Factors Influencing Neurodevelopment after Cardiac Surgery during Infancy
- Pediatric Cardiology, University Hospital RWTH Aachen, Aachen, Germany
Short- and long-term neurodevelopmental (ND) disabilities with negative impact on psychosocial and academic performance, quality of life, and independence in adulthood are known to be the most common sequelae for surviving children after surgery for congenital heart disease (CHD). This article reviews influences and risk factors for ND impairment. For a long time, the search for independent risk factors was focused on the perioperative period and modalities of cardiopulmonary bypass (CPB). CPB operations to ensure intraoperative vital organ perfusion and oxygen supply with or without circulatory arrest or regional cerebral perfusion bear specific risks. Examples of such risks are embolization, deep hypothermia, flow rate, hemodilution, blood gas management, postoperative hyperthermia, systemic inflammatory response, and capillary leak syndrome. However, influences of these procedure-specific risk factors on ND outcome have not been found as strong as expected. Furthermore, modifications have not been found to support the effectiveness of the currently used neuroprotective strategies. Postoperative factors, such as need for extracorporal membrane oxygenation or assist device support and duration of hospital stay, significantly influence ND parameters. On the other hand, the so-called “innate,” less modifiable patient-specific risk factors have been found to exert significant influences on ND outcomes. Examples are type and severity of CHD, genetic or syndromic abnormalities, as well as prematurity and low birth weight. Structural and hemodynamic characteristics of different CHDs are assumed to result in impaired brain growth and delayed maturation with respect to the white matter. Beginning in the fetal period, this so-called “encephalopathy of CHD” is suggested a major innate risk factor for pre-, peri-, and postoperative additional hypoxic or ischemic brain injury and subsequent ND impairment. Furthermore, MRI studies on brain volume, structure, and function in adolescents have been found correlated with cognitive, motor, and executive dysfunctions. Finally, family and environmental factors independently moderate against ND outcomes. In conclusion, the different mediating factors may exert independent effects on ND and interactive influences. Implications for the future comprise modifying clinical risk factors, such as perioperative cerebral oxygen delivery, conducting brain MRI studies in correlation to ND outcomes, and extending psychosocial interventions leading to adequate resilience.
Introduction
The prevalence of congenital heart disease (CHD) is about 1 in every 100 live births. About one-third of CHD cases is in critical need of surgical intervention in neonatal or infant age (1). Since the 1980s, advanced diagnostic technologies, neonatal cardiopulmonary bypass (CPB) operations enabling early correction of complex congenital heart defects, and improved postoperative care have markedly increased life expectancy: today more than 90% of CHD patients survive into adulthood. At the same time, these patients are at remarkable risk of short- and long-term neurodevelopmental (ND) impairment. This can have negative impact on psychosocial and academic performance, quality of life, and independence in adulthood (2–6).
The present article discusses causes and factors mediating risk for ND disabilities from neonatal to adolescent age in CHD patients after cardiac surgery during infancy.
Influencing Factors on Neurodevelopment: Innate and Modifiable Parameters
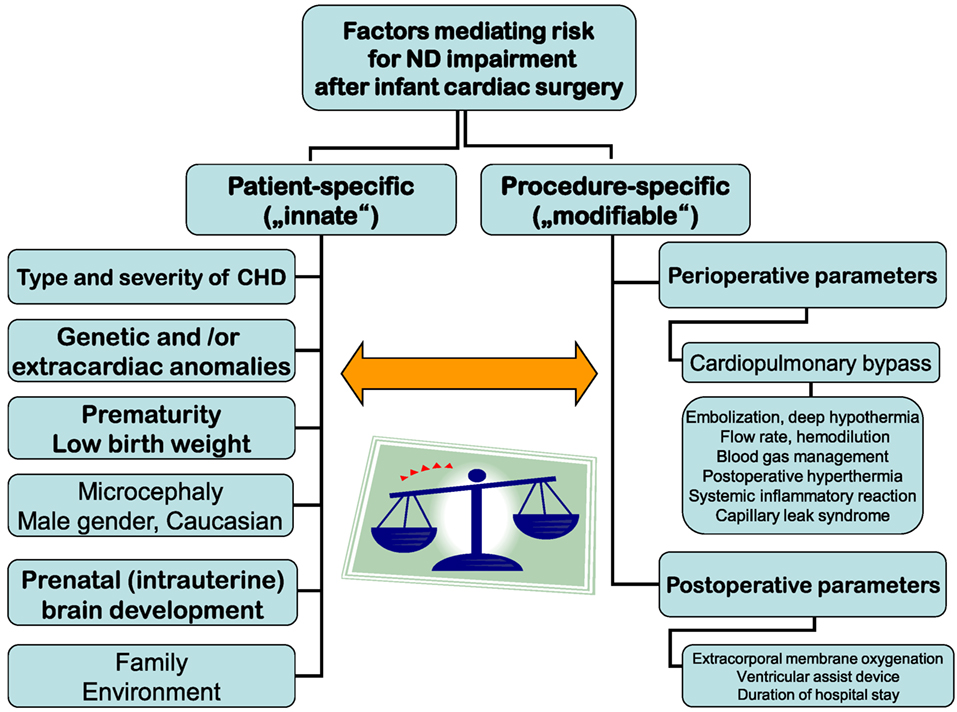
Risk factors for brain injury and consecutive ND disabilities in infants, children, and adolescents with CHD after cardiac surgery in infancy may exert independent, cumulative, and synergistic influences. They comprise patient-specific (mostly innate and not modifiable) and procedure-specific (in part modifiable) parameters (Figure 1).
For a long time, the search for independent factors mediating risk for ND impairment has focused on the perioperative period and modalities of CPB. CPB operations to ensure intraoperative vital organ perfusion and oxygen supply with or without circulatory arrest or regional cerebral perfusion bear specific risks. Examples for such risks are embolization, deep hypothermia, flow rate, hemodilution, blood gas management, postoperative hyperthermia, systemic inflammatory response, and capillary leak syndrome (7–11). Though numerous modifications of these factors have been performed over time, ND outcomes have not improved accordingly (12, 13). In a recent analysis of >1,700 CHD patients from across the world who were born between 1996 and 2009 and had cardiac surgery at age <9 months, only modest improvements in the significantly reduced ND outcomes (psychomotor developmental index—PDI and mental developmental index—MDI) of the Bayley Scales of Infant Development-II at a mean age 14 months have been observed (14). Moreover, CPB management factors explained only about 1% of test results’ variance. Longer support time was hypothesized to be a surrogate for operative complexity. Postoperative parameters like need for extracorporal membrane oxygenation or ventricular assist device support and longer postoperative length of hospital stay were associated with lower ND results. In total, measured intraoperative and postoperative factors accounted for 5% of the variances in PDI and MDI (15).
There is a strong evidence that less modifiable innate patient characteristics and socioeconomic environmental factors have an important impact on ND outcomes (14, 16–19). In addition to type and severity of CHD, prematurity, lower birth weight, white race, and genetic or extracardiac anomalies have been assessed as predicting lower PDI. Lower birth weight, male gender, less maternal education, and genetic or extracardiac anomalies were independent risk factors for lower MDI (14). In general, genetic disorders are found in about 30% of patients with CHD. This includes chromosomal disorders, microdeletions, or mutations. However, only about one-third of the variance in ND outcomes early after cardiac surgery in infancy can be explained by the known innate patient and preoperative risk factors. Further genetic and epigenetic factors (changes in proteins affecting gene regulation) (20) or genetic polymorphisms such as the apolipoprotein E affecting the resilience capability of the brain (18) may exert influences on ND outcomes.
In addition, psychosocial factors comprising family and environmental parameters moderate against, or augment adverse ND behavioral and school outcomes. Family factors comprise parenting style such as overprotection, maternal mental health, and worry. They are able to exert significant influence on cognitive outcomes. Socioeconomic status is considered the most important environmental factor (21, 22).
Encephalopathy of CHD: Delayed Maturation, Injury, and Neurodevelopment
There is evidence that structural and hemodynamic characteristics of different congenital heart defects lead to autoregulation mechanisms in the brain in case of hypoperfusion or hypoxia by vasodilatation of the cerebral arteries with increased diastolic flow and decreased cerebrovascular resistance (23, 24). It has been assumed that prolonged periods of this autoregulation may lead to delayed maturation of the fetal oligodendrocytes, reduced myelinization, and increased vulnerability of the brain (25–28). However, in fetuses with single ventricular heart, decreased cerebrovascular resistance has been found associated with higher PDI scores at the age of 14 months (29, 30). It remains unclear whether fetal cerebral blood flow alterations predict ND outcomes later in childhood.
Fetal brain perfusion disturbance has been supposed to result in impaired brain growth and maturation with respect to the white matter. Neuropathological studies indicate that the brain disturbance of infants with CHD consists predominantly of cerebral white matter injury (WMI). This result is comparable to periventricular leucomalacia as described in preterm infants (31, 32).
During the last decade, structural and functional brain MRI studies in CHD fetuses, neonates before and after cardiac surgery, and adolescents have given increasing evidence of brain abnormalities in relation to factors mediating ND disabilites (Figure 2). In MRI studies, the term “cerebral white matter immaturity” has been suggested (33–35), and a rate of 20–50% of WMI in newborns prior to surgery, which is dependent on the severity of the underlying CHD, has been detected (36–39). Brain MRI studies have also shown smaller brain volumes, abnormal brain metabolism and decreases in cortical folding, and gyral development in CHD fetuses (27, 40). However, the predictive value of brain abnormalities detected in utero MRI on postnatal preoperative brain injury is limited. Further postnatal studies prior to cardiac surgery are needed (41). In newborns with complex CHD prior to surgery (42–46), smaller preoperative brain volumes, abnormal brain metabolism and decreases in cortical folding, and gyral development were also detected. These were associated with a poor behavioral state regulation (43). Brain maturation has been found delayed by 1 month in newborns with transposition of the great arteries or hypoplastic left heart syndrome (34). Associations between lower brain maturity at birth and increased preoperative and postoperative brain injury (47) as well as ND impairment at the age of 2 years (38) suggest that the so-called “encephalopathy of CHD” (48, 49) may increase the vulnerability of the brain to hypoxia or ischemia. This is especially true in the setting of the surgical and perioperative management, and also in terms of a longer preoperative period between birth and surgery (50). After cardiac surgery, more than 50% of the neonates provide MRI signs of WMI (36–38).
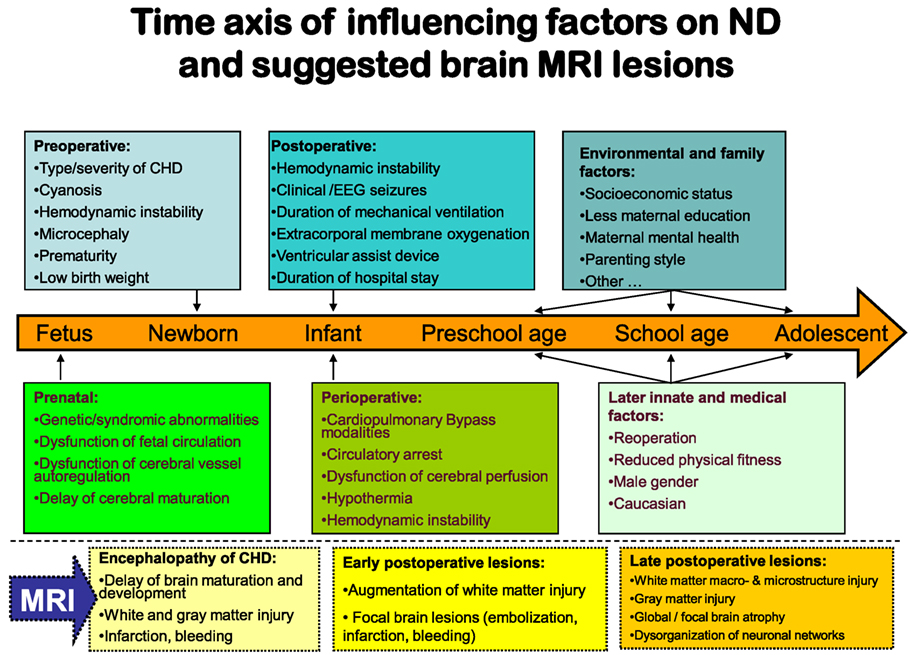
Figure 2. Factors-influencing neurodevelopmental outcomes in relation to brain magnetic resonance imaging.
In summary, the brain immaturity and abnormality in infants with CHD seem to be a complex disturbance with destructive and developmental elements, similar to the encephalopathy first described in premature infants (51). Beginning in the fetal period, the encephalopathy of CHD is a major innate risk factor for preoperative, perioperative, and postoperative additional hypoxic or ischemic brain injury and subsequent ND impairment.
There are also important implications in long-term follow-up linking brain abnormalities in CHD to later ND delay. Brain volumes, including hippocampal volume, remain smaller into adolescence. They are accompanied by reduced ND outcomes (52, 53). MRI macrostructural brain abnormalities (54) and regions of reduced white matter microstructure (55) in TGA adolescents have been found correlated with neurocognitive decline. Diminished white matter microstructure may contribute to cognitive compromise in adolescents who underwent open-heart surgery in infancy. Recently, special brain MRI investigations have suggested that disorganization of neuronal networks may contribute to increased attention deficiency hyperactivity disease symptoms in adolescents with TGA (56).
Conclusion
Besides physical morbidity, ND and psychosocial disabilities are the most common long-term risks of critical CHD. Surgical factors seem to be less important than innate patient and preoperative factors and postoperative events in predicting ND outcomes after cardiac surgery in infancy. Since the variance in percentage explained by the considered clinical variables is quite low, as-yet unknown intrauterine and genetic factors should be investigated. The risk of delayed brain maturation and brain injury evaluated by MRI in fetuses and in neonates with CHD prior to cardiac surgery is of importance.
While the predisposing factors for ND disorders are predominantly innate and while only a few of them are modifiable, research focuses on new approaches for neuroprotection. Examples for such research are cerebral vascular autoregulation monitoring, new perioperative brain biomarkers, and perioperative EEG monitoring. Sophisticated longitudinal brain MRI studies with systematic correlation to ND outcomes aim at an improved risk stratification and therapy for the CHD population.
Author Contributions
HH-G is the only author of the manuscript and contributed concept, text, and reference list.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Schwedler G, Lindinger A, Lange PE, Sax U, Olchvary J, Peters B, et al. Frequency and spectrum of congenital heart defects among live births in Germany: a study of the competence network for congenital heart defects. Clin Res Cardiol (2011) 100(12):1111–7. doi: 10.1007/s00392-011-0355-7
2. Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation (2012) 126(9):1143–72. doi:10.1161/CIR.0b013e318265ee8a
3. Marino BS. New concepts in predicting, evaluating, and managing neurodevelopmental outcomes in children with congenital heart disease. Curr Opin Pediatr (2013) 25(5):574–84. doi:10.1097/MOP.0b013e328365342e
4. Hövels-Gürich HH. Psychomotor development of children with congenital heart defects. Causes, prevalence and prevention of developmental disorders after cardiac surgery in childhood. Monatsschr Kinderheilkd (2012) 160:118–28. doi:10.1007/s00112-011-2498-z
5. Herberg U, Hövels-Gürich H. Neurological and psychomotor development of foetuses and children with congenital heart disease – causes and prevalence of disorders and long-term prognosis. Z Geburtshilfe Neonatol (2012) 216(3):132–40. doi:10.1055/s-0032-1312670
6. Gatzoulis MA. Adult congenital heart disease: education, education, education. Nat Clin Pract Cardiovasc Med (2006) 3(1):2–3. doi:10.1038/ncpcardio0382
7. Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med (1995) 332(9):549–55. doi:10.1056/NEJM199503023320901
8. Bellinger DC, Wypij D, Kuban KC, Rappaport LA, Hickey PR, Wernovsky G, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation (1999) 100(5):526–32. doi:10.1161/01.CIR.100.5.526
9. Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg (2003) 126(5):1385–96. doi:10.1016/S0022-5223(03)00711-6
10. Hövels-Gürich HH, Seghaye MC, Schnitker R, Wiesner M, Huber W, Minkenberg R, et al. Long-term neurodevelopmental outcomes in school-aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg (2002) 124(3):448–58. doi:10.1067/mtc.2002.122307
11. Hövels-Gürich HH, Bauer SB, Schnitker R, Willmes-von Hinckeldey K, Messmer BJ, Seghaye MC, et al. Long-term outcome of speech and language in children after corrective surgery for cyanotic or acyanotic cardiac defects in infancy. Eur J Paediatr Neurol (2008) 12(5):378–86. doi:10.1016/j.ejpn.2007.10.004
12. Hirsch JC, Jacobs ML, Andropoulos D, Austin EH, Jacobs JP, Licht DJ, et al. Protecting the infant brain during cardiac surgery: a systematic review. Ann Thorac Surg (2012) 94(4):1365–73; discussion 1373. doi:10.1016/j.athoracsur.2012.05.135
13. Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL Jr, Dunbar-Masterson C, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation (2011) 124(12):1361–9. doi:10.1161/CIRCULATIONAHA.111.026963
14. Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics (2015) 135(5):816–25. doi:10.1542/peds.2014-3825
15. International Cardiac Collaborative on Neurodevelopment (ICCON) Investigators. Impact of operative and postoperative factors on neurodevelopmental outcomes after cardiac operations. Ann Thorac Surg (2016) 102(3):843–9. doi:10.1016/j.athoracsur.2016.05.081
16. Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation (2012) 125(17):2081–91. doi:10.1161/CIRCULATIONAHA.111.064113
17. Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg (2007) 133(5):1344–53, 1353.e1–3. doi:10.1016/j.jtcvs.2006.10.087
18. Gaynor JW, Kim DS, Arrington CB, Atz AM, Bellinger DC, Burt AA, et al. Validation of association of the apolipoprotein E ε2 allele with neurodevelopmental dysfunction after cardiac surgery in neonates and infants. J Thorac Cardiovasc Surg (2014) 148(6):2560–6. doi:10.1016/j.jtcvs.2014.07.052
19. Atallah J, Joffe AR, Robertson CM, Leonard N, Blakley PM, Nettel-Aguirre A, et al. Two-year general and neurodevelopmental outcome after neonatal complex cardiac surgery in patients with deletion 22q11.2: a comparative study. J Thorac Cardiovasc Surg (2007) 134(3):772–9. doi:10.1016/j.jtcvs.2007.03.007
20. Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, Overton JD, et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature (2013) 498(7453):220–3. doi:10.1038/nature12141
21. McCusker CG, Doherty NN, Molloy B, Casey F, Rooney N, Mulholland C, et al. Determinants of neuropsychological and behavioural outcomes in early childhood survivors of congenital heart disease. Arch Dis Child (2007) 92(2):137–41. doi:10.1136/adc.2005.092320
22. McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, et al. A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child Care Health Dev (2010) 36(1):110–7. doi:10.1111/j.1365-2214.2009.01026.x
23. Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol (2003) 4(5):436–43. doi:10.1007/s00246-002-0404-0
24. Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol (2005) 25(1):32–6. doi:10.1002/uog.1785
25. McQuillen PS, Goff DA, Licht DJ. Effects of congenital heart disease on brain development. Prog Pediatr Cardiol (2010) 29(2):79–85. doi:10.1016/j.ppedcard.2010.06.011
26. Donofrio MT, Duplessis AJ, Limperopoulos C. Impact of congenital heart disease on fetal brain development and injury. Curr Opin Pediatr (2011) 23(5):502–11. doi:10.1097/MOP.0b013e32834aa583
27. Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL Jr, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation (2010) 121(1):26–33. doi:10.1161/CIRCULATIONAHA.109.865568
28. Sanz-Cortés M, Figueras F, Bargalló N, Padilla N, Amat-Roldan I, Gratacós E. Abnormal brain microstructure and metabolism in small-for-gestational-age term fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol (2010) 36(2):159–65. doi:10.1002/uog.7724
29. Williams IA, Fifer C, Jaeggi E, Levine JC, Michelfelder EC, Szwast AL. The association of fetal cerebrovascular resistance with early neurodevelopment in single ventricle congenital heart disease. Am Heart J (2013) 165(4):544.e–50.e. doi:10.1016/j.ahj.2012.11.013
30. Hahn E, Szwast A, Cnota J II, Levine JC, Fifer CG, Jaeggi E, et al. Association between fetal growth, cerebral blood flow and neurodevelopmental outcome in univentricular fetuses. Ultrasound Obstet Gynecol (2016) 47(4):460–5. doi:10.1002/uog.14881
31. Kinney HC, Panigrahy A, Newburger JW, Jonas RA, Sleeper LA. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol (2005) 110(6):563–78. doi:10.1007/s00401-005-1077-6
32. Hinton RB, Andelfinger G, Sekar P, Hinton AC, Gendron RL, Michelfelder EC, et al. Prenatal head growth and white matter injury in hypoplastic left heart syndrome. Pediatr Res (2008) 64(4):364–9. doi:10.1203/PDR.0b013e3181827bf4
33. Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med (2007) 357(19):1928–38. doi:10.1056/NEJMoa067393
34. Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg (2009) 137(3):529–36; discussion 536–7. doi:10.1016/j.jtcvs.2008.10.025
35. Dimitropoulos A, McQuillen PS, Sethi V, Moosa A, Chau V, Xu D, et al. Brain injury and development in newborns with critical congenital heart disease. Neurology (2013) 81(3):241–8. doi:10.1212/WNL.0b013e31829bfdcf
36. Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation (2002) 106(12 Suppl 1):I109–14.
37. Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg (2004) 127(3):692–704. doi:10.1016/j.jtcvs.2003.09.053
38. Beca J, Gunn JK, Coleman L, Hope A, Reed PW, Hunt RW, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation (2013) 127(9):971–9. doi:10.1161/CIRCULATIONAHA.112.001089
39. Khalil A, Suff N, Thilaganathan B, Hurrell A, Cooper D, Carvalho JS. Brain abnormalities and neurodevelopmental delay in congenital heart disease: systematic review and meta-analysis. Ultrasound Obstet Gynecol (2014) 43(1):14–24. doi:10.1002/uog.12526
40. Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, Tworetzky W, McElhinney DB, Brown DW, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex (2013) 23(12):2932–43. doi:10.1093/cercor/bhs281
41. Brossard-Racine M, du Plessis A, Vezina G, Robertson R, Donofrio M, Tworetzky W, et al. Brain injury in neonates with complex congenital heart disease: what is the predictive value of MRI in the fetal period? AJNR Am J Neuroradiol (2016) 37(7):1338–46. doi:10.3174/ajnr.A4716
42. Ortinau C, Alexopoulos D, Dierker D, Van Essen D, Beca J, Inder T. Cortical folding is altered before surgery in infants with congenital heart disease. J Pediatr (2013) 163(5):1507–10. doi:10.1016/j.jpeds.2013.06.045
43. Owen M, Shevell M, Donofrio M, Majnemer A, McCarter R, Vezina G, et al. Brain volume and neurobehavior in newborns with complex congenital heart defects. J Pediatr (2014) 164(5):1121.e–7.e. doi:10.1016/j.jpeds.2013.11.033
44. Sethi V, Tabbutt S, Dimitropoulos A, Harris KC, Chau V, Poskitt K, et al. Single-ventricle anatomy predicts delayed microstructural brain development. Pediatr Res (2013) 73(5):661–7. doi:10.1038/pr.2013.29
45. von Rhein M, Buchmann A, Hagmann C, Dave H, Bernet V, Scheer I, et al. Severe congenital heart defects are associated with global reduction of neonatal brain volumes. J Pediatr (2015) 167(6):1259.e–63.e. doi:10.1016/j.jpeds.2015.07.006
46. Claessens NH, Moeskops P, Buchmann A, Latal B, Knirsch W, Scheer I, et al. Delayed cortical gray matter development in neonates with severe congenital heart disease. Pediatr Res (2016) 80(5):668–74. doi:10.1038/pr.2016.145
47. Andropoulos DB, Hunter JV, Nelson DP, Stayer SA, Stark AR, McKenzie ED, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg (2010) 139(3):543–56. doi:10.1016/j.jtcvs.2009.08.022
48. Volpe JJ. Encephalopathy of congenital heart disease- destructive and developmental effects intertwined. J Pediatr (2014) 164(5):962–5. doi:10.1016/j.jpeds.2014.01.002
49. Gaynor JW. The encephalopathy of congenital heart disease. J Thorac Cardiovasc Surg (2014) 148(5):1790–1. doi:10.1016/j.jtcvs.2014.09.061
50. Lynch JM, Buckley EM, Schwab PJ, McCarthy AL, Winters ME, Busch DR, et al. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg (2014) 148(5):2181–8. doi:10.1016/j.jtcvs.2014.05.081
51. Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol (2009) 8(1):110–24. doi:10.1016/S1474-4422(08)70294-1
52. von Rhein M, Buchmann A, Hagmann C, Huber R, Klaver P, Knirsch W, et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain (2014) 137(Pt 1):268–76. doi:10.1093/brain/awt322
53. Latal B, Patel P, Liamlahi R, Knirsch W, O’Gorman Tuura R, von Rhein M. Hippocampal volume reduction is associated with intellectual functions in adolescents with congenital heart disease. Pediatr Res (2016) 80(4):531–7. doi:10.1038/pr.2016.122
54. Heinrichs AK, Holschen A, Krings T, Messmer BJ, Schnitker R, Minkenberg R, et al. Neurologic and psycho-intellectual outcome related to structural brain imaging in adolescents and young adults after neonatal arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg (2014) 148(5):2190–9. doi:10.1016/j.jtcvs.2013.10.087
55. Rollins CK, Watson CG, Asaro LA, Wypij D, Vajapeyam S, Bellinger DC, et al. White matter microstructure and cognition in adolescents with congenital heart disease. J Pediatr (2014) 165(5):936–44.e1–2. doi:10.1016/j.jpeds.2014.07.028
Keywords: risk factors, neurodevelopment, congenital heart disease, cardiac surgical procedures, cardiopulmonary bypass, brain MRI, encephalopathy
Citation: Hövels-Gürich HH (2016) Factors Influencing Neurodevelopment after Cardiac Surgery during Infancy. Front. Pediatr. 4:137. doi: 10.3389/fped.2016.00137
Received: 11 October 2016; Accepted: 01 December 2016;
Published: 15 December 2016
Edited by:
Elisabeth Utens, Sophia Children’s Hospital, NetherlandsReviewed by:
Elumalai Appachi, Baylor College of Medicine, USAMassimo Caputo, University of Bristol, UK
Copyright: © 2016 Hövels-Gürich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hedwig Hubertine Hövels-Gürich, hhoevels-guerich@ukaachen.de
 Hedwig Hubertine Hövels-Gürich
Hedwig Hubertine Hövels-Gürich