- Non-Clinical Safety, Pharma Research, F. Hoffmann-La Roche Ltd., Basel, Switzerland
The Patchliner® temperature-controlled automated patch clamp system was evaluated for testing drug effects on potassium currents through human ether-à-go-go related gene (hERG) channels expressed in Chinese hamster ovary cells at 35–37°C. IC50 values for a set of reference drugs were compared with those obtained using the conventional voltage clamp technique. The results showed good correlation between the data obtained using automated and conventional electrophysiology. Based on these results, the Patchliner® represents an innovative automated electrophysiology platform for conducting the hERG assay that substantially increases throughput and has the advantage of operating at physiological temperature. It allows fast, accurate, and direct assessment of channel function to identify potential proarrhythmic side effects and sets a new standard in ion channel research for drug safety testing.
Introduction
The cardiac proarrhythmic liability of new pharmacological entities is a major concern for pharmaceutical companies and regulatory agencies. Remarkably, many if not most compounds exert their arrhythmogenic effects through the same electrophysiological mechanism: blockade of repolarizing currents through human ether-à-go-go related gene (hERG) K+ channels located in ventricular myocytes (Sanguinetti and Tristani-Firouzi, 2006). As a result, in-vitro hERG assay has been introduced into the drug development process to test the proarrhythmic potential of small molecules (International Conference on Harmonisation, 2005). The assay evaluates the acute effects of new therapeutic agents on hERG channels stably expressed in mammalian cell lines (such as HEK293 and CHO cells). By directly measuring a compound’s ability to block hERG K+ channels it evaluates the proarrhythmic liability associated with this fairly common arrhythmogenic mechanism.
The gold standard for assessing ion channel functionality is the patch clamp technique. The high-resolution conventional manual method involves a glass micropipette filled with an ionic solution that electrically connects an electrode wire to a small patch of cell membrane. Considerable expertise is needed and throughput is low as only one cell can be recorded at a time. In addition, accurate drug safety assessment requires a highly sensitive temperature-controlled patch clamp since the current kinetics is temperature-dependent and inappropriate experimental temperature may compromise the pharmacological properties of the hERG current (Vandenberg et al., 2006). Thus thorough analysis of hERG liabilities under physiological conditions has only been possible to date for advanced compounds using the conventional manual patch clamp technique. However, the high attrition rate due to cardiovascular adverse effects indicated a strong need for a high-quality electrophysiological method for early hERG profiling of a larger number of drug candidates.
Fortunately, automation has entered the area of experimental electrophysiology over the past few years. The first automated patch clamp instruments focused on increasing throughput by the parallel formation of high-resistance seals using the planar patch clamp at ambient temperature (reviewed in Möller, 2010). Various companies have recently launched second-generation automated platforms, including the Patchliner® (Nanion Technologies GmbH, Munich, Germany), a fully automated patch clamp system with integrated temperature control. The planar NPC® chip electrode combined with temperature control add-on is designed to control giga-Ohm seal formation at 35–37°C and accurately maintain temperatures over an experiment. By recording from up to eight cells simultaneously at physiological temperature, the Patchliner® claims to substantially increase through put without compromising data quality.
The objective of the present study was to transfer the hERG assay at physiological temperature to the Patchliner® and evaluate the automated platform for its ability to correctly determine potency and IC50 values for reference compounds including known hERG blockers and negative controls.
Materials and Methods
Cell Culture
The CHO crelox hERG cell line (ATCC reference Nr. PTA-6812) was generated and validated at Roche (Guthrie et al., 2005). Recombinant hERG K+ channels originally cloned from human heart were stably expressed in CHO cells grown in sterile tissue flasks in DMEM/F-12 (1:1) medium (Invitrogen, USA) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS) (Hyclone, USA) and 500 μg/ml gentamycin solution (Gibco, UK) at 35–37°C in 5% CO2.
Confluent cell cultures were subcultured every 2–3 days using Accumax (Innovative Cell Technologies Inc., USA). Before electrophysiological experiments, cells were treated with Accumax for 3–5 min at 42°C, harvested, and centrifuged at 1000 rpm for 1 min. The pellet was gently resuspended in the extracellular solution and directly used for electrophysiological recording.
Solutions
The extracellular solution contained (mM): NaCl 150; KCl 4; CaCl2 1.2; MgCl2 1; HEPES 10; pH 7.2–7.6 with NaOH, osmolarity 290–330 mOsm. The internal solution contained (mM): KCl, 50; KF, 60; NaCl, 10; HEPES, 10; EGTA, 20; pH 7.0–7.4 with KOH, osmolarity 260–300 mOsm.
All solutions were filtered through 0.2 μM GP Millipore Express PLUS membrane and stored in aliquots at +2–8°C.
Compounds
Olanzapine was synthesized by Roche (Basel, Switzerland). Amiodarone, astemizole, bepridil, clemastine, haloperidol, terfenadine, and verapamil were purchased from Sigma (USA), cisapride from Research Diagnostic Inc. (USA), E-4031 from Wako Chemicals (Japan), quinidine from Aldrich Chemicals (USA), D,L-Sotalol from Bristol-Myers-Squibb (USA), amoxicillin, fluoxetine, isradipine, and tamoxifen from USP (USA), glyburide/glibenclamide from Calbiochem (USA), ibuprofen from ICN Biomedicals Inc. (USA), moxifloxacin from APIChem Technologies Co. (China), and erythromycin and captopril from Fluka (Switzerland).
Selection of test concentrations for each compound was done based on the hERG potency data and the solubility in the extracellular solution. Stock solutions of compounds were freshly prepared in DMSO. Test solutions were made such that solvent concentrations were kept constant throughout the experiment (0.1%). Compound solutions were transferred to 4 mL glass vials that were placed in the compound plate holder of the patch clamp system (Figure 1).
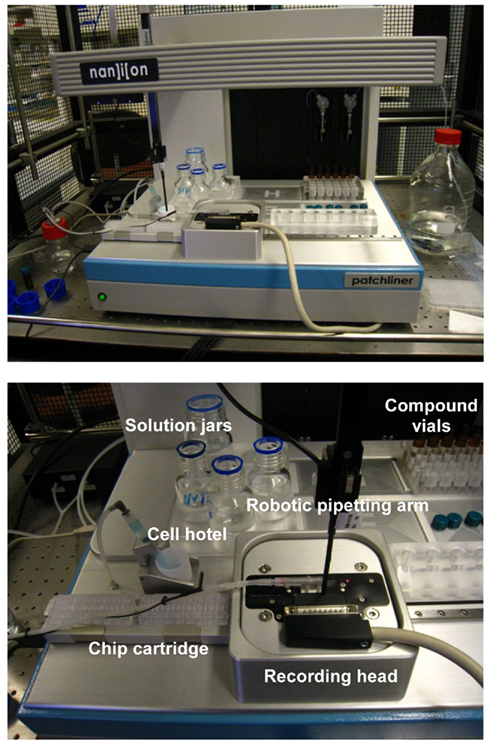
Figure 1. The Patchliner® is shown at the top panel and a closer view of the working area is presented below.
Automated Patch Clamp Procedures on the Patchliner®
On the day of the experiment, an aliquot of the cell suspension in the extracellular solution was placed in the cell hotel (Figure 1). Cells were added to each chip well and attracted to the holes by suction applied by a pressure controller. Once a stable whole-cell configuration was obtained, the experimental protocol was initiated. K+ currents were measured with a patch–voltage-clamp technique in the whole-cell configuration at 35–37°C using the QuadroEPC-10 amplifier (HEKA Elektronik GmbH, Germany) and associated software (Patch MasterPro). Currents were low-pass filtered using the internal Bessel filter of the EPC-10 and digitized at 50 kHz. Series resistance was typically 2–4 MΩ and compensated 70–80%.
Cells were held at a resting voltage of −80 mV and stimulated by a voltage pattern to activate hERG channels and conduct outward IKhERG current (Figure 2, bottom panel), at a stimulation frequency of 0.1 Hz (6 bpm). The reported current amplitudes represent the maximal peak tail current. After the cells stabilized for a few minutes and currents were steady, IKhERG amplitude and kinetics were recorded under control conditions (vehicle control) for 3–5 min. Thereafter, a single test item concentration was added and incubated for at least 3 min until a new steady state current level was reached (see Figures 3 and 4 for the experimental procedure). At the end of the compound incubation, a 1 μM solution of standard IKhERG blocker E-4031 was applied twice to each cell for 1 min to suppress IKhERG entirely.
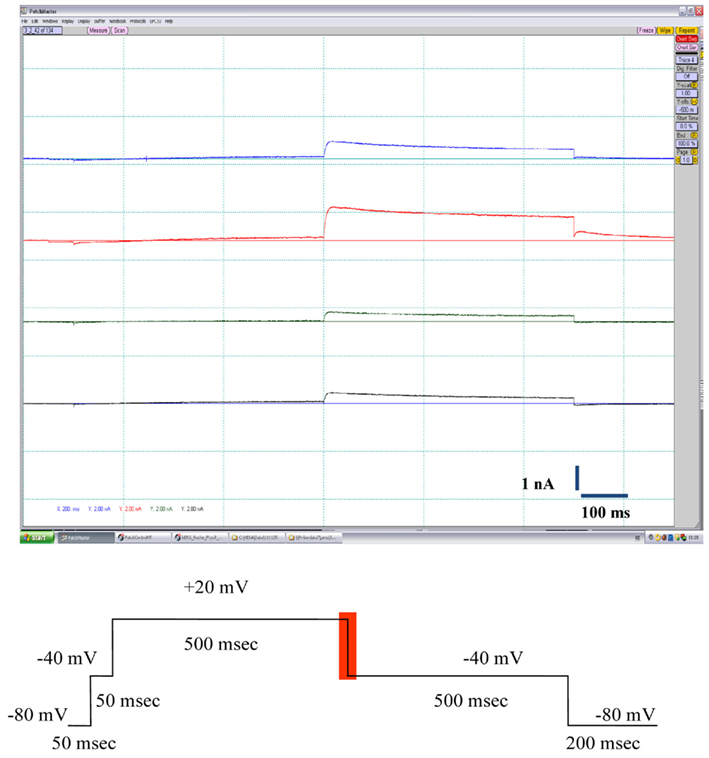
Figure 2. The top panel shows typical outward K+ current traces elicited in CHO-hERG cells by the voltage pulse depicted at the bottom panel (the area for the peak current measurement is highlighted in red).
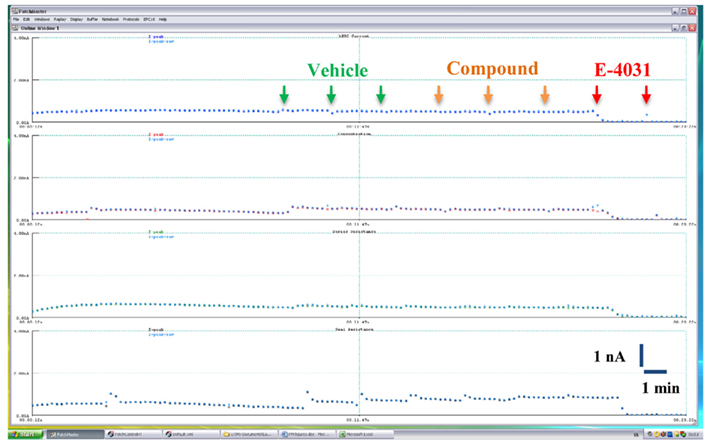
Figure 3. Time course of hERG K+ current amplitude recorded in a representative vehicle control experiment on the Patchliner®. Cells were exposed sequentially to vehicle (0.1% v/v DMSO), compound (0.1% v/v DMSO), and 1 μM E-4031. Arrows indicate compound additions.
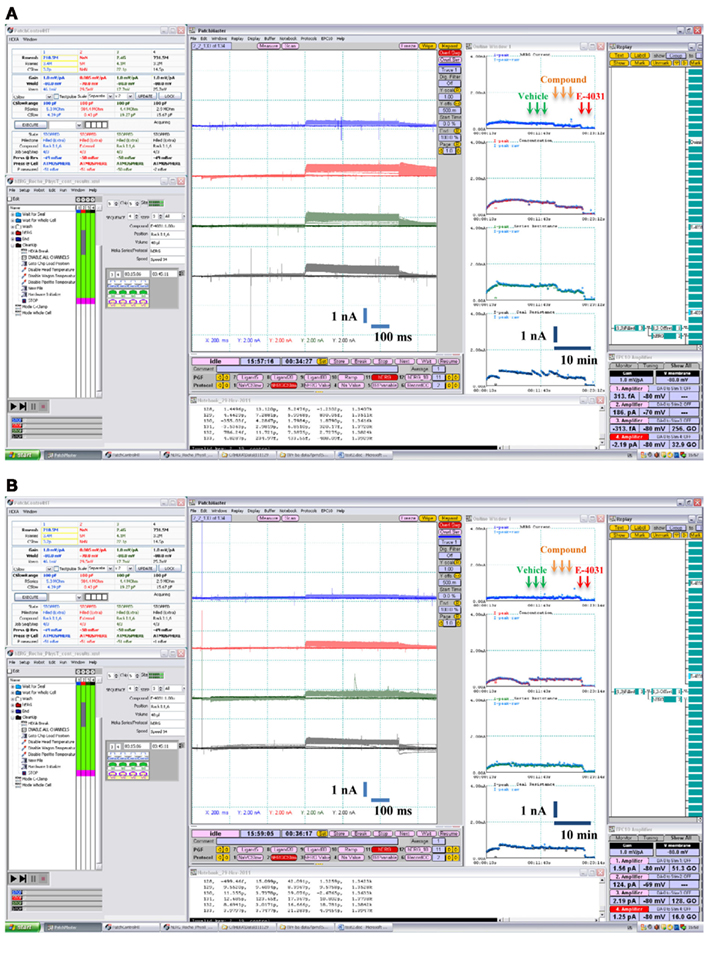
Figure 4. Examples of pharmacological experiments on the Patchliner®. Recordings from four cells were performed in parallel. Original traces are shown in the middle with the on-line analysis window showing the time course of a compound effect on the peak hERG K+ current to the right. Arrows indicate compound additions for vehicle (0.1% v/v DMSO), test item single concentration [0.3 μM quinidine in (A) and 6 μM glibenclamide in (B)] and positive control (1 μM E-4031).
IKhERG amplitudes were recorded at four to five drug concentrations. The hERG current was measured as the average current from five sweeps collected at the end of vehicle or compound addition. After subtracting current levels from that of full block (i.e., positive control) they were compared to the vehicle control values (taken as 100%) to define fractional blocks. The fractional block values at single concentrations were saved in the appropriate.xls file generated by PatchControlHT software (Nanion). Data were expressed as mean ± SEM for each drug concentration and were used to determine the concentration–response relationship for the test compounds. The effect of the solvent (DMSO) on the hERG K+ current was studied in a vehicle control group.
Data Analysis
If the effect of a drug on the currents was ≤20% at the highest concentration tested, IC50 was reported as exceeding the highest concentration value. If the effect of a drug on the currents exceeded 20% at the highest concentration tested, then concentration–response data from three to five cells were normalized to vehicle and 100% blocking levels and the resulting data fitted with the following relationship:
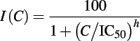
where C was the concentration, IC50 the 50% inhibitory concentration, h the Hill coefficient.
Concentration–response curves were fitted by non-linear regression analysis using the XL fit add-in (IDBS Ltd., UK). Data were fitted using a four-parameter logistic model [fit = (A + (B/(1 + ((x/C)∧D)))], where A = 0 and B = 100).
Statistical analysis was performed using two-way ANOVA with the Bonferroni post-test, applying the correction for multiple comparison at a significance level of p < 0.05 with GraphPad Prism 5 for Windows (GraphPad Software, USA). Correlation between assays [Pearson’s coefficient (r)] and values for statistical significance (p) were determined using GraphPad Prism.
Results
The Patchliner® work station shown in Figure 1 uses NPC-16 borosilicate glass chips with a microfluidic cartridge for parallel seal formation and patch clamp recordings from up to eight different wells. In this study, a simultaneous recording from four cells exposed to the same compound concentration was performed, resulting in a four-point dose–response curve with one chip cartridge, containing 16 recording wells in total. After priming, cells were captured on the holes of the chip by applying continuous vacuum pressure. After allowing current to stabilize in the whole-cell configuration, a quality control check was performed before starting treatment to ensure that only recordings with true giga-Ohm-seals and a minimum current of 300 pA were included in the experiment. The seal resistance varied between 3.9 ± 2.6 GΩ at the start and 3.2 ± 2.1 GΩ at the end of the experiment while the average current amplitude was 0.9 ± 0.6 nA (mean ± SD, n = 297 cells). Typical examples of the hERG current traces are shown in Figure 2. The recordings remained stable for over 30 min at physiological temperature enabling subsequent conduction of compound incubation followed by exposure to the positive control at each concentration with an overall success rate >70%. Cells were maintained in suspension for over 2 h in a cell hotel with no associated decline in patch properties. The sealing quality was very high with the giga-Ohm-seal formation and the whole-cell transition rates almost reaching 100% on the Patchliner®. On average, three out of four cells maintained giga-Ohm-seals and stable currents until the end of the experiments.
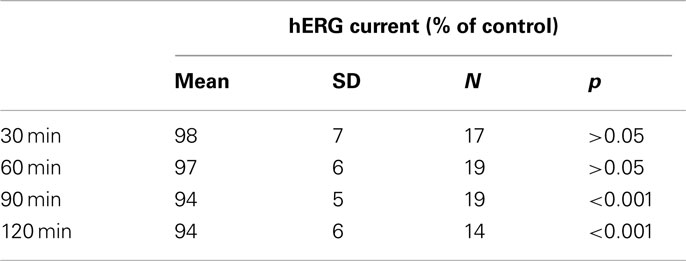
Vehicle effect (0.1% v/v DMSO) on the hERG current was studied in time-matched vehicle control groups (Figure 3). These control measurements were performed once a week during the validation period. The largest reduction in the outward K+ current magnitude (mean ± SEM) produced by the subsequent application of a vehicle alone to the CHO cells expressing recombinant hERG K+ channels was 6 ± 1% (Table 1). This indicates that exposure to solvent may produce only a slight significant effect on the current with time. Therefore, the relative current values were corrected appropriately to the mean vehicle effect at each test item concentration as follows:
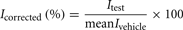
where I was the relative current amplitude.
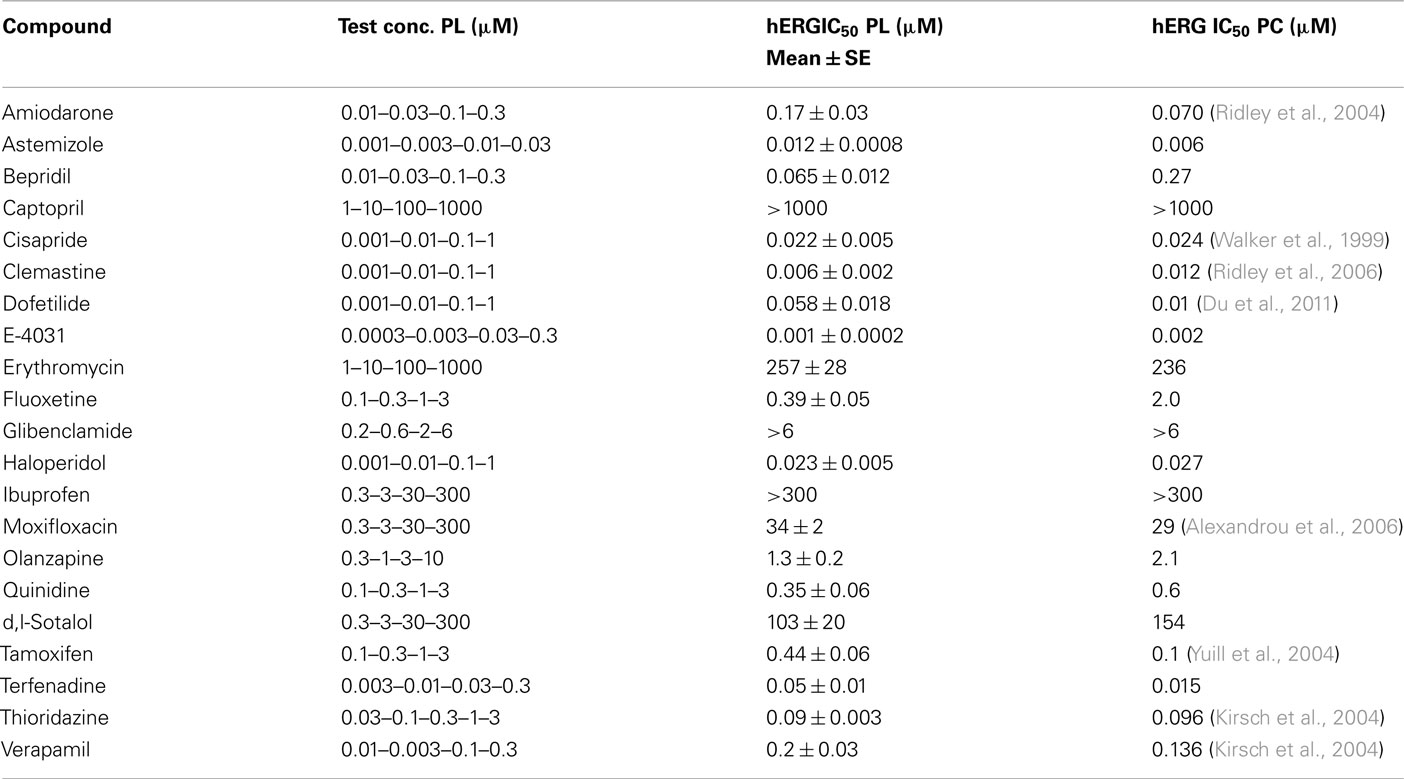
To validate the utility of the Patchliner® for the hERG assay, the actions of reference drugs on the hERG current were examined at physiological temperature. A set of 21 compounds including known hERG blockers and negative controls was evaluated. Examples of pharmacological experiments on the Patchliner® are shown in Figure 4. Obtained IC50 values (Table 2) were plotted against those obtained using the conventional voltage clamp technique. Manual patch clamp data for reference drugs were either generated in-house using the CHO–hERG cells and identical experimental conditions (solutions and voltage step protocol) or taken from published literature. Literature hERGIC50 values were carefully selected to match such key experimental parameters as temperature, expression system, and voltage protocol.
The hERG potencies of all reference drugs were correctly identified. Amiodarone, astemizole, cisapride, clemastine, E-4031, and terfenadine were by far the strongest hERG inhibitors of the compounds tested, with IC50 values in the low and mid-nanomolar range. Some drugs, such as olanzapine, moxifloxacin, sotalol, tamoxifen, and verapamil, showed an intermediate effect on the hERG current. Captopril, ibuprofen, and glibenclamide did not affect the hERG current up to the highest concentration tested. Comparison of the results showed statistically significant correlation (r = 0.9989, p < 0.0001) between the data obtained at physiological temperature on the Patchliner® and those using the conventional patch clamp rigat the same temperature (Figure 5).
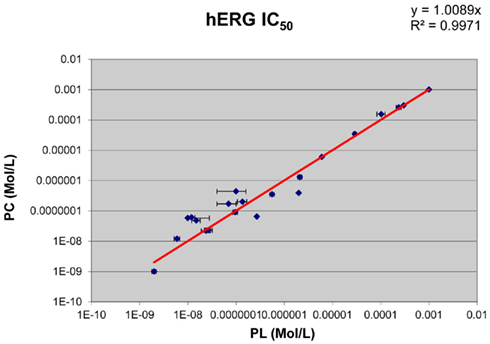
Figure 5. Correlation of the hERG IC50 values obtained in the automated temperature-controlled Patchliner® system (PL) with those measured using the conventional manual patch clamp (PC) at 37°C.
Discussion
The hERG test has become the routine in vitro approach for assessing the cardiac safety of new drugs in early development. Since some compounds exhibit temperature-dependent potency shifts (Yao et al., 2005), performing experiments at physiological temperature improves the relevance of pharmacological data and ensures more accurate cardiac risk assessment in vivo. Until recently, the manual patch clamp was the only technique for conducting temperature-controlled electrophysiological measurements. However, its low throughput hampered functional early profiling of large compound series for proper clinical candidate selection. The availability of a predictive cost–effective hERG assay based on efficient high-resolution technology is thus of major importance for the pharmaceutical industry. A recent publication by Golden et al. (2011) described a first attempt to evaluate the hERG channel physiology at 37°C using an automated sub-giga-Ohm planar patch clamp IonFlux system from Fluxion with a satisfactory outcome. The present study aimed to evaluate the Nanion Patchliner®, a novel automated electrophysiological workstation, for hERG testing under physiological conditions.
The Patchliner® has been successfully introduced as a high-quality medium throughput automated planar patch clamp platform for ion channel research in drug discovery and safety (review in Farre et al., 2009). A unique feature is its ability to conduct stable recordings at different temperatures in the 22–60°C range. The results of the present study showed that the Patchliner® gives high-quality patch clamp recordings in CHO-hERG cells, with a high success rate of giga-Ohm-seals lasting up to an hour at 37°C. Such performance makes the system a powerful tool for studying hERG channel pharmacology at physiological temperature.
In this work, a systematic comparison of diverse panel of reference drugs spanning a broad hERG potency range was performed to validate the Patchliner® for temperature-controlled hERG assay. The obtained IC50s values were compared with their manual patch clamp counterparts. The main finding was that the potency data generated on the Patchliner® matched those obtained by the conventional method (Pearson r = 0.9989, p < 0.0001). It is important to note that among the drugs tested, the inhibitory effects of D,L-sotalol and erythromycin (Kirsch et al., 2004) were correctly identified at 37°C. The Patchliner® system allowed fast and accurate electrophysiological characterization of ion channels, e.g., for determining the IC50 values of ion channel blockers.
In addition to the standard test, the Patchliner® could be a useful tool for studying temperature-dependent potency shifts and new mechanisms of compound action, e.g., the kinetics of recovery after full inhibition of the hERG current that may provide valuable guidance for drug candidate identification (Swinney, 2009). An additional advantage of the system is that experiments can be preprogrammed and executed in a stand-alone mode. Thus the results of the present study indicate that the Patchliner® can be successfully used to produce high-quality data similar to those obtained by means of the current gold standard, the manual patch clamp. The system is unique in combining the features of high-quality patch clamp recordings with increased physiological relevance of the collected data, resulting in the enhanced safety profiling of new chemical entities.
Conclusion
The Patchliner® workstation provides stable currents and high-quality giga-Ohm-seal recordings at 37°C. Based on the validation results, this automated electrophysiology workstation can be used for conducting an advanced hERG assay for new compounds at physiological temperature and may eventually become a new gold standard for ion channel research in safety pharmacology.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author would like to thank the Nanion Technologies GmbH team, especially Drs. David Guinot and Alison Haythornthwaite, for their help. I am also grateful to Drs. Silvio Albertini, Claudia McGinnis, Susanne Mohr, and Thomas Weiser from Roche Non-Clinical Safety for supporting the acquisition and validation of the system, and to Fabian Häusermann for technical assistance.
References
Alexandrou, A., Duncan, R., Sullivan, A., Hancox, J., Leishman, D., Witchel, H., and Leaney, J. (2006). Mechanism of hERG K+ channel blockade by the fluoroquinolone antibiotic moxifloxacin. Br. J. Pharmacol. 147, 905–916.
Du, C. Y., El Harchi, A., Zhang, Y. H., Orchard, C. H., and Hancox, J. C. (2011). Pharmacological inhibition of the HERG potassium channel is modulated by extracellular but not intracellular acidosis. J. Cardiovasc. Electrophysiol. 22, 1163–1670.
Farre, C., Haythornthwaite, A., Haarmann, C., Stoelzle, S., Kreir, M., George, M., Brüggemann, A., and Fertig, N. (2009). Port-a-patch and Patchliner: high fidelity electrophysiology for secondary screening and safety pharmacology. Comb. Chem. High Throughput Screen. 12, 24–37.
Golden, A. P., Li, N., Chen, Q., Lee, T., Nevill, T., Cao, X., Johnson, J., Erdemli, G., Ionescu-Zanetti, C., Urban, L., and Holmqvist, M. (2011). IonFlux: a microfluidic patch clamp system evaluated with human ether-à-go-go related gene channel physiology and pharmacology. Assay Drug Dev. Technol. 9, 608–619.
Guthrie, H., Livingston, F. S., Gubler, U., and Garippa, R. (2005). A place for high-throughput electrophysiology in cardiac safety: screening hERG cell lines and novel compounds with the ion works HTTM system. J. Biomol. Screen. 10, 832–840.
International Conference on Harmonisation. (2005). ICH S7B: the nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. CHMP/ICH/423/02. Fed. Regist. 70, 61133–61134.
Kirsch, G. E., Trepakova, E. S., Brimecombe, J. C., Sidach, S. S., Erickson, H. D., Kochan, M. C., Shyjka, L. M., Lacerda, A. E., and Brown, A. M. (2004). Variability in the measurement of hERG potassium channel inhibition: effects of temperature and stimulus pattern. J. Pharmacol. Toxicol. Methods 50, 93–101.
Möller, C. (2010). Impact of new technologies for cellular screening along the drug value chain. Drug Discov. Today 15, 384–390.
Ridley, J., Milnes, J., Hancox, J., and Witchel, H. (2006). Clemastine, a conventional antihistamine, is a high potency inhibitor of the HERG K+ channel. J. Mol. Cell Cardiol. 40, 107–118.
Ridley, J., Milnes, J., Witchel, H., and Hancox, J. (2004). High affinity HERG K+ channel blockade by the antiarrhythmic agent dronedarone: resistance to mutations of the S6 residues Y652 and F656. Biochem. Biophys. Res. Commun. 325, 883–891.
Sanguinetti, M. C., and Tristani-Firouzi, M. (2006). hERG potassium channels and cardiac arrhythmia. Nature 440, 463–469.
Swinney, D. C. (2009). The role of binding kinetics in therapeutically useful drug action. Curr. Opin. Drug Discov. Devel. 12, 31–39.
Vandenberg, J. I., Varghese, A., Lu, Y., Bursill, J. A., Mahaut-Smith, M. P., and Huang, C. L. (2006). Temperature dependence of ether-a-go-go-related gene K+ currents. Am. J. Physiol. Cell Physiol. 291, C165–C175.
Walker, B., Singleton, C., Bursill, J., Wyse, K., Valenzuela, S., Qiu, M., Breit, S., and Campbell, T. (1999). Inhibition of the human ether-a-go-go-related gene (HERG) potassium channel by cisapride: affinity for open and inactivated states. Br. J. Pharmacol. 128, 444–450.
Yao, J. A., Du, X., Lu, D., Daharsh, E., and Atterson, P. (2005). Estimation of potency of hERG channel blockers: impact of voltage protocol and temperature. J. Pharmacol. Toxicol. Methods 52, 146–153.
Yuill, K. H., Borg, J. J., Ridley, J. M., Milnes, J. T., Witchel, H. J., Paul, A. A., Kozlowski, R. Z., and Hancox, J. C. (2004). Potent inhibition of human cardiac potassium (HERG) channels by the anti-estrogen agent clomiphene-without QT interval prolongation. Biochem. Biophys. Res. Commun. 318, 556–561.
Keywords: hERG, Patchliner®, automated patch clamp, physiological temperature
Citation: Polonchuk L (2012) Toward a new gold standard for early safety: automated temperature-controlled hERG test on the PatchLiner®. Front. Pharmacol. 3:3. doi: 10.3389/fphar.2012.00003
Received: 16 August 2011;
Accepted: 06 January 2012;
Published online: 26 January 2012.
Edited by:
Ralf Franz Kettenhofen, Axiogenesis AG, GermanyReviewed by:
Clemens Möller, Albstadt-Sigmaringen University, GermanyJean-Marie Chambard, Sanofi, France
Aurore Colomar, UCB Pharma, Belgium
Copyright: © 2012 Polonchuk. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Liudmila Polonchuk, Non-Clinical Safety, Pharma Research, F. Hoffmann-La Roche Ltd., Grenzacherstr. 124, Building, 73/R. 103b, CH-4070 Basel, Switzerland. e-mail: liudmila.polonchuk@roche.com