- 1Department of Animal Science, School of Life Sciences, Bharathidasan University, Tiruchirappalli, India
- 2Department of Biotechnology, Alagappa University, Karaikudi, India
It is well established that Cronobacter sakazakii infection cause septicemia, necrotizing enterocolitis and meningitis. In the present study, we tested whether the C. sakazakii infection alter the learning and memory through serotonin transporter (SERT). To investigate the possible effect on SERT, on postnatal day-15 (PND-15), wistar rat pups were administered with single dose of C. sakazakii culture (infected group; 107 CFU) or 100 μL of Luria-Bertani broth (medium control) or without any treatment (naïve control). All the individuals were subjected to passive avoidance test on PND-30 to test their fear memory. We show that single dose of C. sakazakii infection improved fear memory retention. Subsequently, we show that C. sakazakii infection induced the activation of toll-like receptor-3 and heat-shock proteins-90 (Hsp-90). On the other hand, level of serotonin (5-hydroxytryptamine) and SERT protein was down-regulated. Furthermore, we show that C. sakazakii infection up-regulate microRNA-16 (miR-16) expression. The observed results highlight that C. sakazakii infections was responsible for improved fear memory retention and may have reduced the level of SERT protein, which is possibly associated with the interaction of up-regulated Hsp-90 with SERT protein or miR-16 with SERT mRNA. Taken together, observed results suggest that C. sakazakii infection alter the fear memory possibly through SERT. Hence, this model may be effective to test the C. sakazakii infection induced changes in synaptic plasticity through SERT and effect of other pharmacological agents against pathogen induced memory disorder.
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) has been implicated as the modulator of learning and memory with special preference to consolidation of new information into long-term memory (Kandel, 2001). 5-HT play a key role in memory formation by interacting with other neurotransmitters/exerts its effect through their seven (5-HT1–5-HT7) subclass of receptors (Meneses, 1999, 2007, 2013; Meneses et al., 2011; Perez-Garcia and Meneses, 2009; Hoyer et al., 2002). Although evidence from Aplysia to human points at a functional role of serotonergic transmission in learning and memory, the underlying mechanism is depends on the level of 5-HT (Barbas et al., 2003; Meyer et al., 2009), and the depletion of 5-HT could affect the memory formation (Kaang et al., 1993; Barbas et al., 2003; Seyedabadi et al., 2014; Stansley and Yamamoto, 2015).
Serotonin transporters (SERT) play a key role in clearance of the released 5-HT through transport across pre-synaptic membrane and maintain the homeostasis of 5-HT level. In addition, expression status of SERT protein could control the duration and intensity of 5-HT activity at synapse (Gainetdinov and Caron, 2003; Tellez et al., 2012; Yoon et al., 2013; Bravo et al., 2014). Earlier studies reported that expression of SERT protein regulated by the interacting molecules such as ribonucleoprotein (RNP) and sequence specific microRNA (miR; Standart and Jackson, 1994; Wilkie et al., 2003; Bartel, 2009; Croce, 2009; Gyawali et al., 2010; Goldie and Cairns, 2012; Hartley et al., 2012). At this point, heterogeneous nuclear ribonucleoprotein K (hnRNPK) and miR-16 appears to negotiate for the binding site at 3′-untranslated region (UTR) of SERT and regulate the repression/depression of translation (Baudry et al., 2010; Yoon et al., 2013).
Over the past few years, research has been conducted to understand the pathogenicity mechanism, genetic, nature of survival and molecular characterization of virulence in Cronobacter spp. (Jaradat et al., 2014). Reports have shown that source of Cronobacter infection was the powder infant formula (PIF; Yan et al., 2012), apart from that significant association was found with contaminated home environment (Kandhai et al., 2004), packed foods (Friedemann, 2007) and drinking water (Liu et al., 2013). In parallel, Clinical and laboratory studies reported that they have resistance to heat, desiccation and acid stress growth condition (Breeuwer et al., 2003; Edelson-Mammel et al., 2005; Dancer et al., 2009), and Cronobacter infection in neonates and infants cause meningitis, necrotizing enterocolitis (NEC) and sepsis with case fatality rate ranging from 40 to 80% (Muytjens et al., 1983; Block et al., 2002; Hyun-Lee et al., 2011; Yan et al., 2012; Hunter and Bean, 2013). However, Cronobacter infection also have been reported in elder patients or immunocompromised persons (Healy et al., 2010), among them 50% had an underlying malignancy (Lai, 2001; See et al., 2007). In addition, Cronobacter infection have been linked to conjunctivitis, osteomyelitis, diarrhea, acute cholecystitis, and wound infection (Gosney et al., 2006; Flores et al., 2011; Yan et al., 2012; Tsai et al., 2013). Pathogen induced neuroinflammation can alter the behavior possibly either through hypothalamus pituitary-adrenal (HPA) axis or neurotransmitter system through the interacting molecules (Pérez et al., 2009; Herrero et al., 2015). In fact, several line of studies reporting that responding to the endotoxin (lipopolysaccharide, LPS) produced by the pathogenic bacteria, the host system activate innate immune response, in which different toll-like receptors (TLRs) and heat-shock proteins (Hsp) are part of it (Pandey and Agrawal, 2006; Okun et al., 2009; Chen et al., 2014), TLRs in dendritic cells play critical role (Stanislawska et al., 2004) and 5-HT transmission (Desbonnet et al., 2008; van Heesch et al., 2014; Depino, 2015). Currently, very little information is available on the pathogen infection mediated effect on serotonergic system. Therefore, the present study is designed to examine the effect of Cronobacter sakazakii infection on postnatal rats’ serotonergic system particularly on SERT and associated changes in learning and memory.
Materials and Methods
Bacterial Strain and Media
The bacterial strain C. sakazakii was obtained from American Type Cell Culture (ATCC BAA-894). The obtained bacterial strain was cultured on the selective chromogenic Enterobacter sakazakii agar medium (Song et al., 2008). The positive blue-green colonies were picked and grown on 1.5% Luria-Bertani (LB) agar. The overnight culture was prepared in the LB broth, which was maintained at 37°C in an incubator shaking at the rate of 145 rpm. Serial dilution and plating method was used to assess the bacterial concentration (Miller, 1972). In detail, 3 h culture of C. sakazakii was examined through biophotometer (Eppendorf Inc) at O.D600· The bacterial culture was serially diluted and plated on LB agar for colony counting. Based on colony counting assay result, concentration of bacterial cells was calculated. Bacterial concentration of 107 CFU was fixed as infectious dose for the present study based on LC50 analysis.
Animals
Timed-pregnant wistar rats at gestation day-15 were acquired (Sri Venkateshwara Enterprise, Bangalore, India), acclimated and maintained under controlled ambiance (12 h light/dark cycle; temperature: 22 ± 2°C; humidity: 50 ± 5%). The pregnant rats were housed individually in a standard laboratory cage (43 cm × 27 cm × 15 cm) with saw dust as bedding material, and food and water provided ad libitum. This study was carried out in accordance with the recommendation of Institutional Animal Ethics Committee (IAEC), Bharathidasan University (BDU). The animal experimental protocol was approved by IAEC, BDU.
Experimental Groups
Wistar rat pups at the age of postnatal day-15 (PND-15) were used as host system for the present study. Pups from different litters were randomly divided into three different groups: naïve control (NC), medium control (MC), and infected (IF) group. Rat pups in NC groups were maintained at normal condition without treatment. MC groups were treated with single dose of LB (100 μL) and IF pups with C. sakazakii culture (107 CFU) on PND-15 by oral gavage. Then the animals were maintained at typical condition with mother.
Confirmation of Infection
On PND-30, the amygdala region was dissected out as described by Kalin et al. (1994) from NC and IF group rats (n = 3 from each group) and homogenized in phosphate buffer saline (PBS). The homogenate was serially diluted up to 10–4 with PBS and plated on specific medium to identify C. sakazakii (Hicrome E. sakazakii agar; Himedia cat. No. M1641-100G) and incubated at 37°C for overnight to observe the presence of C. sakazakii in brain tissue.
Behavioral Test
Passive avoidance test
Passive avoidance apparatus was constructed following the specification of Zare et al. (2015). The apparatus consisted of equally sized light and dark compartments (20 cm × 40 cm × 20 cm) made up of Plexiglas separated by a guillotine door (12 cm × 12 cm). The floor of both chambers were made up of stainless steel rods (3 mm diameter) spaced 1 cm apart but the gridded floor of the dark chamber could be electrified using a shock generator. All the experiments were conducted between 09:00 and 18:00 h. All groups (NC, n = 14; MC, n = 20; IF, n = 29) were subjected to step-through passive avoidance test, in which the rats were trained to the criterion and tested for their retention 24 h post-training. Each time after removing the animal, the apparatus was wiped with 70% ethanol to remove odor. During each experiment the experimenter handle the animals for <60 s.
Exploration and training
On PND-31, each animal was placed in the light compartment of the apparatus facing away from the door and 10 s later the guillotine was raised. The animal was left for 5 min to habituate the apparatus. On PND-32, each animal was trained for the criterion. The rat was placed in the light compartment of the apparatus facing away from the door and 10 s later the guillotine was raised. When the animal had placed at all four paws in the dark compartment, entrance latency to the dark compartment was recorded. Once the animal entered into the dark compartment, the door was closed and an inescapable foot shock (0.5 mA) was applied for 5 s. After 20 s, the animal was retrieved from the dark box and placed back into their home cage. After 2 min, the procedure was repeated. The rat received foot shock each time it placed its four paws into the dark compartment. The training was terminated when the rat remained in light compartment for 120 s consecutively. Number of trials required for training the animal was recorded.
Retention test
On PND-33, retention test was performed 24 h post training. The rat was placed on the light compartment and 10 s later the door was raised. The step-through latency and time spent in dark compartment was recorded up to 300 s. If the rat did not enter the dark compartment within 300 s, a score of 300 s was assigned.
Neurotransmitter Analysis
On PND-30, group of rats from NC (n = 5), MC (n = 5), and IF (n = 5) were euthanized, and the amygdala region was dissected as described elsewhere (Kalin et al., 1994) and frozen on dry ice. The tissue samples were weighed and homogenized in a glass homogenizer with 0.1 M perchloric acid containing 4.5 mM Na2EDTA and 1.6 mM reduced glutathione. The homogenates were centrifuged at 12,000 rpm for 20 min at 4°C. The supernatants were collected in a fresh tube and stored at –70°C. The level of 5-HT was estimated with a 5-HT ELISA kit (Biosource, Europe S.A., Belgium) by following the manufacturer’s instructions. The concentrations of 5-HT in each tissue samples were calculated by comparing the optical density of the sample (mean for duplicates) with that of the standard curve.
Sample Preparation
On PND-30, group of rats from NC (n = 5), MC (n = 5), and IF (n = 5) groups were euthanized and amygdala region was dissected out from and divided into two part for the preparation of total RNA and protein. Total RNA was isolated from the tissue samples following the manufacture’ instructions (Trizol method; Merck, Bangalore, India) and stored at –70°C with RNase inhibitor (1U/μL; Rnasin, Promega, Madison, WI, USA). Total RNA (1 μg) was converted into cDNA by following manufacture’ instructions (QuantiTect® Reverse Transcription Kit; catalog no. 205311, Qiagen, Germany). Tissue samples were homogenized in 300–400 μL of ice cold lysis buffer (150 mM NaCl, 50 mM Tris–HCl; pH 7.5, 5 mM EDTA, 0.1% v/v NP-40, 1 mM DTT, 0.2 mM sodium orthovanadate, 0.023 mM PMSF) with protease inhibitor cocktail (10 mg/mL; Sigma-Aldrich, USA), and incubated on ice for 30 min. The homogenate was centrifuged at 10,000 g for 30 min at 4°C. The supernatant was collected in a fresh tube and again centrifuged at 12,000 g for 30 min at 4°C. The supernatant was extracted and stored at –70°C.
Quantitative Real-Time PCR
The quantitative real-time PCR (qRT-PCR) was performed in CFX-96 Touch™ Real-time PCR detection system using SSoAdvanced™ SYBR® green mix (Bio-Rad Laboratories, Inc., USA). The level of mRNA of the selected genes were assessed through qPCR using specific primers: Tlr-3 (for 5′-ACAATGCCCAACTGAACCTC-3′ and rev 5′-CGGAGGCTGTTGTAGGAAAG-3′) and miR-16 (for 5′-CCGCTCTAGCAGCACGTAAA-3′ and rev 5′-CCCTGTCACACT AAAGCAGC-3′). The level of Tlr-3 and miR-16 was normalized with internal control GAPDH (for 5′-AACATCATCCCTGCATCCAC-3′ and rev 5′-AGGAACACGGAAGGCCAT GC-3′) and U6 SnRNA (for 5′-CTCGCTTCGGCAGCACA-3′ and rev 5′-AACGCTTCACGAATT TGCGT-3′), respectively. Thermo cycling conditions for qPCR were as follows: initial denaturation at 92°C for 3 min and then denaturation at 92°C for 5 s, annealing (at 59°C for GAPDH, 62°C for tlr-3, 60°C for U6 SnRNA, and 64°C for miR-16), for 5 s, extension at 72°C for 5 s, and melt curve analysis at 65–95°C. Amplification of the single PCR product was confirmed by monitoring the dissociation curve followed by melting curve analysis. Each reaction was performed in triplicates with threefold serial dilution of cDNA with normalizing internal control GAPDH/U6 SnRNA. The data are presented as mean fold change of the normalized expression (CFX Manager™ version 2 software; Bio-Rad Laboratories, Inc., USA).
Western Blotting
An equal concentration of protein (40 μg) was mixed with loading buffer (glycerol, 125 mM Tris–HCl pH 6.8, 4% SDS, 0.006% bromophenol blue, 2% mercaptoethanol) and resolved on 10% polyacrylamide gel (PAGE). The separated proteins were transferred electrophoretically on to the PVDF membrane (Millipore India Pvt. Ltd., India). The membranes were then placed in the blocking solution [5% non-fat dry milk in Tris-buffered saline (TBS) containing 0.1% Tween-20: TBS-T] for 3 h at room temperature (RT). The blocking solution was discarded and the membranes incubated at 4°C overnight with one of the following primary antibodies (Santa-Cruz Biotech, Germany/BD Biosciences, USA): SERT (SC-1458, 1:200), anti-β-actin (SC-130656; 1:1000) affinity purified rabbit polyclonal antibody and Hsp-90 (SC-5977, 1:200) mouse monoclonal antibody. β-actin was used as control for each samples. The membrane was washed and bound antibodies were detected by incubating for 3 h either with the mouse anti-rabbit (Cat # 621100180011730; 1:2000; MERCK, Bangalore, India) or goat anti-mouse (Cat # 621100480011730; 1:2000; MERCK, Bangalore, India) alkaline phosphatase conjugated antibody. The membrane was washed three times with TBS-T, and alkaline phosphatase activity was detected with 5-bromo-4-chloro-3-indolylphosphate disodium salt (BCIP)/nitro-blue tetrazolium chloride (NBT) following the instructions from the manufacturer (Invitrogen, USA). The images were acquired with Molecular Imager ChemiDoc XRS system (Bio-Rad Laboratories, Inc., USA) and the trace quantity for each band was measured using Quantity One image analysis software (Bio-Rad Laboratories, Inc., USA). The obtained Hsp90 and SERT levels were normalized to β-actin for respective samples.
Statistical Analysis
Data were presented as a mean ± standard error of the mean (SEM) and plotted with KyPlot (version 1.0) for graphical representation. The obtained data were evaluated by one-way analysis of variance (ANOVA) to detect differences between groups (SigmaStat; version 3.1) followed by Bonferroni post hoc test was performed. Differences were considered significant if p < 0.05.
Results
C. sakazakii Infection Alters the Fear Memory Retention
To determine whether the C. sakazakii infection affects cognitive function, we compare the fear memory retention between the experimental groups. We first assessed the performance of experimental groups during the training session of the light-dark passive avoidance task, there was no significant difference in the number of acquisition trials between NC (1.42 ± 0.17) and MC (1.15 ± 0.08) groups [F(1,33) = 2.56; P > 0.05]. Similarly, C. sakazakii infection did not change the number of trials in IF group (1.35 ± 0.15) from NC [F(1,42) = 0.113; P > 0.05] and MC group [F(1,47) = 1.00; P > 0.05; Figure 1A]. Further, Bonferroni test revealed that the acquisition trials required by IF group was not significantly different from NC (P = 0.739) and MC group (P = 0.322). In comparison, there was no significant difference between NC and MC groups (P = 0.011). Similarly, there was no significant difference in entrance latency between NC (23.21 ± 3.36 s) and MC (35.10 ± 8.13 s) group [F(1,32) = 1.36; P > 0.05]. When the IF group (30.62 ± 3.0 s) compared to NC [F(1,42) = 2.26; P > 0.05] and MC [F(1,47) = 0.344; P > 0.05], no significant difference was found (Figure 1B). Bonferroni test confirmed that the entrance latency of IF group was not significantly different from NC (P = 0.144) and MC group (P = 0.62). In addition, it showed that NC group was not significantly different from MC group (P = 0.160). However, there was a significant difference between groups in step-through latency during testing. Our analysis revealed that the IF group (237.34 ± 19.35 s) rats showed significantly higher latencies to enter the dark box compared to NC (150.92 ± 30.5 s) [F(1,42) = 4.883; P < 0.05] and MC (171.8 ± 26.38 s) group [F(1,47) = 4.26; P < 0.05]. When we compare the latency exhibited by the NC and MC groups, there was no significant difference between them [F(1,32) = 0.344; P > 0.05]. Further, Bonferroni test showed that the IF group took significantly more time to step into the dark box than NC (P = 0.021) and MC group (P = 0.044), but there was no significant difference between NC and MC group (P = 0.596). The observed data showed that C. sakazakii infection did not alter their learning during acquisition but IF group exhibited higher step-through latency during retention test, which showed the persistence of fear memory.
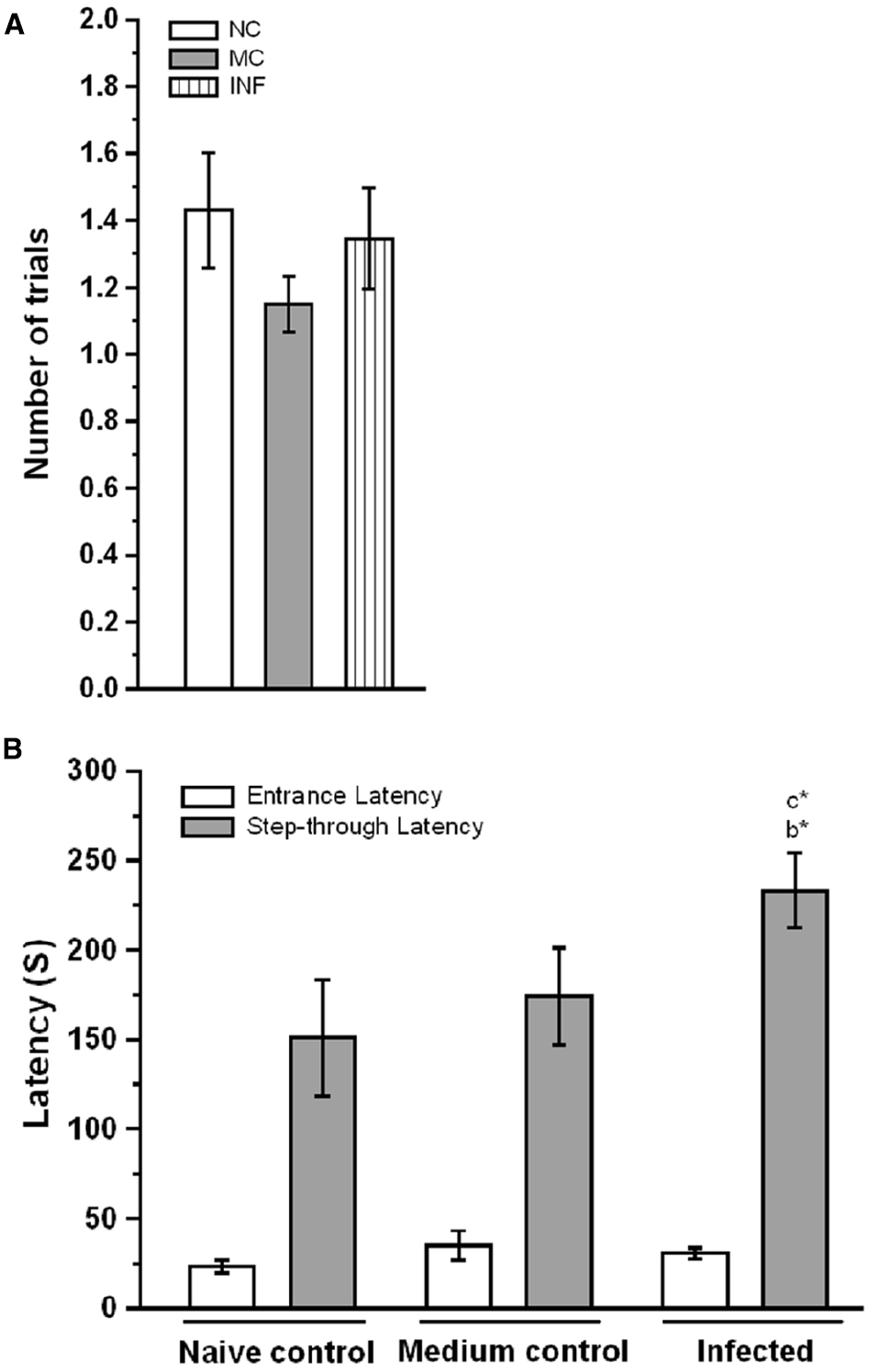
Figure 1. Cronobacter sakazakii infection improved the fear memory retention in passive avoidance (PA) test. (A) The number of trials to acquisition, naïve control (NC), medium control (MC), and infected (IF) group did not show significant difference in the number of trials to acquisition. (B) Step-through latency in the retention test. IF group rats spend more in the light-chamber and taken more time to step-down into the dark-chamber considered as indices of improvement in fear memory. Values are represented as the mean ± SEM; *P < 0.05. Asterisk indicates significant difference respect to comparison between groups (b = NC verses IF; c = MC verses IF).
C. sakazakii Entered into the Brain
To confirm the observed behavioral phenotype was due to the single dose of C. sakazakii infection on PND-15, we tested the presence of C. sakazakii in brain. When we plated the brain tissue homogenates in Enterobacter medium plate, we found the growth of blue-green color colonies from the IF group samples but not in the naïve control (Figure 2). This result suggested that single dose of oral treatment of C. sakazakii during post-natal day is enough to induce the infection at brain.
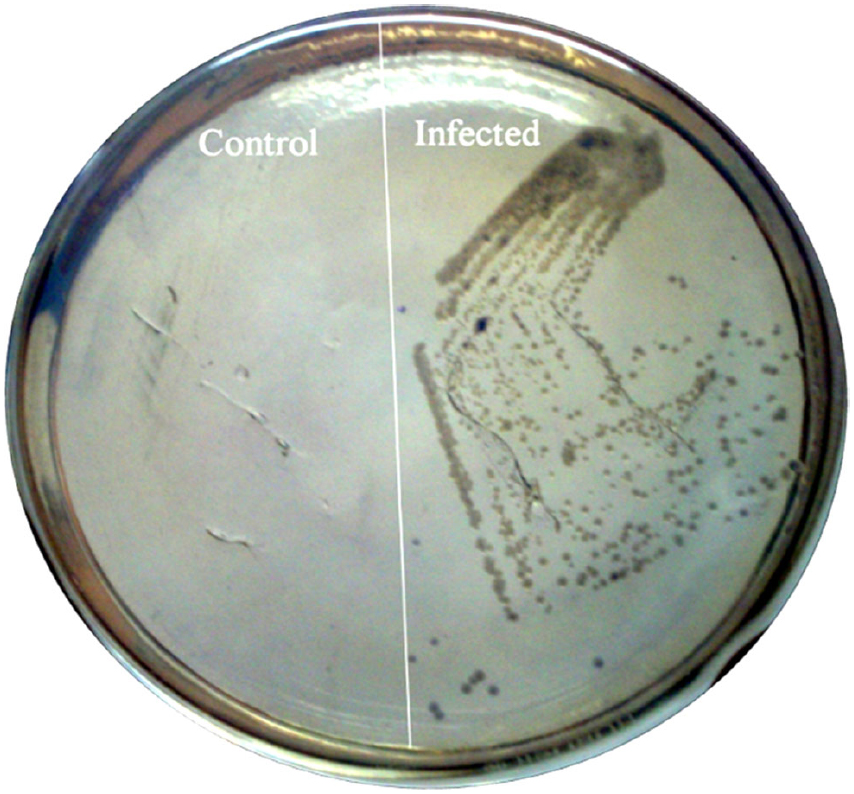
Figure 2. Cronobacter sakazakii colonization in the brain tissue of rat pups. Brain tissues were obtained from experimental groups (Control, Infected) on PND-30. Equal weights of brain tissues were homogenized and homogenates were plated on specific medium. Specific medium showing the presence of C. sakazakii in infected group.
C. sakazakii Infection Activates TLR-3
To further evaluate the effect of C. sakazakii infection on activation of TLR-3. Our analysis revealed that C. sakazakii infection significantly increased the expression level of TLR-3 (Figure 3), the Ct values of TLR-3 for each group followed by GAPDH (NC: 23.24 ± 0.038; 12.11 ± 0.041; MC: 23.24 ± 0.026; 11.77 ± 0.064; IF: 22.36 ± 0.122; 12.12 ± 0.054). The estimated level of TLR-3 was significantly higher in IF group than MC group [F(1,9) = 167.19; P < 0.001] and NC group [F(1,9) = 103.78; P < 0.001]. Similarly, there was a significant difference between MC and NC groups, but the difference obtained by the reduction of TLR-3 expression was significant in MC group than NC group [F(1,9) = 23.68; P < 0.01]. These results suggesting that C. sakazakii infection activated TLR-3 expression.
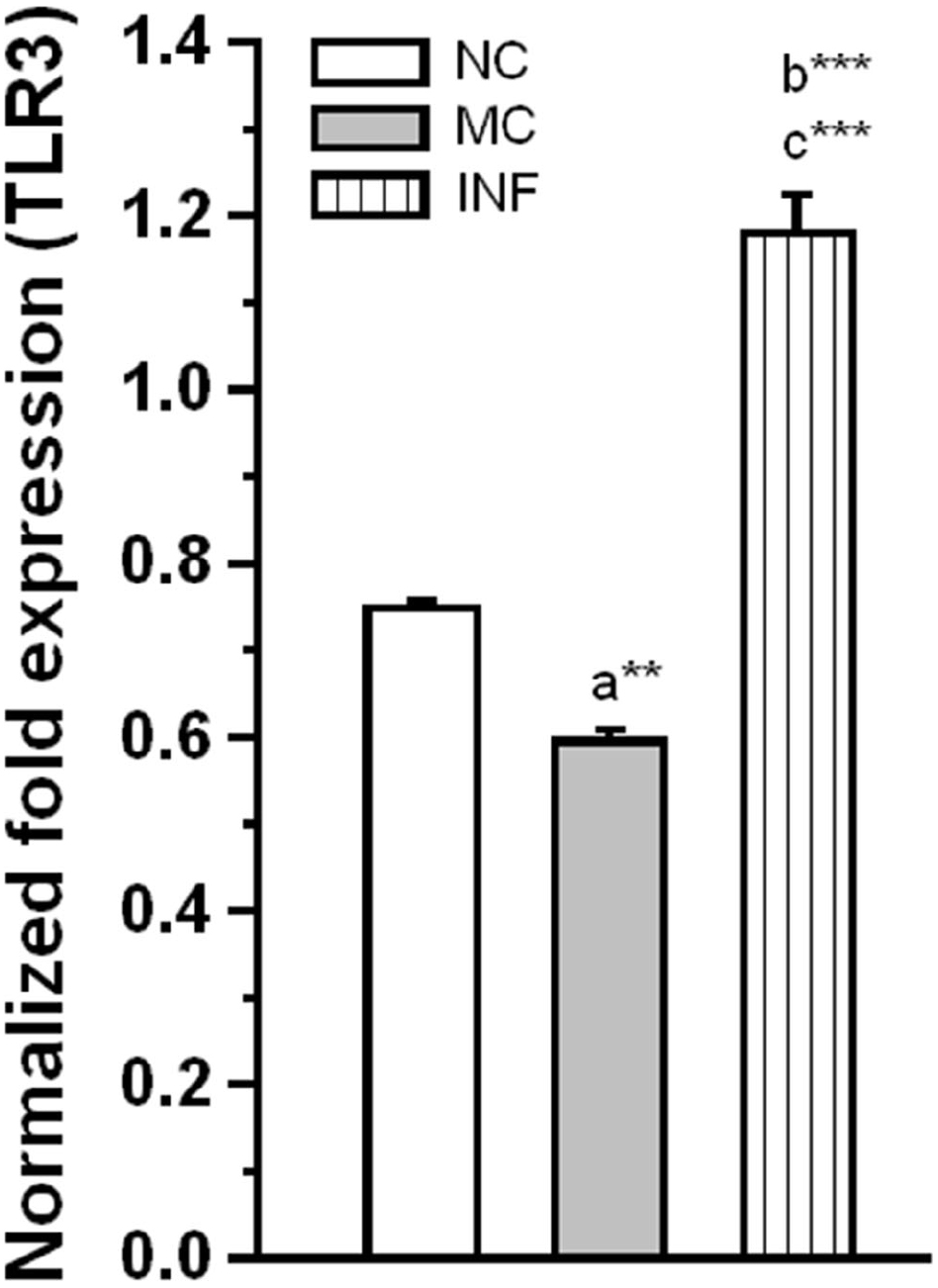
Figure 3. Rats received C. sakazakii infection showed up-regulated expression of TLR-3. The level of TLR-3 expression was significantly increased in infected (IF) group than medium control (MC) and naïve control (NC). The normalized fold variation shown as mean ± SEM. Asterisk indicates significant difference (**P < 0.01; ***P < 0.001) respect to comparison between groups (a = NC verses MC; b = NC verses IF; c = MC verses IF).
C. sakazakii Infection Up-Regulate Hsp-90
We next examined along with activation TLR-3, whether the Hsp-90 also activated following C. sakazakii infection. When examined the level of Hsp-90 in the experimental groups (Figure 4), we found that the C. sakazakii infection significantly alter the Hsp-90. The estimated level was significantly high in IF group than MC [F(1,9) = 188.64; P < 0.001] and NC group [F(1,9) = 1424.96; P < 0.001]. However, there was no significant difference between MC and NC groups [F(1,9) = 6.28; P = 0.052]. Our analysis revealed that C. sakazakii increased Hsp-90 expression.
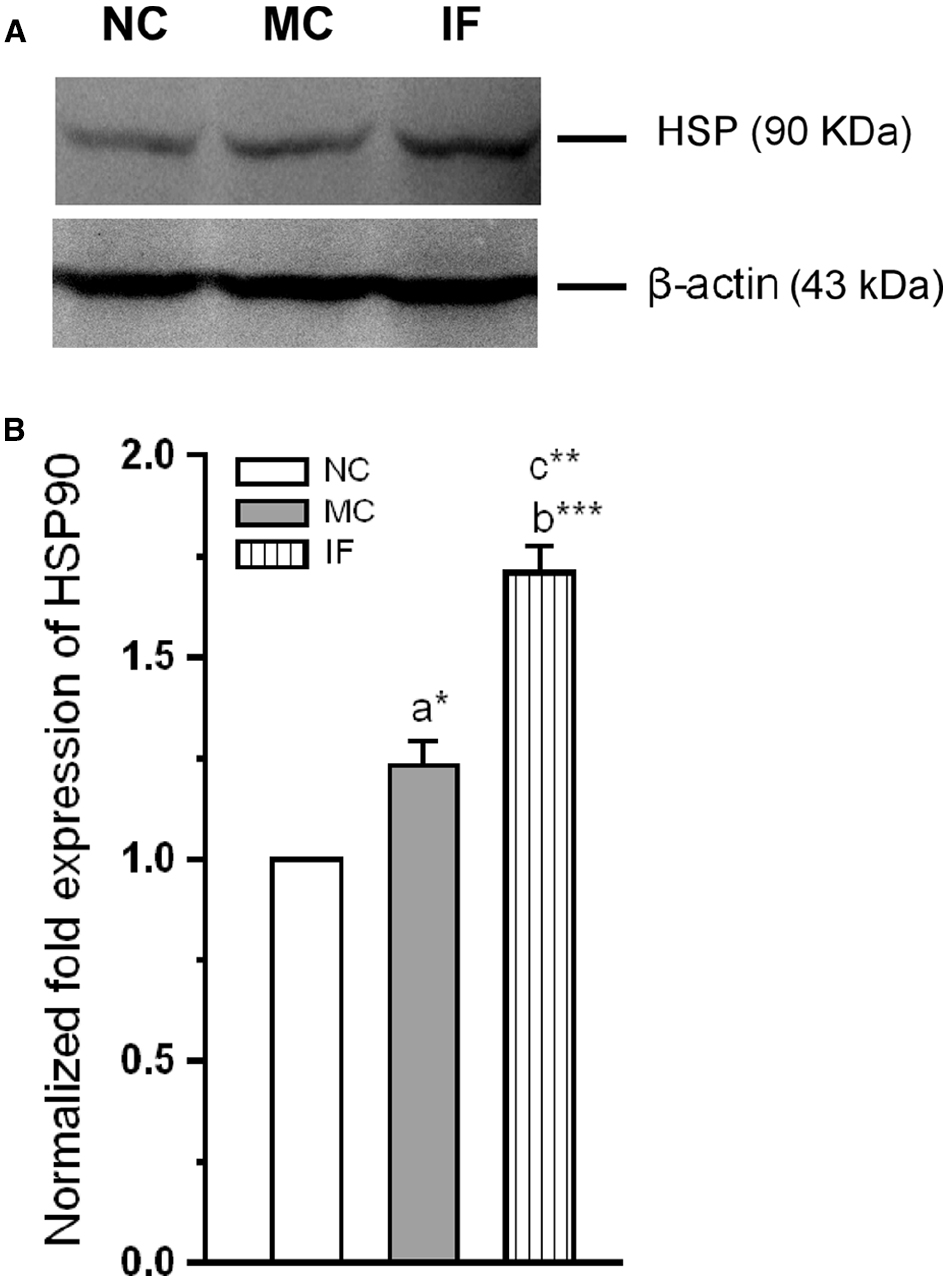
Figure 4. Cronobacter sakazakii infection increases the expression of heat-shock protein-90. (A) Representative western blot showing variation in the expression of HSP-90 levels in experimental groups. (B) HSP-90 level was significantly increased in infected (IF) group than control (MC) and naïve control (NC) groups. The fold variation shown as mean ± SEM. Asterisk indicates significant difference (*P < 0.05; **P < 0.01; ***P < 0.001) respect to comparison between groups (a = NC verses MC; b = NC verses IF; c = MC verses IF).
C. sakazakii Infection Modulates Serotonin and SERT Protein Level
In addition to the activation of TLR-3 and Hsp-90, we estimated the level of 5-HT, and expression level of SERT in experimental group rats. As shown in Figure 5, the basal levels of 5-HT was significantly affected by C. sakazakii infection [F(1,9) = 9735.27; P < 0.001] compared to NC and MC [F(1,9) = 236.78; P < 0.001]. In addition, levels of 5-HT was significantly lower in MC group than NC group [F(1,9) = 9.12; P < 0.05]. Further, our analysis revealed that the expression of SERT was significantly reduced in IF group [F(1,9) = 51.85; P < 0.001] than NC group, but not significantly different from MC group [F(1,9) = 4.8; P = 0.07]. When we compare the expression level of MC and NC groups, they were not significantly different [F(1,9) = 4.1; P = 0.074]. Our analysis suggests that C. sakazakii infection reduced the levels of 5-HT and expression of SERT protein.
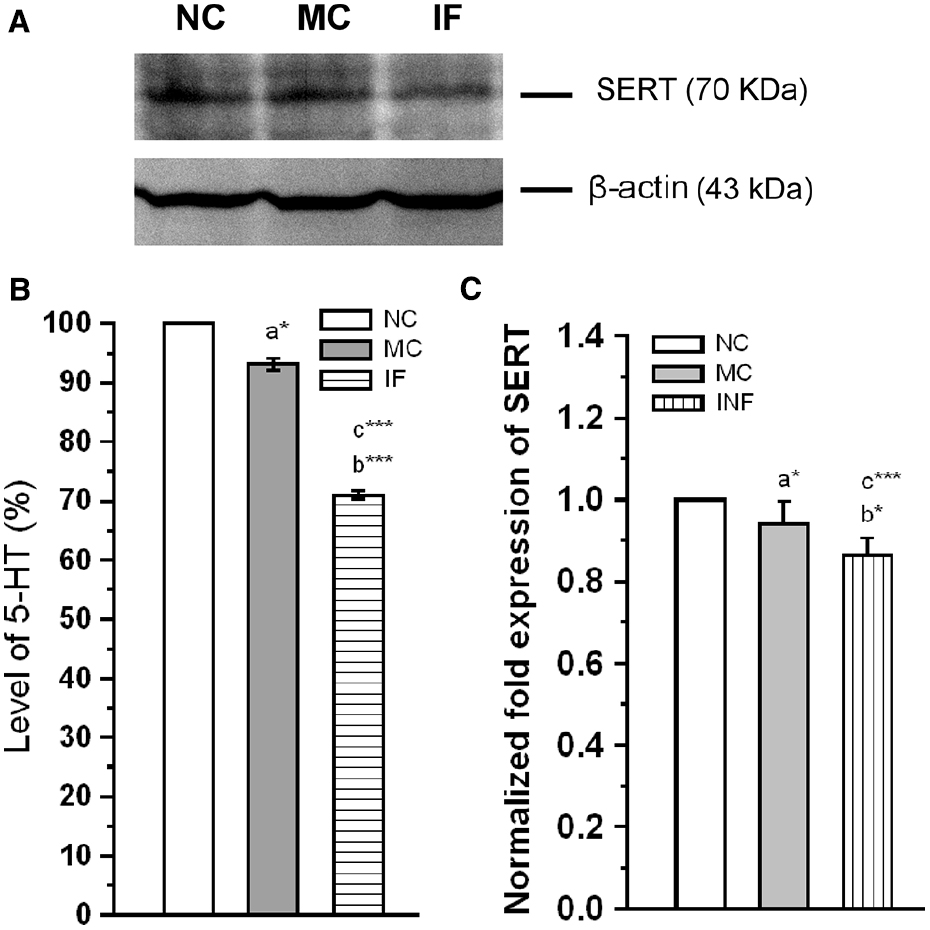
Figure 5. Cronobacter sakazakii infection alters the serotonergic system. (A) Representative western blot showing the differential expression of SERT in experimental groups. (B) There was a significant decrease in 5-HT level in C. sakazakii infected (IF) group than medium control (MC) and naïve control (NC) groups. (C) SERT protein level was significantly decreased in IF group than MC and NC mice. Data were shown as mean ± SEM. Asterisk indicates significant difference (*P < 0.05; ***P < 0.001) respect to comparison between groups (a = NC verses MC; b = NC verses IF; c = MC verses IF).
C. sakazakii Infection Modulates miR-16 Expression
Subsequently, we explored the possible role of miR-16 in C. sakazakii mediated regulation of SERT protein expression. We observed that the level of miR-16 expression was significantly elevated in IF (Ct: 23.21 ± 0.054) group than NC [F(1,9) = 8348.15; P < 0.001] and MC group [F(1,9) = 8117.98; P < 0.001]. However, there was no significant difference between the MC (Ct: 24.73 ± 0.103) and NC (Ct: 25.34 ± 0.260) groups [F(1,9) = 1.68; P = 0.084; Figure 6]. Our results suggested that up-regulation of miR-16 by C. sakazakii infection possibly suppressed the translation of SERT.
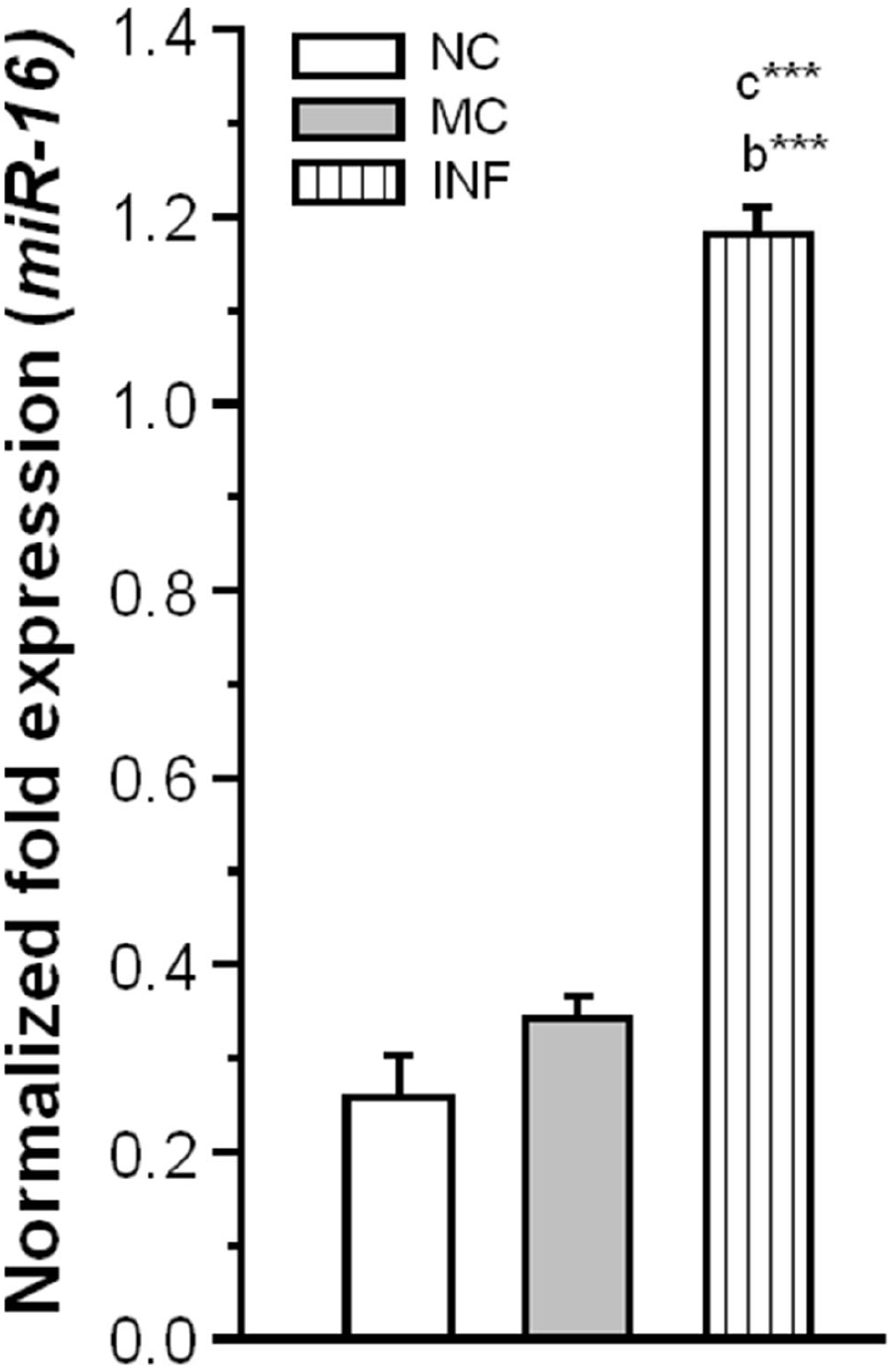
Figure 6. Cronobacter sakazakii infection alters the miR-16 expression. The level of miR-16 expression was significantly increased in infected (IF) group than control (MC) and naïve control (NC) groups. The normalized fold variation shown as mean ± SEM. Asterisk indicates significant difference (***P < 0.001) respect to comparison between groups (a = NC verses MC; b = NC verses IF; c = MC verses IF).
Discussion
Cronobacter sakazakii has been associated with human infection especially in newborn and infants (Joseph and Forsythe, 2011; Cruz-Córdova et al., 2012). Earlier, Hyun-Lee et al. (2011) demonstrated that C. sakazakii can cross the blood–brain barrier (BBB) in postnatal mice (PND-3.5), possibly through exploiting immature dendritic cells (Townsend et al., 2007; Mittal et al., 2009; Emami et al., 2011). In the present study, we IF the postnatal rats on PND-15, during the onset of “brain growth spurt” period (Dobbing and Sands, 1979). During this period, changes like axonal outgrowth and dendritic maturation, establishment of neuronal connections and proliferation of glial cells occurred accompanying with myelinization (Kolb and Whishaw, 1989). At first, we showed the presence of C. sakazakii in PND-30 rats’ brain. Further, we demonstrated that single dose of C. sakazakii infection in postnatal rats did not alter their learning efficiency but improved the fear memory retention. Our results adding support to the earlier study and suggest that postnatal Wistar rats may be used as animal model for human neonatal C. sakazakii infections. Earlier studies discussed how the infection and inflammation lead to changes in brain (Goehler et al., 2007; Jaradat et al., 2014), in which TLRs are a part. TLRs are conserved from sponges to human and very much present in neuronal cells (Barton, 2007; Tang et al., 2007; Wiens et al., 2007). It has been stated that as a pro-inflammatory or a comprehensive neuroprotective response, TLR-3 is activated (Bsibsi et al., 2006; Kim et al., 2008), whereas its activation has not yet been established under normal condition (Okun et al., 2011). In brain, TLR-3 has broad effect on the cognitive function based on injury and/or disease. Studies in animal models reported that TLR-3 deficient mice showed improved contextual and extinction of fear memory (Okun et al., 2010). Interestingly, we found that rats with C. sakazakii infection showed elevated level of TLR-3 expression compared to other groups and they displayed improved fear memory. Supporting to this, earlier study demonstrated that TLR-3 activation possibly negatively regulate ERK-CREB signaling, thus, activation of TLR-3 contribute to cognitive impairment and other behavioral disorders (Okun et al., 2010).
Earlier studies reported that as a innate immune response exposure to pathogen/LPS activate TLR and Hsp90 (Stanislawska et al., 2004; Xie et al., 2015), in many observations expression of Hsp90 facilitates the pathogenesis (Qin et al., 2010; Smith et al., 2010; Shapiro et al., 2012). TLR-3 can also respond to the endogenous ligands such as Hsp-90, especially in dendritic cells during pathogenesis (Stanislawska et al., 2004). Similarly, we found that C. sakazakii infection induced the expression of Hsp90, the estimated level was higher than the other experimental group. Hsp90 is one of the molecules that interact with serotonergic system, especially with SERT. In fact, N- or C-terminus of SERT protein known to interact with many regulatory proteins, they play critical role in folding of SERT protein (El-Kasaby et al., 2010, 2014; Zhong et al., 2012). When we tested the expression pattern of SERT, the level of SERT protein was significantly low in IF group than other experimental groups. Although, it is interesting that the infection appears to have changed 5-HT level and SERT protein, the SERT effect appeared to be rather modest and it is unclear whether the alternation of SERT or any other interacting molecules in altering the individual’s behavior in IF group. However, earlier in vitro report demonstrated that over expression of Hsp90 interact with SERT protein and alter the folding trajectory of SERT protein (El-Kasaby et al., 2014). On the other hand, expression of SERT could be exerted by microRNAs, particularly miR-16 (Baudry et al., 2010; Yoon et al., 2013). Specific miRNAs activation/inactivation patterns are critically regulated by the presence of bacterial effector proteins and localization of the pathogen (Zhu et al., 2010; Al-Quraishy et al., 2012; Izar et al., 2012). Although, there is a differential expression of miR-16 following pathogen infection, we found that miR-16 expression was increased after C. sakazakii infection. Further, our analysis suggests that C. sakazakii infection up-regulate the expression of miR-16, which also interact with the 3′UTR of SERT and down-regulate the translation process. Supporting to our behavioral observations, SERT knock-out animals showed impaired fear extinction (Wellman et al., 2007; Narayanan et al., 2011; Hartley et al., 2012). The down-regulated SERT expression could affect the reuptake of released 5-HT, and then the level of 5-HT. Our analysis revealed that the level of 5-HT significantly decreased following C. sakazakii infection. Supporting to this, in vivo and in vitro studies demonstrating that exposure to pathogens/pathogen produced endotoxin alter the level of 5-HT and behavior (Esmaili et al., 2009; Martin et al., 2009; Shin and Liberzon, 2010; van Heesch et al., 2014). In addition, we observed difference between the naïve control and MC in molecules we tested in this study but not in the behavior. The observed difference in this study is possibly by the micronutrients in the bacterial medium, which may alter the gut microbiota of the individuals. They have the capacity to can influence precursor pool for 5-HT (Desbonnet et al., 2008).
In conclusion, our results demonstrates that C. sakazakii infection enhanced the fear memory retention. Although, further study needed to establish the mechanism of this effect, based on our data, we hypothesize that observed changes in SERT expression may have caused this effect, possibly through the interaction of Hsp-90 and miR-16. Further, the present study suggest that C. sakazakii infection in postnatal rats may be used an animal model to examine the effect of bacterial infection mediated changes in synaptic plasticity through SERT and effect of other pharmacological agents against pathogen induced memory disorder.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the editor Dr. Alfredo Meneses and two referees for their valuable comments and suggestions that improved the final version of this manuscript. This research was partially supported by the Council of Scientific and Industrial Research (CSIR) through a major project to KER [37(1426)/10/EMR-II/2010] and BSS through UGC- Dr. D.S. Kothari Post-doctoral fellowship [Ref. No.F4-2/2006/(BSR)/13-876/2013] Department of Animal Science supported by UGC-SAP-DRS-II.
References
Al-Quraishy, S., Dkhil, M. A., Delic, D., Abdel-Baki, A. A., and Wunderlich, F. (2012). Organ specific testosterone-insensitive response of miRNA expression of C57BL/6 mice to Plasmodium chabaudi malaria. Parasitol. Res. 111, 1093–101. doi: 10.1007/s00436-012-2937-3
Barbas, D., DesGroseillers, L., Castellucci, V. F., Carew, T. J., and Marinesco, S. (2003). Multiple serotonergic mechanisms contributing to sensitization in Aplysia: evidence of diverse serotonin receptor subtypes. Learn. Mem. 10, 373–386. doi: 10.1101/lm.66103
Bartel, D. P. (2009). MicroRNA target recognition and regulatory functions. Cell 136, 215–233. doi: 10.1016/j.cell.2009.01.002
Barton, G. M. (2007). Viral recognition of Toll-like receptors. Semin. Immunol. 19, 33–40. doi: 10.1016/j.smim.2007.01.003
Baudry, A., Mouillet-Richard, S., Schneider, B., Launay, J. M., and Kellermann, O. (2010). miR-16 targets the serotonin transporter: a new fact for adaptive responses to antidepressants. Science 329, 1537–1541. doi: 10.1126/science.1193692
Block, C., Peleg, O., Minster, N., Bar-Oz, B., Simhon, A., Arad, I., et al. (2002). Cluster of neonatal infections in Jerusalem due to unusual biochemical variant of Enterobacter sakazakii. Eur. J. Clin. Microbiol. Infect. Dis. 21, 613–616. doi: 10.1007/s10096-002-0774-5
Bravo, J. A., Dinan, T. G., and Cryan, J. F. (2014). Early-life stress induces persistent alterations in 5-HT1A receptor and serotonin transporter mRNA expression in adult rat brain. Front. Mol. Neurosci. 7:24. doi: 10.3389/fnmol.2014.00024
Breeuwer, P., Lardeau, A., Peterz, M., and Joosten, H. M. (2003). Desiccation and heat tolerance of Enterobacter sakazakii. J. Appl. Microbiol. 95, 967–973. doi: 10.1046/j.1365-2672.2003.02067.x
Bsibsi, M., Persoon-Deen, C., Verwer, R. W., Meeuwsen, S., Ravid, R., and Noort, J. M. V. (2006). Toll like receptor-3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 53, 688–695. doi: 10.1002/glia.20328
Chen, Y., Wang, B., Liu, D., Li, J. J., Xue, Y., Sakata, K., et al. (2014). Hsp90 chaperone inhibitor 17-AAG attenuates Aβ-induced synaptic toxicity and memory impairment. J. Neurosci. 34, 2464–2470. doi: 10.1523/JNEUROSCI.0151-13.2014
Croce, C. M. (2009). Causes and consequences of microRNA deregulation in cancer. Nat. Rev. Genet. 10, 704–714. doi: 10.1038/nrg2634
Cruz-Córdova, A., Rocha-Ramirez, L. M., Ochoa, S. A., Gónzalez-Pedrajo, B., Espinosa, N., Eslava, C., et al. (2012). Flagella from five Cronobacter species induces pro-inflammatory cytokines in macrophage derivatives from human monocytes. PLoS ONE 7:e52091. doi: 10.1371/journal.pone.0052091
Dancer, G. I., Mah, J. H., Rhee, M. S., Hwang, I. G., and Kang, D. H. (2009). Resistance of Enterobacter sakazakii (Cronobacter spp.) to environmental stresses. J. Appl. Microbiol. 107, 1606–1614. doi: 10.1111/j.1365-2672.2009.04347.x
Depino, A. M. (2015). Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience 299, 56–65. doi: 10.1016/j.neuroscience.2015.04.065
Desbonnet, L., Garrett, L., Clarke, G., Bienenstock, J., and Dinan, T. G. (2008). The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 43, 164–174. doi: 10.1016/j.jpsychires.2008.03.009
Dobbing, J., and Sands, J. (1979). Comparative aspects of the brain growth spurt. Early. Hum. Dev. 3, 79–83.
Edelson-Mammel, S. G., Porteous, M. K., and Buchanan, R. L. (2005). Survival of Enterobacter sakazakii in a dehydrated powdered infant formula. J. Food. Prot. 68, 1900–1902.
El-Kasaby, A., Just, H., Malle, E., Stolt-Bergner, P. C., Sitte, H. H., Freissmuth, M., et al. (2010). Mutations in the carboxy-terminal SEC24 binding motif of the serotonin transporter impair folding of the transporter. J. Biol. Chem. 285, 39201–39210. doi: 10.1074/jbc.M110.118000
El-Kasaby, A., Koban, F., Sitte, H. H., Freissmuth, M., and Sucic, S. (2014). A cytosolic relay of heat shock proteins HSP70-1A and HSP90β monitors the folding trajectory of the serotonin transporter. J. Biol. Chem. 289, 28987–29000. doi: 10.1074/jbc.M114.595090
Emami, C. N., Mittal, R., Wang, L., Ford, H. R., and Prasadarao, N. V. (2011). Recruitment of dendritic cells is responsible for intestinal epithelial damage in the pathogenesis of necrotizing enterocolitis caused by Cronobacter sakazakii. J. Biol. Chem. 186, 7067–7079. doi: 10.4049/jimmunol.1100108
Esmaili, A., Nazir, S. F., Borthakur, A., Yu, D., Turner, J. R., Saksena, S., et al. (2009). Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology 137, 2074–2083. doi: 10.1053/j.gastro.2009.09.002
Flores, J. P., Medrano, S. A., Sánchez, J. S., and Fernández-Escartín, E. (2011). Two cases of hemorrhagic diarrhea caused by Cronobacter sakazakii in hospitalized nursing infants associated with the consumption of powdered infant formula. J. Food. Prot. 74, 2177–2181. doi: 10.4315/0362-028X.JFP-11-257
Friedemann, M. (2007). Enterobacter sakazakii in food and beverages (other than infant formula and milk powder). Int. J. Food. Microbiol. 116, 1–10. doi: 10.1016/j.ijfoodmicro.2006.12.018
Gainetdinov, R. R., and Caron, M. G. (2003). Monoamine transporters: from genes to behavior. Annu. Rev. Pharmacol. Toxicol. 43, 261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309
Goehler, L. E., Lyte, M., and Gaykema, R. P. A. (2007). Infection-induced viscerosensory signals from the gut enhance anxiety: implications for psychoneuroimmunology. Brain Behav. Immun. 21, 721–726. doi: 10.1016/j.bbi.2007.02.005
Goldie, B. J., and Cairns, M. J. (2012). Post-transcriptional trafficking and regulation of neuronal gene expression. Mol. Neurobiol. 45, 99–108. doi: 10.1007/s12035-011-8222-0
Gosney, M. A., Martin, M. V., Wright, A. E., and Gallagher, M. (2006). Enterobacter sakazakii in the mouths of stroke patients and its association with aspiration pneumonia. Eur. J. Intern. Med. 17, 185–188. doi: 10.1016/j.ejim.2005.11.010
Gyawali, S., Subaran, R., Weissman, M. M., Hershkowitz, D., McKenna, M. C., Talati, A., et al. (2010). Association of polyadenylation polymorphism in the serotonin transporter and panic disorder. Biol. Psychiatry 67, 331–338. doi: 10.1016/j.biopsych.2009.10.015
Hartley, C. A., McKenna, M. C., Salman, R., Holmes, A., Casey, B. J., Phelps, E. A., et al. (2012). Serotonin transporter polyadenylation polymorphism modulates the retention of fear extension memory. Proc. Natl. Acad. Sci. U.S.A. 109, 5493–5498. doi: 10.1073/pnas.1202044109
Healy, B., Cooney, S., O’Brien, S., Iversen, C., Whyte, P., Nally, J., et al. (2010). Cronobacter (Enterobacter sakazakii): an opportunistic foodborne pathogen. Foodborne Pathog. Dis. 7, 339–350. doi: 10.1089/fpd.2009.0379
Herrero, M. T., Estrada, C., Maatouk, L., and Vyas, S. (2015). Inflammation in Parkinson’s disease: role of glucocorticoids. Front. Neuroanat. 9:32. doi: 10.3389/fnana.2015.00032
Hoyer, D., Hannon, J. P., and Martin, G. R. (2002). Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 71, 533–554. doi: 10.1016/S0091-3057(01)00746-8
Hunter, C. J., and Bean, J. F. (2013). Cronobacter: an emerging opportunistic pathogen associated with neonatal meningitis, sepsis and necrotizing enterocolitis. J. Perinatol. 33, 581–585. doi: 10.1038/jp.2013.26
Hyun-Lee, H. A., Hong, S., Park, H., Kim, H., and Kim, O. (2011). Cronobacter sakazakii infection induced fatal clinical sequels including meningitis in neonatal ICR mice. Lab. Anim. Res. 27, 59–62. doi: 10.5625/lar.2011.27.1.59
Izar, B., Mannala, G. K., Mraheil, M, A., Chakraborthy, T., and Hain, T. (2012). MicroRNA response to Listeria monocytogenes infection in epithelial cells. Int. J. Mol. Sci. 13, 1173–1185. doi: 10.3390/ijms13011173
Jaradat, Z. W., Mousa, W. A., Elbetieha, A., Nabulsi, A. A., and Tall, B. D. (2014). Cronobacter spp.—opportunistic food-borne pathogens. A review of their virulence and environmental—adaptive traits. J. Med. Microbiol. 63, 1023–1037. doi: 10.1099/jmm.0.073742-0
Joseph, S., and Forsythe, S. J. (2011). Predominance of Cronobacter Sakazakii sequence type 4 in neonatal infections. Emerg. Infect. Dis. 17, 1713–1715. doi: 10.3201/eid1709.110260
Kaang, B. K., Kandel, E. R., and Grant, S. G. (1993). Activation of cAMP-responsive genes by stimuli that produce long-term facilitation in Aplysia sensory neurons. Neuron 10, 427–435.
Kalin, N. H., Takahashi, L. K., and Chen, F. L. (1994). Restraint stress increases corticotropin—releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 656, 182–186.
Kandel, E. R. (2001). The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038. doi: 10.1126/science.1067020
Kandhai, M. C., Reij, M. W., Gorris, L. G. M., Guillaume-Gentil, O., and van Schothorst, M. (2004). Occurrence of Enterobacter sakazakii in food production environments and households. Lancet 363, 39–40. doi: 10.1016/S0140-6736(03)15169-0
Kim, H., Yang, E., Lee, J., Kim, S. H., Shin, J. S., Park, J. Y., et al. (2008). Double-stranded RNA mediates interferon regulatory factor 3 activation and interleukin-6 production by engaging Toll-like receptors 3 in the human brain astrocytes. Immunology 124, 480–488. doi: 10.1111/j.1365-2567.2007.02799.x
Kolb, B., and Whishaw, I. Q. (1989). Plasticity in the neocortex: mechanisms underlying recovery from early brain damage. Prog. Neurobiol. 32, 235–276.
Lai, K. K. (2001). Enterobacter sakazakii infections among neonates, infants, children, and adults. Case reports and a review of the literature. Medicine (Baltimore). 80, 113–122. doi: 10.1097/00005792-200103000-00004
Liu, L., Yang, Y., Cui, J., Liu, L., Liu, H., Hu, G., et al. (2013). Evaluation and implementation of a membrane filter method for Cronobacter detection in drinking water. FEMS Microbiol. Lett. 344, 60–68. doi: 10.1111/1574-6968.12155
Martin, E. I., Ressler, K. J., Binder, E., and Nemeroff, C. B. (2009). The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Clin. Lab. Med. 30, 865–891. doi: 10.1016/j.psc.2009.05.004
Meneses, A. (2007). Stimulation of 5-HT1A, 5-HT1B, 5-HT2A/2C, 5-HT3 and 5-HT4 receptors, or 5-HT uptake inhibition: short and long memory. Behav. Brain Res. 184, 81–90. doi: 10.1016/j.bbr.2007.06.026
Meneses, A. (2013). 5-HT systems: emergent targets for memory formation and memory alterations. Rev. Neurosci. 24, 629–664. doi: 10.1515/revneuro-2013-0026
Meneses, A., Perez-Garcia, G., Ponce-Lopez, T., Tellez, R., and Castillo, C. (2011). Serotonin and memory. Neuropharmocology 61, 355–363. doi: 10.1016/j.neuropharm.2011.01.018
Meyer, J. H., Wilson, A. A., Sagrati, S., Miler, L., Rusjan, P., Bloomfield, P. M., et al. (2009). Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch. Gen. Pyschiatry 66, 1304–1312. doi: 10.1001/archgenpsychiatry.2009.156
Mittal, R., Bulgheresi, S., Emami, C., and Prasadarao, N. V. (2009). Enterobacter sakazakii targets DC-SIGN to induce immunosuppressive responses in dendritic cells by modulating MAPKs. J. Immunol. 183, 6588–6599. doi: 10.4049/jimmunol.0902029
Muytjens, H. L., Zanen, H. C., Sonderkamp, H. J., Kollée, L. A., Wachsmuth, I. K., and Farmer, J. (1983). Analysis of eight cases of neonatal meningitis and sepsis due to Enterobacter sakazakii. J. Clin. Microbiol. 18, 115–120.
Narayanan, V., Heiming, R. S., Janses, F., Lesting, J., Sachser, N., Pape, H.-C., et al. (2011). Social defeat: impact on fear extinction and amygdala-prefrontal cortical theta synchrony in 5-HTT deficient mice. PLoS ONE 6:e22600. doi: 10.1371/journal.pone.0022600
Okun, E., Griffioen, K. J., and Mattson, M. P. (2011). Toll-like receptor in neural plasticity and disease. Trends Neurosci. 34, 269–281. doi: 10.1016/j.tins.2011.02.005
Okun, E., Griffioen, K. J., Lathia, J. D., Tang, S. C., Mattson, M. P., and Arumugam, T. V. (2009). Toll-like receptors in neurodegeneration. Brain. Res. Rev. 59, 278–292. doi: 10.1016/j.brainresrev.2008.09.001
Okun, E., Griffioen, K., Barak, B., Roberts, N. J., Castro, K., Pita, M. A., et al. (2010). Toll—like receptor 3 inhibits memory retention and constrains hippocampal neurogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 15625–15630. doi: 10.1073/pnas.1005807107
Pandey, S., and Agrawal, D. K. (2006). Immunobiology of toll like receptors: emerging trends. Immunol. Cell. Biol. 84, 333–341. doi: 10.1111/j.1440-1711.2006.01444.x
Pérez, A, R., Bottasso, O., and Savino, W. (2009). The impact of infectious diseases upon neuroendocrine circuits. Neuroimmunomodulation 16, 96–105. doi: 10.1159/000180264
Perez-Garcia, G., and Meneses, A. (2009). Memory time-course: mRNA 5-HT1A and 5-HT7 receptors. Behav. Brain Res. 202, 102–113. doi: 10.1016/j.bbr.2009.03.027
Qin, Z., DeFee, M., Isaacs, J, S., and Parsons, C. (2010). Extracellular Hsp90 serves as co-factor for MAPK activation and latent viral gene expression during de novo infection by KSHV. Virology 403, 92–102. doi: 10.1016/j.virol.2010.03.052
See, K. C., Than, H. A., and Tang, T. (2007). Enterobacter sakazakii bacteraemia with multiple splenic abscesses in a 75-year-old woman: a case report. Age Ageing 36, 595–596. doi: 10.1093/ageing/afm092
Seyedabadi, M., Fakhfouri, G., Ramezani, V., Mehr, S. E., and Rahimian, R. (2014). The role of serotonin in memory: interactions with neurotransmitters and downstream signaling. Exp. Brain. Res. 232, 723–738. doi: 10.1007/s00221-013-3818-4
Shapiro, R. S., Sellam, A., Tebbji, F., Whiteway, M., Nantel, A., and Cowen, L. E. (2012). Pho85, Pcl 1, and Hms1 Signaling governs Candida albicans morphogenesis induced high temperature or Hsp90 compromise. Curr. Biol. 22, 461–470. doi: 10.1016/j.cub.2012.01.062
Shin, L. M., and Liberzon, I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191. doi: 10.1038/npp.2009.83
Smith, D. R., McCarthy, S., Chrovian, A., Olinger, G., Stossel, A., Geisbert, T. W., et al. (2010). Inhibition of heat shock protein 90 reduces Ebola virus replication. Antiviral. Res. 87, 187–194. doi: 10.1016/j.antiviral.2010.04.015
Song, K.-Y., Hyeon, J. Y., Shin, H. C., Park, C. K., Choi, I. S., and Seo, K. H. (2008). Evaluation of a chromogenic medium supplemented with glucose for detecting Enterobacter sakazakii. J. Microbiol. Biotechnol. 18, 579–584.
Standart, N., and Jackson, R. J. (1994). Regulation of translation by specific protein/m-RNA interactions. Biochimie 76, 867–879. doi: 10.1016/0300-9084(94)90189-9
Stanislawska, J., Interewicz, B., and Olszewski, W. L. (2004). Influence of bacterial antigens on activation of human splenic dendritic cells. Ann. Transplant. 9, 54–57.
Stansley, B. J., and Yamamoto, B. K. (2015). Behavioral impairments and serotonin reductions in rats after chronic L-dopa. Psychopharmacology (Berl). 232, 3203–3213. doi: 10.1007/s00213-015-3980-4
Tang, S. C., Arumugam, T. V., Xu, X., Cheng, A., Mughal, M. R., Jo, D. G., et al. (2007). Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc. Natl. Acad. Sci. U.S.A. 104, 13798–13803. doi: 10.1073/pnas.0702553104
Tellez, R., Gómez-Víquez, L., and Meneses, A. (2012). GABA, Glutamate, dopamine and serotonin transporter expression on memory formation and amnesia. Neurobiol. Learn. Mem. 97, 189–201. doi: 10.1016/j.nlm.2011.12.002
Townsend, S. M., Hurrell, E., Gonzalez-Gomez, I., Lowe, J., Frye, J. G., Forsythe, S., et al. (2007). Enterobacter sakazakii invades brain capillary endothelial cells, persists in human macrophages influencing cytokine secretion and induces severe brain pathology in the neonatal rat. Microbiology 153, 3538–3547. doi: 10.1099/mic.0.2007/009316-0
Tsai, H.-Y., Liao, C. H., Huang, Y.-T., Lee, P.-I., and Hsueh, P.-R. (2013). Cronobacter infections not from infant formula, Taiwan. Emerg. Infect. Dis. 19, 167–169. doi: 10.3201/eid1901.120774
van Heesch, F., Prins, J., Konsman, J. P., Korte-Bouws, G. A., Westphal, K. G., Rybka, J., et al. (2014). Lipopolysaccharide increases degradation of central monoamines: an in vivo microdialysis study in the nucleus accumbens and medial prefrontal cortex of mice. Eur. J. Pharmacol. 725, 55–63. doi: 10.1016/j.ejphar.2014.01.014
Wellman, C. L., Izquierdo, A., Garrett, J. E., Martin, K. P., Carroll, J., Millstein, R., et al. (2007). Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J. Neurosci. 27, 684–691. doi: 10.1523/JNEUROSCI.4595-06.2007
Wiens, M., Korzhev, M., Perovic-Ottstadt, S., Luthringer, B., Brandt, D., Klein, S., et al. (2007). Toll like receptors are part of innate defense system of Sponges (Demospongiae: Porifera). Mol. Bio. Evol. 24, 792–804. doi: 10.1093/molbev/msl208
Wilkie, G. S., Dickson, K. S., and Gray, N. K. (2003). Regulation of mRNA translation by 5′- and 3′-UTR binding factors. Trends. Biochem. Sci. 28, 182–188.
Xie, Y., Song, L., Weng, Z., Liu, S., and Liu, Z. (2015). Hsp90, Hsp 60, sHsp families of heat shock protein genes in channel catfish and their expression after bacterial infection. Fish Shellfish. Immunol. 44, 642–651. doi: 10.1016/j.fsi.2015.03.027
Yan, Q. Q., Condell, O., Power, K., Butler, F., Tall, B. D., and Fanning, S. (2012). Cronobacter species (formerly known as Enterobacter Sakazakii) in powdered infant formula: a review of our current understanding of the biology of this bacterium. J. Appl. Microbiol. 113, 1–15. doi: 10.1111/j.1365-2672.2012.05281.x
Yoon, Y., McKenna, M. C., Rollins, D. A., Song, M., Nuriel, T., Gross, S. S., et al. (2013). Anxiety—associated alternative polyadenylation of the serotonin transporter mRNA confers translational regulation by hnRNPK. Proc. Natl. Acad. Sci. U.S.A. 110, 11624–11629. doi: 10.1073/pnas.1301485110
Zare, N., Motamedi, F., Digaleh, H., Khodagholi, F., and Maghsoudi, N. (2015). Collaboration of geldanamycin-activated P70S6K and Hsp70 against beta-amyloid-induced hippocampal apoptosis: an approach to long-term memory and learning. Cell Stress Chaperones 20, 309–319. doi: 10.1007/s12192-014-0550-3
Zhong, H., Sánchez, C., and Caron, M. G. (2012). Consideration of allosterism and interacting proteins in the physiological functions of the serotonin transporter. Biochem. Pharmacol. 83, 435–442. doi: 10.1016/j.bcp.2011.09.020
Keywords: Cronobacter sakazakii, animal model, fear memory, Hsp-90, SERT, microRNA-16
Citation: Sivamaruthi BS, Madhumita R, Balamurugan K and Rajan KE (2015) Cronobacter sakazakii infection alters serotonin transporter and improved fear memory retention in the rat. Front. Pharmacol. 6:188. doi: 10.3389/fphar.2015.00188
Received: 29 June 2015; Accepted: 19 August 2015;
Published: 04 September 2015.
Edited by:
Alfredo Meneses, Center for Research and Advanced Studies, MexicoReviewed by:
Santiago J. Ballaz, University of Navarra, SpainJohn P. Dougherty, National Institute of Diabetes and Digestive and Kidney Diseases, USA
Copyright © 2015 Sivamaruthi, Madhumita, Balamurugan and Emmanuvel Rajan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Koilmani E. Rajan, Department of Animal Science, School of Life Sciences, Bharathidasan University, Palkalaiperur, Tiruchirappalli 620024, India, emmanuvel1972@yahoo.com
 Bhagavathi S. Sivamaruthi
Bhagavathi S. Sivamaruthi