Influences and Barriers on Physical Activity in Pediatric Oncology Patients
- Boonshoft School of Medicine, Wright State University, Dayton, OH, USA
Objectives: To determine the influence of family, peers, school, and physicians on exercise in pediatric oncology patients and evaluate the barriers to physical activity (PA) levels in this population.
Methods: A search of PubMed and Google Scholar resulted in 12 related articles. The articles were assessed for the influence of school systems, family, peers, self-efficacy, and physicians on exercise. Additionally, barriers and interventions to PA were also assessed. Limitations and research methodologies of each article were also evaluated.
Results: Many school systems were unsure of expectations in regards to PA for their returning students with cancer. Most schools acknowledged willingness to increase exercise for these students; however, there is a communication gap between the medical field and the school system on what expectations should be. Family is associated with increased PA levels and healthier diets in this population with children preferring mothers as exercise partners more than fathers. While physician interventions have been shown to positively impact PA, it has been reported that physicians are not engaging in exercise counseling with their patients.
Conclusion: Several issues and barriers related to PA in pediatric oncology population were identified. Studies have demonstrated that it is feasible to increase PA and self-efficacy in this population. Further research is needed to better understand and quantify these issues as well as further test the interventions that have been suggested in this review and have been successful in other pediatric populations.
Introduction
In 2014, it was projected that the incidence of cancer would be 10,450 in children aged 0–14 and 5,330 new cases in adolescents aged 15–19 in the United States (1). There are an estimated 379,112 survivors of childhood and adolescent cancer as of 2010 in the United States (1). With improved cancer treatments, the 5-year relative survival rates have increased substantially over the past 40 years from approximately 49% in 1975–1979 to 68.1% in 2003–2009 (2). However, 62.3% of cancer survivors suffer from at least one chronic condition, and 27.5% have a severe or life-threatening condition by 29.2 years old (3). Some of these chronic conditions include obesity (4), type 2 diabetes mellitus (5), and cardiovascular disease (6). Physical inactivity is a risk factor for these chronic diseases. The American Cancer Society Institute recommends children engage in moderate to vigorous exercise such as running, aerobics, or heavy yard work for at least 60 min 5 days a week (7). Childhood cancer survivor (CCS) patients aged 9–18 that had a high rate of physical activity (PA) have been shown to have lower percent body fat mass, subcutaneous fat, abdominal visceral fat, and greater lean body mass compared with CCS patients that engaged in low PA (8). Götte et al. discovered that diagnosed pediatric patients undergoing cancer treatment in Germany self-reported less PA compared to before treatment began (9). This was especially apparent in patients staying in the hospital. Additionally, 60–70% of CCSs were found to be physically inactive when compared with their siblings or healthy controls (10). Thus, increasing PA in this population is critical.
Many cancer patients do not meet exercise requirements and suffer from comorbidities later in life that exercise helps to combat. Since this population is at risk for low exercise rates, it is imperative to understand the perspective and influence that family, friends, physicians, and educators have toward exercise and overcoming identified barriers. By understanding these influences, interventions can be incorporated to promote exercise in pediatric oncology patients.
Physical activity programs implemented in school systems have been reported to increase PA and overall fitness in children (11, 12). Since children spend a third of their day in school, it is important that the school system has knowledge of what to expect from these students. Additionally, school children with parental support engaged in more moderate–vigorous exercise when compared with their controls, highlighting the importance of having parental involvement in this process (13). Another influence on pediatric oncology patient PA is their physician. Physician advice and encouragement have been shown to bring about modifications in behavior and increased PA when compared to control groups (14).
The objective of this review is to evaluate the literature regarding current barriers to exercise identified by pediatric oncology patients. We also set out to ascertain, through thorough literature review, the various influences on this population including peer, parental, health-care, and the school administration and how these influences impact PA levels. Additionally, we will provide several ideas for how to overcome these barriers.
Materials and Methods
For this review, PubMed and Google Scholar databases were searched using the following terms in various combinations: PA, exercise, motivation, influences, school, parent perspective, physician, pediatric cancer, and childhood cancer. Due to the limited literature on these subjects, inclusion criteria were broad. Only studies involving children under 18 years of age were included for review. Reference lists included in reviewed studies were used to obtain other relevant studies. The articles were assessed for the influence of school systems, family, peers, self-efficacy, and physicians on exercise. Barriers and interventions to PA were also examined. Additionally, the studies were evaluated for the methodologies used for data collection and limitations. Finally, additional intervention programs utilized in other pediatric populations were also included as suggestions that could be potentially beneficial in the pediatric population.
Results
Influences on Physical Exercise
Assessment Tools
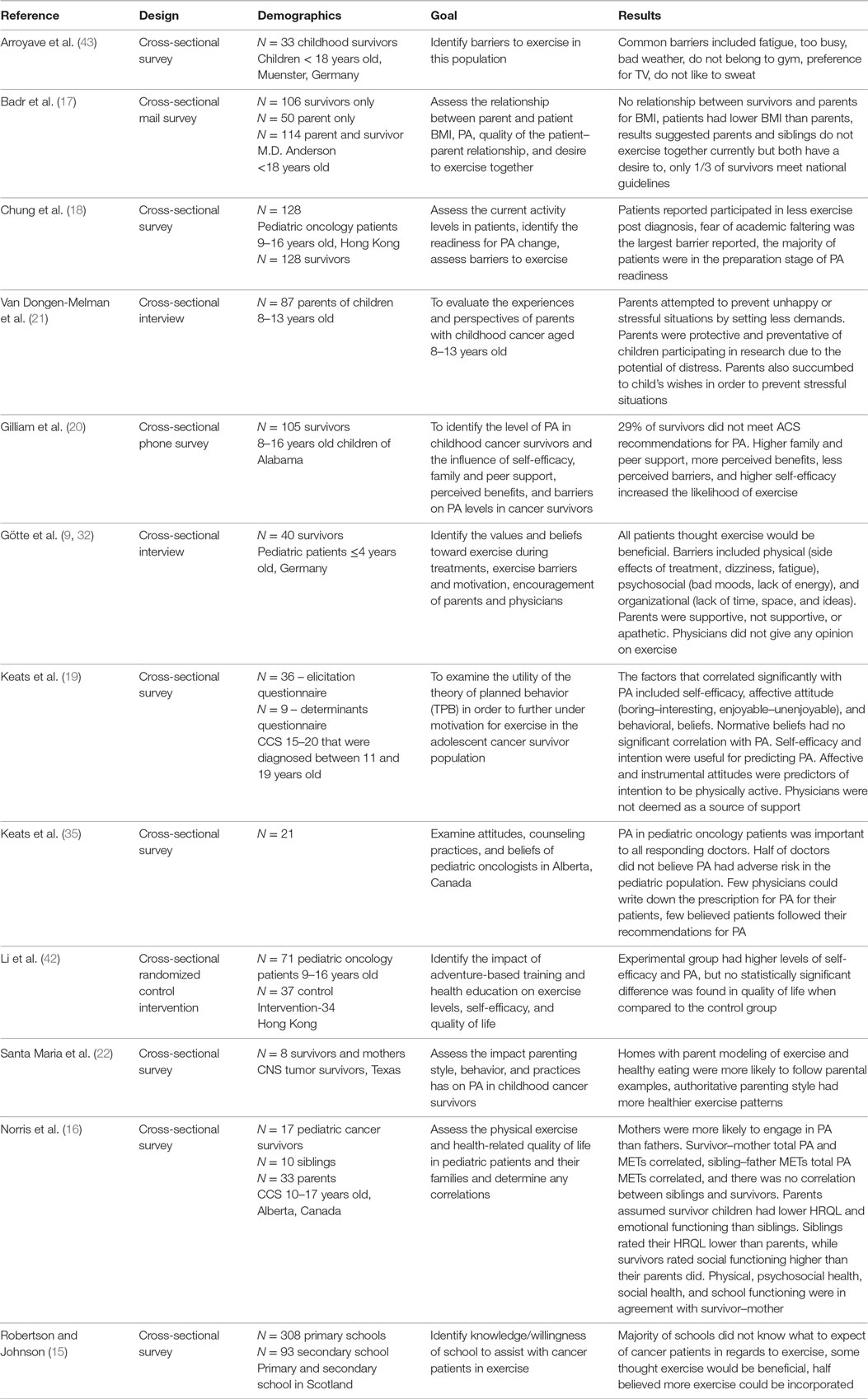
A summary of all of the literature reviewed can be found in Table A1. Robertson and Johnson (15) mailed a questionnaire to all Local Education Authority regions (LEAs) in Scotland. Example questions include: “For a pupil who has returned to school during/after treatment for cancer how much would you expect that pupil to participate in PA (PE)?,” and “If extra PE were prescribed for a pupil returning to school during or after successful treatment for cancer, e.g. an additional 25 minutes of PE per day, could your school accommodate?” Several studies implemented the use of cross-sectional self-reported surveys (16–19). The Godwin Leisure Time Exercise Questionnaire was used by several studies to evaluate the PA levels in pediatric oncology patients (16, 17, 20). The Pediatric Quality of Life Inventory Version 4.0 was used to measure the health-related quality of life (HRQL) (16). PA metabolic equivalent (METs) hours per week were assessed by measuring oxygen consumption. To determine if respondents were receptive to diet and PA interventions, the questions “How interested are you in learning about weight control?” and “Would you want others to join in the intervention with you-if so, who?” were utilized (17). To assess the quality of the caregiver–recipient relationship, a Likert-type scale adapted from Lawrence Cross-sectional interviews was utilized in several studies (20–22). A Likert-like scale was used to assess family support for PA, peer support, perceived benefits to exercise, perceived barriers to exercise, and self-efficacy in regards to PA (20). The theory of planned behavior (TPB), which evaluates attitudes to gage the readiness for an individual to engage in a specific behavior, was utilized to further elicit motivation for exercise (19). Parenting practices, style, and behavior were analyzed through the implementation of an interview (22). The experiences and perspectives of parents were assessed through the use of an interview (21). The PA Stages of Change Questionnaire was implemented to gage the readiness of participants to alter PA behavior (18). This model groups individuals into five stages including the following: (1) precontemplation, where the individual does not engage in exercise and does not intend in changing within the next 6 months, (2) contemplation, where the individual does not exercise but is considering starting within the next 6 months, (3) preparation, where the individual currently engages in PA but not regularly, (4) action, where the individual exercise regularly but has begun so only within the last 6 months, and (5) maintenance, where the individual currently exercises regularly and has done so for more than 6 months. A previously used and validated self-reported survey from Abrahamson et al. was used to determine the beliefs, attitudes, and exercise counseling practices of pediatric oncologists in Alberta.
School Systems’ Perspective and Influence on Exercise
Only one study has been reported, which evaluated academic expectations, past experiences, and physical expectations of pediatric oncology students returning to school in Scotland. Over half (n = 224) of LEAs responded to the survey. Additionally, only 20% of schools that responded to the survey reported they had experience with one or more cancer patient. LEAs did not anticipate increased PE for cancer patients returning to school. The majority of LEAs were uncertain of what to expect in regards to the PA of the child and believed there would be medical guidance provided for each child upon school reentry. The sources of information reported by the schools about education on the child included parents, hospital nurses, and students in descending order of commonality. Almost all schools were willing to increase PA if prescribed for these patients, preferably substituting PA for other lessons or at the start or end of the day. The largest barrier to overcome to accommodate increased PE was supervision difficulties. Other challenges reported after these children returned to school included: behavior issues, extra emotional support needed for the child and staff interacting with the child, and other student’s being “overly protective” upon the return of their peer. Primary schools were unsure how exercise would impact academic progress; in contrast, secondary schools thought increased PE would increase academic performance. Both primary and secondary schools believed increased PE would enhance social development of the returning pupil.
While this study had a substantially large sample size and decreased bias by sampling the entire population in Scotland, the questionnaire was sent via mail. Problems that may have arisen from this method of distribution include: the lack of ability for participants to ask questions if parts of the survey were ambiguous/not understood and that the survey was not piloted prior to the start of the study. Additionally, this article is 13 years old, which may limit the generalizability to current populations due to the vast increase in technology that now makes access to education on any topic readily available. Moreover, a further limitation to this study is the location and the school systems studied being in Scotland, which may not be generalizable to the United States School systems. Also, this study did not identify the role of the individual completing the survey; therefore, different administrative roles may have more or less knowledge on exercise guidelines in pediatric oncology patients.
In summary, this one study demonstrated a self-reported willingness on the part of schools to accommodate pediatric oncology patients returning to school; however, as reported by the majority of schools, there is a vast educational gap on what should be expected and implemented for these children in regards to PA and how PA will impact students’ academic performance. Previously used school-based educational interventions may help to overcome many of these gaps/barriers. For example, it was shown that implementing an after-school exercise program in third grade students without cancer was not only feasible but also resulted in increased physical fitness and body composition (23). Additionally, schools implementing PA time during school have been reported to increase PA, especially when parents were involved (13). Both primary and secondary schools reported increased PE modifications are feasible and can be implemented. To encourage the implementation of these interventions, more communication is needed between the medical team and school system. This may be done through the implementation of reentry school programs that have been previously reported to be successful (24). While schools were concerned how PA would impact academic performance, several studies have revealed that exercise does not impair academic performance (25) but rather promotes performance, concentration, memory, and classroom behavior (26). Further research should investigate intervention programs in schools that not only increase PA but also educate school administration on physical and academic expectations in these students.
Family Influence
Family has been reported to be an influence on PA behavior for a number of years (27). Norris et al. (16) reported survivor total PA and mother total PA metabolic equivalents (PA METs) were significantly correlated as were father–sibling total PA METs; however, sibling–survivor PA did not have a significant correlation. While exercise rates were not different between siblings, cancer patients exercised less intensely when compared to siblings, findings that were similar to other reports (10). Interestingly, parents typically rated their cancer-surviving child lower for HRQL than the child rated himself or herself in areas of school functioning and emotional functioning. Siblings rated their HRQL lower than their parents’ proxy rating. This indicates a gap between perspectives of children and parents. This gap may explain why parents of cancer survivors often are less demanding when it comes to scholastics and other situations to avoid upsetting their child with cancer (21). Substantiating the contrasting HRQL rating between parents and children, parents HRQL corresponded with PA levels: CCSs were rated as having a lower HRQL and had lower PA (16).
Another way parents can influence their children is by engaging in physical exercise with them, behavior modeling, and assuming more authoritative parenting styles (22). A study by Santa Maria and colleagues reported that children displayed better exercise habits if their parents exhibited greater authoritative styles. Additionally, parents who exercised with their children, thus implementing good parent modeling, had children that were more likely to engage in exercise. Patients reported it was more difficult to exercise and maintain a healthy diet and weight when their parents did not model these behaviors and felt it was hypocritical (22). Some mothers reported being protective and did not want their child to obsess over losing weight due to everything else they had recently been subjected to. Finally, children reported it was difficult to exercise with the lack of a supportive home environment. Limitations of this study include the fact that it was completed only in central nervous system (CNS) tumor survivors, which may not be representative of families of children with other cancer types.
It has been reported for a number of years that exercise partners can increase the engagement in PA (28). Additionally, parental exercise engagement has been associated with child exercise engagement (29). Badr et al. (17) evaluated this correlation by asking parents and children “would you want others to join in the intervention, if so, who?” (17). They found that mothers were both more likely to exercise and were reported by their child with cancer to be more preferred (45.9%) as exercise partners when compared to fathers (30.2%). Some limitations of this study include the fact that 80% of participants were mothers and 72% of respondents were white. Therefore, this may not be a complete representation of the populations. Additionally, participants were financially compensated for their time, which may have also affected the population of respondents. Gilliam et al. also found that family support had both a direct and indirect effect on moderate and vigorous PA engagement (20). They reported that the younger the child was at diagnosis, the more family support they received. Given the support of this influence, interventions, such as those developed in Cousins et al., can be implemented to increase PA in this population. In this study, families attended a class and obtained educational material about exercise and diet. Participants that attended the class as a family had the highest reduction in weight-loss compared to those who just received information and did not have the class. The importance of cultural engagement was also reported in this study (30).
Social and Peer Support
In addition to school being an important means to an education and PA, social and peer support can also influence PA in children. As mentioned in Badr et al. exercise partners and socialization can impact PA levels (17). In Gilliam et al. peer support was analyzed and found to have a positive association with PA and self-efficacy (20). Friends were also identified as a critical part of support in Keats et al. (19). A limitation of this study was the lack of assessment on how much the childhood cancer patients’ peers were exercising in total. Furthermore, this study was only performed in one hospital and thus is not representative of the entire population.
A recent protocol was published in order to educate schools and peers about a child with cancer’s condition and expectations with PA (31). It requires peers of the child with cancer to act as ambassadors for the child to improve PA. This study would not only encourage social interaction but also would enhance the school’s understanding of what to expect and allow administration to develop proper PA interventions for these students.
Physician Influence
Many pediatric oncology patients have reported that doctors do not engage in educating pediatric oncology patients and families about exercise (19, 32). Unfortunately, this is a recurring theme nationwide, even in patients who are healthy or who have other chronic illnesses (33, 34). Keats et al. in a study of 21 pediatric oncology physicians in Alberta, Canada, found that of physicians that responded (n = 11) all believed exercise to be important for their patients (35). Fifty percent believed there was no adverse risk with PA. Most physicians reported they counseled their patients on exercise but did not believe their patients followed the recommendations. Physicians reported very few patients inquired about exercise recommendations. The major barriers to PA counseling for physicians were lack of knowledge/resources and lack of time. The time spent on PA counseling was 1–5 min by 73% of responding physicians. All physicians said they verbally counsel patients, while few offered written materials or would refer to a specialist. Only one physician provided details for his or her recommendation of the frequency, intensity, time, and type of PA. A limitation of this study is the small sample size and the lack of generalizability to the United States. However, this study either suggests a knowledge gap in recommendations for pediatric oncology patients or suggests physicians prescribe different recommendations to various patients, which should be elucidated in further studies. The only current guidelines available are those by the Children’s Oncology Group for pediatric cancer survivors for healthy living and the American Cancer Society’s Guidelines to for PA to prevent cancer (36). Future studies should address the knowledge physicians have of these current recommendations and guidelines should be developed as well as resources for patients and their families.
Götte et al. reported that pediatric oncology patients and their families value their doctors’ opinions (32). Several physician intervention programs in healthy children have been shown to be successful in increasing PA in patients (14, 37). By adding just 3–5 min of exercise counseling with the physician and having a health educator call for a few minute “booster” 2 weeks later, exercise was shown to improve by 37 min per week in patients without cancer that received this counseling intervention (14). This is an intervention that pediatric oncologists, with the help of their medical team, could implement in order to increase the PA of their patients. Few studies have evaluated the pediatric hematologist/oncologist perspective on exercise in this population. No studies have assessed physician knowledge of the current PA guidelines as stated by the American Cancer Society. Additionally, few studies have been performed evaluating the pediatric hematologists’/oncologists’ communication about PA with patients and families. Thus, more research should be completed in this area so physicians can better counsel their patients on PA.
Self-Efficacy and Attitudes Influence
Self-efficacy – the confidence an individual has in his or her abilities to successfully complete a task – has also been reported to be positively associated with exercise in many different age-groups (38). In the study by Gilliam et al. higher self-efficacy increased the likelihood of engaging in moderate to vigorous PA (20). One intervention that could improve self-efficacy and PA is the Lifestyle and Education for Activity Program (LEAP). Dishman et al. (39) reported that self-efficacy was promoted with the LEAP program that was implemented in adolescent girls without cancer. This increased self-efficacy, in addition to the program itself, increased PA (39). The LEAP program was a 2-year intervention that increased PA in schools and was supported by the entire school system. Implementations like this would not only benefit children with cancer but also all children attending school. Another intervention that could be implemented in pediatric oncology patients is exercise goal-setting, which mediates the relationship between self-efficacy and PA (40).
In addition to self-efficacy, attitudes also impact engagement in PA (41). The TPB is one way in which attitudes or intentions are analyzed. It states that “attitudes toward the behavior, subjective norm, and perceived behavior control can gage the readiness of an individual to engage in that behavior.” In this study, Keats et al. assessed various beliefs and attitudes to find factors that correlated significantly with PA. Significant correlations included: self-efficacy, affective attitude (boring–interesting, enjoyable–unenjoyable), and behavioral beliefs (i.e., staying fit and looking good). There was no correlation between normative beliefs – the impact of the beliefs of those around the participant have on the participant’s behavior – and PA. This study was completed in Canada, which may not be generalizable to the United States. Additionally, patients were asked about their parent’s attitude on PA to determine how that impacted the child’s attitude toward PA; however, it was not assessed whether or not patients ever actually had conversations about these topics with their parents. Another limitation is the data from all of the questions were not reported. Some items in normative beliefs may have skewed the data to being less significant for various individuals (i.e., a parent’s belief may have more influence than a coach on a child). When PA readiness was assessed in pediatric oncology patients, it was found that the majority of participants were in the preparation stage and many others were in either the precontemplation or contemplation stage (18). In order to increase the engagement in PA, self-efficacy, attitudes, and readiness for PA need to be maximized in this population.
Barriers to PA and Interventions to Resolve Them
Assessment Tools
Arroyave et al. implemented the use of a cross-sectional questionnaire with the implementation of the Likert-scale and open-ended questions to elicit barriers to exercise. Götte et al. implemented interviews to assess barriers to exercise qualitatively. Self-confidence was measured using PA self-efficacy, which asks the participants’ level of sureness on a variety of tasks (42). Quality of life was assessed using the Pediatric Quality of Life Inventory.
Barriers
Barriers to exercise are another reason this population often does not engage in recommended levels of PA. The interviews conducted by Götte et al. in pediatric cancer survivors reported that the average patient only engaged in 24.3 min of exercise with the majority occurring in the cancer ward. Barriers included physical aspects such as: fatigue (15, 18, 43), concern for increased risk of infection (18, 32), side effects of treatment, GI problems, pain, dizziness, and weakness (32). Many patients associated these barriers with chemotherapy. Some of these barriers, such as fatigue, may be improved by exercise (44). Psychological barriers included: lack of energy, bad moods/not feeling like it, preference for other things like staying in bed (32, 43), concern for injury, fear of soreness and sweating (43), falling behind academically (15, 18), and preference for sleeping to not feel side effects (32). Organizational barriers included: lack of time, lack of sports equipment, bad weather, lack of ideas (32, 43), lack of space for physical activities (especially when on infusion), and no one to play with (32). A limitation of this study was the small sample size.
One suggestion by patients to resolve some of these issues was to have access to an all-day sports therapist (32). Additionally, boredom and lack of ideas for PA can be resolved with the implementation of interventions such as those used in the LEAP program, which has been reported to increase enjoyment of physical exercise (45). A recent study showed that supervised-, targeted-exercise interventions increased PA more than encouraging participants to continue with exercise regimes at home suggesting that physical therapy-targeted interventions may be more efficacious than home-based programs (31). Additionally, an adventure-based training program and health education program has also been shown to increase PA and self-efficacy in this population, which may prevent several barriers identified such as boredom and lack of sports equipment. Future studies should address how different interventions can overcome these identified barriers.
Conclusion
In conclusion, this review identified a number of issues related to appropriate PA for pediatric oncology patients. Barriers to exercise identified were psychological, organizational, and physical. Additionally, schools and educators do not demonstrate experience with CCSs and do not know what to expect from patients in regards to exercise. Family support is associated with both increased self-efficacy and increased activity levels with child preference for mothers as exercise partners. In other populations, family has been shown to be the most important social factor affecting weight-loss and exercise. While patients and families report to value their physicians’ opinion in regards to PA, it has been reported that physicians are not providing exercise counseling to their patients and their patients’ families. There have been no new updated studies on the influences of pediatric cancer or exercise counseling by physicians; thus, future research is needed to ascertain if pediatric oncologists are participating in exercise counseling and how interventions can be developed to overcome barriers.
Author Contributions
LY completed and synthesized the literature search and drafted the manuscript. SF contributed to the manuscript and reviewed, critiqued, and revised the manuscript.
Conflict of Interest Statement
This manuscript was not funded by any organization. None of the authors have any financial interests invested in this project. The authors declare no conflicts of interest.
Acknowledgments
Jeffrey Travers, MD, PhD, aided in revising this manuscript and was a great mentor of this project.
References
1. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin (2014) 64:83–103. doi:10.3322/caac.21219
2. Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute (2012). Available from: http://seer.cancer.gov/csr/1975_2012/
3. Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med (2006) 355:1572–82. doi:10.1056/NEJMsa060185
4. Veringa SJ, van Dulmen-den Broeder E, Kaspers GJ, Veening MA. Blood pressure and body composition in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer (2012) 58:278–82. doi:10.1002/pbc.23251
5. Meacham LR, Sklar CA, Li S, Liu Q, Gimpel N, Yasui Y, et al. Diabetes mellitus in long-term survivors of childhood cancer: increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med (2009) 169:1381–8. doi:10.1001/archinternmed.2009.209
6. Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ (2009) 339:b4606. doi:10.1136/bmj.b4606
7. Children’s Oncology Group. Long-Term Follow-up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 4.0. Monrovia, CA: Children’s Oncology Group (2013). Available from: www.survivorshipguidelines.org
8. Slater ME, Ross JA, Kelly AS, Dengel DR, Hodges JS, Sinaiko AR, et al. Physical activity and cardiovascular risk factors in childhood cancer survivors. Pediatr Blood Cancer (2015) 62:305–10. doi:10.1002/pbc.25276
9. Götte M, Kesting S, Winter C, Rosenbaum D, Boos J. Comparison of self-reported physical activity in children and adolescents before and during cancer treatment. Pediatr Blood Cancer (2014) 61:1023–8. doi:10.1002/pbc.24898
10. Zhang FF, Saltzman E, Must A, Parsons SK. Do childhood cancer survivors meet the diet and physical activity guidelines? A review of guidelines and literature. Int J Child Health Nutr (2012) 1:44–58. doi:10.6000/1929-4247.2012.01.01.06
11. Kriemler S, Zahner L, Schindler C, Meyer U, Hartmann T, Hebestreit H, et al. Effect of school based physical activity programme (KISS) on fitness and adiposity in primary schoolchildren: cluster randomised controlled trial. BMJ (2010) 340:c785. doi:10.1136/bmj.c785
12. Sallis JF, McKenzie TL, Alcaraz JE, Kolody B, Faucette N, Hovell MF. The effects of a 2-year physical education program (SPARK) on physical activity and fitness in elementary school students. Sports, play and active recreation for kids. Am J Public Health (1997) 87:1328–34. doi:10.2105/AJPH.87.8.1328
13. Haerens L, De Bourdeaudhuij I, Maes L, Cardon G, Deforche B. School-based randomized controlled trial of a physical activity intervention among adolescents. J Adolesc Health (2007) 40:258–65. doi:10.1016/j.jadohealth.2006.09.028
14. Calfas KJ, Long BJ, Sallis JF, Wooten WJ, Pratt M, Patrick K. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med (1996) 25:225–33. doi:10.1006/pmed.1996.0050
15. Robertson AR, Johnson DA. Rehabilitation and development after childhood cancer: can the need for physical exercise be met? Dev Neurorehabil (2002) 5:235–40. doi:10.1080/1363849031000094072
16. Norris JM, Moules NJ, Pelletier G, Culos-Reed SN. Families of young pediatric cancer survivors: a cross-sectional survey examining physical activity behavior and health-related quality of life. J Pediatr Oncol Nurs (2010) 27:196–208. doi:10.1177/1043454209358411
17. Badr H, Paxton RJ, Ater JL, Urbauer D, Demark-Wahnefried W. Health behaviors and weight status of childhood cancer survivors and their parents: similarities and opportunities for joint interventions. J Am Diet Assoc (2011) 111:1917–23. doi:10.1016/j.jada.2011.09.004
18. Chung OK, Li HC, Chiu SY, Ho KY, Lopez V. The impact of cancer and its treatment on physical activity levels and behavior in Hong Kong Chinese childhood cancer survivors. Cancer Nurs (2014) 37:E43–51. doi:10.1097/NCC.0b013e3182980255
19. Keats MR, Culos-Reed SN, Courneya KS, McBride M. Understanding physical activity in adolescent cancer survivors: an application of the theory of planned behavior. Psychooncology (2007) 16:448–57. doi:10.1002/pon.1075
20. Gilliam MB, Madan-Swain A, Whelan K, Tucker DC, Demark-Wahnefried W, Schwebel DC. Cognitive influences as mediators of family and peer support for pediatric cancer survivors’ physical activity. Psychooncology (2013) 22:1361–8. doi:10.1002/pon.3140
21. Van Dongen-Melman J, Van Zuuren F, Verhulst F. Experiences of parents of childhood cancer survivors: a qualitative analysis. Patient Educ Couns (1998) 34:185–200. doi:10.1016/S0738-3991(98)00031-7
22. Santa Maria D, Swartz MC, Markham C, Chandra J, McCurdy S, Basen-Engquist K. Exploring parental factors related to weight management in survivors of childhood central nervous system tumors. J Pediatr Oncol Nurs (2014) 31:84–94. doi:10.1177/1043454213518112
23. Gutin B, Yin Z, Johnson M, Barbeau P. Preliminary findings of the effect of a 3-year after-school physical activity intervention on fitness and body fat: the Medical College of Georgia Fitkid Project. Int J Pediatr Obes (2008) 3:3–9. doi:10.1080/17477160801896457
24. Canter KS, Roberts MC. A systematic and quantitative review of interventions to facilitate school reentry for children with chronic health conditions. J Pediatr Psychol (2012) 37:1065–75. doi:10.1093/jpepsy/jss071
25. Ahamed Y, Macdonald H, Reed K, Naylor P, Liu-Ambrose T, Mckay H. School-based physical activity does not compromise children’s academic performance. Med Sci Sports Exerc (2007) 39:371. doi:10.1249/01.mss.0000241654.45500.8e
26. Trudeau F, Shephard RJ. Physical education, school physical activity, school sports and academic performance. Int J Behav Nutr Phys Act (2008) 5:10. doi:10.1186/1479-5868-5-10
27. DiLorenzo TM, Stucky-Ropp RC, Vander Wal JS, Gotham HJ. Determinants of exercise among children. II. A longitudinal analysis. Prev Med (1998) 27:470–7. doi:10.1006/pmed.1998.0307
28. Dishman RK, Sallis JF, Orenstein DR. The determinants of physical activity and exercise. Public Health Rep (1985) 100:158–71.
29. Shropshire J, Carroll B. Family variables and children’s physical activity: influence of parental exercise and socio-economic status. Sport Educ Soc (1997) 2:95–116. doi:10.1080/1357332970020106
30. Cousins JH, Rubovits DS, Dunn JK, Reeves RS, Ramirez AG, Foreyt JP. Family versus individually oriented intervention for weight loss in Mexican American women. Public Health Rep (1992) 107:549–55.
31. Thorsteinsson T, Helms AS, Adamsen L, Andersen LB, Andersen KV, Christensen KB, et al. Study protocol: rehabilitation including social and physical activity and education in children and teenagers with cancer (RESPECT). BMC Cancer (2013) 13:544. doi:10.1186/1471-2407-13-544
32. Götte M, Kesting S, Winter C, Rosenbaum D, Boos J. Experience of barriers and motivations for physical activities and exercise during treatment of pediatric patients with cancer. Pediatr Blood Cancer (2014) 61:1632–7. doi:10.1002/pbc.25071
33. Anis NA, Lee RE, Ellerbeck EF, Nazir N, Greiner KA, Ahluwalia JS. Direct observation of physician counseling on dietary habits and exercise: patient, physician, and office correlates. Prev Med (2004) 38:198–202. doi:10.1016/j.ypmed.2003.09.046
34. Wee CC, McCarthy EP, Davis RB, Phillips RS. Physician counseling about exercise. JAMA (1999) 282:1583–8. doi:10.1001/jama.282.16.1583
35. Keats MR, Culos-Reed SN, Courneya KS. An examination of the beliefs, attitudes and counselling practices of paediatric oncologists toward physical activity: a provincial survey. Paediatr Child Health (2007) 12:289–93.
36. Vina CC, Wurz AJ, Culos-Reed SN. Promoting physical activity in pediatric oncology. Where do we go from here? Front Oncol (2013) 3:173. doi:10.3389/fonc.2013.00173
37. Goldstein MG, Pinto BM, Marcus BH, Lynn H, Jette AM, Rakowski W, et al. Physician-based physical activity counseling for middle-aged and older adults: a randomized trial. Ann Behav Med (1999) 21:40–7. doi:10.1007/BF02895032
38. McAuley E, Blissmer B. Self-efficacy determinants and consequences of physical activity. Exerc Sport Sci Rev (2000) 28:85–8.
39. Dishman RK, Motl RW, Saunders R, Felton G, Ward DS, Dowda M, et al. Self-efficacy partially mediates the effect of a school-based physical-activity intervention among adolescent girls. Prev Med (2004) 38:628–36. doi:10.1016/j.ypmed.2003.12.007
40. Dishman RK, Saunders RP, Felton G, Ward DS, Dowda M, Pate RR. Goals and intentions mediate efficacy beliefs and declining physical activity in high school girls. Am J Prev Med (2006) 31:475–83. doi:10.1016/j.amepre.2006.08.002
41. Hagger MS, Chatzisarantis N, Barkoukis V, Wang JC, Hein V, Pihu M, et al. Cross-cultural generalizability of the theory of planned behavior among young people in a physical activity context. J Sport Exerc Psychol (2007) 29:2–20. doi:10.1123/jsep.29.1.2
42. Li H, Chung OKJ, Ho KY, Chiu SY, Lopez V. Effectiveness of an integrated adventure-based training and health education program in promoting regular physical activity among childhood cancer survivors. Psychooncology (2013) 22:2601–10. doi:10.1002/pon.3326
43. Arroyave WD, Clipp EC, Miller PE, Jones LW, Ward DS, Bonner MJ, et al. Childhood cancer survivors’ perceived barriers to improving exercise and dietary behaviors. Oncol Nurs Forum (2008) 35:121–30. doi:10.1188/08.ONF.121-130
44. Blaauwbroek R, Bouma MJ, Tuinier W, Groenier KH, de Greef MH, Meyboom-de Jong B, et al. The effect of exercise counselling with feedback from a pedometer on fatigue in adult survivors of childhood cancer: a pilot study. Support Care Cancer (2009) 17:1041–8. doi:10.1007/s00520-008-0533-y
45. Dishman RK, Motl RW, Saunders R, Felton G, Ward DS, Dowda M, et al. Enjoyment mediates effects of a school-based physical-activity intervention. Med Sci Sports Exerc (2005) 37:478–87. doi:10.1249/01.MSS.0000155391.62733.A7
Appendix
Keywords: childhood cancer, pediatric oncology, exercise, influence, perspective, intervention, physical activity
Citation: Yelton L and Forbis S (2016) Influences and Barriers on Physical Activity in Pediatric Oncology Patients. Front. Pediatr. 4:131. doi: 10.3389/fped.2016.00131
Received: 23 August 2016; Accepted: 21 November 2016;
Published: 19 December 2016
Edited by:
Peter Bader, University Hospital Frankfurt, GermanyReviewed by:
Hema Dave, Children’s National Medical Center, USARachel Rau, Baylor College of Medicine, USA
Copyright: © 2016 Yelton and Forbis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Larrilyn Yelton, yelton.3@wright.edu
 Larrilyn Yelton
Larrilyn Yelton Shalini Forbis
Shalini Forbis