- 1National School of Public Health/FIOCRUZ, Rio de Janeiro, Brazil
- 2International Agency of Research on Cancer (IARC/WHO), Lyon, France
- 3Institute for Translational Epidemiology and Tisch Cancer Institute, Mount Sinai School of Medicine, New York, NY, USA
Introduction: Oral cancer is a public health problem worldwide, being tobacco and alcohol consumption their main risk factors. Sulfotransferase (SULT) 1A1 (encoded by SULT1A1) is involved in procarcinogens metabolism, such as polycyclic aromatic hydrocarbons (PAHs) present in tobacco smoke. Objective: The aim of this study was to explore the magnitude of association between SULT1A1 gene Arg213His polymorphism and oral cancer, and to explore the interaction between such polymorphism and smoking. Methods: A hospital-based case-control study was carried out in Rio de Janeiro, Brazil, during 1999–2002. Epidemiological data and biological samples were obtained from 202 oral cancer patients and 196 sex and age-frequency matched controls without cancer antecedents. Results: No association was observed between Arg213His SULT1A1 polymorphism and oral cancer risk in overall analysis (OR = 1.06, 95% CI = 0.71–1.57). The magnitude of association between cigarette smoking and oral cancer was higher in individuals with a SULT1A1*1 isoform (wild type, genotype Arg/Arg) (OR = 10.19, 95% CI = 3.90–26.61) than in those with at least one SULT1A1*2 allele (genotypes Arg/His + His/His) (OR = 4.50, 95% CI =2.09–9.69). Conclusion: Our results suggest that Arg213His SULT1A1 polymorphism may modulate the association between smoking and oral cancer. However, this association needs to be replicated in other studies: due to modest number of cases and controls, the role of chance in the observed association cannot be ruled out.
Introduction
Oral cancer is a major public health problem worldwide, resulting in 263,020 new cases (3.8 cases/100,000) and 127,654 deaths (1.9/100,000) per year, with the highest incidence and mortality rates occurring in developing countries (Ferlay et al., 2010). In Brazil, according to 2012 estimates, oral cavity cancer will result in 9990 new cases in males (10 cases per 100,000 men) and 4180 in females (4/100,000 women). However, a marked regional variation is expected, with incidence rates ranging from 3/100,000 men (2/100,000 women) in the North region to 15/100,000 men (6/100,000 women) in the Southeast (INCA, 2011).
Tobacco and alcohol consumption are described as the major environmental risk factors to oral cancer (Anantharaman et al., 2011; Lubin et al., 2011). Other environmental factors are diet, pollution (Johnson et al., 2011), oral hygiene (Guha et al., 2007), and certain virus strains, such as human papillomavirus (HPV) (Lambert et al., 2011). Regarding genetic risk factors, some authors have described an association between xenobiotic metabolizing enzymes polymorphisms and the development of oral cancer (Jefferies et al., 1999; Tripathy and Roy, 2006). However, the basis of this genetic susceptibility is not well understood (Ram et al., 2011).
The sulfotransferases (SULTs) are phase II detoxification enzymes involved in the biotransformation of a wide variety of xenobiotics and endogenous steroid (Nowell and Falany, 2006). The SULT1A1 isoform is involved in toxic substances inactivation, but it can also bioactive pro-carcinogens such as heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs) (Bardakci et al., 2008; Koike et al., 2008), both present in tobacco smoke (International Agency for Research on Cancer: Tobacco smoke, 2004). SULT1A1 is a polymorphic gene, and its Arg213His most studied polymorphism results from G to A nucleotide replacement at exon 7, generating SULT1A1*2 isoform (Nagar et al., 2006). The protein derived from SULT1A1*2 (213His variant allele), comparatively to that produced by the wild type (SULT1A1*1), has a twice lower catalytic activity and more reduced thermal stability (Koike et al., 2008).
SULT1A1*2 isoform is associated with increased risks of lung (Pachouri et al., 2006; Liao et al., 2012), stomach cancer (Liang et al., 2004; Boccia et al., 2005), urothelial cancer (Roupret et al., 2007), and breast cancer (Lee et al., 2012). However, others studies reported that SULT1A1*2 isoform could confer not statistically significant reduced risks to bladder cancer (Hung et al., 2004) and colorectal cancer (Nowell et al., 2002), as well as a statistically significant lung cancer risk reduction in heterozygous individuals (Ihsan et al., 2011).
With regard to oral cancer, SULT1A1*2 isoform has only been studied in Taiwan. No association between Arg213His SULT1A1 polymorphisms and oral cancer was observed in that study, but oral cancer risk in betel quid chewers and smokers seemed to be lower in individuals with a SULT1A1*2 isoform compared to those with the wild type genotype (Chung et al., 2009).
The aim of this study was to investigate the association between Arg213His SULT1A1 gene polymorphism and oral cancer, and to explore any interaction between this polymorphism and smoking with regard to oral cancer risk.
Methods
This investigation is part of a multicentric hospital-based case-control study carried out in Brazil, Argentina, and Cuba aiming to explore the association between several environmental and genetic risk factors with oral, larynx, and esophageal cancers (Guha et al., 2007). The city of Rio de Janeiro was one of the places wherein the study was performed during 1999–2002, and as previously described (Marques et al., 2006), cases were 202 patients between 15 and 79-year-old with an histopathological confirmed diagnosis of oral cavity squamous cell carcinoma without previous treatment. Cases were diagnosed in Rio de Janeiro at the public and free care coverage Brazilian National Cancer Institute (INCA). Controls (196 patients) were gender and age-frequency matched to cases, being enrolled among hospitalized patients with no-neoplastic diseases (alcohol- or tobacco-related illnesses excluded) in two public general hospitals, the Institute of Trauma—Orthopaedics (INTO) and the Souza Aguiar Municipality General Hospital, both offering free and universal care in the same city. Hospitalization causes distribution among controls were: injury, poisoning, and certain other consequences of external causes 28.5%; digestive system diseases, 19.4%; genitourinary system diseases, 17.4%; musculoskeletal system and connective tissue diseases, 12.8%; respiratory system diseases, 8.7%; infectious and parasitic diseases, 6.1%; skin and subcutaneous tissue diseases, 2.6%; and 0.5%, others.
Therefore, control group included a variety of ill individuals enrolled in general hospitals, all of them presenting illnesses unrelated to smoking and alcohol intake in their natural history (i.e., emphysema, alcoholic cirrhosis, and others).
All participants were residents in the Metropolitan Region of Rio de Janeiro. Trained interviewers conducted in-person interviews to elicit information on demographic background, tobacco, and alcohol consumption and other lifestyle habits. Participating rate was 95% for cases and 86% for controls.
Peripheral blood samples collected in EDTA Vacutainer tubes were used for genomic DNA extraction following a standard protocol (Lahiri and Nurnberger, 1991). All proceedings were approved by the Ethics Research Committees of all involved institutions.
Genetic polymorphisms were assessed by previously described PCR-RFLP protocols (Coughtrie et al., 1999), with minor modifications. In brief, the amplification of target DNA was achieved by PCR optimized conditions as follows: a final reaction volume of 25 μL was composed of 100–200 ng of DNA, 0.2 mM of each dTNP, 4 mM of MgCl2, 0.75 U of Platinum Taq DNA polymerase (Invitrogen), 1× PCR buffer (Invitrogen), and 10 pmol of each primer (forward 5′gttggctctgcagggtttctagga3′ and reverse 5′cccaaacccccgtgctggccagcaccc3′). The reaction conditions used were: a pre-denaturation at 94°C for 5 min followed by 50 cycles with three steps each (94°C for 30 s, 68°C for 30 s, and 72°C for 45 s), and a cycle of 7 min at 72°C. Negative controls were included in every run, and the success of amplification was confirmed in agarose 1.5% gels, stained with Gel Red (Biotium), and visualized under ultraviolet (UV) light. Endonuclease digestions were performed as follows: a final reaction volume of 20 μL composed of 5 μL of PCR products, 5 U of HaeII enzyme (BioLabs), and 1× reactin buffer (BioLabs), using overnight 37°C incubation conditions. Determination of genotypes was performed in agarose 3% gels, visualized under UV light.
Goodness-of-fit of genotype distribution to Hardy–Weinberg equilibrium was ascertained for controls. Unconditional logistic regression models were used to calculate unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (95% CI) for the association between SULT1A1 polymorphism and oral cancer and to explore any interaction between this polymorphism and environmental risk factors. All statistical analyses were done using STATA 10.0 software.
Results
The distribution of oral cancer cases and controls according to sex, age, skin color, smoking, alcohol consumption, and Arg213His SULT1A1 genotype are presented at Table 1. About 82.7% of cases and 76.5% of controls were male. Mean age was 55.9 years (±9.9) among cases and 55.1 years (±11.7) among controls. Regarding skin color, whites accounted for 41.6% of cases and 53.6% of controls (p = 0.02). Smoking antecedents were more frequent among oral cancer cases than controls: among the former, 76.7% were smokers and 14.4% ex-smokers (respectively, 41.3 and 28.1% among controls, p < 0.01). Alcohol intake antecedents were reported by 67.3% of cases and 51.0% of controls (p < 0.01). Data analysis did not show an association between the presence of at least one SULT1A1*2 allele (genotypes Arg/His + His/His) and oral cancer (OR = 1.06, 95% CI 0.71–1.57). The OR adjustment for selected confounders (smoking, skin color, age, and sex) revealed similar results (OR = 1.07, 95% CI = 0.69–1.65).
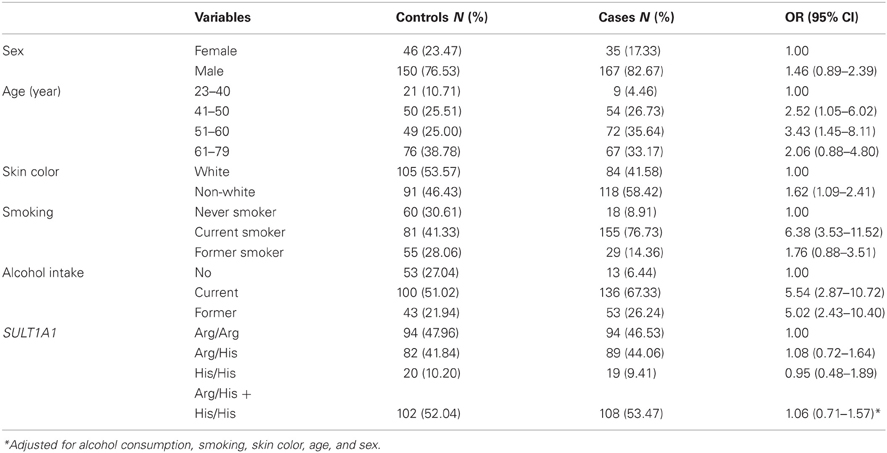
Table 1. Distribution of oral cancer cases and controls according to sex, age, skin color, smoking, alcohol intake, and Arg213His SULT1A1 genotype, Rio de Janeiro, Brazil, 1999–2002.
The association between smoking and oral cancer according to Arg213His SULT1A1 gene polymorphisms is presented at Table 2. Among subjects with an Arg/Arg genotype, an estimated 10-folds higher risk of developing oral cancer was observed among smokers (OR = 10.2, 95% CI = 3.90–26.61) comparatively to no-smokers. Among former smokers, the estimated risk was OR = 2.98 (95% CI = 1.01–8.78). Among individuals who had at least one SULT1A1*2 allele (genotypes Arg/His and His/His), the risk of oral cancer associated with smoking revealed an OR = 4.50 (95% CI = 2.09–9.69) for smokers, and OR = 1.17 (95% CI = 0.46–2.95) for former smokers. When adjusted by age, alcohol consumption, skin color, and sex, such heterogeneity between genotype groups became even higher (Table 2).
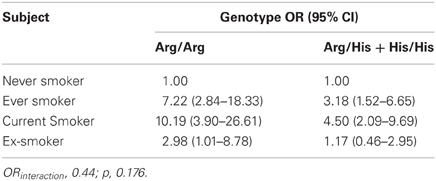
Table 2. Association between smoking and oral cancer according to Arg213His SULT1A1 polymorphism, oral cancer cases and controls, Rio de Janeiro, Brazil, 1999–2002.
Discussion
SULT1A1 is a phase II enzyme involved in the metabolism of a wide variety of xenobiotics and pro-carcinogens bioactivation, such as PAHs as benzopyrene, which are present in cigarette smoke (Bardakci et al., 2008).
Several epidemiological studies have consistently shown tobacco smoking as a major oral cancer risk factor (Garrote et al., 2001; Lubin et al., 2011). Moreover, a marked oral cancer risk reduction has been observed after quitting smoking, thus highlighting the role carcinogenicity of tobacco smoke to oral cavity (Castellsagué et al., 2004).
Genetic polymorphisms participating in cigarette compounds metabolism could affect individual cancer susceptibility by altering the enzymatic expression or function, which would result in increased or decreased carcinogens activation. The reactive intermediate metabolites formed during smoke compounds metabolism, if not eliminated, can eventually form covalent reactions (adducts) with DNA, RNA, or proteins. Therefore, extensive DNA damage may occur, increasing cancer risk (Miller et al., 2001).
Thus, although tobacco carcinogens can promote a variety of genetic alterations alone, there is evidence of the involvement of xenobiotics metabolism genes in the process of transformation of benign oral lesions into oral carcinomas (Lichtenstein et al., 2000).
Since SULT1A1 is involved in bioactivation of tobacco smoke pro-carcinogens and smoking is a major oral cancer risk factor, Arg213His SULT1A1 polymorphism can hypothetically be associated with oral carcinogenesis in smokers. In our study, the magnitude of association between cigarette smoking and oral cancer was higher in individuals with a SULT1A1*1 isoform (wild type, genotype Arg/Arg) (OR = 10.19, 95% CI = 3.90–26.61) than in individuals with at least one SULT1A1*2 allele (genotypes Arg/His + His/His) (OR = 4.50 95% CI = 2.09–9.69), (Table 2, OR interaction p > 0.05). These results suggest that the low enzyme activity variant SULT1A1*2 (213His) (Koike et al., 2008) may lead to a decreased smoking procarcinogens (such as PAHs) bioactivation, thereby reducing the risk of smoking-related cancers. However, considering the small studied sample size, the occurrence of chance as an explanatory reason for this association cannot be ruled out. Additionally, it could also result from other unanalyzed SULT1A1 polymorphisms, or other involved genes in cigarette smoke pro-carcinogens metabolism. In a small sample size case-control study, Chung et al. (2009) explored the association between SUTT1A1 polymorphisms and oral squamous cell carcinoma (OSCC) susceptibility in male Taiwanese. They also reported that the presence of Arg213His SULT1A1 polymorphisms was not associated with the risk of developing oral cancer (OR = 1.04, 95% CI = 0.19–5.12, when no other SULT1A1 SNP was present). However, the risk of developing oral cancer in betel quid chewers and smokers seemed to be lower in SULT1A1*2 isoform patients comparatively to those with a wild type (OR = 0.58, 95% CI = 0.15–2.28).
The association between Arg213His SULT1A1 polymorphism and other cancer types has been mixed (Wang et al., 2003). SULT1A1*2 (213His) allele has been associated with an increased risk of prostate cancer (OR = 1.60, 95% CI = 0.46–5.62) (Nowell et al., 2004), stomach cancer (OR = 3.32; 95% CI = 1.17–9.45) (Liang et al., 2004; Boccia et al., 2005), urothelial cancer (OR = 2.18, 95% CI = 1.28–3.69) (Roupret et al., 2007), and breast cancer (OR = 1.12, 95% CI = 1.02–1.24). On the other hand, a statistically non-significant reduced risks of bladder cancer (OR = 0.67, 95% CI = 0.45–1.03) (Hung et al., 2004) and colorectal cancer (OR = 0.6, 95% CI = 0.3–1.1) (Nowell et al., 2002) have been reported. With regard to lung cancer, Pachouri et al. (2006) reported an increased risk of lung cancer associated with His/His (SULT1A1*2 isoform) genotype (OR = 1.40, 95% CI = 0.48–4.06), which was higher among smokers (OR = 3.9, 95% CI = 1.99–7.81). In contrast, Ihsan et al. (2011) found an inverse association between SULT1A1 Arg213His heterozygous genotype (Arg/His) and lung cancer (OR = 0.51, 95% CI = 0.33–0.78).
Results of this study suggest that Arg213His SULT1A1 polymorphism may modulate susceptibility to oral cancer in smokers. However, as this study had a modest sample size, the role of chance cannot be excluded. Another limitation of this study was that we investigated a single polymorphism with limited data on smoking. Therefore, these results need to be replicated in further studies. Due to heterogeneous results on the role of Arg213His SULT1A1 polymorphism in the activation of smoke procarcinogens and the consequent susceptibility to various cancers, GWAS exploring multiple genes involved in the metabolism of tobacco smoke compounds are needed to obtain more comprehensive evidence of possible interactions between these genes and smoking with regard to cancer risk.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Sabrina S. Santos is a PhD student at the Environment and Public Health Post-graduation Program, National School of Public Health, Oswaldo Cruz Foundation, and supported with a fellowship from the Brazilian Ministry of Education Postgraduation Board (CAPES). Rafaela M. Ferreira and Lilian F. Diniz are graduation students sponsored with PIBIC Scientific Initiation fellowships from the Brazilian Science and Technology Ministry. Rosalina J. Koifman and Sergio Koifman have their research activity supported by the Brazilian National Research Council, CNPq. Data and biological samples collection in this investigation was partly supported by the European Commission and the Ministry of Health of Brazil. The authors are thankful to INCA, INTO, and Souza Aguiar Hospital patients and health personnel for their kind support and collaboration which enabled this study execution.
References
Anantharaman, D., Marron, M., Lagiou, P., Samoli, E., Ahrens, W., Pohlabeln, H., et al. (2011). Population attributable risk of tobacco and alcohol for upper aerodigestive tract cancer. Oral Oncol. 47, 725–731.
Bardakci, F., Arslan, S., Bardakci, S., Binatli, A. O., and Budak, M. (2008). Sulfotransferase 1A1 (SULT1A1) polymorphism and susceptibility to primary brain tumors. J. Cancer Res. Clin. Oncol. 134, 109–114.
Boccia, S., Persiani, R., La Torre, G., Rausei, S., Arzani, D., Gianfagna, F., et al. (2005). Sulfotransferase 1A1 polymorphism and gastric cancer risk: a pilot case-control study. Cancer Lett. 229, 235–243.
Castellsagué, X., Quintana, M. J., Martínez, M. C., Nieto, A., Sánchez, M. J., Juan, A., et al. (2004). The role of type of tobacco and type of alcoholic beverage in oral carcinogenesis. Int. J. Cancer 108, 741–749.
Chung, Y. T., Hsieh, L. L., Chen, I. H., Liao, C. T., Liou, S. H., Chi, C. W., et al. (2009). Sulfotransferase 1A1 haplotypes associated with oral squamous cell carcinoma susceptibility in male Taiwanese. Carcinogenesis 30, 286–294.
Coughtrie, M. W. H., Gilissen, R. A. H. J., Shek, B., Strange, R. C., Fryer, A. A., Jones, P. W., et al. (1999). Phenol sulphotransferase SULT1A1 polymorphism: molecular diagnosisand allele frequencies in Caucasian and African populations. Biochem. J. 337, 45–49.
Ferlay, J., Shin, H. R., Bray, F., Forman, D., Mathers, C., and Parkin, D. M. (2010). GLOBOCAN (2008). v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer. Available online at: http://globocan.iarc.fr, (Accessed on August 2012).
Garrote, L. F., Herrero, R., Reyes, R. M., Vaccarella, S., Anta, J. L., Ferbeye, L., et al. (2001). Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br. J. Cancer 85, 46–54.
Guha, N., Boffetta, P., Wünsch, Filho, V., Eluf Neto, J., Shangina, O., Zaridze, D., et al. (2007). Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am. J. Epidemiol. 166, 1159–1173.
Hung, R. J., Boffetta, P., Brennan, P., Malaveille, P., Hautefeuille, A., Donato, F., et al. (2004). GST, NAT, SULT1A1, CYP1B1 genetic polymorphisms, interactions with environmental exposures and bladder cancer risk in a high-risk population. Int. J. Cancer 110, 598–604.
Ihsan, R., Chauhan, P. S., Mishra, A. K., Yadav, D. S., Kaushal, M., Sharma, J. D., et al. (2011). Multiple analytical approaches reveal distinct gene-environment interactions in smokers and non smokers in lung cancer. PLoS ONE 6:e29431. doi: 10.1371/journal.pone.0029431
INCA. (2011). Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva. Coordenação Geral de Ações Estratégicas. Coordenação de Prevenção e Vigilância. Estimativa 2012: incidência de câncer no Brasil (Rio de Janeiro).
International Agency for Research on Cancer: Tobacco smoke. (2004). Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 83 (Lyon, France), 1187.
Jefferies, S., Eeles, R., Goldgar, D., A'Hern, R., Henk, J. M., and Gore, M. (1999). The role of genetic factors in predisposition to squamous cell cancer of the head and neck. Br. J. Cancer 79, 865–867.
Johnson, N. W., Warnakulasuriya, S., Gupta, P. C., Dimba, E., Chindia, M., Otoh, E. C., et al. (2011). Global oral health inequalities in incidence and outcomes for oral cancer: causes and solutions. Adv. Dent. Res. 23, 237–246.
Koike, H., Nakazato, H., Ohtake, N., Matsui, H., Okugi, H., Shibata, Y., et al. (2008). Further evidence for null association of phenol sulfotransferase SULT1A1 polymorphism with prostate cancer risk: a case–control study of familial prostate cancer in a Japanese population. Int. Urol. Nephrol. 40, 947–951.
Lahiri, D. K., and Nurnberger, J. I. Jr. (1991). A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 19, 5444.
Lambert, R., Sauvaget, C., de Camargo Cancela, M., and Sankaranarayanan, R. (2011). Epidemiology of cancer from the oral cavity and oropharynx. Eur. J. Gastroenterol. Hepatol. 23, 633–641.
Lee, H., Wang, Q., Yang, F., Tao, P., Li, H., Huang, Y., et al. (2012). SULT1A1 Arg213His polymorphism, smoked meat, and breast cancer risk: a case-control study and meta-analysis. DNA Cell Biol. 31, 688–699.
Liang, G., Miao, X., Zhou, Y., Tan, W., and Lin, D. (2004). A functional polymorphism in the SULT1A1 gene (G638A) is associated with risk of lung cancer in relation to tobacco smoking. Carcinogenesis 25, 773–778.
Liao, S. G., Liu, L., Zhang, Y. Y., Wang, Y., and Wang, Y. J. (2012). SULT1A1 Arg213His polymorphism and lung cancer risk: a meta-analysis. Asian Pac. J. Cancer Prev. 13, 579–583.
Lichtenstein, P., Holm, N. V., Verkasalo, P. K., Iliadou, A., Kaprio, J., Koskenvuo, M., et al. (2000). Environmental and heritable factors in the causation of cancer: analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 343, 78–85.
Lubin, J. H., Muscat, J., Gaudet, M. M., Olshan, A. F., Curado, M. P., Dal Maso, L., et al. (2011). An examination of male and female odds ratios by BMI, cigarette smoking, and alcohol consumption for cancers of the oral cavity, pharynx, and larynx in pooled data from 15 case-control studies. Cancer Causes Control 22, 1217–1231.
Marques, C. F., Koifman, S., Koifman, R. J., Boffetta, P., Brennan, P., and Hatagima, A. (2006). Influence of CYP1A1, CYP2E1, GSTM3 and NAT2 genetic polymorphisms in oral cancer susceptibility: results from a case-control study in Rio de Janeiro. Oral Oncol. 42, 632–637.
Miller, M. C., Mohrenweiser, H. W., and Bell, D. A. (2001). Genetic variability in susceptibility and response to toxicants. Toxicol. Lett. 120, 269–280.
Nagar, S., Walther, S., and Blanchard, R. L. (2006). Sulfotransferase (SULT) 1A1 polymorphic variants *1, *2, and *3 are associated with altered enzymatic activity, cellular phenotype, and protein degradation. Mol. Pharmacol. 69, 2084–2092.
Nowell, S., Coles, B., Sinha, R., MacLeod, S., Luke Ratnasinghe, D., Stotts, C., et al. (2002). Analysis of total meat intake and exposure to individual heterocyclic amines in a case-control study of colorectal cancer: contribution of metabolic variation to risk. Mutat. Res. 506–507, 175–185.
Nowell, S., and Falany, C. N. (2006). Pharmacogenetics of human cytosolic sulfotransferases. Oncogene 25, 1673–1678.
Nowell, S., Ratnasinghe, D. L., Ambrosone, C. B., Williams, S., Teague-Ross, T., Trimble, L., et al. (2004). Association of SULT1A1 phenotype and genotype with prostate cancer risk in African-Americans and Caucasians. Cancer Epidemiol. Biomarkers Prev. 13, 270–276.
Pachouri, S. S., Sobti, R. C., Kaur, P., Singh, J., and Gupta, S. K. (2006). Impact of polymorphism in sulfotransferase gene on the risk of lung cancer. Cancer Genet. Cytogenet. 171, 39–43.
Ram, H., Sarkar, J., Kumar, H., Konwar, R., Bhatt, M. L., and Mohammad, S. (2011). Oral cancer: risk factors and molecular pathogenesis. J. Maxillofac. Oral Surg. 10, 132–137.
Rouprêt, M., Cancel-Tassin, G., Comperat, E., Fromont, G., Sibony, M., Molinié, V., et al. (2007). Phenol sulfotransferase SULT1A1*2 allele and enhanced risk of upper urinary tract urothelial cell carcinoma. Cancer Epidemiol. Biomarkers Prev. 16, 2500–2503.
Tripathy, C. B., and Roy, N. (2006). Meta analysis of glutathione S-transeferase M1 genotype and risk toward head and neck cancer. Head Neck 28, 217–224.
Keywords: SULT 1A1 gene, polymorphisms, oral cancer, smoking, alcohol
Citation: Santos SS, Koifman RJ, Ferreira RM, Diniz LF, Brennan P, Boffetta P and Koifman S (2012) SULT1A1 genetic polymorphisms and the association between smoking and oral cancer in a case-control study in Brazil. Front. Oncol. 2:183. doi: 10.3389/fonc.2012.00183
Received: 25 July 2012; Accepted: 14 November 2012;
Published online: 18 December 2012.
Edited by:
Farhad Islami, Mount Sinai School of Medicine, USAReviewed by:
Farhad Islami, Mount Sinai School of Medicine, USAQian Li, Chinese Academy of Medical Sciences, China
Copyright © 2012 Santos, Koifman, Ferreira, Diniz, Brennan, Boffetta and Koifman. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Sergio Koifman, Environment and Public Health Post-graduation Program, National School of Public Health, Oswaldo Cruz Foundation (FIOCRUZ), Rua Leopoldo Bulhões 1480, Rio de Janeiro, 21041-210, Brazil. e-mail: koifman@ensp.fiocruz.br
