- Department of Radiation Oncology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
Proton therapy is associated with significant benefit in terms of normal tissue sparing and potential radiation dose escalation for many patients with malignant diseases. Due to recognition of these qualities, the availability of this technology is increasing rapidly, both through increased availability of large centers, and with the possibility of smaller, lower cost proton therapy centers. Such expansion is associated with increased opportunity to provide this beneficial technology to larger numbers of patients; however, the importance of careful treatment planning and delivery, deliberate patient selection, rigorous scientific investigation including comparison to other technologies when possible, and mindfulness of ethical issues and cost effectiveness must not be forgotten. The obligation to move forward responsibly rests on the shoulders of radiation oncologists around the world. In this article, we discuss current use of proton therapy worldwide, as well as many of the factors that must be taken into account during rapid expansion of this exciting technology.
Introduction
Although proton therapy for treatment of malignant disease is not a new concept, and has been in fact employed clinically since the 1970s, interest in its use is rapidly gaining momentum as clinical technology becomes more widespread and available. Currently, proton therapy centers designed specifically for treatment of cancer patients exist in most regions of the United States, as well as several areas in Europe and Asia. Accompanying the availability of these centers is the promise of smaller, more economical machines that may soon be available in numerous other centers. As the radiation oncology community prepares for rapid changes that are occurring and promise to continue to occur in the field of proton therapy, understanding of the many complexities of center design, treatment planning, and treatment delivery is essential. In the following pages, several of these issues will be outlined, along with current use and data with regard to proton therapy, future goals with regard to this technology, and limitations that may be foreseen at this point.
The Physical Benefit of the Proton Beam
The physical properties of protons allow achievement of a very simple benefit: reduction in radiation delivered outside of the target volume. Photons have neither mass nor charge, and as a result traverse the patient, depositing both entrance and exit dose. In contrast, protons are relatively heavy and carry a positive charge. Together, these properties result in significant stopping power of the proton beam. When a given proton stops within tissue, it deposits the majority of its energy, creating the proverbial Bragg-peak, and eliminating exit dose entirely. With careful planning, Bragg-peaks may be aligned to create a spread-out Bragg-peak (SOBP; Figure 1). The SOBP may be carefully positioned so that the vast majority of dose is delivered to the tumor, with no radiation delivered distally. The implications of this unique quality are quite far reaching. Reducing exit dose within a child’s brain may allow radiation to be delivered to many fewer developing neurons than has been previously possible. Use of proton therapy for spinal lesions may allow elimination of dose to visceral organs, including heart, lung, bowel, and organs of fertility and reproduction. Proton therapy for treatment of sarcomas may allow delivery of high radiation doses with essential sparing of normal tissue. Due to increased tissue sparing with use of proton therapy, this modality may also be used to escalate radiation dose delivered. Certainly, these opportunities are extremely appealing and, in combination with increased accessibility, have prompted increasing use of proton therapy. An analysis of the currently available literature examining clinical use of proton therapy, as well as certain of the scientific and treatment limitations of proton therapy are essential to our delineation of future needs.
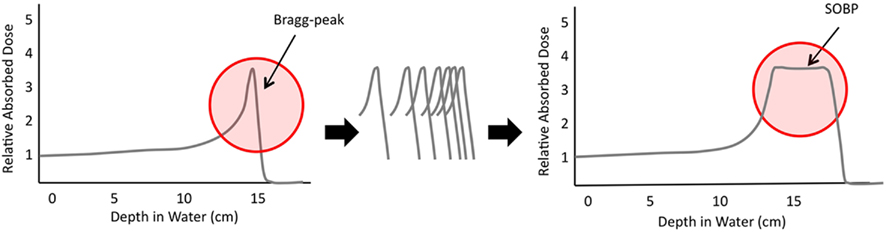
Figure 1. Graphic demonstration of the rapid dosimetric peak and fall-off of the Bragg-peak; the alignment of many Bragg-peaks may allow creation of the spread-out Bragg-peak (SOBP) that allows deposition of radiation in a specific location with essentially no dose deposited beyond this.
Current Use and Published Data Regarding Use of Proton Therapy
Limitations to Data Collection and Analysis
Certainly, the advent of any new technology warrants rigorous scientific investigation of risks and benefits associated with its use. To most radiation oncologists and physicists, the benefit of proton therapy in many clinical cases is without question; protons allow sparing of normal tissue, which is unarguably beneficial to patients. Indeed, to a radiation oncologist experienced in delivery of proton therapy, the decision of whether or not to employ it in a given case is analogous to a surgeon’s decision regarding the choice of a particular scalpel or cautery device. Having said this, a certain onus rests on practitioners within the field of radiation oncology to provide scientific support for treatment decisions; additionally, the sheer cost of proton therapy requires that evidence of benefit be provided to justify the use of this technology for patients who may benefit from it, and to limit the use of this resource to use for those who will achieve improved outcomes as a result.
Although proton therapy has been used clinically for the past 40 years (Skarsgard, 1998), small patient numbers have historically limited publication of large studies. Most published data examining clinical use of proton therapy are single institution series. While these publications, many of which will be discussed further below, are of great value, they do not represent randomized controlled trials (RCT) – held to be the gold standard for evaluation and comparison of treatment techniques. Limitations to conducting RCT evaluating the role of proton therapy compared to photon therapy are numerous, and include limitations in patient numbers, resource access, and ethics of randomization to a modality felt to be inferior by much of the radiation oncology community.
In addition to these limitations is the sheer amount of time that may be required to fully assess outcomes for patients after any type of radiation therapy. This limitation may apply to both retrospective and prospective analyses, which remain relatively sparse within the literature. Assessing disease-related outcomes is critical for any oncologic trial; the evaluation of long-term and late effects is, however, potentially more pertinent to evaluation of a technology whose main benefit is reduction of normal tissue dose. Long-term and late effects may take many years to develop, and so careful, long-term follow-up is required and may be particularly complex for patients who are treated at a tertiary referral proton center and then return to their home institutions. In reality, as use of proton therapy becomes rapidly more widespread, long-term, and late effects data will simply not be available as quickly as they are needed.
Fortunately, many large institutions and cooperative groups have come to formally recognize the need for rigorous data collection and publication, and great progress is being made in this arena. The Radiation Therapy Oncology Group (RTOG) and Children’s Oncology Group (COG) are employing proton therapy in several cooperative trials, and many large proton centers are focused on publishing high-quality data regarding proton therapy. At our own institution, for example, every patient treated with proton therapy is enrolled on a clinical trial; some patients may be enrolled on cooperative group studies, while others are enrolled on single institution experimental protocols or data registries. Furthermore, representatives from several large institutions are dedicated to collaborative work with one another in order to facilitate gathering and publication of robust data from multiple institutions. In the future, these resources may hopefully allow for analysis and publication of large comparative datasets; a discussion of the currently available literature, with focus on publications that are clinical in nature, rather than dosimetric or physics comparisons follows below.
Current Technical Use and Limitations
The challenges in conducting randomized studies extend beyond the above issues due to different stages of development of proton versus photon therapy. The vast majority of institutions currently delivering proton therapy make use of double scattered technology. Scattered beam techniques allow delivery of three-dimensional conformal proton therapy (3D-CPT), somewhat analogous to standard three-dimensional conformal radiation (3D-CRT), with the addition of increased distal stopping power. Although double scattering offers significant tissue sparing at the distal edge of the beam, proximal and lateral beam conformality may be reduced in some cases compared to intensity modulated radiation therapy (IMRT) using photons. This may be of significance in clinical situations in which concave tumor targets are situated next to neighboring organs, such as the liver, kidney, or brainstem. In contrast, intensity modulated proton therapy (IMPT), employing a scanning beam, allows distribution of Bragg-peaks in three-dimensions through the target volumes, allowing improved conformality, and sparing of normal structures (Lomax et al., 2004a). The majority of proton therapy centers worldwide offer scattered proton beams, although the number with access to scanning beam is increasing. The vast majority of published data, as such, regards use of scattered beam technology, although the Paul Scherrer Institute (PSI) in Villagen, Switzerland has published their experience treating several tumor types with scanning proton beams/IMPT (Lomax et al., 2004b; Timmermann et al., 2006). As scanning beam technology becomes more widely available, a lag is expected to exist within the literature as institutions and cooperative groups gather data with its use, analogous to the delay observed in publication of data after use of IMRT compared to 3D conformal radiotherapy in the photon literature.
Additionally, availability of improved imaging in proton treatment room may create new opportunities in terms of increasing the accuracy of proton therapy due to better understanding and management of uncertainties related to the proton beam. As randomized studies tend to be multicentered, the availability of equally advanced technologies at all centers participating is optimal, however, centers are unique in terms of the technologies that they are able to offer. As a result, true assessment of the potential of proton therapy may not be possible until a time when the most advanced technologies may be broadly available for use in the randomized trials meant to evaluate them. Again, this must be expected to introduce a delay within the published literature.
Published Clinical Data
Central nervous system
The brain and spinal cord are currently the sites for which use of proton therapy is most well-supported by the literature and used clinically. In 2004, St Clair and colleagues demonstrated clear dosimetric advantage of proton therapy for treatment of patients with medulloblastoma requiring craniospinal irradiation and boost to the posterior fossa. Advantages included reduction of dose to the cochlea and heart, with expectations for translation to improved outcome with regard to hearing, endocrine, and cardiac function (St Clair et al., 2004). In 2008, Merchant and colleagues demonstrated through dose–cognitive effects models that reduction in integral radiation dose to the pediatric brain could be expected to translate to reduction in IQ deficit long recognized to result from radiation for childhood brain tumors (Merchant et al., 2008). Publications such as these have prompted great interest in use of proton therapy for central nervous system (CNS) tumors. Since that time, numerous dosimetric studies have been published demonstrating reduction of dose to normal brain and nearby organs at risk with proton therapy as compared to photon therapy. Clinical data have become more available over the past 3–5 years, particularly within the pediatric population. MacDonald and colleagues have recently published early clinical outcomes of children treated with 3D-CPT at the Massachusetts General Hospital (MGH) for ependymoma (MacDonald et al., 2008) and CNS germ cell tumors (MacDonald et al., 2011). Excellent clinical outcomes were demonstrated compared to historical controls, including overall survival of 89 and 100%, respectively, with median follow-up greater than 2 years, accompanied by substantial normal tissue sparing with proton therapy as compared to IMRT. Craniopharyngioma is a particularly attractive tumor for use of proton therapy due to its slow-growing nature, development in children, and potential to cause severe damage. The dosimetric superiority of proton therapy for treatment of craniopharyngioma has been demonstrated (Boehling et al., 2011), and multiple groups have published clinical data supporting use of proton therapy in this clinical setting and demonstrating 5 year local control rates of over 90% (Fitzek et al., 2006; Luu et al., 2006). Winkfield et al. (2009) specifically investigated changes in cyst size and dimension during proton radiotherapy and reported need for changes in proton planning due to cyst changes in 6 of 17 patients. Certainly, this report should be kept in mind during treatment of this complex tumor, with routine surveillance of cyst size and dimension performed throughout the course of therapy.
Certainly use of proton therapy for treatment of the entire craniospinal axis is highly appealing because it results in reduction of radiation dose to numerous normal structures (Yuh et al., 2004; Krejcarek et al., 2007). This technique may be quite complicated, requiring matching of multiple fields, and impacted by limitations of field length. Modification of a 3D-CPT technique to reduce lens dose has recently been reported (Cochran et al., 2008), and a single case in the literature using spot-scanning proton radiation to deliver CSI is available (Timmermann et al., 2007).
Data supporting use of proton therapy for adult CNS tumors is somewhat more limited. Ronson et al. (2006) have demonstrated excellent outcomes after proton therapy for pituitary adenomas, and data from the PSI support use of proton therapy for treatment of meningioma (Weber et al., 2004). Mizumoto et al. (2010) have recently reported the results of a phase I/II trial evaluating the role of concomitant proton boost therapy with dose escalation to 96.6 Gy (RBE) for glioblastoma multiforme (GBM) in adult patients. This group demonstrated excellent median and overall survival rates compared to historical controls, and this study represents one of the few available examining clinical dose escalation with proton therapy in the CNS.
Skull base and head and neck tumors
Radiation to the head and neck using any available technology remains complex, due mainly to complex target shape and delineation, close proximity of organs at risk to areas needing treatment, and need for relatively high-dose delivery. Again, several dosimetric studies have been performed evaluating proton therapy, for the most part 3D-CPT, compared to IMRT. These support the ability of proton therapy to result in sparing of normal tissue when radiation is required to the paranasal sinuses (Miralbell et al., 1992; Mock et al., 2004; Chera et al., 2009), nasopharynx (Brown et al., 1989; Taheri-Kadkhoda et al., 2008; Widescott et al., 2008), and oropharynx/hypopharynx/larynx (Slater et al., 1992; Cozzi et al., 2001; Johansson et al., 2004; Steneker et al., 2006). Lomax et al. (2003) have also demonstrated the potential for dose escalation during treatment of head and neck cancers with proton therapy based on dosimetric work.
Strong clinical data exists with regard to treatment of skull base chordoma and chondrosarcoma with proton therapy, with investigators from PSI having published reports of experiences at their center treating both pediatric and adult patients with spot-scanning proton therapy: In 2009, Ares and colleagues reported on 64 patients treated with spot-scanning technique for skull base tumors, with median follow-up of 38 months. The authors report local control rates at 5 years of 81% (chordomas) and 94% (chondrosarcomas), with 94% rate of freedom from high-grade toxicity (Ares et al., 2009). A report of children and adolescents treated with similar techniques demonstrates no treatment failures and no severe late toxicities (Rutz et al., 2008). Interestingly, dosimetric work by Torres et al. (2009) suggests that use of combined proton and IMRT plans for skull base chordomas may result in the best tumor coverage and conformality compared to 3D-CPT alone. Ares and colleagues include a detailed review of the literature within their report, in which they compare their findings to those from several other institutions and report inferior 5-year progression-free survival (PFS) with use of photon irradiation compared to their own findings after proton therapy; specifically, Zorlu et al. (2000) have reported 5-year PFS of 23% using modern diagnostic and photon radiotherapy techniques for treatment of skull base tumors. An exception to this may be the use of fractionated stereotactic radiosurgery for treatment of small chondrosarcomas. Local control rates of 100% are reported in the literature in this specific clinical setting, but remain more disappointing for chordomas (30–50%; Debus et al., 2000; Krishnan et al., 2005). Of note, Nikoghosyan et al. (2010) report that a phase III trial of proton versus carbon ion therapy in patients with chordoma of the skull base is currently underway at the University of Heidelberg.
Clinically, a pilot study by Zenda et al. (2010) has recently demonstrated that hypofractionated proton therapy may be clinically promising in treatment of mucosal melanomas of the head and neck. This data is supported by recent meta-analysis of particle therapy for mucosal melanomas of the head and neck, demonstrating significantly improved 5-year OS with particle therapy versus photon therapy (Ramaekers et al., 2011).
Clinical data is available regarding use of proton therapy for pediatric cancers of the head and neck, most commonly sarcomas. Investigators from MGH have demonstrated improved normal tissue sparing during treatment of parameningeal and orbital rhabdomyosarcoma (Yock et al., 2005; Kozak et al., 2009); particularly of note is dose reduction of contralateral tissues, including the orbit, cochlea, and parotid, when protons are employed versus IMRT.
Clinical data with regard to treatment of squamous cell carcinoma of the head and neck is available in small numbers. Treatment outcomes were reported for 20 patients with primary sphenoid sinus malignancy, treated to a median dose of 76 Gy (RBE). The authors report 2-year local control of 86%, and conclude that proton therapy may result in excellent LC for these patients (Truong et al., 2009). A recent meta-analysis demonstrates improved LC for paranasal and sinonasal cancers with use of proton therapy compared to IMRT; however, the same group found no improvement for patients with cancers of other sites within the head and neck (Ramaekers et al., 2011). The complex nature of treatment volumes for head and neck cancers, which are often large, convex volumes which change significantly from superior most to inferior most extent of treatment, makes the conformal nature of IMRT appealing for their treatment. In many cases, IMRT allows significantly improved conformality compared to 3D-CPT; however, if and when IMPT matures and becomes more widely available, the full benefits of proton therapy for normal tissue sparing are expected to be observed for this group of patients. IMPT will allow both reduction in exit dose and shaping of the proton beam in a way that is not possible with 3D-CPT and promises maximal gain at the oral cavity level, potentially increasing patient tolerance of concomitant chemotherapy. Further investigation of clinical outcomes using IMPT for treatment of head and neck tumors will be essential for proper evaluation of proton therapy in this clinical setting.
Abdomen and pelvis
The diverse nature of tumors of the abdomen and pelvis requires heterogeneous treatment methodology; however, many tumors occur in these regions that may be well-suited to proton therapy. These include, and are not limited to, tumors of the prostate gland, as well as localized tumors within the abdomen and pelvis and tumors of bone and muscle. Prostate cancer is certainly an appealing use of this modality, due to a combination of relatively high required dose of >70 Gy and the proximity of the prostate to normal, more radiosensitive structures, most notably the rectum, but including the bladder, femoral heads, and bowel. Additionally, dose escalation trials have demonstrated potential benefit for prolonging biochemical failure free survival (BFFS), and the possibility of delivering higher doses with decreased risk of damage certainly exists with proton therapy. In a collaborative randomized controlled trial, Proton Radiation Oncology Group (PROG) 95-09, MGH, and Loma Linda University Medical Center conducted an investigation of dose escalation using proton therapy for men with clinically localized prostate cancer; men were treated with combination of proton and photon radiation to a total dose of either 70.2 or 79.2 Gy (RBE). The authors demonstrated a 49% reduction in BFFS with the use of dose escalation with only a 1% associated increase in acute or late urinary or rectal toxicity (Zietman et al., 2005). No increase in patient-reported symptoms in the high-dose arm compared to the lower dose arm was reported in a separate quality of life analysis (Talcott et al., 2010). Men with low- to intermediate-risk prostate cancer treated on the high-dose arm of PROG 95-09 were recently compared in a case-matched analysis to men treated with brachytherapy at the MGH. No difference in BFFS was demonstrated between the two groups; however, the authors note that no quality of life analysis was possible, and that brachytherapy has historically been associated with increase in obstructive voiding symptoms compared to external beam radiation (Chen et al., 2009). In light of excellent quality of life data with proton-based dose escalation reported by Talcott et al. (2010), patient-reported outcomes following brachytherapy are particularly important during any meaningful comparison. Also within the setting of prostate cancer, other groups have demonstrated very low incidence of grade 2 or greater rectal and/or (Nihei et al., 2010), and feasibility of dose escalation to >80 Gy (Coen et al., 2010). Several centers in the US and worldwide routinely employ proton therapy for treatment of localized prostate cancer at this point, and results of larger trials are currently pending.
Radiation of the pediatric pelvis poses significantly greater risk than is seen with adults due to the growing nature of the bony pelvis in a child. In addition, reproductive and endocrine organs may be of particular importance for sparing in young children. In a small group of children treated with proton therapy for rhabdomyosarcoma of the bladder and prostate, significant decrease in mean organ dose to the bladder, testes, femoral heads, growth plates, and pelvic bones has been observed (Cotter et al., 2010), and similar results have been demonstrated dosimetrically by other groups, including decreased ovarian dose, during treatment of pelvic sarcomas (Lee et al., 2005). Radiotherapy is currently widely employed for treatment of most rhabdomyosarcomas, and unresectable, pelvic Ewing’s sarcoma. Scant evidence exists that high-doses of radiation may have curative potential for osteosarcoma. A trial is currently underway examining the potential of heavy ion therapy in this clinical situation (Blattmann et al., 2010).
Chest/lung
Several clinical studies have investigated the role of proton therapy in treatment of non-small cell lung cancer (NSCLC). Hypofractionated, dose escalated, proton beam therapy for patients with early stage lung cancer has been shown to provide excellent clinical results in several phase I and II trials (Hata et al., 2007; Iwata et al., 2010; Nakayama et al., 2010). For early stage patients, dosimetric comparison of proton-based stereotactic body radiotherapy (SBRT) to photon-based SBRT demonstrates decreased radiation to organs at risk with use of protons, including uninvolved lung tissue (Hoppe et al., 2010). In a recent meta-analysis comparing radiation with photons, protons, and carbon-ions for treatment of stage I NSCLC, 5 year OS was 20% after conventional photon radiotherapy compared to 40–42% after proton therapy, carbon ion therapy, or stereotactic body irradiation. The authors caution that their results are limited by small patient numbers and follow-up time (Grutters et al., 2009). The role of proton therapy for treatment of more advanced NSCLC, requiring concurrent chemotherapy, is evolving. Sejpal et al. (2011) compared such patients receiving proton therapy to historical results after IMRT or 3D-CRT and demonstrated decreased risk of severe pneumonitis and esophagitis in the former group. The authors note that a prospective, randomized trial investigating the same question is underway.
The role of proton therapy in treatment of other cancers of the mediastinum and lung is emerging with an investigative role. Clinical data from MD Anderson Cancer Center demonstrates reduced dose to lung, esophagus, heart, and coronary arteries using 3D-PBT for treatment of mediastinal lymphoma (Li et al., 2010). Certainly, use of proton therapy to treat mediastinal disease may be particularly attractive if it is also found to reduce breast dose; this may be of particular importance for young girls and women, recognized to be at significantly increased risk for breast cancer following treatment for Hodgkin’s disease. A dosimetric planning study from the University of Zurich has demonstrated that proton beam therapy may allow reduction in dose to organs at risk during treatment of mesothelioma after extrapleural pneumonectomy. Although this technique is promising from a dosimetric standpoint, the authors note that changing air cavities had significant impact on proton plans; the role of proton therapy in this complex treatment paradigm has yet to be defined clinically.
Delivery of proton therapy to tumors in the chest brings to light many issues of concern with regard to target motion and heterogeneity, both of which will be discussed further below.
Breast
Data with regard to use of proton therapy in treatment of breast cancer are relatively limited. Some dosimetric justification may exist for delivery of “boost” radiation to the tumor bed after radiation to the intact breast (Toscas et al., 2010), and for use of IMPT for delivery of whole breast radiation for patients with left sided breast cancer (Ares et al., 2010). This technique may allow improved dose delivery to the whole breast with sparing of heart and lung; however, it must be employed with great caution to avoid under-dosing of the chest wall and proximal breast tissue during normal respiration. The MGH has published their experience treating 20 early stage breast cancer patients with accelerated partial breast proton irradiation (APBI). With limited follow-up, disease-related outcomes were excellent; however, severe moist desquamation was described in 22% of cases (Kozak et al., 2006). Although further investigation of proton therapy in this specific clinical setting may be warranted, no clear benefit for use of protons over other methods of delivery of APBI has been demonstrated at this point.
A word on pediatrics
Throughout the above discussion, the benefit of proton therapy in treatment of pediatric tumors is noted again and again. When considered both clinically and dosimetrically, the benefit of sparing normal, growing tissue in this unique population is inarguable. It is the opinion of these authors that proton therapy should be considered and utilized for all pediatric patients needing curative irradiation, providing that this is technically feasible. The pediatric oncology community widely recognizes the negative impact of radiation to normal tissues in childhood, and use of technology to reduce the volume of normal tissue exposed is warranted without question.
Potential Limitations: Cost Effectiveness and Issues in Treatment Planning and Delivery
Cost Effectiveness
The message from many of the published clinical and dosimetric studies described above is consistent: In many cases, proton therapy offers the ability to deliver equivalent or higher radiation doses with sparing of normal tissue. This benefit is expected to be magnified as scanning beam technology becomes more widely available and allows delivery of IMPT. This statement likely appears redundant to the reader by this point, and begs the question of why proton therapy is not routinely employed for all patients. Certainly, the cost effectiveness of this modality has yet to be fully elucidated, and is much more complex than a question with a dichotomous answer. For example, proton therapy for childhood medulloblastoma has been associated with 23,600€ in cost-savings per patient, mainly due to decreased IQ losses and growth hormone deficiency (Lundkvist et al., 2005a). In contrast, some groups have raised concerns that proton therapy may not be cost effective for men with prostate cancer (Konski et al., 2007). Analysis of the cost effectiveness of a center treating patients with multiple types of cancer has demonstrated potential for cost-savings; however, the authors note that multiple uncertainties are included in this analysis, including the amount of value placed on quality of life year (QALY) per patient (Lundkvist et al., 2005b). Ultimately, of course, cost effectiveness can only be completely understood when comparative outcomes analyses are available; as described above, we as a community, eagerly await much of this data. Certainly, accurate cost-effectiveness analyses may be useful for consideration not only by treating centers but by third-party payers. Other types of cost-effectiveness analyses that would be useful moving forward include examination of cost effectiveness of centers in close proximity to one another, the most appropriate worldwide distribution of proton and particle therapy centers, and changes in cost effectiveness based on age, which may be impacted by life expectancy and second malignancy risk.
Treatment Planning and Delivery
The complicated nature of treatment planning and delivery must be taken into account by any center delivering or planning to deliver proton therapy. Unique patient immobilization devices compatible with both simulation and treatment delivery hardware must be utilized; although these may vary among centers, their essential nature must not be undervalued. Devices employed generally need to be more robust than those utilized during photon therapy due to the precise nature of dose deposition when protons are used. These authors advocate strong physics presence at the time of simulation and during the entire treatment planning process for proton or particle therapy in order to assist with optimal patient immobilization and accurate treatment plan development. We also recommend strict adherence to the guidelines for treatment planning outlined within the International Commission on Radiation Units and Measurements Report 78 (2007).
Beyond immobilization of the patient is the need for deliberate limitation of motion of the treatment target. Potential risks of under and overdosing are of perhaps greatest importance during consideration of use of proton therapy for moving targets such as prostate and lung tumors. During treatment of the former, changes in bladder and rectal position may greatly impact the position of the prostate itself. The use of a rectal balloon has been demonstrated to decrease the clinical significance of any such motion (Both et al., 2010). During treatment of thoracic tumors, technology must be employed to account for respiratory motion, and may include breath holding techniques, target expansion to create an internal target volume (ITV), and/or four-dimensional CT scanning, planning, and treatment delivery.
Treatment of tumors within the chest may also be made complicated by the heterogeneous nature of tissues within the thorax. Density variations within the path of the proton beam may cause widening of the distal fall-off of the Bragg-peak. This may be particularly problematic within lung tissue, and may result in greater exposure of normal lung tissue than desired or expected (Sawakuchi et al., 2008). Novel methods of accounting for heterogeneity have been proposed and treating centers must verse themselves in these methods in order to deliver the best and most precise treatment in any location of the body. Other circumstances in which heterogeneity may be particularly problematic include treatment of the head and neck near an air-filled sinus, treatment in the presence of hardware that may affect beam distribution, and treatment of the pelvis when air may be variably present within the bowel. During the lattermost circumstance, significant intrafraction variation may further complicate treatment delivery and must be accounted for.
Variability in target position may be significantly impacted by the length of time required to deliver a single fraction. Interestingly, the complicated natures of treatment planning and delivery, as well as cost effectiveness, converge at the point of discussion of the time required to deliver treatment to an individual patient. Increased time for fraction delivery has been demonstrated to result in potentially increased target motion (Both et al., 2010). Additionally, longer table time per patient per day results in decreased numbers of patients who may be treated on a given day at a given center, limiting throughput. In response to both of these factors, many centers employ a methodology in which only certain subsets of fields that are part of a proton plan are treated each day. The appropriateness of this technique is somewhat unique to proton/particle therapy; in contrast to photon radiation plans, when multiple beams are required to provide target coverage, proton plans make use of several beams, each of which is robust in and of itself to provide adequate dose to the target volume, although dose to normal structures may differ significantly from field to field within a given plan. Treating a subset of fields limits daily table time per patient, which may be beneficial in terms of target motion, patient quality of life, and cost effectiveness. Engelsman et al. (2010) recently analyzed the radiobiologic impact of this technique for patients being treated with proton therapy for skull base, lung, pancreatic, and prostate tumors. For many patients, significant variability in dose to organs at risk was described, which may require that specific variability among treatment fields be taken into account in the future when this technique is employed. Treating a subset of fields is not an option when pencil beam scanning is employed, and therefore automated imaging and alignment along with fast delivery techniques are required in order to achieve quality safety and cost effectiveness of treatments with pencil beam scanning across the entire body.
Ethical Issues: Use of Limited Resource
Due in part to the limitations discussed above, proton therapy remains a limited resource that cannot be made available to all patients. In addition, proton therapy is not expected to offer benefit to all patients, and clinicians must be constantly mindful of this fact when determining treatment technique. At our own center, including many others, no patient is planned to receive proton therapy without the approval of a multidisciplinary committee that meets weekly. The committee’s goals are to take into account the relative benefit (or lack thereof) of protons for a certain patient, as well as overall prognosis and other factors that may determine suitability such as technical feasibility. These authors have found this approach to optimize equitable and proper distribution of this limited resource, while minimizing individual physician bias.
The Future of Proton Therapy
Proton therapy is a technology that offers clear benefit to many cancer patients; however, its use is complicated by technical issues, as well as cost and availability worldwide. As availability of scanning beam technology increases, so will potential for increased conformality. This will very likely further increase the proportion of patients who may benefit from treatment with proton therapy. As a field, we will need to address the rapidly growing disparity between clinical need for proton therapy, and the number of centers where robust, safe, and technically advanced therapy is delivered. Delivery of this complex treatment requires massive investment in not only treatment machines, but software and personnel (both physicists and clinicians) with deep understanding and training with regard to its complexities. The nature of the proton beam and the uncertainties associated with it mandate that physicians and physicists work as a team during each treatment site implementation, and also to routinely analyze particularities of clinical cases which may warrant different technical approaches. Our obligation as a field is to remain attentive to these issues as proton therapy burgeons from a tiny part of our field to a technology that may soon be available to benefit large numbers of patients. We must remain intellectually dedicated to examining the risks and benefits of this treatment with careful scientific methodology, and clinically dedicated to utilizing the best technology available for each of our patients.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ares, C., Hug, E. B., Lomax, A. J., Bolsi, A., Timmermann, B., Rutz, H. P., Schuller, J. C., Pedroni, E., and Goitein, G. (2009). Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int. J. Radiat. Oncol. Biol. Phys. 75, 1111–1118.
Ares, C., Khan, S., Macartain, A. M., Heuberger, J., Goitein, G., Gruber, G., Lutters, G., Hug, E. B., Bodis, S., and Lomax, A. J. (2010). Postoperative proton radiotherapy for localized and locoregional breast cancer: potential for clinically relevant improvements? Int. J. Radiat. Oncol. Biol. Phys. 76, 685–697.
Blattmann, C., Oertel, S., Schulz-Ertner, D., Rieken, S., Haufe, S., Ewerbeck, V., Unterberg, A., Karapanagiotou-Schenkel, I., Combs, S. E., Nikoghosyan, A., Bischof, M., Jäkel, O., Huber, P., Kulozik, A. E., and Debus, J. (2010). Non-randomized therapy trial to determine the safety and efficacy of heavy ion radiotherapy in patients with non-resectable osteosarcoma. BMC Cancer 10, 96. doi: 10.1186/1471-2407-10-96
Boehling, N. S., Grosshans, D. R., Bluett, J. B., Palmer, M. T., Song, X., Amos, R., Sahoo, N., Meyer, J. J., Mahajan, A., and Woo, S. Y. (2011). Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity modulated radiotherapy for treatment of pediatric craniopharyngioma. Int. J. Radiat. Oncol. Biol. Phys. doi: 10.1016/j.ijrobp.2010.11.027. [Epub ahead of print].
Both, S., Wang, K. K., Plastaras, J. P., Deville, C., Bar Ad, V., Tochner, Z., and Vapiwala, N. (2010). Real-time study of prostate intrafraction motion during external beam radiotherapy with daily endorectal balloon. Int. J. Radiat. Oncol. Biol. Phys. doi: 10.1016/j.ijrobp.2010.08.052. doi: 10.1016/j.ijrobp.2010.06.047. [Epub ahead of print].
Brown, A. P., Urie, M. M., Chisin, R., and Suit, H. D. (1989). Proton therapy for carcinoma of the nasopharynx: a study in comparative treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 16, 1607–1614.
Chen, R. C., Clark, J. A., and Talcott, J. A. (2009). Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J. Clin. Oncol. 27, 3916–3922.
Chera, B. S., Malyapa, R., Louis, D., Mendenhall, W. M., Li, Z., Lanza, D. C., Yeung, D., and Mendenhall, N. P. (2009). Proton therapy for maxillary sinus carcinoma. Am. J. Clin. Oncol. 32, 296–303.
Cochran, D. M., Yock, T. I., Adams, J. A., and Tarbell, N. J. (2008). Radiation dose to the lens during craniospinal irradiation-an improvement in proton radiotherapy technique. Int. J. Radiat. Oncol. Biol. Phys. 70, 1336–1342.
Coen, J. J., Bae, K., Zietman, A. L., Patel, B., Shipley, W. U., Slater, J. D., and Rossi, C. J. (2010). Acute and late toxicity after dose escalation to 82 gye using conformal proton radiation for localized prostate cancer: initial report of american college of radiology phase ii study 03-12. Int. J. Radiat. Oncol. Biol. Phys. doi: 10.1016/j.ijrobp.2010.07.1989. [Epub ahead of print].
Cotter, S. E., Herrup, D. A., Friedmann, A., MacDonald, S. M., Pieretti, R. V., Robinson, G., Adams, J., Tarbell, N. J., and Yock, T. I. (2010). Proton radiotherapy for pediatric bladder/prostate rhabdomyosarcoma: clinical outcomes and dosimetry compared to intensity modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. [Epub ahead of print].
Cozzi, L., Fogliata, A., Lomax, A., and Bolsi, A. (2001). A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother. Oncol. 61, 287–297.
Debus, J., Schulz-Ertner, D., Schad, L., Essig, M., Rhein, B., Thillman, C., and Wannenmacher, M. (2000) , Stereotactic fractionated radiotherapy for chordomas and chondrosarcomas of the skull base. Int. J. Radiat. Oncol. Biol. Phys. 47, 591–596.
Engelsman, M., DeLaney, T. F., and Hong, T. S. (2010). Proton radiotherapy: the biological effect of treating alternating subsets of fields for different treatment fractions. Int. J. Radiat. Oncol. Biol. Phys. 79, 616–622.
Fitzek, M. M., Linggood, R. M., Adams, J., and Munzenrider, J. E. (2006). Combined proton and photon irradiation for craniopharyngioma: long-term results of the early cohort of patients treated at Harvard Cyclotron Laboratory and Massachusetts General Hospital. Int. J. Radiat. Oncol. Biol. Phys. 64, 1348–1354.
Grutters, J. P. C., Kessels, A. G. H., Pijls-Johannesma, M., De Ruyssher, D., Joore, M. A., and Lambin, P. (2009). Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother. Oncol. 95, 32–40.
Hata, M., Tokuuye, K., Kagei, K., Sugahara, S., Nakayama, H., Fukumitsu, N., Hashimoto, T., Mizumoto, M., Ohara, K., and Akine, Y. (2007). Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: preliminary results of a phase I/II clinical study. Int. J. Radiat. Oncol. Biol. Phys. 68, 786–793.
Hoppe, B. S., Huh, S., Flampouri, S., Nichols, R. C., Oliver, K. R., Morris, C. G., Mendenhall, N. P., and Li, Z. (2010). Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: a dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother. Oncol. 97, 425–520.
International Commission on Radiation Units and Measurements Report 78. (2007). Prescribing, recording, and reporting proton-beam therapy. J. ICRU 7.
Iwata, H., Murakami, M., Demizu, Y., Miyawaki, D., Terashima, K., Niwa, Y., Mima, M., Akagi, T., Hishikawa, Y., and Shibamoto, Y. (2010). High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer 116, 2476–2485.
Johansson, J., Blomquist, E., Montelius, A., Isacsson, U., and Glimelius, B. (2004). Potential outcomes of modalities and techniques in radiotherapy for patients with hypopharyngeal carcinoma. Radiother. Oncol. 72,129–138.
Konski, A., Speier, W., Hanlon, A., Beck, J. R., and Pollack, A. (2007). Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J. Clin. Oncol. 25, 3603–3608.
Kozak, K. R., Adams, J., Krejcarek, S. J., Tarbell, N. J., and Yock, T. I. (2009). A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int. J. Radiat. Oncol. Biol. Phys. 74, 179–186.
Kozak, K. R., Smith, B. L., Adams, J., Kornmehl, E., Katz, A., Gadd, M., Specht, M., Hughes, K., Gioioso, V., Lu, H. M., Braaten, K., Recht, A., Powell, S. N., DeLaney, T. F., and Taghian, A. G. (2006). Accelerated partial-breast irradiation using proton beams: initial clinical experience. Int. J. Radiat. Oncol. Biol. Phys. 66, 691–698.
Krejcarek, S. C., Grant, P. E., Henson, J. W., Tarbell, N. J., and Yock, T. I. (2007). Physiologic and radiographic evidence of the distal edge of the proton beam in craniospinal irradiation. Int. J. Radiat. Oncol. Biol. Phys. 68, 646–649.
Krishnan, S., Foote, R. L., Brown, P. D., Pollock, B. E., Link, M. J., and Garces, Y. I. (2005). Radiosurgery for cranial base chordomas and chondrosarcomas. Neurosurgery 56, 777–784.
Lee, C. T., Bilton, S. D., Famiglietti, R. M., Riley, B. A., Mahajan, A., Chang, E. L., Maor, M. H., Woo, S. Y., Cox, J. D., and Smith, A. R. (2005). Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int. J. Radiat. Oncol. Biol. Phys. 63, 362–372.
Li, J., Dabaja, B., Reed, V., Allen, P. K., Cai, H., Amin, M. V., Garcia, J. A., and Cox, J. D. (2010). Rationale for and preliminary results of proton beam therapy for mediastinal lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 81, 167–174.
Lomax, A. J., Goitein, M., and Adams, J. (2003). Intensity modulation in radiotherapy: photons versus protons in the paranasal sinus. Radiother. Oncol. 66, 11–18.
Lomax, A. J., Pedroni, E., Rutz, H., and Goitein, G. (2004a). The clinical potential of intensity modulated proton therapy. Z. Med. Phys.14, 147–152.
Lomax, A. J., Böhringer, T., Bolsi, A., Coray, D., Emert, F., Goitein, G., Jermann, M., Lin, S., Pedroni, E., Rutz, H., Stadelmann, O., Timmermann, B., Verwey, J., and Weber, D. C. (2004b). Treatment planning and verification of proton therapy using spot scanning: initial experiences. Med. Phys. 31, 3150–3157.
Lundkvist, J., Ekman, M., Ericsson, S. R., Jönsson, B., and Glimelius, B. (2005a). Cost-effectiveness of proton radiation in the treatment of childhood medulloblastoma. Cancer 103, 793–801.
Lundkvist, J., Ekman, M., Ericsson, S. R., Jönsson, B., and Glimelius, B. (2005b). Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncol. 44, 850–861.
Luu, Q. T., Loredo, L. N., Archambeau, J. O., Yonemoto, L. T., Slater, J. M., and Slater, J. D. (2006). Fractionated proton radiation treatment for pediatric craniopharyngioma: preliminary report. Cancer J. 12, 155–159.
MacDonald, S. M., Safai, S., Trofimov, A., Wolfgang, J., Fullerton, B., Yeap, B. Y., Bortfeld, T., Tarbell, N. J., and Yock, T. (2008). Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int. J. Radiat. Oncol. Biol. Phys. 71, 979–986.
MacDonald, S. M., Trofimov, A., Safai, S., Adams, J., Fullerton, B., Ebb, D., Tarbell, N. J., and Yock, T. I. (2011). Proton radiotherapy for pediatric central nervous system germ cell tumors: early clinical outcomes. Int. J. Radiat. Oncol. Biol. Phys. 79, 121–129.
Merchant, T. E., Hua, C. H., Shukla, H., Ying, X., Nill, S., and Oelfke, U. (2008). Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr. Blood Cancer 51, 110–117.
Miralbell, R., Crowell, C., and Suit, H. D. (1992). Potential improvement of three dimension treatment planning and proton therapy in the outcome of maxillary sinus cancer. Int. J. Radiat. Oncol. Biol. Phys. 22, 305–310.
Mizumoto, M., Tsuboi, K., Igaki, H., Yamamoto, T., Takano, S., Oshiro, Y., Hayashi, Y., Hashii, H., Kanemoto, A., Nakayama, H., Sugahara, S., Sakurai, H., Matsumura, A., and Tokuuye, K. (2010). Phase I/II trial of hyperfractionated concomitant boost proton radiotherapy for supratentorial glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 77, 98–105.
Mock, U., Georg, D., Bogner, J., Auberger, T., and Potter, R. (2004). Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 58, 147–154.
Nakayama, H., Sugahara, S., Tokita, M., Satoh, H., Tsuboi, K., Ishikawa, S., and Tokuuye, K. (2010). Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the University of Tsukuba. Int. J. Radiat. Oncol. Biol. Phys. 78, 467–471.
Nihei, K., Ogino, T., Onozawa, M., Murayama, S., Fuji, H., Murakami, M., and Hishikawa, Y. (2010). Multi-institutional phase ii study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. Int. J. Radiat. Oncol. Biol. Phys. 81, 390–396.
Nikoghosyan, A. V., Karapanagiotou-Schenkel, I., Munter, M. W., Jensen, A. D., Combs, S. E., and Debus, J. (2010). Randomised trial of proton vs carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-study. BMC Cancer 10, 607. doi: 10.1186/1471-2407-10-607
Ramaekers, B. L., Pijls-Johannesma, M., Joore, M. A., van den Ende, P., Langendijk, J. A., Lambin, P., Kessels, A. G., and Grutters, J. P. (2011). Systematic review and meta-analysis of radiotherapy in various head and neck cancers: comparing photons, carbon-ions and protons. Cancer Treat. Rev. 37, 185–201.
Ronson, B. B., Schulte, R. W., Han, K. P., Loredo, L. N., Slater, J. M., and Slater, J. D. (2006). Fractionated proton beam irradiation of pituitary adenomas. Int. J. Radiat. Oncol. Biol. Phys. 64, 425–434.
Rutz, H. P., Weber, D. C., Goitein, G., Ares, C., Bolsi, A., Lomax, A. J., Pedroni, E., Coray, A., Hug, E. B., and Timmermann, B. (2008). Postoperative spot-scanning proton radiation therapy for chordoma and chondrosarcoma in children and adolescents: initial experience at Paul Scherrer Institute. Int. J. Radiat. Oncol. Biol. Phys. 71, 220–225.
Sawakuchi, G. O., Titt, U., Mirkovic, D., and Mohan, R. (2008). Density heterogeneities and the influence of multiple Coulomb and nuclear scatterings on the Bragg peak distal edge of proton therapy beams. Phys. Med. Biol. 53, 4605–4619.
Sejpal, S., Komaki, R., Tsao, A., Chang, J. Y., Liao, Z., Wei, X., Allen, P. K., Lu, C., Gillin, M., and Cox, J. D. (2011). Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer 117, 3004–3013.
Skarsgard, L. D. (1998). Radiobiology with heavy charged particles: a historical review. Phys. Med. 14(Suppl. 1), 1–19.
Slater, J. M., Slater, J. D., and Archambeau, J. O. (1992). Carcinoma of the tonsillar region: potential for use of proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 22, 311–319.
St Clair, W. H., Adams, J. A., Bues, M., Fullerton, B. C., La Shell, S., Kooy, H. M., Loeffler, J. S., and Tarbell, N. J. (2004). Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 58, 727–734.
Steneker, M., Lomax, A., and Schneider, U. (2006). Intensity modulated photon and proton therapy for the treatment of head and neck tumors. Radiother. Oncol. 80, 263–267.
Taheri-Kadkhoda, Z., Björk-Eriksson, T., Nill, S., Wilkens, J. J., Oelfke, U., Johansson, K. A., Huber, P. E., and Munter, M. W. (2008). Intensity-modulated radiotherapy of nasopharyngeal carcinoma: a comparative treatment planning study of photons and protons. Radiat. Oncol. 3, 4.
Talcott, J. A., Rossi, C., Shipley, W. U., Clark, J. A., Slater, J. D., Niemierko, A., and Zietman, A. L. (2010). Patient-reported long-term outcomes after conventional and high-dose combined proton and photon radiation for early prostate cancer. JAMA 303, 1046–1053.
Timmermann, B., Lomax, A. J., Nobile, L., Grotzer, M. A., Weiss, M., Kortmann, R. D., Bolsi, A., and Goitein, G. (2007). Novel technique of craniospinal axis proton therapy with the spot-scanning system: avoidance of patching multiple fields and optimized ventral dose distribution. Strahlenther. Onkol. 183, 685–688.
Timmermann, B., Schuck, A., Niggli, F., Weiss, M., Lomax, A., and Goitein, G. (2006). “Spot-scanning” proton therapy for rhabdomyosarcomas of early childhood. First experiences at PSI. Strahlenther. Onkol. 182, 653–659.
Torres, M. A., Chang, E. L., Mahajan, A., Lege, D. G., Riley, B. A., Zhang, X., Lii, M., Kornguth, D. G., Pelloski, C. E., and Woo, S. Y. (2009). Optimal treatment planning for skull base chordoma: photons, protons, or a combination of both? Int. J. Radiat. Oncol. Biol. Phys. 74, 1033–1039.
Toscas, J. I., Linero, D., Rubio, I., Hidalgo, A., Arnalte, R., Escudé, L., Cozzi, L., Fogliata, A., and Miralbell, R. (2010). Boosting the tumor bed from deep-seated tumors in early-stage breast cancer: a planning study between electron, photon, and proton beams. Radiother. Oncol. 96, 192–198.
Truong, M. T., Kamat, U. R., Liebsch, N. J., Curry, W. T., Lin, D. T., Barker, F. G. 2nd, Loeffler, J. S., and Chan, A. W. (2009). Proton radiation therapy for primary sphenoid sinus malignancies: treatment outcome and prognostic factors. Head Neck 10, 1297–1308.
Weber, D. C., Lomax, A. J., Rutz, H. P., Stadelmann, O., Egger, E., Timmermann, B., Pedroni, E. S., Verwey, J., Miralbell, R., Goitein, G., and Swiss Proton Users Group. (2004). Spot-scanning proton radiation therapy for recurrent, residual or untreated intracranial meningiomas. Radiother. Oncol. 71, 251–258.
Widescott, L., Pierelli, A., Fiorino, C., Dell’oca, I., Broggi, S., Cattaneo, G. M., Di Muzio, N., Fazio, F., Calandrino, R., and Schwarz, M. (2008). Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and NTCP evaluation. Int. J. Radiat. Oncol. Biol. Phys. 72, 589–596.
Winkfield, K. M., Linsenmeier, C., Yock, T. I., Grant, P. E., Yeap, B. Y., Butler, W. E., and Tarbell, N. J. (2009). Surveillance of craniopharyngioma cyst growth in children treated with proton radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 73, 716–721.
Yock, T., Schneider, R., Friedmann, A., Adams, J., Fullerton, B., and Tarbell, N. (2005). Proton radiotherapy for orbital rhabdomyosarcoma: clinical outcome and a dosimetric comparison with photons. Int. J. Radiat. Oncol. Biol. Phys. 63, 1161–1168.
Yuh, G. E., Loredo, L. N., Yonemoto, L. T., Bush, D. A., Shahnazi, K., Preston, W., Slater, J. M., and Slater, J. D. (2004). Reducing toxicity from craniospinal irradiation: using proton beams to treat medulloblastoma in young children. Cancer J. 10, 386–390.
Zenda, S., Kawashima, M., Nishio, T., Kohno, R., Nihei, K., Onozawa, M., Arahira, S., and Ogino, T. (2010). Proton beam therapy as a nonsurgical approach to mucosal melanoma of the head and neck: a pilot study. Int. J. Radiat. Oncol. Biol. Phys. 81, 135–139.
Zietman, A. L., DeSilvio, M. L., Slater, J. D., Rossi, C. J. Jr., Miller, D. W., Adams, J. A., and Shipley, W. U. (2005). Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized clinical trial. JAMA 294, 1233–1239.
Keywords: proton therapy, radiation, radiation treatment planning, Bragg-peak
Citation: Hill-Kayser CE, Both S and Tochner Z (2011) Proton therapy: ever shifting sands and the opportunities and obligations within. Front. Oncol. 1:24. doi: 10.3389/fonc.2011.00024
Received: 12 July 2011; Accepted: 11 August 2011;
Published online: 06 September 2011.
Edited by:
Brian James Davis, Mayo Clinic and Foundation, USAReviewed by:
Joel S. Greenberger, University of Pittsburgh Medical Center-Shadyside, USAO. Kenneth Macdonald, Providence Medical Center, USA
Copyright: © 2011 Hill-Kayser, Both and Tochner. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Christine E. Hill-Kayser, Department of Radiation Oncology, Perelman School of Medicine at the University of Pennsylvania, Administrative Offices, 3400 Civic Center Boulevard, TRC-2 Philadelphia, PA 19104, USA. e-mail: hill@uphs.upenn.edu
