- 1Department of Pharmaceutical Biology, Johannes Gutenberg University, Mainz, Germany
- 2Medical and Clinical Affairs Phytomedicines, Steigerwald Arzneimittelwerk GmbH, Bayer Consumer Health, Darmstadt, Germany
Inflammatory bowel diseases (IBD) are chronic relapsing intestinal disorders characterized by up-regulation of pro-inflammatory cytokines followed by invasion of immune cells to the intestinal lamina propria. Standard therapies consist of anti-inflammatory or immunosuppressive drugs. Since clinical efficiency is not satisfactory and the established drugs have massive side effects, new strategies to treat IBD are required. Herein, we investigate the protective effect of the fixed combination herbal preparations STW5 and STW5-II and the contribution of the corresponding single components in an in vitro inflammation model. The normal human colon epithelial cell line, NCM460, was treated with STW5, STW5-II or their single components for 4 h followed by experimental conditions comparable to induction of colitis. A pro-inflammatory cytokine cocktail consisting of TNF-α, IL-β, and IFN-γ was used to simulate inflammatory stimuli normally caused by immune cells. The effects on NCM460 cells were investigated by enzyme-linked immunoassay and Proteome Profiler®. Levels of IP-10, MCP-1, I-TAC, Groα, and IL-8 were elevated in chemokine-treated cells compared to untreated cells, but significantly reduced upon pretreatment with STW5 or STW5-II. However, the single compounds revealed only little effects on protein expression. Furthermore, we investigated the effect of both combination preparations on pro-inflammatory transcription factors of the STAT family using Western blot. In addition, we tested the effects on upstream MAPK p38. Both, STW5 and STW5-II did not show any effect on MAPK p38, but were effective in reducing phosphorylated levels of STAT1. In conclusion, both combination preparations act in an anti-inflammatory manner by influencing cytokine secretion via reduced activity of the JAK/STAT1 pathway. Relevant differences between STW5 and STW5-II were not found indicating similar efficacies.
Introduction
Crohn’s disease and UC are the most common forms of IBD with high incidence and prevalence in western civilizations (Karlinger et al., 2000). Both are characterized by a dysregulated balance between pro- and anti-inflammatory processes in the intestinal tract leading to chronic inflammation (Vecchi Brumatti et al., 2014). Although the etiology of IBD remains unclear, genetic, and environmental factors have been implicated in disease pathogenesis (Ferguson et al., 2007; Xavier and Podolsky, 2007).
Medicinal standard therapies comprise anti-inflammatory agents such as 5-aminosalicylate, glucocorticoids, immuno-suppressant drugs, or therapeutic antibodies (Abraham and Cho, 2009; Danese and Fiocchi, 2011). Diagnosis of IBD is based on symptoms and endoscopic evaluation (Kugathasan et al., 2003). The intestinal inflammation is often monitored by clinical indices and invasive techniques (Crama-Bohbouth et al., 1989; Langhorst et al., 2008). Cytokines may act as surrogate markers, since elevated levels have been found in the intestinal mucosa of patients with IBD.
Cytokines are biologically active compounds involved in immune reactions and can be classified as being pro- or anti-inflammatory. They are produced by both resident and migrating cells. A subset of cytokines, called chemokines, is responsible for the migration of immune cells to the site of inflammation (Calixto et al., 2004). Targeting cytokine pathways is a promising target for treating IBD and is already established in the case of anti-tumor necrosis factor-alpha (TNF-α) antibodies. Side effects associated with these therapies, e.g., high risk of infections (Khanna and Feagan, 2015) underscore the need of novel improved treatment strategies.
Some natural products showed promising activities in the treatment of intestinal inflammation (Schneider et al., 2014), and could present an additional treatment option for IBD. STW5 (Iberogast®) consists of a fixed combination of nine hydro-alcoholic extracts of medicinal plants which have been traditionally used in Europe to treat diverse gastrointestinal illnesses. It is a well-tolerated herbal medicinal product, clinically used for the treatment of functional dyspepsia and irritable bowel syndrome (Holtmann et al., 2004; Allescher and Wagner, 2007) and shown to be effective in gastroesophageal reflux disease models (Abdel-Aziz et al., 2010, 2015; Patrick, 2011). In animal models of UC, STW5 was able to reduce inflammatory markers and histological changes (Michael et al., 2012; Wadie et al., 2012).
The aim of the present study was to investigate the mechanisms of action of STW5 (fixed combination of nine herbal extracts; Iberogast®), the investigational preparation STW5-II (fixed combination of six herbal extracts, lacking three extracts of STW5), and their corresponding single extracts in terms of cytokine secretion from inflamed intestinal cells.
Materials and Methods
Substances
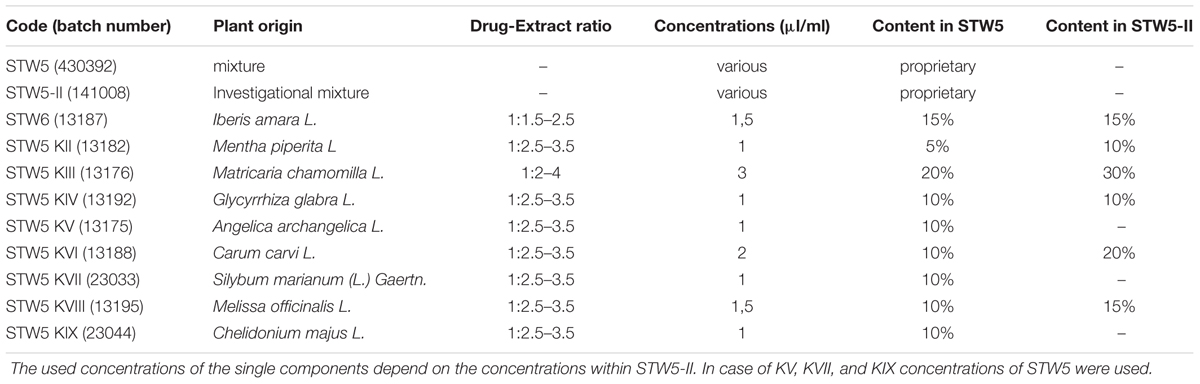
STW5 was provided in the form of lyophylisates by Steigerwald Arzneimittelwerk GmbH (Darmstadt, Germany). The preparation consists of the 50% (v/v) hydroethanolic fresh plant extract of Iberis amara L. (Brassicaceae) whole plants and the 30% hydroethanolic extracts of dried Melissa officinalis L. (Lamiaceae) leaves, Matricaria chamomilla (Compositae) flowers, Carum carvi L. (Apiaceae) fruits, Mentha piperita L. (Lamiaceae), Angelica archangelica L. (Apiaceae) roots, Silybum marianum (L.) Gaertn. (Compositae) fruits, Chelidonium majus L. (Papaveraceae) herbs, and Glycyrrhiza glabra L. (Leguminosae) roots. STW5-II (investigational mixture) and the single extracts were also provided as lyophylisates (Table 1). The preparations and their single extracts are well characterized according to validated analytical methods. They belong according to the guidelines of the European Medicines Agency to “other herbal substances.” The extraction processes as well as the quality controls were previously described in detail (Kroll and Cordes, 2006; Allam et al., 2015). Briefly, the extracts were produced and their quality controlled according to Good Manufacturing Practice and Good Agricultural Practice of Medicinal and Aromatic Plants (cited and outlined in Kroll and Cordes, 2006). The quality of each extract is tested according to individual specifications, among which is the identity of the used drug (chromatographic fingerprint) and the content of marker substances (Allam et al., 2015) within a defined range, as measured by high performance liquid chromatography or gas-liquid chromatography.
Lyophilisates were freshly re-suspended in Dulbecco’s modified eagle medium (DMEM; Life Technology, Darmstadt, Germany) supplemented with 1% non-essential amino acids (NEAA, Life Technology, Darmstadt, Germany) shortly before use.
Cell Culture
NCM460 cells were purchased from INCELL Corporation (San Antonio, TX, USA) and cultured in M3 media (San Antonio, TX, USA) supplemented with 10% FBS (fetal bovine serum), 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a 95% humidified atmosphere with 5% CO2. Cells were passaged at pre-confluent densities using 0.05% trypsin and 0.5 mm EDTA (Invitrogen, Karlsbad, Germany).
Cell Viability Assay
The cytotoxic effects of the test extracts against NCM460 were assessed after 24 h using a resazurin reduction assay (RRA) according to O’Brien et al. (2000). Cells were seeded in 96-well plates at a density of 10,000 cells per well in 0.1 ml medium. After 24 h cultivation, cells were treated with different concentrations of the plant extracts or DMEM (negative control). Following 24 h incubation at 37°C and 5% CO2, 20 μl resazurin working solution (0.01% w/v) were added per well. After another 4 h incubation, the resazurin solution was analyzed by fluorometry using the Infinite M2000 ProTM plate reader (excitation at 544 nm and emission at 590 nm, Tecan, Crailsheim, Germany). The cytotoxicity of the test extracts was evaluated by comparing values of emission at 590 nm for the treated probes to normalized medium controls. The mean absorbance for the negative control (Medium) was normalized as 100%.
Proteome ProfilerTM Human Cytokine Antibody Array
To evaluate anti-inflammatory effects of plant extracts on NCM460 cells, the cytokine and chemokine production was investigated using the ‘Human Cytokine Array Panel A’ array system obtained from R&D Systems GmbH (Wiesbaden-Nordenstadt, Germany). Following 3-days cultivation of NCM460 cells in a 12 well plate under standard conditions, medium was removed and cells were washed with PBS. Cells were pre-treated with plant extracts for 4 h and subsequently inflammation was induced for 24 h using a human CM consisting of 10 ng/ml TNF-α, 5 ng/ml IL-1β, and 10 ng/ml IFN-γ. After centrifugation, the supernatant was collected and analyzed according to the manufacturer’s instructions.
Enzyme-Linked Immunoassay
As the “Proteome ProfilerTM” delivers only semi-quantitative data, enzyme-linked immunosorbent assays were performed using DuoSet Kits from R&D Systems according to the manufacturer’s instructions. Aliquots of 100 μl were pipetted onto the plates coated with the capture antibody. Plates were analyzed using the Infinite M2000 ProTM plate reader (Tecan) at a wavelength of 450 nm.
HEK-Blue Reporter Cell Line
HEK293 cell lines stably expressing HEK-Blue-Null1 vector for secreted alkaline phosphatase on a NF-κB promoter were purchased from InvivoGen (San Diego, CA, USA). The cells were cultured according to the manufacturer’s recommendations. The cells were treated with varying concentrations of STW5 or STW5-II for 4 h and then inflammation was induced with TNF-α (10 ng/ml) for 24 h. NF-κB activation was spectrophotometrically detected by measuring secreted embryonic alkaline phosphatase at 630 nm upon Quanti Blue addition (InvivoGen, San Diego, CA, USA). A known NF-κB inhibitor, MG-132 was used as positive control (Zanotto-Filho et al., 2011).
Cell Extracts
NCM460 cells were seeded at 20,000 cells/cm2 in a 6-well plate and grown for 3 days in RPMI media supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 95% humidified atmosphere with 5% CO2. Medium was removed and replaced with DMEM supplemented with 1% NEAA containing or not containing the STW extracts. After 4 h incubation, the medium was removed and cells were induced for various times with human CM consisting of 10 ng/ml TNF-α, 5 ng/ml IL-1β, and 10 ng/ml IFN-γ. Cells were washed with PBS, incubated with cell lysis buffer (New England Biolabs) for 5 min and collected. Lysated cells were sonicated four times for 5 min and centrifuged at 14,000 × g for 10 min. The supernatants were used for further analysis.
TransAM ELISA for NF-κB Activity
NF-κB activity was determined using a commercial ELISA kit (TransAM® NF-κB p65, active motif, La Hulpe, Belgium) according to the manufacturer’s instructions. In brief, cell extracts were added to a 96-well plate in which multiple copies of double stranded DNA were immobilized. After the binding to DNA, NF-κB was detected with HRP-linked antibody. Plates were analyzed using the Infinite M2000 ProTM plate reader (Tecan) at a wavelength of 450 nm.
Western Blot
Western Blot was performed as described before (Esquivel-Solís et al., 2009) Protein aliquots (60 μg/well) were loaded on a discontinuous SDS-PAGE for separation. After blotting proteins to a PVDF-membrane, the membrane was incubated overnight with primary antibody; anti-pSTAT1, anti-actin (New England Biolabs). After washing with TBST, membranes were incubated for 1 h with secondary HRP-linked antibody. Before the proteins were detected with LuminataTM Classico Western HRP substrate, the membrane was washed again three times for 5 min with TBST. Documentation of the membranes was performed with ImageJ2 v 2.0 Snap 1.49d software (NIH, Bethesda, MD, USA).
AP-1 Reporter Assay
NCM460 cells were transfected with signal AP-1 reporter kit (Qiagen) according to manufacturer’s instructions. In brief, cells were suspended and added to a mixture of reporter and transfection reagent. After 24 h of transfection, medium was changed to assay medium (Opti-MEM + 0.5% FBS + 0.1mM NEAA + 1 mM Sodium pyruvate + 100 U/ml penicillin + 100 μg/ml streptomycin) and cells were treated with 10 ng/ml of PMA or CM in mentioned concentrations. PMA is a known inducer of AP-1 activity and was used as a positive control. The AP-1 reporter contains a mixture of inducible AP-1-responsive firefly luciferase construct and constitutively expressing renilla luciferase construct (40:1). The assay was developed by using Dual-Luciferase Reporter Assay System from Promega. Cells were lysed within the wells and luciferase assay reagent was added. Luminescence was measured on an Infinite M2000 ProTM plate reader (Tecan). After measuring firefly luciferase activity, the stop solution was added and the measurement was repeated to measure renilla luciferase activity. Relative Luciferase activity was determined as luminescence signal of firefly luciferase activity normalized to the signal of renilla luciferase activity.
MAPK p38 Activity Assay
MAPK p38 activity assay was performed as described before (Huang et al., 1999). NCM460 cells were seeded at 20.000 cells/cm2 in 6-well plates and grown for 3 days before treatment. For kinetic measurements, cells were treated with the CM for different periods. For evaluation of STW5 and STW5-II effects, cells were preincubated with the combination preparations for 4 h before adding the CM. Lysed cells were incubated overnight with immobilized anti-pp38 antibody beads. Kinase assays were performed by incubating the immunoprecipitates with ATF-2 fusion protein and ATP for 30 min at 30°C. The pATF-2 content was determined by western blot.
Results
Cytotoxicity of STW5 and STW5-II
Both STW5 and STW5-II did not show cytotoxic effects in a concentration of up to 20 μl/ml (Figure 1). For further experiments, only non-cytotoxic concentrations were used.
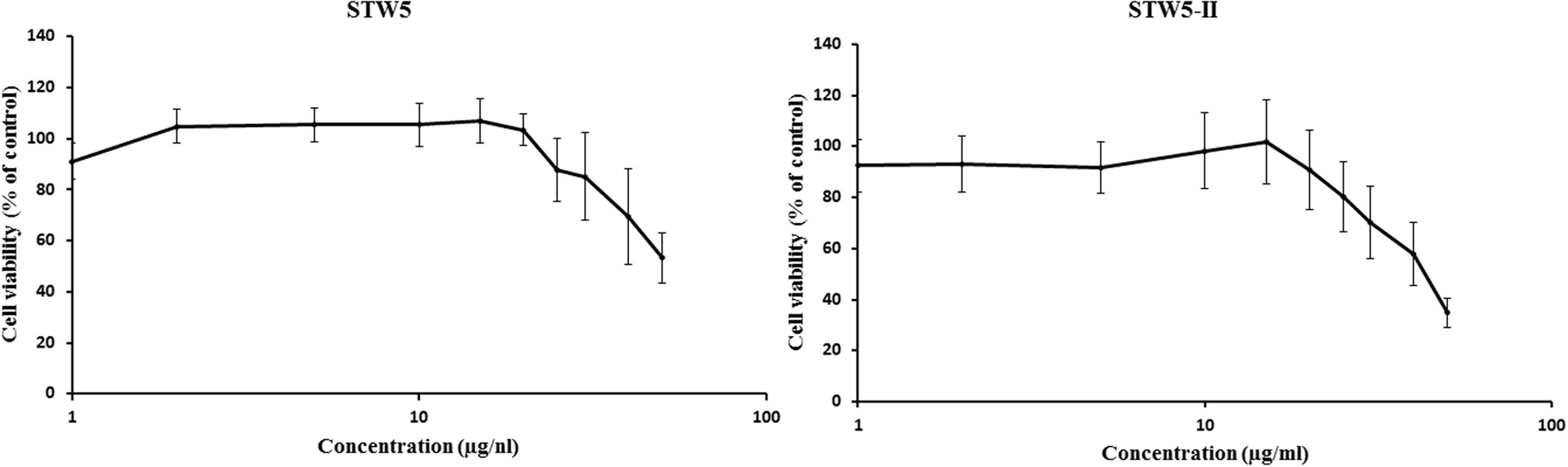
FIGURE 1. Cytotoxic effects of STW5 and STW5-II: NCM460 cells were treated with STW5 or STW5-II for 24 h. Cytotoxicity was measured by resazurin assays by normalizing to untreated control.
STW5 Inhibited Cytokine Release in a Dose-Dependent Manner
Cytokine-challenged NCM460 cells showed elevated levels of IL-8, IP-10, MCP-1, and I-TAC. Treatment with both STW5 and STW5-II led to significantly reduced cytokine levels compared to inflamed control as shown by Proteome Profiler. IP-10 showed the highest induction after cytokine treatment. IL-8, MCP-1, and I-TAC were most effectively reduced by STW5 and STW5-II. These four chemokines were chosen for quantitative examination. ELISA analyses revealed dose-dependent effects of STW5 and STW5-II. Inhibition of I-TAC and MCP-1 secretion was most effective and could be reduced at high doses to the level of untreated cells. Due to the quantitative nature of the ELISA, STW5-II showed a dose-dependent effect over 1–20 μl/ml, which was not seen in the Proteome Profiler (Figure 2). The single plant extracts showed much weaker effects than the two combination preparations (Figure 3).
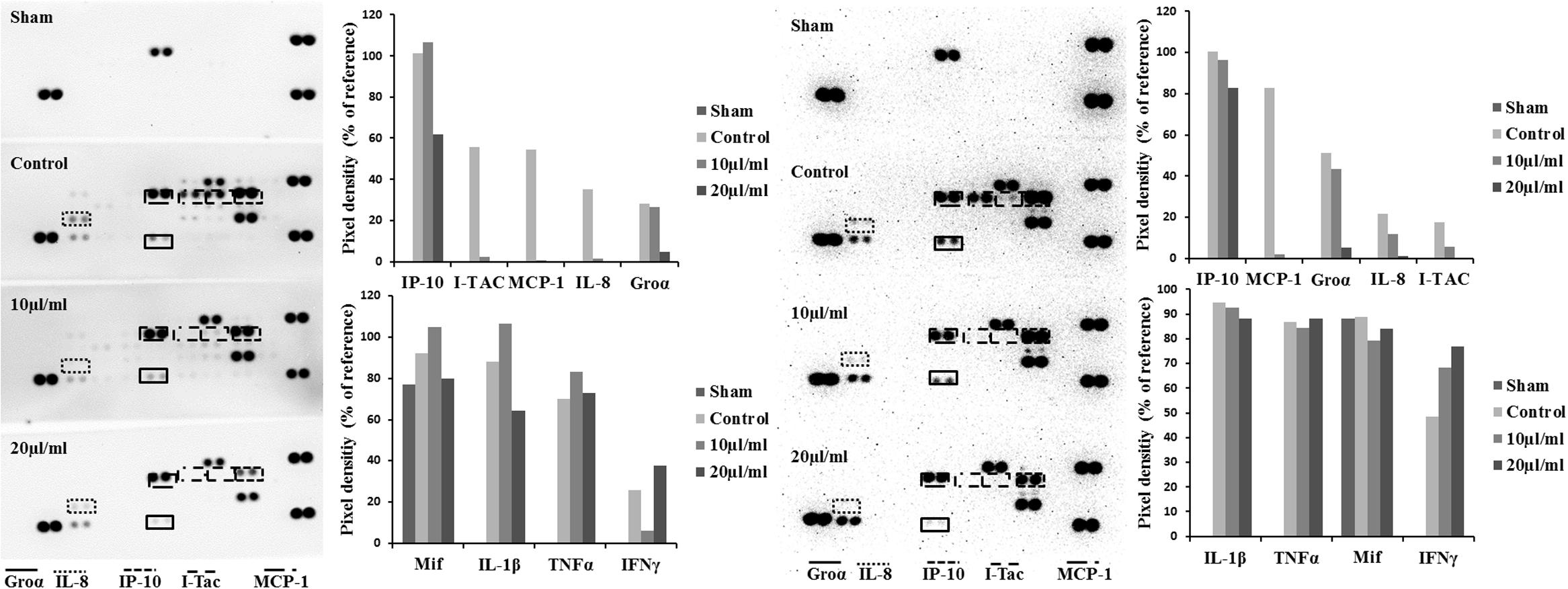
FIGURE 2. Effect of STW5 and STW5 on the cytokine profile of NCM460 cells. Cells were treated with STW5 (left) or STW5-II (right) (10 or 20 μl/ml) for 4 h prior to induction with the CM (10 ng/ml TNF-α, 5 ng/ml IL-1β, and 10 ng/ml IFN-γ) for 24 h. Supernatants were collected and analyzed by Proteome Profiler® and evaluated for Pixel density using ImageJ software.
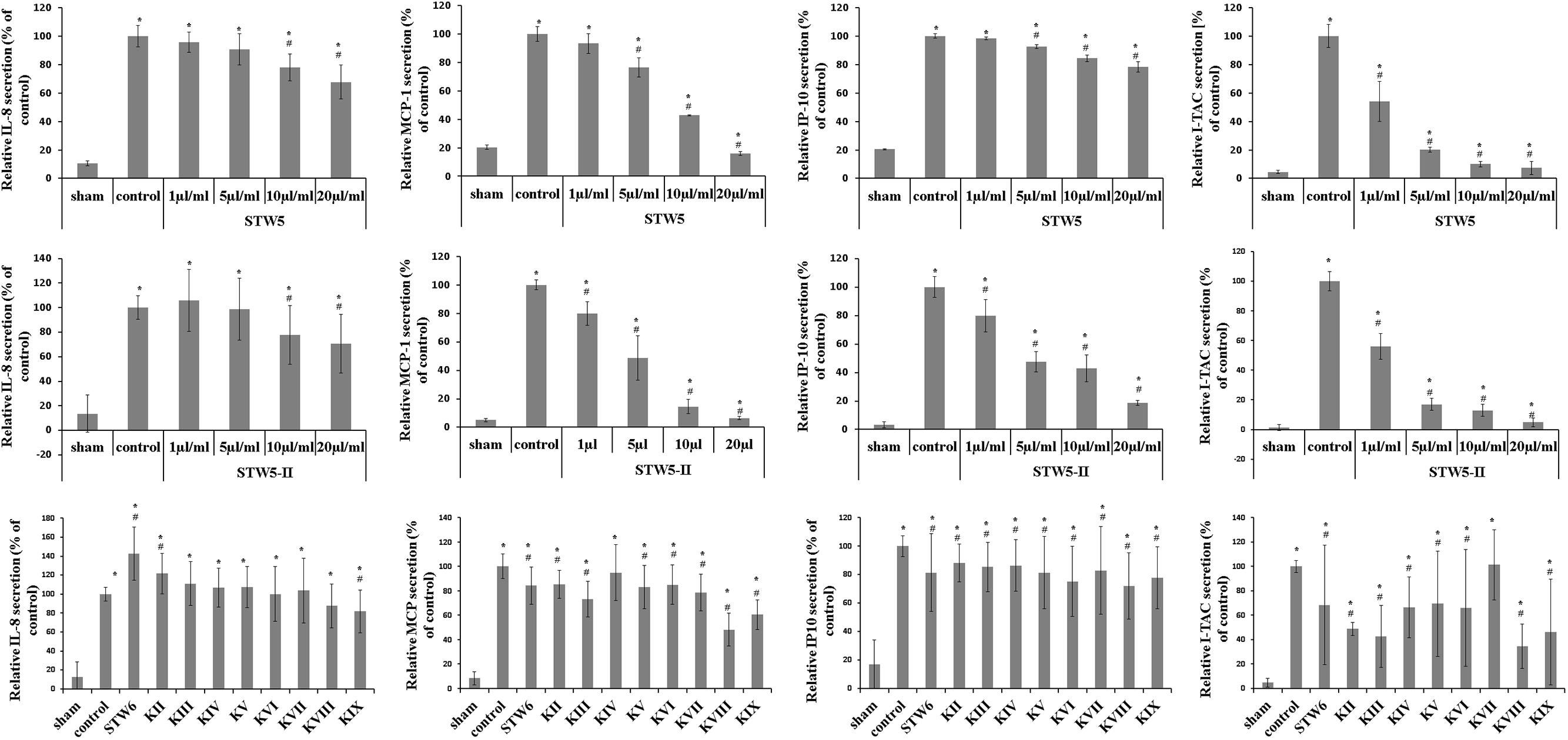
FIGURE 3. Dose-dependent effects of STW5, STW5-II, and single plant extracts on cytokine release. NCM460 cells were treated with STW5 or STW5-II (1; 5; 10; 20 μl/ml) or single plant extract (percentage of STW5-II calculated for 10 μl/ml according to content in herbal preparation) for 4 h prior to induction with the CM (10 ng/ml TNF-α, 5 ng/ml IL-1β, and 10 ng/ml IFN-γ) for 24 h. Supernatants were collected and analyzed by ELISA. # denotes statistical significance at p < 0.05 vs. control, * denotes statistical significance at p < 0.05 vs. sham.
Effect on NF-κB Activation
Cytokine-stimulated NCM460 and HEK Blue Null1 reporter cells showed increased NF-κB activity compared to non-stimulated control cells (sham). The levels of activated NF-κB were slightly reduced by both extract combinations (Figure 4).
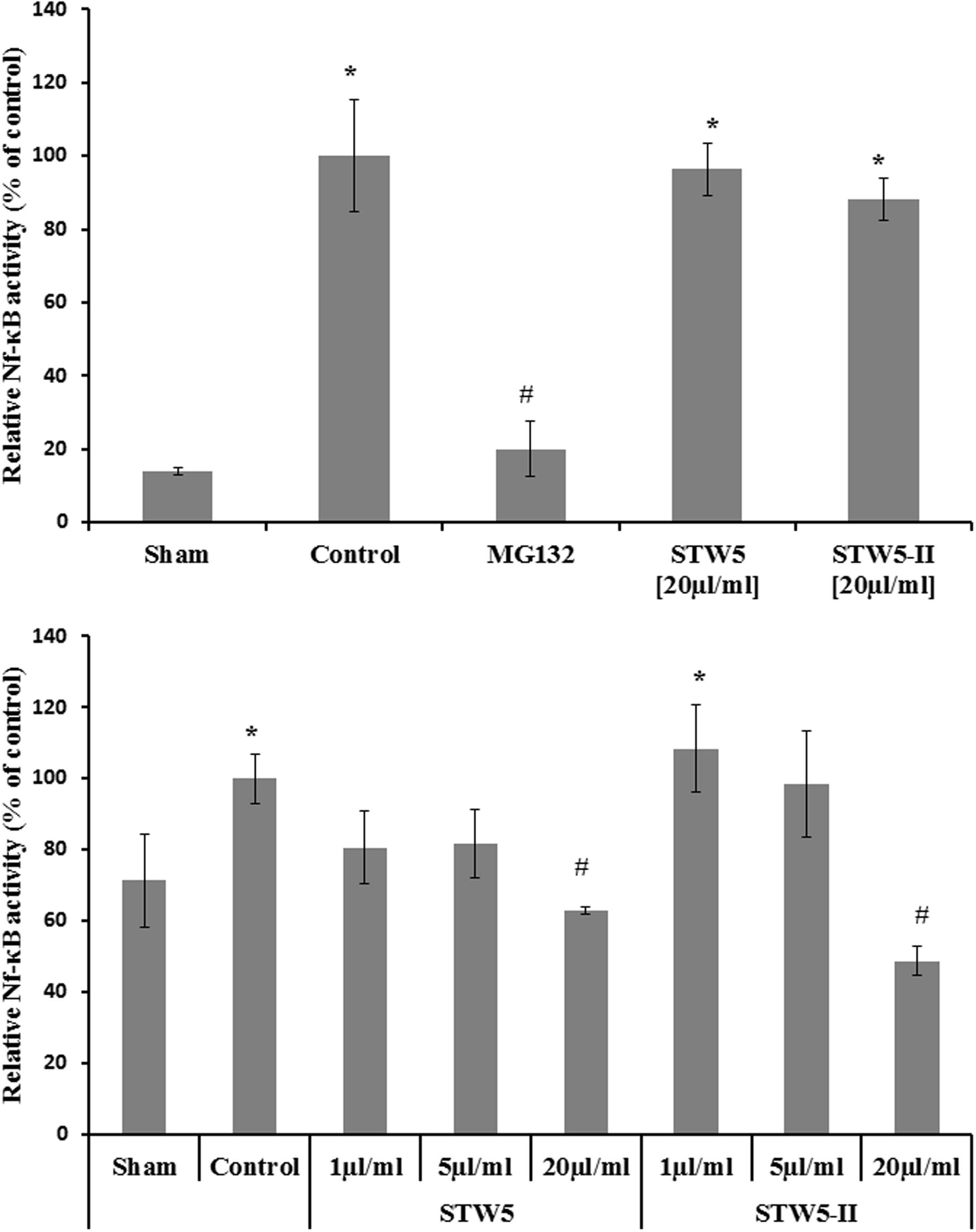
FIGURE 4. Effects of STW5 and STW5-II on NF-κB activity: The upper panel displays the NF-κB activity measured by Quanti Blue assay in HEK reporter cells. The lower panel shows the level of activated NF-κB in NCM460 cell extracts. # denotes statistical significance at p < 0.05 vs. control, * denotes statistical significance at p < 0.05 vs. sham.
Effect of Cytokine Mixture on AP-1 Activation
Cells transfected with an AP-1 reporter plasmid showed increased luciferase activity. Treatment with inflammatory cytokines reduced the signal (Figure 5), rather than increasing it, and therefore did not lead to AP-1 activation. The reporter was also tested with PMA, a standard inducer of AP-1, which led to a marked increase in luciferase activity.
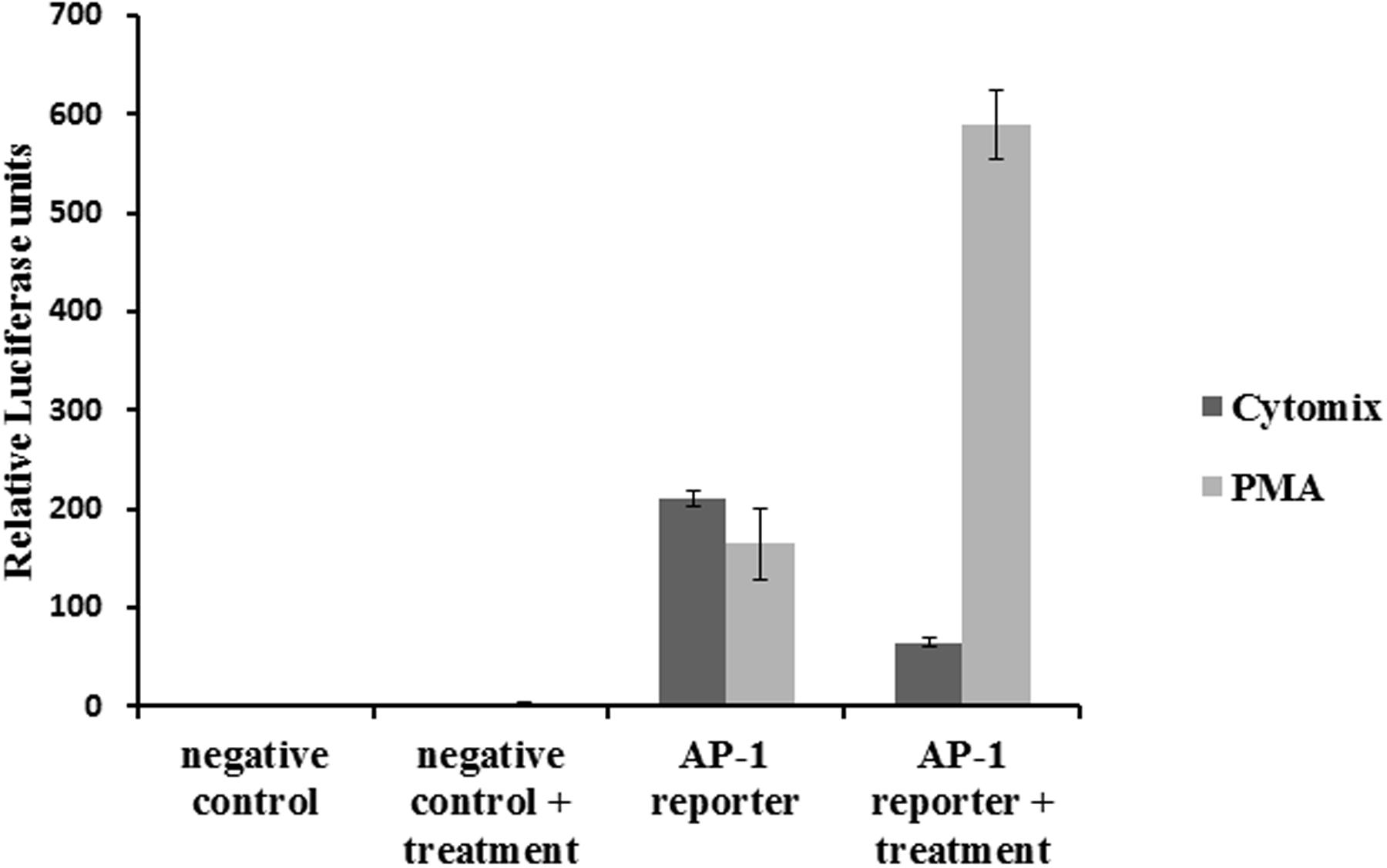
FIGURE 5. Effect of the CM on AP-1 activity: NCM460 cells were transfected with luciferase plasmid under the control of AP-1. Cells were treated with PMA (10 ng/ml) or the CM (10 ng/ml TNF-α, 5 ng/ml IL-1β, and 10 ng/ml IFN-γ) for 24 h. Cell lysates were analyzed for luciferase activity.
Effect on MAPK p38
The activity of p38 was determined at different time points after cytokine treatment. It was five times more active after 10 min compared to the untreated control (Figure 6). Longer incubation times led to reduced activities. Pretreatment with STW5 or STW5-II did not alter the activity (Figure 7).
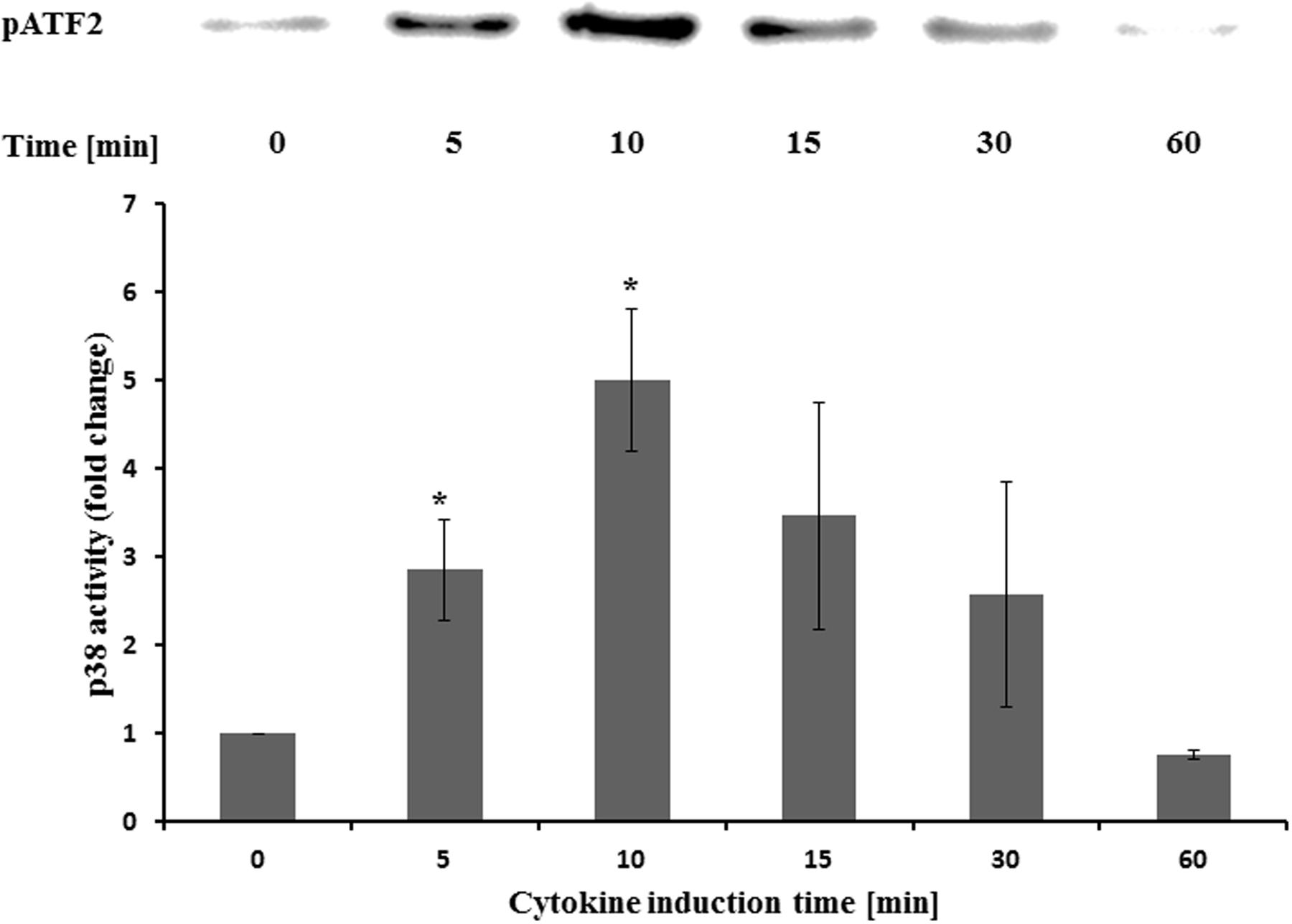
FIGURE 6. Effects of STW5 and STW5-II on p38 activity NCM460 cells were treated with the CM (10 ng/ml TNF-α, 5 ng/ml IL-1β, and 10 ng/ml IFN-γ) for the indicated time points prior to harvesting and p38 activity measurement by ATF-2 phosphorylation. The upper panel shows a representative western blot for pATF-2. The lower panel displays the quantification of three independent western blot experiments (pixel density fold change compared to 0 min; three independent experiments; * denotes statistical significance at p < 0.05 vs. 0 min).
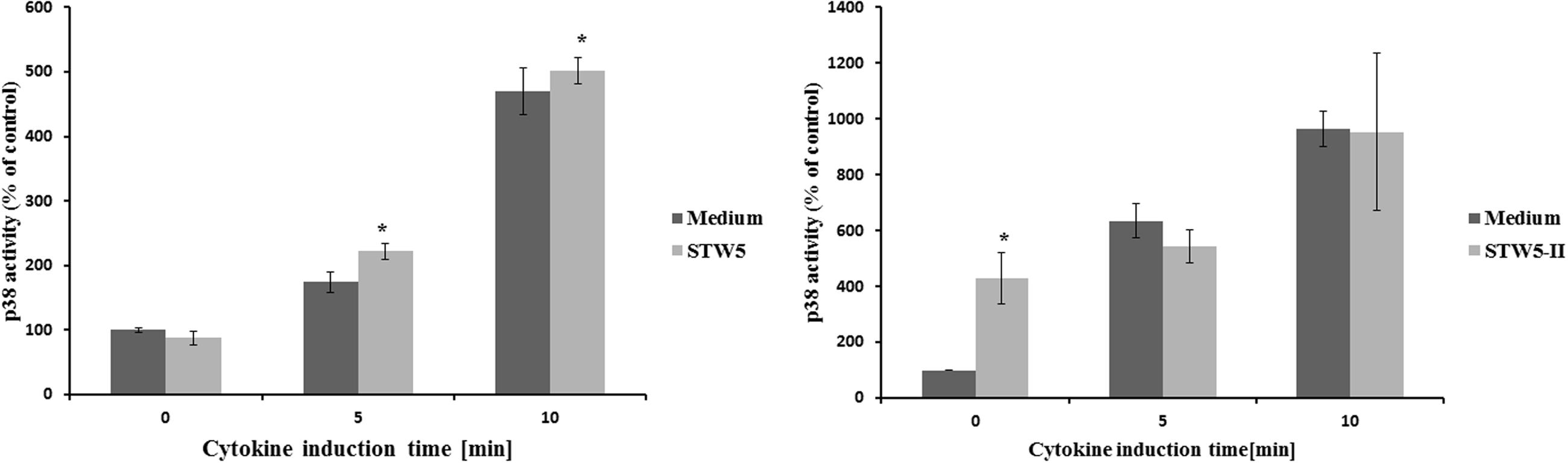
FIGURE 7. Effects of single plant extracts on p38 activity: NCM460 cells were treated with STW5-II or STW5 at 20 μl/ml for 1 h prior to induction with the CM (10 ng/ml TNF-α, 5 ng/ml IL-1β, and 10 ng/ml IFN-γ) for the indicated time points. Cell lysates were used to measure p38 activity by ATF-2 phosphorylation (pixel density fold change compared to 0 min medium treatment; three independent experiments; * denotes statistical significance at p < 0.05 vs. medium treatment at the same induction time).
Effect on STAT1
Cytokine treatment led to an increased phosphorylation of STAT1 protein compared to non-treated normal controls. Treatment with STW5-II or STW5 reversed this effect in a dose-dependent manner. STW5-II was slightly more effective than STW5 (Figure 8). None of the single extracts was able to mimic these effects.
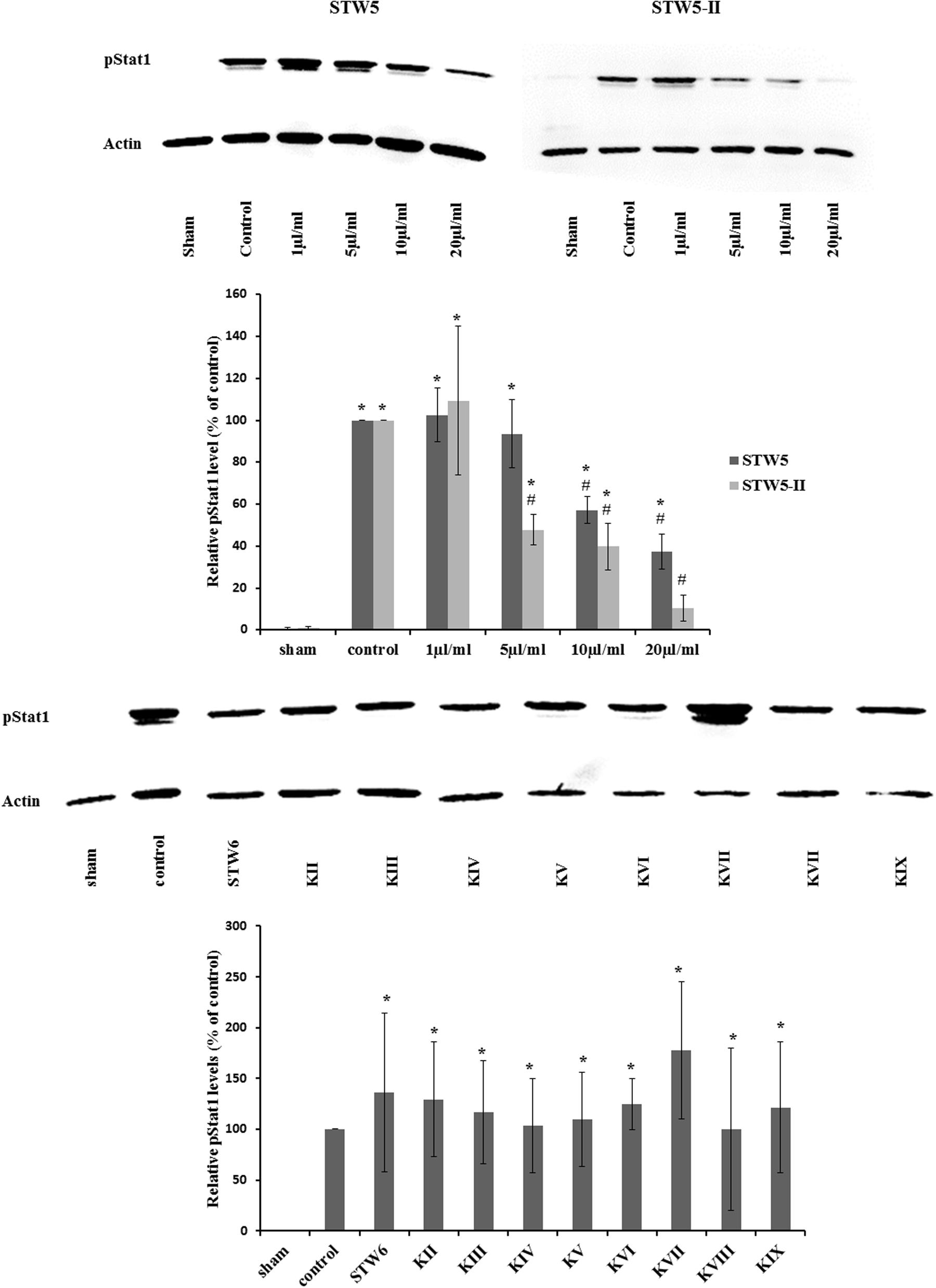
FIGURE 8. Effect of STW5, STW5-II, and single plant extracts on Stat1 phosphorylation: NCM460 cells were treated with STW5 or STW5-II at indicated concentrations or single plant extract (percentage of STW5-II calculated for 10 μl/ml according to content in the herbal preparation) for 4 h prior to induction with the CM (10 ng/ml TNF-α, 5 ng/ml IL-1β, and 10 ng/ml IFN-γ) for 30 min. Cell extracts were analyzed for phosphorylated Stat1 protein by western blotting. The lower panel displays the ratio of pStat1 to actin in relation to the control sample (n = three independent experiments; # denotes statistical significance at p < 0.05 vs. control, * denotes statistical significance at p < 0.05 vs. sham).
Statistical Analysis
Results are represented as mean ± SEM. P-values were calculated by Student’s t-test. Differences were considered as significant at P < 0.05.
Discussion
STW5 (Iberogast®) was shown to be effective in a TNBS model of intestinal inflammation via Adenosine A2A receptor activation, and hence inhibition of the TNF-alpha pathway. Furthermore, STW5 increased IL-10 levels in mucosal preparations of mice and reduced TNF-α secretion in LPS-induced human monocytes (Michael et al., 2009, 2012). In a DSS model of inflammation STW5 was able to reduce biochemical markers and attenuated histological parameters. Nevertheless, its influence on the epithelial cells and their contribution to the inflammation process has yet to be investigated.
In the present study, we have evaluated the effects of the clinically established fixed combination preparation STW5 (Iberogast®) and the modified STW5-II extract combination, which contains only six of the nine herbal extracts in STW5, for their immunomodulatory effects on human colonic epithelial cells and the underlying molecular mechanisms.
Cell models can be used to mimic an inflammatory state usually by treating the cells with an appropriate stimulus (Romier-Crouzet et al., 2009; Fernandes et al., 2016). To avoid artifacts, we used the normal human colon cell line NCM460 (Moyer et al., 1996) rather than colon cancer cell lines. Therefore, we examined whether NCM460 is responsive to stimuli and useful as a model of inflammation. As inflammatory stimulus, a CM consisting of TNF-α, IL-1β, and IFN-γ was used. These cytokines are known to be released in IBD (Beck and Wallace, 1997; Ciccia et al., 2012) and are effective in inducing inflammatory reactions in cell models (Mueller et al., 2013). First, we profiled the cytokine release of the cells by a membrane-based sandwich immunoassay. NCM460 cells were able to respond and secrete a variety of chemokines, some of which are known to be involved in chemotaxis and were linked to IBD. We decided to focus on IL-8; MCP-1; I-TAC, and IP-10, as those were the ones most strongly modulated in our experimental setting. IL-8 is a powerful neutrophil chemoattractant and activator, whose levels are increased in the mucosa of IBD patients (Mitsuyama et al., 1994). It is also an established inflammatory marker in other cell lines (Ren et al., 2014; Akbari et al., 2015; Noh et al., 2015). Levels of MCP-1 were shown to be increased in IBD (Banks et al., 2003) and were associated with the response toward infliximab therapy (Magnusson et al., 2015). I-TAC facilitates the development of Th17 cells and stimulates Th1 cells to produce IL-6 (Liu et al., 2011). Human intestinal cell lines were shown to produce I-TAC after cytokine stimulation (Marion et al., 2004). IP-10 plays a role in the inflammatory process, as it was shown to be increased in mucosal biopsies from patients with UC or CD (Ostvik et al., 2013) and was induced in vitro after IFN-γ stimulation in epithelial cell lines (Dwinell et al., 2001; Marion et al., 2004). It also presents a promising target for treatment of IBD, as a phase II study demonstrated efficacy of anti-IP-10 antibodies in patients suffering from moderate to severe UC (Mayer et al., 2014).
To investigate a dose-effect relationship, we tested the effect of the herbal preparations on chemokine release via ELISA. STW5 was able to reduce IL-8 in a dose-dependent manner, while STW5-II pretreatment led to an increased secretion at low concentrations and decreased release at higher ones. C. majus L. (STW5 KIX) was the only single extract that significantly reduced IL-8 levels. MCP-1 release from colon cells was significantly reduced after pretreatment with both STW5 and STW5-II. Furthermore, almost all single extracts except for G. glabra L. (STW5 KIV) decreased the secretion of MCP-1 significantly, but not as effectively as STW5 or STW5-II. Both combination preparations reduced the cytokine induced IP-10 secretion. Here STW5-II was a lot more effective than STW5. Interestingly, STW5-II showed dose-dependent effects on the IP-10 release over 1–20 μg/ml, but no great difference between 5 and 10 μg/ml and an immense decrease between 10 and 20 μg/ml. This inconsistency indicates further mechanisms of IP-10 inhibition which occurs only in high doses of STW5-II. All single extracts inhibited IP-10 release comparable to STW5 but not to STW5-II. The secretion of I-TAC was inhibited dose-dependently over 1–20 μg/ml for both extract combinations. Again, chemokine levels were stable over 5–10 μg/ml for STW5-II, supporting the theory of various mechanisms involved in the inhibition.
To examine which signaling pathway might be involved in the anti-inflammatory mechanism of STW5 and STW5-II, we first identified molecules that are affected by the cytokine treatment. Since we used TNF-α, IL-1β, and IFN-γ as stimuli, activated pathways should lead to the activation of transcription factors like AP-1, NF-κB, and STAT proteins. Activation of the IL-1β receptor (IL-1R) or the TNF-α receptor (TNFR) results in recruitment of adaptor proteins which in turn activate secondary proteins. The mitogen-activated protein kinase kinase 3 (MEKK3) is a central kinase in both receptor signaling pathways and is able to activate the MAPKs p38 and c-Jun NH(2)-terminal kinase (JNK; Chen and Goeddel, 2002; Wajant et al., 2003). JNK subsequently activates the transcription factor AP-1 (Weston and Davis, 2002). MEKK3 also plays a pivotal role in activating the IκB kinase [IKK, a kinase that phosphorylates the Inhibitor of kappa B (IκB) and thus causes its degradation and subsequent activation of NF-κB]. Other adaptor proteins in the TNF-α signaling pathway also lead to activation of IKK. Activation of the IFN-γ receptor results in the activation of STAT proteins (Ramana et al., 2015). We decided to focus on these key points in the signaling pathways (Figure 9) to gain insights in the molecular mechanisms of STW5. Most of the investigated proteins were shown to be involved in IBD. The MAPK p38 is a key regulator in the activation process of different transcription factors (Chen and Goeddel, 2002; Liacini et al., 2002; Delgado, 2003).
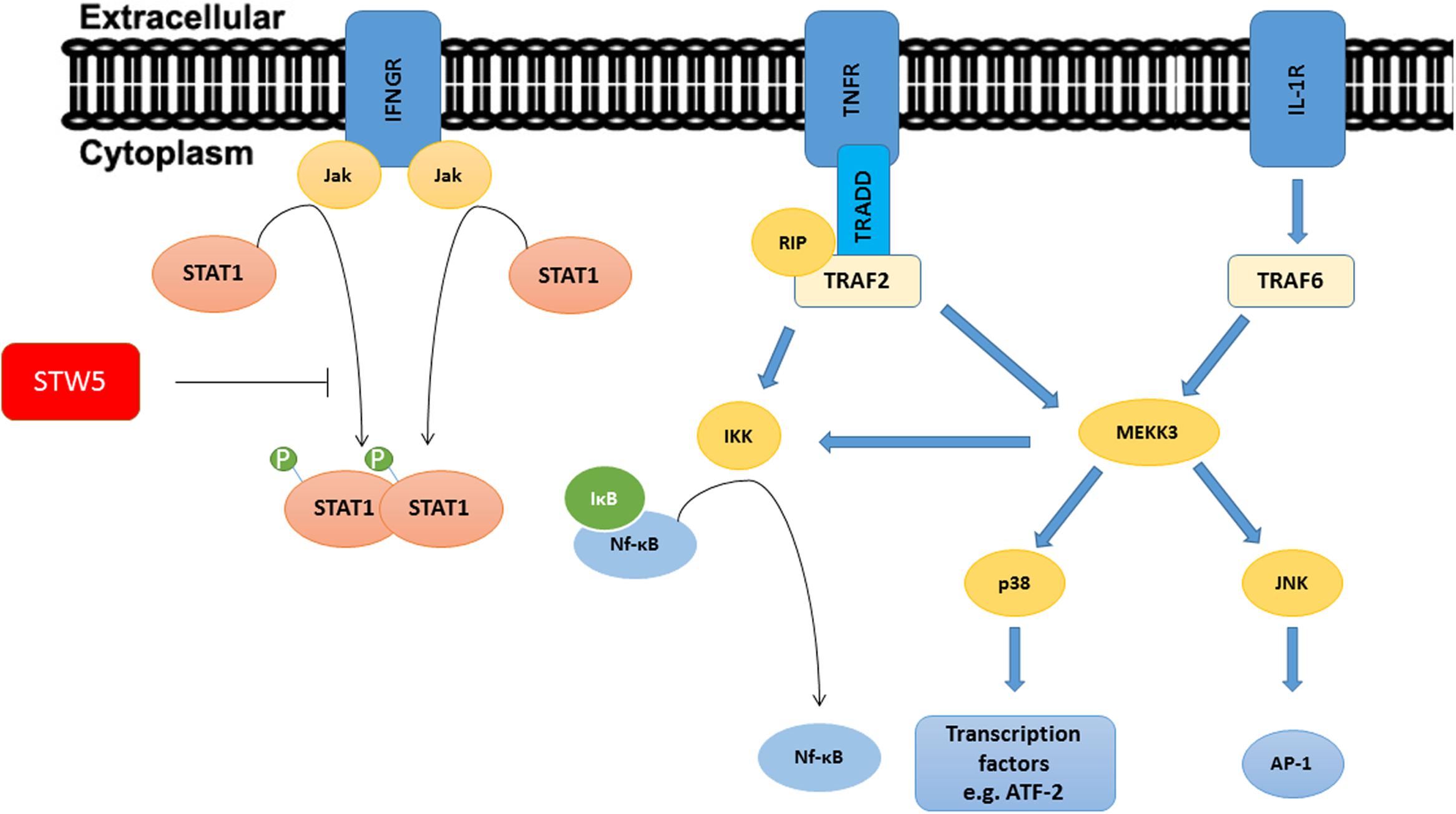
FIGURE 9. Signaling pathways of TNF-α, IL-1β, and IFN-γ: The picture shows a simplified representation of the involved pathways. Recruitment and activation of adaptor proteins lead to the activation of Transcription factors. STW5 and STW5-II reduced the phosphorylation of Stat1.
AP-1 regulates cell responses to a variety of stimuli including cytokines (Hess et al., 2004). Because AP-1 was previously shown to be involved in IL-8 release in vivo and in vitro (Moriyama et al., 2008; Grbic et al., 2012), we examined if AP-1 is also induced with the CM in our experimental setting. The CM was not able to increase the activity of AP-1, indicating that it is not responsible for the cytokine release.
NF-κB is a central transcription factor involved in many cell functions including the regulation of cytokine expression. Cytokines found to be involved in IBD like IL-8 are influenced by NF-κB. Inhibition of the latter was shown to attenuate signs of experimental colitis in mice (Neurath et al., 1996). To determine the activity of NF-κB in NCM460 cells, protein extracts were added to plates in which multiple copies of double stranded consensus DNA were immobilized. Induction of NF-κB was insufficient in this setting, but the results hinted to some activity of STW5 and previous literature data showed a potent effect on NF-κB in colon adenocarcinoma cells (Bonaterra et al., 2013). Therefore, we further examined the influence of cytokines and extract in commercial available HEK-BlueTM Null1 cells which express the SEAP (secreted alkaline phosphatase) reporter gene under the control of the IFN-β minimal promoter fused to five NF-κB and AP-1 binding sites. In these cells, NF-κB activity was sufficiently increased after cytokine treatment. Nevertheless, STW5 and STW5-II had only week effects, not sufficient to explain the inhibition of cytokine release. We concluded that NF-κB activity is not affected by the plant extract combinations in our model.
p38 mitogen-activated protein kinase (MAPK) signaling pathways were shown to be involved in IBD and can be induced by inflammatory cytokines like IL-1β and TNF-α (Feng and Li, 2011). Furthermore, p38 is involved in the activation of transcription factors. To measure the activation of p38, protein extracts were precipitated with immobilized anti-pp38 antibody beads. Activity was measured by the ability of p38 to phosphorylate ATF-2. As expected, the treatment with cytokines increased the activity of p38 which peaked after 10 min induction time. Pretreatment with the extract combinations showed no effect at all during the activation of p38. This indicates that p38 is not affected by STW5 or STW5-II.
STAT proteins are cytoplasmic transcription factors, which dimerize and translocate to the nucleus upon phosphorylation, where they interact with DNA and induce transcription. STAT1 protein expression and phosphorylation were increased in mucosal samples from IBD patients (Schreiber et al., 2002). Also, increased phosphorylation of STAT1 was observed in cell models of IBD (Li et al., 2012; Wang et al., 2012). STAT1 phosphorylation can be induced by IFN-γ, making STAT1 a possible target of the plant extract combinations. We showed that pSTAT1 levels increased after cytokine treatment. Furthermore, pretreatment with STW5 or STW5-II revealed a dose-dependent inhibition over 1–20 μg/ml. STAT3 and STAT5 were also inhibited by the combination preparation. However, their induction was rather week in our experimental setting, therefore their contribution to the overall inflammatory state is probably minor.
Thus, inhibiting STAT1 might explain the reduced cytokine expression, which in turn could partly explain the anti-inflammatory effects of STW5 and STW5-II.
Conclusion
Human epithelial cells react to inflammatory stimuli (e.g., Th1 cytokines) by releasing a subset of chemokines, which in turn attract immune cells such as macrophages. Our study revealed that the herbal preparation STW5 (Iberogast®) and the modified investigational combination STW5-II reversed these effects without affecting NF-κB activity. Further studies showed that the extract combinations reduced STAT1 phosphorylation. We conclude that these herbal preparations may have a therapeutic potential for patients with IBD.
Author Contributions
MS: Performance of experiments; Writing of Paper. TE: Design of Project; Revision of Paper; Supervision of MS. HA-A: Design of Project; Revision of Paper.
Funding
The Project was funded by: Steigerwald Arzneimittelwerk GmbH, Havelstrasse 5, 64295 Darmstadt, Germany.
Conflict of Interest Statement
HA-A is employed by Steigerwald Arzneimittelwerk GmbH.
All the other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CD, Crohn’s disease; CM, cytokine mixture; ELISA, enzyme-linked immunoassay; IBD, inflammatory bowel disease; IFN, interferon; IL, interleukin; PMA, phorbol 12-myristate 13-acetate; TNF, tumor necrosis factor; UC, ulcerative colitis.
References
Abdel-Aziz, H., Schneider, M., Neuhuber, W., Kassem, A. M., Khailah, S., Müller, J., et al. (2015). GPR84 and TREM-1 signaling contribute to the pathogenesis of reflux esophagitis. Mol. Med. 21, 1011–1024. doi: 10.2119/molmed.2015.00098
Abdel-Aziz, H., Zaki, H. F., Neuhuber, W., Kelber, O., Weiser, D., and Khayyal, M. T. (2010). Effect of an herbal preparation, STW 5, in an acute model of reflux oesophagitis in rats. J. Pharmacol. Sci. 113, 134–142. doi: 10.1254/jphs.09355FP
Abraham, C., and Cho, J. H. (2009). Inflammatory bowel disease. N. Engl. J. Med. 361, 2066–2078. doi: 10.1056/NEJMra0804647
Akbari, P., Braber, S., Alizadeh, A., Verheijden, K. A., Schoterman, M. H., Kraneveld, A. D., et al. (2015). Galacto-oligosaccharides protect the intestinal barrier by maintaining the tight junction network and modulating the inflammatory responses after a challenge with the mycotoxin deoxynivalenol in human caco-2 cell monolayers and B6C3F1 mice. J. Nutr. 145, 1604–1613. doi: 10.3945/jn.114.209486
Allam, S., Krueger, D., Demir, I. E., Ceyhan, G., Zeller, F., and Schemann, M. (2015). Extracts from peppermint leaves, lemon balm leaves and in particular angelica roots mimic the pro-secretory action of the herbal preparation STW 5 in the human intestine. Phytomedicine 22, 1063–1070. doi: 10.1016/j.phymed.2015.08.008
Allescher, H. D., and Wagner, H. (2007). STW 5/Iberogast: multi-target-action for treatment of functional dyspepsia and irritable bowel syndrome. Wien. Med. Wochenschr. 157, 301–307. doi: 10.1007/s10354-007-0429-3
Banks, C., Bateman, A., Payne, R., Johnson, P., and Sheron, N. (2003). Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J. Pathol. 199, 28–35. doi: 10.1002/path.1245
Beck, P. L., and Wallace, J. L. (1997). Cytokines in inflammatory bowel disease. Mediators Inflamm. 6, 95–103. doi: 10.1080/09629359791785
Bonaterra, G. A., Kelber, O., Weiser, D., and Kinscherf, R. (2013). Mechanisms of the anti-proliferative and anti-inflammatory effects of the herbal fixed combination STW 5 (Iberogast§)® on colon adenocarcinoma (HT29) cells in vitro. Phytomedicine 20, 691–698. doi: 10.1016/j.phymed.2013.02.011
Calixto, J. B., Campos, M. M., Otuki, M. F., and Santos, A. R. (2004). Anti-inflammatory compounds of plant origin. Part II. modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 70, 93–103. doi: 10.1055/s-2004-815483
Chen, G., and Goeddel, D. V. (2002). TNF-R1 signaling: a beautiful pathway. Science 296, 1634–1635. doi: 10.1126/science.1071924
Ciccia, F., Rizzo, A., Accardo-Palumbo, A., Giardina, A., Bombardieri, M., Guggino, G., et al. (2012). Increased expression of interleukin-32 in the inflamed ileum of ankylosing spondylitis patients. Rheumatology 51, 1966–1972. doi: 10.1093/rheumatology/kes170
Crama-Bohbouth, G., Pena, A. S., Biemond, I., Verspaget, H. W., Blok, D., Arndt, J. W., et al. (1989). Are activity indices helpful in assessing active intestinal inflammation in Crohn’s disease? Gut 30, 1236–1240. doi: 10.1136/gut.30.9.1236
Danese, S., and Fiocchi, C. (2011). Ulcerative colitis. N. Engl. J. Med. 365, 1713–1725. doi: 10.1056/NEJMra1102942
Delgado, M. (2003). Inhibition of interferon (IFN) gamma-induced Jak-STAT1 activation in microglia by vasoactive intestinal peptide: inhibitory effect on CD40, IFN-induced protein-10, and inducible nitric-oxide synthase expression. J. Biol. Chem. 278, 27620–27629. doi: 10.1074/jbc.M303199200
Dwinell, M. B., Lugering, N., Eckmann, L., and Kagnoff, M. F. (2001). Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology 120, 49–59. doi: 10.1053/gast.2001.20914
Esquivel-Solís, H., Quiñones-Falconi, F., Zarain-Herzberg, A., Amieva-Fernández, R. I., and López-Vidal, Y. (2009). Impaired activation of Stat1 and c-Jun as a possible defect in macrophages of patients with active tuberculosis. Clin. Exp. Immunol. 158, 45–54. doi: 10.1111/j.1365-2249.2009.03985.x
Feng, Y. J., and Li, Y. Y. (2011). The role of p38 mitogen-activated protein kinase in the pathogenesis of inflammatory bowel disease. J. Dig. Dis. 12, 327–332. doi: 10.1111/j.1751-2980.2011.00525.x
Ferguson, L. R., Shelling, A. N., Browning, B. L., Huebner, C., and Petermann, I. (2007). Genes, diet and inflammatory bowel disease. Mutat. Res. 622, 70–83. doi: 10.1016/j.mrfmmm.2007.05.011
Fernandes, P., MacSharry, J., Darby, T., Fanning, A., Shanahan, F., Houston, A., et al. (2016). Differential expression of key regulators of Toll-like receptors in ulcerative colitis and Crohn’s disease: a role for Tollip and peroxisome proliferator-activated receptor gamma? Clin. Exp. Immunol. 183, 358–368. doi: 10.1111/cei.12732
Grbic, D. M., Degagne, E., Larrivee, J. F., Bilodeau, M. S., Vinette, V., Arguin, G., et al. (2012). P2Y6 receptor contributes to neutrophil recruitment to inflamed intestinal mucosa by increasing CXC chemokine ligand 8 expression in an AP-1-dependent manner in epithelial cells. Inflamm. Bowel Dis. 18, 1456–1469. doi: 10.1002/ibd.21931
Hess, J., Angel, P., and Schorpp-Kistner, M. (2004). AP-1 subunits: quarrel and harmony among siblings. J. Cell Sci. 117(Pt. 25), 5965–5973. doi: 10.1242/jcs.01589
Holtmann, G., Adam, B., and Vinson, B. (2004). [Evidence-based medicine and phytotherapy for functional dyspepsia and irritable bowel syndrome: a systematic analysis of evidence for the herbal preparation Iberogast]. Wien. Med. Wochenschr. 154, 528–534. doi: 10.1007/s10354-004-0119-3
Huang, C., Ma, W. Y., Maxiner, A., Sun, Y., and Dong, Z. (1999). p38 kinase mediates UV-induced phosphorylation of p53 protein at serine 389. J. Biol. Chem. 274, 12229–12235. doi: 10.1074/jbc.274.18.12229
Karlinger, K., Gyorke, T., Mako, E., Mester, A., and Tarjan, Z. (2000). The epidemiology and the pathogenesis of inflammatory bowel disease. Eur. J. Radiol. 35, 154–167. doi: 10.1016/S0720-048X(00)00238-2
Khanna, R., and Feagan, B. G. (2015). Safety of infliximab for the treatment of inflammatory bowel disease: current understanding of the potential for serious adverse events. Expert Opin. Drug Saf. 14, 987–997. doi: 10.1517/14740338.2015.1029915
Kroll, U., and Cordes, C. (2006). Pharmaceutical prerequisites for a multi-target therapy. Phytomedicine 13(Suppl. 5), 12–19. doi: 10.1016/j.phymed.2006.03.016
Kugathasan, S., Judd, R. H., Hoffmann, R. G., Heikenen, J., Telega, G., Khan, F., et al. (2003). Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: A statewide population-based study. J. Pediatr. 143, 525–531. doi: 10.1067/S0022-3476(03)00444-X
Langhorst, J., Elsenbruch, S., Koelzer, J., Rueffer, A., Michalsen, A., and Dobos, G. J. (2008). Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: Performance of fecal lactoferrin, calprotectin, and PMN-Elastase, CRP, and clinical indices. Am. J. Gastroenterol. 103, 162–169. doi: 10.1111/j.1572-0241.2007.01556.x
Li, Y., Deuring, J., Peppelenbosch, M. P., Kuipers, E. J., de Haar, C., and van der Woude, C. J. (2012). STAT1, STAT6 and adenosine 3’,5’-cyclic monophosphate (cAMP) signaling drive SOCS3 expression in inactive ulcerative colitis. Mol. Med. 18, 1412–1419. doi: 10.2119/molmed.2012.00277
Liacini, A., Sylvester, J., Li, W. Q., and Zafarullah, M. (2002). Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 21, 251–262. doi: 10.1016/S0945-053X(02)00007-0
Liu, Z., Chen, X., Wang, X., Chen, X., Song, C. H., Du, Y., et al. (2011). Chemokine CXCL11 links microbial stimuli to intestinal inflammation. Clin. Exp. Immunol. 164, 396–406. doi: 10.1111/j.1365-2249.2011.04382.x
Magnusson, M. K., Strid, H., Isaksson, S., Bajor, A., Lasson, A., Ung, K. A., et al. (2015). Response to infliximab therapy in ulcerative colitis is associated with decreased monocyte activation, reduced CCL2 expression and downregulation of Tenascin C. J. Crohns Colitis 9, 56–65. doi: 10.1093/ecco-jcc/jju008
Marion, R., Coeffier, M. M., Gargala, G., Ducrotte, P., and Dechelotte, P. P. (2004). Glutamine and CXC chemokines IL-8, Mig, IP-10 and I-TAC in human intestinal epithelial cells. Clin. Nutr. 23, 579–585. doi: 10.1016/j.clnu.2003.10.011
Mayer, L., Sandborn, W. J., Stepanov, Y., Geboes, K., Hardi, R., Yellin, M., et al. (2014). Anti-IP-10 antibody (BMS-936557) for ulcerative colitis: a phase II randomised study. Gut 63, 442–450. doi: 10.1136/gutjnl-2012-303424
Michael, S., Abdel-Aziz, H., Weiser, D., Muller, C. E., Kelber, O., and Nieber, K. (2012). Adenosine A2A receptor contributes to the anti-inflammatory effect of the fixed herbal combination STW 5 (Iberogast(R)) in rat small intestinal preparations. Naunyn Schmiedebergs Arch. Pharmacol. 385, 411–421. doi: 10.1007/s00210-011-0714-y
Michael, S., Kelber, O., Hauschildt, S., Spanel-Borowski, K., and Nieber, K. (2009). Inhibition of inflammation-induced alterations in rat small intestine by the herbal preparations STW 5 and STW 6. Phytomedicine 16, 161–171. doi: 10.1016/j.phymed.2008.10.011
Mitsuyama, K., Toyonaga, A., Sasaki, E., Watanabe, K., Tateishi, H., Nishiyama, T., et al. (1994). IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn’s disease. Clin. Exp. Immunol. 96, 432–436. doi: 10.1111/j.1365-2249.1994.tb06047.x
Moriyama, I., Ishihara, S., Rumi, M. A., Aziz, M. D., Mishima, Y., Oshima, N., et al. (2008). Decoy oligodeoxynucleotide targeting activator protein-1 (AP-1) attenuates intestinal inflammation in murine experimental colitis. Lab. Invest. 88, 652–663. doi: 10.1038/labinvest.2008.38
Moyer, M. P., Manzano, L. A., Merriman, R. L., Stauffer, J. S., and Tanzer, L. R. (1996). NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell. Dev. Biol. Anim. 32, 315–317. doi: 10.1007/BF02722955
Mueller, D., Triebel, S., Rudakovski, O., and Richling, E. (2013). Influence of triterpenoids present in apple peel on inflammatory gene expression associated with inflammatory bowel disease (IBD). Food Chem. 139, 339–346. doi: 10.1016/j.foodchem.2013.01.101
Neurath, M. F., Pettersson, S., Meyer zum Buschenfelde, K. H., and Strober, W. (1996). Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat. Med. 2, 998–1004. doi: 10.1038/nm0996-998
Noh, S. Y., Kang, S. S., Yun, C. H., and Han, S. H. (2015). Lipoteichoic acid from Lactobacillus plantarum inhibits Pam2CSK4-induced IL-8 production in human intestinal epithelial cells. Mol. Immunol. 64, 183–189. doi: 10.1016/j.molimm.2014.11.014
O’Brien, J., Wilson, I., Orton, T., and Pognan, F. (2000). Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267, 5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x
Ostvik, A. E., Granlund, A. V., Bugge, M., Nilsen, N. J., Torp, S. H., Waldum, H. L., et al. (2013). Enhanced expression of CXCL10 in inflammatory bowel disease: potential role of mucosal Toll-like receptor 3 stimulation. Inflamm. Bowel Dis. 19, 265–274. doi: 10.1002/ibd.23034
Patrick, L. (2011). Gastroesophageal reflux disease (GERD): a review of conventional and alternative treatments. Altern. Med. Rev. 16, 116–133.
Ramana, C. V., DeBerge, M. P., Kumar, A., Alia, C. S., Durbin, J. E., and Enelow, R. I. (2015). Inflammatory impact of IFN-gamma in CD8+ T cell-mediated lung injury is mediated by both Stat1-dependent and -independent pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L650–L657. doi: 10.1152/ajplung.00360.2014
Ren, T., Qiu, Y., Wu, W., Feng, X., Ye, S., Wang, Z., et al. (2014). Activation of adenosine A3 receptor alleviates TNF-alpha-induced inflammation through inhibition of the NF-kappaB signaling pathway in human colonic epithelial cells. Mediat. Inflamm. 2014:818251. doi: 10.1155/2014/818251
Romier-Crouzet, B., Van De Walle, J., During, A., Joly, A., Rousseau, C., Henry, O., et al. (2009). Inhibition of inflammatory mediators by polyphenolic plant extracts in human intestinal Caco-2 cells. Food Chem. Toxicol. 47, 1221–1230. doi: 10.1016/j.fct.2009.02.015
Schneider, M. J., Abdel-Aziz, H., and Efferth, T. (2014). Phytochemicals for the treatment of inflammatory bowel diseases. Phytochemistry Rev. 13, 629–642. doi: 10.1007/s11101-013-9320-6
Schreiber, S., Rosenstiel, P., Hampe, J., Nikolaus, S., Groessner, B., Schottelius, A., et al. (2002). Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut 51, 379–385. doi: 10.1136/gut.51.3.379
Vecchi Brumatti, L., Marcuzzi, A., Tricarico, P. M., Zanin, V., Girardelli, M., and Bianco, A. M. (2014). Curcumin and inflammatory bowel disease: potential and limits of innovative treatments. Molecules 19, 21127–21153. doi: 10.3390/molecules191221127
Wadie, W., Abdel-Aziz, H., Zaki, H. F., Kelber, O., Weiser, D., and Khayyal, M. T. (2012). STW 5 is effective in dextran sulfate sodium-induced colitis in rats. Int. J. Colorectal Dis. 27, 1445–1453. doi: 10.1007/s00384-012-1473-z
Wajant, H., Pfizenmaier, K., and Scheurich, P. (2003). Tumor necrosis factor signaling. Cell Death. Differ. 10, 45–65. doi: 10.1038/sj.cdd.4401189
Wang, Q., Huber, N., Noel, G., Haar, L., Shan, Y., Pritts, T. A., et al. (2012). NF-kappaBeta inhibition is ineffective in blocking cytokine-induced IL-8 production but P38 and STAT1 inhibitors are effective. Inflamm. Res. 61, 977–985. doi: 10.1007/s00011-012-0490-2
Weston, C. R., and Davis, R. J. (2002). The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12, 14–21. doi: 10.1016/S0959-437X(01)00258-1
Xavier, R. J., and Podolsky, D. K. (2007). Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434. doi: 10.1038/nature06005
Keywords: Inflammatory bowel disease, Phytotherapy, Proteomics
Citation: Schneider M, Efferth T and Abdel - Aziz H (2016) Anti-inflammatory Effects of Herbal Preparations STW5 and STW5-II in Cytokine-Challenged Normal Human Colon Cells. Front. Pharmacol. 7:393. doi: 10.3389/fphar.2016.00393
Received: 10 June 2016; Accepted: 07 October 2016;
Published: 26 October 2016.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Fang-Rong Chang, Kaohsiung Medical University, TaiwanJian Li, Beijing University of Chinese Medicine, China
Salvador Cañigueral, University of Barcelona, Spain
Copyright © 2016 Schneider, Efferth and Abdel-Aziz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heba Abdel-Aziz, heba.abdelaziz@gmail.com
 Mathias Schneider
Mathias Schneider Thomas Efferth
Thomas Efferth Heba Abdel-Aziz
Heba Abdel-Aziz