- Dipartimento di Biotecnologie e Bioscienze, Università di Milano-Bicocca, Milano, Italy
ACH1 encodes a mitochondrial enzyme of Saccharomyces cerevisiae endowed with CoA-transferase activity. It catalyzes the CoASH transfer from succinyl-CoA to acetate generating acetyl-CoA. It is known that ACH1 inactivation results in growth defects on media containing acetate as a sole carbon and energy source which are particularly severe at low pH. Here, we show that chronological aging ach1Δ cells which accumulate a high amount of extracellular acetic acid display a reduced chronological lifespan. The faster drop of cell survival is completely abrogated by alleviating the acid stress either by a calorie restricted regimen that prevents acetic acid production or by transferring chronologically aging mutant cells to water. Moreover, the short-lived phenotype of ach1Δ cells is accompanied by reactive oxygen species accumulation, severe mitochondrial damage, and an early insurgence of apoptosis. A similar pattern of endogenous severe oxidative stress is observed when ach1Δ cells are cultured using acetic acid as a carbon source under acidic conditions. On the whole, our data provide further evidence of the role of acetic acid as cell-extrinsic mediator of cell death during chronological aging and highlight a primary role of Ach1 enzymatic activity in acetic acid detoxification which is important for mitochondrial functionality.
Introduction
In the single-celled yeast Saccharomyces cerevisiae, the replicative and chronological aging paradigms have been described. In the latter, chronological lifespan (CLS) is the mean and maximum survival period of a population of non-dividing cells in postmitotic stationary phase. Viability over time is defined as the ability to resume mitotic growth upon return to rich fresh medium (Fabrizio and Longo, 2003). This growth arrest simulates the postmitotic quiescent state of multicellular organisms.
Yeast cells respond to nutrient scarcity by inducing a series of metabolic, physiological, and morphological changes which mainly increase stress resistance in order to survive starvation (Smets et al., 2010). Moreover, in this context, unfit cells can undergo apoptosis for the benefit of the whole population (Longo et al., 2005; Fabrizio and Longo, 2008). Apoptosis is a highly regulated cellular “suicide” program whose activation can rely on different exogenous or endogenous stimuli (Carmona-Gutierrez et al., 2010). Chronological aging is an example of an endogenous, physiological trigger (Herker et al., 2004), while treatment of yeast cells with a harsh environmental stress, such as acetic acid, is an example of an exogenous one (Ludovico et al., 2001). Acetic acid which is also a by-product of the yeast metabolism and in some settings has been reported to restrict CLS (Burtner et al., 2009; Murakami et al., 2010). In both chronological aging and acetic acid-induced apoptosis, mitochondria play an active and fundamental role (Ludovico et al., 2002; Bonawitz et al., 2006; Aerts et al., 2009; Pan, 2011). In addition, among the different mitochondrial proteins involved in the execution of the acetic acid-induced apoptotic program, Dnm1 has been shown to be also implicated in chronological aging. This protein is required for mitochondrial fission and its lack of function impairs not only mitochondrial apoptotic fragmentation but also increases CLS (Scheckhuber et al., 2007), underlying a connection among mitochondrial dynamics, apoptosis, and aging.
Mitochondria are also important organelles for yeast carbon metabolism and become essential for growth on non-fermentable substrates such as acetate. Acetate metabolism requires acetate activation to acetyl-CoA by acetyl-CoA synthetase isoenzymes, the mitochondrial Acs1, and cytosolic Acs2 which are known as the gluconeogenic and glycolytic isoforms, respectively (Verduyn et al., 1992; van den Berg et al., 1996). Once generated, acetyl-CoA can be used to fuel the glyoxylate and TCA cycles and also for the synthesis of macromolecules which requires active gluconeogenesis (dos Santos et al., 2003). The concentration of cellular acetyl-CoA is primarily controlled by the balance between its synthesis and utilization in the different metabolic pathways.
The mitochondrial enzyme Ach1 was initially assumed to act as acetyl-CoA hydrolase, probably involved in reducing mitochondrial accumulation of acetyl-CoA during growth on acetate to avoid toxic effects (Lee et al., 1990; Buu et al., 2003). However, hydrolysis of a high energy thioester bond has no apparent metabolic advantage since would result in losing two ATP molecules which have been consumed for acetate activation in the ester. Similarly, the ortholog Acu-8 from Neurospora crassa had been classified as an acetyl-CoA hydrolase (Connerton et al., 1992), giving no further possible explanation for the physiological role of this “energy wasting” process. Thus, the existence of such an enzyme has been denoted as a biochemical conundrum (Buu et al., 2003). Successively, the mitochondrial enzyme CoaT from Aspergillus nidulans, which is involved in propionyl-CoA detoxification in the presence of acetate, was characterized (Fleck and Brock, 2009). This protein shows a high aminoacidic identity to Ach1 and Acu-8, but displays a CoA-transferase activity being able to transfer the CoASH moiety from propionil-CoA to acetate (Fleck and Brock, 2009). A re-characterization of Ach1 has been performed indicating that this enzyme acts as a CoA-transferase by catalyzing the transfer of the CoASH moiety from succinyl-CoA to acetate. Thus, it could detoxify mitochondria from acetate by an enzymatic reaction which would save one ATP (Fleck and Brock, 2009).
In this work we provide evidence that ACH1 inactivation severely impairs mitochondrial functions. This influences the acetate metabolism, the CLS which is restricted, and the occurrence of apoptosis.
Materials and Methods
Yeast Strains and Growth Conditions
All haploid strains with null mutations were generated by PCR-based methods in a W303-1A background (MAT a ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100): ach1Δ (ach1Δ::KlLEU2), yca1Δ (yca1Δ::URA3; Bettiga et al., 2004) and ach1Δ yca1Δ (ach1Δ::KlLEU2 yca1Δ::URA3). The accuracy of gene replacement was verified by PCR with flanking and internal primers. Standard methods were used for DNA manipulation and yeast transformation. Yeast cells were grown in batches at 30°C in minimal medium (Difco Yeast Nitrogen Base without amino acids, 6.7 g/l), supplemented with 2% w/v or 0.05% w/v (Calorie Restriction, CR) glucose. Auxotrophies were compensated for with a fourfold excess of supplements (Fabrizio et al., 2005). For shift experiments in acetate-containing medium, cells were grown in minimal medium 2% glucose up to exponential phase (107 cells/ml), centrifuged, and resuspended in fresh acetate medium. For acetate medium, pre-calculated amounts of 0.2 M acetic acid and 0.2 M sodium acetate solutions were mixed and added to minimal medium to obtain the required pH and molarity. All strains were inoculated at the same cellular density (culture volume no more than 20% of the flask volume) and growth was monitored by determining cell number using a Coulter Counter-Particle Count and Size Analyser, as described (Vanoni et al., 1983). Duplication times (Td) were obtained by linear regression of the cell number increase over time on a semi-logarithmic plot.
For growth assays on agar plates, exponentially growing cells were dropped (5 μl from a concentrated solution of 108 cells/ml and from serial 10-fold dilutions) onto rich medium (YEP, 1% w/v yeast extract, 2% w/v bacto peptone) agar plates supplemented with acetic acid (YEPA) at the indicated pH and molarity. Plates were incubated at 30°C for 3–5 days.
Metabolite Measurements
At designated time-points, aliquots of the yeast cultures were centrifuged and supernatants were frozen at −20°C until used. Glucose, ethanol, and acetate concentrations in the growth medium were determined using enzymatic assays (K-HKGLU, K-ETOH, and K-ACET kits from Megazyme). Values represent the average of three independent experiments.
CLS Determination
Survival experiments in expired medium were performed on cells grown in minimal medium (with a fourfold excess of supplements) containing 2% glucose as described by (Fabrizio and Longo, 2003; Fabrizio et al., 2005). During growth, cell number and extracellular glucose, ethanol, and acetic acid were measured in order to define the growth profile (exponential phase, diauxic shift, post-diauxic phase, and stationary phase) of the culture. Cell survival was monitored by harvesting aliquots of cells starting 72 h (Day 3, first age-point) after the diauxic shift, when cells stopped dividing and cell density reached a plateau value. Subsequent age-points were taken every 2–3 days. Cells were plated onto rich medium/2% glucose (YEPD) plates and viability was scored by counting colony-forming units (CFUs). The number of CFUs at Day 3 was considered the initial survival (100%). For survival experiments in water, post-diauxic cells (at Day 1) were harvested, washed with sterile distilled water, and resuspended in a volume of water equal to the initial culture volume. Every 48 h, cells were washed with water and resuspended in fresh water to remove nutrients released by dead cells (Fabrizio and Longo, 2003). Survival experiments in water containing acetic acid were performed essentially as described by (Fabrizio et al., 2005) for survival in water/ethanol. In our setting, acetic acid (10 mM) substituted for ethanol and the pH of the water was adjusted to 2.8 for both the control and acetic acid add-back cultures (Burtner et al., 2009). Viability was measured as described above. Survival experiments in expired medium were also performed on cells grown in minimal medium (with a fourfold excess of supplements) containing 0.05% glucose.
Index of respiratory competence (IRC) was also measured according to Parrella and Longo (2008) by plating identical samples on YEPD plates and on rich medium 3% glycerol (YEPG) plates. IRC was calculated as colonies on YEPG divided by colonies on YEPD times 100%.
Tests for Oxidative Stress and Cell Death Markers
Reactive oxygen species (ROS) were detected with dihydrorhodamine 123 (DHR123, Sigma) and dihydroethidium (DHE, Sigma; Madeo et al., 1999). TdT-mediated dUTP nick end labeling (TUNEL, Roche) and Annexin V (ApoAlert Annexin V Apoptosis Kit, Clontech) assay for apoptotic markers as well as propidium iodide (PI, Sigma) staining for necrotic ones were performed as described (Orlandi et al., 2004). Caspase activity was measured with 50 μM FITC-VAD-fmk (CaspACE, Promega) as described (Bettiga et al., 2004). The mitochondrial membrane potential was assessed by staining with rhodamine 123 (RH123) and 3,3′′-dihexyloxacarbocyanine iodide (DiOC6; both from Molecular Probes, Invitrogen), according to (Koning et al., 1993; Du et al., 2008).
A Nikon Eclipse E600 fluorescence microscope equipped with a Leica DC 350 F ccd camera was used. Digital images were acquired using FW4000 software (Leica).
Statistical Analysis
All values are presented as the mean ± standard error of the means (SEM). Differences in measurements were assessed by Student’s t-test. The level of statistical significance was set at a P value of ≤ 0.001.
Results
Loss of ACH1 Restricts CLS and Increases Apoptosis
Since acetic acid is a factor whose presence in the growth medium promotes chronological aging and Ach1 is a mitochondrial CoA-transferase which has been proposed to be involved in acetic acid detoxification (Fleck and Brock, 2009), we decided to analyze the effects of ACH1 inactivation on CLS. In the context of a standard CLS experiment (Fabrizio and Longo, 2003), after the diauxic shift, when cells switched from fermentation- to a respiration-based metabolism, we also measured extracellular ethanol and acetic acid concentrations (see Material and Methods). As a by-product of glucose fermentation, the wild type (wt) strain and ach1Δ mutant produced a similar maximal amount of ethanol which, during the post-diauxic phase, decreased at a similar rate in both culture media (Figure 1A). Acetic acid concentration, in the wt culture, initially increased and then rapidly decreased because it is utilized by the cells for the respiratory metabolism during the post-diauxic phase (Figure 1B). On the contrary, ach1Δ mutant showed a prolonged accumulation of this C2 compound. In fact, high levels of acetic acid were still present 6 days following the entry in post-diauxic phase (Figure 1B) indicating a severe impairment in its utilization in line with a previous study (Fleck and Brock, 2009). Moreover, ACH1 inactivation significantly reduced CLS (Figure 1C).
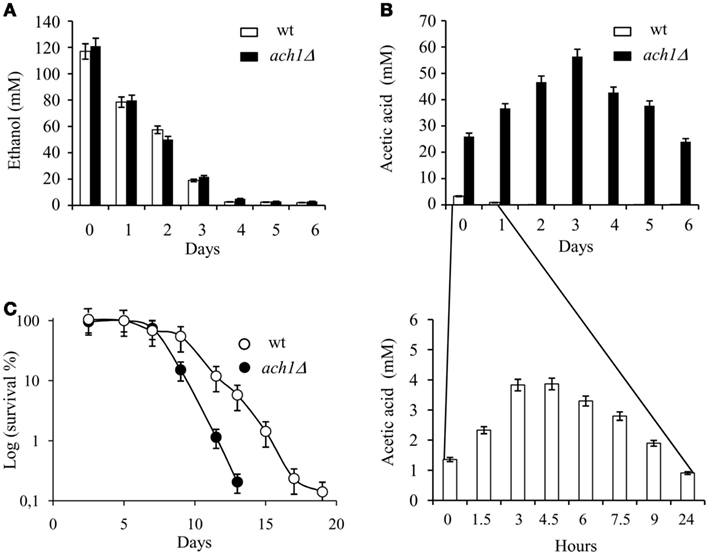
Figure 1. ACH1 inactivation shortens CLS in concert with increased extracellular acetic acid. Wild type (wt) and ach1Δ mutant cells were grown in minimal medium (with a fourfold excess of supplements) containing 2% glucose and followed up to stationary phase. Bar charts of ethanol (A) and acetic acid (B) concentrations measured in the medium of both cultures at the indicated time-points. Day 0, diauxic shift. Inset: time-scale blow-up. Error bars are the standard deviation of three replicates. (C) CLS of wt and ach1Δ mutant cells. At every time-point, viability was determined by counting CFUs on YEPD plates. 72 h after the diauxic shift (Day 3), was considered the first age-point (see Materials and Methods). Error bars are the standard deviation of three replicates.
Yeast cells undergo apoptosis during chronological aging as well as upon exposure to acetic acid (Ludovico et al., 2001; Herker et al., 2004; Fabrizio and Longo, 2008; Rockenfeller and Madeo, 2008). Thus, we assessed different apoptotic features in the short-lived ach1Δ cells. DNA fragmentation was detected by TUNEL assay, exposure of phosphatidylserine at the outer leaflet of the plasma membrane and membrane integrity were evaluated by combined Annexin V/PI staining which allows for the identification of early apoptotic events (Annexin V+) and necrotic cell death (PI+; Madeo et al., 1997). At Day 6, when survival of ach1Δ cells began to decrease (Figure 1C), in these cells DNA strand breakage occurred and the percentage of TUNEL+ was fourfold higher (24 ± 1%) as compared to wt ones (6 ± 1%; Figure 2A). At the same time-point, Annexin V staining, under conditions where plasma membrane was not compromised, as indicated by exclusion of PI co-staining (data not shown), detected about 18.4 ± 3.1% of Annexin V+ach1Δ cells in comparison to 7.3 ± 1.9% in the wt (Figure 2B). All these data indicate that the ach1Δ mutant is subject to a much faster chronological aging process accompanied by an early insurgence of apoptosis.
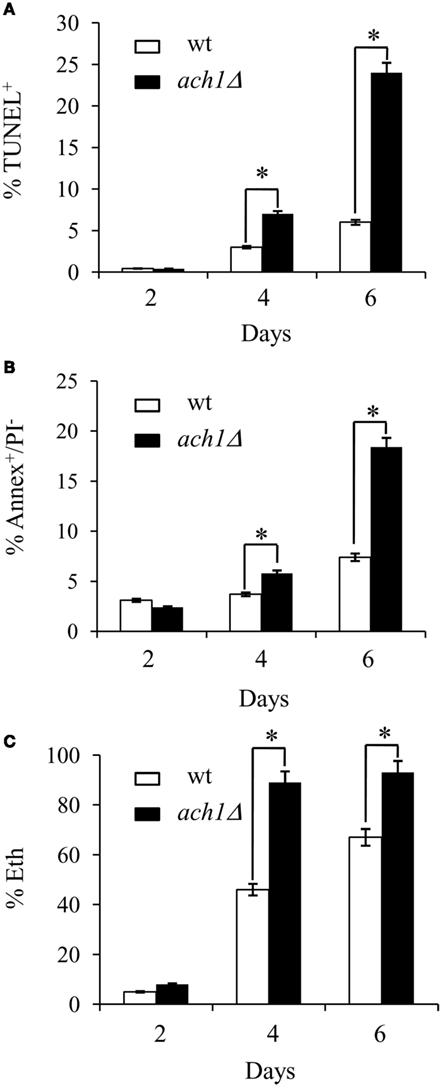
Figure 2. Chronological aging ach1Δ cells display an early insurgence of apoptosis. Chronological aging cultures of Figure 1 at the indicated time-points were assessed for DNA fragmentation by TUNEL assay (A), phosphatidylserine externalization and membrane integrity by Annexin V/propidium iodide (PI) co-staining (Annex V+/PI−, (B) and intracellular superoxide by dihydroethidium conversion into fluorescent ethidium (Eth), (C). Summary graphs of the percentage of positive cells for each test are indicated. Evaluation of about 1000 cells for each sample in three independent experiments was performed. Standard deviations are indicated. Statistical significance between the strains is indicated (*P ≤ 0.001, Student’s t-test).
In the ach1Δ Mutant Mitochondrial Functionality is Impaired
ROS accumulation is an important endogenous trigger which has been associated with apoptosis during chronological aging and acetic acid treatment (see Carmona-Gutierrez et al., 2010, for a review). ROS content, measured as the superoxide-driven conversion of non-fluorescent DHE into fluorescent ethidium (Eth), was already significantly enhanced in the ach1Δ mutant (89 ± 3%) as compared to the wt strain (46 ± 4%) at Day 4 (Figure 2C) revealing an endogenous situation of higher oxidative stress in the mutant.
Mitochondria are key organelles in superoxide generation. This radical can directly induce oxidative damage or can be converted to other ROS which, in turn, induce aging-associated damage (Pan, 2011). In addition, a direct correlation between reduced CLS and dysfunctional mitochondria has been reported (Bonawitz et al., 2006; Fabrizio and Longo, 2007). Consequently, we decided to analyze whether ACH1 inactivation could affect mitochondrial functionality. S. cerevisiae can grow by either fermentation on glucose as carbon source or by respiration by using different non-fermentable substrates such as glycerol. The growth on the latter can take place only when mitochondria are functional. This feature is exploited to evaluate whether mitochondria are extensively damaged at a point when the cell is still viable. The percentage of viable cells which are competent to respire defines the IRC (Parrella and Longo, 2008). At Day 1, both the wt and ach1Δ chronologically aging cells had an IRC of about 100% (Figure 3A) indicating that all the cells are respiration-competent. During the following days, this value never dropped below about 80% for the wt, while it decreased quickly for the ach1Δ mutant reaching about 15–20% by Day 6 which is indicative of a time-dependent loss of mitochondrial functionality. In parallel, mitochondrial morphology was examined by using DiOC6 dye. In fact, at low concentrations (20–100 ng/ml), this dye accumulates specifically at mitochondrial membranes and can be observed by fluorescence microscopy. However, cells with low mitochondrial membrane potential will fail to accumulate DiOC6 (Koning et al., 1993). Balanced fusion and fission of mitochondria results in tubular mitochondrial morphology, as was the case for wt cells, whereas for ach1Δ cells punctiform formations were observed at Day 2 (Figure 3B). The conversion of mitochondrial morphology from tubular structures to punctuate ones is also referred to as mitochondrial fragmentation. It is presumed to occur by excessive mitochondrial fission (Fabrizio and Longo, 2008) and has been observed in yeast apoptosis induced by different stimuli including acetic acid treatment (Fabrizio and Longo, 2008). Moreover, at Day 4, DiOC6 staining was greatly reduced in ach1Δ cells (Figure 3B) revealing a reduction in mitochondrial membrane potential. Taken together these data indicate that the early insurgence of apoptosis in chronological aging ach1Δ cells is preceded by a severe damage of mitochondria.
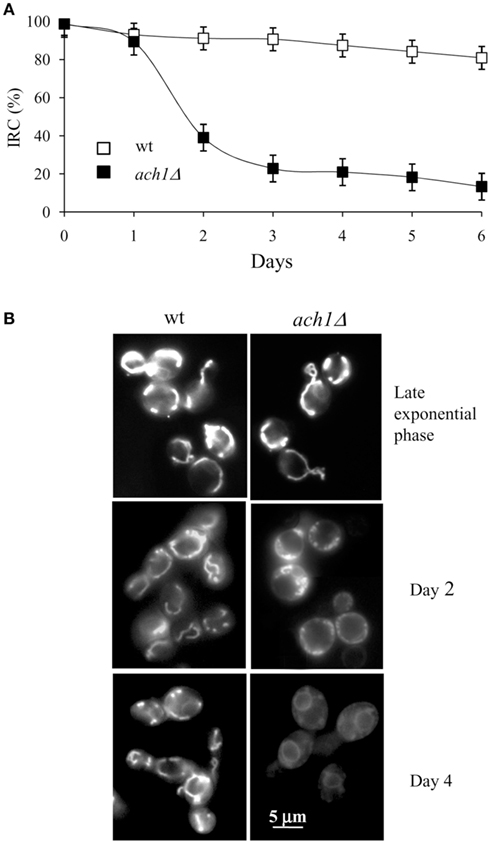
Figure 3. Chronological aging ach1Δ cells display compromised mitochondrial activity. Chronological aging cultures of Figure 1 at the indicated time-points were serially diluted, plated onto YEPD and YEPG plates and the index of respiratory competence (IRC) was determined (A). Standard deviations of three independent experiments are indicated. (B) Same cells stained with DiOC6 to visualize mitochondrial membranes. Morphologies of the mitochondria of cells in late exponential phase are also reported. Representative images are shown. Bar: 5 μm.
Acetic Acid is Responsible for the Reduced CLS of ach1Δ Mutant
In order to determine whether the reduced CLS of ach1Δ mutant was linked to the excess of acetic acid in the extracellular environment, we first examined the effects of lowering glucose concentration from 2 to 0.05% in the initial culture medium. This is a growth condition of CR which reduces acetic acid production in the chronological aging culture and extends CLS (Burtner et al., 2009). As expected, almost undetectable extracellular acetic acid was present in wt cultures grown in CR (Figure 4A) which displayed an enhanced survival relative to wt cultures grown on 2% glucose (Figure 4B and Figure 1C). Growth in a CR regimen for the ach1Δ mutant produced undetectable extracellular acetic acid as well (Figure 4A). Additionally, it was also sufficient to avoid completely the deleterious effect on cell viability associated with growth on 2% glucose. In fact, CR ach1Δ cells had a CLS comparable to CR wt cells (Figures 4B and 1C).
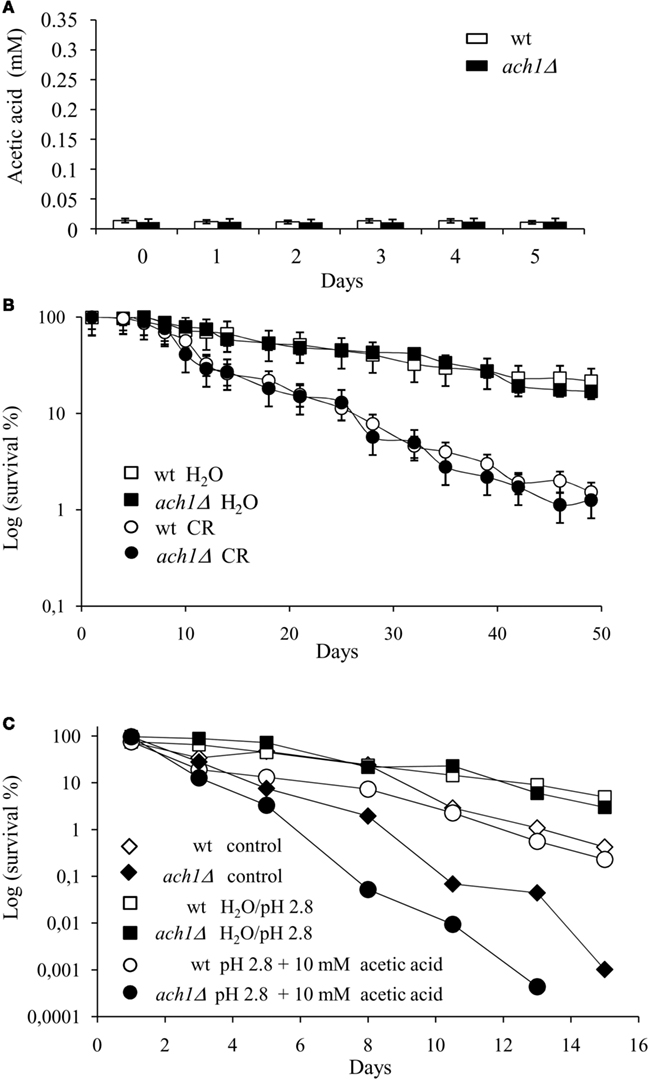
Figure 4. CR suppresses the CLS-shortening effect of ACH1 inactivation. Wild type and ach1Δ cells were grown on 0.05% glucose (CR) and extracellular acetic acid concentration was measured for both cultures at the indicated time-points. Day 0, diauxic shift (A). In parallel, cell survival in the exhausted medium was determined by counting CFUs on YEPD plates (B). Wild type and ach1Δ chronological aging cells grown on 2% glucose were switched to water (extreme CR) at Day 1. Every 48 h, cultures were resuspended in fresh water and at every time-point, viability was measured by counting CFUs on YEPD plates (B). Survival of cells in their exhausted medium was also monitored as control (reported in Figures 1C and 4C). Error bars are the standard deviation of three replicates. (C) Wild type and ach1Δ chronological aging cells grown on 2% glucose were switched to water adjusted to pH 2.8 and water/pH 2.8 containing 10 mM acetic acid at Day 1. Every 48 h, cultures were resuspended in fresh water/pH 2.8 and each time 10 mM acetic acid was added. At every time-point, viability was measured as in (B). Survival of cells in their exhausted medium was also monitored (control). One representative experiment is shown.
We next monitored survival of cells switched from the expired medium to water. Incubation in water is an extreme form of CR which is known to dramatically extend CLS (Fabrizio et al., 2005). Wt and ach1Δ mutant cells were grown on 2% glucose and after the diauxic shift switched to water (see Materials and Methods). As shown in Figure 4B, both wt and ach1Δ cells, incubated in water, increased CLS to the same extent. Thus, the short-lived phenotype of the ach1Δ mutant seems to be mainly due to the toxicity of acetic acid which is accumulated in the environment of chronologically aging ach1Δ cells.
Starting from the aforementioned results, we wondered whether the addition of acetic acid could influence the chronological survival of ach1Δ cells. It has been previously reported that the addition of acetic acid (10 mM) to low pH (2.8) water can prevent CLS extension of chronologically aging cells associated with their transfer to water (Burtner et al., 2009). Wt and ach1Δ cultures grown in 2% glucose were transferred to low pH water supplemented with acetic acid after the diauxic shift and cell viability monitored. In line with acetic acid pro-aging role, acetic acid add-back cultures had a reduced CLS compared to cells incubated in water alone (Figure 4C). Notably, acetic acid affected chronological survival of ach1Δ cells to a higher extent relative to wt cells. In fact, acetic acid add-back wt cultures displayed a CLS similar to that of wt cells aged in their exhausted medium in agreement with (Burtner et al., 2009), while for the acetic acid add-back mutant cultures the CLS was much more reduced (Figure 4C). Together all these data are fully consistent with the notion that the extracellular acetic acid is responsible for the reduced CLS of the ach1Δ mutant and also suggest that the lack of Ach1 makes cells more sensitive to acetic acid.
The ach1Δ Mutant Displays an Apoptotic Caspase-Dependent Phenotype
Since ACH1 deletion results in growth defects on media containing acetic acid as a sole carbon and energy source which are particularly severe under acidic conditions (Fleck and Brock, 2009), to further refine our investigation, we examined whether such a growth impairment could be ascribed to mitochondrial damages. For this purpose, wt and ach1Δ cells exponentially growing on 2% glucose were harvested and transferred to 50 mM acetic acid medium, pH 4.5. In this way, cells were released from glucose repression and were able to express all the genes involved in acetate catabolism and assimilation (Paiva et al., 2004), including ACH1 (Lee et al., 1996). Moreover, the combination 50 mM acetic acid/pH 4.5 is still a permissive growth condition for wt cells but not for the mutant (Fleck and Brock, 2009). Analyses were performed 4 and 16 h after the metabolic shift corresponding to time-windows during which gene expression changes required for acetate metabolism (Paiva et al., 2004) and a significant CoA-transferase activity (Fleck and Brock, 2009) were respectively detected. At these time-points, wt and ach1Δ cells were incubated with RH123 in order to visualize active mitochondria, and with the ROS-sensing dye, DHR123. After 4 h, in both strains similar mitochondrial patterns were observed with RH123 associated with DHR123-negative staining indicating that mitochondria retain their membrane potential and no ROS accumulation took place (Figure 5A). This was still the case for wt cells at 16 h, while in the mutant a severe reduction in mitochondrial membrane potential and increase in ROS-accumulating cells (about 80%) were observed (Figures 5A,B). These features were also accompanied by the appearance of Annexin V+ (about 29%) and TUNEL+ (about 37%) cells (Figure 5B) suggesting the onset of apoptosis.
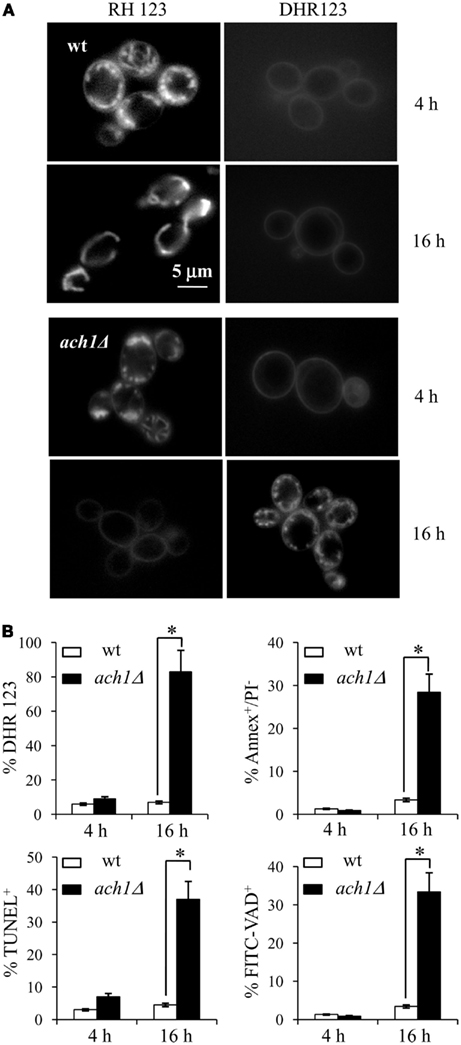
Figure 5. The presence of acetic acid induces apoptosis in ach1Δ cells. Wild type and ach1Δ cells exponentially growing on 2% glucose were shifted to acetate (50 mM) medium, pH 4.5 as sole carbon source. At the indicated time-points after the shift, cells were stained with Rhodamine 123 (RH123) to visualize functional mitochondria and with DHR123 for the presence of intracellular ROS (A). Representative images are shown. Bar: 5μm. The same cultures were analyzed for apoptotic markers by Annexin V/PI co-staining and TUNEL assay, and for caspase activity by FITC-VAD-fmk. Summary graphs of the percentage of ROS-accumulating cells, of Annexin V+/PI−, of TUNEL+ and FITC-VAD+ are reported (B). Evaluation of about 500 cells for each sample in three independent experiments was performed. Standard deviations are indicated. Statistical significance between the strains is indicated (*P ≤ 0.001, Student’s t-test).
Both caspase-dependent and caspase-independent cell death pathways have been described in yeast (Madeo et al., 2009). The Yca1 metacaspase is a yeast functional ortholog of mammalian caspases which mediates the apoptotic process triggered by several intrinsic and extrinsic inducers including acetic acid (Guaragnella et al., 2006; Madeo et al., 2009). To evaluate whether ach1Δ cells shifted to the acetate medium showed an endogenous metacaspase activity, the FITC-labeled caspase inhibitor VAD-fmk, which binds to activated caspase, was used. 16 h after the shift, about 32 ± 2.3% of ach1Δ cells were FITC-positive (Figure 5B) suggesting that apoptosis in ach1Δ cells occurs through a caspase-dependent cascade. Consequently, we analyzed the effects of YCA1 inactivation in the ach1Δ background. Initially, we assessed cellular growth on 50 mM acetic acid media whose pH had been adjusted to 5.8 or 4.5: a permissive and a restrictive growth condition for the ach1Δ mutant, respectively (Fleck and Brock, 2009). As depicted in Figure 6A, both wt and yca1Δ cells grew on all the acetic acid-containing media while acidification of the medium affected severely the ach1Δ mutant viability. Notably, this effect was almost completely prevented by deleting YCA1 (Figure 6A). Changes in the pH of glucose-containing media did not influence the growth of all strains (data not shown). Moreover, on 50 mM acetic acid medium, pH 4.5, the measurement of apoptotic markers (Annexin V+/PI−) and ROS showed that YCA1 inactivation partly rescued the ach1Δ mutant from apoptosis, as well as from ROS accumulation (Figure 6B). Decreases in Annexin V+/PI− cells and ROS were also observed in the ach1Δ yca1Δ mutant compared to the ach1Δ mutant in 35 mM acetic acid medium, pH 4.5 (Figure 6B). This was accompanied by an improvement of cellular growth (Figure 6C) confirming the results obtained for 50 mM acetic acid medium. Together all these data point to an involvement of Yca1 in the caspase-dependent apoptosis of the ach1Δ mutant promoted by acetic acid.
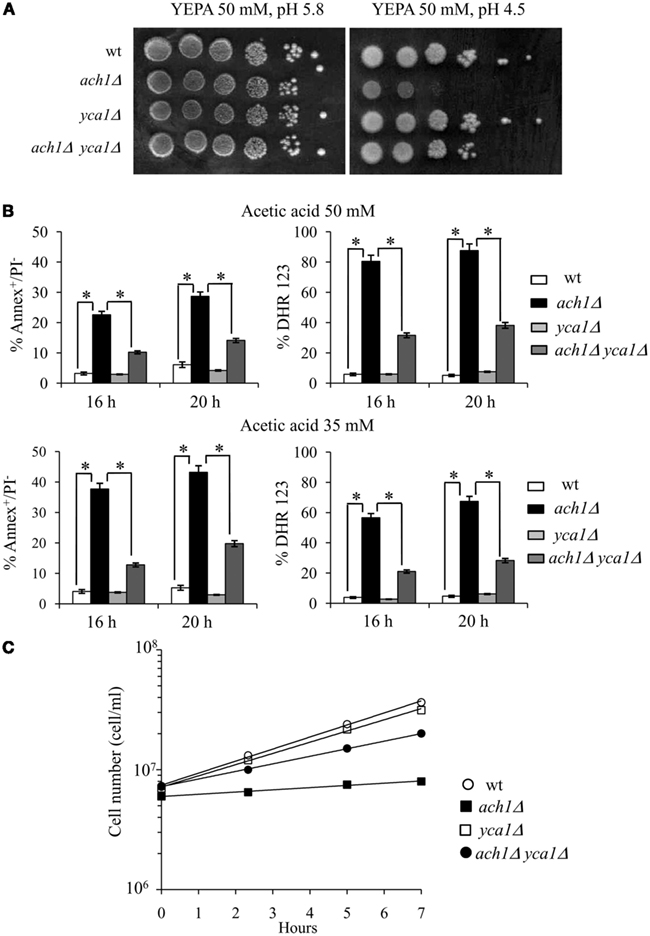
Figure 6. Effects of YCA1 inactivation on ach1Δ cells. (A) The indicated strains exponentially growing on 2% glucose were spotted (10-fold serial dilutions) onto plates containing 50 mM acetic acid (YEPA) as a carbon source at pH 5.8 and pH 4.5. Plates were incubated at 30°C for 4 days. One significant experiment out of 3 is shown. (B) The indicated strains exponentially growing on 2% glucose were shifted to 35 mM and 50 mM acetic acid-containing media, pH 4.5. At the indicated time-points after the shift, cells were analyzed for apoptotic markers by Annexin V/PI co-staining and stained with DHR123 for the presence of intracellular ROS. Summary graphs of the percentage of Annexin V+/PI− and of ROS-accumulating cells are reported. Evaluation of about 500 cells for each sample in three independent experiments was performed. Standard deviations are indicated. Statistical significance between the strains is indicated (*P ≤ 0.001, Student’s t-test). (C) Growth curves of the indicated strains grown in 35 mM acetic acid-containing medium, pH 4.5. Growth was measured as increase in cell number over time. One representative experiment is shown.
Discussion
Acetic acid, a well known by-product of yeast glucose fermentation, has been identified as a cell-extrinsic mediator of cell death during chronological aging (Burtner et al., 2009). Data reported in this paper provide more experimental evidence of a role for acetic acid toxicity as a determinant of CLS and of the positive feed-forward connection between mitochondrial damage and apoptosis. In fact, we show that inactivation of ACH1 encoding a mitochondrial CoASH transferase which catalyzes the transfer of the CoASH moiety from succinyl-CoA to acetate (Fleck and Brock, 2009), reduces CLS. The short-lived phenotype relies on an excess of extracellular acetic acid which is accumulated in the medium of chronologically aging ach1Δ cells. In fact, the faster chronological aging process can be completely abrogated either by transferring chronologically aging ach1Δ cells from the expired medium to water, thus alleviating the acid stress experienced by the cells, or by growing them under a CR regimen (0.05% glucose) and consequently avoiding acetic acid production (Burtner et al., 2009; this work). Since 0.05% is a glucose concentration which is well below what is normally needed to relieve glucose repression (Yin et al., 2003), yeast metabolism switches from fermentation to respiration. Acetyl-CoA is synthesized directly from pyruvate by oxidative decarboxylation, catalyzed by the mitochondrial pyruvate dehydrogenase complex, and in such a way glycolysis is coupled to the TCA cycle (Pronk et al., 1996).
In parallel with the faster drop of cell survival, chronologically aging ach1Δ cells undergo an early insurgence of apoptosis. In addition, ach1Δ cells also undergo apoptosis when they are inoculated in 35 mM or 50 mM acetate medium at pH 4.5; a growth condition which has a detrimental effect on the viability of this mutant. It is well known that acetic acid represents a compound commonly used to induce yeast apoptosis when applied in the presence of glucose at pH 3 (Ludovico et al., 2001, 2002; Giannattasio et al., 2005). Here, we show that acetic acid alone triggers apoptosis in glucose-derepressed ach1Δ cells. Uptake of acetate is linked to a proton symport mechanism (subjected to glucose repression) accompanied by passive/facilitated diffusion of the uncharged/undissociated acid through the Fps1 aquaglyceroporin channel (see Casal et al., 2008, for a review). Acetic acid displays toxicity at low extracellular pH primarily due to the undissociated acid diffusion. In fact, the acetic/acetate couple forms a buffer system; at pH values below the pKa of the acid (4.75) the undissociated form prevails and diffuses through the plasma membrane. Once inside the cell (pH close to neutral), the acid dissociates causing anion accumulation and intracellular acidification that, in turn, is thought to have negative effects on yeast metabolic activity. Additionally, free radical production is also affected leading to severe oxidative stress (Piper et al., 2001). In line with this, ach1Δ cells on acetate display ROS accumulation and a strong reduction in mitochondrial membrane potential similar to that elicited by acetic acid in glucose-repressed wild type cells (Ludovico et al., 2002) supporting the notion of a role for Ach1 as a mitochondrial detoxifying enzyme (Fleck and Brock, 2009). Moreover, as in the case of the acetic acid-induced apoptosis of glucose-repressed cells, occurrence of apoptotic markers in the ach1Δ mutant is accompanied by caspase activation. So far, four proteases have been described in the yeast apoptotic scenarios: the separase Esp1 (Yang et al., 2008), the nuclear HtrA-like protein Nma111 (Fahrenkrog et al., 2004; Wissing et al., 2004), Kex1 (Hauptmann et al., 2006), and the metacaspase Yca1 (Madeo et al., 2002). Here, evidence is provided that Yca1 is involved in the caspase-dependent apoptosis of the ach1Δ mutant promoted by acetic acid since YCA1 deletion decreases apoptotic markers, as well as ROS accumulation in the ach1Δ mutant and, conversely, improves its cellular growth on acetic acid-containing media at low pH. Such an involvement is fully consistent with the requirement of this metacaspase for the ROS-dependent acetic acid-induced apoptosis (Guaragnella et al., 2010a,b).
A pattern of endogenous severe oxidative stress is also observed in chronologically aging ach1Δ cells in concert with elevated levels of extracellular acetic acid. Here, a precocious increase of mitochondrial superoxide which is well known to target primarily several mitochondrial enzymes (Longo et al., 1999; Fabrizio et al., 2001) and to play a major role in chronological aging (Fabrizio and Longo, 2003; Mesquita et al., 2010; Pan, 2011), is associated with loss of respiratory competence which precedes apoptotic death suggesting that Ach1 is required to protect mitochondrial function during chronological aging. Fleck and Brock (2009) proposed that under extracellular acidic conditions, the diffusional entry of the undissociated acid through the plasma membrane into the cell might also lead to an influx of acetic acid over the mitochondrial membrane. This would result in acetate accumulation and mitochondrial acidification affecting negatively mitochondrial functionality. Ach1 enzymatic activity would prevent this acetate accumulation by a CoASH transfer from succinyl-CoA (produced by the TCA cycle) to acetate generating acetyl-CoA. Ach1 has a low Km for both succinyl-CoA and acetate (Fleck and Brock, 2009). In addition, the CoA-transferase reaction saving ATP, compared with the acetate activation to acetyl-CoA by acetyl-CoA synthetase, would be favored in a condition where in order to counteract intracellular acidification induced by acetic acid, cells are already consuming energy (Piper et al., 2001). Taken together our data are fully consistent with this hypothesis since they indicate that mitochondria are the main target of acetic acid toxic effects in ach1Δ cells in extracellular acidic conditions such as acetate medium at pH 4.5 and during chronological aging (pH about 3; Burtner et al., 2009). This implies that Ach1 can function as a mitochondrial detoxifying enzyme. Moreover, the mitochondrial damage resulting from Ach1 loss of function can account for the growth impairment on acetate and the CLS decrease.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Neil Campbell for English revision. This work was supported by FAR 2010 to Marina Vai.
References
Aerts, A. M., Zabrocki, P., Govaert, G., Mathys, J., Carmona-Gutierrez, D., Madeo, F., Winderickx, J., Cammue, B. P., and Thevissen, K. (2009). Mitochondrial dysfunction leads to reduced chronological lifespan and increased apoptosis in yeast. FEBS Lett. 583, 113–117.
Bettiga, M., Calzari, L., Orlandi, I., Alberghina, L., and Vai, M. (2004). Involvement of the yeast metacaspase Yca1 in ubp10Delta-programmed cell death. FEMS Yeast Res. 5, 141–147.
Bonawitz, N. D., Rodeheffer, M. S., and Shadel, G. S. (2006). Defective mitochondrial gene expression results in reactive oxygen species-mediated inhibition of respiration and reduction of yeast life span. Mol. Cell. Biol. 26, 4818–4829.
Burtner, C. R., Murakami, C. J., Kennedy, B. K., and Kaeberlein, M. (2009). A molecular mechanism of chronological aging in yeast. Cell Cycle 8, 1256–1270.
Buu, L. M., Chen, Y. C., and Lee, F. J. (2003). Functional characterization and localization of acetyl-CoA hydrolase, Ach1p, in Saccharomyces cerevisiae. J. Biol. Chem. 278, 17203–17209.
Carmona-Gutierrez, D., Eisenberg, T., Buttner, S., Meisinger, C., Kroemer, G., and Madeo, F. (2010). Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ. 17, 763–773.
Casal, M., Paiva, S., Queiros, O., and Soares-Silva, I. (2008). Transport of carboxylic acids in yeasts. FEMS Microbiol. Rev. 32, 974–994.
Connerton, I. F., McCullough, W., and Fincham, J. R. (1992). An acetate-sensitive mutant of Neurospora crassa deficient in acetyl-CoA hydrolase. J. Gen. Microbiol. 138, 1797–1800.
dos Santos, M. M., Gombert, A. K., Christensen, B., Olsson, L., and Nielsen, J. (2003). Identification of in vivo enzyme activities in the cometabolism of glucose and acetate by Saccharomyces cerevisiae by using 13C-labeled substrates. Eukaryot. Cell 2, 599–608.
Du, L., Su, Y., Sun, D., Zhu, W., Wang, J., Zhuang, X., Zhou, S., and Lu, Y. (2008). Formic acid induces Yca1p-independent apoptosis-like cell death in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 8, 531–539.
Fabrizio, P., Gattazzo, C., Battistella, L., Wei, M., Cheng, C., McGrew, K., and Longo, V. D. (2005). Sir2 blocks extreme life-span extension. Cell 123, 655–667.
Fabrizio, P., and Longo, V. D. (2003). The chronological life span of Saccharomyces cerevisiae. Aging Cell 2, 73–81.
Fabrizio, P., and Longo, V. D. (2007). The chronological life span of Saccharomyces cerevisiae. Methods Mol. Biol. 371, 89–95.
Fabrizio, P., and Longo, V. D. (2008). Chronological aging-induced apoptosis in yeast. Biochim. Biophys. Acta 1783, 1280–1285.
Fabrizio, P., Pozza, F., Pletcher, S. D., Gendron, C. M., and Longo, V. D. (2001). Regulation of longevity and stress resistance by Sch9 in yeast. Science 292, 288–290.
Fahrenkrog, B., Sauder, U., and Aebi, U. (2004). The S. cerevisiae HtrA-like protein Nma111p is a nuclear serine protease that mediates yeast apoptosis. J. Cell Sci. 117, 115–126.
Fleck, C. B., and Brock, M. (2009). Re-characterisation of Saccharomyces cerevisiae Ach1p: fungal CoA-transferases are involved in acetic acid detoxification. Fungal Genet. Biol. 46, 473–485.
Giannattasio, S., Guaragnella, N., Corte-Real, M., Passarella, S., and Marra, E. (2005). Acid stress adaptation protects Saccharomyces cerevisiae from acetic acid-induced programmed cell death. Gene 354, 93–98.
Guaragnella, N., Bobba, A., Passarella, S., Marra, E., and Giannattasio, S. (2010a). Yeast acetic acid-induced programmed cell death can occur without cytochrome c release which requires metacaspase YCA1. FEBS Lett. 584, 224–228.
Guaragnella, N., Passarella, S., Marra, E., and Giannattasio, S. (2010b). Knock-out of metacaspase and/or cytochrome c results in the activation of a ROS-independent acetic acid-induced programmed cell death pathway in yeast. FEBS Lett. 584, 3655–3660.
Guaragnella, N., Pereira, C., Sousa, M. J., Antonacci, L., Passarella, S., Corte-Real, M., Marra, E., and Giannattasio, S. (2006). YCA1 participates in the acetic acid induced yeast programmed cell death also in a manner unrelated to its caspase-like activity. FEBS Lett. 580, 6880–6884.
Hauptmann, P., Riel, C., Kunz-Schughart, L. A., Frohlich, K. U., Madeo, F., and Lehle, L. (2006). Defects in N-glycosylation induce apoptosis in yeast. Mol. Microbiol. 59, 765–778.
Herker, E., Jungwirth, H., Lehmann, K. A., Maldener, C., Frohlich, K. U., Wissing, S., Buttner, S., Fehr, M., Sigrist, S., and Madeo, F. (2004). Chronological aging leads to apoptosis in yeast. J. Cell Biol. 164, 501–507.
Koning, A. J., Lum, P. Y., Williams, J. M., and Wright, R. (1993). DiOC6 staining reveals organelle structure and dynamics in living yeast cells. Cell Motil. Cytoskeleton 25, 111–128.
Lee, F. J., Lin, L. W., and Smith, J. A. (1990). A glucose-repressible gene encodes acetyl-CoA hydrolase from Saccharomyces cerevisiae. J. Biol. Chem. 265, 7413–7418.
Lee, F. J., Lin, L. W., and Smith, J. A. (1996). Acetyl-CoA hydrolase involved in acetate utilization in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1297, 105–109.
Longo, V. D., Liou, L. L., Valentine, J. S., and Gralla, E. B. (1999). Mitochondrial superoxide decreases yeast survival in stationary phase. Arch. Biochem. Biophys. 365, 131–142.
Longo, V. D., Mitteldorf, J., and Skulachev, V. P. (2005). Programmed and altruistic ageing. Nat. Rev. Genet. 6, 866–872.
Ludovico, P., Rodrigues, F., Almeida, A., Silva, M. T., Barrientos, A., and Corte-Real, M. (2002). Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 2598–2606.
Ludovico, P., Sousa, M. J., Silva, M. T., Leao, C., and Corte-Real, M. (2001). Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology 147, 2409–2415.
Madeo, F., Carmona-Gutierrez, D., Ring, J., Buttner, S., Eisenberg, T., and Kroemer, G. (2009). Caspase-dependent and caspase-independent cell death pathways in yeast. Biochem. Biophys. Res. Commun. 382, 227–231.
Madeo, F., Frohlich, E., and Frohlich, K. U. (1997). A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 139, 729–734.
Madeo, F., Frohlich, E., Ligr, M., Grey, M., Sigrist, S. J., Wolf, D. H., and Frohlich, K. U. (1999). Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145, 757–767.
Madeo, F., Herker, E., Maldener, C., Wissing, S., Lachelt, S., Herlan, M., Fehr, M., Lauber, K., Sigrist, S. J., Wesselborg, S., and Frohlich, K. U. (2002). A caspase-related protease regulates apoptosis in yeast. Mol. Cell 9, 911–917.
Mesquita, A., Weinberger, M., Silva, A., Sampaio-Marques, B., Almeida, B., Leao, C., Costa, V., Rodrigues, F., Burhans, W. C., and Ludovico, P. (2010). Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc. Natl. Acad. Sci. U.S.A. 107, 15123–15128.
Murakami, C. J., Wall, V., Basisty, N., and Kaeberlein, M. (2010). Composition and acidification of the culture medium influences chronological aging similarly in vineyard and laboratory yeast. PLoS ONE 6, e24530. doi:10.1371/journal.pone.0024530
Orlandi, I., Bettiga, M., Alberghina, L., and Vai, M. (2004). Transcriptional profiling of ubp10 null mutant reveals altered subtelomeric gene expression and insurgence of oxidative stress response. J. Biol. Chem. 279, 6414–6425.
Paiva, S., Devaux, F., Barbosa, S., Jacq, C., and Casal, M. (2004). Ady2p is essential for the acetate permease activity in the yeast Saccharomyces cerevisiae. Yeast 21, 201–210.
Pan, Y. (2011). Mitochondria, reactive oxygen species, and chronological aging: a message from yeast. Exp. Gerontol. 46, 847–852.
Parrella, E., and Longo, V. D. (2008). The chronological life span of Saccharomyces cerevisiae to study mitochondrial dysfunction and disease. Methods 46, 256–262.
Piper, P., Calderon, C. O., Hatzixanthis, K., and Mollapour, M. (2001). Weak acid adaptation: the stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 147, 2635–2642.
Pronk, J. T., Steensma, H. Y., and van Dijken, J. P. (1996). Pyruvate Metabolism in Saccharomyces cerevisiae. Yeast 12, 1607–1633.
Rockenfeller, P., and Madeo, F. (2008). Apoptotic death of ageing yeast. Exp. Gerontol. 43, 876–881.
Scheckhuber, C. Q., Erjavec, N., Tinazli, A., Hamann, A., Nystrom, T., and Osiewacz, H. D. (2007). Reducing mitochondrial fission results in increased life span and fitness of two fungal ageing models. Nat. Cell Biol. 9, 99–105.
Smets, B., Ghillebert, R., De Snijder, P., Binda, M., Swinnen, E., De Virgilio, C., and Winderickx, J. (2010). Life in the midst of scarcity: adaptations to nutrient availability in Saccharomyces cerevisiae. Curr. Genet. 56, 1–32.
van den Berg, M. A., de Jong-Gubbels, P., Kortland, C. J., van Dijken, J. P., Pronk, J. T., and Steensma, H. Y. (1996). The two acetyl-coenzyme A synthetases of Saccharomyces cerevisiae differ with respect to kinetic properties and transcriptional regulation. J. Biol. Chem. 271, 28953–28959.
Vanoni, M., Vai, M., Popolo, L., and Alberghina, L. (1983). Structural heterogeneity in populations of the budding yeast Saccharomyces cerevisiae. J. Bacteriol. 156, 1282–1291.
Verduyn, C., Postma, E., Scheffers, W. A., and Van Dijken, J. P. (1992). Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8, 501–517.
Wissing, S., Ludovico, P., Herker, E., Buttner, S., Engelhardt, S. M., Decker, T., Link, A., Proksch, A., Rodrigues, F., Corte-Real, M., Frohlich, K. U., Manns, J., Cande, C., Sigrist, S. J., Kroemer, G., and Madeo, F. (2004). An AIF orthologue regulates apoptosis in yeast. J. Cell Biol. 166, 969–974.
Yang, H., Ren, Q., and Zhang, Z. (2008). Cleavage of Mcd1 by caspase-like protease Esp1 promotes apoptosis in budding yeast. Mol. Biol. Cell 19, 2127–2134.
Keywords: Saccharomyces cerevisiae, Ach1, acetic acid, mitochondria, chronological aging, apoptosis
Citation: Orlandi I, Casatta N and Vai M (2012) Lack of Ach1 CoA-transferase triggers apoptosis and decreases chronological lifespan in yeast. Front. Oncol. 2:67. doi: 10.3389/fonc.2012.00067
Received: 11 April 2012; Accepted: 11 June 2012;
Published online: 29 June 2012.
Edited by:
Manuela Côrte-Real, Universidade do Minho, PortugalReviewed by:
Gavin McStay, Columbia University, USAVítor Costa, Instituto de Biologia Molecular e Celular, Portugal
Copyright: © 2012 Orlandi, Casatta and Vai. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Marina Vai, Dipartimento di Biotecnologie e Bioscienze, Università di Milano-Bicocca, Piazza della Scienza 2, 20126 Milano, Italy. e-mail: marina.vai@unimib.it
