- 1Department of Radiation Oncology, Mary Babb Randolph Cancer Center of West Virginia University, Morgantown, WV, USA
- 2The International Geriatric Radiotherapy Group, Tucson, AZ, USA
- 3Northern Illinois University Institute for Neutron Therapy at Fermilab, Batavia, IL, USA
- 4Division of Hematology and Oncology, Mary Babb Randolph Cancer Center of West Virginia University, Morgantown, WV, USA
- 5Thoracic Surgery, Mary Babb Randolph Cancer Center of West Virginia University, Morgantown, WV, USA
- 6Department of Radiation Oncology, Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, IL, USA
Radiation dose in the setting of chemo-radiation for locally advanced non-small cell lung cancer (NSCLC) has been historically limited by the risk of normal tissue toxicity and this has been hypothesized to correlate with the poor results in regard to local tumor recurrences. Dose escalation, as a means to improve local control, with concurrent chemotherapy has been shown to be feasible with three-dimensional conformal radiotherapy in early phase studies with good clinical outcome. However, the potential superiority of moderate dose escalation to 74 Gy has not been shown in phase III randomized studies. In this review, the limitations in target volume definition in previous studies; and the factors that may be critical to safe dose escalation in the treatment of locally advanced NSCLC, such as respiratory motion management, image guidance, intensity modulation, FDG–positron emission tomography incorporation in the treatment planning process, and adaptive radiotherapy, are discussed. These factors, along with novel treatment approaches that have emerged in recent years, are proposed to warrant further investigation in future trials in a more comprehensive and integrated fashion.
Introduction
Concurrent chemo-radiation is the standard of care in the management of non-small cell lung cancer (NSCLC) after its superiority over radiotherapy alone or sequential chemo-radiation has been demonstrated in multiple phase III randomized trials (1–5). In a meta-analysis of 1205 patients with locally advanced NSCLC from six randomized studies, concurrent chemo-radiation decreased loco-regional progression by 6.1% at 5 years when compared with sequential chemo-radiation (28.9 vs. 35.0%, p = 0.01) (6). This resulted in an improvement in overall survival of 4.5% at 5 years (15.1 vs. 10.6%, p = 0.004) and as suggested by the authors of this study, survival may be directly related to loco-regional control. The risk of regional failure, i.e., in the mediastinum, is relatively low after chemo-radiation to a dose of approximately 60 Gy with conventional fractionation (5, 7); in contrast, the rate of local failure remains relatively high. As such, it seems reasonable to propose that techniques to improve primary tumor control through dose escalation may be one strategy to improve treatment outcome in locally advanced NSCLC.
Dose escalation in the treatment of stage I–III NSCLC has been shown to be feasible in multiple institutional and early phase prospective studies (8–11). Among them, the radiation dose has been found to be a significant predictor of local control and survival by many (8–10). Based on the clinical outcome from a University of Michigan study, 84.5 Gy was found to be required to achieve a local progression free survival (LPFS) of 50% at 30 months in patients with NSCLC (12). Further radiobiological modeling has suggested that a biologically effective dose (BED) of over 100 Gy10 is required to achieve a LPFS of ≥80% at 30 months in the treatment of NSCLC (13). This has been validated clinically in the treatment of early stage NSCLC with stereotactic ablative radiotherapy (SABR), which is also referred to as stereotactic body radiation therapy (SBRT) (14–16). In locally advanced NSCLC, every 1 Gy10 in dose escalation was found to be associated with a 4% increase in survival and a 3% increase in loco-regional control in past chemo-radiation trials conducted by the Radiation Therapy Oncology Group (RTOG) (17). When combined with weekly carboplatin and paclitaxel, a maximally tolerated dose (MTD) of 74 Gy delivered with three-dimensional (3D) technique was found in a phase I/II RTOG study, RTOG 0117 (18). Inoperable patients with stage I–III NSCLC were included in this study, and a median survival of 21.6 months was observed in stage III patients. In a similar phase II study, a median survival of 24.3 months in stage III NSCLC patients was observed after induction carboplatin/paclitaxel followed by concurrent carboplatin/paclitaxel and radiation to 74 Gy (19).
Based on early clinical evidence, a phase III randomized dose escalation trial (RTOG 0617) was conducted by the RTOG. In this study, patients were randomized to chemo-radiation to 60 vs. 74 Gy, and with or without Cetuximab (four arms) (20). Despite the anticipated improvement in clinical outcome with dose escalation, it actually resulted in inferior median survival (19.5 vs. 28.7 months, p = 0.0007) and an increase in local failure at 18 months (34.3 vs. 25.1%, p = 0.0319). In addition, dose escalation also resulted in an increase in grade 3 esophagitis (20.9 vs. 7.0%, p = 0.0003). The causes of poorer outcome in the 74-Gy arms remain to be discerned. However, several factors may potentially contribute to this finding, such as, larger-than-necessary planning target volume (PTV) in patients receiving 3D conformal radiotherapy (3D-CRT), which potentially leads to increased normal tissue toxicity; suboptimal target volume delineation when 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) information is not incorporated in the treatment planning process; failure to account for tumor shrinkage during the course of radiotherapy; and delayed tumor cell repopulation associated with prolonged overall treatment time. These can potentially be minimized with image-guided, intensity-modulated radiotherapy (IG-IMRT), and adaptive radiotherapy (ART), which may improve the efficacy of dose escalation in the treatment of locally advanced NSCLC in future clinical trials.
Target Volume in Relation to Clinical Outcome and Toxicities
In the treatment of locally advanced NSCLC with chemo-radiation, large tumor margins are often used to account for respiratory motion and set up uncertainties in the era of conventional and 3D radiotherapy (Table 1). With the addition of elective nodal irradiation (ENI), an even larger volume of normal tissue is included within the treated volume. As shown in Table 1, multiple clinical trials investigating the efficacy of sequential and concurrent chemo-radiation frequently used margins of 1.5–2 cm for the primary tumor. ENI was often carried out in these studies with inclusion of the bilateral hilum, mediastinum, and the ipsilateral supra-clavicular fossa in the initial radiation field, which extended 4–5 cm below the carina (3–5, 21–23). In these studies, dose to the gross disease has been limited to approximately 60 Gy with poor clinical outcome and fatal treatment related toxicities reported. Concurrent chemo-radiation prior to RTOG 0617 often led to a local control of 60–83%, while the median survival with concurrent chemo-radiation often increased to >15 months (3–5). Among them, seven late fatal pulmonary toxicities were observed in RTOG 9410, which demonstrated no significant improvement in local control and only marginal improvement in median survival with concurrent chemo-radiation over sequential chemo-radiation (5). These findings suggest that the large tumor volumes treated in the past not only precludes dose escalation to the primary tumor, but also increase the risk of severe treatment related toxicity.
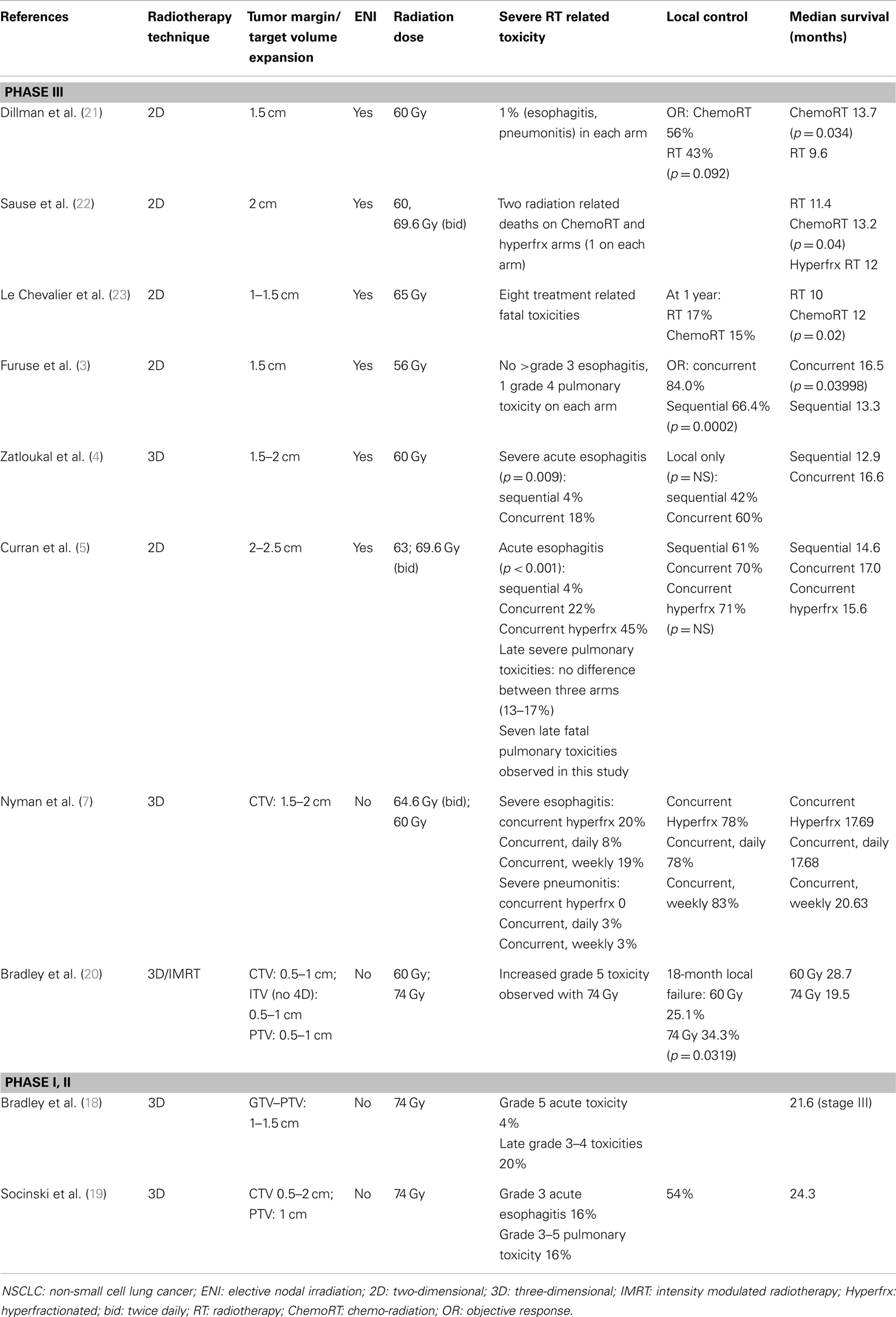
Table 1. Target volume, toxicity, and clinical outcome in selected phase III randomized trials and phase I/II dose escalation trials combining chemotherapy and radiation for locally advanced NSCLC.
Elective nodal irradiation, which for the most part involves treating areas of mediastinum that do not exhibit tumor as determined by imaging, has been shown to be unnecessary in the era of 3D-CRT. For example, a study by Rosenzweig et al. only identified a 6.1% elective nodal failure among 524 NSCLC patients who underwent radiotherapy to a mean dose of 66 Gy after a median follow up of 41 months (24). In a randomized prospective study by Yuan et al. no statistically significant difference in the rate of elective nodal failure at 5 years following ENI (4%) and involved field irradiation (7%) was observed in stage III NSCLC patients who received concurrent chemo-radiation (25). However, involved field irradiation led to a reduction in radiation pneumonitis (RP) (mainly grade 2–3) from 29 to 17% (p = 0.044), and dose escalation from 60–64 to 68–74 Gy. This dose escalation led to statistically significant improvement in local control (55 vs. 38%, p = 0.016) and median survival (20.0 vs. 15.0 months, p = 0.048). With omission of ENI, dose escalation to 74 Gy with 3D techniques was found to be feasible in RTOG 0117 and CALGB 30105 (18, 19). However, a high rate of severe toxicity was still observed (Table 1). In this regard, the increased toxicity associated with dose escalation was corroborated by findings in RTOG 0617. This may be associated with the limitations of 3D techniques as 3D-CRT without image guidance was allowed in RTOG 0617, which often resulted in sizable gross tumor volume (GTV) to PTV expansion margins (26). Based on these studies, it is proposed that increasing expansion size may also increase the risk of severe treatment related toxicity in the high dose arm of RTOG 0617, which could potentially contribute to the observed decrease in patient survival.
Strategies to Improve Target Volume Definition through Adaptive Image-Guided IMRT
Respiratory Motion and IMRT
The high incidence of loco-regional failures in NSCLC following radiotherapy may be associated with a high rate of tumor localization error during treatment (27). Such geometric errors may be minimized when respiratory motion is taken into consideration. Lung tumor motion associated with respiration has been well illustrated in multiple studies. In this regard, Seppenwoolde et al. showed that lower lobe tumors, not attached to any rigid thoracic structures, had increased cranial–caudal motion, as compared to upper lobe tumors or tumors attached to rigid structures during treatment (12 ± 6 vs. 2 ± 2 mm, p = 0.005) (28). In addition, anterior–posterior tumor motion of >5 mm could be observed in tumors located in the anterior or middle thorax and tumor motion was further complicated by hysteresis. In another study by Liu et al., cranial–caudal motion of >5 mm and ≥1 cm can be observed in 30 and 10% of patients with stage III NSCLC, which is associated with diaphragmatic movement of 1.53 cm on average (29). In a report by the AAPM task group 76, several strategies have been recommended to account for and control respiratory motion, which may be distinct for each individual patient (30).
One commonly used strategy to account for respiratory motion in the treatment of locally advanced NSCLC is 4D CT based treatment planning. Through 4D CT based planning, the range of tumor motion and changes in tumor volume through the entire breathing cycle can be more appropriately and reliably accounted for (29, 31–33). Also, it can potentially decrease the volume of normal tissue included in the PTV and this might be one technique to safely allow for dose escalation to the primary tumor (31). As such, it seems reasonable to suggest that improved accuracy in tumor localization throughout the respiratory cycle could improve tumor control probability (TCP) as “geometric tumor miss” is reduced. In this regard, the maximum intensity projections (MIP) reconstructed from a 4D data set, which reflects the brightest object along each ray on the projection image, may be used for the generation of the internal target volume (ITV) (34) that includes tumor motion into the radiation planning process. However, the outer excursion of respiratory motion may be underestimated by MIP in more advanced tumors and in the setting of irregular breathing patterns by approximately 20% as reported in previous studies (35–37). Thus, the ITV should be generated based on all available 4D CT data.
The utility of 4D CT combined with thoracic IMRT in the treatment of locally advanced NSCLC with chemo-radiation was shown to lower treatment related toxicity and improve patient survival when compared to 3D-CRT in a study from MD Anderson Cancer Center (38). This may be associated with dosimetric advantages of IMRT over 3D-CRT in dose conformity and the sparing of normal organs at risk (OARs) (39–41). As shown by Lievens et al., IMRT can result in significant reduction in the dose to the normal lungs and the spinal cord when compared to 3D-CRT (41). This led to the safe escalation of the prescribed dose (66 Gy in 33 daily fractions) by 8.6–14.2 Gy. Therefore, IMRT may be a strategy for dose escalation in the treatment of locally advanced NSCLC in selected patients through its ability to control radiation dose to critical OARs. This is also suggested by a quality of life (QoL) analysis of RTOG 0617, which demonstrated that the use of IMRT in the setting of dose escalation may improve patients’ QoL, which has been correlated with overall survival (42). Dose escalation with the use of IMRT alone remains to be further investigated in future studies.
Image Guidance with Cone-Beam CT (CBCT)
Intensity modulated radiotherapy can be used to achieve highly conformal dose distribution with sharp dose gradients and careful daily tumor localization should ensure accurate dose delivery with maximal reduction of treatment margins used for target volume delineation (43–45). This is especially important for dose escalation as radiotherapy often requires 6 weeks or greater to complete in the setting of chemo-radiation for locally advanced NSCLC. In this regard, various image guidance strategies have been proposed and are in clinical use currently (30, 46). Tumor volume, its geometric relation to surrounding OARs, and any anatomical changes during a course of radiotherapy are best imaged with volumetric imaging techniques such as kV or MV cone-beam CT (CBCT). KV CBCT often provides superior soft tissue resolution with compared with MV CBCT due to the prevalence of photoelectric absorption interactions associated with lower beam energies (47). However, MV CBCT may be more helpful in the imaging of regions with high density materials, which produce artifacts in kV CT images (46, 47). As shown by Bissonnette et al., kV CBCT can reduce the set up margin for geometric uncertainties to 3 mm for locally advanced NSCLC patients (48). Such small margin can be safely used under daily image guidance, which reduce set up errors of ≥5 mm from 20–43 to 6% when compared with less-than-daily image guidance (49). Thus, daily image guidance is critical for the safe maximal reduction of set up margins when IGRT, or IG-IMRT is delivered; which will also maximize the possibility of safe dose escalation to the highest dose possible. When compared to other forms of image guidance, kV CBCT was found to be associated with more reliable tumor localization and smaller set up errors (50, 51) and this advantage appears to be most prominent with image registration based on soft tissue in addition to rigid bony registration (51, 52). However, tissue resolution and motion artifacts continue to be a concern for the use of CBCT in the setting of locally advanced NSCLC. These issues may be minimized by 4D CBCT, which remains to be further investigated in the clinical setting (53). One caveat for the clinical adaptation of image-guided IMRT is that there is always a risk for underestimating the range of tumor motion at the time of CT simulation due to the random occurrence of irregular breathing patterns (e.g., random deep inspiration) during the actual delivery of a course of conventionally fractionated radiotherapy. This may potentially lead to the under-dosing of the gross tumor in selected situations of dose painting as very sharp dose gradients are generated at the edge of the tumor targets with intensity modulation. Respiration motion management strategies, such as forced shallow breathing with abdominal compression, may reduce the risk for such geometric misses by limiting diaphragmatic motion to within 5 mm during daily treatment.
The Role of FDG–PET in Target Volume Delineation
FDG–PET/CT has been increasingly incorporated into the treatment planning process to further increase the accuracy of target volume delineation in recent years. FDG–PET imaging is achieved through the detection of a pair of γ rays (511 keV each) that are produced in positron annihilation, which are emitted in 180° to each other (54). FDG–PET is associated with superior sensitivity and specificity (83 and 91%) for tumor detection when compared to CT (64 and 74%) (55). Therefore, PET may provide information for target volume delineation that may be otherwise not available with CT alone.
When compared with CT based treatment planning, PET registered to the planning CT was shown to alter tumor staging in 31% of the patients with stage I–III NSCLC by Bradley et al. (56). In this study, the addition of FDG–PET information resulted in alterations of the treatment volumes in 58% of the patients who were planned for 3D-CRT. Such alterations resulted from the identification of additional regional or parenchymal disease; and the improved tumor definition in the setting of atelectasis, which decreases the unnecessary inclusion of normal lung tissue in the GTV and these findings were corroborated in other studies (57, 58). Also, increased PTV volume, due to the additional nodal disease found with FDG–PET, does not necessarily increase the normal tissue complication probability; while PET data may improve the chance of local control by reducing the likelihood of geometric misses (57). Therefore, the inclusion of FDG–PET information in the treatment planning improves the accuracy of tumor volume delineation, which is critical in the optimization of TCP through IG-IMRT. In addition, PET may lead to nodal GTV reduction and lower doses to the OARs for stage III NSCLC when compared with treatment planning with CT alone, possibly leading to significant iso-toxic dose escalation (59, 60). Based on the evidence illustrated above, FDG–PET inclusion in the treatment planning process may be required in future dose escalation trials for locally advanced NSCLC.
Adaptive Image-Guided IMRT
Tumor shrinkage through a course of conventionally fractionated radiotherapy has been well characterized in multiple studies (61–64). In one study, a median GTV reduction of 24.7% after 30 Gy, and 44.3% after 50 Gy was observed in 22 patients with stage I–III NSCLC (78% stage III, 68% squamous cell carcinoma, and 68% underwent concurrent chemo-radiation) (63). In an analysis of 4D CT data collected before and during radiotherapy of at least 6 weeks from patients with stage I–III NSCLC, tumor shrinkage of ≥40% by the end of radiotherapy was observed in 50% of the patients (mainly stage III) (64). Also, increased tumor motion in the cranio-caudal direction increased in half of the patients as tumors shrunk. The observed changes may have an impact on PTV dose coverage and OAR sparing in the treatment of locally advanced NSCLC (62, 65). Therefore, re-planning or ART based on changes observed through daily image guidance is warranted in the treatment of locally advanced NSCLC, which may further maximize safe dose escalation to the gross disease. This may be especially important for IG-IMRT due to the sharp dose gradient generated and small margins for geometric uncertainties used.
Altered fractionation has been previously shown to improve local control and overall survival in the treatment of locally advanced NSCLC (66). This is also supported by the observed clinical outcome following hypo-fractionated radiotherapy for early stage NSCLC (16, 67). Altered fractionation is especially appealing in the setting of dose escalation, as prolonged course of radiotherapy can lead to increased geometric uncertainties, and impairment of local control due to accelerated tumor cell repopulation, which may partially explain the results observed in RTOG 0617 (13, 65). This is also supported by previous RTOG studies on concurrent chemo-radiation, which demonstrated a 2% increase in the risk of death for each day in the prolongation of therapy (68). A dose-per-fraction escalation strategy has been previously proposed to overcome the negative impact of tumor cell repopulation encountered when the total tumor dose is escalated with conventional fractionation. As modeled by Welsh et al., the TCP can be increased to >80% with this strategy in approximately 5 weeks, which would require 100 Gy to be delivered over 10 weeks with conventional fractionation (69).
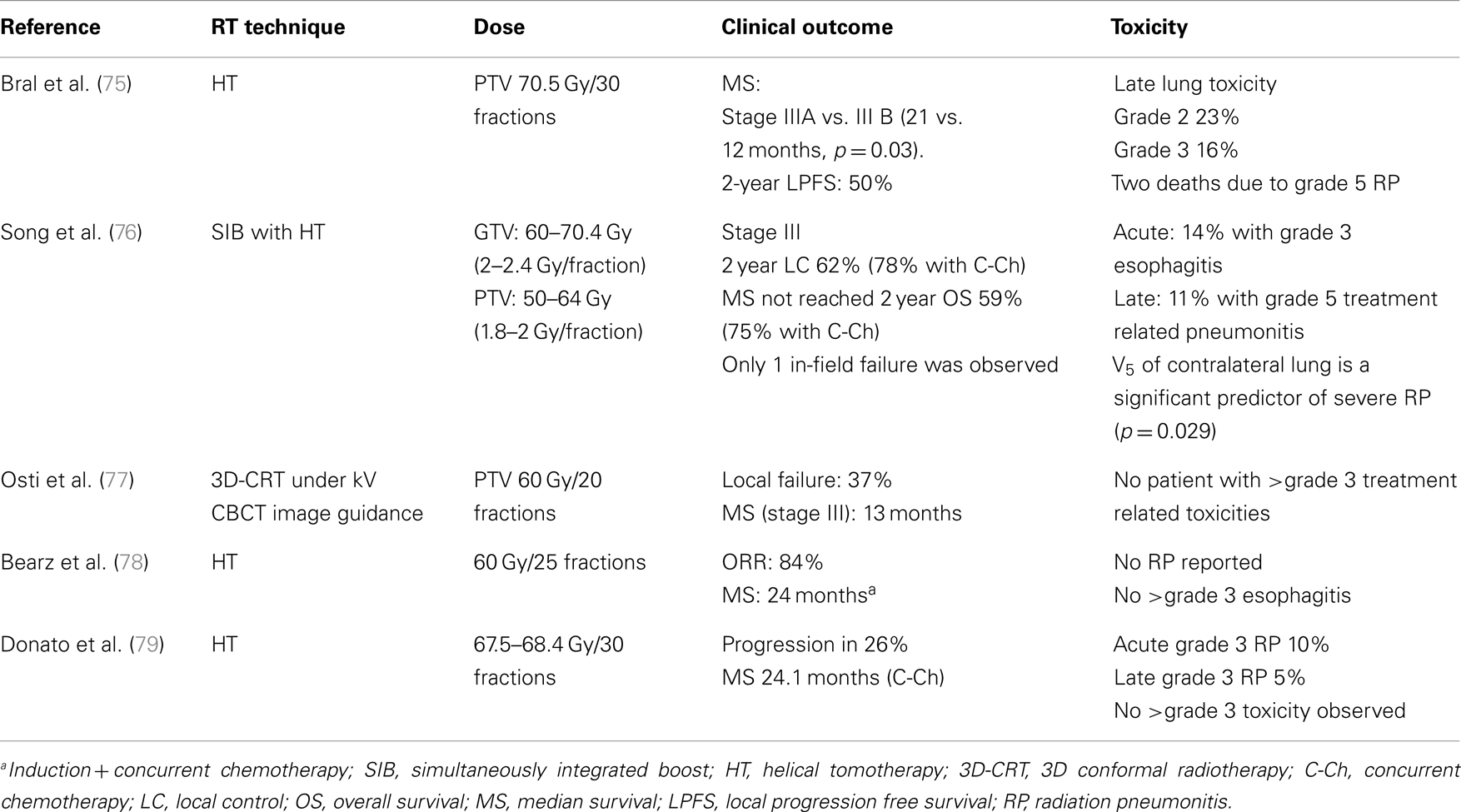
Early phase and retrospective studies on altered fractionation for radiotherapy alone or combined with chemotherapy in the treatment of stage III NSCLC have shown the feasibility of this strategy (70–78). This is especially true for chemo-radiation delivered with IGRT (74–78). As shown in Table 2, excellent median survival and local control have been frequently observed when hypofractionated, image-guided IMRT was delivered with concurrent chemotherapy. However, severe RP may occur with this approach if low dose irradiation of the normal lungs was not carefully constrained (74, 75). This was shown by Song et al., who observed four RP related deaths following image-guided IMRT delivered with helical tomotherapy (HT) (75). In this study, the rate of ≥grade 3 RP increased from 0 to 35%, when the volume of the contralateral lung receiving 5 Gy (V5) was increased to above 60% (p = 0.010). This is corroborated in a study by Kim et al., which identified the ipsilateral V5, V10, V15, and the contralateral V5 to be significant predictors of RP following HT based IGRT (79). This observation may be partially due to the fan-beam nature of IMRT delivery with HT, which leads to increased low dose irradiation of the normal tissue (80). However, the importance of normal lung sparing from low dose irradiation was observed following linac based IMRT as well, suggesting this to be independent from methods of radiation delivery (81).
One strategy to reduce treatment related toxicities associated with hypo-fractionated radiotherapy may be simultaneously integrated boost (SIB) to the gross tumor through FDG–PET based dose painting and the utility of adaptive IG-IMRT. This is suggested in a dosimetric study of 13 patients with stage III NSCLC (82). In this study, IMRT was shown to reduce the mean lung dose; furthermore, adaptive IMRT with SIB was shown to be superior to 3D-CRT or IMRT alone in dose escalation for larger GTVs, and was able to achieve maximal iso-toxic dose escalation of 17.1 ± 10.1%, which increased TCP by 17.2% on average. This is consistent with image-guided IMRT with the SIB technique that has been previously shown to be feasible by Song et al. (75), and the utilization of adaptive IG-IMRT with SIB in the treatment of locally advanced NSCLC warrants further investigation in future dose escalation trials.
Novel Use of a Stereotactic Boost and Proton Therapy
Tumor shrinkage after a course of conventionally fractionated radiotherapy may allow for the delivery of a stereotactic boost to the primary tumor in selected patients. In a study by Feddock et al., 10 Gy × 2 fractions, or 6.5 Gy × 3 fractions were delivered following chemo-radiation to a median dose of 59.4 Gy in the treatment of stage II–III NSCLC (83). Although well tolerated in patients with peripheral primary tumors, two deaths due to fatal pulmonary hemorrhage occurred in patients with central lesions. Six local recurrences were observed among 35 patients after a median follow up of 13 months, which corresponds to an actuarial local control of 82.9%. These preliminary results suggest the feasibility of this treatment approach in selected patients, however, patient selection and the most optimal dose fractionation schedule to be used in this setting remains to be further investigated.
Proton therapy has been increasingly investigated in the treatment of lung cancer in recent years due to its advantage in normal tissue sparing over photon therapy (84). In the interaction with tissue, protons enter with lower dose than photon, and deposit the majority of their energy at a certain depth (Bragg peak) with very little exit dose. To be clinically useful, several Bragg peaks can be super-positioned to create a spread-out Bragg peak (SOBP) to cover a specific tumor volume with a desired dose. When compared with photon therapy (3D-CRT or IMRT), proton therapy was shown to significantly reduce the dose to the thoracic OARs, which allowed dose escalation from 63 to 74 Gy within the boundaries of accepted normal tissue dose constraints in stage III NSCLC (85). Intensity-modulated proton therapy (IMPT) may further improve OAR sparing in stage IIIB NSCLC patients, which increases the possibility of dose escalation when compared to IMRT (63–83.5 Gy), and passive scattering proton therapy (PSPT) (74–84.4 Gy) (86). ART may also be indicated in the delivery of PT to improve OAR sparing and target volume coverage (87). In a retrospective study of PT for patients with stage II–III NSCLC, local control of 88.6% was achieved following a median follow up of 16.9 months (88). In this study, a median dose of 78.3 Gy was delivered without any ≥grade 3 toxicity observed. In a phase II study of concurrent chemotherapy and PSPT (74 Gy) for stage III NSCLC, local control of 79.5% and a median overall survival of 29.4 months were observed after a median follow up of 19.7 months (89). In this study, isolated local failure was observed in only 9.1% of the patients, while no grade 4–5 toxicity was observed. While comparable to what is observed in the standard arm of RTOG 0617 in median survival, this may be further improved with adaptive IG-IMPT, which needs to be further investigated in future studies. Due to the unique physical properties of protons, dose distribution in IMPT is very sensitive to tumor motion in relation to the treatment beam scanning motion, which is known as the interplay effect (90). Range uncertainties produced by the interplay effect may lead to under-dosing of the tumor or increased dose to the OARs immediately beyond the range of the proton beam. To account for interplay uncertainties, 4D treatment planning, larger spot size, and fractionated dose schedules have been advocated (91–93). Recently, image guidance for range verification during proton therapy has been shown to be feasible with in-room PET imaging (94). This along with strategies to overcome interplay uncertainties in proton therapy warrants further investigation. However, early clinical experience with proton therapy in the setting of dose escalation for locally advanced NSCLC appears promising.
Although not widely studied, delivering SABR to small peripheral primary tumors and conventionally fractionated radiotherapy combined with concurrent chemotherapy to regional nodal disease in selected cases of locally advanced NSCLC has been endorsed and clinically used by many thoracic Radiation Oncologists. The local control of the primary tumor may be potentially improved as suggested by clinical outcome from SABR for early stage NSCLC (16). At the same time, the regional disease can be addressed with concurrent chemo-radiation. SABR combined with concurrent chemo-radiation, as definitive treatment or a boost to the primary disease, will be suitable for a selected group of patients only. As the mediastinum contains many critical normal structures, which may be at a risk for overdosing along with the lungs if the primary tumor is in their proximity.
Chemotherapy in the Setting of Dose Escalation
Combining chemotherapy with radiotherapy has been shown to improve local control and patients’ overall survival in previous trials (1, 95). However, chemotherapy, especially taxane-based regimens, has been associated with increased incidence of severe RP (96–99). Severe RP was observed in 47% of the patients treated with concurrent weekly docetaxel and conventionally fractionated radiotherapy to 60–66 Gy delivered with 2D techniques in a phase II study (96). This observation was dose independent, but correlated with the size of initial radiation portals. Consolidation docetaxel was also found to be correlated with increased risk of grade 2–5 RP (14.6 vs. 3.6%, p = 0.015) following concurrent chemo-radiation in the retrospective review of the dosimetric data from a randomized prospective study (97). In this study, the mean lung dose was also significantly correlated with the incidence of RP. Furthermore, the increased risk of RP with chemotherapy may be more prominent in patients who are older (98, 99). Therefore, special attention may be necessary to keep the treated volume to as small as possible to minimize the amount of normal lung tissue irradiated to the full dose, which may be especially important in patients who are older than 65 years (99). For the purpose of dose escalation in the setting of chemo-radiation, this may be best accomplished with modern techniques of respiratory motion management and image guidance used in the context of various emerging treatment strategies discussed above.
Conclusion
Adaptive, image-guided IMRT, when delivered with appropriate respiratory motion management strategies, can effectively reduce the tumor target volume while accurately localize the tumor during a course of chemo-radiation. This allows for dose escalation to the gross disease in the treatment of locally advanced NSCLC as less normal tissue is being irradiated to the prescribed dose. This strategy can be further enhanced with the incorporation of FDG–PET in target volume definition. The utility of stereotactic ablative therapy as a boost or definitive treatment for the primary tumor appears to be feasible in selected patients, while proton therapy, and especially IMPT, appears to be promising and may be superior to photon therapy in dose escalation for the treatment of locally advanced NSCLC. These new treatment approaches remain to be further studied in future clinical trials. Here, we propose the following strategies to be further investigated in selected patients in future trials with respiratory motion management and the incorporation of FDG–PET in the treatment planning required:
(1) Adaptive image-guided IMRT or IMPT delivered in a simultaneously integrated fashion with concurrent chemotherapy.
(2) Stereotactic boost to the primary tumor to be delivered prior or after a standard course of concurrent chemo-radiation to 60 Gy, or given in between a split course of concurrent chemo-radiation that is hypofractionated, image-guided, and intensity modulated.
(3) Stereotactic ablative radiotherapy to the primary tumor to be followed by concurrent chemotherapy and image-guided IMRT to the regional nodal disease.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Schaake-Konning C, Van Den Bogaert W, Dalesio O, Festen J, Hoogenhout J, van Houtte P, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med (1992) 326:524–30. doi:10.1056/NEJM199202203260805
2. Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Hyperfractionated radiation therapy with or without concurrent low-dose daily carboplatin/etoposide for stage III non-small-cell lung cancer: a randomized study. J Clin Oncol (1996) 14:1065–70.
3. Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small cell lung cancer. J Clin Oncol (1999) 17:2692–9.
4. Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer (2004) 46:87–98. doi:10.1016/j.lungcan.2004.03.004
5. Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst (2011) 103:1452–60. doi:10.1093/jnci/djr325
6. Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol (2010) 28:2181–90. doi:10.1200/JCO.2009.26.2543
7. Nyman J, Friesland S, Hallqvist A, Seke M, Bergström S, Thaning L, et al. How to improve loco-regional control in stages IIIa-b NSCLC? Results of a three-armed randomized trial from the Swedish Lung Cancer Study Group. Lung Cancer (2009) 65:62–7. doi:10.1016/j.lungcan.2008.10.021
8. Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, et al. High-dose radiation improved local control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys (2005) 63:324–33. doi:10.1016/j.ijrobp.2005.02.010
9. Rosenzweig KE, Fox JL, Yorke E, Amols H, Jackson A, Rusch V, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer (2005) 103:2118–27. doi:10.1002/cncr.21007
10. Willner J, Baier K, Caragiani E, Tschammler A, Flentje M. Dose, volume, and tumor control predictions in primary radiotherapy of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys (2002) 52:382–9. doi:10.1016/S0360-3016(01)01823-5
11. Bradley J, Graham MV, Winter K, Purdy JA, Komaki R, Roa WH, et al. Toxcity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys (2005) 61:318–28. doi:10.1016/j.ijrobp.2004.06.260
12. Martel MK, Ten Haken RK, Hazuka MB, Kessler ML, Strawderman M, Turrisi AT, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer (1999) 24:31–7. doi:10.1016/S0169-5002(99)00019-7
13. Fowler JF, Tomé W, Fenwick JD, Mehta MP. A challenge to traditional radiation oncology. Int J Radiat Oncol Biol Phys (2004) 60:1241–56. doi:10.1016/j.ijrobp.2004.07.691
14. Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol (2007) 2:S94–100. doi:10.1097/JTO.0b013e318074de34
15. Grills IS, Hope AJ, Guckenberger M, Kestin LL, Werner-Wasik M, Yan D, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol (2012) 7:1382–93. doi:10.1097/JTO.0b013e318260e00d
16. Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol (2010) 94:1–11. doi:10.1016/j.radonc.2009.12.008
17. Machtay M, Bae K, Movsas B, Paulus R, Gore EM, Komaki R, et al. Higher biologically effective dose of radiotherapy is associated with improve outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys (2012) 82:425–34. doi:10.1016/j.ijrobp.2010.09.004
18. Bradley JD, Bae K, Graham MV, Byhardt R, Govindan R, Fowler J, et al. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol (2010) 28:2475–80. doi:10.1200/JCO.2009.27.1205
19. Socinski MA, Blackstock AW, Bogart JA, Wang X, Munley M, Rosenman J, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol (2008) 26:2457–63. doi:10.1200/JCO.2007.14.7371
20. Bradley JD, Paulus R, Komaki R, Masters GA, Forster K, Schild SE, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer. Results on radiation dose in RTOG 0617. J Clin Oncol (2013) 31:(Suppl 7501).
21. Dillman RO, Seagren SL, Propert KJ, Guerra J, Eaton WL, Perry MC, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med (1990) 323:940–5. doi:10.1056/NEJM199010043231403
22. Sause W, Kolesar P, Taylor S, Johnson D, Livingston R, Komaki R, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest (2000) 117:358–64. doi:10.1378/chest.117.2.358
23. Le Chevalier T, Arriagada R, Quoix E, Ruffie P, Martin M, Douillard JY, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in unresectable non-small cell lung carcinoma. Lung Cancer (1994) 10:S239–44. doi:10.1016/0169-5002(94)91687-X
24. Rosenzweig KE, Sura S, Jackson A, Yorke E. Involved-field radiation therapy for inoperable non-small-cell lung cancer. J Clin Oncol (2007) 25:5557–61. doi:10.1200/JCO.2007.13.2191
25. Yuan S, Sun X, Li M, Yu J, Ren R, Yu Y, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol (2007) 30:239–44. doi:10.1097/01.coc.0000256691.27796.24
26. Available from: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0617
27. Rosenman JG, Halle JS, Socinski MA, Deschesne K, Moore DT, Johnson H, et al. High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys (2002) 54:348–56. doi:10.1016/S0360-3016(02)02958-9
28. Seppenwoolde Y, Shirato H, Kitamura K, Shimizu S, van Herk M, Lebesque JV, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys (2002) 53:822–34. doi:10.1016/S0360-3016(02)02803-1
29. Liu HH, Balter P, Tutt T, Choi B, Zhang J, Wang C, et al. Assessing respiration-induced tumor motion and internal target volume using four-dimensional computed tomography for radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys (2007) 68:531–40. doi:10.1016/j.ijrobp.2006.12.066
30. Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys (2006) 33:3874–900. doi:10.1118/1.2349696
31. Rietzel E, Liu AK, Doppke KP, Wolfgang JA, Chen AB, Chen GT, et al. Design of 4D treatment planning target volumes. Int J Radiat Oncol Biol Phys (2006) 66:287–95. doi:10.1016/j.ijrobp.2006.05.024
32. Pantarotto JR, Piet AH, Vincent A, van Sörnsen de Koste JR, Senan S. Motion analysis of 100 mediastinal lymph nodes: potential pitfalls in treatment planning and adaptive strategies. Int J Radiat Oncol Biol Phys (2009) 74:1092–9. doi:10.1016/j.ijrobp.2008.09.031
33. Redmond KJ, Song DY, Fox JL, Zhou J, Rosenzweig CN, Ford E. Respiratory motion changes of lung tumors over the course of radiation therapy based on respiration-correlated four-dimensional computed tomography scans. Int J Radiat Oncol Biol Phys (2009) 75:1605–12. doi:10.1016/j.ijrobp.2009.05.024
34. Underberg RW, Lagerwaard FJ, Slotman BJ, Cuijpers JP, Senan S. Use of maximum intensity projections (MIP) for target volume generation in 4DCT scans for lung cancer. Int J Radiat Oncol Biol Phys (2005) 63:253–60. doi:10.1016/j.ijrobp.2005.05.045
35. Muirhead R, McNee SG, Featherstone C, Moore K, Muscat S. Use of maximum intensity projections (MIPs) for target outlining in 4DCT radiotherapy planning. J Thorac Oncol (2008) 3:1433–8. doi:10.1097/JTO.0b013e31818e5db7
36. Cai J, Read PW, Baisden JM, Larner JM, Benedict SH, Sheng K. Estimation of error in maximal intensity projection-based internal target volume of lung tumors: a simulation and comparison study using dynamic magnetic resonance imaging. Int J Radiat Oncol Biol Phys (2007) 69:895–902. doi:10.1016/j.ijrobp.2007.07.2322
37. Park K, Huang L, Gagne H, Papiez L. Do maximum intensity projection images truly capture tumor motion? Int J Radiat Oncol Biol Phys (2009) 73:618–25. doi:10.1016/j.ijrobp.2008.10.008
38. Liao ZX, Komaki RR, Thames HD, Liu HH, Tucker SL, Mohan R, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys (2010) 76:775–81. doi:10.1016/j.ijrobp.2009.02.032
39. Liu HH, Wang X, Dong L, Wu Q, Liao Z, Stevens CW, et al. Feasibility of sparing lung and other thoracic structures with intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys (2004) 58:1268–79. doi:10.1016/j.ijrobp.2003.09.085
40. Christian JA, Bedford JL, Webb S, Brada M. Comparison in inverse-planned three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys (2007) 67:735–41. doi:10.1016/j.ijrobp.2006.09.047
41. Lievens Y, Nulens A, Gaber MA, Defraene G, De Wever W, Stroobants S, et al. Intensity-modulated radiotherapy for locally advanced non-small-cell lung cancer: a dose-escalation planning study. Int J Radiat Oncol Biol Phys (2011) 80:306–13. doi:10.1016/j.ijrobp.2010.06.025
42. Movsas B, Hu C, Sloan J, Bradley JD, Kavadi VS, Narayan S, et al. Quality of life (QOL) analysis of the randomized radiation (RT) dose-escalation NSCLC trial (RTOG 0617): the rest of the story. Int J Radiat Oncol Biol Phys (2013) 87:S1–2. doi:10.1016/j.ijrobp.2013.06.012
43. Verellen D, De Ridder M, Linthout N, Tournel K, Soete G, Storme G. Innovations in image-guided radiotherapy. Nat Rev Cancer (2007) 7:949–60. doi:10.1038/nrc2288
44. Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol (2012) 9:688–99. doi:10.1038/nrclinonc.2012.194
45. Yeung AR, Li JG, Shi W, Newlin HE, Chvetsov A, Liu C, et al. Tumor localization using cone-beam CT reduces setup margins in conventionally fractionated radiotherapy for lung tumors. Int J Radiat Oncol Biol Phys (2009) 74:1100–7. doi:10.1016/j.ijrobp.2008.09.048
46. Dawson LA, Jaffray DA. Advances in image-guided radiation therapy. J Clin Oncol (2007) 25:938–46. doi:10.1200/JCO.2006.09.9515
47. Korreman S, Rasch C, McNair H, Verellen D, Oelfke U, Maingon P, et al. The European Society of Therapeutic Radiology and Oncology-European Institute of Radiotherapy (ESTRO-EIR) report on 3D CT-based in-room image guidance systems: a practical and technical review and guide. Radiother Oncol (2010) 94:129–44. doi:10.1016/j.radonc.2010.01.004
48. Bissonnette JP, Purdie TG, Higgins JA, Li W, Bezjak A. Cone-beam computed tomographic image guidance for lung cancer radiation therapy. Int J Radiat Oncol Biol Phys (2009) 73:927–34. doi:10.1016/j.ijrobp.2008.08.059
49. Higgins J, Bezjak A, Hope A, Panzarella T, Li W, Cho JB, et al. Effect of image-guidance frequency on geometric accuracy and setup margins in radiotherapy for locally advanced lung cancer. Int J Radiat Oncol Biol Phys (2011) 80:1330–7. doi:10.1016/j.ijrobp.2010.04.006
50. Borst GR, Sonke JJ, Betgen A, Remeijer P, van Herk M, Lebesque JV. Kilo-voltage cone-beam computed tomography setup measurements for lung cancer patients; first clinical results and comparison with electronic portal-imaging device. Int J Radiat Oncol Biol Phys (2007) 68:555–61. doi:10.1016/j.ijrobp.2007.01.014
51. Roman NO, Shepherd W, Mukhopadhyay N, Hugo GD, Weiss E. Interfractional positional variability of fiducial markers and primary tumors in locally advanced non-small-cell lung cancer during audiovisual biofeedback radiotherapy. Int J Radiat Oncol Biol Phys (2012) 83:1566–72. doi:10.1016/j.ijrobp.2011.10.051
52. Sonke JJ, Lebesque J, van Herk M. Variability of four-dimensional computed tomography patient models. Int J Radiat Oncol Biol Phys (2008) 70:590–8. doi:10.1016/j.ijrobp.2007.08.067
53. Sonke JJ, Zijp L, Remeijer P, van Herk M. Respiratory correlated cone beam CT. Med Phys (2005) 32:1176–86. doi:10.1118/1.1869074
54. Nestle U, Kremp S, Grosu AL. Practical integration of [18F]-FDG-PET and PET-CT in the planning of radiotherapy for non-small cell lung cancer (NSCLC): the technical basis, ICRU-target volumes, problems, perspectives. Radiother Oncol (2006) 81:209–25. doi:10.1016/j.radonc.2006.09.011
55. Gambhir SS, Czemin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med (2001) 42:S1–93.
56. Bradley J, Thorstad WL, Mutic S, Miller TR, Dehdashti F, Siegel BA, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys (2004) 59:78–86. doi:10.1016/j.ijrobp.2003.10.044
57. Erdi YE, Rosenzweig K, Erdi AK, Macapinlac HA, Hu YC, Braban LE, et al. Radiotherapy treatment planning for patients with non-small cell lung cancer using positron emission tomography (PET). Radiother Oncol (2002) 62:51–60. doi:10.1016/S0167-8140(01)00470-4
58. Mah K, Caldwell CB, Ung YC, Danjoux CE, Balogh JM, Ganguli SN, et al. The impact of (18) FDG-PET on target and critical organs in CT-based treatment planning of patients with poorly defined non-small-cell lung carcinoma: a prospective study. Int J Radiat Oncol Biol Phys (2002) 52:339–50. doi:10.1016/S0360-3016(01)01824-7
59. van Der Wel A, Nijsten S, Hochstenbag M, Lamers R, Boersma L, Wanders R, et al. Increased therapeutic ratio by 18FDG-PET CT planning in patients with clinical CT stage N2-N3M0 non-small-cell lung cancer: a modeling study. Int J Radiat Oncol Biol Phys (2005) 61:649–55. doi:10.1016/j.ijrobp.2004.06.205
60. Møller DS, Khalil AA, Knap MM, Muren LP, Hoffmann L. A planning study of radiotherapy dose escalation of PET-active tumour volumes in non-small cell lung cancer patients. Acta Oncol (2011) 50:883–8. doi:10.3109/0284186X.2011.581694
61. Kupelian PA, Ramsey C, Meeks SL, Willoughby TR, Forbes A, Wagner TH, et al. Serial megavoltage CT imaging during external beam radiotherapy for non-small-cell lung cancer: observations on tumor regression during treatment. Int J Radiat Oncol Biol Phys (2005) 63:1024–8. doi:10.1016/j.ijrobp.2005.04.046
62. Ramsey CR, Langen KM, Kupelian PA, Scaperoth DD, Meeks SL, Mahan SL, et al. A technique for adaptive image-guided helical tomotherapy for lung cancer. Int J Radiat Oncol Biol Phys (2006) 64:1237–44. doi:10.1016/j.ijrobp.2005.11.012
63. Fox J, Ford E, Redmond K, Zhou J, Wong J, Song DY. Quantification of tumor volume changes during radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys (2009) 74:341–8. doi:10.1016/j.ijrobp.2008.07.063
64. Britton KR, Starkschall G, Tucker SL, Pan T, Nelson C, Chang JY, et al. Assessment of gross tumor volume regression and motion changes during radiotherapy for non-small-cell lung cancer as measured by four-dimensional computed tomography. Int J Radiat Oncol Biol Phys (2007) 68:1036–46. doi:10.1016/j.ijrobp.2007.01.021
65. Britton KR, Starkschall G, Liu H, Chang JY, Bilton S, Ezhil M, et al. Consequences of anatomic changes and respiratory motion on radiation dose distributions in conformal radiotherapy for locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys (2009) 73:94–102. doi:10.1016/j.ijrobp.2008.04.016
66. Saunders M, Dische S, Barrett A, Harvey A, Griffiths G, Palmar M. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol (1999) 52:137–48. doi:10.1016/S0167-8140(99)00087-0
67. Bogart JA, Hodgson L, Seagren SL, Blackstock AW, Wang X, Lenox R, et al. Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol (2010) 28:202–6. doi:10.1200/JCO.2009.25.0753
68. Machtay M, Hsu C, Komaki R, Sause WT, Swann S, Langer CJ, et al. Effect of overall treatment time on outcomes after concurrent chemoradiation for locally advanced non-small-cell lung carcinoma: analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int J Radiat Oncol Biol Phys (2005) 63:667–71. doi:10.1016/j.ijrobp.2005.03.037
69. Welsh JS, Lock M, Harari PM, Tomé WA, Fowler J, Mackie TR, et al. Clinical implementation of adaptive helical tomotherapy: a unique approach to image-guided intensity modulated radiotherapy. Technol Cancer Res Treat (2006) 5:465–79.
70. Kepka L, Tyc-Szczepaniak D, Bujko K. Dose-per-fraction escalation of accelerated hypofractionated three-dimensional conformal radiotherapy in locally advanced non-small cell lung cancer. J Thorac Oncol (2009) 4:853–61. doi:10.1097/JTO.0b013e3181a97dda
71. Cho KH, Ahn SJ, Pyo HR, Kim KS, Kim YC, Moon SH, et al. A phase II study of synchronous three-dimensional conformal boost to the gross tumor volume for patients with unresectable stage III non-small-cell lung cancer: results of Korean Radiation Oncology Group 0301 study. Int J Radiat Oncol Biol Phys (2009) 74:1397–404. doi:10.1016/j.ijrobp.2008.10.020
72. Cannon DM, Mehta MP, Adkinson JB, Khuntia D, Traynor AM, Tomé W, et al. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol (2013) 31:4343–8. doi:10.1200/JCO.2013.51.5353
73. Zhu ZF, Fan M, Wu KL, Zhao KL, Yang HJ, Chen GY, et al. A phase II trial of accelerated hypofractionated three-dimensional conformal radiation therapy in locally advanced non-small cell lung cancer. Radiother Oncol (2011) 98:304–8. doi:10.1016/j.radonc.2011.01.022
74. Bral S, Duchateau M, Versmessen H, Engels B, Tournel K, Vinh-Hung V, et al. Toxicity and outcome results of a class solution with moderately hypofractionated radiotherapy in inoperable stage III non-small cell lung cancer using helical tomotherapy. Int J Radiat Oncol Biol Phys (2010) 77:1352–9. doi:10.1016/j.ijrobp.2009.06.075
75. Song CH, Pyo H, Moon SH, Kim TH, Kim DW, Cho KH. Treatment-related pneumonitis and acute esophagitis in non-small cell lung cancer patients treated with chemotherapy and helical tomotherapy. Int J Radiat Oncol Biol Phys (2010) 78:651–8. doi:10.1016/j.ijrobp.2009.08.068
76. Osti MF, Agolli L, Valeriani M, Falco T, Bracci S, De Sanctis V, et al. Image guided hypofractionated 3-dimensional radiation therapy in patients with inoperable advanced stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys (2013) 85:e157–63. doi:10.1016/j.ijrobp.2012.10.012
77. Bearz A, Minatel E, Rumeileh IA, Borsatti E, Talamini R, Franchin G, et al. Concurrent chemoradiotherapy with tomotherapy in locally advanced non-small cell lung cancer: a phase I, docetaxel dose-escalation study with hypofractionated radiation regimen. BMC Cancer (2013) 13:513. doi:10.1186/1471-2407-13-513
78. Donato V, Arcangeli S, Monaco A, Caruso C, Cianciulli M, Boboc G, et al. Moderately escalated hypofractionated (chemo) radiotherapy delivered with helical intensity-modulated technique in stage III unresectable non-small cell lung cancer. Front Oncol (2013) 3:286. doi:10.3389/fonc.2013.00286
79. Kim Y, Hong SE, Kong M, Choi J. Predictive factors for radiation pneumonitis in lung cancer treated with helical tomotherapy. Cancer Res Treat (2013) 45:295–302. doi:10.4143/crt.2013.45.4.295
80. Chi A, Jang SY, Welsh JS, Nguyen NP, Ong E, Gobar L, et al. Feasibility of helical tomography in stereotactic body radiation therapy for centrally located early stage non-small-cell lung cancer or lung metastases. Int J Radiat Oncol Biol Phys (2011) 81:856–62. doi:10.1016/j.ijrobp.2010.11.051
81. Shi A, Zhu G, Wu H, Yu R, Li F, Xu B. Analysis of clinical and dosimetric factors associated with severe acute radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent chemotherapy and intensity-modulated radiotherapy. Radiat Oncol (2010) 5:35. doi:10.1186/1748-717X-5-35
82. Guckenberger M, Kavanagh A, Patridge M. Combining advanced radiotherapy technologies to maximize safety and tumor control probability in stage III non-small cell lung cancer. Strahlenther Onkol (2012) 188:894–900. doi:10.1007/s00066-012-0161-9
83. Feddock J, Arnold SM, Shelton BJ, Sinha P, Conrad G, Chen L, et al. Stereotactic body radiation therapy can be used safely to boost residual disease in locally advanced non-small cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys (2013) 85:1325–31. doi:10.1016/j.ijrobp.2012.11.011
84. Liao Z, Lin SH, Cox JD. Status of particle therapy for lung cancer. Acta Oncol (2011) 50:745–56. doi:10.3109/0284186X.2011.590148
85. Chang JY, Zhang X, Wang X, Kang Y, Riley B, Bilton S, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys (2006) 65:1087–96. doi:10.1016/j.ijrobp.2006.01.052
86. Zhang X, Li Y, Pan X, Li X, Mohan R, Komaki R, et al. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: a virtual clinical study. Int J Radiat Oncol Biol Phys (2010) 77:357–66. doi:10.1016/j.ijrobp.2009.04.028
87. Koay EJ, Lege D, Mohan R, Komaki R, Cox JD, Chang JY. Adaptive/nonadaptive proton radiation planning and outcomes in a phase II trial for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys (2012) 84:1093–100. doi:10.1016/j.ijrobp.2012.02.041
88. Nakayama H, Satoh H, Sugahara S, Kurishima K, Tsuboi K, Sakural H, et al. Proton beam therapy of stage II and III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys (2011) 81:979–84. doi:10.1016/j.ijrobp.2010.06.024
89. Chang JY, Komaki R, Lu C, Wen HY, Allen PK, Tsao A, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer (2011) 117:4707–13. doi:10.1002/cncr.26080
90. Bert C, Durante M. Motion in radiotherapy: particle therapy. Phys Med Biol (2011) 56:R113–44. doi:10.1088/0031-9155/56/16/R01
91. Engelsman M, Rietzel E, Kooy HM. Four-dimensional proton treatment planning for lung tumors. Int J Radiat Oncol Biol Phys (2006) 64:1589–95. doi:10.1016/j.ijrobp.2005.12.026
92. Kang Y, Zhang X, Chang JY, Wang H, Wei X, Liao Z, et al. 4D proton treatment planning strategy for mobile lung tumors. Int J Radiat Oncol Biol Phys (2007) 67:906–14. doi:10.1016/j.ijrobp.2006.10.045
93. Grassberger C, Dowdell S, Lomax A, Sharp G, Shackleford J, Choi N, et al. Motion interplay as a function of patient parameters and spot size in spot scanning proton therapy for lung cancer. Int J Radiat Oncol Biol Phys (2013) 86:380–6. doi:10.1016/j.ijrobp.2013.01.024
94. Min CH, Zhu X, Winey BA, Grogg K, Testa M, El Fakhri G, et al. Clinical application of in-room positron emission tomography for in vivo treatment monitoring in proton radiation therapy. Int J Radiat Oncol Biol Phys (2013) 86:183–9. doi:10.1016/j.ijrobp.2012.12.010
95. Aupérin A, Le Péchoux C, Pignon JP, Koning C, Jeremic B, Clamon G, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol (2006) 17:473–83. doi:10.1093/annonc/mdj117
96. Onishi H, Kuriyama K, Yamaguchi M, Komiyama T, Tanaka S, Araki T, et al. Concurrent two-dimensional radiotherapy and weekly docetaxel in the treatment of stage III non-small cell lung cancer: a good local response but no good survival due to radiation pneumonitis. Lung Cancer (2003) 40:79–84. doi:10.1016/S0169-5002(02)00532-9
97. Barriger RB, Fakiris AJ, Hanna N, Yu M, Mantravadi P, McGarry RC. Dose-volume analysis of radiation pneumonitis in non-small-cell lung cancer patients treated with concurrent cisplatinum and etoposide with or without consolidation docetaxel. Int J Radiat Oncol Biol Phys (2010) 78:1381–6. doi:10.1016/j.ijrobp.2009.09.030
98. Dang J, Li G, Ma L, Diao R, Zang S, Han C, et al. Predictors of grade ≥ 2 and grade ≥ 3 radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with three-dimensional conformal radiotherapy. Acta Oncol (2013) 52:1175–80. doi:10.3109/0284186X.2012.747696
Keywords: image guidance, intensity-modulated radiotherapy, NSCLC, adaptive radiotherapy, proton therapy
Citation: Chi A, Nguyen NP, Welsh JS, Tse W, Monga M, Oduntan O, Almubarak M, Rogers J, Remick SC and Gius D (2014) Strategies of dose escalation in the treatment of locally advanced non-small cell lung cancer: image guidance and beyond. Front. Oncol. 4:156. doi: 10.3389/fonc.2014.00156
Received: 13 March 2014; Paper pending published: 24 April 2014;
Accepted: 04 June 2014; Published online: 20 June 2014.
Edited by:
William Small, Stritch School of Medicine Loyola University Chicago, USACopyright: © 2014 Chi, Nguyen, Welsh, Tse, Monga, Oduntan, Almubarak, Rogers, Remick and Gius. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Chi, Mary Babb Randolph Cancer Center of West Virginia University, PO Box 9234, 1 Medical Center Drive Morgantown, WV 26505, USA e-mail: achiaz2010@gmail.com
 Alexander Chi
Alexander Chi Nam Phong Nguyen
Nam Phong Nguyen James S. Welsh3
James S. Welsh3 Olusola Oduntan
Olusola Oduntan