurons, and striatal neurons in mixed cortex and striatal cultures, generated bursts that were also extremely sensitive to DNQX. The effect was rapid and returned the striatal neurons to resting “DOWN” state membrane potential with complete reversal after only 2 min in normal perfusion medium (Figure 6).
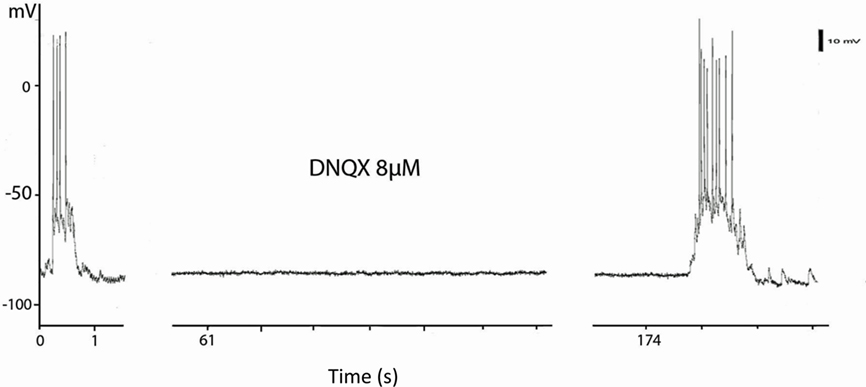
Figure 6. Blockade of UP states by blockade of AMPA receptors The current clamp records are taken from a continuous record of a striatal neuron in a mixed corticostriatal culture. The culture was treated with artificial CSF containing 8 μM DNQX for 1 min and the bursts were abolished for the next 2 min. The first burst of the recovery is shown at 174 s after DNQX application.
Discussion
Spontaneous Activity
Isolated cortical neurons have been shown to support burst-like activity even when isolated in the cat’s skull from surrounding tissue (Burns, 1950, 1951; Timofeev et al., 2000). In cortical slices (Boakes et al., 1988; Allene et al., 2008) as well as in dissociated cell culture (Potter and DeMarse, 2001; Wagenaar et al., 2005; Rolston et al., 2007) and slice culture (Plenz and Kitai, 1998; Johnson and Buonomano, 2007; Plenz and Thiagarajan, 2007) cortical neurons exhibit bursts of activity associated with depolarized membrane potentials. These intermittent bursts are maintained even in the absence of the thalamic input to the cortex (Burns, 1950, 1951), though in vivo and in slices in which thalamocortical fibers are preserved these UP states in cortex are driven by thalamic input and inhibited by synchronous activity resulting from cortical stimulation (Rigas and Castro-Alamancos, 2007; Gibson et al., 2008; Haider and McCormick, 2009). Clearly in the organotypic cultures they must have another origin and intrinsic cortical activity seems the likeliest explanation. Viewed in multielectrode arrays the bursts are synchronous over many of the electrodes suggesting that the property is one of the assembly rather than a single neuron property (Wagenaar et al., 2005; Rolston et al., 2007) especially since timed stimuli to the cortical network can influence activity in all of the culture (Wagenaar et al., 2005).
Furthermore the bursts in cortex influence strongly the following of striatal neurons in vivo and in vitro (Schlosser et al., 1999). Evidence from other situations suggests that striatal neurons will follow such cortical activity in a fairly stereotyped fashion. In anesthetized rats, for instance, the cortex fires synchronous bursts that underlie the dominant voltage changes in the EEG (Stern et al., 1997, 1998; Mahon et al., 2001; Kasanetz et al., 2002). Although Blackwell et al. (2003) suggest that at least half of the depolarization that forms the UP states in striatal neurons in organotypic culture is contributed through GABA mediated chloride channel opening, it is not thought that the inhibition comes directly from cortex, but that it may result from the cortical driving of fast-spiking striatal interneurons. These neurons are certainly present in our cultures (Schock et al., 2010) and so the UP states we record may have a similar mixture of synaptic origins. We have not knowingly recorded from “fast-spiking interneurons” they can be a little hard to recognize in cultures where the morphology of the neurons is less obviously diagnostic of cell types, we certainly have not filled any with the well described physiology and anatomical characteristics. They are also relatively rare even in vivo though their importance in supporting the striatal firing patterns are a consequence of their powerful synaptic inputs on to the striatal output neurons, rather than their numbers (Koos and Tepper, 1999; Koos et al., 2004).
Our dissociated cell cultures with identifiable cortical neurons in close association with striatal neurons show that this “minimal” system will support the kind of UP/DOWN activity typical of their behavior in vivo. That already suggests that although dopamine may modulate the behavior it is not necessary for its expression in this system since no dopamine neurons from substantia nigra are present in the cultures. We have seen occasional tyrosine hydroxylase positive interneurons but they do not heavily innervate the cultures (Schock et al., 2010). Although no dopamine is present in our cultures we have not encountered giant spontaneous postsynaptic GABAergic currents either. We have explored the electrophysiological characteristics of striatal neurons in a series of different neuronal culture systems that have both cortical and striatal neurons harvested from rat or mouse embryos. In these preparations too the cortex produces bursts of activity nearly synchronously across the cultures (Arbuthnott et al., 2005). The striatal neurons follow those bursts (Garcia-Munoz et al., 2010). Bursting in cortical and striatal neurons is blocked by application of glutamate antagonists of the AMPA type.
Single Neuron Interactions
Synaptic activity between pairs of neurons was seen in 55% of recorded pairs, slightly less than the proportion found in cortical slices by Debanne et al. (2008). Corticostriatal EPSCs varied in size in individual pairs; sometimes they were large enough to trigger action potentials. We stimulated only one cortical neuron enough to fire an action potential but we have no control over how many neurons were connected to the stimulated neuron and then synapsed with the striatal neuron from which we were recording. We have developed electron-microscopic methods that will allow us to measure how many synapses are made by the stimulated neuron on the recorded one but have not so far been able to use them on a connected pair of neurons. However, if there is spread from the activated neuron to many cortical neurons the rise time of the subsequent EPSP on the striatal neuron ought to be slower – even if the effects of different inputs to the neurons cannot be distinguished. In many cases we can see clearly discontinuous rise times on the EPSPs, but the relationship between time to peak and size was not straightforward, therefore there is no obvious correlation between the two measures. The answer to the question with which we started “How big is the response to a single corticostriatal synapse?” is not at all obvious. The average size of the EPSC’s with short enough latencies to be supposed monosynaptic is 100 pA, but the range includes currents smaller (10 pA) and larger (371 pA) than the estimates from previous work (e.g., Mori et al., 1994a,b,c; Blackwell et al., 2003; Ding et al., 2008) quoted in the introduction.
Some nine of the corticostriatal responses were inhibitory in nature. This abnormal connectivity is easy to explain if the cortical interneurons that we know are in the cultures (Schock et al., 2010), make synapses with the striatal neurons nearby. This seems indeed to be the case for many of the corticostriatal inhibitory pairs since there are clear cases of “intermittent bursting” responses in the records from the cortical neurons involved. This is not true in every case, however. In a few cases (two pairs so far) we have clear evidence for both excitatory and inhibitory input evoked by cortical neuron firing. Such cases make it likely that at least some of the responses are polysynaptic and may involve either inhibitory cortical or striatal neurons if our interpretation is correct. A more detailed analysis awaits more recorded pairs so that latencies can be separated and the input neurons characterized in more detail.
The other class of pairwise interactions is also not seen in vivo. An inhibitory action of striatal neurons on cortical neurons was observed. These synaptic actions have latencies compatible with monosynaptic activation and are often large at −80 mV (−14 to −598 pA peak: median 120: mean 168 ± 148: N = 53). They were not seen in the organotypic cultures described by Plenz and Aertsen (1996). Striatal neurons, having many of the characteristics of spiny output neurons, produce IPSCs considerably larger than those seen between pairs of striatal neurons in acute slices (Koos and Tepper, 1999; Koos et al., 2004). There may be several reasons for this, not only because the synapses are unusually numerous or otherwise abnormal, but also that the neurons at the striatal electrode might not be MSNs. Although unlikely, some of the activity we record might be from pallidal neurons (since globus pallidus is also formed from the ganglionic eminence that is the source of our neurons). It is, however, hard to believe that all of these pairs are of that nature since parvalbumin and calretinin immunopositive neurons are not much more common in the cultures than they are in the striatum in vivo (Schock et al., 2010) and there they are interneurons, not pallidal in origin. Similarly the typical spontaneous activity and whole neuron physiology of pallidal neurons are not obvious in the neurons from which we recorded. We looked for a bimodal distribution of the sizes of the inhibitory synaptic currents in striatocortical pairs we recorded but did not find it, although there is a long tail of very large currents that may suggest that there was a subgroup of synaptic currents of a different source.
Conclusion
We have developed a co-culture of dissociated cells from cortex and striatum in which pairs of neurons that are interconnected can be recorded. In such cultures we observed excitatory corticostriatal pairs, a few inhibitory pairs and a recurrent connectivity in the opposite direction with striatal neurons powerfully inhibiting cortical ones. In spite of this complex synaptology the cultures have a robust spontaneous activity pattern that is very like that observed in the corticostriatal system in vivo. Quantitative comparisons between this set of data and those from in vivo recordings may perhaps be misleading, in spite of the qualitative similarity of activity in the cultures to in vivo patterns of activity.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Allene, C., Cattani, A., Ackman, J. B., Bonifazi, P., Aniksztejn, L., Ben-Ari, Y., and Cossart, R. (2008). Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J. Neurosci. 28, 12851–12863.
Arbuthnott, G. W., Poulter, M. O., and Staines, W. A. (2005) “Corticostiatal co-cultures; a minimal system for “up” states in striatal cells?” in British Neuroscience Association Annual Meeting, Brighton.
Blackwell, K. T., Czubayko, U., and Plenz, D. (2003) Quantitative estimate of synaptic inputs to striatal neurons during up and down states in vitro. J. Neurosci. 23, 9123–9132.
Boakes, R. J., Burns, B. D., and Webb, A. C. (1988). Transmission of burst responses through slices of rat cerebral cortex. J. Physiol. (Lond.) 404, 467–478.
Burns, B. D. (1950). Some properties of the cat’s isolated cerebral cortex. J. Physiol. (Lond.) 111, 50–68.
Burns, B. D. (1951). Some properties of the isolated cerebral cortex in the unanaesthetized cat. J. Physiol. (Lond.) 112, 156–175.
Corner, M. A., van Pelt, J., Wolters, P. S., Baker, R. E., and Nuytinck, R. H. (2002). Physiological effects of sustained blockade of excitatory synaptic transmission on spontaneously active developing neuronal networks – an inquiry into the reciprocal linkage between intrinsic biorhythms and neuroplasticity in early ontogeny. Neurosci. Biobehav. Rev. 26, 127–185.
Cowan, R. L., and Wilson, C. J. (1994). Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J. Neurophysiol. 71, 17–32.
Debanne, D., Boudkkazi, S., Campanac, E., Cudmore, R. H., Giraud, P., Fronzaroli-Molinieres, L., Carlier, E., and Caillard, O. (2008). Paired-recordings from synaptically coupled cortical and hippocampal neurons in acute and cultured brain slices. Nat. Protoc. 3, 1559–1568.
Ding, J., Peterson, J. D., and Surmeier, D. J. (2008). Corticostriatal and thalamostriatal synapses have distinctive properties. J. Neurosci. 28, 6483–6492.
Garcia-Munoz, M., Carrillo-Reid, L., and Arbuthnott, G. W. (2010). Functional anatomy: dynamic states in basal ganglia circuits. Front. Neuroanat. 4:144. doi: 10.3389/fnana.2010.00144
Gibson, J. R., Bartley, A. F., Hays, S. A., and Huber, K. M. (2008). Imbalance of neocortical excitation and inhibition and altered up states reflect network hyperexcitability in the mouse model of fragile x syndrome. J. Neurophysiol. 100, 2615–2626.
Haider, B., and McCormick, D. A. (2009). Rapid neocortical dynamics: cellular and network mechanisms. Neuron 62, 171–189.
Helmstaedter, M., de Kock, C. P. J., Feldmeyer, D., Bruno, R. M., and Sakmann, B. (2007). Reconstruction of an average cortical column in silico. Brain Res. Rev. 55, 193–203.
Higley, M. J., and Contreras, D. (2005) Integration of synaptic responses to neighboring whiskers in rat barrel cortex in vivo. J. Neurophysiol. 93, 1920–1934.
Johnson, H. A., and Buonomano, D. V. (2007). Development and plasticity of spontaneous activity and up states in cortical organotypic slices. J. Neurosci. 27, 5915–5925.
Kasanetz, F., Riquelme, L. A., and Murer, M. G. (2002). Disruption of the two-state membrane potential of striatal neurones during cortical desynchronisation in anaesthetised rats. J. Physiol. (Lond.) 543, 577–589.
Kincaid, A. E., Zheng, T., and Wilson, C. J. (1998). Connectivity and convergence of single corticostriatal axons. J. Neurosci. 18, 4722–4731.
Koos, T., and Tepper, J. M. (1999). Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat. Neurosci. 2, 467–472.
Koos, T., Tepper, J. M., and Wilson, C. J. (2004). Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J. Neurosci. 24, 7916–7922.
Mahon, S., Deniau, J. M., and Charpier, S. (2001) Relationship between EEG potentials and intracellular activity of striatal and cortico-striatal neurons: an in vivo study under different anesthetics. Cereb. Cortex 11, 360–373.
Mori, A., Takahashi, T., Miyashita, Y., and Kasai, H. (1994a). Quantal properties of H-type glutamatergic synaptic input to the striatal medium spiny neurons. Brain Res. 654, 177–179.
Mori, A., Takahashi, T., Miyashita, Y., and Kasai, H. (1994b). Quantal properties of S-type glutamatergic synaptic input to the striatal medium spiny neuron from neonate rat. Neurosci. Lett. 169, 199–202.
Mori, A., Takahashi, T., Miyashita, Y., and Kasai, H. (1994c). Two distinct glutamatergic synaptic inputs to the striatal medium spiny neurones of neonatal rats and paired-pulse depression. J. Physiol. 476, 217–228.
Plenz, D., and Aertsen, A. (1996). Neural dynamics in cortex-striatum co-cultures.1. Anatomy and electrophysiology of neuronal cell types. Neuroscience 70, 861–891.
Plenz, D., and Kitai, S. T. (1998). Up and down states in striatal medium spiny neurons simultaneously recorded with spontaneous activity in fast-spiking interneurons studied in cortex-striatum-substantia nigra organotypic cultures. J. Neurosci. 18, 266–283.
Plenz, D., and Thiagarajan, T. C. (2007). The organizing principles of neuronal avalanches: cell assemblies in the cortex? Trends Neurosci. 30, 101–110.
Potter, S. M., and DeMarse, T. B. (2001). A new approach to neural cell culture for long-term studies. J. Neurosci. Methods 110, 17–24.
Rigas, P., and Castro-Alamancos, M. A. (2007). Thalamocortical up states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J. Neurosci. 27, 4261–4272.
Rolston, J. D., Wagenaar, D. A., and Potter, S. M. (2007). Precisely timed spatiotemporal patterns of neural activity in dissociated cortical cultures. Neuroscience 148, 294–303.
Sachdev, R. N. S., Ebner, F. F., and Wilson, C. J. (2004). Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J. Neurophysiol. 92, 3511–3521.
Schlosser, B., ten Bruggencate, G., and Sutor, B. (1999). Local disinhibition of neocortical neuronal circuits causes augmentation of glutamatergic and GABAergic synaptic transmission in the rat neostriatumin vitro. Exp. Neurol. 157, 180–193.
Schock, S., Jolin-Dahel, K., Schock, P., Staines, W., Garcia-Munoz, M., and Arbuthnott, G. (2010). Striatal interneurons in dissociated cell culture. Histochem. Cell Biol. 134, 1–12.
Stern, E. A., Jaeger, D., and Wilson, C. J. (1998). Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo Nature 394, 475–478.
Stern, E. A., Kincaid, A. E., and Wilson, C. J. (1997). Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal neurons in vivo. J. Neurophysiol. 77, 1697–1715.
Timofeev, I., Grenier, F., Bazhenov, M., Sejnowski, T. J., and Steriade, M. (2000). Origin of slow cortical oscillations in deafferented cortical slabs. Cereb. Cortex 10, 1185–1199.
Tsirigotis, M., Thurig, S., Dube, M., Vanderhyden, B. C., Zhang, M., and Gray, D. A. (2001). Analysis of ubiquitination in vivo using a transgenic mouse model. Biotechniques 31, 120–130.
Vergara, R., Rick, C., Hern·ndez-LÛpez, S., Laville, J. A., Guzman, J. N., Galarraga, E., Surmeier, D. J., and Bargas, J. (2003). Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice. J. Physiol. (Lond.) 553, 169–182.
Wagenaar, D. A., Madhavan, R., Pine, J., and Potter, S. M. (2005). Controlling bursting in cortical cultures with closed-loop multi-electrode stimulation. J. Neurosci. 25, 680–688.
Wickens, J. R., and Arbuthnott, G. W. (2010) “Gating of cortical input to the striatum,” in Handbook of Basal Ganglia Structure and Function, a Decade of Progress, eds H. Steiner, and K. Y. Tseng (Amsterdam: Elsevier), 341–352.
Wilson, C. J. (1993). The generation of natural firing patterns in neostriatal neurons. Prog. Brain Res. 99, 277–297.
Wilson, C. J., Chang, H. T., and Kitai, S. T. (1983). Disfacilitation and long-lasting inhibition of neostriatal neurons in the rat. Exp. Br. Res. 51, 227–235.
Wilson, C. J., and Groves, P. M. (1980). Fine structure and synaptic connections of the common spiny neuron in the rat neostriatum. A study employing intracellular injection of horseradish peroxidase. J. Comp. Neurology 194, 599–615.
Wilson, C. J., and Groves, P. M. (1981). Spontaneous firing patterns of identified spiny neurons in the rat neostriatum. Brain Res. 220, 67–80.
Wilson, C. J., and Kawaguchi, Y. (1996). The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 16, 2397–2410.
Keywords: dissociated neuronal culture, cryopreserved neurons, corticostriatal, basal ganglia, synaptic physiology
Citation: Randall FE, Garcia-Munoz M, Vickers C, Schock SC, Staines WA and Arbuthnott GW (2011) The corticostriatal system in dissociated cell culture. Front. Syst. Neurosci. 5:52. doi: 10.3389/fnsys.2011.00052
Received: 31 January 2011; Paper pending published: 08 March 2011;
Accepted: 08 June 2011; Published online: 28 June 2011.
Edited by:
Jose Bargas, Universidad Nacional Autónoma de México, MexicoReviewed by:
Enrico Bracci, University of Manchester, UKM. Gustavo Murer, Universidad de Buenos Aires, Argentina
Paul Bolam, University of Oxford, UK
Copyright: © 2011 Randall, Garcia-Munoz, Vickers, Schock, Staines and Arbuthnott. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Gordon W. Arbuthnott, Brain Mechanisms for Behaviour Unit, Okinawa Institute of Science and Technology, 1919-1 Tancha, Kunigami-gun 904-0412, Okinawa, Japan.e-mail: gordon@oist.jp