How man-made interference might cause gas bubble emboli in deep diving whales
- 1 Maritime Systems Division, Norwegian Defence Research Establishment (FFI), Horten, Norway
- 2 Sea Mammal Research Unit, University of St. Andrews, St. Andrews, Scotland
- 3 Woods Hole Oceanographic Institution, Woods Hole, MA, USA
- 4 Institute of Marine Research, Bergen, Norway
- 5 Acoustics and Sonar Research Group, Netherlands Organisation for Applied Scientific Research (TNO), Hague, Netherlands
- 6 Department of Life Sciences, Texas A&M Corpus Christi, Corpus Christi, TX, USA
Naval sonar has been accused of causing whale stranding by a mechanism which increases formation of tissue N2 gas bubbles. Increased tissue and blood N2 levels, and thereby increased risk of decompression sickness (DCS), is thought to result from changes in behavior or physiological responses during diving. Previous theoretical studies have used hypothetical sonar-induced changes in both behavior and physiology to model blood and tissue N2 tension , but this is the first attempt to estimate the changes during actual behavioral responses to sonar. We used an existing mathematical model to estimate blood and tissue N2 tension from dive data recorded from sperm, killer, long-finned pilot, Blainville’s beaked, and Cuvier’s beaked whales before and during exposure to Low- (1–2 kHz) and Mid- (2–7 kHz) frequency active sonar. Our objectives were: (1) to determine if differences in dive behavior affects risk of bubble formation, and if (2) behavioral- or (3) physiological responses to sonar are plausible risk factors. Our results suggest that all species have natural high N2 levels, with deep diving generally resulting in higher end-dive as compared with shallow diving. Sonar exposure caused some changes in dive behavior in both killer whales, pilot whales and beaked whales, but this did not lead to any increased risk of DCS. However, in three of eight exposure session with sperm whales, the animal changed to shallower diving, and in all these cases this seem to result in an increased risk of DCS, although risk was still within the normal risk range of this species. When a hypothetical removal of the normal dive response (bradycardia and peripheral vasoconstriction), was added to the behavioral response during model simulations, this led to an increased variance in the estimated end-dive N2 levels, but no consistent change of risk. In conclusion, we cannot rule out the possibility that a combination of behavioral and physiological responses to sonar have the potential to alter the blood and tissue end-dive N2 tension to levels which could cause DCS and formation of in vivo bubbles, but the actually observed behavioral responses of cetaceans to sonar in our study, do not imply any significantly increased risk of DCS.
Introduction
It has been suggested that anthropogenic sound, such as naval sonar, might lead to development of tissue N2 gas bubbles and decompression sickness (DCS; Jepson et al., 2003), and that relationships between sound and DCS could explain some unusual whale strandings (Jepson et al., 2003). Increased blood or tissue N2 tensions could either be caused by a change in dive behavior in response to sonar (Jepson et al., 2003), by changes in physiological responses to diving (Hooker et al., 2012) or directly by an acoustically enhanced bubble growth (Crum and Mao, 1996). While logistical and ethical constraints have prevented physiological studies on large whales, gas exchange models have indicated that the cardiac output, blood flow distribution, and pulmonary shunt are important variables that determine the level of blood and tissue (Fahlman et al., 2006, 2009). Theoretical studies have also indicated certain behavioral changes that may affect risk (Houser et al., 2001; Zimmer and Tyack, 2007; Hooker et al., 2009). It has been suggested that N2 loading is managed by the animals through different physiological trade offs, and if a behavioral response to an unanticipated acute threat (such as man-made noise) over-rides behaviors adapted to manage N2, the result may be decompression injury (Hooker et al., 2012). Until recently, no data existed on behavioral changes associated with sonar exposure. Previous theoretical studies attempting to estimate the effect of physiology and behavior on tissue and blood N2 levels in marine mammals tested a range of plausible behavioral responses, such as changes in the ascent and descent rates (Houser et al., 2001; Zimmer and Tyack, 2007; Hooker et al., 2009), the ratio between surface interval and dive duration (Fahlman et al., 2006), deep diving (Houser et al., 2001; Zimmer and Tyack, 2007; Hooker et al., 2009), and repetitive shallow diving (Houser et al., 2001; Zimmer and Tyack, 2007; Hooker et al., 2009).
Recent behavioral response studies have investigated how exposure to naval sonar signals affects the natural dive behavior in a range of species: Blainville’s beaked whales (Mesoplodon densirostris; Tyack et al., 2011), Cuvier’s beaked whales (Ziphius cavirostris; Southall et al., 2011), sperm whales (Physeter macrocephalus), long-finned pilot whales (Globicephala melas), and killer whales (Orcinus orca; (Miller et al., 2011; Sivle et al., submitted). Beaked whales and sperm whales are expert deep divers which regularly descend to depths of >1000 m for more than 60 min (Tyack et al., 2006, 2011; Watwood et al., 2006; Sivle et al., submitted), pilot whales are intermediate divers, typically performing dives to 300–600 m but of relatively short durations (<15 min; Baird et al., 2002; Aguilar Soto et al., 2008; Sivle et al., submitted), while killer whales are shallow divers that hardly ever exceed 100 m depth and dive durations of 10 min (Baird et al., 2005; Miller et al., 2010; Sivle et al., submitted). During these behavioral response studies, the whale was equipped with a suction cup attached digital tag (Johnson and Tyack, 2003). Following tag attachment, the whale was allowed to continue diving without sound exposure for between 1 and 7 h, followed by pre-determined periods of sonar exposures. The collected data allow comparison of the natural dive behavior during the pre-exposure as compared with that during sonar exposure. These data, therefore, provide species-specific cases which can be used to estimate how changes in dive behavior may affect blood and tissue levels.
We have used a previously published mathematical model (e.g., Fahlman et al., 2009) to estimate blood and tissue N2 tension from dive data recorded from sperm-, killer-, long-finned pilot-, Blainville’s beaked-, and Cuvier’s beaked whales before, during and after exposure to sonar signals. Our objectives were: (1) to determine if differences in natural behavior make some species more prone to DCS (i.e., higher end-dive levels), (2) to investigate if the measured sonar-induced changes in dive behavior make odontocetes vulnerable to anthropogenic disturbance, and finally (3) to investigate how a hypothetical sonar-induced physiological flight response, involving changes in cardiac output on top of the behavioral response, would affect the risk of DCS.
Materials and Methods
Permits
Animal experiments on sperm whales (Physeter macrocephalus, sw), long-finned pilot whales (Globicephala melas, Gm), and killer whales (Orcinus orca, Oo) were conducted in Norwegian waters under permits issued by the Norwegian Animal Research Authority to Dr. Petter Kvadsheim (permits no 2004/20607 and S-2007/61201), and in compliance with ethical use of animals in experimentation. The research on Blainville’s beaked whales (Mesoplodon densirostris, Md) was conducted under permits for marine mammal research issued by the U.S. National Marine Fisheries Service (NMFS) to Dr. Peter Tyack (Permit #981-1578), and issued by the Government of the Bahamas to the Bahamas Marine Mammal Research Organisation (Bahamas permit #01/09) and Dr. Ian Boyd (Bahamas permit #02/07 and #02/08). The research on Cuvier’s beaked whales (Ziphius cavirostris, Cv) were conducted in U.S. waters under U.S. NMFS research permit (#14534), as well as Channel Islands National Marine Sanctuary (CINMS) permit (#2010/004) for operations within the boundaries of the CINMS. All research protocols were also approved by the University of St. Andrews Animal Welfare and Ethics Committee as well as the Woods Hole Oceanographic Institution Animal Care and Use Committee.
Dive Data
Dive data for this research were collected in conjunction with several different research projects studying behavioral responses of cetaceans to naval sonar signals using very similar methodology. The “3S-project” collected data on sperm whales, pilot whale and killer whales in the Norwegian Sea, off the coast of Northern Norway, in 2006–2009 (Miller et al., 2011). The “AUTEC BRS-project” collected data on Blainville’s beaked whales off Andro’s Island, Bahamas, in 2007–2008 (Tyack et al., 2011). The “SOCAL BRS-project” collected data on Cuvier’s beaked whales off the coast of California, USA, in 2010 (Southall et al., 2011). In all these projects, time versus depth records were collected at 50 Hz sampling rate using a digital tag (Johnson and Tyack, 2003) attached to the whale by suction cups. In addition to the depth sensor the tag also contains acoustic sensors that can be used to measure the level of sound exposures. Following tag attachment, the whale was allowed to continue diving without sound exposure during a pre-exposure period of 1–7 h duration. This was followed by pre-determined periods of sonar exposures. During exposure the ship carrying the sonar source gradually approached the position of the whale and/or gradually increased the transmitted source level to achieve an escalation of the received sound pressure levels from initial values of 60–120 dB to maximum levels of 147–180 dB re 1 μPa (RMS values). This procedure was used to simulate an approaching naval vessel. Complete dive profiles and details of experimental procedures and calculations of received sonar levels are given in Miller et al. (2011) for sperm whales, pilot whales, and killer whales, in Tyack et al. (2011) for Blainville’s beaked whales and in Southall et al. (2011) for Cuviers’s beaked whales. A total of 21 dive records of >8 h were gathered (Table 1). Thirteen whales in the data set were exposed to LFAS (1–2 kHz) and/or MFAS (3–4 or 6–7 kHz) sonar signals and eight records contain undisturbed baseline behavior only (Table 1).
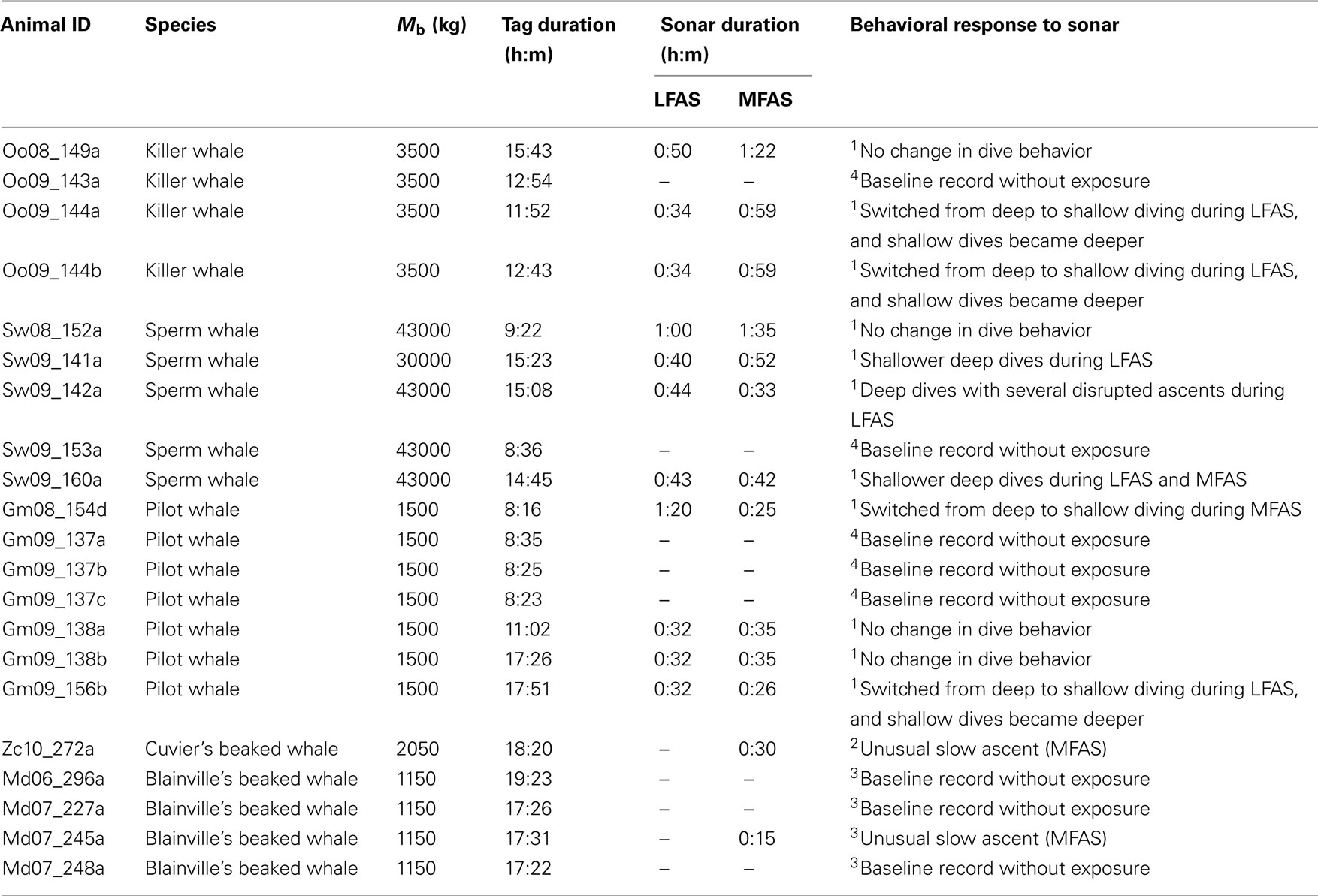
Table 1. Animal ID, species, assumed body size (Mb), total dive record duration, sonar exposure duration (LFAS and/or MFAS) and description of behavioral responses to sonar as reported by 1Sivle et al. (submitted), 2 Southall et al. (2011), or 3 Tyack et al. (2011). In addition some baseline data records without sonar exposures are also included as reported by 3 Tyack et al. (2011) and 4 Miller et al. (2011).
Gas Exchange Model
The dive records were entered into a gas exchange model in order to estimate blood and tissue N2 tension throughout the dives. The model was adapted from a previous breath-hold model which included exchange of N2, O2, and CO2 and also the effect of pressure on pulmonary gas exchange as previously detailed in Bostrom et al. (2008), Fahlman et al. (2009), and Hooker et al. (2009) with the revisions for the current analysis summarized below. The body was partitioned into four different tissue compartments (brain, fat, muscle, and central circulation) and one blood compartment (arterial and mixed venous). The parameters used for this model is the best available information from literature survey for each species, when available information was insufficient we applied information for other relevant species. In the current study, bone was included in the fat compartment as the bone of deep diving whales appears to be high in fat content (Higgs et al., 2010). The central circulatory compartment included heart, kidney, liver, and alimentary tract while the muscle compartment included muscle, skin, connective tissue, and all other tissues (Fahlman et al., 2009). The size of each compartment was taken from Hooker et al. (2009) for beaked whales and for the sperm whale, killer whale and pilot whale was based on available data for the sperm whale (Omura, 1950; McAlpine, 1985; Rice, 1989). Body mass for each species was estimated based on data recorded from stranded animals or from length-weight equations and length estimates (beaked whale; Hooker et al. (2009), sperm whale; Lockyer, 1991); killer whale; Clark et al. (2000)).
Gas exchange was assumed to occur between lung and blood and between blood and each compartment. The same assumptions were used for the blood N2 stores as those detailed in Fahlman et al. (2009). The total – (Qtot) and fractional blood flow to each tissue were not fixed, and could be varied to mimic diving bradycardia and changes in regional blood flow due to peripheral vasoconstriction (Butler and Jones, 1997). Hence, cardiovascular changes seen in freely diving animals could be simulated.
As in previous studies (Fahlman et al., 2006, 2009; Hooker et al., 2009), in the instances in which we had no direct anatomical or physiological data for the species in this study, we used data reported for the Weddell seal (Davis and Kanatous, 1999). The model included pulmonary shunting which varied with depth and diving lung volume (Bostrom et al., 2008; Fahlman et al., 2009; Hooker et al., 2009; see section below for details). For the sperm, killer, and pilot whale, the relative size of each compartment, expressed as a per cent weight of the body mass, was 3.3% for central circulation, 0.18% for brain, 50.02% for blubber, 26.5% for muscle, and 20% for blood. For the beaked whale the muscle was 57%, central circulation 3%, brain 0.2%, blubber 19.8%, and blood 20% of the total body mass. When calculating the O2 stores, it was assumed that the lean muscle mass was 23.9% of the body mass for the killer, sperm, and pilot whale and 49% for the beaked whales. The mass specific cardiac output was calculated according to Fahlman et al. (2009) and was 80 ml O2·min−1·kg−1 for a 43 ton sperm whale, 151 ml O2·min−1·kg−1 for a 3500 kg killer whale, and 186 ml O2·min−1·kg−1 for a 1500 kg pilot whale. While at the surface 31% of the cardiac output was directed to the central circulation, 67% to the muscle, 1.3% to the brain, and 0.7% to the blubber. During diving, the cardiac output was decreased to half and the blood distribution was changed such that 80% was directed to the central circulation, 1% to the muscle, 12% to the brain, and 7% to the blubber (Fahlman et al., 2006, 2009; Hooker et al., 2009).
Tissue Metabolic Rate and Gas Stores
While we do not report the blood and tissue O2 and CO2 levels in the current study, estimates of these parameters are included in the model as they affect the uptake and removal of N2 from the lungs and thereby the overall blood and tissue (Fahlman et al., 2009). The initial lung, blood, and tissue gas stores were assumed to be similar to those used in Fahlman et al. (2009). The metabolic rates for each tissue compartment were estimated from the data presented in Davis and Kanatous (1999). The O2 available during a dive came from lung, blood and tissue stores (mainly muscle, see below). The Ostwald solubility coefficient was used to calculate the dissolved O2 content in blood and we used a value of 0.0261 l O2·l−1 blood (Weathersby and Homer, 1980). The same solubility coefficient was used to estimate O2 content of muscle and central circulation. For the fat and brain compartment we used a value of 0.133 l O2·l−1 tissue.
In addition to dissolved O2, the muscle compartment was assumed to contain a significant amount of endogenous O2 bound to myoglobin and available for muscle metabolism. When calculating the total O2 stored in the muscle compartment, we assumed that for the pilot whale, sperm whale, and killer whale, 23.9% of the total Mb was skeletal muscle, an estimate based on data for the sperm whale (Omura, 1950; McAlpine, 1985; Rice, 1989). The same parameter was estimated at 49% for the beaked whales, i.e., the muscle compartments for the different species were composed of a variety of tissues. For beaked whales, we used the reported myoglobin concentration for Hyperoodon (63 g·kg−1 muscle; Butler and Jones, 1997), and for pilot, sperm, and killer whales we used the value reported for P. macrocephalus (57 g·kg−1 muscle, Dolar et al., 1999). For all species, an O2-binding capacity of 1.34 ml O2 (STPD)·g−1 muscle tissue (Stephenson, 2005) was assumed. The muscle was assumed to be completely saturated at the beginning of a trial run, i.e., initial conditions. The blood was assumed to have a hemoglobin (Hb) concentration of 0.26 kg·l−1 of blood and the same O2-binding capacity as myoglobin (Stephenson, 2005). Initially, it was assumed that arterial blood was 97% saturated and venous blood 87% saturated.
Lung Compression and Pulmonary Shunt
The lung collapse model presented by Bostrom et al. (2008) was used to estimate alveolar volume at depth (DVA). Initial parameters used to estimate DVA were: total lung capacity (TLC, total respiratory volume), the volume of the upper respiratory system including trachea and bronchi (VT), and maximal alveolar volume (VA), i.e., TLC = VT + VA. TLC was estimated as TLC = 0.135·Mb0.92 (Kooyman, 1973; Fahlman et al., 2011). It was assumed that gas exchange occurred only in the alveoli and when DVA = 0, no gas exchange occurred. Dead space volume was assumed to be 1/15 (6.7%) of TLC, the value reported for the bottlenose whale (Kooyman, 1973). It was assumed that all species dived with a lung volume (DVL) lower than TLC and the reduction in gas volume was taken from the alveolar gas space. That is, DVA = DVL − VT. For sperm, killer, and pilot whales, we used a DVL = 26.4 ml·kg−1 estimated for the sperm whale (Miller et al., 2004). Thus, for a 43000 kg sperm whale diving on a DVL = 26.4 ml·kg−1: TLC = 2472 l, VT = 165 l, DVL = 1135 l, DVA = 970 l. For Blainville’s beaked whale, we assumed a DVL estimated for this species of 13.1 ml·kg−1 (Zimmer and Tyack, 2007).
Estimated Levels during Diving
A dive was defined as a submergence for >10 s to a depth >1 m. Dives were categorized as shallow (depth >1 m and ≤30), intermediate (depth >30 m and ≤200 m) or deep (depth >200 m) based on the maximum depth of the dive. These categories were based on the assumption that shallow dives <30 m may serve to reduce PN2 and be potentially helpful as decompression dives (Fahlman et al., 2007). Intermediate dives are dives where there is still significant gas exchange and thus N2 is being absorbed by the body (Kooyman and Sinnett, 1982; Fahlman et al., 2008, 2009; Hooker et al., 2009) because of the hydrostatic pressure, whereas during deep dives the alveoli will most likely be collapsed and gas exchange will have ceased (Kooyman and Sinnett, 1982; Fahlman et al., 2008, 2009; Hooker et al., 2009). Within these categories we present average maximum dive depth (the maximum depth reached during the dive), average dive depth (the average depth of the dive), and average dive duration (the time spent submerged; Table 2).
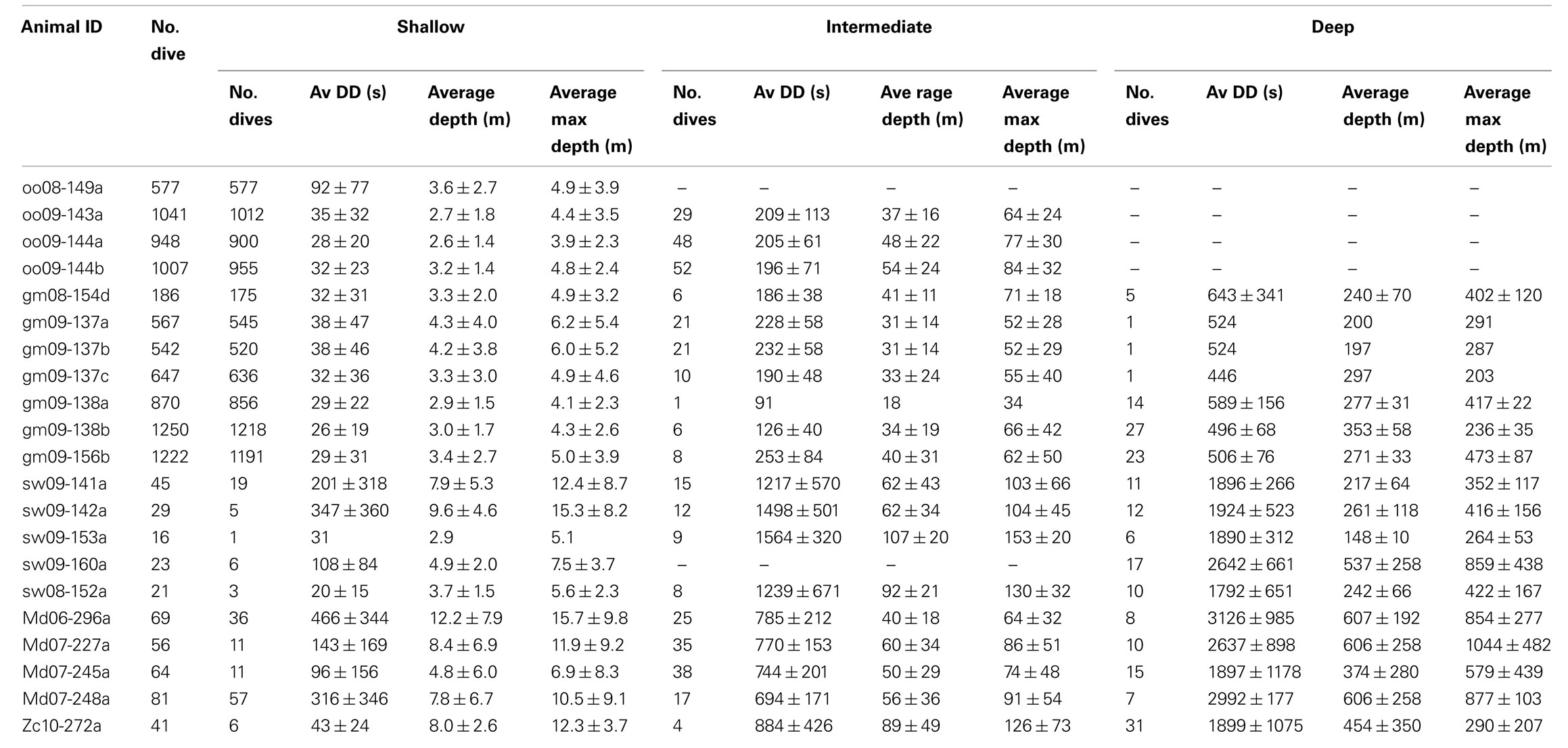
Table 2. Summary of animal dive series used in the current study. Shallow dives are to depths of 1–30 m, intermediate dives to depths of 30–200 m, and deep dives are deeper than 200 m. Values are mean ± SD.
Tissue and blood partial pressure of N2 were estimated throughout the entire duration of each dive series. As the N2 equilibrium state of a diving whale is not known at the start of a dive trace, the starting tissue and blood N2 must therefore be assumed (Zimmer and Tyack, 2007). Alternatively, the dive trace needs to be long enough such that a “quasi-equilibrium” is reached, which depends on the size of the animal and the specific dive behavior (Hooker et al., 2009). The time to equilibrium was shown to be approximately 4 h for a 1000 kg whale and 13 h for a 5000 kg whale. Consequently, none of the dive series used in this study were long enough for the sperm whales to reach equilibrium. For that reason, we initialized all tissues to two times the surface , which provided us reasonable equilibrium values for all tissues. This was based on testing a range of starting -values, were we determined that initializing the blood and tissue to two times ambient minimized variability of the model output. Dive records with less than 2 h of pre-exposure data still had to be removed from the analysis as the pre-exposure estimates became too uncertain.
Risk of decompression sickness (R)
The end-dive values were extracted for each dive category (shallow–intermediate–deep) as the average value of the first 10 s after the animal reached the surface. Risk of DCS following each dive was estimated as the instantaneous mixed venous supersaturation level (R):
where is given in Atmospheres Absolute (ATA) corresponding to the pressure at the sea surface (1 ATA = 101.3 kPa). The mixed venous levels were chosen because they represent the overall saturation level of the animals and have previously been used as a measure of risk of DCS in other species (Berghage et al., 1979) including humans (Weathersby et al., 1984). R was extracted for each dive during the pre-exposure period and compared to the estimated R-levels during LFAS and MFAS sonar exposure. This tested the effect of potential changes in behavior on the overall risk. To test the effect of a hypothetical physiological response to sonar, we removed the dive response during sonar exposure and re-ran the model. This implied that the model was run assuming that total cardiac output and blood distribution between tissue compartments were the same during diving as before diving. The change in R was again estimated and the pre-exposure compared with the exposure period. This tested the combined effect of changes in both behavioral and physiological responses.
Behavioral “Responders”
Analyses of changes in dive behavior in response to sonar exposure have been conducted on the same dataset used here to study potential changes in risk of DCS in beaked whales, sperm whales, pilot whales, and killer whales (Table 1). The Blainville’s beaked whale (md07_296a) and the Cuvier’s beaked whale (zc10_272a) were both exposed at depth and responded in much the same manner. Echolocation based foraging ceased and the animals broke off the deep dive prematurely before performing an unusually slow ascent to the surface (Southall et al., 2011; Tyack et al., 2011; Figure 1). In sperm whales responses were less clear, but there was an overall trend that deep dives were shorter and shallower during LFAS exposure (Sivle et al., submitted; e.g., sw09_160a in Figure 1), and this was often associated with reduced echolocation rates (Miller et al., 2011). Sperm whales generally performed normal deep dives with echolocations sounds during MFAS exposure (Sivle et al., submitted). When killer whales and pilot whales were engaged in deep diving foraging behavior at the time of exposure onset, they typically ended foraging and switched to shallow diving traveling mode. Interestingly, the shallow dives also became deeper during exposure than the shallow resting dives performed between deep dives prior to exposure (Sivle et al., submitted; e.g., gm09_156b and oo09_144a in Figure 1). Animals that were already in shallow diving traveling mode at exposure onset, just continued without changes in the dive pattern (Sivle et al., submitted). This response was consistent during LFAS exposure but less consistent during MFAS exposure (Sivle et al., submitted).
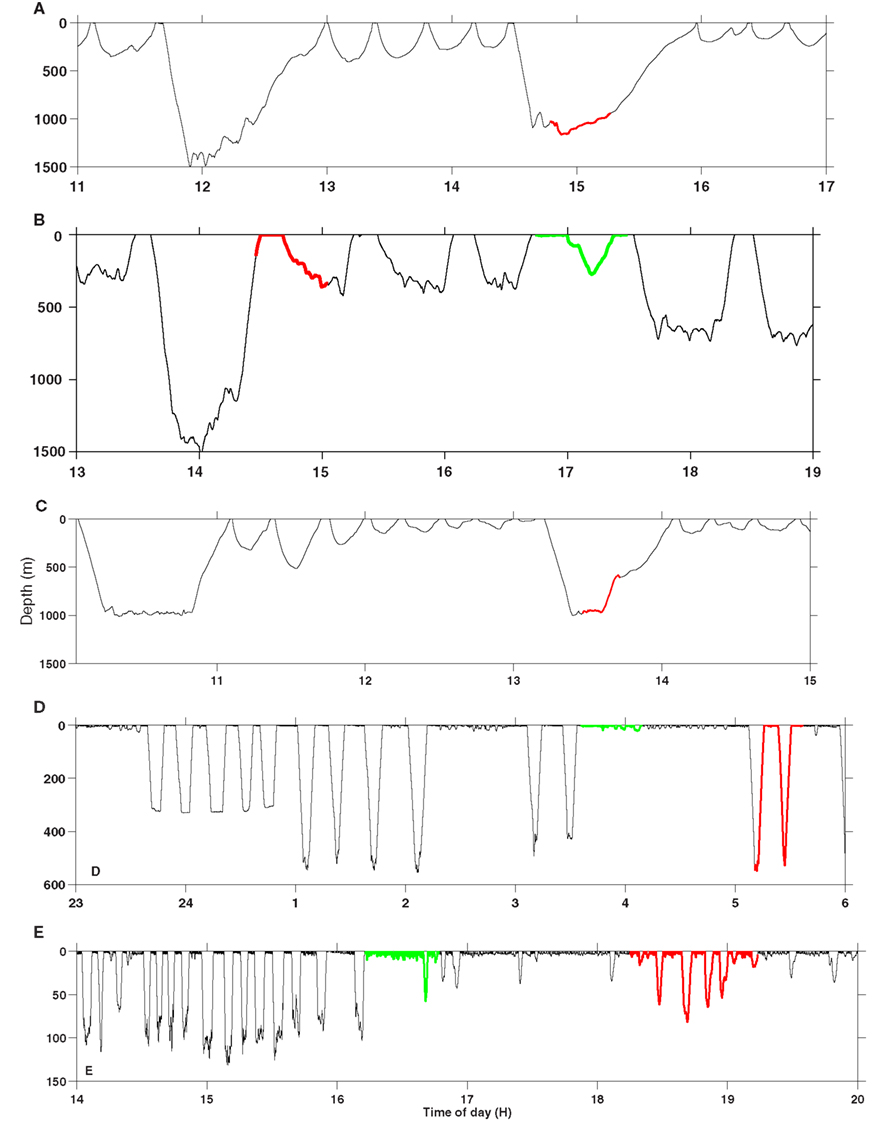
Figure 1. Typical examples of changes in dive behavior in response to sonar. (A) Cuvier’s beaked whale (zc10_272a), (B) sperm whale (sw09_160a), (C) Blainville’s beaked whale (md07_245a), (D) pilot whale (gm09_156b), (E) killer whale (oo09_144a). The red part of the dive profile is exposure to MFAS sonar and the green to LFAS sonar. Time is in hours GMT and depth is in meters. Note the differences in depth scale between the different panels.
In addition to the comparison of risk of DCS (R, Eq. 1) between the exposure- and pre-exposure periods within each dive category (shallow–intermediate–deep; Figure 3), we have also used the gas exchange model to look at sequential changes from pre-exposure to exposure in behavioral “responders” without considering dive categories (Figure 4). This analysis will capture effects of subtle behavioral change within a dive category as well as effect of behavioral changes were the animal changes dive category in response to sonar (e.g., going from deep to shallow diving). In animals which are supersaturated even a single event of having a high R, even for a short period might be enough to trigger a cascade of bubble formation. Therefore we have calculated both average and maximum R-values for dives during exposure and compared those values to maximum and average values for dives during the pre-exposure period in the behavioral “responders” (Figure 4).
Results
Summary statistics for each species and dive series are presented in Table 2. Each dive trace is indicated by the species abbreviation (oo: killer whale, sw: sperm whale, gm: pilot whale, zc: cuvier’s beaked whale, md: Blainville’s beaked whale) and an animal ID.
Estimated Blood and Tissue during Normal Diving
The blood and tissue end-dive as well as the variation between tissues increased as the dive depth increased (Figure 2). This increase in end-dive levels and tissue variance is caused by the increase in the “fast” tissues, which has low tissue time constants (brain and central circulation) with depth, while fat and muscle end-dive levels were less variable with dive depth. The correlation between dive depth and end-dive levels implied a higher risk to the deep divers (sperm whales and beaked whales) than the shallower divers (killer whales; Figure 2). Except for sperm whales, the end-dive during shallow dives was highest for the fat compartment (Figure 2). For deep and intermediate depth dives, end-dive was highest for the fast tissues (central circulation and brain) and lowest for the muscle compartment for all animals (Figure 2).
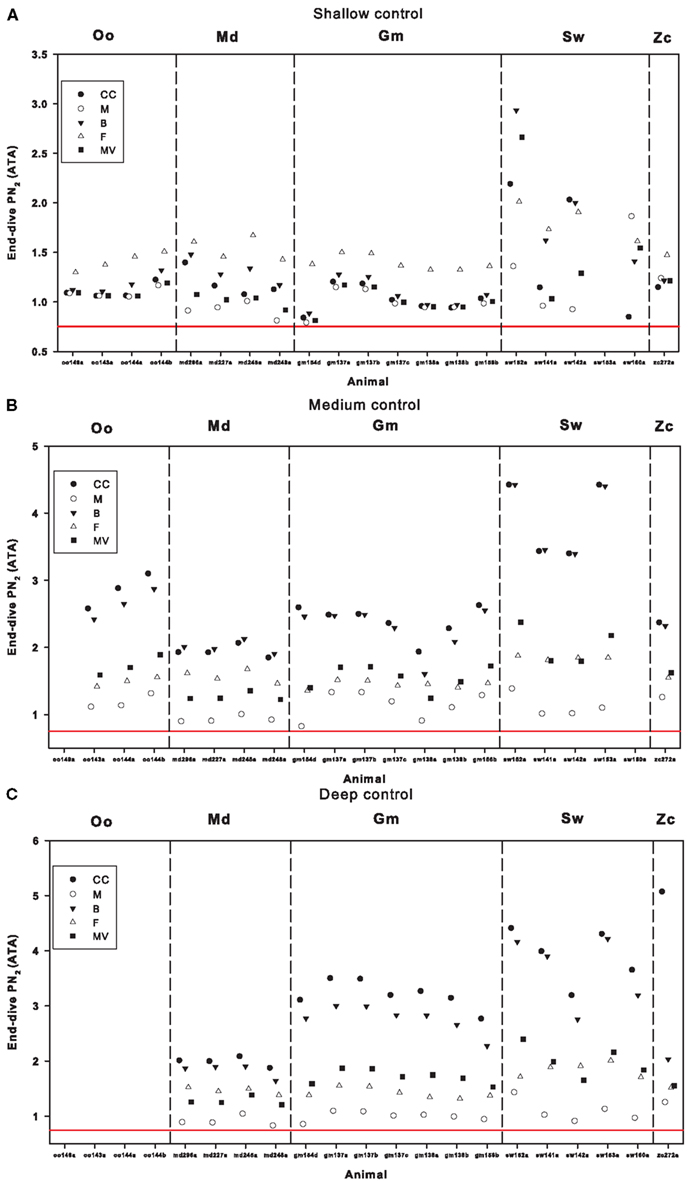
Figure 2. End-dive tissue and blood N2 tension following (A) shallow- (<30 m), (B) intermediate- (>30 m but <200 m), or (C) deep (>200 m) dives in the pre-exposure control period for killer whales (Oo), Blainville’s beaked whales (Md), pilot whales (Gm), sperm whales (Sw), and Cuvier’s beaked whale (Zc). Values are given for different tissue compartments; central circulation (CC), muscle (M), brain (B), fat (F), and mixed venous (MV). The red line at 0.75 ATA indicate 100% saturation at the surface (i.e., no risk of DCS).
Changes in Risk of DCS during LFAS Sonar Exposure
The maximum change in risk of DCS (R, Eq.1) during exposure as compared with the pre-exposure period is shown in Figure 3 on the left hand panels, for shallow, intermediate, and deep dives. For shallow dives the changes in R were not consistent and very minor for the killer whales, pilot whales and for all but one sperm whale. R decreased significantly for sperm whale sw08_152a during the sonar exposure, but there is very few shallow dives in this record and this might therefore be a coincidence. When the dive response was removed during sonar exposure, R increased somewhat for three of the four sperm whales, but decreased for the fourth one. For dives to intermediate depth, R decreased for the killer whales oo09_144a and oo09_144b, and removal of the dive response further decreased R for oo09_144a. For the pilot whale gm08_154d and the sperm whales sw08_152a and sw09_141a, removal of the dive response during sonar increased R. During deep dives, the behavior caused varying changes in R for the sperm whales and removal of the dive response increased R.
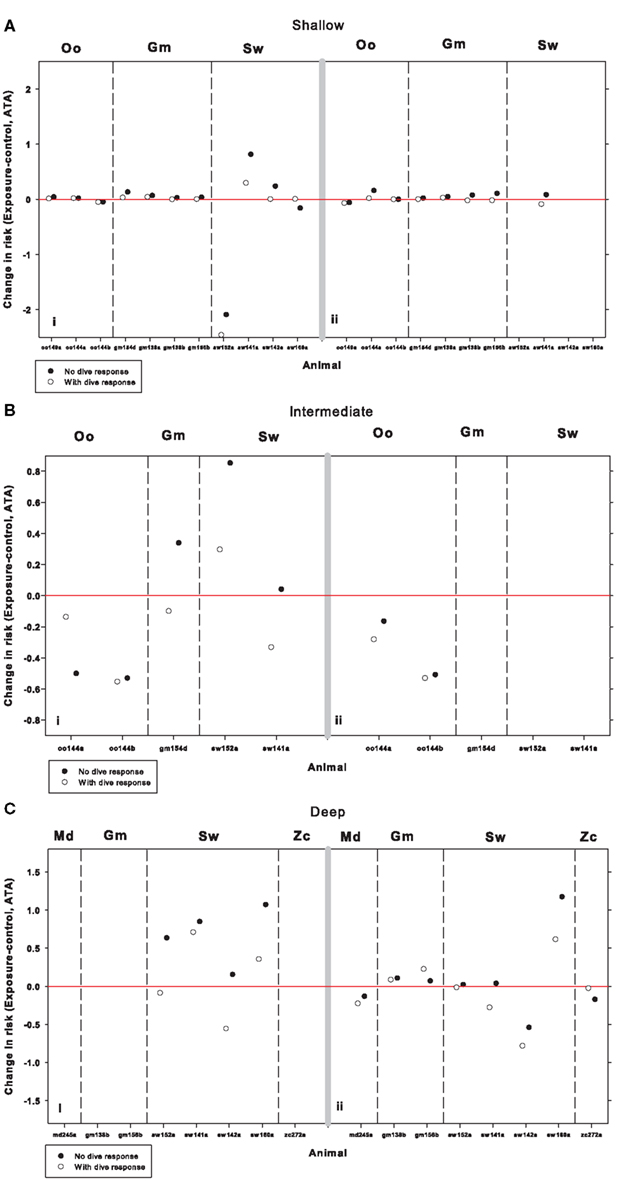
Figure 3. Change in risk of DCS (R, Eq. 1) during sonar exposure as compared with pre-sonar control period during (A) shallow-, (B) intermediate-, and (C) deep-dives for killer whales (Oo), pilot whales (Gm), and sperm whales (Sw). The left panels are LFAS exposures (i) and right panel MFAS exposures (ii). Open symbols indicate model output assuming normal physiological dive response, and solid symbols indicate model output when assuming a hypothetical removal of the dive response (no reduction in cardiac output and no redistribution of blood flow) in addition to the behavioral response during sonar exposure. Risk is defined as the end-dive mixed venous N2 tension minus the ambient N2 tension (Eq. 1). The red line indicates zero change in risk.
Changes in Risk of DCS during MFAS Sonar Exposure
For shallow dives, there was large variation in risk of DCS (R, Eq.1), and overall R decreased during MFAS exposure (Figure 3). However, removal of the dive response increased R for oo09_144a, gm09_138b, gm09_156b, and sw09_141a. For intermediate dives, the change in behavior reduced R and only a slight effect was noticed in oo09_144a when the dive response was removed during sonar exposure. For the deep dives, MFAS exposure mostly caused a slight decrease in R in all species, but removal of the dive response increased R, especially for sw09_160a.
Changes in Risk of DCS in “Behavioral Responders”
Typical examples of dive records of behavioral “responders” performing typical change in dive behavior in response to sonar are presented in Figure 1 for each of the studied species. Of 13 whales exposed to LFAS and/or MFAS, 10 showed a change in dive behavior apparently in response to the sonar (Table 1). This response varied from unusual slow or disrupted ascents of the deep divers to complete shifts from deep dive to shallow dive mode seen in pilot whales and killer whales (Figure 1). Except for the sperm whales reported to respond to sonar by shallower deep diving, R decreased during sonar exposure in all behavioral “responders” (Figure 4). In sw09_141a during LFAS exposure and for sw09_160a during both LFAS and MFAS exposure both maximum and average R increased (Figure 4).
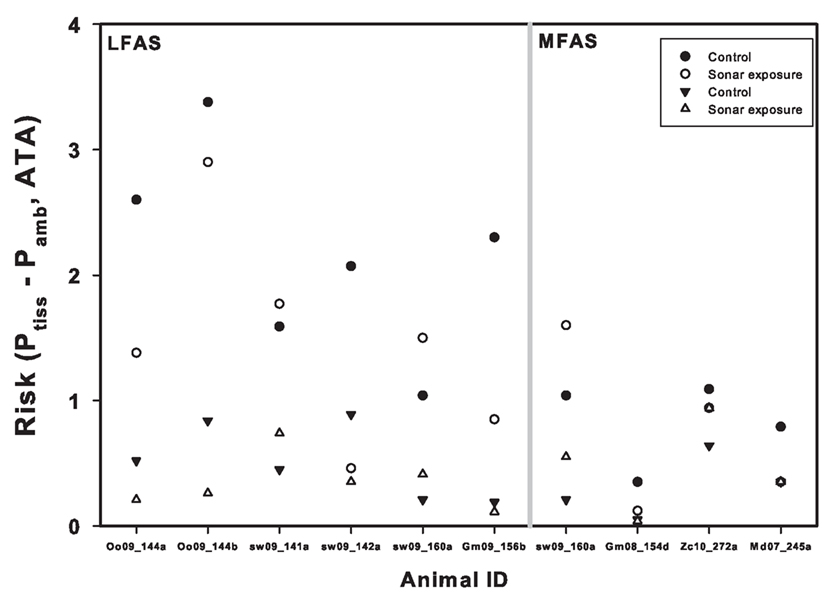
Figure 4. Average (▽) and maximum (◯) risk of DCS (R, Eq. 1) during pre-sonar control (solid symbols) and sonar exposure periods (open symbols) in behavioral “responders”. LFAS (left) and MFAS (right). Killer whales (Oo), pilot whales (Gm), sperm whales (Sw), Blainville’s beaked whales (Md), and Cuvier’s beaked whales (Zc). Risk is defined as the end-dive mixed venous N2 tension minus the ambient N2 tension (Eq. 1).
Discussion
Our model estimates suggest that shallow (killer whales), intermediate (pilot whales) and deep diving whales (sperm whales, Cuvier’s beaked whale, and Blainville’s beaked whale) all live with high blood and tissue levels, but the deep divers seem to experience the most extreme values (Figure 2). The deep diving sperm whales which respond to sonar exposure by shallower but still deep diving, were found to increase risk of DCS (R, Eq. 1), but not beyond the normal risk range of sperm whales. We found no systematic changes in R during sonar exposure in the other species, thus for some animals R appeared to increase slightly, while for others it decreased. However, the variation in R increased with dive depth. Also, removal of the dive response during sonar exposure increased R for most whales except in a few instances, e.g., oo09_144a during LFAS exposure, but also increased the variation of R.
Effect of Dive Depth on End-Dive Levels
We have shown that the estimated end-dive values increased with maximum dive depth (Figure 2). The largest increase in levels between dive categories happens between the shallow and intermediate dives, with only a moderate further increase in some tissues between intermediate and deep dives. Shallow dives (1–30 m) includes the decompression depth zone where tissue and blood exceed the ambient partial pressure of N2 and the direction of N2 flux is therefore from the blood into the lung (N2 removal; Fahlman et al., 2007). Intermediate dives (30–200 m) extend into the compression depth zone where pulmonary exchange still occurs (Hooker et al., 2009), but ambient pressure now exceeds tissue and blood and therefore the direction of N2 flux changes and N2 is now being absorbed. However, in this region depth related pulmonary shunting begins to impede gas exchange (Kooyman and Sinnett, 1982; Bostrom et al., 2008). Thus, variation in dive behavior and physiological responses may cause large variation in end-dive tissue and blood in this zone. The deep dives (>200 m) extend into the no-compression depth zone where lungs are completely collapsed and gas exchange ceased (Bostrom et al., 2008; Fahlman et al., 2009). Consequently, the total body N2 load will be determined by the ratio of time spent within the compression zone and the decompression zone, whereas time spent into the no-compression zone will not add to the total body N2 load, but may allow time for redistribution of N2 between different tissues.
For shallow dives, end-dive mixed venous ranged between 0.8 ATA for pilot whales to values >1.5 ATA for sperm whales. For dives to medium and deep depths, mixed venous were >1.0 ATA for all whales (Figure 2) and were close to or exceeding 2 ATA for the sperm whale and pilot whales. Although difficult to compare directly because of differences in how shallow dives where defined, these results appear to disagree with the suggestion made by Zimmer and Tyack (2007) that shallow dives increase the risk of inert gas bubbles and DCS. One possible reason for these divergent results could be related to the different assumptions on how pulmonary gas exchange is altered during diving. Empirical data in both the California sea lion and harbor seal have indicated that a pulmonary shunt develops that is related to the dive depth and diving lung volume (Kooyman and Sinnett, 1982). Despite this, previous studies made the simplistic assumption that gas exchange was perfusion limited until the alveoli collapsed, and the collapse depth was assumed to be at a pre-determined depth, e.g., 70 m (Fahlman et al., 2006; Zimmer and Tyack, 2007). It was suggested that this was a conservative approach and considered a worst-case scenario. More recent work has developed a model that predicts air volumes in the upper and lower airways, based on the structural properties of the respiratory system (Bostrom et al., 2008). The lung compression model was later coupled with the empirically derived pulmonary shunt data for pinnipeds (Kooyman and Sinnett, 1982). This made it possible to include the effect of pressure and diving lung volume on gas exchange (Fahlman et al., 2009; Hooker et al., 2009). When the lung compression/pulmonary shunt model was included in gas exchange models, the models output agreed well with measured blood and tissue N2, CO2 and O2 levels (Fahlman et al., 2009). The differences in model estimates vary substantially with these varying assumptions in gas exchange models used (Fahlman et al., 2009) and may be one reason for the divergent results.
Effect of Body Mass on End-Dive Levels
A previous study showed a positive correlation between predicted end-dive and body mass, when the body mass was varied for each species (Hooker et al., 2009). However, when the tissue and blood levels were estimated with the species-specific body mass, there were little differences in predicted N2 levels between species. It was suggested that these results may indicate behavioral adjustments within each species that limits the end-dive (Hooker et al., 2009). In the current study, there were no clear differences in end-dive blood or tissue with animal size (body mass), not even between the expert deep divers (sperm whale and beaked whales). However, the variation in estimated values was much greater in sperm whales at all depths (Figure 2).
Changes in Risk of DCS due to Behavioral Responses to Sonar
The behavioral responses to sonar differed both within species and between the species in this study. The beaked whales (Md and Zc) displayed an unusually slow ascent from the deep dive (Southall et al., 2011; Tyack et al., 2011), while sperm whales tended to continue deep diving during exposure, but shallower than before (Sivle et al., submitted). Pilot whales are intermediate divers and killer whales shallow divers as compared with the expert deep diving sperm- and beaked whales. Pilot whales typically perform bouts of relatively deep dives in between periods of very shallow diving (Sivle et al., submitted). Sonar responses in killer whales and pilot whales that were in deep diving mode prior to exposure typically involved a shift to shallow diving mode, but the shallow dives also became deeper than during normal undisturbed shallow diving (Sivle et al., submitted). These differences in response is probably largely explained by differences between species, but could also partly be explained by differences in the experimental procedures. Sperm whales, pilot whales and killer whales were all exposed using the same protocol (Miller et al., 2011), involving multiple exposures in a random behavioral context (feeding, resting, traveling) using a moving source. The beaked whales were exposed using a different and stationary source, and exposures were always conducted in a fixed behavioral context during deep feeding dives (Southall et al., 2011; Tyack et al., 2011).
Beaked Whales
Zimmer and Tyack (2007) reported that increased ascent rates from deep dives would decrease end-dive . The actual observed response of Zc and Md to sonar was an unusually slow ascent (Southall et al., 2011; Tyack et al., 2011; Figure 1), and this could increase R because of the additional time spent in the compression zone. However, theoretical studies have suggested that a reduced ascent rate in the decompression zone coupled with a pre-surface tachycardia may reduce end-dive by as much as 45% (Fahlman et al., 2006). Our results indicate that even without this physiological adjustment the actual observed decrease in ascent rate resulted in a slightly decreased R (Figure 4 ).
Sperm Whales
Sperm whales sw09_141a and sw09_160a were both reported to respond to the LFAS by continuing to perform deep dives, but the deep dives became shallower (Sivle et al., submitted; Figure 1). In the two animals which responded this way the shallower deep dives implied switching from dives at maximum depth of 1200–1500 m pre-exposure to about 300–400 m during exposure in sw09_160a (Figure 1), and from 250–400 m pre-exposure to only 50 m, during exposure in sw09_141a (Miller et al., 2011). Both these animals showed an increased R during LFAS exposure (Figures 3 and 4), while for the other two sperm whales, which did not respond by shallower deep dives, R did not increase (Figure 3). During the MFAS exposure, again the sw09_160a responded by shallower deep diving (Sivle et al., submitted) and again R increased (Figure 4), while for the other three, who did not display shallower deep diving, R did not increase. For sw09_160a the shallower deep dives during sonar exposure were still deep enough to extend well into the no-compression zone (300–400 m), but the descent phases of these dives were much slower than for the deep dives during pre-exposure (Figure 1). The increase in R is thereby explained by the increased time spent in the compression zone during the descent phase of these dives. The shallower deep diving response of sw09_141a to the LFAS exposure is similar to the hypothetical response described to result in higher R also in beaked whales by Zimmer and Tyack (2007). This animal switched from dives to depth well within the no-compression zone (250–400 m) to shallower dives during sonar exposure where most of the time was spent in the compression zone and never extended into the no-compression zone, and therefore resulted in increased R.
It has been proposed that the deep diving species are more at risk of suffering from decompression injury than shallower diving species (Hooker et al., 2009). Our results support this hypothesis. Even though the increase in R during sonar exposure was within the normal risk range of sperm whales, it is still a conspicuous observation that this increase happened in all three cases where the whales also changed dive behavior. Deep divers such as beaked whales and sperm whales probably push the physiological limits of diving in mammals and this might make them more vulnerable to human disturbance such as naval sonar (Hooker et al., 2012). Dysbaric osteonecrosis progressing with age has been reported in sperm whales (Moore and Early, 2004), and a recent study by Bernaldo de Quirós et al. (in press) showed that at necropsy of stranded animals there was a higher prevalence of gas bubbles in deep divers compared to non-deep divers.
Killer Whales and Pilot Whales
The change from deep dive mode to shallow diving mode in response to sonar seen in killer whales and pilot whales (Sivle et al., submitted; Figure 1), did not seem to increase the R (Figures 3 and 4). Indeed, an increase in R is not expected from such behavioral change since it implies that animals spend more time in the decompression zone, where N2 may be removed, instead of in the compression zone, where N2 is taken up. However, the deeper shallow dives which were also associated with this response could potentially increase R if they extended into the compression zone. Even if the dives were deeper (Sivle et al., submitted), they were still quite shallow (<10 m) and therefore probably still within the decompression zone. Thus, our results showed no consistent change in R in killer and pilot whales. The responses seen in the behavioral “responders” indicate that R was actually reduced.
Changes in Risk of DCS due to Hypothetical Physiological Response to Sonar
It was previously suggested that the dive response may be useful to reduce N2 uptake during diving and thereby minimize R (Fahlman et al., 2006). The results in this study concur, as R increased for most whales when the dive response was hypothetically removed during sonar exposure, thereby increasing N2 uptake during the dive. Still, in a few occasions the elevated cardiac output reduced R. This agrees with more recent work that indicate that the diving bradycardia does not always reduce N2 levels during repeated diving, but that there are certain tissue time constants (τ) that should be avoided to reduce N2 levels (Fahlman et al., 2007; Hooker et al., 2009). Inert gas loading is probably managed through complex trade offs between physiological and behavioral responses (Hooker et al., 2012). If a behavioral response to an unanticipated acute threat (such as man-made noise) is perceived as more immediately critical than management of N2, it might result in decompression injury (Hooker et al., 2012). For example, metabolic demand limits the ability to adjust blood flow, and there is therefore a trade-off between the need to supply sufficient O2 and reducing CO2 and N2 accumulation. As the cardiac output and blood flow distribution alter the tissue time constant (see Eq. 3 in Fahlman et al., 2006), studies are required to determine the physiological responses in deep diving whales both during undisturbed condition and during sonar exposure.
Methodological Considerations
Using mathematical models to investigate complex problems offers important insight but is also limited in scope as models are only an abstraction of the real world. For example, the model used in this study uses a pre-determined blood flow at the surface and while diving, but it is known that the heart rate, and therefore most likely the cardiac output, changes throughout a dive (Thompson and Fedak, 1993; Ponganis et al., 1997). The estimates for some of the physiological variables in the model are also taken from studies on pinnipeds, and may differ for cetaceans. In addition, understanding the effect of pressure on gas exchange is rudimentary and recent studies have suggested that there may be species variation in the depth-dependent pulmonary shunt (Bostrom et al., 2008; Fahlman et al., 2011; Moore et al., 2011). While the parameter estimates and compartment sizes for this model were not always species specific, the model has been calibrated against known blood and tissue , PO2 and PCO2 values and resulted in good agreement between observed and predicted values (Fahlman et al., 2009). Furthermore, we have published several studies using this model where sensitivity analyses were conducted (Fahlman et al., 2006, 2007, 2009; Hooker et al., 2009). These sensitivity analysis consistently show that the variables that had the greatest impact on the model outcome were changes in rate of pulmonary gas exchange, cardiac output, and blood flow distribution with depth, all variables where data only exist in pinnipeds and shallow diving odontocetes. The results from these previous sensitivity analyses contributed to the hypotheses that sonar could cause a startle response which could affect blood flow and thereby risk of DCS. We therefore tested the effect of this potential response in the current study.
Previous theoretical studies have used hypothetical sonar-induced changes in both behavior and physiology to model blood and tissue (Hooker and Baird, 1999; Houser et al., 2001; Fahlman et al., 2006, 2009; Zimmer and Tyack, 2007), but this is the first attempt to estimate the changes during actual behavioral responses to sonar. The behavioral response data were collected to determine how different species respond to anthropogenic sound. Of special interest was to determine the behavioral responses to LFAS and MFAS sonar signals, as studies have suggested that their use is related to mass-strandings (Cox et al., 2006; D’Amico et al., 2009). Jepson et al. (2003) and Fernández et al. (2005) expanded on this correlation and suggested that sonar related strandings may be associated with in vivo bubble formation. The large variation in decompression risk in response to sonar exposure may partly be due to the experimental design, with relative short pre-exposure periods followed by short exposures. This design is chosen to generate dose response functions, where the key is to determine acoustic dose at the threshold of response (Miller et al., 2011; Tyack et al., 2011). In addition, for some animals the sonar exposures to MFAS and/or LFAS were repeated during a single tag deployment to investigate frequency specificity of responses and habituation or sensitization during repeated exposures (Miller et al., 2011).
The uptake and removal of inert gas is commonly modeled using single exponential models where the kinetics is determined by a time constant (τ) that determines the time to equilibrium. The time constant is physiologically relevant and related to the solubility of the gas and the blood flow rate (see Eq. 3 in Fahlman et al., 2006). For tissues with a high perfusion rate, e.g., heart and brain, τ is short and time to equilibrium faster than for other tissues. For a diving animal, this means that these tissues may experience extreme during the dive, but removal is also so fast that the supersaturation seldom reaches dangerous levels, and the tissue soon equilibrate when the animal has reached the surface (Kooyman et al., 1972; Fahlman et al., 2006; Houser et al., 2010). Slow tissues such as blubber, are those where the surface interval duration between repeated dives in a bout are too short for the tissues to return to equilibrium with the surface atmosphere. For these tissues, N2 slowly accumulates to reach considerable levels. It has also been suggested that these tissues may limit the length of a dive bout, and it may be those slow tissues that put animals at risk of developing DCS (Fahlman et al., 2007). Eventually, the animal will reach a state of quasi-equilibrium where the saturation state is more or less constant between dives, but the time to this equilibrium depends on the size, physiology, and dive behavior of the animal (Hooker et al., 2009). Thus, large animals and tissues with a long τ will take longer time to respond but they will also show less variation between dives. Therefore, to accurately estimate tissue and blood N2 levels, it is important to have data sets that contain a representative sample of the natural dive behavior and exposures that are long enough to clearly indicate the behavioral responses. The data used in the current study are therefore not ideal for modeling gas management. For the type of analysis conducted here a more optimal design would be to increase the duration of the pre-exposure and sonar exposure periods. In particular, larger animals and slow responding tissues (e.g., fat) have very long response times (Fahlman et al., 2006), and therefore short exposure durations may not allow for such tissues to reach maximum values.
Conclusion
We conclude that there is great variation in the behavioral responses to sonar exposure and in most cases the response does not increase decompression risk, but there may be certain situations where the risk is increased, such as the shallower deep dives seen in sperm whales. The hypothetical removal of dive response during sonar exposure increased the variation in risk of DCS (R, Eq. 1), suggesting that physiological responses to anthropogenic sound may lead to altered tissue and blood N2 levels. Cetaceans seem to live with natural high N2 levels, and since both behavioral and physiological responses have the potential to alter R, we have to assume that N2 levels are managed through complex interactions between behavioral and physiological responses. We therefore can not rule out the possibility that a combination of behavioral and physiological responses to sonar have the potential to alter the blood and tissue end-dive N2 tension to levels which could cause DCS. Our results support previous suggestions that deep divers might be more at risk of suffering from decompression injury than shallower diving species. As little is known concerning the physiological adjustments associated with diving in large whales, future work should improve our knowledge in these areas.
Conflict of Interest Statement
This study is mainly funded by three naval organizations; The US Office of Naval Research, The Norwegian Ministry of Defence, and the Netherlands Ministry of Defence. In addition the Norwegian Research Council, and WWF-Norway have also contributed financially. Funders had no role in study design, data analysis, or preparation of the manuscript. Authors are employed by government (Norwegian Defence Research Establishment and Institute of Marine Research), independent no-profit (Woods Hole Oceanographic Institution and Netherlands Organisation for Applied Scientific Research), or academic (University of St. Andrews and Texas A&M Corpus Christi) research organizations. No authors are employed by naval organizations.
Acknowledgments
We thank all colleagues involved in collecting the data at sea. This study was funded by the Norwegian Ministry of Defence, US-Office of Naval Research, the Netherlands Ministry of Defence, the Norwegian Research Council and WWF-Norway. We thank Ann Allen for help with getting the dive data. We thank Brandon Southall and the SOCAL team for giving us access to their beaked whale data.
References
Aguilar Soto, N., Johnson, M. P., Madsen, P. T., Diaz, F., Dominguez, I., Brito, A., and Tyack, P. (2008). Cheetahs of the deep sea: deep foraging sprints in short-finned pilot whales off Tenerife (Canary Islands). J. Anim. Ecol. 77, 936–947.
Baird, R. W., Borsani, J. F., Hanson, M. B., and Tyack, P. L. (2002). Diving and night time behavior of long-finned pilot whales in the Ligurian Sea. Mar. Ecol. Prog. Ser. 237, 301–305.
Baird, R. W., Hanson, M. B., and Dill, L. M. (2005). Factors influencing the diving behaviour of fish-eating killer whales: sex differences and diel and interannual variation in diving rates. Can. J. Zool. 83, 257–267.
Berghage, T. E., David, T. D., and Dyson, C. V. (1979). Species differences in decompression. Undersea Biomed. Res. 6, 1–13.
Bernaldo de Quirós, Y., Gonzales-Diaz, O., Arbelo, M., Sierra, E., Sacchini, S., and Fernández, A. (in press). Decompression versus decomposition: distribution, quantity and gas composition of bubbles in stranded marine mammals. Front. Aquat. Physiol.
Bostrom, B. L., Fahlman, A., and Jones, D. R. (2008). Tracheal compression delays alveolar collapse during deep diving in marine mammals. Respir. Physiol. Neurobiol. 161, 298–305.
Butler, P. J., and Jones, D. R. (1997). Physiology of diving birds and mammals. Physiol. Rev. 77, 837–899.
Clark, S. T., Odell, D. K., and Lacinak, C. T. (2000). Aspects of growth in captive killer whales (Orcinus orca). Mar. Mamm. Sci. 16, 110–123.
Cox, T. M., Ragen, T. J., Read, A. J., Vos, E., Baird, R. W., Balcomb, K., Barlow, J., Caldwell, J., Cranford, T., Crum, L., D’amico, A., D’spain, G., Fernández, A., Finneran, J., Gentry, R., Gerth, W., Gulland, F., Hildebrand, J., Houser, D., Hullar, T., Jepson, P. D., Ketten, D. R., Macleod, C. D., Miller, P., Moore, S., Mountain, D., Palka, D., Ponganis, P., Rommel, S., Rowles, T., Taylor, B., Tyack, P., Wartzok, D., Gisiner, R., Mead, J., and Benner, L. (2006). Understanding the impacts of anthropogenic sound on beaked whales. J. Cetacean Res. Manag. 7, 177–187.
Crum, L. A., and Mao, Y. (1996). Acoustically enhanced bubble growth at low frequencies and its implications for human diver and marine mammal safety. J. Acoust. Soc. Am. 99, 2898–2907.
D’Amico, A. D., Gisiner, R., Ketten, D. R., Hammock, J. A., Johnson, C., Tyack, P., and Mead, J. (2009). Beaked whale strandings and naval exercises. Aquat. Mamm. 35, 452–472.
Davis, R. W., and Kanatous, S. B. (1999). Convective oxygen transport and tissue oxygen consumption in Weddell seals during aerobic dives. J. Exp. Biol. 202, 1091–1113.
Dolar, M. L. L., Suarez, P., Ponganis, P. J., and Kooyman, G. L. (1999). Myoglobin in pelagic small cetaceans. J. Exp. Biol. 202, 227–236.
Fahlman, A., Hooker, S. K., Olszowka, A., Bostrom, B. L., and Jones, D. R. (2009). Estimating the effect of lung collapse and pulmonary shunt on gas exchange during breath-hold diving: the Scholander and Kooyman legacy. Respir. Physiol. Neurobiol. 165, 28–39.
Fahlman, A., Loring, S. H., Ferrigno, M., Moore, C., Early, G., Niemeyer, M., Lentell, B., Wenzel, F., Joy, R., and Moore, M. J. (2011). Inflation and deflation pressure–volume loops in breath-hold diving marine mammals. J. Exp. Biol. 214, 3822–3828.
Fahlman, A., Olszowka, A., Bostrom, B., and Jones, D. R. (2006). Deep diving mammals: dive behavior and circulatory adjustments contribute to bends avoidance. Respir. Physiol. Neurobiol. 153, 66–77.
Fahlman, A., Schmidt, A., Jones, D. R., Bostrom, B. L., and Handrich, Y. (2007). To what extent does N2 limit dive performance in king penguins? J. Exp. Biol. 210, 3344–3355.
Fahlman, A., Svärd, C., Rosen, D. A. S., Jones, D. R., and Trites, A. W. (2008). Metabolic costs of foraging and the management of O2 and CO2 stores in Steller sea lions. J. Exp. Biol. 211, 3573–3580.
Fernández, A., Edwards, J. F., Rodriguez, F., Espinosa De Los Monteros, A., Herraez, P., Castro, P., Jaber, J. R., Martin, V., and Arbelo, M. (2005). Gas and fat embolic syndrome involving a mass stranding of beaked whales (family ziphiidae) exposed to anthropogenic sonar signals. Vet. Pathol. 42, 446–457.
Higgs, N. D., Little, C. T. S., and Glover, A. G. (2010). Bones as biofuel: a review of whale bone composition with implications for deep-sea biology and palaeoanthropology. Proc. R. Soc. Lon. B Biol. Sci. 278, 9–17.
Hooker, S. K., and Baird, R. W. (1999). Deep-diving behaviour of the northern bottlenose whale, Hyperoodon ampullatus (cetacea: ziphiidae). Proc. R. Soc. Lon. B Biol. Sci. 266, 671–676.
Hooker, S. K., Baird, R. W., and Fahlman, A. (2009). Could beaked whales get the bends? Effect of diving behaviour and physiology on modelled gas exchange for three species: Ziphius cavirostris, Mesoplodon densirostris and Hyperoodon ampullatus. Respir. Physiol. Neurobiol. 167, 235–246.
Hooker, S. K., Fahlman, A., Moore, M. J., Aguilar De Soto, N., Bernaldo De Quiros, Y., Brubakk, A. O., Costa, D. P., Costidis, A. M., Dennison, S., Falke, K. J., Fernandez, A., Ferrigno, M., Fitz-Clarke, J. R., Garner, M. M., Houser, D. S., Jepson, P. D., Ketten, D. R., Kvadsheim, P. H., Madsen, P. T., Pollock, N. W., Rotstein, D. S., Rowles, T. K., Simmons, S. E., Van Bonn, W., Weathersby, P. K., Weise, M. J., Williams, T. M., and Tyack, P. L. (2012). Deadly diving? Physiological and behavioural management of decompression stress in diving mammals. Proc. R. Soc. Lon. B Biol. Sci. 279, 1041–1050.
Houser, D. S., Dankiewicz-Talmadge, L. A., Stockard, T. K., and Ponganis, P. J. (2010). Investigation of the potential for vascular bubble formation in a repetitively diving dolphin. J. Exp. Biol. 213, 52–62.
Houser, D. S., Howard, R., and Ridgway, S. (2001). Can diving-induced tissue nitrogen supersaturation increase the chance of acoustically driven bubble growth in marine mammals? J. Theor. Biol. 213, 183–195.
Jepson, P. D., Arbelo, M., Deaville, R., Patterson, I. A. P., Castro, P., Baker, J. R., Degollada, E., Ross, H. M., Herraez, P., Pocknell, A. M., Rodriguez, F., Howie, F. E., Espinosa, A., Reid, R. J., Jaber, J. R., Martin, V., Cunningham, A. A., and Fernandez, A. (2003). Gas-bubble lesions in stranded cetaceans. Nature 425, 575–576.
Johnson, M., and Tyack, P. L. (2003). A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Ocean Eng. 28, 3–12.
Kooyman, G. L., Schroeder, J. P., Denison, D. M., Hammond, D. D., Wright, J. J., and Bergman, W. P. (1972). Blood nitrogen tensions of seals during simulated deep dives. Am. J. Physiol. 223, 1016–1020.
Kooyman, G. L., and Sinnett, E. E. (1982). Pulmonary shunts in Harbor sels and sea lions during simulated dives to depth. Physiol. Zool. 55, 105–111.
Lockyer, C. (1991). Body composition of the sperm whale, Physter catodon, with special references to the possible functions of fat depots. Rit Fiskidelidar 12, 1–12.
McAlpine, D. F. (1985). Size and growth of heart, liver, and kidneys in North Atlantic fin (Balaenoptera physalus), sei (B. borealis), and sperm (Physeter macrocephalus) whales. Can. J. Zool. 63, 1402–1409.
Miller, P. J., Johnson, M. P., Tyack, P. L., and Terray, E. A. (2004). Swimming gaits, passive drag and buoyancy of diving sperm whales Physeter macrocephalus. J. Exp. Biol. 207, 1953–1967.
Miller, P. J. O., Antunes, R., Alves, A. C., Wensveen, P., Kvadsheim, P. H. L. K., Nordlund, N., Lam, F. P., Ijsselmuide, S., Visser, F., and Tyack, P. (2011). The 3S experiments: studying the behavioral effects of sonar on killer whales (Orcinus orca), sperm whales (Physeter macrocephalus), and long-finned pilot whales (Globicephala melas) in Norwegian waters. Scottich Ocean Inst. Tech. Rept. SOI-2011–2011.
Miller, P. J. O., Shapiro, A. D., and Deecke, V. B. (2010). The diving behaviour of mammal-eating killer whales (Orcinus orca): variations with ecological not physiological factors. Can. J. Zool. 88, 1103–1112.
Moore, M. J., and Early, G. A. (2004). Cumulative sperm whale bone damage and the bends. Science 306, 2215.
Moore, M. J., Hammar, T., Arruda, J., Cramer, S., Dennison, S., Montie, E., and Fahlman, A. (2011). Hyperbaric computed tomographic measurement of lung compression in seals and dolphins. J. Exp. Biol. 214, 2390–2397.
Omura, H. (1950). “On the body weight of sperm and sei whales located in the adjacent waters of Japa,” in Scientific Reports of the Whales Research Institute, 1–13.
Ponganis, P. J., Kooyman, G. L., Winter, L. M., and Starke, L. N. (1997). Heart rate and plasma lactate responses during submerged swimming and trained diving in California sea lions, Zalophus californianus. J. Comp. Physiol. B 167, 9–16.
Rice, D. W. (1989). “Sperm whale- Physeter macrocephalus Linneaus 1758,” in Handbook of Marine Mammals-River Dolphins and the Larger Toothed Whales, eds S. H. Ridgway, and R. J. Harrison (London: Academic Press), 177–233.
Southall, B., Calambokidis, J., Tyack, P., Moretti, D., Hildebrand, J., Kyburg, C., Carlson, R., Friedlander, A., Falcone, E., Schorr, G., Douglas, A., Deruiter, S., Goldbogen, J., and Barlow, J. (2011). “Biological and behavioral responses of marine mammals in southern California 2010 (SOCAL-10).” SOCAL-10 project report.
Stephenson, R. (2005). Physiological control of diving behaviour in the Weddell seal Leptonychotes weddelli: a model based on cardiorespiratory control theory. J. Exp. Biol. 208, 1971–1991.
Thompson, D., and Fedak, M. A. (1993). Cardiac responses of grey seals during diving at sea. J. Exp. Biol. 174, 139–154.
Tyack, P. L., Johnson, M., Soto, N. A., Sturlese, A., and Madsen, P. T. (2006). Extreme diving of beaked whales. J. Exp. Biol. 209, 4238–4253.
Tyack, P. L., Zimmer, W. M., Moretti, D., Southall, B. L., Claridge, D. E., Durban, J. W., Clark, C. W., D’amico, A., Dimarzio, N., Jarvis, S., Mccarthy, E., Morrissey, R., Ward, J., and Boyd, I. L. (2011). Beaked whales respond to simulated and actual navy sonar. PLoS ONE 6, e17009.
Watwood, S., Miller, P. J. O., Johnson, M., Madsen, P. T., and Tyack, P. L. (2006). Deep-diving foraging behaviour of the sperm whale, Physeter macrocephalus. J. Anim. Ecol. 75, 814–825.
Weathersby, P. K., and Homer, L. (1980). Solubility of inert gases in biological fluids and tissues: a review. Undersea Biomed. Res. 7, 277–296.
Weathersby, P. K., Homer, L. D., and Flynn, E. T. (1984). On the likelihood of decompression sickness. J. Appl. Physiol. 57, 815–825.
Keywords: decompression sickness, diving physiology, marine mammals, gas exchange, modeling
Citation: Kvadsheim PH, Miller PJO, Tyack PL, Sivle LD, Lam FPA, and Fahlman A (2012) Estimated tissue and blood N2 levels and risk of decompression sickness in deep-, intermediate-, and shallow-diving toothed whales during exposure to naval sonar. Front. Physio. 3:125. doi: 10.3389/fphys.2012.00125
Received: 03 February 2012; Accepted: 14 April 2012;
Published online: 10 May 2012.
Edited by:
Michael Castellini, University of Alaska Fairbanks, USAReviewed by:
Stephen J. Trumble, Baylor University, USAMelinda Fowler, University of California Santa Cruz, USA
Copyright: © 2012 Kvadsheim, Miller, Tyack, Sivle, Lam and Fahlman. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: A. Fahlman, Department of Life Sciences, Texas A&M Corpus Christi, Corpus Christi, TX 78412, USA. e-mail: andreas.fahlman@tamucc.edu
