- 1 Laboratório de Endocrinologia Experimental e Eletrofisiologia Endócrina, Departamento de Fisiologia, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil
- 2 Biomedicina, Centro Universitário Metodista do IPA, Porto Alegre, Rio Grande do Sul, Brazil
The aim of this study was to evaluate the effect of pertussis toxin (PTX) on the depolarizing component of the action of follicle stimulating hormone (FSH) on the membrane potential (MP) of Sertoli cells, which is linked to the rapid entry of Ca2+ into cells and to the Ca2+-dependent transport of neutral amino acids by the A system. This model allowed us to analyze the involvement of Gi proteins in the action of FSH in these phenomena. In parallel, using an inactive analog of insulin-like growth factor type I (IGF-1), JB1, and an anti-IGF-I antibody we investigated the possible mediating role of IGF-I on these effects of FSH because IGF-I is produced and released by testicular cells in response to stimulation by FSH and shows depolarization effects on MP similar to those from FSH. Our results have the following implications: (a) the rapid membrane actions of FSH, which occur in a time-frame of seconds to minutes and include the depolarization of the MP, and stimulation of 45Ca2+ uptake and [14C]-methyl aminoisobutyric acid ([14C]-MeAIB) transport, are nullified by the action of PTX and, therefore, are probably mediated by GiPCR activation; (b) the effects of FSH were also nullified by verapamil, an L-type voltage-dependent Ca2+ channel blocker; (c) wortmannin, an inhibitor of phosphoinositide 3-kinase (PI3K), prevented FSH stimulation of 45Ca2+ entry and [14C]-MeAIB transport; and (d) these FSH actions are independent of the IGF-I effects. In conclusion, these results strongly suggest that the rapid action of FSH on L-type Ca2+ channel activity in Sertoli cells from 10- to 12-day-old rats is mediated by the Gi/βγ/PI3Kγ pathway, independent of the effects of IGF-I.
Introduction
Follicle stimulating hormone (FSH) regulates a vast array of Sertoli cell functions by acting through its transmembrane G protein-coupled receptor (GPCR). The complexity of signaling pathways activated by FSH after receptor binding has been emphasized in a number of recent studies (Griswold, 1998; Cheng and Mruk, 2002; Silva et al., 2002; Walker and Cheng, 2005; Loss et al., 2007; Ulloa-Aguirre et al., 2007). FSH induces the rapid activation of multiple signaling cascades, especially cAMP-adenylate cyclase-protein kinase A signaling, that impact diverse biological processes in Sertoli cells, such as those involving Ca2+ uptake and phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB) (Marinissen and Gutkind, 2001; Cunnigham et al., 2003; Meroni et al., 2004; Ulloa-Aguirre et al., 2007). Some of these effects occur very rapidly, within seconds to minutes, as illustrated by the effect of FSH on membrane potential (MP; Wassermann et al., 1992b) and Ca2+ uptake through L-type voltage-dependent Ca2+ channels (VDCC) in the Sertoli cells of immature rat testes (Grasso and Reichert Jr., 1989 Gorczynska and Handelsman, 1991; Gorczynska et al., 1994; Sharma et al., 1994). The FSH stimulatory actions on Ca2+ uptake (Dahia and Rao, 2006) and on insulin-like growth factor type I (IGF-I) synthesis (Khan et al., 2002; Meroni et al., 2004) differ between pre-pubertal and pubertal rat testes, being more active during the proliferative phase of the Sertoli cells. It has also been reported that during this phase FSH greatly induces the Ca2+-dependent Na+-amino acid co-transport (Wassermann et al., 1992a).
In immature rat testes, FSH also induces biphasic MP changes in Sertoli cells that are specific to the rapid effects of the hormone: a very short hyperpolarization phase (seconds) followed by a prolonged depolarization phase (more than 6 min) (Wassermann et al., 1992b). The hyperpolarization is blocked by tolbutamide, an inhibitor of ATP-sensitive K+ channels (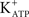 ) (Loss et al., 2007). Forskolin [an adenylate cyclase (AC) activator] and isoproterenol (a β-adrenergic agonist) also produce a rapid hyperpolarization of the immature Sertoli cell membrane. These actions are inhibited by the
) (Loss et al., 2007). Forskolin [an adenylate cyclase (AC) activator] and isoproterenol (a β-adrenergic agonist) also produce a rapid hyperpolarization of the immature Sertoli cell membrane. These actions are inhibited by the 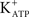 channel-blocking agent, glibenclamide (Jacobus et al., 2005). FSH-induced depolarization is also prevented by verapamil, a blocker of L-type VDCCs. These findings indicate that FSH-induced depolarization is related to the uptake of Ca2+ through VDCCs (Gorczynska and Handelsman, 1991; Wassermann et al., 1992b). The rapid effect of FSH on the immature Sertoli cell membrane has been widely reported (Grasso and Reichert Jr., 1989; Gorczynska and Handelsman, 1991; Wassermann et al., 1992b; Sharma et al., 1994; Dahia and Rao, 2006; Loss et al., 2007; Ulloa-Aguirre et al., 2007), but there is as yet no satisfactory explanation for its mechanism of action.
channel-blocking agent, glibenclamide (Jacobus et al., 2005). FSH-induced depolarization is also prevented by verapamil, a blocker of L-type VDCCs. These findings indicate that FSH-induced depolarization is related to the uptake of Ca2+ through VDCCs (Gorczynska and Handelsman, 1991; Wassermann et al., 1992b). The rapid effect of FSH on the immature Sertoli cell membrane has been widely reported (Grasso and Reichert Jr., 1989; Gorczynska and Handelsman, 1991; Wassermann et al., 1992b; Sharma et al., 1994; Dahia and Rao, 2006; Loss et al., 2007; Ulloa-Aguirre et al., 2007), but there is as yet no satisfactory explanation for its mechanism of action.
Follicle stimulating hormone stimulation of Sertoli cells leads to an increase of intracellular Ca2+ through a biphasic kinetic process (Grasso et al., 1992). The first phase, called the “surge phase”, is very rapid (seconds) and is associated with a Ca2+ current through L-type VDCC (Wassermann et al., 1992b). The second phase, known as the “sustained phase” is slower (minutes to hours) and involves mobilization of intracellular Ca2+ associated with cAMP production (Grasso and Reichert, 1990; Gorczynska et al., 1994; Sharma et al., 1994).
Ulloa-Aguirre et al. (2007) reported that [Ca2+]i increases may occur in either a cAMP-dependent or cAMP-independent manner. During the sustained phase, cAMP appears to increase intracellular calcium by promoting calcium mobilization from intracellular stores, whereas in the cAMP-independent surge phase, different G proteins or βγ heterodimers might be involved.
The mechanism of the FSH-stimulated rapid 45Ca2+ current/inflow (1 min; Dahia and Rao, 2006) in the surge phase is not clear. However, this phenomenon is an important component of Sertoli cell membrane signaling during the proliferative phase. Kinetic analysis of FSH-stimulated calcium-dependent [14C]-MeAIB uptake in Sertoli cells revealed that [14C]MeAIB transport is very rapid (seconds), and is caused by an increase in Vmax, indicating a fast calcium-dependent increase in the activity of functional transporters (Silva et al., 2002).
Earlier studies established that Sertoli cells secrete IGF-I following FSH stimulation. IGF-I synthesis only occurs in the Sertoli cells of immature animals. Both FSH and IGF-I are important determinants of testicular development and Sertoli cell function. It is currently thought that FSH affects the proliferation and differentiation of Sertoli cells from 10-day-old rats by mediating IGF-I production, at least in part (Khan et al., 2002). Some of the biological effects of IGF-I in Sertoli cells depend on activation of the PI3K/Akt signaling pathway. FSH not only stimulates the secretion of IGF-I in immature rat Sertoli cells, but it also enhances the effects of IGF-I on the PI3K/Akt pathway (Khan et al., 2002). However, it has also been reported that FSH can activate PKB/Akt independently of IGF-I in a PI3K-dependent manner in Sertoli cells from 20-day-old rats (Meroni et al., 2004).
Hormone receptors are known to bind more than one type of G protein (Hubbard and Hepler, 2006; Nechamen et al., 2007). For example, the β2 adrenergic receptor activates AC via Gs stimulation and can also powerfully stimulate Gi in murine cardiac myocytes (Ford et al., 1998; Xiao et al., 1999). Furthermore, in HEK 293 cells, adrenergic stimulation of MAPK by the β2 adrenergic receptor is mediated by the βγ subunits of pertussis toxin (PTX)-sensitive G protein (Daaka et al., 1997).
Gorczynska et al. (1994) reported that the relative roles of Gs and Gi in FSH signal transduction in Sertoli cells were unclear. They observed that the rapid (60–240 s) Ca2+ increase produced by FSH in isolated Sertoli cells from immature rats was significantly inhibited by pre-incubation with 1 mg/l PTX for 90 min (Figure 4 from Gorczynska et al., 1994). They proposed that, in addition to FSH stimulating AC via coupling to a Gs protein, the FSH receptor may also be linked to a Gi protein (Gorczynska and Handelsman, 1991).
Other authors have also reported the presence of active Gi subunits in Sertoli cells (Monaco et al., 1988; Krantic and Benahmed, 2000). Crépieux et al. (2001) demonstrated that FSH activates the ERK/MAPK pathway following coupling of the FSH receptor to both the Gs and Gi heterotrimeric proteins in primary cultures of Sertoli cells from 5- to 12-day-old rats. This dual signaling mechanism could explain the different electrophysiological effects of FSH on Sertoli cells during the proliferative phase. FSH action on Gs proteins could induce hyperpolarization of the MP, probably through the conversion of ATP to cAMP, opening 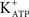 channels (Nakashima and Vanhoutte, 1995), whereas the depolarization phase associated with Ca2+ entry (current) through the L-type VDCC (Wassermann et al., 1992b) and the consequent increase on the MeAIB transport (Wassermann et al., 1992a) could be caused by Gi protein activation.
channels (Nakashima and Vanhoutte, 1995), whereas the depolarization phase associated with Ca2+ entry (current) through the L-type VDCC (Wassermann et al., 1992b) and the consequent increase on the MeAIB transport (Wassermann et al., 1992a) could be caused by Gi protein activation.
In light of these previous studies, we investigated the action of PTX on (a) FSH-induced changes in the MP of Sertoli cells from 10- to 12-day-old rats; (b) the rapid initial phase (seconds) of Ca2+ inflow or entry trough L-type VDCCs stimulated by FSH, and (c) [14C]-MeAIB transport by the Ca2+-dependent A system that is activated by FSH. The possible mediation of IGF-I in these phenomena was also investigated.
Materials and Methods
Follicle stimulating hormone, IGF-I, wortmannin, PTX, verapamil, tolbutamide, and forskolin were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). JB1 was purchased from Bachem Bioscience, Inc. (King of Prussia, PA, USA). 45Ca2+ (specific activity 444 GBq/g), and α-[1-14C] methylamino isobutyric acid ([14C]-MeAIB, specific activity 1.85 GBq/mmol) were acquired from Perkin ElmerNEN® (Waltham, MA, USA). The anti-IGF-I polyclonal antibody we used was acquired from the National Hormone and Peptide Program (NHPP) of the National Institute of Diabetes and Digestive and Kidney Disease (NIDDKD-NIH) at the UCLA Medical Center (Torrance, CA, USA).
The experimental animals were immature, 10- to 12-day-old Wistar rats. The animals were bred in our animal facility and housed in an air-conditioned room (approximately 24°C) with controlled lighting (lights on from 06:00 to 20:00). Pelleted food (Purina, Nutripal, Porto Alegre, Rio Grande do Sul, Brazil) and tap water were available to the mothers ad libitum. The suckling rats were kept with their mothers until they were sacrificed by cervical dislocation.
Solutions and Drugs Used
Krebs Ringer bicarbonate buffer solution (KRb): NaCl (146 mM), KCl (4.7 mM), KH2PO4 (1.2 mM), NaHCO3 (25 mM), MgSO4·7H2O (1.2 mM), CaCl2·2H2O (2.5 mM), Glucose (5.5 mM). This solution was gassed with O2:CO2 (95:5, v/v) until it reached pH 7.4. Stock solutions of wortmannin and tolbutamide were prepared in dimethyl sulfoxide (DMSO) and stored at −20°C until required. These stock solutions were diluted in KRb to achieve the final concentration at the time of use. The final concentration of DMSO did not exceed 0.1% or affect the analyzed parameters. The drugs concentrations used in each experiment are included in the figures and legends.
Electrophysiological Experiments
In each experiment, whole testes were decapsulated and carefully stretched with two callipers, exposing 3–10 undisrupted seminiferous tubules. They were then fixed to the bottom of a superfusion chamber, incubated with 1 ml/min KRb buffer with glucose (5 mmol/l), pH 7.4, at 34°C and equilibrated with O2:CO2 (95:5; v/v). Standard single microelectrode recording was performed according to the method described in von Ledebur et al. (2002). Microelectrode borosilicate pipettes were filled with KCl (3 M) and had a tip resistance of 15–25 MΩ. Intracellular recording was amplified using an intra 767 WPI (World Precision Instruments, Inc., USA). Square current pulses of 0.5 nA, 0.5 Hz, and 250-ms duration were applied through the intracellular electrode to estimate membrane resistance (R0) using the S48 stimulator (Grass Instrument, West Warwick, RI, USA). To register the recording, we used an oscilloscope (Tektronix, 2 Channel Digital Oscilloscope TDS 210, Beaverton, OR, USA) and Wavestar Lite Version 1.0.10 software. FSH (4 mU/ml), IGF-I (100 or 25 ng/ml) and forskolin (100 nM) were applied topically to the bath after the resting potential had stabilized for at least 2 min. Tolbutamide (10 μM) or verapamil (100 μM) solutions were perfused for 10 min before the topical application of FSH or IGF-I. After decapsulation, testes were pre-incubated in KRb buffer with glucose (5 mM/l) and 1 μg/ml of PTX for 60 min, processed as described above and fixed in the superfusion chamber before the MP was recorded and the FSH was applied topically. Each treatment was repeated at least five times with different cells, and variations in MP and input membrane resistance (R0) were recorded. The results are given as mean ± SEM.
45Ca2+ uptake experiments
Testes were removed and one gonad from each rat, alternately the left or right, was used as the experimental gonad; the contralateral gonad was used as the control. The testes (n = 5 in each group) were weighed, decapsulated and pre-incubated in KRb with 45Ca2+(2.7 kBq/sample) for 60 min in a Dubnoff metabolic incubator to equilibrate intra- and extracellular 45Ca2+ levels until they reached a plateau, which was carried out at 34°C, pH 7.4, with O2:CO2 (95:5; v/v). Following equilibration, the gonads were incubated for 2 min in KRb with 45Ca2+, with or without FSH (4 mU/ml), IGF-I (25 ng/ml), or forskolin (100 nM). In some experiments, testes were pre-incubated for 10 min with JB1 or verapamil, or for 1 h with wortmannin or PTX. To end the experiment and stop calcium flux, 1 ml of cold buffer (0°C) containing lanthanum chloride (LaCl3) (10 mM) was added to the samples (Batra and Sjogren, 1983). This solution was calcium-free and, in addition to LaCl3, contained (in mM): NaCl, 127.5; KCl, 4.6; MgCl2, 1.2; LaCl3, 10; HEPES, 10; and glucose, 11 (Batra and Sjogren, 1983). The supernatant was preserved, and the testes were removed to screw-cap tubes containing 1 ml of distilled water and stored at −20°C for further analysis. The testes were frozen and subsequently boiled. Aliquots of 100 μl were taken from each sample and placed in Aquasol 2® (Perkin Elmer, Inc, USA) for measurement of radioactivity using an LKB rack beta liquid scintillation spectrometer, model 1215 (LKB-Producer AB, Bromma, Sweden). The counting efficiency was 85–90%.
[14C]-MeAIB Transport Experiments
Testes were processed as described above. The samples (n = 5 in each group) were weighed, decapsulated and pre-incubated in KRb buffer for 60 min in a Dubnoff metabolic incubator at 34°C, pH 7.4, and gassed with O2:CO2 (95:5; v/v). The gonads were then incubated for 45 min in KRb with [14C]-MeAIB (2.7 kBq/sample), with or without FSH (4 mU/ml) or IGF-I (different doses, see Figure 6B). In experiments utilizing wortmannin, PTX, or the anti-IGF-I antibody, the testes were pre-incubated for 60 min and then incubated in the presence of the appropriate drug. The supernatant was preserved, and the testes were removed to screw-cap tubes containing 1 ml of distilled water and stored at −20°C until further analysis as above.
Statistical analyses were performed using unpaired Student’s t-test, a two-way ANOVA with Bonferroni’s post test or a two-way ANOVA of repeated measures with Bonferroni’s post test, which were carried out using GraphPad InStat version 3.01, 32 bit for Windows 95/NT (GraphPad Software, San Diego, California, USA, www.graphpad.com). Differences were considered to be significant when p < 0.05. In brackets, number of samples from one or more experiments.
Results
Basal Electrophysiological Values of 10- to 12-Day-Old-Rats
In our experimental conditions, the basal electrical characteristics of impaled Sertoli cells were as follows: resting potential −44 ± 1.1 mV (n = 73) and membrane resistance 10 ± 0.5 MΩ (n = 73). These conditions were stable for at least 30 min before the experiment (Figure 1A).
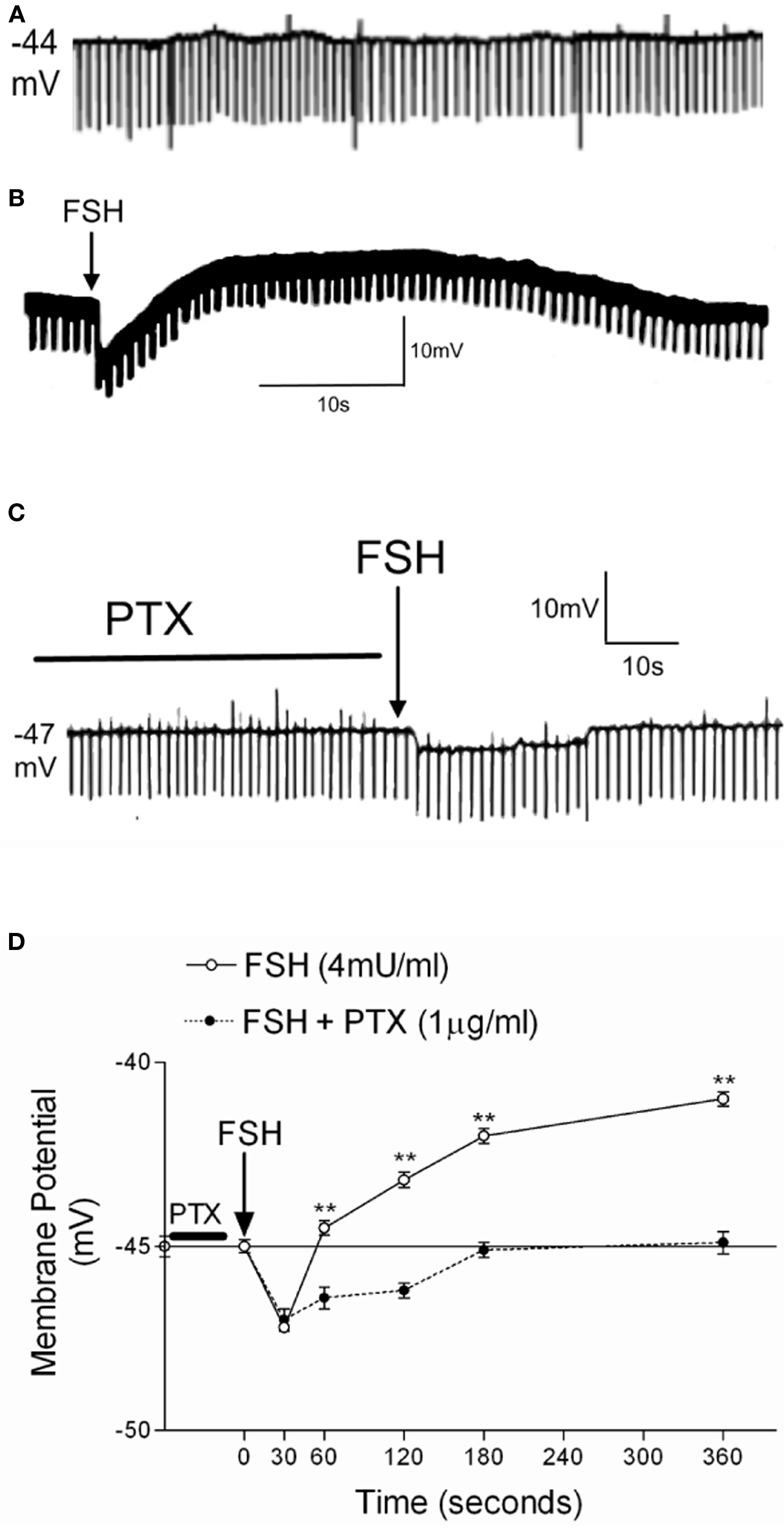
Figure 1. Effect of 1 h of PTX pre-incubation (1 μg/ml) on the action of FSH (4 mU/ml) on the membrane potential of 10- to 12-day-old rat Sertoli cells. Register of a Sertoli cell with resting potential of Em = −44 mV (A). Typical response of cells treated with FSH without (B) and with PTX (C). (D) Changes of membrane potential produced by FSH application with or without PTX. Statistical analysis: two-way ANOVA followed by Bonferroni post test, **p < 0.001; n = 5 in each treatment (FSH or FSH plus PTX) from five separated experiments.
Effect of PTX on the Electrophysiological Action of FSH on Sertoli Cells
Figure 1B shows the response to a topical application of 4 mU/ml of FSH on the seminiferous tubules in the superfusion chamber (without stopping the flow) after at least 2 min of MP stabilization. FSH produces a biphasic effect characterized by rapid hyperpolarization followed by depolarization. Pre-incubation (60 min) with PTX prevents the depolarization phase induced by FSH (Figure 1C). Figure 1D shows the time course of the action of FSH and FSH plus PTX on the MP of Sertoli cells. The statistical differences in all times of the depolarization response were highly significant (**p < 0.001; n = 5). This depolarization was also previously shown to be inhibited by verapamil, an L-type VDCC blocker (Wassermann et al., 1992b; Loss et al., 2007).
FSH and Forskolin Induced Hyperpolarization in Sertoli Cell Membranes Related to 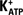 Channels
Channels
Activation of the AC-cAMP pathway by forskolin produces hyperpolarization of Sertoli cell membranes (p < 0.001; n = 5) (Figures 2C,D). The hyperpolarization produced by forskolin was inhibited by glibenclamide, which blocks 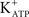 channels, as was previously reported by Jacobus et al. (2005). FSH-induced rapid hyperpolarization phase was also inhibited by pre-treatment with tolbutamide, an inhibitor of
channels, as was previously reported by Jacobus et al. (2005). FSH-induced rapid hyperpolarization phase was also inhibited by pre-treatment with tolbutamide, an inhibitor of 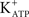 channels (**p < 0.001; n = 5) (Figures 2A,B).
channels (**p < 0.001; n = 5) (Figures 2A,B).
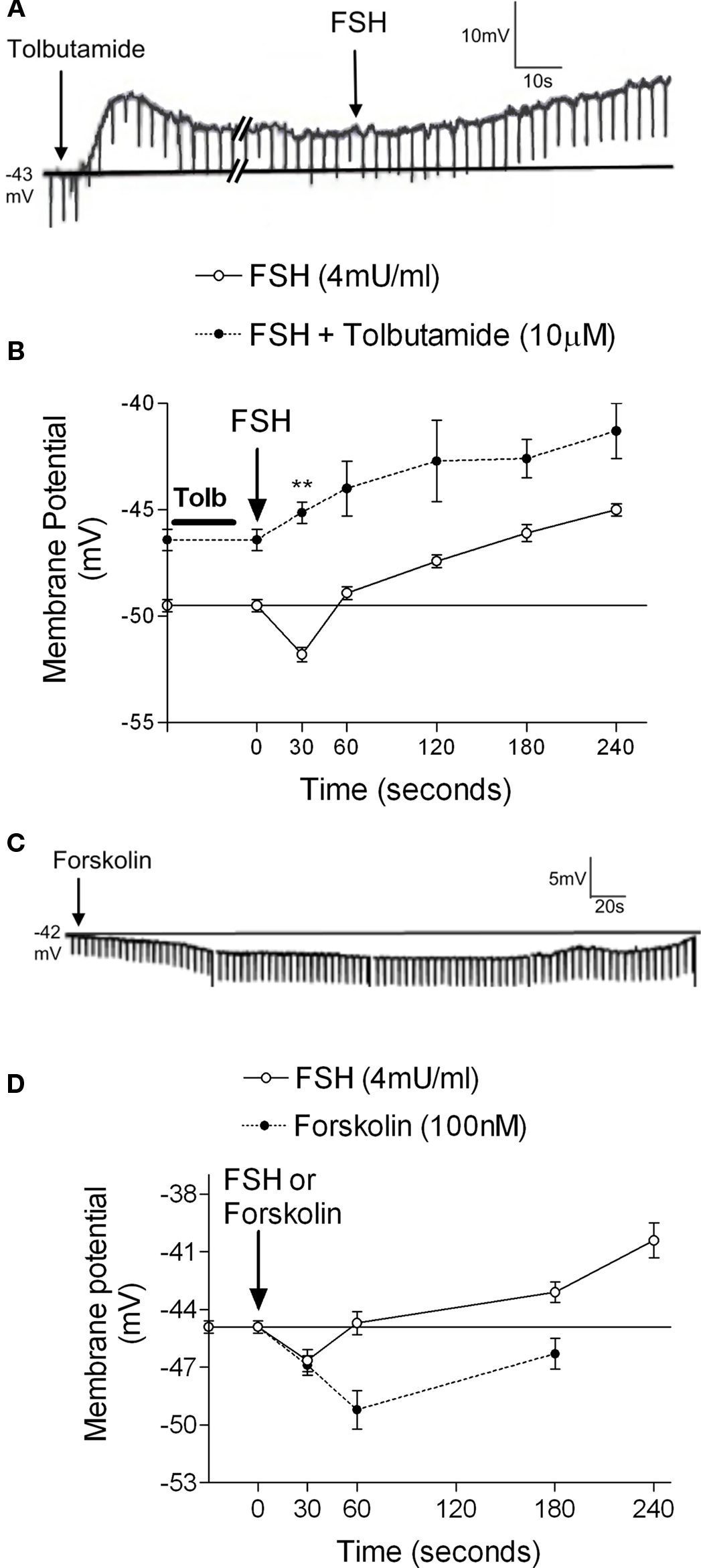
Figure 2. 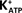 channel involvement in the hyperpolarization produced by FSH and forskolin on the Sertoli cell membrane. Pre-treatment with tolbutamide inhibited the rapid hyperpolarization response without affecting the depolarization phase, register of a representative cell (A). Changes produced by tolbutamide on the FSH response (B). Topical application of forskolin (100 nM) produced hyperpolarization on Sertoli cell membrane (C). Comparison of the FSH and forskolin action on Sertoli cell membrane potential (D). **p < 0.001; n = 5 in each treatment (FSH or FSH plus tolbutamide) from five separated experiments.
channel involvement in the hyperpolarization produced by FSH and forskolin on the Sertoli cell membrane. Pre-treatment with tolbutamide inhibited the rapid hyperpolarization response without affecting the depolarization phase, register of a representative cell (A). Changes produced by tolbutamide on the FSH response (B). Topical application of forskolin (100 nM) produced hyperpolarization on Sertoli cell membrane (C). Comparison of the FSH and forskolin action on Sertoli cell membrane potential (D). **p < 0.001; n = 5 in each treatment (FSH or FSH plus tolbutamide) from five separated experiments.
Electrophysiological Effect of IGF-I on the Sertoli Cell Membrane
The topical administration of IGF-I (25 ng/ml) on seminiferous tubules from immature rat testes induced an immediate depolarization in the MP of impaled Sertoli cells (*p < 0.05; n = 10) (Figure 3A, inset). This response increased with time, reaching a maximum after approximately 2 min. The depolarization produced by IGF-I (100 ng/ml) was not significantly changed by pre-incubating the Sertoli cells with PTX (1 μg/ml) for 60 min (Figure 3A). However, IGF-I-induced depolarization was inhibited in the presence of verapamil (100 μM) (an inhibitor of L-type VDCC) at all times studied (**p < 0.001; n = 6) (Figure 3B).
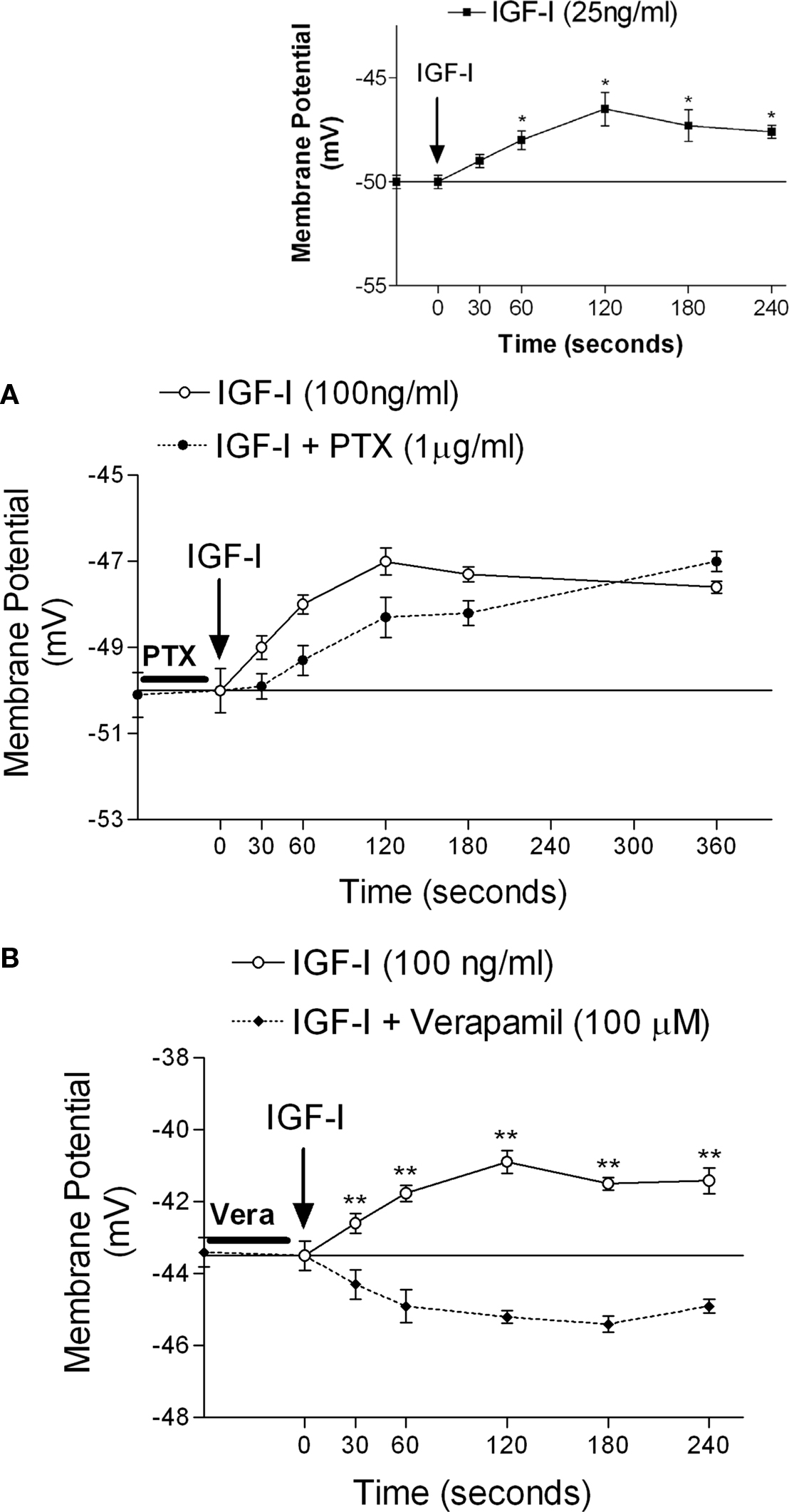
Figure 3. Rapid depolarization of immature Sertoli cells produced by topical application of IGF-I (25 ng/ml) (A, inset). The IGF-I depolarization time course was not affected by pre-incubation with PTX (1 μg/ml) (A), but was nullified by the presence of verapamil (100 μM) (B). Sertoli cells were pre-incubated with PTX for 1 h before IGF-I application, and verapamil was perfused 10 min before IGF-I application. *p < 0.05; n = 5 in each time from five independent experiments; **p < 0.001; n = 6 in each treatment (IGF-I or IGF-I plus verapamil) from five separated experiments.
FSH-Induced Stimulation of 45Ca2+ Entry
Follicle stimulating hormone stimulated 45Ca2+ uptake within 2 min of its addition to testes. This stimulatory effect of FSH on 45Ca2+ uptake was blocked by 60 min pre-incubation with PTX (Figure 4A), suggesting that the rapid effect of FSH is mediated by Gi proteins. FSH-stimulated 45Ca2+ entry was also inhibited by 60 min pre-incubation with wortmannin (Figure 4B), indicating that the PI3K pathway is also required for this action of the hormone. Pre-incubation for 10 min with verapamil also inhibited FSH stimulation of 45Ca2+ entry (Figure 4D). However, stimulation of AC by forskolin (100 nM) during the 2 min of incubation did not change 45Ca2+ uptake in immature rat testes (Figure 4C).
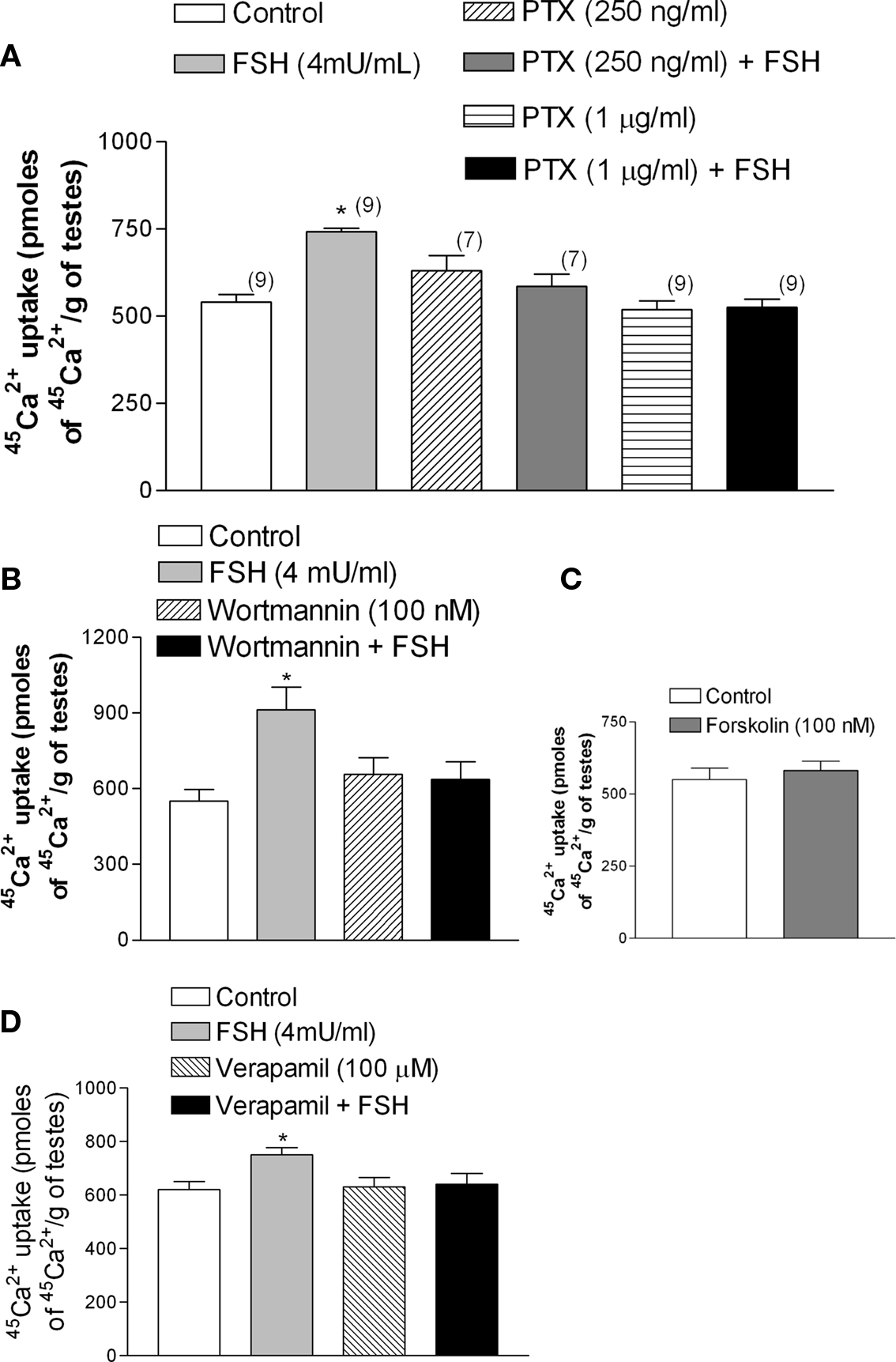
Figure 4. Follicle stimulating hormone produces an increase in 45Ca2+ uptake within 2 min of incubation. This increase was inhibited by PTX (A), wortmannin (B), and verapamil (D). Forskolin was not able to increase 45Ca2+ in 2 min (C). (A)*p < 0.05, as compared with the other groups, n in brackets from two separated experiments; (B,D) *p < 0.05, as compared with the other groups, n = 5 from one experiment.
FSH-Stimulated [14C]-MeAIB Transport was Inhibited by PTX and Wortmannin
Follicle stimulating hormone, through the increase of calcium uptake and the consequent stimulation of microtubules activity (Wassermann et al., 1992a), produces a fast increase (seconds) (Silva et al., 2002) in amino acid transport in Sertoli cells during the proliferative phase (between 10- to 22-day-old rats) (Pérez-Sánches and Wassermann, 1981). The stimulatory effect of FSH (4 mU/ml) on the transport of the model amino acid [14C]-MeAIB was abolished by the pre-incubation with PTX (Figure 5A) (*p < 0.05; n = 5). This stimulatory action of FSH was also blocked by wortmannin, an inhibitor of PI3K (Figure 5B) (*p < 0.05; n = 5).
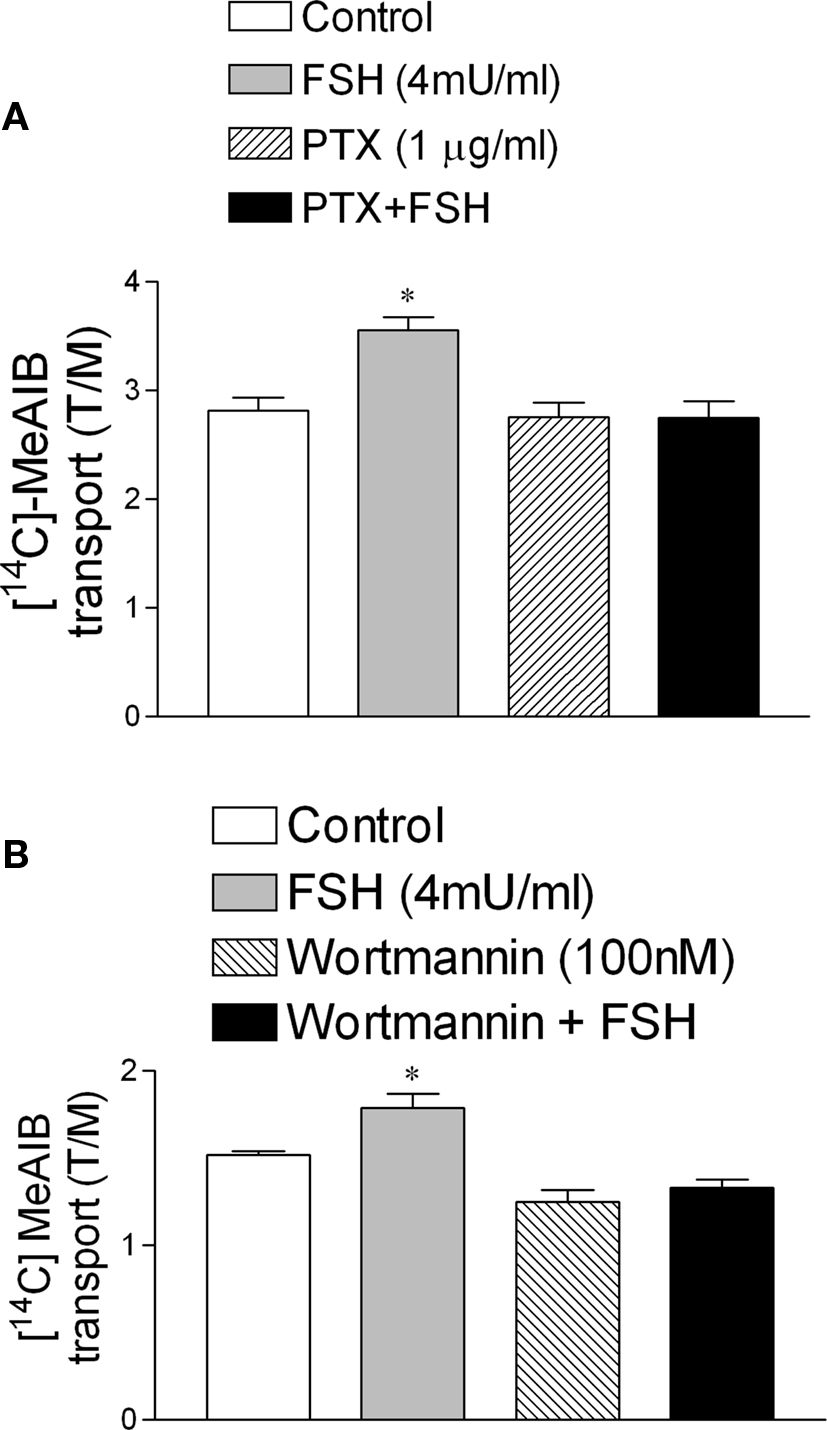
Figure 5. Follicle stimulating hormone stimulation of [14C]-MeAIB transport was blocked by PTX (1 μg/ml) (A) and by wortmannin (100 nM). (B) *p < 0.05, as compared with the other groups, n = 5 from one experiment.
Actions of IGF-I On 45Ca2+ uptake and [14C]-MeAIB Transport in Immature Sertoli Cells
The incubation of immature rat testes with IGF-I (25 ng/ml) for 2 min increased 45Ca2+ uptake (*p < 0.05; n = 9) (Figure 6A). A similar stimulatory action of IGF-I was observed using [14C]-MeAIB in testes incubated for 45 min with different concentrations of the growth factor (Figure 6B). The stimulatory action of IGF-I on [14C]-MeAIB was also observed in testes pre-incubated for 60 min with PTX (250 ng/ml) (Figure 6C). This result indicates that, unlike FSH, the stimulatory effect of IGF-I on [14C]-MeAIB transport was not affected by the presence of PTX (Figure 6C) (*p < 0.05; n = 5 as compared with the other groups), suggesting that IGF-I and FSH utilize different signaling pathways to elicit this response.
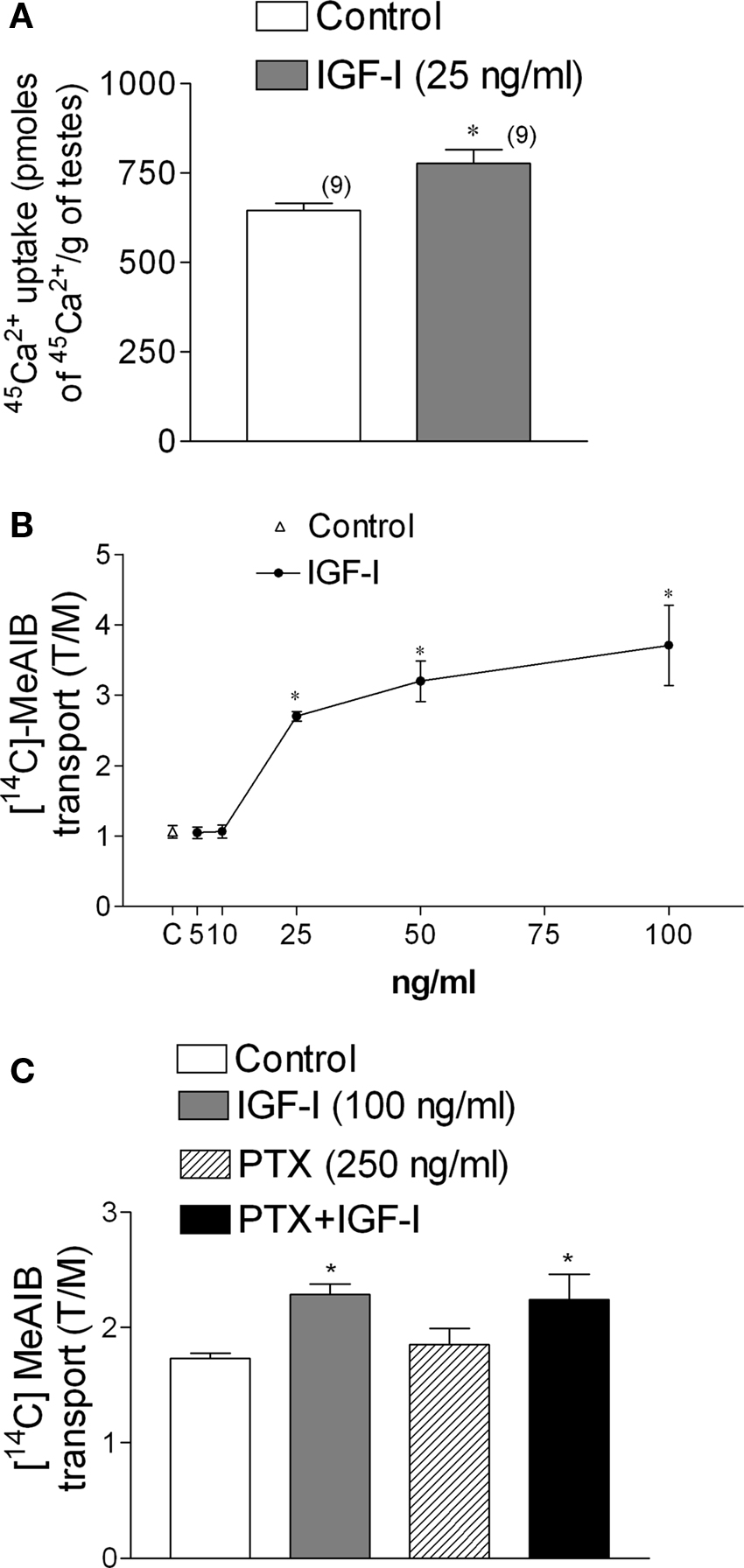
Figure 6. IGF-I (25 ng/ml) increases 45Ca2+ uptake within 2 min of incubation (A) and [14C]-MeAIB transport at different doses of IGF-I (B). PTX (250 ng/ml) did not affect [14C]-MeAIB transport stimulated by IGF-I (100 ng/ml) (C). Samples were pre-incubated for 60 min with PTX and incubated for 45 min with [14C]-MeAIB and IGF-I. Statistical analysis: a Student’s t-test was performed in (A) (*p < 0.05; n in brackets from two independent experiments); a two-way ANOVA followed by Bonferroni post test. (B) *p < 0.05; n = 5 in each concentration from five separated experiments, (C) *p < 0.05 as compared with the other groups, n = 5 from one experiment.
Effect of the IGF-I Receptor Blocking Agent, JB1, on the Actions of IGF-I and FSH
The stimulatory effect of IGF-I (25 ng/ml) on 45Ca2+ entry was inhibited by JB1 (1 μg/ml), an inactive analog of IGF-I (Figure 7A). However, this analog had no effect when applied in combination with FSH (4 mU/ml) (Figure 7B) (*p < 0.05; n = 5 as compared with the other groups).
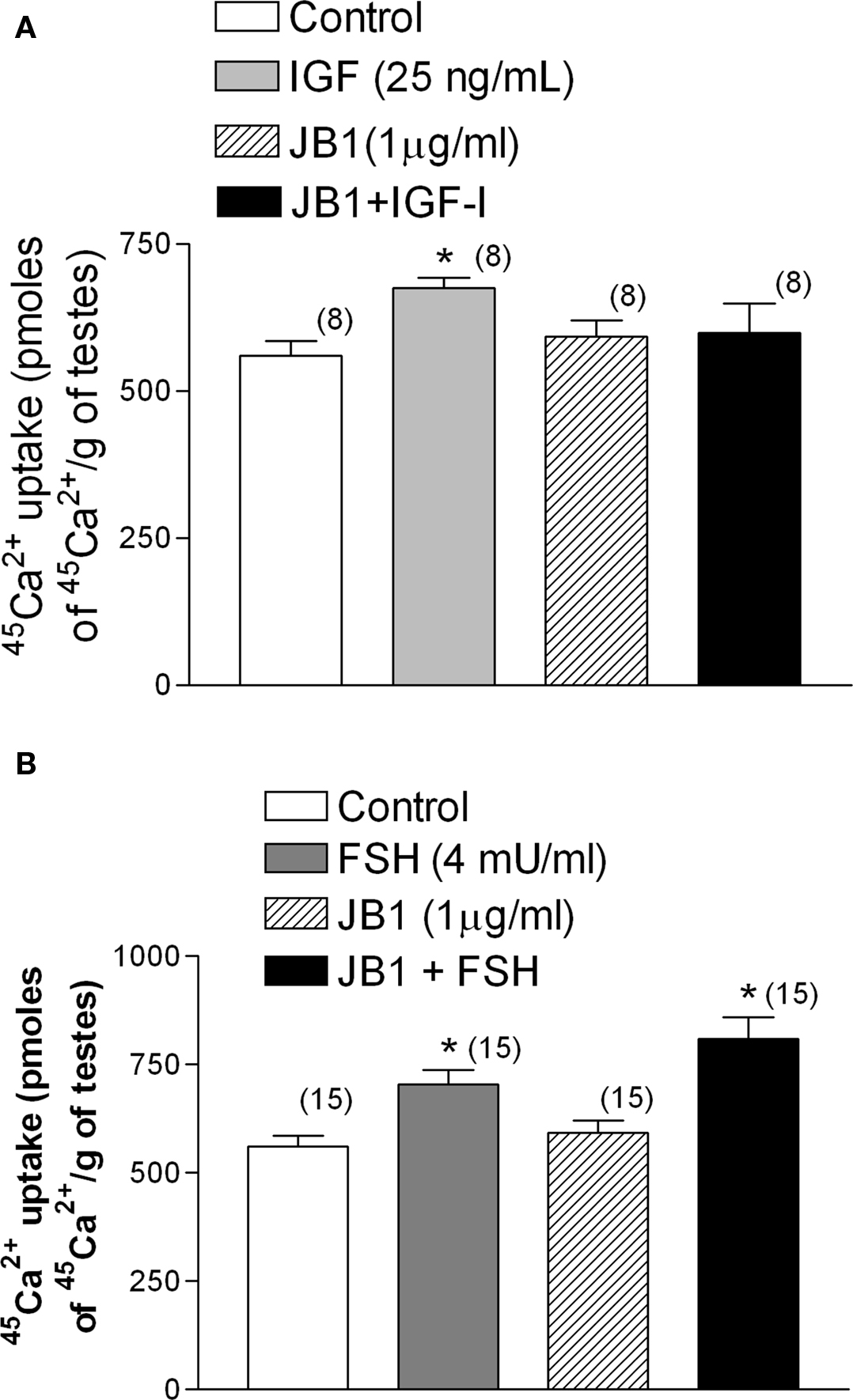
Figure 7. Effect of the inactive analog of IGF-I, JB1 (1 μg/ml), on IGF-I (A) and FSH (B) stimulation of 45Ca2+ entry after 2 min of incubation. JB1 inhibited the stimulatory effect of IGF-I on 45Ca2+ entry, but not the action of FSH, on 45Ca2+ uptake. (A) *p < 0.05 as compared with the other groups, n in brackets from two independent experiments; (B) *p < 0.05 as compared with the other groups, n in brackets from three separated experiments.
Effect of an anti-IGF-I Antibody on the Actions of IGF-I and FSH on Membrane Potential and [14C]-MeAIB Transport
Anti-IGF-I antibody (500 ng/ml) abolished the depolarization caused by IGF-I (100 ng/ml) (Figure 8A), but did not interfere with the typical MP changes induced by FSH (Figure 8B). Similarly, the anti-IGF-I antibody inhibited IGF-I-stimulated [14C]-MeAIB transport at all concentrations tested (Figure 8C), but did not inhibit the FSH response (Figure 8D) (*p < 0.05; n = 5 as compared with the other groups).
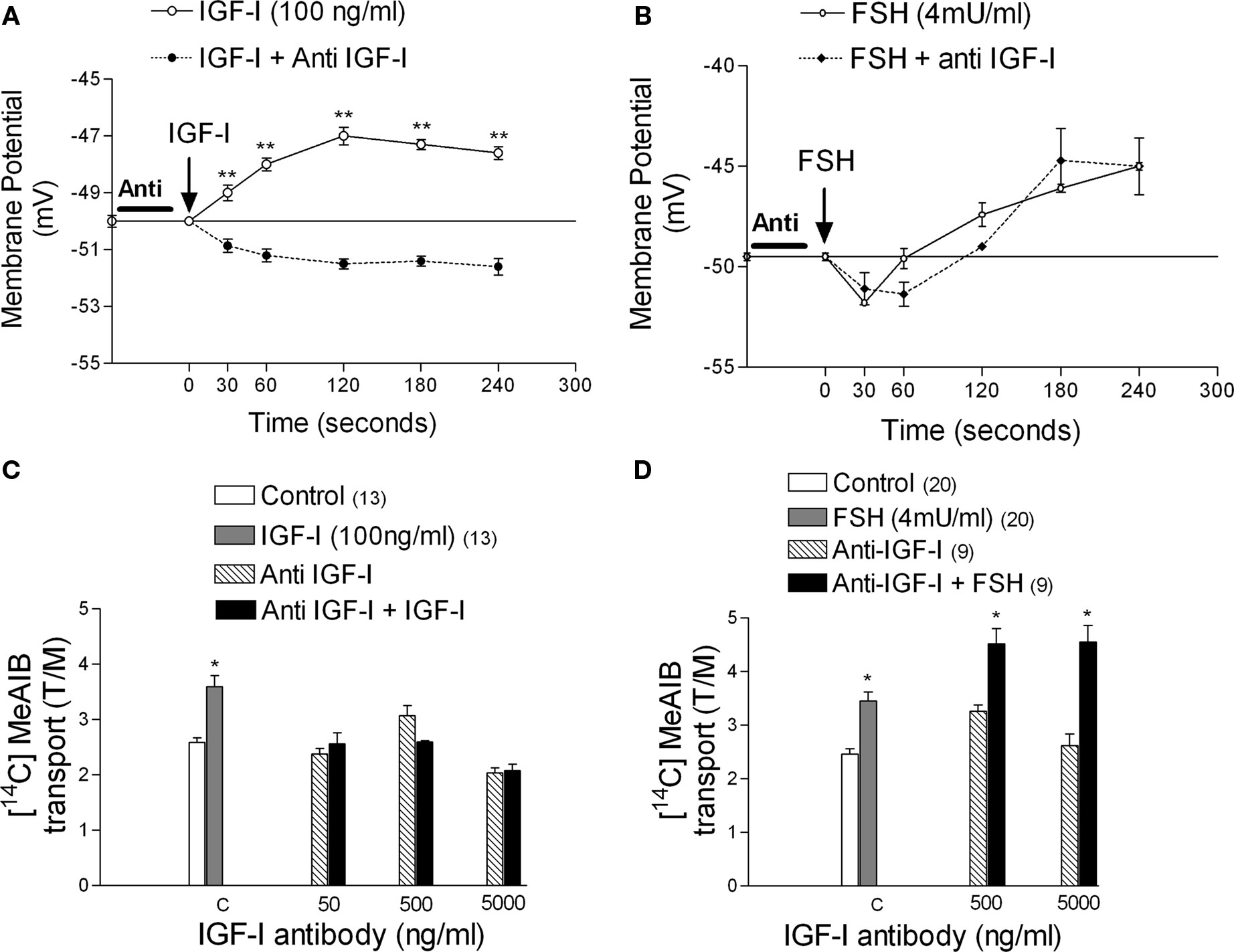
Figure 8. Effect of anti-IGF-I antibody (500 ng/ml) on the action of IGF-I and FSH on membrane potential and [14C]-MeAIB transport. The anti-IGF-I antibody inhibited the depolarization produced by IGF-I on MP (A) but it did not interfere with the MP modifications produced by FSH (B). The antibody inhibited the action of IGF-I on [14C]-MeAIB transport at all concentrations tested (C) but did not inhibit the effect of FSH (D). Sertoli cells were pre-incubated with anti-IGF-I antibody for 1 h before IGF-I or FSH application. (A) **p < 0.001; n = 5 in each treatment (IGF-I or IGF-I plus anti-IGF-I) from five separated experiments; (C) *p < 0.05; n = 5 or in brackets, one separated experiments for each concentration; (D) *p < 0.05; n in brackets, two separated experiments for each concentration.
Discussion
The aim of this study was to evaluate the effect of PTX on the depolarizing phase of FSH stimulation on the MP of Sertoli cells from 10- to 12-day-old rats, which is linked to the rapid entry of Ca2+ (Wassermann et al., 1992b; Loss et al., 2007) and the Ca2+-dependent transport of neutral amino acids by the A system (Wassermann et al., 1992a). In these experiments we analyzed the involvement of Gi proteins in FSH-induced effects. In parallel, we investigated the possible mediating role of IGF-I on the effects of FSH, as IGF-I is produced and released by testicular cells in response to FSH stimulation (Khan et al., 2002) and has a depolarization effect on the MP of Sertoli cells that is similar to FSH (Jacobus et al., 2010).
We found that the rapid membrane actions of FSH that occur in a time-frame of seconds to minutes, including membrane depolarization, stimulation of 45Ca2+ uptake and [14C]-MeAIB transport, were prevented by PTX and, therefore, it is likely that these effects are mediated by GiPCR activation. Furthermore, these effects were also inhibited by verapamil, a blocker of the L-type VDCC. Finally, FSH stimulation of 45Ca2+ uptake and [14C]-MeAIB transport were inhibited by wortmannin, an inhibitor of PI3K. These FSH effects were independent of IGF-I.
In earlier studies, Grasso and Reichert Jr. (1989) observed a suppression of FSH-stimulated 45Ca2+ uptake by methoxy-verapamil and nifedipine, specific blockers of voltage-activated calcium channels. These authors also reported that FSH stimulates 45Ca2+ entry within 1 min (surge phase) (Grasso et al., 1992). In another experiment, after 72 h in culture, cells were pre-incubated (4 h) with PTX and then incubated for 24 h with FSH plus 0.4 μCi of 45Ca2+. Then, the cells were washed and 45Ca2+ was measured. In this experiment, PTX was ineffective at blocking the effect of FSH. However, this experimental model is suitable for studying the sustained phase of FSH action, which is probably mediated by cAMP, but not the surge phase of 45Ca2+ entry (Figure 6B from Grasso and Reichert, 1990). In a supplementary experiment, the authors incubated with FSH for 2 min and added 45Ca2+ at the same time (Table 4 from Grasso and Reichert, 1990), but did not observe an effect of PTX under these experimental conditions.
The results of PTX treatment in isolated Sertoli cells by Gorczynska et al. (1994) and in our experiments (Figures 1C, 4A and 5A) conflict with this last result of Grasso and Reichert (1990). Other than the differences in time and treatment methods used, as described, the reason for this discrepancy is not entirely clear, but may be due to differences in the experimental approach or technical procedures. In our 45Ca2+ experiments, we pre-incubated with 45Ca2+ until an intracellular plateau was reached, after approximately 60 min. At that time, we added FSH (4 mU/ml) and incubated the samples for an additional 2 min. At the end of the incubations, we were very careful to avoid losing intracellular 45Ca2+ during washes, using LaCl3 in the washing solutions (Grasso and Reichert Jr., 1994; Batra and Sjogren, 1983; Grasso et al., 1992; ).
Using a different approach, measuring cytoplasmic Ca2+ through a Fura2-fluorescence technique, Sharma et al. (1994) observed that the immediate FSH-induced rise in [Ca2+]i was due to Ca2+ entry. Additionally, they showed that verapamil and Co2+ both abolished the FSH-triggered increase in [Ca2+]i. Furthermore, Ca2+-free medium prevented this FSH effect. They suggested that PTX does not affect the FSH-induced [Ca2+]i rise. However, this interpretation is based on only one representative record (Figure 7A from Sharma et al., 1994) without statistical processing, which they did carry out for the PTX-potentiated FSH-stimulated accumulation of cAMP (Figure 7B from Sharma et al., 1994).
In the absence of extracellular Ca2+, cAMP analog (8-Br-cAMP and dbcAMP) also induce a rise in [Ca2+]i, which is probably due to the mobilization of Ca2+ from internal stores (Sharma et al., 1994) in the cAMP-dependent sustained phase (Grasso et al., 1992). Gorczynska et al. (1994) also reported differences between the FSH- and cAMP-mediated cytosolic increases in Ca2+. They found that verapamil abolished the FSH-stimulated transmembrane Ca2+ flux (surge phase) in Sertoli cells, but a calcium channel-blocking agent or the absence of extracellular Ca2+ only partially blocked the cytosolic increase produced by cAMP, indicating an intracellular source of calcium for this increase (sustained phase). Lalevée et al. (1999) reported that the peak of calcium induced by cAMP was suppressed in Sertoli cells exposed to thapsigargin, suggesting the release of calcium from smooth endoplasmic reticulum. The relationship between cAMP and calcium following FSH stimulation is clearly complex. Differences in the origin of calcium in the FSH- versus cAMP-induced cytosolic calcium increase have been observed. The FSH-stimulated cytosolic calcium rise in freshly isolated Sertoli cells is dependent on the availability of extracellular calcium (Gorczynska and Handelsman, 1991), but a large proportion of the calcium participating in the cAMP-induced calcium rise is derived from the intracellular calcium sources (Gorczynska et al., 1994). These results indicate that a cAMP-independent pathway for increasing cytosolic calcium might be used by Sertoli cells. Therefore, the physiological responses of Sertoli cells to FSH might be dependent on the continuous cross-talk of both cAMP-dependent and cAMP-independent pathways (Gorczynska-Fjalling, 2004).
The average resting potential of seminiferous tubules in our experiments on 10- to 12-day-old rats was –44 ± 1.1 mV (n = 73), and the membrane input resistance was 10 ± 0.5 MΩ (n = 73). The basal electrical characteristics in seminiferous tubules of 12 and the membrane input resistance 9.3 ± 0.7 MΩ (n = 30). To avoid working with germ cells, only cells with MP lower than −35 mV were included in the experiments, as this MP is commonly found in Sertoli cells from normal (Eusebi et al., 1983) or Sertoli cell enriched seminiferous tubules of pre-pubertal rats (Wassermann et al., 1992b). The tip resistance that we used (15–25 MΩ) was appropriate for the preferential impalement of cells with a size similar to that of Sertoli cells (von Ledebur et al., 2002). This tip diameter makes the impalement of smaller cells, such as peritubular myoid cells, difficult. The membrane input resistance in Sertoli cells from normal seminiferous tubules is relatively lower than that found in cells from Sertoli cells-enriched seminiferous tubules (Eusebi et al., 1983) or isolated Sertoli cells (50 ± 5.2 MΩ; n = 10) (data not shown). The low membrane resistance is probably due to the fact that Sertoli cells in normal seminiferous epithelia are electrically coupled to each other and to germ cells. To preserve the gap junctions of immature Sertoli cells, whole seminiferous tubules were used in the present work, which meant that normal functional integrity of the environment was preserved as well as possible (Eusebi et al., 1983; von Ledebur et al., 2002).
In our experimental conditions, topical application of FSH produced changes in the MP of Sertoli cells. An immediate hyperpolarization was followed by depolarization (more than 6 min), returning to the resting potential after this period (Figure 1B). The depolarizing response was blocked by pre-treatment with verapamil (Wassermann et al., 1992b), indicating the involvement of L-type VDCC on the action of FSH.
Gorczynska and Handelsman (1991) reported that exchanging sodium and potassium concentrations in the extracellular medium (high potassium and low sodium, an ionic solution that produces MP depolarization) led to an immediate increase in cytosolic calcium, and subsequent FSH administration did not further increase these levels. The absence of external calcium in the incubation medium abolished the initial rise in the cytosolic free calcium concentration that was induced by FSH.
The gating of L-type calcium channels can be regulated by the α1 subunit of the channel through voltage changes in the cellular membrane, i.e., voltage clamp or ionic manipulation (Gorczynska and Handelsman, 1991), or by specific phosphorylation of the β subunit by PKB (Dolphin, 2003; Viard et al., 2004).
Phosphoinositide 3-kinases are enzymes that catalyze the phosphorylation of membrane phosphoinositides at the inositol 3-OH position. Class I, receptor-regulated PI3Ks connect many extracellular stimuli to intracellular responses. Class I PI3Ks are subdivided into class 1A (PI3K α, β, δ) kinases, which are activated by tyrosine kinase receptors (TKRs) or non-receptor tyrosine kinases, and the class 1B (PI3Kγ), which is uniquely activated by GPCRs and regulated by free Gβγ subunits of heterotrimeric G proteins, usually of the Gi subtype (Hirsch et al., 2007; Musnier et al., 2010). The PI3K downstream pathway leads to PIP2 phosphorylation producing PIP3, and this phosphoinositide then enhances PKB/Akt activation (Hirsch et al., 2007).
Active PKB/Akt is able to phosphorylate a vast number of proteins, thus representing a key effector of PI3K signaling. One of the actions reported for PKB/Akt is the control of extracellular Ca2+ entry due to a direct effect on the L-type Ca2+ channel leading to its plasma membrane exposure (Viard et al., 2004). These data allow us to speculate that PI3Kγ, activated by the βγ subunits of Gi proteins, could mediate the FSH stimulation of L-type calcium channels. Despite the fact that the molecular mechanism of this hypothetical stimulation is not clear, it could be achieved through PIP3 generation by activated PI3Kγ isoforms, which in turn could activate the β-subunit (regulatory unit) of the L-type Ca2+ channels through PKB, as was suggested by Viard et al. (2004) for adrenergic receptors, and by Le Blanc et al. (2004) for angiotensin II receptors in vascular cells.
It has been also reported that FSH produces a fast enhancement (seconds) in the activity (Vmax) of functional transporters of neutral amino acids in the membrane of Sertoli cells (Silva and Wassermann, 1999; Silva et al., 2002) through the increase of Ca2+ uptake and consequent stimulation of microtubule activity (Wassermann et al., 1992a). This FSH stimulatory action was independent of its effect on AC-cAMP activity and was observed using FSH from different species and sources (NHPP: ovine, porcine, and rat; Sigma-Aldrich: ovine) (Irusta and Wassermann, 1974; Pérez-Sánches and Wassermann, 1981; Cruz Curte and Wassermann, 1985; Spritzer and Wassermann, 1985; Silva and Wassermann, 1999; Silva et al., 2002).
The stimulatory action of the IGF-I on [14C]-MeAIB transport observed in immature Sertoli cells (Figures 6A,B) is probably processed through similar Ca2+-dependent mechanism activated by class 1A (PI3K) kinases (Figure 9). In addition to the complex role of IGF-I signaling in pre-pubertal testes, studies in neuronal cells have demonstrated that PTX inhibits IGF-I-mediated activation of MAPK, with a specific role for Gβγ subunits in IGF-I signaling (Hallak et al., 2000). These results show an association of heterotrimeric Gi with the IGF-I receptor. Inhibitory effects of PTX have also been reported on other cellular actions of IGF-I (Jin et al., 1993; Poiraudeau et al., 1997; Sarbassov et al., 1997; Uehara et al., 1999), but this was not seen in all cases (Stracke et al., 1988; Langlois et al., 1990; Linder et al., 1994; Sieble et al., 1996). Thus, in different cell types, Gβγ subunits can mediate cross-talk between GPCR and TKR signaling. However, this interrelationship can be discounted for the effects studied here in Sertoli cells, as PTX did not inhibit or modify the IGF-I depolarization of the MP (Figure 3A) or stimulation of [14C]-MeAIB transport (Figure 6C), unlike FSH (Figure 5A).
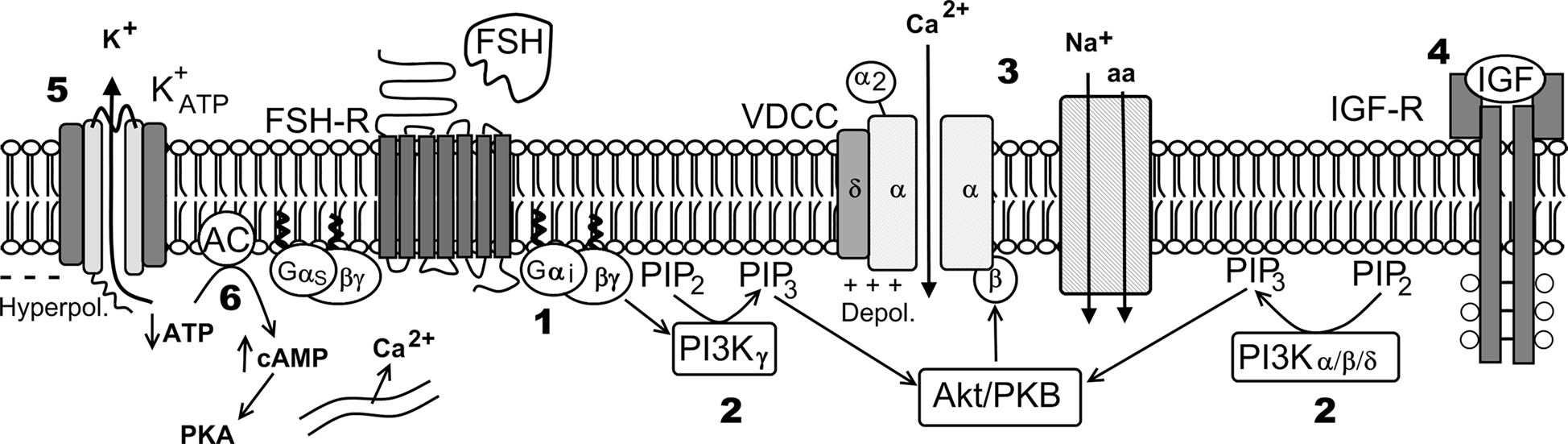
Figure 9. Hypothetical model of FSH action on Ca2+ uptake and amino acids transport. Numbers indicate pharmacological agent effects on different steps of the FSH action: 1 – PTX blocks Gi protein; 2 – Wortmannin blocks PI3K; 3 – Verapamil inhibits Ca2+ uptake and amino acid transport; 4 – JB1 and anti-IGF-I antibody block IGF-I action but not the FSH action; 5 – tolbutamide inhibits the 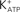 channel; 6 – Forskolin, that activates adenylate cyclase, produces hyperpolarization and did not affect Ca2+ uptake. VDCC, voltage-dependent Ca2+ channel (surge phase); Na+-aa, neutral amino acid co-transport; ER, endoplasmic reticulum (sustained phase).
channel; 6 – Forskolin, that activates adenylate cyclase, produces hyperpolarization and did not affect Ca2+ uptake. VDCC, voltage-dependent Ca2+ channel (surge phase); Na+-aa, neutral amino acid co-transport; ER, endoplasmic reticulum (sustained phase).
Because of the close relationship between FSH and IGF-I in immature testis (Loss et al., 2007), we examined the effects of an anti-IGF-I antibody and an inhibitory analog of IGF-I, JB1, on the effects of FSH and IGF-I. The anti-IGF-I antibody abolished the depolarization caused by IGF-I (Figure 8A) but did not interfere with the typical MP modification produced by FSH (Figure 8B). Similarly, the anti-IGF-I antibody inhibited IGF-I-stimulated [14C]-MeAIB transport at all concentrations tested (Figure 8C), but it was ineffective in the inhibition of FSH activity (Figure 8D). The same effects on 45Ca2+ uptake were observed using JB1. These results indicate that the effects of FSH that we observed were unrelated to IGF-I.
In summary, the calcium related rapid effects of FSH on Sertoli cell membranes from 10- to 12-day-old rats were blocked by specific pharmacological inhibitors: PTX (250 ng/ml, 1000 ng/ml), wortmannin (100 nM), and verapamil (100 μM). These results suggest the following pathway for the rapid action of FSH on Sertoli cell membranes: Gi protein activates PI3Kγ through its βγ subunits, by means of an unknown mechanism (most likely through PIP3-activated PKB/Akt (Viard et al., 2004; Vecchione et al., 2005; Hirsch et al., 2007), and stimulates extracellular 45Ca2+ entry (current) through the voltage-dependent L-type Ca2+ channel, which depolarizes the membrane and activates the A system for neutral amino acid transport (Figure 9). This hypothesis deserves further investigation to clarify the different steps involved in this pathway.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from CNPq. The authors are indebted to Prof. Luis Carlos Kusharski for the generous supply of 45Ca2+ and Dr. A. Parlow from the National Hormone and Peptide Program (NHPP), National Institute of Diabetes and Digestive and Kidney Disease (NIDDKD) at the UCLA Medical Center (Torrance, CA, USA) for the drug contribution.
References
Batra, S., and Sjogren, C. (1983). Effect of estrogen treatment on calcium uptake by the rat uterine smooth muscle. Life Sci. 32, 315–319.
Cheng, C. Y., and Mruk, D. D. (2002). Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 82, 825–874.
Crépieux, P., Marion, S., Martinat, N., Fafeur, V., Vern, Y. L., Kerboeuf, D., Guillou, F., and Reiter, E. (2001). The ERK-dependent signalling is stage-specifically modulated by FSH, during primary Sertoli cell maturation. Oncogene 20, 4696–4709.
Cruz Curte, A., and Wassermann, G. F. (1985). Identification of amino acid transport systems stimulated by FSH in rat testes. J. Endocrinol. 106, 291–294.
Cunnigham, M. A., Zhu, Q., Unterman, T. G., and Hammond, J. M. (2003). Follicle-stimulating hormone promotes nuclear exclusion of the forkhead transcription factor Fox01a via phosphatidylinositol 3-kinase in porcine granulose cells. Endocrinology 144, 5585–5594.
Daaka, Y., Luttrell, L. M., and Lefkowitz, R. J. (1997). Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390, 88–91.
Dahia, C. L., and Rao, A. J. (2006). Regulation of FSH receptor, PKIβ, IL-6 and calcium mobilization: possible mediators of differential action of FSH. Mol. Cell. Endocrinol. 247, 73–81.
Dolphin, A. C. (2003). G protein modulation of voltage-gated calcium channels. Pharmacol. Rev. 55, 607–627.
Eusebi, F., Ziparo, E., Fratamico, G., Russo, M. A., and Stefanini, M. (1983). Intercellular communication in rat seminiferous tubules. Dev. Biol. 100, 249–255.
Ford, C. E., Skiba, N. P., Bae, H., Daaka, Y., Reuveny, E., Shekter, L. R., Rosal, R., Weng, G., Yang, C. S., Iyengar, R., Miller, R. J., Jan, L. Y., Lefkowitz, R. J., and Hamm, H. E. (1998). Molecular basis for interactions of G protein betagamma subunits with effectors. Science 280, 1271–1274.
Gorczynska, E., and Handelsman, D. J. (1991). The role of calcium in follicle-stimulating hormone signal transduction in Sertoli cells. J. Biol. Chem. 266, 23739–23744.
Gorczynska, E., Spaliverio J., and Handelsman D. J. (1994). The relationship between 3′,5′-cyclic adenosine monophosphate and calcium in mediating follicle-stimulating hormone signal transduction in Sertoli cells. Endocrinology 134, 293–300.
Gorczynska-Fjalling, E. (2004). The role of calcium in signal transduction processes in Sertoli cells. Reprod. Biol. 4, 219–241.
Grasso, P., Santa-Coloma, T. A., and Reichert, L. E. Jr. (1992). Correlation of follicle-stimulating hormone (FSH)-receptor complex internalization with the sustained phase of FSH-induced calcium uptake by cultured rat Sertoli cells. Endocrinology 131, 2622–2628.
Grasso, P., and Reichert, L. E. Jr. (1989). Follicle-stimulating hormone receptor-mediated uptake of 45Ca2+ by proteoliposomes and culture rat Sertoli cells: evidence for involvement of voltage-activated and voltage independent calcium channels. Endocrinology 125, 3029–3036.
Grasso, P., and Reichert, L. E. (1990). Follicle-stimulating hormone receptor-mediated uptake of 45Ca2+ by cultured rat Sertoli cells does not require activation of cholera toxin- or pertussis toxin-sensitive guanine nucleotide binding proteins or adenylate cyclase. Endocrinology 127, 949–956.
Grasso, P., and Reichert, L. E. Jr. (1994). Evidence that a calmodulin-like calcium-binding domain of the FSH beta-subunit is involved in FSH-induced calcium uptake by Sertoli cells. J. Mol. Endocrinol. 13, 149–155.
Griswold, M. D. (1998). The central role of Sertoli cells in Spermatogenesis. Semin. Cell Dev. Biol. 9, 411–416.
Hallak, H., Seiler, A. E., Green, J. S., Ross, B. N., and Rubin, R. (2000). Association of heterotrimeric Gi with the insulin-like growth factor-I receptor. J. Biol. Chem. 275, 2255–2258.
Hirsch, E., Costa, C., and Ciraolo, E. (2007). Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J. Endocrinol. 194, 243–256.
Hubbard, K. B., and Hepler, J. R. (2006). Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell. Signal. 18, 135–150.
Irusta, O., and Wassermann, G. F. (1974). Factors influencing the uptake of [α-14C] aminoisobutyric acid by rat testes. J. Endocrinol. 60, 463–471.
Jacobus, A. P., Loss, E. S., and Wassermann, G. F. (2010). “The rapid FSH stimulation of L-type Ca2+ channels in rat Sertoli cells during proliferative phase is independent of IGF-I action,” in Keystone Symposia on Molecular and Cellular Discovery. G-Protein Coupled Receptors, April 12, Breckenridge, Colorado, USA.
Jacobus, A. P., Rodrigues, D. O., Borba, P. F., Loss, E. S., and Wassermann, G. F. (2005). Isoproterenol opens K+ ATP channels via a beta2-adrenoceptor-linked mechanism in Sertoli cells from immature rats. Horm. Metab. Res. 37, 198–204.
Jin, G. F., Guo, Y. S., Ball, C., and Houston, C. W. (1993). Insulin-like growth factors enhance phagocytosis by human neutrophils in vitro. Regul. Pept. 49, 125–131.
Khan, S. A., Ndjountche, L., Pratchard, L., Spicer, L. J., and Davis, J. S. (2002). Follicle-stimulating hormone amplifies insulin-like growth factor I-mediated activation of AKT/protein kinase B signaling in immature rat Sertoli cells. Endocrinology 143, 2259–2267.
Krantic, S., and Benahmed M. (2000). Somatostatin inhibits follicle-stimulating hormone-induced adenylyl cyclase activity and proliferation in immature porcine Sertoli cell via sst2 receptor. Biol. Reprod. 62, 1835–1843.
Lalevée, N., Rogier, C., Becq, F., and Joffre, M. (1999). Acute effects of adenosine triphosphates, cyclic 3′,5′-adenosine monophosphates, and follicle-stimulating hormone on cytosolic calcium level in cultured immature rat Sertoli cells. Biol. Reprod. 61, 343–352.
Langlois, D., Hinsch, K. D., Saez, J. M., and Begeot, M. (1990). Stimulatory effect of insulin and insulin-like growth factor I on Gi proteins and angiotensin-II-induced phosphoinositide breakdown in cultured bovine adrenal cells. Endocrinology 126, 1867–1872.
Le Blanc, C., Mironneau, C., Barbot, C., Henaff, M., Bondeva, T., Wetzker, R., and Macrez, N. (2004). Regulation of vascular L-type Ca2+ channels by phosphatidylinositol 3,4,5-trisphosphate. Circ. Res. 95, 300–307.
Linder, B., Harris, S., Eisen, A., and Nissley, P. (1994). Evidence against roles for pertussis toxin sensitive G proteins or diacylglycerol generation in insulin-like growth factor-1 stimulated DNA synthesis in MG-63 osteosarcoma cells. Mol. Cell. Endocrinol. 105, 111–118.
Loss, E. S., Jacobus, A. P., and Wassermann, G. F. (2007). Diverse FSH and testosterone signaling pathways in the Sertoli cell. Horm. Metab. Res. 39, 806–812.
Marinissen, M. J., and Gutkind, J. S. (2001). G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol. Sci. 22, 368–376.
Meroni, S. B., Riera, M. F., Pellizzari, E. H., Galardo, M. N., and Cigorraga, S. B. (2004). FSH activates phosphatidylinositol 3-kinase/protein kinase B signaling pathway in 20-day-old Sertoli cells independently of IGF-I. J. Endocrinol. 180, 257–265.
Monaco, L., DeManno, D. A., Martin, M. W., and Conti, M. (1988). Adenosine inhibition of the hormonal response in the Sertoli cell is reversed by pertussis toxin. Endocrinology 122, 2692–2698.
Musnier, A., Blanchot. B., Reiter. E., and Crépieux, P. (2010). GPCR signalling to the translation machinery. Cell. Signal. 22, 707–716.
Nakashima, M., and Vanhoutte, P. M. (1995). Isoproterenol causes hyperpolarization through opening of ATP-sensitive potassium channels in vascular smooth muscle of the canine saphenous vein. J. Pharmacol. Exp. Ther. 272, 379–384.
Nechamen, C. A., Thomas, R. M., and Dias, J. A. (2007). APPL1, APPL2, Akt2 and FOXO1a interact with FSHR in a potential signaling complex. Mol. Cell. Endocrinol. 260–262, 93–99.
Pérez-Sánches, V. H., and Wassermann, G. F. (1981). Amino acid transport in rat Sertoli cell enriched testes: studies on the mechanism of action of follicle stimulating hormone. Braz. J. Med. Biol. Res. 14, 11–17.
Poiraudeau, S., Lieberherr, M., Kergosie, N., and Corvol, M. T. (1997). Different mechanisms are involved in intracellular calcium increase by insulin-like growth factors 1 and 2 in articular chondrocytes: voltage-gated calcium channels, and/or phospholipase C coupled to a pertussis-sensitive G-protein. J. Cell. Biochem. 64, 414–422.
Sarbassov, D. D., Jones, L. G., and Peterson, C. A. (1997). Extracellular signal-regulated kinase-1 and -2 respond differently to mitogenic and differentiative signaling pathways in myoblasts. Mol. Endocrinol. 11, 2038–2047.
Sharma, O. P., Flores, J. A., Leong, D. A., and Veldhuis, J. D. (1994). Cellular basis for follicle-stimulating hormone-stimulated calcium signaling in single rat Sertoli cells: possible dissociation from effects of adenosine 3′,5′-monophosphate. Endocrinology 134, 1915–1923.
Sieble, T., Kiess, W., Linder, B., Kessler, U., Schwarz, H. P., and Nissley, S. P. (1996). Pertussis toxin sensitive G-proteins are not involved in the mitogenic signaling pathway of insulin-like growth factor-I in normal rat kidney epithelial (NRKE) cells. Regul. Pept. 62, 65–71.
Silva, F. R., Leite, L. D., and Wassermann, G. F. (2002). Rapid signal transduction in Sertoli cells. Eur. J. Endocrinol. 147, 425–433.
Silva, F. R. M. B., and Wassermann, G. F. (1999). Kinetics of FSH stimulation of methylaminoisobutyric acid uptake in Sertoli cells in culture from testes of 15-day-old rats. Med. Sci. Res. 27, 627–630.
Spritzer, P. M., and Wassermann, G. F. (1985). Amino acid uptake and protein synthesis in rat testes: stimulation by dissociable factors. Horm. Metab. Res. 17, 237–240.
Stracke, M. L., Kohn, E. C., Aznavoorian, S. A., Wilson, L. L., Salomon, D., Krutzsch, H. C., Liotta, L. A., and Schiffmann, E. (1988). Insulin-like growth factors stimulate chemotaxis in human melanoma cells. Biochem. Biophys. Res. Commun. 153, 1076–1083.
Uehara, T., Tokumitsu, Y., and Nomura, Y. (1999). Pertussis toxin-sensitive and insensitive intracellular signalling pathways in undifferentiated 3T3-L1 cells stimulated by insulin converge with phosphatidylinositol 3-kinase upstream of the Ras mitogen-activated protein kinase cascade. Eur. J. Biochem. 259, 801–808.
Ulloa-Aguirre, A., Zarinán, T., Pasapera, A. M., Casas-González, P., and Dias, J. A. (2007). Multiple facets of follicle-stimulating hormone receptor function. Endocrine 32, 251–263.
Vecchione, C., Patrucco, E., Marino, G., Barberis, L., Poulet, R., Aretini, A., Maffei, A., Gentile, M. T., Storto, M., Azzolino, O., Brancaccio, M., Colussi, G. L., Bettarini, U., Altruda, F., Silengo, L., Tarone, G,, Wymann, M. P., Hirsch, E., and Lembo, G. (2005). Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3Kgamma. J. Exp. Med. 201, 1217-1228.
Viard, P., Butcher, A. J., Halet, G., Davies, A., Nürnberg, B., Heblich, F., and Dolphin, A. C. (2004). PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat. Neurosci. 7, 939–946.
von Ledebur, E. I., Almeida, J. P., Loss, E. S., and Wassermann, G. F. (2002). Rapid effect of testosterone on rat Sertoli cell membrane potential. Relationship with K+ ATP channels. Horm. Metab. Res. 34, 550–555.
Walker, W. H., and Cheng, J. (2005). FSH and testosterone signaling in Sertoli cells. Reproduction 130, 15–28.
Wassermann, G. F., Monti Bloch, L., Grillo, M. L., Silva, F. R., Loss, E. S., and Mcconnell, L. L. (1992a). Biochemical factors involved in the FSH action on amino acid transport in immature rat testes. Horm. Metab. Res. 24, 276–279.
Wassermann, G. F., Monti Bloch, L., Grillo, M. L., Silva, F. R., Loss, E. S., and Mcconnell, L. L. (1992b). Electrophysiological changes of Sertoli cells produced by acute administration of aminoacid and FSH. Horm. Metab. Res. 24, 326–328.
Keywords: Sertoli cell, follicle stimulating hormone, Gi protein, membrane potential, L-type Ca2+ channels, immature testes
Citation: Jacobus AP, Loss ES and Wassermann GF (2010) Pertussis toxin nullifies the depolarization of the membrane potential and the stimulation of the rapid phase of 45Ca2+ entry through L-type calcium channels that are produced by follicle stimulating hormone in 10- to 12-day-old rat Sertoli cells. Front. Physio. 1:138. doi: 10.3389/fphys.2010.00138
Received: 24 June 2010;
Accepted: 15 September 2010;
Published online: 21 October 2010.
Edited by:
Angel Nadal, Universidad Miguel Hernandez de Elche, SpainReviewed by:
Martyn P Mahaut-Smith, University of Leicester, UKPascale Crépieux, Institut National de la Recherche Agronomique- Centre de Tours, France
Copyright: © 2010 Jacobus, Loss and Wassermann. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Guillermo Federico Wassermann, Departamento de Fisiologia, ICBS, UFRGS. Rua Sarmento Leite, 500, 90050-170 Porto Alegre, RS, Brasil. e-mail: gwass@ufrgs.br
