- 1Department of Molecular Biophysics and Physiology, Rush University Medical Center, Chicago, IL, USA
- 2Department of Bioengineering, University of California San Diego, La Jolla, CA, USA
Calcium plays a crucial role in excitation-contraction coupling (ECC), but it is also a pivotal second messenger activating Ca2+-dependent transcription factors in a process termed excitation-transcription coupling (ETC). Evidence accumulated over the past decade indicates a pivotal role of inositol 1,4,5-trisphosphate receptor (IP3R)-mediated Ca2+ release in the regulation of cytosolic and nuclear Ca2+ signals. IP3 is generated by stimulation of plasma membrane receptors that couple to phospholipase C (PLC), liberating IP3 from phosphatidylinositol 4,5-bisphosphate (PIP2). An intriguing aspect of IP3 signaling is the presence of the entire PIP2-PLC-IP3 signaling cascade as well as the presence of IP3Rs at the inner and outer membranes of the nuclear envelope (NE) which functions as a Ca2+ store. The observation that the nucleus is surrounded by its own putative Ca2+ store raises the possibility that nuclear IP3-dependent Ca2+ release plays a critical role in ETC. This provides a potential mechanism of regulation that acts locally and autonomously from the global cytosolic Ca2+ signal underlying ECC. Moreover, there is evidence that: (i) the sarcoplasmic reticulum (SR) and NE are a single contiguous Ca2+ store; (ii) the nuclear pore complex is the major gateway for Ca2+ and macromolecules to pass between the cytosol and the nucleoplasm; (iii) the inner membrane of the NE hosts key Ca2+ handling proteins including the Na+/Ca2+ exchanger (NCX)/GM1 complex, ryanodine receptors (RyRs), nicotinic acid adenine dinucleotide phosphate receptors (NAADPRs), Na+/K+ ATPase, and Na+/H+ exchanger. Thus, it appears that the nucleus represents a Ca2+ signaling domain equipped with its own ion channels and transporters that allow for complex local Ca2+ signals. Many experimental and modeling approaches have been used for the study of intracellular Ca2+ signaling but the key to the understanding of the dual role of Ca2+ mediating ECC and ECT lays in quantitative differences of local [Ca2+] in the nuclear and cytosolic compartment. In this review, we discuss the state of knowledge regarding the origin and the physiological implications of nuclear Ca2+ transients in different cardiac cell types (adult atrial and ventricular myocytes) as well as experimental and mathematical approaches to study Ca2+ and IP3 signaling in the cytosol and nucleus. In particular, we focus on the concept that highly localized Ca2+ signals are required to translocate and activate Ca2+-dependent transcription factors (e.g., nuclear factor of activated T-cells, NFAT; histone deacetylase, HDAC) through phosphorylation/dephosphorylation processes.
Introduction
Calcium is a pivotal signaling molecule and its intracellular concentration ([Ca2+]i) is precisely regulated in different subcellular domains. The modulation of [Ca2+]is a crucial factor for a variety of physiological functions of living cells. In cardiac myocytes, including ventricular and atrial cells, Ca2+ release through channels located in the sarcoplasmic reticulum (SR) membrane and termed ryanodine receptors (RyRs), is a key event linking membrane depolarization and mechanical activity during excitation-contraction coupling (ECC) (Bers, 2001). The amount of Ca2+ release with each heart beat and by that the force of contraction is also modulated by hormonal action, e.g., by Endothelin I and Angiotensin II (Proven et al., 2006). These two hormones stimulate plasma membrane receptors (G protein coupled receptors, GPCRs) that couple to phospholipase C (PLC), liberating IP3 from phosphatidylinositol 4,5-bisphosphate (PIP2). IP3 freely diffuses within the cytoplasm to bind to a second type of SR Ca2+ release channels, the inositol 1,4,5-trisphosphate receptor (IP3R) (Roderick and Bootman, 2003; Kockskämper et al., 2008; Berridge, 2009). IP3Rs, albeit at a much smaller density compared to ryanodine receptors (RyR:IP3R ~100:1), are expressed in the SR membrane and nuclear envelope (NE) (Bootman et al., 2009). The activation of IP3Rs upon binding of IP3 can modulate ECC by sensitizing nearby RyRs leading to positive inotropic but also pro-arrhythmic effects (Petersen et al., 1994; Vogelsand et al., 1994; Zima and Blatter, 2004; Harzheim et al., 2009). Experimental evidence accumulated over the past decade also indicates an important role of IP3R-mediated Ca2+ release in excitation-transcription coupling (ETC) and pro-hypertrophic signaling (Arantes et al., 2012). The entire PIP2-PLC-IP3 cascade, including GPCRs and IP3Rs, can be found in the NE (Bkaily et al., 2011; Vaniotis et al., 2011; Tadevosyan et al., 2012). The presence of nuclear GPCRs in combination with highly localized nuclear IP3R-mediated Ca2+ release and Ca2+ removal might provide for a putative distinct signaling domain that regulates nuclear Ca2+ dynamics (e.g., for autocrine signaling), whereas the cytosolic Ca2+ is regulated separately via sarcolemmal GPCR signaling and IP3R-mediated SR Ca2+ release in conjunction with Ca2+ release and removal by the set of proteins involved in ECC (e.g., RyR, SERCA, troponin C). Sarcolemmal GPCRs allow for paracrine signaling and positive inotropic effects mediated by hormonal stimulation (e.g., with Angiotensin II or Endothelin I), (Kockskämper et al., 2008; Bootman et al., 2009). A comprehensive understanding of the mechanisms regulating nuclear IP3 and Ca2+ signals and the impact of alterations of cytosolic Ca2+ and IP3 signals on nuclear functions requires well-characterized experimental approaches, but also whole-cell system mathematical models. In this review, we discuss quantitative aspects of IP3-dependent Ca2+ homeostasis in adult ventricular and atrial myocytes. In particular, we focus on novel modeling and experimental approaches to support the concept that IP3R-mediated Ca2+-release and the Ca2+ removal machinery in the SR and NE allow for highly localized and independent cellular signaling.
Excitation-Contraction Coupling in Ventricular and Atrial Myocytes and the Role of IP3
In cardiomyocytes, ECC describes the process of action potential (AP) triggered Ca2+-induced Ca2+ release (CICR) providing sufficient Ca2+ for the activation of the proteins regulating muscle contraction and to induce active muscle force (Bers, 2001). Membrane depolarization during an AP allows Ca2+ influx through voltage-dependent L-type Ca2+ channels (LTCC) which triggers CICR and thereby amplifies the cytosolic Ca2+ signal to levels required for the activation of the contractile proteins. An important feature of all ventricular myocytes, setting them apart from most atrial cells, is the presence of plasma membrane invaginations throughout the cytosol (transverse or t-tubules), putting LTCC in close vicinity to RyRs (Figure 1). The SR containing RyRs that oppose LTCC is called junctional SR (jSR). The jSR is crucial for the spatiotemporal homogeneity of Ca2+ release leading to largely uniform cytosolic Ca2+ transients ([Ca2+]i) during a ventricular cell twitch (Figure 2), (Franzini-Armstrong et al., 1999; Heinzel et al., 2002; Louch et al., 2004; Crossman et al., 2011; Hake et al., 2012; Signore et al., 2013). Unlike in ventricular cells, the t-tubular system in atrial myocytes is either absent (Figure 1) (Hüser et al., 1996; Kockskämper et al., 2001) or poorly developed (Kirk et al., 2003). However more recent work in sheep and human has provided evidence that atrial cells from larger animals tend to have a higher density of t-tubules (Dibb et al., 2009; Richards et al., 2011), and even in rodent atrial cells an irregular internal transverse-axial tubular system has been identified that affects kinetics of SR Ca2+ release (Kirk et al., 2003).The absence or paucity of t-tubules in atrial cells leads to great differences in the shape and kinetics of local Ca2+ transients and gradients in subcellular regions where Ca2+ is provided by release from jSR and non-junctional SR (njSR) (Figure 2). Subsarcolemmal Ca2+ transients rise faster, have a higher Ca2+ peak and are initiated by Ca2+ currents through LTCCs, followed by RyR-mediated Ca2+ release from the jSR. These local jSR Ca2+ transients resemble Ca2+ release in ventricular cells. Central cytosolic Ca2+ transients, however, have a slower rise time and a lower peak, and result from CICR that propagates in a Ca2+ wave-like fashion from the periphery to the center of the cell. (Blatter et al., 2003; Maxwell and Blatter, 2012). Furthermore, the specific topological organization of the plasma membrane in atrial myocytes leads not only to different spatial [Ca2+]i distribution as compared to the ventricle, it also affects nuclear Ca2+ transients by further delaying their onset due to the wave-like propagation of Ca2+ toward the nucleus (Figure 2). Interestingly, for both atrial and ventricular cells, a role of cytosolic IP3 ([IP3]i) has been reported for the modulation of cytosolic Ca2+ transients in a variety of animal models (Zima and Blatter, 2004; Proven et al., 2006; Domeier et al., 2008; Harzheim et al., 2009; Kim et al., 2010). IP3R channel activity, with type-2 IP3Rs as the most prevalent isoform in cardiac myocytes, depends on [IP3]i and [Ca2+]i (Michell et al., 1981; Domeier et al., 2008; Kockskämper et al., 2008). There is evidence that atrial myocytes express functional IP3Rs at higher densities than ventricular myocytes (Figure 1; in ventricular cell the IP3Rs are not shown in the junctional space due their relatively low density) (Mackenzie et al., 2004; Zima and Blatter, 2004). As shown in Figure 2, the acute increase in cytosolic IP3, induced by photolytic release of IP3 from a caged IP3 compound, increases cytosolic Ca2+ transient peak amplitudes during field stimulation in atrial cells in contrast to ventricular cells. In ventricular cells only increased expression levels of IP3R, as it occurs in cardiac hypertrophy, could experimentally be tied to enhanced cytosolic SR Ca2+ release (Harzheim et al., 2009). The neurohumoral stimulation with Endothelin I or Angiotensin II, however, has been shown to have similar positive inotropic effects in both ventricular and atrial cells, indicating a role of IP3-mediated Ca2+ release in the enhancement of cytosolic Ca2+ release (Zima and Blatter, 2004).
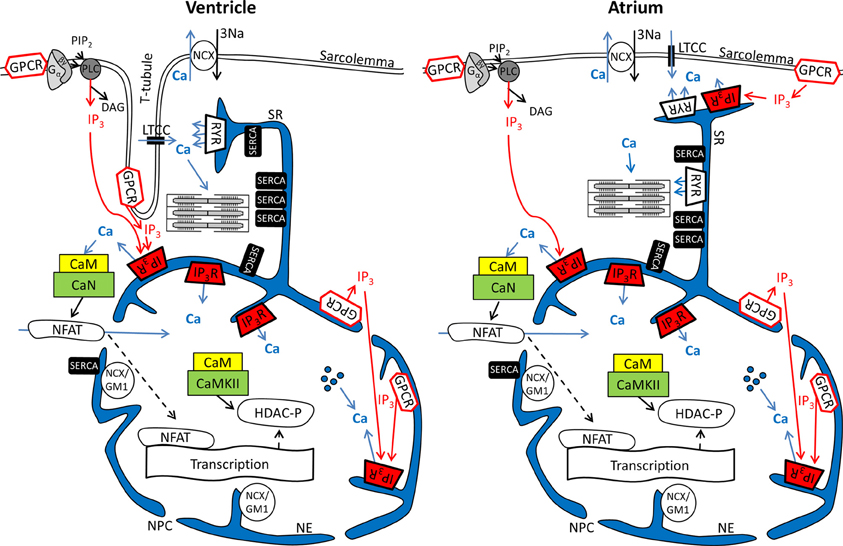
Figure 1. Ca2+ and IP3 and their involvement in cardiac excitation-contraction and excitation-transcription coupling in ventricular and atrial myocytes. Schematics depict parts of the sarcolemmal plasma membrane as well sarcoplasmic reticulum and nuclear envelope as a contiguous Ca2+ store. Abbreviations: GPCR, G protein-coupled receptor; PIP2, phosphatidylinositol 4,5-bisphosphate; PLC, phospholipase C; LTCC, L-type Ca2+ channel; NCX, Na+/Ca2+ exchanger; RyR, ryanodine receptor; IP3R, IP3 receptor; SERCA, SR Ca2+ ATPase; NCX/GM1, NCX ganglioside complex; CaM, Calmodulin; CaN, Calcineurin; CaMKII, Ca-Calmodulin dependent kinase; NFAT, nuclear factor of activated t-cells; DAG, Diacylglycerol; HDAC, histone deacetylase; NPC, nuclear pore complex;  Ca2+ released from intra-nuclear pools.
Ca2+ released from intra-nuclear pools.
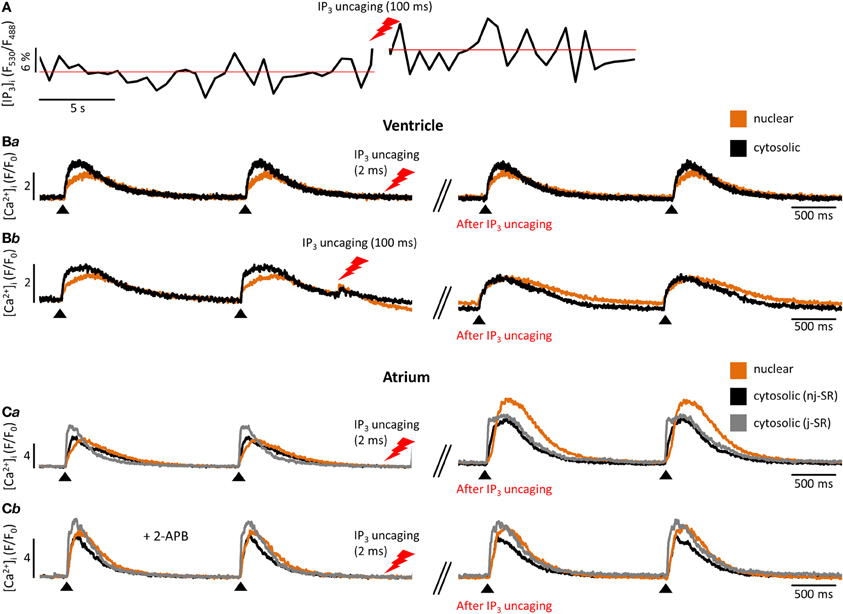
Figure 2. Experimental measurement of cytosolic and nuclear Ca2+ and IP3. (A) Cardiomyocyte loaded with the caged IP3 compound cag-IP3 PM and expressing cytosolic FIRE-1. Whole cell [IP3] signal shown as changes in FIRE-1 FRET signal (expressed as F530/F488) after global IP3 uncaging by exposure to 405 nm laser illumination for 100 ms. (Ba,b) Effects of global IP3 uncaging (2 ms or 100 ms illumination) in field-stimulated ventricular myocytes on global nuclear and cytosolic Ca2+ transients. (Ca) The effect of global IP3 uncaging (2 ms) on global nuclear, subsarcolemmal (j-SR) and central (nj-SR) Ca2+ transients in field stimulated atrial myocyte. (Cb) The effect of global IP3 uncaging (2 ms) on global nuclear, j-SR and central Ca2+ transients in field stimulated atrial myocyte pre-incubated with the IP3R blocker 2-APB. Pacing frequency 0.5 Hz. Black arrowheads indicate application of electrical stimuli.
Excitation-Transcription Coupling in Ventricular and Atrial Myocytes and the Role of IP3
Nuclear Ca2+ signals however are different with regards to kinetics during action potential induced Ca2+ transients. This can largely be attributed to the fact that the nucleus is surrounded by the nuclear envelope (Kockskämper et al., 2008; Alonso and García-Sancho, 2011), consisting of the outer and inner nuclear membranes and the space between them that is contiguous with the SR (Wu et al., 2006; Shkryl et al., 2012). The nuclear membranes fuse at many locations to form pores (diameter ~100, length ~50 nm) that harbor the nuclear pore complexes (NPCs). The NPCs are the major gateway for ions (including Ca2+) to diffuse along the gradient between the cytosol and nucleoplasm. It has been proposed that NPCs can act as diffusion filter and introduce a kinetic delay in the equilibration of nucleoplasmic Ca2+ concentration ([Ca2+]nuc) and [Ca2+]i (Bootman et al., 2009). The extent of the kinetic delay might be subject to modulation. Although NPCs do not close, their conductance can change in response to factors such as Ca2+ and ATP. The density of NPCs can vary from 1 to 5 NPCs per μm2, depending on the cell type (Wang and Clapham, 1999). A greater expression of NPCs would allow for a more rapid equilibration of [Ca2+]i and [Ca2+]nuc. Recent data from Alonso and García-Sancho (2011) also suggest a role for NE invaginations (nucleoplasmic reticulum) and intra-nuclear Ca2+ pools for the regulation of nuclear Ca2+ (Figure 1). More evidence that nuclear Ca2+ dynamics are not just a function of cytosolic Ca2+ transients can be found in structural and functional differences of NE Ca2+ handling proteins as compared to the SR. Even though the NE is an extension of the SR (Wu et al., 2006; Shkryl et al., 2012) SERCA presumably is not expressed at the inner NE membrane (Malviya and Klein, 2006; Bootman et al., 2009). Nonetheless, other putative Ca2+ handling and ion transporting proteins have been suggested to be present in the NE, including a splice variant of the type-1 Na+/Ca2+ exchanger associated with ganglioside (NCX/GM1 complex, typical for non-excitable cells), RyRs, NAADPR (nicotinic acid adenine dinucleotide phosphate receptor), Na+/K+ ATPase and Na+/H+ exchanger (Gerasimenko et al., 2003; Irvine, 2003; Bkaily et al., 2006; Ledeen and Wu, 2007; Zima et al., 2007; Guatimosim et al., 2008; Wu et al., 2009).
Even more important seems the preferential expression of IP3Rs in the NE (Bare et al., 2005). Using Fluo-5N Zima et al. observed a depletion of the nuclear envelope upon experimental stimulation of IP3Rs with IP3 in isolated nuclei (Zima et al., 2007) that was paralleled by an increase of [Ca2+]nuc. Wu and colleagues obtained similar results with Fluo-5N on IP3 dependent NE Ca2+ depletion in permeabilized cells (Wu et al., 2006). The importance of IP3 for the regulation of [Ca2+]nuc is underscored by the results shown in Figure 2: Following cell-wide IP3 uncaging, nuclear Ca2+ transients are consistently and preferentially altered in atrial and ventricular cells. However since IP3 is buffered (i.e., by IP3Rs) and degraded over time (Woodcock and Matkovich, 2005), the subcellular localization of IP3Rs and the site of IP3 generation (i.e., GPCR) are important to generate highly localized Ca2+ signals to control Ca2+-dependent transcription (Bers, 2013; Ibarra et al., 2013). The traditional view on the positioning of GPCRs in cardiac myocytes sees their main site of expression in the sarcolemmal and nuclear membrane (Figure 1). Only recently, work from Ibarra et al. (2013) suggested a third type of localization for GPCRs in t-tubules close to the nuclear envelope (Figure 1, ventricular cell). The positioning of IP3 production and IP3Rs is important since differences in the kinetics of local [Ca2+] can lead to altered activation of transcription factors. A pronounced local elevation of [Ca2+] for instance, can activate calmodulin dependent-protein kinase II (CaMKII) and promote histone deacetylases (HDAC) phosphorylation (Wu et al., 2006), whereas a sustained smaller [Ca2+] elevation increases nuclear factor of activated T-cells (NFAT) dephosphorylation via the Ca2+ sensitive phosphatase calcineurin (CaN). This ultimately leads to the activation of different sets of transcription factors, e.g., myocyte enhancer factor 2 (MEF2) for HDAC and GATA for NFAT (Molkentin et al., 1998). The separate set of Ca2+ release and removal proteins in the NE, with IP3Rs as the most prominent example, as well as the specific expression of GPCRs in the sarcolemmal and nuclear membranes might be key to understanding the conundrum of Ca2+ being a modulator of contraction and transcription at the same time (Bootman et al., 2009). Mathematical modeling of nuclear and cytosolic Ca2+ homeostasis, accounting for different expression levels of sarcolemmal, cytosolic and nuclear Ca2+ handling proteins, paralleled by experimental approaches might provide a better understanding of functional differences of nuclear and cytosolic Ca2+.
Experimental Tools for Measuring Cytosolic and Nuclear Ca2+ and IP3 Signals
Confocal laser microscopy, multiphoton imaging and conventional microscopy provide the basis for visualization of whole cell and subcellular ion concentration distributions, and the development of chemical fluorescent Ca2+ indicators (Grynkiewicz et al., 1985) made imaging of Ca2+ movements inside living cells feasible. Nowadays a variety of ratiometric and non-ratiometric Ca2+ indicators, with Indo-1 and Fluo-4 among the most prominent examples, are being used. In principle, upon excitation, these indicators emit light at particular wave lengths and the emitted fluorescence intensity or the emission spectrum is changed in a Ca2+ bound state (Takahashi et al., 1999). The dissociation constant (Kd) as a measure of Ca2+ binding affinity is crucial for the selection of the appropriate Ca2+ dye for a particular cellular compartment of interest. Low affinity, high Kd dyes (like Fluo-5N) are used for the visualization of changes in SR [Ca2+] or nuclear envelope [Ca2+], whereas, e.g., Fluo-4 (Kd of 345 nM) is one of the preferred dyes for imaging of changes in cytosolic free [Ca2+], which varies roughly between 100 nM and values at times exceeding 1 μM during ECC. Since the nucleoplasm and the cytoplasm are interconnected compartments with similar global [Ca2+] characteristics, dyes suitable to show changes in [Ca2+]i can be used for the detection of changes in [Ca2+]nuc as well. Using Ca2+ sensitive dyes, Zima and Blatter (2004) were able to visualize cytosolic IP3R-mediated Ca2+ release events (Ca2+ puffs) and show a positive inotropic effect of neurohumoral stimulation with Endothelin-1 in cardiac myocytes. As mentioned above, the same group was also able to show changes of local nuclear envelope [Ca2+] in isolated nuclei upon stimulation with IP3, using Fluo-5N (Zima et al., 2007).
A variety of pharmacologic interventions can be used to influence the IP3-dependent signaling cascade. Tools for stimulation of the neurohumoral GPCR pathway in cardiomyocytes include for example Angiotensin II and Endothelin-1. PLC-inhibitors like U73122 and IP3R blockers like 2-Aminoethoxydiphenyl borate (2-APB) or heparin are widely used IP3R blockers to study the GPCR/PLC/IP3 pathway. More recent molecular techniques and the generation of transgenic animals complement these tools. Noteworthy are the generation of IP3R knock-out and IP3R overexpressing mice as well as the development of IP3-sponges that allows the cellular overexpression of IP3 buffering proteins. The generation of IP3R overexpressing mice combined with the adenoviral expression of an IP3 sponge provided novel insights into the importance of this pathway in cardiac physiology and pathophysiology. Ca2+ transients in IP3R overexpressing mice were increased and showed a higher potential for arrhythmias after Endothelin-1 treatment. These effects were abrogated after expression of the IP3 sponge (Nakayama et al., 2010). Insensitivity toward GPCR stimulation and IP3R-mediated pro-arrhythmic effects were confirmed in IP3R knock-out mice (Li et al., 2005).
An approach to directly visualize cellular [IP3] would allow for a more complete picture of cell physiology. Only recently Remus et al. (2006) developed biosensors termed FIRE to dynamically study [IP3] in living cells. Briefly, FIRE is incorporated into an adenoviral vector, expressed in target cells, and utilizes fluorescence resonance energy transfer (FRET) between cyan and yellow fluorescent protein (CFP and YFP) upon binding of IP3. For that purpose FIRE contains a fusion protein of CFP, YFP, and the IP3 binding domain of the IP3 receptor type 1, 2, or 3 and can be targeted to the cytosolic or nuclear compartment. An increase in [IP3] is detected by an increase in FRET signals and a change in the YFP/CFP fluorescence ratio.
Further progress in the study of IP3-dependent Ca2+ signaling became possible with the development of caged IP3 compounds (Smith et al., 2009). Upon UV-light dependent photolysis, IP3 is released in its biological active form and can be readily used to study this signaling pathway without possible additional effects of GPCR stimulation other than IP3 generation (i.e., effects mediated by diacylglycerol that is generated concomitantly with IP3 by PLC). These approaches can be used in parallel, as shown in Figure 2A: a cardiomyocyte expressing FIRE-1-cyt exhibits an increase in the FRET signal of ~6% upon IP3 uncaging, indicative of a detectable change of global cytosolic [IP3]. Moreover Figure 2 depicts the influence of IP3 uncaging on different cellular compartments in atrial and ventricular cells. Figure 2B exemplifies the small impact of IP3 uncaging on local cytosolic and nuclear Ca2+ transients in field stimulated (0.5 Hz) ventricular cells (Fluo-4). Only prolonged exposure to the IP3 uncaging signal (100 ms laser illumination) has immediate visible effects on local Ca2+ release (Figure 2C). The IP3 effects on diastolic [Ca2+]i and the Ca2+ transient amplitude are particularly pronounced for the nuclear region. As compared to ventricular cells, atrial myocytes are more sensitive to IP3 uncaging at smaller laser exposure durations (2 ms; i.e., smaller [IP3]) and the overall effect on cytosolic, nuclear and subsarcolemmal Ca2+ transient amplitudes is higher upon IP3 uncaging. Note also the altered Ca2+ transient kinetics with a prolongation of the Ca2+ transient's amplitude following IP3 uncaging (Figure 2Ca). Figure 2Cb shows the effect of the IP3R blocker 2-APB (10 μM). The effect of IP3 uncaging on Ca2+ transients in an atrial cell, pre-incubated with 2-APB, was abolished.
Mathematical Approaches for Simulating Cytosolic and Nuclear Ca2+ and IP3 Signals
Computational modeling has proven to be a powerful approach to study cardiac physiology and its implications for disease. With increasing availability of biophysical and physiological data, mathematical models have also become more sophisticated. They provided new insights into how cellular structures, channels and receptor distributions or Ca2+/IP3 signaling regulate cardiac ECC. A number of deterministic models of ventricular and atrial myocyte electrophysiology, intracellular Ca2+ handling and bioenergetics have been published. For a more complete review on successes and failures in these modeling pursuits we refer the reader to some excellent recently published articles (Noble, 2011; Jafri, 2012; Noble et al., 2012; Sobie and Lederer, 2012; Poláková and Sobie, 2013; Wilhelms et al., 2013). Several computational models have been constructed to investigate IP3 synthesis and the sub-cellular mechanisms regulating IP3R-mediated Ca2+ signaling. The first model of an IP3 signaling system, built to simulate IP3 signals in response to stimulation with cardiac hypertrophic neurohumoral agonists like Endothelin-1 and Angiotensin II, was published by Cooling et al. (2007). The key controlling parameters with respect to the resultant cytosolic [IP3] in atrial cells were identified, including phosphorylation of membrane receptors, ligand strength, binding kinetics to pre-coupled (with GαGDP) receptors and kinetics associated with pre-coupling the receptors. In 1992, De Young and Keizer (1992) constructed the first simplified model of the IP3 receptor. Subsequent theoretical studies, based on new experimental data, have investigated the complex dynamic properties of type 1, 2, or 3 IP3Rs (Li and Rinzel, 1994; Laurent and Claret, 1997; LeBeau et al., 1999; Moraru et al., 1999; Mak et al., 2001; Sneyd and Dufour, 2002; Dawson et al., 2003; Siekmann et al., 2012). Based on quantitative measurements of IP3R properties, several stochastic models of the single channel and channel-clusters have been constructed (Swillens et al., 1998; Shuai and Jung, 2002; Falcke, 2003; Fraiman and Dawson, 2004; Thul and Falcke, 2004; Gin et al., 2009). Fraiman and Dawson (2004) were the first to include an explicit dependence of IP3R gating on SR-luminal Ca2+. To investigate the mechanisms underlying pacemaker cell activity, Youm et al. (2006) developed a deterministic model that includes ion channels, NCX, pumps, the intracellular machinery for Ca2+ regulation, cytosolic IP3 production and IP3-mediated Ca2+ release activity. Their model supports the idea that the cyclic changes in cytosolic Ca2+ and IP3 play a key role in the generation of regenerative pacemaker potentials. Spatiotemporal continuum models, seeking to investigate the mechanisms of IP3-mediated Ca2+ signaling in cells where IP3Rs are known to be the dominant Ca2+ release channels, have been published as well. Jafri and Keizer, combining a realistic model of IP3-induced Ca2+ oscillations with the diffusion of IP3 and buffered diffusion of Ca2+, developed a reaction-diffusion continuum model in Xenopus oocytes (Jafri and Keizer, 1994, 1995). Their results suggest that Ca2+ diffusion, which was much slower than that of IP3 because of endogenous Ca2+ buffers, had only a small effect on predicted Ca2+ transients. These findings imply a possible previous undisclosed role for IP3 in cell signaling. Means et al. (2006) used a reaction-diffusion model to simulate Ca2+ and IP3 dynamics in mast cells. The model was built upon a 3D reconstruction of the endoplasmic reticulum (ER) geometry from electron-tomography series. This model simultaneously tracks the changes in cytoplasmic and ER [Ca2+], includes luminal and cytoplasmic Ca2+ buffers, plasma membrane Ca2+ fluxes, SERCA, ER leakage, and type-2 IP3R. A unique feature of the model is the inclusion of the stochastic behavior of type-2 IP3R. The results showed that IP3Rs in close proximity modulate the activity of their neighbors through local Ca2+ feedback effects. Finally, in 1999 an analysis performed by fluorescence measurements of [Ca2+]i and [Ca2+]nuc in ventricular myocytes revealed that [Ca2+]nuc increases concomitantly with [Ca2+]i upon electrical stimulation, but the pattern of [Ca2+]nuc increase was biphasic (rapid and slow) (Genka et al., 1999). Both sets of [Ca2+]i and [Ca2+]nuc data were well fitted by predictions derived from a simplified model of Ca2+ diffusion across the NPCs with two different Ca2+ diffusion constants. A plausible explanation of this finding is that the change in [Ca2+]nuc is caused by Ca2+ diffusion from the cytosol to the nucleus through NPCs, but the permeability of the NPCs shifts from free to moderately restricted during contraction (Genka et al., 1999). The partial restriction of Ca2+ diffusion into the nucleus at high [Ca2+]i may support the idea of a defense mechanism protecting the nucleus against Ca2+ overload during cell contraction.
Taken together, the aforementioned modeling efforts fill a number of specific gaps of knowledge with respect to cell electrophysiology and cytosolic Ca2+ and IP3 signaling. To date, however, no quantitative model coupling the cell electrophysiology with Ca2+ and IP3 signaling in the cytosol and nucleus in cardiomyocytes exists. The development of a new system model, coupling ECC and ETC is important because: (a) this tool would provide fundamental new information on the role of IP3R-mediated Ca2+ signaling during ECC for arrhythmogenesis, for electrophysiological changes and for nuclear Ca2+ signaling in normal and failing cardiac cells; (b) as more experimental details on the complexity of IP3 regulation in myocytes accumulates, the intuitive interpretation of new findings becomes increasingly impractical and sometimes controversial. In pursuing this goal we extended the Shannon-Bers model in rabbit ventricular myocytes (Shannon et al., 2004). New equations, describing nuclear Ca2+ dynamics and its dependence on [Ca]i, nuclear Ca2+ buffering and transport via NPCs and NE (i.e., SR) were incorporated (see Figure 1; Michailova et al. unpublished data). Preliminary results (Figures 3A,B) show that the model predictions are in qualitative agreement with our Ca2+ transient measurements at 0.5 Hz electrical stimulation (see Figure 2B) and published experimental data (Ljubojevic et al., 2011) of global cytosolic and nuclear Ca2+ transients under control conditions, i.e., in absence of activation of IP3 signaling. The predicted [Ca2+]i and [Ca2+]nuc transients (and action potentials and [Ca2+]SR; not shown) are stable during 10 min stimulation at 0.5, 1, or 2 Hz. The model mimics also the frequency-dependent increases in the diastolic [Ca2+]i (Shannon et al., 2004), but no obvious differences in diastolic levels of [Ca2+]nuc vs. [Ca2+]i at any given frequency were predicted. At each frequency the systolic Ca2+ peaks were lower in the nuclei and positive force-frequency increases in systolic [Ca2+]i and [Ca2+]nuc were predicted. The kinetic parameters of Ca2+ transients (time to peak and time to 50% [Ca2+] relaxation; RT50) were slower in the nucleus as compared to the cytosol. The physiological utility of the model was tested further by applying different frequencies to simulate the positive force-frequency relationship (Figure 3C). In agreement with experiments (Ljubojevic et al., 2011), upon increasing the rate from 0.5 to 2 Hz diastolic [Ca2+] and systolic Ca2+ peaks in the nucleus and cytoplasm increased in magnitude and the predicted amplitude of the Ca2+ transients were smaller in the nucleus compared to the cytosol.
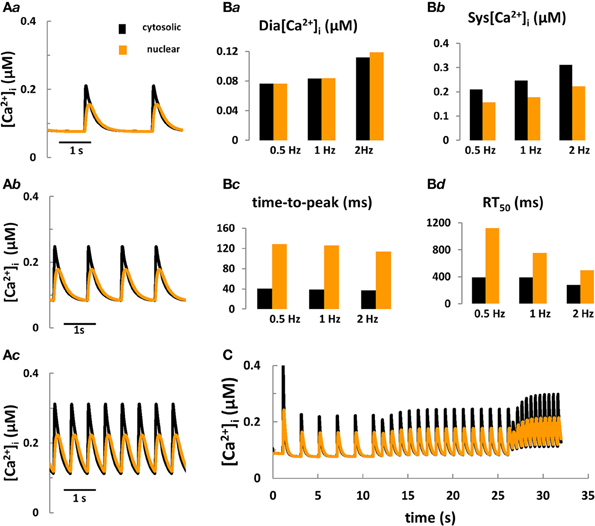
Figure 3. Computer modeling of cytosolic and nuclear Ca2+ signals under control conditions. (Aa–Ac) Predicted cytosolic and nuclear steady-state Ca2+ transients under control conditions in rabbit ventricular myocytes stimulated for 10 min at frequencies of 0.5, 1, and 2 Hz, respectively. (Ba–Bd) Predicted kinetic parameters of [Ca2+]i and [Ca2+]nuc transients: Dia [Ca2+], diastolic Ca2+; Sys [Ca2+], systolic Ca2+; RT50, time to 50% [Ca2+] relaxation. (C) Frequency-dependent changes in [Ca2+] in the nucleus vs. the cytoplasm during stepwise increases of the stimulation frequency from 0.5 to 2 Hz.
Conclusions and Future Perspectives
In this review we discussed the current state of experimental and modeling approaches to investigate nuclear and cytosolic Ca2+ homeostasis, whereby we focused on IP3-dependent Ca2+ signaling in adult myocytes. We presented experimental data from ventricular and atrial cells, showing the effects of sudden increases in [IP3] on nuclear and cytosolic Ca2+ transients during field stimulation as well as different approaches to study IP3-mediated Ca2+ release (i.e., FIRE-1-cyt as a tool to quantify [IP3], IP3 uncaging to mimic physiological increases in [IP3] and 2-APB to block IP3R mediated Ca2+ release). Moreover we compared experimentally the influence of IP3 uncaging on different compartments (nucleoplasm, cytosol) and were able to show that ventricular cells need a stronger IP3 stimulus to elicit a nuclear response, whereas atrial cells display substantial increases in nuclear and cytosolic Ca2+ transient amplitude upon a weaker IP3 uncaging stimulus, consistent with their higher total expression of IP3Rs as compared to ventricle. The recent development of FRET-based probes used for the detection of [IP3] as well as approaches to alter nuclear and/or cytosolic [IP3] provide experimental tools for the study of IP3-dependent Ca2+ release and its importance in ECC and ETC.
We also presented our recent efforts of a first attempt to develop an electrophysiological and Ca2+ signaling model that integrates three different cellular subsystems (cytosol, SR, nucleus) and couples Ca2+ dynamics in the cytosol and nucleus. This new tool is under development and will undergo further testing in its prediction of experimental [Ca2+]nuc and [Ca2+]i data in rabbit ventricular cells. The proposed model will also be extended to investigate how the complex dynamics of type-2 IP3 receptors (Sneyd and Dufour, 2002; Siekmann et al., 2012), the stochastic behavior of IP3R channel (Fraiman and Dawson, 2004) and/or the stimulation of IP3 signal transduction pathway with neurohumoral agonists (Cooling et al., 2007) regulate ventricular ECC and ETC. Furthermore, the mechanisms underlying IP3-induced positive inotropy in cardiomyocytes continue to be controversial with numerous cellular targets being implicated in the response, including L-type Ca2+ channels, K+ channels, and Na+/Ca2+ exchange (Lauer et al., 1992; Watanabe and Endoh, 1999; Woo and Lee, 1999; Yang et al., 1999; He et al., 2000; James et al., 2001; Zhang et al., 2001; Puglisi et al., 2011; Signore et al., 2013). The current model can be extended to investigate these effects as well. This model also provides a good quantitative framework to integrate reactions for calmodulin (CaM), calcineurin (CaN), CaMKII, and CaM buffering in the nucleus and can be coupled to the previously described and validated ECC models of CaM-CaMKII-CaN in rabbit ventricular cells (Hund and Rudy, 2004; Grandi et al., 2007; Saucerman and Bers, 2008; Bers and Grandi, 2009; Kraeuter et al., 2010; Soltis and Saucerman, 2010). This will allow testing hypotheses on how the interactions between Ca2+, IP3, and CaMKII signaling pathways contribute to heart failure phenotypes. Finally, the tools and insights our group develops will be useful to investigate how perturbations in cytosolic and nuclear Ca2+ and IP3 signaling affect ECC and ETC in atrial myocytes (Grandi et al., 2011; Koivumäki et al., 2011).
Author and Contributors
Designed the work: Anushka P. Michailova and Felix Hohendanner. Performed the experiments: Felix Hohendanner and Lothar A. Blatter. Performed the simulations: Anushka P. Michailova. Analyzed the data: Anushka P. Michailova, Felix Hohendanner, Lothar A. Blatter, and Andrew D. McCulloch. Contributed reagents/materials/analysis tools: Anushka P. Michailova, Felix Hohendanner, and Lothar A. Blatter. Wrote the paper: Anushka P. Michailova, Felix Hohendanner, Lothar A. Blatter, and Andrew D. McCulloch.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work is supported by NIBIB 1R03EB014593-01 (Michailova), National Biomedical Computational Resource (NIH grant 8P41 GM103426-19), NHLBI Systems Biology Collaboration 1R01HL105242-01 (McCulloch), and National Institutes of Health grants HL62231, HL80101 and HL101235 and the Leducq Foundation (Blatter).
References
Alonso, M. T., and García-Sancho, J. (2011). Nuclear Ca(2+) signalling. Cell Calcium 49, 280–289. doi: 10.1016/j.ceca.2010.11.004
Arantes, L. A., Aguiar, C. J., Amaya, M. J., Figueiró, N. C., Andrade, L. M., Rocha-Resende, C., et al. (2012). Nuclear inositol 1,4,5-trisphosphate is a necessary and conserved signal for the induction of both pathological and physiological cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. 53, 475–486. doi: 10.1016/j.yjmcc.2012.06.017
Bare, D. J., Kettlun, C. S., Liang, M., Bers, D. M., and Mignery, G. A. (2005). Cardiac type 2 inositol 1,4,5-triphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 280, 15912–15920. doi: 10.1074/jbc.M414212200
Berridge, M. J. (2009). Inositol trisphosphate and calcium signaling mechanisms. Biochem. Biophys. Acta 1793, 933–940. doi: 10.1016/j.bbamcr.2008.10.005
Bers, D. (2001). Excitation-Contraction Coupling and Cardiac Contractile Force. Dortrecht; Boston, MA; London: Kluwer Academic Publishers. doi: 10.1007/978-94-010-0658-3
Bers, D. (2013). Membrane receptor neighborhoods: snuggling up to the nucleus. Circ. Res. 112, 224–226. doi: 10.1161/CIRCRESAHA.112.300494
Bers, D. M., and Grandi, E. (2009). CaMKII regulation of cardiac ion channels. J. Cardiovasc. Pharmacol. 54, 180–187. doi: 10.1097/FJC.0b013e3181a25078
Bkaily, G., Avedanian, L., Al-Khoury, J., Provost, C., Nader, M., D'Orléans-Juste, P., et al. (2011). Nuclear membrane receptors for ET-1 in cardiovascular function. Am. J. Physiol. Regul. Integr. Comp. Physiol., 300, R251–R263. doi: 10.1152/ajpregu.00736.2009
Bkaily, G., Nader, M., Avedanian, L., Choufani, S., Jacques, D., D'Orléans-Juste, P., et al. (2006). G-protein-coupled receptors, channels, and Na+-H+ exchanger in nuclear membranes of heart, hepatic, vascular endothelial, and smooth muscle cells. J. Can. Physiol. Pharmacol. 84, 431–441. doi: 10.1139/y06-002
Blatter, L. A., Kockskamper, J., Sheehan, K. A., Zima, A. V., Huser, J., and Lipsius, S. (2003). Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J. Physiol. 546, 19–31. doi: 10.1113/jphysiol.2002.025239
Bootman, M. D., Fearnley, C., Smyrnias, I., MacDonald, F., and Roderick, J. L. (2009). An update on nuclear calcium signaling. J. Cell Sci. 122, 2337–2350. doi: 10.1242/jcs.028100
Cooling, M., Hunter, P., and Crampin, E. (2007). Modeling hypertrophic IP3 transients in the cardiac myocyte. Biophys. J. 93, 3421–3433. doi: 10.1529/biophysj.107.110031
Crossman, D. J., Ruygrok, P. N., Soeller, C., and Cannell, M. B. (2011). Changes in the organization of excitation-contraction coupling structures in failing human heart. PLoS ONE 6:e17901. doi: 10.1371/journal.pone.0017901
Dawson, A. P., Lea, E. J., and Irvine, R. F. (2003). Kinetic model of inositol triphosphate receptor that shows both steady-state and quantal patterns of Ca2+ release from intracellular stores. Biochem. J. 370, 621–629. doi: 10.1042/BJ20021289
De Young, G. W., and Keizer, J. (1992). A single-pool inositol 1,4,5-triphosphate receptor-based model for agonist-simulated oscillations in Ca2+ concentration. Proc. Natl. Acad. Sci. U.S.A. 89, 9895–9899. doi: 10.1073/pnas.89.20.9895
Dibb, K. M., Clarke, J. D., Horn, M. A., Richards, M. A., Graham, H. K., Eisner, D. A., et al. (2009). Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ. Heart Fail. 2, 482–489. doi: 10.1161/CIRCHEARTFAILURE.109.852228
Domeier, T. L., Zima, A. V., Maxwell, J. T., Huke, S., Mignery, G. A., and Blatter, L. A. (2008). IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 294, H596–H604. doi: 10.1152/ajpheart.01155.2007
Falcke, M. (2003). On the role of stochastic channel behavior in intracellular dynamics. Biophys. J. 84, 42–56. doi: 10.1016/S0006-3495(03)74831-0
Fraiman, D., and Dawson, S. P. (2004). A model of the IP3 receptor with a luminal calcium binding site: stochastic simulations and analysis. Cell Calcium 35, 403–413. doi: 10.1016/j.ceca.2003.10.004
Franzini-Armstrong, C., Protasi, F., and Ramesh, V. (1999). Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys. J. 77, 1528–1539. doi: 10.1016/S0006-3495(99)77000-1
Genka, C., Ishida, H., Ichimori, K., Hirota, Y., Tanaami, T., and Nakazawa, H. (1999). Visualization of biphasic Ca2+ diffusion from cytosol to nucleus in contracting adult rat cardiac myocytes with an ultra-fast confocal imaging system. Cell Calcium 25, 199–208. doi: 10.1054/ceca.1999.0026
Gerasimenko, J. V., Maruyama, Y., Yano, K., Dolman, N. J., Tepikin, A. V., Petersen, O. H., et al. (2003). NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J. Cell Biol. 163, 271–282. doi: 10.1083/jcb.200306134
Gin, E., Falcke, M., Wagner, L. E. 2nd. Yule, D. I., and Sneyd, J. (2009). A kinetic model of the inositol triphosphate receptor based of single-channel data. Biophys. J. 96, 4053–4062. doi: 10.1016/j.bpj.2008.12.3964
Grandi, E., Pandit, S. V., Voigt, N., Workman, A. J., Dobrev, D., Jalife, J., et al. (2011). Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ. Res. 109, 1055–1066. doi: 10.1161/CIRCRESAHA.111.253955
Grandi, E., Puglisi, J. L., Wagner, S., Maier, L. S., Severi, S., and Bers, D. M. (2007). Simulation of Ca-calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potential. Biophys. J. 93, 3835–3847. doi: 10.1529/biophysj.107.114868
Grynkiewicz, G., Poenie, M., and Tsien, R. Y. (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450.
Guatimosim, S., Amaya, M. J., Guerra, M. T., Aguiar, C. J., Goes, A. M., Gómez-Viquez, N. L., et al. (2008). Nuclear Ca2+ regulates cardiomyocyte function. Cell Calcium 44, 230–242. doi: 10.1016/j.ceca.2007.11.016
Hake, J. E., Edwards, A. G., Yu, Z., Kekenes-Huskey, P., Michailova, A. P., McCammon, A., et al. (2012). Modeling cardiac calcium sparks in a three-dimensional reconstruction of a calcium release unit. J. Physiol. 590, 4403–4422. doi: 10.1113/jphysiol.2012.227926
Harzheim, D., Movassagh, M., Foo, R., Ritter, O., Tashfeen, A., Conway, S. J., et al. (2009). Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 106, 11406–11411. doi: 10.1073/pnas.0905485106
He, J. Q., Pi, Y., Walker, J. W., and Kamp, T. J. (2000). Endothelin-1 and photoreleased diacylglycerol increse L-type Ca2+ current by activation of protein kinase C in rat ventricular myocytes. J. Physiol. 524, 807–820. doi: 10.1111/j.1469-7793.2000.00807.x
Heinzel, F. R., Bito, V., Volders, P. G., Antoons, G., Mubagwa, K., and Sipido, K. R. (2002). Spatial and temporal inhomogeneities during Ca2+ release from the sarcoplasmic reticulum in pig ventricular myocytes. Circ. Res. 91, 1023–1030. doi: 10.1161/01.RES.0000045940.67060.DD
Hund, T. J., and Rudy, Y. (2004). Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation 110, 3168–3174. doi: 10.1161/01.CIR.0000147231.69595.D3
Hüser, J., Lipsius, S. L., and Blatter, L. A. (1996). Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J. Physiol. 494, 641–651.
Ibarra, C. C., Vicencio, J. M., Estrada, M., Lin, Y., Rocco, P., Rebellato, P., et al. (2013). Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors. Circ. Res. 112, 236–245. doi: 10.1161/CIRCRESAHA.112.273839
Irvine, R. F. (2003). Nuclear lipid signaling. Nat. Rev. Mol. Cell Biol. 4, 349–360. doi: 10.1038/nrm1100
Jafri, M. S. (2012). Models of excitation-contraction coupling in cardiac ventricular myocytes. Methods Mol. Biol. 910, 309–335. doi: 10.1007/978-1-61779-965-5_14
Jafri, M. S., and Keizer, J. (1994). Diffusion of inositol 1,4,5-triphosphate but not Ca2+ is necessary for class of inositol 1,4,5-triphosphate-iduced Ca2+ waves. Proc. Natl. Acad. Sci. U.S.A. 91, 9485–9489. doi: 10.1073/pnas.91.20.9485
Jafri, M. S., and Keizer, J. (1995). On the roles of Ca2+ diffusion, Ca2+ buffers, and the endoplasmic reticulum in IP3-induced Ca2+ waves. Biophys. J. 69, 2139–2153. doi: 10.1016/S0006-3495(95)80088-3
James, A. F., Ramsey, J. E., Reynolds, A. M., Hendry, B. M., and Shattock, M. J. (2001). Effects of endothelin-1 on K+ currents from rat ventricular myocytes. Biochem. Biophys. Res. Commun. 284, 1048–1055. doi: 10.1006/bbrc.2001.5083
Kim, J. C., Son, M. J., Subedi, K. P., Li, Y., Ahn, J. R., and Woo, S. H. (2010). Atrial local Ca2+ signaling and inositol 1,4,5-trisphosphate receptors. Prog. Biophys. Mol. Biol. 103, 59–70. doi: 10.1016/j.pbiomolbio.2010.02.002
Kirk, M. M., Izu, L. T., Chen-Izu, Y., McCulle, S. L., Wier, W. G., Balke, C. W., et al. (2003). Role of transverse-axial tubule system in generating calcium sparks and calcium transients in rat atrial myocytes. J. Physiol. 547, 441–451. doi: 10.1113/jphysiol.2002.034355
Kockskämper, J., Sheehan, K. A., Bare, D. J., Lipsius, S. L., Mignery, G. A., and Blatter, L. A. (2001). Activation and propagation of Ca2+ release during excitation-contraction coupling in atrial myocytes. Biophys. J. 81, 2590–2595. doi: 10.1016/S0006-3495(01)75903-6
Kockskämper, J., Zima, A. V., Roderick, H. L., Pieske, B., Blatter, L. A., and Bootman, M. D. (2008). Emerging roles of inositol 1,4,5-thriphosphate signaling in cardiac myocytes. J. Mol. Cell. Cardiol. 45, 128–147. doi: 10.1016/j.yjmcc.2008.05.014
Koivumäki, J. T., Korhonen, T., and Tavi, P. (2011). Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: a computational study. PLoS Comp. Biol. 7:e1001067. doi: 10.1371/journal.pcbi.1001067
Kraeuter, M. J., Soltis, A. R., and Saucerman, J. (2010). Modeling cardiac β-adrenergic signaling with normalized-Hill differential equations: comparison with a biochemical model. BMC Syst. Biol. 4:157. doi: 10.1186/1752-0509-4-157
Lauer, M. R., Gunn, M. D., and Clusin, W. T. (1992). Endothelin-1 activates voltage-dependent Ca2+ current by a G protein-dependent mechanism in rabbit cardiac myocytes. J. Physiol. 448, 729–747.
Laurent, M., and Claret, M. (1997). Single-induced Ca2+ oscillations through the regulation of the inositol 1,4,5-triphosphate-gared Ca2+ channel: an allosteric model. J. Theor. Biol. 186, 307–326. doi: 10.1006/jtbi.1996.0365
LeBeau, A. P., Yule, D. I., Groblewski, G. E., and Sneyd, J. (1999). Agonist-dependent phosphorylation of 1,4,5-triphosphate receptor: a possible mechanism for agonist-specific calcium oscillations in pancreatic acinar cells. J. Gen. Physiol. 113, 851–872. doi: 10.1085/jgp.113.6.851
Ledeen, R., and Wu, G. (2007). GM1 in the nuclear envelope regulates nuclear calcium through association with a nuclear sodium-calcium exchanger. J. Neurochem. Suppl. 1, 126–134. doi: 10.1111/j.1471-4159.2007.04722.x
Li, X., Zima, A. V., Sheikh, F., Blatter, L. A., and Chen, J. (2005). Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ. Res. 96, 1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c
Li, Y. X., and Rinzel, J. (1994). Equations for InsP3 receptor-mediated [Ca2+]i oscillations derived from a detailed kinetic model: a Hodgkin-Huxley like formalism. J. Theor. Biol. 166, 461–473. doi: 10.1006/jtbi.1994.1041
Ljubojevic, S., Walther, S., Asgarzoei, M., Sedej, S., Pieske, B., and Kockskämper, J. (2011). In situ calibration of nucleoplasmic versus cytoplasmic Ca2+ concentration in adult cardiomyocytes. Biophys. J. 100, 2356–2366. doi: 10.1016/j.bpj.2011.03.060
Louch, W. E., Bito, V., Heinzel, F. R., Macianskiene, R., Vanhaecke, J., Flameng, W., et al. (2004). Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc. Res. 62, 63–73. doi: 10.1016/j.cardiores.2003.12.031
Mackenzie, L., Roderick, H. I., Proven, A., Conway, S. J., and Bootman, M. D. (2004). Inositol 1,4,5,-triphosphate receptors in the heart. Biol. Res. 37, 553–557. doi: 10.4067/S0716-97602004000400008
Mak, D., McBride, S., and Foskett, J. K. (2001). Regulation by Ca2+ and inositol 1,4,5-triphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels: Ca2+ activation uniquely distinguishes types 1 and 3 InsP3 receptors. J. Gen. Physiol. 117, 435–446. doi: 10.1085/jgp.117.5.435
Malviya, A. N., and Klein, C. (2006). Mechanism regulating nuclear calcium signaling. Can. J. Physiol. Pharmacol. 84, 403–422. doi: 10.1139/y05-130
Maxwell, J. T., and Blatter, L. A. (2012). Facilitation of cytosolic calcium wave propagation by local calcium uptake into the sarcoplasmic reticulum in cardiac myocytes. J. Physiol. 590, 6037–6045. doi: 10.1113/jphysiol.2012.239434
Means, S., Smith, A. J., Shepherd, J., Shadid, J., Fowler, J., Wojcikiewicz, R. H. J., et al. (2006). Reaction diffufion modeling of calcium dynamics with realistic ER geometry. Biophys. J. 91, 537–557. doi: 10.1529/biophysj.105.075036
Michell, R. H., Kirk, C. J., Jones, L. M., Downes, C. P., and Creba, J. A. (1981). The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos. Trans. R. Soc. Lond. Biol. Sci. 296, 123–138. doi: 10.1098/rstb.1981.0177
Molkentin, J. D., Lu, J. R., Antos, C. L., Markham, B., Richardson, J., Robbins, J., et al. (1998). A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93, 215–228. doi: 10.1016/S0092-8674(00)81573-1
Moraru, I., Kaftan, E. J., Ehrlich, B. E., and Watras, J. (1999). Regulation of type 1 inositol 1,4,5-triphosphate-gated calcium channels by InsP3 and calcium: simulation of single channel kinetics based on ligand binding and electro physiological analysis. J. Gen. Physiol. 113, 837–849. doi: 10.1085/jgp.113.6.837
Nakayama, H., Bodi, I., Maillet, M., DeSantiago, J., Domeier, T. L., Mikoshiba, K., et al. (2010). The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ. Res. 107, 659–666. doi: 10.1161/CIRCRESAHA.110.220038
Noble, D. (2011). Successes and failures in modeling heart cell electrophysiology. Heart Rhythm 8, 1798–1803. doi: 10.1016/j.hrthm.2011.06.014
Noble, D., Garny, A., and Noble, P. J. (2012). How the Hodgkin-Huxley equations inspired the cardiac physiome project. J. Physiol. 590, 2613–2628. doi: 10.1113/jphysiol.2011.224238
Petersen, O. H., Petersen, C. C., and Kasai, H. (1994). Calcium and hormone action. Annu. Rev. Physiol. 56, 297–319. doi: 10.1146/annurev.ph.56.030194.001501
Poláková, E., and Sobie, E. A. (2013). Alterations in T-tubule and dyad structure in heart disease: challenges and opportunities for computational analyses. Cardiovasc. Res. 98, 233–239. doi: 10.1093/cvr/cvt026
Proven, A., Roderick, H. L., Conway, S. J., Berridge, M. J., Horton, J. K., Capper, S. J., et al. (2006). Inositol 1,4,5,-supports the arrhythmogenic action of endothelin-1 on ventricular cardiac myocytes. J. Cell Sci. 119, 3363–3375. doi: 10.1242/jcs.03073
Puglisi, J. L., Yuan, W., Timofeyev, V., Myers, R. E., Chiamvimonvat, N., Samarel, A. M., et al. (2011). Phorbol ester and endothelin-1 alter functional expression of Na+/Ca2+ exchange, K+, and Ca2+ currents in cultured neonatal rat myocytes. Am. J. Physiol. Heart Circ. Physiol. 300, H617–H626. doi: 10.1152/ajpheart.00388.2010
Remus, T. P., Zima, A. V., Bossuyt, J., Bare, D. J., Martin, J. L., and Blatter, L. A. (2006). Biosensors to measure inositol 1,4,5-triphosphate concentration in living cells with spatiotemporal resolution. J. Biol. Chem. 281, 608–616. doi: 10.1074/jbc.M509645200
Richards, M. A., Clarke, J. D., Saravanan, P., Voigt, N., Dobrev, D., Eisner, D. A., et al. (2011). Transverse tubules are a common feature in large mammalian atrial myocytes including human. Am. J. Physiol. Heart Circ. Physiol. 301, H1996–H2005. doi: 10.1152/ajpheart.00284.2011
Roderick, H. L., and Bootman, M. D. (2003). Bi-directional signaling from the InsP3 receptor: regulation by calcium and accessory factors. Biochem. Soc. Trans. 31, 950–953. doi: 10.1042/BST0310950
Saucerman, J., and Bers, D. M. (2008). Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes. Biophys. J. 95, 4597–4612. doi: 10.1529/biophysj.108.128728
Shannon, T., Wang, F., Puglisi, J., Weber, C. H. R., and Bers, D. B. (2004). A mathematical treatment of integrated Ca dynamic within the ventricular myocyte. Biophys. J. 87, 3351–3371. doi: 10.1529/biophysj.104.047449
Shkryl, V. M., Maxwell, J. T., and Blatter, L. A. (2012). A novel method for spatially complex diffraction-limited photoactivation and photobleaching in living cells. J. Physiol. 590, 1093–1100. doi: 10.1113/jphysiol.2011.223446
Shuai, J. W., and Jung, P. (2002). Stochastic properties of Ca2+ release of inositol 1,4,5-trisphosphate receptor clusters. Biophys. J. 83, 87–97. doi: 10.1016/S0006-3495(02)75151-5
Siekmann, I., Wagner, L. E. 2nd. Yule, D., Crampin, E. J., and Sneyd, J. (2012). A kinetic model for type I and II IP3R accounting for mode changes. Biophys. J. 103, 658–668. doi: 10.1016/j.bpj.2012.07.016
Signore, S., Sorrentino, A., Ferreira-Martins, J., Kannappan, R., Shafaie, M., Del Ben, F., et al. (2013). Inositol 1, 4, 5-trisphosphate receptors and human left ventricular myocytes. Circulation 128, 1286–1297. doi: 10.1161/CIRCULATIONAHA.113.002764
Smith, I. F., Wiltgen, S. M., and Parker, I. (2009). Localization of puff sites adjacent to the plasma membrane: functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium 45, 65–76. doi: 10.1016/j.ceca.2008.06.001
Sneyd, J., and Dufour, J. F. (2002). A dynamic model of the type-2 inositol triphosphate receptor. Proc. Natl. Acad. Sci. U.S.A. 99, 2398–2403. doi: 10.1073/pnas.032281999
Sobie, E. A., and Lederer, W. J. (2012). Dynamic local changes in sarcoplasmic reticulum calcium: physiological and pathophysiological roles. J. Mol. Cell. Cardiol. 52, 304–311. doi: 10.1016/j.yjmcc.2011.06.024
Soltis, A. R., and Saucerman, J. (2010). Synergy between CaMKII substrates and β-adrenergic signaling in regulation of cardiac myocyte Ca2+ handling. Biophys. J. 99, 2038–2047. doi: 10.1016/j.bpj.2010.08.016
Swillens, S., Champeil, P., Combettes, L., and Dupont, G. (1998). Stochastic simulation of a single inositol 1,4,5-trisphosphate-sensitive Ca2+ channel reveals repetitive openings during ‘blip-like’ Ca2+ transients. Cell Calcium 23, 291–302. doi: 10.1016/S0143-4160(98)90025-2
Tadevosyan, A., Vaniotis, G., Allen, B. G., Hebert, T. E., and Nattel, S. (2012). G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J. Physiol. 590, 1313–1330. doi: 10.1113/jphysiol.2011.222794
Takahashi, A., Camacho, P., Lechleiter, J. D., and Herman, B. (1999). Measurement of intracellular calcium. Physiol. Rev. 79, 1089–1125.
Thul, R., and Falcke, M. (2004). Release currents of IP3 receptor channel clusters and concentration profiles. Biophys. J. 86, 2660–2673. doi: 10.1016/S0006-3495(04)74322-2
Vaniotis, G., Allen, B. G., and Hebert, T. E. (2011). Nuclear GPCRs in cardiomyocytes: an insider's view of beta-adrenergic receptor signaling. Am. J. Physiol. Heart Circ. Physiol. 301, H1754–H1764. doi: 10.1152/ajpheart.00657.2011
Vogelsand, M., Broede-Sitz, A., Schafer, E., Zerkowski, H. R., and Brodde, O. E. (1994). Endothelin ETA-receptors couple to inositol phosphate formation and inhibition of adenylate cyclase in human right atrium. J. Cardiovasc. Pharmacol. 23, 344–347. doi: 10.1097/00005344-199402000-00025
Wang, H., and Clapham, D. E. (1999). Conformational changes of the in situ nuclear pore complex. Biophys. J. 77, 241–247. doi: 10.1016/S0006-3495(99)76885-2
Watanabe, T., and Endoh, M. (1999). Characterization of the endothelin-1 induced regulation of L-type Ca2+ current in rabbit ventricular myocytes. Naunyn Schmiedebergs Arch. Pharmacol. 360, 654–664. doi: 10.1007/s002109900130
Wilhelms, M., Hettmann, H., Maleckar, M. M., Koivumäki, J. T., Dössel, O., and Seemann, G. (2013). Benchmarking electrophysiological models of human atrial myocytes. Front. Physiol. 3:487. doi: 10.3389/fphys.2012.00487
Woo, S. H., and Lee, C. (1999). Effects of endothelin-1 on Ca2+ signaling in guinea-pig ventricular myocytes: role of protein kinase C. J. Mol. Cell. Cardiol. 31, 613–643. doi: 10.1006/jmcc.1998.0899
Woodcock, E. A., and Matkovich, S. J. (2005). Ins(1,4,5)P3 receptors and inositol phosphates in the heart-evolutionary artefacts or active signal transducers? Pharmacol. Ther. 107, 240–251. doi: 10.1016/j.pharmthera.2005.04.002
Wu, G., Xie, X., Lu, Z. H., and Ledeen, R. W. (2009). Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 106, 10829–10834. doi: 10.1073/pnas.0903408106
Wu, X., Zhang, T., Bossuyt, J., Li, X., McKinsey, T. A., Dedman, J. R., et al. (2006). Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J. Clin. Invest. 116, 657–682. doi: 10.1172/JCI27374
Yang, H. T., Sakurai, K., Sugawara, H., Watanabe, T., Norota, I., and Endoh, M. (1999). Role of Na+/Ca2+ exchange in endothelin-1-induced increases in Ca2+ transient and contractility in rabbit ventricular myocytes: pharmacological analysis with KB-R7943. Br. J. Pharmacol. 126, 1785–1795. doi: 10.1038/sj.bjp.0702454
Youm, J. B., Kim, N., Han, J., Kim, E., Joo, H., Leem, C. H., et al. (2006). A mathematical model of pacemaker activity recorded from mouse small intestine. Philos. Trans. A Math. Phys. Eng. Sci. 364, 1135–1154. doi: 10.1098/rsta.2006.1759
Zhang, Y. H., James, A. F., and Hancox, J. C. (2001). Regulation by endothelin-1 of Na+-Ca2+ exchange current (INaCa) from guinea-pig isolated ventricular myocytes. Cell Calcium 30, 351–360. doi: 10.1054/ceca.2001.0244
Zima, A. V., Bare, D. J., Mignery, G. A., and Blatter, L. A. (2007). IP3-dependent nuclear Ca2+ signaling in the mammalian heart. J. Physiol. 584, 601–611. doi: 10.1113/jphysiol.2007.140731
Keywords: Ca2+, IP3, excitation-contraction coupling, excitation-transcription coupling, cardiomyocyte
Citation: Hohendanner F, McCulloch AD, Blatter LA and Michailova AP (2014) Calcium and IP3 dynamics in cardiac myocytes: experimental and computational perspectives and approaches. Front. Pharmacol. 5:35. doi: 10.3389/fphar.2014.00035
Received: 18 December 2013; Accepted: 18 February 2014;
Published online: 06 March 2014.
Edited by:
Donald M. Bers, University of California, Davis, USAReviewed by:
Baruch Minke, The Hebrew University, IsraelClemens Möller, Albstadt-Sigmaringen University, Germany
Copyright © 2014 Hohendanner, McCulloch, Blatter and Michailova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anushka P. Michailova, Department of Bioengineering, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0412, USA e-mail: amihaylova@eng.ucsd.edu
 Felix Hohendanner
Felix Hohendanner Andrew D. McCulloch
Andrew D. McCulloch Lothar A. Blatter
Lothar A. Blatter Anushka P. Michailova
Anushka P. Michailova