- 1 Rowett Institute of Nutrition and Health, University of Aberdeen, Aberdeen, UK
- 2 Food and Health Research Centre, University of Eastern Finland, Kuopio, Finland
- 3 School of Biological Sciences, University of Hong Kong, Hong Kong, China
DNA damage is an essential component of the genesis of colonic cancer. Gut microbial products and food components are thought to be principally responsible for the damage that initiates disease progression. Modified Ames tests and Comet assays have been developed for measuring mutagenicity and genotoxicity. Their relevance to oncogenesis remains to be confirmed, as does the relative importance of different mutagenic and genotoxic compounds present in fecal water and the bacteria involved in their metabolism. Dietary intervention studies provide clues to the likely risks of oncogenesis. High-protein diets lead to increases in N-nitroso compounds in fecal water and greater DNA damage as measured by the Comet assay, for example. Other dietary interventions, such as non-digestible carbohydrates and probiotics, may lead to lower fecal genotoxicity. In order to make recommendations to the general public, we must develop a better understanding of how genotoxic compounds are formed in the colon, how accurate the Ames and Comet assays are, and how diet affects genotoxicity.
Colorectal cancer (CRC) is a major cause of cancer death in affluent countries, and diet plays an important role in its development (WCRF, 2007). The human colon is exposed to a vast array of potentially mutagenic chemicals deriving either from dietary residues including cured meats and burnt protein rich foods (Nader et al., 1981; Gill and Rowland, 2002) or from endogenous excretion of digestive compounds (Reddy et al., 1980). Both classes of chemicals will undergo extensive microbial fermentation and modification which complicates assessment of the exposure of the colonic epithelium to pro-carcinogens and carcinogens. Five major groups of colonic carcinogens have been identified in diet and feces, including polycyclic aromatic hydrocarbons, heterocyclic amines, N-nitroso compounds (NOC), bile acids, and fecapentaenes (for an extensive review see de Kok and van Maanen, 2000). It has been known for decades that DNA damage and subsequent mutation are the key events in initiating the process of carcinogenesis which ultimately leads to disease (Preston and Williams, 2005). Historically, the discovery of mutagenic properties of human feces by Bruce et al. (1977) has initiated research aiming to describe the carcinogen exposure of the intestinal tract and the role of diet and microbiota. This review will examine the potential use of fecal water to assess mutagenicity/genotoxicity in the large intestine, and to predict the impact of diet on the risk to develop colon cancer.
Bacterial Mutation Assays
Bacterial mutation assays are used widely to predict the mutagenicity of chemical compounds. The challenges of determining the mutagenicity of a pure, probably sterile chemical compound are quite different to the problems of measuring mutagenesis in biological samples.
Methodological Considerations
The two main bacterial mutagenicity tests are the Ames test and the SOS Chromotest (Figure 1). The reverse mutation test (Ames et al., 1975; Green et al., 1976) measures the reversion rate of amino acid auxotrophs when exposed to test materials (Hubbard et al., 1976; Gatehouse et al., 1994). Sustained research and method development led to recommendations for standard operating procedures (Gatehouse et al., 1994; Organisation for Economic Co-operation and Development, 1997) which specify assay conditions and especially the strains of bacteria to be used. In the literature, the most commonly used tester strains are S. typhimurium TA98 and TA100. Various kits are available commercially, which enable the mutagenic hazards posed by chemical compounds in particular to be measured much more conveniently than the original methods, using 96-well plate technologies termed Ames II test (Fluckiger-Isler et al., 2004; Kamber et al., 2009). The SOS chromotest is an elegant alternative to the reverse mutation test (Quillardet et al., 1982; Quillardet and Hofnung, 1985, 1993). The SOS response in bacteria (E. coli strain PQ37) occurs as a response to genotoxic agents and is linked to the synthesis of β-galactosidase, which can be readily measured colorimetrically. The genetic background of strain PQ37 is that its cell envelope has been rendered lipopolysaccharide-deficient and more permeable by an rfa mutation, its DNA excision repair capability has been made deficient by a mutation of uvrA, and a sfiA::lacZ fusion has been introduced. Thus, possible mutagens can permeate the cell more easily, and if DNA damage occurs it is not repaired. This leads to the induction of sfiA as part of the SOS response. The fused lacZ gene then expresses β-galactosidase. To monitor potential toxic effects of the test material, bacterial biomass is monitored by measuring alkaline phosphatase activity in another colorimetric assay.
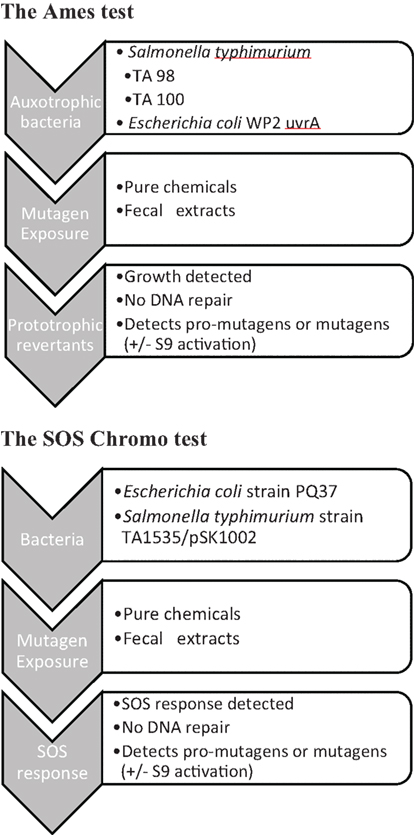
Figure 1. Schematic illustration of the bacterial mutation assays [(A) Ames test and (B) SOS Chromo test].
Mutagenicity of fecal material has most often been measured in organic extracts with many fewer investigations being carried out with fecal water, in spite of the latter being considered to be of greater relevance to events at the epithelial mucosa (Schiffman, 1986). Fecal water is a term that is used both for aqueous extracts of feces, generally prepared by adding buffer to fresh feces, or the liquid expressed from feces in the supernatant following high-speed centrifugation. A major problem in using fecal water for mutagenicity testing in comparison with pure chemicals or solvent extracts of feces is that fecal water will always be contaminated by fecal bacteria (de Kok and van Maanen, 2000). Autoclaving has been used by some workers (Mower et al., 1982) to eliminate microbiological contamination in the Ames test, but possible heat-labile mutagens would be destroyed by such a procedure. In some studies, bacterial contamination was removed by ultrafiltration (Kuhnlein et al., 1983; Johansson et al., 1998). In our experience, ultrafiltration is indeed a satisfactory method for preparing fecal waters, reducing bacterial numbers sufficiently not to interfere in the final “incubation” phase of the Ames test. The presence of amino acids in the samples was considered to be a likely problem in the reverse mutation test, thereby enabling auxotrophs to grow without reversion, giving false positives. An elaborate system for the detection of “auxotrophic growth enhancement” was devised to compensate (Venitt and Bosworth, 1986; Venitt et al., 1986; Bosworth and Venitt, 1989). In our experience, free histidine in fecal water is present at concentrations that do not interfere with the assay. However, histidine is generated from proteins and peptides present in the samples. This can be problematic again during the “incubation” phase. The solution to this problem is to centrifuge the sample + bacteria mixture after the “exposure” step, thereby removing the source of the histidine prior to the “incubation” phase. When assessing results published in the literature, studies, where the elimination of bacterial and proteolytic contamination is unclear, would therefore have to be viewed with caution.
The SOS chromotest has been used to assay mutagens extracted from feces (Nair et al., 1991, 2000), but relatively infrequently in fecal water samples. Earlier measurements appeared to show that fecal water tested negative in the SOS chromotest (Venitt and Bosworth, 1986), but it was subsequently discovered that enzymic activity present in two samples compromised the results (Bosworth and Venitt, 1986). A “washing step,” the method not specified, minimized this problem. In our own experiments in this area, the SOS chromotest was confirmed to be compromised by the presence of large amounts of endogenous enzymic activities from lysed fecal bacteria, which proved impossible to eliminate entirely. Thus, we do not recommend the SOS Chromotest for use with fecal water. The umu-test (Matsumoto and Benno, 2004) is a test based on the ability of genotoxins to induce expression of the umuC gene, one of the SOS genes responsible for error-prone repair; this gene is more involved in mutagenesis than other known SOS genes in bacteria. The tester strain (S. typhimurium TA1535/pSK1002) carries a fused umuC–lacZ gene, allowing for the monitoring of umuC expression once again by measuring β-galactosidase activity in a colorimetric assay. Thus, it will be subject to the same limitations as the Chromotest. On the other hand, the centrifugation and washing steps following exposure to fecal water enables the Ames II test to be performed satisfactorily and this test is therefore preferable over the SOS Chromotest and the umu-test.
Whole Diet Interventions on Fecal Water Mutagenicity
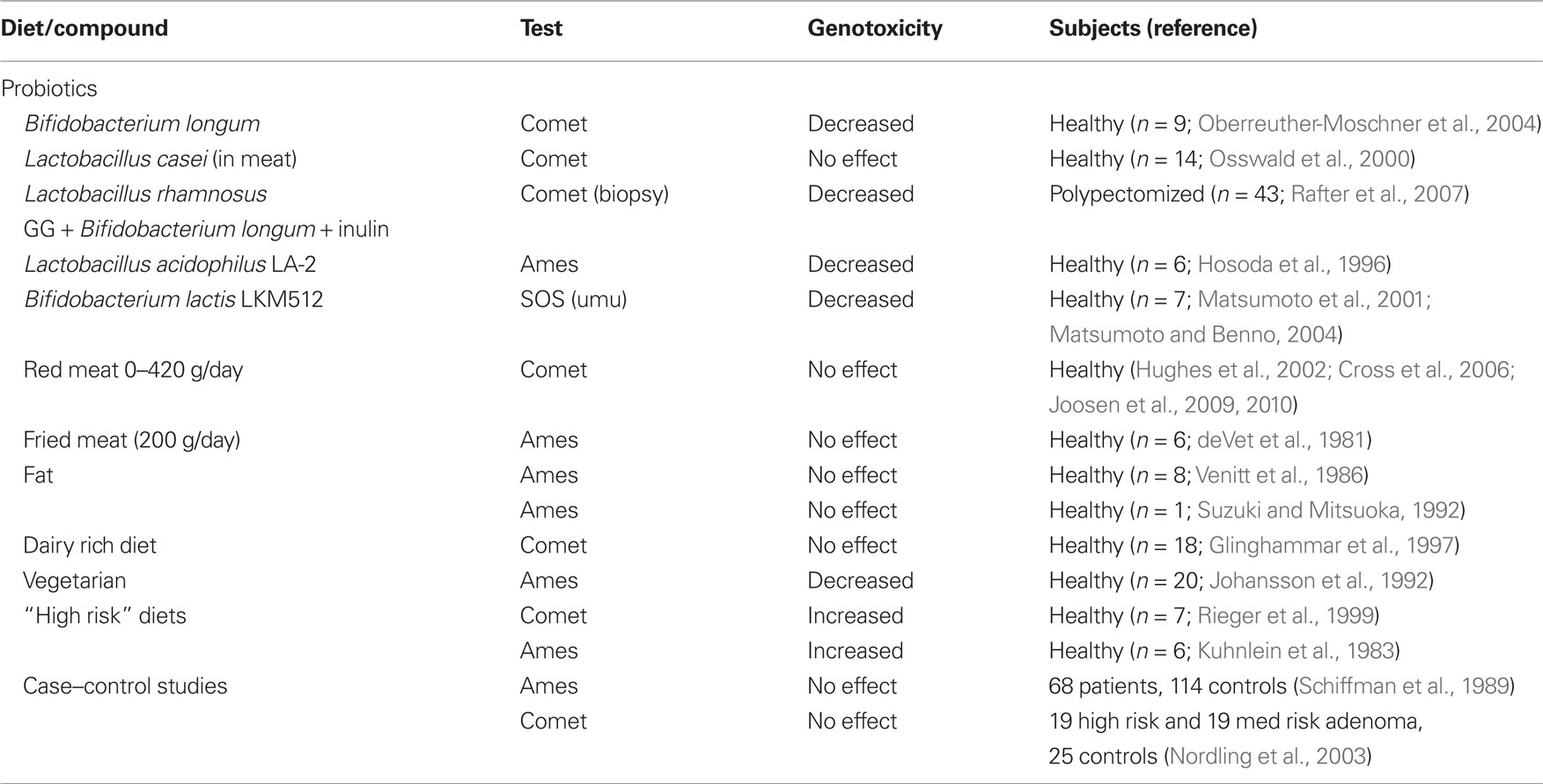
Using bacterial mutation assays, valuable information has been gathered from human samples in dietary intervention trials, population-based comparisons of high and low risk populations, and a case–control study in CRC patients (de Kok and van Maanen, 2000). This study showed no differences in fecal mutagenicity between cases and controls, which may be due to the fact that fecal composition is reflecting very recent dietary intakes, whereas cancer sufferers passed their initiation and progression stages of disease development at some point in the distant past (Schiffman et al., 1989). The population-based studies found that population groups with low risk of CRC (vegetarians, rural populations) excreted mutagenic feces less frequently than high risk groups (omnivores, urban populations). A dietary intervention study found that 2 weeks on a low risk vegetarian diet led to the excretion of less mutagenic stools in six volunteers compared to a high risk mixed diet (Kuhnlein et al., 1983). Another study using one volunteer observed that the addition of 150 g/day of fat to the normal diet did not influence fecal mutagenicity, but addition of 30 g/day wheat fiber decreased mutagenicity (Venitt et al., 1986). Shifting from a normal mixed diet to an ovo-lacto vegetarian diet was studied in a longitudinal study in 20 volunteers. After 3 months, mutagenicity of fecal aqueous extracts was slightly but significantly reduced (from average 5 to 3 positive wells in a multiwell assay, p < 0.05) using E. coli WP uvrA tester strain without S9 activation. This change was maintained for 12 months on the vegetarian diet, but fecal mutagenicity increased again when subjects shifted back to their normal mixed diet. Authors also stated that change in fecal mutagenicity was only observed when expressed per gram feces but not per 24 h feces, and conclude that the effect was most likely due to increased fecal water content following increased fiber intake on vegetarian diets (Johansson et al., 1992, 1998). However, this study was performed without taking into account the auxotrophic potential of fecal waters and might have therefore overestimated fecal mutagenicity. A study using a shift from low fat to high fat diets in eight healthy volunteers showed no change in fecal mutagenicity using the Salmonella T100 tester strain (Suzuki and Mitsuoka, 1992). Furthermore, in a study assessing the fecal mutagenicity from 199 self collected fecal samples in healthy volunteers using the umu-test, authors found no correlation between fecal mutagenicity and any food group (Kosaka et al., 2001), but saw an positive correlation between mutagenicity and zinc and iron content in feces and a negative correlation with sodium under +S9 conditions.
Probiotic Interventions
Administration of milk fermented with Lactobacillus acidophilus LA-2 for 7 days decreased the fecal mutagenicity of six volunteers by 72% compared to initial values and this change might have been due to increased fecal lactobacilli and bifidobacteria (Hosoda et al., 1996). A probiotic intervention with a yogurt containing Bifidobacterium lactis LKM512 also found a significant reduction in fecal mutagenicity (in the umu-test) of subjects consuming probiotics for 2 weeks when compared to a placebo group (Matsumoto and Benno, 2004). Authors also suggest that production of spermidine by probiotic LKM512 is protective against mutagenicity of aqueous fecal extracts. Similar effects of LKM512 were found in an elderly population by the same authors (Matsumoto et al., 2001). The positive effect of some bacterial groups on decreasing fecal mutagenicity is further supported by the finding that depressed populations of bifidobacteria and lactobacilli were associated with increased fecal mutagenicity in a study of 52 subjects (Savitskaia and Bondarenko, 2008).
Conclusion
Determination of mutagenicity of fecal water is fraught with technical difficulties, such that trusting all published results is unwise. Bacterial contamination and potential amino acid residues need to be considered when testing fecal material for its mutagenicity. The variation in assays used further complicates the interpretation of findings. However, it appears that individuals on vegetarian diets might excrete less mutagenic feces and that probiotics might have a potential in decreasing fecal mutagenicity. We believe that modified methods are now good enough to further evaluate how diet and intestinal bacteria affect mutagenicity of fecal water.
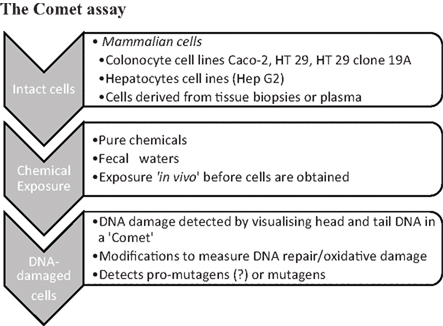
The Comet Assay
Despite some very valuable information generated using bacterial mutation assays, these studies have been almost completely replaced by assays using mammalian cells as targets. Over the last 15 years, the Comet assay has become commonly used to study the effect of diet on DNA damage and to identify harmful and protective dietary components. After rigorous validation, the Comet assay is now well established and widely used to determine oxidative DNA damage and antioxidant properties of food components in peripheral blood cells, and a vast variety of dietary studies have been conducted and reviewed (Collins et al., 2004; Wasson et al., 2008). This method has also been used to assess the DNA damaging capacity of human feces.
Methodological Considerations
The most commonly used method is to separate fecal water from homogenized feces by high-speed centrifugation (usually 2 h at 14,000–50,000 g), and subsequently expose enterocyte cell lines (usually Caco-2, HT 29, or HT 29 clone 19A) to fecal water at 5–50% dilutions for 30 min at 37°C (Figure 2). Only one study (Cross et al., 2006) compared the standard incubation of HT 29 cells for 30 min at 37°C, with a modified incubation for 5 min at 4°C to reduce DNA repair occurring in cells during exposure. Using the latter assay, none of the fecal waters induced any DNA damage whereas fecal waters induced low DNA damage in the first protocol. In our own work however, we were able to detect DNA damage in HT 29 cells following incubation with fecal water for 5 min on ice, and we also found best repeatability of results using this incubation.
To increase the information generated by the Comet assay, lesion specific repair enzymes can be included to detect oxidized pyrimidines (Endonuclease III) and oxidized purines (formamidopyrimidine DNA N-glycosylase, FPG). Different methods are used to illustrate the proportion of DNA remaining in the Comet Head (i.e., undamaged DNA) and in the Comet tail (i.e., damaged DNA). To quantify the amount of damage, Comets can be assessed visually or by using a scoring software. Visual scoring into four comet categories (undamaged – highly damaged) can be used where no scoring software is available and there is very good agreement between the two methods (Woods et al., 2002). However, minor differences in Comet tails, which are likely to be linked to dietary changes, could be missed by the visual scoring method. Using scoring software, commonly used measures to quantify DNA damage are percentage tail intensity (fluorescence intensity of head and tail fraction), tail length (in micrometer), and the tail moment (tail length × tail intensity; Tice et al., 2000). It appears most appropriate to include the measures of tail intensity (either percentage tail or tail moment) into the scoring of Comet results, as this method best describes the ratio of undamaged to damaged DNA and is less prone to variation with differences in electrophoresis conditions.
One of the major obstacles in using fecal water is the vast variability in genotoxicity between fecal samples and individuals. Venturi et al. (1997) screened 35 fecal samples from free living Swedish and English volunteers to describe the variability occurring between fecal water samples. They found that based on measurements of tail moments, 54.3% of samples were non-genotoxic, 2.8% were low, 11.4% were medium, and 31.4% of fecal waters were highly genotoxic toward Caco-2 cells. They also found that treatment of cells with Endonuclease III increased genotoxicity of all positive samples, whereas non-genotoxic samples remained negative. A similar study in free living Irish volunteers found 52.2% of fecal water samples to be non-genotoxic, 19.6% showing low-moderate damage, and 28.3% of samples being highly genotoxic (Woods et al., 2002). These studies show that about half of the individuals excrete genotoxic feces on their habitual diets. Osswald et al. (2000) performed further studies to describe individual and experimental variability in fecal water genotoxicity using HT29 clone 19A cells in the Comet assay. They found all repeated fecal samples from six individuals to be more genotoxic than the negative controls. Interexperimental variability between six repeat experiments on the same FW sample was relatively low (CV range 6.9–31) whereas variability within different fecal samples from the same subject was high (intraindividual variation, 29.9–76.6) and non-genotoxic as well as highly genotoxic samples were found from the same individual. Interindividual variation comparing different subjects was also very high (CV 21.3–64.0) and was not significantly decreased when subjects consumed identical diets. With this background variation it appears a challenge to assess the effect of diet on fecal water genotoxicity.
Another point hampering the use of fecal water in dietary intervention studies is the variable amount of fecal water generated from each fecal sample. Klinder et al. (2007) compared three different preparation methods for fecal water (direct centrifugation, aqueous buffer extraction, and freeze drying). Fifty-seven percent of fecal waters were non-genotoxic when prepared with all three preparation methods and 28% were similarly genotoxic when prepared by method A and B. Method B yielded higher volumes of fecal water and would be preferable. However, most studies in the literature use direct centrifugation of feces, as this is believed to better represent the portion of the fecal material that will interact with the intestinal epithelium.
In summary, variability of results generated by the Comet assay is the major concern, and great care has to be taken to generate repeatable results for each individual fecal sample. Furthermore, each individual investigator performing the assay has to be properly trained. Repeat experiments assessing the same sample and inclusion of negative controls and reference fecal water samples into each assay might improve results. Positive controls most often use hydrogen peroxide to induce DNA damage in target cells. This is an appropriate control when oxidative DNA damage is the suspected mode of action, but in the case of alkylating DNA damage, other positive controls should be used to ensure that the target cells used are susceptible to this kind of DNA damage. Furthermore, the design of human intervention studies has to be strictly controlled and allow for large enough sample sizes. This might help to overcome the issue of variable genotoxicity of individual samples and enable researchers to detect any potential effect of changes in diet.
The use of colon carcinoma derived cell lines is common practice in the studies presented here and raises the question of relevance of results for assessing events occurring in the normal gut epithelium. However, the availability and common use of these cell lines and the literature available on them drives further investigations using the same model. Great care has to be taken when interpreting these results, as the cells’ capacity to activate pro-carcinogens and to repair damage might influence the findings.
Whole Diet Interventions
The first study to assess the effect of diet on fecal water genotoxicity was carried out by Glinghammar et al. (1997) in 18 healthy volunteers. In a crossover design, volunteers shifted from their habitual dairy product-rich diet (1 week) to a dairy-free diet for a subsequent week. Volunteers were advised on how to substitute their dairy products with other food items. On average, no effect of diet was observed on fecal water genotoxicity in Caco-2 cells, but results were very variable. In a more controlled pilot trial in seven healthy volunteers shifted from a high risk or low risk diet for 12 days each with 1 week washout (Rieger et al., 1999). Subjects received either diet 1 high in animal fat, protein, and sugar and low in dietary fiber or diet 2, low in animal fat, protein, and sugar and high in fiber. Fecal water genotoxicity induced by diet 1 was almost twice as high as compared to diet 2 (28.7 vs 17.5% tail DNA), but fecal waters of subjects on both diets induced significant DNA damage in HT 29 clone 19A cells. Further experiments using lesion specific enzymes (Endonuclease III and FPG) did not show any significant difference between the two diets. This study showed a very clear and promising result in such a small study population. It appears that strictly controlled dietary trials are more suitable to study the effect of diet on fecal water genotoxicity.
Meat and Fish Interventions
Hughes et al. (2002) studied whether supplementing a high meat diet (320 g/day) with additional vegetables (400 g/day), tea or soy (200 g/day) for 15 days each would change fecal water genotoxicity using the standard comet assay protocol with Caco-2 cells. Fecal waters induced low to moderate DNA damage, but no changes due to diet were reported. Two strictly controlled dietary trials, compared vegetarian and red meat diets (60, 120, or 420 g red meat/day, iron supplements and heme iron supplements), all fed to 21 healthy males for 15 days (Cross et al., 2006). None of the diets altered fecal water genotoxicity using the standard Comet assay on HT 29 cells. It is surprising that these carefully conducted studies failed to show any effect of meat consumption on fecal water genotoxicity, but again, interindividual variation might have masked any effect. Similarly, diets high in red and processed meat (420 or 360 g/day for males and females) fed for 14 days did not increase fecal water genotoxicity toward Caco-2 cells, but rather decreased it when compared to a vegetarian diet (Joosen et al., 2009). This surprising finding that fecal waters of vegetarians are more genotoxic will need further investigation. In a subsequent study from the same group (Joosen et al., 2010) the effect of high red meat (325 or 260 g/day for males and females), high fish (375 or 300 g/day for males and females), or a combined meat and fish diet on fecal water genotoxicity was studied. Thirteen volunteers received above diets for 14 days in a crossover design, but again no changes in fecal water genotoxicity (including strand breaks and oxidized pyrimidines or purines) were observed between subjects on any diet. Another recent study using oil rich and lean fish (Pot et al., 2010) also failed to show any change in fecal water genotoxicity (89 subjects) or DNA damage in colonocytes from biopsies (70 subjects) before and after the fish interventions (150 g of additional fish/week for 6 months). Additionally, no correlation between genotoxicity of fecal waters and DNA damage in colonocytes (n = 34, Spearman correlation 0.06) was found. Authors conclude that these two assays do not measure the same end point, as fecal water genotoxicity measures the genotoxic burden of excreted feces, whereas colonocyte DNA damage reflects the effects in cells, which additionally depends on the expression of biotransformation enzymes and DNA repair mechanisms. However, if there is no correlation between genotoxicity of feces and DNA damage in the intestinal epithelium, this would suggest that fecal genotoxicity is not involved in damaging intestinal DNA and contributing to cancer risk. These results clearly need further consideration in the future.
Interventions with Bread
In a pilot dietary intervention, seven volunteers followed their habitual diet supplemented with lignin-containing bread (200 g/day) for 4 weeks (Osswald et al., 2000). Fecal water genotoxicity was significantly decreased when volunteers consumed the bread as compared to the unsupplemented diet despite the large variability of data. It is unclear whether the observed benefit derived from the bread or the linseed supplementation. In a better controlled parallel trial, 38 healthy subjects received control bread (2 weeks) followed by 5 weeks of bread supplemented with either probiotics or probiotics and antioxidants (Glei et al., 2005). DNA damage in peripheral lymphocytes was measured to assess systemic carcinogen exposure, DNA damage in buccal lymphocytes was measured as a reflection of direct exposure via the oral route, and the genotoxicity of fecal water was used to estimate the effects caused by gut fermentation of the fiber ingredients in bread. DNA damage in buccal cells did not change with dietary change, but supplemented bread did decrease DNA damage in peripheral lymphocytes of smokers (not observed in non-smokers) and decreased fecal water genotoxicity in non-smokers (not in smokers). This led to the conclusion that smokers and non-smokers benefited differently from the intervention with probiotic bread ± antioxidants. A subsequent study from the same group (Helbig et al., 2009) examined the effect of 250 g/day of bread supplemented with tocopherol-rich blackcurrant seed press residue on stool tocopherol, antioxidant capacity, and fecal water genotoxicity (n = 36 women, 4 weeks intervention). Tocopherol in plasma and stool were increased and genotoxicity of fecal water was also increased after consumption of supplemented bread compared to control bread. Also, several urinary markers of oxidative stress increased with supplemented bread, and authors conclude that consumption of bread supplemented with ground berry seeds may not be of advantage.
Pro/Prebiotic Interventions
One of the clearest results of a protective effect was obtained in a very small study where nine healthy volunteers consumed either control yogurt or probiotic yogurt (Lactobacillus acidophilus 145 and Bifidobacterium longum 913 and oligofructose) for a total of 7 weeks (Oberreuther-Moschner et al., 2004). During the first 6 weeks of intervention, subjects followed their normal diet, but during week 7 subjects received a controlled diet to minimize variability at the time of fecal sample collection. Fecal water genotoxicity was reduced to less than half in the probiotic group compared to controls. In a short term intervention (Lactobacillus casei 5 × 109 CFU in 50 g meat daily for 9 days in 14 subjects), probiotics had no effect on fecal water genotoxicity (Osswald et al., 2000). In a mechanistic study, Burns and Rowland (2004) showed that incubating genotoxic fecal water with probiotic bacteria reduces the sample’s genotoxic potential toward HT 29 cells (Bifidobacterium Bb12 and Lactobacillus plantarum were most effective), and non-viable bacteria were not capable of this effect. They then showed that culture supernatants of various probiotics grown on prebiotic substrates (Lactobacillus plantarum on FOS-based probiotics were most effective) can also strengthen the resistance of HT 29 cell to withstand genotoxicity of fecal water. These studies suggest that probiotics might either degrade genotoxic agents present in fecal water, or might produce protective substances released into the culture supernatant. Rafter et al. (2007) conducted a placebo-controlled randomized trial in polypectomized and colon cancer patients receiving 12 weeks of a synbiotic preparation (Lactobacillus rhamnosus GG, Bifidobacterium lactis Bb12, inulin) and looked at DNA damage in colonoscopies of these patients. In polypectomized patients, DNA damage was slightly decreased post-intervention when compared to baseline, but this effect was not seen in colon cancer patients. Fecal water genotoxicity was not measured, but other fecal water related toxicities (necrosis induction, barrier function) were decreased due to symbiotic intervention.
Other Studies
In a case–control study in patients with colorectal adenomas (n = 25 controls, 19 medium risk adenoma patients, and 20 high risk adenoma patients) no differences were found in fecal water genotoxicity between cases and controls in the Comet assay and no correlation between strand breaks and fecal pH or total bile acids was found (Nordling et al., 2003). In a second genotoxicity assay using naked double stranded DNA authors observed significantly increased DNA damage induced by fecal waters from medium risk adenoma patients compared to controls, and this variance of fecal water genotoxicity could be partly explained by concentrations of lithocholic acid (15% of variance) and fecapentaene-12 (7%) in a regression model. This finding illustrates the importance in choosing the target DNA and cells, and the difficulty in interpreting the DNA damage results generated by the Comet assay.
The only population-based study to compare fecal water genotoxicity of populations investigated African American at high risk of CRC (n = 52) and Caucasian Americans at low risk CRC (n = 46), and tried to link results to dietary information and microbiota composition in these subjects. No difference in fecal water genotoxicity was found between the two racial groups, but significantly higher genotoxicity of fecal waters of women compared to men was found in both groups. No association was found with dietary fat intake, heterocyclic amine intake, age, or BMI. However, African Americans reported increased intake of heterocyclic amines and reduced intake of Vitamin D and showed differences in fecal microbiota (Mai et al., 2009).
Summary and Recommendations
A number of studies have examined the relationship between diet and fecal mutagenicity/genotoxicity using the Ames and Comet assay (Table 1). When summarizing the results, we find that most dietary interventions failed to change fecal mutagenicity or genotoxicity. The only dietary modification that did convincingly alter fecal potential to damage DNA is probiotics, although different strains have been used in each study. Dietary shifts from high risk to low risk diets might also alter the DNA damaging capacity of feces in the Comet and the Ames assay. However, studies on meat have not been successful in establishing a relationship between meat intake and fecal genotoxicity. It is therefore difficult to draw a firm conclusion on whether dietary changes really change the genotoxic and mutagenic potency of fecal material.
Technical difficulties and variations in the assay conditions used in these studies make it difficult to compare study outcomes. It is impossible to judge from the literature, what the impact of these differences in assay conditions on the published results might be. However, with modified methods and incorporation of rigorous controls, both assays should be used to assess fecal material for its potency to induce DNA damage in mammalian cells and mutations in a well established and rapid bacterial test. We believe that the bacterial mutation assays and the Comet assay can be used in combination to answer fundamental questions in the future, including
• How does diet influence the mutagenicity/genotoxicity of fecal water?
• What are the most important mutagens/genotoxic agents under different dietary regimes?
• What is the role of different endogenous bacteria in producing and detoxifying these mutagens/genotoxic agents?
• Can pre- or probiotics influence the mutagenicity/genotoxicity caused by the diet and intestinal bacteria?
The Link between Chemical Carcinogens and Cancer Risk – The Case of N-Nitroso Compounds
Several NOC have been classified as 2A probable human carcinogens by the International Agency for the Research on Cancer (IARC) due to their DNA-alkylating properties. DNA adducts have been found in various human samples and are suggested good biomarkers to assess recent exposure to NOC (Gallo et al., 2008).
Human exposure to NOC has been studied extensively and the relation to stomach cancer risk is widely accepted in the literature (Gonzalez et al., 2006; Jakszyn et al., 2006). Stricter regulations in food processing limiting the use of nitrite in meat processing have led to a significant reduction in NOC intake from food (Santarelli et al., 2008). However, exposure to NOC also occurs from endogenous formation inside the intestinal tract. Under acidic conditions in the stomach, amine and amide precursors will react with nitrite to form NOC. The role of the small and large intestine in formation of NOC is still unclear. In vitro incubations have shown that precursor substances such as nitrosothiols and nitrosoheme are formed under acidic conditions present in the stomach (Kuhnle et al., 2007; Lunn et al., 2007). These could then form NOC by nitrosating suitable substrates present in the large intestine which can derive from the diet or from bacterial protein fermentation in the lower gut (Hughes et al., 2000). There is strong evidence from dietary interventions in humans that the endogenous formation of NOC from diet is related to meat intake and especially intake of red meat (Hughes et al., 2001; Bingham et al., 2002; Cross et al., 2003; Kuhnle and Bingham, 2007; Kuhnle et al., 2007). Dietary heme has also been shown to increase endogenous formation of NOC, most likely though the formation of nitrosoheme, which is suggested to be a precursor of endogenous N-nitrosation of other molecules (Cross et al., 2003).
It is well accepted that NOC and iron intake play a role in explaining the epidemiological link between high red meat intake and increased risk of CRC development. Several carefully conducted dietary intervention studies (Cross et al., 2006; Joosen et al., 2009, 2010) with increased intake of red and white meat clearly showed that red meat and heme content greatly contributed to the endogenous formation of NOC in humans measured in fecal samples. However, these studies failed to detect any difference in fecal water genotoxicity in relation to meat intake, and no correlation was found between fecal NOC concentrations and DNA damage induced by fecal water in the Comet assay. These results are surprising as pure NOC have been shown to induce DNA damage in the Comet assay in hepatocytes (Arranz et al., 2007a,b), and they have also been found to be Ames test positive (Quillardet and Hofnung, 1993). Furthermore, the NOC specific DNA adduct O6-carboxymethyl guanine has been detected in shed human colonocytes extracted from stool samples, and these DNA adducts was found to be increased in a high meat intervention (Lewin et al., 2006). In an in vitro yeast assay, N-nitrosoglycine induced a mutation spectrum in p53 tumor suppressor gene that was highly similar to mutation spectra observed in human gastric and colonic tumors (Gottschalg et al., 2007), which further supports the theory that NOC are able to attack mucosal DNA and might cause mutations that are relevant to intestinal cancers. It is therefore likely that NOCs at the concentrations present in the colonic lumen contribute to DNA damage in the colon and possibly to human cancer risk. However, the lack of DNA damage induced by fecal waters using the Comet assay might suggest that either the Comet assay in human-derived colonocyte cell lines is not suitable to detect genotoxic chemicals in fecal water or that the complex mixture of genotoxic and genoprotective compounds present in fecal water might mask any possible effect.
Alternative Approaches and Future Outlooks
Several studies using fecal water to assess diet-related changes in genotoxicity also incorporate other assays to assess the potential of fecal water to contribute to CRC development. These include cell based assays to study cytotoxicity, cell cycle and apoptosis, and genotoxicity. The Micronucleus assay has been recently used as an alternative test to describe fecal water genotoxicity (Benassi-Evans et al., 2010). Intestinal barrier function measurements and cell invasion assays have been suggested to describe the potential of fecal water to alter processes in tumor promotion and formation of metastasis (Klinder et al., 2007). A recent study also used fecal calprotectin as an inflammation marker (Joosen et al., 2010). Maybe a whole battery of test assays would be needed to describe “fecal water activity” toward intestinal cells (Pearson et al., 2009).
DNA adducts are established markers to assess exposure to carcinogens including polycyclic aromatic hydrocarbons, heterocyclic amines, and NOC in human urine samples and lymphocyte DNA (Gallo et al., 2008), but the feasibility of this approach in fecal samples is questionable. However, a functional assay to study DNA adducts induced by fecal samples has been developed. This polymerase arrest assay detects DNA adducts in the p53 tumor suppressor that gene occur adjacent to a guanine residue, indicating that guanosine adducts are present. As most known fecal mutagens (NOC, polycyclic aromatic hydrocarbons, heterocyclic amines) predominantly form guanosine adducts, this is indirect evidence that these adducts might occur. This is further supported by the correlation between these adduct sites found in vitro and known mutated sense-strand guanines found in colorectal tumors (CRC mutation hot spots), highlighting the potential carcinogenicity of fecal material (Greetham et al., 2007). Microarray technology has recently been used to assess changes in gene expression following exposure of Caco-2 cells to pure NOC and found several cancer related pathways were affected (apoptosis, cell cycle blockage, DNA repair, oxidative stress; Hebels et al., 2009). This method might be applied to study cellular responses to fecal water in the future.
Conclusion
Already Schiffman (1987) suggested in his review on diet and fecal genotoxicity that the fecal contents provides the best available non-invasive way of studying “exposures” of the colorectal mucosa and suggests fecal genotoxicity as a potential intermediate between dietary genotoxins and CRC. Then and now, variability of measurements severely hampers the assessment of dietary components and their potential to enhance or reduce fecal genotoxicity. These complex changes in chemical composition of feces might only induce subtle changes in the genotoxic potential which could remain undetected. However, the study of fecal samples is still considered an important field. The use of various cell based assays to describe fecal water activity combined with sophisticated analytical methods detecting harmful and beneficial chemicals and DNA adducts might be a way forward. This way we could better describe the nature of this complex sample and assess the role of diet and microbiota on its potential to affect the colonic mucosa. If this approach will ever be robust enough to allow statements on the effect of diet on the risk to develop colon cancer remains to be seen in the future.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ames, B. N., McCann, J., and Yamasaki, E. (1975). Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 31, 347–364.
Arranz, N., Haza, A. I., Garcia, A., Moller, L., Rafter, J., and Morales, P. (2007a). Protective effects of organosulfur compounds towards N-nitrosamine-induced DNA damage in the single-cell gel electrophoresis (SCGE)/HepG2 assay. Food Chem. Toxicol. 45, 1662–1669.
Arranz, N., Haza, A. I., Garcia, A., Rafter, J., and Morales, P. (2007b). Protective effect of vitamin C towards N-nitrosamine-induced DNA damage in the single-cell gel electrophoresis (SCGE)/HepG2 assay. Toxicol. In Vitro 21, 1311–1317.
Benassi-Evans, B., Clifton, P., Noakes, M., and Fenech, M. (2010). High-protein/high red meat and high-carbohydrate weight-loss diets do not differ in their effect on faecal water genotoxicity tested by use of the WIL2-NS cell line and with other biomarkers of bowel health. Mutat. Res. 703, 130–136.
Bingham, S. A., Hughes, R., and Cross, A. J. (2002). Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J. Nutr. 132, 3522S–3525S.
Bosworth, D., and Venitt, S. (1986). Testing human faecal extracts for genotoxic activity with the SOS chromotest: the importance of controlling for faecal enzyme activity. Mutagenesis 1, 143–149.
Bosworth, D., and Venitt, S. (1989). A study of the heterogeneity of bacterial fluctuation-test data and the effects of auxotrophic-growth enhancement. Mutagenesis 4, 126–132.
Bruce, W. R., Varghese, A. J., Furrer, R., and Land, P. C. (1977). “A mutagen in the feces of normal humans,” in Origins of Human Cancer, eds H. H. Hiatt, J. D. Watson, and J. A. Winsten (New York: Cold Spring Harbor), 1641–1646.
Burns, A. J., and Rowland, I. R. (2004). Antigenotoxicity of probiotics and prebiotics on faecal water-induced DNA damage in human colon adenocarcinoma cells. Mutat. Res. 551, 233–243.
Collins, A. R., Cadet, J., Moller, L., Poulsen, H. E., and Vina, J. (2004). Are we sure we know how to measure 8-oxo-7,8-dihydroguanine in DNA from human cells? Arch. Biochem. Biophys. 423, 57–65.
Cross, A. J., Greetham, H. L., Pollock, J. R., Rowland, I. R., and Bingham, S. A. (2006). Variability in fecal water genotoxicity, determined using the Comet assay, is independent of endogenous N-nitroso compound formation attributed to red meat consumption. Environ. Mol. Mutagen. 47, 179–184.
Cross, A. J., Pollock, J. R., and Bingham, S. A. (2003). Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 63, 2358–2360.
de Kok, T. M., and van Maanen, J. M. (2000). Evaluation of fecal mutagenicity and colorectal cancer risk. Mutat. Res. 463, 53–101.
deVet, H. C. W., Shrma, C., and Reddy, B. S. (1981). Effect of dietary fried meat on fecal mutagenic and co-mutagenic activity in humans. Nutr. Rep. Int. 23, 653–660.
Fluckiger-Isler, S., Baumeister, M., Braun, K., Gervais, V., Hasler-Nguyen, N., Reimann, R., Van Gompel, J., Wunderlich, H. G., and Engelhardt, G. (2004). Assessment of the performance of the Ames II assay: a collaborative study with 19 coded compounds. Mutat. Res. 558, 181–197.
Gallo, V., Khan, A., Gonzales, C., Phillips, D. H., Schoket, B., Györffy, E., Anna, L., Kovács, K., Møller, P., Loft, S., Kyrtopoulos, S., Matullo, G., and Vineis, P. (2008). Validation of biomarkers for the study of environmental carcinogens: a review. Biomarkers 13, 505–534.
Gatehouse, D., Haworth, S., Cebula, T., Gocke, E., Kier, L., Matsushima, T., Melcion, C., Nohmi, T., Ohta, T., and Venitt, S. (1994). Recommendations for the performance of bacterial mutation assays. Mutat. Res. 312, 217–233.
Gill, C. I., and Rowland, I. R. (2002). Diet and cancer: assessing the risk. Br. J. Nutr. 88(Suppl. 1), S73–87.
Glei, M., Habermann, N., Osswald, K., Seidel, C., Persin, C., Jahreis, G., and Pool-Zobel, B. L. (2005). Assessment of DNA damage and its modulation by dietary and genetic factors in smokers using the Comet assay: a biomarker model. Biomarkers 10, 203–217.
Glinghammar, B., Venturi, M., Rowland, I. R., and Rafter, J. J. (1997). Shift from a dairy product-rich to a dairy product-free diet: influence on cytotoxicity and genotoxicity of fecal water--potential risk factors for colon cancer. Am. J. Clin. Nutr. 66, 1277–1282.
Gonzalez, C. A., Jakszyn, P., Pera, G., Agudo, A., Bingham, S., Palli, D., Ferrari, P., Boeing, H., del Giudice, G., Plebani, M., Carneiro, F., Nesi, G., Berrino, F., Sacerdote, C., Tumino, R., Panico, S., Berglund, G., Siman, H., Nyren, O., Hallmans, G., Martinez, C., Dorronsoro, M., Barricarte, A., Navarro, C., Quiros, J. R., Allen, N., Key, T. J., Day, N. E., Linseisen, J., Nagel, G., Bergmann, M. M., Overvad, K., Jensen, M. K., Tjonneland, A., Olsen, A., Bueno-de-Mesquita, H. B., Ocke, M., Peeters, P. H., Numans, M. E., Clavel-Chapelon, F., Boutron-Ruault, M. C., Trichopoulou, A., Psaltopoulou, T., Roukos, D., Lund, E., Hemon, B., Kaaks, R., Norat, T., and Riboli, E. (2006). Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 98, 345–354.
Gottschalg, E., Scott, G. B., Burns, P. A., and Shuker, D. E. (2007). Potassium diazoacetate-induced p53 mutations in vitro in relation to formation of O6-carboxymethyl- and O6-methyl-2′-deoxyguanosine DNA adducts: relevance for gastrointestinal cancer. Carcinogenesis 28, 356–362.
Green, M. H., Muriel, W. J., and Bridges, B. A. (1976). Use of a simplified fluctuation test to detect low levels of mutagens. Mutat. Res. 38, 33–42.
Greetham, H. L., Bingham, S. A., and Burns, P. A. (2007). Adduction of human p53 gene by fecal water: an in vitro biomarker of mutagenesis in the human large bowel. Cancer Epidemiol. Biomarkers Prev. 16, 2681–2685.
Hebels, D. G., Jennen, D. G., Kleinjans, J. C., and de Kok, T. M. (2009). Molecular signatures of N-nitroso compounds in Caco-2 cells: implications for colon carcinogenesis. Toxicol. Sci. 108, 290–300.
Helbig, D., Wagner, A., Glei, M., Basu, S., Schubert, R., and Jahreis, G. (2009). Blackcurrant seed press residue increases tocopherol concentrations in serum and stool whilst biomarkers in stool and urine indicate increased oxidative stress in human subjects. Br. J. Nutr. 102, 554–562.
Hosoda, M., Hashimoto, H., He, F., Morita, H., and Hosono, A. (1996). Effect of administration of milk fermented with Lactobacillus acidophilus LA-2 on fecal mutagenicity and microflora in the human intestine. J. Dairy Sci. 79, 745–749.
Hubbard, S. A., Green, M. H., Gatehouse, D., and Bridges, B. A. (1976). “The fluctuation test in bacteria,” in Handbook of Mutagenesis Procedures, eds B. J. Kilbey, M. Legator, W. Nichols, and C. Ramel (Amsterdam: Elsevier Science Publishers BV), 141–160.
Hughes, R., Cross, A. J., Pollock, J. R., and Bingham, S. (2001). Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis 22, 199–202.
Hughes, R., Magee, E. A., and Bingham, S. (2000). Protein degradation in the large intestine: relevance to colorectal cancer. Curr. Issues Intest. Microbiol. 1, 51–58.
Hughes, R., Pollock, J. R., and Bingham, S. (2002). Effect of vegetables, tea, and soy on endogenous N-nitrosation, fecal ammonia, and fecal water genotoxicity during a high red meat diet in humans. Nutr. Cancer 42, 70–77.
Jakszyn, P., Bingham, S., Pera, G., Agudo, A., Luben, R., Welch, A., Boeing, H., Del Giudice, G., Palli, D., Saieva, C., Krogh, V., Sacerdote, C., Tumino, R., Panico, S., Berglund, G., Siman, H., Hallmans, G., Sanchez, M. J., Larranaga, N., Barricarte, A., Chirlaque, M. D., Quiros, J. R., Key, T. J., Allen, N., Lund, E., Carneiro, F., Linseisen, J., Nagel, G., Overvad, K., Tjonneland, A., Olsen, A., Bueno-de-Mesquita, H. B., Ocke, M. O., Peeters, P. H., Numans, M. E., Clavel-Chapelon, F., Trichopoulou, A., Fenger, C., Stenling, R., Ferrari, P., Jenab, M., Norat, T., Riboli, E., and Gonzalez, C. A. (2006). Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC–EURGAST) study. Carcinogenesis 27, 1497–1501.
Johansson, G., Holmen, A., Persson, L., Hogstedt, B., Wassen, C., Ottova, L., and Gustafsson, J. A. (1998). Long-term effects of a change from a mixed diet to a lacto-vegetarian diet on human urinary and faecal mutagenic activity. Mutagenesis 13, 167–171.
Johansson, G., Holmen, A., Persson, L., Hogstedt, R., Wassen, C., Ottova, L., and Gustafsson, J. A. (1992). The effect of a shift from a mixed diet to a lacto-vegetarian diet on human urinary and fecal mutagenic activity. Carcinogenesis 13, 153–157.
Joosen, A. M., Kuhnle, G. G., Aspinall, S. M., Barrow, T. M., Lecommandeur, E., Azqueta, A., Collins, A. R., and Bingham, S. A. (2009). Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis 30, 1402–1407.
Joosen, A. M., Lecommandeur, E., Kuhnle, G. G., Aspinall, S. M., Kap, L., and Rodwell, S. A. (2010). Effect of dietary meat and fish on endogenous nitrosation, inflammation and genotoxicity of faecal water. Mutagenesis 25, 243–247.
Kamber, M., Fluckiger-Isler, S., Engelhardt, G., Jaeckh, R., and Zeiger, E. (2009). Comparison of the Ames II and traditional Ames test responses with respect to mutagenicity, strain specificities, need for metabolism and correlation with rodent carcinogenicity. Mutagenesis 24, 359–366.
Klinder, A., Karlsson, P. C., Clune, Y., Hughes, R., Glei, M., Rafter, J. J., Rowland, I., Collins, J. K., and Pool-Zobel, B. L. (2007). Fecal water as a non-invasive biomarker in nutritional intervention: comparison of preparation methods and refinement of different endpoints. Nutr. Cancer 57, 158–167.
Kosaka, H., Nakamura, S., Oda, H., Miyajima, T., Sumimoto, T., Murata, H., Hori, S., Komachi, Y., Sato, S., Kiyama, M., Naito, Y., and Iida, M. (2001). Relationship between the fecal mutagenicity and metal content, smoking habit and dietary intake (Abstract only). Nippon Koshu Eisei Zasshi 48, 929–937.
Kuhnle, G. G., and Bingham, S. A. (2007). Dietary meat, endogenous nitrosation and colorectal cancer. Biochem. Soc. Trans. 35, 1355–1357.
Kuhnle, G. G., Story, G. W., Reda, T., Mani, A. R., Moore, K. P., Lunn, J. C., and Bingham, S. A. (2007). Diet-induced endogenous formation of nitroso compounds in the GI tract. Free Radic. Biol. Med. 43, 1040–1047.
Kuhnlein, H. V., Kuhnlein, U., and Bell, P. A. (1983). The effect of short-term dietary modification on human fecal mutagenic activity. Mutat. Res. 113, 1–12.
Lewin, M. H., Bailey, N., Bandaletova, T., Bowman, R., Cross, A. J., Pollock, J., Shuker, D. E., and Bingham, S. A. (2006). Red meat enhances the colonic formation of the DNA adduct O6-carboxymethyl guanine: implications for colorectal cancer risk. Cancer Res. 66, 1859–1865.
Lunn, J. C., Kuhnle, G., Mai, V., Frankenfeld, C., Shuker, D. E., Glen, R. C., Goodman, J. M., Pollock, J. R., and Bingham, S. A. (2007). The effect of haem in red and processed meat on the endogenous formation of N-nitroso compounds in the upper gastrointestinal tract. Carcinogenesis 28, 685–690.
Mai, V., McCrary, Q. M., Sinha, R., and Glei, M. (2009). Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutr. J. 8, 49.
Matsumoto, M., and Benno, Y. (2004). Consumption of Bifidobacterium lactis LKM512 yogurt reduces gut mutagenicity by increasing gut polyamine contents in healthy adult subjects. Mutat. Res. 568, 147–153.
Matsumoto, M., Ohishi, H., and Benno, Y. (2001). Impact of LKM512 yogurt on improvement of intestinal environment of the elderly. FEMS Immunol. Med. Microbiol. 31, 181–186.
Mower, H. F., Ichinotsubo, D., Wang, L. W., Mandel, M., Stemmermann, G., Nomura, A., Heilbrun, L., Kamiyama, S., and Shimada, A. (1982). Fecal mutagens in two Japanese populations with different colon cancer risks. Cancer Res. 42, 1164–1169.
Nader, C. J., Spencer, L. K., and Weller, R. A. (1981). Mutagen production during pan-broiling compared with microwave irradiation of beef. Cancer Lett. 13, 147–152.
Nair, P. P., Davis, K. E., Shami, S., and Lagerholm, S. (2000). The induction of SOS function in Escherichia coli K-12/PQ37 by 4-nitroquinoline oxide (4-NQO) and fecapentaenes-12 and -14 is bile salt sensitive: implications for colon carcinogenesis. Mutat. Res. 447, 179–185.
Nair, P. P., Shami, S., Sainz, E., Judd, J. T., Taylor, P. R., and Schatzkin, A. (1991). Quantitative assessment of the genotoxicity of fecapentaenes. Mutat. Res. 260, 153–157.
Nordling, M. M., Glinghammar, B., Karlsson, P. C., de Kok, T. M., and Rafter, J. J. (2003). Effects on cell proliferation, activator protein-1 and genotoxicity by fecal water from patients with colorectal adenomas. Scand. J. Gastroenterol. 38, 549–555.
Oberreuther-Moschner, D. L., Jahreis, G., Rechkemmer, G., and Pool-Zobel, B. L. (2004). Dietary intervention with the probiotics Lactobacillus acidophilus 145 and Bifidobacterium longum 913 modulates the potential of human faecal water to induce damage in HT29clone19A cells. Br. J. Nutr. 91, 925–932.
Organisation for Economic Co-operation and Development. (1997). “Bacterial muttation test,” in OECD Guidlines for Testing of Chemicals, Paris.
Osswald, K., Becker, T. W., Grimm, M., Jahreis, G., and Pool-Zobel, B. L. (2000). Inter- and intra-individual variation of faecal water - genotoxicity in human colon cells. Mutat. Res. 472, 59–70.
Pearson, J. R., Gill, C. I., and Rowland, I. R. (2009). Diet, fecal water, and colon cancer--development of a biomarker. Nutr. Rev. 67, 509–526.
Pot, G. K., Habermann, N., Majsak-Newman, G., Harvey, L. J., Geelen, A., Przybylska-Philips, K., Nagengast, F. M., Witteman, B. J., van de Meeberg, P. C., Hart, A. R., Schaafsma, G., Hooiveld, G., Glei, M., Lund, E. K., Pool-Zobel, B. L., and Kampman, E. (2010). Increasing fish consumption does not affect genotoxicity markers in the colon in an intervention study. Carcinogenesis 31, 1087–1091.
Preston, R. J., and Williams, G. M. (2005). DNA-reactive carcinogens: mode of action and human cancer hazard. Crit. Rev. Toxicol. 35, 673–683.
Quillardet, P., and Hofnung, M. (1985). The SOS chromotest, a colorimetric bacterial assay for genotoxins: procedures. Mutat. Res. 147, 65–78.
Quillardet, P., Huisman, O., D’Ari, R., and Hofnung, M. (1982). SOS chromotest, a direct assay of induction of an SOS function in Escherichia coli K-12 to measure genotoxicity. Proc. Natl. Acad. Sci. U.S.A. 79, 5971–5975.
Rafter, J., Bennett, M., Caderni, G., Clune, Y., Hughes, R., Karlsson, P. C., Klinder, A., O’Riordan, M., O’Sullivan, G. C., Pool-Zobel, B., Rechkemmer, G., Roller, M., Rowland, I., Salvadori, M., Thijs, H., Van Loo, J., Watzl, B., and Collins, J. K. (2007). Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 85, 488–496.
Reddy, B. S., Sharma, C., Darby, L., Laakso, K., and Wynder, E. L. (1980). Metabolic epidemiology of large bowel cancer. Fecal mutagens in high- and low-risk population for colon cancer. A preliminary report. Mutat. Res. 72, 511–522.
Rieger, M. A., Parlesak, A., Pool-Zobel, B. L., Rechkemmer, G., and Bode, C. (1999). A diet high in fat and meat but low in dietary fibre increases the genotoxic potential of “faecal water”. Carcinogenesis 20, 2311–2316.
Santarelli, R. L., Pierre, F., and Corpet, D. E. (2008). Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr. Cancer 60, 131–144.
Savitskaia, I. S., and Bondarenko, V. M. (2008). Inhibition of mutagen activity of colon metabolites by normal microbiocenosis. Zh. Mikrobiol. Epidemiol. Immunobiol. 3, 53–58.
Schiffman, M. H., Andrews, A. W., Van Tassell, R. L., Smith, L., Daniel, J., Robinson, A., Hoover, R. N., Rosenthal, J., Weil, R., and Nair, P. P. (1989). Case–control study of colorectal cancer and fecal mutagenicity. Cancer Res. 49, 3420–3424.
Suzuki, K., and Mitsuoka, T. (1992). Effect of low-fat, high-fat, and fiber- supplemented high-fat diets on colon cancer risk factors in feces of healthy subjects. Nutr. Cancer 18, 63–71.
Tice, R. R., Agurell, E., Anderson, D., Burlinson, B., Hartmann, A., Kobayashi, H., Miyamae, Y., Rojas, E., Ryu, J. C., and Sasaki, Y. F. (2000). Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 35, 206–221.
Venitt, S., and Bosworth, D. (1986). Further studies on the detection of mutagenic and genotoxic activity in human faeces: aerobic and anaerobic fluctuation tests with S. typhimurium and E. coli, and the SOS chromotest. Mutagenesis 1, 49–64.
Venitt, S., Bosworth, D., and Alldrick, A. J. (1986). Pilot study of the effect of diet on the mutagenicity of human faeces. Mutagenesis 1, 353–358.
Venturi, M., Hambly, R. J., Glinghammar, B., Rafter, J. J., and Rowland, I. R. (1997). Genotoxic activity in human faecal water and the role of bile acids: a study using the alkaline comet assay. Carcinogenesis 18, 2353–2359.
Wasson, G. R., McKelvey-Martin, V. J., and Downes, C. S. (2008). The use of the comet assay in the study of human nutrition and cancer. Mutagenesis 23, 153–162.
WCRF. (2007). Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington DC: AICR.
Keywords: Ames test, comet assay, DNA, feces, colon, diet, N-nitroso compounds, human
Citation: Gratz SW, Wallace RJ and El-Nezami HS (2011) Recent perspectives on the relations between fecal mutagenicity, genotoxicity, and diet. Front. Pharmacol. 2:4. doi: 10.3389/fphar.2011.00004
Received: 31 August 2010;
Accepted: 31 January 2011;
Published online: 03 March 2011.
Edited by:
Erwin L. Roggen, Novozymes, DenmarkReviewed by:
Erwin L. Roggen, Novozymes, DenmarkClaus Thagaard Christophersen, Commonwealth Scientific and Industrial Research Organisation, Australia
Copyright: © 2011 Gratz, Wallace and El-Nezami. This is an open-access article subject to an exclusive license agreement between the authors and Frontiers Media SA, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Silvia W. Gratz, Microbial Biochemistry Group, Rowett Institute of Nutrition and Health, University of Aberdeen, Greenburn Road, Bucksburn, Aberdeen AB21 9SB, UK. e-mail: s.gratz@abdn.ac.uk