- 1 Department of Pharmacology, Eurofins-Product Safety Laboratories, Dayton, NJ, USA
- 2 Department of Physiology and Biophysics, Robert Wood Johnson Medical School, Piscataway, NJ, USA
- 3 Alizee Pathology, Thurmont, MD, USA
- 4 Factors R & D Technologies, Burnaby, BC, Canada
- 5 Canadian Centre for Functional Medicine, Coquitlam, BC, Canada
- 6 Food, Nutrition and Health Program, University of British Columbia, Vancouver, BC, Canada
- 7 Faculty of Kinesiology, Department of Biochemistry and Molecular Biology, University of Calgary, Calgary, AB, Canada
Dietary fiber can reduce insulin resistance, body weight, and hyperlipidemia depending on fiber type, water solubility, and viscosity. PolyGlycopleX® (PGX®) is a natural, novel water soluble, non-starch polysaccharide complex that with water forms a highly viscous gel compared to other naturally occurring dietary fiber. We determined the effect of dietary PGX® vs. cellulose and inulin on the early development of insulin resistance, body weight, hyperlipidemia, and glycemia-induced tissue damage in young Zucker diabetic rats (ZDFs) in fasted and non-fasted states. ZDFs (5 weeks old) were fed a diet containing 5% (wgt/wgt) cellulose, inulin, or PGX® for 8 weeks. Body weight, lipids, insulin, and glucose levels were determined throughout the study and homeostasis model assessment (HOMA) was used to measure insulin sensitivity throughout the study in fasted animals. At study termination, insulin sensitivity (oral glucose tolerance test, OGTT) and kidney, liver, and pancreatic histopathology were determined. Body weight and food intake were significantly reduced by PGX® vs. inulin and cellulose. Serum insulin in fasted and non-fasted states was significantly reduced by PGX® as was non-fasted blood glucose. Insulin resistance, measured as a HOMA score, was significantly reduced by PGX® in weeks 5 through 8 as well as terminal OGTT scores in fed and fasted states. Serum total cholesterol was also significantly reduced by PGX®. PGX® significantly reduced histological kidney and hepatic damage in addition to reduced hepatic steatosis and cholestasis. A greater mass of pancreatic β-cells was found in the PGX® group. PGX® therefore may be a useful dietary additive in the control of the development of the early development of the metabolic syndrome.
Introduction
Dietary fiber is generally accepted as having efficacy in disease states including colorectal cancer, obesity, cardiovascular disease, insulin resistance, and diabetes (Wilson and Wilson, 1984; Hill, 1997; Howarth et al., 2001; Grunberger et al., 2006; Panahi et al., 2007). The effects of non-digestible dietary fiber are variable depending on their physicochemical properties with viscous, water soluble fibers being more efficacious for lipid reduction, weight reduction, and improved glycemic control compared to insoluble, low viscosity fibers (Blackwood et al., 2000; Jenkins et al., 2000; Panahi et al., 2007). Improved post-prandial glycemic control and weight reduction may be due to slowed gastric emptying due to bulk-forming properties, binding of carbohydrates (Walsh et al., 1983; Anderson et al., 1999; Chandala et al., 2000), and increased expression of proglucagon, which is the precursor of glucagon-like peptide-1 (GLP-1; Reimer and McBurney, 1996; Massimino et al., 1998; Wang et al., 2007). GLP-1 inhibits gastric emptying, acid secretion, and glucagon secretion but also increases insulin sensitivity and secretion (Kim and Egan, 2008). For cholesterol reduction, viscosity and water solubility are also critical factors (Venter et al., 1990; Sugatani et al., 2008). High viscosity fibers interfere with ileal bile acid absorption thereby reducing cholesterol re-absorption as well as increasing hepatic cholesterol metabolism and breakdown (Judd and Truswell, 1982; Ganji and Kies, 1994). Fibers such as pectin lower hepatic lipid and triglyceride levels, although these effects are variable, depending on the model and specific fiber involved (Wilson and Wilson, 1984; Williams, 1991; Anderson et al., 1994).
Levels of non-digestible fiber needed to affect critical parameters involved with metabolic syndrome vary from 10 to 20 g per day depending on such factors as viscosity (Rozan et al., 2008) and efforts to increase viscosity through novel methods of synthesis may reduce the amount of fiber needed to be incorporated into food (Rozan et al., 2008). A new fiber complex has been developed that possesses superior gel-forming properties as described in detail by Abdelhameed et al. (2010) and Harding et al. (2010). The viscous polysaccharide used in this study goes under the name PolyGlycopleX® [(α-D-glucurono-α-D-manno-β-D-mannoβ-D-gluco), (α-L-glucurono-β-D mannurono), β-D-gluco-β-D-mannan; PGX®)] (InovoBiologic Inc., Calgary, AB, Canada) and is manufactured from highly purified polysaccharides by a proprietary process (EnviroSimplex®) from konjac, sodium alginate and xanthan gum. These polysaccharides form a complex with a viscosity higher than any currently known individual polysaccharide. Although PGX® complex formation takes place at secondary and tertiary levels (networking and junction zones), the primary structures of the natural polysaccharides remain unchanged (Harding et al., 2010) and PGX® is approved as a Natural Health Product in Canada by Health Canada’s Natural Health Product Directorate (NHPD) with health claims. A recently published study showed PGX® to improve acute and delayed post-prandial glycemic control in healthy human subjects (Brand-Miller et al., 2010) and suggest therapeutic potential for PGX®, although more detailed studies are necessary. A recent study by Reimer et al. (2011a) showed significant reductions in body weight and triglycerides in high sucrose fed Sprague-Dawley rats that were obese but not diabetic but results on these in a model of florid, well developed diabetes in Zucker diabetic rats (ZDFs) were variable, perhaps due to the degree of damage and the progression of the disease already present (Grover et al., 2011). In this model, once diabetes became severe, improvements in glycemic control were observed. PGX® in the later stages of diabetes actually preserved body weight, perhaps due to improved glycemic control and increased efficiency of substrates. In addition, PGX® also increased serum triglycerides in these ZDFs perhaps due to altered mobilization from the liver. No studies have been done in young ZDFs to determine the effect PGX® on the development of metabolic syndrome and this model may be more relevant for observing effects on body weight, triglycerides, and the progression of protein glycation-induced tissue damage.
Our goal was to determine the effects of PGX® on food intake, body weight, glycemic control, and lipids (cholesterol and triglycerides) profile in ZDFs as well as histomorphometric assessment of liver, pancreas, and kidney damage seen in developing diabetes. This was done in young ZDFs to assess the ability of this fiber to inhibit or prevent early development of the sequelae of the metabolic syndrome. Inulin in its variant forms improves glycemic control and reduce body weight (Sugatani et al., 2008), although usually at doses approximating 10% (Rozan et al., 2008). Our objective was to determine if PGX® can reduce the development of aspects of the metabolic syndrome at relatively lower doses.
Materials and Methods
Experimental Design
Male ZDFs were used, as they represent a good model of obesity with co-morbid type 2 diabetes and reduced insulin sensitivity at the early ages used in the present study (Daubioul et al., 2002; Lenhard et al., 2004). We used young ZDFs to determine if PGX® could slow the early progression of obesity and diabetes. Thirty male ZDF/Crl-Leprfa/fa rats obtained from Charles River, (Kingston, NY, USA) at 5 weeks of age were housed singly in suspended wire mesh cages. They conformed to the size recommendations in the most recent Guide for the Care and Use of Laboratory Animals. All studies were approved by the Eurofins Institutional Animal Use and Care Committee. The animals were conditioned for 1 week after arrival and the animals had access to water and food ad libitum. At this age, the ZDFs are not hyperglycemic under fasting conditions, but are insulin resistant, although glucose is poorly controlled under fed conditions.
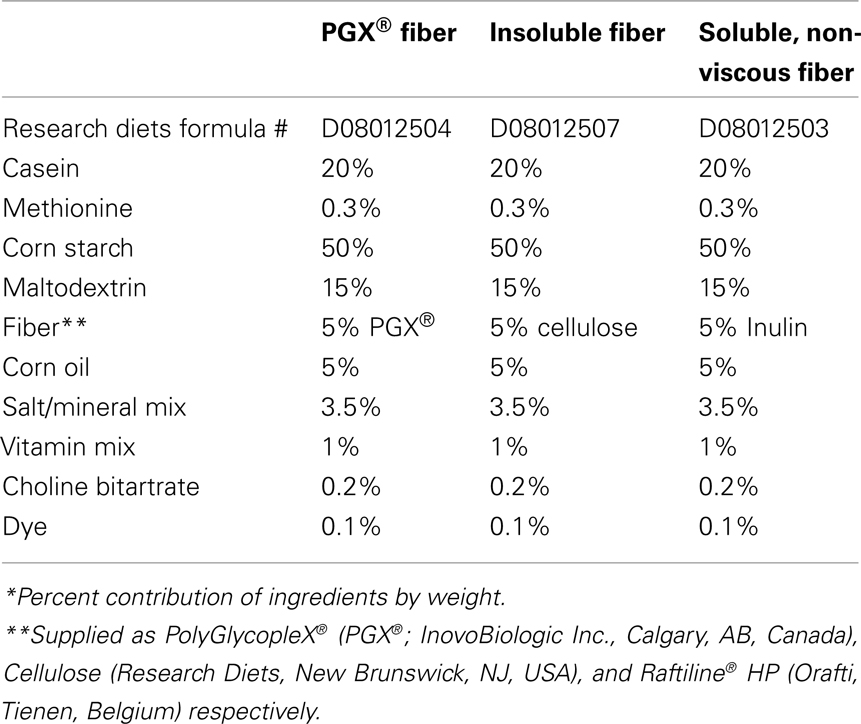
PolyGlycopleX®, cellulose, and inulin were shipped to Research Diets (New Brunswick, NJ, USA) for incorporation into a basic rat chow (D11725) at 5% wgt/wgt (Table 1). All diets were formulated to be isoenergetic (PGX® and inulin diets provided 3.98 kcal/g and cellulose provided 3.90 kcal/g). The rats were randomly assigned to one of three groups each given chow containing either: PGX®, cellulose, or inulin (Raftiline® HP) for 8 weeks. Cellulose was selected as the insoluble reference fiber that is considered to be inert (Anderson et al., 1994). Inulin is water soluble and non-digestible and some papers have shown efficacy for weight reduction, lipid reduction, and glycemic control, usually at 10% wgt/wgt or greater (Rozan et al., 2008). An sample size of 10 was used for each group and no animals were excluded from the study.
Basic monitoring procedures were conducted throughout the study (i.e., thrice weekly measurement of food intake and once per week measurement of body weight). Blood glucose and serum insulin were measured after 16 h fasting once per week and the blood was collected from the retro-orbital plexus under light isoflurane anesthesia and the first measurements were done at the end of week 1 and then throughout the rest of the study. Measurements while in the fed state are important as this is when hyperglycemia is first evident and because the action of the fibers are exerted primarily while they are in the gut, particularly since ZDFs eat continuously through the night. Measurement of non-fasted glucose was also done weekly starting at week 3 and throughout the rest of the study and the measurement of fasted vs. non-fasted glucose levels were separated by 2 days. We recognized within the first 2 weeks that non-fasted glucose values would be better predictors of glycemic control given that these animals feed continuously. Hyperglycemia was only observed in the non-fasted state and this was also when PGX® was in the gastrointestinal tract. In the non-fasted state, serum insulin was only measured at the end of the study [baseline measurement of final non-fasted oral glucose tolerance test (OGTT), see below]. Weekly insulin measurements were not done in the non-fasted state as too much blood would need to be withdrawn per week to do this for both fasted and non-fasted states. We felt that since blood glucose was not elevated in these young ZDFs when fasted, insulin was the critical variable under these conditions and consequently we measured this more thoroughly. Blood glucose was measured using a Bayer Ascensia Elite Glucometer (Bayer Health Care, Tarrytown, NY, USA) and serum insulin was measured using an ELISA (AniLytics, Gaithersburg, MD, USA). In fasted animals, serum triglycerides were measured once weekly (along with the glucose measurement) throughout the study, while in non-fasted animals, only a terminal measurement was taken along with cholesterol (IDEXX, North Grafton, MA, USA).
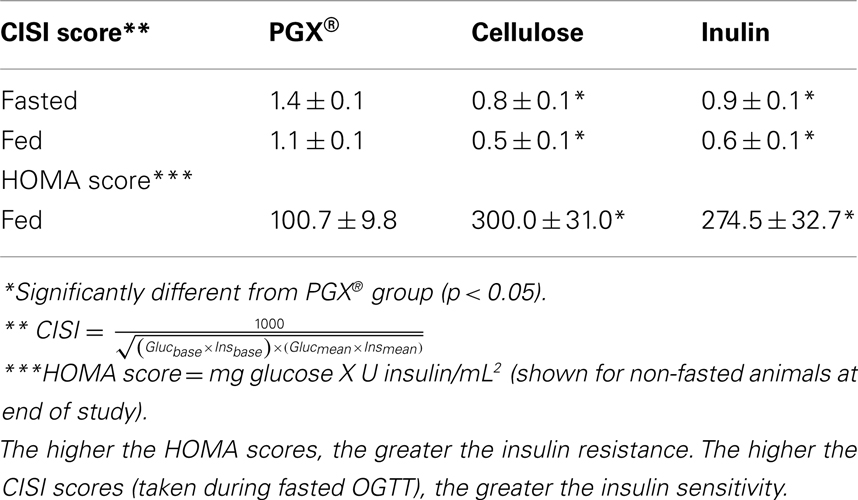
The study was concluded with two OGTTs; fasted and non-fasted with the fasted measurement being done at week 7, the non-fasted OGTT being done at the end of week 8 and glucose and insulin were measured after glucose challenge in both groups. Homeostasis model assessment (HOMA) scores were calculated throughout the study as (mg glucose × U insulin/mL2) for fasted animals and at the end of the study for non-fasted animals (baseline insulin only measured at end of study). Lower HOMA scores are reflective of increased peripheral insulin sensitivity. We also calculated the composite insulin sensitivity index (CISI) scores for the OGTT studies using the following formula:
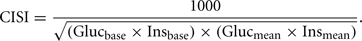
This score takes into account glucose excursion/area under the curve (higher score shows improved insulin sensitivity). The initial (pre-glucose loading) blood sample for the final (non-fasted) OGTT and the final 8 week blood samples were also used for a clinical chemistry panel including: electrolytes, blood urea nitrogen (BUN), blood glucose, creatinine alkaline phosphatase, aspartate aminotransferase (ALT), alanine aminotransferase (AST), and bilirubin (direct + indirect), non-fasted insulin and serum cholesterol (IDEXX, North Grafton, MA, USA).
Oral glucose tolerance tests were performed using an oral 2 g/kg glucose challenge and blood samples from tail nicks were taken at 30, 60, 90, and 120 min after the glucose challenge and were analyzed for blood glucose and serum insulin content for both fasted (7 weeks) and non-fasted (8 weeks) animals. At the conclusion of the second OGTT, all rats were sacrificed by isoflurane overdose and the relevant organs were harvested for histopathological analysis.
Necropsy
One lobe of the liver, one kidney and the pancreas were fixed in 10% neutral buffered formalin. The pancreas was transferred to 70% ethanol after 24 h. Tissues were processed and embedded in paraffin. The liver and kidney were sectioned (5 μm) and stained with hematoxylin and eosin (H&E). The pancreas was serial sectioned twice (5 μm) and were stained with H&E or immunohistochemically stained with a mouse antibody against rat insulin (1:300, Cell Signaling Technology, Danvers, MA, USA). Immunohistochemistry was performed according to the following procedure. Following deparaffinization, antigen retrieval was performed using Declere® solution (Cell Marque™ Corporation, Rocklin, CA, USA). Slides were incubated for 20 min in 5% normal goat serum. The slides were incubated with the primary antibody for 60 min followed by 30 min incubation in biotinylated goat anti-rabbit and hematoxylin counterstaining. Following necropsy, an additional liver lobe was snap-frozen, embedded in OCT, sectioned at 5 μm, and stained with Sudan black for analysis of lipid content.
All slides stained with H&E or Sudan black were evaluated for morphological changes related to those commonly observed in ZDFs and were graded semi-quantitatively on a scale of 0–5 based upon the severity of that finding. The percent of the islet area with insulin positive cells was measured morphometrically on the pancreas slides stained with anti-insulin antibody. The percent of islet area positive for insulin was calculated using ImagePro® Plus imaging software. Previous work suggests that increased GLP-1 will increase or preserve the density of pancreatic insulin-secreting cells, although this has not been looked at in detail for dietary fiber (Kim et al., 2007). Circulating GLP-1 is significantly increased by dietary fiber (Reimer and McBurney, 1996) and preliminary data show PGX® to have similar effects (Grover et al., 2011).
Statistical Methods
Data collected at multiple times were analyzed by two-way repeated measures analysis of variance (ANOVA) and post hoc comparisons using Bonferroni’s multiple comparison test (MCT). Insulin, cholesterol, and blood chemistries measured only at the end of the study in non-fasted rats were analyzed by one-way ANOVA followed by post hoc comparisons using Dunnett’s MCT. Non-interval data (e.g., histology scores) were analyzed by Kruskal Wallis test and Dunn’s MCT. Significance was set at p < 0.05.
Results
Food Consumption and Body Weight
Rats fed a PGX®-containing diet gained weight at a significantly slower rate than rats fed cellulose- or inulin-containing diets (Figure 1A). Food intake was significantly lower for PGX®-treated animals compared to the inulin and cellulose groups up to 4 weeks into the protocol (Figure 1B). Food intake in the PGX® group remained lower for the rest of the protocol but is not statistically different from the other two groups although the reduction is still likely to be physiologically significant. A previous study from our laboratory showed that PGX® increased body weight relative to control, but this was after the animals were floridly diabetic and their body weight growth curves were asymptotic. The obese ZDFs stopped gaining weight much sooner than non-obese rats with the same genetic background (Grover et al., 2011). This suggests the possibility that at the late stage of diabetes, body weight may be difficult to maintain and PGX® helped maintain weight by more efficient use of substrates.
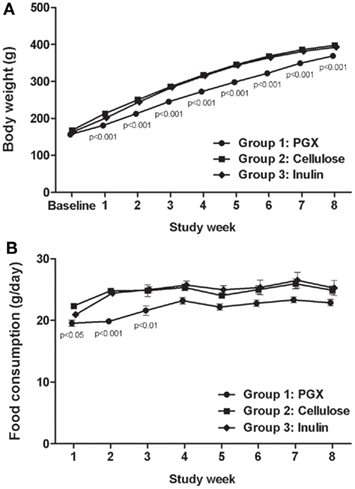
Figure 1. The effect of PGX®, cellulose, or inulin diets on body weight (A) and food consumption (B) in Zucker diabetic rats (ZDFs). The increase in body weight with respect to time was significantly obtunded in the PGX®-treated animals (cellulose-fed vs. PGX®fed animals, from week 1 to 8). Food consumption was significantly reduced in PGX®-treated animals for the first 3–4 weeks but the trend continued throughout the study. p Values marked on the figure are those of PGX® vs. cellulose.
Glycemic Control
Under fasted conditions (16 h), rats had near normal (i.e., non-diabetic) blood glucose concentrations (Figure 2A). Under these conditions, rats fed the PGX®-containing diet maintained lower serum insulin concentrations compared to the other groups, which had elevated insulin (Figure 2B). Significant insulin reductions caused by PGX® were seen starting at week 5 up to study termination. Insulin resistance during the course of this study in fasted rats was assessed by calculating HOMA and CISI scores (Figure 2C; Table 2). HOMA scores rose over the course of the study but significantly less so for the PGX® group from 5 to 8 weeks. CISI scores were calculated for the OGTT in fasted animals (7 weeks) as another test for insulin sensitivity. This score was significantly higher for PGX® treated animals, further showing improved insulin sensitivity (Table 2).
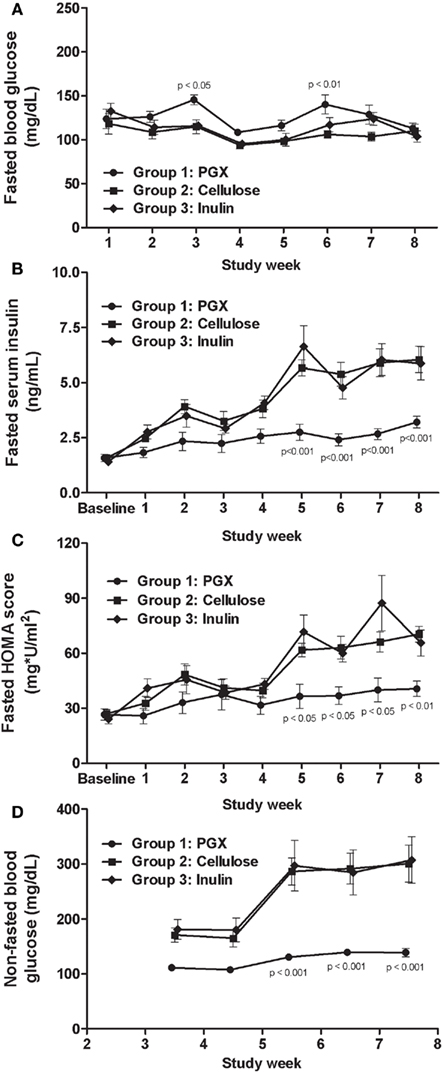
Figure 2. The effect of PGX®, cellulose, or inulin diets on fasted blood glucose (A), fasted serum insulin (B), fasted HOMA scores (C), and non-fasted glucose (D) with respect to time in Zucker diabetic rats (ZDFs). Fasted blood glucose values were not greatly elevated in any group. Fasted serum insulin was reduced throughout the protocol by PGX®, but significance was seen starting at 5 weeks. Fasted HOMA scores throughout the study are shown on (C). PGX® significantly reduced the HOMA score starting at 5 weeks into the procedure. Non-fasted glucose (D) was significantly reduced by PGX® starting at 5 weeks. p Values marked on the figure are those of PGX® vs. cellulose.
Under non-fasted conditions, PGX® treated rats had lower blood glucose compared to rats in the other two groups from week 3 onwards and significantly from weeks 5 through 8 (Figure 2D). In fed animals we only measured serum insulin under resting conditions before glucose challenge as the baseline level for the OGTT done at 8 weeks and observed significantly lower insulin in PGX®-fed rats (PGX-fed, 8.68 ± 0.56 ng/mL; cellulose-fed; 10.23 ± 0.52 ng/mL; inulin-fed, 10.91 ± 0.96 ng/mL; p < 0.05). Insulin resistance (HOMA) was significantly lower in the PGX® group compared to the other groups (Table 2). The CISI scores for fed animals are also shown on Table 2 and PGX® rats showed significantly greater insulin sensitivity compared to the other groups. Glucose levels at 30 min post-glucose challenge were significantly lower for the PGX® group (Figure 3). As can be seen from Figure 3, the early part of the glucose response curve was obtunded by PGX®. After peak glucose concentrations, serum glucose levels did not return to baseline within the 120-min measurement period. This slow return to baseline has been previously reported for ZDF rats (Han et al., 2008) and high fat and high carbohydrate-fed mice under similar conditions (Pederson et al., 1998; Gault et al., 2007) and is likely due to the poor insulin response seen in these animals as well as being in the fed state. The non-fasted OGTT was shown as it was felt to be more relevant for the PGX® study. Insulin areas under the curve (AUC) were not significantly different between groups and remained at higher levels consistent with the elevated glucose but it is possible that insulin responses were limited in the fed state. Imbedded into Figure 3, the insulin AUC is shown and PGX only tended to reduce the insulin AUC. The CISI score incorporated both glucose and insulin data and showed increased insulin sensitivity for PGX.
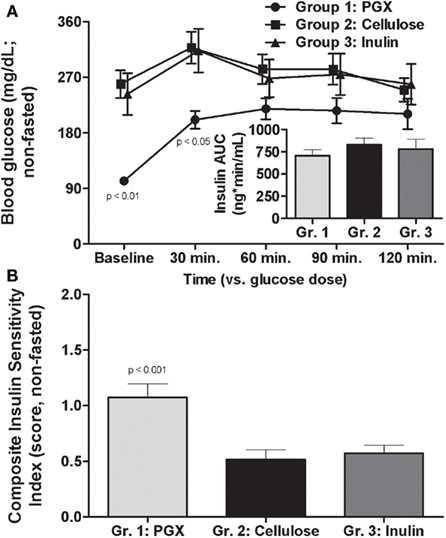
Figure 3. Effects of PGX®, cellulose, or inulin diets on oral glucose excursion in non-fasted animals after administration of 2 g/kg oral glucose (oral glucose tolerance test; OGTT at 8 weeks). PGX® significantly reduced early and peak glucose response to the challenge [(A), main figure; *p < 0.05] compared to the other fibers. The glucose did not return to baseline for any group within the time in which blood glucose was measured for this part of the study. Also shown is the area under the curve (AUC) for the insulin response [(A), inset]. While there were no significant differences, PGX tended to reduce insulin AUC. When a CISI score was determined, taking into account, glucose and insulin excursion (B), PGX significantly increased insulin sensitivity. p Values marked on the figure are those of PGX® vs. cellulose.
Lipid Profile
Serum triglycerides were measured in fasted (throughout study, Figure 4) and non-fasted states, which were measured only at the end of the study in order to reduce excess blood withdrawal (Table 3). In the fasted animals, the results were inconclusive with PGX® showing an early and significant lowering effect on triglycerides. However, after 2–3 weeks, this effect was lost. In non-fasted rats at study end, serum triglycerides were similar and higher for PGX®- and inulin-treated groups while the cellulose-treated group levels were significantly lower than the other two groups (Table 3). At the end of the study, serum cholesterol was measured in the baseline sample before the last OGTT (fed state) or as the last sample for the fasted animals. As seen in Table 3, the animals were hypercholesterolemic and PGX® significantly reduced cholesterol levels compared to cellulose and inulin-fed groups.
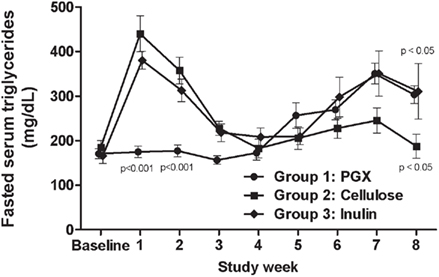
Figure 4. Fasted serum triglycerides for all groups measured throughout the study. While PGX® showed an early reduction in triglycerides, the data became variable and therefore hard to interpret. At the end of the study, inulin and PGX® did significantly increase triglycerides relative to cellulose, butthe mechanism(s) for these changes are presently unknown. p Values marked on the figure are those of PGX® vs. cellulose.
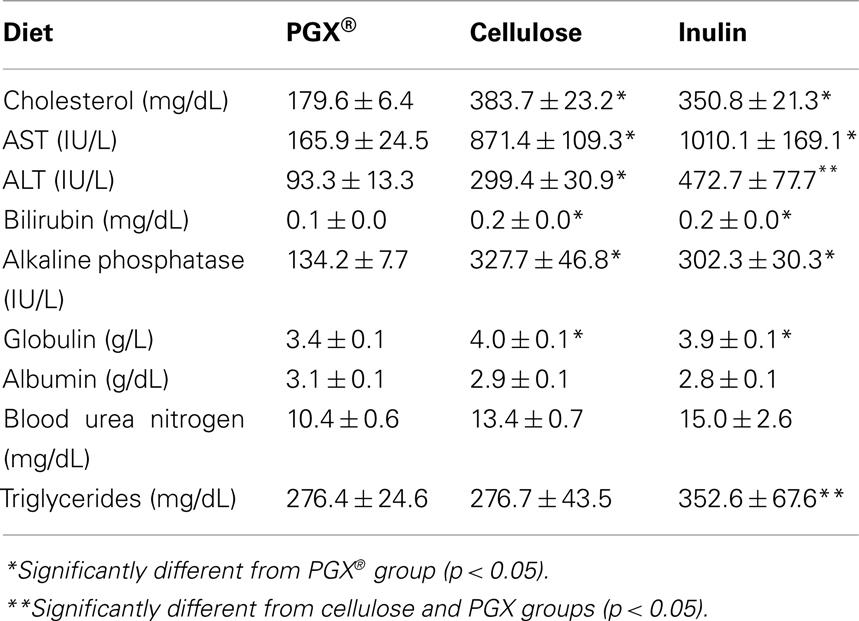
Table 3. Plasma chemistry of key analytes taken at the study termination in non-fasted rats (baseline measurement of final non-fasted OGTT).
Histopathological Evaluation of Liver, Pancreas, and Kidneys
Kidney
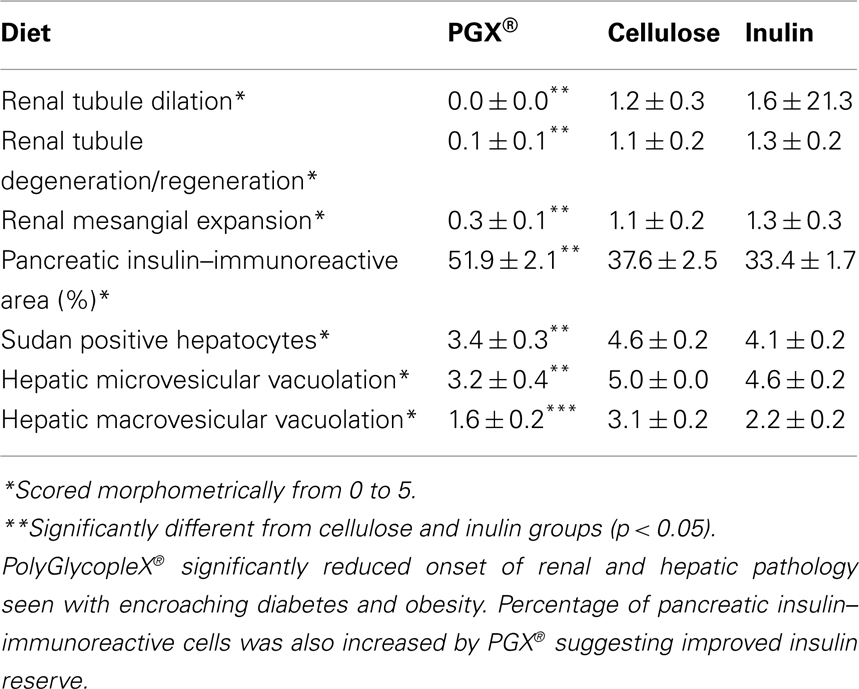
Several renal pathology parameters showed differences between rats fed PGX®-containing diet vs. the other two groups. This was assessed because of nephropathy commonly seen even in early diabetes due to protein glycation (Nonaka et al., 2007; Table 4). The overall degree of renal pathology was mild and is most likely related to the early phase in the pathogenesis of the diabetes in the relatively young ZDFs used in this study. Micrographs were not shown because the differences were subtle and the degree of damage in the cellulose or reference fiber group were so mild as to be difficult to be seen with the naked eye. Tubule dilatation was scored absent in PGX® rats, but was found in rats fed inulin or cellulose and these scores were significantly higher in these groups. Renal tubule degeneration/regeneration scores were significantly higher for rats fed cellulose- or inulin-containing diets compared to PGX®-fed rats. Glomerular mesangial expansion was reduced in the group receiving PGX®-containing diet with significant differences noted between the PGX® group vs. inulin-fed and cellulose-fed rats. Other parameters, including morphological changes in the kidneys and clinical chemistries (electrolytes, creatinine, which are not shown and BUN, Table 3) failed to reach statistical significance. BUN did tend to be lower in the PGX® treated group and it is likely with further progress of the disease, this parameter may have eventually reached significance. The kidney damage was not severe enough to affect some of these parameters such as creatinine which is why we chose to examine tissue histomorphometrically in order to detect the early development of organ damage.
Pancreas
Rats fed a PGX®-containing diet maintained a significantly higher area of insulin immunoreactivity as a percent of total pancreatic islet area compared to rats fed cellulose- or inulin-containing diets (Table 4; Figure 5) suggesting that PGX® rats can maintain a greater reserve capacity for insulin secretion. The remaining morphological changes in the pancreatic islets showed little or no treatment effect (data not shown) although scores for islet hemorrhage or hemosiderin revealed a trend for lower scores in PGX® rats. In ZDFs, increased hemorrhage or hemosiderin in islets are often seen resulting from dilated blood vessels, particularly in more florid diabetes states (Nugent et al., 2008).
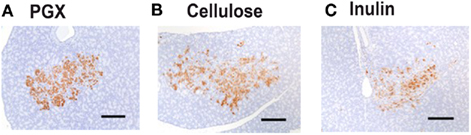
Figure 5. Photomicrograph of the pancreas from PGX® (A), cellulose (B), and inulin (C) immunohistochemically stained for the presence of insulin. The pancreas was serial sectioned at 5 μm, and slides were immunohistochemically stained with a mouse antibody against rat insulin. Sections were evaluated morphometrically for the ratio of insulin to total islet cell area. PGX® significantly increased the morphometrically stained area composed of insulin-secreting cells compared to cellulose or inulin-treated groups (p < 0.05). The scale bar is 100 microns and images taken at 10×.
Liver
Rats fed PGX®-containing diet showed less hepatic steatosis than rats fed cellulose- or inulin-containing diets (Table 4; Figure 6). Rats fed PGX® diet showed less hepatocyte vacuolation than rats fed cellulose or inulin (Table 4). Microvesicular hepatocyte vacuolation was severe in rats fed a cellulose- or inulin-containing diet while rats fed a PGX®-containing diet averaged significantly lower scores. In all treatment groups, macrovesicular hepatocyte vacuolation was less prominent than microvesicular hepatocyte vacuolation (Table 4). There was a significant difference between groups fed PGX®- vs. cellulose-containing and inulin-containing diets but no differences were seen between groups fed inulin- and cellulose-containing diets.
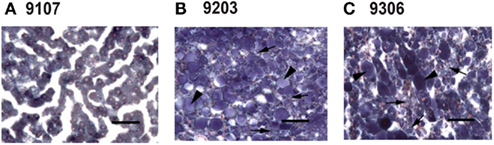
Figure 6. Photomicrograph of livers stained with Sudan black B from PGX® (A), cellulose (B), and inulin (C) treated animals. Using this stain, fat appears as blue–black globules. In the PGX® group any fat is present as very small, rare globules (A). Cellulose (B), and inulin (C) slides contained significantly more lipid globules (p < 0.05) and significantly more (p < 0.05) microvesicular (arrow) and macrovesicular (arrowhead) globules. Images taken at 40× magnification (Bar = 50 microns).
PolyGlycopleX®-treated rats showed significantly lower terminal serum ALT levels compared to rats receiving cellulose- or inulin-containing diets (Table 3). Serum AST showed a similar pattern (Table 3). Rats fed a PGX®-containing diet had lower serum alkaline phosphatase levels than rats fed cellulose- or inulin-containing diets (Table 3). Lower serum alkaline phosphatase levels caused by PGX® suggests a protective effect on cholestasis, while decreases in ALT and AST indicate reduced hepatocellular injury (Pratt and Kaplan, 2001). Conversely, globulin and bilirubin are cleared by the liver and elevations reflect some degree of compromised hepatic function. Rats fed a PGX®-containing diet showed significantly lower concentrations of both analytes vs. Cellulose- and inulin-treated groups, further suggesting improved liver function (Table 3). The inulin-treated group showed higher serum levels of hepatic enzymes compared to all other groups, but it is doubtful if this is meaningful as liver histology showed no significant pathology when compared to cellulose-treated animals.
Discussion
Dietary fiber is considered to be the edible part of plants or analogous carbohydrates that are resistant to digestion and absorption in the small intestine but may be subject to partial or complete fermentation in the colon (Ramberg et al., 2005; Wang et al., 2007). Cellulose is indigestible as well as being poorly water soluble, and is poorly fermented in the colon. Therefore cellulose is considered the control, inert fiber for dietary fiber studies as it is generally without effects on the parameters tested in the present study (Anderson et al., 1994). The biological activities of multiple fiber forms are difficult to quantify systematically but have been variously reported to improve insulin sensitivity, trap nutrients such as lipids and bile acids and thereby reduce serum cholesterol and liver lipid content (Judd and Truswell, 1982; Wilson and Wilson, 1984; Williams, 1991; Anderson et al., 1994; Ganji and Kies, 1994; Grunberger et al., 2006; Parnell and Reimer, 2010), promote weight loss in obese subjects (Howarth et al., 2001; Weickert and Pfeiffer, 2008; Parnell and Reimer, 2009) and improve glycemic control (Anderson et al., 1999; Panahi et al., 2007; Weickert and Pfeiffer, 2008; Brand-Miller et al., 2010) in man as well as animals.
Since water soluble, high viscosity, gel-forming fibers are advantageous for control of lipids, glycemic control, and satiety, some limited albeit interesting efforts have been made to improve or optimize the activity of natural fibers with novel chemical entities (Anderson et al., 1991; Blackwood et al., 2000; Jenkins et al., 2000; Grunberger et al., 2006; Wang et al., 2007; Abdelhameed et al., 2010; Harding et al., 2010). We therefore tested a novel fiber complex: PGX®, a highly viscous polysaccharide that imparts a significant increase in the viscosity of gastrointestinal contents at a lower gravimetric quantity than its fiber constituents tested individually. This property allows the fiber to potentially exert physiological effects at levels lower than other soluble fibers, making it easier to incorporate into food. PGX® is generally regarded as safe (Self Affirmed GRAS) with a NOAEL (no observable adverse effect level) of 50,000 ppm. PGX® showed no toxicity in an OECD 408, 90 day rat (Sprague-Dawley) feeding study (Matulka et al., 2009), in a genotoxicity study (Marone et al., 2009) or in a controlled human tolerance trial (Carabin et al., 2009).
The young ZDFs used in this study were in the early stage of development of diabetes and PGX® reduced the rate of body weight gain by approximately 10% over the course of the study. This may be partially or wholly due to the reduced food intake seen during the study or reduced absorption of nutrients (Schneenman, 1985; Ganji and Kies, 1994; Blackwood et al., 2000). PGX® may have induced satiety either through its volumetric effects in the gut, slowing of gastric emptying, or by increasing secretion of GLP-1 (Reimer and McBurney, 1996; Massimino et al., 1998; Wang et al., 2007; Grover et al., 2011). While weight loss has been shown in various experimental models and clinical trials for several fibers (Walsh et al., 1983; Schneenman, 1985; Reimer et al., 2011a), not all studies have shown weight loss for dietary fibers and there is great variability between fibers (Anderson et al., 1994; Grover et al., 2011) and this may be model dependent and or related to the degree of progression of obesity or the metabolic syndrome.
The manufacture of the highly viscous PGX® was done with the idea of optimizing the activity of dietary fiber and reducing the variability of its efficacy. The present study showed clear reduction of body weight and glycemic control compared to cellulose and inulin on a wgt/wgt basis. Preliminary studies, just recently completed, show that the body weight loss in ZDFs caused by PGX® is associated with a loss of adiposity but also with an increase in lean mass as measured by dual energy X-ray absorptiometry (DEXA, unpublished data). The reduced energy intake and associated decrease in body fat seen in this study could be related to alterations in satiety hormone production. This is consistent with results in man showing increased plasma PYY with PGX® supplementation (Reimer et al., 2011b). Furthermore, preliminary, detailed (unpublished) studies in ZDFs in our lab show that PGX® increases circulating GLP-1 although a less detailed study also showed a reduction in GLP-1 (Grover et al., 2011). The current study represents a broad characterization of PGX® in a specific model of obesity and insulin resistance with developing diabetes and obesity. Multiple mechanisms for the protective effects of PGX® will need to be worked out in further studies in order to explain the improved regulation of glucose homeostasis. It is possible that these actions of PGX® are secondary to its anti-obesity effects. While this may be a component, there are studies with dietary fiber that show improvements in glycemic control even when the animals did not lose weight, so there may be another component to the action of PGX® that is independent of weight loss (Yoshida et al., 1991). Studies show that increased levels of GLP-1 are induced by dietary fiber and as stated above, preliminary evidence as well as published data (Grover et al., 2011) from our laboratories show that PGX® causes increased GLP-1 levels which can increase insulin sensitivity as well as act as an insulin secretagogue. These effects may not be directly related to body weight loss.
Various dietary fibers show beneficial effects on glycemic control in animal models and in man (Anderson et al., 1999; Chandala et al., 2000; Jenkins et al., 2000; Panahi et al., 2007; Parnell and Reimer, 2009). In our studies, the fasted, young ZDFs did not yet have greatly elevated glucose levels likely due to the compensation provided by hyperinsulinemia, showing increased insulin resistance. PGX® probably had no effect on blood glucose in fasted animals as the fiber was not present in the gut under these conditions and the glucose levels in the fasted animals were still at normal levels making it difficult to reduce glucose further. Improvements in glucose handling (insulin sensitivity) were seen with PGX® in both fasted and fed states compared to the other two diets. In fasted animals, PGX® improved insulin sensitivity as measured by CISI and HOMA scores. We also measured blood glucose from 3 weeks after the initiation of the study through to study termination in non-fasted animals. While non-fasted animals in the cellulose and inulin groups were hyperglycemic, glucose levels were reduced or maintained by PGX® to nearly non-diabetic levels. We only measured resting, fed state insulin at study termination (the baseline blood sample before the final non-fasted OGTT) and during the OGTT and the significantly lower levels reflected improved insulin sensitivity caused by PGX®.
In this study, PGX® prevented the development of insulin resistance in ZDF rats; however, other effects were also observed. PGX® inhibited the loss of pancreatic β-cell mass, measured as insulin–immunoreactive area. This suggests that the reserve capacity of the pancreas is preserved by PGX®. The preservation of two sequential steps in glucose response shows a high degree of protection from the early development of diabetes. It is important to note that the stage of diabetes shown in this study was mild and a reduced insulin capacity in control-fiber treated animals was still not a limiting factor. ZDFs show an increased severity of diabetes with time and the animals. The results showing a preservation of insulin reserve (improved pancreatic beta-cell survival) will become critical as the diabetes becomes more severe. GLP-1 improves both insulin reserve and sensitivity (Kim and Egan, 2008) and that was clearly shown in our results. In a much more severe model of type two diabetes (older ZDFs), insulin levels were increased by PGX® compared to control animals so GLP-1 not only improved insulin sensitivity, but also maintained insulin reserve (Grover et al., 2011). Dietary fibers, including PGX® have been shown to release GLP-1 as well as preserving pancreatic-β cells (Merani et al., 2008). While fasted glucose levels were not elevated in this study, post-prandial glucose (non-fasted) appears to have a greater predictive value for the onset of early diabetes. Recent data suggest that post-prandial glucose levels have a higher correlation with cardiovascular events than fasting glucose levels (Beisswenger et al., 2008).
We also determined if PGX® could reduce the appearance of organ damage secondary to poor glycemic control and perhaps act to delay or prevent the progression of diabetes. We found significant, albeit modest organ damage present in our animals at this early stage of diabetes, emphasizing that these changes occur quickly and fasted glucose may not adequately predict developing organ damage. Nephropathy is a clinically important sequela of diabetes, particularly thickening of the glomerular basement membrane and expansion of the mesangium and tubules. Tubular degeneration was also seen in these young animals and this organ damage appeared quickly despite mild diabetes. We showed less renal injury in the PGX®-fed group, in particular mesangial expansion. A reduction in protein glycation likely served as a major factor in reduced renal injury and shows that fasted glucose levels by themselves are not perfect predictors of tissue glycation and further emphasizes the importance of early treatment of type 2 diabetes and insulin resistance.
Serum cholesterol was significantly reduced by PGX® and this is probably related to sequestration of bile acids and subsequent excretion from the body (Anderson et al., 1999, 1994; Jenkins et al., 2000). Reduction of cholesterol is probably not secondary to reductions in weight gain as the mechanism is related to reduced re-uptake of cholesterol via reduced bile acid absorption. Serum triglycerides were more variable and therefore the data were difficult to interpret. Liver steatosis was reduced by PGX® which may be due to several mechanisms. Reduction of body weight and mobilization of hepatic fatty acids and triacylglycerides may have occurred (Daubioul et al., 2002). In another study, Wilson and Wilson (1984) showed increased triglyceride production with subsequent increases in plasma triglycerides in ZDFs with dietary fiber and this may explain our data. Therefore, the increased serum triglyceride levels associated with PGX® may be secondary to body weight reduction. Reduced liver production and output of glucose (gluconeogenesis) has been reported for GLP-1 as well as reduced fatty acid synthesis (Daubioul et al., 2002; Kim and Egan, 2008; Weickert and Pfeiffer, 2008), so more work needs to be done to further elucidate PGX®-induced effects on triglycerides. PGX® reduced indices of hepatocellular injury but also reduced ALT and AST which is consistent with reduced steatosis and liver damage. The effect of PGX® on alkaline phosphatase may also indicate reduced cholestasis.
Obesity and diabetes are on the rise worldwide. Useful treatments for diabetes are still welcomed as both diabetes and obesity will almost certainly require combination therapies in addition to diet restriction and a support program. Based on the data in this report, PGX® may be useful for treatment of diabetes and obesity and may be particularly useful for delaying the progression of diabetes. Of further significance, these studies show that “optimized” dietary fiber combinations such PGX® may show efficacy for diseases such as the metabolic syndrome with much lower amounts in food than for other soluble fibers.
Conflict of Interest Statement
Gary Grover Lee Koetzner and Joan Wicks received funding from InovoBiologic Inc., to perform this study and have no financial interest in PGX®. Roland Gahler is the owner of the Factors Group of Companies, which retains an interest in PGX®. Michael Lyon receives consulting fees from the Factors Group of Companies and Simon Wood and Raylene Reimer receive consulting fees from InovoBiologic Inc.
References
Abdelhameed, A. S., Ang, A., Morris, G. A., Smith, I., Lawson, C., Gahler, R., Wood, S., and Harding, S. E. (2010). An analytical ultracentrifuge study on ternary mixtures of konjac glucomannan supplemented with sodium alginate and xanthan gum. Carbohydr. Polym. 81, 141–148.
Anderson, J. W., Allgodd, L. D., Turner, J., Oeltgen, R. R., and Daggy, B. P. (1999). Effects of psyllium on glucose and serum lipid responses in men with type 2 diabetes. Am. J. Clin. Nutr. 70, 466–473.
Anderson, J. W., Floore, T. L., and Bazel-Geil, P. (1991). Hypocholesterolemic effects of different bulk-forming hydrophilic fibers as adjunts to dietary therapy in mild to moderate hypercholesterolemia. Cholesterol-lowering effects of psylium hydrophilic mucilloid for hypercholesterolemic men. Arch. Intern. Med. 151, 1597–1602.
Anderson, J. W., Jones, A. E., and Riddell-Mason, S. (1994). Ten different dietary fibers have significantly different effects on serum and liver lipids of cholesterol-fed rats. J. Nutr. 124, 78–83.
Beisswenger, P., Heine, R. J., Leiter, L., Moses, A., and Tuomilehto, J. (2008). Prandial glucose regulation in the glucose triad. Endocrine 25, 195–202.
Blackwood, A. D., Salter, J., Dettmar, P. W., and Chaplin, M. F. (2000). Dietary fiber, physicochemical properties and their relationship to health. J. R. Soc. Promot. Health 120, 242–247.
Brand-Miller, J. C., Atkinson, F. S., Gahler, R. J., Kacinik, V., Lyon, M. R., and Wood, S. (2010). Effects of PGX, a novel functional fibre, on acute and delayed postprandial glycaemia. Eur. J. Clin. Nutr. 24, 1–6.
Carabin, I. G., Lyon, M. R., Wood, S., Pelletier, X., Donazzolo, Y., and Burdock, G. A. (2009). Supplementation of the diet with the functional fiber PolyGlycopleX® is well tolerated by healthy subjects in a clinical trial. J. Nutr. 5, 8–9.
Chandala, M., Garg, A., Lutjohann, D., von Bergmann, K., Grundy, S. M., and Brinkley, L. J. (2000). Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 342,1392–1398.
Daubioul, C., Rousseau, N., Demeure, R., Gallez, B., Taper, H., Declerck, B., and Delzenne, N. (2002). Dietary fructans, but not cellulose, decrease triglyceride accumulation in the liver of obese Zucker fa/fa rats. J. Nutr. 132, 967–973.
Ganji, V., and Kies, C. V. (1994). Psyllium husk fiber supplementation to soybean and coconut oil diets of humans: effect on fat digestibility and fecal fatty acid excretion. Eur. J. Clin. Nutr. 48, 595–597.
Gault, V. A., McClean, P. L., Cassidy, R. S., Irwin, N., and Flatt, P. R. (2007). Chemical gastric inhibitory polypeptide receptor antagonism protects against obesity, insulin resistance, glucose intolerance and associated disturbances in mice fed high-fat and cafeteria diets. Diabetologia 50, 1752–1762.
Grover, G. J., Koetzner, L., Wicks, J., Gahler, R. J., Lyon, M. R., Reimer, R. A., and Wood, S. (2011). Effects of the soluble fiber complex PolyGlycopex on glycemic control, insulin secretion, and GLP-1 levels in Zucker diabetic rats. Life Sci. 88, 392–399.
Grunberger, G., Jen, K.-L., Catherine, D., and Artiss, J. D. (2006). The benefits of early intervention in obese diabetic patients with FBCX. Diabetes Metab. Res. Rev. 23, 56–62.
Han, S., Hagan, D., Taylor, J., Xin, L., Meng, W., Biller, S., Wetterau, J. R., Washburn, W. N., and Whaley, J. M. (2008). Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 57, 1723–1729.
Harding, S. E., Smith, I. H., Lawson, C. J., Gahler, R. J., and Wood, S. (2010). Studies on macromolecular interactions in ternary mixtures of konjac glucomannan, xanthan gum and sodium alginate. J. Carbpol 10, 1016–1020.
Hill, M. J. (1997). Cereals, cereal fiber and colorectal cancer risk: a review of the epidemiological literature. Eur. J. Cancer Prev. 6, 69–72.
Howarth, N. C., Salzman, E., and Roberts, S. B. (2001). Dietary fiber and weight regulation. Nutr. Rev. 59, 163–169.
Jenkins, D. J. A., Kendal, C. W. C., Axelson, M., Augustin, L. S. A., and Vuksan, V. (2000). Viscous and nonviscous fibers, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr. Opin. Lipidol. 11, 49–56.
Judd, P. A., and Truswell, A. S. (1982). Comparison of the effects of high- and low-methoxy pectins on blood and fecal lipids in man. Br. J. Nutr. 48, 451–458.
Kim, S.-J., Nian, C., Doudet, D. J., and McIntosh, C. H. S. (2007). Inhibition of dipeptidyl peptidase IV with sitagliptin prolongs graft survival in streptozotocin-induced diabetic mice. Diabetes 57, 1331–1339.
Kim, W., and Egan, J. M. (2008). The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 60, 470–412.
Lenhard, J. M., Croom, D. K., and Minnick, D. T. (2004). Reduced serum dipeptidyl peptidase-IV after metformin and pioglitazone treatments. Biochem. Biophys. Res. Commun. 324, 92–97.
Marone, P. A., Lyon, M., Gahler, R., Doneth, C., Hofman-Huther, A., and Wood, S. (2009). Genotoxicity studies of PolyGlycoplex. Int. J. Toxicol. 28, 318–331.
Massimino, S. P., McBurney, M. I., Field, C. J., Thomson, A. B. R., Keelan, M., Hayek, M. G., and Sunvold, G. C. (1998). Fermentable dietary fiber increases GLP-1 secretion and improves glucose homeostasis despite increased intestinal glucose transport capacity in healthy dogs. J. Nutr. 128, 1786–1793.
Matulka, R. A., Lyon, M. R., Wood, S., Marone, P. A., Merkel, D. J., and Burdock, G. A. (2009). The safety of PolyGlycopleX® (PGX®) as shown in a 90-day rodent feeding study. Nutr. J. 8, 1–11.
Merani, S., Truong, W., Emamaullee, J. H., Toso, C., Knudsen, L. B., and Shapiro, A. M. J. (2008). Liraglutide, a long acting human glucagon-like peptide 1 analog, improves glucose homeostasis in marginal mass islet transplantation in mice. Endocrinology 149, 4322–4328.
Nonaka, K., Kakikawa, T., Sato, A., Okuyama, K., Fujimoto, G., Kato, N., Suzuki, H., Hirayana, Y., Ahmed, T., Davies, M. J., and Stein, P. P. (2007). Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res. Clin. Pract. 79, 291–298.
Nugent, D. A., Smith, D. M., and Jones, H. B. (2008). A review of islet of Langerhans degeneration in rodent models of type 2 diabetes. Toxicol. Pathol. 36, 529–551.
Panahi, S., Exatagha, A., Temelli, F., Vasanthan, T., and Vuksan, V. (2007). β-Glucan from two sources of oat concentrates effect postprandial glycemia in relation to the level of viscosity. J. Am. Coll. Nutr. 26, 639–644.
Parnell, J. A., and Reimer, R. A. (2009). Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 89, 1751–1759.
Parnell, J. A., and Reimer, R. A. (2010). Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: a dose-response study in JCR: LA-cp rats. Br. J. Nutr. 103, 1577–1584.
Pederson, R. A., Satkunarajah, M., and NcIntosh, C. H. (1998). Enhanced glucose-dependent insulinotropic polypeptide secrtion and insulinotropic action in glucogaon-like peptide 1 receptor −/− mice. Diabetes 47, 1046–1052.
Pratt, D. S., and Kaplan, M. M. (2001). “Evaluation of liver function,” in Harrison’s Principles of Internal Medicine 15th Edn, eds E. Braunwald, A. Fauci, D. Kasper, S. Hauser, D. Longo, and J. Jameson (New York, NY: MacGraw-Hill Professional Publishing), 1711–1715.
Ramberg, J., Murray, R. K., Vennum, E., and McAnalley, B. (2005). Dietary carbohydrates in the human GI tract: the established and emerging science. Glycoscience & Nutrition 6, 1–10.
Reimer, R. A., Grover, G. J., Koetzner, L., Gahler, R. J., Lyon, M. R., and Wood, S. (2011a). The soluble fiber complex PolyGlycoplex lowers serum triglycerides and reduces hepatic steatosis in high-sucrose-fed rats. Nutr. Res. 31 296–301.
Reimer, R. A., Pelletier, X., Carabin, I. G., Lyon, M., Gahler, R., and Wood, S. (2011b). Increased plasma PYY levels following supplementation with the functional fiber PolyGlycopleX in healthy adults. Eur. J. Clin. Nutr. 141, 1–6.
Reimer, R. A., and McBurney, M. (1996). Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology 137, 3948–3956.
Rozan, P., Nejdi, A., Hidalgo, S., Bisson, J.-F., and Desor, D. (2008). Effects of lifelong intervention with an oligofructose-enriched inulin in rats on general health and lifespan. Br. J. Nutr. 99, 1–8.
Schneenman, B. O. (1985). Effects of nutrients and nonnutrients on food intake. Am. J. Clin. Nutr. 42, 966–972.
Sugatani, J., Osabe, M., Wada, T., Yamakawa, K., Yamazaki, Y., Takahashi, T., Ikari, A., and Miwa, M. (2008). Comparison of enzymatically synthesized inulin, resistant maltodextrin and clofibrate effects on biomarkers of metabolic disease in rats fed a high-fat and high-sucrose (cafeteria) diet. Eur. J. Nutr. 47, 192–200.
Venter, C. S., Vorster, H. H., and Cummings, J. H. (1990). Effects of dietary propionate on carbohydrate and lipid metabolism in healthy volunteers. Am. J. Gastroenterol. 85, 549–553.
Walsh, D. E., Yaghoubian, V., and Behforooz, T. (1983). Effect of glucomannan on obese patients: a clinical study. Int. J. Obes. 8, 289–293.
Wang, Z. Q., Zuberi, A. R., Zhang, X. H., Macgowan, J., Qin, J., Ye, X., Son, L., Wu, Q., Lian, K., and Cefalu, W. T. (2007). Effects of dietary fibers on weight gain, carbohydrate metabolism and gastric ghrelin gene expression in mice fed a high fat diet. Metab. Clin. Exp. 56, 1635–1642.
Weickert, M. O., and Pfeiffer, A. F. (2008). Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 138, 439–442.
Williams, C. M. (1991). Effects of inulin on lipid parameters in humans. J. Nutr. 128(Suppl.), 1471S–1473S.
Wilson, J. N., and Wilson, S. P. (1984). Eaton RP. Dietary fiber and lipoprotein metabolism in the genetically obese Zucker rat. Arteriosclerosis 4, 147–153.
Keywords: dietary fiber, obesity, insulin resistance, insulin, hepatic steatosis, PGX®
Citation: Grover GJ, Koetzner L, Wicks J, Gahler RJ, Lyon MR, Reimer RA and Wood S (2011) Effects of the soluble fiber complex PolyGlycopleX® on glucose homeostasis and body weight in young Zucker diabetic rats. Front. Pharmacol. 2:47. doi: 10.3389/fphar.2011.00047
Received: 29 May 2011; Paper pending published: 01 July 2011;
Accepted: 26 July 2011; Published online: 07 September 2011.
Edited by:
Dongsheng Cai, Albert Einstein College of Medicine, USAReviewed by:
Guo-Jun Cai, Second Military Medical University, ChinaYi Feng, Shanghai University of Traditional Chinese Medicine, China
Copyright: © 2011 Grover, Koetzner, Wicks, Gahler, Lyon, Reimer and Wood. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Gary James Grover, Department of Physiology and Biophysics, Robert Wood Johnson Medical School, University of Medicine and Dentistry of New Jersey, 675 Hoes Lane, Piscataway, NJ 732-235-4552, USA; Department of Pharmacology, Eurofins-Product Safety Laboratories, 2394 Highway 130, Suite E, Dayton, NJ 08810, USA. e-mail: grovergj@umdnj.edu