- 1Cellular Signaling Laboratory, Key Laboratory of Molecular Biophysics of Ministry of Education, College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, China
- 2Institut de Génomique Fonctionnelle, CNRS UMR5203, INSERM U661, Universités de Montpellier I & II, Montpellier, France
The main inhibitory neurotransmitter, GABA, acts on both ligand-gated and G protein-coupled receptors, the GABA A/C and GABA B receptors, respectively. The later play important roles in modulating many synapses, both at the pre- and post-synaptic levels, and are then still considered as interesting targets to treat a number of brain diseases, including addiction. For many years, several subtypes of GABA B receptors were expected, but cloning revealed only two genes that work in concert to generate a single type of GABA B receptor composed of two subunits. Here we will show that the signaling complexity of this unit receptor type can be largely increased through various ways, including receptor stoichiometry, subunit isoforms, cell-surface expression and localization, crosstalk with other receptors, or interacting proteins. These recent data revealed how complexity of a receptor unit can be increased, observation that certainly are not unique to the GABA B receptor.
Introduction
Neurons communicate with others to form network in the brain through the release and detection of neurotransmitters. Many receptors participate in the detection of neurotransmitters including ionotropic receptors which mediate fast responses and metabotropic receptors which induce slow and long-term plasticity regulations. Metabotropic glutamate receptors (mGluRs), which are activated by glutamate, the major excitatory neurotransmitter of the central nervous system (CNS), consist of eight subtypes named from mGluR1 to mGluR8 show different localization and signaling in synapse (Kniazeff et al., 2011). Other receptors such as serotonin receptors and dopamine receptors, which are activated by serotonin or dopamine, also have several variants (Lee et al., 2000). However, as the main inhibitory neurotransmitter in the CNS, gamma aminobutyric acid (GABA) has only one metabotropic receptor subtype, GABA B receptor (Kaupmann et al., 1998; Benke et al., 1999; Marshall et al., 1999). Located both pre-synaptically and post-synaptically, GABA B receptor is thought to play a role in CNS disorders such as epilepsy, spasticity, schizophrenia, anxiety, depression, cognitive deficits, and addiction (Bettler et al., 2004). It is also shown to be involved in cell survival, nerve growth cone guidance, migration and position of neurons (Xiang et al., 2002; McClellan et al., 2008; Zhou et al., 2008). How one GABA B receptor induces multiple downstream functions remains to be discussed. Here, we show how multiple functions can be generated from a single GABA B receptor through: (1) the oligomeric state in dimers or larger complexes; (2) subunit and isoform variants; (3) cell surface expression and localization; (4) crosstalk with other receptors GABA B receptor interacting proteins.
Dimerization and Large Oligomization of GABAB Receptor
As a member of GPCR class C, GABA B receptor consists of two subunits: GABA B1 and GABA B2, and functions as a heterodimer (Kaupmann et al., 1998; Marshall et al., 1999). As shown in Figure 1, each subunit is composed of a large extracellular domain (Venus flytrap, VFT), a seven-transmembrane domain, and an intracellular C-terminal. Although GABA B2 shares 54% similarity with GABA B1, only the VFT domain of GABA B1 can bind ligands (such as GABA and baclofen) and orthosteric antagonists (such as CGP54626, CGP64213; Pin et al., 2004; Geng et al., 2012; Geng et al., 2013). Due to the presence of endoplasmic reticulum (ER) retention sequence (RSRR) in the C-terminal, GABA B1 cannot reach the plasma membrane by itself. GABA B2 masks GABA B1 ER retention sequence via a coiled-coil domain to escort GABA B1 to the cell surface (Galvez et al., 2001). GABA B2 ectodomain does not bind GABA, but interacts with the GABA B1 ectodomain to increase agonist affinity by stabilizing the agonist-bound conformation of GABAB1 (Liu et al., 2004; Rondard et al., 2008; Geng et al., 2012). GABA B2 is also responsible for G protein coupling (Duthey et al., 2002; Havlickova et al., 2002). Following activation of Gi/o protein, Gαi/o subunits inhibit adenylyl cyclase to reduce cAMP levels while Gβγ subunits inhibit Ca2+ channels and activate K+ channels (Bowery et al., 2002; Ulrich and Bettler, 2007; Chalifoux and Carter, 2011).
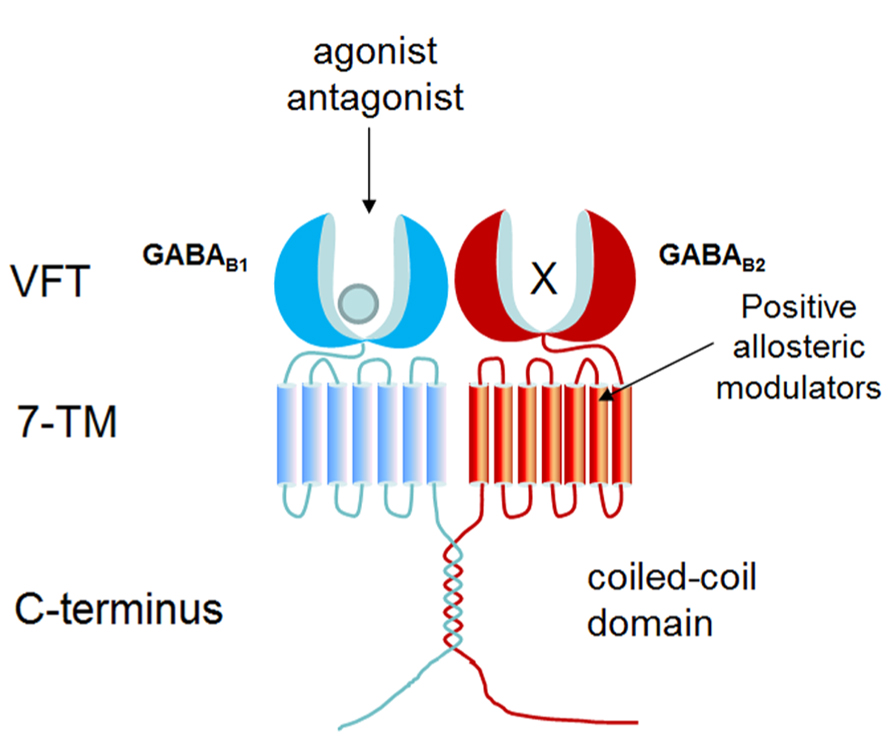
FIGURE 1. Structure organization of GABAB receptor. GABA B receptor forms heterodimer composed by GABA B1 and GABA B2. GABA B1 is responsible for ligand binding in N-terminal VFT domain, whereas the VFT of GABA B2 fails to bind any known ligand. PAMs bind to GABA B2 transmembrane domain to potentiate the effect of agonist.
Up to now, baclofen is the only drug targeting GABA B receptor in the market, which is used as a muscle relaxant to treat spasticity (Froestl, 2010). Positive allosteric modulators (PAMs), such as CGP7930 and GS39783, bind within GABA B2 transmembrane domain to strengthen the effect of agonists (Urwyler et al., 2001). CGP7930 acts as a PAM and partial agonist through GABA B2 which can facilitate agonist response at low concentration and activate the receptor alone at higher concentration (Urwyler et al., 2001; Onali et al., 2003; Binet et al., 2004; Tu et al., 2007).
Dimers, tetramers, or higher order oligomers of GABA B receptor can be detected both in heterologous system (Maurel et al., 2008; Calebiro et al., 2013) and in native neurons (Schwenk et al., 2010; Comps-Agrar et al., 2011). GABA B receptor is present in equilibrium between heterodimers and higher-order oligomers, with a relative preference for tetramers (dimers of dimers) and octamers (tetramers of dimers; Calebiro et al., 2013). Whereas GABA B receptor heterodimers are stable due to strong non-covalent interactions, the higher-order oligomers are the result of weaker and likely transient interactions among heterodimers (Calebiro et al., 2013). Although agonist stimulation did not alter receptor di-/oligomerization (Calebiro et al., 2013), destabilizing the oligomers by a competitor or a GABA B1 mutant revealed different G protein coupling efficiencies depending on the oligomeric state of the receptor (Comps-Agrar et al., 2012), suggesting a negative functional cooperativity between the GABA B receptor heterodimers within the large oligomers.
GABAB Receptor Subunits and Isoforms
The GABA B receptor subunits GABA B1 and GABA B2 are co-expressed throughout the brain (Bettler et al., 2004; Lujan et al., 2004; Bettler and Tiao, 2006). GABA B1 knock-out mice displayed seizures, hyperalgesia, hyperlocomotion, memory impairment, anxiety, and immobility decrease (Schuler et al., 2001; Ruttimann et al., 2004; Catalano et al., 2005) while inactivation of the GABA B2 induced similar phenotype (Mombereau et al., 2005). Both the GABA B1 and GABA B2 subunits are essential for normal function of GABA B receptor. However, baclofen was able to inhibit K+ channels in the CA1 pyramidal neurons of GABA B2-/- mice (Gassmann et al., 2004), suggesting specific properties of GABA B1 in the absence of GABA B2. Mutation of the GABA B1 ER retention sequence RSRR to ASAR allows GABA B1 to reach the cell surface by itself (Couve et al., 1998). GABA B receptor agonist, baclofen, could induce ERK phosphorylation in cerebellar granule cells and HEK cells overexpressed GABA B1 and GABA B2 (Tu et al., 2007). The GABA B1-ASAR mutant could also increase ERK phosphorylation through Gβγ in the absence of GABA B2 (Baloucoune et al., 2012). Although only GABA B2 was reported to be important for Gi/o coupling, Gβγ were found to pre-couple to C-terminal of GABA B for presynaptic inhibition (Laviv et al., 2011). These observations suggested the direct coupling from GABA B1 to G protein signaling. On the other hand, GABA B2 alone co-precipitated and co-expressed with M2 muscarinic receptor (M2R) in cortical neurons. Co-expression of the GABA B2 rescued internalization of M2R and desensitization of GIRK channels induced by chronic stimulation (Boyer et al., 2009). Since GABA B1 and GABA B2 do not always exhibit the same expression pattern (Bettler and Tiao, 2006), the interaction between M2R and the GABA B2 provides a possible mechanism for signaling induced by GABA B2 alone. Overall, though it is well accepted GABA B1 and GABA B2 form a functional receptor together, it is still possible that each subunit plays individual roles, no matter when they act in heterodimer or alone.
Furthermore, 14 isoforms of the GABA B1 can be generated by differential transcription or splicing of the mRNA named from GABA B1a to GABA B1n (Bettler et al., 2004). GABA B1a and GABA B1b are the most abundant isoforms expressed in brain (Benke et al., 1999). GABA B1c has a single sushi-domain and widely expressed in brain and form functional receptors in HEK cells co-expressed with GABA B2 (Pfaff et al., 1999). GABA B1e /g/h/i/ j/ l /m /n do not have transmembrane domains. Been secreted, GABA B1e strongly interacted with GABA B2 and disturbed normal GABA B1/GABA B2 association, but failed to disrupt G-protein coupled inwardly rectifying potassium activation (Schwarz et al., 2000). Purified sushi domains of GABA B1j could impair the inhibitory effect of GABA B heteroreceptors on evoked and spontaneous glutamate release (Tiao et al., 2008). GABA B1g/h/i show similar sequence with GABA B1j containing the sushi domains followed by a unique C-terminal sequence (Jiang et al., 2012), but the function remains to be detected. The inhibitory effect of GABA B heteroreceptors-induced potassium current was also found in GABA B1l and GABA B1m, but not for GABA B1k (Lee et al., 2010). Other isoforms like GABA B1d/f were mostly detected in transcription expression profile and no function was confirmed yet (Jiang et al., 2012). GABAB1a and GABA B1b are well studied compared with others. GABA B1a has two additional sushi domains in the N-terminal region, compared with GABA B1b (Blein et al., 2004). Due to the presence of these sushi domains, GABA B1a preferentially targets to the axon terminals of excitatory synapses. Post-synaptically, both isoforms were found in dendrites, but only GABA B1b could localize in spine heads (Vigot et al., 2006; Biermann et al., 2010). GABA B1b was responsible for mediating postsynaptic inhibition of Ca2+ spikes, whereas presynaptic inhibition of GABA release was mediated by GABA B1a (Perez-Garci et al., 2006). GABA B1a, but not GABA B1b, was involved in impaired synaptic plasticity in hippocampus long-term potentiation (Vigot et al., 2006), emphasizing the molecular differences in synaptic GABA B functions. GABA B1a and GABA B1b also contributed differentially to GABA B receptor-mediated cognitive processes such as spontaneous alternation, object recognition and passive avoidance (Jacobson et al., 2007). Till now, no difference has been shown on molecular pharmacology between GABA B1a and GABA B1b (Billinton et al., 2001). However, CHOP was found to subtype-selective interact with GABA B1a but not GABA B1b and reduced GABA B 1a/GABA B2 receptor cell surface expression (Sauter et al., 2005), suggesting the functional diversity mediated by GABA B1a and GABA B1b through different protein-protein interactions.
GABAB Receptor Cell Surface Expression and Localization
Control of cell surface GABA B receptor expression plays an important role in the regulation of receptor efficacy. GABA B receptor cell surface expression is remarkably stable and baclofen treatment did not elicit conventional β-arrestin recruitment (Couve et al., 2002; Fairfax et al., 2004). However, GABA B receptor undergoes rapid constitutive receptor internalization (Grampp et al., 2007). The balance between sorting and degradation after internalization and rapid recycling process maintains its cell surface expression stability (Grampp et al., 2008). Phosphorylation of serine892 in the C-terminus of GABA B2 is important for cell surface expression stability. Chronic agonist stimulation de-phosphorylates serine892 in GABA B2 and decreases GABA B receptor cell surface expression (Couve et al., 2002; Fairfax et al., 2004). The interaction between endogenous protein Mupp1 and GABA B2 also plays a role to maintain GABA B receptor membrane stability (Balasubramanian et al., 2007).
Lipid rafts are specialized microdomains compartmentalize cellular processes by serving as organizing centers for the assembly of signaling molecules to regulate signal transduction. The GABA B receptor and its downstream effectors, Gα i and Gα o proteins, are all localized in lipid rafts (Becher et al., 2001, 2004). Interestingly, GABA B receptors exhibited a lower GTPγS response to agonist binding in raft-enriched fractions than in whole membranes (Becher et al., 2004), suggesting that changes in membrane environment may regulate its function. Activation of 5-HT 1a receptor could target it to lipid rafts and facilitated receptor-mediated signal transduction (Renner et al., 2007), whereas mu-opioid receptor agonists promoted receptor exiting from lipid rafts (Zheng et al., 2008). Examination of the dynamic lateral diffusion of GABA B receptors at the cell surface revealed restricted mobility of GABA B2. After activation by baclofen, levels of the mobile fraction were significantly increased (Pooler and McIlhinney, 2007). Furthermore, by using single-molecule analysis of fluorescently labeled GPCR revealed that larger oligomers of GABA B receptor were prevalently organized into ordered arrays (Calebiro et al., 2013). Agonist stimulation increased the mobility of large oligomer of GABA B receptor on the cell surface (Calebiro et al., 2013). These data suggested the possibility of GABA B receptor mobility between lipid raft and non-lipid raft domains. Given that the level of cell surface GABA B receptors is highly stable following activation, lateral diffusion of GABA B receptor might provide another mechanism for controlling its signal strength.
Crosstalk of GABAB Receptor with other Receptors
GABAB and GABAA Receptors
Both receptors are located pre-synaptically and post-synaptically. GABA A receptors are Cl- ion channels which produce fast electrical signals, whereas GABA B receptor induced long-term modulation through G protein-regulated gene transcription and protein synthesis (Luscher et al., 2011). Crosstalk between them was identified in several cell types. Due to variants of GABA A receptor subtype in different neurons, GABA B receptor showed multiple functions. In developing hypothalamic neurons, GABA B receptor activation can depress GABA A receptor-mediated Ca2+ rise by both reducing the synaptic release of GABA presynaptically and decreasing the postsynaptic Ca2+ responsiveness (Obrietan and van den Pol, 1998). In dentate gyrus granule cells, GABA B receptors showed remarkable distribution overlap with GABA A receptor on post-synaptic dendritic and somatic membranes. GABA B receptors enhanced tonic inhibition induced by extrasynaptic GABA A receptor (Tao et al., 2013). This was also observed in ventrobasal thalamus and cerebellar granule cells, but absent in CA1 pyramidal cells or layer 2/3 cortical pyramidal neurons (Connelly et al., 2013; Tao et al., 2013). One explanation is that postsynaptic GABA B receptor is possible to preferentially modulate δ–type subunit containing GABA A receptor which is dominant in dentate gyrus granule cells, compared with α5–type subunit containing GABA A receptor, which is expressed in CA1 pyramidal cells (Caraiscos et al., 2004; Glykys et al., 2008). The γ2 subunit of GABA A was found to interact with GABA B receptors and regulate GABA B receptor internalization (Balasubramanian et al., 2004). On the other hand, activation of GABA B receptor promotes GABA A receptor cell surface expression through increasing secreting brain-derived neurotrophic factor (BDNF) and PLC/DAG/PKC activation (Kuczewski et al., 2011). The crosstalk between GABA B and GABA A receptors shows possibility for drug co-application in disease treatment. In animal model, co-application of both of their agonists: muscimol and baclofen protected hippocampal CA1 neurons in cerebral ischemic injury (Zhang et al., 2007). Tiagabine and vigabatrin which increase GABA level in the brain and affect both GABA B and GABA A receptor activity, are effective in treating alcohol addiction (Tyacke et al., 2010).
GABAB Receptors and mGluR1s
Metabotropic glutamate receptors 1 also belongs to GPCR class C as GABA B receptor. It is coupled to Gq protein to increase IP3 production and Ca2+ flux when activated by glutamate (Mao et al., 2005). Both receptors exhibited a high co-localization in the dendritic spine of Purkinje cells (Kamikubo et al., 2007; Rives et al., 2009) and co-immunoprecipitated from brain lysates (Tabata et al., 2004), but no oligomerization of GABA B receptor and mGluR1a was observed (Rives et al., 2009), suggesting the existence of a GABA B-mGluR1 receptor complex but no direct physical contact. GABA B receptor enhanced the long-term depression of a glutamate-evoked current and increased the magnitude of depression in cerebellar parallel fiber–Purkinje cell synapses (Kamikubo et al., 2007). PLC, Gαi/o and Gβγ subunits was involved in GABA B receptor potentiated mGluR1 signaling (Rives et al., 2009). Baclofen regulated mGluR1-current was concentration dependent: a low concentration of baclofen showed augment effect while higher concentration showed inhibition (Hirono et al., 2001). The crosstalk was also related to mGluR1a receptor expression: more potentiation by GABA B receptor when mGluR1a receptors were less expressed (Rives et al., 2009). It suggests a precise control of two receptors for the balance of neuronal inhibition and activation. The crosstalk between G i/o-coupled and G q-coupled receptors which is independent of direct interaction was also observed in other receptors such as mGluR1a and mGluR2 or GABA B and 5-HT 2c receptor (Rives et al., 2009). The co-compartmentalization of these receptors and other scaffold proteins like G proteins, homer to assemble in platforms ensures the crosstalk specificity (Bockaert et al., 2004).
GABAB and NMDA Receptors
Gamma aminobutyric acid(B) receptor cell surface expression is independent of agonist stimulation but controlled by glutamate. Application of glutamate, mainly through NMDA receptor, decreased GABA B receptor cell surface expression and GABA B receptor activated K+ channel current (Guetg et al., 2010; Maier et al., 2010; Terunuma et al., 2010). Activated by NMDA receptors, CaMKII can directly interact with and phosphorylates serine867 in the C-terminus of GABA B1 to trigger GABA B receptor endocytosis (Guetg et al., 2010). CaMKII might be a key signal molecular to modulate the crosstalk between GABA B receptor signaling and glutamate signaling as CaMKII was shown to interact with the NMDA receptor and regulate NMDA receptor controlled plasticity (Bayer et al., 2001; El Gaamouch et al., 2012). Upon NMDA receptor activation, the phosphorylation of serine783 in GABA B2 was increased by AMP-dependent protein kinase (Terunuma et al., 2010). Furthermore, both presynaptic and postsynaptic GABA B receptor can regulate NMDA-mediated excitatory currents (Morrisett et al., 1991; Sun et al., 2006). Baclofen improved NMDA hypofunction-related social function and spatial memory deficient in knockout mice model (Gandal et al., 2012), suggesting the crosstalk between GABA B and NMDA receptors in two directions.
GABAB and Tyrosine Kinase Receptors
The transactivation of GPCR to RTK is an important signaling pathway which contributes to growth promotion activity (Delcourt et al., 2007). GABA B receptor could trigger secretion of BDNF and subsequent activation of the BDNF-related kinase TrkB receptor signaling pathway to promote the development of GABAergic synapses which is called ligand-dependent transactivation (Fiorentino et al., 2009). GABA B receptor could transactivate insulin-like growth factor-1 (IGF-1) receptor to induce Akt phosphorylation and protect cerebellar granule cells from apoptosis. This was independent of ligand IGF-1 (Tu et al., 2010). The first mechanism leads to the RTK activation in cells surrounding the activated GPCR due to a diffusion of the ligand, whereas the second mechanism is mediated by intracellular event that is limited to the signaling protein complex dynamically regulated upon receptor activation. Gi/o proteins were found to be pre-associated with the GABA B receptor. Upon activation, the Gαi/o and Gβγ subunits were released from GABA B receptor, followed by recruitment of FAK1, IGF-1 receptor, and Akt to GABA B receptor. FAK1 played a key role in coordinating this dynamic process. This dynamic of the GABA B receptor-associated complex is critical for signaling transduction and transactivation-dependent neuronal survival (Lin et al., 2012). Other RTKs such as epidermal growth factor receptor, neurotrophin receptor, platelet derived growth factor receptor, and fibroblast growth factor receptor have been investigated for other GPCRs (Peavy et al., 2001; Shah and Catt, 2004). Whether GABA B receptor transactivates other RTKs remains to be identified.
GABAB Receptor Interacting Proteins
The intracellular GABA B receptor interacting proteins are involved in GABA B receptor functions such as cell surface expression (e.g., CHOP, MUPP1) (Sauter et al., 2005; Balasubramanian et al., 2007), desensitization (e.g., GRK4, NSF) (Perroy et al., 2003; Pontier et al., 2006) and signaling transduction (e.g., G proteins, ATF4, FAK1) (Vernon et al., 2001; Lin et al., 2012). Several new proteins have been identified recently to modulate GABA B receptor heterodimer function. 14-3-3ς interacting with GABA B1 coiled-coil domain can partially bind to GABA B1 coiled-coil domain and disrupt association of GABA B1/GABA B2 heterodimers (Couve et al., 2001). Disruption of 14-3-3ς/GABA B1 interaction provides a strategy to enhance the effect of antinociceptive drugs (Laffray et al., 2012). The potassium channel tetramerization domain-containing (KCTD) protein family members KCTD8, KCTD12, KCTD12b, and KCTD16 are tightly associated with the C-terminus of GABA B2 as auxiliary subunits in tetramers (Bartoi et al., 2010; Schwenk et al., 2010). This co-assembly changes the properties of the GABA B1 and GABA B2 core receptor in a KCTD subtype-specific manner. KCTD16 and KCTD8 led to persistent inhibition of Ca v channels activity, whereas KCTD12 and KCTD12b receptors transiently decreased Ca v channels activity (Schwenk et al., 2010; Seddik et al., 2012). Except regulating agonist potency and kinetics, KCTD12 reduced constitutive receptor internalization to increase the magnitude of receptor signaling (Ivankova et al., 2013). The expression levels of individual KCTD transcripts vary during postnatal brain development. KCTD12 and KCTD16 are widely expressed in most neurons whereas KCTD8 and KCTD12b show a restricted expression pattern (Metz et al., 2011). The distinct spatial and temporal KCTD distribution patterns might underlie functional differences in native GABA B receptor responses.
Conclusion
In all, we summarize how one single GABA B receptor generates multiple functions through the following aspects: (1) the composition of tetramer and large oligomer increased the complexity of the receptor; (2) Variants of subunits and isoforms contribute to functional diversity. Differential compartmentalization of the receptor variants participate in distinct function; (3) Cell surface expression and localization in lipid raft are involved in regulating receptor signaling efficacy; (4) Novel functions are generated through crosstalk with interacting proteins, auxiliary subunits or other membrane receptors as shown in Figure 2. The observation of the complexity generated from a single GPCR such as GABA B receptor will provide new strategy for drug development.
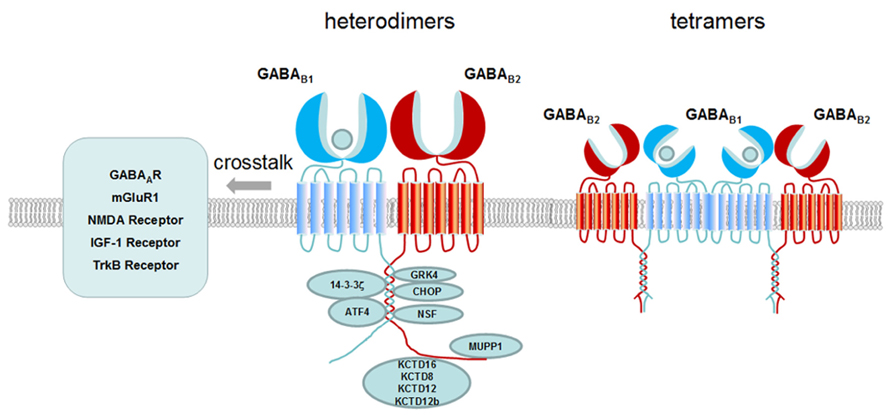
FIGURE 2. Schematic presentation of GABAB receptor interacting proteins and crosstalk of GABAB receptor with other receptors. GABA B receptor interacting proteins are binding to the C-terminus of GABA B receptor modulating the receptor membrane expression (e.g., CHOP, MUPP1), desensitization (e.g., GRK4, NSF), signaling transduction (e.g., G proteins, ATF4) and diverse function (e.g., 14-3-3ς, KCTD8, KCTD12, KCTD12b, and KCTD16). At the membrane, GABA B receptors have crosstalk with other receptors such as GABA A receptor, mGluR1, NMDA receptor, TrkB receptor and IGF-1 receptor. Both heterodimers and tetramers of GABA B receptors exist at the membrane. Dimers and dimers of GABA B receptors form tetramers through GABA B1 and GABA B1 interaction.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) [grant numbers 31130028 and 31225011] and the Ministry of Science and Technology [grant number 2012CB518000], the Program of Introducing Talents of Discipline to the Universities of the Ministry of Education [grant number B08029], and the Mérieux Research Grants Program of Institut-Mérieux (to Jianfeng Liu) and the grants from CNRS, INSERM, ANR (ANR-12-BSV2-0015-01, GABAplEx) and FRM (Equipe FRM DEQ20130326522)(to Jean-Philippe Pin and Philippe Rondard).
References
Balasubramanian, S., Fam, S. R., and Hall, R. A. (2007). GABAB receptor association with the PDZ scaffold Mupp1 alters receptor stability and function. J. Biol. Chem. 282, 4162–4171. doi: 10.1074/jbc.M607695200
Balasubramanian, S., Teissere, J. A., Raju, D. V., and Hall, R. A. (2004). Hetero-oligomerization between GABAA and GABAB receptors regulates GABA B receptor trafficking. J. Biol. Chem. 279, 18840–18850. doi: 10.1074/jbc.M313470200
Baloucoune, G. A., Chun, L., Zhang, W., Xu, C., Huang, S., Sun, Q., et al. (2012). GABAB receptor subunit GB1 at the cell surface independently activates ERK1/2 through IGF-1R transactivation. PLoS ONE 7:e39698. doi: 10.1371/journal.pone.0039698
Bartoi, T., Rigbolt, K. T., Du, D., Kohr, G., Blagoev, B., and Kornau, H. C. (2010). GABAB receptor constituents revealed by tandem affinity purification from transgenic mice. J. Biol. Chem. 285, 20625–20633. doi: 10.1074/jbc.M109.049700
Bayer, K. U., De Koninck, P., Leonard, A. S., Hell, J. W., and Schulman H. (2001). Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411, 801–805. doi: 10.1038/35081080
Becher, A., Green, A., Ige, A. O., Wise, A., White, J. H., and McIlhinney, R. A. (2004). Ectopically expressed gamma-aminobutyric acid receptor B is functionally down-regulated in isolated lipid raft-enriched membranes. Biochem. Biophys. Res. Commun. 321, 981–987. doi: 10.1016/j.bbrc.2004.07.057
Becher, A., White, J. H., and McIlhinney, R. A. (2001). The gamma-aminobutyric acid receptor B, but not the metabotropic glutamate receptor type-1, associates with lipid rafts in the rat cerebellum. J. Neurochem. 79, 787–795. doi: 10.1046/j.1471-4159.2001.00614.x
Benke, D., Honer, M., Michel, C., Bettler, B., and Mohler H. (1999). gamma-aminobutyric acid type B receptor splice variant proteins GBR1a and GBR1b are both associated with GBR2 in situ and display differential regional and subcellular distribution. J. Biol. Chem. 274, 27323–27330. doi: 10.1074/jbc.274.38.27323
Bettler, B., Kaupmann, K., Mosbacher, J., and Gassmann M. (2004). Molecular structure and physiological functions of GABA(B) receptors. Physiol. Rev. 84, 835–867. doi: 10.1152/physrev.00036.2003
Bettler, B., and Tiao, J. Y. (2006). Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol. Ther. 110, 533–543. doi: 10.1016/j.pharmthera.2006.03.006
Biermann, B., Ivankova-Susankova, K., Bradaia, A., Abdel Aziz, S., Besseyrias, V., Kapfhammer, J. P., et al. (2010). The Sushi domains of GABAB receptors function as axonal targeting signals. J. Neurosci. 30, 1385–1394. doi: 10.1523/JNEUROSCI.3172-09.2010
Billinton, A., Ige, A. O., Bolam, J. P., White, J. H., Marshall, F. H., and Emson, P. C. (2001). Advances in the molecular understanding of GABA(B) receptors. Trends Neurosci. 24, 277–282. doi: 10.1016/S0166-2236(00)01815-4
Binet, V., Brajon, C., Le Corre, L., Acher, F., Pin, J. P., and Prezeau L. (2004). The heptahelical domain of GABA(B2) is activated directly by CGP7930, a positive allosteric modulator of the GABA(B) receptor. J. Biol. Chem. 279, 29085–29091. doi: 10.1074/jbc.M400930200
Blein, S., Ginham, R., Uhrin, D., Smith, B. O., Soares, D. C., Veltel, S., et al. (2004). Structural analysis of the complement control protein (CCP) modules of GABA(B) receptor 1a: only one of the two CCP modules is compactly folded. J. Biol. Chem. 279, 48292–48306. doi: 10.1074/jbc.M406540200
Bockaert, J., Fagni, L., Dumuis, A., and Marin P. (2004). GPCR interacting proteins (GIP). Pharmacol. Ther. 103, 203–221. doi: 10.1016/j.pharmthera.2004.06.004
Bowery, N. G., Bettler, B., Froestl, W., Gallagher, J. P., Marshall, F., Raiteri, M., et al. (2002). International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol. Rev. 54, 247–264. doi: 10.1124/pr.54.2.247
Boyer, S. B., Clancy, S. M., Terunuma, M., Revilla-Sanchez, R., Thomas, S. M., Moss, S. J., et al. (2009). Direct interaction of GABAB receptors with M2 muscarinic receptors enhances muscarinic signaling. J. Neurosci. 29, 15796–15809. doi: 10.1523/JNEUROSCI.4103-09.2009
Calebiro, D., Rieken, F., Wagner, J., Sungkaworn, T., Zabel, U., Borzi, A., et al. (2013). Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc. Natl. Acad. Sci. U.S.A. 110, 743–748. doi: 10.1073/pnas.1205798110
Caraiscos, V. B., Elliott, E. M., You-Ten, K. E., Cheng, V. Y., Belelli, D., Newell, J. G., et al. (2004). Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. U.S.A. 101, 3662–3667. doi: 10.1073/pnas.0307231101
Catalano, P. N., Bonaventura, M. M., Silveyra, P., Bettler, B., Libertun, C., Lux-Lantos, V. A. (2005). GABA(B1) knockout mice reveal alterations in prolactin levels, gonadotropic axis, and reproductive function. Neuroendocrinology 82, 294–305. doi: 10.1159/000093128
Chalifoux, J. R., and Carter, A. G. (2011). GABAB receptor modulation of synaptic function. Curr. Opin. Neurobiol. 21, 339–344. doi: 10.1016/j.conb.2011.02.004
Comps-Agrar, L., Kniazeff, J., Brock, C., Trinquet, E., and Pin, J. P. (2012). Stability of GABAB receptor oligomers revealed by dual TR-FRET and drug-induced cell surface targeting. FASEB J. 26, 3430–3439. doi: 10.1096/fj.12-203646
Comps-Agrar, L., Kniazeff, J., Norskov-Lauritsen, L., Maurel, D., Gassmann, M., Gregor, N., et al. (2011). The oligomeric state sets GABA(B) receptor signalling efficacy. EMBO J. 30, 2336–2349. doi: 10.1038/emboj.2011.143
Connelly, W. M., Fyson, S. J., Errington, A. C., McCafferty, C. P., Cope, D. W., Di Giovanni, G., et al. (2013). GABA B Receptors Regulate Extrasynaptic GABAA Receptors. J. Neurosci. 33, 3780–3785. doi: 10.1523/JNEUROSCI.4989-12.2013
Couve, A., Filippov, A. K., Connolly, C. N., Bettler, B., Brown, D. A., and Moss, S. J. (1998). Intracellular retention of recombinant GABA B receptors. J. Biol. Chem. 273, 26361–26367. doi: 10.1074/jbc.273.41.26361
Couve, A., Kittler, J. T., Uren, J. M., Calver, A. R., Pangalos, M. N., Walsh, F. S., et al. (2001). Association of GABA(B) receptors and members of the 14-3-3 family of signaling proteins. Mol. Cell. Neurosci. 17, 317–328. doi: 10.1006/mcne.2000.0938
Couve, A., Thomas, P., Calver, A. R., Hirst, W. D., Pangalos, M. N., Walsh, F. S., et al. (2002). Cyclic AMP-dependent protein kinase phosphorylation facilitates GABA(B) receptor-effector coupling. Nat. Neurosci. 5, 415–424. doi: 10.1038/nn833
Delcourt, N., Bockaert, J., and Marin P. (2007). GPCR-jacking: from a new route in RTK signalling to a new concept in GPCR activation. Trends Pharmacol. Sci. 28, 602–607. doi: 10.1016/j.tips.2007.09.007
Duthey, B., Caudron, S., Perroy, J., Bettler, B., Fagni, L., Pin, J. P., et al. (2002). A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor. J. Biol. Chem. 277, 3236–3241. doi: 10.1074/jbc.M108900200
El Gaamouch, F., Buisson, A., Moustie, O., Lemieux, M., Labrecque, S., Bontempi, B., et al. (2012). Interaction between alphaCaMKII and GluN2B controls ERK-dependent plasticity. J. Neurosci. 32, 10767–10779. doi: 10.1523/JNEUROSCI.5622-11.2012
Fairfax, B. P., Pitcher, J. A., Scott, M. G., Calver, A. R., Pangalos, M. N., Moss, S. J., et al. (2004). Phosphorylation and chronic agonist treatment atypically modulate GABAB receptor cell surface stability. J. Biol. Chem. 279, 12565–12573. doi: 10.1074/jbc.M311389200
Fiorentino, H., Kuczewski, N., Diabira, D., Ferrand, N., Pangalos, M. N., Porcher, C., et al. (2009). GABA(B) receptor activation triggers BDNF release and promotes the maturation of GABAergic synapses. J. Neurosci. 29, 11650–11661. doi: 10.1523/JNEUROSCI.3587-09.2009
Froestl W. (2010). Chemistry and pharmacology of GABAB receptor ligands. Adv. Pharmacol. 58, 19–62. doi: 10.1016/S1054-3589(10)58002-5
Galvez, T., Duthey, B., Kniazeff, J., Blahos, J., Rovelli, G., Bettler, B., et al. (2001). Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J. 20, 2152–2159. doi: 10.1093/emboj/20.9.2152
Gandal, M. J., Sisti, J., Klook, K., Ortinski, P. I., Leitman, V., Liang, Y., et al. (2012). GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl. Psychiatry 2, e142. doi: 10.1038/tp.2012.69
Gassmann, M., Shaban, H., Vigot, R., Sansig, G., Haller, C., Barbieri, S., et al. (2004). Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice. J. Neurosci. 24, 6086–6097. doi: 10.1523/JNEUROSCI.5635-03.2004
Geng, Y., Bush, M., Mosyak, L., Wang, F., and Fan, Q. R. (2013). Structural mechanism of ligand activation in human GABAB receptor. Nature 504, 254–259. doi: 10.1038/nature12725
Geng, Y., Xiong, D., Mosyak, L., Malito, D. L., Kniazeff, J., Chen, Y., et al. (2012). Structure and functional interaction of the extracellular domain of human GABA(B) receptor GBR2. Nat. Neurosci. 15, 970–978. doi: 10.1038/nn.3133
Glykys, J., Mann, E. O., and Mody, I. (2008). Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 28, 1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008
Grampp, T., Notz, V., Broll, I., Fischer, N., and Benke, D. (2008). Constitutive, agonist-accelerated, recycling and lysosomal degradation of GABA(B) receptors in cortical neurons. Mol. Cell. Neurosci. 39, 628–637. doi: 10.1016/j.mcn.2008.09.004
Grampp, T., Sauter, K., Markovic, B., and Benke, D. (2007). Gamma-aminobutyric acid type B receptors are constitutively internalized via the clathrin-dependent pathway and targeted to lysosomes for degradation. J. Biol. Chem. 282, 24157–24165. doi: 10.1074/jbc.M702626200
Guetg, N., Abdel Aziz, S., Holbro, N., Turecek, R., Rose, T., Seddik, R., et al. (2010). NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1. Proc. Natl. Acad. Sci. U.S.A. 107, 13924–13929. doi: 10.1073/pnas.1000909107
Havlickova, M., Prezeau, L., Duthey, B., Bettler, B., Pin, J. P., and Blahos, J. (2002). The intracellular loops of the GB2 subunit are crucial for G-protein coupling of the heteromeric gamma-aminobutyrate B receptor. Mol. Pharmacol. 62, 343–350. doi: 10.1124/mol.62.2.343
Hirono, M., Yoshioka, T., and Konishi S. (2001). GABA(B) receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nat. Neurosci. 4, 1207–1216. doi: 10.1038/nn764
Ivankova, K., Turecek, R., Fritzius, T., Seddik, R., Prezeau, L., Comps-Agrar, L., et al. (2013). Up-regulation of GABA(B) receptor signaling by constitutive assembly with the K+ channel tetramerization domain-containing protein 12 (KCTD12). J. Biol. Chem. 288, 24848–24856. doi: 10.1074/jbc.M113.476770
Jacobson, L. H., Bettler, B., Kaupmann, K., and Cryan, J. F. (2007). Behavioral evaluation of mice deficient in GABA(B(1)) receptor isoforms in tests of unconditioned anxiety. Psychopharmacology (Berl) 190, 541–553. doi: 10.1007/s00213-006-0631-9
Jiang, X., Su, L., Zhang, Q., He, C., Zhang, Z., Yi, P., et al. (2012). GABAB receptor complex as a potential target for tumor therapy. J. Histochem. Cytochem. 60, 269–279. doi: 10.1369/0022155412438105
Kamikubo, Y., Tabata, T., Kakizawa, S., Kawakami, D., Watanabe, M., Ogura, A., et al. (2007). Postsynaptic GABAB receptor signalling enhances LTD in mouse cerebellar Purkinje cells. J. Physiol. 585, 549–563. doi: 10.1113/jphysiol.2007.141010
Kaupmann, K., Malitschek, B., Schuler, V., Heid, J., Froestl, W., Beck, P., et al. (1998). GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687. doi: 10.1038/25360
Kniazeff, J., Prezeau, L., Rondard, P., Pin, J. P., and Goudet, C. (2011). Dimers and beyond: The functional puzzles of class C GPCRs. Pharmacol. Ther. 130, 9–25. doi: 10.1016/j.pharmthera.2011.01.006
Kuczewski, N., Fuchs, C., Ferrand, N., Jovanovic, J. N., Gaiarsa, J. L., and Porcher, C. (2011). Mechanism of GABAB receptor-induced BDNF secretion and promotion of GABAA receptor membrane expression. J. Neurochem. 118, 533–545. doi: 10.1111/j.1471-4159.2011.07192.x
Laffray, S., Bouali-Benazzouz, R., Papon, M. A., Favereaux, A., Jiang, Y., Holm, T., et al. (2012). Impairment of GABAB receptor dimer by endogenous 14-3-3zeta in chronic pain conditions. EMBO J. 31, 3239–3251. doi: 10.1038/emboj.2012.161
Laviv, T., Vertkin, I., Berdichevsky, Y., Fogel, H., Riven, I., Bettler, B., et al. (2011). Compartmentalization of the GABAB receptor signaling complex is required for presynaptic inhibition at hippocampal synapses. J. Neurosci. 31, 12523–12532. doi: 10.1523/JNEUROSCI.1527-11.2011
Lee, C., Mayfield, R. D., and Harris, R. A. (2010). Intron 4 containing novel GABAB1 isoforms impair GABAB receptor function. PLoS ONE 5:e14044. doi: 10.1371/journal.pone.0014044
Lee, S. P., Xie, Z., Varghese, G., Nguyen, T., O’Dowd, B. F., and George, S. R. (2000). Oligomerization of dopamine and serotonin receptors. Neuropsychopharmacology 23, S32–S40. doi: 10.1016/S0893-133X(00)00155-X
Lin, X., Li, X., Jiang, M., Chen, L., Xu, C., Zhang, W., et al. (2012). An activity-based probe reveals dynamic protein-protein interactions mediating IGF-1R transactivation by the GABA(B) receptor. Biochem. J. 443, 627–634. doi: 10.1042/BJ20120188
Liu, J., Maurel, D., Etzol, S., Brabet, I., Ansanay, H., Pin, J. P., et al. (2004). Molecular determinants involved in the allosteric control of agonist affinity in the GABAB receptor by the GABAB2 subunit. J. Biol. Chem. 279, 15824–15830. doi: 10.1074/jbc.M313639200
Lujan, R., Shigemoto, R., Kulik, A., and Juiz, J. M. (2004). Localization of the GABAB receptor 1a/b subunit relative to glutamatergic synapses in the dorsal cochlear nucleus of the rat. J. Comp. Neurol. 475, 36–46. doi: 10.1002/cne.20160
Luscher, B., Fuchs, T., and Kilpatrick, C. L. (2011). GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron 70, 385–409. doi: 10.1016/j.neuron.2011.03.024
Maier, P. J., Marin, I., Grampp, T., Sommer, A., and Benke, D. (2010). Sustained glutamate receptor activation down-regulates GABAB receptors by shifting the balance from recycling to lysosomal degradation. J. Biol. Chem. 285, 35606–35614. doi: 10.1074/jbc.M110.142406
Mao, L., Yang, L., Tang, Q., Samdani, S., Zhang, G., and Wang, J. Q. (2005). The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J. Neurosci. 25, 2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005
Marshall, F. H., Jones, K. A., Kaupmann, K., and Bettler, B. (1999). GABAB receptors – the first 7TM heterodimers. Trends Pharmacol. Sci. 20, 396–399. doi: 10.1016/S0165-6147(99)01383-8
Maurel, D., Comps-Agrar, L., Brock, C., Rives, M. L., Bourrier, E., Ayoub, M. A., et al. (2008). Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat. Methods 5, 561–567. doi: 10.1038/nmeth.1213
McClellan, K. M., Calver, A. R., and Tobet, S. A. (2008). GABAB receptors role in cell migration and positioning within the ventromedial nucleus of the hypothalamus. Neuroscience 151, 1119–1131. doi: 10.1016/j.neuroscience.2007.11.048
Metz, M., Gassmann, M., Fakler, B., Schaeren-Wiemers, N., and Bettler, B. (2011). Distribution of the auxiliary GABAB receptor subunits KCTD8, 12, 12b, and 16 in the mouse brain. J. Comp. Neurol. 519, 1435–1454. doi: 10.1002/cne.22610
Mombereau, C., Kaupmann, K., Gassmann, M., Bettler, B., van der Putten, H., and Cryan JF. (2005). Altered anxiety and depression-related behaviour in mice lacking GABAB(2) receptor subunits. Neuroreport 16, 307–310. doi: 10.1097/00001756-200502280-00021
Morrisett, R. A., Mott, D. D., Lewis, D. V., Swartzwelder, H. S., and Wilson, W. A. (1991). GABAB-receptor-mediated inhibition of the N-methyl-D-aspartate component of synaptic transmission in the rat hippocampus. J. Neurosci. 11, 203–209.
Obrietan, K., and van den Pol, A. N. (1998). GABAB receptor-mediated inhibition of GABA A receptor calcium elevations in developing hypothalamic neurons. J. Neurophysiol. 79, 1360–1370.
Onali, P., Mascia, F. M., and Olianas, M. C. (2003). Positive regulation of GABA(B) receptors dually coupled to cyclic AMP by the allosteric agent CGP7930. Eur. J. Pharmacol. 471, 77–84. doi: 10.1016/S0014-2999(03)01823-5
Peavy, R. D., Chang, M. S., Sanders-Bush, E., and Conn, P. J. (2001). Metabotropic glutamate receptor 5-induced phosphorylation of extracellular signal-regulated kinase in astrocytes depends on transactivation of the epidermal growth factor receptor. J. Neurosci. 21, 9619–9628.
Perez-Garci, E., Gassmann, M., Bettler, B., and Larkum, M. E. (2006). The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron 50, 603–616. doi: 10.1016/j.neuron.2006.04.019
Perroy, J., Adam, L., Qanbar, R., Chenier, S., and Bouvier M. (2003). Phosphorylation-independent desensitization of GABA(B) receptor by GRK4. EMBO J. 22, 3816–3824. doi: 10.1093/emboj/cdg383
Pfaff, T., Malitschek, B., Kaupmann, K., Prezeau, L., Pin, J. P., Bettler, B., et al. (1999). Alternative splicing generates a novel isoform of the rat metabotropic GABA(B)R1 receptor. Eur. J. Neurosci. 11, 2874–2882. doi: 10.1046/j.1460-9568.1999.00704.x
Pin, J. P., Kniazeff, J., Binet, V., Liu, J., Maurel, D., Galvez, T., et al. (2004). Activation mechanism of the heterodimeric GABA(B) receptor. Biochem. Pharmacol. 68, 1565–1572. doi: 10.1016/j.bcp.2004.06.035
Pontier, S. M., Lahaie, N., Ginham, R., St-Gelais, F., Bonin, H., Bell, D. J., et al. (2006). Coordinated action of NSF and PKC regulates GABAB receptor signaling efficacy. EMBO J. 25, 2698–2709. doi: 10.1038/sj.emboj.7601157
Pooler, A. M., and McIlhinney R. A. (2007). Lateral diffusion of the GABAB receptor is regulated by the GABAB2 C terminus. J. Biol. Chem. 282, 25349–25356. doi: 10.1074/jbc.M702358200
Renner, U., Glebov, K., Lang, T., Papusheva, E., Balakrishnan, S., Keller, B., et al. (2007). Localization of the mouse 5-hydroxytryptamine(1A) receptor in lipid microdomains depends on its palmitoylation and is involved in receptor-mediated signaling. Mol. Pharmacol. 72, 502–513. doi: 10.1124/mol.107.037085
Rives, M. L., Vol, C., Fukazawa, Y., Tinel, N., Trinquet, E., Ayoub, M. A., et al. (2009). Crosstalk between GABA(B) and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J. 28, 2195–2208. doi: 10.1038/emboj.2009.177
Rondard, P., Huang, S., Monnier, C., Tu, H., Blanchard, B., Oueslati, N., et al. (2008). Functioning of the dimeric GABA(B) receptor extracellular domain revealed by glycan wedge scanning. EMBO J. 27, 1321–1332. doi: 10.1038/emboj.2008.64
Ruttimann, E., Vacher, C. M., Gassmann, M., Kaupmann, K., Van der Putten, H., and Bettler B. (2004). Altered hippocampal expression of calbindin-D-28k and calretinin in GABA(B(1))-deficient mice. Biochem. Pharmacol. 68, 1613–1620. doi: 10.1016/j.bcp.2004.07.019
Sauter, K., Grampp, T., Fritschy, J. M., Kaupmann, K., Bettler, B., Mohler, H., et al. (2005). Subtype-selective interaction with the transcription factor CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) regulates cell surface expression of GABA(B) receptors. J. Biol. Chem. 280, 33566–33572. doi: 10.1074/jbc.M503482200
Schuler, V., Luscher, C., Blanchet, C., Klix, N., Sansig, G., Klebs, K., et al. (2001). Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 31, 47–58. doi: 10.1016/S0896-6273(01)00345-2
Schwarz, D. A., Barry, G., Eliasof, S. D., Petroski, R. E., Conlon, P. J., and Maki, R. A. (2000). Characterization of gamma-aminobutyric acid receptor GABAB(1e), a GABAB(1) splice variant encoding a truncated receptor. J. Biol. Chem. 275, 32174–32181. doi: 10.1074/jbc.M005333200
Schwenk, J., Metz, M., Zolles, G., Turecek, R., Fritzius, T., Bildl, W., et al. (2010). Native GABA(B) receptors are heteromultimers with a family of auxiliary subunits. Nature 465, 231–235. doi: 10.1038/nature08964
Seddik, R., Jungblut, S. P., Silander, O. K., Rajalu, M., Fritzius, T., Besseyrias, V., et al. (2012). Opposite effects of KCTD subunit domains on GABA(B) receptor-mediated desensitization. J. Biol. Chem. 287, 39869–39877. doi: 10.1074/jbc.M112.412767
Shah, B. H., and Catt, K. J. (2004). GPCR-mediated transactivation of RTKs in the CNS: mechanisms and consequences. Trends Neurosci. 27, 48–53. doi: 10.1016/j.tins.2003.11.003
Sun, H., Ma, C. L., Kelly, J. B., and Wu SH. (2006). GABAB receptor-mediated presynaptic inhibition of glutamatergic transmission in the inferior colliculus. Neurosci. Lett. 399, 151–156. doi: 10.1016/j.neulet.2006.01.049
Tabata, T., Araishi, K., Hashimoto, K., Hashimotodani, Y., van der Putten, H., Bettler, B., et al. (2004). Ca2+ activity at GABAB receptors constitutively promotes metabotropic glutamate signaling in the absence of GABA. Proc. Natl. Acad. Sci. U.S.A. 101, 16952–16957. doi: 10.1073/pnas.0405387101
Tao, W., Higgs, M. H., Spain, W. J., and Ransom, C. B. (2013). Postsynaptic GABAB receptors enhance extrasynaptic GABAA receptor function in dentate gyrus granule cells. J. Neurosci. 33, 3738–3743. doi: 10.1523/JNEUROSCI.4829-12.2013
Terunuma, M., Vargas, K. J., Wilkins, M. E., Ramirez, O. A., Jaureguiberry-Bravo, M., Pangalos, M. N., et al. (2010). Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc. Natl. Acad. Sci. U.S.A. 107, 13918–13923. doi: 10.1073/pnas.1000853107
Tiao, J. Y., Bradaia, A., Biermann, B., Kaupmann, K., Metz, M., Haller, C., et al. (2008). The sushi domains of secreted GABA(B1) isoforms selectively impair GABA(B) heteroreceptor function. J. Biol. Chem. 283, 31005–31011. doi: 10.1074/jbc.M804464200
Tu, H., Rondard, P., Xu, C., Bertaso, F., Cao, F., Zhang, X., et al. (2007). Dominant role of GABAB2 and Gbetagamma for GABAB receptor-mediated-ERK1/2/CREB pathway in cerebellar neurons. Cell. Signal. 19, 1996–2002. doi: 10.1016/j.cellsig.2007.05.004
Tu, H., Xu, C., Zhang, W., Liu, Q., Rondard, P., Pin, J. P., et al. (2010). GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation. J. Neurosci. 30, 749–759. doi: 10.1523/JNEUROSCI.2343-09.2010
Tyacke, R. J., Lingford-Hughes, A., Reed, L. J., and Nutt, D. J. (2010). GABAB receptors in addiction and its treatment. Adv. Pharmacol. 58, 373–396. doi: 10.1016/S1054-3589(10)58014-1
Ulrich, D., and Bettler B. (2007). GABA(B) receptors: synaptic functions and mechanisms of diversity. Curr. Opin. Neurobiol. 17, 298–303. doi: 10.1016/j.conb.2007.04.001
Urwyler, S., Mosbacher, J., Lingenhoehl, K., Heid, J., Hofstetter, K., Froestl, W., et al. (2001). Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol. Pharmacol. 60, 963–971.
Vernon, E., Meyer, G., Pickard, L., Dev, K., Molnar, E., Collingridge, G. L., et al. (2001). GABA(B) receptors couple directly to the transcription factor ATF4. Mol. Cell. Neurosci. 17, 637–645. doi: 10.1006/mcne.2000.0960
Vigot, R., Barbieri, S., Brauner-Osborne, H., Turecek, R., Shigemoto, R., Zhang, Y. P., et al. (2006). Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron 50, 589–601. doi: 10.1016/j.neuron.2006.04.014
Xiang, Y., Li, Y., Zhang, Z., Cui, K., Wang, S., Yuan, X. B., et al. (2002). Nerve growth cone guidance mediated by G protein-coupled receptors. Nat. Neurosci. 5, 843–848. doi: 10.1038/nn899
Zhang, F., Li, C., Wang, R., Han, D., Zhang, Q. G., Zhou, C., et al. (2007). Activation of GABA receptors attenuates neuronal apoptosis through inhibiting the tyrosine phosphorylation of NR2A by Src after cerebral ischemia and reperfusion. Neuroscience 150, 938–949. doi: 10.1016/j.neuroscience.2007.09.070
Zheng, H., Chu, J., Qiu, Y., Loh, H. H., and Law PY. (2008). Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc. Natl. Acad. Sci. U.S.A. 105, 9421–9426. doi: 10.1073/pnas.0802253105
Zhou, C., Li, C., Yu, H. M., Zhang, F., Han, D., and Zhang GY. (2008). Neuroprotection of gamma-aminobutyric acid receptor agonists via enhancing neuronal nitric oxide synthase (Ser847) phosphorylation through increased neuronal nitric oxide synthase and PSD95 interaction and inhibited protein phosphatase activity in cerebral ischemia. J. Neurosci. Res. 86, 2973–2983. doi: 10.1002/jnr.21728
Keywords: GABAB receptor, dimers, large oligomers, G-protein coupled receptor interacting proteins, signal transduction
Citation: Xu C, Zhang W, Rondard P, Pin J-P and Liu J (2014) Complex GABAB receptor complexes: how to generate multiple functionally distinct units from a single receptor. Front. Pharmacol. 5:12. doi: 10.3389/fphar.2014.00012
Received: 14 November 2013; Accepted: 22 January 2014;
Published online: 11 February 2014.
Edited by:
Pietro Marini, University of Aberdeen, UKReviewed by:
Victoria Risbrough, University of California at San Diego, USAChanghoon Lee, University of California at Los Angeles, USA
Copyright © 2014 Xu, Zhang, Rondard, Pin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianfeng Liu, Cellular Signaling Laboratory, Key Laboratory of Molecular Biophysics of Ministry of Education, College of Life Science and Technology, Huazhong University of Science and Technology, Luoyu road 1037, Wuhan, Hubei 430074, China e-mail: jfliu@mail.hust.edu.cn
 Chanjuan Xu
Chanjuan Xu Wenhua Zhang
Wenhua Zhang Philippe Rondard
Philippe Rondard Jean-Philippe Pin
Jean-Philippe Pin Jianfeng Liu
Jianfeng Liu