- 1 Blood-Brain Barrier Research Group, Department of Molecular Cell Biology and Immunology, VU University Medical Center, Amsterdam, Netherlands
- 2 Division of Neuroimmunology and Neurological Infections, Department of Neurology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 3 Division of Neuroimmunology and Neurological Infections, Department of Psychiatry, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
ATP-binding cassette (ABC) transporters are highly expressed by brain endothelial cells that form the blood–brain barrier (BBB). These efflux pumps play an important role in maintaining brain homeostasis as they actively hinder the entry of unwanted blood-derived compounds into the central nervous system (CNS). Consequently, their high activity at the BBB has been a major hurdle for the treatment of several brain diseases, as they prevent numerous drugs to reach their site of action within the brain. Importantly, recent data indicate that endogenous substrates for ABC transporters may include inflammatory mediators, such as prostaglandins, leukotrienes, cytokines, chemokines, and bioactive lipids, suggesting a potential role for ABC transporters in immunological responses, and more specifically in inflammatory brain disorders, such as multiple sclerosis (MS). In this review, we will give a comprehensive overview of recent findings that illustrate this novel role for ABC transporters in neuro-inflammatory processes. Moreover, we will provide first insights into underlying mechanisms and focus on the importance for bioactive lipids, in particular platelet-activating factor, herein. A thorough understanding of these events may form the basis for the development for selective treatment modalities to dampen the neuro-inflammatory attack in MS and thereby reducing tissue damage.
ABC Transporters at the Blood–Brain Barrier
The blood–brain barrier (BBB) protects the central nervous system (CNS) from the entry of unwanted compounds and leukocytes, thereby maintaining brain homeostasis. However, during neuro-inflammatory diseases like multiple sclerosis (MS), immune cells traverse the endothelial barrier and accumulate within the brain parenchyma where they cause extensive tissue damage leading to neurological deficits (Frohman et al., 2006; Comabella and Khoury, 2012). The BBB is in essence formed by specialized brain endothelial cells, however surrounding cells, like astrocytes and pericytes also play a key role in maintenance of barrier features (Abbott et al., 2006). Next to the presence of complex tight junctions, barrier properties of the BBB are instated by the presence of specific endothelial ATP-binding cassette (ABC) transporters, which actively remove unwanted compounds from the brain. The ABC transporter family consists of a variety of efflux pumps (Loscher and Potschka, 2005a), of which a few are generally regarded as key BBB transporters, including P-glycoprotein (P-gp), breast-cancer resistance protein (BCRP), and the multi-drug resistance-associated proteins-1 and -2 (MRP-1, -2). In general, ABC transporters drive cellular exclusion of a variety of exogenous compounds and drugs through the cell membrane against a concentration gradient at the cost of ATP hydrolysis (Loscher and Potschka, 2005b). Expression and function of these transporters can be regulated by a broad variety of endogenous and exogenous factors, including nuclear receptors like steroid and xenobiotic receptors (Loscher and Potschka, 2005b) or a variety of inflammatory molecules (see review Miller, 2010).
ABC Transporters and Neuro-Inflammation
Most data on the function of ABC transporters originates from oncology research and is based on their capacity to cause multi-drug resistance (MDR; Lage, 2008). To date, evidence is accumulating that ABC transporters may also contribute to immunological processes based on their expression on various immune cells like antigen presenting cells (Randolph et al., 1998; van de Ven et al., 2006) and their potential to secrete inflammatory molecules like leukotrienes and prostaglandins (see review van de Ven et al., 2009). Together with their high expression on the BBB, ABC transporters may modulate inflammatory processes that occur in CNS disorders, including MS, a chronic inflammatory disorder of the CNS leading to severe neurological deficits. Pathologically, active MS lesions contain abundant cellular infiltrates, which mainly consist of T-cells and macrophages with a foamy appearance (Bruck et al., 1996). The latter acquire their distinctive morphology by ingestion and accumulation of vast amounts of myelin-derived lipids and cellular debris. Foamy macrophages originate from both resident microglia and infiltrating monocytes (Li et al., 1996) and are thought to contribute to myelin sheaths damage, resulting in neuronal dysfunction. In the course of lesion progression, enlarged proliferative astrocytes become the most predominant cell type. These reactive astrocytes secrete neurotrophic factors for neuronal survival, but also contribute to pathology by producing pro-inflammatory cytokines and chemokines (Tani et al., 1996; Speth et al., 2005; Sofroniew, 2009), which subsequently attract leukocytes into MS lesions.
ATP-binding cassette transporters have been implicated in various neurological disorders, including Alzheimer’s disease (Vogelgesang et al., 2002), HIV encephalitis (Langford et al., 2004), Parkinson’s disease (Bartels et al., 2008), and epilepsy (Sisodiya et al., 2002; Aronica et al., 2011). However, as these disorders lack a profound inflammatory component, the potential involvement for ABC transporters in neuro-inflammation has been long overseen. Conversely, we recently identified an important role for ABC transporters in neuro-inflammatory processes underlying MS pathogenesis (Kooij et al., 2009, 2010, 2011). We demonstrated that P-gp, MRP-1, and -2 are absent on healthy brain astrocytes, but their expression is highly increased on reactive astrocytes in MS lesions. In contrast, the expression of P-gp on endothelial cells of the BBB was slightly reduced in active lesions (Kooij et al., 2010), whereas the expression of endothelial BCRP, MRP-1 and -2 remained unaffected. Moreover, we showed that the astrocytic ABC transporters P-gp and MRP-1 mediated the secretion of chemokine (C–C motif) ligand 2 (CCL2), which in turn promotes immune cell migration in an in vitro model of the BBB (Kooij et al., 2011). In vivo evidence supporting a role for P-gp in immunomodulation was obtained using animals that lack P-gp (mdr1a/1b−/− animals), as they experience a significant reduction in clinical symptoms of experimental autoimmune encephalomyelitis (EAE; Kooij et al., 2009), a validated animal model for MS. Overall, these results strengthen the hypothesis that in addition to exporting unwanted compounds from cells, ABC transporters can actively interfere in immune processes by secreting inflammatory molecules, thereby illustrating a novel (patho)physiological role for these transporters in neuro-inflammatory disorders.
Bioactive Lipids
A controversial issue remains whether ABC transporters themselves are capable to transport inflammatory molecules as suggested by some groups (Gsur et al., 1996; Frank et al., 2001) or that ABC transporters mediate the secretion of other relevant physiological substrates, such as bioactive lipids (Raggers et al., 2001) that in turn affect cytokine secretion as a secondary effect (Huang et al., 1999). The latter seems plausible as a large group of ABC transporter substrates have a lipophilic nature (Lagas et al., 2008). To date, the ABC transporter mediated secretome of endogenous molecules remains to be determined, but likely include various bioactive lipids, which are known to regulate a variety of cellular signaling events through biophysical interactions with proteins that modify location, scaffolding, and signal transduction (Wiegmann et al., 1994; Ballou et al., 1996; Bradshaw et al., 1996; dam-Klages et al., 1998; Kronke, 1999). For example, rapid and transient alterations in the ceramide content of neuronal membranes have been shown to regulate neuronal excitability, synaptic transmitter release, plasma membrane insertion, and removal of transmembrane receptors (Inokuchi et al., 1997; Rohrbough et al., 2004; Kajimoto et al., 2007; Wheeler et al., 2009; Norman et al., 2010). Similarly, ceramide metabolism has been shown to direct immune activation through regulating the formation and function of the immunological synapse, T-cell activation, and cell death (Ballou et al., 1996; D’Souza et al., 1996; Solomon et al., 2003; Falcone et al., 2004; Detre et al., 2006). Other lipids such as sphingosine-1-phosphate, prostaglandins, arachidonic acid, and platelet-activating factor (PAF) act directly as second messengers. Many of these lipid metabolites are released from cells and act as ligands to regulate function through specific receptor interactions. Although many of these lipid metabolites are released from cells in a regulated manner, the exact underlying mechanisms are currently not well defined. Understanding how these lipid-derived second messengers are exported from cells will further increase our understanding of immune regulation and could identify specific therapeutic targets to dampen aberrant immune function in disease settings such as MS.
Mechanism of Action: Platelet-Activating Factor
Although the etiology of MS remains elusive, a crucial role has been established for the immune system, including a broad repertoire of inflammatory agents (Steinman, 2001; Hohlfeld and Wekerle, 2004; Comabella and Khoury, 2012). One of these agents is PAF, a potent pro-inflammatory phospholipid mediator with a wide range of biological activities (Stafforini et al., 2003). Several lines of evidence suggest a potential role for PAF in MS and EAE pathogenesis (Desai and Barton, 1990; Pedotti et al., 2003). Bioactivities of PAF are elicited by binding to the PAF receptor (PAFR), which belongs to the family of G-protein-coupled receptors (Honda et al., 1991). Interestingly, animals that lack the PAFR displayed lower incidence of disease and less severe clinical symptoms in the chronic phase of EAE compared to control mice (Kihara et al., 2005). Strikingly, no differences were observed in peripheral immune parameters that are associated with EAE, highlighting an important role for the PAFR in the neuropathology of EAE. Importantly, it has been demonstrated that PAF levels are elevated in the cerebrospinal fluid and plasma of MS patients (Callea et al., 1999). Together, these findings suggest that PAF likely contributes to MS pathogenesis; however, its cellular source in the CNS is yet unknown and data on the expression of PAFR in MS brain tissue remains elusive.
To first gain insight into the PAFR expression profile we performed a comprehensive immunohistochemical analysis to examine the distribution pattern of PAFR in various MS lesion stages. Classification of MS lesions was based on standard immunohistochemical analysis for myelin (proteolipid protein, PLP, Figure 1A). Active demyelination was demonstrated by the presence of phagocytic perivascular and parenchymal macrophages containing myelin degradation products (Figure 1A, insert) as described previously (van der Valk and De Groot, 2000; van Horssen et al., 2006a,b). In white matter from non-neurological control brain tissue (data not shown) and normal appearing white matter (NAWM) PAFR (Figure 1B) immunoreactivity was mainly restricted to glial cells (arrowheads). In active demyelinating lesions (Figure 1C) abundant PAFR staining was observed in foamy macrophages (arrows) and hypertrophic astrocytes (arrowhead), whereas endothelial cells only weakly express PAFR. In chronic inactive MS lesions, PAFR was consistently upregulated in reactive astrocytes (data not shown). Using double immunofluorescence analysis we confirmed the cellular localization of PAFR in active MS lesions and showed that it is expressed by CD86-positive macrophages (Figure 1D) and GFAP-positive astrocytes (Figure 1E). These results are in line with microarray data showing increased levels of PAFR transcripts in MS lesions (Lock et al., 2002), but illustrate for the first time the cellular origin of PAFR. Kihara and co-workers (Kihara et al., 2005) have shown that binding of PAF to its receptor increases the phagocytic activity of macrophages, a process that may likely occur in MS lesion development. However, the cellular source of PAF in the CNS during neuro-inflammation is to date unknown.
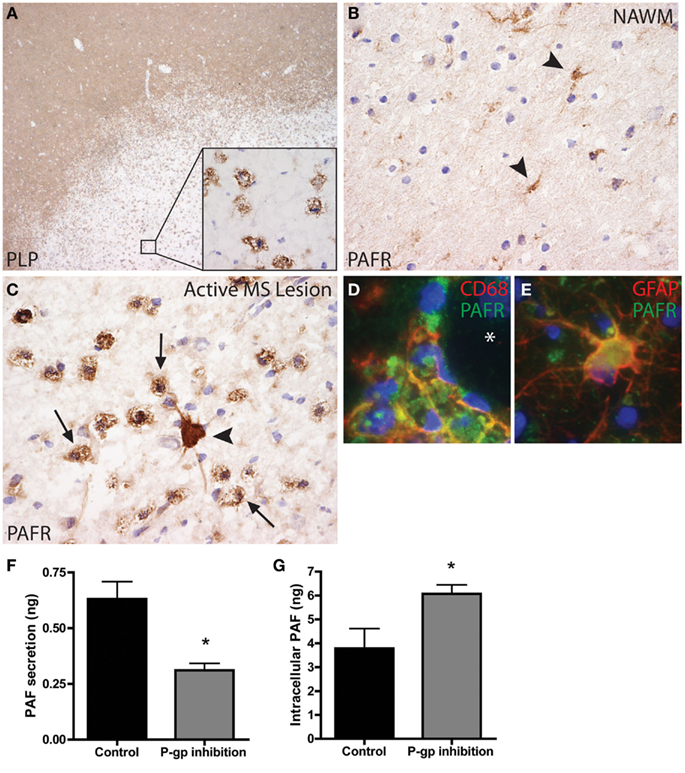
Figure 1. Platelet-activating factor receptor (PAFR) expression in active MS lesions. (A) Active MS lesions are characterized by a loss of myelin (proteolipid protein, PLP), which is ingested by foamy macrophages (insert). (B) In normal appearing white matter (NAWM), PAFR expression was mainly restricted to glial cells (arrowheads). (C) In active lesions PAFR immunostaining was intense in reactive astrocytes (arrowhead) and foamy macrophages (arrows). Co-localization of PAFR immunoreactivity (green) with CD68 (D) and GFAP (E) immunoreactivity (in red) confirms the morphological observations. Original magnification: (A) 20×, (B,C) 200×, (D,E) 250×. PAF C16:0 levels were determined in supernatants and cell pellets derived from primary human reactive astrocytes from MS lesions using liquid chromatography electro spray ionization mass spectrometry (LC/ESI/MS/MS). P-gp inhibition reduced PAF secretion from reactive astrocytes (F), whereas intracellular levels were increased (G). *p < 0.05 by Student’s t-test.
In general, PAF is produced by a variety of cells, including neutrophils, eosinophils, monocytes, macrophages, and vascular endothelial cells (Montrucchio et al., 2000). Although it is currently unknown how PAF is secreted from these cells, an important role has been suggested for ABC transporters like P-gp (Raggers et al., 2001). Our findings suggest an important role for P-gp on reactive astrocytes, as P-gp expression and function are highly increased early in the course of MS lesion formation (Kooij et al., 2011). Astrocytic P-gp may mediate the release of leukocyte attracting chemokines, a process that might be mediated by PAF. We isolated human primary reactive astrocytes directly from active MS lesions (as described previously by De Groot et al., 1997; Elkord et al., 2005) and cultured them in the presence or absence of the specific P-gp inhibitor reversin 121 for 6 h. Supernatants and cell pellets were harvested and PAF C16:0 levels were determined using liquid chromatography electro spray ionization mass spectrometry (LC/ESI/MS/MS; Wheeler et al., 2008). Notably, PAF secretion was significantly reduced in the presence of P-gp inhibition, whereas intracellular levels were increased (Figures 1F,G). These data strongly suggest a direct role for P-gp in the release of PAF form reactive astrocytes, and provide the first mechanistic insights in the role of bioactive lipids and ABC transporters in MS lesion formation.
Future Perspectives
Over the last decade, a novel role for ABC transporters in regulating immune responses is emerging. These new findings are challenging the current view of ABC transporters as gatekeepers of the BBB, but instead indicate that these transporters are able to contribute to degenerative and inflammatory responses under pathological conditions (Figure 2, model). Hence, further research is warranted to gain insight into the ABC transporter secretome under normal and inflammatory conditions to define a set of bioactive lipids that regulate the release of inflammatory mediators from cells. Moreover, future experiments need to define the exact mechanisms by which bioactive lipids induce cytokine/chemokine release from cells, or if they act as chemoattractants themselves (i.e., G-protein-coupled PAFR has many similarities with chemokine receptors). From a therapeutical point of view, targeting cell-specific ABC transporter substrates may be an attractive approach to dampen inflammation, as systemic inhibition of ABC transporters will likely cause major side effects. Next to that, more research is warranted to delineate the contribution of bioactive lipids in immune responses and neuro-inflammatory diseases like MS, using specific knock-out animals and/or selective inhibitors. Ultimately, this will open new therapeutic avenues to limit the neuro-inflammatory attack and prevent brain tissue damage.
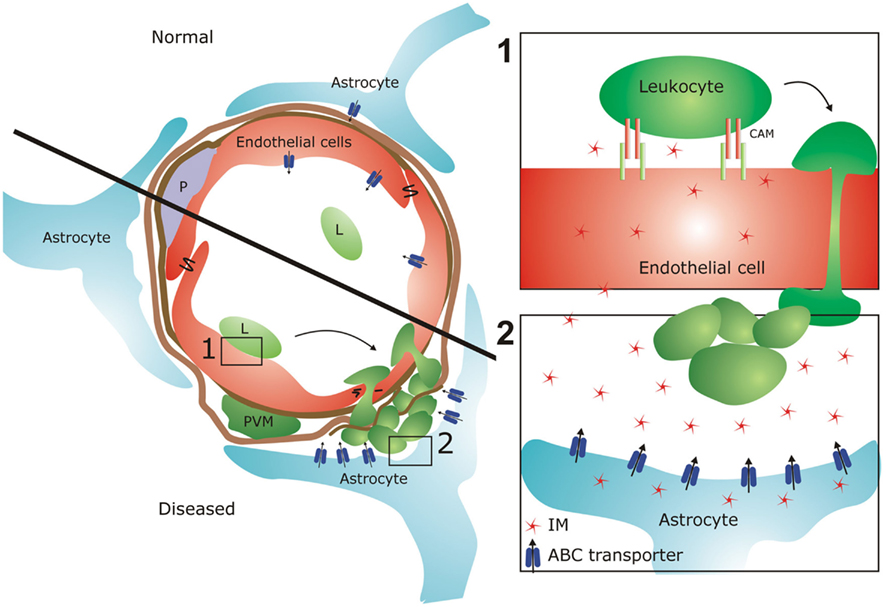
Figure 2. ATP-binding cassette (ABC) transporters at the BBB in health and disease. The BBB is formed by brain endothelial cells, instated by astrocyte endfeet and pericytes (P) and surrounded by perivascular macrophages (PVM). Under normal conditions, astrocytes only marginally express ABC transporters. In diseased state, leukocytes (L) adhere to brain EC via cell adhesion molecules (CAMs), which in turn lead to transmigration of these cells into the CNS where they cause tissue damage. Our preliminary data indicate that reactive astrocytes highly increase their ABC transporter expression and function, which may result in increased efflux of inflammatory mediators (IM). These molecules in turn may (1) increase endothelial CAM expression and subsequent leukocyte adhesion and (2) stimulate leukocyte attraction into the CNS via chemokine secretion. In that way, ABC transporters can regulate leukocyte migration into the CNS at various cellular levels, thereby contributing to tissue damage in MS.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ABC, ATP-binding cassette; BBB, blood–brain barrier; BCRP, breast-cancer resistance protein; CCL2: chemokine (C–C motif) ligand 2; CNS, central nervous system; EAE, experimental autoimmune encephalomyelitis; MDR, multi-drug resistance; MRP, multi-drug resistance-associated protein; MS, multiple sclerosis; PAF, platelet-activating factor; PAFR, platelet-activating factor receptor; P-gp, P-glycoprotein.
References
Abbott, N. J., Ronnback, L., and Hansson, E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53.
Aronica, E., Sisodiya, S. M., and Gorter, J. A. (2011). Cerebral expression of drug transporters in epilepsy. Adv. Drug Deliv. Rev. (in press).
Ballou, L. R., Laulederkind, S. J., Rosloniec, E. F., and Raghow, R. (1996). Ceramide signalling and the immune response. Biochim. Biophys. Acta 1301, 273–287.
Bartels, A. L., Willemsen, A. T., Kortekaas, R., de Jong, B. M., de Vries, R., de Klerk, O., van Oostrom, J. C., Portman, A., and Leenders, K. L. (2008). Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J. Neural Transm. 115, 1001–1009.
Bradshaw, C. D., Ella, K. M., Thomas, A. L., Qi, C., and Meier, K. E. (1996). Effects of Ara-C on neutral sphingomyelinase and mitogen- and stress-activated protein kinases in T-lymphocyte cell lines. Biochem. Mol. Biol. Int. 40, 709–719.
Bruck, W., Sommermeier, N., Bergmann, M., Zettl, U., Goebel, H. H., Kretzschmar, H. A., and Lassmann, H. (1996). Macrophages in multiple sclerosis. Immunobiology 195, 588–600.
Callea, L., Arese, M., Orlandini, A., Bargnani, C., Priori, A., and Bussolino, F. (1999). Platelet activating factor is elevated in cerebral spinal fluid and plasma of patients with relapsing-remitting multiple sclerosis. J. Neuroimmunol. 94, 212–221.
Comabella, M., and Khoury, S. J. (2012). Immunopathogenesis of multiple sclerosis. Clin. Immunol. 142, 2–8.
dam-Klages, S., Schwandner, R., Adam, D., Kreder, D., Bernardo, K., and Krönke, M. (1998). Distinct adapter proteins mediate acid versus neutral sphingomyelinase activation through the p55 receptor for tumor necrosis factor. J. Leukoc. Biol. 63, 678–682.
De Groot, C. J., Langeveld, C. H., Jongenelen, C. A., Montagne, L., Van Der Valk, P., and Dijkstra, C. D. (1997). Establishment of human adult astrocyte cultures derived from postmortem multiple sclerosis and control brain and spinal cord regions: immunophenotypical and functional characterization. J. Neurosci. Res. 49, 342–354.
Desai, S., and Barton, R. (1990). Role of platelet activating factor in the pathogenesis of active and passive models of experimental allergic encephalomyelitis in the rat. Agents Actions 31, 43–46.
Detre, C., Kiss, E., Varga, Z., Ludányi, K., Pászty, K., Enyedi, A., Kövesdi, D., Panyi, G., Rajnavölgyi, E., and Matkó, J. (2006). Death or survival: membrane ceramide controls the fate and activation of antigen-specific T-cells depending on signal strength and duration. Cell. Signal. 18, 294–306.
D’Souza, S. D., Bonetti, B., Balasingam, V., Cashman, N. R., Barker, P. A., Troutt, A. B., Raine, C. S., and Antel, J. P. (1996). Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J. Exp. Med. 184, 2361–2370.
Elkord, E., Williams, P. E., Kynaston, H., and Rowbottom, A. W. (2005). Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology 114, 204–212.
Falcone, S., Perrotta, C., De Palma, C., Pisconti, A., Sciorati, C., Capobianco, A., Rovere-Querini, P., Manfredi, A. A., and Clementi, E. (2004). Activation of acid sphingomyelinase and its inhibition by the nitric oxide/cyclic guanosine 3′,5′-monophosphate pathway: key events in Escherichia coli – elicited apoptosis of dendritic cells. J. Immunol. 173, 4452–4463.
Frank, M. H., Denton, M. D., Alexander, S. I., Khoury, S. J., Sayegh, M. H., and Briscoe, D. M. (2001). Specific MDR1 P-glycoprotein blockade inhibits human alloimmune T cell activation in vitro. J. Immunol. 166, 2451–2459.
Frohman, E. M., Racke, M. K., and Raine, C. S. (2006). Multiple sclerosis–the plaque and its pathogenesis. N. Engl. J. Med. 354, 942–955.
Gsur, A., Hamilton, G., Zhao, S., Angerler, J., Fiegl, M., Zojer, N., Raderer, M., Haberl, I., Andreeff, M., and Huber, H. (1996). Involvement of P-glycoprotein in the transmembrane transport of interleukin-2 (IL-2), IL-4, and interferon-gamma in normal human T lymphocytes. Blood 88, 1747–1754.
Hohlfeld, R., and Wekerle, H. (2004). Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc. Natl. Acad. Sci. U.S.A. 101(Suppl. 2), 14599–14606.
Honda, Z., Nakamura, M., Miki, I., Minami, M., Watanabe, T., Seyama, Y., Okado, H., Toh, H., Ito, K., Miyamoto, T., and Shimizu, T. (1991). Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature 349, 342–346.
Huang, Y. H., Schafer-Elinder, L., Wu, R., Claesson, H. E., and Frostegard, J. (1999). Lysophosphatidylcholine (LPC) induces proinflammatory cytokines by a platelet-activating factor (PAF) receptor-dependent mechanism. Clin. Exp. Immunol. 116, 326–331.
Inokuchi, J., Mizutani, A., Jimbo, M., Usuki, S., Yamagishi, K., Mochizuki, H., Muramoto, K., Kobayashi, K., Kuroda, Y., Iwasaki, K., Ohgami, Y., and Fujiwara, M. (1997). Up-regulation of ganglioside biosynthesis, functional synapse formation, and memory retention by a synthetic ceramide analog (L-PDMP). Biochem. Biophys. Res. Commun. 237, 595–600.
Kajimoto, T., Okada, T., Yu, H., Goparaju, S. K., Jahangeer, S., and Nakamura, S. (2007). Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol. Cell. Biol. 27, 3429–3440.
Kihara, Y., Ishii, S., Kita, Y., Toda, A., Shimada, A., and Shimizu, T. (2005). Dual phase regulation of experimental allergic encephalomyelitis by platelet-activating factor. J. Exp. Med. 202, 853–863.
Kooij, G., Mizee, M. R., van Horssen, J., Reijerkerk, A., Witte, M. E., Drexhage, J. A. R., van der Pol, S. M. A., van het Hof, B., Scheffer, G., Scheper, R., Dijkstra, C. D., van der Valk, P., and de Vries, H. E. (2011). Adenosine triphosphate-binding cassette transporters mediate chemokine (C-C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis. Brain 134, 555–570.
Kooij, G., van Horssen, J., de Lange, E. C., Reijerkerk, A., van der Pol, S. M., van Het Hof, B., Drexhage, J., Vennegoor, A., Killestein, J., Scheffer, G., Oerlemans, R., Scheper, R., van der Valk, P., Dijkstra, C. D., and de Vries, H. E. (2010). T lymphocytes impair P-glycoprotein function during neuroinflammation. J. Autoimmun. 34, 416–425.
Kooij, G., Backer, R., Koning, J. J., Reijerkerk, A., van Horssen, J., van der Pol, S. M. A., Drexhage, J., Schinkel, A., Dijkstra, C. D., den Haan, J. M. M., Geijtenbeek, T. B. H., and de Vries, H. E. (2009). P-glycoprotein acts as an immunomodulator during neuroinflammation. PLoS ONE 4, e8212. doi:10.1371/journal.pone.0008212
Kronke, M. (1999). Biophysics of ceramide signaling: interaction with proteins and phase transition of membranes. Chem. Phys. Lipids 101, 109–121.
Lagas, J. S., Sparidans, R. W., van Waterschoot, R. A., Wagenaar, E., Beijnen, J. H., and Schinkel, A. H. (2008). P-glycoprotein limits oral availability, brain penetration, and toxicity of an anionic drug, the antibiotic salinomycin. Antimicrob. Agents Chemother. 52, 1034–1039.
Lage, H. (2008). An overview of cancer multidrug resistance: a still unsolved problem. Cell. Mol. Life Sci. 65, 3145–3167.
Langford, D., Grigorian, A., Hurford, R., Adame, A., Ellis, R. J., Hansen, L., and Masliah, E. (2004). Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J. Neuropathol. Exp. Neurol. 63, 1038–1047.
Li, H., Cuzner, M. L., and Newcombe, J. (1996). Microglia-derived macrophages in early multiple sclerosis plaques. Neuropathol. Appl. Neurobiol. 22, 207–215.
Lock, C., Hermans, G., Pedotti, R., Brendolan, A., Schadt, E., Garren, H., Langer-Gould, A., Strober, S., Cannella, B., Allard, J., Klonowski, P., Austin, A., Lad, N., Kaminski, N., Galli, S. J., Oksenberg, J. R., Raine, C. S., Heller, R., and Steinman, L. (2002). Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 8, 500–508.
Loscher, W., and Potschka, H. (2005a). Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2, 86–98.
Loscher, W., and Potschka, H. (2005b). Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 6, 591–602.
Miller, D. S. (2010). Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol. Sci. 31, 246–254.
Montrucchio, G., Alloatti, G., and Camussi, G. (2000). Role of platelet-activating factor in cardiovascular pathophysiology. Physiol. Rev. 80, 1669–1699.
Norman, E., Cutler, R. G., Flannery, R., Wang, Y., and Mattson, M. P. (2010). Plasma membrane sphingomyelin hydrolysis increases hippocampal neuron excitability by sphingosine-1-phosphate mediated mechanisms. J. Neurochem. 114, 430–439.
Pedotti, R., De Voss, J. J., Steinman, L., and Galli, S. J. (2003). Involvement of both “allergic” and “autoimmune” mechanisms in EAE, MS and other autoimmune diseases. Trends Immunol. 24, 479–484.
Raggers, R. J., Vogels, I., and van, M. G. (2001). Multidrug-resistance P-glycoprotein (MDR1) secretes platelet-activating factor. Biochem. J. 357, 859–865.
Randolph, G. J., Beaulieu, S., Pope, M., Sugawara, I., Hoffman, L., Steinman, R. M., and Muller, W. A. (1998). A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc. Natl. Acad. Sci. U.S.A. 95, 6924–6929.
Rohrbough, J., Rushton, E., Palanker, L., Woodruff, E., Matthies, H. J. G., Acharya, U., Acharya, J. K., and Broadie, K. (2004). Ceramidase regulates synaptic vesicle exocytosis and trafficking. J. Neurosci. 24, 7789–7803.
Sisodiya, S. M., Lin, W. R., Harding, B. N., Squier, M. V., and Thom, M. (2002). Drug resistance in epilepsy: expression of drug resistance proteins in common causes of refractory epilepsy. Brain 125, 22–31.
Sofroniew, M. V. (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647.
Solomon, J. C., Sharma, K., Wei, L. X., Fujita, T., and Shi, Y. F. (2003). A novel role for sphingolipid intermediates in activation-induced cell death in T cells. Cell Death Differ. 10, 193–202.
Speth, C., Dierich, M. P., and Sopper, S. (2005). HIV-infection of the central nervous system: the tightrope walk of innate immunity. Mol. Immunol. 42, 213–228.
Stafforini, D. M., McIntyre, T. M., Zimmerman, G. A., and Prescott, S. M. (2003). Platelet-activating factor, a pleiotropic mediator of physiological and pathological processes. Crit. Rev. Clin. Lab. Sci. 40, 643–672.
Tani, M., Glabinski, A. R., Tuohy, V. K., Stoler, M. H., Estes, M. L., and Ransohoff, R. M. (1996). In situ hybridization analysis of glial fibrillary acidic protein mRNA reveals evidence of biphasic astrocyte activation during acute experimental autoimmune encephalomyelitis. Am. J. Pathol. 148, 889–896.
van de Ven, R., de Jong, M. C., Reurs, A. W., Schoonderwoerd, A. J., Jansen, G., Hooijberg, J. H., Scheffer, G. L., de Gruijl, T. D., and Scheper, R. J. (2006). Dendritic cells require multidrug resistance protein 1 (ABCC1) transporter activity for differentiation. J. Immunol. 176, 5191–5198.
van de Ven, R., Oerlemans, R., van der Heijden, J. W., Scheffer, G. L., de Gruijl, T. D., Jansen, G., and Scheper, R. J. (2009). ABC drug transporters and immunity: novel therapeutic targets in autoimmunity and cancer. J. Leukoc. Biol. 86, 1075–1087.
van der Valk, P., and De Groot, C. J. (2000). Staging of multiple sclerosis (MS) lesions: pathology of the time frame of MS. Neuropathol. Appl. Neurobiol. 26, 2–10.
van Horssen, J., Bo, L., Dijkstra, C. D., and de Vries, H. E. (2006a). Extensive extracellular matrix depositions in active multiple sclerosis lesions. Neurobiol. Dis. 24, 484–491.
van Horssen, J., Vos, C. M., Admiraal, L., van Haastert, E. S., Montagne, L., van der Valk, P., and de Vries, H. E. (2006b). Matrix metalloproteinase-19 is highly expressed in active multiple sclerosis lesions. Neuropathol. Appl. Neurobiol. 32, 585–593.
Vogelgesang, S., Cascorbi, I., Schroeder, E., Pahnke, J., Kroemer, H. K., Siegmund, W., Kunert-Keil, C., Walker, L. C., and Warzok, R. W. (2002). Deposition of Alzheimer’s beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics 12, 535–541.
Wheeler, D., Bandaru, V. V., Calabresi, P. A., Nath, A., and Haughey, N. J. (2008). A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain 131, 3092–3102.
Wheeler, D., Knapp, E., Bandaru, V. V., Wang, Y., Knorr, D., Poirier, C., Mattson, M. P., Geiger, J. D., and Haughey, N. J. (2009). Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J. Neurochem. 109, 1237–1249.
Keywords: ATP-binding cassette transporters, blood–brain barrier, multiple sclerosis, astrocytes, bioactive lipids, platelet-activating factor, chemokines
Citation: Kooij G, van Horssen J, Bandaru VVR, Haughey NJ and de Vries HE (2012) The role of ATP-binding cassette transporters in neuro-inflammation: relevance for bioactive lipids. Front. Pharmacol. 3:74. doi: 10.3389/fphar.2012.00074
Received: 12 March 2012; Accepted: 10 April 2012;
Published online: 30 April 2012.
Edited by:
Joana A. Palha, University of Minho, PortugalReviewed by:
Muzamil Ahmad, Indian Institute of Integrative Medicine, IndiaRaja S. Settivari, The Dow Chemical Company, USA
Copyright: © 2012 Kooij, van Horssen, Bandaru, Haughey and de Vries. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Helga E. de Vries, Blood-Brain Barrier Research Group, Department of Molecular Cell Biology and Immunology, VU University Medical Center, P.O. Box 7057, 1007 MB Amsterdam, Netherlands. e-mail: he.devries@vumc.nl
