
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr. , 02 August 2016
Sec. Neonatology
Volume 4 - 2016 | https://doi.org/10.3389/fped.2016.00075
Arginine vasopressin (AVP) plays a major role in the homeostasis of fluid balance, vascular tonus, and the regulation of the endocrine stress response. The measurement of AVP levels is difficult due to its short half-life and laborious method of detection. Copeptin is a more stable peptide derived from the same precursor molecule, is released in an equimolar ratio to AVP, and has a very similar response to osmotic, hemodynamic, and stress-related stimuli. In fact, copeptin has been propagated as surrogate marker to indirectly determine circulating AVP concentrations in various conditions. Here, we present an overview of the current knowledge on AVP and copeptin in perinatology with a particular focus on the baby’s transition from placenta to lung breathing. We performed a systematic review of the literature on fetal stress hormone levels, including norepinephrine, cortisol, AVP, and copeptin, in regard to birth stress. Finally, diagnostic and therapeutic options for copeptin measurement and AVP functions are discussed.
The nature of stress hormones is comparable to that of firefighters: they act fast on demand and pull back as soon as possible. While firefighters return to the firehouse for recreation once their task is finished, the activity of stress hormones is naturally limited due to their short half-life, which allows for a targeted and time-limited action but complicates their detection in the circulatory system. One such stress hormone is arginine vasopressin (AVP), also known as antidiuretic hormone (ADH), which is a small peptide with a multitude of functions in many systems, including the central nervous system (CNS). Fortunately, AVP is derived together with three other peptides from a larger precursor peptide, and one of these peptides, copeptin, is more stable than AVP itself and is released in a 1:1 ratio to AVP. One decade ago, a quantitative copeptin sandwich immunoassay was developed (1), and since then, a large variety of clinical studies demonstrated the usefulness of copeptin to indirectly determine circulating AVP concentrations.
This review briefly summarizes the basic information on AVP and copeptin in general, including their production and action via AVP receptors, followed by analysis of the relationship between labor and stress hormone release and the action of AVP in healthy neonates and in newborn diseases. The particular focus of this review is on the role of AVP in the transition from placenta to lung breathing and in acute and chronic stress responses. We performed a systematic review of the literature on various stress hormones at birth, such as norepinephrine, cortisol, AVP, and copeptin, and present them here. Finally, the therapeutic and diagnostic avenues of AVP and copeptin are outlined.
Arginine vasopressin is produced as a larger precursor pre-proAVP by magnocellular and parvocellular neurons within the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) of the hypothalamus (2). Pre-proAVP is produced by magnocellular neurons of the PVN and SON, is packaged into neurosecretory granules, and is transported axonally to the posterior pituitary, also called the neurohypophysis. En route, pre-proAVP is enzymatically processed into four peptides: the N-terminal signal peptide, the active hormone AVP, neurophysin 2, and the C-terminal copeptin. Upon activation with various stimuli, the stored peptides AVP, neurophysin 2, and copeptin are secreted into the circulatory system in equimolar amounts. The pre-proAVP produced in parvocellular neurons in the PVN is transported by axons projecting to the median eminence where it is processed and secreted into the hypothalamic–hypophysial portal vessels and ultimately reaches its destination, the anterior pituitary. A third portion is produced by parvocellular neurons of the PVN, the medial amygdala, the bed nucleus of the stria terminalis, and the suprachiasmatic nucleus, with projections toward distinct brain regions (3). Together, there are at least three distinct pathways by which AVP exerts its functions. First, AVP regulates water absorption via the posterior pituitary. Second, AVP is critically involved in the hypothalamic-pituitary-adrenal (HPA) stress axis via the posterior pituitary. Third, AVP remaining in the CNS contributes to behavior and cognitive functions.
The receptors for AVP have been divided into three major types, V1a, V1b (or V3), and V2, according to their pharmacological and G-protein-coupled properties (2). AVP released into the circulatory system functions as a peripheral hormone by binding to its receptors located at the plasma membrane of various target cells. The V1a receptor is predominantly found in vascular smooth muscle and is involved in the control of vasoconstrictor effects and blood pressure regulation. V1b receptors are primarily located on specialized cells, called corticotrophs, in the anterior pituitary gland, where they stimulate the release of adrenocorticotropic hormone (ACTH) synergistically with corticotropin-releasing hormone (CRH). The V2 receptor expressed on kidney cells is responsible for water reabsorption in the collecting ducts by activating aquaporin-2 channels, whereas its expression on endothelial cells of the vasculature and platelets makes AVP an important hormone in hemostasis. V1a and V1b receptors are found in the brain. Finally, AVP binds to the oxytocin receptor, which further increases its complexity (4).
The measurement of AVP is cumbersome and complex due to several pre-analytical obstacles; thus, the detection of AVP is unsuited for clinical diagnostics and is limited to a few specialized laboratories (5). For example, 90% of AVP in the circulatory system is bound to platelets, which falsifies the actual amounts of circulating AVP (6). AVP is a bioactive peptide hormone that is tightly regulated and rapidly cleared from the circulation, with an in vivo half-life of less than 30 min (7). And to make matters worse, AVP is unstable in isolated plasma, even when stored at −20°C (8). In contrast, copeptin does not have such limitations (5). Several copeptin assays are currently available, but the only assays with sufficient technical descriptions and clinical data to justify their routine clinical use are the original sandwich immunoluminometric assay (1) and its automated immunofluorescent successor (on the KRYPTOR platform). In conclusion, the great advantages of copeptin measurement are the remarkably high sensitivity of this robust AVP surrogate marker, its extreme stability once collected in blood sampling tubes, and the fact that only 50 μl of serum or plasma are needed for the assay (9).
An important “side effect” of vaginal delivery is the surge in fetal stress hormones, including catecholamines, cortisol, and AVP. These stress hormones are widely recognized to facilitate the transition of the newborn to air breathing, cardiovascular adaptation, thermogenesis, glucose, and water homeostasis. It has been four decades since the first results on AVP concentrations in an infant’s umbilical cord blood at birth were published, which indicated strongly elevated AVP concentrations (10–16). These initial studies have led to the following key findings: (1) cord blood AVP is of fetal origin because AVP concentrations in arterial cord blood are higher than values reported in venous cord blood (arteriovenous gradient) and because AVP is absent in cord blood of anencephalic infants (10–12, 14, 16, 17), (2) cord blood AVP levels in infants delivered vaginally are much higher than those in infants delivered by cesarean section performed before the onset of labor (15, 16), (3) cord blood AVP levels correlate positively with fetal distress measured as cord blood pH (17) and have been reported to be exceedingly high in asphyxiated infants (10, 14, 18), leading to the assumption of “pituitary exhaustion” (10), and (4) following delivery, AVP levels drop rapidly within a few hours to adult basal levels (16, 19).
With the goal to study systematically the surge of AVP and copeptin levels at birth in comparison to other stress hormones, namely cortisol and norepinephrine, we performed a systematic review of the literature. The authors independently searched the database Medline, available via PubMed, retrieved, and reviewed studies for eligibility and extracted data. All disagreements encountered in the review process were resolved through consensus. We included studies that met the following criteria: (1) study design: cohort, cross-sectional, or case-control studies in humans, which evaluated the relationship between different delivery modes and concentrations of stress hormones in healthy term-born infants at birth. Only studies comparing unassisted vaginal delivery with primary cesarean section were included. Studies were excluded from this review if they reported emergency or secondary cesarean sections, the latter are cesareans with contractions or rupture of the membranes, and if a study reported only data on either vaginal deliveries or primary cesarean sections. (2) Outcome measures: plasma or serum concentrations of cortisol, norepinephrine, AVP, and copeptin in arterial or venous umbilical cord blood collected at birth. (3) Statistical analysis: the authors of the studies must have presented hormone concentrations as medians or means as well as patient numbers of each delivery group separately. Searches were carried out to identify studies for each hormone concerned with: (1) neonate, (2) umbilical cord blood, and (3) delivery or birth. These three concepts were linked by the “AND” operator. A variety of search terms was used within each concept, e.g., (1) newborn, baby, infant, fetus; (2) cord blood, serum, plasma; and (3) parturition, delivery mode, birth stress. Both free text and subject headings (MeSH terms) were used.
For cortisol, 47 studies, norepinephrine 19, AVP 9, and for copeptin, 7 studies were eligible and data were retrieved. Data are presented in Figure 1 as fold change between vaginal delivery and primary cesarean section of cortisol, norepinephrine, AVP, and copeptin in separate panels. For each hormone, a mean fold change is given weighted with respect to sample size. Studies are numbered chronologically, and the corresponding references are as follows:
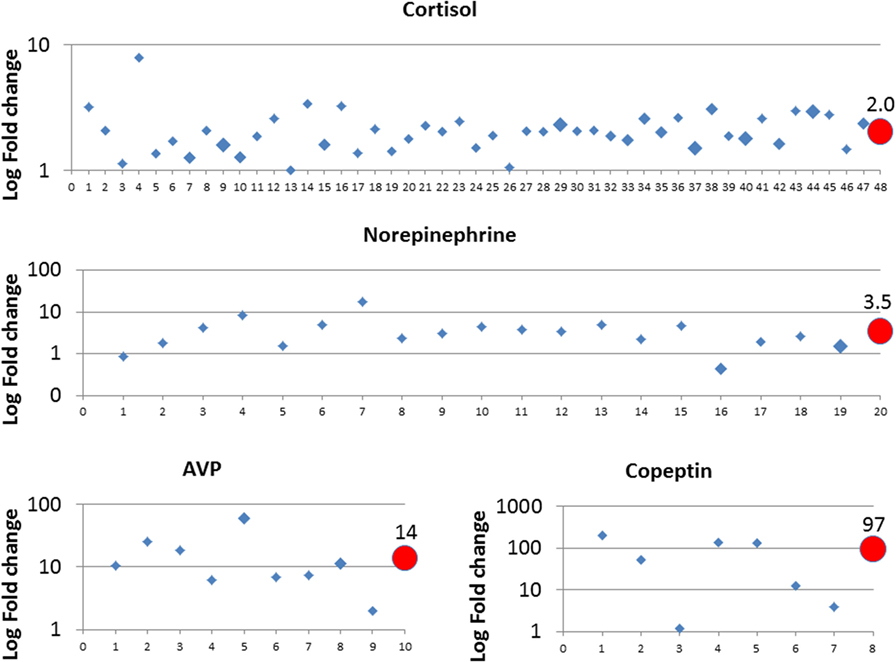
Figure 1. Fold change of norepinephrine, cortisol, AVP, and copeptin between primary cesarean section and vaginal delivery. Study data were retrieved from the published literature and listed in chronologic order from oldest to newest on x-axis, corresponding references are given in Section “Surge in Fetal Stress Hormones” for all four hormones. The size of the diamonds reflects the study sample size: small corresponds to n = 0–50, middle n = 50–100, and large n = 100–170. Circles reflect the weighted mean values depending on sample size and are given at the final position of the x-axis.
Cortisol: 1 (20), 2 (21), 3 (22), 4 (23), 5 (24), 6 (25), 7 (26), 8 (27), 9 (28), 10 (29), 11 (30), 12 (31), 13 (32), 14 (33), 15 (34), 16 (35), 17 (36), 18 (37), 19 (38), 20 (39), 21 (40), 22 (41), 23 (42), 24 (43), 25 (44), 26 (45), 27 (46), 28 (47), 29 (48), 30 (49), 31 (50), 32 (51), 33 (52), 34 (53), 35 (54), 36 (55), 37 (56), 38 (57), 39 (58), 40 (59), 41 (60), 42 (61), 43 (62), 44 (63), 45 (64), 46 (65), and 47 (66); Norepinephrine: 1 (67), 2 (68), 3 (33), 4 (69), 5 (70), 6 (71), 7 (72), 8 (73), 9 (74), 10 (36), 11 (75), 12 (76), 13 (77), 14 (78), 15 (79), 16 (80), 17 (81), 18 (55), and 19 (66); and AVP: 1 (11), 2 (12), 3 (15), 4 (16), 5 (82), 6 (83), 7 (41), 8 (84), and 9 (85). Copeptin: 1 (86), 2 (87), 3 (88), 4 (89), 5 (90), 6 (91), and 7 (92).
In summary, all four hormones were higher in the cord blood of infants after vaginal delivery than after primary cesarean section, with highest values observed for AVP (14-fold) and copeptin (97-fold). Of note, we identified a few studies that reported no difference (cortisol study number 13 and 26, copeptin study number 3) or lower values after vaginal delivery (norepinephrine study 1 and 16) (Figure 1). For the studies on cortisol and norepinephrine, we could not identify a specific reason. With respect to copeptin, five out of seven included studies conducted copeptin measurements using the validated BRAHMS KRYPTOR immunoanalyzer (Thermo Scientific Brahms GmbH, Hennigsdorf, Germany). Study number 3 used the human copeptin ELISA kit from Cusabio Inc. (Cusabio Biotech Co., USA) and study number 7 used the ELISA kit from Phoenix Pharmaceuticals Inc. (Phoenix Pharmaceuticals Inc., Karlsruhe, Germany), where both studies showed fold change below fivefold.
Both AVP and copeptin concentrations are significantly higher in infants after secondary cesarean section and assisted vaginal delivery as compared to primary cesarean and spontaneous delivery, respectively (11, 15, 16, 86, 90, 93). In addition, AVP and copeptin are closely related with metabolic stress markers pH, negative base excess, and lactate at birth, with highest concentrations after perinatal hypoxia ischemia, see also below (86, 87, 94, 95). Together, these findings support the pivotal role of AVP and copeptin as fetal stress markers.
Birth is more than just the few minutes of delivery. It is a complex process that involves the preparation of the mother’s and infant’s bodies. Maternal labor is the driving force in vaginal delivery, as it encourages the mother’s body to dilate the cervix and to push the fetus through the birth canal (96). Normal labor is initiated by the removal of the inhibitory effects of pregnancy on the myometrium rather than an active process governed by uterine stimulants (97). The uterus is usually maintained in a state of quiescence during pregnancy. Myometrium inhibition occurs via the integrated action of different inhibitors, and their effect diminishes near term due to the activation of the uterus with regular uterine contractions accompanied by slow cervical dilation. This process represents the latent phase of the first stage of labor, which ultimately results in the descent of the fetal head into the pelvis. The second stage of labor begins when the cervix is fully dilated and ends with the delivery of the infant. Stage three events (uterine involution) occur after delivery. During the first and second stages of labor, each uterine contraction leads to a brief pO2 drop in the fetus, often without noticeable changes in fetal heart rate tracings (98–100). Data from animal studies show a direct and dose-dependent link between uterine contractions and fetal arterial pO2 changes (101, 102), as well as a relationship between arterial pO2 and plasma AVP in fetal sheep (103–105). Furthermore, AVP stimulates ACTH release and cortisol secretion, as demonstrated repeatedly in fetal sheep (106–109). Even small changes in fetal arterial pO2 of 2.5 mmHg were sufficient to cause ACTH release, a key hormone of the HPA axis downstream of AVP and CRH (110). Arterial hypoxia is a strong trigger of AVP (111) and copeptin release (112), and brief events of a few minutes of hypoxia are sufficient to cause a fivefold increase in copeptin levels (113). Very recently, we completed a randomized controlled trial with a truncated oxytocin challenge test 2 h prior to elective cesarean section with subsequent arterial umbilical cord blood sampling at birth. Plasma copeptin levels were found to be threefold higher in the newborn babies of the oxytocin group than in the placebo infants without any evidence of fetal acidosis (114). Interestingly, only half the women in the oxytocin group noticed the contractions, indicating that the mild subclinical contractions are sufficient to trigger fetal AVP/copeptin release.
Thus, we conclude that uterine contractions precondition the fetus by activating the fetal HPA axis hours and days before birth. AVP plays a key role in this cascade. After small fetal arterial pO2 changes due to uterine contractions, fetal AVP is released with subsequent ACTH and cortisol secretion.
Arginine vasopressin is related to a variety of transitional processes at birth, including water homeostasis, breathing, peripheral analgesia, regulation of the vasculature resistance, and platelet function, which are summarized below and shown in Figure 2.
Increased AVP levels at birth have been linked to delayed first voiding in newborns (93, 115), and copeptin concentrations at birth are inversely related to postnatal weight loss by day 3 of life (86). Reducing urinary water loss at birth until the enteral supply picks with the establishment of lactation appears to be a prudent strategy, and cesarean section without any previous labor deprives the newborn infant of the wave of fetal AVP, which serves as a preemptive adaptation. In fact, the extent of weight loss is much greater in newborns born by cesarean than by vaginal delivery (116, 117).
In vitro studies showed that cultivated type II pneumocytes express AVP receptor-2 and secrete phospholipoproteins after AVP binding, the constituents of surfactant (118, 119). Pulmonary surfactant is a surface-active phospholipoprotein complex, is produced by type II pneumocytes, and acts at the air–water interface of alveoli to reduce surface tension. Primary surfactant deficiency is a key feature in the pathogenesis of neonatal respiratory distress syndrome (RDS). Of interest, a pilot trial in extremely preterm infants to evaluate AVP versus dopamine as initial therapy in hypotension during the first 24 h of life showed that the AVP group received significantly fewer doses of surfactant and had lower PaCO2 values than the dopamine group (120). Compared to vaginal delivery, infants delivered by cesarean section have a significantly increased risk of neonatal admission because of respiratory complications mainly due to delayed lung fluid reabsorption after birth, namely wet lung (121). AVP has been shown to decrease the production of fetal lung liquid in ovine fetuses (122), and it has been shown that AVP is involved in liquid removal from the lungs at birth (123–125). Thus, it might be speculated that, in the absence of preconditioning, when primary cesarean section was performed, AVP administration shortly after birth to the baby, e.g., via nasal spray, might represent a novel strategy to support postnatal adaptation.
Peripheral analgesia has been observed in vaginally delivered infants on the first day of life and has been linked to AVP signaling (4). In particular, animal studies have shown that the vasopressin-1A receptor mediates oxytocin and AVP-induced analgesia (126, 127).
Apart from water reabsorption in the kidney, vasoconstriction is a key function of AVP and has been reviewed elsewhere (128). Vasoconstriction is of vital importance to the neonate immediately after delivery to maintain blood pressure by increasing peripheral resistance and to close the umbilical cord blood vessels. AVP induces a redistribution of fetal blood flow from peripheral areas to vital organs (129). On a molar basis, AVP is even more potent than norepinephrine and angiotensin II (130). In contrast to the peripheral arterioles, AVP reduces pulmonary artery resistance through the stimulation of V1 receptors, which induces the release of the very potent vasodilator endothelial-derived nitric oxide (131). This opposing effect of AVP on the vascular bed is particularly advantageous in the transition from placenta to lung breathing and makes AVP a highly attractive drug in neonatology (120).
Arginine vasopressin stimulates the release of coagulation factor, von Willebrand factor (VWF), and tissue plasminogen activator (t-PA) via V2 receptors on endothelial cells and platelets. AVP constitutes an important factor in prothrombotic activity, and the AVP analog desmopressin (DDAVP) is widely used in a variety of bleeding diseases (132). Interestingly, thrombotic sealing is a crucial event for the closure of the ductus arteriosus, promoting vascular obliteration and subsequent remodeling (133). Apart from the absolute platelet count, platelet function may also play an important role in the pathobiology of the ductus arteriosus (134). Based on this finding, we speculate that AVP released at birth may contribute to the thrombotic sealing of the ductus arteriosus.
Both in healthy adults as well as in healthy neonates, AVP/copeptin plasma values are higher in males than in females (1, 90, 135–139).
When birth stress is increased because the fetus is compromised by prolonged hypoxic events, perinatal hypoxia ischemia (asphyxia) may result in which the highest fetal AVP and copeptin concentrations have been measured (87, 94, 95). While the difference between copeptin concentrations at birth of healthy neonates after elective cesarean delivery and vaginal delivery is about 100-fold (Figure 1), the difference between healthy vaginally delivered neonates and neonates with asphyxia is small (only twofold on average) (87). A major complication of asphyxia is brain edema, which may lead to increased intracranial pressure and is associated with poor outcome (140). The blood–brain barrier (BBB) Na/H exchanger is stimulated by brief exposure to hypoxia, aglycemia, and AVP (141) involving the V1 receptor, which is present in brain microvessels (142–144). Brain edema in animal models could be reduced by an AVP blocker (Conivaptan) (145, 146) and aggravated by AVP delivery (147, 148). Moreover, in the AVP-deficient Brattleboro rat, brain edema was attenuated compared to the control Long–Evans strain (149). Importantly, either intranasal AVP or AVP receptor antagonist did not evoke edema in the non-ischemic cortex of Mongolian gerbils (148). This result could indicate that increased AVP concentrations alone, namely after normal vaginal delivery, are insufficient to deteriorate the integrity of the BBB and that a severe lack of oxygen must be present as well.
A second sequela of asphyxia is the syndrome of inappropriate ADH secretion (SIADH), which is also a consequence of exceedingly high levels of AVP/ADH, renal failure, or both (150–154). Again, increased AVP concentrations alone, namely after normal vaginal delivery, have never been shown to cause SIADH, but they cause delayed voiding as described above.
Syndrome of inappropriate ADH secretion is also known as a complication of neonatal sepsis. In fact, sudden hyponatremia is a typical early sign in the early course of a severe neonatal infection. The pro-inflammatory cytokine interleukin-6 directly triggers the release of AVP (155, 156).
Chronic stress is associated with chronically elevated AVP/copeptin levels in rodent as well as human fetuses suffering from intrauterine growth restriction (82, 157, 158). After birth, neonates exposed to chronic stress, such as mechanical respiratory support, have elevated plasma copeptin concentrations (159).
Arginine vasopressin and its analogs have not yet been studied systematically in neonates (160). Various series of case reports indicate that AVP and its analogs are well tolerated and effective in neonates as rescue therapy for refractory arterial hypotension and particularly for persistent pulmonary hypertension (161–164). The recently published results of the first prospective randomized, double-blinded, pilot trial comparing AVP and dopamine in the treatment of hypotension in extremely low birth weight infants demonstrated the safety of AVP in these tiny infants (120). Although there were no differences between the two groups concerning time to adequate mean blood pressure, it was shown that infants who received vasopressin had lower mean PaCO2 levels and needed fewer doses of surfactant than infants who received dopamine; additionally, infants who received vasopressin did not show tachycardia during drug administration compared to infants who received dopamine. The authors believe that these effects may be due to the pulmonary vasodilatory effects and lack of cardiac effects of vasopressin (120).
In view of the various actions AVP is involved (Figure 2) and the close relationship between fetal AVP and arterial pO2, we would like to conclude that the surge in fetal stress hormones prior to vaginal delivery is the beneficial result of intermittent hypoxic preconditioning of the fetus due to the uterine contractions required to dilate the cervix and to push the fetus through the birth canal.
In respect to diagnostic implications and the data presented here (Figure 1), copeptin is apparently the most sensitive birth stress biomarker when measured with a clinically established assay. Future research will investigate copeptin in the diagnosis of AVP-dependent disorders of fluid homeostasis in infants and children as well, including nephrogenic and cerebral reasons such as increased intracerebral pressure after intraventricular hemorrhage, stroke, or traumatic brain injury. Given the sensitivity of copeptin to brief hypoxic episodes in animal models, copeptin might also be of diagnostic value in unclear clinical situations such as apneas or apparent life threatening events.
Together, the availability of quantitative copeptin measurements opens new avenues to study copeptin as an adjunct in the diagnosis of various diseases and to further understand AVP action.
SW conceived and designed the review. KE and SW performed the literature search. KE analyzed the data and prepared the graphs. SW drafted the initial manuscript. KE and SW critically revised the manuscript for important intellectual content, agreed on the final manuscript, and approved its submission for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem (2006) 52(1):112–9. doi: 10.1373/clinchem.2005.060038
2. Koshimizu TA, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev (2012) 92(4):1813–64. doi:10.1152/physrev.00035.2011
3. Rood BD, De Vries GJ. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol (2011) 519(12):2434–74. doi:10.1002/cne.22635
4. Wellmann S, Buhrer C. Who plays the strings in newborn analgesia at birth, vasopressin or oxytocin? Front Neurosci (2012) 6:78. doi:10.3389/fnins.2012.00078
5. Morgenthaler NG, Struck J, Jochberger S, Dunser MW. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab (2008) 19(2):43–9. doi:10.1016/j.tem.2007.11.001
6. Preibisz JJ, Sealey JE, Laragh JH, Cody RJ, Weksler BB. Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension (1983) 5(2 Pt 2):I129–38. doi:10.1161/01.HYP.5.2_Pt_2.I129
7. Baumann G, Dingman JF. Distribution, blood transport, and degradation of antidiuretic hormone in man. J Clin Invest (1976) 57(5):1109–16. doi:10.1172/jci108377
8. Robertson GL, Mahr EA, Athar S, Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest (1973) 52(9):2340–52. doi:10.1172/jci107423
9. Christ-Crain M, Fenske W. Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat Rev Endocrinol (2016) 12(3):168–76. doi:10.1038/nrendo.2015.224
10. Hoppenstein JM, Miltenberger FW, Moran WH Jr. The increase in blood levels of vasopressin in infants during birth and surgical procedures. Surg Gynecol Obstet (1968) 127(5):966–74.
11. Chard T, Hudson CN, Edwards CR, Boyd NR. Release of oxytocin and vasopressin by the human foetus during labour. Nature (1971) 234(5328):352–4. doi:10.1038/234352a0
12. Polin RA, Husain MK, James LS, Frantz AG. High vasopressin concentrations in human umbilical cord blood – lack of correlation with stress. J Perinat Med (1977) 5(3):114–9. doi:10.1515/jpme.1977.5.3.114
13. Czernichow P, Pattin AM. [Plasma vasopressin in paired maternal-cord sera (proceedings) (author’s transl)]. Ann Endocrinol (Paris) (1978) 39(3):225–6.
14. Hadeed AJ, Leake RD, Weitzman RE, Fisher DA. Possible mechanisms of high blood levels of vasopressin during the neonatal period. J Pediatr (1979) 94(5):805–8. doi:10.1016/S0022-3476(79)80162-6
15. DeVane GW, Porter JC. An apparent stress-induced release or arginine vasopressin by human neonates. J Clin Endocrinol Metab (1980) 51(6):1412–6. doi:10.1210/jcem-51-6-1412
16. Leung AK, McArthur RG, McMillan DD, Ko D, Deacon JS, Parboosingh JT, et al. Circulating antidiuretic hormone during labour and in the newborn. Acta Paediatr Scand (1980) 69(4):505–10. doi:10.1111/j.1651-2227.1980.tb07122.x
17. Parboosingh J, Lederis K, Ko D, Singh N. Vasopressin concentration in cord blood: correlation with method of delivery and cord pH. Obstet Gynecol (1982) 60(2):179–83.
18. Feldman W, Drummond KN, Klein M. Hyponatremia following asphyxia neonatorum. Acta Paediatr Scand (1970) 59(1):52–7. doi:10.1111/j.1651-2227.1970.tb15514.x
19. Rees L, Forsling ML, Brook CG. Vasopressin concentrations in the neonatal period. Clin Endocrinol (Oxf) (1980) 12(4):357–62. doi:10.1111/j.1365-2265.1980.tb02720.x
20. Murphy BE. Does the human fetal adrenal play a role in parturition? Am J Obstet Gynecol (1973) 115(4):521–5. doi:10.1016/0002-9378(73)90400-6
21. Pokoly TB. The role of cortisol in human parturition. Am J Obstet Gynecol (1973) 117(4):549–53. doi:10.1016/0002-9378(73)90119-1
22. Talbert LM, Easterling WE Jr, Potter HD. Maternal and fetal plasma levels of adrenal corticoids in spontaneous vaginal delivery and cesarean section. Am J Obstet Gynecol (1973) 117(4):554–9. doi:10.1016/0002-9378(73)90120-8
23. Cawson MJ, Anderson AB, Turnbull AC, Lampe L. Cortisol, cortisone, and 11-deoxycortisol levels in human umbilical and maternal plasma in relation to the onset of labour. J Obstet Gynaecol Br Commonw (1974) 81(10):737–45. doi:10.1111/j.1471-0528.1974.tb00373.x
24. Simmer HH, Frankland MV, Greipel M. Unbound unconjugated cortisol in umbilical cord and corresponding maternal plasma. Materno-fetal gradient: comparison of methods. Gynecol Invest (1974) 5(4):199–221. doi:10.1159/000301652
25. Toaff R, Toaff ME, Peyser MR, Ayalon D, Cordova T. Responsiveness of the human fetus to stress. Isr J Med Sci (1974) 10(12):1487–92.
26. Goldkrand JW, Schulte RL, Messer RH. Maternal and fetal plasma cortisol levels at parturition. Obstet Gynecol (1976) 47(1):41–5.
27. Ohrlander S, Gennser G, Eneroth P. Plasma cortisol levels in human fetus during parturition. Obstet Gynecol (1976) 48(4):381–7.
28. Sybulski S, Maughan GB. Cortisol levels in umbilical cord plasma in relation to labor and delivery. Am J Obstet Gynecol (1976) 125(2):236–8. doi:10.1016/0002-9378(76)90599-8
29. Weekes AR, Wade AP, West CR. Umbilical vein cortisol after spontaneous and induced labour and at elective Caesarean section. Br J Obstet Gynaecol (1976) 83(11):870–2. doi:10.1111/j.1471-0528.1976.tb00763.x
30. Talbert LM, Pearlman WH, Potter HD. Maternal and fetal serum levels of total cortisol and cortisone, unbound cortisol, and corticosteroid-binding globulin in vaginal delivery and cesarean section. Am J Obstet Gynecol (1977) 129(7):781–7. doi:10.1016/0002-9378(77)90397-0
31. Kauppila A, Koivisto M, Pukka M, Tuimala R. Umbilical cord and neonatal cortisol levels. Effect of gestational and neonatal factors. Obstet Gynecol (1978) 52(6):666–72.
32. Laatikainen T, Pelkonen J, Apter D, Ranta T. Fetal and maternal serum levels of steroid sulfates, unconjugated steroids, and prolactin at term pregnancy and in early spontaneous labor. J Clin Endocrinol Metab (1980) 50(3):489–94. doi:10.1210/jcem-50-3-489
33. Faxelius G, Hagnevik K, Lagercrantz H, Lundell B, Irestedt L. Catecholamine surge and lung function after delivery. Arch Dis Child (1983) 58(4):262–6. doi:10.1136/adc.58.4.262
34. Kohno H, Furuhashi N, Fukaya T, Tachibana Y, Shinkawa O, Suzuki M. Serum cortisol levels of maternal vein, umbilical artery, and umbilical vein classified by mode of delivery. Gynecol Obstet Invest (1984) 17(6):301–8. doi:10.1159/000299167
35. Pohjavuori M, Rovamo L, Laatikainen T. Plasma immunoreactive beta-endorphin and cortisol in the newborn infant after elective caesarean section and after spontaneous labour. Eur J Obstet Gynecol Reprod Biol (1985) 19(2):67–74. doi:10.1016/0028-2243(85)90021-8
36. Falconer AD, Poyser LM. Fetal sympatho-adrenal mediated metabolic responses to parturition. Br J Obstet Gynaecol (1986) 93(7):747–53. doi:10.1111/j.1471-0528.1986.tb07976.x
37. Lao TT, Chin RK, Swaminathan R, Mak YT. Plasma and erythrocyte zinc concentrations in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol (1989) 30(2):117–22. doi:10.1016/0028-2243(89)90057-9
38. Okamoto E, Takagi T, Makino T, Sata H, Iwata I, Nishino E, et al. Immunoreactive corticotropin-releasing hormone, adrenocorticotropin and cortisol in human plasma during pregnancy and delivery and postpartum. Horm Metab Res (1989) 21(10):566–72. doi:10.1055/s-2007-1009289
39. Hercz P, Siklos P, Ungar L. Serum dehydroepiandrosterone and cortisol concentration in the maternal-fetoplacental hormonal system in elective caesarean section and spontaneous vaginal delivery in the 28th to 36th and 40th weeks of pregnancy. Gynecol Obstet Invest (1990) 29(2):112–4. doi:10.1159/000293314
40. Gasparoni A, Chirico G, De Amici D, Marconi M, Belloni C, Mingrat G, et al. Neutrophil chemotaxis in infants delivered by caesarean section. Eur J Pediatr (1991) 150(7):481–2. doi:10.1007/BF01958427
41. Ramin SM, Porter JC, Gilstrap LC III, Rosenfeld CR. Stress hormones and acid-base status of human fetuses at delivery. J Clin Endocrinol Metab (1991) 73(1):182–6. doi:10.1210/jcem-73-1-182
42. Gasparoni A, Maccario R, Chirico G, Belloni C, Mingrat G, De Amici D, et al. Neonatal B lymphocyte subpopulations and method of delivery. Biol Neonate (1992) 61(3):137–41. doi:10.1159/000243735
43. Ruth V, Hallman M, Laatikainen T. Corticotropin-releasing hormone and cortisol in cord plasma in relation to gestational age, labor, and fetal distress. Am J Perinatol (1993) 10(2):115–8. doi:10.1055/s-2007-994641
44. Bird JA, Spencer JA, Mould T, Symonds ME. Endocrine and metabolic adaptation following caesarean section or vaginal delivery. Arch Dis Child Fetal Neonatal Ed (1996) 74(2):F132–4. doi:10.1136/fn.74.2.F132
45. Posaci C, Guney M, Erata YE, Demir N, Onvural A. Stress hormones and acid-base status in human fetuses at term delivery: the effect of delivery method. J Pak Med Assoc (1996) 46(6):123–6.
46. Kazda H, Taylor N, Healy D, Walker D. Maternal, umbilical, and amniotic fluid concentrations of tryptophan and kynurenine after labor or cesarean section. Pediatr Res (1998) 44(3):368–73. doi:10.1203/00006450-199809000-00017
47. Gasparoni A, De Amici D, Ciardelli L, Autelli M, Regazzi-Bonora M, Bartoli A, et al. Effect of lidocaine on neutrophil chemotaxis in newborn infants. J Clin Immunol (1998) 18(3):210–3. doi:10.1023/A:1020583022614
48. Chirico G, Gasparoni A, Ciardelli L, Martinotti L, Rondini G. Leukocyte counts in relation to the method of delivery during the first five days of life. Biol Neonate (1999) 75(5):294–9. doi:10.1159/000014107
49. De Amici D, Gasparoni A, Chirico G, Ceriana P, Bartoli A, Ramajoli I, et al. Natural killer cell activity and delivery: possible influence of cortisol and anesthetic agents. A study on newborn cord blood. Biol Neonate (1999) 76(6):348–54. doi:10.1159/000014178
50. Gitau R, Menson E, Pickles V, Fisk NM, Glover V, MacLachlan N. Umbilical cortisol levels as an indicator of the fetal stress response to assisted vaginal delivery. Eur J Obstet Gynecol Reprod Biol (2001) 98(1):14–7. doi:10.1016/S0301-2115(01)00298-6
51. Mears K, McAuliffe F, Grimes H, Morrison JJ. Fetal cortisol in relation to labour, intrapartum events and mode of delivery. J Obstet Gynaecol (2004) 24(2):129–32. doi:10.1080/01443610410001645389
52. Miller NM, Fisk NM, Modi N, Glover V. Stress responses at birth: determinants of cord arterial cortisol and links with cortisol response in infancy. BJOG (2005) 112(7):921–6. doi:10.1111/j.1471-0528.2005.00620.x
53. Marchini G, Hagenas L, Kocoska-Maras L, Berggren V, Hansson LO. Insulin-like growth factor binding protein-1 and interleukin-6 are markers of fetal stress during parturition at term gestation. J Pediatr Endocrinol Metab (2005) 18(8):777–83. doi:10.1515/JPEM.2005.18.8.777
54. Oh SY, Romero R, Shim SS, Park JS, Jun JK, Yoon BH. Fetal plasma cortisol and dehydroepiandrosterone sulfate concentrations in pregnancy and term parturition. J Matern Fetal Neonatal Med (2006) 19(9):529–36. doi:10.1080/14767050600853179
55. Vogl SE, Worda C, Egarter C, Bieglmayer C, Szekeres T, Huber J, et al. Mode of delivery is associated with maternal and fetal endocrine stress response. BJOG (2006) 113(4):441–5. doi:10.1111/j.1471-0528.2006.00865.x
56. Varvarigou AA, Petsali M, Vassilakos P, Beratis NG. Increased cortisol concentrations in the cord blood of newborns whose mothers smoked during pregnancy. J Perinat Med (2006) 34(6):466–70. doi:10.1515/JPM.2006.091
57. Yektaei-Karin E, Moshfegh A, Lundahl J, Berggren V, Hansson LO, Marchini G. The stress of birth enhances in vitro spontaneous and IL-8-induced neutrophil chemotaxis in the human newborn. Pediatr Allergy Immunol (2007) 18(8):643–51. doi:10.1111/j.1399-3038.2007.00578.x
58. Clifton VL, Bisits A, Zarzycki PK. Characterization of human fetal cord blood steroid profiles in relation to fetal sex and mode of delivery using temperature-dependent inclusion chromatography and principal component analysis (PCA). J Chromatogr B Analyt Technol Biomed Life Sci (2007) 855(2):249–54. doi:10.1016/j.jchromb.2007.05.041
59. Smith AK, Newport DJ, Ashe MP, Brennan PA, Laprairie JL, Calamaras M, et al. Predictors of neonatal hypothalamic-pituitary-adrenal axis activity at delivery. Clin Endocrinol (Oxf) (2011) 75(1):90–5. doi:10.1111/j.1365-2265.2011.03998.x
60. Yildiran A, Yurdakul E, Guloglu D, Dogu F, Arsan S, Arikan M, et al. The effect of mode of delivery on T regulatory (Treg) cells of cord blood. Indian J Pediatr (2011) 78(10):1234–8. doi:10.1007/s12098-011-0400-6
61. Ortega-Senovilla H, Schaefer-Graf U, Meitzner K, Graf K, Abou-Dakn M, Herrera E. Lack of relationship between cord serum angiopoietin-like protein 4 (ANGPTL4) and lipolytic activity in human neonates born by spontaneous delivery. PLoS One (2013) 8(12):e81201. doi:10.1371/journal.pone.0081201
62. Bagnoli F, Mori A, Fommei C, Coriolani G, Badii S, Tomasini B. ACTH and cortisol cord plasma concentrations in preterm and term infants. J Perinatol (2013) 33(7):520–4. doi:10.1038/jp.2012.165
63. Wynne-Edwards KE, Edwards HE, Hancock TM. The human fetus preferentially secretes corticosterone, rather than cortisol, in response to intra-partum stressors. PLoS One (2013) 8(6):e63684. doi:10.1371/journal.pone.0063684
64. Olza Fernandez I, Marin Gabriel MA, Garcia Murillo L, Malalana Martinez AM, Costarelli V, Millan Santos I. Mode of delivery may influence neonatal responsiveness to maternal separation. Early Hum Dev (2013) 89(5):339–42. doi:10.1016/j.earlhumdev.2012.11.005
65. Janer C, Pitkanen OM, Suvari L, Turpeinen U, Palojarvi A, Andersson S, et al. Duration of gestation and mode of delivery affect the genes of transepithelial sodium transport in pulmonary adaptation. Neonatology (2015) 107(1):27–33. doi:10.1159/000363729
66. Elbay A, Celik U, Celik B, Ozer OF, Kilic G, Akkan JC, et al. Intraocular pressure in infants and its association with hormonal changes with vaginal birth versus cesarean section. Int Ophthalmol (2016). doi:10.1007/s10792-016-0215-6
67. Eliot RJ, Lam R, Leake RD, Hobel CJ, Fisher DA. Plasma catecholamine concentrations in infants at birth and during the first 48 hours of life. J Pediatr (1980) 96(2):311–5. doi:10.1016/S0022-3476(80)80836-5
68. Jones CM III, Greiss FC Jr. The effect of labor on maternal and fetal circulating catecholamines. Am J Obstet Gynecol (1982) 144(2):149–53. doi:10.1016/0002-9378(82)90616-0
69. Puolakka J, Kauppila A, Tuimala R, Jouppila R, Vuori J. The effect of parturition on umbilical blood plasma levels of norepinephrine. Obstet Gynecol (1983) 61(1):19–21.
70. Faxelius G, Lagercrantz H, Yao A. Sympathoadrenal activity and peripheral blood flow after birth: comparison in infants delivered vaginally and by cesarean section. J Pediatr (1984) 105(1):144–8. doi:10.1016/S0022-3476(84)80381-9
71. Hagnevik K, Faxelius G, Irestedt L, Lagercrantz H, Lundell B, Persson B. Catecholamine surge and metabolic adaptation in the newborn after vaginal delivery and caesarean section. Acta Paediatr Scand (1984) 73(5):602–9. doi:10.1111/j.1651-2227.1984.tb09982.x
72. Jouppila R, Puolakka J, Kauppila A, Vuori J. Maternal and umbilical cord plasma noradrenaline concentrations during labour with and without segmental extradural analgesia, and during caesarean section. Br J Anaesth (1984) 56(3):251–5. doi:10.1093/bja/56.3.251
73. Lundell BP, Hagnevik K, Faxelius G, Irestedt L, Lagercrantz H. Neonatal left ventricular performance after vaginal delivery and cesarean section under general or epidural anesthesia. Am J Perinatol (1984) 1(2):152–7. doi:10.1055/s-2007-999992
74. Jones CR, McCullouch J, Butters L, Hamilton CA, Rubin PC, Reid JL. Plasma catecholamines and modes of delivery: the relation between catecholamine levels and in-vitro platelet aggregation and adrenoreceptor radioligand binding characteristics. Br J Obstet Gynaecol (1985) 92(6):593–9. doi:10.1111/j.1471-0528.1985.tb01397.x
75. Irestedt L, Dahlin I, Hertzberg T, Sollevi A, Lagercrantz H. Adenosine concentration in umbilical cord blood of newborn infants after vaginal delivery and cesarean section. Pediatr Res (1989) 26(2):106–8. doi:10.1203/00006450-198908000-00007
76. Otamiri G, Berg G, Ledin T, Leijon I, Lagercrantz H. Delayed neurological adaptation in infants delivered by elective cesarean section and the relation to catecholamine levels. Early Hum Dev (1991) 26(1):51–60. doi:10.1016/0378-3782(91)90043-3
77. Hirsimaki H, Kero P, Ekblad H, Scheinin M, Saraste M, Erkkola R. Mode of delivery, plasma catecholamines and Doppler-derived cardiac output in healthy term newborn infants. Biol Neonate (1992) 61(5):285–93. doi:10.1159/000243756
78. Agata Y, Hiraishi S, Misawa H, Han JH, Oguchi K, Horiguchi Y, et al. Hemodynamic adaptations at birth and neonates delivered vaginally and by Cesarean section. Biol Neonate (1995) 68(6):404–11. doi:10.1159/000244263
79. Moftaquir-Handaj A, Barbe F, Barbarino-Monnier P, Aunis D, Boutroy MJ. Circulating chromogranin A and catecholamines in human fetuses at uneventful birth. Pediatr Res (1995) 37(1):101–5. doi:10.1203/00006450-199501000-00019
80. Hata T, Manabe A, Makihara K, Hata K, Miyazaki K. Plasma catecholamines and Doppler-derived cardiac time intervals in vaginally and cesarean delivered neonates. Gynecol Obstet Invest (1997) 44(3):173–6. doi:10.1159/000291513
81. Wang L, Zhang W, Zhao Y. The study of maternal and fetal plasma catecholamines levels during pregnancy and delivery. J Perinat Med (1999) 27(3):195–8. doi:10.1515/JPM.1999.027
82. Pohjavuori M, Raivio KO. The effects of acute and chronic perinatal stress on plasma vasopressin concentration and renin activity at birth. Biol Neonate (1985) 47(5):259–64. doi:10.1159/000242126
83. Pochard JL, Lutz-Bucher B. Vasopressin and oxytocin levels in human neonates. Relationships with the evolution of labour and beta-endorphins. Acta Paediatr Scand (1986) 75(5):774–8. doi:10.1111/j.1651-2227.1986.tb10289.x
84. Ohno Y, Mizutani S, Kurauchi O, Nishida Y, Arii Y, Tomoda Y. Umbilical plasma concentration of endothelin-1 in intrapartum fetal stress: effect of fetal heart rate abnormalities. Obstet Gynecol (1995) 86(5):822–5. doi:10.1016/0029-7844(95)00242-j
85. Briana DD, Boutsikou M, Boutsikou T, Dodopoulos T, Gourgiotis D, Malamitsi-Puchner A. Plasma copeptin may not be a sensitive marker of perinatal stress in healthy full-term growth-restricted fetuses. J Matern Fetal Neonatal Med (2016):1–5. doi:10.1080/14767058.2016.1183632
86. Wellmann S, Benzing J, Cippa G, Admaty D, Creutzfeldt R, Mieth RA, et al. High copeptin concentrations in umbilical cord blood after vaginal delivery and birth acidosis. J Clin Endocrinol Metab (2010) 95(11):5091–6. doi:10.1210/jc.2010-1331
87. Schlapbach LJ, Frey S, Bigler S, Manh-Nhi C, Aebi C, Nelle M, et al. Copeptin concentration in cord blood in infants with early-onset sepsis, chorioamnionitis and perinatal asphyxia. BMC Pediatr (2011) 11:38. doi:10.1186/1471-2431-11-38
88. Foda AA, Abdel Aal IA. Maternal and neonatal copeptin levels at cesarean section and vaginal delivery. Eur J Obstet Gynecol Reprod Biol (2012) 165(2):215–8. doi:10.1016/j.ejogrb.2012.08.012
89. Smith J, Halse KG, Damm P, Lindegaard ML, Amer-Wahlin I, Hertel S, et al. Copeptin and MR-proADM in umbilical cord plasma reflect perinatal stress in neonates born to mothers with diabetes and MR-proANP reflects maternal diabetes. Biomark Med (2013) 7(1):139–46. doi:10.2217/bmm.12.79
90. Burckhardt MA, Wellmann M, Fouzas S, Lapaire O, Burkhardt T, Benzing J, et al. Sexual disparity of copeptin in healthy newborn infants. J Clin Endocrinol Metab (2014) 99(9):E1750–3. doi:10.1210/jc.2014-2244
91. Blohm ME, Arndt F, Sandig J, Diehl W, Zeller T, Mueller GC, et al. Cardiovascular biomarkers in paired maternal and umbilical cord blood samples at term and near term delivery. Early Hum Dev (2016) 94:7–12. doi:10.1016/j.earlhumdev.2016.01.001
92. Briana DD, Baka S, Boutsikou M, Boutsikou T, Xagorari M, Gourgiotis D, et al. Cord blood copeptin concentrations in fetal macrosomia. Metabolism (2016) 65(1):89–94. doi:10.1016/j.metabol.2015.09.018
93. Vuohelainen T, Ojala R, Virtanen A, Laatta J, Morsky P, Uotila J, et al. Predictors of AVP and TSH levels and the timing of first voiding in the newborn. Pediatr Res (2007) 62(1):106–10. doi:10.1203/PDR.0b013e3180674d92
94. Speer ME, Gorman WA, Kaplan SL, Rudolph AJ. Elevation of plasma concentrations of arginine vasopressin following perinatal asphyxia. Acta Paediatr Scand (1984) 73(5):610–4. doi:10.1111/j.1651-2227.1984.tb09983.x
95. Ruth V, Fyhrquist F, Clemons G, Raivio KO. Cord plasma vasopressin, erythropoietin, and hypoxanthine as indices of asphyxia at birth. Pediatr Res (1988) 24(4):490–4. doi:10.1203/00006450-198810000-00015
96. Liao JB, Buhimschi CS, Norwitz ER. Normal labor: mechanism and duration. Obstet Gynecol Clin North Am (2005) 32(2):145–164,vii. doi:10.1016/j.ogc.2005.01.001
97. Lopez Bernal A, Rivera J, Europe-Finner GN, Phaneuf S, Asboth G. Parturition: activation of stimulatory pathways or loss of uterine quiescence? Adv Exp Med Biol (1995) 395:435–51.
98. Rooth G, Fall O, Huch A, Huch R. Integrated interpretation of fetal heart rate, intrauterine pressure and fetal transcutaneous pO2. Gynecol Obstet Invest (1979) 10(6):265–80. doi:10.1159/000299977
99. Klink F, Grosspietzsch R, Klitzing LV, Oberheuser F. Uterine contraction intervals and transcutaneous levels of fetal oxygen pressure. Obstet Gynecol (1981) 57(4):437–40.
100. Schmidt S, Langner K, Dudenhausen JW, Saling E. Measurement of transcutaneous pCO2 and pO2 in the fetus during labor. Arch Gynecol (1985) 236(3):145–51. doi:10.1007/BF02133957
101. Pimentel G, Poore ER, Nathanielsz PW. The effect of continuous or pulsatile administration of oxytocin to ewes at 126 to 136 days’ gestation on myometrial activity and fetal oxygenation. Am J Obstet Gynecol (1989) 160(1):242–7. doi:10.1016/0002-9378(89)90129-4
102. Shinozuka N, Yen A, Nathanielsz PW. Alteration of fetal oxygenation and responses to acute hypoxemia by increased myometrial contracture frequency produced by pulse administration of oxytocin to the pregnant ewe from 96 to 131 days’ gestation. Am J Obstet Gynecol (1999) 180(5):1202–8. doi:10.1016/S0002-9378(99)70617-4
103. Alexander DP, Forsling ML, Martin MJ, Nixon DA, Ratcliffe JG, Redstone D, et al. The effect of maternal hypoxia on fetal pituitary hormone release in the sheep. Biol Neonate (1972) 21(3):219–28. doi:10.1159/000240510
104. Rurak DW. Plasma vasopressin levels during hypoxaemia and the cardiovascular effects of exogenous vasopressin in foetal and adult sheep. J Physiol (1978) 277:341–57. doi:10.1113/jphysiol.1978.sp012275
105. DeVane GW, Naden RP, Porter JC, Rosenfeld CR. Mechanism of arginine vasopressin release in the sheep fetus. Pediatr Res (1982) 16(6):504–7. doi:10.1203/00006450-198206000-00021
106. Durand P, Cathiard AM, Dacheux F, Naaman E, Saez JM. In vitro stimulation and inhibition of adrenocorticotropin release by pituitary cells from ovine fetuses and lambs. Endocrinology (1986) 118(4):1387–94. doi:10.1210/endo-118-4-1387
107. Levidiotis M, Oldfield B, Wintour EM. Corticotrophin-releasing factor and arginine vasopressin fibre projections to the median eminence of fetal sheep. Neuroendocrinology (1987) 46(5):453–6. doi:10.1159/000124860
108. Norman LJ, Challis JR. Synergism between systemic corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin release in vivo varies as a function of gestational age in the ovine fetus. Endocrinology (1987) 120(3):1052–8. doi:10.1210/endo-120-3-1052
109. Faucher DJ, Laptook AR, Parker CR, Porter JC, Rosenfeld CR. Increased fetal secretion of ACTH and cortisol by arginine vasopressin. Am J Physiol (1988) 254(3 Pt 2):R410–6.
110. Woudstra BR, Kim C, Aarnoudse JG, Nathanielsz PW. Myometrial contracture-related increases in plasma adrenocorticotropin in fetal sheep in the last third of gestation are abolished by maintaining fetal normoxemia. Endocrinology (1991) 129(4):1709–13. doi:10.1210/endo-129-4-1709
112. L’Abate P, Wiegert S, Struck J, Wellmann S, Cannizzaro V. Determinants of plasma copeptin: a systematic investigation in a pediatric mechanical ventilation model. Respir Physiol Neurobiol (2013) 185(2):222–7. doi:10.1016/j.resp.2012.10.011
113. Ostergaard L, Rudiger A, Wellmann S, Gammella E, Beck-Schimmer B, Struck J, et al. Arginine-vasopressin marker copeptin is a sensitive plasma surrogate of hypoxic exposure. Hypoxia (2014) 2:143–51. doi:10.2147/HP.S57894
114. Wellmann S, Koslowski A, Spanaus K, Zimmermann R, Burkhardt T. Fetal release of copeptin in response to maternal oxytocin administration: a randomized controlled trial. Obstet Gynecol (2016). doi:10.1097/AOG.0000000000001594
115. Vuohelainen T, Ojala R, Virtanen A, Holm P, Tammela O. Predictors of delayed first voiding in newborn. Acta Paediatr (2008) 97(7):904–8. doi:10.1111/j.1651-2227.2008.00809.x
116. Flaherman VJ, Schaefer EW, Kuzniewicz MW, Li SX, Walsh EM, Paul IM. Early weight loss nomograms for exclusively breastfed newborns. Pediatrics (2015) 135(1):e16–23. doi:10.1542/peds.2014-1532
117. Wilbaux M, Kasser S, Wellmann S, Lapaire O, van den Anker JN, Pfister M. Characterizing and forecasting individual weight changes in term neonates. J Pediatr (2016) 173, 101–7.e110. doi:10.1016/j.jpeds.2016.02.044
118. Brown LA, Wood LH. Stimulation of surfactant secretion by vasopressin in primary cultures of adult rat type II pneumocytes. Biochim Biophys Acta (1989) 1001(1):76–81. doi:10.1016/0005-2760(89)90309-3
119. Brown LA, Chen M. Vasopressin signal transduction in rat type II pneumocytes. Am J Physiol (1990) 258(6 Pt 1):L301–7.
120. Rios DR, Kaiser JR. Vasopressin versus dopamine for treatment of hypotension in extremely low birth weight infants: a randomized, blinded pilot study. J Pediatr (2015) 166(4):850–5. doi:10.1016/j.jpeds.2014.12.027
121. Glavind J, Uldbjerg N. Elective cesarean delivery at 38 and 39 weeks: neonatal and maternal risks. Curr Opin Obstet Gynecol (2015) 27(2):121–7. doi:10.1097/gco.0000000000000158
122. Albuquerque CA, Nijland MJ, Ross MG. Mechanism of arginine vasopressin suppression of ovine fetal lung fluid secretion: lack of V2-receptor effect. J Matern Fetal Med (1998) 7(4):177–82. doi:10.1002/(SICI)1520-6661(199807/08)7:4<177::AID-MFM3>3.3.CO;2-Q
123. Ross MG, Ervin G, Leake RD, Fu P, Fisher DA. Fetal lung liquid regulation by neuropeptides. Am J Obstet Gynecol (1984) 150(4):421–5. doi:10.1016/S0002-9378(84)80151-9
124. Perks AM, Kindler PM, Marshall J, Woods B, Craddock M, Vonder Muhll I. Lung liquid production by in vitro lungs from fetal guinea pigs: effects of arginine vasopressin and arginine vasotocin. J Dev Physiol (1993) 19(5):203–12.
125. Cummings JJ, Carlton DP, Poulain FR, Fike CD, Keil LC, Bland RD. Vasopressin effects on lung liquid volume in fetal sheep. Pediatr Res (1995) 38(1):30–5. doi:10.1203/00006450-199507000-00006
126. Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci (2010) 30(24):8274–84. doi:10.1523/jneurosci.1594-10.2010
127. Juif PE, Poisbeau P. Neurohormonal effects of oxytocin and vasopressin receptor agonists on spinal pain processing in male rats. Pain (2013) 154(8):1449–56. doi:10.1016/j.pain.2013.05.003
128. Mavani GP, DeVita MV, Michelis MF. A review of the nonpressor and nonantidiuretic actions of the hormone vasopressin. Front Med (2015) 2:19. doi:10.3389/fmed.2015.00019
129. Iwamoto HS, Rudolph AM, Keil LC, Heymann MA. Hemodynamic responses of the sheep fetus to vasopressin infusion. Circ Res (1979) 44(3):430–6. doi:10.1161/01.RES.44.3.430
130. Altura BM, Altura BT. Actions of vasopressin, oxytocin, and synthetic analogs on vascular smooth muscle. Fed Proc (1984) 43(1):80–6.
131. Holmes CL, Patel BM, Russell JA, Walley KR. Physiology of vasopressin relevant to management of septic shock. Chest (2001) 120(3):989–1002. doi:10.1378/chest.120.3.989
132. Svensson PJ, Bergqvist PB, Juul KV, Berntorp E. Desmopressin in treatment of haematological disorders and in prevention of surgical bleeding. Blood Rev (2014) 28(3):95–102. doi:10.1016/j.blre.2014.03.001
133. Echtler K, Stark K, Lorenz M, Kerstan S, Walch A, Jennen L, et al. Platelets contribute to postnatal occlusion of the ductus arteriosus. Nat Med (2010) 16(1):75–82. doi:10.1038/nm.2060
134. Sallmon H, Weber SC, von Gise A, Koehne P, Hansmann G. Ductal closure in neonates: a developmental perspective on platelet–endothelial interactions. Blood Coagul Fibrinolysis (2011) 22(3):242–4. doi:10.1097/MBC.0b013e328344c5ed
135. Szinnai G, Morgenthaler NG, Berneis K, Struck J, Muller B, Keller U, et al. Changes in plasma copeptin, the c-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab (2007) 92(10):3973–8. doi:10.1210/jc.2007-0232
136. Bhandari SS, Loke I, Davies JE, Squire IB, Struck J, Ng LL. Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci (Lond) (2009) 116(3):257–63. doi:10.1042/cs20080140
137. Meijer E, de Jong PE, Gansevoort RT. Vasopressin and microalbuminuria: is it vasopressin per se or is it salt intake? Kidney Int (2010) 77(9):832–3. doi:10.1038/ki.2010.46
138. Schnabel RB, Wild PS, Schulz A, Zeller T, Sinning CR, Wilde S, et al. Multiple endothelial biomarkers and noninvasive vascular function in the general population: the Gutenberg Health Study. Hypertension (2012) 60(2):288–95. doi:10.1161/hypertensionaha.112.191874
139. Blanchard A, Steichen O, De Mota N, Curis E, Gauci C, Frank M, et al. An abnormal apelin/vasopressin balance may contribute to water retention in patients with the syndrome of inappropriate antidiuretic hormone (SIADH) and heart failure. J Clin Endocrinol Metab (2013) 98(5):2084–9. doi:10.1210/jc.2012-3794
140. Lupton BA, Hill A, Roland EH, Whitfield MF, Flodmark O. Brain swelling in the asphyxiated term newborn: pathogenesis and outcome. Pediatrics (1988) 82(2):139–46.
141. Lam TI, Wise PM, O’Donnell ME. Cerebral microvascular endothelial cell Na/H exchange: evidence for the presence of NHE1 and NHE2 isoforms and regulation by arginine vasopressin. Am J Physiol Cell Physiol (2009) 297(2):C278–89. doi:10.1152/ajpcell.00093.2009
142. Pearlmutter AF, Szkrybalo M, Kim Y, Harik SI. Arginine vasopressin receptors in pig cerebral microvessels, cerebral cortex and hippocampus. Neurosci Lett (1988) 87(1–2):121–6. doi:10.1016/0304-3940(88)90156-5
143. Ostrowski NL, Lolait SJ, Bradley DJ, O’Carroll AM, Brownstein MJ, Young WS III. Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinology (1992) 131(1):533–5. doi:10.1210/endo.131.1.1535312
144. Ostrowski NL, Lolait SJ, Young WS III. Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology (1994) 135(4):1511–28. doi:10.1210/endo.135.4.7925112
145. Zeynalov E, Jones SM, Seo JW, Snell LD, Elliott JP. Arginine-vasopressin receptor blocker conivaptan reduces brain edema and blood-brain barrier disruption after experimental stroke in mice. PLoS One (2015) 10(8):e0136121. doi:10.1371/journal.pone.0136121
146. Nakayama S, Amiry-Moghaddam M, Ottersen OP, Bhardwaj A. Conivaptan, a selective arginine vasopressin V and V Receptor antagonist attenuates global cerebral edema following experimental cardiac arrest via perivascular pool of aquaporin-4. Neurocrit Care (2016) 24(2):273–82. doi:10.1007/s12028-015-0236-4
147. Shuaib A, Xu Wang C, Yang T, Noor R. Effects of nonpeptide V(1) vasopressin receptor antagonist SR-49059 on infarction volume and recovery of function in a focal embolic stroke model. Stroke (2002) 33(12):3033–7. doi:10.1161/01.STR.0000039405.31526.06
148. Zhao XY, Zhang QS, Yang J, Sun FJ, Wang DX, Wang CH, et al. The role of arginine vasopressin in electroacupuncture treatment of primary sciatica in human. Neuropeptides (2015) 52:61–5. doi:10.1016/j.npep.2015.06.002
149. Dickinson LD, Betz AL. Attenuated development of ischemic brain edema in vasopressin-deficient rats. J Cereb Blood Flow Metab (1992) 12(4):681–90. doi:10.1038/jcbfm.1992.93
150. Kaplan SL, Feigin RD. Inappropriate secretion of antidiuretic hormone complicating neonatal hypoxic-ischemic encephalopathy. J Pediatr (1978) 92(3):431–3. doi:10.1016/S0022-3476(78)80437-5
151. Oh W. Renal function and fluid therapy in high risk infants. Biol Neonate (1988) 53(4):230–6. doi:10.1159/000242795
152. Kojima T, Isozaki Y, Hirata Y, Matsuzaki S, Kobayashi Y. Urinary arginine vasopressin excretion and hyponatremia in the sick neonates. Am J Perinatol (1992) 9(5–6):329–33. doi:10.1055/s-2007-999257
153. Gupta BD, Sharma P, Bagla J, Parakh M, Soni JP. Renal failure in asphyxiated neonates. Indian Pediatr (2005) 42(9):928–34.
154. Basu P, Som S, Das H, Choudhuri N. Electrolyte status in birth asphyxia. Indian J Pediatr (2010) 77(3):259–62. doi:10.1007/s12098-010-0034-0
155. Buckingham JC, Loxley HD, Christian HC, Philip JG. Activation of the HPA axis by immune insults: roles and interactions of cytokines, eicosanoids, glucocorticoids. Pharmacol Biochem Behav (1996) 54(1):285–98. doi:10.1016/0091-3057(95)02127-2
156. Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev (1999) 79(1):1–71.
157. Oosterbaan HP, Swaab DF, Boer GJ. Increased amniotic vasopressin levels in experimentally growth-retarded rat fetuses. J Dev Physiol (1985) 7(2):89–97.
158. Burkhardt T, Schwabe S, Morgenthaler NG, Natalucci G, Zimmermann R, Wellmann S. Copeptin: a marker for stress reaction in fetuses with intrauterine growth restriction. Am J Obstet Gynecol (2012) 207(6):.e491–5. doi:10.1016/j.ajog.2012.09.024
159. Benzing J, Wellmann S, Achini F, Letzner J, Burkhardt T, Beinder E, et al. Plasma copeptin in preterm infants: a highly sensitive marker of fetal and neonatal stress. J Clin Endocrinol Metab (2011) 96(6):E982–5. doi:10.1210/jc.2010-2858
160. Shivanna B, Rios D, Rossano J, Fernandes CJ, Pammi M. Vasopressin and its analogues for the treatment of refractory hypotension in neonates. Cochrane Database Syst Rev (2013) 3:Cd009171. doi:10.1002/14651858.CD009171.pub2
161. Scheurer MA, Bradley SM, Atz AM. Vasopressin to attenuate pulmonary hypertension and improve systemic blood pressure after correction of obstructed total anomalous pulmonary venous return. J Thorac Cardiovasc Surg (2005) 129(2):464–6. doi:10.1016/j.jtcvs.2004.06.043
162. Papoff P, Caresta E, Versacci P, Grossi R, Midulla F, Moretti C. The role of terlipressin in the management of severe pulmonary hypertension in congenital diaphragmatic hernia. Paediatr Anaesth (2009) 19(8):805–6. doi:10.1111/j.1460-9592.2009.03078.x
163. Bidegain M, Greenberg R, Simmons C, Dang C, Cotten CM, Smith PB. Vasopressin for refractory hypotension in extremely low birth weight infants. J Pediatr (2010) 157(3):502–4. doi:10.1016/j.jpeds.2010.04.038
Keywords: antidiuretic hormone, HPA axis, asphyxia, respiratory distress, pain, stress, cesarean section, neonate
Citation: Evers KS and Wellmann S (2016) Arginine Vasopressin and Copeptin in Perinatology. Front. Pediatr. 4:75. doi: 10.3389/fped.2016.00075
Received: 14 April 2016; Accepted: 08 July 2016;
Published: 02 August 2016
Edited by:
Offer Erez, Soroka University Medical Center, IsraelReviewed by:
Jill L. Maron, Tufts Medical Center, USACopyright: © 2016 Evers and Wellmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sven Wellmann, sven.wellmann@ukbb.ch
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.