- 1Wake Forest Baptist Medical Center, Medical Center Boulevard, Winston-Salem, NC, USA
- 2Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA
The vas deferens, a muscular conduit conveying spermatozoa from the epididymis to the urethra, has been used as a model tissue for smooth muscle pharmacological and physiological advancements. Many drugs, notably α-adrenergic antagonists, have effects on contractility and thus normal ejaculation, incurring significant side effects for patients that may interfere with compliance. A more thorough understanding of the innervation and neurotransmitter pharmacology of the vas has indicated that this is a highly complex structure and a model for co-transmission at the synapse. Recent models have shown clinical scenarios that alter the vas contraction. This review covers structure, receptors, neurotransmitters, smooth muscle physiology, and clinical implications of the vas deferens.
Introduction
In the treatment of male sexual disorders, focus has often been on erectile disorders and premature ejaculation (PE), the latter probably the most common disorder of male sexual function (Abdel-Hamid et al., 2009). Ejaculation consists of two distinct phases, emission and expulsion. Emission denotes the ejection into the posterior urethra of spermatozoa mixed with products secreted by accessory sexual glands. During the emission phase, both epithelial secretion and smooth muscle cell contraction take place throughout the seminal tract in a sequential manner. The function of the vas (ductus) deferens is to convey spermatozoa from the epididymis to the urethra. During emission, its coordinated muscular contractions propel the spermatozoa toward the urethra. However, the vas does not serve only as a conduit, but also contributes to secretion of fluid for sperm transport and possibly to resorption of spermatozoan remnants from the duct lumen. Adrenergic mechanisms play a major role for vas smooth muscle contraction, but many substances are capable of altering its contractility by modulating neurotransmitter release or the basal tone of the smooth muscle layers. Interference with the contractile function by, e.g., metabolic disorders and drugs used for lower urinary tract disorders, may lead to ejaculatory dysfunction, and ultimately anejaculation. The mechanisms regulating the contractile behavior of the vas may therefore be of interest as targets for drugs meant for control of ejaculation (e.g., contraception). In addition, these mechanisms may have general physiological/pharmacological interest since the isolated vas deferens has proven to be one of the most useful preparations for the study of basic physiological mechanisms and the effects of drugs. It has been used to study the electrophysiology of the smooth muscle myocytes and the release and inactivation of neurotransmitters, receptors and receptor-mediated mechanisms, and signaling pathways.
The present review gives an update on some of the mechanisms involved in the generation, propagation, and transduction of signals in the vas deferens. Some examples of the clinical consequences of interference with its contractile function are also given.
General Structure of Vas Deferens
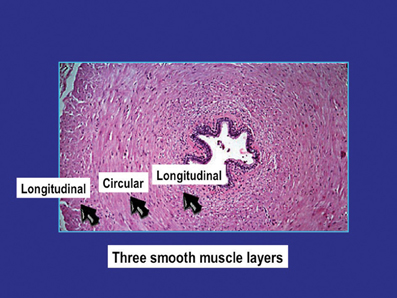
The general structure and function of the vas deferens from humans and different animal species have many similarities (Steers, 1994; Dixon et al., 1998; Kaleczyc, 1998; Westfall and Westfall, 2001; Burnstock and Verkhratsky, 2010). The vas is a tubular structure consisting of a muscle coat, an inner mucosa and an outer adventitia. The smooth muscle coat, which may have a thickness of 1–1.5 mm, consists of a circular layer surrounded by inner and outer longitudinal layers. The circular layer is the most prominent and forms a tightly wound spiral, whereas the longitudinal layers are formed by muscle bundles slightly helical in their arrangement. The outer longitudinal smooth muscle cells are up to 30–40 μm in length and 2–5 μm in diameter (Figure 1). Each smooth muscle cell is closely associated with 6–12 other cells, with gaps as close as 15–20 nm (Elbadawi and Goodman, 1980). The cells are electrically coupled allowing electronic spread and depolarization to travel from one cell to the next. This intercellular coupling can be suppressed by heptanol (Manchanda and Venkateswarlu, 1997, 1999), believed to interact with gap junction function (Christ, 1995).
The lumen of the vas deferens is lined by columnar epithelial cells with microvilli extending into the lumen (Dixon et al., 1998). Blood supply comes from the inferior vesical artery. The vas is innervated by autonomic postganglionic nerve fibers originating primarily from neurons in pelvic ganglia, and to a lesser extent, from neurons in the caudal mesenteric ganglion and sympathetic chain ganglia, and also by sensory nerve fibers arising from dorsal root ganglia (Kaleczyc, 1998; Kihara et al., 1998; Burnstock and Verkhratsky, 2010). In rodents the hypogastric nerve provides bilateral innervation to the vas, and contractile responses can be elicited with hypogastric stimulation from either side (Kihara et al., 1996; Harji et al., 1998).
Signal Generation
Autonomic Effector Mechanisms
Adrenergic nerves
Adrenergic nerves are the most common among the nerve fiber groups supplying the mammalian vas deferens. Early studies using fluorescence histochemistry and biochemical detection of catecholamines have revealed numerous adrenergic nerve fibers innervating the vas of many mammalian species including the rat, guinea-pig, rabbit, cat, opossum, bull, and pig. The vas deferens of man, other primates, dog, and possibly the fox receives a less dense adrenergic innervation than that of other species (see overviews by Dixon et al., 1998; Kaleczyc, 1998). More recent immunohistochemical investigations have confirmed the results of the histochemical studies. Kaleczyc et al. (1997), showed in the pig vas deferens, similar to what has been found in other mammals, that the adrenergic nerves were distributed in the lamina propria and throughout the circular and longitudinal muscle layers. In the lamina propria, the adrenergic axons formed a loose network with the nerve terminals sometimes found beneath, but never penetrating into, the epithelium. In the muscle layers, the nerves were usually more numerous and run chiefly along the smooth muscle cells.
There is morphological, physiological, and pharmacological evidence that the adrenergic neurons supplying the mammalian vas deferens can utilize adenosine triphosphate (ATP) and/or a related purine as a possible co-transmitter with noradrenaline (NA) (see below).
In human vas, varicose nerve terminals able to take up and bind quinacrine have been demonstrated. These nerves may represent purinergic nerves (Alm, 1982). It may be assumed that these nerves also contain NA, but this does not seem to have been established (Figure 2).
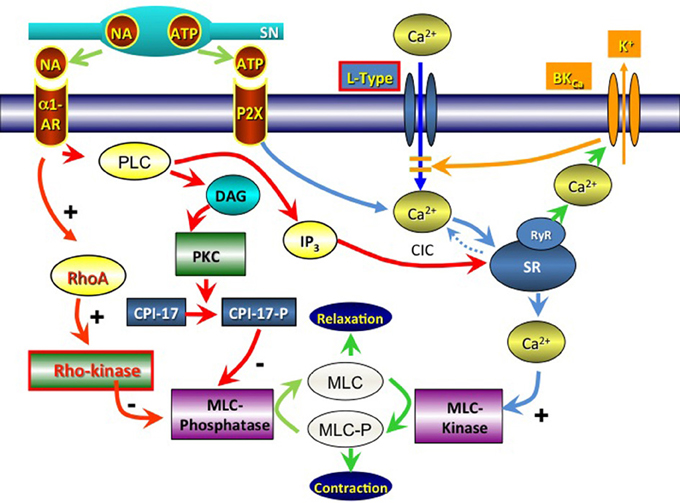
Figure 2. Signal pathways involved in contractile activation of the vas deferens via noradrenaline (NA; α1-Adrenoceptors) and adenosine triphosphate (ATP; P2X receptors). SN, sympathetic neuron; PLC, phospholipase C; DAG, diacylglycerol; PKC, protein kinase C; RhoA, ras homolog gene family, member A; CPI-17, protein phosphatase 1 regulatory subunit 14A; MLC, myosin light chain; IP3, inositol trisphosphate; SR, sarcoplasmic reticulum; CIC, calcium-induced calcium release; RyR, ryanodine receptor; BKCa, big K+ activated Ca2+ channel; L-type, L-type voltage dependent Ca2+-channel.
There is also evidence suggesting the coexistence of neuropeptides in noradrenergic nerve fibers supplying the mammalian vas deferens. Although there may be species differences, many noradrenergic nerves innervating the vas deferens muscle coat also express immunoreactivity to somatostatin, Leu-enkephalin, and neuropeptide Y (Kaleczyc, 1998; Burnstock and Verkhratsky, 2010). The majority of adrenergic (tyrosine hydroxylase-immunoreactive: IR) nerves supplying the muscle of the human vas deferens (Jen et al., 1997) were shown to contain neuropeptide Y.
Cholinergic nerves
Although the histochemical localization of acetylcholinesterase (AChE) is thought not to be specific for cholinergic nerve fibers (Lincoln and Burnstock, 1993), most information on cholinergic innervation of the vas is based on this methodology. The general impression is that cholinergic (AChE positive or choline acetyltransferase IR) nerve fibers supplying the mammalian vas deferens are fewer than the adrenergic ones and are mostly concentrated within the lamina propria (in contrast to adrenergic nerves that are present especially in the muscle coat). Such an innervation pattern has been found in many mammals including the rat, guinea-pig, dog, bull, monkey, and man (for review, see Kaleczyc, 1998; Burnstock and Verkhratsky, 2010).
The functional significance of cholinergic input to the innervation of the vas deferens has not been established. Sjöstrand (1962) suggested that the main action of the cholinergic innervation was to suppress adrenergic neurotransmission. In addition to such a function, there is some evidence that cholinergic nerves may act on the epithelial cells of the vas deferens (Sjöstrand, 1965), which may be responsible for fluid resorption from the lumen or for active secretion of certain components necessary for sperm maturation. Cholinergic nerve fibers supplying the mammalian vas deferens contain neuropeptides, particularly vasoactive intestinal polypeptide (VIP), NPY, and/or somatostatin, as well as other active substances such as nitric oxide synthase (Kaleczyc, 1998).
Afferent nerves
Neurons in the dorsal root ganglion contain a number of different substances including tachykinins, especially substance P (SP), and calcitonin gene-related peptide (CGRP). The mammalian vas deferens is supplied with some CGRP- and/or SP-IR nerve terminals that are presumed to derive from DRG (see, Kaleczyc, 1998), which is consistent with observations of Kolbeck and Steers (1993) in the rat that many DRG neurons project to the vas deferens. In the pig, double-labeling immunofluorescence has revealed almost a complete colocalization of SP and CGRP in some nerve fibers supplying the vas deferens (Kaleczyc et al., 1997). SP/CGRP-IR nerve terminals were located mainly in the longitudinal muscle layer where they sometimes appeared as very long, fine varicose fibers running parallel to the smooth muscle bundles. In the lamina propria, these fibers were occasionally discovered. CGRP-IR fibers supplying the vas deferens have been found in some other mammals including the guinea-pig, rat, and man. SP-IR nerves have been revealed in the mouse, guinea-pig, cat, rabbit, and man, but are absent from the vas deferens of the rat (see Kaleczyc, 1998; Burnstock and Verkhratsky, 2010). GCRP, widely distributed in peripheral and central sensory afferents throughout the body, are released in the vas deferens from various stimuli, notably capsaicin (Sheykhzade et al., 2011), which induces GCRP release through the transient receptor potential cation channel vanilloid subfamily member 1 (TRPV1) agonism. As a brief review, transient receptor channels are omnipresent in the body, typically allowing various cation passage with appropriate stimuli (Nilius et al., 2007). In the vas, vanilloid are the only subtypes to our knowledge.
Responses to nerve stimulation
Electrical field stimulation of the sympathetic nerves in the nonhuman vas deferens results in a contraction with two distinct components. The twitch or phasic component is transient, while the secondary tonic component is maintained for the duration of the stimulation. These biphasic responses have been found in the guinea pig, rat, mouse, and rabbit vas deferens (Ambache and Zar, 1971; Swedin, 1971, see review by Westfall and Westfall, 2001). Ambache and Zar (1971) suggested that the biphasic response was due to the involvement of a second neurotransmitter, and the inability of phentolamine and pretreatment with reserpine to block the phasic portion of the neurogenic response (Swedin, 1971) supported this suggestion. Burnstock (1972) proposed that the second transmitter was ATP, and Westfall et al. (1978) first demonstrated that stimulation of the vas deferens results in the release of purines. Since then, neuronal release of endogenous ATP and NA has been convincingly confirmed in several species using various techniques (see Westfall and Westfall, 2001). In the human vas, adrenergic mechanisms were considered primarily responsible for contraction of the smooth muscle, since the response to nerve stimulation was more or less completely blocked by α-adrenoceptor (AR) antagonists (Figure 2) (Anton and McGrath, 1977; Hedlund et al., 1985; Smith and Bray, 1990; Steers, 1994). However, Banks et al. (2006) demonstrated that the human vas deferens smooth muscle contracts in response to both adrenergic and purinergic agonists. They considered the adrenergic system functionally dominant, but that purinergic co-transmission was also functionally significant. While not certain, there have been studies suggesting the presence of pacemaker cells in the vas that initiate spontaneous contractions in a similar manner to the Interstitial Cells of Cajal (Metzger et al., 2008; Burnstock and Lavin, 2002). These cells may be c-kit+ interepithelial cells.
It has not been definitely established whether ATP and NA are co-stored and released from the same vesicles or stored and released from different vesicles. As pointed out by Knight et al. (2003), four possible scenarios for the storage and release of these two neurotransmitters exist: (1) ATP and NA may be stored and released from the same vesicles, (2) ATP and NA may be stored and released from separate vesicles, (3) ATP and NA might be stored and released from different sets of varicosities, (4) ATP and NA may be stored and released from the same vesicles but in different proportions in different varicosities. The concept of separate storage and differential release of ATP and NA seems to have growing support (Westfall et al., 2002). Additional studies indicate that the epithelium may play a role in the regulation of vas contractility. Ruan et al. (2008), demonstrated that exogenous ATP could inhibit EFS smooth muscle contraction in an epithelium dependent mechanism, likely through its induction of PGE2 synthesis. This was posed to be due to P2Y receptor activation by ATP, and calcium release from epithelium, which leads to cAMP-dependent K+ channel activation and membrane hyperpolarization of smooth muscle.
While ATP and NA are the primary affectors released from nerve terminals upon stimulation, other neurotransmitters have been posed to influence the neuromuscular relationship in the vas deferens. Li et al. (2003, 2006) and Hu (2007) have demonstrated that histamine coexists with NA in sympathetic nerves, is released with nervous stimulation, and may have sympathetic affects.
Postjunctional receptor mechanisms
As indicated above, contraction of the smooth muscle myocytes in the nonhuman vas deferens is elicited by at least two neurotransmitters, NA, which evokes a contraction mediated by α1-Adrenoceptors (Minneman et al., 1988; Honner and Docherty, 1999), and ATP, which evokes a faster contraction mediated by ligand-gated P2X1-receptors (Liang et al., 2000; Mulryan et al., 2000). Neurotransmitter release from sympathetic varicosities is highly intermittent (Brock and Cunnane, 1988), and non-uniform between varicosities (Lavidis and Bennett, 1992).
Purinergic receptors
Genetic as well as pharmacological and developmental studies provide strong evidence that P2X1 receptors, probably forming homomeric channels, are primarily responsible for fast purinergic transmission in the mouse vas deferens (Liang et al., 2000, 2001; Mulryan et al., 2000). It has been suggested that P2X receptors in the mouse vas deferens and other sympathetically innervated smooth muscles exist in clusters beneath sympathetic varicosities (Barden et al., 1999). However, only a small proportion of the P2X1-receptors located on a smooth muscle cell contribute to spontaneous EJCs, suggesting a diffuse distribution of P2X1-purinoceptors on the smooth muscle myocytes (Liang et al., 2001). Immunostaining results by several groups also support the notion that P2X1-purinoceptors in the mouse vas deferens are diffusely distributed over the entire surface of the smooth muscle cells (Vulchanova et al., 1996; Lee et al., 2000; Liang et al., 2001). Recent analysis of the P2X1 receptor in human vas deferens (Amobi et al., 2012) indicated P2X1-purinoceptor stimulation elicits excitatory effects that lead to longitudinal muscle contraction. There was a secondary activation of 4-aminopyridine-sensitive (KV), and iberiotoxin-sensitive (BKCa) K+ channels. The contraction mediated by P2X1-purinoceptor stimulation was subcontractile in circular muscle due to the ancillary activation of BKCa channels. These differences in activation between longitudinal and circular muscle were considered to have functional implication in terms of the purinergic contribution to overall contractile function of human vas deferens. Amobi et al. (2012) also considered the modulatory effects of KV and BKCa channels following P2X1-purinoceptor activation to be pivotal in providing the crucial physiological mechanism that ensures temporal co-ordination of longitudinal and circular muscle contractility. Interestingly, the BKCa channels have also been found to mediate vas smooth muscle relaxation when stimulated with sodium hydrosulfide (NaHS) (Li et al., 2012). Li et al. demonstrated that NaHS induced relaxation did not involve the nitric oxide pathway, nor transient receptor potential channels.
The most conclusive evidence to date that the P2X1-receptor mediates the postjunctional excitatory response to ATP in the vas deferens comes from mice lacking P2X1-receptors. The vas deferens from P2X1-receptor−/− mice did not respond to exogenously applied ATP or α, β-meATP, and these tissues lacked spontaneous and evoked EJPs (Mulryan et al., 2000). A consequence of this gene deletion was a 90% reduction in the fertility of male animals, which resulted from a low sperm count in the ejaculated semen. Thus mutant females did not become pregnant when mated with mutant males, but normal rates of conception were observed when they mated with wild-type or heterozygous males. The sperm from the mutant male mice was, however, viable and able to fertilize ova in vitro. Mulryan et al. (2000) suggested that selective pharmacological blockade of P2X1 receptors should produce a similar effect, and might thus provide the means for developing a non-hormonal male contraceptive pill. To this effect, sildenafil, known to reduce vas contractility has been posed to inhibit contractions by way of the purinergic receptor system (Bilge et al., 2005). In addition, agents that potentiate the actions of ATP at P2X1 receptors may be useful in the treatment of male infertility.
α-Adrenoceptors
The myocytes from both human and non-human vas deferens express both α1- and α2-ARs (Hedlund et al., 1985; Salles and Badia, 1991; Ventura and Pennefather, 1994). It has been suggested that contractions of rat vas deferens smooth muscle cells to exogenous NA or adrenaline are mediated predominantly by α1A-ARs (Aboud et al., 1993; Honner and Docherty, 1999; Campos et al., 2003), or the postulated α1L-AR in addition to α1A-ARs (Ohmura et al., 1992). In a study of rat vas deferens, Honner and Docherty (1999) found that contractions to exogenous NA were mediated predominantly by α1A-adrenoceptors, and contractions to endogenous NA by α1D-ARs. Cleary et al. (2004) confirmed that the predominant α1-AR in rat vas deferens is the α1A-AR, both in terms of ligand binding and contractions to exogenous agonists. The α1D-AR was only detectable by ligand binding following chemical sympathectomy, but seemed to be involved in NA-evoked contractions.
The human vas deferens can be contracted by NA; this effect is mediated by α1-ARs, and the motility of the vas deferens can be effectively inhibited by α1-AR antagonists (Holmquist et al., 1990). In the human vas deferens, Furukawa et al. (1995) reported that the contractile response to l-phenylephrine is mediated by the α1A-AR subtype, a finding confirmed by several other investigators. Using RNase protection assay, in situ hybridization, and a functional study, Moriyama et al. (1997) confirmed that both the epididymal and pelvic portions of the human vas contained α1A-ARs mediating the contraction of phenylephrine. This was also found by Amobi et al. (1999a,b), who demonstrated that contractions evoked by NA in both longitudinal and circular smooth muscle from human vas deferens are mediated via activation of α1A-ARs. However the involvement of α1A-AR variants, such as the α1L-AR subtype may explain demonstrated differences in effects on longitudinal and circular muscle between some α1A-AR antagonists.
α1-AR antagonists are extensively used in the treatment of hypertension and lower urinary tract symptoms associated with benign prostatic hyperplasia. Among the side effects, ejaculatory dysfunction occurs more frequently with drugs that are relatively selective for α1A-ARs compared with other drugs of this class. Sanbe et al. (2007) explored physiological contribution of each α1-AR subtype using α1-AR subtype-selective knockout (KO) mice (α1A-, α1B-, and α1D-AR KO mice). They found that contractile tension of the vas deferens in response to NA was markedly decreased in α1-AR KO mice, and this contraction was completely abolished in α1-AR triple-KO mice. This attenuation of contractility was also observed in the electrically stimulated vas deferens. They concluded that α1-ARs, particularly α1A-ARs are required for normal contractility of the vas deferens and consequent sperm ejaculation as well as having a function in fertility. These findings seem to be valid also for humans, and the functional and clinical importance of the α1A-AR in the vas can be illustrated by the effects of silodosin, which has a high selectivity for the this receptor (Yamada et al., 2001).
Fifteen healthy male volunteers (urologists) took silodosin or a placebo twice daily for 3 days in a randomized, double-blind crossover design (Kobayashi et al., 2008). When on silodosin, all the subjects had a complete lack of ejaculation. Three days after completion of silodosin, the mean ejaculatory volume recovered to the baseline level. There was no sperm in urine after ejaculation under silodosin administration in any volunteer, and it was concluded that the mechanism of ejaculatory dysfunction caused by silodosin was a loss of seminal emission (anejaculation). Nagai et al. (2008) performed a real-time observation of ejaculation by healthy males sing color Doppler ultrasound in three healthy males. They concluded that the mechanism of ejaculatory dysfunction after silodosin was intricately related to retrograde ejaculation (retrograde inflow of seminal fluid), insufficient contraction of the seminal vesicles, and insufficient rhythmic contraction of the muscles of the pelvic floor. In a double-blind crossover study (Shimizu et al., 2010), 50 healthy volunteer men were randomly assigned to receive either a single dose of silodosin or placebo with 3 days of washout before crossover. Subjects masturbated 4 h after administering agents. Eleven men overall (22%) on silodosin administration had less than a 50% decrease from baseline in the amount of semen. It was concluded that silodosin may adversely affect the subjective orgasmic function by causing an abnormal ejaculation with decreased (or no) semen discharge and a decrease in the number of bulbocavernosus/pelvic floor muscle contractions. Anejaculation rather that retrograde ejaculation was produced. This has been confirmed in a number of clinical studies on patients with lower urinary tract symptoms associated with benign prostatic hyperplasia where the rate of abnormal ejaculation has been up to 28% (Kawabe et al., 2006).
In human vas deferens, Birowo et al. (2010) found that phophodiesterase inhibitors (PDEs), such as rolipram and RO-1724 (PDE4), milrinone (PDE3), and sildenafil (PDE5) effectively antagonized contraction induced by NA—this was accompanied by an up to 2–8-fold increases in tissue cAMP concentrations. Sildenafil produced a 12-fold increase in the cGMP concentration of the preparations. Whether or not this inhibitory action has any effects on ejaculation in men taking, e.g., PDE5 inhibitors remains to be established.
Other receptors
Substances other than ATP and NA can influence the contractility of the vas deferens, presumably via receptors located on the smooth muscle. Muscarinic receptor stimulation (carbachol) causes an M2-receptor mediated contraction of the vas deferens (Eltze, 1994). Vasopressin contracts the human vas via stimulation of V1 receptors (Andersson et al., 1988). β2-Adrenoceptors can influence sympathetic neuroeffector transmission both prejunctionally, where they facilitate equally well the release of sympathetic cotransmitters (see below) and postjunctionally, where they inhibit smooth muscle contractions evoked by ATP (Todorov et al., 2001). Other established receptors include serotonin (5HT) have been demonstrated in several studies. Kose et al. (2012) showed that a rat varicocele model showed decreased contractile response to 5HT. Given that there are several other substances that can modify the vas contractile response, including neuropeptide Y (Torres et al., 1992), endothelin (Telemaque and d'Orleans-Juste, 1991), vasopressin (Medina et al., 1998), and angiotensin II (Ellis and Burnstock, 1989; Maletìnská et al., 1998), it is possible that there are still unidentified receptors in vas deferens smooth muscle, or that the identified ones are promiscuous in their agonist recognition.
Prejunctional receptors
In the vas deferens of humans and various animal species, it has been amply demonstrated that a number of prejunctional receptors can modulate the release of NA and ATP. As in many other tissues, adrenergic nerves in the vas have prejunctional α2-ARs which, when stimulated, reduce the release of NA. In the vas deferens of various species, including the mouse, rat, and guinea pig, stimulation of the prejunctional α2-ARs not only reduces the release of NA, but that of ATP as well (Sneddon and Westfall, 1984; Driessen et al., 1993). ATP can also produce an inhibition of transmitter release in vas deferens (Von Kugelgen et al., 1989; Forsyth et al., 1991). In the prostatic portion of the rat vas deferens, endogenous ATP was found to exert a dual and opposite modulation of NA release: an inhibition through activation of P2Y receptors with a pharmacological profile similar to that of the P2Y12 and P2Y13 receptors and a facilitation through activation of P2X receptors with a pharmacological profile similar to that of P2X1 and P2X3, or PX2/P2X3 receptors (Queiroz et al., 2003). Some of the effects of ATP may be due to formation of adenosine. Adenosine reduced the amount of nerve-stimulated 3H-NA release, suggesting the involvement of a prejunctional P1 receptor of the A1 type (Hedqvist and Fredholm, 1976). Adenosine A2A receptors were found to facilitate NA release by a mechanism that involves a protein kinase C-mediated attenuation of effects mediated by presynaptic inhibitory receptors, i.e., α2-ARs, adenosine A1 and P2Y receptors (Queiroz et al., 2003). Queiroz et al. (2004) found that adenosine A(2B) receptors are involved in a facilitation of NA release in the prostatic portion of rat vas deferens.
Many other prejunctional receptors in the vas deferens from various species have been found to affect neurotransmitter release including opioid, cannabinoid, bradykinin receptors Trendelenburg et al. (2000), the β2-AR (Driessen et al., 1993, 1996; Todorov et al., 2001), the cholinergic nicotinic receptor (Todorov et al., 1991; Von Kugelgen and Starke, 1991), the NPY receptor Y2 (Bitran et al., 1991), GABAB receptor (Strobel et al., 1989), histamine receptors (Zamfirova and Todorov, 1995; Poli et al., 1994), and receptors for endothelins and natriuretic peptides (Mutafova-Yambolieva and Radomirov, 1993; Mutafova-Yambolieva et al., 1993).
Regional variation in purinergic and adrenergic responses
It is well established that various regions of the vas deferens respond differently to nerve stimulation and exogenous agonists (Ventura, 1998). Segments from both ends of the vas deferens respond to ATP and NA however, segments from the prostatic end are more responsive to ATP and segments from the epididymal end are more responsive to NA (French and Scott, 1983; Schomig et al., 1990; Sneddon and Machaly, 1992). The density of adrenergic nerves and catecholamine content is higher in the prostatic than in the epididymal part of the vas. However, no differences in the distribution of P2X1 receptors (Knight et al., 2003) were demonstrated in the mouse vas, or in α1-Adrenoceptors in the human (Hedlund et al., 1985) or rat vas (Salles and Badia, 1991; Ventura and Pennefather, 1994). There is, however, evidence in a rat model that the density and mRNA level of α1-receptors, as well as maximal response to phenylephrine in the epididymal vas may decrease with age (Yono et al., 2008). In the mouse, the difference in response to ATP was attributed to insufficient nerve-terminal release of ATP in the epididymal part (Knight et al., 2003). Terradas et al. (2001) confirmed that the two portions of rat vas deferens differed in the postjunctional sensitivity to NA. Western blot analysis indicated a smaller concentration of Gq/11 protein in the prostatic half, and the authors suggested that the different sensitivity to NA could be due to the higher availability of this sort of G protein in the epididymal portion. The functional importance of this regional variation remains to be established.
Signal Propagation/Spread
Electrophysiology
Burnstock and Holman (1961, 1966) made the first recordings of EJPs produced by sympathetic nerves innervating the smooth muscle of the guinea-pig vas deferens (see, Sneddon, 2000). This led to the identification of ATP as the mediator of EJPs in this tissue. The EJPs are mediated solely by ATP acting on P2X receptors leading to action potentials and a rapid phasic contraction, whilst NA mediates a slower, tonic contraction which is not dependent on membrane depolarization.
In single smooth muscle cells from the human vas, Park et al. (2004) recorded and characterized two types of Ca2+ currents, the L and T-type. The importance of L type Ca2+ currents for vas contractility is well established (Ohya et al., 2001; Shishido et al., 2009), whereas the role and action of the T-type currents are not well defined. Park et al. (2004) also characterized two types of K+ channel currents, namely BKCa and delayed rectifier currents. Voltage-gated K+ currents (a fast-inactivating transient current and a delayed rectifier current) have also been demonstrated in rat vas deferens smooth muscle cells (Harhun et al., 2003). Their physiological importance has not been established.
Intercellular Communication
Paton et al. (1976), using electron microscope, was unable to demonstrate gap junctions in the vas deferens. However, there are reasons to believe that the smooth muscle cells of the vas are electrically coupled. Neurogenic contractions such as those evoked in the guinea pig vas deferens by stimulation of adrenergic nerves, only a small proportion of cells are directly influenced by transmitter released from the sympathetic motor innervation, because only about a fifth of the cells receive direct innervation by close-contact axonal varicosities (Merrillees, 1968; Bennett, 1973), and because varicosities do not release transmitter in response to every invasion by the axonal action potential because of the low probability of evoked transmitter release (Cunnane and Stjarne, 1984; Brock and Cunnane, 1988). Therefore, spread and co-ordination of excitation from the few directly activated cells to other cells probably requires the involvement of gap junctions. As mentioned previously, in the smooth muscle cells of the vas deferens, EJPs are produced following stimulations of adrenergic nerves. EJPs are thought to reflect not just depolarization of the cell being recorded from, but the summed activity of several cells in the neighborhood, by virtue of intercellular electrical coupling (Cunnane and Manchanda, 1990).
Manchanda and Venkateswarlu (1997) investigated the effects on EJPs of heptanol, a presumptive gap junction blocking agent (Christ, 1995), with a view to determining the influence of intercellular electrical coupling on smooth muscle junction potentials. Heptanol abolished rapidly and reversibly the EJP of the guinea pig vas deferens. Further investigation showed that heptanol inhibited both EJP-dependent and non EJP-dependent contractions of the vas, and that a postjunctional site of action of heptanol, probably intercellular uncoupling of smooth muscle cells, contributed to the inhibition of contraction (Venkateswarlu et al., 1999).
Signal Transduction
In the vas deferens, as in other types of smooth muscle, the most commonly used explanation for excitation–contraction coupling in smooth muscle cells is an increase in intracellular Ca2+ through either L-type Ca2+ channels or the release of Ca2+ from intracellular stores (Berridge, 1993, 2008). It has been shown that blockade of L-type calcium channels by nifedipine abolishes the purinergic component of contraction in mouse vas deferens (Cleary et al., 2003), suggesting that activation of the P2X1 receptors are dependent on Ca2+ influx. Brain et al. (2003), investigating the sources and sequestration of Ca2+ to neuroeffector Ca2+ transients in the mouse vas deferens, suggested that Ca2+ stores initially amplify and the sequester Ca2+ that enters through P2X receptors.
The contractile response of rat vas deferens myocytes to exogenous NA has been reported to be associated with the efflux of Ca2+ from the sarcoplasmic reticulum (SR) rather than the influx of Ca2+ via the plasmalemma. α-1-Adrenoceptors couple with phospholipase C (PLC) (Summers and McMartin, 1993; Burt et al., 1998) which produces inositol (1,4,5)-triphosphate (IP3) and diacylglycerol (DAG) using phospholipids from the plasma membrane (Berridge and Irvine, 1984). IP3 induces Ca2+ release from the SR, allowing the activation of myosin light chain kinase (MLCK) with ultimate phosphorylation of myosin with subsequent smooth muscle contraction (Somlyo and Somlyo, 1994). Khoyi et al. (1988, 1993) suggested that the NA response relies mainly on intracellular Ca2+ and that nifedipine-sensitive calcium entry may function as a trigger for calcium-induced calcium release (CICR) from the SR. However, in the rat vas deferens, there is apparently little contribution to NA-induced contraction from intracellular stores, as exposing intracellular Ca2+ stores to ryanodine, cyclopiazonic acid or thapsigargin had little effect on NA induced contraction (Amobi et al., 1999a,b). Amobi et al. (1999a,b) suggested that the NA-induced contraction may involve an increase in the sensitivity of the contractile apparatus to Ca2+, possibly through a Rho-kinase-mediated pathway (Büyükafar et al., 2003). In brief, the Rho is a GTPase and its downstream protein Rho-kinase is a downstream protein that mediates calcium sensitivity of smooth muscles via inhibition of myosin phosphatase, effectively maintaining smooth muscle contraction (Sward et al., 2000; Fukata et al., 2001); Rho kinase (ROCK-2) is expressed in mouse vas deferens, and inhibitors of Rho-kinase reduce contractions induced by NA (Amobi et al., 2006), phenylephrine, ATP, and KCl (Büyükafar et al., 2003).
There may be differences in the mechanisms for mobilizing intracellular Ca2+ in the prostatic and epididymal parts of the rat vas. Amobi and Smith (1999) suggested that, during stimulation of the epididymal part, the SR functions mainly to buffer calcium entering through nifedipine-sensitive voltage-gated calcium channels. In contrast, in the prostatic part, the SR serves mainly as a source of calcium and contributes more to contractions evoked by higher concentrations of the agonist.
It has been established that Ca2+ sparks are local and due to transient Ca2+ release events from the SR through ryanodine receptors (Jaggar et al., 2000). A spontaneous Ca2+ spark in the superficial area activates BKCa channels nearby and induces membrane hyperpolarization, which reduces Ca2+ channel activity. BKCa channels and ryanodine receptors may co-localize densely at the junctional areas of plasmalemma and SR fragments, where Ca2+ sparks occur to elicit spontaneous transient outward currents (STOCs). In single smooth muscle cells of guinea-pig vas deferens, Ca2+ entry through voltage-dependent Ca2+ channels in the early stages of an action potential may evoke calcium induced calcium release from discrete subplasmalemmal Ca2+ storage sites and generate local Ca2+ transients that spread over the cell to initiate a contraction (Imaizumi et al., 1998). In addition, the subplasmalemmal Ca2+ transients activate BK channels nearby, which results in the activation of Ca2+-dependent K+ current, a major outward current responsible for action potential repolarization and afterhyperpolarization. These two local Ca2+ release events, Ca2+ sparks at rest and Ca2+ transients upon depolarization, share physiological roles to activate BK channels and induce membrane hyperpolarization.
Ohi et al. (2001) studied local Ca2+ transient and distribution of BKCa channels and ryanodine receptors in the guinea pig vas deferens myocytes. They found that a limited number of discrete SR fragments in the subplasmalemmal area play key roles in the control of BKCa channel activity by generating Ca2+ sparks at rest to activate STOCs. These fragments also generate Ca2+ transients presumably triggered by sparks during an action potential to activate a large Ca2+-dependent K+ current and also induce a contraction. White and McGeown (2003) found in guinea pig vas myocytes that IP3 receptors Ca2+ regulate store content and modulate Ca2+ sparks, and that blockade of these receptors increases SR Ca2+ store content promoting Ca2+ sparks and STOC activity.
Medina et al. (2010) investigated the effects of K+ channel inhibitors on ring segments of the epididymal part of the human vas deferens. They found that charybdotoxin and tetraethylammonium (inhibiting non-selectively BKCa and IKCa channels), but not iberiotoxin (inhibiting selectively BKCa channels), apamin (inhibiting SKCa channels) and glibenclamide (inhibiting ATP sensitive K+channels) increased contraction induced by NA and electrical field stimulation. Theys suggested that the effects of charybdotoxin were mediated via L-type Ca2+ channels and an increase in Ca2+ influx.
Models of Altered Contractility
While an array of studies have attempted to understand the physiologic nature of the vas with chemical and electrical stimulation, several models have been created to understand how different clinically applicable scenarios influence the vas. In an attempt to understand the influence of acute ischemia, models of torsion using rats have demonstrated a decrease in contractile response in the ipsilateral vas deferens (Karacay et al., 2011) Interestingly, spontaneous hypertensive rats have shown an INCREASE in contractile response to EFS and NA (Katsuragi et al., 1991), and Gur et al. (2010) showed increased contractile respone to purinergic stimulation in L-NG-Nitroarginine Methyl Ester (L-NAME) induced hypertensive rat vas deferens. In conjuction with this study, Gur et al. (2010) also demonstrated that rats co-treated with sildenafil and L-NAME reversed this EFS and α−β-methylATP hypercontractile property of the vas deferens. Additionally, a varicocele model has resulted in a decrease vas contractile response (Ozen et al., 2007). Taken together, alterations in vas are clinically applicable, and may have implications for fertility, ejaculation abnormalities, and possibly vasectomy associated pain (Granitsiotis and Kirk, 2004; Tandon and Sabanegh, 2008). Posed mechanisms for this supersensitivity are thought to be denervation related, and range from increased receptor density (Hata et al., 1981), partial resting membrane potential depolarization (Fleming et al., 1973; Fleming, 1975; Fleming and Westfall, 1975; Hershman et al., 1992, 1993, 1995) and changes in intracellular secondary messenger transduction and calcium sensitivity (Minneman et al., 1988; Abraham et al., 2003; Quintas et al., 2005; Amobi et al., 2006).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdel-Hamid, I. A., Jannini, E. A., and Andersson, K. E. (2009). Premature ejaculation: focus on therapeutic targets. Expert. Opin. Ther. Targets 13, 175–193. doi: 10.1517/14728220802663549
Aboud, R., Shafii, M., and Docherty, J. R. (1993). Investigation of the subtypes of alpha 1-adrenoceptor mediating contractions of rat aorta, vas deferens and spleen. Br. J. Pharmacol. 109, 80–87. doi: 10.1111/j.1476-5381.1993.tb13534.x
Abraham, S. T., Robinson, M., and Rice, P. J. (2003). A role for protein kinase C in thesupersensitivity of the rat vas deferens following chronic surgical denervation. Pharmacology 67, 32–40. doi: 10.1159/000066784
Alm, P. (1982). On the autonomic innervation of the human vas deferens. Brain Res. Bull. 9, 673–677. doi: 10.1016/0361-9230(82)90172-1
Ambache, N., and Zar, M. A. (1971). Some physiological and pharmacological characteristics of the motor transmission in the guinea-pig vas deferens. J. Physiol. 212, 15P–16P.
Amobi, N. I., Chung, I. P., and Smith, I. C. (2006). Attenuation of contractility in rat epididymal vas deferens by Rho kinase inhibitors. Auton. Autocoid Pharmacol. 26, 169–181. doi: 10.1111/j.1474-8673.2006.00367.x
Amobi, N. I., Guillebaud, J., Coker, C., Mulvin, D., and Smith, I. C. (1999a). Functional characterization of alpha1-adrenoceptor subtypes in longitudinal and circular muscle of human vas deferens. Eur. J. Pharmacol. 367, 291–298. doi: 10.1016/S0014-2999(98)00989-3
Amobi, N. I., Sugden, D., and Smith, I. C. (1999b). Pharmacomechanical coupling in rat vas deferens, effects of agents that modulate intracellular release of calcium and protein kinase C activation. Life Sci. 65, 145–156. doi: 10.1016/S0024-3205(99)00231-3
Amobi, N. I., Guillebaud, J., and Smith, I. C. (2012). Perspective on the role of P2X-purinoceptor activation in human vas deferens contractility. Exp. Physiol. 97, 583–602. doi: 10.1113/expphysiol.2011.063206
Amobi, N. I., and Smith, I. C. (1999). Different actions in the rat prostatic and epididymal vas deferens of cyclopiazonic acid or ryanodine on noradrenaline-induced contractions. Gen. Pharmacol. 32, 271–278. doi: 10.1016/S0306-3623(98)00209-2
Andersson, K. E., Fovaeus, M., Hedlund, H., Holmquist, F., and Lundin, S. (1988). Immunoreactive arginine vasopressin (AVP) and effects of AVP in the human vas deferens. J. Urol. 140, 1054–1057.
Anton, P. G., and McGrath, J. C. (1977). Further evidence for adrenergic transmission in the human vas deferens. J. Physiol. 273, 45–55.
Banks, F. C., Knight, G. E., Calvert, R. C., Thompson, C. S., Morgan, R. J., and Burnstock, G. (2006). The purinergic component of human vas deferens contraction. Fertil. Steril. 85, 932–939. doi: 10.1016/j.fertnstert.2005.09.024
Barden, J. A., Cottee, L. J., and Bennett, M. R. (1999). Vesicle-associated proteins and P2X receptor clusters at single sympathetic varicosities in mouse vas deferens. J. Neurocytol. 28, 469–480. doi: 10.1023/A:1007053004771
Bennett, M. R. (1973). Structure and electrical properties of the autonomic neuromuscular junction. Philo. Trans. Roy. Soc. Lond. B Biol. Sci. 265, 25–34. doi: 10.1098/rstb.1973.0006
Berridge, M. J. (1993). Inositol trisphosphate and Calcium signalling. Nature 361, 315–325. doi: 10.1038/361315a0
Berridge, M. J. (2008). Smooth muscle cell calcium activation mechanisms. J. Physiol. 586, 5047–5061. doi: 10.1113/jphysiol.2008.160440
Berridge, M. J., and Irvine, R. F. (1984). Inositol triphosphate, a novel second messenger in cellular signal transduction. Nature 312, 315–321. doi: 10.1038/312315a0
Bilge, S., Kesim, Y., Kurt, M., Aksoz, E., and Celik, S. (2005). Possible role of sildenafil in inhibiting rat vas deferens contractions by influencing the purinergic system. Int. J. Urol. 12, 829–834. doi: 10.1111/j.1442-2042.2005.01127.x
Birowo, P., Uckert, S., Kedia, G. T., Sonnenberg, J. E., Sandner, P., Thon, W. F., et al. (2010). Exposure of human seminal vesicle tissue to phosphodiesterase (PDE) inhibitors antagonizes the contraction induced by norepinephrine and increases production of cyclic nucleotides. Urology 76, 1518.e1–6. doi: 10.1016/j.urology.2010.07.461
Bitran, M., Torres, G., Fournier, A., St Pierre, S., and Huidobro-Toro, J. P. (1991). Age and castration modulate the inhibitory action of neuropeptide Y on neurotransmission in the rat vas deferens. Eur. J. Pharmacol. 203, 267–274. doi: 10.1016/0014-2999(91)90723-4
Brain, K. L., Cuprian, A. M., Williams, D. J., and Cunnane, T. C. (2003). The sources and sequestration of Ca2+ transients in the mouse vas deferens. J. Physiol. 533, 627–635. doi: 10.1113/jphysiol.2003.049734
Brock, J. A., and Cunnane, T. C. (1988). Electrical activity at the sympathetic neuroeffector junction in the guinea-pig vas deferens. J. Physiol. 399, 607–632.
Burnstock, G., and Holman, M. E. (1961). The transmission of excitation from autonomic nerve to smooth muscle. J. Physiol. 155, 115–133.
Burnstock, G., and Holman, M. E. (1966). Junction potentials at adrenergic synapses. Pharmacol. Rev. 18, 481–493.
Burnstock, G., and Lavin, S. (2002). Interstitial cells of Cajal and purinergic signaling. Auton. Neurosci. 97, 68–72. doi: 10.1016/S1566-0702(02)00005-X
Burnstock, G., and Verkhratsky, A. (2010). Vas deferens–a model used to establish sympathetic cotransmission. Trends Pharmacol. Sci. 31, 131–139. doi: 10.1016/j.tips.2009.12.002
Burt, R. P., Chapple, C. R., and Marshall, I. (1998). Alpha 1A-adrenoceptor mediated contraction of rat prostatic vas deferens and the involvement of ryanodine stores and Ca2+ influx stimulated by diacylglycerol and PKC. Br. J. Pharmacol. 123, 317–325. doi: 10.1038/sj.bjp.0701588
Büyükafar, K., Levent, A., and Ark, M. (2003). Expression of Rho-kinase and its functional role in the contractile activity of the mouse vas deferens. Br. J. Pharmacol. 140, 743–749. doi: 10.1038/sj.bjp.0705479
Campos, M., de Lucena Morais, P., and Pupo, A. S. (2003). Functional characterisation of alpha(1)-adrenoceptors in denervated rat vas deferens. Naunyn Schmiedebergs Arch. Pharmacol. 368, 72–78. doi: 10.1007/s00210-003-0770-z
Christ, G. J. (1995). Modulation of alpha 1-adrenergic contractility in isolated vascular tissues by heptanol: a functional demonstration of the potential importance of intercellular communication to vascular response generation. Life Sci. 56, 709–721. doi: 10.1016/0024-3205(95)00001-M
Cleary, L., Slattery, J., Bexis, S., and Docherty, J. R. (2004). Sympathectomy reveals alpha 1A- and alpha 1D-adrenoceptor components to contractions to noradrenaline in rat vas deferens. Br. J. Pharmacol. 143, 745–752. doi: 10.1038/sj.bjp.0705987
Cleary, L., Vandeputte, C., and Docherty, J. R. (2003). Investigation of postjunctional α1- and α2 adrenoceptor subtypes in vas deferens from wild-type and α2A/D-adrenoceptor knockout mice. Br. J. Pharmacol. 138, 1069–1076. doi: 10.1038/sj.bjp.0705137
Cunnane, T. C., and Manchanda, R. (1990). On the factors which determine the time-courses of junction potentials in the guinea-pig vas deferens. Neuroscience 37, 507–516. doi: 10.1016/0306-4522(90)90418-4
Cunnane, T. C., and Stjarne, L. (1984). Transmitter secretion from individual varicosities of guinea-pig and mouse vas deferens: highly intermittent and monoquantal. Neuroscience 13, 1–20. doi: 10.1016/0306-4522(84)90255-0
Dixon, J. S., Jen, P. Y. P., and Gosling, J. A. (1998). Structure and autonomic innervation of the human vas defererens: a review. Micros. Res. Tech. 42, 423–432.
Driessen, B., Bultmann, R., Goncalves, J., and Starke, K. (1996). Opposite modulation of noradrenaline and ATP release in guinea-pig vas deferens through prejunctional beta-adrenoceptors: evidence for the beta 2 subtype. Naunyn Schmiedebergs Arch. Pharmacol. 353, 564–571. doi: 10.1007/BF00169177
Driessen, B., von Kugelgen, I., and Starke, K. (1993). Neural ATP release and its alpha 2-adrenoceptor-mediated modulation in guinea-pig vas deferens. Naunyn Schmiedebergs Arch. Pharmacol. 348, 358–366. doi: 10.1007/BF00171334
Elbadawi, A., and Goodman, D. C. (1980). “Autonomic innervation of the accessory male genital glands,” in Male Accessory Sex Glands, eds E. Spring-Mills and E. S. E. Hafez (New York, NY: Elsevier/North Holland), 101–128.
Ellis, J. L., and Burnstock, G. (1989). Angiotensin neuromodulation of adrenergic and purinergic co-transmission in the guinea-pig vas deferens. Br. J. Pharmacol. 97, 1157–1164. doi: 10.1111/j.1476-5381.1989.tb12574.x
Eltze, M. (1994). Pathways involved in muscarinic M1 and M2 receptor stimulation in rabbit vas deferens. Eur. J. Pharmacol. 263, 31–7. doi: 10.1016/0014-2999(94)90520-7
Fleming, W. W. (1975). Supersensitivity in smooth muscle. Introduction and historical perspective. Fed. Proc. 34, 1968–1969.
Fleming, W. W., McPhillips, J. J., and Westfall, D. P. (1973). Postjunctional supersensitivity and subsensitivity of excitable tissues to drugs. Ergeb. Physiol. Biol. Chem. Exp. Pharmakol. 68, 55–119.
Fleming, W. W., and Westfall, D. P. (1975). Altered resting membrane potential in the supersensitive vas deferens of the guinea pig. J. Pharmacol. Exp. Ther. 192, 381–389.
Forsyth, K. M., Bjur, R. A., and Westfall, D. P. (1991). Nucleotide modulation of norepinephrine release from sympathetic nerves in the rat vas deferens. J. Pharmacol. Exp. Ther. 256, 821–826.
French, A. M., and Scott, N. C. (1983). Evidence to support the hypothesis that ATP is a co-transmitter in rat vas deferens. Experientia 39, 264–266. doi: 10.1007/BF01955295
Fukata, Y., Amano, M., and Kaibuchi, K. (2001). Rho – Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 22, 32–39. doi: 10.1016/S0165-6147(00)01596-0
Furukawa, K., Rosario, D. J., Smith, D. J., Chapple, C. R., Uchiyama, T., and Chess-Williams, R. (1995). Alpha 1A-adrenoceptor-mediated contractile responses of the human vas deferens. Br. J. Pharmacol. 116, 1605–1610. doi: 10.1111/j.1476-5381.1995.tb16380.x
Granitsiotis, P., and Kirk, D. (2004). Chronic testicular pain: an overview. Eur. Urol. 45, 430–436. doi: 10.1016/j.eururo.2003.11.004
Gur, S., Sikka, S., Knight, G., Burnstock, G., and Hellstrom, W. (2010). Purinergic contraction of the rat vas deferens in L-NAME-induced hypertension: effects of sildenafil. Asian J. Androl. 12, 415–421. doi: 10.1038/aja.2009.70
Harhun, M. I., Jurkiewics, A., Jurkiewics, N. H., Kryshtal, D. O., Shuba, M. F., and Vladimirova, I. A. (2003). Voltage-gated potassium currents in rat vas deferens smooth muscle. Flugers Arch. Eur. J. Physiol. 446, 380–386.
Harji, F., Gonzales, J., Glaindo, R., and Dail, W. G. (1998). Preganglionic fibers in the rat hypogastric nerve project bilaterally to pelvic ganglia. Anat. Rec. 252, 229–234.
Hata, F., Takeyasu, K., Morikawa, Y., Lai, R. T., Ishida, H., and Yoshida, H. (1981). Role of alpha-adrenergic receptors in denervation supersensitivity of rat vasdeferens. Jpn. J. Pharmacol. 31, 383–390. doi: 10.1254/jjp.31.383
Hedlund, H., Andersson, K. E., and Larsson, B. (1985). Effects of drugs interacting with adrenoreceptors and Muscarinic receptors in the epididymal and prostatic parts of the human isolated vas deferens. J. Auton. Pharmacol. 5, 261–270. doi: 10.1111/j.1474-8673.1985.tb00127.x
Hedqvist, P., and Fredholm, B. B. (1976). Effects of adenosine on adrenergicneurotransmission, prejunctional inhibition and postjunctional enhancement. Naunyn Schmeidebergs Arch. Pharmacol. 293, 217–223. doi: 10.1007/BF00507344
Hershman, K. M., Taylor, D. A., and Fleming, W. W. (1992). Identification of Na+/K+ pump alpha subunits in the guinea-pig vas deferens. FASEB J. 5, 1190.
Hershman, K. M., Taylor, D. A., and Fleming, W. W. (1993). Adaptive supersensitivity in the guinea pig vas deferens is associated with a reduction in the abundance of the alpha 2 subunit isoform of Na+/K+-ATPase. Mol. Pharmacol. 43, 833–837.
Hershman, K. M., Taylor, D. A., and Fleming, W. W. (1995). Adaptive supersensitivity and the Na+/K+ pump in the guinea pig vas deferens: time course of the decline in the alpha 2 subunit. Mol. Pharmacol. 47, 726–729.
Holmquist, F., Hedlund, H., and Andersson, K. E. (1990). Effects of the alpha 1-adrenoceptor antagonist R-(-)-YM12617 on isolated human penile erectile tissue and vas deferens. Eur. J. Pharmacol. 186, 87–93. doi: 10.1016/0014-2999(90)94063-4
Honner, V., and Docherty, J. R. (1999). Investigation of the subtypes of alpha1-adrenoceptor mediating contractions of rat vas deferens. Br. J. Pharmacol. 128, 1323–1331. doi: 10.1038/sj.bjp.0702913
Hu, J. (2007). Wide distribution and subcellular localization of histamine in sympathetic nervous systems of different species. Neurosci. Res. 59, 231–236. doi: 10.1016/j.neures.2007.06.1481
Imaizumi, Y., Torii, Y., Ohi, Y., Nagano, N., Atsuki, K., Yamamura, H., et al. (1998). Ca2+ images and K+ current during depolarization in smooth muscle cells of the guinea-pig vas deferens and urinary bladder. J. Physiol. 510(Pt 3), 705–719.
Jaggar, J. H., Porter, V. A., Lederer, W. J., and Nelson, M. T. (2000). Calcium sparks in smooth muscle. Am. J. Physiol. Cell Physiol. 278, C235–C256.
Jen, P. Y., Dixon, J. S., and Gosling, J. A. (1997). Co-localization of nitric oxide synthase, neuropeptides and tyrosine hydroxylase in nerves supplying the human post-natal vas deferens and seminal vesicle. Br. J. Urol. 80, 291–299. doi: 10.1046/j.1464-410X.1997.00219.x
Kaleczyc, J. (1998). Origin and neurochemical characteristics of nerve fibres supplying the mammalian vas deferens. Microsc. Res. Tech. 42, 409–422.
Kaleczyc, J., Timmermans, J. P., Majewski, M., Lakomy, M., and Scheuermann, D. W. (1997). Immunohistochemical characteristics of nerve fibres supplying the porcine vas deferens. A colocalisation study. Histochem. Cell Biol. 107, 229–241. doi: 10.1007/s004180050108
Karacay, S., Sözübir, S., Bilge, S. S., Aksoz, E., Ekingen, G., and Guvenç, B. H. (2011). Subsequent alterations in the contractile property of the vas deferens according to duration of spermatic cord torsion. Fertil. Steril. 96, 1234–1238. doi: 10.1016/j.fertnstert.2011.08.008
Katsuragi, T., Kuratomi, L., Sato, C., and Furukawa, T. (1991). Hyperreactivity of α1-adrenoceptors, but not of P2X-purinoceptors, in vas deferens of spontaneously hypertensive rats. Eur. J. Pharmacol. 199, 303–307. doi: 10.1016/0014-2999(91)90493-A
Kawabe, K., Yoshida, M., Homma, Y., and Silodosin Clinical Study Group. (2006). Silodosin, a new alpha1A-adrenoceptor- selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 98, 1019–1024. doi: 10.1111/j.1464-410X.2006.06448.x
Khoyi, M. A., Dalziel, H. H., Zhang, L., Bjur, R. A., Gerthoffer, W. T., Buxton, I. L. O., et al. (1993). [Ca21]i-sensitive, IP3-independent Ca21 influx in smooth muscle of rat vas deferens revealed by procaine. Br. J. Pharmacol. 110, 1353–1358. doi: 10.1111/j.1476-5381.1993.tb13968.x
Khoyi, M. A., Westfall, D. P., Buxton, I. L. O., Akhtar-Khavari, F., Rezaei, E., Salaices, M., et al. (1988). Norepinephrine and potassium induced calcium translocation in rat vas deferens. J. Pharmacol. Exp. Ther. 246, 917–923.
Kihara, K., Kakizaki, H., and deGroat, W. C. (1996). Reorganization of the innervation of the vas deferens after sympathetic decentralization. J. Auton. Nerv. Syst. 40, 1–12.
Kihara, K., Sato, K., and Oshima, H. (1998). Sympathetic efferent pathways projecting to the vas deferens. Microsc. Res. Tech. 42, 398–408.
Knight, D., D'Arbe, M., Liang, S., Phillips, W. D., and Lavidis, N. A. (2003). Regional differences in sympathetic purinergic transmission along the length of the mouse vas deferens. Synapse 47, 225–235. doi: 10.1002/syn.10119
Kobayashi, K., Masumori, N., Hisasue, S., Kato, R., Hashimoto, K., Itoh, N., et al. (2008). Inhibition of Seminal emission is the main cause of anejaculation induced by a new highly selective alpha1A-blocker in normal volunteers. J. Sex. Med. 5, 2185–2190. doi: 10.1111/j.1743-6109.2008.00779.x
Kolbeck, S. C., and Steers, W. D. (1993). Origin of neurons supplying the vas deferens of the rat. J. Urol. 149, 918–921.
Kose, M. G., Erdem, S. R., Peskircioglu, C. L., and Caylak, B. (2012). Effects of angiogenesis inhibition by spironolactone on isolated vas deferens contractility in an experimental varicocele model in rats. Urology 80, 816–821. doi: 10.1016/j.urology.2012.07.017
Lavidis, N. A., and Bennett, M. R. (1992). Probabilistic secretion of quanta from visualized sympathetic nerve varicosities in mouse vas deferens. J. Physiol. 454, 9–26.
Lee, H. Y., Bardini, M., and Burnstock, G. (2000). P2X receptor immunoreactivity in the male genital organs of the rat. Cell Tissue Res. 300, 321–330. doi: 10.1007/s004410000207
Li, M. (2007). Histamine in Macaca mulatto monkey cardiac sympathetic nerve system: a morphological and functional assessment Auton. Neuroscience 137, 37–43. doi: 10.1016/j.autneu.2007.06.285
Li, M., Hu, J., Chen, Z., Meng, J., Wang, H., Ma, X., et al. (2006). Evidence for histamine as a neurotransmitter in the cardiac sympathetic nervous system. Am. J. Physiol. Heart Circ. Physiol. 291, H45–H51. doi: 10.1152/ajpheart.00939.2005
Li, M., Luo, X., Chen, L., Zhang, J., Hu, J., and Lu, B. (2003). Co-localization of histamine and dopamine-β-hydroxylase in sympathetic ganglion and release of histamine from cardiac sympathetic terminals of guinea-pig. Auton. Autacoid. Pharmacol. I23, 327–333. doi: 10.1111/j.1474-8673.2004.00305.x
Li, Y., Fu, S., Zhang, H., Gao, L., and Li, J. (2012). H2S relaxes vas deferens smooth muscle by modulating the large conductance Ca2+ - activated K+ (BKCa). channels via a redox mechanism. J. Sex Med. 9, 2806–2813. doi: 10.1111/j.1743-6109.2012.02879.x
Liang, S. X., D'arbe, M., Phillips, W. D., and Lavidis, N. A. (2000). Development of fast purinergic transmission in the mouse vas deferens. Synapse 37, 283–291.
Liang, S. X., Motin, L., Moussa, C. E., Lavidis, N. A., and Phillips, W. D. (2001). Spatial distribution and developmental appearance of postjunctional P2X1 receptors on smooth muscle cells of the mouse vas deferens. Synapse 42, 1–11. doi: 10.1002/syn.1094
Lincoln, J., and Burnstock, G. (1993). “Autonomic innervation of the urinary bladder and urethra,” in The Autonomic Nervous System, Chapter 2, Nervous Control of the Urogenital System, Vol. 6, ed C. A. Maggi (London: Harwood Academic Publisher), 33–68.
Maletìnská, L., Slaninova, J., Kunes, J., and Zelezna, B. (1998). Direct evidence for an angiotensin AT1 receptor type in rat vas deferens. Eur. J. Pharmacol. 351, 371–375.
Manchanda, R., and Venkateswarlu, K. (1997). Effects of heptanol on electrical activity in the guinea-pig vas deferens. Br. J. Pharmacol. 120, 367–370. doi: 10.1038/sj.bjp.0700900
Manchanda, R., and Venkateswarlu, K. (1999). Quantal evoked depolarizations underlying the excitatory junction potential of the guinea-pig isolated vas deferens. J. Physiol. 15(Pt 2), 527–537.
Medina, P., Segarra, G., Chuan, P., Domenech, C., Vila, J. M., Aldasoro, M., et al. (1998). Vasopressin receptors involved in adrenergic neurotransmission in the circular muscle of the human vas deferens. Eur. J. Pharmacol. 355, 41–49. doi: 10.1016/S0014-2999(98)00470-1
Medina, P., Segarra, G., Mauricio, M. D., Vila, J. M., Chuan, P., and Lluch, S. (2010). Modulation of adrenergic responses of human vas deferens by K+ channel inhibitors. Urology 76, 1518.e7–12. doi: 10.1016/j.urology.2010.07.475
Merrillees, N. C. R. (1968). The nervous environment of individual smooth muscle cells of the guinea pig vas deferens. J. Cell Biol. 37, 794–817. doi: 10.1083/jcb.37.3.794
Metzger, R., Rolle, U., Fiegel, H. C., Folker, F. E., Muenstedt, K., and Till, H. (2008). C-kit receptor in the human vas deferens: distinction of mast cells, interstitial cells and interepithelial cells. Reproduction 135, 377–384. doi: 10.1530/REP-07-0346
Minneman, K. P., Mumford, G. K., and Abel, P. W. (1988). High efficiency coupling of alpha-1 adrenergic receptors to inositol phospholipid metabolism revealed by denervation of rat vas deferens. J. Pharmacol. Exp. Ther. 244, 226–230.
Moriyama, N., Nasu, K., Takeuchi, T., Akiyama, K., Murata, S., Nishimatsu, H., et al. (1997). Quantification and distribution of alpha 1-adrenoceptor subtype mRNAs in human vas deferens: comparison with those of epididymal and pelvic portions. Br. J. Pharmacol. 122, 1009–1014. doi: 10.1038/sj.bjp.0701485
Mulryan, K., Gitterman, D. P., Lewis, C. J.Vial, C., Leckie, B. J., Cobb, A., et al. (2000). Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptor. Nature 403, 86–89. doi: 10.1038/47495
Mutafova-Yambolieva, V., and Radomirov, R. (1993). Effects of endothelin-1 on postjunctionally-mediated purinergic and adrenergic components of rat vas deferens contractile responses. Neuropeptides 24, 35–42. doi: 10.1016/0143-4179(93)90038-C
Mutafova-Yambolieva, V. N., Venkova, K. M., and Lasova, L. S. (1993). Atrial natriuretic peptide inhibits the purinergic and not the adrenergic component of electrically induced contractile responses in guinea pig vas deferens. J. Pharmacol. Exp. Ther. 265, 920–926.
Nagai, A., Hara, R., Yokoyama, T., Jo, Y., Fujii, T., and Miyaji, Y. (2008). Ejaculatory dysfunction caused by the new alpha1-blocker silodosin: a preliminary study to analyze human ejaculation using color Doppler ultrasonography. Int. J. Urol. 15, 915–918. doi: 10.1111/j.1442-2042.2008.02136.x
Nilius, B., Owsianik, G., Voets, T., and Peters, J. A. (2007). Transient receptor potential cation channels in disease. Physiol. Rev. 87, 165–217. doi: 10.1152/physrev.00021.2006
Ohi, Y., Yamamura, H., Nagano, N., Ohya, S., Muraki, K., Watanabe, M., et al. (2001). Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J. Physiol. 534, 313–326. doi: 10.1111/j.1469-7793.2001.t01-3-00313.x
Ohmura, T., Oshita, M., Kigoshi, S., and Muramatsu, I. (1992). Identification of alpha 1-adrenoceptor subtypes in the rat vas deferens: binding and functional studies. Br. J. Pharmacol. 107, 697–704. doi: 10.1111/j.1476-5381.1992.tb14509.x
Ohya, S., Yamamura, H., Muraki, K., Watanabe, M., and Imaizumi, Y. (2001). Comparative study of the molecular and functional expression of L-type Ca2+ channels and large-conductance, Ca2+-activated K+ channels in rabbit aorta and vas deferens smooth muscle. Pflugers Arch. 441, 611–620. doi: 10.1007/s004240000463
Ozen, I. O., Moralioglu, S., Vural, I. M., Ozturk, G. S., Ozkan, M. H., Demirtola, A., et al. (2007). Effects of varicocele on electrical field stimulation-induced biphasic twitch responses in the ipsilateral and contralateral rat vasa deferentia. Eur. Surg. Res. 39, 269–274. doi: 10.1159/000102592
Park, S. Y., Lee, M. Y., Keum, E. M., Myung, S. C., and Kim, S. C. (2004). Ionic currents in single smooth muscle cells of the human vas deferens. J. Urol. 172, 628–633. doi: 10.1097/01.ju.0000131252.99041.dd
Paton, D. M., Buckland-Nicks, J., and Johns, A. (1976). Postjunctional supersensitivityof the rat vas deferens and gap junctions. Can. J. Physiol. Pharmacol. 54, 412–416. doi: 10.1139/y76-058
Poli, E., Todorov, S., Pozzoli, C., and Bertaccini, G. (1994). Presynaptic histamine H2 receptors modulate the sympathetic nerve transmission in the isolated rat vas deferens; no role for H3-receptors. Agents Actions 42, 95–100. doi: 10.1007/BF01983472
Queiroz, G., Quintas, C., Talaia, C., and Goncalves, J. (2004). Coupling to protein kinases A and C of adenosine A2B receptors involved in the facilitation of noradrenaline release in the prostatic portion of rat vas deferens. Neuropharmacology 47, 216–224. doi: 10.1016/j.neuropharm.2004.03.015
Queiroz, G., Talaia, C., and Goncalves, J. (2003). ATP modulates noradrenaline release by activation of inhibitory P2Y receptors and facilitatory P2X receptors in the rat vas deferens. J. Pharmacol. Exp. Ther. 307, 809–815. doi: 10.1124/jpet.103.054809
Quintas, L. E., Cunha, V. M., Scaramello, C. B., da Silva, C. L., Caricati-Neto, A., Lafayette, S. S., et al. (2005). Adaptive expression pattern of different proteins involved in cellular calcium homeostasis in denervated rat vas deferens Eur. J. Pharmacol. 525, 54–59. doi: 10.1016/j.ejphar.2005.10.006
Ruan, Y., Wang, Z., Du, J. Y., Zuo, W. L., Guo, J. H., Zhang, J., et al. (2008). Regulation of smooth muscle contractility by the epithelium in rat vas deferens: role of ATP-induced release of PGE2. J. Physiol. 4843–4857. doi: 10.1113/jphysiol.2008.154096
Salles, J., and Badia, A. (1991). Mechanisms underlying the differential sensitivity to alpha 1-adrenoceptor activation in the bisected rat vas deferens. Br. J. Pharmacol. 102, 439–445. doi: 10.1111/j.1476-5381.1991.tb12192.x
Sanbe, A., Tanaka, Y., Fujiwara, Y., Tsumura, H., Yamauchi, J., Cotecchia, S., et al. (2007). Alpha1-adrenoceptors are required for normal male sexual function. Br. J. Pharmacol. 152, 332–340. doi: 10.1038/sj.bjp.0707366
Schomig, E., Schonfeld, C. L., Halbrugge, T., Graefe, K. H., and Trendelenburg, U. (1990). The heterogeneity of the neuronal distribution of exogenous noradrenaline in the rat vas deferens. Naunyn Schmiedebergs Arch. Pharmacol. 342, 160–170. doi: 10.1007/BF00166959
Sheykhzade, M., Gupta, S., Sorensen, T., Sorensen, O. A., Koch, H., Boonen, H. C. M., et al. (2011). Characterization of capsaicin induced responses in mice vas deferens: Evidence of CGRP uptake. Eur. J. Pharm. 667, 375–382. doi: 10.1016/j.ejphar.2011.06.031
Shimizu, F., Taguri, M., Harada, Y., Matsuyama, Y., Sase, K., and Fujime, M. (2010). Impact of dry ejaculation caused by highly selective alpha1A-blocker: randomized, double-blind, placebo-controlled crossover pilot study in healthy volunteer men. J. Sex Med. 7, 1277–1283. doi: 10.1111/j.1743-6109.2009.01663.x
Shishido, T., Sakai, S., and Tosaka, T. (2009). T- and L-type calcium channels mediate α1-adrenoceptor-evoked contraction in the guinea-pig vas deferens. Neurourol. Urodynam. 28, 447–454. doi: 10.1002/nau.20654
Sjöstrand, N. O. (1962). Inhibition by ganglionic blocking agents of the motor response of the isolated guinea-pig vas deferens to hypogastric nerve stimulation. Acta Physiol. Scand. 54, 306–315. doi: 10.1111/j.1748-1716.1962.tb02354.x
Sjöstrand, N. O. (1965). The adrenergic innervation of the vas deferens and the accessory male genital glands. An experimental and comparative study of its anatomical and functional organization in some mammals, including the presence of adrenaline and chromaftin cells in these organs. Acta Physiol. Scand. 65(Suppl. 257), 1–82.
Smith, I. C., and Bray, M. (1990). Direct and indirect contractile responses of the human vas deferens and actions of noradrenaline and of calcium antagonists. Exp. Physiol. 75, 33–43.
Sneddon, P. (2000). Electrophysiology of autonomic neuromuscular transmission involving ATP. J. Auton. Nerv. Syst. 81, 218–24. doi: 10.1016/S0165-1838(00)00141-7
Sneddon, P., and Machaly, M. (1992). Regional variation in purinergic and adrenergic responses in isolated vas deferens of rat, rabbit and guinea-pig. J. Auton. Pharmacol. 12, 421–428. doi: 10.1111/j.1474-8673.1992.tb00390.x
Sneddon, P., and Westfall, D. P. (1984). Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J. Physiol. (Lond.). 347, 561–580.
Somlyo, A. P., and Somlyo, A. V. (1994). Signal transduction and regulation in smooth muscle. Nature 372, 231–236. doi: 10.1038/372231a0
Steers, W. D. (1994). Physiology of the vas deferens. World J. Urol. 12, 281–285. doi: 10.1007/BF00191208
Strobel, G. H., Calixto, J. B., and Ballejo, G. (1989). Decreased gamma-aminobutyric acid (GABA) modulatory effect on rat vas deferens neurotransmission after chronic administration of imipramine. Cell. Mol. Neurobiol. 9, 469–473. doi: 10.1007/BF00712794
Summers, R. J., and McMartin, L. R. (1993). Adrenoceptors and their second messenger systems. J. Neurochem. 60, 12–23. doi: 10.1111/j.1471-4159.1993.tb05817.x
Sward, K., Dreja, K., Susnjar, M., Hellstrand, P., Hartshorne, D. J., and Walsh, M. P. (2000). Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum. J. Physiol. 522, 33–49. doi: 10.1111/j.1469-7793.2000.0033m.x
Swedin, G. (1971). Biphasic mechanical response of the isolated vas deferens to nerve stimulation. Acta Physiol. Scand. 81, 574–576. doi: 10.1111/j.1748-1716.1971.tb04936.x
Tandon, S., and Sabanegh, E. Jr. (2008). Chronic pain after vasectomy: a diagnostic andtreatment dilemma Br. J. Urol. Int. 102, 166–169. doi: 10.1111/j.1464-410X.2008.07602.x
Telemaque, S., and d'Orleans-Juste, P. (1991). Presence of phosphoramidon-sensitive endothelin-converting enzyme which converts big-endothelin-1, but not big-endothelin-3, in the rat vas deferens. Naunyn Schmiedeberg's Arch. Pharmacol. 344, 505–507. doi: 10.1007/BF00172593
Terradas, D., Tabernero, A., Badia, A., and Vivas, N. M. (2001). Different alpha1-adrenoceptor-induced inositol phosphate formation in the two portions of rat vas deferens. Naunyn Schmiedebergs Arch. Pharmacol. 363, 11–15. doi: 10.1007/s002100000322
Todorov, L., Windisch, K., Shersen, H., Lajtha, A., Papasova, M., and Vizi, E. S. (1991). Prejunctional nicotinic receptors involved in facilitation of stimulation-evoked noradrenaline release from the vas deferens of the guinea-pig. Br. J. Pharmacol. 102, 186–190. doi: 10.1111/j.1476-5381.1991.tb12151.x
Todorov, L. D., Clerkin, R., Mihaylova-Todorova, S. T., Khoyi, M. A., and Westfall, D. P. (2001). Beta2-adrenoceptor-mediated prejunctional facilitation and postjunctional inhibition of sympathetic neuroeffector transmission in the guinea pig vas deferens. J. Pharmacol. Exp. Ther. 298, 623–633.
Torres, G., Bitran, M., and Huidobro-Toro, J. P. (1992). Co-release of neuropeptide Y (NPY) and noradrenaline from the sympathetic nerve terminals supplying the rat vas deferens; influence of calcium and the stimulation intensity. Neurosci. Lett. 148, 39–42. doi: 10.1016/0304-3940(92)90799-D
Trendelenburg, A. U., Cox, S. L., Schelb, V., Klebroff, W., Khairallah, L., and Starke, K. (2000). Modulation of (3)H-noradrenaline release by presynaptic opioid, cannabinoid and bradykinin receptors and beta-adrenoceptors in mouse tissues. Br. J. Pharmacol. 130, 321–330. doi: 10.1038/sj.bjp.0703305
Venkateswarlu, K., Dange, S. Y., and Manchanda, R. (1999). Effects of heptanol on the neurogenic and myogenic contractions of the guinea-pig vas deferens. Br. J. Pharmacol. 126, 227–234. doi: 10.1038/sj.bjp.0702307
Ventura, S. (1998). Autoinhibition, sympathetic cotransmission and biphasic contractile responses to trains of nerve stimulation in the rodent vas deferens. Clin. Exp. Pharmacol. Physiol. 25, 965–973. doi: 10.1111/j.1440-1681.1998.tb02169.x
Ventura, S., and Pennefather, J. N. (1994). Alpha 2-adrenoceptor binding sites vary along the length of the male reproductive tract: a possible basis for the regional variation in response to field stimulation. Eur. J. Pharmacol. 254, 167–173. doi: 10.1016/0014-2999(94)90384-0
Von Kugelgen, I., Schoffel, E., and Starke, K. (1989). Inhibition by nucleotides acting at presynaptic P2-receptors of sympathetic neuroeffector transmission in the mouse isolated vas deferens. Naunyn Schmiedeberg's Arch. Pharmacol. 340, 522–532.
Von Kugelgen, I., and Starke, K. (1991). Release of noradrenaline and ATP by electrical stimulation and nicotine in guinea-pig vas deferens. Naunyn Schmiedebergs Arch. Pharmacol. 344, 419–429.
Vulchanova, L., Arvidsson, U., Riedl, M.Wang, J., Buell, G., Surprenant, A., et al. (1996). Differential distribution of two ATPgated channels (P2X receptors) determined by immunocytochemistry. Proc. Natl. Acad. Sci. U.S.A. 93, 8063–8067. doi: 10.1073/pnas.93.15.8063
Westfall, D. P., Stitzel, R. E., and Rowe, J. N. (1978). The postjunctional effects and neural release of purine compounds in the guinea-pig vas deferens. Eur. J. Pharmacol. 50, 27–38. doi: 10.1016/0014-2999(78)90250-9
Westfall, D. P., Todorov, L. D., and Mihailova-Todorova, S. T. (2002). ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J. Pharmacol. Exp. Ther. 302, 439–444. doi: 10.1124/jpet.102.035113
Westfall, T. D., and Westfall, D. P. (2001). Pharmacological techniques for the in vitro study of the vas deferens. J. Pharmacol. Toxicol. Methods 45, 109–122. doi: 10.1016/S1056-8719(01)00144-7
White, C., and McGeown, J. G. (2003). Inositol 1,4,5-trisphosphate receptors modulate Ca2+ sparks and Ca2+ store content in vas deferens myocytes. Am. J. Physiol. Cell Physiol. 285, C195–C204. doi: 10.1152/ajpcell.00374.2002
Yamada, S., Okura, T., and Kimura, R. (2001). In vivo demonstration of alpha(1A)-adrenoceptor subtype selectivity of KMD-3213 in rat tissues. J. Pharmacol. Exp. Ther. 296, 160–167.
Yono, M., Latifpour, J., Yamamoto, Y., Imanishi, A., and Yoshida, M. (2008). Region and age dependent differences in α1-adrenergic responsiveness of rat seminal vesicle and vas deferens Eur. J. Pharm. 587, 291–295.
Keywords: vas deferens, smooth muscle, adrenergic receptors, contraction, fertility, purinergic receptors
Citation: Koslov DS and Andersson K-E (2013) Physiological and pharmacological aspects of the vas deferens—an update. Front. Pharmacol. 4:101. doi: 10.3389/fphar.2013.00101
Received: 21 May 2013; Paper pending published: 18 June 2013;
Accepted: 29 July 2013; Published online: 22 August 2013.
Edited by:
Ulf Simonsen, Aarhus University, DenmarkReviewed by:
Yoh Takuwa, Kanazawa University Graduate School of Medical Sciences, JapanRuth A. Elliott, University of Leicester, UK
Copyright © 2013 Koslov and Andersson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karl-Erik Andersson, Institute for Regenerative Medicine, Wake Forest University School of Medicine, 391 Technology Way, Winston-Salem, NC 27101, USA e-mail: karl-erik.andersson@med.lu.se