- Department of Human Physiology and Centre for Neuroscience, Flinders University, Adelaide, SA, Australia
The pacemaker and pattern generator that underlies the cyclical generation of spontaneous colonic migrating motor complexes (CMMCs) has recently been identified to lie within the myenteric plexus and/or muscularis externa. Neither the mucosa, nor the release of substances from the mucosa were found to be required for the spontaneous generation of CMMCs. However, it is known that stretch applied to the colonic wall can also evoke CMMCs and since stretch of the gut wall is known to stimulate the mucosa, it is not clear whether release of substances from the mucosa and/or submucosal plexus are required for stretch-evoked CMMCs. Therefore, the aim of this study was to determine whether circumferential stretch-evoked CMMCs require the presence of the mucosa and/or submucosal plexus in isolated mouse colon. Spontaneous CMMCs were recorded from full length sheet preparations of colon in vitro. Graded circumferential stretch (at a rate of 100 μm/s) applied to a 15-mm segment of mid–distal colon reliably evoked a CMMC, which propagated to the oral recording site. Sharp dissection to remove the mucosa and submucosal plexus from the entire colon did not prevent spontaneous CMMCs and circumferential stretch-evoked CMMCs were still reliably evoked by circumferential stretch, even at significantly lower thresholds. In contrast, in intact preparations, direct stimulation of the mucosa (without accompanying stretch) proved highly inconsistent and rarely evoked a CMMC. These observations lead to the inescapable conclusion that the sensory neurons activated by colonic stretch to initiate CMMCs lie in the myenteric plexus, while the mechanoreceptors activated by stretch, lie in the myenteric ganglia and/or muscularis externa. Stretch activation of these mechanoreceptors does not require release of any substance(s) from the mucosa, or neural inputs arising from submucosal ganglia.
Introduction
Colonic migrating motor complexes (CMMCs) are one of the major types of colonic motor pattern that occur spontaneously or, can be evoked by physiological stimuli in the large intestine of mammals and are thought to facilitate colonic transit (Sarna, 1991b; Bywater et al., 1998; Spencer et al., 1998a; Brierley et al., 2001; Spencer, 2001; Roberts et al., 2007, 2008; Heredia et al., 2010). In early in vivo studies, attempts to identify control mechanisms underlying CMMC generation proved challenging, probably due to the limitations of studying colonic motility in living animals (Sarna, 1986, 1991a,b; Karaus et al., 1987). However, in the mid 1970s it was discovered that colonic motor complexes could be preserved during in vitro recordings made from the whole isolated colon of mice (Wood, 1973; Wood et al., 1986) and cats (Christensen et al., 1974). Since this discovery, many laboratories have recorded CMMCs in vitro and much has been learnt about the mechanisms underlying their cyclical generation (Bywater et al., 1998; Spencer et al., 1998a,b, 2005, 2007; Brierley et al., 2001; Roberts et al., 2007, 2008; Keating and Spencer, 2010).
One of the recent discoveries regarding spontaneous CMMCs has been the localization of the pacemaker and pattern generator underlying their cyclical generation (Keating and Spencer, 2010); and the basic intrinsic circuitry that underlies their generation and propagation (Spencer et al., 2005). It was found that careful removal of the mucosa and submucosal plexus from the entire mouse colon did not prevent spontaneous CMMCs (Keating and Spencer, 2010) – an observation verified by other laboratories (H. Sjovall, University of Gothenburg, personal communication). This important result showed that release of substances from the mucosa, or submucosal ganglia were not required for spontaneous CMMC generation or propagation.
Whilst much has now been learnt about the generation of spontaneous CMMCs, it is also known that CMMCs can be evoked by external stimuli, such as transmural electrical nerve stimulation (Spencer and Bywater, 2002; Powell et al., 2003), or circumferential stretch of the colon wall (Powell et al., 2003; Heredia et al., 2009). It is well known that stretch of the colonic wall potently activates intrinsic polarized neural pathways (Spencer and Smith, 2001; Thornton and Bornstein, 2002; Bian et al., 2004). However, it is unclear whether release of substances from the mucosa, such as 5-HT, are required for the generation of stretch-evoked CMMCs. In previous studies, it has been shown that when the mucosa and submucosal ganglia are removed from the colon (Spencer et al., 2002), or small intestine (Smith et al., 1991) they have not prevented stretch-evoked peristaltic reflex pathways. Interestingly, despite these studies, it was recently concluded that release of serotonin (5-HT) from the mucosa was critical for distension-evoked CMMCs in mouse colon (Heredia et al., 2009). In that study, it was concluded that 5-HT was released from the mucosa following distension, and this 5-HT release was suggested to activate sensory nerve terminals in the mucosa, which then activated CMMCs (Heredia et al., 2009). These conclusions were made without any recordings of 5-HT release from the mucosa.
If the mucosa, or release of substances (such as 5-HT) from the mucosa are indeed essential for stretch-evoked CMMCs, as has been proposed, then removal of the mucosa would be expected to prevent stretch from evoking CMMCs. We have investigated this notion by directly testing whether distension-evoked CMMCs can still be evoked following removal of the mucosa and submucosal plexus from the entire colon in vitro. Secondly, we have investigated whether mucosal stimulation (independent of direct circumferential stretch) is a reliable stimulus to evoke CMMCs.
Materials and Methods
Preparation of Tissues
C57BL/6 mice (30–90 days of age) of either sex were euthanized by isoflurane inhalation overdose, followed by cervical dislocation, as approved by the animal welfare committee at Flinders University. The entire colon was removed from mice and placed in room temperature Krebs solution, which was constantly bubbled with carbogen gas (95% O2/5% CO2).
Dissection to Remove the Mucosa and Submucosa in Sheet Preparations of Colon
We have previously reported that in order to preserve spontaneous CMMCs following sharp dissection to remove the mucosa requires care to prevent damage to the underlying myenteric plexus (Keating and Spencer, 2010). In this study, we removed the mucosa and submucosa by firstly making a longitudinal incision along the mesenteric border and pinning the entire colon as a flat sheet preparation, with the mucosal surface facing uppermost. We then pinched the mucosal surface with fine dissecting forceps (see below) and physically cut off the mucosa and underlying submucosa from the myenteric plexus with fine surgical scissors. In general, peeling the mucosa and submucosa off the underlying myenteric plexus rarely preserved CMMCs. The forceps we used for dissections to pinch small segments of mucosa were: catalog # 5SF inox (code: 11252-00) or 55SF (code: 11255-20) from Fine Science Tools, Canada. The scissors we used to cut off the mucosa and submucosa were Vannas, Tubingen, Germany; code # 15003-08, which were 8.5 cm in length. The dissection process to remove the mucosa and submucosal plexus was performed in a small Petri dish containing ice cold Krebs solution that was constantly bubbled with carbogen gas (95% O2/5% CO2).
Method for Recording Motor Activity from the Circular Muscle Layer and Applying Controlled Levels of Circumferential Stretch
Colonic preparations used for recording and evoking CMMCs in response to stretch ranged in length from 36 to 48 mm (under resting slack conditions). This distance equated to approximately 70% of the entire length of colon. The terminal region of anal sphincter was not attached to the colon. To record motor activity from the circular muscle layer, two independent hooks were used (see Figure 1) to record and stretch the colon from a single site in the mid to distal region of colon. One hook (12 mm long) was applied to the mid–distal region of colon, while the proximal hook was 9 mm long and was used only for recording spontaneous changes in muscle tension. A distance of 6–9 mm separated the closest oral to anal edges of the two hooks. A basal resting tension was applied to the distal hook of 300 mg and 400–500 mg to the proximal hook (see Figure 1). Circumferential stretch was applied at a rate of 100 μm/s to a level that measured as a 30% increase in circumferential diameter, calculated for each preparation to take into account preparation variability. This level of stretch was maintained for 5 s by a stepper motor controlled tissue stretcher. The circumferential diameter of each preparation slack varied between 7 and 8 mm. The tissue stretcher used to apply controlled stretches was set to apply stretch at an time point that was 20% shorter than the spontaneously occurring CMMCs. In intact control colons, stretch was applied approximately every 50–60 s and every 80–90 s for colonic preparations devoid of mucosa and submucosal plexus.
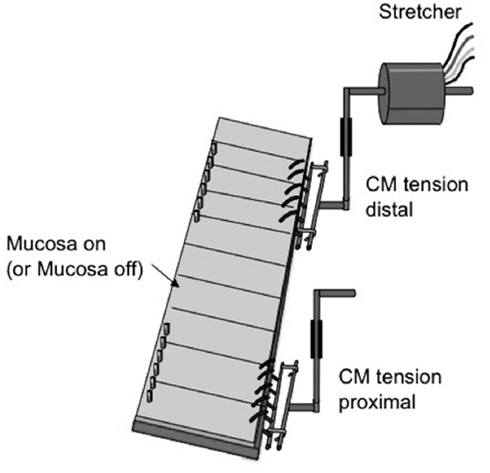
Figure 1. Diagrammatic representation of the preparation used to elicit and record distension-evoked CMMCs. A stepper motor controlled tissue stretcher was used to apply controlled distensions to a 15-mm segment of distal colon. In the proximal colon, an isometric tension transducer was used to record dynamic mechanical activities of the oral region of colon. Stretch was only applied to distal colon.
Protocol for Mucosal Stimulation
The protocol used for stimulation of the mucosa was identical to that used in our previous study on guinea-pig distal colon (Spencer et al., 1999). In brief, the isolated whole mouse colon was pinned mucosal side uppermost and mechanical recordings made simultaneously from the proximal mid and distal regions. The mucosal surface was stimulated by three consecutive brush strokes, applied via a fine artists paint brush, over an area ~7 mm in length across the full circumference, applied ~2–4 mm from the anus.
Measurements and Statistics
Measurements of the half duration and peak amplitude of CMMCs were measured from tension recordings, as was the interval between CMMCs. The propagation velocity of CMMCs was not possible to ascertain with only two recording sites, as the actual direction of propagation was not possible to determine with confidence. Data in the Section “Results” are presented as means ± SEM. The use of “N” in the Section “Results” refers to the number of animals on which observations were made. Data sets were considered statistically significant if P values < 0.05 were reached. Student’s unpaired t-test were used for comparison of data.
Drugs and Solutions
The Krebs solution used contained (in mM): NaCl, 118; KCl, 4.7; NaHPO4·2H20, 1.0; NaHCO3, 25; MgCl·6H20, 1.2; D-Glucose, 11; CaCl2·2H20, 2.5. Hexamethonium was obtained from Sigma Chemical Co., MO, USA and made up as a stock solution of 100 mM in deionized water.
Results
In isolated sheet preparations of whole mouse colon, mechanical recordings made simultaneously from the proximal and distal colon revealed spontaneous CMMCs occurred with a mean interval between of 78 ± 8 s (N = 8), where the mean peak amplitude of CMMC contractions was 3.9 ± 1.1 g (N = 8). When the mucosa and submucosal plexus were removed from the whole isolated colon, spontaneous CMMCs were still recorded (Figure 2). Histological staining of these preparations for Hematoxylin and Eosin (H and E) confirmed the removal of the submucosal ganglia and mucosa (Figure 3). Overall, there was no significant difference in the amplitude (control: 3.9 ± 1.1 g.s; N = 5, cf. mucosa removed: 2.4 ± 0.6 g.s) or area-under-contraction (control: 20.9 ± 4.9 g.s, cf. 16.9 ± 4.9 g.s; see Figure 4; P < 0.05; N = 5) of spontaneous CMMCs in intact preparations, compared with spontaneous CMMCs following removal of the mucosa and submucosal plexus (Figure 4). However, the interval between CMMCs was significantly longer. CMMCs in mucosa-free preparations occurred every 131 ± 11 s, which equated to an increase in interval of 68%, P < 0.005; N = 5, Figure 4, consistent with our previous study (Keating and Spencer, 2010).
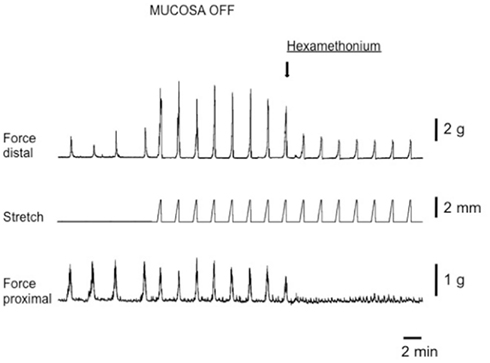
Figure 2. Circumferential stretch-evoked CMMCs in isolated whole mouse colon devoid of mucosa and submucosal plexus. On the left side of the figure four spontaneous CMMCs occur prior to circumferential stretch applied. Circumferential stretch was applied to the distal colon (see stretch trace) and evoked CMMCs at an increased frequency than the spontaneous frequency. The amplitude of spontaneous and evoked CMMCs in the proximal colon was no different. Upon addition of Hexamethonium (300 μM) stretch-evoked CMMCs were abolished in the proximal colon. During imposed stretches in the presence of hexamethonium a passive increase in tension occurs in the distal colon, but no CMMCs are evoked.
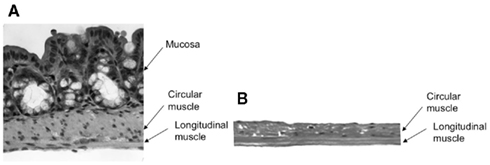
Figure 3. Hematoxylin and Eosin (H and E) stain of control (undissected) (A) colon, and dissected colon (B), with mucosa and submucosal ganglia removed.
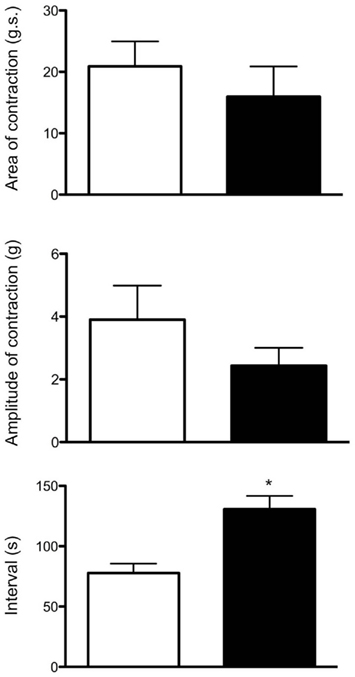
Figure 4. Similarities between CMMCs evoked in intact colons compared with those evoked in mucosa and submucosal plexus-free colons. The area-under-contraction and amplitude of evoked CMMCs was no different between either preparation. Interestingly, the threshold levels of circumferential stretch (in mm) required to evoked CMMCs was actually lower in preparations that had had their mucosa and submucosal plexus removed (N = 5; P < 0.05). White boxes refer to control colon, while black bars refer to mucosa and submucosal plexus-free colons.
In intact preparations, when graded increases in circumferential stretch (of 30% beyond resting slack width) were applied to the distal colon, at a controlled rate of 100 μm/s, a CMMC was evoked in the distal colon that propagated to the proximal colon (Figure 2). When the identical rate of circumferential stretch was applied to the same region of colon in mucosa and submucosal plexus-free preparations, the stretch threshold required to evoke a CMMC actually decreased by 23%, since the stretch threshold length in control preparations was 2.2 ± 0.1 mm (N = 5), but was 1.7 ± 0.1 mm (N = 5) in mucosa and submucosal plexus-free preparations (Figure 5; N = 5, P < 0.01). There was no significant difference in the area-under-contraction of evoked CMMCs in intact preparations compared with stretch-evoked CMMCs in mucosa and submucosal plexus-free preparations (Figures 4 and 5; N = 5). Hexamethonium (200 μM) was used to test the role of nicotinic transmission in spontaneous and evoked CMMCs. Hexamethonium always abolished spontaneous CMMCs (N = 5; Figure 2) and stretch-evoked CMMCs in intact preparations (N = 5; Figure 6), or preparations with their mucosa and submucosal plexus removed (Figure 7).
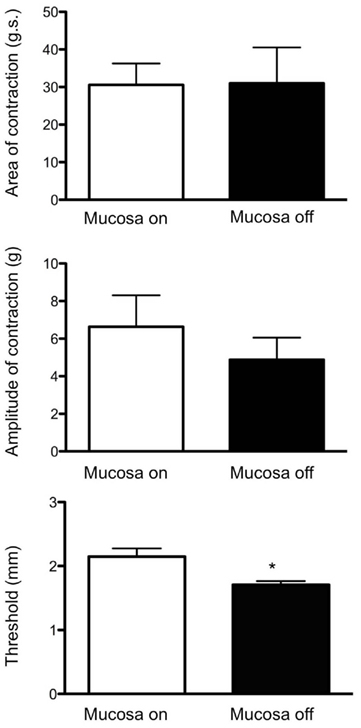
Figure 5. Similarities and differences between spontaneous CMMCs in intact whole colon compared with spontaneous CMMCs occurring following removal of the mucosa and submucosal plexus. Following removal of the mucosa and submucosal plexus, there was no significant difference between the amplitude or area-under-contraction of spontaneous CMMCs before or after removal of the mucosa or submucosal plexus. The only significant difference was the interval between spontaneous CMMCs was significantly slower following removal of the mucosa and submucosal plexus. White boxes refer to control colon, while black bars refer to mucosa and submucosal plexus-free colons.
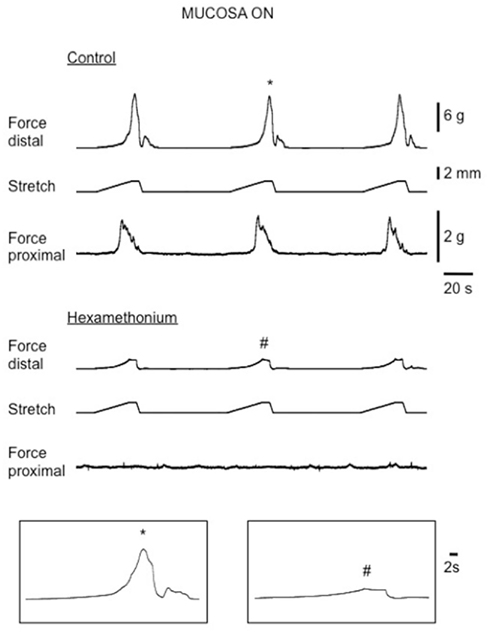
Figure 6. Recordings of evoked CMMCs in response to circumferential stretch of the distal colon in an intact (mucosa on) control mouse colon. Top traces show passive and active increases in resting tension in the distal recording in response to graded stretch at a rate of 100 μm s−1. In the bottom traces, hexamethonium abolished evoked CMMCs in the proximal colon whilst small passive increases in tension were recorded in the distal colon, where the stretch was applied.
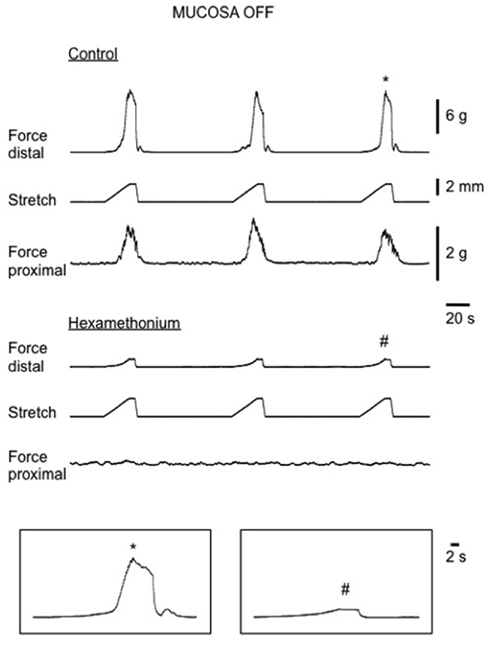
Figure 7. Stretch-evoked CMMCs in isolated mouse colon with mucosa and submucosal plexus removed. Top traces show control CMMCs, evoked following each graded distension stimulus. Bottom traces show in the presence of hexamethonium stretch fails to evoke CMMCs in the proximal or distal colon. Passive increases in tension in the circular muscle layers are detected in the distal colon when each stretch stimulus is delivered.
Overall, in intact preparations of colon, there were no significant differences in the mean area-under-contraction of evoked CMMCs (control: 30.6 ± 5.7 g.s; N = 5) compared with those evoked by stretch in preparations with mucosa and submucosal plexus removed (31 ± 9.6 g.s, N = 6; see Figure 5; P > 0.05; unpaired t-test). Similarly, there was no difference in the mean peak amplitude of CMMCs evoked in intact preparations, compared with those evoked following removal of the mucosa and submucosal plexus (control: 6.6 ± 1.7 g; Mucosa off 4.8 ± 1.4, NS). Taken together, these results suggest that once initiated by stretch, evoked CMMCs in intact segments of colon are indistinguishable from CMMCs evoked in preparations devoid of mucosa and submucosal plexus.
Effects of Mucosal Stimulation in the Absence of Circumferential Stretch
The experiments above showed that acute circumferential stretch of sheet preparations of colon, without direct compression of the mucosa, readily evokes CMMCs. However, we sought to determine if direct mucosal stimulation (without circumferential stretch) would also evoke CMMCs. To do this, we recorded spontaneous CMMCs in isolated full length sheet preparations of whole colon, with the mucosa present and facing uppermost. Once stable spontaneous CMMCs were recorded, we applied three brush strokes to the mucosa, using a fine artists paint brush, as previously described (Spencer et al., 1999). Overall, we found CMMCs rarely evoked by direct mucosal stimulation. In only 2 of 50 trials (N = 9) was a CMMC evoked in the distal colon which propagated to the proximal colon. Commonly no response was evoked (Table 1). In fact, in 32 of these 50 trials, no contractile response was elicited (see Table 1; N = 6). In the two trials where mucosal stimulation did evoke a CMMC, we removed the mucosa from the site of stimulation to expose the underlying circular muscle. In this case, reapplication of the identical mucosal stimulus to the circular muscle revealed that a CMMC could still be evoked, suggesting that the initial response was unlikely to be due to the mucosal stimulation, but rather inadvertent stimulation of the underlying myenteric plexus or muscularis externa.
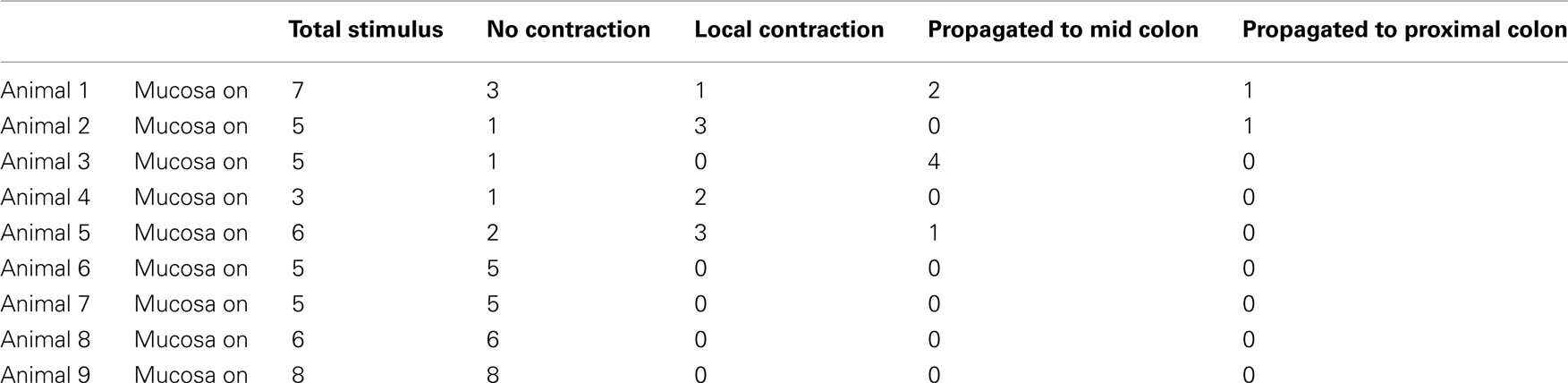
Table 1. Proportion of mucosal stimuli that evoked a local or propagating contraction, or no response.
Discussion
The aim of this study was to determine if the mucosa and submucosal plexus were required for the generation and propagation of CMMCs evoked by circumferential stretch. The major finding of the current study shows that circumferential stretch, but not mucosal stimulation, readily evokes CMMCs in isolated mouse colon; and that removal of the mucosa and submucosal plexus does not prevent their initiation or propagation following circumferential stretch. Mucosal stimulation alone, in the absence of stretch rarely evoked CMMCs (2 of 50 trials) and proved highly unreliable.
Possible Mechanisms Underlying Evoked CMMCs
Under normal physiological conditions in vivo, when an intraluminal distension stimulus is applied to the gut wall, it imposes at least two major types of stimuli to the gut wall. Firstly, it distorts and compresses the mucosa, which is known to stimulate release of many different substances such as 5-HT, and secondly, distension of the gut wall imposes stretch to the muscularis externa and enteric ganglia. Whether one, or both of these different types of stimuli are required for the initiation of distension-evoked CMMCs was unknown and had been difficult to reconcile. The experimental approach we employed in flat sheet preparations was chosen to test independently whether direct mucosal compression or stretch were able to evoke CMMCs.
Current Understanding about 5-HT Release from EC Cells during Intestinal Motor Patterns
It has been well characterized that endogenous 5-HT is released from EC cells in both the small intestine (Büllbring and Lin, 1958; Bertrand, 2006) and colon (Foxx-Orenstein et al., 1996; Grider et al., 1996) following mechanical stimulation of the mucosa. These findings have led to the popular conclusion that release of 5-HT from EC cells following luminal distension is responsible for the initiation of peristalsis in small intestine (Büllbring and Lin, 1958) and colon (Grider et al., 1996; Kadowaki et al., 1996; Jin et al., 1999) or CMMCs in mouse colon. Until recently, no studies had ever actually recorded release of 5-HT from the mucosa to verify these hypotheses. Recent studies have now used real time amperometry to record 5-HT release directly from the mucosa. When the first direct recordings of 5-HT release were made from the mouse colon (Keating and Spencer, 2010), it was found that 5-HT is released as a consequence of the contractions underling CMMCs and that any mucosal release of 5-HT is not their underlying cause. This was demonstrated when recent studies showed that removal of the mucosa abolished all 5-HT release, but did not abolish spontaneous CMMCs (Keating and Spencer, 2010). Similar conclusions have now also been demonstrated for peristalsis in the guinea-pig colon, where removal of the mucosa and submucosal plexus in tubular preparations abolished all release of 5-HT, but did not prevent peristalsis, nor the natural propulsion of fecal pellets along the colon (Spencer et al., 2011). These conclusions are entirely consistent with the work of Bertrand (2006) who also concluded that EC cells released 5-HT as a consequence of contraction induced during peristalsis and deformation of the ECs, rather than the EC cells releasing 5-HT to initiate peristalsis.
Previous Hypotheses Regarding Stretch-Evoked CMMCs in Mouse Colon
It was recently concluded that distension of the mouse colon activates CMMCs by releasing 5-HT from EC cells, and that this release of 5-HT was suggested to activate sensory nerve endings in the mucosa itself (Heredia et al., 2009). These conclusions were not supported by any recordings of 5-HT release (Heredia et al., 2009). If these conclusions are accurate, then removal of the mucosal layer would be expected to prevent distension or stretch from evoking CMMCs. We found that following removal of the mucosa and submucosal plexus, circumferential stretch still readily evoked CMMCs. This suggests that the mucosa itself, or release of substances from the mucosa are not required for stretch-evoked or spontaneous CMMCs. In further support of this, when we applied direct mucosal stroking to the mouse colon (in the absence of acute stretch or distension), CMMCs were rarely evoked (2 of 50 trials from nine animals) and on the two occasions they were evoked, they could still be evoked by stimulating the same site after removal of the mucosa. Taken together, these results strongly suggest that distension of the mouse colon activates stretch-receptors in the myenteric plexus and/or muscularis externa and that activation of these stretch-receptors do not require release of substances from the mucosa, nor could it be possible that they require activation of sensory nerve endings in the mucosa, or submucosal ganglia. We confirmed that no mucosa was present in our preparations by immunohistochemical staining for H and E which showed that not only the mucosa, but the entire submucous plexus had been excised. In these preparations, we showed previously that all detectable release of 5-HT was prevented (Keating and Spencer, 2010). In support of our conclusions, the recent work of Gershon and colleagues has shown that selective blockade of 5-HT release from EC cells does not have any effect on gastric emptying or colonic motility in live mice (Yadav et al., 2010). Our recent findings in the guinea-pig colon are also highly consistent with our findings in this current study; and the work of Gershon and colleagues. In guinea-pig distal colon we also recently showed that removal of the mucosa or submucosal plexus did not prevent peristalsis, nor prevent fecal pellet propulsion evoked by natural distension (fecal pellets; Spencer et al., 2011).
We are unable to explain how mucosal stimulation in the past has been claimed to evoke CMMCs (Heredia et al., 2009). It is presumed that by stroking the mucosa with a brush activates only sensory nerve endings in the mucosa. However, forceful compression of the mucosa also inadvertently compresses the underlying myenteric ganglia, which in itself is known to be a potent stimulus for activating intrinsic neural circuitry (Spencer et al., 2003). We found that mucosal stimulation rarely evoked a CMMC and in the two of 50 trials where a CMMC was evoked, it continued to do so after removal of the mucosa and submucosal plexus when the stimulus was applied to the circular muscle. Taken together, we found no evidence that mucosal stimulation alone reliably evokes CMMCs.
Intrinsic Sensory Neurons and Evoked CMMCs
A number of major types of mechanosensitive enteric neurons have been described, including myenteric and submucosal Dogiel type II neurons (Furness et al., 1998) and different classes of Dogiel type I neurons (Spencer and Smith, 2004; Mazzuoli and Schemann, 2009). Of these sensory neurons, Dogiel type II cells have been shown to project to the lamina propria and mucosa (Song et al., 1994) and are potently activated by exogenous 5-HT applied to their cell bodies (Wood and Mayer, 1979) and mucosal terminals, largely via 5HT3 receptors (Bertrand et al., 2000). The results of the present study suggest that the submucosal intrinsic primary afferent neurons are not required for the initiation or propagation of CMMCs in mouse colon. It is likely they are more closely involved in reflexes activated by nutrients (Gwynne and Bornstein, 2007) or in secretomotor reflexes. The sensory neurons that are activated by distension must be located within the outer muscle layers (myenteric plexus) of the colon, and are sufficient to trigger and maintain fully functional CMMCs. Whilst there is sound evidence that mucosally projecting Dogiel type II neurons in the myenteric plexus are intrinsic sensory neurons (Bornstein et al., 2004; Bornstein, 2006), our results suggest that activation of the mucosally projecting processes of Dogiel type II neurons are clearly not required for spontaneous or evoked CMMC generation in mouse colon, nor the initiation of colonic peristalsis in guinea-pig colon (Spencer et al., 2011).
Conclusion
The findings of the current study show that circumferential stretch, but not mucosal stimulation reliably evokes CMMCs in isolated mouse colon. The mechanisms that initiate CMMCs in response to circumferential stretch do not require neurons in the submucosal plexus, release of substances from the mucosa, or activation of nerve endings in the mucosa. The intrinsic sensory neurons that initiate stretch-evoked CMMCs lie in the myenteric plexus; and their mechanoreceptive terminals lie either in the myenteric ganglia and/or muscularis externa.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The experiments carried out in this study were funded by grants to NJS (grant # 535034 & #1025766) from the National Health and Medical Research Council (NH and MRC) of Australia.
References
Bertrand, P. P. (2006). Real-time measurement of serotonin release and motility in guinea pig ileum. J. Physiol. (Lond.) 577, 689–704.
Bertrand, P. P., Kunze, W. A., Furness, J. B., and Bornstein, J. C. (2000). The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 101, 459–469.
Bian, X. C., Heffer, L. F., Gwynne, R. M., Bornstein, J. C., and Bertrand, P. P. (2004). Synaptic transmission in simple motility reflex pathways excited by distension in guinea pig distal colon. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1017–G1027.
Bornstein, J. C. (2006). Intrinsic sensory neurons of mouse gut – toward a detailed knowledge of enteric neural circuitry across species. Focus on “characterization of myenteric sensory neurons in the mouse small intestine.” J. Neurophysiol. 96, 973–974.
Bornstein, J. C., Costa, M., and Grider, J. R. (2004). Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol. Motil. 16(Suppl. 1), 34–38.
Brierley, S. M., Nichols, K., Grasby, D. J., and Waterman, S. A. (2001). Neural mechanisms underlying migrating motor complex formation in mouse isolated colon. Br. J. Pharmacol. 132, 507–517.
Büllbring, E., and Lin, R. C. Y. (1958). The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J. Physiol. (Lond.) 140, 381–407.
Bywater, R. A., Spencer, N. J., Fida, R., and Taylor, G. S. (1998). Second-, minute- and hour-metronomes of intestinal pacemakers. Clin. Exp. Pharmacol. Physiol. 25, 857–861.
Christensen, J., Anuras, S., and Hauser, R. L. (1974). Migrating spike bursts and electrical slow waves in the cat colon: effect of sectioning. Gastroenterology 66, 240–247.
Foxx-Orenstein, A. E., Kuemmerle, J. F., and Grider, J. R. (1996). Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology 111, 1281–1290.
Furness, J. B., Kunze, W. A., Bertrand, P. P., Clerc, N., and Bornstein, J. C. (1998). Intrinsic primary afferent neurons of the intestine. Prog. Neurobiol. 54, 1–18.
Grider, J. R., Kuemmerle, J. F., and Jin, J. G. (1996). 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am. J. Physiol. 270, G778–G782.
Gwynne, R. M., and Bornstein, J. C. (2007). Mechanisms underlying nutrient-induced segmentation in isolated guinea pig small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1162–G1172.
Heredia, D. J., Dickson, E. J., Bayguinov, P. O., Hennig, G. W., and Smith, T. K. (2009). Localized release of serotonin (5-Hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136, 1328–1338.
Heredia, D. J., Dickson, E. J., Bayguinov, P. O., Hennig, G. W., and Smith, T. K. (2010). Colonic elongation inhibits pellet propulsion and migrating motor complexes in the murine large bowel. J. Physiol. (Lond.) 588, 2919–2934.
Jin, J. G., Foxx-Orenstein, A. E., and Grider, J. R. (1999). Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J. Pharmacol. Exp. Ther. 288, 93–97.
Kadowaki, M., Wade, P. R., and Gershon, M. D. (1996). Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am. J. Physiol. 271, G849–G857.
Karaus, M., Sarna, S. K., Ammon, H. V., and Wienbeck, M. (1987). Effects of oral laxatives on colonic motor complexes in dogs. Gut 28, 1112–1119.
Keating, D. J., and Spencer, N. J. (2010a). Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 138, 659–670; e651–e652.
Mazzuoli, G., and Schemann, M. (2009). Multifunctional rapidly adapting mechanosensitive enteric neurons (RAMEN) in the myenteric plexus of the guinea pig ileum. J. Physiol. 587, 4681–4694.
Powell, A. K., Fida, R., and Bywater, R. A. (2003). Motility in the isolated mouse colon: migrating motor complexes, myoelectric complexes and pressure waves. Neurogastroenterol. Motil. 15, 257–266.
Roberts, R. R., Bornstein, J. C., Bergner, A. J., and Young, H. M. (2008). Disturbances of colonic motility in mouse models of Hirschsprung’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G996–G1008.
Roberts, R. R., Murphy, J. F., Young, H. M., and Bornstein, J. C. (2007). Development of colonic motility in the neonatal mouse-studies using spatiotemporal maps. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G930–G938.
Sarna, S. K. (1986). Myoelectric correlates of colonic motor complexes and contractile activity. Am. J. Physiol. 250, G213–G220.
Sarna, S. K. (1991b). Physiology and pathophysiology of colonic motor activity (2). Dig. Dis. Sci. 36, 998–1018.
Smith, T. K., Bornstein, J. C., and Furness, J. B. (1991). Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea-pig ileum. J. Auton. Nerv. Syst. 34, 69–75.
Song, Z. M., Brookes, S. J., Gibbins, I. L., and Costa, M. (1994). All calbindin-immunoreactive myenteric neurons project to the mucosa of the guinea-pig small intestine. Neurosci. Lett. 180, 219–222.
Spencer, N., Mccarron, S. L., and Smith, T. K. (1999). Sympathetic inhibition of ascending and descending interneurones during the peristaltic reflex in the isolated guinea-pig distal colon. J. Physiol. (Lond.) 519(Pt 2), 539–550.
Spencer, N. J. (2001). Control of migrating motor activity in the colon. Curr. Opin. Pharmacol. 1, 604–610.
Spencer, N. J., Bayguinov, P., Hennig, G. W., Park, K. J., Lee, H. T., Sanders, K. M., and Smith, T. K. (2007). Activation of neural circuitry and Ca2+ waves in longitudinal and circular muscle during CMMCs and the consequences of rectal aganglionosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G546–G555.
Spencer, N. J., and Bywater, R. A. (2002). Enteric nerve stimulation evokes a premature colonic migrating motor complex in mouse. Neurogastroenterol. Motil. 14, 657–665.
Spencer, N. J., Bywater, R. A., and Taylor, G. S. (1998a). Disinhibition during myoelectric complexes in the mouse colon. J. Auton. Nerv. Syst. 71, 37–47.
Spencer, N. J., Bywater, R. A., and Taylor, G. S. (1998b). Evidence that myoelectric complexes in the isolated mouse colon may not be of myogenic origin. Neurosci. Lett. 250, 153–156.
Spencer, N. J., Hennig, G. W., Dickson, E., and Smith, T. K. (2005). Synchronization of enteric neuronal firing during the murine colonic MMC. J. Physiol. (Lond.) 564, 829–847.
Spencer, N. J., Hennig, G. W., and Smith, T. K. (2002). A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J. Physiol. 545, 629–648.
Spencer, N. J., Hennig, G. W., and Smith, T. K. (2003). Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea pig distal colon. Am. J. Physiol. Gastrointest. Liver Physiol. 284, G231–G241.
Spencer, N. J., Nicholas, S., Robinson, L., Flack, N., Brookes, S. J., Zagorodnyuk, V. P., and Keating, D. J. (2011). Mechanisms underlying distension-evoked peristalsis in guinea-pig distal colon: Is there a role for enterochromaffin cells? Am. J. Physiol. 301, G519–G527.
Spencer, N. J., and Smith, T. K. (2001). Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J. Physiol. (Lond.) 533, 787–799.
Spencer, N. J., and Smith, T. K. (2004). Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J. Physiol. 558, 577–596.
Thornton, P. D., and Bornstein, J. C. (2002). Slow excitatory synaptic potentials evoked by distension in myenteric descending interneurones of guinea-pig ileum. J. Physiol. (Lond.) 539, 589–602.
Wood, J. D. (1973). Electrical activity of the intestine of mice with hereditary megacolon and absence of enteric ganglion cells. Am. J. Dig. Dis. 18, 477–488.
Wood, J. D., Brann, L. R., and Vermillion, D. L. (1986). Electrical and contractile behavior of large intestinal musculature of piebald mouse model for Hirschsprung’s disease. Dig. Dis. Sci. 31, 638–650.
Wood, J. D., and Mayer, C. J. (1979). Serotonergic activation of tonic-type enteric neurons in guinea pig small bowel. J. Neurophysiol. 42, 582–593.
Yadav, V. K., Balaji, S., Suresh, P. S., Liu, X. S., Lu, X., Li, Z., Guo, X. E., Mann, J. J., Balapure, A. K., Gershon, M. D., Medhamurthy, R., Vidal, M., Karsenty, G., and Ducy, P. (2010). Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat. Med. 16, 308–312.
Keywords: migrating motor complex, colon, peristalsis, enteric, sensory neuron, pacemaker
Citation: Zagorodnyuk VP and Spencer NJ (2011) Localization of the sensory neurons and mechanoreceptors required for stretch-evoked colonic migrating motor complexes in mouse colon. Front. Physio. 2:98. doi: 10.3389/fphys.2011.00098
Received: 14 September 2011;
Accepted: 24 November 2011;
Published online: 21 December 2011.
Edited by:
Paul P. Bertrand, University of New South Wales, AustraliaReviewed by:
Paul P. Bertrand, University of New South Wales, AustraliaXiaochun Bian, Michigan State University, USA
Copyright: © 2011 Zagorodnyuk and Spencer. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Nick J. Spencer, Department of Human Physiology, School of Medicine, Flinders University, Adelaide, SA, Australia. e-mail: nicholas.spencer@flinders.edu.au
