Duration differences of corticostriatal responses in striatal projection neurons depend on calcium activated potassium currents
- Departamento de Neurociencia Cognitiva, División de Neurociencias, Instituto de Fisiología Celular, Universidad Nacional Autónoma de México, México City DF, México
The firing of striatal projection neurons (SPNs) exhibits afterhyperpolarizing potentials (AHPs) that determine discharge frequency. They are in part generated by Ca2+-activated K+-currents involving BK and SK components. It has previously been shown that suprathreshold corticostriatal responses are more prolonged and evoke more action potentials in direct pathway SPNs (dSPNs) than in indirect pathway SPNs (iSPNs). In contrast, iSPNs generate dendritic autoregenerative responses. Using whole cell recordings in brain slices, we asked whether the participation of Ca2+-activated K+-currents plays a role in these responses. Secondly, we asked if these currents may explain some differences in synaptic integration between dSPNs and iSPNs. Neurons obtained from BAC D1 and D2 GFP mice were recorded. We used charybdotoxin and apamin to block BK and SK channels, respectively. Both antagonists increased the depolarization and delayed the repolarization of suprathreshold corticostriatal responses in both neuron classes. We also used NS 1619 and NS 309 (CyPPA), to enhance BK and SK channels, respectively. Current enhancers hyperpolarized and accelerated the repolarization of corticostriatal responses in both neuron classes. Nevertheless, these drugs made evident that the contribution of Ca2+-activated K+-currents was different in dSPNs as compared to iSPNs: in dSPNs their activation was slower as though calcium took a diffusion delay to activate them. In contrast, their activation was fast and then sustained in iSPNs as though calcium flux activates them at the moment of entry. The blockade of Ca2+-activated K+-currents made iSPNs to look as dSPNs. Conversely, their enhancement made dSPNs to look as iSPNs. It is concluded that Ca2+-activated K+-currents are a main intrinsic determinant causing the differences in synaptic integration between corticostriatal polysynaptic responses between dSPNs and iSPNs.
Introduction
Ca2+-activated K+-currents are important regulators of excitability: they control firing frequency and synaptic integration (Bond et al., 2005; Salkoff et al., 2006; Faber, 2009). In striatal projection neurons (SPNs), Ca2+-activated K+-currents contribute to action potential repolarization and the afterhyperpolarization that makes up a great part of the interspike interval during repetitive firing (Pineda et al., 1992; Vilchis et al., 2000; Pérez-Garci et al., 2003; Pérez-Rosello et al., 2005; Wolf et al., 2005; Galarraga et al., 2007; Flores-Barrera et al., 2009). Selective peptidic toxins made clear that Ca2+-activated K+ currents in SPNs comprise “small” SK and “large” BK conductance channels (Pineda et al., 1992; Bargas et al., 1999). These channels are important targets for the actions of neurotransmitters, e.g.: dopamine, acetylcholine and somatostatin, among others (Pineda et al., 1995; Hernández-López et al., 1996; Vilchis et al., 2002; Pérez-Rosello et al., 2005; Galarraga et al., 2007). In dissociated neurons, Ca2+-activated K+-currents are preferentially activated by calcium entry through CaV2.1 and CaV2.2 channels. CaV1 and CaV2.3 calcium channel represent a much smaller Ca2+ source (Vilchis et al., 2000).
However, Ca2+ entry in the dendrites of SPNs is not mainly due CaV2.1 and CaV2.2 channels. Near synaptic sites of SPNs, NMDA, CaV1, CaV2.3, and CaV3 channels become a main source of Ca2+ (Galarraga et al., 1997; Carter and Sabatini, 2004; Higley and Sabatini, 2008; Flores-Barrera et al., 2011). Because Ca2+-activated K+-channels could be present in the synaptic regions of dendrites (Ngo-Anh et al., 2005; Gu et al., 2008; Benhassine and Berger, 2009; Wynne et al., 2009; Faber, 2010; Grewe et al., 2010; Allen et al., 2011; Hosy et al., 2011; Tonini et al., 2013, e.g., Womack et al., 2004), one question to ask is whether Ca2+-activated K+ currents are involved in dendritic synaptic integration and the repolarization of polysynaptic corticostriatal responses (Vizcarra-Chacon et al., 2013), given that Ca2+ sources could be different than in the soma. Different sources of Ca2+ to activate Ca2+-dependent K+-currents in the dendrites and the soma-axon hillock regions could imply that synaptic inputs and the generation of action potentials may be regulated differentially. Thus, in the present work we describe the role of Ca2+-activated K+-currents in the corticostriatal responses and use them as reporters of the importance of Ca2+ influx into the dendrites during synaptic integration.
SK class channels are widely expressed in the brain and are found in dendritic spines near synaptic contacts (Ngo-Anh et al., 2005; Gu et al., 2008; Faber, 2010; Allen et al., 2011; Hosy et al., 2011; Tonini et al., 2013). BK class channels are functional in each neuronal compartment: soma, dendrites and terminals (Gu et al., 2008; Benhassine and Berger, 2009; Wynne et al., 2009; Grewe et al., 2010). Ca2+-activated K+ channels on neuronal dendrites can be activated by calcium influx through synaptic glutamate receptors and/or voltage-activated Ca2+-channels (Faber, 2009).
Because polysynaptic corticostriatal responses after a single stimulus are more prolonged, and evoke more action potentials, in direct pathway SPNs (dSPNs) than in indirect pathway SPNs (iSPNs) (Bargas et al., 1991; Flores-Barrera et al., 2010, 2011), a logical follow up of our previous studies had the following goals: first, identify whether Ca2+-activated K+-currents participate in the polysynaptic corticostriatal response of SPNs (Vizcarra-Chacon et al., 2013). Second, determine the relative importance of these currents in suprathreshold synaptic integration. Third, observe whether there is a difference in the roles of BK and SK currents in these responses. And finally, find out if these currents can explain the difference in duration between the corticostriatal polysynaptic responses of dSPNs and iSPNs.
Materials and Methods
Slice Preparation
All experiments were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care Committee of the Universidad Nacional Autónoma de México. D1 and D2 dopamine receptors-eGFP BAC transgenic mice, between postnatal days 30–60 (PD30-60; FVB background, developed by the GENSAT project) were used. Adult Wistar rats were also used to detect possible inconsistencies. The number of animals employed in the experimental samples was near the minimal possible to attain robust reproducible results and statistical significance. Animals were anesthetized with ketamine/xylazine. Their brains were quickly removed and placed into ice cold 4°C) cerebrospinal fluid (CSF) containing (in mM): 126 NaCl, 3 KCl, 25 NaHCO3, 1 MgCl2, 2 CaCl2, 11 glucose, 300 mOsm/L, pH = 7.4 with 95% O2 and 5% CO2. Parasagittal neostriatal slices (250–300 μm thick) were cut using a vibratome and stored in oxygenated bath CSF at room temperature for at least 1 h before recording.
Electrophysiological Recordings
Whole-cell patch-clamp recordings were performed with micropipettes made with borosilicate glass, fire polished for D.C. resistances of about 3–6 MΩ. Internal solution was (in mM): 120 KMeSO4, 2 MgCl2, 10 HEPES, 10 EGTA, 1 CaCl2, 0.2 Na2ATP, 0.2 Na3GTP, and 1% biocytin. Some recordings were carried out using sharp microelectrodes (80–120 MΩ) filled with 1% biocytin and 3M potassium acetate fabricated from borosilicate-glass (Flores-Barrera et al., 2010). Slices were superfused with CSF at 2 ml/min (34–36°C). Recordings were digitized and stored with the aid of software designed in the laboratory by one of the co-authors in the LabView environment (National Ins., Austin, TX, USA). To have an approximation of whole neuronal input resistance (RN) and changes in bridge balance, a small current pulse of −20 mV was delivered to the soma before the orthodromic responses were obtained at intervals over 20 s. Changes over 20% in the responses to this pulse or in the D.C. current to maintain the holding potential in current-clamp mode made us to discard the experiments. Sampling rate or digitizing procedures made difficult to have both the slow corticostriatal responses and full-blown trains of sodium spikes at the same time, so that some spikes look truncated in some traces. Cells with resting potential more negative than −70 mV (at zero current) and RN about 100–200 MΩ were chosen (e.g., Salgado et al., 2005).
Drugs were dissolved in the CSF from stock solutions made daily. Recordings were carried out in the dorsal striatum. Stimulation was performed with concentric bipolar electrodes (tip = 50 μm) in the cortex (>250 μm from the callosum). Synaptic responses were evoked by a single square pulse of 0.1 ms. The cell membrane potential was held at −80 mV. A series of current pulses of increasing intensities were used. Responses obtained with suprathreshold stimulus strength (2× threshold) were compared (Vizcarra-Chacon et al., 2013). Current-clamp data were obtained to observe the most physiological response. eGFP-positive and negative neurons from D1 and D2 eGFP animals were compared. After recordings, neurons were injected with biocytin. eGFP-positive visualization was observed on a confocal microscope as previously described (Flores-Barrera et al., 2010).
The following drugs: apamin and charybdotoxin were obtained from Alomone Labs (Israel). NS 1619 (BK channel activator), CyPPA, and NS 309 (SK channel activators) were acquired from TOCRIS (R&D Systems, Minneapolis, MN, USA).
Statistics
Statistical values are presented as mean ± s.e.m. Digital subtraction was used to obtain time courses of the components enhanced or decreased by the different peptides and drugs. Main parameter reported was “half width” (duration at 50% of maximal response amplitude) because it comprises both the increase in response duration and the increase in response amplitude. Kruskal-Wallis tests with post-hoc Dunn tests were used to compare samples with different treatments. Statistical difference was accepted when P < 0.05. Intensity-response (I-R) plots where built using as response the average magnitude of the half width as a function of stimulus strength. Data were fitted to the following sigmoid function using a non-linear Marquardt algorithm:
where R(i) is the response as a function of stimulus intensity normalized to threshold units, Rmax is the maximal half width attained, k is the slope factor and ih is the stimulus intensity necessary to attain a response of half magnitude. In this work, the value reported is Rmax. The function fits to average experimental values and their 95% confidence intervals are plotted as colored bands (Tecuapetla et al., 2005; López-Huerta et al., 2013) while standard errors of average responses are plotted as usual: lines with the symbols.
Results
SPNs were recorded from PD30-60 BAC D1 or D2 eGFP mice and from rats of equivalent age (n = 77). We previously reported that suprathreshold corticostriatal responses are more prolonged and evoke more action potentials in dSPNs than in iSPNs (Flores-Barrera et al., 2010) and that their duration depends on polysynaptic activity (Vizcarra-Chacon et al., 2013) involving both cortical and striatal neurons (a feed-forward circuit) as well as intrinsic currents (Vergara et al., 2003; Flores-Barrera et al., 2009, 2011).
Blockade of BK and SK Channels Depolarize and Prolong Corticostriatal Responses in Striatal Projection Neurons
Red trace in Figure 1A is a control corticostriatal response in a dSPN. The superimposed blue trace is a response obtained with the same stimulus and in the same cell after adding 20 nM charybdotoxin (ChTx), a blocker of BK-channels, to the bath CSF: the response exhibited an additional depolarization that prolonged the response and the number of spikes. The superimposed black trace is the corticostriatal response to the same stimulus after adding 100 nM apamin, a blocker of SK-channels, in the continuous presence of ChTx. This response was even more depolarized, more prolonged and the number of action potentials fired increased, showing that Ca2+-activated K+-currents control the level and duration of corticostriatal responses which physiologically compose the depolarized up-states (Stern et al., 1997; Vergara et al., 2003; Vautrelle et al., 2009) of SPNs. At the bottom, digital subtractions of the hyperpolarizing influences elicited by Ca2+-activated K+-currents that were suppressed by the actions of these peptides are illustrated (ChTx in blue and apamin plus ChTx in black). Note that hyperpolarizations induced by Ca2+-activated K+-currents rise slowly and lasts hundreds of milliseconds, suggesting that, after synaptic activation, Ca-entry takes some time to activate BK- and SK-channels in dSPNs. The parameter that encompasses both the depolarization and the increase in duration produced by the peptides is the half width of the response (duration at half amplitude). Figure 1B shows that half width as a function of stimulus intensity (in threshold units) could be used to build I-R plots, which could be fitted to sigmoid functions (see Materials and Methods): continuous traces represent the average fit of several similar experiments and surrounding shadowed areas represent 95% confidence intervals. Symbols with lines represent individual average responses ± s.e.m. to each stimulus. Note that peptides, administered jointly, or independently, change I-R plots with respect to the control, although all plots tend to reach saturation. Box plots in Figure 1C summarize the actions of both peptides in the experimental samples (because not all distributions approached normality, free-distribution statistical tests were used for comparisons; see Materials and Methods), including a sample where both peptides were applied together in either order at 2× threshold strength (saturation). Half width in the control was (mean ± s.e.m.): 296 ± 26 ms (n = 24, in Red), after ChTx it was 474 ± 40 ms or 60% increase (n = 10; in blue, *P < 0.05), after apamin it was 370 ± 53 ms or an increase of 25% (in purple, n = 10; *P < 0.05), and with both peptides applied together it was 773 ± 26 ms or a 161% increase (in black, n = 6; **P < 0.02). Fitted values of the same parameter (e.g., Rmax for maximal half width; see Materials and Methods) were not significantly different to experimental measurements (Figure 1B) and therefore are not included here to avoid redundancy.
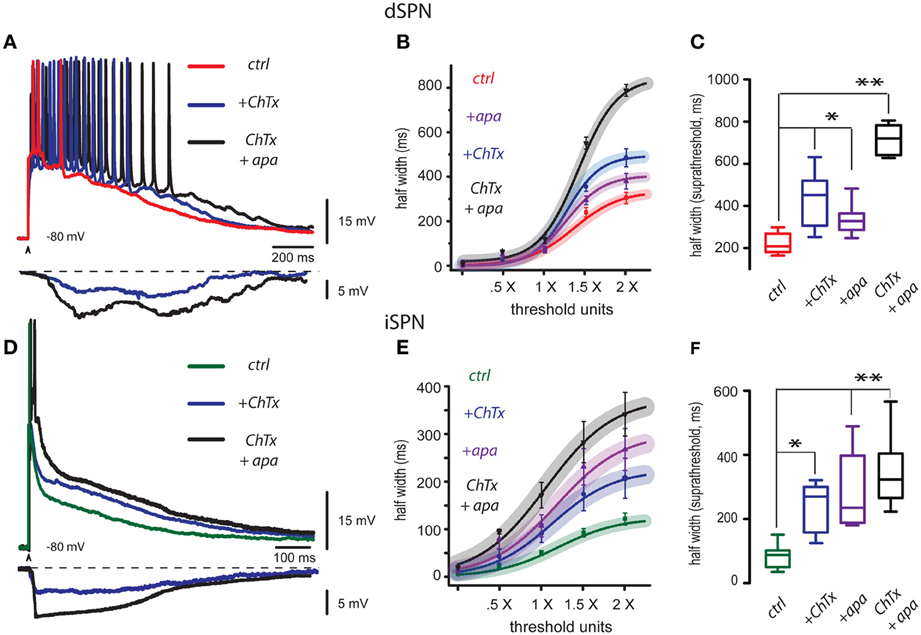
Figure 1. Blockade of BK and SK channels depolarize and prolong corticostriatal responses in striatal projection neurons. (A) Three superimposed records from a dSPN were obtained without changing stimulus strength: they show a corticostriatal response in control conditions (red trace), the same response in the presence of the blocker of BK-channels, 20 nM charybdotoxin (ChTx, blue trace), and finally, the same response in the presence of both ChTx and the blocker of SK channels, 100 nM apamin (ChTx + apamin, black trace). Each blocker depolarized and prolonged the response, and also increased the number of action potentials that were fired. Digital subtractions at bottom illustrate the hyperpolarizing influence that Ca2+-activated K+-currents exerted over the corticostriatal responses and that were suppressed by the blockade of ChTx (blue) and apamin (black). Note that the hyperpolarizing influence of Ca2+-activated K+-currents rise slowly and last hundreds of milliseconds thus contributing to restrain the build up of the corticostriatal response. (B) Intensity-response (I-R) graphs obtained by plotting average half width (duration at 50% of the peak amplitude of the response) as a function of threshold intensity including: minimal, subthreshold (0.5×), threshold (1.0×) and suprathreshold responses (2.0×). A sigmoid function was fitted. Curves with the action of each blocker are plotted individually as well as the administration of both blockers together. Shadowed colored areas denote 95% confidence intervals, symbols depict average values of the samples for each stimulus strength ± s.e.m. (C) Tukey box plots illustrate the distributions of measurements for suprathreshold responses (half width at 2× threshold strength), in control, in the presence of each blocker, and in the presence of both blockers. Differences are significant: *P < 0.5 and **P < 0.01 with respect to the controls. (D) Three superimposed records from an iSPN were obtained without changing stimulus strength: they show a corticostriatal response in control conditions (green trace), the same response in the presence of the blocker of BK channels, 20 nM charybdotoxin (ChTx, blue trace), and finally, the same response in the presence of both ChTx and apamin (ChTx plus apamin, black trace). As in the case of dSPNs, each blocker increased response duration and depolarization (half width). Subtractions at the bottom show the hyperpolarizing influences exerted by Ca2+-activated K+-currents that were suppressed by charybdotoxin (ChTx, blue trace) and apamin plus ChTx (black trace). Note that hyperpolarization induced by Ca2+-activated K+-currents rise fast and then produced a sustained plateau hyperpolarization that restrains the corticostriatal response by hundreds of milliseconds until final return to holding potential. (E) Fitted I-R plots (half width) were graphed individually for each blocker and for both blockers administered together as in (B). (F) Box plots summarize the distributions of measurements for suprathreshold response in control, with each blocker, and with both blockers administered together. Differences are significant: *P < 0.5 and **P < 0.01 with respect to the control. Due to the intent of showing the slow responses some fast spikes are truncated by the digitizing procedure.
The same experiments were performed in iSPNs. Half width of iSPNs is known to be briefer than that from dSPNs (Flores-Barrera et al., 2010). These neurons commonly fire a single or brief high frequency burst of spikes at the beginning of the synaptic response. As above, Figure 1D shows three superimposed records: the green one was taken in control conditions, the blue trace was obtained with the same stimulus and in the same cell after addition of 20 nM charybdotoxin (+ChTx) and the black trace was obtained after addition of 100 nM apamin (+apa) in the continuous presence of charybdotoxin. Each peptide depolarized and prolonged the response (half width). At the bottom, subtractions of the hyperpolarizing influences elicited by Ca2+-activated K+-currents blocked by these peptides are illustrated (charybdotoxin in blue and ChTx plus apamin in black). Note that hyperpolarizions induced by Ca2+-activated K+-currents in iSPNs show a major difference when compared to those obtained from dSPNs: hyperpolarizations begin quickly, and then are followed by a plateau-like hyperpolarization that restrains the corticostriatal response by hundreds of milliseconds to slowly return to holding potential during the last half of the response. These responses suggest that in contrast to dSPNs, Ca-entry activates BK- and SK-channels immediately at the beginning of the synaptic response in iSPNs, suggesting that Ca-entry that induces them probably comes from all-or-none dendritic phenomena that produce the Ca-influx (Bargas et al., 1991; Flores-Barrera et al., 2010; Higley and Sabatini, 2010; Plotkin et al., 2011; Vizcarra-Chacon et al., 2013). Figure 1E shows fitted I-R plots for each peptide acting independently or together in either order. Continuous traces are the function fits for the set of experiments and surrounding shadowed correspond to 95% confidence intervals. Symbols with lines are average measurements ± s.e.m. Box plots in Figure 1F summarize the actions of both peptides in different samples, including the sample where both peptides were applied together in either order: at 2 × threshold strength, the control was 115 ± 13 ms (in green, n = 19), while after ChTx it was 204 ± 44 ms or a 77% increase (in blue, n = 7; *P < 0.05), and after apamin it was 267 ± 46 ms or a 132% increase (in purple, n = 8; **P < 0.02). Both peptides applied together rendered a half width of 344 ± 48 ms or a 199% increase (in black, n = 6; **P < 0.02).
Enhancement of BK and SK Channels Hyperpolarize and Shorten Corticostriatal Responses in Striatal Projection Neurons
Red trace in Figure 2A is a control corticostriatal response in a dSPN. The superimposed orange trace is the same response obtained with the same stimulus and in the same cell after adding 2.5 μM NS 309, an activator of SK-channels, to the bath CSF: response depolarization decreased and duration became briefer. 2.5 μM CyPPA had the same actions (not illustrated). Finally, the same response is shown in the presence of both NS 309 and the activator of BK-channels: 2.5 μM NS 1619 (black trace): there was a larger reduction of the response, repolarization was faster and number of action potentials fired was reduced to one. These actions show that a positive modulation of Ca2+-activated K+-currents may control the magnitude of the corticostriatal responses in dSPNs. At the bottom, subtractions illustrate the cortical-induced depolarizations suppressed by these drugs (NS 309 in orange and NS 1619 plus NS 309 in black). That is, enhancement of Ca2+-activated K+-currents may suppress a great part of the prolonged depolarizing corticostriatal response. Nonetheless, note that these depolarizing components begin and end slowly in dSPNs. Figure 2B shows that I-R plots produced by any enhancer or a combination of them were depressed with respect to the control. Box plots in Figure 2C summarize the actions of both enhancers in different experimental samples, including a sample where both drugs were applied together in either order at 2× threshold strength. Half width in the control was (mean ± s.e.m.): 302 ± 31 (n = 18, in red), after NS 1619 it was 57 ± 2 ms or a 81% decrease (n = 6; in pink, *P < 0.05), after NS 309 it was 65 ± 6 ms or a 78% decrease (in orange, n = 6; *P < 0.05), and with both activators applied together it was 54 ± 16 ms or a 82% decrease (in black, n = 5; *P < 0.05).
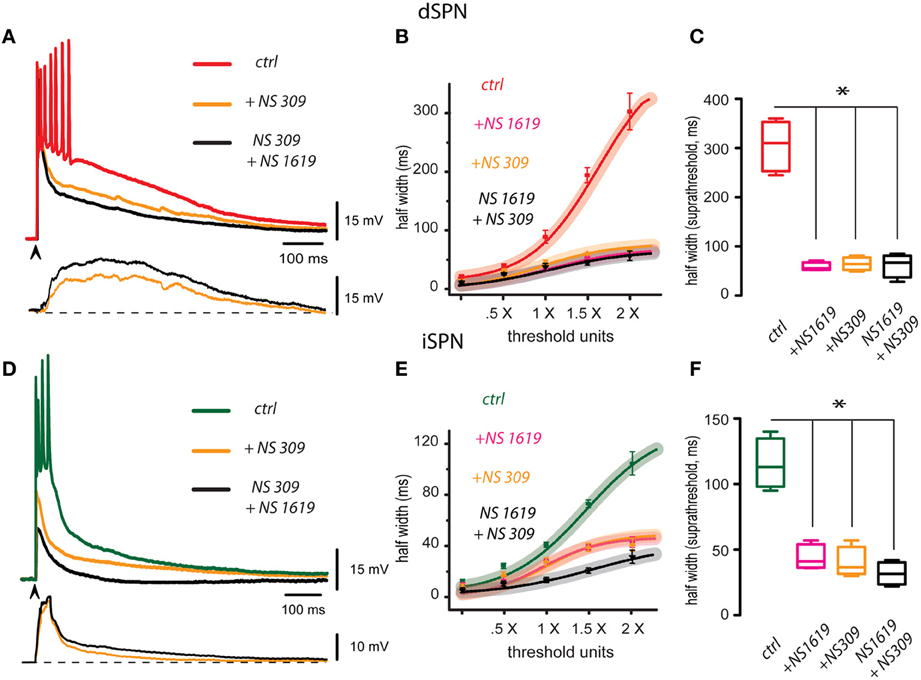
Figure 2. Enhancement of BK and SK channels hyperpolarizes and shortens corticostriatal responses in striatal projection neurons. (A) Three representative records from a dSPN were obtained in the same cell without changing stimulus strength (superimposed): the red trace shows a corticostriatal response in control conditions, the same response is shown in the presence of 2.5 μM NS 309 (orange trace), an activator of SK-channels, and finally, the same response is shown in the presence of both NS 309 and the activator of BK-channels, 2.5 μM NS 1619 (black trace). Each activator hyperpolarized the response, shortened its duration, and decreased the number of action potentials fired. Subtractions of the response depolarizations blocked by NS 309 (orange) and NS 309 plus NS 1619 (black) are shown at the bottom. Note that depolarizations blocked by these drugs begin slowly and last hundreds of milliseconds. Therefore, their blockade greatly reduced the suprathreshold responses (B). Fitted I-R plots with 95% confidence intervals and experimental averages ± s.e.m. Action of each drug is plotted individually as well as the administration of both activators together. (C) Box plots summarize the distribution of measurements for suprathreshold response (half width at 2×) in different samples: control, with each activator, and with both activators administered together. Samples differed significantly from the control (*P < 0.05). (D) Three superimposed records from an iSPN were obtained without changing stimulus strength: they show a corticostriatal response in control conditions (green trace), the same response in the presence of the activators of SK channels, 2.5 μM NS309 (orange trace), and finally, the same response in the presence of both NS309 and 2.5μM NS1619 (black trace). As in the case of dSPNs, each blocker decreased response duration and depolarization (half width). Subtractions of the depolarizations blocked by NS309 (orange) and NS309 plus NS1619 (black) are shown at the bottom. Note that depolarizations in this case had a sudden initiation and repolarization. (E) Intensity-response (I-R) graphs obtained by plotting half width (duration) at 50% of the peak amplitude of the response as a function of threshold intensity including subthreshold (0.5×), threshold (1.0×), and suprathreshold responses (2.0×). Action of each activator is plotted individually as well as the administration of both drugs together. (F) Box plots summarize the distribution of samples for suprathreshold response (half width at 2×) in control, with each activator, and with both drugs administered together. Differences were significant (*P < 0.05). Although some fast spikes are truncated by the digitizing procedure, note that in these cases some initial spikes exhibit inactivation due to their relative refractory periods.
Green trace in Figure 2D is a control corticostriatal response in an iSPN. The superimposed orange trace is the same response after adding 2.5 μM NS 309 (SK-channels enhancer). Note that response depolarization decreased, duration became briefer and less action potentials were fired. 2.5 μM CyPPA had the same actions (not illustrated). Also superimposed, the response of the same cell is shown in the presence of both NS 309 and 2.5 μM NS 1619 (black trace). Note that cell response was reduced to the point of becoming subthreshold. These actions show that Ca2+-activated K+-currents may modulate corticostriatal responses in iSPNs. At the bottom, subtractions showing the depolarizing components of the corticostriatal responses shunted by the enhancement of Ca2+-activated K+-currents are illustrated (NS 309 in orange and NS 1619 plus NS 309 in black). Note that actions of these drugs begin and return faster, as compared to those from dSPNs. Figure 2E shows that I-R plots produced by any enhancer or a combination of them were depressed with respect to the control. Box plots in Figure 2F summarize the actions of both enhancers in the experimental samples, including a sample where both drugs were applied together in either order at 2× threshold strength. Half width in the control was (mean ± s.e.m.): 110 ± 9 ms (n = 10, in green), after NS 1619 it was 44 ± 4 ms or a 60% decrease (n = 6; in pink, *P < 0.05), after NS 309 it was 40 ± 5 ms or a 62% decrease (in orange, n = 6; *P < 0.05), and with both activators applied together it was 38 ± 16 ms or a 64% decrease (in black, n = 4; *P < 0.05).
To conclude, in both classes of SPNs Ca2+-activated K+-currents control the level of depolarization and duration of the prolonged polysynaptic corticostriatal response during synaptic integration. These results strongly suggest that intrinsic currents such as Ca2+-activated K+-currents may control the influence of polysynaptic entries and their consequent activation of intrinsic currents contributing to up-states duration. However, a main difference between both classes of SPNs is that in iSPNs, a much larger influence of Ca2+-activated K+-currents appears to be present, triggered at the initiation of the synaptic inputs and immediately as in iSPNs. Next, we studied the influence of Ca2+-activated K+-currents in subthreshold synaptic responses, before action potentials were elicited, to see if there is a difference between both neurons classes.
Ca2+-Activated K+-Currents Control Subthreshold Synaptic Inputs in iSPNs
Figures 3A,B illustrate subthreshold synaptic potentials recorded after cortical stimulation in a dSPN and an iSPN in three different conditions: control (red and green traces for dSPN and iSPN, respectively), after addition of 100 nM apamin to block SK-channels (purple trace), and after addition of 20 nM ChTx in the continuous presence of apamin to block both SK- and BK-components of Ca2+-activated K+-currents (black traces). Note that in the case of dSPNs, calcium influx during subthreshold synaptic events does not appear to activate much Ca2+-activated K+-currents since the subthreshold synaptic potentials are very little affected. In comparison, blockade of Ca2+-activated K+-currents in iSPNs greatly alters the amplitude of subthreshold synaptic events, suggesting that calcium-influx capable to activate these currents can be generated with a minimum of active synapses (Higley and Sabatini, 2010). Because these events most probably occur in dendritic spines, we conclude that calcium influx during subthreshold synaptic events is enough to activate Ca2+-activated K+-currents in iSPNs, but not in dSPNs. Figures 3C,D show similar experiments with the enhancers of Ca2+-activated K+-currents. First, subthreshold synaptic potentials were recorded in control conditions (red and green traces for dSPN and iSPN, respectively), then the same events were recorded during enhancement of SK-channels (orange traces, NS 309), and finally, the same events were recorded during enhancement of both SK- and BK-current components (black traces, with NS 309 plus NS 1619). Enhancers of Ca2+-activated K+-currents do affect the amplitude of subthreshold synaptic potentials in dSPNs, showing that, if activated by some other means (more or larger synaptic potentials or spatial summation, as in suprathreshold responses), the channels that carry these currents are near enough to shunt synaptic inputs. However, enhancers of Ca2+-activated K+-currents reduce subthreshold synaptic potentials induced in iSPNs in such an extent as to almost completely block the synaptic event. These results suggest that in iSPNs, synaptic inputs may activate Ca2+-activated K+-currents augmenting the action of the enhancers and exerting a much larger effect as compared to dSPNs.
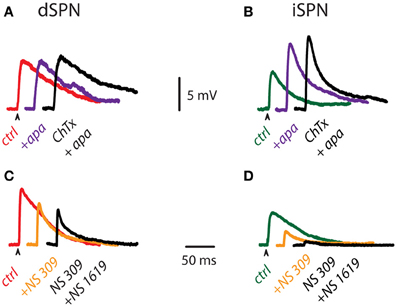
Figure 3. Influence of Ca2+-activated K+-currents on subthreshold corticostriatal responses. (A) Subthreshold synaptic potentials recorded after cortical stimulation in a dSPN in control conditions (red), after addition of 100 nM apamin (purple) and after addition of 20 nM ChTx in the continuous presence of apamin (black). (B) The same experiment was performed on an iSPN (control condition is in green). Note that Ca2+-activated K+-currents appear to exert more influence on subthreshold synaptic events of iSPNs. (C) Subthreshold synaptic potentials recorded after cortical stimulation in a dSPN in control conditions (red), after addition of 2.5 μM NS 309 (orange), an enhancer of SK-channels, and after addition of 2.5 μM NS 1619, an enhancer of BK-channels, in the continuous presence of NS 309 (black). (D) The same experiment performed on an iSPN (control condition is in green). Enhancers of Ca2+-activated K+-currents also indicate more influence of these currents in subthreshold synaptic events of iSPNs.
We conclude that even at subthreshold levels, Ca-entry during synaptic potentials in iSPNs is powerful enough to activate Ca2+-activated K+-currents and then shunt synaptic entries, to a degree that, during suprathreshold polysynaptic events (Vizcarra-Chacon et al., 2013) their duration and depolarization (half width) are greatly reduced in comparison to the same events generated in dSPNs. To further support these conclusions, we tried to control the corticostriatal suprathreshold responses in these neurons by manipulating their Ca2+-activated K+-currents.
Controlling Voltage Trajectories of Corticostriatal Responses
The question underlying this series of experiments is: what are the main determinants of the different voltage trajectories found in dSPNs, as compared with iSPNs, during corticostriatal responses? Figure 4A shows that when the corticostriatal response from a dSPN is subject to an enhancer of a Ca2+-activated K+-current (BK-channels component enhanced by NS 1619 in this case), the response acquires a faster repolarization, shortens its train of spikes and becomes similar to a typical corticostriatal response from an iSPN. This result suggests that the reason why dSPNs responses are slower than those from iSPNs is a decreased activation of Ca2+-activated K+-currents. Conversely, Figure 4B shows that when the corticostriatal response from an iSPN, exhibiting a brief train of spikes and a faster repolarization, is subject to a blocker a Ca2+-activated K+-current (SK-channels component blocked by apamin in this case), the response is prolonged, its repolarization retarded and its train of spikes increases in duration, becoming similar to a corticostriatal response from a dSPN. This result support the inference that the reason why iSPN responses are faster than those from dSPNs is an increased activation of Ca2+-activated K+-currents. Superimposition of the traces in each condition supports these hypotheses (Figures 4C,D).
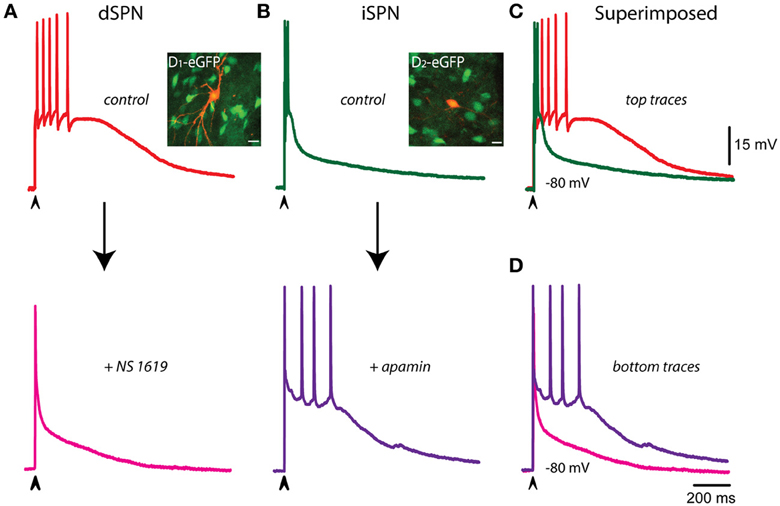
Figure 4. Controlling voltage trajectories of corticostriatal responses: converting responses from dSPNs or iSPNs into one another. (A) Control corticostriatal response in a dSPN (top). The arrow indicates the addition of 2.5 μM of the of the BK-channel activator NS 1619. Note that repolarization was enhanced and half width decreased, the corticostriatal response becoming comparable to that of an iSPN in control conditions (bottom). Inset: immunocytochemical preparation showing neurons from a BAC-D1 eGFP mouse (green) and the recorded neuron filled with biocytin (orange). (B) Control corticostriatal response in an iSPN. The arrow indicates the addition of 100 nM of the SK-channel blocker apamin. Note that fast repolarization and the firing of a brief burst of action potentials at the beginning of the response were changed by a response with increased duration (half width) and a more prolonged train of action potentials firing at lower frequency; comparable to those seen in control dSPNs. Inset: immunocytochemical preparation showing neurons from a BAC-D2 eGFP mouse (green) and the recorded neuron filled with biocytin (orange). (C) Superimposition of control dSPNs and iSPNs responses clearly shows larger half width for dSPNs. (D) After the actions of NS 1619 and apamin in dSPNs and iSPNs, respectively, half width of iSPNs became larger than that of a dSPNs, strongly suggesting that the shape of the corticostriatal responses in these neurons depend on Ca2+-activated K+-currents. Experiment made by triplicate and with different blockers and enhancers. Although the digitizing procedure may show incomplete fast spikes, the increase in the amplitude of some spikes in the dSPN recording are due to longer interspike intervals (A, top). Note constancy of spikes amplitude in (B), bottom.
Ca2+-Activated K+-Currents and Propagation of Dendritic Autorregenerative Events in iSPNs
It has been shown that the reason why iSPNs fire a brief train of action potentials at the beginning of the corticostriatal response and then repolarize faster than dSPNs, is the presence of autoregenerative events in their dendrites: the brief train of action potentials rides on top of autoregenerative events that are elicited as local dendritic responses (Carter and Sabatini, 2004; Day et al., 2008; Flores-Barrera et al., 2010). However, in some occasions dendritic autoregenerative evens propagate to the somatic compartment inactivating the brief burst of spikes (Flores-Barrera et al., 2010). It is out of the scope of the present report to find out what is the origin and ionic constitution of these regenerative events in iSPNs (but see: Higley and Sabatini, 2010). However, several independent reports using different methods have posited that different classes of voltage-activated calcium channels as well as NMDA channels may be the origin of these events (Sabatini and Svoboda, 2000; Sabatini et al., 2002; Carter and Sabatini, 2004; Carter et al., 2007; Higley and Sabatini, 2008; Flores-Barrera et al., 2009; Plotkin et al., 2011). Moreover, channels that carry Ca2+-activated K+-currents have been shown to be present in dendritic spines or nearby dendrites in many neuron classes (Wolfart and Roeper, 2002; Cai et al., 2004; Bond et al., 2005; Ngo-Anh et al., 2005; Gu et al., 2008; Benhassine and Berger, 2009; Faber, 2009, 2010; Lujan et al., 2009; Hopf et al., 2010; Allen et al., 2011; Hosy et al., 2011), and data from striatal neurons is very suggestive that, at least in iSPNs, a tight synaptic association between synaptic, voltage-activated Ca2+-channels and Ca2+-activated K+-channels may be present (Day et al., 2008; Higley and Sabatini, 2008; Hopf et al., 2010).
The above described phenomena suggest that Ca2+-activated K+-currents may control autoregenerative events initiated by dendritic synaptic inputs, thus interfering with their propagation to the somatic-axonal compartments at most times. An experiment showing that this inference may be true is shown in Figure 5A: the green trace shows a couple of actions potentials riding on top of a suspected dendritic regenerative event taking place during synaptic activation. The subsequent addition of 100 nM apamin discloses the regenerative event by promoting its propagation to the soma (purple trace). Further addition of 20 nM ChTx in the continuous presence of apamin (black trace) makes this event more prolonged and capable to induce the firing of fast spikes. These results suggest that Ca2+-activated K+-currents were preventing autoregenerative events in the dendrites to propagate to the somatic compartment. In addition, in iSPNs, autoregenerative events sometimes take the place instead of the initial bursts of sodium spikes (Flores-Barrera et al., 2010). Green trace in Figure 5B illustrates one such a case. It is also shown that addition of NS 309 (orange trace) rapidly abolished the propagation of the regenerative event producing a fast repolarization. These experiments suggest that Ca2+-activated K+-currents control synaptic integration in SPNs, in particular, they avoid propagation of regenerative events to the somatic area, secluding them within dendritic compartments. Their propagation would make inefficient the generation and propagation of a burst of action potentials out to the axon.
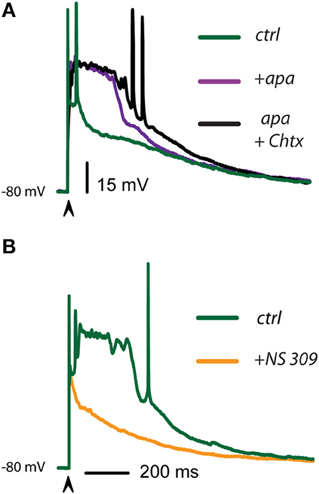
Figure 5. Ca2+-activated K+-currents control the propagation of autoregenerative potentials in iSPNs. (A) Green trace shows a control suprathreshold corticostriatal response in an iSPN. Addition of 100 nM apamin discloses an autoregenerative and propagated action potential whose main ionic component is Ca2+ (purple trace; Bargas et al., 1991). Subsequent addition of 20 nM ChTx in the continuous presence of apamin further prolongs the duration of the regenerative event without increasing its amplitude, illustrating its all-or-none properties (black trace). (B) Green trace is a spontaneous regenerative event that sometimes appears in control suprathreshold responses in iSPNs (alternating with the initial burst of spikes). Addition of 2.5 μM NS 309 hindered the propagation of this event to the somatic area and accelerated the repolarization (orange trace). Clearly, fast spikes inactivate when calcium autoregenerative potentials propagate.
Discussion
Briefly, the present work shows that: (1) The SK and BK components of Ca2+-activated K+ currents present in SPNs (Pineda et al., 1992; Bargas et al., 1999; Vilchis et al., 2000), participate in shaping the corticostriatal polysynaptic responses. Basically, these currents participate in synaptic integration controlling the duration and depolarization of the responses. (2) The role of Ca2+-activated K+-currents is different in dSPNs as compared to iSPNs: in dSPNs, Ca2+-activated K+ currents produce slow gradual responses, suggesting that a diffusion delay occurs between Ca-entry and K+-currents activation. In contrast, Ca2+-activated K+-current action measured in the same way in iSPNs is fast rising and thereafter maintained during a large portion of the response, suggesting that they are activated immediately at the beginning of the synaptic inputs. In fact, Ca2+-activated K+ currents appeared to regulate small subthreshold synaptic potentials in iSPNs (Higley and Sabatini, 2010) and much less so in dSPNs. (3) We show that Ca2+-activated K+-currents are main determinants of the time courses of the corticostriatal responses in SPNs: enhancement of these currents accelerated the repolarization of dSPNs making them to look as iSPNs. Correspondingly, their blockade prolongs the corticostriatal responses in iSPNs making them to look as dSPNs. Finally, (4) a main role is played by Ca2+-activated K+ currents in iSPNs. It is known that when these neurons are activated, they may elicit regenerative events, seen as all-or-none events that, when propagation ensues, can become full-blown calcium action potentials as recorded from the soma. When this occurs, the burst of action potentials that should go out to the axon is obliterated.
Outputs of dSPNs and iSPNs have Different Durations
Before BAC-mice were available, investigators averaged the output of SPNs thinking that different durations were a part of the same spectrum. However, BAC-D1/2 GFP mice have taught us that iSPNs and dSPNs have different excitable properties (Shen et al., 2007; Day et al., 2008; Kravitz et al., 2010; Gerfen and Surmeier, 2011), and that their responses to intracortical stimulation are differentially integrated (Flores-Barrera et al., 2010), even though responses to stimulation at the dendrites, with uncaged glutamate, and the glutamate receptors employed, do not seem different (Plotkin et al., 2011; Vizcarra-Chacon et al., 2013). However, once SPNs classes were sorted out, differences in duration of their physiological responses—trains of action potentials—were easily observed, even during extracellular recordings under certain conditions (optogenetic stimulation; e.g., see Figures 2E–H in: Kravitz et al., 2010). How to explain these differences in duration? In the present work we show that Ca2+-activated K+-currents are a main factor in explaining the duration of corticostriatal responses.
Apparently, dendritic Ca2+-activated K+-currents limit calcium influx and depolarization, making a negative feedback loop (Faber, 2009). Since a stronger calcium entry has been previously reported in iSPNs (Day et al., 2008), the functional importance of Ca2+-activated K+-currents in determining the threshold and duration of dendritic autoregenerative potentials (most probably due to Ca2+: Bargas et al., 1991; Carter and Sabatini, 2004) is fundamental to avoid their propagation in order to be able to generate the brief train of action potentials that goes out to the axon. The action of these currents at subthreshold levels shows that the calcium to activate them is available even with weak synaptic stimuli (Higley and Sabatini, 2010). However, at the level of dendritic spines, blockage of some Ca2+ sources could have paradoxical effects increasing the amplitude of synaptic events (Higley and Sabatini, 2010). But at suprathreshold levels, where up-states could arise, NMDA receptors, and SK channels are functionally coupled in the dendritic spines of hippocampal, amygdala, and striatal neurons (Bloodgood and Sabatini, 2005; Ngo-Anh et al., 2005; Lujan et al., 2009; Faber, 2010; Higley and Sabatini, 2010) to control the duration and depolarizing level of plateau potentials (Cai et al., 2004), limiting the influx of calcium through NMDA receptors and controlling the amount of excitation (Bond et al., 2005; Ngo-Anh et al., 2005; Faber, 2010; Tonini et al., 2013).
But why the activation of Ca2+-dependent K+-currents is faster in iSPNs than in dSPNs so that repolarization of responses in iSPNs makes them much briefer? Apparently, eliciting local autoregenerative responses in the dendrites, at the time of synaptic inputs, is more common in iSPNs than in dSPNs (Flores-Barrera et al., 2010; Higley and Sabatini, 2010). A brisk Ca2+ entry may explain the fast rise in the activation and actions of Ca2+-activated K+-currents, and therefore the faster repolarization of iSPNs after a brief burst of action potentials.
However, these are not the only intrinsic currents that participate in shaping the suprathreshold corticostriatal response or in eliciting autoregenerative responses in SPNs. Other inward currents participate. As for example, the slow inward current carried by CaV1 channels (Galarraga et al., 1997; Vergara et al., 2003; Carter and Sabatini, 2004; Flores-Barrera et al., 2011), a slow sodium component blocked by phenytoin and riluzole (Carrillo-Reid et al., 2009b), inward currents carried by CaV3 and CaV2.3 channels (Carter and Sabatini, 2004; Higley and Sabatini, 2010; Plotkin et al., 2011), all them besides the synaptically activated NMDA and kainate slow current components (Schiller and Schiller, 2001; Carter and Sabatini, 2004; Plotkin et al., 2011; Vizcarra-Chacon et al., 2013). But analyses have not been complete, other inward currents need to be confirmed or discarded in mature neurons as for example currents carried by TRP channels (Hill et al., 2006) or calcium-activated non-selective cation currents (ICAN; Mrejeru et al., 2011).
Further research is needed to see if different synapses (proximal, distal) use the same calcium source, or if there could be many sources being either the same or diverse for different synapses. It is known that voltage-gated Ca2+-channels and NMDA receptors differ in their distribution over spines and dendritic shafts. High calcium concentrations can only be reached within a few nanometers around the channel and dissipate within microseconds (Sabatini et al., 2002). So that the Ca2+-source used to activate Ca2+-dependent K+-currents may be very specific and precise (Vilchis et al., 2000). In contrast, NMDA-channels lead to a longer lasting calcium gradient extending across the spine during an excitatory postsynaptic potential (Sabatini and Svoboda, 2000; Keller et al., 2008). In any case, the Ca2+ source should be able to generate autoregenerative events to explain the differences in duration of the responses between dSPNs and iSPNs.
In view of the above, it should not seem strange that intrinsic outward currents should also participate in suprathreshold corticostriatal responses to damp or stop inward currents from reaching too strong depolarizations. In the present work we show that Ca2+-activated K+-currents are a component of the outward currents that fulfill this role. They have been described at the somatic (Bargas et al., 1999) and the dendritic levels (Ngo-Anh et al., 2005). However, several other outward currents may participate together with the synaptic GABAergic currents (Flores-Barrera et al., 2010) in order to control suprathreshold responses originated by converging polysynaptic cortical inputs known to outlast the initial stimulus and the monosynaptic responses by several hundreds of milliseconds (Vizcarra-Chacon et al., 2013).
It is known that these prolonged responses arise from the polysynaptic convergence of cortical and feed-forward striatal inputs because in current-clamp mode the glutamatergic response can clearly be divided into two parts, an early part, which is monosynaptic, and a late part, which by definition cannot be monosynaptic, but is blocked by AMPA-receptor antagonists (Vizcarra-Chacon et al., 2013), that is, it has to be polysynaptic. In addition, in voltage-clamp mode, at certain holding potentials, a late barrage of synaptic inputs keeps arriving during several hundreds of milliseconds after the initial monosynaptic response (Goldberg et al., 2012; Vizcarra-Chacon et al., 2013). Either stimulus strength or holding potential have to be changed for the activation of unclamped intrinsic currents by this synaptic barrage (Flores-Barrera et al., 2009). Finally, several striatal interneurons can be activated from the cortex (e.g., Vizcarra-Chacon et al., 2013) and the feed-forward circuit that involves other striatal cellular elements in the suprathreshold corticostriatal response of SPNs is well known (rev. in: Surmeier et al., 2011).
Conclusion
Ca2+-activated K+ channels, most probably located at the dendrites, explain the different durations between corticostriatal responses in dSPNs and iSPNs. Because polysynaptic corticostriatal responses (Vizcarra-Chacon et al., 2013) are the components of more prolonged up-states (Stern et al., 1997), an inference can be made: transmitters that modulate Ca2+-influx or Ca2+-activated K+-currents intervene in the regulation of up-states duration. This in turn regulates correlated firing among neuronal ensembles to produce network dynamics (Carrilo-Reid et al., 2008). Therefore, they may be a part of the explanation of the actions of modulatory transmitters at the striatal microcircuit (Carrillo-Reid et al., 2009a; Jáidar et al., 2010; Carrillo-Reid et al., 2011).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Antonio Laville, Gabriela X Ayala, and Ariadna Aparicio for technical support and advice and Dr. Claudia Rivera for animal care. This work was supported by the Consejo Nacional de Ciencia y Tecnología (México) grants 98004 and 154131 and by grants from Dirección General de Asuntos del Personal Académico. Universidad Nacional Autónoma de México: IN202914 and IN202814 to Elvira Galarraga and José Bargas, respectively. Mario A Arias-García has a CONACyT doctoral fellowship and data in this work are part of his doctoral dissertation in the Posgrado en Ciencias Biomédicas de la Universidad Nacional Autónoma de México. Jesús Pérez-Ortega was the author of the acquisition program used for stimulation and recording.
References
Allen, D., Bond, C. T., Luján, R., Ballesteros-Merino, C., Lin, M. T., Wang, K., et al. (2011). The SK2-Long isoform directs synaptic localization and function of K2-containing channels. Nat. Neurosci. 14, 744–749. doi: 10.1038/nn.2832
Bargas, J., Ayala, C. X., Vilchis, C., Pineda, J. C., and Galarraga, E. (1999). Ca2+-activated outward currents in neostriatal neurons. Neuroscience 88, 479–488. doi: 10.1016/S0306-4522(98)00211-5
Bargas, J., Galarraga, E., and Aceves, J. (1991). Dendritic activity on neostriatal neurons as inferred from somatic intracellular recordings. Brain Res. 539, 159–163. doi: 10.1016/0006-8993(91)90700-6
Benhassine, N., and Berger, T. (2009). Large-conductance calcium-dependent potassium channels prevent dendritic excitability in neocortical pyramidal neurons. Pflugers Arch. 457, 1133–1145. doi: 10.1007/s00424-008-0569-3
Bloodgood, B. L., and Sabatini, B. L. (2005). Neuronal activity regulates diffusion across the neck of dendritic spines. Science 310, 866–869. doi: 10.1126/science.1114816
Bond, C. T., Maylie, J., and Adelman, J. P. (2005). SK channels in excitability, pacemaking and synaptic integration. Curr. Opin. Neurobiol. 15, 305–311. doi: 10.1016/j.conb.2005.05.001
Cai, X., Liang, C. W., Muralidharan, S., Muralidharan, S., Kao, J. P., Tang, C. M., et al. (2004). Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44, 351–364. doi: 10.1016/j.neuron.2004.09.026
Carrillo-Reid, L., Hernández-Lopez, S., Tapia, D., Galarraga, E., and Bargas, J. (2011). Dopaminergic modulation of the striatal microcircuit: receptor-specific configuration of cell assemblies. J. Neurophysiol. 31, 4972–4983. doi: 10.1523/JNEUROSCI.3226-11.2011
Carrilo-Reid, L., Tecuapetla, F., Tapia, D., Hernández-Cruz, A., Galarraga, E., Drucker-Colin, R., et al. (2008). Encoding network states by striatal cell assemblies. J. Neurophysiol. 99, 1435–1450. doi: 10.1152/jn.01131.2007
Carrillo-Reid, L., Tecuapetla, F., Ibañez-Sandoval, O., Hernández-Cruz, A., Galarraga, E., and Bargas, J. (2009a). Activation of the cholinergic system endows compositional properties to striatal cell assemblies. J. Neurophysiol. 101, 737–749. doi: 10.1152/jn.90975.2008
Carrillo-Reid, L., Tecuapetla, F., Vautrelle, N., Hernandez, A., Vergara, R., Galarraga, E., et al. (2009b). Muscarinic enhancement of persistent sodium current synchronizes striatal medium spiny neurons. J. Neurophysiol. 102, 682–690. doi: 10.1152/jn.00134.2009
Carter, A. G., and Sabatini, B. L. (2004). State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron 44, 483–493. doi: 10.1016/j.neuron.2004.10.013
Carter, A. G., Soler-Llavina, G. J., and Sabatini, B. L. (2007). Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons. J. Neurosci. 27, 8967–8977. doi: 10.1523/JNEUROSCI.2798-07.2007
Day, M., Wokosin, D., Plotkin, J. L., Tian, X., and Surmeier, D. J. (2008). Differential excitability and modulation of striatal medium spiny neuron dendrites. J. Neurosci. 28, 11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008
Faber, E. S. (2009). Functions and modulation of neuronal SK channels. Cell Biochem. Biophys. 55, 127–139. doi: 10.1007/s12013-009-9062-7
Faber, E. S. (2010). Functional interplay between NMDA receptors, SK channels and voltage-gated Ca2+ channels regulates synaptic excitability in the medial prefrontal cortex. J. Physiol. 588, 1281–1292. doi: 10.1113/jphysiol.2009.185645
Flores-Barrera, E., Laville, A., Plata, V., Tapia, D., Bargas, J., and Galarraga, E. (2009). Inhibitory contribution to suprathreshold corticostriatal responses: an experimental and modeling study. Cell. Mol. Neurobiol. 29, 719–731. doi: 10.1007/s10571-009-9394-2
Flores-Barrera, E., Vizcarra-Chacón, B. J., Bargas, J., Tapia, D., and Galarraga, E. (2011). Dopaminergic modulation of corticostriatal responses in medium spiny projection neurons from direct and indirect pathways. Front. Syst. Neurosci. 5:15. doi: 10.3389/fnsys.2011.00015
Flores-Barrera, E., Vizcarra-Chacon, B. J., Tapia, D., Bargas, J., and Galarraga, E. (2010). Different corticostriatal integration in spiny projectionneurons from direct and indirect pathways. Front. Syst. Neurosci. 4:15. doi: 10.3389/fnsys.2010.00015
Galarraga, E., Hernández-López, S., Reyes, A., Barral, J., and Bargas, J. (1997). Dopamine facilitates striatal EPSPs through an L-type Ca2+ conductance. Neuroreport 8, 2183–2186. doi: 10.1097/00001756-199707070-00019
Galarraga, E., Vilchis, C., Tkatch, T., Salgado, H., Perez-Rosello, T., Tecuapetla, F., et al. (2007). Somatostatinergic modulation of firing pattern and calcium activated potassium currents in medium spiny neostriatal neurons. Neuroscience 146, 537–554. doi: 10.1016/j.neuroscience.2007.01.032
Gerfen, C. R., and Surmeier, D. J. (2011). Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466. doi: 10.1146/annurev-neuro-061010-113641
Goldberg, J. A., Ding, J. B., and Surmeier, D. J. (2012). Muscarinic modulation of striatal function and circuitry. Handb. Exp. Pharmacol. 208, 223–241. doi: 10.1007/978-3-642-23274-9_10
Grewe, B. F., Bonnan, A., and Frick, A. (2010). Back-propagation of physiological action potential output in dendrites of slender-tufted L5A pyramidal neurons. Front. Cell. Neurosci. 4:13. doi: 10.3389/fncel.2010.00013
Gu, N., Hu, H., Vervaeke, K., and Storm, J. F. (2008). SK (KCa2) channels do not control somatic excitability in CA1 pyramidal neurons but can be activated by dendritic excitatory synapses and regulate their impact. J. Neurophysiol. 100, 2589–2604. doi: 10.1152/jn.90433.2008
Hernández-López, S., Bargas, J., Reyes, A., and Galarraga, E. (1996). Dopamine modulates the afterhyperpolarization in neostriatal neurons. Neuroreport 7, 454–456. doi: 10.1097/00001756-199601310-00019
Higley, M. J., and Sabatini, B. L. (2008). Calcium signaling in dendrites and spines: practical and functional considerations. Neuron 59, 902–913. doi: 10.1016/j.neuron.2008.08.020
Higley, M. J., and Sabatini, B. L. (2010). Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat. Neurosci. 13, 958–967. doi: 10.1038/nn.2592
Hill, K., Tigue, N. J., Kelsell, R. E., Benham, C. D., McNulty, S., Schaefer, M., et al. (2006). Characterisation of recombinant rat TRPM2 and a TRPM2-like conductance in cultured rat striatal neurones. Neuropharmacology 50, 89–97. doi: 10.1016/j.neuropharm.2005.08.021
Hopf, F. W., Seif, T., Mohamedi, M. L., Chen, B. T., and Bonci, A. (2010). The small-conductance calcium-activated potassium channel is a key modulator of firing and long-term depression in the dorsal striatum. Eur. J. Neurosci. 31, 1946–1959. doi: 10.1111/j.1460-9568.2010.07231.x
Hosy, E., Piochon, C.Teuling, E., Rinaldo, L., and Hansel, C. (2011). SK2 channel expression and function in cerebellar Purkinje cells. J. Physiol. 589, 3433–3440. doi: 10.1113/jphysiol.2011.205823
Jáidar, O., Carrillo-Reid, L., Hernández, A., Drucker-Colín, R., Bargas, J., and Hernández-Cruz, A. (2010). Dynamics of the parkinsonian striatal microcircuit: entrainment into a dominant network state. J. Neurosci. 30, 11326–11336. doi: 10.1523/JNEUROSCI.1380-10.2010
Keller, D. X., Franks, K. M., Bartol, T. M. Jr., and Sejnowski, T. J. (2008). Calmodulin activation by calcium transients in the postsynaptic density of dendritic spines. PLoS ONE 3:e2045. doi: 10.1371/journal.pone.0002045
Kravitz, A. V., Freeze, B. S., Parker, P. R., Kay, K., Thwin, M. T., Deisseroth, K., et al. (2010). Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626. doi: 10.1038/nature09159
López-Huerta, V. G., Carrillo-Reid, L., Galarraga, E., Tapia, D., Fiordelisio, T., Drucker-Colín, R., et al. (2013). The balance of striatal feedback transmission is disrupted in a model of parkinsonism. J. Neurosci. 33, 4964–4975. doi: 10.1523/JNEUROSCI.4721-12.2013
Lujan, R., Maylie, J., and Adelman, J. P. (2009). New sites of action for GIRK and SKchannels. Nat. Rev. Neurosci. 10, 475–480. doi: 10.1038/nrn2668
Mrejeru, A., Wei, A., and Ramirez, J. M. (2011). Calcium-activated non-selective cation currents are involved in generation of tonic and bursting activity in dopamine neurons of the substantia nigra pars compacta. J. Physiol. 589, 2497–2514. doi: 10.1113/jphysiol.2011.206631
Ngo-Anh, T. J., Bloodgood, B. L., Lin, M., Sabatini, B. L., Maylie, J., and Adelman, J. P. (2005). SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat. Neurosci. 8, 642–649. doi: 10.1038/nn1449
Pérez-Garci, E., Bargas, J., and Galarraga, E. (2003). The role of Ca2+ channels in the repetitive firing of striatal projection neurons. Neuroreport 14, 1253–1256. doi: 10.1097/01.wnr.0000081861.45938.71
Pérez-Rosello, T., Figueroa, A., Salgado, H., Vilchis, C., Tecuapetla, F., Guzman, J. N., et al. (2005). The cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J. Neurophysiol. 93, 2507–2519. doi: 10.1152/jn.00853.2004
Pineda, J. C., Bargas, J., Flores-Hernández, J., and Galarraga, E. (1995). Muscarinic receptors modulate the afterhyperpolarizing potential in neostriatal neurons. Eur. J. Pharmacol. 281, 271–277. doi: 10.1016/0014-2999(95)00263-K
Pineda, J. C., Galarraga, E., Bargas, J., Cristancho, J. M., and Aceves, J. (1992). Charybdotoxin and apamin sensitivity of the calcium-dependent repolarization and the afterhyperpolarization in neostriatal neurons. J. Neurophysiol. 68, 287–294.
Plotkin, J. L., Day, M., and Surmeier, D. J. (2011). Synaptically driven state transitions in distal dendrites of striatal spiny neurons. Nat. Neurosci. 12, 881–888. doi: 10.1038/nn.2848
Sabatini, B. L., Oertner, T. G., and Svoboda, K. (2002). The life cycle of (Ca2+) ions in dendritic spines. Neuron 33, 439–452. doi: 10.1016/S0896-6273(02)00573-1
Sabatini, B. L., and Svoboda, K. (2000). Analysis of calcium channels in single spines using optical fluctuation analysis. Nature 408, 589–593. doi: 10.1038/35046076
Salgado, H., Tecuapetla, F., Perez-Rosello, T., Perez-Burgos, A., Perez-Garci, E., Galarraga, E., et al. (2005). A reconfiguration of CaV2 Ca2+ channels current and its dopaminergic D2 modulation in developing neostriatal neurons. J. Neurophysiol. 94, 3771–3787. doi: 10.1152/jn.00455.2005
Salkoff, L., Butler, A., Ferreira, G., Santi, C., and Wei, A. (2006). High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. 7, 921–931. doi: 10.1038/nrn1992
Schiller, J., and Schiller, Y. (2001). NMDA receptor-mediated dendritic spikes and coincident signal amplification. Curr. Opin. Neurobiol. 11, 343–348. doi: 10.1016/S0959-4388(00)00217-8
Shen, W., Tian, X., Day, M., Ulrich, S., Tkatch, T., Nathanson, N. M., et al. (2007). Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat. Neurosci. 10, 1458–1466. doi: 10.1038/nn1972
Stern, E. A., Kincaid, A. E., and Wilson, C. J. (1997). Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J. Neurophysiol. 77, 1697–1715.
Surmeier, D. J., Carrillo-Reid, L., and Bargas, J. (2011). Dopaminergic modulation of striatal neurons, circuits, and assemblies. Neuroscience 19, 3–18. doi: 10.1016/j.neuroscience.2011.08.051
Tecuapetla, F., Carrillo-Reid, L., Guzman, J. N., Galarraga, E., and Bargas, J. (2005). Different inhibitory inputs onto neostriatal projection neurons as revealed by field stimulation. J. Neurophysiol. 93, 1119–1126. doi: 10.1152/jn.00657.2004
Tonini, R., Ferraro, T., Sampedro-Castañeda, M., Cavaccini, A., Stocker, M., ichards, C. D., et al. (2013). Small-conductance Ca2+-activated K+ channels modulate action potential- induced Ca2+ transients in hippocampal neurons. J. Neurophysiol. 109, 1514–1524. doi: 10.1152/jn.00346.2012
Vautrelle, N., Carrillo-Reid, L., and Bargas, J. (2009). “Diversity of up-state voltage transitions during different network states,” in Cortico-Subcortical Dynamics in Parkinson Disease, ed K. Y. Tseng (New York, NY: Humana/Springer), 73–85. doi: 10.1007/978-1-60327-252-0_5
Vergara, R., Rick, C., Hernández-López, S., Laville, J. A., Guzman, J. N., Galarraga, E., et al. (2003). Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice. J. Physiol. 15, 169–182. doi: 10.1113/jphysiol.2003.050799
Vilchis, C., Bargas, J., Ayala, G. X., Galván, E., and Galarraga, E. (2000). Ca2+ channels that activate Ca2+ dependent K+ currents in neostriatal neurons. Neuroscience 95, 745–752. doi: 10.1016/S0306-4522(99)00493-5
Vilchis, C., Bargas, J., Pérez-Roselló, T., Salgado, H., and Galarraga, E. (2002). Somatostatin modulates Ca2+ currents in neostriatal neurons. Neuroscience 109, 555–567. doi: 10.1016/S0306-4522(01)00503-6
Vizcarra-Chacon, V. J., Arias-García, M. A., Pérez-Ramírez, M. B., Flores-Barrera, E., Tapia, D., Drucker-Colín, R., et al. (2013). Contribution of different classes of glutamate receptors in the corticostriatal polysynaptic responses from striatal direct and indirect projection neurons. BMC Neurosci. 14:60. doi: 10.1186/1471-2202-14-60
Wolf, J. A., Moyer, J. T., Lazarewicz, M. T., Contreras, D., Benoit-Marand, M., O'Donnell, P., et al. (2005). NMDA/AMPA ratio impacts state transitions and entrainment to oscillations in a computational model of the nucleus accumbens medium spiny projection neuron. J. Neurosci. 25, 9080–9095. doi: 10.1523/JNEUROSCI.2220-05.2005
Wolfart, J., and Roeper, J. (2002). Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J. Neurosci. 22, 3404–3413.
Womack, M. D., Chevez, C., and Khodakhah, K. (2004). Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar purkinje neurons. J. Neursci. 24, 8818–8822. doi: 10.1523/JNEUROSCI.2915-04.2004
Wynne, P. M., Puig, S. I., Martin, G. E., and Treistman, S. N. (2009). Compartmentalized β subunit distribution determines characteristics and ethanol sensitivity of somatic, dendritic, and terminal large conductance calcium-activated potassium channels in the rat central nervous system. J. Pharmacol. Exp. Ther. 329, 978–986. doi: 10.1124/jpet.108.146175
Keywords: Ca2+-activated K+-currents, BK channels, SK channels, corticostriatal pathway, medium spiny neurons, striatum, synaptic integration
Citation: Arias-García MA, Tapia D, Flores-Barrera E, Pérez-Ortega JE, Bargas J and Galarraga E (2013) Duration differences of corticostriatal responses in striatal projection neurons depend on calcium activated potassium currents. Front. Syst. Neurosci. 7:63. doi: 10.3389/fnsys.2013.00063
Received: 05 August 2013; Accepted: 15 September 2013;
Published online: 04 October 2013.
Edited by:
Alon Korngreen, BarIlan University, IsraelReviewed by:
James M. Tepper, Rutgers, The State University of NJ, USAEdward A. Stern, BarIlan University, Israel
Copyright © 2013 Arias-García, Tapia, Flores-Barrera, Pérez-Ortega, Bargas and Galarraga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elvira Galarraga, Departamento de Neurociencia Cognitiva, División de Neurociencias, Instituto de Fisiología Celular, Universidad Nacional Autónoma de México, Circuito Exterior S/N, Ciudad Universitaria, 04510 México City DF, México e-mail: egalarra@ifc.unam.mx