Resting state low-frequency fluctuations in prefrontal cortex reflect degrees of harm avoidance and novelty seeking: an exploratory NIRS study
- 1Department of Psychology, Graduate School of Education, Hiroshima University, Hiroshima, Japan
- 2Department of Psychiatry and Neurosciences, Institute of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan
- 3Faculty of Medicine, Hiroshima University, Hiroshima, Japan
- 4Department of Psychology, Faculty of Psychology, Otemon Gakuin University, Osaka, Japan
- 5Institute of Mental Health Research, University of Ottawa, Ottawa, ON, Canada
- 6Department of Neuropsychiatry, Kochi University, Kochi, Japan
Harm avoidance (HA) and novelty seeking (NS) are temperament dimensions defined by Temperament and Character Inventory (TCI), respectively, reflecting a heritable bias for intense response to aversive stimuli or for excitement in response to novel stimuli. High HA is regarded as a risk factor for major depressive disorder and anxiety disorder. In contrast, higher NS is linked to increased risk for substance abuse and pathological gambling disorder. A growing body of evidence suggests that patients with these disorders show abnormality in the power of slow oscillations of resting-state brain activity. It is particularly interesting that previous studies have demonstrated that resting state activities in medial prefrontal cortex (MPFC) are associated with HA or NS scores, although the relation between the power of resting state slow oscillations and these temperament dimensions remains poorly elucidated. This preliminary study investigated the biological bases of these temperament traits by particularly addressing the resting state low-frequency fluctuations in MPFC. Regional hemodynamic changes in channels covering MPFC during 5-min resting states were measured from 22 healthy participants using near-infrared spectroscopy (NIRS). These data were used for correlation analyses. Results show that the power of slow oscillations during resting state around the dorsal part of MPFC is negatively correlated with the HA score. In contrast, NS was positively correlated with the power of resting state slow oscillations around the ventral part of MPFC. These results suggest that the powers of slow oscillation at rest in dorsal or ventral MPFC, respectively, reflect the degrees of HA and NS. This exploratory study therefore uncovers novel neural bases of HA and NS. We discuss a neural mechanism underlying aversion-related and reward-related processing based on results obtained from this study.
Introduction
Temperament and character are the basic elements of personality that vary among individuals. In contrast to character, which is strongly influenced by experiential factors, temperament is probably more biologically based and stable across a person's life span. Harm avoidance (HA) and novelty seeking (NS) are temperament dimensions defined by the Temperament and Character Inventory (TCI), reflecting a heritable bias for responding intensely to aversive stimuli or for excitement in response to novel stimuli, respectively, (Cloninger, 1987; Cloninger et al., 1993). It is particularly interesting that extreme expression on these temperaments is associated with vulnerability to psychiatric disorders (Richter and Brandstrom, 2009). Increased levels of HA are thought to play a role as a risk factor for development of depression (Joffe et al., 1993; Richter et al., 2000; Farmer et al., 2003; Abrams et al., 2004; Smith et al., 2005; Celikel et al., 2009; Quilty et al., 2010) and anxiety disorders (Jylha and Isometsa, 2006; Mertol and Alkin, 2012). In contrast, a high level of NS is associated with increased risk of exhibiting substance abuse (Cloninger et al., 1988; Gerra et al., 2003) and pathological gambling disorder (Won Kim and Grant, 2001). Therefore, it is important to characterize the biological bases of these temperament traits widely, not only in terms of psychology but of psychiatry.
Neurally, HA and NS are known to be associated with resting state activities in various brain regions including prefrontal cortex (PFC). Positron-emission tomography (PET) reports have described that medial PFC (MPFC) glucose metabolism during resting state is negatively correlated with the HA score (Youn et al., 2002; Hakamata et al., 2006, 2009). Studies measuring cerebral blood flow (Sugiura et al., 2000; O'gorman et al., 2006) also tend to show negative correlation between HA score and activities within frontal regions including MPFC. Functional magnetic resonance imaging (fMRI) studies have demonstrated that functional connectivity between MPFC and amygdala is negatively correlated with the HA score (Li et al., 2012). In contrast, only a few studies have currently addressed the neural characteristics of NS trait from the perspective of resting-state activity. A single photon emission computed tomography (SPECT) study demonstrated that the resting state cerebral blood flow in anterior cingulate and insula are positively correlated with the NS score (Sugiura et al., 2000). Youn and colleagues reported that the NS score is positively associated with the glucose metabolic rate in the right PFC including MPFC (Youn et al., 2002). Taken together, resting state brain activity within MPFC is apparently an important neural basis underlying the temperament traits: HA and NS.
In recent years, interest in the brain's synchronous slow oscillations during a resting state has increased immensely, particularly in the field of psychiatry. Slow oscillations have been observed using measurements of different types, fMRI (Biswal et al., 1995; Fransson, 2006; Chepenik et al., 2010) and electroencephalography (Horovitz et al., 2008; Helps et al., 2010; Broyd et al., 2011; EEG). Although the mechanisms underlying the slow oscillations are not fully understood, slow oscillations of the fMRI blood oxygenation level-dependent (BOLD) signal are known to correlate with local field potentials (LFPs) in a broad frequency range (1–100 Hz) (He et al., 2008; Scholvinck et al., 2010; Pan et al., 2011, 2013; Wang et al., 2012b). Moreover, slow oscillations reportedly modulate higher-frequency activity (Canolty and Knight, 2010; Wang et al., 2012b; Valencia et al., 2013). It is particularly interesting that the slow oscillations have been used to identify the neural characteristics of psychiatric disorders such as major depression disorder (Wang et al., 2012a; Fan et al., 2013; Liu et al., 2013), anxiety disorders (Yin et al., 2011; Hou et al., 2012; Bing et al., 2013), and substance abuse (Jiang et al., 2011). Considering that HA and NS are reported as risk factors for these disorders, it would be interesting to address the question of whether these temperament traits correlate to the slow oscillation activities at rest. However, this question remains to be answered.
This preliminary study was undertaken to characterize the neural bases of temperament dimensions (i.e., HA and NS) by particularly addressing resting state low-frequency fluctuations using near-infrared spectroscopy (NIRS). This non-invasive technique uses near-infrared light to evaluate spatiotemporal characteristics of brain functions near the brain surface. As with fMRI and EEG, NIRS enables the detection of spontaneous slow oscillations in oxygenated hemoglobin (oxy-Hb) (Obrig et al., 2000). Based on earlier studies described above, we specifically focused on the examination of MPFC resting state activity. It is noteworthy that MPFC is characterized by large amplitudes of spontaneous slow oscillations during a resting state (Raichle et al., 2001; Fransson, 2005; Zou et al., 2008). TCI (Cloninger et al., 1993) was used to assess HA and NS temperament traits. We examined whether HA or NS is related with the power of resting-state slow oscillations in the MPFC.
Method
Participants
Twenty two healthy volunteer participants (12 males; age range = 21–27 years, mean age = 22.7 years) were recruited from Hiroshima University. All participants were right-handed, with normal or corrected-to-normal vision. All were free of neurological and psychiatric disorders. To control possible confounding factors of brain activity (Duncan and Northoff, 2012), participants who were habitual drinkers or taking medication were not recruited. Participants were not permitted to smoke tobacco from 3 h before the experiment started. Written informed consent was obtained from each participant before the investigation, in line with a protocol approved by the Research Ethics Committee of Hiroshima University. Each participant was paid a small fee for participating.
Self-Report Measures
Temperament traits including HA and NS were quantified using the TCI (Cloninger et al., 1993). The TCI is a 240-item questionnaire that assumes a human personality consisting of four temperament and three character dimensions. The temperament dimensions include HA, NS, reward dependence, and persistence. The character dimensions include self-directedness, cooperativeness, and self-transcendence. In this study, the measures of HA and NS were particularly addressed.
Resting States
After NIRS probe placement, participants were seated on a comfortable chair facing a computer screen in a dark shielded room. During recording, a chin rest was used to help participants maintain the head position. Participants performed counterbalanced resting eyes-closed (EC) and eyes-open (EO) baseline periods of 5 min each. Each participant was instructed to relax and allow the mind to disengage during these periods. During the EO resting state, participants were asked to gaze with fixation at a cross presented at the center of the computer screen, but were allowed to blink normally. Because the EC and EO resting states were thought to reflect baseline brain activity of different types (Marx et al., 2004; Barry et al., 2009; Yan et al., 2009), we included resting states of these two types in the present study. After each type of resting state measurement, participants were asked to fill out a questionnaire that included the question: “Did you fall asleep during the resting state scan?” No participant reported that they had fallen asleep during resting state recordings.
NIRS Data Acquisitions
Relative changes in the concentration of oxy-Hb and deoxy-Hb were measured using a multichannel NIRS imaging system (FOIRE-3000; Shimadzu Corp., Kyoto, Japan) with three wavelengths (780, 805, and 830 nm) of infrared light based on Matcher et al. (1995). The data sampling time was 115 ms. The source–detector probes were placed in fronto-temporal regions. The probe set was mounted on a cap for fixation (Figure 1B). The lower frontal probes were positioned along the Fp1–Fp2 line according to the international 10–20 system used for electroencephalography. The distance between pairs of source–detector probes was set at 3 cm. Each measuring area between the pairs of source–detector probes was defined as a channel. It is inferred that the machine, with source–detector spacing of 3 cm, measures points at 2–3 cm depth from the scalp [i.e., measurements are taken from the surface of the cerebral cortex; Hock et al. (1997); Toronov et al. (2001); Okada and Delpy (2003a,b)]. Because the exact optical path length is unknown, the unit used to measure these values is the molar concentration multiplied by length (mM•cm). The 43 measuring points were labeled as ch1–ch43 (see Figure 1A). Of 43 channels, 15 channels in MPFC regions (ch3, ch4, ch5, ch9, ch10, ch17, ch18, ch19, ch25, ch26, ch32, ch33, ch34, ch40, ch41) were used in correlation analyses (see below) for reasons described in the Introduction. Because of a technical problem, data of three channels (ch25, ch28, and ch41) from eight participants failed to record a signal. Unless otherwise indicated, 22 participants' data were used. Three-dimensional locations of the NIRS probe were measured using a Fastrak System (TX-2; Polhemus, USA). Using the MATLAB toolbox NFRI functions (http://www.jichi.ac.jp/brainlab/tools.html), statistical results for each channel were shown for the surface of a standardized brain (Singh et al., 2005).

Figure 1. (A) Approximate location of the NIRS channel positions in MNI space. The channel number shown in yellow denotes channels of interest for this study, where (B) shows the NIRS probe position.
NIRS Analysis
The NIRS data analysis was conducted using software (MATLAB 8.0; The MathWorks Inc., Natick, MA, USA). Resting state oxy-Hb data were filtered using a low-pass filter of 0.4 Hz. The linear trend caused by drift was removed (Tachtsidis et al., 2004). A Fast Fourier Transform (FFT) was performed on oxy-Hb data EC and EO resting state data. The Welch technique with a Hanning window of 1024 sample points (117.76 s sliding window) and an overlap of 512 points was used. Power spectral density (mM•cm2/Hz) was calculated for each channel over the range of 0.02–0.15 Hz. The Welch technique (Welch, 1967) involves sectioning the time-series data into many sub-sections and converting them to a modified estimate of the spectral density before averaging the signals of the sections. Subsequently, the band-limited power in the following two frequency bands was calculated based on previous studies (Obrig et al., 2000; Tachtsidis et al., 2004; Näsi et al., 2011; Pierro et al., 2012): very low-frequency oscillations (VLFO; 0.02–0.04 Hz) and low-frequency oscillations (LFO; 0.04–0.15 Hz). The VLFO and LFO are lower frequency ranges known to be differentiated from other oscillatory phenomena such as eye blinking, heart beat, and respiratory cycles (Obrig et al., 2000; Aminoff, 2012; Pierro et al., 2012; Sassaroli et al., 2012; Li et al., 2012).
Correlation Analysis
To investigate the relations between the temperament traits and resting state activity derived from 15 channels covering MPFC, we performed separate correlation analyses for each combination among temperament traits (HA, NS), different frequency band (VLFO, LFO), and resting states of two types (EC, EO). Before calculating Pearson correlation coefficients, outliers of each datum were excluded from the correlation analysis using an upper limit of the mean ± 3 SD of the participants' data. For cases in which there were outliers for Pearson's correlation analysis, we also calculated Spearman's rank correlation coefficient, which is insensitive to outliers, using all participants' data. In both correlation analyses, Benjamini and Hochberg (BH) false discovery rate (FDR) (Benjamini and Hochberg, 1995) was applied to avoid an increase in false positives for the 15 channels. A bootstrap procedure (Efron and Tibshirani, 1986) with n = 1000 resamples was used to establish 95% confidence intervals (CI) around the r value.
Results
Self-Report Data
The mean scores of HA and NS were, respectively, 51.41 (SD = 7.48, range = 35–65) and 48.73 (SD = 7.03, range = 36–63). No significant correlation was found between the HA and NS score (r = −0.37, p = 0.09, CI = –0.78–0.13).
Resting State Data
Resting state power spectrum density
Table 1 presents the averaged power across all NIRS channels for each resting-state condition (EC and EO) and for each frequency band (VLFO and LFO). The mean VLFO power of the EC resting state was 0.0005 mM•cm2/Hz (SD = 0.0002). That of the EO resting state was 0.0007 mM•cm2/Hz (SD = 0.0006). The mean LFO power of the EC resting state was 0.00008 mM•cm2/Hz (SD = 0.00004). That of the EO resting state was 0.0001 mM•cm2/Hz (SD = 0.00006). In both frequency bands, the EO resting state showed significantly greater power than the EC resting state did [VLFO, t(21) = 2.15, p = 0.04; LFO, t(21) = 2.98, p = 0.007]. These results resemble those reported from earlier studies (Obrig et al., 2000; Tachtsidis et al., 2004; Yan et al., 2009).
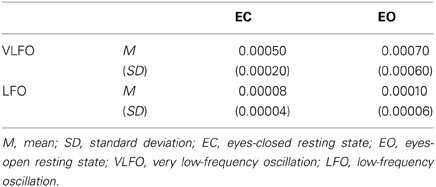
Table 1. Summary of averaged power (mM•cm2/Hz) across all NIRS channels for each resting state condition (EC and EO) and for each frequency band (VLFO and LFO).
These resting state data reported in this manuscript have been published previously (Nakao et al., 2013) and were included as part of a larger data collection. Nakao et al. (2013) reported the results for relations among the power of resting state slow oscillations, early life stress, and frontal activation during decision making tasks. The present manuscript describes a specific examination of the relations between the power of resting state slow oscillations and temperament traits (HA or NS).
Correlation between resting state and temperament scores
Figure 2A presents some correlation results between the powers of VLFO during EC resting state and the HA score. The power of VLFO at right dorsal MPFC (ch9, Brodmann area: BA9) was negatively correlated with the HA score (Pearson r = −0.61, FDR adjusted p < 0.05, CI = –0.80 to –0.34, N = 21; Spearman rs = –0.71, FDR adjusted p < 0.01CI = –0.88 to –0.41, N = 22). The power of VLFO or LFO during EC or EO resting state in other channels showed no significant correlation with the HA score.
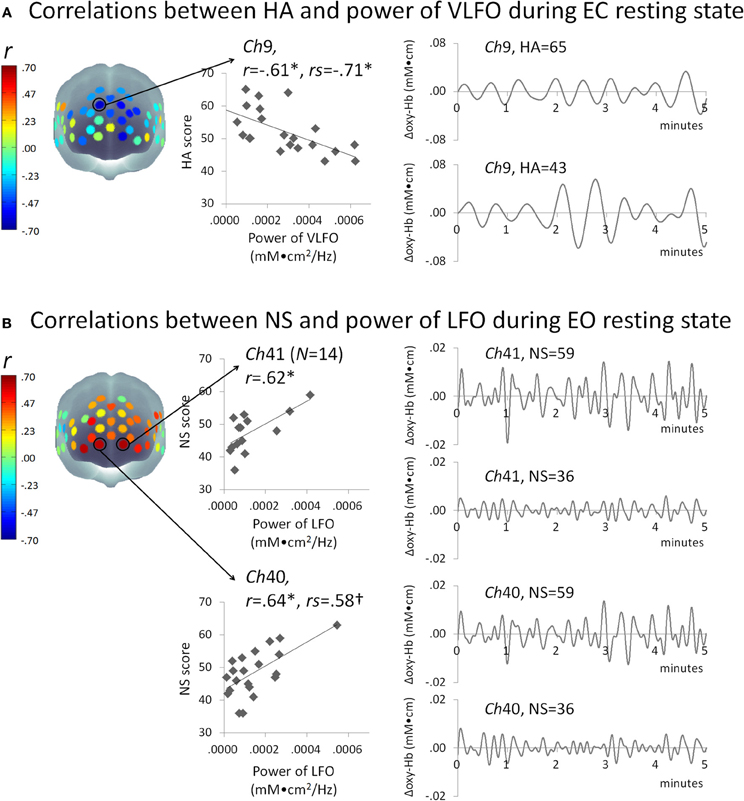
Figure 2. Schematic figure of correlation results and scatter plots between the powers of resting state slow oscillations (mM•cm2/Hz) and (A) HA or (B) NS score. Waveform plots shown at right are examples of time series data of each frequency range (VLFO, 0.02–0.04 Hz; LFO, 0.04–0.15 Hz) from individuals with high or low temperament trait scores. *FDR adjusted P < 0.05; †FDR adjusted P < 0.07; HA, harm avoidance; NS, novelty seeking; VLFO, very low-frequency oscillation; LFO, low-frequency oscillation; Ch, channel; r, Pearson's correlation coefficient; rs, Spearman's correlation coefficient.
Figure 2B presents the correlation results between the powers of LFO during EO resting state and NS score. The powers of LFO at bilateral ventral MPFC (ch40, BA10; ch41 BA10) were positively correlated with the NS score (ch40, Pearson r = 0.64, FDR adjusted p < 0.05, CI = 0.33–0.85, N = 21; Spearman rs = 0.58, FDR adjusted p < 0.07 CI = 0.19–0.79, N = 22; ch41, Pearson r= 0.62, FDR adjusted p < 0.05, CI = 0.21–0.90, N = 14). The power of VLFO or LFO during EC or EO resting state in other channels showed no significant correlation with NS score.
Discussion
This study was undertaken to investigate the relations between the power of slow oscillation during resting state and HA or NS. As Figure 2 shows, slow oscillations during resting state at the dorsal MPFC were negatively correlated with the HA score. In contrast, NS was correlated positively with resting-state slow oscillations around the ventral MPFC. These results provide new insights into the neural bases of HA or NS by particularly addressing low-frequency fluctuations.
Previous reports have described that HA is associated with decreased resting state cerebral blood flow (Sugiura et al., 2000; O'gorman et al., 2006) within frontal regions including dorsal MPFC. Although our index of resting state brain activity (i.e., the power of NIRS oxy-Hb slow oscillations) differed from those earlier studies, our results were consistent with those in that HA was found to be associated with the attenuated resting state activity in the dorsal regions of MPFC (Figure 2A). In contrast, our results showed that NS is associated with amplified resting state activation within ventral regions of the MPFC (Figure 2B). These results are consistent with those of previous studies which reported that the NS was associated with increased resting state glucose metabolism in the prefrontal regions including ventral MPFC (Youn et al., 2002). Consequently, these exploratory data provide new evidence that the neural bases of HA or NS can be assessed by low-frequency fluctuations during a resting state measured by NIRS, in addition to other indexes such as the glucose metabolism and cerebral blood flow. It would be interesting to investigate the relations among NIRS low frequency fluctuations and other measurements of brain activity (e.g., the glucose metabolism and cerebral blood flow) in terms of neural bases of temperament traits.
Considering our finding about the relation between HA and the power of resting state slow oscillation, resting state activity in dorsal MPFC might be related to aversion-related processing. Indeed, dorsal MPFC is known as a part of neural network activated by aversive stimuli (Hayes and Northoff, 2011, 2012). The dorsal MPFC is reported to serve an important role in sustaining fear response (Vidal-Gonzalez et al., 2006; Laurent and Westbrook, 2009; Furlong et al., 2010; Robinson et al., 2012). Laurent and Westbrook (2009) demonstrated that inactivation of the rat's paralimbic neurons, which are thought to have similar function with human dorsal MPFC in fear conditioning (Milad et al., 2007, 2009; Robinson et al., 2012), prevents fear response to conditioned aversive stimulus. In addition, Vidal-Gonzalez et al. (2006) demonstrated that microstimulation of that region increased fear response. Robinson et al. (2012) conducted a human fMRI study that showed that the functional connectivity between dorsal MPFC and amygdala was increased during the processing of fearful faces under anxious conditions, and that the amount of coupling was stronger in participants with higher trait anxiety. Based on this evidence, people with high HA personality are expected to show sustained fear response and greater activity in dorsal MPFC under aversive conditions. It would be interesting to examine whether and how the attenuated resting state activity in dorsal MPFC relates to the enhanced aversive-stimulus-induced activity in the same region in high HA people.
Ventral PFC, resting state activity of which correlated positively with NS, is known as a part of the reward-related network (Liu et al., 2011). The activity of ventral PFC is thought to represent the expected value of the outcome which guides reward-based decision making (Hampton and O'Doherty, 2007; O'doherty, 2007; Nakao et al., 2012). Bermpohl et al. (2008) revealed that people with high NS showed enhanced ventral MPFC activity during the expectancy of emotional stimuli. In the relation with resting state brain activity, Li et al. (2013) reported that the resting state functional connectivity in the reward-related network including ventral MPFC was associated with high impulsivity in decision making (i.e., higher preference for an immediate small reward than a larger delayed reward). It is possible that enhanced activity of ventral MPFC at rest observed in people with higher NS scores influences the intensity of the response to rewarding stimuli. Future studies must be undertaken to elucidate how resting state activity in ventral MPFC influences reward-based decision making.
Although we used TCI, which was developed to assess the seven dimensions of the psychobiological model of personality, another line of personality model exists: the five factor model (FFM; Costa and Maccrae, 1992). Neuroticism and extroversion are dimensions of the FFM. These are known to correlate, respectively, with HA and NS (Zuckerman and Cloninger, 1996; De Fruyt et al., 2000; Sher et al., 2000). Like HA, neuroticism is known to be associated with depression and anxiety disorders (Boyce et al., 1991; Rosellini and Brown, 2011). Similarly to NS, a higher extroversion score is associated with alcohol abuse (Flory et al., 2002; Merenäkk et al., 2003). Kunisato et al. (2011) and Wei et al. (2012) examined the relation between resting-state slow oscillation and neuroticism or extroversion using fMRI. They reported that extroversion correlated positively with the amplitude of slow oscillation in the prefrontal regions including ventral MPFC, which are similar to our results for NS. However, they reported no significant correlation between neuroticism and prefrontal regions, which is inconsistent with our results for HA. De Fruyt et al. (2000) reported that 23–51% of the variance of the TCI scales is explainable using the FFM, and concluded that although a substantial overlap exists between the TCI and the FFM, these two cannot be regarded as an equivalent tool to assess individual differences of personality. It would be interesting to examine the differences and similarities between the two personality models in terms of resting state brain activity.
Despite the importance of our data for revealing the neural bases of temperament traits, these findings leave several questions unresolved. First, although NIRS is expected to be useful to assess the bases of HA traits, it was impossible to address the question of how changes of the frontal power of slow oscillation in relation with HA traits are associated with the resting-state activity in the amygdala, where functional connectivity to the MPFC regions was reported previously to correlate to HA (Li et al., 2012; Wang et al., 2013). Additional fMRI studies are expected to be useful to provide further integrative understanding about the neural basis of temperament traits. Second, our data demonstrate that the HA correlated strongly with VLFO power during the EC resting state (Figure 2A), whereas the NS score correlated strongly with LFO power during the EO resting state (Figure 2B). However, although several studies addressed the differences in the frequencies of slow oscillation (Schroeter et al., 2004; Harrison et al., 2008) and the resting state eye conditions (Yang et al., 2007; Qin et al., 2013; Tan et al., 2013), the characteristics in brain function related to these frequencies/conditions remain poorly understood. Further studies investigating the characteristics of VLFO and LFO, and those of EC and EO resting states in the brain function are expected to contribute to the elucidation of the neural bases of temperament traits. Third, we did not record physiological data of eye blink, heat rate, or respiratory cycles because the ranges of slow oscillation can be differentiated from these artifacts (Obrig et al., 2000; Aminoff, 2012; Li et al., 2012; Pierro et al., 2012; Sassaroli et al., 2012). However, recording these artifact data and careful assessment of the pollution on cortical activity data are preferred for future study.
Conclusion
This study was undertaken to investigate the relations between temperament dimensions (i.e., HA and NS) and the power of slow oscillation in a resting state. We demonstrated a unique relation between them in that HA and NS are oppositely associated, respectively, with the power of slow oscillations in different subregions in the MPFC. These results suggest that the degrees of HA and NS might be predicted by the power of low-frequency fluctuations at rest. Further research on this matter must be conducted using data of more participants. Considering that both slow oscillation activity and temperament traits are involved in the pathophysiology of various psychiatric disorders, the results of this study are expected to be of great interest in the field not only of personality research but also that of psychiatric research. It would therefore be interesting to extend this study to the assessment of patients with such disorders. Beyond elucidating the neural bases of the temperament traits, this line of investigation is expected to contribute to improvement of our understanding of resting-state brain activity.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank N. Kanayama for his useful comments. This work was supported by the following grants: Hiroshima University Grants-in-Aid for a scientific research project to overcome stress-vulnerability; the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science and Technology of Japan; JSPS KAKENHI Grant Numbers 24390284 and 25870467.
References
Abrams, K. Y., Yune, S. K., Kim, S. J., Jeon, H. J., Han, S. J., Hwang, J., et al. (2004). Trait and state aspects of harm avoidance and its implication for treatment in major depressive disorder, dysthymic disorder, and depressive personality disorder. Psychiatry Clin. Neurosci. 58, 240–248. doi: 10.1111/j.1440-1819.2004.01226.x
Aminoff, M. J. (2012). Aminoff's Electrodiagnosis in Clinical Neurology,
Barry, R. J., Clarke, A. R., Johnstone, S. J., and Brown, C. R. (2009). EEG differences in children between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 120, 1806–1811. doi: 10.1016/j.clinph.2009.08.006
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300.
Bermpohl, F., Pascual-Leone, A., Amedi, A., Merabet, L. B., Fregni, F., Wrase, J., et al. (2008). Novelty seeking modulates medial prefrontal activity during the anticipation of emotional stimuli. Psychiatry Res. 164, 81–85. doi: 10.1016/j.pscychresns.2007.12.019
Bing, X., Ming-Guo, Q., Ye, Z., Jing-Na, Z., Min, L., Han, C., et al. (2013). Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Res. 1490, 225–232. doi: 10.1016/j.brainres.2012.10.048
Biswal, B., Yetkin, F. Z., Haughton, V. M., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541. doi: 10.1002/mrm.1910340409
Boyce, P., Parker, G., Barnett, B., Cooney, M., and Smith, F. (1991). Personality as a vulnerability factor to depression. Br. J. Psychiatry 159, 106–114. doi: 10.1192/bjp.159.1.106
Broyd, S. J., Helps, S. K., and Sonuga-Barke, E. J. S. (2011). Attention-induced deactivations in very low frequency EEG oscillations: differential localisation according to ADHD symptom status. PLoS ONE 6:e17325. doi: 10.1371/journal.pone.0017325
Canolty, R. T., and Knight, R. T. (2010). The functional role of cross-frequency coupling. Trends. Cogn. Sci. 14, 506–515. doi: 10.1016/j.tics.2010.09.001
Celikel, F. C., Kose, S., Cumurcu, B. E., Erkorkmaz, U., Sayar, K., Borckardt, J. J., et al. (2009). Cloninger's temperament and character dimensions of personality in patients with major depressive disorder. Compr. Psychiatry 50, 556–561. doi: 10.1016/j.comppsych.2008.11.012
Chepenik, L. G., Raffo, M., Hampson, M., Lacadie, C., Wang, F., Jones, M. M., et al. (2010). Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Res. 182, 207–210. doi: 10.1016/j.pscychresns.2010.04.002
Cloninger, C. (1987). A systematic method for clinical description and classification of personality variants: a proposal. Arch. Gen. Psychiatry 44, 573–588. doi: 10.1001/archpsyc.1987.01800180093014
Cloninger, C. R., Sigvardsson, S., and Bohman, M. (1988). Childhood personality predicts alcohol abuse in young adults. Alcohol. Clin. Exp. Res. 12, 494–505. doi: 10.1111/j.1530-0277.1988.tb00232.x
Cloninger, C. R., Svrakic, D. M., and Przybeck, T. R. (1993). A psychobiological model of temperament and character. Arch. Gen. Psychiatry 50, 975–990. doi: 10.1001/archpsyc.1993.01820240059008
Costa, P. T., and Maccrae, R. R. (1992). Revised NEO Personality Inventory (NEO PI-R) and NEO Five-Factor Inventory (NEO FFI): Professional Manual. Odessa, FL: Psychological Assessment Resources.
De Fruyt, F., Van De Wiele, L., and Van Heeringen, C. (2000). Cloninger's psychobiological model of temperament and character and the five-factor model of personality. Pers. Individ. Dif. 29, 441–452. doi: 10.1016/S0191-8869(99)00204-4
Duncan, N. W., and Northoff, G. (2012). Overview of potential procedural and participant-related confounds for neuroimaging of the resting state. J. Psychiatry Neurosci. 37:120059. doi: 10.1503/jpn.120059
Efron, B., and Tibshirani, R. (1986). Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1, 54–75. doi: 10.1214/ss/1177013815
Fan, T., Wu, X., Yao, L., and Dong, J. (2013). Abnormal baseline brain activity in suicidal and non-suicidal patients with major depressive disorder. Neurosci. Lett. 534, 35–40. doi: 10.1016/j.neulet.2012.11.032
Farmer, A., Mahmood, A., Redman, K., Harris, T., Sadler, S., and McGuffin, P. (2003). A sib-pair study of the temperament and character inventory scales in major depression. Arch. Gen. Psychiatry 60, 490–496. doi: 10.1001/archpsyc.60.5.490
Flory, K., Lynam, D., Milich, R., Leukefeld, C., and Clayton, R. (2002). The relations among personality, symptoms of alcohol and marijuana abuse, and symptoms of comorbid psychopathology: results from a community sample. Exp. Clin. Psychopharmacol. 10, 425–434. doi: 10.1037/1064-1297.10.4.425
Fransson, P. (2005). Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 26, 15–29. doi: 10.1002/hbm.20113
Fransson, P. (2006). How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44, 2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017
Furlong, T. M., Cole, S., Hamlin, A. S., and McNally, G. P. (2010). The role of prefrontal cortex in predictive fear learning. Behav. Neurosci. 124, 574–586. doi: 10.1037/a0020739
Gerra, G., Bassignana, S., Zaimovic, A., Moi, G., Bussandri, M., Caccavari, R., et al. (2003). Hypothalamic–pituitary–adrenal axis responses to stress in subjects with 3,4-methylenedioxy-methamphetamine (‘ecstasy’) use history: correlation with dopamine receptor sensitivity. Psychiatry Res. 120, 115–124. doi: 10.1016/S0165-1781(03)00175-6
Hakamata, Y., Iwase, M., Iwata, H., Kobayashi, T., Tamaki, T., Nishio, M., et al. (2006). Regional brain cerebral glucose metabolism and temperament: a positron emission tomography study. Neurosci. Lett. 396, 33–37. doi: 10.1016/j.neulet.2005.11.017
Hakamata, Y., Iwase, M., Iwata, H., Kobayashi, T., Tamaki, T., Nishio, M., et al. (2009). Gender difference in relationship between anxiety-related personality traits and cerebral brain glucose metabolism. Psychiatry Res. 173, 206–211. doi: 10.1016/j.pscychresns.2008.10.002
Hampton, A. N., and O'Doherty, J. P. (2007). Decoding the neural substrates of reward-related decision making with functional MRI. Proc. Natl. Acad. Sci. U.S.A. 104, 1377–1382. doi: 10.1073/pnas.0606297104
Harrison, B. J., Pujol, J., Ortiz, H., Fornito, A., Pantelis, C., and Yücel, M. (2008). Modulation of brain resting-state networks by sad mood induction. PLoS ONE 3:e1794. doi: 10.1371/journal.pone.0001794
Hayes, D. J., and Northoff, G. (2011). Identifying a network of brain regions involved in aversion-related processing: a cross-species translational investigation. Front. Integr. Neurosci. 5:49. doi: 10.3389/fnint.2011.00049
Hayes, D. J., and Northoff, G. (2012). Common brain activations for painful and non-painful aversive stimuli. BMC Neurosci. 13:60. doi: 10.1186/1471-2202-13-60
He, B. J., Snyder, A. Z., Zempel, J. M., Smyth, M. D., and Raichle, M. E. (2008). Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc. Natl. Acad. Sci. U.S.A. 105, 16039–16044. doi: 10.1073/pnas.0807010105
Helps, S. K., Broyd, S. J., James, C. J., Karl, A., Chen, W., and Sonuga-Barke, E. J. (2010). Altered spontaneous low frequency brain activity in attention deficit/hyperactivity disorder. Brain Res. 1322, 134–143. doi: 10.1016/j.brainres.2010.01.057
Hock, C., Villringer, K., Müller-Spahn, F., Wenzel, R., Heekeren, H., Schuh-Hofer, S., et al. (1997). Decrease in parietal cerebral hemoglobin oxygenation during performance of a verbal fluency task in patients with Alzheimer's disease monitored using near-infrared spectroscopy (NIRS)—correlation with simultaneous rCBF-PET measurements. Brain Res. 755, 293–303. doi: 10.1016/S0006-8993(97)00122-4
Horovitz, S. G., Fukunaga, M., De Zwart, J. A., Van Gelderen, P., Fulton, S. C., Balkin, T. J., et al. (2008). Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum. Brain Mapp. 29, 671–682. doi: 10.1002/hbm.20428
Hou, J., Wu, W., Lin, Y., Wang, J., Zhou, D., Guo, J., et al. (2012). Localization of cerebral functional deficits in patients with obsessive-compulsive disorder: a resting-state fMRI study. J. Affect. Disord. 138, 313–321. doi: 10.1016/j.jad.2012.01.022
Jiang, G. H., Qiu, Y. W., Zhang, X. L., Han, L. J., Lv, X. F., Li, L. M., et al. (2011). Amplitude low-frequency oscillation abnormalities in the heroin users: a resting state fMRI study. Neuroimage 57, 149–154. doi: 10.1016/j.neuroimage.2011.04.004
Joffe, R. T., Bagby, R. M., Levitt, A. J., Regan, J. J., and Parker, J. D. (1993). The tridimensional personality questionnaire in major depression. Am. J. Psychiatry 150, 959–960.
Jylha, P., and Isometsa, E. (2006). Temperament, character and symptoms of anxiety and depression in the general population. Eur. Psychiatry 21, 389–395. doi: 10.1016/j.eurpsy.2005.09.003
Kunisato, Y., Okamoto, Y., Okada, G., Aoyama, S., Nishiyama, Y., Onoda, K., et al. (2011). Personality traits and the amplitude of spontaneous low-frequency oscillations during resting state. Neurosci. Lett. 492, 109–113. doi: 10.1016/j.neulet.2011.01.067
Laurent, V., and Westbrook, R. F. (2009). Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn. Mem. 16, 520–529. doi: 10.1101/lm.1474609
Li, N., Ma, N., Liu, Y., He, X. S., Sun, D. L., Fu, X. M., et al. (2013). Resting-state functional connectivity predicts impulsivity in economic decision-making. J. Neurosci. 33, 4886–4895. doi: 10.1523/JNEUROSCI.1342-12.2013
Li, Y., Qin, W., Jiang, T., Zhang, Y., and Yu, C. (2012). Sex-dependent correlations between the personality dimension of harm avoidance and the resting-state functional connectivity of amygdala subregions. PLoS ONE 7:e35925. doi: 10.1371/journal.pone.0035925
Liu, C.-H., Ma, X., Wu, X., Fan, T.-T., Zhang, Y., Zhou, F.-C., et al. (2013). Resting-state brain activity in major depressive disorder patients and their siblings. J. Affect. Disord. 149, 299–306. doi: 10.1016/j.jad.2013.02.002
Liu, X., Hairston, J., Schrier, M., and Fan, J. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 35, 1219–1236. doi: 10.1016/j.neubiorev.2010.12.012
Marx, E., Deutschlander, A., Stephan, T., Dieterich, M., Wiesmann, M., and Brandt, T. (2004). Eyes open and eyes closed as rest conditions: impact on brain activation patterns. Neuroimage 21, 1818–1824. doi: 10.1016/j.neuroimage.2003.12.026
Matcher, S. J., Elwell, C. E., Cooper, C. E., Cope, M., and Delpy, D. T. (1995). Performance comparison of several published tissue near-infrared spectroscopy algorithms. Anal. Biochem. 227, 54–68. doi: 10.1006/abio.1995.1252
Merenäkk, L., Harro, M., Kiive, E., Laidra, K., Eensoo, D., Allik, J., et al. (2003). Association between substance use, personality traits, and platelet MAO activity in preadolescents and adolescents. Addict. Behav. 28, 1507–1514. doi: 10.1016/S0306-4603(02)00270-8
Mertol, S., and Alkin, T. (2012). Temperament and character dimensions of patients with adult separation anxiety disorder. J. Affect. Disord. 139, 199–203. doi: 10.1016/j.jad.2012.02.034
Milad, M. R., Pitman, R. K., Ellis, C. B., Gold, A. L., Shin, L. M., Lasko, N. B., et al. (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry 66, 1075–1082. doi: 10.1016/j.biopsych.2009.06.026
Milad, M. R., Quirk, G. J., Pitman, R. K., Orr, S. P., Fischl, B., and Rauch, S. L. (2007). A role for the human dorsal anterior cingulate cortex in fear expression. Biol. Psychiatry 62, 1191–1194. doi: 10.1016/j.biopsych.2007.04.032
Nakao, T., Matsumoto, T., Morita, M., Shimizu, D., Yoshimura, S., Northoff, G., et al. (2013). The degree of early life stress predicts decreased medial prefrontal activations and the shift from internally to externally guided decision making: an exploratory NIRS study during resting state and self-oriented task. Front. Hum. Neurosci. 7:339. doi: 10.3389/fnhum.2013.00339
Nakao, T., Ohira, H., and Northoff, G. (2012). Distinction between externally vs. internally guided decision-making: operational differences, meta-analytical comparisons and their theoretical implications. Front. Neurosci. 6:31. doi: 10.3389/fnins.2012.00031
Näsi, T., Virtanen, J., Noponen, T., Toppila, J., Salmi, T., and Ilmoniemi, R. J. (2011). Spontaneous hemodynamic oscillations during human sleep and sleep stage transitions characterized with near-infrared spectroscopy. PLoS ONE 6:e25415. doi: 10.1371/journal.pone.0025415
Obrig, H., Neufang, M., Wenzel, R., Kohl, M., Steinbrink, J., Einhaupl, K., et al. (2000). Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage 12, 623–639. doi: 10.1006/nimg.2000.0657
O'doherty, J. P. (2007). Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann. N.Y. Acad. Sci. 1121, 254–272. doi: 10.1196/annals.1401.036
O'gorman, R. L., Kumari, V., Williams, S. C., Zelaya, F. O., Connor, S. E., Alsop, D. C., et al. (2006). Personality factors correlate with regional cerebral perfusion. Neuroimage 31, 489–495. doi: 10.1016/j.neuroimage.2005.12.048
Okada, E., and Delpy, D. T. (2003a). Near-infrared light propagation in an adult head model. I. Modeling of low-level scattering in the cerebrospinal fluid layer. Appl. Opt. 42, 2906–2914. doi: 10.1364/AO.42.002906
Okada, E., and Delpy, D. T. (2003b). Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Appl. Opt. 42, 2915–2922. doi: 10.1364/AO.42.002915
Pan, W. J., Thompson, G. J., Magnuson, M. E., Jaeger, D., and Keilholz, S. (2013). Infraslow LFP correlates to resting-state fMRI BOLD signals. Neuroimage 74, 288–297. doi: 10.1016/j.neuroimage.2013.02.035
Pan, W. J., Thompson, G., Magnuson, M., Majeed, W., Jaeger, D., and Keilholz, S. (2011). Broadband local field potentials correlate with spontaneous fluctuations in functional magnetic resonance imaging signals in the rat somatosensory cortex under isoflurane anesthesia. Brain Connect. 1, 119–131. doi: 10.1089/brain.2011.0014
Pierro, M. L., Sassaroli, A., Bergethon, P. R., Ehrenberg, B. L., and Fantini, S. (2012). Phase-amplitude investigation of spontaneous low-frequency oscillations of cerebral hemodynamics with near-infrared spectroscopy: a sleep study in human subjects. Neuroimage 63, 1571–1584. doi: 10.1016/j.neuroimage.2012.07.015
Qin, P., Grimm, S., Duncan, N. W., Holland, G., Guo, J. S., Fan, Y., et al. (2013). Self-specific stimuli interact differently than non-self-specific stimuli with eyes-open versus eyes-closed spontaneous activity in auditory cortex. Front. Hum. Neurosci. 7:437. doi: 10.3389/fnhum.2013.00437
Quilty, L. C., Godfrey, K. M., Kennedy, S. H., and Bagby, R. M. (2010). Harm avoidance as a mediator of treatment response to antidepressant treatment of patients with major depression. Psychother. Psychosom. 79, 116–122. doi: 10.1159/000276372
Raichle, M. E., Macleod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., and Shulman, G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682. doi: 10.1073/pnas.98.2.676
Richter, J., and Brandstrom, S. (2009). Personality disorder diagnosis using the temperament and character inventory. Compr. Psychiatry 50, 347–352. doi: 10.1016/j.comppsych.2008.09.002
Richter, J., Eisemann, M., and Richter, G. (2000). Temperament and character during the course of unipolar depression among inpatients. Eur. Arch. Psychiatry Clin. Neurosci. 250, 40–47. doi: 10.1007/PL00007538
Robinson, O. J., Charney, D. R., Overstreet, C., Vytal, K., and Grillon, C. (2012). The adaptive threat bias in anxiety: amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. Neuroimage 60, 523–529. doi: 10.1016/j.neuroimage.2011.11.096
Rosellini, A. J., and Brown, T. A. (2011). The NEO Five-Factor Inventory: latent structure and relationships with dimensions of anxiety and depressive disorders in a large clinical sample. Assessment 18, 27–38. doi: 10.1177/1073191110382848
Sassaroli, A., Pierro, M., Bergethon, P. R., and Fantini, S. (2012). Low-frequency spontaneous oscillations of cerebral hemodynamics investigated with near-infrared spectroscopy: a review. IEEE J. Sel. Top. Quantum Electron. 18, 1478–1492. doi: 10.1109/JSTQE.2012.2183581
Scholvinck, M. L., Maier, A., Ye, F. Q., Duyn, J. H., and Leopold, D. A. (2010). Neural basis of global resting-state fMRI activity. Proc. Natl. Acad. Sci. U.S.A. 107, 10238–10243. doi: 10.1073/pnas.0913110107
Schroeter, M. L., Schmiedel, O., and Von Cramon, D. Y. (2004). Spontaneous low-frequency oscillations decline in the aging brain. J. Cereb. Blood Flow Metab. 24, 1183–1191. doi: 10.1097/01.WCB.0000135231.90164.40
Sher, K. J., Bartholow, B. D., and Wood, M. D. (2000). Personality and substance use disorders: a prospective study. J. Consult. Clin. Psychol. 68, 818–829. doi: 10.1037/0022-006X.68.5.818
Singh, A. K., Okamoto, M., Dan, H., Jurcak, V., and Dan, I. (2005). Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage 27, 842–851. doi: 10.1016/j.neuroimage.2005.05.019
Smith, D. J., Duffy, L., Stewart, M. E., Muir, W. J., and Blackwood, D. H. (2005). High harm avoidance and low self-directedness in euthymic young adults with recurrent, early onset depression. J. Affect. Disord. 87, 83–89. doi: 10.1016/j.jad.2005.03.014
Sugiura, M., Kawashima, R., Nakagawa, M., Okada, K., Sato, T., Goto, R., et al. (2000). Correlation between human personality and neural activity in cerebral cortex. Neuroimage 11, 541–546. doi: 10.1006/nimg.2000.0564
Tachtsidis, I., Elwell, C. E., Leung, T. S., Lee, C.-W., Smith, M., and Delpy, D. T. (2004). Investigation of cerebral haemodynamics by near-infrared spectroscopy in young healthy volunteers reveals posture-dependent spontaneous oscillations. Physiol. Meas. 25, 437–445. doi: 10.1088/0967-3334/25/2/003
Tan, B., Kong, X., Yang, P., Jin, Z., and Li, L. (2013). The difference of brain functional connectivity between eyes-closed and eyes-open using graph theoretical analysis. Comput. Math. Methods Med. 2013, 976365. doi: 10.1155/2013/976365
Toronov, V., Webb, A., Choi, J. H., Wolf, M., Michalos, A., Gratton, E., et al. (2001). Investigation of human brain hemodynamics by simultaneous near-infrared spectroscopy and functional magnetic resonance imaging. Med. Phys. 28, 521–527. doi: 10.1118/1.1354627
Valencia, M., Artieda, J., Bolam, J. P., and Mena-Segovia, J. (2013). Dynamic interaction of spindles and gamma activity during cortical slow oscillations and its modulation by subcortical afferents. PLoS ONE 8:e67540. doi: 10.1371/journal.pone.0067540
Vidal-Gonzalez, I., Vidal-Gonzalez, B., Rauch, S. L., and Quirk, G. J. (2006). Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn. Mem. 13, 728–733. doi: 10.1101/lm.306106
Wang, J., Qin, W., Liu, B., Zhou, Y., Wang, D., Zhang, Y., et al. (2013). Neural mechanisms of oxytocin receptor gene mediating anxiety-related temperament. Brain Struct. Funct. doi: 10.1007/s00429-013-0584-9. [Epub ahead of print].
Wang, L., Dai, W., Su, Y., Wang, G., Tan, Y., Jin, Z., et al. (2012a). Amplitude of low-frequency oscillations in first-episode, treatment-naive patients with major depressive disorder: a resting-state functional MRI Study. PLoS ONE 7:e48658. doi: 10.1371/journal.pone.0048658
Wang, L., Saalmann, Y. B., Pinsk, M. A., Arcaro, M. J., and Kastner, S. (2012b). Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron 76, 1010–1020. doi: 10.1016/j.neuron.2012.09.033
Wei, L., Duan, X., Zheng, C., Wang, S., Gao, Q., Zhang, Z., et al. (2012). Specific frequency bands of amplitude low-frequency oscillation encodes personality. Hum. Brain Mapp. doi: 10.1002/hbm.22176. [Epub ahead of print].
Welch, P. (1967). The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoustics 15, 70–73. doi: 10.1109/TAU.1967.1161901
Won Kim, S., and Grant, J. E. (2001). Personality dimensions in pathological gambling disorder and obsessive–compulsive disorder. Psychiatry Res. 104, 205–212. doi: 10.1016/S0165-1781(01)00327-4
Yan, C., Liu, D., He, Y., Zou, Q., Zhu, C., Zuo, X., et al. (2009). Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS ONE 4:e5743. doi: 10.1371/journal.pone.0005743
Yang, H., Long, X. Y., Yang, Y., Yan, H., Zhu, C. Z., Zhou, X. P., et al. (2007). Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage 36, 144–152. doi: 10.1016/j.neuroimage.2007.01.054
Yin, Y., Li, L., Jin, C., Hu, X., Duan, L., Eyler, L. T., et al. (2011). Abnormal baseline brain activity in posttraumatic stress disorder: a resting-state functional magnetic resonance imaging study. Neurosci. Lett. 498, 185–189. doi: 10.1016/j.neulet.2011.02.069
Youn, T., Lyoo, I. K., Kim, J. K., Park, H. J., Ha, K. S., Lee, D. S., et al. (2002). Relationship between personality trait and regional cerebral glucose metabolism assessed with positron emission tomography. Biol. Psychol. 60, 109–120. doi: 10.1016/S0301-0511(02)00047-9
Zou, Q. H., Zhu, C. Z., Yang, Y., Zuo, X. N., Long, X. Y., Cao, Q. J., et al. (2008). An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods 172, 137–141. doi: 10.1016/j.jneumeth.2008.04.012
Keywords: low-frequency fluctuations, resting state, medial prefrontal cortex (MPFC), personality, reward, aversion, harm avoidance, novelty seeking
Citation: Nakao T, Matsumoto T, Shimizu D, Morita M, Yoshimura S, Northoff G, Morinobu S, Okamoto Y and Yamawaki S (2013) Resting state low-frequency fluctuations in prefrontal cortex reflect degrees of harm avoidance and novelty seeking: an exploratory NIRS study. Front. Syst. Neurosci. 7:115. doi: 10.3389/fnsys.2013.00115
Received: 30 September 2013; Accepted: 02 December 2013;
Published online: 17 December 2013.
Edited by:
Andrew J. Greenshaw, University of Alberta, CanadaReviewed by:
Takashi Yamamoto, Osaka University Graduate School of Dentistry, JapanMatthew R. G. Brown, University of Alberta, Canada
Copyright © 2013 Nakao, Matsumoto, Shimizu, Morita, Yoshimura, Northoff, Morinobu, Okamoto and Yamawaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takashi Nakao, Department of Psychology, Graduate School of Education, Hiroshima University, 1-1-1, Kagamiyama, Higashi-Hiroshima, Hiroshima 739-8524, Japan e-mail: takana818@gmail.com
†These authors have contributed equally to this work.
 Takashi Nakao
Takashi Nakao Tomoya Matsumoto
Tomoya Matsumoto Daisuke Shimizu3
Daisuke Shimizu3  Shinpei Yoshimura
Shinpei Yoshimura Georg Northoff
Georg Northoff Shigeru Morinobu
Shigeru Morinobu Yasumasa Okamoto
Yasumasa Okamoto Shigeto Yamawaki
Shigeto Yamawaki