- 1Department of Pathology and Laboratory Medicine, Mount Sinai Hospital and University of Toronto, Toronto, ON, Canada
- 2Clinical Pathology Division, Institute of Pathology, University of Bern, Bern, Switzerland
- 3Department of Pathology, London Health Sciences Centre, Western University, London, ON, Canada
- 4Division of General Surgery, Taunton and Somerset NHS Foundation Trust, Taunton, UK
Venous invasion (VI) is a well-established independent prognostic indicator in colorectal cancer (CRC). Its accurate detection is particularly important in stage II CRC as it may influence the decision to administer adjuvant therapy. The Royal College of Pathologists (RCPath) of the United Kingdom state that VI should be detected in at least 30% of CRC resection specimens. However, our experience in Ontario, Canada suggests that this (conservative) benchmark is rarely met. This article highlights the “Ontario experience” with respect to VI reporting and the key role that careful morphologic assessment, elastin staining and knowledge transfer has played in improving VI detection provincially and beyond.
Introduction
Venous invasion (VI) is a well-established independent predictor of hematogenous spread and mortality in colorectal cancer (CRC) (1–8). Although the prognostic importance of VI has been recognized since the late 1930s (1, 9), its assessment remains one of the most poorly performed aspects of colorectal pathology reporting. Indeed it is surprising that many of the “giants” in the field that authored the early papers on colorectal carcinoma, and introduced the classification of colorectal carcinoma that forms the basis of the TNM classification, chose to use lymph node metastasis as a surrogate marker of the risk of metastatic disease rather than VI, a more direct measure. Unless tumor spreads via the lymphatics and thoracic duct, it needs to get into veins to disseminate, especially to the liver. Had they thought this way, the subject of this review may well have been completely obsolete, and the surprise (and power of “tradition”) is that it has taken us another 80 years to rethink what we do and how we do it, as reflected in this review.
Extramural venous invasion (EMVI) is a strong predictor of adverse outcome (4, 6, 10–14), while the prognostic significance of intramural venous invasion (IMVI) remains unclear. Several studies suggest that IMVI may impact outcome, albeit to a lesser degree than EMVI (6, 10, 15).
Venous invasion is of particular importance in stage II CRC as its detection may prompt oncologists to consider adjuvant chemotherapy. The importance of accurate risk stratification in stage II CRC will become increasingly relevant as the proportion of node-negative CRC is set to increase with the expansion of screening colonoscopy programs. Current evidence suggests that at least 70% of screening detected CRCs are node negative (16). Thus, the interest in measures other than nodal status to stratify risk of disease progression in CRC is likely to grow.
The importance of VI is recognized by the UK Royal College of Pathologists (RCPath), which has recently adjusted its minimum audit standard of VI detection to 30% in CRC resections (17). However, data from population-based studies indicate that this standard is rarely achieved (5, 8, 13, 18) with marked variability in the reported incidence of VI that ranges from 9 to 90% (8). Such variability is most likely to be attributed to differences in case mix, reporting criteria, sampling, use of special stains, and the diligence and skill of the reporting pathologist. Under-reporting of VI is particularly common outside of sub-specialist units (7, 10, 18, 19).
Accurate detection of VI can be challenging on routine H&E slides, especially when the muscular wall of the vein is obliterated beyond morphologic recognition or altered by pre-operative radiation (Figures 1G,H). In these circumstances, VI is easily overlooked unless key morphologic clues are sought. These include the so-called “orphan arteriole” sign (a circumscribed tumor nodule adjacent to muscularized artery without an obvious accompanying vein) (Figures 1A,B) and the “protruding tongue sign” (a smooth bordered protrusion of tumor into pericolic fat adjacent to an artery) (Figure 1E). Either of these findings, in the absence of a clearly visualized vessel wall, should prompt an elastin stain (Figures 1C,F) and/or a smooth muscle immunostain (Figure 1D), which will resolve the vast majority of equivocal cases (19, 20).
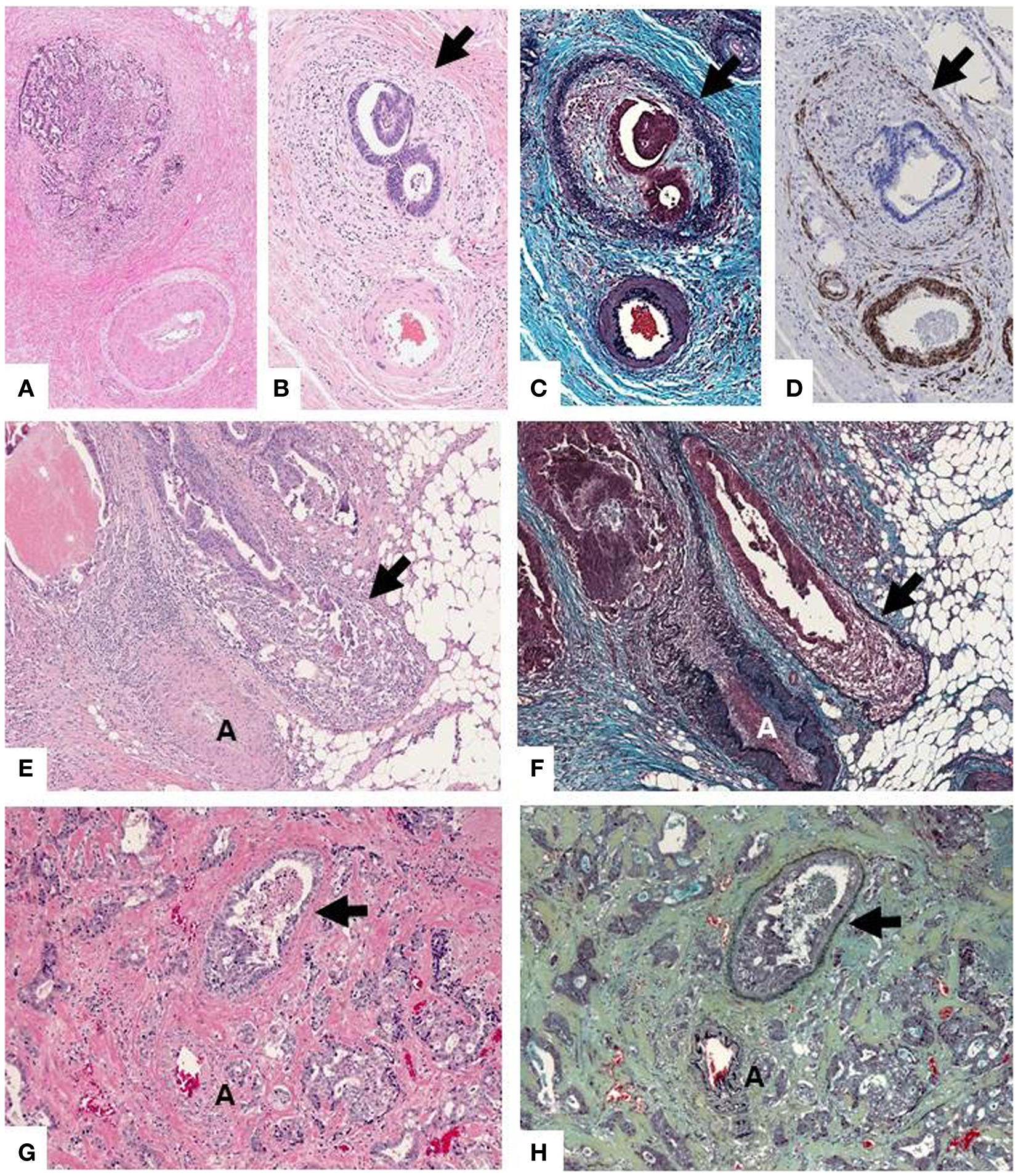
Figure 1. (A,B) “Orphan arteriole” sign (circumscribed tumor nodule adjacent to muscularized artery without an obvious accompanying vein). The residual vessel wall (arrow) can be highlighted with elastin trichrome stain (C) or immunohistochemical stain for caldesmon (smooth muscle marker) (D), facilitating the detection of VI. (E) “Protruding tongue sign” (rounded tongue-like protrusion of tumor into pericolic fat adjacent to an artery [A]). (F) An elastin stain highlights elastin fibers of the residual vessel wall which has been partially obliterated by tumor. (G) In some instances detection of VI can be virtually impossible on (H,E) but easily recognized on the elastin stain (H).
The Role of Elastin Staining
There is a growing body of evidence that routine elastin staining substantially increases VI detection. Many studies have demonstrated the superiority of elastin staining compared to H&E alone in the assessment of VI, with the majority reporting a two to threefold increase in VI detection with elastin stains (15, 19, 21–24).
Furthermore, evidence suggests that VI assessed with an elastin stain may be a far better predictor of cancer survival than VI assessed by H&E alone. Roxburgh et al. (15) studied 419 patients undergoing curative CRC resection (before and after the introduction of routine elastin staining at their institution, n = 194 and 225, respectively) and found elastin-detected VI to be superior at predicting 3-year cancer-specific survival than VI detected by H&E alone. A follow-up study of 631 CRC resections found elastin-detected VI to be associated with a decrease in 5-year cancer-specific survival from 92 to 67% in node-negative disease (T1-4,N0) and from 79 to 42% in node positive disease (T1-4,N1/2). The combination of T-stage and elastin-detected VI was at least equivalent to T-stage and nodal status in predicting cancer-specific survival, and superior to TNM in node-negative disease (14). This led the authors to propose a “TVI” staging system as a simple alterative to TNM. Suzuki et al. (25), in a study of 124 patients with stage I CRC, found only elastin-detected VI to be an independent predictor of distant metastasis (VI detected on H&E alone was not significant on either univariate or multivariate analysis). Baumhoer et al. (26) failed to demonstrate a difference in 5-year survival between patients with and without elastin-detected VI in 185 patients with stage I and II CRC, but the study was limited by type II error due to the relatively small sample size.
Not surprisingly, the accuracy of VI detection on H&E has been shown to affect its prognostic power. Betge et al. (10) compared the prognostic significance of original vs. review diagnoses of VI (the latter by two experienced GI pathologists) in 381 CRC resection specimens. Review diagnoses of VI had a much stronger influence on both progression free survival [HR 3.97 (95% CI 2.71–5.81), p < 0.001 vs. HR 1.01 (95% CI 0.60–1.71), p = 0.96] and cancer-specific survival [HR 4.45 (95% CI 2.96–6.68), p < 0.001 vs. HR 1.05 (95% CI 0.60–1.84), p = 0.87] than did diagnoses made in the routine setting.
The Ontario Experience
Institutional and provincial audits in the province of Ontario, Canada have revealed under-reporting of VI in CRC resections (18, 27) with a provincial VI detection rate of just 14% in 2010 (11% if academic hospitals were excluded)1. In order to determine the reasons for such under-reporting, a population-based survey of 361 Ontario pathologists was undertaken (18). A 15-item survey addressed reporting criteria (n = 6), the use of special stains (n = 5), and demographic information (n = 4). Pathologists were also asked to provide a self-estimate of their VI detection rates in CRC resection specimens (<10%; 10–19%; 20–29%; >30%). The overall response rate was 65%, which is relatively high for a survey of physicians. A majority of pathologists (70.2%) considered their VI detection rates to be <10%, with only 9% reporting VI detection rates >20%. Factors independently associated with estimated VI detection rates of ≥10% included (1) practice in a university-affiliated center, (2) a sub-specialist interest in GI pathology, and (3) application of the “orphan artery criterion” (i.e., tumor nodule adjacent to an artery where residual smooth muscle or elastin can be demonstrated on H&E or special stains) (18). Higher rates of VI detection among GI1 pathologists and those practicing in university-affiliated centers were also noted in institutional and provincial audits (18, 27) as well as in a study using a pre-defined set of cases (19). In the latter, the use of an elastin stain more than doubled VI detection by both GI and general pathologists (p = 0.001) and increased interobserver agreement for the detection of EMVI (H&E: κ = 0.23 vs. Elastin: κ = 0.41) (19).
The under-reporting of VI in Ontario, together with the heightened awareness generated by the population-based survey and the availability of a provincial mailing list presented a unique opportunity for practice improvement through knowledge transfer. Initially, an educational document was sent to all Ontario pathologists on the provincial mailing list providing the following information:
(1) Detailed feedback on the results of the VI survey
(2) Information regarding the prognostic importance of VI and the RC Path UK minimum standard for VI and
(3) A set of micrographs illustrating both the morphologic clues for VI detection (i.e., “orphan arteriole” and “protruding tongue” signs) and the utility of elastin staining in enhancing VI detection.
Broader national exposure was achieved by publication of a similar document in the Journal of Canadian Pathology (28). The impact of these initiatives is beginning to emerge at an institutional, provincial and national level. Many institutions in Ontario have since implemented routine elastin staining on all CRC resections. At our institution, elastin trichrome stains are performed on a minimum of five tumor-containing blocks per case. The associated costs are modest (about $3–4 per slide) and there has been no impact on turn-around time as stains are performed up front on pre-selected blocks. A recent audit of VI detection rates at Mount Sinai Hospital for the year prior to and the year following implementation of routine elastin staining revealed a twofold increase in VI detection rates (20.0–42.0%) that was independent of other clinicopathologic variables [adjusted Odds Ratio, 3.54 (95% confidence interval, 1.89–6.63); p < 0.001]. Comparable VI detection rates were achieved by both gastrointestinal (GI) and non-gastrointestinal (non-GI) pathologists (41 and 44%, respectively) (unpublished data).
Similar improvements in VI detection were observed among Ontario pathologists who had participated in the interobserver variability study VI in CRC (19, 29). Participants of this study were invited to submit pathology reports from all CRC resections issued in the 18 months prior to, and 18 months following completion of the study. Nine of the 12 pathologists (5 GI, 4 non-GI) submitted reports they had signed out (n = 233 pre-study and n = 216 post-study). Again, a two-fold increase in VI detection rates in the post-study period was noted [18.5–39.8% overall (p < 0.0001), 18.2–38.4% for GI pathologists (p < 0.001), and 19.1–45.6% for non-GI pathologists (p < 0.002)]. In addition, the mean number of elastin stains per case increased from 0.10 (SE, 0.41) to 2.51 (2.06) (p < 0.001), while the mean number of tumor-containing blocks per case remained constant [8.28 (6.69) vs. 8.12 (4.07); p = 0.761]. There were no significant differences in small vessel invasion, TNM stage, tumor location or proportion of patients receiving neoadjuvant therapy pre- and post-study.
The broader impact of knowledge transfer on VI detection at a provincial level was addressed by means of a follow-up survey of pathologists in Ontario. Participants were asked whether or not they considered their VI detection rates and use of elastin stains in CRC resections to have increased since the original 2010 VI practice pattern survey and receipt of the feedback/educational material that followed. Despite a relatively low response rate of 24%, 33 different hospitals were represented. Overall 67.5% of respondents considered their use of elastin stains to have increased, including 85% (11/13) of GI pathologists and 62% (38/61) of non-GI pathologists. Of those who reported increased use of elastin stains, 72% (36/50) felt that their VI detection rates had increased; this included 91% (10/11) of GI pathologists and 67% (26/39) of non-GI pathologists. Of those who reported no increase in elastin staining, only 8% (2/26) felt that their VI detection rates had increased. There was a highly significant association between the increased use of elastin staining and a perceived increase in VI detection (p < 0.0001). Finally, unpublished results from a survey of North American pathologists revealed that 46.5% of Canadian GI pathologists (20/43) routinely performed an elastin stain on at least one tumor-containing block on every CRC resection compared to less than 2% (1/58) of US GI pathologists. Differences were also seen between Canadian and US non-GI pathologists [19% (15/79) vs. 2% (3/139), respectively]. It would appear that knowledge transfer initiatives have influenced pathology practice with respect to VI reporting at both a provincial and national level in Canada.
International Criteria for the Reporting of Vessel Invasion
Colorectal cancer reporting protocols of the RC Path (UK), Royal College of Pathologists of Australasia (RCPA), College of American Pathologists (CAP), and Japanese Society of Cancer of the Colon and Rectum (JSCCR) vary considerably with respect to blood vessel and lymphatic vessel invasion reporting.
The recently updated RC Path dataset (17) includes only venous (large vessel) invasion as a mandatory data element (reported as extramural, intramuscular or submucosal) and regards the evidence as insufficient for mandatory reporting of lymphatic and small vessel invasion. Talbot’s definition of VI as “tumor present in an endothelium-lined space surrounding a rim of smooth muscle or containing red blood cells” remains in use, but in addition the demonstration of a convincing elastic lamina around a tumor nodule is now considered sufficient to categorize as positive for VI, even if an endothelial-lined space is not visible. The guidelines recommend that VI is detected in at least 30% of CRC resections and that individual centers should monitor VI detection rates and consider routine elastin staining to facilitate its detection if the 30% minimum standard is not met (Table 1).
The RCPA guidelines (30) recognize the significance of both large vessel (venous) and small vessel (lymphatic and capillary) invasion and recommend separate documentation of each as well as the distinction between EMVI and IMVI. The use of histochemical and immunohistochemical stains is encouraged if there is a suspicion of VI on H&E.
While the CAP reporting guidelines (31) recognize the superior prognostic significance of EMVI, all forms of blood vessel and lymphatic invasion (large and small vessel) are grouped under the broad term “lymph-vascular invasion.” Currently there are no recommendations regarding the use of special stains to identify blood vessel or lymphatic invasion.
Consensus guidelines for the assessment of lymphatic and blood vessel invasion from the Pathology Working Group of the JSCCR have recently been developed using the Delphi method (32). Diagnostic criteria for elastica-detected VI and D2-40-detected LI include an elastic membrane covering more than half of the circumference of a tumor cluster even without an accompanying artery or vessel structure and D2-40 positive endothelial cells covering at least half of the circumference of a tumor cluster, respectively. However, a recent international study group found that attempts to apply these criteria were associated with a decrease in interobserver agreement (33). JSCCR guidelines suggest the separate reporting of blood vessel and lymphatic vessel invasion, but do not distinguish the size of the vessel.
The variation in national/regional pathology reporting guidelines for blood and lymphatic vessel invasion may influence patient management. While most national guidelines recommend that adjuvant chemotherapy be offered in “high-risk” stage II disease (34, 35), precisely what defines “high-risk” in terms of vessel invasion differs regionally. For instance, the US-based National Comprehensive Cancer Network considers any type of blood or lymphatic vessel invasion (34) as a high-risk feature in Stage II CRC; this is in line with the CAP guidelines grouping of all forms of blood and lymphatic vessel invasion in single category (“lymph-vascular invasion”). In contrast, EMVI but not other types of blood/lymphatic vessel invasion are considered “high-risk” by the National Institute for Health and Care Excellence in the UK (35) in line with UK pathology guidelines requiring separate reporting of EMVI and which do not include small vessel invasion as a mandatory data element.
Finally, while current guidelines recommend chemotherapy be offered in stage II CRC with any “high-risk” feature, the relative benefit of chemotherapy for individual “high-risk” features (such as VI) remains to be determined. Attempts to determine the relative benefit of chemotherapy for VI in stage II CRC are currently limited by (1) low VI detection rates and lack of central review in some major trials [e.g., VI rate of 13% in the QUASAR (36) and 8.9% of Stage II patients in the MOSAIC trial (37)], (2) insensitive methods for VI detection (i.e., H&E stain alone), and (3) relatively small numbers when subgroup analyses are performed. Therefore prospective studies to determine the relative benefit of chemotherapy in patients with elastin-detected VI would be of considerable interest and importance.
Conclusion
Venous invasion, particularly when in an extramural location, is a powerful prognostic factor in CRC. Its presence in stage II CRC may prompt oncologists to offer adjuvant chemotherapy. Institutional and provincial audits in Ontario identified widespread under-reporting of VI, which led to several knowledge transfer initiatives in the province. These appear to have had an impact on the detection and reporting of VI both at a provincial and national level. In particular, recognition of key diagnostic clues, such as the “orphan artery sign” and “protruding tongue sign” and use of elastin staining can substantially increase VI detection rates. In turn, the more accurate/sensitive detection of VI has been shown to increase its prognostic power. Future challenges include establishing evidence based internationally accepted guidelines for the definition and reporting of blood vessel and lymphatic invasion, and implementing of methods to improve their detection.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnote
- ^During this period, provincial reporting was performed according to the College of American Pathologists checklist (version 6) based on AJCC/UICC TNM 6th edition which required separate reporting of large vessel (venous) and small vessel invasion.
References
1. Seefeld PH, Bargen JA. The spread of carcinoma of the rectum: invasion of lymphatics, veins and nerves. Ann Surg (1943) 118:76–90. doi: 10.1097/00000658-194307000-00005
2. Talbot IC, Ritchie S, Leighton M, Hughes AO, Bussey HJ, Morson BC. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology (1981) 5:141–63. doi:10.1111/j.1365-2559.1981.tb01774.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Chapuis PH, Dent OF, Fisher R, Newland RC, Pheils MT, Smyth E, et al. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg (1985) 72:698–702. doi:10.1002/bjs.1800720909
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Freedman LS, Macaskill P, Smith AN. Multivariate analysis of prognostic factors for operable rectal cancer. Lancet (1984) 2:733–6. doi:10.1016/S0140-6736(84)92636-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Morris M, Platell C, de Boer B, McCaul K, Iacopetta B. Population-based study of prognostic factors in stage II colonic cancer. Br J Surg (2006) 93:866–71. doi:10.1002/bjs.5345
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Petersen VC, Baxter KJ, Love SB, Shepherd NA. Identification of objective pathological prognostic determinants and models of prognosis in Dukes’ B colon cancer. Gut (2002) 51:65–9. doi:10.1136/gut.51.1.65
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Quirke P, Morris E. Reporting colorectal cancer. Histopathology (2007) 50:103–12. doi:10.1111/j.1365-2559.2006.02543.x
8. Messenger DE, Driman DK, Kirsch R. Developments in the assessment of venous invasion in colorectal cancer: implications for future practice and patient outcome. Hum Pathol (2012) 43:965–73. doi:10.1016/j.humpath.2011.11.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Brown CE, Warren S. Visceral metastases from rectal carcinoma. Surg Gynecol Obstet (1938) 66:611–21.
10. Betge J, Pollheimer MJ, Lindtner RA, Kornprat P, Schlemmer A, Rehak P, et al. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer (2012) 118:628–38. doi:10.1002/cncr.26310
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Roxburgh CS, McMillan DC, Richards CH, Atwan M, Anderson JH, Harvey T, et al. The clinical utility of the combination of T stage and venous invasion to predict survival in patients undergoing surgery for colorectal cancer. Ann Surg (2014) 259(6):1156–65. doi:10.1097/SLA.0000000000000229
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. van Wyk HC, Roxburgh CS, Horgan PG, Foulis AF, McMillan DC. The detection and role of lymphatic and blood vessel invasion in predicting survival in patients with node negative operable primary colorectal cancer. Crit Rev Oncol Hematol (2014) 90:77–90. doi:10.1016/j.critrevonc.2013.11.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Maughan NJ, Morris E, Forman D, Quirke P. The validity of the Royal College of Pathologists’ colorectal cancer minimum dataset within a population. Br J Cancer (2007) 97:1393–8. doi:10.1038/sj.bjc.6604036
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Roxburgh CS, McMillan DC, Richards CH, Atwan M, Anderson JH, Harvey T, et al. The clinical utility of the combination of T stage and venous invasion to predict survival in patients undergoing surgery for colorectal cancer. Ann Surg (2014) 259:1156–65. doi:10.1097/SLA.0000000000000229
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Roxburgh CS, McMillan DC, Anderson JH, McKee RF, Horgan PG, Foulis AK. Elastica staining for venous invasion results in superior prediction of cancer-specific survival in colorectal cancer. Ann Surg (2010) 252:989–97. doi:10.1097/SLA.0b013e3181f1c60d
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Horgan PG, McMillan DC. Surgeons and selection of adjuvant therapy for node-negative colonic cancer. Br J Surg (2010) 97:1459–60. doi:10.1002/bjs.7254
17. Loughrey MB, Quirke P, Shepherd NA. Dataset for Colorectal Cancer Histopathology Reports. Available from: http://www.rcpath.org/Resources/RCPath/Migrated%20Resources/Documents/G/G049_ColorectalDataset_July14.pdf
18. Messenger DE, Driman DK, McLeod RS, Riddell RH, Kirsch R. Current practice patterns among pathologists in the assessment of venous invasion in colorectal cancer. J Clin Pathol (2011) 64:983–9. doi:10.1136/jclinpath-2011-200156
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Kirsch R, Messenger DE, Riddell RH, Pollett A, Cook M, Al-Haddad S, et al. Venous invasion in colorectal cancer: impact of an elastin stain on detection and interobserver agreement among gastrointestinal and nongastrointestinal pathologists. Am J Surg Pathol (2013) 37:200–10. doi:10.1097/PAS.0b013e31826a92cd
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Howlett CJ, Tweedie EJ, Driman DK. Use of an elastic stain to show venous invasion in colorectal carcinoma: a simple technique for detection of an important prognostic factor. J Clin Pathol (2009) 62:1021–5. doi:10.1136/jcp.2009.065615
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Kingston EF, Goulding H, Bateman AC. Vascular invasion is underrecognized in colorectal cancer using conventional hematoxylin and eosin staining. Dis Colon Rectum (2007) 50:1867–72. doi:10.1007/s10350-007-9021-6
22. Inoue T, Mori M, Shimono R, Kuwano H, Sugimachi K. Vascular invasion of colorectal carcinoma readily visible with certain stains. Dis Colon Rectum (1992) 35:34–9. doi:10.1007/BF02053336
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Vass DG, Ainsworth R, Anderson JH, Murray D, Foulis AK. The value of an elastic tissue stain in detecting venous invasion in colorectal cancer. J Clin Pathol (2004) 57:769–72. doi:10.1136/jcp.2003.015826
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Sejben I, Bori R, Cserni G. Venous invasion demonstrated by orcein staining of colorectal carcinoma specimens is associated with the development of distant metastasis. J Clin Pathol (2010) 63:575–8. doi:10.1136/jcp.2010.075846
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Suzuki A, Togashi K, Nokubi M, Koinuma K, Miyakura Y, Horie H, et al. Evaluation of venous invasion by Elastica van Gieson stain and tumor budding predicts local and distant metastases in patients with T1 stage colorectal cancer. Am J Surg Pathol (2009) 33:1601–7. doi:10.1097/PAS.0b013e3181ae29d6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Baumhoer D, Thiesler T, Maurer CA, Huber A, Cathomas G. Impact of using elastic stains for detection of venous invasion in the prognosis of patients with lymph node negative colorectal cancer. Int J Colorectal Dis (2010) 25:741–6. doi:10.1007/s00384-010-0890-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Messenger DE, McLeod RS, Kirsch R. What impact has the introduction of a synoptic report for rectal cancer had on reporting outcomes for specialist gastrointestinal and nongastrointestinal pathologists? Arch Pathol Lab Med (2011) 135:1471–5. doi:10.5858/arpa.2010-0558-OA
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Messenger DE, Driman DK, McLeod RS, Riddell RH, Kirsch R. Is venous invasion an underreported finding among Canadian pathologists? Results of a population based survey of Ontario pathologists. Can J Pathol (2012) 4:46–9.
29. Kirsch R, Assarzadegan N, Messenger DE, Riddell RH, Pollen A, Streutker CJ, et al. The Impact of Knowledge Transfer Strategies on the Detection of Venous Invasion in Colorectal Cancer Laboratory Investigation. Nature Publishing Group (2014). p. 506A.
30. Stephen A, Brown I, Ellis D, Hawkins N, Hicks S, Hunter A, et al. Colorectal Cancer Structured Reporting Protocol. (2012). Available from: http://www.rcpa.edu.au/getattachment/95109a49-9e15-4038-9858-cf314d9c7422/Protocol-colorectal-cancer.aspx
31. Tang L, Berlin J, Branton P, Burgart LJ, Carter DK, Compton CC, et al. Protocol for the Examination of Specimens from Patients with Primary Carcinoma of the Colon and Rectum. (2013). Available from: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2013/Colon_13protocol_3300.pdf
32. Kojima M, Shimazaki H, Iwaya K, Kage M, Akiba J, Ohkura Y, et al. Pathological diagnostic criterion of blood and lymphatic vessel invasion in colorectal cancer: a framework for developing an objective pathological diagnostic system using the Delphi method, from the Pathology Working Group of the Japanese Society for Cancer of the Colon and Rectum. J Clin Pathol (2013) 66:551–8. doi:10.1136/jclinpath-2012-201076
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Kojima M, Kirsch R, Basturk O, Frankel WL, Vieth M, Lugli A, et al. A Ochiai, blood and lymphatic vessel invasion in pT1 colorectal cancer. Int Concord Study (2014). Available from: https://www.nccn.org/store/login/login.aspx?
34. Benson AB III, Venook AP, Bekaii-Saab T, Chan E, Chen Y, Cooper HS, et al. Clinical practice guidelines in oncology (NCCN guidelines): colon cancer. NCCN (2014).
35. Bewley S, Bhutani G, Bostock J, Corbett S, Graham J, Hoskin P, et al. Colorectal Cancer. The Diagnosis and Management of Colorectal Cancer. National Institute for Health and Clinical Excellence (NICE) (2014). Available from: http://www.nice.org.uk/guidance/cg11/resources/guidance-colorectal-cancer-pdf
36. Quasar Collaborative G, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet (2007) 370:2020–9. doi:10.1016/S0140-6736(07)61866-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Tournigand C, Andre T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol (2012) 30:3353–60. doi:10.1200/JCO.2012.42.5645
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: venous invasion, colorectal cancer, elastin stain, prognostic marker, stage II colorectal cancer
Citation: Dawson H, Kirsch R, Driman DK, Messenger DE, Assarzadegan N and Riddell RH (2015) Optimizing the detection of venous invasion in colorectal cancer: the Ontario, Canada, experience and beyond. Front. Oncol. 4:354. doi: 10.3389/fonc.2014.00354
Received: 30 September 2014; Paper pending published: 22 October 2014;
Accepted: 26 November 2014; Published online: 05 January 2015.
Edited by:
Kieran Sheahan, ST Vincent’s University Hospital, IrelandReviewed by:
Giacomo Puppa, ‘G. Fracastoro’ City Hospital, ItalyMotohiro Kojima, National Cancer Center Hospital East, Japan
Aoife Maguire, Memorial Sloan Kettering Cancer Center, USA
Copyright: © 2015 Dawson, Kirsch, Driman, Messenger, Assarzadegan and Riddell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heather Dawson, Clinical Pathology Division, Institute of Pathology, University of Bern, Murtenstrasse 31, 3010 Bern, Switzerland e-mail: heather.dawson@pathology.unibe.ch
 Heather Dawson
Heather Dawson Richard Kirsch1
Richard Kirsch1 David E. Messenger
David E. Messenger