
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 20 May 2015
Sec. Experimental Pharmacology and Drug Discovery
Volume 6 - 2015 | https://doi.org/10.3389/fphar.2015.00093
This article is part of the Research Topic Cocoa as a medicine; The Present and Future of Cocoa Research View all 8 articles
 Laura A. Massee1
Laura A. Massee1 Karin Ried2
Karin Ried2 Matthew Pase1,3
Matthew Pase1,3 Nikolaj Travica2
Nikolaj Travica2 Jaesshanth Yoganathan1
Jaesshanth Yoganathan1 Andrew Scholey1
Andrew Scholey1 Helen Macpherson1
Helen Macpherson1 Greg Kennedy1
Greg Kennedy1 Avni Sali2
Avni Sali2 Andrew Pipingas1*
Andrew Pipingas1*Cocoa supplementation has been associated with benefits to cardiovascular health. However, cocoa's effects on cognition are less clear. A randomized, placebo-controlled, double-blind clinical trial (n = 40, age M = 24.13 years, SD = 4.47 years) was conducted to investigate the effects of both acute (same-day) and sub-chronic (daily for four-weeks) 250 mg cocoa supplementation on mood and mental fatigue, cognitive performance and cardiovascular functioning in young, healthy adults. Assessment involved repeated 10-min cycles of the Cognitive Demand Battery (CDB) encompassing two serial subtraction tasks (Serial Threes and Sevens), a Rapid Visual Information Processing task, and a mental fatigue scale over the course of half an hour. The Swinburne University Computerized Cognitive Assessment Battery (SUCCAB) was also completed to evaluate cognition. Cardiovascular function included measuring both peripheral and central blood pressure and cerebral blood flow. At the acute time point, consumption of cocoa significantly improved self-reported mental fatigue and performance on the Serial Sevens task in cycle one of the CDB. No other significant effects were found. This trial was registered with the Australian and New Zealand Clinical Trial Registry (Trial ID: ACTRN12613000626763). Accessible via http://www.anzctr.org.au/TrialSearch.aspx?searchTxt=ACTRN12613000626763&ddlSearch=Registered.
In recent years, there has been increasing attention given to the potential cognitive enhancing benefits of natural interventions, specifically foods containing flavonoids, the largest subclass of polyphenol antioxidants. Flavonoids and flavanols are abundant in the human diet, with popular dietary sources including tea, fruits, berries and cocoa (Bravo, 1998). The consumption of flavanol rich foods has been shown to result in neurobiological effects, improving aspects of learning, memory, and overall cognitive performance (Letenneur et al., 2007; Macready et al., 2009; Nurk et al., 2009; Williams and Spencer, 2012). In particular, cocoa and cocoa-containing products comprise many different forms of beneficial flavonoids (Gu et al., 2004) and as such, there has been increasing research aimed at uncovering the cognitive enhancing potential of high-flavanol cocoa.
It is important to differentiate between the terms cacao, cocoa and chocolate. The raw seeds obtained from the Theobroma cacao tree are referred to as cacao, however once these seeds are processed by way of grinding or roasting the name changes to cocoa (Latif, 2013). Chocolate confectionary involves further processing and the addition of multiple other ingredients including sugar and fat, resulting in a solid edible product (Cooper et al., 2008). The seeds of the T. cacao tree are rich in a subclass of polyphenol antioxidants known as flavonoids, specifically catechin and epicatechin, as well as polymeric units which comprise both catechin and epicatechin subunits, which are collectively referred to as proanthocyanidins (or procyanidins) (Steinberg et al., 2003; Nehlig, 2013). Cacao seeds have been used medicinally for centuries. Writings of the Mesoamerican civilizations describe the use of a beverage made from cacao seeds for the treatment of many ailments (Katz et al., 2011; Lippi, 2013). Cacao seeds were brought to Europe by Spanish explorers (Lippi, 2009), where cocoa was recognized for its health benefits until the 20th century when modern day manufacturing enabled the production of chocolate confectionary to begin. Since then, chocolate has been perceived as lacking nutritional value due to additional components such as fats and sugar, however, if certain controlled processing standards are implemented, substantial amounts of antioxidants from the original cacao seed can be retained in some modern day chocolates (Steinberg et al., 2003).
The human digestive system is able to readily absorb the epicatechin component of flavanol-rich cocoa, with blood plasma concentrations peaking at 2–3 h following consumption (Nehlig, 2013). Epicatechin has been shown to cross the blood brain barrier in animal studies (Abd El Mohsen et al., 2002), suggesting that flavonoids from the cacao bean have the ability to act directly on the brain, which could potentially lead to cognitive enhancement (Nehlig, 2013). Additional evidence from animal studies on flavanols in fruits such as grapes and berries found that flavanol consumption boosts memory and learning (Shukitt-Hale et al., 2006, 2008; Joseph et al., 2007) by acting directly on numerous receptors, kinases and transcription factors (Spencer, 2007, 2009). As such, flavanols in cocoa may also have the potential to act directly on the human brain and improve aspects of cognitive performance through direct enhancement of memory systems, just as the flavanols in fruit have been shown to do.
Cocoa may not only exert direct effects on the brain, but could potentially act indirectly through effects to other bodily systems. It has long been known that cocoa has beneficial effects on the cardiovascular system, with initial evidence coming from observational studies on the Kuna Indians, residing off the coast of Panama. The Kuna are one of few remaining tribes who are protected from vascular ailments, such as arterial hypertension (Corti et al., 2009). It has been suggested that this is due to the prominent role cocoa plays in the Kuna diet (Galleano et al., 2009; Hollenberg et al., 2009), where they consume the equivalent of five cups of liquid cocoa, or approximately 900 mg cocoa flavanols, per day (Pucciarelli, 2013). Research demonstrated that when members of the Kuna relocated to Panama, changing their culture and diet (in particular a reduced dietary intake of flavanol-rich cocoa), significant increases in blood pressure were subsequently observed (Galleano et al., 2009; Fraga et al., 2011). Clinical investigations have revealed that supplementation with flavanol-rich cocoa reliably improves a variety of cardiovascular variables, including peripheral blood pressure in pre-hypertensive and hypertensive patients (Taubert et al., 2007; Hooper et al., 2008; Ried et al., 2010), as well as in normotensive populations (Ried et al., 2012). Changes to cerebral blood flow (CBF) (Fisher et al., 2006; Sorond et al., 2008) and flow-mediated dilation (Faridi et al., 2008; Grassi et al., 2008; Monahan et al., 2011; Njike et al., 2011) have been observed following cocoa supplementation. Given the strong links between vascular health and cognitive function (Dinges, 2006), it is possible that cocoa may improve cognitive performance indirectly through improvements to CBF and vascular health.
Randomized controlled trials have investigated the possibility that cognitive function may be improved following cocoa consumption. A short term neuroimaging investigation in young, healthy, female participants revealed that acute administration of a high flavanol cocoa beverage (172 mg/day for 5 days) enhanced the Blood Oxygen Level Dependent (BOLD) signal following completion of a cognitive switching task (Francis et al., 2006). Also using an acute intervention, Field et al. (2011) found that 2 h after cocoa-flavanol supplementation, young participants showed enhanced performance on the accuracy component of a spatial working memory task, as well as performance improvements on a choice reaction time task. Cognitive performance has been enhanced following cocoa beverage supplementation in elderly people with mild cognitive impairment (Desideri et al., 2012). In contrast, some studies have found no such cognitive effects, for instance, Pase et al. (2013) found no changes to cognitive performance acutely or sub-chronically for either a 250 or 500 mg cocoa flavanol dose in healthy participants (40–65 years). Crews et al. (2008) found no cognitive effects in a healthy, older sample (>60 years) following a 6 week intervention with a cocoa beverage (397.30 mg flavanols) and bar (357.41 mg flavanols). More recently, however, Scholey et al. (2010) found acute improvements in cognitive performance following a 520 mg cocoa flavanol dose, in response to a high level of cognitive demand. In this same study, cocoa flavanols also reduced self-reported mental fatigue. Both Pase et al. (2013) and Crews et al. (2008) did not implement a mentally demanding task, suggesting that cocoa may only improve cognitive performance in healthy populations when participants are subjected to sustained effortful processing.
The aim of the current randomized, controlled, double-blind clinical trial was to assess both the acute (2 h post dose), and sub-chronic (30 day post dose) effects of cocoa supplementation on mood and mental fatigue, cognitive performance and cardiovascular function in a young, healthy sample. Unlike previous clinical trials investigating cocoa and cognition, the current trial provided participants with either a visually identical cocoa or true placebo tablet without flavanols. By providing the supplement in tablet form, participant expectation is removed as they cannot visually decipher which treatment is the placebo and which is active, compared to other trials which used dark and white chocolate supplements (Field et al., 2011). Additionally, by making use of a tablet, other potentially active ingredients like sugars and fats can be excluded in order to make sure that any resulting effects can reliably be attributed to cocoa flavanols, and not to any of the other components found in commercial chocolate or other styles of intervention such as chocolate drinks (Francis et al., 2006; Crews et al., 2008; Scholey et al., 2010; Desideri et al., 2012; Pase et al., 2013). Participants were randomly allocated to receive tablets containing either 250 or 0 mg (placebo) cocoa-flavanols. It was hypothesized that, compared to placebo, cocoa would improve cognitive performance and mood.
Young, healthy participants aged 18–40 years (M = 24.13, SD = 4.47), residing in Melbourne, Australia, were recruited through magazine and social media advertisements, phone calls, and emails. Potential participants expressing interest in the study were screened for the following inclusion criteria: not currently suffering medically diagnosed cardiovascular or cognitive impairment, bleeding disorders, or gastrointestinal disorders; no clinically significant pulmonary, cardiovascular, psychiatric, or neurological conditions in the past 12 months; not taking any illicit drugs, cognitive enhancing medication, or herbal supplements; not pregnant or lactating; not color blind; not taking antidepressants, antipsychotics, anxiolytics, or anticoagulants; hold a good working knowledge of English language. Of the 40 recruited participants, 38 returned for sub-chronic assessment (see Figure 1).
The current trial investigated the effects of cocoa supplementation on cognitive and cardiovascular functioning using a randomized, placebo-controlled, double-blind, parallel design over a four-week period. Participants were randomly allocated to receive one of two daily treatments:
(1) Active cocoa tablet (3058 mg T. cacao seed extract standardized to contain 250 mg catechin polyphenols and 5.56 mg caffeine).
(2) Placebo tablet (Identical in appearance, size, texture and color to cocoa tablet, containing inert cellulose powder).
The active and matching placebo supplements used in this clinical trial were provided by Swisse Wellness Pty. Ltd. (Melbourne, Australia). Participants were required to take one active (or matching placebo) tablet daily for 4 weeks. The tablets were presented in visually identical bottles, differing only in the labels, which depicted the participant ID number as well as a code relating to the relevant group allocation. The placebo tablets were identical to the active tablets, and blinding success of participants was evaluated at the conclusion of the study using a questionnaire. Participants were randomly assigned to receive either active or placebo tablets using a computer generated permuted block randomization schedule. Randomization was performed by an independent statistical consultant that had no involvement in the trial, and supplement bottles were labeled according to the randomization schedule. The blinding code was only revealed after analysis of the main study outcomes.
The present study investigated the effect of cocoa supplementation on mood, cognitive and cardiovascular function between baseline and acute (same day, 2 h after ingestion of trial tablets), and baseline and sub-chronic (daily for 4 weeks) testing sessions. The primary outcomes were cognitive performance, measured using the Swinburne University Computerized Cognitive Assessment Battery (SUCCAB) (Pipingas et al., 2010), mood, mental fatigue and stress, using the Cognitive Demand Battery (CDB). Secondary outcomes were cardiovascular markers, including blood pressure and cerebral blood flow.
Participants completed eight computer-based tasks from the SUCCAB to assess various aspects of cognitive performance (Pipingas et al., 2010). A 4-button response box was used to complete the tasks, with each button representing a color (red, blue, green or yellow), “yes” or “no,” or the spatial location of objects on the screen (top, bottom, left or right).
Instructions were presented at the beginning of each of the tasks, which were read aloud to participants by the experimenter. All tasks except the delayed recall task were preceded by a practice trial where participants could experience an example of the task and ask any questions. Each of the eight tasks was scored by calculating the average reaction time and per cent accuracy, and are described below in further detail.
(1) Simple reaction time: A single white square appeared in the center of the computer screen 30 times at randomized intervals. The “yes” button was required to be pressed as soon as the square stimulus was visible to participants.
(2) Choice reaction time: Either a blue triangle or red square appeared in the center of the computer screen with the order and timing of the stimuli being randomized. Responses were recorded by the pressing of either the blue or red buttons corresponding with the stimuli's color. This task assessed participants' visual perception decision time.
(3) Immediate recognition: Forty successive images were presented in the center of the computer screen. After the viewing period, participants were required to respond a second series of successive images with either “yes” or “no” buttons, signaling whether or not they had just seen a given image during the earlier viewing period. Half of these images in the second series were from the original 40, and half were novel stimuli. This task was a non-verbal assessment of recognition memory.
(4) Congruent Stroop color word: Randomized alternating trials of stimulus words (red, yellow, blue, or green) in congruent colors appeared on the computer screen (I.E. the word blue would be presented in blue ink). To respond, the color button that corresponded to the color of the word, not the physical presentation of the word itself needed to be pressed. This task assessed participants' executive functioning and inhibition, as they had to ignore the reflex of reading the word and focus instead on the color it was presented in.
(5) Incongruent Stroop color word: Conducted in the same way as congruent Stroop color word, except that the stimulus words (red, yellow, blue, or green) appeared in incongruent colors on the computer screen. (I.E. the word blue could be presented in red, green or yellow ink, but not blue ink). Participants' skills in executive functioning and inhibition are more vigorously tested in these incongruent trials.
(6) Spatial working memory: A 4 × 4 white grid was displayed against a black background, with six of the grid positions filled with solid white squares. The aim was to remember where these squares were located during a brief viewing period. The grid then became blank, and a series of four white squares were presented one at a time in varying grid locations. Participants responded with either the “yes” or “no” buttons indicating if the new squares were presented in the same locations as the original set. This task assessed participants' ability to hold spatial information in working memory.
(7) Contextual memory: A series of 20 everyday pictures that were positioned at either the top, bottom, left, or right of the computer screen were shown against a black background. After the viewing period, the same images were randomly presented in the center of the computer screen. Participants responded using either the top, bottom, left, or right buttons to indicate the image's original location on the screen. This task assessed participants' episodic memory capabilities, as they needed to recall the spatial location of the original stimuli.
(8) Delayed recognition: The immediate recognition task described earlier was repeated at the end of the testing battery without a practice trial. Participants were shown the remaining original 20 images and another 20 novel images, and were again required to respond “yes” or “no” as to whether or not they had viewed them earlier. As in the immediate recognition task, this also assessed recognition memory.
The CDB is used to evaluate cognitive function by placing participants under mental load. Moreover, this battery is designed to assess mental fatigue (and mood) resulting from the ongoing mental load. This battery involves the completion of two serial subtraction tasks (serial threes and serial sevens), the Bakan Rapid Visual Information Processing Task (RVIP) and a mental fatigue visual analog scale (Kennedy and Scholey, 2004). Research has shown that while completing this battery, participants' task performance gradually declines whilst they report feeling more mentally fatigued. Many studies looking at the cognitive effects of various dietary interventions have shown that this decline can be somewhat mitigated by supplementation. Figure 2 displays the structure of the CDB. There are three main components to the CDB (Serial Threes, Sevens and RVIP), which are both preceded and followed by a mental fatigue assessment.
(1) Mental fatigue scales: Initially, participants are allocated 1 min to rate their current state of mental fatigue and stress, by marking a point on a computerized 100 mm Visual Analog Scale (VAS), with the left hand end of the scale labeled “not at all,” and the right hand end of the scale labeled “very much so.”
(2) Serial Threes subtraction task: Participants are presented with a given number ranging between 800 and 999, and are required to use the number keys on the keyboard to count backwards by threes from this number as quickly and as accurately as possible. The original starting number is cleared from the screen upon entry of participants' first response. Two minutes are allocated for this task and participants are scored based on the number of correct responses, errors and speed of response.
(3) Serial Sevens subtraction task: This task is identical in format to the Serial Threes subtraction task, however it required participants to count backwards by sevens. Two minutes were also allocated for this task.
(4) Rapid Visual Information Processing Task (RVIP): Participants are required to observe a continuous presentation of single digits for targets of any three consecutive odd or even digits. One hundred digits were presented each minute and participants responded to the detection of targets by pressing the spacebar as quickly as possible. This task runs for 5 min and is scored based on the number of correctly detected targets, number of errors and number of false alarms.
(5) Mental fatigue scales: Participants were again required to rate their current subjective mental fatigue and stress on the same 100 mm VAS used at the beginning of the CDB.
The duration for one cycle of the CDB is 10 min. Participants in the present study completed three cycles, resulting in a total duration of 30 min.
Blood pressure was assessed in a quiet, dedicated university laboratory following a five-minute rest period completed by participants in the supine position on an examination bed. The non-invasive SphygmoCor XCEL (AtCor Medical, Sydney, Australia) device and software was used to attain participants' peripheral and central aortic blood pressures, by applying the cuff to participants' right arm, over the brachial artery. The SphygmoCor XCEL takes three measurements of brachial blood pressure for each participant, each separated by a thirty second delay. The first measurement is discarded, while the second and third are averaged to provide a more accurate final reading. The SphygmoCor XCEL provides a valid estimation of central aortic blood pressure using the brachial cuff volume displacement waveform method (Butlin et al., 2012).
Also using a dedicated university laboratory, a non-invasive transcranial Doppler (Compumedics DWL, CA, USA) with a 4-MHz probe was used to record cerebral blood flow velocity in the common carotid artery (CCA). Experimenters recorded the flow through the CCA for approximately 2 min in order to calculate the resting mean CCA blood flow velocity in centimeters per second.
Participants completed a total of three testing sessions; baseline and acute assessment which took place on the same day (2–3.5 h after tablet ingestion), and sub-chronic assessment which was completed four-weeks after the initial testing date. Participants consumed their allocated treatment daily for 30 days. Participants were instructed to abstain from caffeinated products from the night prior to each testing session, as well as abstaining from consuming any food or drink with the exception of water for the duration of each of the testing days. The current trial implemented procedures, which were conducted in accordance with the Declaration of Helsinki (as revised in 2004). The Swinburne University Human Research Ethics Committee approved the trial, and written informed consent was obtained from all participants prior to the commencement of testing.
The first testing day included both baseline and acute assessment and ran for approximately 5 h, while the follow-up testing day involved sub-chronic assessment, which required approximately one and a half hours. All three testing sessions are outlined in Figure 3.
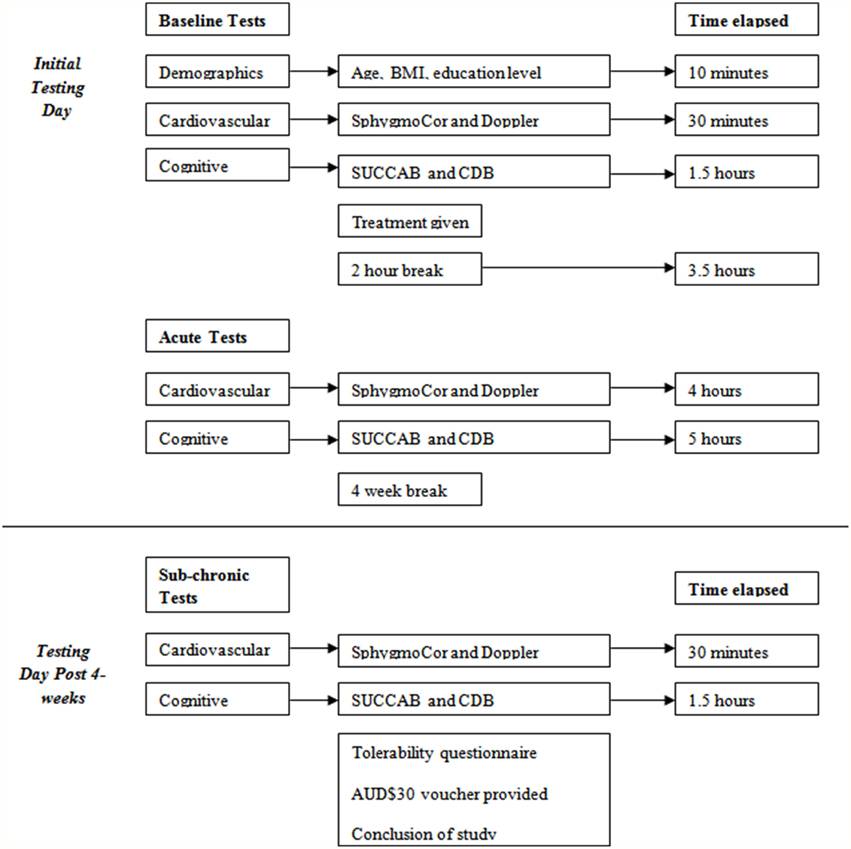
Figure 3. Testing day structure. BMI, Body Mass Index; SUCCAB, Swinburne University Computerized Cognitive Assessment Battery; CDB, Cognitive Demand Battery.
At the completion of the acute session, an appointment time for the post four-week testing session was scheduled, planned for the same time of day as the first testing session. Additionally, participants were instructed to take their final supplement tablet the day prior to the scheduled final testing session.
The follow up sub-chronic testing session was conducted exactly 4 weeks after the initial session. The session was conducted in a way synonymous to the baseline session. Participants were reminded to not consume any supplement tablets on the day of sub-chronic testing. This way, experimenters could be certain that they were measuring the sub-chronic, and not acute effects of cocoa. At the completion of the final testing session participants completed a questionnaire that evaluated their tolerability to the supplement over the four-week period, as well as the blinding success of group allocation. Participants were then reimbursed for their time and travel expenses with a $30 voucher.
A power-analysis was performed based on effect sizes obtained from Scholey et al. (2010)'s study. For mental fatigue, Scholey et al. (2010) reported effect sizes ranging from 0.33 to 0.42. To be 95% confident in finding a significant effect (d = 0.33) of cocoa supplementation on mental fatigue, a sample size of 26 participants (13 per group) was deemed sufficient. For cognitive performance Scholey et al. (2010) reported effect sizes ranging from 0.30 to 0.53. For this measure, a sample size of 32 participants (16 per group) was considered sufficient to be 95% confident in detecting a significant effect (d = 0.30). It was decided that a sample of 40 participants (20 per group) would allow for a 20% attrition rate, while also being powerful enough to have 95% confidence in detecting significant effects.
SPSS version 21 (IBM, New York, NY, USA) was used to compile and analyse all data. The data was first screened for any potential outliers, which were subsequently removed. Analyses of co-variance (ANCOVAs) were used to examine potentially significant differences between groups at the both the two-hour acute, and four-week sub-chronic time points, while taking baseline measures into account as a covariate. Additionally, post hoc independent samples t-tests were used to compare the characteristics of the treatment groups at baseline, while post hoc paired samples t-tests were used to compare any significant differences found when comparing baseline to acute or to sub-chronic assessment time points. All results were considered significant at p < 0.05. All p-values are calculated two-tailed.
Table 1 displays the means and standard deviations for the two groups across baseline demographic variables. ANCOVAs revealed no significant between-group differences at baseline. Also noteworthy is that the sample had normal blood pressure levels at baseline.
No significant between-group differences were found for accuracy or reaction time in any SUCCAB task at the acute time point when co-varying for baseline data (Table 2).
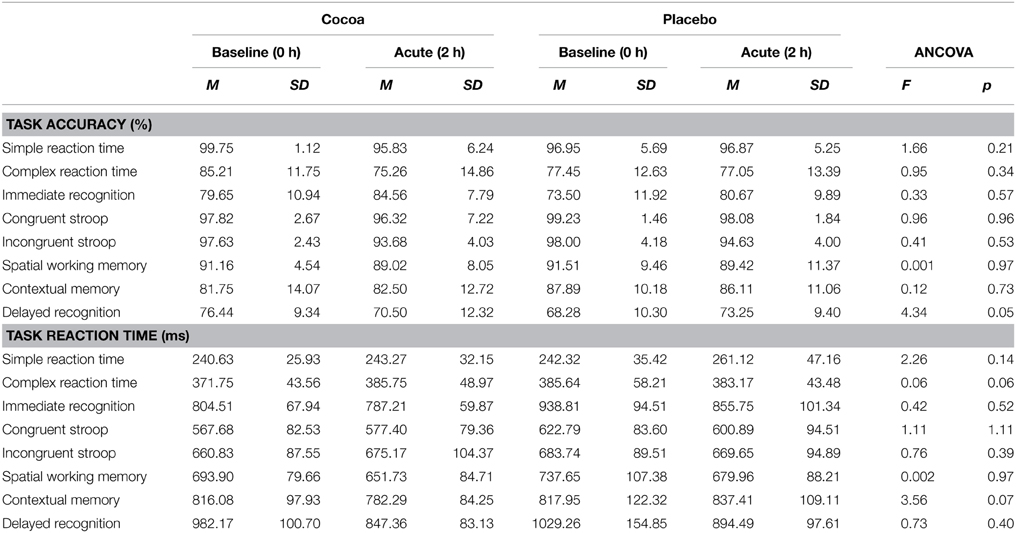
Table 2. Acute effects of cocoa supplementation on cognition: SUCCAB task accuracy and reaction time.
Prior to commencing the CDB, participants receiving cocoa supplementation reported feeling significantly less mentally fatigued at the beginning of the battery, compared to participants receiving placebo supplementation (Table 3).
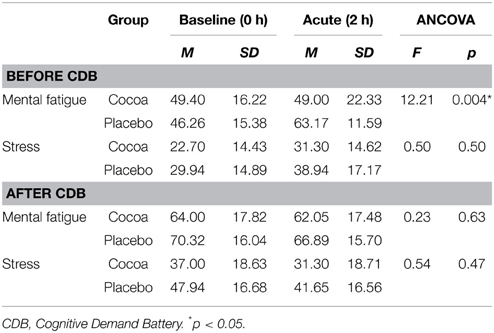
Table 3. Acute effects of cocoa supplementation on self-reported mental fatigue and stress, before and after the cognitive demand battery.
Figure 4 displays participants responses to the question “How mentally fatigued do you feel right now,” which were found to be significantly different at the acute-pre CDB time point in the ANCOVA analysis presented in Table 3. Post hoc independent samples t-tests revealed that while the treatment groups were not significantly different at baseline [t(37) = 0.62, p = 0.54], participants receiving cocoa reported being significantly less mentally fatigued (M = 49.00, SD = 22.33) than participants receiving placebo supplementation (M = 63.17, SD = 11.59) at the acute pre-CDB time point [t(29.16) = −2.49, p = 0.02]. While the groups were not significantly different in the ANCOVA analysis at the acute post-CDB time point, Figure 4 shows that both groups did report being more mentally fatigued after completing the acute CDB compared to previous time points, with participants in the placebo group reporting feeling more mentally fatigued than participants receiving cocoa.
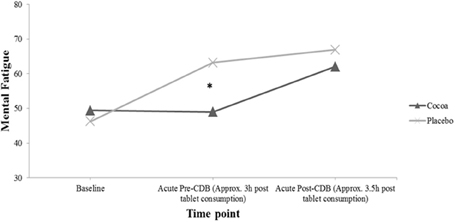
Figure 4. Participants average response to the question “how mentally fatigued do you feel right now” both before, and after acute tablet consumption. (For mental fatigue, responses to the question range from 1 = “not at all” to 100 = “very much so”). Significant differences compared to placebo are indicated (*p < 0.05). CDB, Cognitive Demand Battery.
Significant between-group differences were found for the Serial Sevens task at the acute time point when co-varying for baseline, with participants receiving cocoa providing significantly more correct answers than those in receiving placebo. No significant between-group differences were observed during cycles two and three of the CDB (Table 4).
No significant effects were found for any of the cardiovascular measures at the acute time point when co-varying for baseline data (Table 5).
No significant between-group differences were found for accuracy or reaction time in any SUCCAB task at the sub-chronic time point when co-varying for baseline data (Table 6).
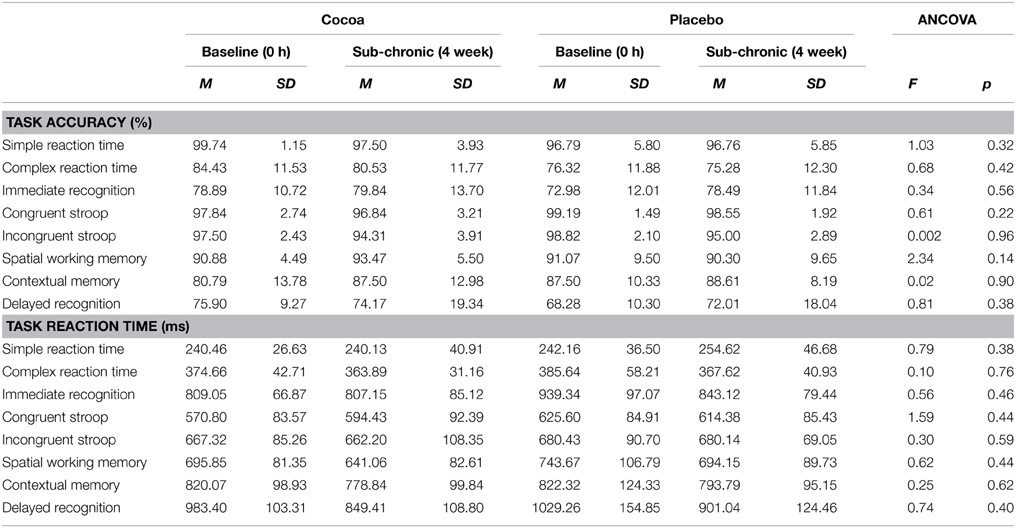
Table 6. Sub-chronic effects of cocoa supplementation on cognition: SUCCAB task accuracy and reaction time.
Looking at sub-chronic data after the third cycle of the CDB, participants receiving the placebo reported feeling significantly less stressed compared to those receiving cocoa (Table 7). Post-hoc independent samples t-tests revealed that the treatment groups were not significantly different at baseline [t(34) = −1.83, p = 0.08] or sub-chronic [t(34) = 0.54, p = 0.59] assessment. Further post hoc paired t-test analyses were subsequently completed to compare stress levels over time separately for each of the groups. Results revealed that while the participants receiving cocoa did not report any significant changes in subjective stress levels over time [t(17) = −0.88, p = 0.39], participants receiving placebo supplementation did report significantly lower levels of stress after completing the CDB at sub-chronic assessment relative to baseline [t(16) = 3.43, p = 0.003].
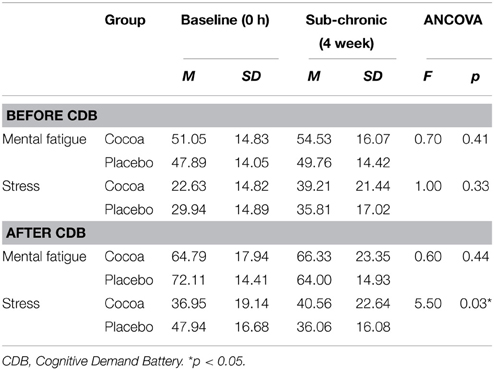
Table 7. Sub-chronic effects of cocoa supplementation on self-reported mental fatigue and stress, before and after the cognitive demand battery.
During the first, second and third cycles of the CBD, there were no significant between-group differences for any of the tasks at the sub-chronic time point when co-varying for baseline data (Table 8).
No significant effects were found for any of the cardiovascular measures at the sub-chronic time point when co-varying for baseline data (Table 9).
This randomized, placebo-controlled, double-blind clinical trial investigated both the acute and sub-chronic effects of cocoa supplementation on cognitive performance. Consumption of 250 mg cocoa flavanols improved performance acutely on the Serial component of the Cognitive Demand Battery (CDB) in cycle one only. Furthermore, acute cocoa supplementation significantly reduced participants' self-reported mental fatigue prior to commencing the CDB testing battery. Following completion of the CDB at sub-chronic assessment, participants receiving the placebo supplement reported feeling significantly less stressed compared to participants supplemented with cocoa. No significant effects were found for cognition measured with the SUCCAB or cardiovascular function.
The acute improvements found in cognitive performance and mental fatigue are in line with the acute findings of Scholey et al. (2010). However, the present improvements in cognitive performance were found during the Serial Sevens component rather than for Serial Threes. Using a 520 mg cocoa beverage intervention, Scholey et al. (2010) found that participants produced more correct responses during the Serial Threes task throughout each of the six cycles (lasting 1 h, in comparison to the present study's three cycles lasting half an hour), with the most significant improvement seen during the fourth cycle at just over 2 h post cocoa consumption. Significant effects were also found for a 940 mg beverage; however the effect was weaker, producing significant improvements for the first four cycles only.
We found CDB effects only in the first cycle, whereas Scholey et al. (2010) found effects throughout six cycles. In the current study, participants did not begin the CDB until 3 h after taking the cocoa supplement, whereas participants in Scholey et al.'s (2010) study began the CDB one and a half hours post-consumption. Participants in our study completed the SUCCAB testing battery first, as a way of mentally taxing participants, before later completing the CDB. As the epicatechin component of cocoa flavanols peaks in concentration in human blood plasma at 2–3 h post consumption (Francis et al., 2006; Nehlig, 2013), it is possible that by completing the SUCCAB first, the CDB was pushed into a time frame where there were diminished levels of epicatechin. Additionally, as Scholey et al. (2010) used a much higher dose than was investigated in the current study, it is possible that participants in the current study had insufficient levels of epicatechin during the testing period.
At the acute time point, participants supplemented with cocoa reported feeling significantly less mentally fatigued prior to completing the CDB, than those receiving the placebo supplement. As the CDB was the final task in the testing sequence and was undertaken four and a half hours after the commencement of the experimental session, it is likely that participants may have been feeling fatigued at this point. It appears that at the time that mental fatigue was assessed, at 3 h post consumption, cocoa flavanols significantly attenuated this fatigue. While this effect was not carried through to the fatigue assessment after the CDB was completed, it is possible that this too relates to the fact that epicatechin levels were likely to have declined well past their peak effect by the end of the CDB (Francis et al., 2006; Nehlig, 2013),
Similar to results found by Crews et al. (2008), supplementation with cocoa flavanols did not produce any cognitive sub-chronic effects, with no significant changes found to performance on any of the CDB tasks or the SUCCAB test battery. Additionally, no significant changes to participants' self-reported mental fatigue were observed. The lack of sub-chronic effects on cognitive performance is also consistent with the findings of Pase et al.'s (2013). Interestingly, following completion of the CDB, participants that received placebo supplementation sub-chronically, reported feeling significantly less stressed than their cocoa counterparts. This result is unexpected, and in the absence of other sub-chronic effects, it may simply be a chance finding. It is noteworthy that participants had been instructed to abstain from the daily trial supplement on the morning of the sub-chronic testing (1 month follow up). In this way, any potential acute effects were eliminated. Therefore, our conclusions regarding the lack of sub-chronic effects are further bolstered by this aspect of the experimental design. However, this does not eliminate the possibility that there may be positive benefits with a longer cumulative supplementation period. Future studies should also consider the potential effects of acute, chronic and acute on chronic effects.
The exact mechanism behind the action of cocoa flavanols is yet to be conclusively determined. One study suggested that improvements in vascular health may partly underpin improvements in cognition following cocoa supplementation in adults who are in poor health (Desideri et al., 2012). However, in young healthy adults, increases in cerebral blood flow have been reported in the absence of significant behavioral effects (Francis et al., 2006). Although the present study did not find any effects on blood flow velocity through the common carotid artery using Doppler, this measure may be relatively insensitive, as compared to the more costly neuroimaging methods utilized by Francis et al. It is also possible that the 250 mg dose of cocoa flavanols used in this study was insufficient to influence cardiovascular and blood flow changes.
The present study had a number of limitations. Despite participants completing a practice run for each SUCCAB sub-test during testing sessions, there was not a separate testing day devoted to practice testing prior to the assessment of baseline performance for all tasks. As such, the possibility of practice effects occurring in this sample cannot be eliminated. Larger studies making use of a practice testing day are recommended to confirm the findings presented here. Additionally, the duration of this trial was only 30 days. The possibility that longer intervention periods may result in different effects cannot be ruled out. Future studies may also benefit from investigating the effects of cocoa supplementation using a higher dose, rather than the 250 mg dose used here. It is possible that the lack of cardiovascular effects, and subsequent lack of cognitive effects seen here may be a result of too low a dose of cocoa flavanols. A study by Brickman et al. (2014) for example, established a chronic, 3 month intervention using a diet containing either high (900 mg) or low (10 mg) cocoa flavanols in healthy, older participants aged 50–69 years. It was found that in the high flavanol diet group, both dentate gyrus function and cognitive performance on the ModBent task were enhanced. It is possible that these findings were a result of the higher dose used (900 mg compared to 250 mg in the present study). It should also be noted that the cocoa supplement used in the current study included 5.56 mg of caffeine, whereas the placebo did not. Although the effects of such a small dose of caffeine on cognition are unclear, future research could match the placebo for caffeine content. Furthermore, the young age and size of the present cohort may also have contributed to our lack of cognitive and vascular findings.
In conclusion, a 250 mg dose of cocoa flavanols was found to attenuate mental fatigue and improve minor aspects of cognitive performance acutely but not sub-chronically during a highly demanding task. Future research should assess a higher dose of cocoa as well as cognitive, mood and cardiovascular effects in an older cohort.
Sponsors were not involved in study design, data collection, analysis, interpretation and writing of the article. Swisse Wellness Pty Ltd. provided cocoa and placebo tablets for the study, however there was no commercial interest in the study. Any affiliations of authors with sponsors of the study are independent of the research project. All authors declare no conflicts of interest. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank The University of Adelaide, Discipline of General Practice, for funding of the participant vouchers, the National Institute of Integrative Medicine for funding of the Therapeutic Goods Association and Clinical Trial Notification application, and Swisse Wellness Pty. Ltd. for providing the cocoa and placebo tablets. Dr Pase is funded by an Australian National Health and Medical Research Council (NHMRC) Fellowship.
Abd El Mohsen, M. M., Kuhnle, G., Rechner, A. R., Schroeter, H., Rose, S., Jenner, P., et al. (2002). Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 33, 1693–1702. doi: 10.1016/S0891-5849(02)01137-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bravo, L. (1998). Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 56, 317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brickman, A. M., Khan, U. A., Provenzano, F. A., Yeung, L. K., Suzuki, W., Schroeter, H., et al. (2014). Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 17, 1798–1803. doi: 10.1038/nn.3850
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Butlin, M., Qasem, A., and Avolio, A. P. (2012). Estimation of central aortic pressure waveform features derived from the brachial cuff volume displacement waveform. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 2591–2594. doi: 10.1109/EMBC.2012.6346494
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cooper, K. A., Donovan, J. L., Waterhouse, A. I., and Williamson, G. (2008). Cocoa and health: a decade of research. Br. J. Nutr. 99, 1–11. doi: 10.1017/S0007114507795296
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Corti, R., Flammer, A. J., Hollenberg, N. K., and Luscher, T. F. (2009). Cocoa and cardiovascular health. Circulation 119, 1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Crews, W. D. Jr., Harrison, D. W., and Wright, J. W. (2008). A double-blind, placebo- controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: clinical findings from a sample of healthy, cognitively intact older adults. Am. J. Clin. Nutr. 87, 872–880.
Desideri, G., Kwik-Uribe, C., Grassi, D., Necozione, S., Ghiadoni, L., Mastroiacovo, D., et al. (2012). Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the cocoa, cognition, and aging (CoCoA) study. Hypertension 60, 794–801. doi: 10.1161/HYPERTENSIONAHA.112.193060
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dinges, D. F. (2006). Cocoa flavanols, cerebral blood flow, cognition, and health: going forward. J. Cardiovasc. Pharmacol. 47, S221–S223. doi: 10.1097/00005344-200606001-00019
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Faridi, Z., Njike, V. Y., Dutta, S., Ali, A., and Katz, D. L. (2008). Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. Am. J. Clin. Nutr. 88, 58–63.
Field, D. T., Williams, C. M., and Butler, L. T. (2011). Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol. Behav. 103, 255–260. doi: 10.1016/j.physbeh.2011.02.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fisher, N. D. L., Sorond, F. A., and Hollenberg, N. K. (2006). Cocoa flavanols and brain perfusion. J. Cardiovasc. Pharmacol. 47, S210–S214. doi: 10.1097/00005344-200606001-00017
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fraga, C. G., Litterio, M. C., Prince, P. D., Calabro, V., Piotrkowski, B., and Galleano, M. (2011). Cocoa flavanols: effects on vascular nitric oxide and blood pressure. J. Clin. Biochem. Nutr. 48, 63–67. doi: 10.3164/jcbn.11-010FR
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Francis, S. T., Head, K., Morris, P. G., and Macdonald, I. A. (2006). The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 47, S215–S220. doi: 10.1097/00005344-200606001-00018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Galleano, M., Oteiza, P. I., and Fraga, C. G. (2009). Cocoa, chocolate, and cardiovascular disease. J. Cardiovasc. Pharmacol. 54, 483–449. doi: 10.1097/FJC.0b013e3181b76787
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grassi, D., Desideri, G., Necozione, S., Lippi, C., Casale, R., Properzi, G., et al. (2008). Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 138, 1671–1676.
Gu, L., Kelm, M. A., Hammerstone, J. F., Beecher, G., Holden, J., Haytowitz, D., et al. (2004). Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 134, 613–617.
Hollenberg, N. K., Fisher, N. D. L., and McCullough, M. L. (2009). Flavanols, the Kuna, cocoa consumption, and nitric oxide. J. Am. Soc. Hypertens. 3, 105–112. doi: 10.1016/j.jash.2008.11.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hooper, L., Kroon, P. A., Rimm, E. B., Cohn, J. S., Harvey, I., Le Cornu, K. A., et al. (2008). Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 88, 38–50.
Joseph, J. A., Shukitt-Hale, B., and Lau, F. C. (2007). Fruit polyphenols and their effects on neuronal signalling and behavior in senescence. Annu. N.Y. Acad. Sci. 1100, 470–485. doi: 10.1196/annals.1395.052
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Katz, D. L., Doughty, K., and Ali, A. (2011). Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 15, 2779–2811. doi: 10.1089/ars.2010.3697
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kennedy, D. O., and Scholey, A. B. (2004). A glucose-caffeine ‘energy drink’ ameliorates subjective and performance deficits during prolonged cognitive demand. Appetite 42, 331–333. doi: 10.1016/j.appet.2004.03.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Letenneur, L., Proust-Lima, C., Le Gouge, A., Dartigues, J. F., and Barberger-Gateau, P. (2007). Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 16, 1364–1371. doi: 10.1093/aje/kwm036
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lippi, D. (2009). Chocolate and medicine: dangerous liasions? Nutrition 25, 1100–1113. doi: 10.1016/j.nut.2009.08.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lippi, D. (2013). Chocolate in history: food, medicine, medi-food. Nutrients 5, 1573–1584. doi: 10.3390/nu5051573
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Macready, A. L., Kennedy, O. B., Ellis, J. A., Williams, C. M., Spencer, J. P., and Butler, L. T. (2009). Flavonoids and cognitive function: a review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 4, 227–242. doi: 10.1007/s12263-009-0135-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Monahan, K. D., Feehan, R. P., Kunselman, A. R., Preston, A. G., Miller, D. L., and Lott, M. E. (2011). Dose-dependent increases in flow-mediated dilation following acute cocoa ingestion in healthy older adults. J. Appl. Physiol. 111, 1568–1574. doi: 10.1152/japplphysiol.00865.2011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nehlig, A. (2013). The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br. J. Clin. Pharmacol. 75, 716–727. doi: 10.1111/j.1365-2125.2012.04378.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Njike, V. Y., Faridi, Z., Shuval, K., Dutta, S., Kay, C. D., West, S. G., et al. (2011). Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int. J. Cardiol. 149, 83–88. doi: 10.1016/j.ijcard.2009.12.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nurk, E., Refsum, H., Drevon, C. A., Tell, G. S., Nygaard, H. A., Engedal, K., et al. (2009). Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J. Nutr. 139, 120–127. doi: 10.3945/jn.108.095182
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pase, M. P., Scholey, A. B., Pipingas, A., Kras, M., Noldin, K., Gibbs, A., et al. (2013). Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo-controlled trial. J. Psychopharmacol. 27, 451–458. doi: 10.1177/0269881112473791
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pipingas, A., Harris, E., Tournier, E., King, R., Kras, M., and Stough, C. K. (2010). Assessing the efficacy of nutraceutical interventions on cognitive functioning in the elderly. Curr. Top. Nutraceutical Res. 8, 79–88.
Pucciarelli, D. L. (2013). Cocoa and heart health: a historical review of the science. Nutrients 5, 3854–3870. doi: 10.3390/nu5103854
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ried, K., Sullivan, T., Fakler, P., Frank, O. R., and Stocks, N. P. (2010). Does chocolate reduce blood pressure? A meta-analysis. BMC Med. 8:39. doi: 10.1186/1741-7015-8-39
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ried, K., Sullivan, T. R., Fakler, P., Frank, O. R., and Stocks, N. P. (2012). Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 8:CD008893. doi: 10.1002/14651858.CD008893.pub2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Scholey, A. B., French, S. J., Morris, P. J., Kennedy, D. O., Milne, A. L., and Haskell, C. F. (2010). Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J. Psychopharmacol. 24, 1505–1514. doi: 10.1177/0269881109106923
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shukitt-Hale, B., Carey, A., Simon, L., Mark, D. A., and Joseph, J. A. (2006). Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition 22, 295–302. doi: 10.1016/j.nut.2005.07.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shukitt-Hale, B., Lau, F. C., and Joseph, J. A. (2008). Berry fruit supple- mentation and the aging brain. J. Agric. Food Chem. 56, 636–641. doi: 10.1021/jf072505f
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sorond, F. A., Lipsitz, L. A., Hollenberg, N. K., and Fisher, N. D. L. (2008). Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 4, 433–440.
Spencer, J. P. E. (2007). The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2, 257–273. doi: 10.1007/s12263-007-0056-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Spencer, J. P. E. (2009). Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 4, 243–250. doi: 10.1007/s12263-009-0136-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Steinberg, F. M., Bearden, M. M., and Keen, C. L. (2003). Cocoa and chocolate flavonoids: implications for cardiovascular health. J. Am. Diet. Assoc. 103, 215–223. doi: 10.1053/jada.2003.50028
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Taubert, D., Roesen, R., Lehmann, C., Jung, N., and Schomig, E. (2007). Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. J. Am. Med. Assoc. 298, 49–60. doi: 10.1001/jama.298.1.49
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Williams, R. J., and Spencer, J. P. E. (2012). Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 52, 35–45. doi: 10.1016/j.freeradbiomed.2011.09.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: cocoa, chocolate, flavanols, cognition, mental fatigue, cardiovascular, mood
Citation: Massee LA, Ried K, Pase M, Travica N, Yoganathan J, Scholey A, Macpherson H, Kennedy G, Sali A and Pipingas A (2015) The acute and sub-chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: a randomized, controlled trial. Front. Pharmacol. 6:93. doi: 10.3389/fphar.2015.00093
Received: 12 March 2015; Accepted: 16 April 2015;
Published: 20 May 2015.
Edited by:
Rabia Latif, University of Dammam, Saudi ArabiaReviewed by:
Filippo Caraci, University of Catania, ItalyCopyright © 2015 Massee, Ried, Pase, Travica, Yoganathan, Scholey, Macpherson, Kennedy, Sali and Pipingas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew Pipingas, Centre for Human Psychopharmacology, Swinburne University of Technology, PO Box 218, Hawthorn, VIC 3122, Australia,apipingas@swin.edu.au
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.