Monitoring oxygenation and gas exchange in neonatal intensive care units: current practice in the Netherlands
- Department of Pediatrics, Division of Neonatology, Leiden University Medical Center, Leiden, Netherlands
Background: Although recommendations in oxygenation and gas exchange monitoring in the neonatal intensive care unit (NICU) are available, little is known of the current practice.
Aim: To evaluate the current practice in oxygenation and gas exchange monitoring of the NICUs in the Netherlands.
Methods: An online survey-based questionnaire concerning preferences and current practice of monitoring oxygenation and gas exchange was sent out to all 107 neonatal staff members (neonatologists, neonatal fellows, and physician assistants) of the 10 NICUs in the Netherlands.
Results: The response rate was 42%. Pulse oximetry (PO), partial pressure of oxygen in arterial blood gas (paO2), and oxygen saturation in arterial blood gas (saO2) was used by, respectively, 100, 80, and 27% of the staff members for monitoring oxygenation. Of all staff members, 76% considered PO as the best parameter for monitoring oxygenation, 22% paO2, and 2% saO2. Blood gas, transcutaneous gas monitoring, endotracheal gas monitoring, and near-infrared spectroscopy was used by, respectively, 100, 82, 40, and 18% of the staff members for monitoring gas exchange. During endotracheal ventilation, 67% of the caregivers would exclusively accept arterial blood gas for gas exchange monitoring. In contrast, during non-invasive ventilation, 68% of the caregivers did not prefer arterial or capillary blood gas (CBG). CBG is found reliable in infants with warm extremities by 76% of the caregivers. Venous blood gas would be accepted by 60% of the caregivers, independent of the mode of respiratory support, and only when venous blood sample was needed for other reasons.
Conclusion: This survey identified a wide variation in preference in monitoring oxygenation and gas exchange monitoring among Dutch neonatal staff members.
Introduction
Monitoring of gas exchange and oxygenation is daily practice in infants admitted in the neonatal intensive care unit (NICU). Several methods for monitoring are available and several studies compared invasive and non-invasive methods: arterial blood gas (ABG), capillary blood gas (CBG), and venous blood gas (VBG) analyses (1–6), pulse oximetry (PO) (7–11), transcutaneous (tc) gas monitoring (i.e., tcO2, tcCO2) (9, 12–17), end-tidal gas monitoring (etCO2) (18–21), and near-infrared spectroscopy (NIRS) (22).
The ABG is considered the gold standard, but there is 10–25% failure of placing of an arterial line and also severe complication (as limb ischemia, necrosis) can occur (1, 4, 22, 23). The best alternative is the CBG in a well-perfused infant (1, 2, 4–6, 24–26). However, VBG is not recommended because various (small) studies show conflicting results (1, 3, 6, 23, 27, 28). Transcutaneous O2 and CO2 are considered the best non-invasive method; studies have shown that the values correlate well with ABG values (9, 12–17). PO has become standard of care to monitor oxygenation in the NICU; it is easy to use, and monitoring is continuous. However, studies showed that saturation of PO is not interchangeable with saturation in ABG (7, 9, 11, 29, 30). End-tidal CO2 measurement is only recommended for confirming endotracheal tube position (18–21, 31, 32).
Although all studies reported the advantages and disadvantages of the different methods and methods are recommended, the choice of the caregiver on what to use in daily practice is also influenced on the availability, practicality, and local preferences. Guidelines are needed to aim for a uniform policy, but first it is important to know how the current daily practice corresponds with the available recommendations. To investigate this, we performed a survey to inventory the current practice in monitoring oxygenation and gas exchange in all NICUs in the Netherlands.
Materials and Methods
We sent a questionnaire to the 107 neonatal staff members [84 neonatologists, 18 neonatal fellows, and 5 physician assistants/nurse practitioners (PA/NP)] working in the 10 Dutch tertiary NICU centers in 2012, who are registered by the Dutch Association of Pediatrics. The questionnaire included questions concerning the daily practice in monitoring oxygenation and gas exchange (see Table 1). The units in the Netherlands are all up-to-date level III NICUs, and there are very little difference in resources and employees. For this reason, we aimed for a national overview. Most equipment for monitoring is available in all Dutch NICUs. We aimed for at least three responders per NICU, a first general reminder was sent after 4 weeks, and the last reminder was sent specific to NICUs when the response rate was less than three. The local institutional ethical review board approved the questionnaire study.
Statistical Analysis
All replies were collected in MS Excel 2010 and expressed as percentages of total participants.
Results
From the 10 NICU centers, 45/107 (42%) neonatal staff members responded and returned the questionnaire; of which 35 (42%) neonatologists, 8(44%) neonatal fellows, and 2(40%) PA/NP. From each unit at least three staff members responded. All staff members indicated that there were no specific guidelines available in the unit concerning monitoring oxygenation and gas exchange.
Oxygenation
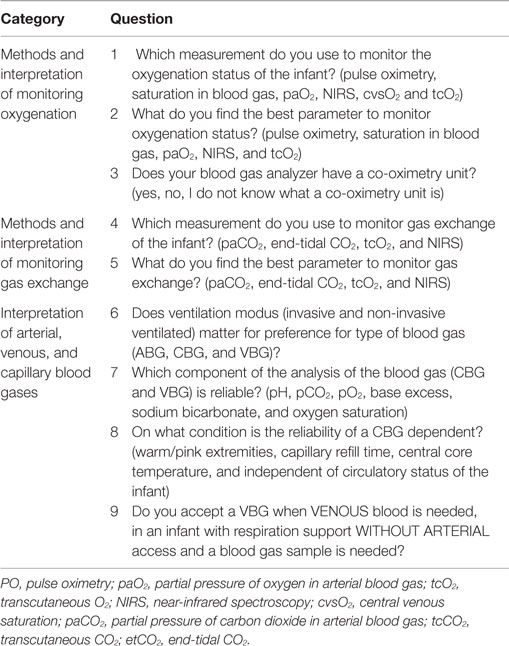
To monitor oxygenation, 100% of the staff members used PO, 80% used partial pressure of oxygen in ABG (paO2), and 27% oxygen saturation in ABG (saO2); while tcO2 (7%), NIRS (2%), lactate (2%), and central venous saturation oxygenation (2%) were rarely used (Table 2). Most staff members (76%) considered PO the best parameter for monitoring oxygenation status, 22% paO2, and 2% saO2. The staff members using saO2, 75% of them were not aware whether co-oximetry measures saO2, or estimates it, based on paO2. While most staff members in all units use PO and paO2, the use of saO2, tcO2, NIRS, and lactate was variable in and between units (Table 2).
Gas Exchange
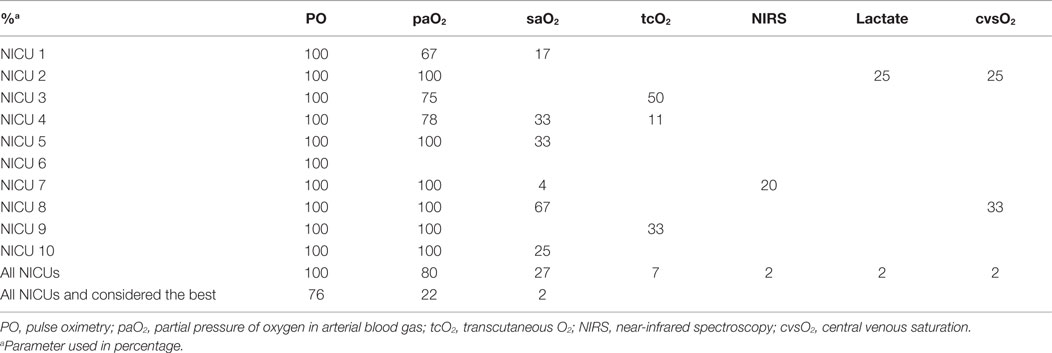
To monitor gas exchange, 100% of the staff members used paCO2, 82% tcCO2, 40% etCO2, 18% NIRS, and 7% tidal volume (Table 3). Most staff members (91%) considered paCO2 the best parameter for monitoring gas exchange, in contrast to tcCO2 (9%). The use of tcCO2 was very variable in and between units (Table 4).
Blood Gas
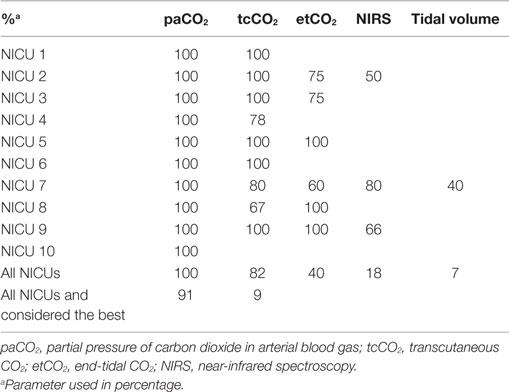
The type of blood gas (ABG, CBG, or VBG) used depended on the ventilation modus of the infant (Figure 1). In all units, most often ABG is used in intubated and mechanically ventilated infants, while in non-invasive ventilated infants, both ABG and CBG were preferential. Staff members considered the reliability of CBG depended on the presence of warm extremities (76%), capillary refill time <2 s (44%), pink extremities (37%), and normal central body temperature (22%). Overall, staff members considered CBG more reliable than VBG (Table 5); nevertheless, 11% uses VBG in ventilated infants and 13% in non-invasive ventilated infants (Figure 1). VBG and CBG were not considered reliable at all by 13 and 4% of the caregivers, respectively (Table 5). In contrast, this opinion changes when a blood gas was due when venous blood sampling was simultaneously needed; the majority (60%) of the clinicians would then accept a VBG.
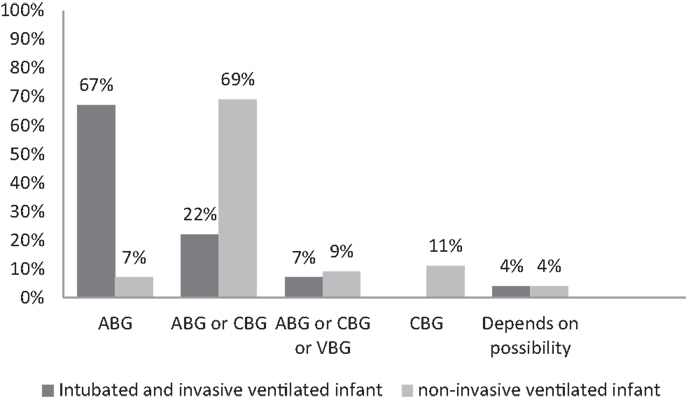
Figure 1. Percentage used blood gas in an invasive and non-invasive ventilated patients. ABG, arterial blood gas; CBG, capillary blood gas; VBG, venous blood gas.
Discussion
This study provided an overview of the current clinical practice. Although most recommendations concerning monitoring gas exchange are followed, there is a variance in and between units in opinion about reliability and interpretation of the measurements. Oxygenation and gas exchange were most often monitored by PO, tcCO2, and ABG, while other current available measurements are infrequently or rarely used. Factors like practicality, personal experiences and aiming for less cumbersome measurements probably influenced current practice in monitoring.
The response rate was low (42%), but this is higher than the average response rate of online voluntary survey in physician specialists (33). We anticipated a low response, and we therefore aimed for at least three responders of each NICU. We reached this target with a mix of inexperienced and experienced users. We believe that the respondents give a good reflection of the Dutch neonatal staff members.
According to the literature, the best method for monitoring hypoxia and hyperoxia is an ABG (analyzed with a co-oximetry unit), but we observed that staff members infrequently used the oxygen saturation in the ABG (27%). In addition, most caregivers were not aware whether the blood gas analyzer uses co-oximetry or is an estimation. While co-oximetry would be more accurate than PO; caregivers considered PO as the best and most commonly used monitoring for oxygenation. PO is non-invasive, non-cumbersome, easy to apply, and will provide continuous measurements. However, oxygen saturation can be overestimated by PO (7) and is inaccurate at oxygen saturation levels <70% and in hyperoxia >95% (29, 34). The other disadvantages of the PO are motion artifacts, even in the motion resistant types, and low perfusion quality disturbances depending on probe site placement (29).
While studies recommended continuous tcO2 monitoring for preventing hypoxemia/hyperoxemia (15, 32), few caregivers (7%) used this. In contrast, most caregivers considered tcCO2 the best non-invasive continuous monitoring of CO2, which is consistent with a recent German survey (35). Studies demonstrated that tcO2/CO2 is well correlated with paO2/CO2, but tcO2 often underestimated paO2 while tcCO2 overestimated paCO2 (12, 15–17, 32, 35–37). In contrast to the literature, most caregivers considered tc measurements not reliable and is possibly mostly used for trend monitoring. It is possible that the frequency of replacement and calibrations has influenced the caregiver’s experience in the reliability. While in the performed studies, the sensor is frequently replaced and calibrated, in daily practice this is not always possible. In addition, dislocated adhesive rings, skin burns, and decubitus can discourage caregiver in using tc monitoring. In this view, the preference of staff members to use tc monitoring in preterm infants was surprising, especially considering the fact that correlation is poor in preterm infants when compared to term infants (32, 38). End-tidal CO2 monitoring is not frequently used in the Netherlands, most probably because the inaccuracy due to high respiratory rate, the low-tidal volume, and the increase of dead space volume (19).
The staff members considered ABG analysis the gold standard for monitoring gas exchange, which is consistent with the literature (23, 31, 36). The preference of performing ABG in ventilated infants probably reflects that most of the ventilated infants have an arterial catheter for hemodynamic monitoring, while CBG is well accepted in non-invasive ventilated infants. The reliability of CBG can be poor by disturbed perfusion, which is more likely to occur in the more critical and mechanical ventilated infants. The staff members considered the CBG for pH, pCO2, and sodium bicarbonate in a child with warm extremities reliable, which is consistent with the current literature (1, 24, 26, 36).
The use of VBG is currently not recommended, as the studies performed are small and showed conflicting results (1, 3, 6, 23, 27, 28). Nevertheless, most staff members will accept a VBG when venous blood withdrawing will occur, most likely due to practical considerations. However, so far there is no data whether VBG and CBG can be interchangeably used. Gillivray et al. concluded that VBG would be useful for decision making in the need for intubation, but not for monitoring ventilation (6).
Conclusion
This survey identified a large variation in oxygenation and gas exchange monitoring in the Dutch NICUs. Although guidelines are absent in the units, recommendations are followed combined with practical considerations. In current daily practice, PO is considered the best method of monitoring oxygenation and blood gasses for monitoring gas exchange. The preference for the type of blood gas analysis depends on circumstances: type of respiratory support and the opportunity of simultaneously venous blood withdrawing. Tc gas monitoring is then considered the best non-invasive measurement for gas exchange but is infrequently used. These results can be helpful when developing a guideline that is evidence based as well as practical.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all participants for answering our questionnaire.
References
1. Yildizdas D, Yapicioglu H, Yilmaz HL, Sertdemir Y. Correlation of simultaneously obtained capillary, venous, and arterial blood gases of patients in a paediatric intensive care unit. Arch Dis Child (2004) 89:176–80. doi:10.1136/adc.2002.016261
2. Escalante-Kanashiro R, Tantalean-Da-Fieno J. Capillary blood gases in a pediatric intensive care unit. Crit Care Med (2000) 28:224–6. doi:10.1097/00003246-200001000-00037
3. Kirubakaran C, Gnananayagam JE, Sundaravalli EK. Comparison of blood gas values in arterial and venous blood. Indian J Pediatr (2003) 70:781–5. doi:10.1007/BF02723794
4. Koch G, Wendel H. Comparison of pH, carbon dioxide tension, standard bicarbonate and oxygen tension in capillary blood and in arterial blood during neonatal period. Acta Paediatr Scand (1967) 56:10–6. doi:10.1111/j.1651-2227.1967.tb15339.x
5. Saili A, Dutta AK, Sarna MS. Reliability of capillary blood gas estimation in neonates. Indian Pediatr (1992) 29:567–70.
6. McGillivray D, Ducharme FM, Charron Y, Mattimoe C, Treherne S. Clinical decisionmaking based on venous versus capillary blood gas values in the well-perfused child. Ann Emerg Med (1999) 34:58–63. doi:10.1016/S0196-0644(99)70272-6
7. Poets CF, Wilken M, Seidenberg J, Southall DP, von der HH. Reliability of a pulse oximeter in the detection of hyperoxemia. J Pediatr (1993) 122:87–90. doi:10.1016/S0022-3476(05)83494-8
8. Gerstmann D, Berg R, Haskell R, Brower C, Wood K, Yoder B, et al. Operational evaluation of pulse oximetry in NICU patients with arterial access. J Perinatol (2003) 23:378–83. doi:10.1038/sj.jp.7210944
9. Southall DP, Bignall S, Stebbens VA, Alexander JR, Rivers RP, Lissauer T. Pulse oximeter and transcutaneous arterial oxygen measurements in neonatal and paediatric intensive care. Arch Dis Child (1987) 62:882–8. doi:10.1136/adc.62.9.882
10. Shiao SY, Ou CN. Validation of oxygen saturation monitoring in neonates. Am J Crit Care (2007) 16:168–78.
11. Kugelman A, Wasserman Y, Mor F, Goldinov L, Geller Y, Bader D. Reflectance pulse oximetry from core body in neonates and infants: comparison to arterial blood oxygen saturation and to transmission pulse oximetry. J Perinatol (2004) 24:366–71. doi:10.1038/sj.jp.7211102
12. Rome ES, Stork EK, Carlo WA, Martin RJ. Limitations of transcutaneous PO2 and PCO2 monitoring in infants with bronchopulmonary dysplasia. Pediatrics (1984) 74:217–20.
13. Tobias JD. Transcutaneous carbon dioxide monitoring in infants and children. Paediatr Anaesth (2009) 19:434–44. doi:10.1111/j.1460-9592.2009.02930.x
14. Sandberg KL, Brynjarsson H, Hjalmarson O. Transcutaneous blood gas monitoring during neonatal intensive care. Acta Paediatr (2011) 100:676–9. doi:10.1111/j.1651-2227.2011.02164.x
15. Carter B, Hochmann M, Osborne A, Nisbet A, Campbell N. A comparison of two transcutaneous monitors for the measurement of arterial PO2 and PCO2 in neonates. Anaesth Intensive Care (1995) 23:708–14.
16. Kost GJ, Chow JL, Kenny MA. Transcutaneous carbon dioxide for short-term monitoring of neonates. Clin Chem (1983) 29:1534–6.
17. Fanconi S, Sigrist H. Transcutaneous carbon dioxide and oxygen tension in newborn infants: reliability of a combined monitor of oxygen tension and carbon dioxide tension. J Clin Monit (1988) 4:103–6. doi:10.1007/BF01641810
18. Wu CH, Chou HC, Hsieh WS, Chen WK, Huang PY, Tsao PN. Good estimation of arterial carbon dioxide by end-tidal carbon dioxide monitoring in the neonatal intensive care unit. Pediatr Pulmonol (2003) 35:292–5. doi:10.1002/ppul.10260
19. Molloy EJ, Deakins K. Are carbon dioxide detectors useful in neonates? Arch Dis Child Fetal Neonatal Ed (2006) 91:F295–8. doi:10.1136/adc.2005.082008
20. Trevisanuto D, Giuliotto S, Cavallin F, Doglioni N, Toniazzo S, Zanardo V. End-tidal carbon dioxide monitoring in very low birth weight infants: correlation and agreement with arterial carbon dioxide. Pediatr Pulmonol (2012) 47:367–72. doi:10.1002/ppul.21558
21. Lopez E, Grabar S, Barbier A, Krauss B, Jarreau PH, Moriette G. Detection of carbon dioxide thresholds using low-flow sidestream capnography in ventilated preterm infants. Intensive Care Med (2009) 35:1942–9. doi:10.1007/s00134-009-1647-5
22. van Bel F, Lemmers P, Naulaers G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology (2008) 94:237–44. doi:10.1159/000151642
23. Goldsmith JP, Karotkin EH. Assisted Ventilation of the Neonate. 4th ed. St. Louis, Missouri: Elsevier Saunders (2003).
24. Courtney SE, Weber KR, Breakie LA, Malin SW, Bender CV, Guo SM, et al. Capillary blood gases in the neonate. A reassessment and review of the literature. Am J Dis Child (1990) 144:168–72. doi:10.1001/archpedi.1990.02150260046025
25. McLain BI, Evans J, Dear PR. Comparison of capillary and arterial blood gas measurements in neonates. Arch Dis Child (1988) 63:743–7. doi:10.1136/adc.63.7_Spec_No.743
26. Harrison AM, Lynch JM, Dean JM, Witte MK. Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care unit patients. Crit Care Med (1997) 25:1904–8. doi:10.1097/00003246-199711000-00032
27. Bilan N, Behbahan AG, Khosroshahi AJ. Validity of venous blood gas analysis for diagnosis of acid-base imbalance in children admitted to pediatric intensive care unit. World J Pediatr (2008) 4:114–7. doi:10.1007/s12519-008-0022-x
28. Chu YC, Chen CZ, Lee CH, Chen CW, Chang HY, Hsiue TR. Prediction of arterial blood gas values from venous blood gas values in patients with acute respiratory failure receiving mechanical ventilation. J Formos Med Assoc (2003) 102:539–43.
29. Nitzan M, Romem A, Koppel R. Pulse oximetry: fundamentals and technology update. Med Devices (Auckl) (2014) 7:231–9. doi:10.2147/MDER.S47319
30. Rosychuk RJ, Hudson-Mason A, Eklund D, Lacaze-Masmonteil T. Discrepancies between arterial oxygen saturation and functional oxygen saturation measured with pulse oximetry in very preterm infants. Neonatology (2012) 101:14–9. doi:10.1159/000326797
31. Bosman S, Beyers R, Beganovic N, Vader HL. [Non-invasive method of arterial oxygen saturation monitoring in the neonatal intensive care unit (with a review of blood oxygen monitoring methods)]. Tijdschr Kindergeneeskd (1988) 56:20–6.
32. Aliwalas LL, Noble L, Nesbitt K, Fallah S, Shah V, Shah PS. Agreement of carbon dioxide levels measured by arterial, transcutaneous and end tidal methods in preterm infants < or = 28 weeks gestation. J Perinatol (2005) 25:26–9. doi:10.1038/sj.jp.7211202
33. Cunningham CT, Quan H, Hemmelgarn B, Noseworthy T, Beck CA, Dixon E, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol (2015) 15:32. doi:10.1186/s12874-015-0016-z
34. Dawson JA, Bastrenta P, Cavigioli F, Thio M, Ong T, Siew ML, et al. The precision and accuracy of Nellcor and Masimo oximeters at low oxygen saturations (70%) in newborn lambs. Arch Dis Child Fetal Neonatal Ed (2014) 99:F278–81. doi:10.1136/archdischild-2013-305091
35. Rudiger M, Topfer K, Hammer H, Schmalisch G, Wauer RR. A survey of transcutaneous blood gas monitoring among European neonatal intensive care units. BMC Pediatr (2005) 5:30. doi:10.1186/1471-2431-5-30
36. Quine D, Stenson BJ. Arterial oxygen tension (paO2) values in infants <29 weeks of gestation at currently targeted saturations. Arch Dis Child Fetal Neonatal Ed (2009) 94:F51–3. doi:10.1136/adc.2007.135285
37. Crane LD, Snyder JE, Knight P, Philips JB, Cassady G. Effects of position changes on transcutaneous carbon dioxide tension in neonates with respiratory distress. J Perinatol (1990) 10:35–7.
Keywords: questionnaire, oxygenation monitoring, gas exchange monitoring, neonatal intensive care unit, current practice
Citation: Tan RNGB, Mulder EEM, Lopriore E and te Pas AB (2015) Monitoring oxygenation and gas exchange in neonatal intensive care units: current practice in the Netherlands. Front. Pediatr. 3:94. doi: 10.3389/fped.2015.00094
Received: 16 July 2015; Accepted: 20 October 2015;
Published: 03 November 2015
Edited by:
Maximo Vento, University and Polytechnic Hospital and Health Research Center La Fe, SpainReviewed by:
Drucilla Jane Roberts, Massachusetts General Hospital, USAThurman Allen Merritt, Faculty Physicians and Surgeons of Loma Linda University, USA
Copyright: © 2015 Tan, Mulder, Lopriore and te Pas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ratna N. G. B. Tan, r.n.g.b.tan@lumc.nl
 Ratna N. G. B. Tan
Ratna N. G. B. Tan Estelle E. M. Mulder
Estelle E. M. Mulder Enrico Lopriore
Enrico Lopriore Arjan B. te Pas
Arjan B. te Pas